Abstract
Background and aims
The coronavirus disease 2019 (COVID-19) pandemic has immensely strained healthcare systems worldwide. Diabetes has emerged as a major comorbidity in a large proportion of patients infected with COVID-19 and is associated with poor health outcomes. We aim to provide a practical guidance on screening of hyperglycemia in persons without known diabetes in low resource settings.
Methods
We reviewed the available guidelines on this subject and proposed an algorithm based on simple measures of blood glucose (BG) which can be implemented by healthcare workers with lesser expertise in low resource settings.
Results
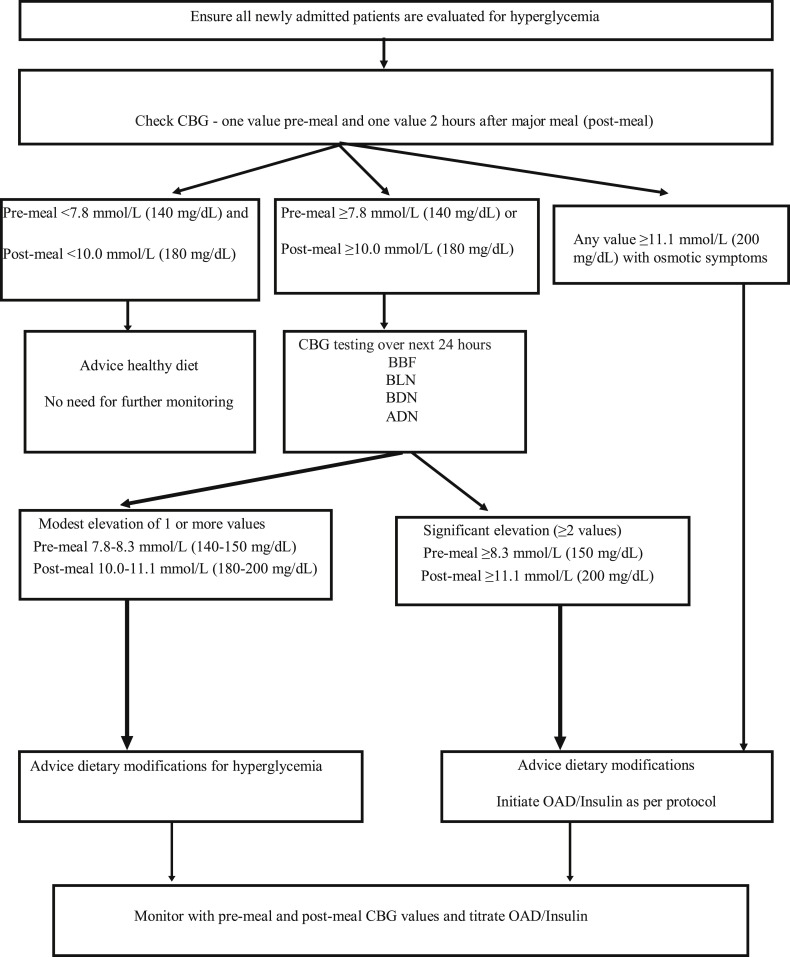
We propose that every hospitalized patient with COVID-19 infection undergo a paired capillary BG assessment (pre-meal and 2-h post-meal). Patients with pre-meal BG < 7.8 mmol/L (140 mg/dL) and post-meal BG < 10.0 mmol/L (180 mg/dL) may not merit further monitoring. On the other hand, those with one or more value above these thresholds should undergo capillary BG monitoring (pre-meals and 2 hours after dinner) for the next 24 hours. When two or more (≥50%) such values are significantly elevated [pre-meal ≥8.3 mmol/L (150 mg/dL) and post-meal ≥11.1 mmol/L (200 mg/dL)], pharmacotherapy should be immediately initiated. On the other hand, in patients with modest elevation of one or more values [pre-meal 7.8–8.3 mmol/L (140–150 mg/dL) and post-meal 10.0–11.1 mmol/L (180–200 mg/dL)], dietary modifications should be initiated and pharmacotherapy considered only if BG control remains suboptimal.
Conclusion
We highlight strategies for screening of hyperglycemia in persons without known diabetes treated for COVID-19 infection in low resource settings. This guidance may well be applied to other settings in the near future.
Keywords: Covid-19, Diabetes, Hyperglycemia, Screening, Low resource setting
Highlights
-
•
In-patient hyperglycemia is associated with poor clinical outcomes, especially in patients with undiagnosed diabetes.
-
•
We propose an algorithm for screening of in-patient hyperglycemia in low resource settings.
-
•
This algorithm should be researched further in low resource and other settings.
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has resulted in an unprecedented rise in admissions in dedicated COVID facilities. A significant proportion of patients with COVID-19 have comorbidities such as diabetes, hypertension and cardiovascular disease, which associate with poor disease outcomes [[1], [2], [3]]. Besides, the COVID-19 infection could itself induce new onset diabetes [4,5]. While the importance of intensive glycemic control in persons with diabetes and COVID-19 infection has been emphasized [[6], [7], [8], [9], [10]], we aim to highlight the strategies for screening of hyperglycemia in persons without known diabetes in low resource settings.
2. Available guidance on the subject
Hyperglycemia is an established risk factor for poor clinical outcomes and mortality in hospitalized patients. However, the prognosis is worse in patients with undiagnosed diabetes compared to those with a known history of diabetes [11]. The Endocrine Society clinical practice guidelines for inpatient management of hyperglycemia recommend that all hospitalized patients regardless of the diagnosis of diabetes undergo laboratory blood glucose (BG) testing on admission [12]. Patients without known diabetes and BG > 7.8 mmol/L (140 mg/dL) should undergo bedside testing by a point-of-care device for the next 24–48 hours, and appropriate treatment initiated in those with persistently high BG (>7.8 mmol/L or 140 mg/dL). Measurement of glycated hemoglobin (HbA1c) is also recommended in such individuals to distinguish stress hyperglycemia (HbA1c <6.5%) from previously unrecognized diabetes (HbA1c ≥ 6.5%) [10]. Similarly, the American Diabetes Association (ADA) guidelines suggest BG testing at admission in all hospitalized patients, HbA1c in those with BG greater than 7.8 mmol/L (140 mg/dL), and initiation of treatment in cases with persistent hyperglycemia (≥10.0 mmol/L or 180 mg/dL) [13].
We need to appropriately tailor the available guidance to low resource healthcare settings, where facilities for testing of HbA1c and plasma glucose may not be readily available. Further, the burden of tests performed may exceed capacity of healthcare staff available to interpret and act on their results, thus highlighting the need for algorithms based on simple measures of BG which can be implemented by healthcare workers with lesser expertise.
3. Proposed method for screening of hyperglycemia in low resource settings and its rationale
We propose that every hospitalized patient with COVID-19 infection undergo a paired capillary blood glucose assessment (pre-meal and 2-h post-meal). We prefer paired BG readings over single random BG measurement because: a) biological variability is greater for post-prandial compared to fasting BG [14], hence, a decision based on random BG performed in post-prandial state alone may be erroneous, b) interpretation of a BG value depends upon its relation to timing of meal intake, hence, adoption of a uniform threshold (RBG >7.8 mmol/L or 140 mg/dL) without relating it to meal intake may not be appropriate.
4. Proposed thresholds for recognition of hyperglycemia and subsequent action
Patients with pre-meal BG < 7.8 mmol/L (140 mg/dL) and post-meal BG < 10.0 mmol/L (180 mg/dL) may not merit further monitoring. On the other hand, those with one or more value above these thresholds should undergo capillary blood glucose monitoring four times (pre-meals and 2 hours after dinner) for the next 24 hours. In a scenario where two or more (≥50%) values are significantly elevated [pre-meal ≥8.3 mmol/L (150 mg/dL) and post-meal ≥11.1 mmol/L (200 mg/dL)], pharmacotherapy should be immediately initiated. On the other hand, in patients with modest elevation of one or more values [pre-meal 7.8–8.3 mmol/L (140–150 mg/dL) and post-meal 10.0–11.1 mmol/L (180–200 mg/dL)], dietary modifications should be initiated and pharmacotherapy considered only if BG control remains suboptimal (Fig. 1 ). Finally, patients with any BG value ≥ 11.1 mmol/L (200 mg/dL) and osmotic symptoms warrant immediate pharmacotherapy.
Fig. 1.
Proposed algorithm for screening of hyperglycemia in persons without known diabetes treated for COVID-19 infection in low resource setting.
Abbreviations: ADN: After dinner, BBF: Before breakfast, BDN: Before dinner, BL: Before lunch, CBG: Capillary blood glucose, OAD: Oral antihyperglycemic drug.
5. Pros and cons of the proposed approach
The proposed algorithm may aid in early recognition and management of in-hospital hyperglycemia and has a potential to improve clinical outcomes associated with COVID-19 infection. While capillary testing may suffer from inaccuracy, especially at extremes of BG values [15], it is easily available, cost-effective and remains a feasible option in low resource settings.
It should be ensured that BG meters used for this purpose are accurate and as per prescribed standards since erroneous results may result in misclassification and wrong treatment. Several pre-analytical, analytical and post-analytical factors affect the performance of BG meters which should be carefully looked into [16]. Our pragmatic approach of using capillary BG testing for diagnosis and management of hyperglycemia could be supplemented with fasting plasma glucose and HbA1c measurement, where feasible.
6. Post-discharge follow-up
The diagnosis and treatment plan should be reviewed again at the time of hospital discharge. Patients with stress hyperglycemia related to the acute viral infection may not require any pharmacotherapy and be discharged on dietary and lifestyle modifications alone. On the other hand, significant down-titration of the existing anti-hyperglycemic medications should be considered for patients with previously unrecognized diabetes. A close vigilance for hypoglycemia should be maintained in patients discharged on agents such as insulin and sulphonylurea. Such patients should also receive appropriate training on recognition and management of hypoglycemia. Home monitoring of capillary BG, maintenance of a proper BG log and self-adjustment of medications should be emphasized, where required. A need for close follow-up with an experienced physician should be highlighted considering the possibility of long-term metabolic alterations induced by the virus [6].
7. Conclusion
To conclude, this commentary highlights strategies for screening of hyperglycemia in persons without known diabetes treated for COVID-19 infection in low resource settings. This guidance may well be applied to other settings in the near future.
Funding
None.
Author contributions
YG conceived the idea of this paper. AG, SG and YG wrote the first draft of the manuscript which was read and edited by NT. All authors approved the final version of the manuscript.
Declaration of competing interest
The authors declare that there is no conflict of interest.
References
- 1.Chen Y., Gong X., Wang L., Guo J. Effects of hypertension, diabetes and coronary heart disease on COVID-19 diseases severity: a systematic review and meta-analysis. medRxiv. 2020 doi: 10.1101/2020.03.25.20043133. published online March 30. (preprint) [DOI] [Google Scholar]
- 2.Zhu L., She X.G., Cheng X. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metabol. 2020 doi: 10.1016/j.cmet.2020.04.021. published online May 1; pii: S1550-4131(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in covid-19 pneumonia - a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14:395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta R., Hussain A., Misra A. Diabetes and COVID-19: evidence, current status and unanswered research questions. Eur J Clin Nutr. 2020:1–7. doi: 10.1038/s41430-020-0652-1. [published online ahead of print, 2020 May 13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang J.K., Lin S.S., Ji X.J., Guo L.M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bornstein S.R., Rubino F., Khunti K. Practical recommendations for the management of diabetes in patients with COVID-19 [published online ahead of print, 2020 Apr 23] Lancet Diabetes Endocrinol. 2020 doi: 10.1016/S2213-8587(20)30152-2. S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaiser U.B., Mirmira R.G., Stewart P.M. Our response to COVID-19 as endocrinologists and diabetologists. J Clin Endocrinol Metab. 2020;105(5) doi: 10.1210/clinem/dgaa148. pii: dgaa148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta R., Ghosh A., Singh A.K., Misra A. Clinical considerations for patients with diabetes in times of COVID-19 epidemic. Diabetes Metab Syndr. 2020;14(3):211–212. doi: 10.1016/j.dsx.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ceriello A., Stoian A.P., Rizzo M. COVID-19 and diabetes management: what should be considered? Diab Res Clin Pract. 2020:108151. doi: 10.1016/j.diabres.2020.108151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W., Lu J., Gu W., Zhang Y., Liu J., Ning G. Care for diabetes with COVID-19: advice from China. J Diabetes. 2020;12(5):417–419. doi: 10.1111/1753-0407.13036. [DOI] [PubMed] [Google Scholar]
- 11.Umpierrez G.E., Isaacs S.D., Bazargan N., You X., Thaler L.M., Kitabchi A.E. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87:978–998. doi: 10.1210/jcem.87.3.8341. [DOI] [PubMed] [Google Scholar]
- 12.Umpierrez G.E., Hellman R., Korytkowski M.T. Management of hyperglycemia in hospitalized patients in non-critical care setting: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:16–38. doi: 10.1210/jc.2011-2098. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association 15. Diabetes care in the hospital. Diabetes Care. 2020;43(Supplement 1):S193–S202. doi: 10.2337/dc20-S015. [DOI] [PubMed] [Google Scholar]
- 14.Chai J.H., Ma S., Heng D. Impact of analytical and biological variations on classification of diabetes using fasting plasma glucose, oral glucose tolerance test and HbA1c. Sci Rep. 2017;7(1):13721. doi: 10.1038/s41598-017-14172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotwal N., Pandit A. Variability of capillary blood glucose monitoring measured on home glucose monitoring devices. Indian J Endocrinol Metab. 2012;16(Suppl 2):S248–S251. doi: 10.4103/2230-8210.104052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klonoff D.C. Point-of-Care blood glucose meter accuracy in the hospital setting. Diabetes Spectr. 2014;27(3):174–179. doi: 10.2337/diaspect.27.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]