Supplemental Digital Content is available in the text.
Abstract
Health care systems are belligerently responding to the new Coronavirus Disease 2019 (COVID-19). The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a specific condition, whose distinctive features are severe hypoxemia associated with (>50% of cases) normal respiratory system compliance.1 When a patient requires intubation and invasive ventilation, the outcome is poor,2–4 and the length of stay in the intensive care unit (ICU) is usually 2or 3 weeks.2 In this article, theauthors review several technological devices, which could support health care providers at the bedside to optimizethe care for COVID-19 patients who are sedated, paralyzed, and ventilated. Particular attention is provided to the use of videolaryngoscopes (VL) because these can assist anesthetists to perform a successful intubation outside the ICU while protecting health care providers from this viral infection. Authors will also review processed electroencephalographic (EEG) monitors which are used to better titrate sedation and the train-of-four monitors which are utilizedto better titrate neuromuscular blocking agents in the view of sparing limited pharmacological resources. COVID-19 can rapidly exhaust human and technological resources too within the ICU. This review features a series of technological advancements that can significantly improve the care of patients requiring isolation. The working conditions in isolation could cause gaps or barriers in communication, fatigue, and poor documentation of provided care. The available technology has several advantages including (a) facilitating appropriate paperless documentation and communication between all health care givers working in isolation rooms or large isolation areas; (b) testing patients and staff at the bedside using smart point-of-care diagnostics (SPOCD) to confirm COVID-19 infection; (c) allowing diagnostics and treatment at the bedside through point-of-care ultrasound (POCUS) and thromboelastography(TEG); (d) adapting the use of anesthetic machines and the use of volatile anesthetics. Implementing technologies for safeguarding health care providers as well as monitoring the limited pharmacological resources are paramount. Only by leveraging new technologies, it will be possible to sustain and support health care systems during the expected long course of this pandemic.
A pandemic is defined as an epidemic occurring worldwide, crossing international boundaries and usually affecting rapidly a large number of people.5 The classical definition includes nothing about population immunity, virology, or disease severity. On the contrary, the most typical feature of a pandemicis the simultaneous global burden for a large proportion of society. Rationalization of human and pharmaceutical resources using technology is fundamental to improve patients’ outcome, to match the increasing number of ventilators installed worldwide and to allow caring for the majority of people infected.
This articlewill feature available technologies to provide a more effective and sustainable care for patients admitted to the intensivecareunit(ICU).1 At the beginning of the Coronavirus Disease 2019(COVID-19) pandemic, special focus conveyed on the need for mechanical ventilators. Unfortunately, these very sophisticated machines are only the tip of the iceberg given that complex patients’ care requires many more resources to be effective. Moreover, the inappropriate use of mechanical ventilators is armful and potentially life-threatening. This is particularly relevant in the case of COVID-19 because the severe acute respiratory syndrome coronavirus 2(SARS-CoV-2) does not reflect the classic definition of acute respiratory distress syndrome (ARDS).1 COVID-19 patients despite sharing a single etiology may present quite differently from one another.1,2 The intensive care doctor routinely assesses critically ill patients and the anesthesiologists position the endotracheal tube to start the invasive ventilation. The patient needs to be sedated from the time the endotracheal tube is inserted until the complete recovery of the lung function and removal of the breathing tube.3 The introduction (intubation) and the removal of the endotracheal tube (extubation) are critical moments that could expose the health care professionals at a risk of infection.6,7
The COVID-19 critically ill patient is frequently very unstable and therefore it is ideal to minimizetransfers for diagnostic purposes. These patients are also kept in isolation areas. The combination of working using personal protective equipment (PPEs) in isolation rooms could potentially cause gaps or barriers in communication, fatigue, and poor documentation of provided care.
Considering all these challenges, the technologies recommended in this articleare used:
a. To enable safe positioning of the endotracheal tube at initiation of the invasive mechanical ventilation, for example, videolaryngoscopy (VL).
b. To spare sedative drugs which are becoming increasingly constrained at this time of global needs, for example, processed EEG monitoring to provide sedation.
c. To better administer neuromuscular blocking agents (NMBA) when needed, for example, train-of-four (TOF) monitoring of neuromuscular blockade (NMB).
d. To facilitate appropriate paperless documentation and communication between all health care providers working in isolation rooms or large isolation areas.
e. To test patients and staff at the bedside using smart point-of-care diagnostics (SPOCD), for example, confirmation of COVID-19 infection.
f. To allow diagnostics and treatment at the bedside, for example, point-of-care ultrasound (POCUS) or thromboelastography(TEG).
g. To adapt the use of anesthetic machines to the ICU and use anesthetic inhalation agents.
DISCUSSION
Safe Intubation Using PPEs and VL
A number of health care professionals have contracted this viral infection due to the spreading through droplets, aerosols,6,7 or contaminated surfaces.7 Anesthesiologists in particular are involved in caring for patients needing airways management including bag-mask ventilation, intubation, extubation, suctioning of secretions, and even cardiopulmonary resuscitation. These maneuvers are aerosol generating and might cause the spread of COVID-19.8–11 High-flow oxygen in a spontaneously breathing COVID-19–positive patient is also associated with the increased transmission of the SARS-CoV-2 and it is limited or avoided in several hospitals.12 Once the patient is intubated and transferred to the ICU, any leak from the tracheal tube cuff, manipulations, or adjustments of the tracheal tube and disconnections of the breathing circuit should be avoided because these can lead to aerosolization. The American Society of Anesthesiologists (ASA)11 as well as other authors13,14 have reinforced the fact that the risk of infection to health care personnel can be reduced by using airborne PPEs, including N95 mask and VL.11 The consensus statementfrom the Society for Neuroscience in Anesthesiology & Critical Care (SNACC) suggests that following a 5-minute preoxygenation with good mask seal, rapid sequence induction should be performed using VL. Sufficient doses of NMBA should be given to ensure no cough reflex during intubation, and for the same reason, awake fibrotic intubation should be avoided if possible.13,14 Normally, VL devices are used for managing difficult intubations or minimize movements in the cervical spine during intubation of trauma patients. Senior anesthesiologists can operate at a higher distance from the mouth of these patients,15,16 can work faster reducing the time needed to complete the procedure and thus minimize aerosolization of secretions.15 In the face of a pandemic, doctors and nurses from different backgrounds are required to work in the ICU.16 These health care professionals have minimal training in the management of the airways and the VL devices use a camera connected to a screen, which is accessible by all bystanders. This has the advantage of facilitating understanding of intubation by non–ICU-trained staff.14,16 The authors will not explore the advantages of a single device but simply highlight the importance of VL in treating COVID-19 cases.17 Studies looking at nonexpert operators using VL have shown increased success rate and decreased time to intubation;this should not encourage nonexpert to intubate COVID-19 patients.17,19 The concept of a dedicated “intubation team” has been promoted in the United Kingdom, North America, and Canada. This team consists of experts (normally 2 anesthesiologists and 1 nurse) using dedicated technology including 1 type of VL device.
Monitoring Sedationvia Processed EEG
Some drugs are becoming increasingly difficult to obtain and technology could help in sparing sedative and NMBA.
Processed EEG monitoring devices have been available for more than 25 years20,21 but infrequently used in the ICU. There are 3 widely used technologies:
the bispectral index (BIS),
the entropy,
the narcotrend-derived variables.
There is no significant difference in the use of these devices at the bedside;they need sensors positioned on the forehead (Figure 1). Good care should be given to apply the sensors in such a way that they cannot easily dislodge. Ideally, freshly apply sensors should be used each change of position, for example, switch from supine to prone position and vice versa.21–23 Some studies have indicated that the prone position might alter the BIS values21–23;however, patients can be in the so-called swimmer’s position during proning23 (Figure 1) or alternative placement options can be found, for example, the postauricular area22 (Figure 1). The care team needs to check regularly if the sensor is properly placed and the skin is intact.21,22 The question that arises during this pandemic is as follows: can processed EEG devices help health care professionals to better titrate sedation when dealing with ventilated COVID-19 patients?24 Can these monitoring systems be helpful to better use scarce resources, to facilitate the administration of sedative drugs by nontrained ICU staff and to prevent side effects?
Figure 1.
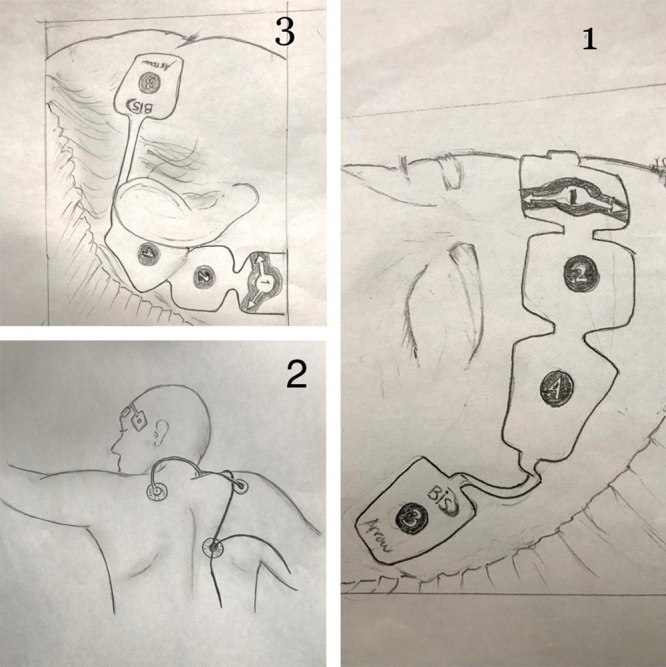
A, Positioning of the BIS sensor on the forehead. B, Swimmer’s position: showing the actual ease with which BIS monitoring can be applied in the prone position. C, Positioning of the BIS sensor postauricular site. BIS indicates bispectral index.
Kaplan and Bailey25 showed that monitoring with the BIS patients on continuous infusion of sedatives reduces drug utilization and costs. Intensive care patients are monitored and treated using a significant number of devices, which unfortunately might cause a myriad of artifacts in the reading of the processed EEG.25 The treating ICU physicians can determine the desired level of sedation, and anyone at the bedspace can target light 75–85, moderate 65–75, or deep 55–65 sedation. This is again a significant advantage provided by this technology when non–ICU-trained staff is requested to support the care of critical patients. Patients under sedation move their forehead more and they have more reactions, such as sweating or perspiration or shivering, compared to patients under general anesthesia. During the initial phases of SARS-CoV-2, most patients are sedated and paralyzed (BIS <60, general anesthesia),1 subsequently patients need only sedation (BIS 65–75) for 2–3 weeks. COVID-19 patients have an increased respiratory drive and they are at risk of “patient self-inflicted lung injury.”24 The BIS could be used to gently increase the level of sedation;it is useful to titrate sedation and to control the work of breathing.
Most monitoring devices integrate the facial electromyography (EMG) to the EEG26 which means that the signal is modified by the use of NMBA.26 The nonexperienced operator should not lower the dose of sedative drugs following the initiation of NMBA because this might lead to potential risks of awareness and posttraumatic distress syndrome.26 Propofol in combination with opioids (eg, fentanyl) are the drugs of choice for sedation in the ICU. The BIS is used in a large number of studies for monitoring propofol administration only;however, the former drug is spared when conjoined with opioids to achieve the same level of sedation.20 The BIS values will take longer time to change when administering midazolam boluses or continuous infusion, compared to propofol.27 BIS values are 20% higher28 utilizingmidazolam and 20% lower29 using dexmedetomidine30 compared to propofol. Ketamine increases to a maximum of 30% for up to 10 minutes the BIS value when it is used alone and in combination with propofol.
The entropy is a device integrated in several anesthetic machines and its use is increased in the COVID-19 cases because such ventilators have been hired in the ICU. The entropy monitoring system can spare (29% and 40%, respectively) the consumption of propofol and sevoflurane in a short period of time (average 74–100 minutes).31 This is a significant saving if done over days and weeks.
In summary, all processed EEG monitors can be a valuable tool to titrate sedation in COVID-19 patients, using the following considerations:
Be careful of noises but don’t be afraid! The noise related to artifacts, for example, interferences with other electronic devices, could increase the BIS values. The operator should not react by overshooting sedation.
NMBA reduce the BIS values. The operator should not react by undershooting sedation.
Propofol is the preferred sedative drug when BIS monitoring is used;however, other agents can easily be managed.
In the ICU, the sedation is provided in combination. The concomitant use of opioids will lower the BIS values and increase the sedation while the use of NMBAs will lower the BIS but it does not increase the sedation.26
Prolonged use of high doses of propofol can cause propofol syndrome,32 which is dose dependent, and this is another reason why non–ICU-trained staff should use this technology to reduce the overall administration of propofol.
Monitoring NMB
NMBAs are frequently used in COVID-19 patients either as “procedural” drugs, for example, for transfer to specific radiological examinations, or as an “adjunct” to support invasive ventilation.1 It is beyond the scope of this review to comment on the usefulness of profound NMB during ventilation of severe ARDS patients.33,34 NMB is certainly needed at the time of endotracheal intubation as well as during invasive mechanical ventilation to improve oxygenation,33 to correct patient-ventilator asynchrony,34–37 to reduce the work of breathing,1,24,38 and to reduce the driving pressure.39 Prolonged NMB increases the incidence of neuromuscular weakness and critical illness neuropathy, which could delay the weaning process and cause various problems during the rehabilitation.35 The duration of the NMB is controversial and this should not be longer than eligibly needed. Gattinoni et al1 suggest that there are 2 COVID-19 pneumonia phenotypes namely L and H. The characteristic of these phenotypes can dictate the ventilation strategy as well as the duration of both sedation and NMB.1 Previous publications on severe ARDS failed to prove any influence of continuous NMB on mortality.1 Patient self-induced ventilator lung injury (P-SILI)24 is suspected as being part of the pathophysiology of SARS-CoV-2 and therefore NMB agents might be needed to prevent progression of the disease.1
There are currently several monitoring systems available.40 Althoughmost anesthesia machines have quantitative monitoring devices integrated, such as the kinemyography, acceleromyography, or electromyography, this is not the case in the ICU. Treating COVID-19 patients, the TOF modes should be selected but a rather important aspect is the choice of the site where to apply the sensors. Standard sites, such as the adductor pollicis muscle, reflect relaxation of skeletal muscles. However, to better assess NMB of the diaphragm or the larynx, the corrugator supercilii muscle, located around the eye, is the location of choice.41 A handheld device needs to be used for the TOF monitoring which should not take more than 2–3 minutes when the electrodes are in place. The limitation of leaving those sensors in place for long time is only the skin integrity. Results of the TOF should be recorded on the patient’s chart on a regular basis and at preset time intervals. The frequency of such monitoring can change depending on the NMBA and the modality of use (bolus versus continuous infusion). If a bolus is used, for example, before proning, the TOF should be recorded just before the procedure. Alternatively, if a continuous infusion is started, the TOF should be done at preset time intervals.
COVID-19 patients are intubated (by the intubation team) using a rapid sequence10–12; therefore, succinylcholine and rocuronium are preferred choices.10,12 The overall safest combination might be to administer a high dose of rocuronium, for example, 1.2 mg/kg, which has a fast onset and,43,44 offers the advantage of being rapidly reversible using 16 mg/kg sugammadex if intubation fails or an allergic reaction (anaphylaxis) occurs. The TOF monitoring handheld device could be difficult to carry and to clean after its use outside the ICU. The TOF monitoring should be used in all COVID-19 patients requiring complete paralysis inside the ICU. In summary, handheld TOF monitoring is safe and can be used by trained nurses at the bedside. The frequency of monitoring depends on the ICU protocol on the mode of administration of NMBAs (bolus versus continuous infusion), plus the workload.43,44 To prevent complications such as critical illness neuropathy, NMBAs should be given for a limited time and should be administered under close monitoring.
Facilitate AppropriatePaperlessElectronicDocumentation, Prescribingand CommunicationBetweenAll HealthCare ProvidersWorkingin IsolationRoomsor LargeIsolationAreas
Reeves et al45 indicated that health care providers and organizationshave invested on technology-based tools which can effectively support institutions during a pandemic by facilitating the immediate widespread distribution of information, tracking transmission in real-time, creating virtual venues for meetings and day-to-day operations, and, perhaps most importantly, offering tools for paperless ward rounds in the COVID-19 areas. The majority of electronic health record (EHR) systems allows screening protocols, system-level EHR personalized templates, emergency department and wards order panels, reports and analytics of give performance and outcomes, communication channels, and patient-centered technology.45 EHR systems have protected workforces from this infection in multiple ways, including allowing work-at-home or having exclusively electronic patients’ records inside the isolation rooms. As a matter of fact, paper charts, prescribing folders, or paper notes have been banned from most isolation rooms because these could accidentally be taken inside cleaned areas45 (Figure 2).
Figure 2.

COVID-19 patient’s isolation room before implementing paperless work. On the computer, there are paper charts, pens, smart cards; all these objects could be accidentally carried outside the isolation area and could spread this infection. EHRs allow clinicians to prescribe and document exclusively on a computer. This has reduced the number of objects that are taken in and out of the isolation rooms. This is a mechanism that could protect health care workers from this infection. This figure is showing a system using smartcards that are needed to access information and enter documents or prescriptions. These cards have been either removed or strictly kept outside the isolation rooms. The limitation with removing those from the bed space is that clinicians cannot see blood results or images at the bedside and they need to leave the room to complete their examination and treatment plan. Clinicians might not be able to document their findings and they have to defer their work. COVID-19 indicates Coronavirus Disease 2019; EHR, electronic health records.
Authors have illustrated some tools that EHR vendors have used in dealing with the pandemic.
Allscripts (Allscripts Company, British Columbia, Canada) is enabling providers to identify and treat patients without requiring them to come to hospitals. Allscripts Virtual Triage (https://www.allscripts.com/2020/03/allscripts-virtual-triage-to-provide-much-needed-support-for-organizations/) can serve as a “digital front door” to triage high-risk patients.
Athenahealth (Athenahealth, Inc Company, Watertown, MA) is promoting virtual care by enabling clinicians to remotely message patients and to respond via an application.
Cerner (Cerner EHR TechnologiesCompany, North Kansas City, MO) has developed an EHR to allow ICU physicians inside the COVID-19 isolation areas to document their ward rounds and care plans. Since the implementation of such electronic records, ICUs could function without using paper notes (Figure 2).
eClinicalWorks (eClinicalWorks LLC Company, Westborough, MA) is integrating virtual care into clinical practice. This includes the use of telehealth to “allow clinicians to do a TeleVisit.”
EPIC (EPIC-userweb company, employee-owned and developer lead, Verona, WI) clinical records systems are used by a number of the nation’s largest health systems. EPIC has appreciated the huge value of sharing data to learn about this new disease. The company is actively engaging with doctors and researchers encouraging organizations to share COVID-19 information through EPIC’s interoperability network. Similarly, EPIC has advised provider customers that MyChart portal can be used to handle questions.
Meditech (Meditech Company, Westwood, MA) has generated a program called “Expanse,” once again to support triage outside the hospital using the Centre for Disease Control recommendations.
Most important features are preventing the use of paper charts which are at avery high risk of spreading the virus among doctors and nurses45 (Figure 2).
Second, communication with PPEs is very challenging and technology can help workers requesting devices, results, medications held outside the isolation areas without physically walk out of the bed space. PPEs are limited and therefore it is important to complete as many tasks as possible at the bedside before leaving the area.
Finally, communicating with masks is challenging, and using phones should be avoided because a phone can come close to the face of health care providers and lead to involuntary transmission of the viral infection (Figure 3). Speaker-phones or computer-based calls are preferable in the isolation space to discuss case, exams ask for help, or consultation without leaving the bed space.
Figure 3.
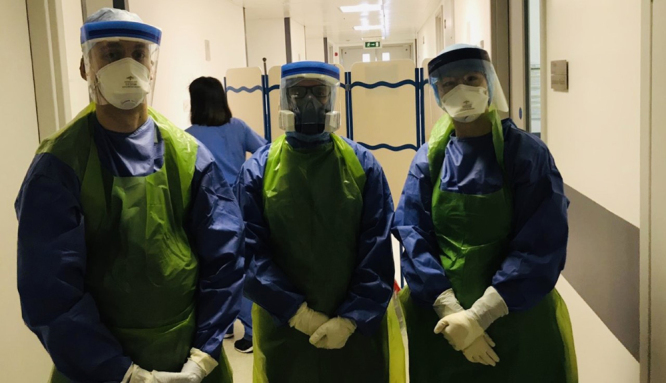
The figure shows that communication is very difficult with PPEs, and normal phones are not efficient because the head is mostly protected and it is very difficult to hear. Furthermore, using landline systems while wearing PPEs might expose to contamination via the contact of the face with infected phones. PPEs indicates personal protective equipments.
Testing Health Care Professional and Patients at the Bedside Using SPOCD for COVID-19 Infection
Clinical laboratory evaluation is essential to care for critically ill patients in general. However, while central laboratories have clear advantages, such as trained technicians, established quality control procedures, and high throughput testing that creates financial efficiencies, SPOCD might revolution patient’s care. In the course of this pandemic,SPOCD are providing early diagnosis, prognostication, and evaluation of clinical response to therapies. D-dimerlevels, for instance, has a very high prognostic value and its trend can predict COVID-19 patient’s outcome.
In the setting of a pandemic testing, the population and the health care providers for COVID-19 is paramount.46 Home testing for COVID-19 as well as testing before admitting patients to hospitals would be beneficial.46,47 Home testing will reduce the spread of the disease by identifying early healthy carriers and prehospital or preoperative testing could allow creation of clean wards. Clean services which could continue supporting emergencies and non-COVID surgery, including trauma and transplant. The detection of SARS-CoV-2 positive is obtained through oropharyngeal swabs,46 which are taken to laboratories for conventional real-time reverse transcription-polymerase chain reaction (RT-PCR) analysis. Unfortunately, such approach is expensive, depends on technical expertise, and results lengthy (up to 24 hours from collection to readout) with up to 30% false negative. The policy of testing all patients before undergoing surgery is limited by the number of false-negative results. The consequence is that full precautions are still taken for all cases. Many units have adopted a hybrid policy, with the application of PPEs depending on the type of surgery. The details of these strategies are beyond the scope of this article;however, during intubation in anesthesia for any type of surgery, full PPEs are applied.
RT-PCR does not provide a clear picture of patients’ immune status, being unable to discriminate between those who already healed (IgM−/IgG+) and those who never had previous contact with the virus (IgM−/IgG−). This latter is the main reason why clinicians have urged governments to complement RT-PCR with serological assays to optimize the epidemiological and clinical management of the outbreak.47,48 In this scenario, the introduction of devices for point-of-care diagnostics enabling a rapid and cost-efficient detection of SARS-CoV-2 in biological samples could represent a game-changing event in the battle against COVID-19 pandemic.47–50 SPOCD could in fact replace standard RT-PCR allowing for a faster detection of SARS-CoV-2 while awaiting serology status for dosage of IgG antibodies. To overcome the current time-consuming and laborious detection technique using RT-PCR, scientists are currently suggested to deploy alternative molecular amplification techniques such as real-time loop-mediated isothermal amplification. Multiplex detection systems based on RT-LAMP diagnostic platforms already exist for detection of RNA fragments of most human influenza viruses,51 laboratory evidence recently published from a study conducted in South Korea revealed a sensitivity of 98.9%, hence much higher than that of conventional RT-PCR analysis.49 In the last few weeks, a few companies have started commercializing also SPOCD for antibodies detection based on lateral flow immunoassays specifically conceived for the ultrarapid detection of SARS-CoV-2 within 1 hour.
Diagnostics at the Bedsideto SupportTreatmentand Care
The authorswill focus on the use of POCUS and TEG.
The introduction of POCUS in clinical practice has been facilitated by the advancements in the technology and the availability of smaller, cheaper handheld ultrasound devices fitted with different ultrasound probes.52–56 In the case of COVID-19, the host response to the viral infection generates a vast series of cardiovascular and respiratory abnormalities, including pleural effusion, myocarditis, pulmonary emboli, cardiac thrombus, as well as pericardial effusions.2,3,57,58 Initial data from COVID-19 patients1–3 show that less than 5% of patients have a normal chest x-ray.1–3 This is particularly relevant given limitations in the ability of clinical examination and chest radiography to differentiate several pulmonary pathologies. The chest CT scan is the gold standard, and radiological patterns of COVID-19 pneumonia have been thoroughly described.1 However, this radiological modality presents several limitations, including the availability of this resource for all patients, the challenging clinical conditions of ventilated COVID-19 patients, and the need for intrahospital transfers (porters, nurses, anesthetists, or intensivists, etc) which carries the risks of spreading the viral infection. Advanced modalities for bedside chest investigations are needed in most of these instable patients.53–56 In 1 meta-analysis, the lung ultrasound (LUS) has a sensitivity of 88% and a specificity of 86% in the diagnosis of pneumonia (subpleural consolidation in Figure 4) compared with the chest CT or the chest radiography.56 The evaluation of B-lines (Figure 5) which are vertical is used to assess the alveolar-interstitial syndrome, which includes pulmonary congestion due to heart failure.53 These lines are basically reverberation artifacts from the pleural line, they move along with the pleural sliding with every breath and they reflect an increase in the extravascular lung water.57–59 The LUS findings in COVID-19 pneumonia1 with predominant phenotype (L)1 are (a)pleural thickening and irregularities (Figures 4, 5); (b) subpleural consolidations (Figure 4); (c) patchy and confluent B-lines (curtain-like) that corresponds to ground-glass opacification on the chest computed tomography (Figure 5); (d) spared area (areas of normal aeration; A-lines;Figure 5). In COVID-19 pneumonia phenotype H1, the presence of lobar consolidation is predominant and this is also true in the setting of superadded bacterial pneumonia (Figure 6).
Figure 4.
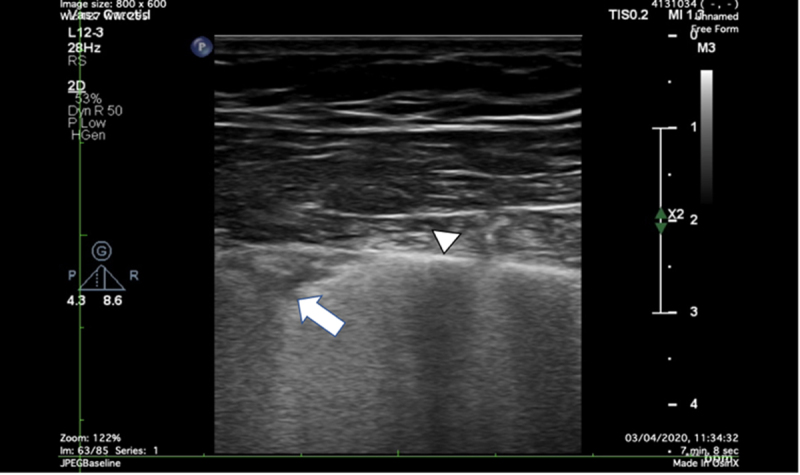
The pleural ultrasound in a patient with COVID-19 by high-frequency linear probe (5–10 MHz). White triangle = pleural line. White arrow = subpleural consolidation. COVID-19 indicates Coronavirus Disease 2019.
Figure 5.
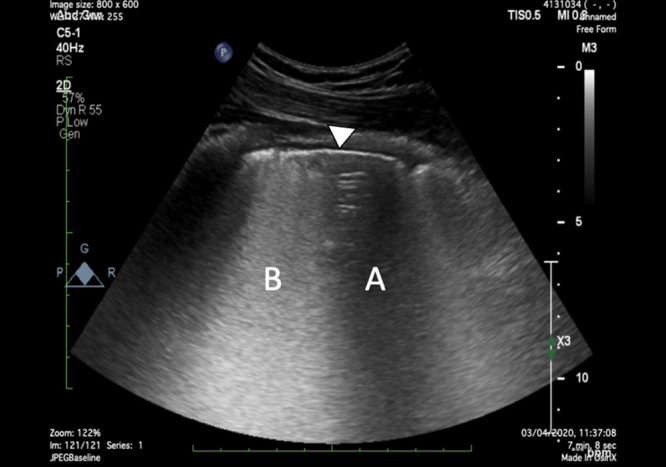
The lung parenchymal ultrasound in a patient with COVID-19 by the curvilinear probe (2–5 MHz). White triangle = pleural line; A = spared area of horizontal A-lines; B = confluent B lines in a curtain-like pattern. COVID-19 indicates Coronavirus Disease 2019.
Figure 6.
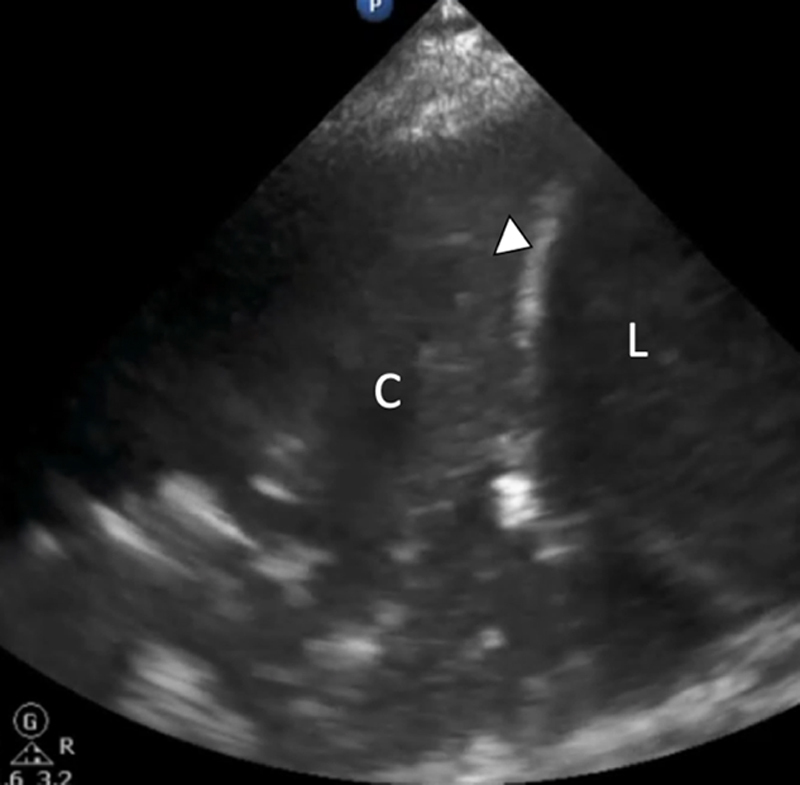
The lung ultrasound performed in a patient with COVID-19 complicated with secondary bacterial pneumonia showing evidence of right lower lobe consolidation = C, the diaphragm = D, and the liver = L. COVID-19 indicates Coronavirus Disease 2019.
LUS could be used to evaluate lung aeration and59 a lung aeration score has been described by Bouhemad et al.59 The assessment of aeration should be used for COVID-19 ventilation strategies which are based on the use of relatively low tidal volume and high positive end-expiratory pressure (PEEP)namely between 8 and 10 cmH2O in those with preserved lung compliance (phenotype L) and around PEEP of 10–15 cmH2O in those with reduced lung compliance (phenotype H).1 Alternatively, the LUS could define the maximum recruitment achieved with the lowest PEEP and therefore prevent barotrauma and timely diagnose pneumothoraxes. LUS is found to be highly sensitive (91%) and specific (98%) for detecting pleural effusions and the development of pleural effusion is a rapidly reversible problem to optimize care and weaning of COVID-19 patients ventilated in the ICU.1–3,60 Outcome data from the UnitedStatesand China show that >33% of COVID-19 patients suffer fromcardiomyopathy.3,58 COVID-19 patients are at risk of pulmonary embolism because they are coagulopathic.2,3 Echocardiography could provide essential real-time information on the cardiac function and exclude myocarditis as well as pericardial effusions.61,65 Although the transoesophageal echocardiography (TOE) is the gold standard, there are limitations in using it in COVID-19 patients, because of the high risk of aerosolization. In the case a TOE is necessary, the patient should be fully paralyzed to avoid cough and the operators should wear full airborne PPEs. The transthoracic echocardiography (TTE) is the modality of choice in COVID-19 even if at times poor acoustic windows might be generated by the mentioned strategy of setting positive pressure ventilation with high PEEP values.65 This issue could be overcome by the use of a subcostal window and a contrast TTE to enhance visualization of the myocardial wall or by temporarily reducing PEEP without disconnecting the ventilator circuit.
The use of POCUS has also been expanded to include confirmation of the endotracheal intubation with the so-called Bullet sign and of the esophageal intubation with the double tract sign.66,67 In a study by Gekle et al,68the average time to confirmation of central venous catheters (CVC) insertion by ultrasound (saline flush visualized and pneumothorax excluded) is shorter 8.80 minutes (95% confidence interval, 7.46–10.14 minutes) compared to the chest radiography, which is the gold standard in non-COVID-19 patients.68 LUS post-CVC insertion can also exclude complications such as pneumothoraxes faster compared to a chest radiography. In general, POCUS is recommended in COVID-19 patients because it is affordable, reproducible, and it prevents complications secondary to other radiological examinations including transfer to scans. The limiting factor is knowledge, training experience, and resources.
The use of TEG is advised for all COVID-19 patients with severe pneumonia. All intubated patients admitted to the ICU with any type of pneumonia receive prophylaxis for venous thromboembolism.69 However, the activation of the coagulation and/or fibrinolysis occurs in COVID-19 as part of the acute inflammatory host response.70 Tang et al71 found that increased D-dimer levels in 183 consecutive patients correlate to overall mortality of 11.5%. All ICU COVID-19 ventilated patients with high D-dimers(<2000 μg/L) are on treatment doses heparin or low molecular weight heparin, if no contraindications. Standard laboratory tests, such as prothrombin time (PT) and partial thromboplastin time (PTT), measure the clotting activity from the plasma, ignoring other components of the coagulation such as the platelets and the fibrinolysis. Unfortunately, the platelet count (usually normal in COVID-193) and the fibrinogen concentrations give static numbers with no information regarding their functionality. The TEG and the rotationalthromboelastometry(ROTEM) are 2 methods of whole blood viscoelastic analysis thatrapidly measure the whole blood capability to make and sustain clot formation. Current technology is evolved and the TEG can be performed at the bedside to monitor any coagulopathy. It is becoming clear that the shape of the TEG in COVID-19 cases (Supplemental Digital Content, Figure, http://links.lww.com/AA/D114) is very similar to a condition reported in sepsis and called disseminated intravascular coagulation (DIC). This is a severe disturbance of the coagulation in which small blood clots develop throughout the bloodstream, blocking small blood vessels. Postmortem data are supporting this hypercoagulative state and the presence of microthrombi in several systems. Bedside technology such as the TEG could guide the clinician exploring, learning about this new disease and treating these patients according to a more specific target.
Use of Anesthesia Machines for the Purpose of ICU Ventilation
This section aims to highlight important factors and offer guidance when using anesthetic machines as long-term ventilators. The following recommendations are based on official statements by manufacturers,72–76 government authorities, and official associations dedicated to patient safety or anesthesiology.77 The general consent among all these entities clearly states that the use of anesthesia machines for long-term ventilation of COVID-19 patients must be considered “off-label use” and can thus not be officially recommended. Nonetheless, anesthesia ventilators are needed and they have interesting monitoring features, which are not normally integrated to ICU ventilators, these include the entropy. According to the FDA’s Emergency Use Authorization, the known and potential benefits of anesthesia gas machines and positive pressure breathing devices modified for use as ventilators when used to treat patients during the COVID-19 pandemic, outweigh the known and potential risks of such products.78 Anesthesia machines must first undergo modifications to ensure effective and secure treatment when used for long-term ventilation (see Supplemental Digital Content, Appendix, http://links.lww.com/AA/D114). If used outside an operating room, there may not be a compatible outlet for the scavenger system, then it should either be disconnected from hoses coming from the breathing system and ventilator or removed completely if it is a closed-scavenger system preventing high airway pressures and unintended PEEP at the airway. Most anesthesia machines are not approved for active humidification, which is crucial to prevent bronchial plugging in long-term ventilation. In this case, passive systems must be used if compatible with the prevention of the spread of the virus. A heat and humidity exchange (HMEF) filtershould be placed between endotracheal tube and the breathing circuit of the machine. A second filter (high-efficiency particulate air [HEPA]) may be added on the expiratory hose where it connects to the anesthesia machine for extra security. This setup might however modify system resistances, it is therefore mandatory to monitor vital and ventilation parameters very closely. The addition of carbon dioxide scrubbers and the enhancement of monitoring equipment (eg, end-tidal CO2, Fico2, Spo2, actual Fio2) may open up additional possibilities in flow regulation and therefore humidification management. The gas sample tubing must be connected to the filter on the side close to the ventilator to prevent contamination and be directed upward to prevent aspiration of condense water into the water trap which may damage the gas module.76–78 All users should be familiar with the anesthesia system user interface, with system testing, controls, functions, configurations, and alarms before using these devices.78 The flow rate should be adjusted according to the level of moisture within the system, as excess moisture can degrade the performance of the ventilator sensors and reduce theability to keep the system clean. Too little moisture may dry out the patients’ airways leading to mucus plugging and epithelial dysfunction.75–78 Further adjustments to the freshgasflowshould be considered in case of an inadequate supply of CO2 absorbent or the lack of a carbon dioxide scrubber in mobile ventilators. In these set-ups, the anesthesia machine's ability to change fresh gas flow can be used to save CO2 absorbent. If the fresh gas flow exceeds minute ventilation, there is little to no rebreathing causing very little usage of CO2 absorbent. On the other hand, the recommendation to minimize fresh gas flow is intended to utilize the unique design of an anesthesia machine to minimize oxygen utilization and maintain humidity in the inspired gas avoiding active humidification. Though reducing fresh gas flow requires a greater use of CO2 absorbents and may lead to flooding of the system.79,80 For the maintenance of anesthesia machines, carbon dioxide scrubbers must be exchanged if Fico2exceeds 5mmHg and breathing circuit filters must be exchanged daily and after every patient. Usually, anesthesia machines are intended to be power cycled (turned on/off), calibrated, and tested at least once every 24hours. Use of the device without appropriate periodic calibration may result in the degradation of device delivery and monitoring performance, including pressure, flow and volumes, and spontaneous breath triggering. If long-term therapy is required, patients should be transferred to an ICU ventilator and if this is not possible, devices must be at least power cycled and checked out between patients.79 Ventilators must be disinfected between patients. In case of incorrect placement or lack of filters, special measures must be taken before using the ventilator on a new patient. The machine must be turned off, disinfected thoroughly as mentioned above, and all single-use items must be removed. The Society of Critical Care Medicine (SCCM), American Association for Respiratory Care (AARC), ASA, AnaesthesiaPatient Safety Foundation (APSF), American Association of Critical-Care Nurses (AACN), and American College of Chest Physicians (CHEST) advise clinicians against the use of anesthesia machines on more than 1 patient simultaneously. The severity of the disease and its complexity make it hard to manage single patient ventilation. Ventilating multiple patients at the same time might just reduce the likelihood of survival of all patients because as suggested human and pharmacological resources are limited. For more details, also look at Supplemental Digital Content, Appendix, http://links.lww.com/AA/D114.
LIMITATIONS
Authors have not explored technologies related to interfaces (eg, helmet, face masks, optiflow) to provide noninvasive ventilation.
Authors have not examined the use of transpulmonary pressure catheters and devices which are very important to measure the driving pressure and the work of breathing in COVID-19 pneumonia.1,24 As a matter of fact, transpulmonary pressure, lung volumes, and related strain and stress values should be measured to guide mechanical ventilation strategies. No mention is given to neurally adjusted ventilatory assist (NAVA) and airway pressure release ventilation (APRV).
Authors have not explored the use of CO2 removal and cytokines-absorbing hemofilters which could be indicated given the inflammatory storm generated by this virus.
The authorshave not described specific indications for ECMO.
All these points have not been presented due to limitations on the length of this article. However, these are highly valuable technologies and worth further reading and implementation in the care of COVID-19 pneumonia.
CONCLUSIONS
Technology is required at the bedside to sustain and support health care professionals during the expected long course of this pandemic. ICU staff is challenged by the risk of contamination and infection as well as limited resources. Technology can facilitate operational tasks, for example, safe intubation using VL as well as monitoring (sedation and NMB for titration of essential drugs). Technology such as the VL or the EEG monitoring could easily be understood by nonintensivists and this is crucial to work efficiently with people from different backgrounds. The level of anxiety and concern health care workers are exposed is enormous. The challenge of using devices and new tools inside the isolation rooms is increased by wearing PPEs and knowing the risk of spreading the disease. EHR vendors are working very hard in collaboration with hospitals to minimizethe need forintroducing unnecessary paper charts and records in the infected space. At the same time, new cutting-edge technology is replacing old fashion laboratory analysis to test patients for COVID-19 earlier and to process useful blood tests at the bedside, for example, D-dimers. Ultrasonography is substituting routine chest radiography and scans and supporting treatment choices by providing real-time assessment of the heart and the lungs. Finally, anesthesia ventilators, which have been adapted off-label to increase the current number of ventilators, can offer interesting new features such as the entropy. Efficiency and sustainability in providing high quality of care for hundreds of severe COVID-19 patients would be impossible without the use of a variety of technological devices. It has been perceived that the pandemic has driven many more transformations in a short time than ever and that the working patterns after this period will change.
ACKNOWLEDGMENTS
The authors thank Umesh Patel for editing the content.
DISCLOSURES
Name: Francesca Rubulotta, MD, PhD, MBA, FRCA, FICM.
Contribution: This author had the original idea.
Name: Hatem Soliman-Aboumarie, MD, FEACVI, FASE, FHEA.
Contribution: This author helped develop the session on point-of-care ultrasound (POCUS).
Name: Kevin Filbey, MD.
Contribution: This author helped develop the session on ventilators.
Name: Goetz Geldner, MD, PhD.
Contribution: This author helped develop the session on anesthesia ventilators.
Name: Kai Kuck, PhD.
Contribution: This author has done the session on ventilators and the appendix.
Name: Mario Ganau, MD, PhD, FEBNS, FACS.
Contribution: This author helped develop the session on smart point-of-care.
Name: Thomas M. Hemmerling, MSc, MD, DEAA.
Contribution: This author helped develop the session on electroencephalographic (EEG) monitoring on NMB monitoring and edit the entire manuscript.
This manuscript was handled by: Thomas R. Vetter, MD, MPH.
Supplementary Material
FOOTNOTES
GLOSSARY
- AACN
- American Association of Critical-Care Nurses
- AARC
- American Association for Respiratory Care
- APRV
- airway pressure release ventilation
- APSF
- Anaesthesia Patient Safety Foundation
- ARDS
- acute respiratory distress syndrome
- ASA
- American Society of Anesthesiologists
- BIS
- bispectral index
- CHEST
- American College of Chest Physicians
- COVID-19
- Coronavirus Disease 2019
- CT
- xxx
- CVC
- central venous catheters
- DIC
- disseminated intravascular coagulation
- ECMO
- xxx
- EEG
- electroencephalographic
- EMG
- electromyography
- EHR
- electronic health record
- FDA
- xxx
- Fico2
- xxx
- Fio2
- xxx
- HEPA
- high-efficiency particulate air
- HMEF
- heat and humidity exchange
- ICU
- intensive care unit
- Ig
- xxx
- LUS
- lung ultrasound
- NAVA
- neurally adjusted ventilatory assist
- NMB
- neuromuscular blockade
- NMBA
- neuromuscular blocking agents
- P-SILI
- patient self-induced ventilator lung injury
- PEEP
- positive end-expiratory pressure
- POCUS
- point-of-care ultrasound
- PPE
- personal protective equipment
- PT
- prothrombin time
- PTT
- partial thromboplastin time
- ROTEM
- rotational thromboelastometry
- RT-LAMP
- xxx
- RT-PCR
- reverse transcription-polymerase chain reaction
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus 2
- SCCM
- Society of Critical Care Medicine
- SNACC
- Society for Neuroscience in Anesthesiology & Critical Care
- Spo2
- xxx
- SPOCD
- smart point-of-care diagnostics
- TEG
- thromboelastography
- TOE
- transoesophageal echocardiography
- TOF
- train-of-four
- TTE
- transthoracic echocardiography
- VL
- videolaryngoscopy
Funding: None.
The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website.
All the authors have equally contributed to this article.
Reprints will not be available from the authors.
REFERENCES
- 1.Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatment for different phenotypes? ICM. 2020;1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ICNARC COVID-19 report 2020-04-04. Source: ICNARC COVID-19 Study case mix programme database 4th April 2020.
- 3.Arentz M, Yim E, KlaffL Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Last JM. A Dictionary of Epidemiology. 20014th ed New York: Oxford University Press [Google Scholar]
- 6.van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng L, Qiu H, Wan L, et al. Intubation and ventilation amid the COVID-19 outbreak: Wuhan’s experience. Anesthesiology. 2020;132:1317–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuo MZ, Huang YG, Ma WH, et al. Expert recommendations for tracheal intubation in critically ill patients with novel Coronavirus Disease 2019. Chin Med Sci J. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenland JR, Michelow MD, Wang L, London MJ. COVID-19 infection: implications for perioperative and critical care physicians. Anesthesiology. 2020;132:1346–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo M, Cao S, Wei L, et al. Precautions for intubating patients with COVID-19. Anesthesiology. 2020;132:1616–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ASA Committee on Occupational Health. Coronavirus Resources for Anesthesiologists. Available at: https://www.asahq.org/about-asa/governance-and-committees/asa-committees/committee-on-occupational-health/coronavirus. Accessed April 2, 2020.
- 12.Yu IT, Xie ZH, Tsoi KK, et al. Why did outbreaks of severe acute respiratory syndrome occur in some hospital wards but not in others? Clin Infect Dis. 2007;44:1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orser B. Recommendations for endo tracheal intubation of COVID-19 patients. Anesth Analg. 2020;130:1109–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma D, Rasmussen M, Han R, et al. Anesthetic Management of Endovascular Treatment of Acute Ischemic Stroke During COVID-19 Pandemic: Consensus Statement from Society for Neuroscience in Anesthesiology & Critical Care (SNACC). Available at: https://www.snacc.org/wp-content/uploads/2020/04/SNACC-Consensus-Statement-on-Anesthetic-Management-of-Endovascular-Treatment-of-Acute-Ischemic-Stroke-During-COVID-19-Pandemic-with-Image.pdf.
- 15.Hall D, Steel A, Heij R, et al. Videolaryngoscopy increases “mouth to mouth” distance compared to direct laryngoscopy. Anaesthesia. 2020;75:822–823. [DOI] [PubMed] [Google Scholar]
- 16.World Federation of Societies of Anaesthesiologists. Coronavirus – guidance for anaesthesia and perioperative care providers. 2020. Available at: https://www.wfsahq.org/latest-news/latestnews/943-coronavirus-staying-safe. Accessed November 3, 2020.
- 17.Niforopoulou P, Pantazopoulos I, Demestiha T, Koudouna E, Xanthos T. Video-laryngoscopes in the adult airway management: a topical review of the literature. Acta Anaesthesiol Scand. 2010;54:1050–1061. [DOI] [PubMed] [Google Scholar]
- 18.Nouruzi-Sedeh P, Schumann M, Groeben H. Laryngoscopy via Macintosh blade versus GlideScope: success rate and time for endotracheal intubation in untrained medical personnel. Anesthesiology. 2009;110:32–37. [DOI] [PubMed] [Google Scholar]
- 19.Brown CA, 3rd, Bair AE, Pallin DJ, Laurin EG, Walls RM; National Emergency Airway Registry (NEAR) Investigators National Emergency Airway Registry (NEAR) Investigators Improved glottic exposure with the Video Macintosh Laryngoscope in adult emergency department tracheal intubations. Ann Emerg Med. 2010;56:83–88. [DOI] [PubMed] [Google Scholar]
- 20.Hajat Z, Ahmad N, Andrzejowski J. The role and limitations of EEG-based depth of anaesthesia monitoring in theatres and intensive care. Anaesthesia. 2017;72suppl 138–47. [DOI] [PubMed] [Google Scholar]
- 21.Mahmood S, Parchani A, El-Menyar A, Zarour A, Al-Thani H, Latifi R. Utility of bispectral index in the management of multiple trauma patients. Surg Neurol Int. 2014;5:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akavipat P, Hungsawanich N, Jansin R. Alternative placement of bispectral index electrode for monitoring depth of anesthesia during neurosurgery. Acta Med Okayama. 2014;68:151–155. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira VM, Piekala DM, Deponti GN. Safe prone checklist: construction and implementation of a tool for performing the prone maneuver. Rev Bras Ter Intensiva. 2017;29:131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to optimise progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195:438–442. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan L, Bailey H. Bispectral index (BIS) monitoring of ICU patients on continuous infusion of sedatives and paralytics reduces sedative drug utilization and cost. Crit Care. 2000;4:P190. [Google Scholar]
- 26.Rubulotta F, Rubulotta G, Occhipinti G, Naimo J, Gullo A. Comment on “Effects of neuromuscular block on systemic and cerebral hemodynamics and bispectral index during moderate or deep sedation in critically ill patients” by Inoue et al. Intensive Care Med. 2007;33:388–389. [DOI] [PubMed] [Google Scholar]
- 27.Ibrahim AE, Taraday JK, Kharasch ED. Bispectral index monitoring during sedation with sevoflurane, midazolam, and propofol. Anesthesiology. 2001;95:1151–1159. [DOI] [PubMed] [Google Scholar]
- 28.Hans P, Dewandre PY, Brichant JF, Bonhomme V. Comparative effects of ketamine on bispectral indexand spectral entropy of the electroencephalogram under sevoflurane anaesthesia. Br J Anaesth. 2005;94:336–340. [DOI] [PubMed] [Google Scholar]
- 29.Kasuya Y, Govinda R, Rauch S, Mascha EJ, Sessler DI, Turan A. The correlation between bispectral index and observational sedation scale in volunteers sedated with dexmedetomidine and propofol. Anesth Analg. 2009;109:1811–1815. [DOI] [PubMed] [Google Scholar]
- 30.Xi C, Sun S, Pan C, Ji F, Cui X, Li T. Different effects of propofol and dexmedetomidine sedation on electroencephalogram patterns: wakefulness, moderate sedation, deep sedation and recovery. PLoS One. 2018;13:e0199120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yli-Hankala A, Vakkuri A, Annila P, Korttila K. EEG bispectral index monitoring in sevoflurane or propofol anaesthesia: analysis of direct costs and immediate recovery. Acta Anaesthesiol Scand. 1999;43:545–549. [DOI] [PubMed] [Google Scholar]
- 32.Mirrakhimov AE, Voore P, Halytskyy O, Khan M, Ali AM. Propofol infusion syndrome in adults: a clinical update. Crit Care Res Pract. 2015;2015:260385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papazian L, Forel JM, Gacouin A; ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–1116. [DOI] [PubMed] [Google Scholar]
- 34.Dodia NN, Richert ME, Deitchman AR. A surveyof academic intensivists’ useof neuromuscular blockadein subjects withARDS. Respir Care. 2020;65:362–368. [DOI] [PubMed] [Google Scholar]
- 35.Moss M, Huang DT, Brower RG, et al. for the National Heart, Lung, and Blood Institute PETAL Clinical Trials Network. Early neuromuscular blockadein the acute respiratory distress syndrome. N Engl J Med. 2019;380:1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho ATN, Patolia S, Guervilly C. Neuromuscular blockade in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. J Intensive Care. 2020;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slutsky AS. Neuromuscular blocking agents in ARDS. N Engl J Med. 2010;363:1176–1180. [DOI] [PubMed] [Google Scholar]
- 38.Bourenne J, Hraiech S, Roch A, Gainnier M, Papazian L, Forel JM. Sedation and neuromuscular blocking agents in acute respiratory distress syndrome. Ann Transl Med. 2017;5:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amato MB, Meade MO, Slutsky AS,, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–755. [DOI] [PubMed] [Google Scholar]
- 40.Naguib M, Brull SJ, Johnson KB. Conceptual and technical insights into the basis of neuromuscular monitoring. Anaesthesia. 2017;72suppl 116–37. [DOI] [PubMed] [Google Scholar]
- 41.Hemmerling TM, Le N. Brief review: neuromuscularmonitoring: an update for the clinician. Can J Anaesth. 2007;54:58–72. [DOI] [PubMed] [Google Scholar]
- 42.Bowdle A, Bussey L, Michaelsen K, et al. Counting train-of-four twitch response: comparison of palpation to mechanomyography, acceleromyography, and electromyography. Br J Anaesth. 2020;124:7122–717. [DOI] [PubMed] [Google Scholar]
- 43.deBacker J, Hart N, Fan E. Neuromuscular blockadein the 21st century managementof the critically ill patient. Chest. 2017;151:697–706. [DOI] [PubMed] [Google Scholar]
- 44.Chambers D, Paulden M, Paton F,, et al. Sugammadex for reversal of neuromuscular block after rapid sequence intubation: a systematic review and economic assessment. Br J Anaesth. 2010;105:568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reeves JJ, Hollandsworth HM, Torriani FJ, et al. Rapid response to COVID-19: health informatics support for outbreak management in an academic health system. J Am Med Inform Assoc. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu X, Yang J, Yu L, Long D. Plasma miRNA-223 correlates with risk, inflammatory markers as well as prognosis in sepsis patients. Medicine (Baltimore). 2018;97:e11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Millot G, Voisin B, Loiez C, Wallet F, Nseir S. The next generation of rapid point-of-care testing identification tools for ventilator-associated pneumonia. Ann Transl Med. 2017;5:451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ganau L, Prisco L, Ligarotti GKI, et al. Understanding the pathological basisof neurological diseases through diagnostic platforms basedon innovationsin biomedical engineering: new conceptsand theranostics perspectives. Medicines (Basel). 2018;5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen T, Duong Bang D, Wolff A. 2019 Novel Coronavirus Disease (COVID-19): pavingthe road for rapid detectionand point-of-care diagnostics. Micromachines (Basel). 2020;11:E306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahn SJ, Baek YH, Lloren KKS. Rapid and simple colorimetric detection of multiple influenza viruses infecting humans using a reverse transcriptional loop-mediated isothermal amplification (RT-LAMP) diagnostic platform. BMC Infect Dis. 2019;19:676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leidi F, Casella F, Cogliati C. Bedside lung ultrasound in the evaluation of acute decompensated heart failure. Intern Emerg Med. 2016;11:597–601. [DOI] [PubMed] [Google Scholar]
- 53.Lichtenstein D, Mézière G, Biderman P, Gepner A, Barré O. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med. 1997;156:1640–1646. [DOI] [PubMed] [Google Scholar]
- 54.Ganau M, Ligarotti GK, Apostolopoulos V. Real-time intraoperative ultrasound in brain surgery: neuronavigation and use of contrast-enhanced image fusion. Quant Imaging Med Surg. 2019;9:350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Long L, Zhao HT, Zhang ZY, Wang GY, Zhao HL. Lung ultrasound for the diagnosis of pneumonia in adults: ameta-analysis. Medicine (Baltimore). 2017;96:e5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parlamento S, Copetti R, Di Bartolomeo S. Evaluation of lung ultrasound for the diagnosis of pneumonia in the ED. Am J Emerg Med. 2009;27:379–384. [DOI] [PubMed] [Google Scholar]
- 57.Gargani L. Lung ultrasound: a new tool for the cardiologist. Cardiovasc Ultrasound. 2011;9:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvementin a patient withCoronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bouhemad B, Brisson H, Le-Guen M, Arbelot C, Lu Q, Rouby JJ. Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment. Am J Respir Crit Care Med. 2011;183:341–347. [DOI] [PubMed] [Google Scholar]
- 60.Rocco M, Carbone I, Morelli A. Diagnostic accuracy of bedside ultrasonography in the ICU: feasibility of detecting pulmonary effusion and lung contusion in patients on respiratory support after severe blunt thoracic trauma. Acta Anaesthesiol Scand. 2008;52:776–784. [DOI] [PubMed] [Google Scholar]
- 61.Canty DJ, Royse CF, Kilpatrick D, Williams DL, Royse AG. The impact of pre-operative focused transthoracic echocardiography in emergency non-cardiac surgery patients with known or risk of cardiac disease. Anaesthesia. 2012;67:714–720. [DOI] [PubMed] [Google Scholar]
- 62.Suriani RJ, Neustein S, Shore-Lesserson L, Konstadt S. Intraoperative transesophageal echocardiography during noncardiac surgery. J Cardiothorac Vasc Anesth. 1998;12:274–280. [DOI] [PubMed] [Google Scholar]
- 63.Schulmeyer C, Farías J, Rajdl E, de La Maza J, Labbé M. Utility of transesophageal echocardiography during severe hypotension in non-cardiac surgery. Rev Bras Anestesiol. 2010;60:513–521. [DOI] [PubMed] [Google Scholar]
- 64.Hofer CK, Zollinger A, Rak M. Therapeutic impact of intra-operative transoesophageal echocardiography during noncardiac surgery. Anaesthesia. 2004;59:3–9. [DOI] [PubMed] [Google Scholar]
- 65.Cowie B. Three years’ experience of focused cardiovascular ultrasound in the peri-operative period. Anaesthesia. 2011;66:268–273. [DOI] [PubMed] [Google Scholar]
- 66.Galicinao J, Bush AJ, Godambe SA. Use of bedside ultrasonography for endotracheal tube placement in pediatric patients: a feasibility study. Pediatrics. 2007;120:1297–1303. [DOI] [PubMed] [Google Scholar]
- 67.Ramsingh D, Frank E, Haughton R. Auscultation versus point-of-care ultrasoundto determine endotrachealversus bronchial intubation: a diagnostic accuracy study. Anesthesiology. 2016;124:1012–1020. [DOI] [PubMed] [Google Scholar]
- 68.Gekle R., Dubensky L, Haddad S, et al. Can bedside sonography replace conventional radiographyfor confirmationof above-the-diaphragm central venous catheter placement? J Ultrasound Med. 2015;34:1295–1299. [DOI] [PubMed] [Google Scholar]
- 69.NICE guidelines Venous thromboembolism: reducing the risk for patients in hospital. Available at: https://www.nice.org.uk/guidance/cg92.
- 70.Rodelo JR, De la Rosa G, Valencia ML. D-dimer is a significant prognostic factor in patients with suspected infection and sepsis. Am J Emerg Med. 2012;30:1991–1999. [DOI] [PubMed] [Google Scholar]
- 71.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia J Thromb Haemost. 2020;18:844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.SARS-CoV-2 und Handhabung von Dräger-Anästhesiearbeitsplätzen (20 February 2020/updated 18.03.2020).
- 73.COVID-19: Einsatz von Dräger Oxylog Notfall- und Transportbeatmungsgeräten zur Langzeitbeatmung (24.03.2020).
- 74.Kundeninformation: Einsatz von Löwenstein Medical Anästhesiegeräten LEON/LEONmri/LEONplus zur Langzeitbeatmung bei COVID-19 (20.03.2020).
- 75.GE Healthcare, Datex-Ohmeda, Inc. Statement: COVID-19 - Requests for information regarding the off-label use of GE Healthcare anaesthesia devices for ICU ventilation (23.03.2020).
- 76.APSF/ASA Guidance on Purposing Anesthesia Machines as ICU Ventilators. Available at: https://www.asahq.org/in-the-spotlight/coronavirus-covid-19-information/purposing-anesthesia-machines-for-ventilators.
- 77.Joint statement on multiple patients per ventilator. Available at:https://www.apsf.org/news-updates/joint-statement-on-multiple-patients-per-ventilator/Accessed March 26, 2020.
- 78.APSF/ASA Guidance on Purposing anaesthesia Machines as ICU Ventilators (26.03.2020)
- 79.Please insert the missing reference details here.
- 80.Please insert the missing reference details here.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


