Abstract
Pulmonary artery smooth muscle cells (PASMCs) and pericytes are NG2+ mural cells that provide structural support to pulmonary arteries and capillaries. In pulmonary arterial hypertension (PAH), both mural cell types contribute to PA muscularization, but whether similar mechanisms are responsible for their behavior is unknown. RNA-seq was used to compare the gene profile of pericytes and PASMCs from PAH and healthy lungs. NG2-Cre-ER mice were used to generate NG2-selective reporter mice (NG2tdT) for cell lineage identification and tamoxifen-inducible mice for NG2-selective SDF1 knockout (SDF1NG2-KO). Hierarchical clustering of RNA-seq data demonstrated that the genetic profile of PAH pericytes and PASMCs is highly similar. Cellular lineage staining studies on NG2tdT mice in chronic hypoxia showed that, similar to PAH, tdT+ cells accumulate in muscularized microvessels and demonstrate significant upregulation of SDF1, a chemokine involved in chemotaxis and angiogenesis. Compared with control mice, SDF1NG2-KO mice in chronic hypoxia had reduced muscularization and lower abundance of NG2+ cells around microvessels. SDF1 stimulation in healthy pericytes induced greater contractility and impaired their capacity to establish endothelial–pericyte communications. In contrast, SDF1 knockdown reduced PAH pericyte contractility and improved their capacity to associate with vascular tubes in coculture. SDF1 is upregulated in NG2+ mural cells and is associated with PA muscularization. Targeting SDF1 could help prevent and/or reverse muscularization in PAH.
Keywords: pulmonary arterial hypertension, pericytes, smooth muscle cells, vascular remodeling, stromal cell–derived factor-1
Clinical Relevance
Pulmonary artery smooth muscle cells (PASMCs) and pericytes are NG2+ mural cells that provide structural support and coordinate vascular repair after injury. Studies have shown that pulmonary arterial hypertension (PAH) PASMCs and PAH pericytes are abundant in muscularized vessels and display higher rates of proliferation and contractility. These data led us to speculate that PASMCs and pericytes may share common molecular mechanisms responsible for their abnormal behavior in PAH. Compared to healthy controls, PAH pericytes and PASMCs exhibit a significant overlap in gene expression patterns. SDF1, a chemokine involved in chemotaxis and angiogenesis, was highly expressed in both PAH PASMCs and PAH pericytes, suggesting a pivotal role in PAH mural cell behavior. NG2-selective SDF1 knockdown was protective against hypoxia-induced pulmonary hypertension in mice. In culture, SDF1 stimulation can trigger hypercontractility and hyperproliferation of healthy pericytes, whereas its knockdown can partially normalize PAH pericyte behavior. We believe that SDF1 is responsible for mural cell dysfunction, and pharmacological inhibition could help prevent and reverse muscularization of small distal vessels in PAH.
Pulmonary arterial hypertension (PAH) is a life-threatening disease characterized by abnormally elevated pulmonary pressures and progressive right heart failure that leads to premature death (1). A major pathological hallmark of PAH is the combined loss and muscularization of microvessels (<50 μm), resulting in their progressive narrowing and reduced perfusion to alveoli (2). At present, there are no disease-modifying therapies for PAH, partly because of the incomplete understanding of mechanisms that orchestrate vessel regeneration and remodeling.
Although the contribution of pulmonary artery smooth muscle cells (PASMCs) to PAH is well established, we are just starting to understand the role that pericytes play in pulmonary vascular remodeling. Pericytes are highly specialized mural cells that directly interact with endothelial cells (ECs) to provide support and promote vessel maturity (3, 4). Lung pericytes are found at precapillary arteries, capillaries, and venules, where they regulate vascular tone and preserve endothelial viability through physical contact and chemokine production (5). As mesenchymal cells, pericytes and PASMCs likely share a common developmental origin, as evidenced by the expression of mural cell surface markers such as NG2 (i.e., chondroitin sulfate proteoglycan 4) and PDGFRβ (6, 7).
Our group and others have shown that the dissociation of pericytes from capillaries and their relocation to muscularized precapillary arterioles is a pathological feature in PAH. Pericyte detection in explanted PAH lungs has shown that most pericytes are relocated to small and medium-sized remodeled pulmonary microvessels (8, 9). Our studies show that PAH pericytes have reduced capacity to associate with pulmonary microvascular endothelial cells because of endogenous deficiencies in Wnt signaling (9, 10). Importantly, these changes correlate with persistent loss of small vessels and redistribution of pericytes to muscularized precapillary arteries, a pathology that is reminiscent of that seen in patients with PAH (2). Our findings have led us to propose a model by which the establishment of endothelial–pericyte interactions is critical for vascular repair.
Our group and others have also documented major similarities between PAH pericytes and PASMCs. Compared with the quiescent phenotype seen in healthy vessels, PAH pericytes are more invasive and exhibit hyperproliferation, apoptosis resistance, and hypercontractility (8, 11). We linked hyperproliferation of PAH pericytes to a metabolic switch to glycolysis (i.e., the Warburg effect) through upregulation of PDK4, an enzyme that reduces acetyl-CoA production. PDK isoforms are also upregulated in PAH PASMCs and drive hyperproliferation through suppression of apoptosis and activation of multiple trophic pathways (12, 13). Given the similarities between PAH pericytes and PASMCs, it is possible that these mural cells act together as part of a pathological response responsible for muscularization and remodeling of precapillary arterioles in PAH.
In this study, we used RNA sequencing (RNA-seq) and cell lineage tracing approaches to show that PAH pericytes and PASMCs share similar transcriptomic profiles and that overexpression of SDF1, a chemokine involved in pathological vascular remodeling and angiogenesis, is associated with mural cell–dependent pulmonary artery (PA) muscularization, pericyte hyperproliferation, and hypercontractility.
Methods
An expanded Methods section containing a detailed description of animals, reagents, techniques, and assays is provided in the data supplement.
Results
RNA-seq Analysis Demonstrates Similar Gene Profiles in PAH Pericytes and PASMCs
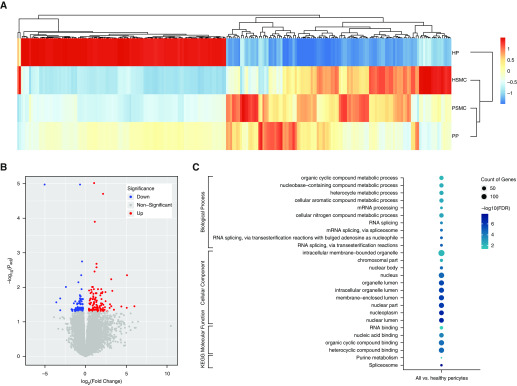
RNA-seq transcriptomic profiling was performed on RNA isolated from healthy donor and PAH PASMCs (n = 3 per group) and pericytes (n = 3 per group) (see Table E1 in the data supplement for patient information). Hierarchical clustering of gene datasets via DEseq2 R package revealed that healthy donor PASMCs have a different transcriptional profile compared with healthy donor pericytes. In contrast, there was more similarity in the transcriptomic profiles of PAH pericytes and PASMCs, as evidenced by the clustering of gene expression patterns (Figure 1A). Volcano plot revealed a total 113 upregulated and 104 downregulated genes in PAH pericytes + healthy and PAH PASMCs versus healthy donor pericytes (Figure 1B; for a full list see Table E2). The commonly altered genes in PAH pericytes + healthy and PAH PASMCs versus healthy donor pericytes are also shown in the Venn diagram (Figure E1 and Table E3). Gene Ontology (GO) functional annotation and Kyoto Encyclopedia of Genes and Genomes pathway analysis using the Database for Annotation Visualization and Integrated Discovery bioinformatics resources demonstrated the enrichment of pathways involved in organelle lumen regulation, nuclear lumen, and nucleoplasm regulation metabolic processes (Figure 1C). Of note, a subset of highly expressed genes belonged to the spliceosome, nuclear machinery composed of small nuclear RNAs involved in mRNA maturation through splicing of pre-mRNA (14, 15). Interestingly, this transcriptional profile has also been documented in neoplasia and correlates with efforts to support metabolic processes and cell activity (16, 17).
Figure 1.
RNA sequencing (RNA-seq) analysis demonstrates similar gene profiles in pulmonary arterial hypertension (PAH) pericytes and pulmonary artery smooth muscle cells (PASMCs). (A) Hierarchical clustering of differentially expressed gene sets from healthy versus PAH pericytes and PASMCs (n = 3 per group). HP = healthy pericytes; HSMC = healthy PASMC; PP = PAH pericytes; PSMC = PAH PASMC. P < 0.05. (B) Volcano plot of differentially expressed genes in PAH pericytes and SMCs compared with healthy controls (FDR [false discovery rate] < 0.05). (C) Gene Ontology and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of differentially expressed genes.
Mice under Chronic Hypoxia Demonstrate Accumulation of NG2+ Mural Cells in Muscularized Microvessels
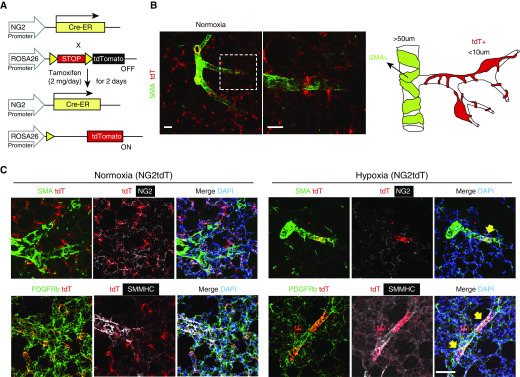
Lineage-tracing studies in mice have shown that resident lung progenitor cells in medium-sized muscularized vessels can act as a source of SMCs in the setting of chronic hypoxia (18, 19), but whether mural cells such as PASMCs and pericytes can also contribute to PA muscularization is unknown. To study mural cell response to hypoxia, we generated an NG2-selective reporter mouse by crossing a murine NG2-Cre-ER line with the ROSA26R-tdTomatoflox/flox (i.e., NG2tdT; Figure 2A) reporter line, such that a small dose of intraperitoneal tamoxifen selectively marks NG2 promoter-driven cells with red (tdT+) fluorescence (Figure 2B, see Methods for more details). NG2 is a mural cell marker expressed by both pericytes and PASMCs, although expression appears to be higher in pericytes (5). Use of confocal microscopy together with array tomography and scanning EM helped confirm mural cell identity of tdT+ cells through their location in the vessel wall and sharing of basement membrane with endothelial cells (Figure E2). In normoxia, most tdT+ cells are localized within the alveolar–capillary space, exhibit long cellular processes reminiscent of pericytes (Figure 2B, higher magnification panel), are α-smooth muscle actin (SMA) negative, and do not express the alveolar marker SPC (Figure E3 and Video E1). However, a small subset of spindle-shaped tdT+ cells is located in middle-sized muscularized (>100 μm) arteries that are SMA+ NG2+ and likely represent PASMCs (Figure 2B). Finally, we ruled out that tdT+ are ECs, because they do not express CD31 and are located outside the endothelial layer (Figure E4).
Figure 2.
Muscularization of distal arterioles with hypoxia in NG2tdT murine line. (A) Diagram showing the strategy for generation of NG2tdT mice. (B) The landscape of the NG2tdT lung with diagram. The dashed box represents the higher magnification of the area. Scale bars: 50 μm. (C) Left lung of normoxia and hypoxia NG2tdT was stained for SMA (SMC marker, green), NG2 (mural cell marker, white), PDGFRb (pericyte and SMC marker, green), SMMHC (SMC marker, white), and nuclei (DAPI, blue). Note that tdTomato (red) is a reporter color and marked with the lineage tag without antibody labeling. The yellow arrows indicate tdT+ cells cover areas of a small arteriole and are PDGFRb+SMMHC+. Scale bar: 50 μm. ER = estrogen receptor; tdT = tdTomato.
Having established the baseline tdT+ distribution, we exposed NG2tdT mice to 3 weeks of hypoxia, a well-established experimental model to induce small vessel muscularization and pulmonary hypertension (PH). After 3 weeks, tdT+ cells are found mostly around muscularized microvessels ∼20 μm in size (Figure 2C; yellow arrows). Close examination reveals that tdT+ cells in direct contact with muscularized microvessels have a less-elongated shape and shorter processes compared with tdT+ cells in normoxia. Given the location of tdT+ cells within muscularized microvessels, we wanted to test whether these cells also expressed mature SMC markers in addition to the mural cell markers NG2 and PDGFRβ. Indeed, tdT+ cells in normoxia are NG2+ PDGFRb+/SMMHC−SMA−, whereas those in hypoxia are NG2low PDGFRb+/SMMHC+SMA+ by immunostaining (Figure 2C). One interesting observation is that NG2 staining appears to be either negative or reduced under hypoxia compared with normoxia. Although we cannot conclude that capillary tdT+ cells become PASMCs under hypoxia, these findings support a significant level of plasticity in mural cells in response to stress.
RNA-seq Profile of Lung tdT+ Cells Reveals Significant Upregulation of SDF1 under Hypoxia
Our lineage staining model demonstrated that similar to PAH lungs, hypoxic NG2-tdT+ cells accumulate within muscularized vessels, raising the possibility that they participate in vascular remodeling. In an effort to identify candidate gene networks associated with the NG2+ tdT+ hypoxic response, we used FACS to isolate tdT+ cells from whole-lung suspensions of normoxic and hypoxic mice followed by RNA-seq analysis. Comparison of transcriptomic profiles of normoxia versus hypoxia tdT+ cells revealed that 292 genes demonstrated significant expression changes (Figure 3A), with a total of 94 upregulated and 198 downregulated genes in hypoxia compared with normoxia, as shown by volcano plot (Figure 3B; for a full list, see Table E4). Interestingly, GO term enrichment analysis demonstrated that many of these genes were involved in cell movement functions, such as locomotion, regulation of organelle transport, and localization, among others (Figure 3C).
Figure 3.
RNA-seq of tdT+ cells purified from normoxic versus hypoxic NG2tdT mice. (A) Heat map represents 13,504 gene expression (z scaled) pattern, which indicates the overall distinct transcriptome profiling between hypoxia (Hx) and normoxia (Norm) condition. (B) Volcano plot of 292 significantly expressed genes. The green circle highlights the coordinate for SDF1 (a.k.a. CXCL12) (log fold change: 1.1187, and −log10[adj P value] = 1.90806). (C) Gene Ontology enrichment performed using Gorilla (http://cbl-gorilla.cs.technion.ac.il/) with 292 significantly expressed genes as target genes and all 13,504 expressed genes as background. (D) SDF1 staining on NG2tdT lung slices in normoxia versus hypoxia. Lungs were stained for SMA (SMC marker, green), SDF1 (white), and nuclei (DAPI, blue). Scale bar: 50 μm.
As a next step, we sought to validate the biological relevance of candidate genes in our experimental system. First, we sought to find common genes that were affected in both human and mouse RNA-seq data. The commonly altered genes are summarized in Table E5. Second, among the highly expressed genes, we were intrigued by Sdf1 (a.k.a. Cxcl12), a cytokine involved in coordinating cellular responses to injury, including motility, angiogenesis, and cell differentiation (20–22). Importantly, SDF1 has recently been identified as a candidate gene responsible for PASMC proliferation and muscularization in animal models (23). In our RNA-seq, Sdf1 was upregulated significantly in hypoxia, with ∼1.12-fold change and a false discovery rate–adjusted P value of 0.012 (Table E6). Moreover, staining NG2tdT lung slices against SDF1 demonstrated that, compared with normoxia, hypoxic lungs exhibited a significant upregulation of SDF1 not only in tdT+ cells but also in muscularized vessels and across the parenchyma (Figure 3D). On the basis of these data, we decided to focus on expanding the role of SDF1 in orchestrating mural cell behavior under hypoxia.
Mice with NG2-Selective SDF1 Knockout Are Protected against Hypoxia-induced PH
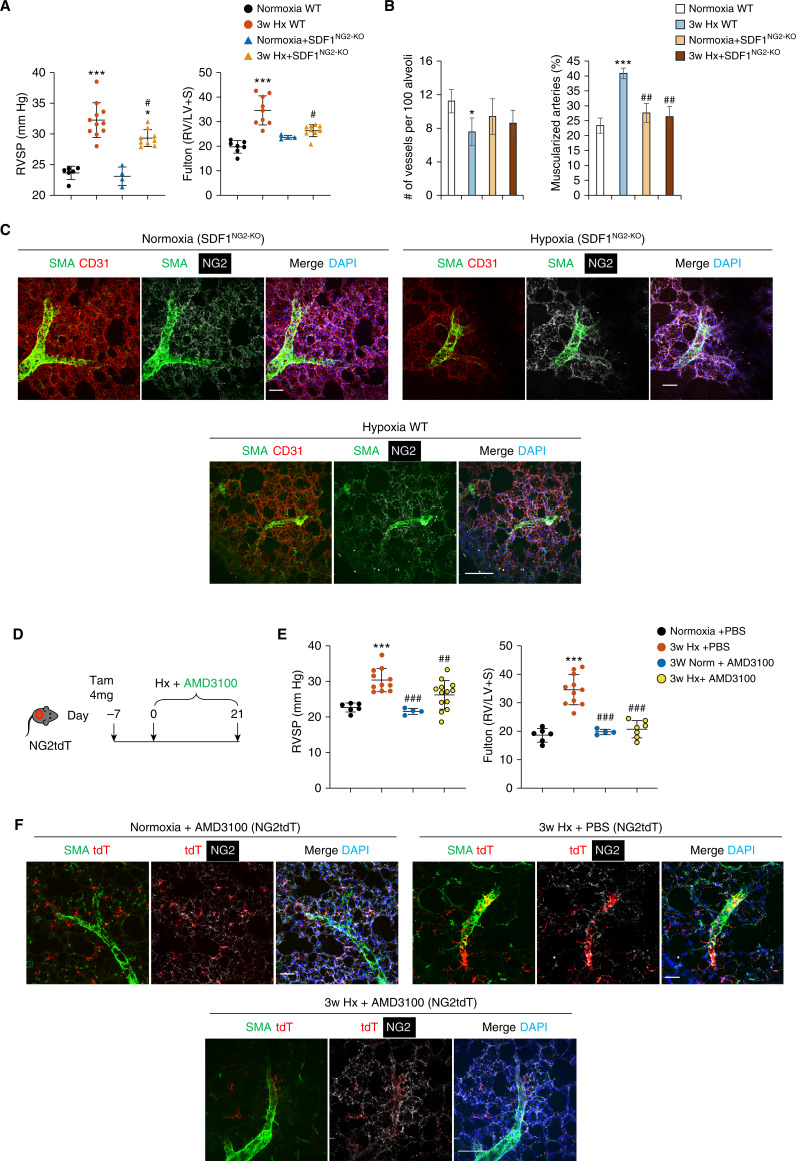
To assess the biological contribution of NG2-derived SDF1 to hypoxia-induced PA muscularization, we generated a conditional transgenic murine line by crossing our NG2-CreER with an SDF1flox/flox line (SDF1NG2-KO). After administration of tamoxifen, animals were allowed to rest for 7 days and then placed in hypoxia for 3 weeks, followed by hemodynamic and morphometric studies. The validation of knockout is shown in Figure E5. Although there were no significant differences between wild-type (WT) and SDF1NG2-KO in normoxia, hypoxic SDF1NG2-KO mice had significantly reduced right ventricular systolic pressure (RVSP) and no change of right ventricular (RV) hypertrophy compared with corresponding control mice (Figure 4A). Furthermore, hypoxic SDF1NG2-KO demonstrated no significant change in small vessel numbers and muscularization compared with both normoxic control mice and wild-type control mice (Figure 4B).
Figure 4.
SDF1NG2-KO are protected against hypoxia-induced pulmonary hypertension (PH). (A) Right ventricular systolic pressure (RVSP) and Fulton index of wild-type (WT) and SDF1NG2-KO mice. (B) Quantification of the number of vessels per 100 alveoli and number of muscularized vessels in SDF1NG2-KO model by optimal cutting temperature cross-section. (C) Left lungs of WT and SDF1NG2-KO mice stained for SMA (SMC marker, green), CD31 (endothelial marker, red), NG2 (mural cell marker, white), and nuclei (DAPI, blue). Scale bar: 50 μm. (D) Diagram shows the prevention strategy using AMD3100 and hypoxia on NG2tdT model. (E) RVSP and Fulton index on NG2tdT mice treated with AMD3100 and hypoxia. (F) Distribution of tdT+ cells under normoxia + AMD3100, hypoxia + PBS, and hypoxia + AMD3100 conditions. Lungs were stained for SMA (SMC marker, green), NG2 (mural cell marker, white), and nuclei (DAPI, blue). tdT is a reporter marker. Scale bar: 50 μm. *Depicts statistically significant difference compared with normoxia WT or normoxia + PBS: *P < 0.05 and ***P < 0.001 (one-way ANOVA with Dunnett’s post hoc test). #Depicts statistical significant difference compared with 3-week hypoxia WT or 3-week hypoxia + PBS: #P < 0.05, ##P < 0.01, and ###P < 0.001 (one-way ANOVA with Bonferroni’s post hoc test). LV = left ventricular; S = septal.
Next, we sought to document the number and distribution of NG2+ cells in lung tissue of normoxia and hypoxia SDF1NG2-KO. In normoxia, NG2+ SMA− cells were predominantly associated with lung capillaries, whereas SMA+ cells were in medium-sized (>50 μm [note: there is no SMA+ cells in vessels <50 μm.]) vessels in both WT (data not shown) and SDF1NG2-KO lungs (Figure 4C, upper left panel). In WT hypoxic lung, there were SMA+ cells coverage in microvessels (∼20 μm). However, hypoxic SDF1NG2-KO lungs demonstrated fewer NG2+ SMA+ cells around the same sized microvessels (Figure 4C, upper right panel) compared with hypoxia WT mice (Figure 4C, lower panel). Interestingly, most NG2+ SMA− cells in SDF1NG2-KO lungs were found to be distributed in the parenchyma in a manner consistent with their regular location under normoxia.
AMD3100 Prevents Pulmonary Hypertension in Mice Exposed to Chronic Hypoxia
SDF1 exerts its biological activity by triggering intracellular signal activity through CXCR4, its cognate receptor (24–26). To determine whether pharmacological inhibition of SDF1 signaling could reduce mural cell accumulation and PA muscularization in hypoxia, we administered AMD3100 (a CXCR4 inhibitor [27]) to NG2tdT hypoxic mice through implantable osmotic pumps. For our study, we selected to use a prevention approach (AMD3100 + hypoxia) (Figure 4D) followed by hemodynamic and morphometric analysis. As predicted, mice that received AMD3100 had significantly lower RVSP and RV hypertrophy compared with those who received PBS alone (Figure 4E). Next, we compared the distribution of tdT+ cells in the lung sections of PBS- and AMD3100-treated NG2tdT mice. No differences between PBS- and AMD3100-treated mice were found in the normoxia groups (data not shown). Compared with PBS-treated mice, hypoxic NG2tdT mice treated with AMD3100 exhibited fewer or absence of tdT+ cells associated with muscularized PAs (Figure 4F, right panel).
SDF1 Is Highly Expressed in PAH Pericytes and Is Associated with Hypercontractility and Reduced Endothelial–Pericyte Communications
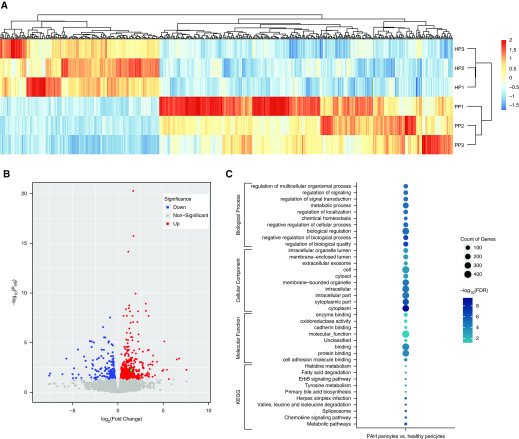
Studies to date have shown that SDF1 expression is increased in PASMCs and correlates with PA muscularization in human PAH samples and rat models of PH, but the role of SDF1 in lung pericyte biology remains unclear (23). As a first step, we analyzed the RNA-seq data of healthy (n = 3) and PAH (n = 3) pericytes to identify the most differentially expressed genes and their involvement in cellular processes. Heat map analysis revealed that healthy and PAH pericytes exhibit different hierarchical clusters (Figure 5A). Furthermore, we found a total of 502 genes (326 upregulated vs. 176 downregulated genes; for a full list see Table E7) with >1.5-fold differential expression in PAH versus healthy donor pericytes (Figure 5B). GO analysis demonstrated that most of these genes belonged to categories such as cytoplasm and regulation of biological activity followed by genes involved in the production of cellular components, biological regulation, and metabolic processes (Figure 5C). As anticipated, SDF1 was found among the significantly upregulated genes, as illustrated in the volcano plot (see green dot, Figure 5B).
Figure 5.
RNA-seq analysis reveals differential gene expression patterns between healthy versus PAH pericytes. (A) Hierarchical clustering of differentially expressed gene sets from healthy pericytes (n = 3) and PAH pericytes (n = 3). (B) Volcano plot of differentially expressed genes between PAH pericytes and healthy controls (FDR < 0.05). The green dot represents SDF1. (C) Gene Ontology and KEGG pathway analysis of differentially upregulated and downregulated expressing genes.
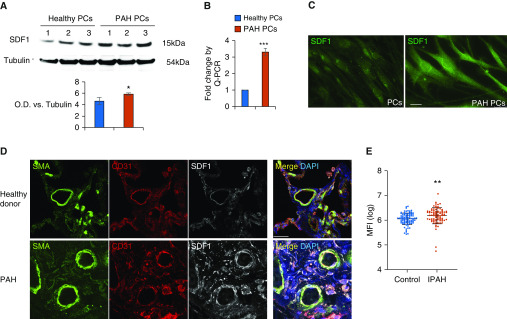
To confirm the results of our RNA-seq, we conducted quantitative PCR, Western blotting (WB), and immunofluorescence analyses on the healthy and PAH pericytes used in the RNA-seq. Indeed, SDF1 was found to be significantly upregulated more than twofold in PAH versus healthy pericytes (Figures 6A–C). Confocal analysis of lung tissue demonstrated high expression of SDF1 in lesions of patients with PAH (Figure 6D), whereas proteomic analysis of patient serum by Luminex demonstrated a significantly higher level of circulating SDF1 in 88 patients with idiopathic PAH compared with 83 healthy donors (Figure 6E).
Figure 6.
SDF1 is increased in PAH pericytes and PAH vascular lesions. (A) Representative Western blotting images of SDF1 in cell lysates of pericytes (PCs) from three healthy donors versus three patients with PAH. O.D. = optical density. (B) TaqMan quantitative PCR for SDF1 in donor control (n = 3) versus PAH pericytes (n = 3). *P < 0.05 and ***P < 0.001 versus controls using the unpaired t test. (C) Immunofluoresence of SDF1 from healthy and PAH pericytes. Scale bar: 10 μm. (D) Confocal images of SDF1 lung sections. Smooth muscle layer was stained with SMA (green), the endothelium with CD31 (red), and lung tissue with SDF1 (white). DAPI labels cell nuclei (blue). Scale bar: 25 μm. (E) Expression of SDF1 in plasma from 83 control subjects versus 88 patients with idiopathic PAH (IPAH). **P = 0.0032, unpaired t test.
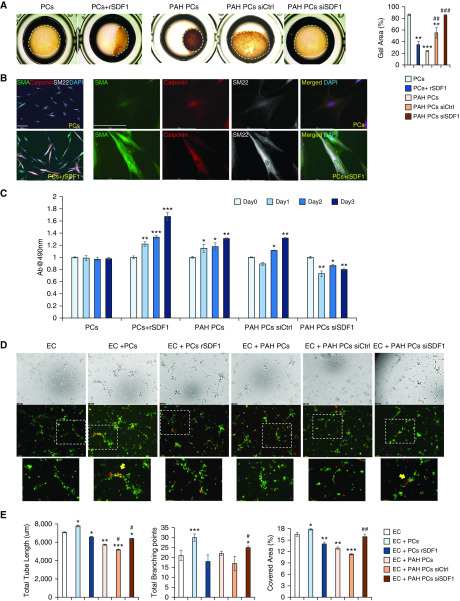
Next, we decided to test whether stimulation of healthy lung pericytes with recombinant SDF1 could induce hypercontractility and cell proliferation and reduce endothelial–pericyte interactions in coculture. These functional endpoints were chosen based on our previous work demonstrating that PAH pericytes exhibit these features in culture (11). Compared with cells exposed to the carrier, SDF1-stimulated healthy pericytes demonstrated significantly increased contractility over 6 hours (Figure 7A). Interestingly, SDF1 stimulation also increased expression of the SMC markers SMA, Calponin, and SM22 after 48 hours (Figure 7B). Using the CellTiter 96 AQueous One Solution assay, we found that SDF1 significantly increased pericyte numbers over 3 days of stimulation (Figure 7C). Finally, preincubation of healthy pericytes with SDF1 for 24 hours, followed by coculture with healthy pulmonary microvascular endothelial cells in Matrigel, resulted in smaller and less-organized tube networks compared with controls (Figures 7D and 7E).
Figure 7.
SDF1 regulates pericyte contractility, proliferation, and association with vascular tubes. (A) Collagen contractility assay. Dashed line represents the size of the collagen gel after 6 hours. The cover area was quantified by ImageJ. *Depicts statistical significant difference compared with PCs: **P < 0.01 and ***P < 0.001 versus WT (one-way ANOVA with Dunnett’s post hoc test). #Depicts statistical significant difference compared with PAH Pc: ##P < 0.01 and ###P < 0.001 (one-way ANOVA with Bonferroni’s post hoc test). (B) IF of unstimulated (top) and SDF1-stimulated (bottom) healthy pericytes. SMA (green), calponin (red), and SM22 (white) expression compared with control. Scale bar: 100 μm. (C) MTS assay to measure healthy and PAH pericyte proliferation over 3 days. Experimental conditions are indicated on the x-axis. *P < 0.05, **P < 0.01, and ***P < 0.001 versus each group Day 0 (one-way ANOVA with Dunnett’s post hoc test). (D) Matrigel assays show tube formation by healthy pulmonary microvascular endothelial cells (PMVECs) (PKH67, green) alone and in the presence of pericytes (PKH26, red) from healthy donors or from patients with PAH. The bottom row shows enlarged images from dashed squares in the top row. Yellow arrows indicate pericytes around tubes. (E) Total tube length (left), total branching points (middle), and covered surface area (right) in PMVECs with pericytes from healthy subjects (control) or from patients with PAH, with and without SDF1. Data are expressed as means ± SEM of three experiments. *Depicts statistical significant difference compared with PCs: **P < 0.01 and ***P < 0.001 versus EC alone (one-way ANOVA with Dunnett’s post hoc test); #depicts statistical significant difference compared with EC + PAH Pc: #P < 0.05 and ##P < 0.01 (one-way ANOVA with Bonferroni’s post hoc test). Scale bar: 100 μm. Ab = absorbance; EC = PMVEC (pulmonary microvascular endothelial cells); rSDF1 = recombinant SDF1; siCtrl = control siRNA; siSDF1 = SDF1 siRNA.
Taken together, our findings support that SDF1 stimulation of healthy pericytes can mimic the phenotypic features of PAH pericytes in culture. As a final step, we sought to reduce SDF1 expression in PAH pericytes to determine whether this could help normalize their phenotype. mRNA expression of SDF1 was reduced sevenfold by siRNA treatment (data not shown). As anticipated, siRNA-mediated SDF1 knockdown reduced PAH contractility (Figure 7A) and proliferation (Figure 7C) and improved endothelial–pericyte interactions and pericyte coverage (Figure 7D, yellow arrows), resulting in broader and more organized tube networks. We noted that pericyte distribution is seen along the length of vascular tubes with healthy pericytes or PAH pericytes with siSDF1, whereas it appears clustered around nodes with healthy pericytes + rSDF1 treatment, PAH pericytes, and PAH with siRNA control.
Discussion
Muscularization and loss of distal vessels are key pathological features of PAH. The natural function of mural cells is to regulate vascular tone, ensure that vessels have adequate structural support, and support endothelial homeostasis (3, 5, 28, 29). Studies using pathological samples and animal models have revealed evidence in support of local proliferation, a distal extension of PASMCs, a local progenitor cell population, and endothelial-to-mesenchymal transition (18, 30–32). We now present compelling evidence in support of a novel role for NG2+ mural cells in small vessel muscularization. We believe that our findings expand our current understanding of vascular remodeling not only in humans but also in murine models of chronic hypoxia (33).
Using a combination of RNA-seq and bioinformatics, we show that the genetic profiles of PAH PASMCs and pericytes are similar compared with their healthy counterparts, supporting the existence of a common genetic landscape responsible for the phenotypic similarities between these cell types in PAH. Moreover, this strategy has also allowed us to begin identifying the genetic differences responsible for pericyte behavior both in PAH and in hypoxia-induced PH. SDF1 was shown to be a key player in driving NG2+ mural cells to participate in vascular remodeling and in triggering pathological PAH pericyte activity. This is in line with recent observations that have shown the therapeutic potential of targeting SDF1 in reducing PA muscularization in PAH rat models (23), while bringing the focus to the contribution of the pericyte in this complex process.
RNA-seq analysis has become a powerful next-generation sequencing tool to analyze the complex genetic networks involved in cellular behavior and in identifying relevant pathways involved in disease states (34, 35). This approach has been successfully used to identify candidate pathways involved in the cellular response to hypoxia and BMPR2 mutations (36, 37). RNA-seq is also a powerful tool to study the evolution of cell identity through the comparison of genetic profiles between cells using bioinformatic approaches (38, 39). In our study, we found that the transcriptomic profile of healthy donor pericytes and PASMCs is distinct, whereas that of PAH cells has a more marked overlap. Compared with their healthy counterparts, the genetic landscape of PAH pericytes provides tantalizing hints concerning the mechanisms behind the phenotypic alterations observed in cell culture. Interestingly, the most altered genes are involved in the regulation of the spliceosome and nuclear structure. Alterations of spliceosome genes appear to be a frequent finding in RNA-seq studies of cancer cells such as leukemia and could represent alterations in the machinery that regulates mRNA processing of key genes involved in cell differentiation and control of proliferation (14, 15). This has potential importance to the field of PAH research, as recent studies using PASMCs, fibroblasts, and endothelial cells have identified dysregulation of pathways relevant to carcinogenesis that appear to be possible therapeutic targets (40–42). Indeed, our own studies have shown that PAH pericytes exhibit a Warburg effect that correlates with high proliferative rates, which further stresses the relevance of this finding to PAH (11).
Despite the power of RNA-seq and bioinformatic analysis in analyzing the genetic landscape of mural cells, it does not tell us how these cells behave under conditions known to induce PA muscularization. Lineage tracing is a powerful genetic tool that allows labeling of specific cell populations in mice to track their behavior under defined experimental conditions (43, 44). Using the NG2-CreER mouse strain, Volz and colleagues used lineage tracing to demonstrate that pericytes act as progenitors for coronary artery SMCs during cardiac development (45). Likewise, work by Sheikh and colleagues made use of a lineage tracing approach to identify an SMC progenitor population located in the junction of the pulmonary arteriole muscular–nonmuscularized border that serves as a pool of SMC cells that migrate distally to contribute to hypoxia-induced muscularization (18). When placed in the context of the work of Sheikh and colleagues, our findings suggest a model in which NG2+ cells accumulate within precapillary arterioles under hypoxia to contribute to muscularization. Although this model is appealing, there are several questions that must be addressed in future studies to establish the validity of the model. Presently, we do not know whether the identity of tdT+ cells in muscularized vessels is mostly NG2+ pericytes versus PASMCs or whether NG2+ pericytes could differentiate into SMC-like cells under hypoxia. To address this, clonal analysis studies would need to be undertaken. Also, we must stress that NG2 is not a marker exclusive of pericytes, because a minority of SMCs in large-sized vessels (>100 μm) also express this marker in the NG2tdT. Therefore, it is impossible to rule out that some of the NG2+ SMMHC+ cells seen in muscularized vessels were originated from this population or that they could represent members of the SMC progenitor pool identified by Sheik and colleagues (18). Until a marker exclusive of pericytes is discovered, this issue will remain unresolved.
The power of RNA-seq lies in the identification of gene candidates involved in cell behavior pertinent to disease. Using RNA-seq of human samples and NG2-tdT+ cells from our animal model, we identified SDF1 as a candidate gene that could orchestrate mural cell behavior in the lung. SDF1 is a major component of a signaling circuit that orchestrates vital processes ranging from cell proliferation and metabolism to stem cell differentiation (20–24, 26). Several studies have shown a correlation between heightened SDF1 expression and PAH, as indicated by elevated circulating levels of SDF1 in patient plasma (46) and high SDF1 expression in pulmonary vascular lesions. In this study, there was abundant and heterogeneous expression of SDF1 in lung parenchyma after hypoxia (Figure 3D). Furthermore, hypoxia-driven activation of SDF1 is a major trigger of pulmonary muscularization in neonatal chronic hypoxia-induced PH, a life-threatening disease that affects premature infants (47). Recently, Bordenave and colleagues used monocrotaline and sugen hypoxia-treated rats to test the therapeutic potential of two SDF1 inhibitors. In this setting, the authors observed that the animals treated with the SDF1 inhibitors demonstrated reduced RV hypertrophy and reduced muscularization that correlated with decreased PASMC and pericyte coverage (23). In this study, pericytes were identified via 3G5, a selective marker that allows separation of pericytes from lung suspension via magnetic beads, while NG2 was relegated to WB probing whole lung lysates. This study supports a key role for SDF1 in PAH muscularization, but it fails to answer how specifically SDF1 is involved in the events that lead to vascular remodeling and how mural cells are specifically affected with this intervention. To address this, we generated an SDF1NG2-KO in NG2+ cell strains followed by exposure to hypoxia. Using this tool, we were able to show that selective knockout of SDF1 was able to significantly reduce the accumulation of NG2+ cells in muscularized microvessels, resulting in improved RVSP and RV hypertrophy. Having documented the behavior of NG2+ cells in the NG2tdT model, these findings stress that SDF1 is necessary to drive these cells to accumulate around precapillary arterioles. Again, it is tempting to speculate that the source of tdT+ cells is pericytes relocating from the parenchyma to muscularized vessels, but this transgenic model prevents us from knocking out SDF1 exclusively in pericytes. Therefore, the definitive role of SDF1 in pericyte contribution to PAH pathogenesis should be further characterized.
A major finding in PAH is that pericytes fail to attach to endothelial cells during vascular tube formation (9, 10). SDF1 stimulation on healthy human pericytes prevents their association with endothelial cells and induces behavior that is reminiscent of that seen in PAH pericytes, whereas inhibition of SDF1 partially restores the capacity of PAH pericytes to associate with endothelial cells and recover their healthy phenotype. Although these findings stress the importance of SDF1 in PAH pericyte biology, it is still unclear how SDF1 works in pericytes and what molecular mechanisms are behind its actions. SDF1 is the only known ligand of the receptor CXCR4, and their interaction is essential for progenitor cell migration during development of the cardiovascular, hematopoietic and central nervous systems (20, 22, 24). SDF1 is a transcriptional target of HIF2a (hypoxia-inducible factor 2a), a member of the HIF signaling pathway that is stabilized on exposure to hypoxia (48). Although studies support a role of HIF2a in SMC-driven pulmonary vascular remodeling (49, 50), it is unclear whether this signaling axis drives SDF1 production in mural cells in PAH.
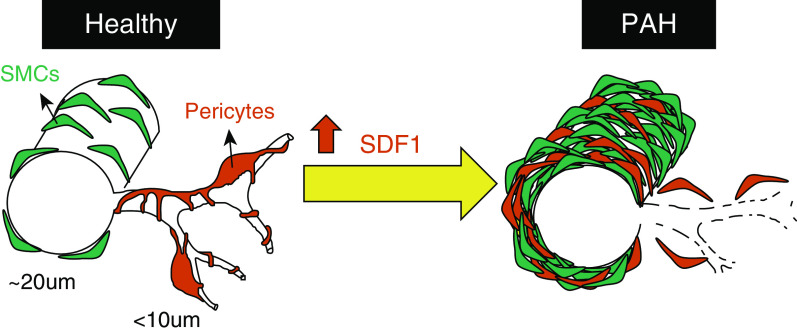
In conclusion, we propose a model in which high SDF1 drives pericyte detachment and PASMC proliferation that contributes to abnormal muscularization and vascular obstruction (see model, Figure 8). Beyond SDF1, the molecular and genetic knowledge gathered through our RNA-seq studies can be exploited for the development of new drugs capable of preventing abnormal vessel remodeling in PAH.
Figure 8.
Proposed model.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Dr. Marlene Rabinovitch for her critical review of this work. Lung tissues from PAH and control patients were provided by the Pulmonary Hypertension Breakthrough Initiative, which is funded by the NIH and managed at Stanford by Drs. Marlene Rabinovitch and R.T.Z. The tissues were procured at the Transplant Procurement Centers at Stanford University, Cleveland Clinic, and Allegheny General Hospital, and deidentified patient data were obtained via the Data Coordinating Center at the University of Michigan. The authors thank all patients and their proxies who participated in this study. They also thank Mrs. Patricia Angeles del Rosario for helping with the collection and processing of blood samples, Mr. Andrew Hsi for helping organize the patient database.
Footnotes
Supported by U.S. National Institutes of Health (NIH) grants R01 HL134776, R01 HL139664, and R03 HL133423-01; Stanford Cardiovascular Institute and Translational Research and Applied Medicine (V.A.d.J.P.); American Heart Association Scientist Development grant 15SDG25710448, the Parker B. Francis Fellowship, and the Pulmonary Hypertension Association Aldrighetti Research Award (K.Y.); NIH grants R01 HL141371, R01 HL145676, and R01 HL133272 (J.C.W.); and NIH grant NS069375 (Stanford Neuroscience Microscopy Service).
Author Contributions: K.Y. and V.A.d.J.P.: conception and design, acquisition of data, analysis and interpretation of data; and drafted and revised the manuscript. The rest of authors made substantial contributions to acquisition, analysis, and interpretation of data. All authors approved the final version of the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2019-0401OC on February 21, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, et al. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:261–272. doi: 10.1164/rccm.201201-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Díaz-Flores L, Gutiérrez R, Madrid JF, Varela H, Valladares F, Acosta E, et al. Pericytes: morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24:909–969. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- 4.Ribatti D, Nico B, Crivellato E. The role of pericytes in angiogenesis. Int J Dev Biol. 2011;55:261–268. doi: 10.1387/ijdb.103167dr. [DOI] [PubMed] [Google Scholar]
- 5.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Winkler EA, Bell RD, Zlokovic BV. Pericyte-specific expression of PDGF beta receptor in mouse models with normal and deficient PDGF beta receptor signaling. Mol Neurodegener. 2010;5:32. doi: 10.1186/1750-1326-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang FJ, You WK, Bonaldo P, Seyfried TN, Pasquale EB, Stallcup WB. Pericyte deficiencies lead to aberrant tumor vascularizaton in the brain of the NG2 null mouse. Dev Biol. 2010;344:1035–1046. doi: 10.1016/j.ydbio.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ricard N, Tu L, Le Hiress M, Huertas A, Phan C, Thuillet R, et al. Increased pericyte coverage mediated by endothelial-derived fibroblast growth factor-2 and interleukin-6 is a source of smooth muscle-like cells in pulmonary hypertension. Circulation. 2014;129:1586–1597. doi: 10.1161/CIRCULATIONAHA.113.007469. [DOI] [PubMed] [Google Scholar]
- 9.Yuan K, Shamskhou EA, Orcholski ME, Nathan A, Reddy S, Honda H, et al. Loss of endothelium-derived Wnt5a is associated with reduced pericyte recruitment and small vessel loss in pulmonary arterial hypertension. Circulation. 2019;139:1710–1724. doi: 10.1161/CIRCULATIONAHA.118.037642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan K, Orcholski ME, Panaroni C, Shuffle EM, Huang NF, Jiang X, et al. Activation of the Wnt/planar cell polarity pathway is required for pericyte recruitment during pulmonary angiogenesis. Am J Pathol. 2015;185:69–84. doi: 10.1016/j.ajpath.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan K, Shao NY, Hennigs JK, Discipulo M, Orcholski ME, Shamskhou E, et al. Increased pyruvate dehydrogenase kinase 4 expression in lung pericytes is associated with reduced endothelial-pericyte interactions and small vessel loss in pulmonary arterial hypertension. Am J Pathol. 2016;186:2500–2514. doi: 10.1016/j.ajpath.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michelakis ED, McMurtry MS, Wu XC, Dyck JR, Moudgil R, Hopkins TA, et al. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: role of increased expression and activity of voltage-gated potassium channels. Circulation. 2002;105:244–250. doi: 10.1161/hc0202.101974. [DOI] [PubMed] [Google Scholar]
- 13.Sutendra G, Dromparis P, Bonnet S, Haromy A, McMurtry MS, Bleackley RC, et al. Pyruvate dehydrogenase inhibition by the inflammatory cytokine TNFα contributes to the pathogenesis of pulmonary arterial hypertension. J Mol Med (Berl) 2011;89:771–783. doi: 10.1007/s00109-011-0762-2. [DOI] [PubMed] [Google Scholar]
- 14.Will CL, Lührmann R. Spliceosome structure and function. Cold Spring Harb Perspect Biol. 2011;3:a003707. doi: 10.1101/cshperspect.a003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cvitkovic I, Jurica MS. Spliceosome database: a tool for tracking components of the spliceosome. Nucleic Acids Res. 2013;41:D132–D141. doi: 10.1093/nar/gks999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Marabti E, Younis I. The cancer spliceome: reprograming of alternative splicing in cancer. Front Mol Biosci. 2018;5:80. doi: 10.3389/fmolb.2018.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seiler M, Peng S, Agrawal AA, Palacino J, Teng T, Zhu P, et al. Cancer Genome Atlas Research Network. Somatic mutational landscape of splicing factor genes and their functional consequences across 33 cancer types. Cell Rep. 2018;23:282–296, e4. doi: 10.1016/j.celrep.2018.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheikh AQ, Misra A, Rosas IO, Adams RH, Greif DM. Smooth muscle cell progenitors are primed to muscularize in pulmonary hypertension. Sci Transl Med. 2015;7:308ra159. doi: 10.1126/scitranslmed.aaa9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheikh AQ, Saddouk FZ, Ntokou A, Mazurek R, Greif DM. Cell autonomous and non-cell autonomous regulation of SMC progenitors in pulmonary hypertension. Cell Reports. 2018;23:1152–1165. doi: 10.1016/j.celrep.2018.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dillenburg-Pilla P, Patel V, Mikelis CM, Zárate-Bladés CR, Doçi CL, Amornphimoltham P, et al. SDF-1/CXCL12 induces directional cell migration and spontaneous metastasis via a CXCR4/Gαi/mTORC1 axis. FASEB J. 2015;29:1056–1068. doi: 10.1096/fj.14-260083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renko O, Tolonen AM, Rysä J, Magga J, Mustonen E, Ruskoaho H, et al. SDF1 gradient associates with the distribution of c-Kit+ cardiac cells in the heart. Sci Rep. 2018;8:1160. doi: 10.1038/s41598-018-19417-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petit I, Jin D, Rafii S. The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol. 2007;28:299–307. doi: 10.1016/j.it.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bordenave J, Thuillet R, Tu L, Phan C, Cumont A, Marsol C, et al. Neutralization of CXCL12 attenuates established pulmonary hypertension in rats. Cardiovasc Res. 2020;116:686–697. doi: 10.1093/cvr/cvz153. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Y, Cao HB, Li WJ, Zhao L. The CXCL12 (SDF-1)/CXCR4 chemokine axis: oncogenic properties, molecular targeting, and synthetic and natural product CXCR4 inhibitors for cancer therapy. Chin J Nat Med. 2018;16:801–810. doi: 10.1016/S1875-5364(18)30122-5. [DOI] [PubMed] [Google Scholar]
- 25.De Filippo K, Rankin SM. CXCR4, the master regulator of neutrophil trafficking in homeostasis and disease. Eur J Clin Invest. 2018;48:e12949. doi: 10.1111/eci.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Lourdes Perim A, Amarante MK, Guembarovski RL, de Oliveira CE, Watanabe MA. CXCL12/CXCR4 axis in the pathogenesis of acute lymphoblastic leukemia (ALL): a possible therapeutic target. Cell Mol Life Sci. 2015;72:1715–1723. doi: 10.1007/s00018-014-1830-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cashen AF, Nervi B, DiPersio J. AMD3100: CXCR4 antagonist and rapid stem cell-mobilizing agent. Future Oncol. 2007;3:19–27. doi: 10.2217/14796694.3.1.19. [DOI] [PubMed] [Google Scholar]
- 28.Lyle MA, Davis JP, Brozovich FV. Regulation of pulmonary vascular smooth muscle contractility in pulmonary arterial hypertension: implications for therapy. Front Physiol. 2017;8:614. doi: 10.3389/fphys.2017.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stenmark KR, Frid MG, Graham BB, Tuder RM. Dynamic and diverse changes in the functional properties of vascular smooth muscle cells in pulmonary hypertension. Cardiovasc Res. 2018;114:551–564. doi: 10.1093/cvr/cvy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hopper RK, Moonen JR, Diebold I, Cao A, Rhodes CJ, Tojais NF, et al. In pulmonary arterial hypertension, reduced BMPR2 promotes endothelial-to-mesenchymal transition via HMGA1 and its target slug. Circulation. 2016;133:1783–1794. doi: 10.1161/CIRCULATIONAHA.115.020617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tajsic T, Morrell NW. Smooth muscle cell hypertrophy, proliferation, migration and apoptosis in pulmonary hypertension. Compr Physiol. 2011;1:295–317. doi: 10.1002/cphy.c100026. [DOI] [PubMed] [Google Scholar]
- 32.Qiao L, Nishimura T, Shi L, Sessions D, Thrasher A, Trudell JR, et al. Endothelial fate mapping in mice with pulmonary hypertension. Circulation. 2014;129:692–703. doi: 10.1161/CIRCULATIONAHA.113.003734. [DOI] [PubMed] [Google Scholar]
- 33.Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1013–L1032. doi: 10.1152/ajplung.00217.2009. [DOI] [PubMed] [Google Scholar]
- 34.Stark R, Grzelak M, Hadfield J. RNA sequencing: the teenage years. Nat Rev Genet. 2019;20:631–656. doi: 10.1038/s41576-019-0150-2. [DOI] [PubMed] [Google Scholar]
- 35.Finotello F, Di Camillo B. Measuring differential gene expression with RNA-seq: challenges and strategies for data analysis. Brief Funct Genomics. 2015;14:130–142. doi: 10.1093/bfgp/elu035. [DOI] [PubMed] [Google Scholar]
- 36.Gu M, Shao NY, Sa S, Li D, Termglinchan V, Ameen M, et al. Patient-specific iPSC-derived endothelial cells uncover pathways that protect against pulmonary hypertension in BMPR2 mutation carriers. Cell Stem Cell. 2017;20:490–504, e5. doi: 10.1016/j.stem.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rhodes CJ, Im H, Cao A, Hennigs JK, Wang L, Sa S, et al. RNA sequencing analysis detection of a novel pathway of endothelial dysfunction in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2015;192:356–366. doi: 10.1164/rccm.201408-1528OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaik S, Martin EC, Hayes DJ, Gimble JM, Devireddy RV. Transcriptomic profiling of adipose derived stem cells undergoing osteogenesis by RNA-seq. Sci Rep. 2019;9:11800. doi: 10.1038/s41598-019-48089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsa E, Burridge PW, Yu KH, Ahrens JH, Termglinchan V, Wu H, et al. Transcriptome profiling of patient-specific human iPSC-cardiomyocytes predicts individual drug safety and efficacy responses in vitro. Cell Stem Cell. 2016;19:311–325. doi: 10.1016/j.stem.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pullamsetti SS, Savai R, Seeger W, Goncharova EA. Translational advances in the field of pulmonary hypertension: from cancer biology to new pulmonary arterial hypertension therapeutics. Targeting cell growth and proliferation signaling hubs. Am J Respir Crit Care Med. 2017;195:425–437. doi: 10.1164/rccm.201606-1226PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ranchoux B, Meloche J, Paulin R, Boucherat O, Provencher S, Bonnet S. DNA damage and pulmonary hypertension. Int J Mol Sci. 2016;17:E990. doi: 10.3390/ijms17060990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meloche J, Potus F, Vaillancourt M, Bourgeois A, Johnson I, Deschamps L, et al. Bromodomain-containing protein 4: the epigenetic origin of pulmonary arterial hypertension. Circ Res. 2015;117:525–535. doi: 10.1161/CIRCRESAHA.115.307004. [DOI] [PubMed] [Google Scholar]
- 43.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014;507:190–194. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen HI, Sharma B, Akerberg BN, Numi HJ, Kivelä R, Saharinen P, et al. The sinus venosus contributes to coronary vasculature through VEGFC-stimulated angiogenesis. Development. 2014;141:4500–4512. doi: 10.1242/dev.113639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Volz KS, Jacobs AH, Chen HI, Poduri A, McKay AS, Riordan DP, et al. Pericytes are progenitors for coronary artery smooth muscle. eLife. 2015;4:e10036. doi: 10.7554/eLife.10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCullagh BN, Costello CM, Li L, O’Connell C, Codd M, Lawrie A, et al. Elevated plasma CXCL12α is associated with a poorer prognosis in pulmonary arterial hypertension. PLoS One. 2015;10:e0123709. doi: 10.1371/journal.pone.0123709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Young KC, Torres E, Hatzistergos KE, Hehre D, Suguihara C, Hare JM. Inhibition of the SDF-1/CXCR4 axis attenuates neonatal hypoxia-induced pulmonary hypertension. Circ Res. 2009;104:1293–1301. doi: 10.1161/CIRCRESAHA.109.197533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 49.Dai Z, Li M, Wharton J, Zhu MM, Zhao YY. Prolyl-4 hydroxylase 2 (PHD2) deficiency in endothelial cells and hematopoietic cells induces obliterative vascular remodeling and severe pulmonary arterial hypertension in mice and humans through hypoxia-inducible factor-2α. Circulation. 2016;133:2447–2458. doi: 10.1161/CIRCULATIONAHA.116.021494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang H, Babicheva A, McDermott KM, Gu Y, Ayon RJ, Song S, et al. Endothelial HIF-2α contributes to severe pulmonary hypertension due to endothelial-to-mesenchymal transition. Am J Physiol Lung Cell Mol Physiol. 2018;314:L256–L275. doi: 10.1152/ajplung.00096.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.