Abstract
Background
Due to the complicated molecular and cellular heterogeneity in hepatocellular carcinoma (HCC), the morbidity and mortality still remains high level in the world. However, the number of novel metabolic biomarkers and prognostic models could be applied to predict the survival of HCC patients is still small. In this study, we constructed a metabolic gene signature by systematically analyzing the data from The Cancer Genome Atlas (TCGA), Gene Expression Omnibus (GEO) and International Cancer Genome Consortium (ICGC).
Methods
Differentially expressed genes (DEGs) between tumors and paired non-tumor samples of 50 patients from TCGA dataset were calculated for subsequent analysis. Univariate cox proportional hazard regression and LASSO analysis were performed to construct a gene signature. The Kaplan–Meier analysis, time-dependent receiver operating characteristic (ROC), Univariate and Multivariate Cox regression analysis, stratification analysis were used to assess the prognostic value of the gene signature. Furthermore, the reliability and validity were validated in four types of testing cohorts. Moreover, the diagnostic capability of the gene signature was investigated to further explore the clinical significance. Finally, Go enrichment analysis and Gene Set Enrichment Analysis (GSEA) have been performed to reveal the different biological processes and signaling pathways which were active in high risk or low risk group.
Results
Ten prognostic genes were identified and a gene signature were constructed to predict overall survival (OS). The gene signature has demonstrated an excellent ability for predicting survival prognosis. Univariate and Multivariate analysis revealed the gene signature was an independent prognostic factor. Furthermore, stratification analysis indicated the model was a clinically and statistically significant for all subgroups. Moreover, the gene signature demonstrated a high diagnostic capability in differentiating normal tissue and HCC. Finally, several significant biological processes and pathways have been identified to provide new insights into the development of HCC.
Conclusion
The study have identified ten metabolic prognostic genes and developed a prognostic gene signature to provide more powerful prognostic information and improve the survival prediction for HCC.
Keywords: Hepatocellular carcinoma, Bioinformatics, Gene signature, Metabolism, Survival, Diagnosis, Prognosis, Biomarker
Introduction
Primary liver cancer is the seventh most commonly occurring cancer in 2018, and the second most common cause of cancer mortality worldwide. The overall 5-year survival of patients with liver cancer is currently 10–20%. Among them, HCC accounts for most of the primary liver cancer (75–85%), which is characterized by high invasiveness, high metastasis potential and low survival rate (Bray et al., 2018; Yu et al., 2017). The situation is even more serious in China; liver cancer has a new incidence of 370,000 in 2015, ranking fourth in the number of malignant tumors, 326,000 deaths, and second in the number of deaths (Zheng et al., 2019). However, there is still a lack of effective biomarkers for prediction of high recurrence populations, death risk and target therapies. Thus, identification of effective biomarker for the prognosis of HCC is urgent for the diagnosis and treatment of HCC.
Traditional serum markers have been proved as potential tumor markers for prognostic in HCC, such as alpha-fetoprotein (AFP) (Hanazaki et al., 2001). However, AFP is only elevated in about half of the HCC patients and significant tumor burden limits its usefulness in screening and operable therapy (Tangkijvanich et al., 2000). C-reactive protein (CRP) and Platelet lymphocyte ratio (PLR) could be considered as tumor markers for low-AFP HCC patients (Suner et al., 2019a), and possess parameter values for tumor growth and invasiveness (Suner et al., 2019b). However, the effects of CRP or PLR on survival reveal unclear. What’s more, traditional prognosis markers for HCC only focused on single biomarker, including enzymes and isoenzymes, growth factors and their receptors, tumor-associated antigens, microRNAs (miRNAs) and long noncoding RNAs (lncRNAs) (Mann et al., 2007; Singhal et al., 2012; Yu et al., 2007; Yu, Chen & Ding, 2010), which may lack sensitivity and specificity. With the development of high-throughput technologies, many new potential biomarkers are easier and the gene prognostic signature is more likely to generated for prognosis in HCC.
Altered cellular metabolism plays a key role for cancerous cells, and cancerous cell metabolism reprograming is considered the novel hallmark of cancer in the future (Hanahan & Weinberg, 2011). Previous studies have indicated that metabolism alteration could promote cell proliferation and progression, and XR, Xu et al. (2001) shown that the transcription level of metabolic genes has changed in HCC. Thus several metabolism-related genes may play a role in the occurrence and development of HCC. However, the number of novel metabolic biomarkers and prognostic models could be applied to predict the survival of HCC patients is still small. Jiang et al. (2019) have constructed a glycolysis gene prognostic signature of HCC, but only one validation cohort have been used to prove the performance of the predicted model, and it lacks of comparison of performance with other different biomarker. Benfeitas et al. (2019) built a four-gene survival signature of HCC; systematic analysis is required to further prove the predicted value. Inspired by all these works, our research combined with clinically significant metabolic genes to make a gene prognosis model, which could provide better guidance for the survival and prognosis of HCC.
Method
Identification and acquisition of TCGC, GEO and ICGC data
Gene expression profiles and clinical data associating with HCC were identified and acquired from TCGA project (https://cancergenome.nih.gov/), GEO database (https://www.ncbi.nlm.nih.gov/gds/) and ICGC project (https://icgc.org/). A total of 415 candidates (365 patients and 50 normal candidates) and the clinical information (age, gender, grade, stage, myometrial invasion, lymph node status, distant metastasis status) were identified and acquired from TCGA dataset. A total of 220 patients and the clinical information (age, gender, ALT, AFP, stage) were identified and acquired from GEO database. A total of 243 patients and the clinical information (gender, age, stage, prior Malignancy) were identified and acquired from ICGC dataset.
Patients characteristics and grouping
A total of 365 patients from TCGA dataset, used as the training cohort. Using the R package Caret, with the ratio of 1:1 in a random manner, 184 patients from TCGA dataset were selected as the internal testing cohort. A total of 243 patients from ICGC dataset and 220 patients from GSE14520 dataset, used as the external testing cohorts. Finally, we integrated all the 828 patients from the TCGA, GEO and ICGC datasets to use as the entire testing cohort. The clinical information of the four cohorts is summarized in Table 1.
Table 1. Summary of patient demographics and clinical characteristics.
| Characteristic | Training cohort | Internal testing cohort | GSE14520 testing cohort | ICGC testing cohort | Entire testing cohort |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 246 (67%) | 121 (66%) | 191 (87%) | 182 (75%) | 619 (75%) |
| Female | 119 (33%) | 63 (34%) | 29 (13%) | 61 (25%) | 209 (25%) |
| Age | |||||
| <60 | 165 (45%) | 83 (45%) | 177 (80%) | 45 (18%) | 387 (47%) |
| >=60 | 200 (55%) | 101 (55%) | 43 (20%) | 198 (82%) | 441 (53%) |
| Stage | |||||
| Stage I–II | 254 (74%) | 136 (78%) | 170 (77%) | 146 (60%) | 570 (70%) |
| Stage III–IV | 88 (26%) | 38 (22%) | 50 (23%) | 97 (40%) | 235 (30%) |
| Vital status | |||||
| Living | 239 (65%) | 125 (68%) | 135 (61%) | 199 (82%) | 573 (69%) |
| Dead | 126 (35%) | 59 (32%) | 85 (39%) | 45 (18%) | 255 (31%) |
Identification of metabolism related genes
Metabolism-related genes in the KEGG pathway associated with metabolism were screened from the GSEA (http://software.broadinstitute.org/gsea/index.jsp), and the overlapping metabolism-related genes were identified from TCGA, GSE14520, and ICGC gene expression profile.
Prognostic genes were identified and gene signature were constructed by utilizing training cohort
Using the Limma version 3.36.2 R package, DEGs were calculated between tumors and paired non-tumor samples of 50 patients from TCGA dataset, the adjusted P-value < 0.05 and absolute log2 fold change (FC) > 1.5 were considered as the selection criterion. By using the R package survival and Coxph function, Univariate Cox proportional hazard regression analysis was performed to discover the prognostic genes in the training cohort, with the adjusted P-value < 0.05 as the significance cutoff. We further narrowed the gene range to construct a gene signature by performing LASSO analysis, R package glmnet was used to perform LASSO analysis.
The performance of gene signature
With the risk-formula: Risk score = expression of gene1 × β1gene1 + expression of gene2 × β2gene2 +…… expression of genen × βngenen, patients in training cohort were divided into low or high risk group basing on the median risk score. The expression level was compared between low and high risk group. Kaplan–Meier analysis was performed to compare the survival rate between the two groups. ROC curve analysis for OS was performed to assess the clinically predictive ability of the gene signature. Next, Univariate and Multivariate Cox proportional hazards analysis were performed to investigate whether the gene signature could be independent of other clinical parameters, including age, gender, grade, stage, myometrial invasion, lymph node status, distant metastasis status. Furthermore, stratification analysis were used to assess the prognostic value of the gene signature in different subgroups stratified clinical variables. Moreover, the diagnostic capability of the gene signature was investigated to further explore the clinical significance, including differentiating normal tissue and HCC, different stages and grades.
Validation of the gene signature
Internal testing cohort, GSE14520 testing cohort, ICGC testing cohort and entire testing cohort were used to validate the reliability and validity of the gene signature. According to the risk-formula and the median risk score, the patients from the four testing cohorts were divided into low or high risk group. And the same analyses were performed to validate the performance, including Kaplan–Meier analysis, the ROC curve analysis, Univariate and Multivariate Cox proportional hazards analysis, stratification analysis.
Go enrichment analysis and Gene set enrichment analysis
Metabolism related DEGs were identified between high- and low-risk groups, with corrected P-value < 0.05 and absolute log fold change (FC) > 1.5 being considered as the cutoff criterion. Next, gene ontology processes were considered as enriched by using the DAVID database (https://david.ncifcrf.gov/). Furthermore, we generated an ordered list of all genes according to their correlation with two subtypes and elucidated the significant survival difference between high- and low-risk groups by GSEA. A total of 1,000 times were performed for gene set permutations. The nominal P value was used to sort the pathways enriched in each phenotype.
Statistical analysis
P < 0.05 was considered statistically significant, The statistical analyses were conducted by employing the R (version 3.4.3) and GraphPad Prism 7.
Result
Identification of prognostic genes
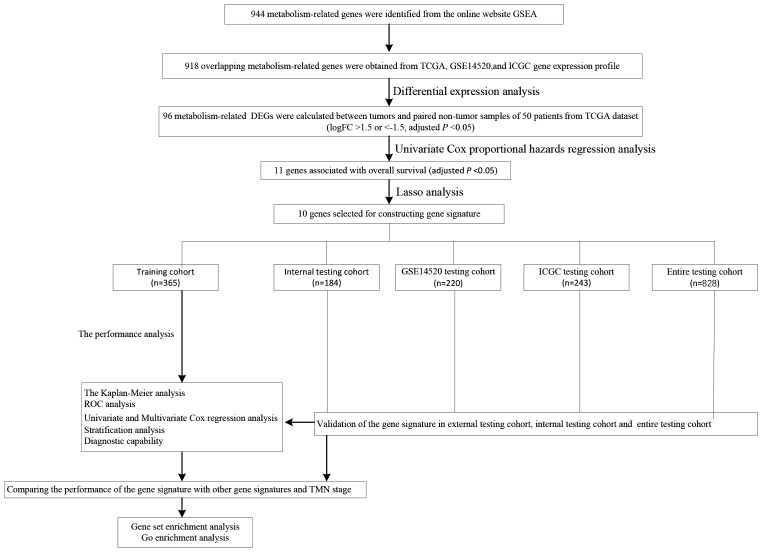
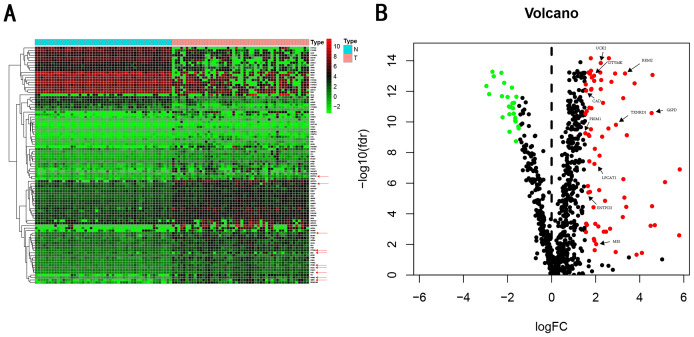
We carry on our study as described in the flow chart (Fig. 1). A total of 944 metabolism-related genes were identified from the online website GSEA (Table S1) and 918 overlapping metabolism-related genes were obtained between TCGA, GSE14520 and ICGC gene expression profile (Table S2). A total of 71 up-regulated genes and 25 down-regulated genes were calculated for subsequent analysis (Fig. 2; Table S3). By conducting Univariate Cox proportional hazards regression analysis, 11 significant genes associated with OS were obtained for further analysis (adjusted P < 0.05) (Table S4).
Figure 1. The schematic workflow of the study.
Figure 2. Heatmap and Volcano plot of metabolism-related DEGs.
(A) Heatmap of metabolism-related DEGs. Red indicates that the gene expression is relatively high, green indicates that the gene expression is relatively low, and white indicates no significant changes in gene expression (FDR < 0.05, absolute log FC > 1.5). A total of 10 prognostic genes were marked using red arrow. (B) Volcano plot of metabolism-related DEGs. The red points represent high expression genes, the green points represent low expression genes, the black points represent genes with no significant difference (FDR < 0.05, absolute log FC > 1.5). A total of 10 prognostic genes were marked using black arrow.
Establishment of gene signature from the training cohort
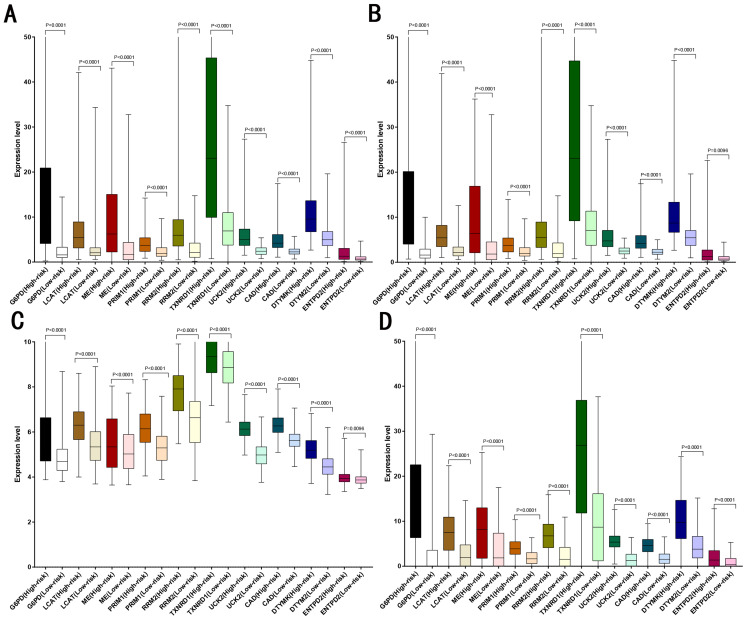
LASSO analysis was performed to narrow the gene range to construct a ten-gene signature from 11 significant genes (Risk-formula: Risk score = expression of G6PD × 0.0015558631423743 + expression of LPCAT1 × 0.00310356967612766 + expression of ME1 × 0.00632401235412166 + expression of PRIM1 × 0.0026285256475841 + expression of RRM2 × 0.00979851158278189 + expression of TXNRD1 × 0.00783495109084 + expression of UCK2 × 0.057281553558209 + expression of CAD × 0.06164749118803 + expression of DTYMK × 0.00849922964649245 + expression of ENTPD2 × 0.03691738437542). The risk score was computed for each patient in the training cohort, 365 patients from training cohort were divided into low risk group (183 patients) and high risk group (182 patients) according to the median risk score: 0.73. A total of 243 patients from ICGC testing cohort were divided into low risk group (100 patients) and high risk group (143 patients). A total of 220 patients in GSE14520 testing cohort were divided into low risk group (112 patients) and high group (108 patients). A total of 184 patients in the internal testing cohort were divided into low risk group (95 patients) and high risk group (89 patients), 828 patients in the entire testing cohort were divided into low risk group (356 patients) and high risk group (432 patients). The 10 prognostic genes expression level distribution between low and high risk group of the training cohort was showed in Fig. 3A, the expression of ten prognostic genes in high risk group was higher than in low risk group, the result was consisted with internal testing cohort in Fig. 3B, GSE14520 testing cohort in Fig. 3C and ICGC testing cohort in Fig. 3D.
Figure 3. Expression of the ten genes in low- and high-risk groups of training cohort, internal testing cohort, GSE14520 testing cohort and ICGC testing cohort.
(A) Training cohort. (B) Internal testing cohort. (C) GSE14520 testing cohort. (D) ICGC testing cohort.
The performance of gene signature
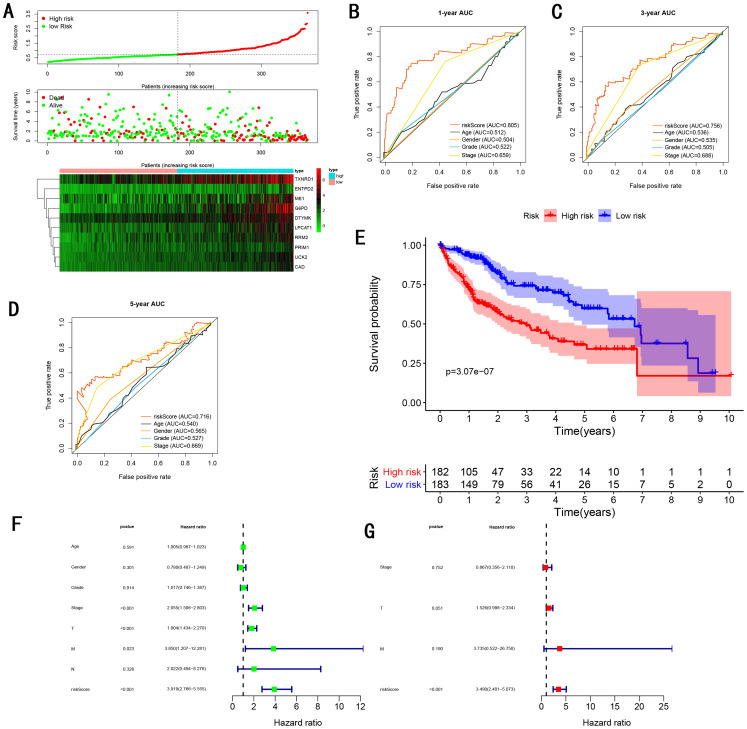
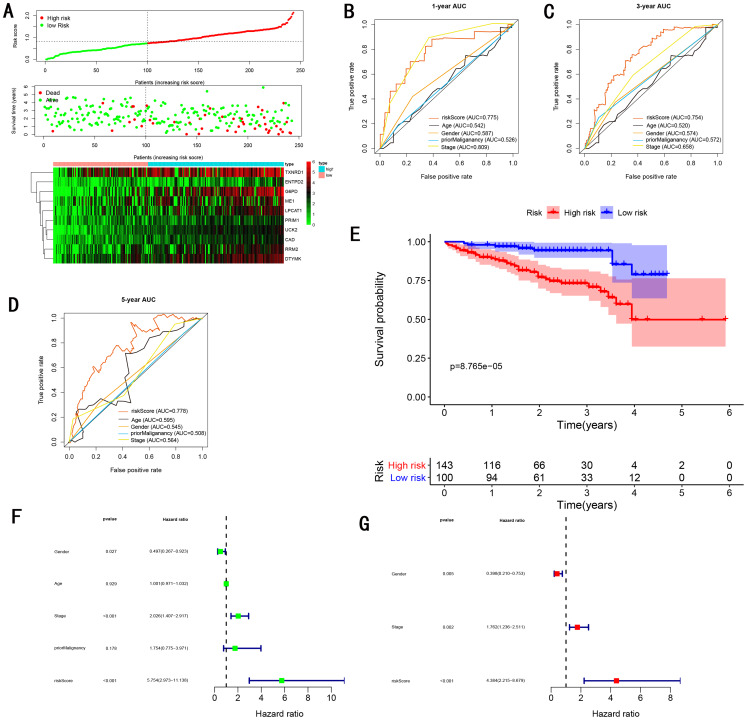
As showed in Fig. 4A, with the increasing risk score, patients in the training cohort have a worse OS, the expression ten prognostic genes increased. The ROC curve was presented to assess the clinically predictive ability of the gene signature, the AUC for 1-year (Fig. 4B), 3-year (Fig. 4C), and 5-year (Fig. 4D) OS were 0.805, 0.756, 0.716 for training cohort, which was higher than other clinical characteristics, including age (0.512, 0.536, 0.540), gender (0.504, 0.535, 0.565), grade (0.522, 0.505, 0.527), stage (0.659, 0.688, 0.669). Besides, we found the gene signature also performed more specific and sensitive than any single gene (Table 2). The OS in the training cohort was significantly different between low and high risk group, in the 1-year, 3-year and 5-year, the OS in the high risk group were 0.576, 0.181, 0.076, the OS in the low risk group were 0.814, 0.306, 0.142, the result indicated that patients with a high-risk score have more poor OS than the patients with low-risk score (P < 0.001), detail was presented in Fig. 4E. By conducting Univariate and Multivariate Cox regression analysis, we noted that gene signature have a significant correlation with worse OS, the HR of the gene signature was 3.919 (95% CI [2.766–5.555]) with P-value < 0.001 in Univariate Cox regression analysis (Fig. 4F), 3.490 (95% CI [2.401–5.073]) with P-value < 0.001 in Multivariate Cox regression analysis (Fig. 4G), thus the gene signature was an independent prognostic factor of other clinical variables.
Figure 4. Gene signature performance analysis using training cohort.
(A) Distribution of 10‐gene‐based risk scores, patient survival durations, gene expression levels. (B) One-year ROC curve analyses of gene signature and clinical parameters. (C) Three-year ROC curve analyses of gene signature and clinical parameters. (D) Five-year ROC curve analyses of gene signature and clinical parameters. (E) Kaplan–Meier curves of OS based on gene signature. (F) Prognostic value detection of the gene signature via univariate survival-related analysis. (G) Prognostic value detection of the gene signature via multivariate survival-related analysis.
Table 2. Comparison of the AUC between gene signature and single gene.
| Characteristic | Training cohort | Internal testing cohort | GSE14520 testing cohort | ICGC testing cohort | Entire testing cohort |
|---|---|---|---|---|---|
| Risk score | 0.786 | 0.773 | 0.707 | 0.775 | 0.732 |
| G6PD | 0.738 | 0.741 | 0.575 | 0.698 | 0.665 |
| LPCAT1 | 0.708 | 0.680 | 0.514 | 0.722 | 0.663 |
| ME1 | 0.630 | 0.642 | 0.514 | 0.597 | 0.586 |
| PRIM1 | 0.706 | 0.748 | 0.534 | 0.707 | 0.634 |
| RRM2 | 0.716 | 0.754 | 0.501 | 0.698 | 0.655 |
| TXNRD1 | 0.674 | 0.663 | 0.532 | 0.611 | 0.599 |
| UCK2 | 0.736 | 0.699 | 0.650 | 0.711 | 0.695 |
| CAD | 0.743 | 0.726 | 0.602 | 0.688 | 0.676 |
| DTYMK | 0.689 | 0.722 | 0.534 | 0.734 | 0.641 |
| ENTPD2 | 0.552 | 0.546 | 0.503 | 0.662 | 0.580 |
Validation of the gene signature
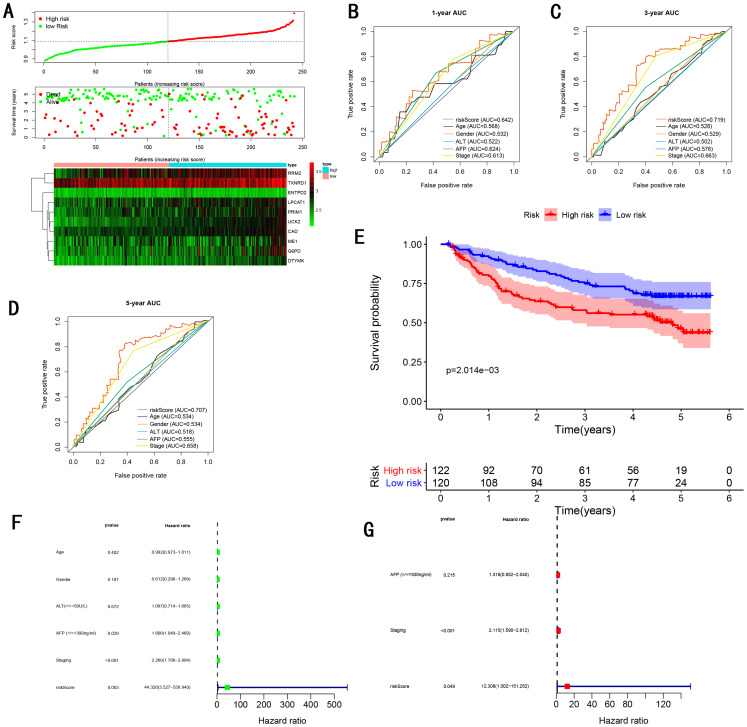
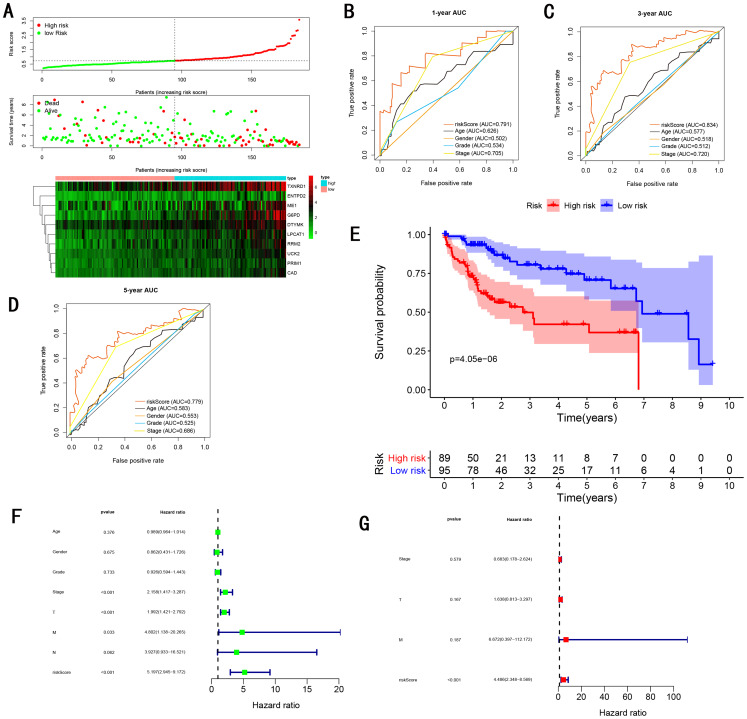
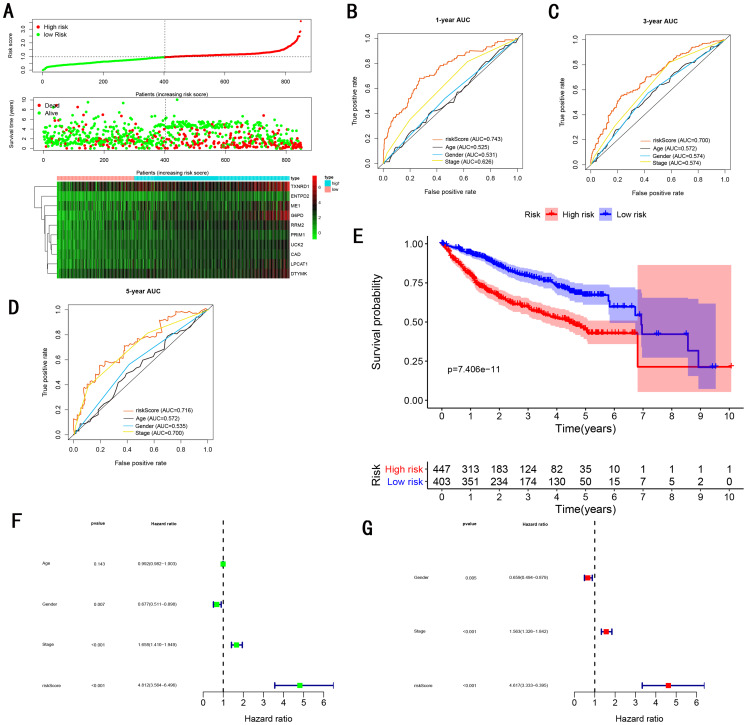
To validate the predictive ability in different HCC populations, we applied the gene signature to ICGC testing cohort, the result was similar to the training cohort. Figure 5A showed the distribution of risk scores for each patients, patients in high risk group had a worse OS than patients with a low-risk group. In addition, the AUC for 1-year (Fig. 5B), 3-year (Fig. 5C), and 5-year (Fig. 5D) OS were 0.775, 0.754, 0.778, which was higher than other clinical characteristics, including age (0.542, 0.520, 0.595), gender (0.587, 0.574, 0.545), prior Maliganancy (0.526, 0.572, 0.508). Even though the AUC of gene signature was a little less than the TNM stage at 1-year OS (0.775 vs 0.809), the AUC of gene signature was much larger than the TNM stage at 3-year OS (0.754 vs 0.658), 5-year OS (0.778 vs 0.564). The AUC of ROC for gene signature was obviously greater than single gene (Table 2). Moreover, the OS for patients in the high risk group was 0.576 at 1-year, 0.181 at 3-year, 0.076 at 5-year, compared with 0.814, 0.306, 0.142 in the low risk group (P < 0.001, Fig. 5E). Further Univariate Cox regression analysis and Multivariate Cox regression analysis displayed gene signature was a powerful and independent factor in external testing cohort (Figs. 5F and 5G). For GSE14520 testing cohort (Fig. 6), internal testing cohort (Fig. 7) and entire testing cohort (Fig. 8), the gene signature had the similar predictive ability. The distribution of risk scores, gene expression were evaluated in GSE14520 testing cohort (Fig. 6A), internal testing cohort (Fig. 7A) and entire testing cohort (Fig. 8A). The ROC curve demonstrated that gene signature was more specific and sensitive than any clinical characteristics and any single gene in GSE14520 testing cohort (Figs. 6B–6D; Table 2), internal testing cohort (Figs. 7B–7D; Table 2) and entire testing cohort (Figs. 8B–8D; Table 2). Patients in a high-risk group have poorer OS than the patients in low-risk group for GSE14520 testing cohort (Fig. 6E), internal testing cohort (Fig. 7E), entire testing cohort (Fig. 8E) (P < 0.001). Univariate and Multivariate Cox regression analysis indicated the gene signature was an independent prognostic factor for GSE14520 testing cohort (Figs. 6F and 6G), internal testing cohort (Figs. 7F and 7G) and entire testing cohort (Figs. 8F and 8G).
Figure 5. Gene signature performance analysis using ICGC testing cohort.
(A) Distribution of 10‐gene‐based risk scores, patient survival durations, gene expression levels. (B) One-year ROC curve analyses of gene signature and clinical parameters. (C) Three-year ROC curve analyses of gene signature and clinical parameters. (D) Five-year ROC curve analyses of gene signature and clinical parameters. (E) Kaplan–Meier curves of OS based on gene signature. (F) Prognostic value detection of the gene signature via univariate survival-related analysis. (G) Prognostic value detection of the gene signature via multivariate survival-related analysis.
Figure 6. Gene signature performance analysis using GSE14520 testing cohort.
(A) Distribution of 10‐gene‐based risk scores, patient survival durations, gene expression levels. (B) One-year ROC curve analyses of gene signature and clinical parameters. (C) Three-year ROC curve analyses of gene signature and clinical parameters. (D) Five-year ROC curve analyses of gene signature and clinical parameters. (E) Kaplan–Meier curves of OS based on gene signature. (F) Pognostic value detection of the gene signature via univariate survival-related analysis. (G) Prognostic value detection of the gene signature via multivariate survival-related analysis.
Figure 7. Gene signature performance analysis using internal testing cohort.
(A) Distribution of 10‐gene‐based risk scores, patient survival durations, gene expression levels. (B) One-year ROC curve analyses of gene signature and clinical parameters. (C) Three-year ROC curve analyses of gene signature and clinical parameters. (D) Five-year ROC curve analyses of gene signature and clinical parameters. (E) Kaplan–Meier curves of OS based on gene signature. (F) Prognostic value detection of the gene signature via univariate survival-related analysis. (G) Prognostic value detection of the gene signature via multivariate survival-related analysis.
Figure 8. Gene signature performance analysis using entire testing cohort.
(A) Distribution of 10‐gene‐based risk scores, patient survival durations, gene expression levels. (B) One-year ROC curve analyses of gene signature and clinical parameters. (C) Three-year ROC curve analyses of gene signature and clinical parameters. (D) Five-year ROC curve analyses of gene signature and clinical parameters. (E) Kaplan–Meier curves of OS based on gene signature. (F) Prognostic value detection of the gene signature via univariate survival-related analysis. (G) Prognostic value detection of the gene signature via multivariate survival-related analysis.
Stratification analysis
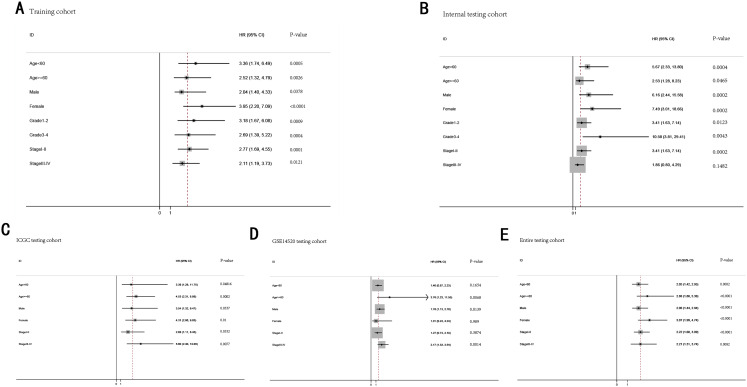
To further demonstrate the clinical significance of the gene signature in HCC, we perform the survival analysis stratified by clinical variables (age, gender, grade, stage) in training cohort and internal testing cohort, by clinical covariates (age, gender, stage) in ICGC testing cohort, GSE14520 testing cohort and entire testing cohort. Patients of stage I–II, stage III–IV, grade 1–2, grade 3–4, age <60, age >=60, female, male were stratified into high risk group and low risk group. The log-rank test indicated that HCC patients in high risk group still had obviously worse OS than patients in low risk group for training cohort (Fig. 9A), internal testing cohort (Fig. 9B), ICGC testing cohort (Fig. 9C), GSE14520 testing cohort (Fig. 9D) and entire testing cohort (Fig. 9E), and the high-risk patients of subgroup subdivided by the signature had poorer survival than the low-risk patients in training cohort (Figs. S1A–S1H), internal testing cohort (Figs. S1I–S1P), GSE14520 testing cohort (Figs. S2A–S2C, S2E and S2F), ICGC testing cohort (Figs. S2G–S2L) and entire testing cohort (Figs. S3A–S3F). There was no different trend between high and low risk group for female patients in GSE14520 testing cohort, small number female patients may be an important reason. Interestingly, subgroup stage I, stage II, stage III in entire testing cohort were stratified into high risk group and low risk group, HCC patients in high risk group had poorer OS than patients in low risk group (Figs. S3G–S3I).
Figure 9. The predictive performance of the gene signature on OS in different subgroups stratified by clinical parameters.
(A) Training cohort. (B) Internal testing cohort. (C) ICGC testing cohort. (D) GSE14520 testing cohort. (E) Entire testing cohort.
Bidkhori et al. (2018) published a article that was proposing 3 sub-types of HCC, including iHCC1, iHCC2 and iHCC3. They reported that the expression of iHCC3 tumors are markedly distinct from those of iHCC2 and iHCC1, and a larger number of genes are differentially expressed between iHCC3 and iHCC1/iHCC2 compared with iHCC1 vs iHCC2. Consistent with the result, we revealed that the expression level of majority of genes of the gene signature were higher in iHCC3 than iHCC1/iHCC2, and the expression level of iHCC1 was similar to iHCC2 (Fig. S4).
Diagnostic capability
The gene signature have been validated the predictive ability in different HCC populations. To further explore diagnostic capability of the gene signature, we compared risk score between normal liver and HCC in training cohort (Fig. S5A, P < 0.0001), ICGC testing cohort (Fig. S5B, P < 0.0001) and GSE14520 testing cohort (Fig. S5C, P < 0.0001), the risk score in HCC was obviously higher in than normal liver. The AUC of ROC curve was 0.98 in training cohort (Fig. S6A), 0.77 in ICGC testing cohort (Fig. S6B) and 0.97 in GSE14520 testing cohort (Fig. S6C), which revealed the strong diagnostic capability for HCC. In addition, we also investigated the distribution of high-risk and low-risk patients in different stages and grades, the proportion of high risk patients is higher in advanced tumor grade (grade 3–4 ) than early tumor grade (grade 1–2) in training cohort (Fig. S5D). The proportion of high risk patients is higher in late stage (TNM III–IV) than early stage (TNM I–II) in training cohort (Fig. S5E), ICGC testing cohort (Fig. S5F) and GSE14520 testing cohort (Fig. S5G). Furthermore, as the TNM stage and tumor grade increased, the risk score increased (Figs. S5H–S5K). For early and advanced tumor grade in training cohort (Figs. S6D and S6E), early and advanced TNM stage in training cohort (Figs. S6F and S6G), ICGC testing cohort (Figs. S6H and S6I), GSE14520 testing cohort (Figs. S6J and S6K), the AUC of ROC curve indicated the modest diagnostic capability.
Comparing the performance of the gene signature with other gene signatures and TMN stage
TMN stage is still significant to predict the survival of HCC patients. In the training cohort, we found the AUC of gene signature was larger than the TNM stage at 1-year, 3-year, 5-year (0.805 vs 0.659 at 1-year, 0.756 vs 0.688 at 3-year, 0.716 vs 0.669 at 5-year ) (Figs. 4B–4D), the result was consistent with GSE14520 testing cohort (Figs. 6B–6D), internal testing cohort (Figs. 7B–7D) and entire testing cohort (Figs. 8B–8D). For ICGC testing cohort (Figs. 5B–5D), even though the AUC of gene signature was a little less than the TNM stage at 1-year OS (0.775 vs 0.809), the AUC of gene signature was much larger than the TNM stage at 3-year OS (0.754 vs 0.658), 5-year OS (0.778 vs 0.564). Thus we believed the gene signature was more specific and sensitive than TNM stage. Next, we also compared the AUC of ROC between the gene signature and all single genes, the AUC of the gene signature was larger than any single gene (Table 2). Moreover, we further compared the gene signature with other gene signatures, the ROC analysis indicated our model had better performance, 0.805 at 1-year, 0.756 at 3-year, 0.716 at 5-year, the AUC of Long et al. (2018) model is 0. 7674, 0.7040, 0.6919 at 1, 3 and 5-year, the AUC of Qiao et al. (2019) model is 0.71, 0.69 at 3 and 5-year, the AUC of Li et al. (2017) model is 0.67, 0.67 at 3 and 5-year, the AUC of Xiao-Hong Xiang et al. (2019) model is 0.708, 0.699, 0.678 at 1, 3 and 5-year, the AUC of Li et al. (2017) model is 0.727, 0.709, 0.604 at 1, 3 and 5-year, the AUC of Yan et al. (2019) model is 0.712, 0.661 at 1, 3-year, indicating that the model had a high sensitivity and specificity for predict the survival.
Go enrichment analysis and Gene set enrichment analysis
We performed a differential expression analysis between low and high risk group to reveal the association between the metabolic genes and two subtypes. We identified 18 upregulated and 14 downregulated genes between patients in the low vs high risk group. Go enrichment analysis revealed that upregulated genes in high risk group were mainly involved in cell cycle regulation, such as DNA replication, chromatid/chromosome segregation and regulation, spindle organization and recombination. Upregulated genes in low risk group were mainly involved metabolic and energy regulation, including metabolism of amino acids, oxidative phosphorylation, fatty acid β oxidation and catabolism (Fig. S7A).
In order to further explore the mechanism of prognostic genes in patients with hepatocellular carcinoma, we conducted GSEA between low and high risk group to identify the significant pathways (FDR < 0.05, NOM P-value < 0.05). The most meaningful pathway were identified according to the FDR standard, we uncovered the most meaning pathways which were active in the high-risk group, including KEGG_CELL_CYCLE, KEGG_NUCLEOTIDE_EXCISION_REPAIR, KEGG_OOCYTE_MEIOSIS, KEGG_PURINE_METABOLISM, KEGG_PYRIMIDINE_METABOLISM. And the most meaning pathways which were active in the low-risk group, including KEGG_DRUG_METABOLISM_CYTOCHROME_P450, KEGG_FATTY_ACID_METABOLISM, KEGG_GLYCINE_SERINE_AND_THREONINE_METABOLISM, KEGG_PRIMARY_BILE_ACID_BIOSYNTHESIS, KEGG_VALINE_LEUCINE_AND_ISOLEUCINE_DEGRADATION. The result was consistent with go enrichment analysis, significant pathways which were active in high risk group were mainly related with cell cycle regulation. In contrast, meaningful pathways which were active in low risk group were mainly associated with metabolic and energy regulation (Fig. S7B).
Discussion
Due to the complicated molecular and cellular heterogeneity in HCC, the morbidity and mortality still remains high level in the world. Novel prognostic biomarkers to predict the survival of HCC patients is urgently needed. Metabolism reprograming is considered the novel hallmark of cancer in the future. However, the number of novel metabolic biomarkers and prognostic models could be applied to predict the survival of HCC patients is still small. In this study, we constructed a metabolic gene signature by performing training cohort from TCGA dataset, the gene signature showed a strong prognostic performance for predicting the survival of HCC patients. Compared with previous studies and TNM stage, the model possessed a higher sensitivity and specificity. Meantime, the gene signature was an independent prognostic factor of other clinical variables, which showed a high value of HR. The prognostic value of the gene signature was validated by performing internal testing cohort, ICGC testing cohort, GSE14520 testing cohort and entire testing cohort. Furthermore, the gene signature demonstrated a high diagnostic capability in differentiating normal tissue and HCC, and showed a modest diagnostic capability in early and advanced TNM stage, early and advanced grade. Finally, several significant biological processes signaling pathways underlying hepatocellular carcinoma were identified for further validation.
We identified ten risky prognostic genes (CAD, DTYMK, ENTPD2, G6PD, ME1, RRM2, TXNRD1, UCK2, LPCAT1, PRIM1). CDA gene encodes a 243-kDa multifunctional protein, consists of carbamyl phosphate synthetase (CPSase), aspartate transcarbamylase (ATCase), glutamine amidotransferase (GLNase), dihydroorotase (DHOase) (Kim, Kelly & Evans, 1992). CDA is the main participant in de novo pyrimidine synthesis, which is very important to provide malignant cells and proliferating cells with nucleotides for DNA replication (Aoki & Weber, 1981; Fairbanks et al., 1995). Therefore, the upregulation of CAD may be considered as a prognosis biomarker and therapeutic target. Uhlen et al. (2015) have reported that the expression level of CAD is high, Morin et al. (2012) indicated the expression level of CAD was associated with local tumor extension and cancer relapse and identified CAD as a potential predictive marker of cancer relapse, Sigoillot, Sigoillot & Guy (2004) have showed the intracellular CAD concentration was 3.5- to 4-fold higher in MCF7 cells than that in normal MCF10A breast cells, and MAP kinase activity and a nonclassical ERalpha/Sp1-mediated pathway may account for the high CAD level (Khan et al., 2003). Previous study have indicated the expression level of CAD is higher in hepatoma carcinoma cell than normal liver cell, however, The prognostic value of CAD for hepatocellular carcinoma has not been validated. DTYMK is a nuclear-encoded deoxythymidylate kinase, which expressed in all tissues and participate in the activity of dTTP production (Caspi et al., 2016), and is a key part for DNA synthesis. Liu et al. (2013b) have indicated the expression of DTYMK is increased in lung adenocarcinomas in comparison to normal lung, and identified elevated DTYMK expression as an unfavorable predictor. Yeh et al. (2017) found the DTYMK was a poor prognostic factor in HCC. DTYMK was observed in the 5-FU resistant colon cancer cells, which may provide a new therapy for the HCC by applying the 5-FU combination therapy. ENTPD2 belongs to ENTPD family (Chiu et al., 2017), have reported only ENTPD1 plays an important role in cancer, however, discloses that ENTPD2 is harnessed by cancer cells to escape immune-mediated destruction and the expression level of ENTPD2 was also high in HCC patients, and the high expression of ENTPD2 was associated with direct liver invasion, tumor microsatellite formation and venous invasion, as well as the absence of tumor encapsulation. The pentose phosphate pathway belongs to major carbohydrate pathways, it could produce ribose and NADPH to protect and promote cells proliferation in hypoxic conditions, such characteristic meets the demand of malignant proliferating cells, therefor, the change of the pentose phosphate pathway may be the landmark of cancer (Sacoman et al., 2012). G6PD is the rate-controlling enzyme of pentose phosphate pathway, previous researches have reported G6PD gene is an oncogene and the expression level upregulates in bladder cancer (Ohl et al., 2006), ESCC (Wang et al., 2016), breast cancer (Pu et al., 2015), Sun et al. (2014) indicated G6PD-deficient women have reduced breast cancer risk, Zhang et al. (2017) indicated overexpression of G6PD increases the risk of colon cancer, Wang et al. (2012a) reported G6PD could promote the progression of gastric cancer cells and is associated with poor clinical outcome for patients with gastric cancer. Munemoto et al. (2019) activated G6PD gene to accelerates carcinogenesis and cancer progression. Previous studies have detected the G6PD is overexpressed in HCC (Li et al., 2012; Xu et al., 2014), and Gao et al. (2017) found G6PD WAS significantly changed by using sequential window acquisition of all theoretical mass spectra (SWATH-MS), Hu H et al found the cell migration and invasion ability decreased when the expression of G6PD was downregulated. miR122 and miR-1 suppress the expression of G6PD to inhibit tumor growth through inhibiting the activity of PPP in hepatocellular cancer (Barajas et al., 2018). Zhao et al. (2018) found G6PD promotes migration and invasion of hepatocellular carcinoma cells through inducing epithelial-mesenchymal transition by activating of transcription 3 (STAT3) pathway. ME1 is multifunctional protein, which relates glycolytic and citric acid cycles. ME1 plays an important role in tumor development, it have reported that the expression level of ME1 is high in various cancers and promotes growth and metastasis, including colorectal cancer, 39 breast cancer (Liao et al., 2018), bladder cancer (Liu et al., 2018), gastric cancer (Lu et al., 2018), nasopharyngeal carcinoma (Zheng et al., 2012). Several studies indicates that ME1 is associated with poor prognosis in hepatocellular carcinomas and OSCC (Knoblich et al., 2014; Wen et al., 2015), ME1 promotes HCC metastasis through influencing epithelial-mesenchymal transition (EMT) processes (Wen et al., 2015), and ME1 also could reduce the sensibility of radiation (Chakrabarti, 2015; Woo et al., 2016). As a vital subunit of rate-limiting catalyzes (ribonucleotide reductase) (RNR), which is necessary for DNA replication and DNA damage repair (Aye et al., 2015). RRM2 was reported to be associated with various cancers, including ovarian cancer (Wang et al., 2012b), bladder cancer (Morikawa et al., 2010b), colorectal cancers (Liu et al., 2013a; Lu et al., 2012), gastric cancer (Morikawa et al., 2010a). Meantime, several showed up-regulated RRM2 promotes tumorigenesis, proliferation, and inhibits apoptosis, and is associated with poor prognosis (Das et al., 2019; Kolberg et al., 2017; Liang et al., 2019; Liu et al., 2013a; Souglakos et al., 2008; Wang et al., 2012b). It is known that RRM2 promotes drug resistance in various cancers (Goan et al., 1999; Nakano et al., 2007; Shah et al., 2014). Thus RRM2 is consider a novel drug target (Aye et al., 2015; Minami et al., 2015) including Trans-4,4′-Dihydroxystilbene (Chen et al., 2019), COH29 (Chen et al., 2015), GW8510 (Hsieh et al., 2016). For hepatocellular carcinoma, many bioinformatics analysis indicated RRM2 is a clinical prognostic markers (Dawany, Dampier & Tozeren, 2011; He et al., 2017; Wu et al., 2019). Lee et al. (2014) showed the expression level of RRM2 is up-regulated and RRM2 is a significant marker for predicting clinical prognosis. Several drug may target RRM2 to suppress hepatocellular carcinoma cells (Gao et al., 2013; Kosakowska-Cholody et al., 2009). Cancerous cells have to face the increased oxidative stress due to the high metabolism and metabolic disorders, the activation of glutathione (GSH) and thioredoxin (TXN) systems could compensate the severe stress (Arner & Holmgren, 2006), thus cancerous cells overactivated GSH and TXN systems to adapt to the oxidative stress, the cytosolic TXN reductase 1 (TXNRD1) is a vital part of thioredoxin (TXN) system, which is up-regulated in various cancers (Cadenas et al., 2010; Hughes et al., 2018; Lincoln et al., 2003) and high expression is associated with poor clinical prognosis in multiply types of cancers (Bhatia et al., 2016; Leone et al., 2017). In addition, TXNRD1 is also consider as a drug target with high efficacy and low toxicity. Recently, several studies found that the expression of TXNRD1 is high (Cadenas et al., 2010; Cho et al., 2019; Fu et al., 2017) and is related with poorer clinicopathological features, meantime, inhibition of TXNRD1 Inhibits the development and progression of hepatocellular carcinoma cells (Lee et al., 2019a). UCK2 gene encodes uridine-cytidine kinase 2, which plays vital role in biosynthesis of the pyrimidine nucleotide (Schumacher et al., 2013; Tomoike et al., 2017). It have reported that UCK2 is overexpressed in multiple type of cancers and is associated with poor prognosis, including breast cancer, pancreatic cancer, colon cancer. Yu et al. (2019) showed that high UCK2 expression is associated with clinicopathologic feature and is a independent marker for predicting OS and RFS in hepatocellular carcinoma, the expression level may be influenced by the methylation of status cg0927774. In addition, knockdown of UCK2 suppressed proliferation, migration and invasion (Huang et al., 2019), UCK2 may promotes HCC cell progress through stat3 signaling pathway. The alterations of membrane phospholipid levels could influence membrane fluidity and facilitate metastases because they affect motility, basement membrane invasion, and adhesion (Taraboletti et al., 1989). LPCAT1 is a cytosolic enzyme that converts lysophosphatidylcholine (LPC) to phosphatidylcholine (PC). As an important subtype belongs to the 4 LPCAT subtypes (Shindou & Shimizu, 2009), LPCAT1 has been obtained much attention for cancers, LPCAT1 could contribute the progression, metastasis, and recurrence of cancer. To date, LPCAT1 over-expression have been reported in multiple types of cancers, including cell renal cell carcinoma (Du et al., 2017), gastric cancer (Uehara et al., 2016), breast cancer (Abdelzaher & Mostafa, 2015), oral squamous cell carcinoma (Shida-Sakazume et al., 2015), hepatocellular carcinoma (Morita et al., 2013). The DNA primase polypeptide 1 (PRIM1) is responsible for synthesizing small RNA primers for Okazaki fragments generated during discontinuous DNA replication, the DNA replication cannot proceed without the catalytic function of PRIM1, thus PRIM1 is an vital role in the initiation (priming) of the DNA replication, and its aberrations may play a key tumorigenic factor by affecting the cell cycle transition from G1 to S phase (Yotov et al., 1999). So far, PRIM1 have been reported be associated with the formation of breast cancer (Lee et al., 2019b).
Several significant biological process and signaling pathway which were active in high risk or low risk group have been revealed to provide a new insights of the development of HCC. We have divided patients from training cohort into two subtypes by performing our gene signature. Next, we conducted differential expression analysis between high and low group to the association between the metabolic genes and two subtypes. We found that upregulated genes in high risk group were mainly involved in cell cycle regulation, and upregulated genes in low risk group were mainly involved metabolic and energy regulation. Likely, GSEA analysis have demonstrated the similar result. Significant pathways which were active in high risk group were mainly related with cell cycle regulation. In contrast, meaningful pathways which were active in low risk group were mainly associated with metabolic and energy regulation.
Conclusion
The study identified ten prognostic genes that participate aberrant metabolism in HCC, and developed a metabolic ten-gene signature which provides more powerful prognostic information and improve the survival prediction for HCC. We could also score each patient according to the metabolic nine-gene signature and thus identified high-risk HCC patients. The prognostic ability of ten-gene signature was validated by internal testing cohort, GSE14520 testing cohort, ICGC testing cohort, entire testing cohort. Moreover, several significant biological process and signaling pathway have been identified to provide a new insights of the development of HCC. However, further biological experiments should performed to validate our results.
Supplemental Information
(A-B) Age <60 and >=60 in training cohort (C-D) Male and female in training cohort (E-F) Grade 1-2 and grade 3-4 in training cohort (G-H) Stage I-II and stage III-IV in training cohort (I-J) Age <60 and >=60 in internal testing cohort (K-L) Male and female in internal testing cohort (M-N) Grade 1-2 and grade 3-4 in internal testing cohort (O-P) Stage I-II and stage III-IV in internal testing cohort
(A-B) Age <60 and >=60 in GSE14520 testing cohort (C-D) Male and female in GSE14520 testing cohort (E-F) Stage I-II and stage III-IV in GSE14520 testing cohort (G-H) Age <60 and >=60 in ICGC testing cohort (I-J) Male and female in ICGC testing cohort (K-L) Stage I-II and stage III-IV in ICGC testing cohort
(A-B) Age <60 and >=60 in entire testing cohort (C-D) Male and female in entire testing cohort (E-F) Stage I-II and stage III-IV in entire testing cohort (G-I) Stage I, stage II and stage III in entire testing cohort
The risk score was grouped by (A-C) Tissue type (H) Tumor grade (I-K) TNM stage. The distribution of high-risk and low-risk patients in different stages and grades (D) Tumor grade (E-G) TNM stage A, D, E, H, I were from training cohort. B, F, J were from ICGC testing cohort. C, G, K were from GSE14520 testing cohort
The capacity in differentiating between normal and HCC(A-C), different grade (D-E), different stage ( F-K). A, D, E, F, G were from training cohort. B,H,I were from ICGC testing cohort. C,J,K were from GSE14520 testing cohort
(A) Biological processes (B) KEGG signaling pathway
Acknowledgments
We are grateful to the reviewers for their constructive comments which led to improvements in this manuscript. In addition, thanks to Bin Zhao (Official Wechat Account: Bio_Med2017) of Xiamen University and Hexin Lin of Fujian Medical University for suggestions on the manuscripts.
Funding Statement
This work was supported by Xiamen Scientific and Technological Plan (No. 3502Z20194005, 3502Z20184020). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Zhipeng Zhu conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Lulu Li performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Jiuhua Xu performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Weipeng Ye performed the experiments, prepared figures and/or tables, and approved the final draft.
Borong Chen analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Junjie Zeng performed the experiments, prepared figures and/or tables, and approved the final draft.
Zhengjie Huang conceived and designed the experiments, performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The data and code files are available as Supplemental Files.
The raw data is available from TCGA (search terms: HCC), NCBI GEO (GSE14520) and from the ICGC project (https://icgc.org/; specific datasets used: “Liver Cancer - NCC, JP”).
References
- Abdelzaher & Mostafa (2015).Abdelzaher E, Mostafa MF. Lysophosphatidylcholine acyltransferase 1 (LPCAT1) upregulation in breast carcinoma contributes to tumor progression and predicts early tumor recurrence. Tumour Biology. 2015;36(7):5473–5483. doi: 10.1007/s13277-015-3214-8. [DOI] [PubMed] [Google Scholar]
- Aoki & Weber (1981).Aoki T, Weber G. Carbamoyl phosphate synthetase (glutamine-hydrolyzing): increased activity in cancer cells. Science. 1981;212(4493):463–465. doi: 10.1126/science.7209543. [DOI] [PubMed] [Google Scholar]
- Arner & Holmgren (2006).Arner ES, Holmgren A. The thioredoxin system in cancer. Seminars in Cancer Biology. 2006;16(6):420–426. doi: 10.1016/j.semcancer.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Aye et al. (2015).Aye Y, Li M, Long MJ, Weiss RS. Ribonucleotide reductase and cancer: biological mechanisms and targeted therapies. Oncogene. 2015;34(16):2011–2021. doi: 10.1038/onc.2014.155. [DOI] [PubMed] [Google Scholar]
- Barajas et al. (2018).Barajas JM, Reyes R, Guerrero MJ, Jacob ST, Motiwala T, Ghoshal K. The role of miR-122 in the dysregulation of glucose-6-phosphate dehydrogenase (G6PD) expression in hepatocellular cancer. Scientific Reports. 2018;8(1):9105. doi: 10.1038/s41598-018-27358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfeitas et al. (2019).Benfeitas R, Bidkhori G, Mukhopadhyay B, Klevstig M, Arif M, Zhang C, Lee S, Cinar R, Nielsen J, Uhlen M, Boren J, Kunos G, Mardinoglu A. Characterization of heterogeneous redox responses in hepatocellular carcinoma patients using network analysis. EBioMedicine. 2019;40:471–487. doi: 10.1016/j.ebiom.2018.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia et al. (2016).Bhatia M, McGrath KL, Di Trapani G, Charoentong P, Shah F, King MM, Clarke FM, Tonissen KF. The thioredoxin system in breast cancer cell invasion and migration. Redox Biology. 2016;8:68–78. doi: 10.1016/j.redox.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidkhori et al. (2018).Bidkhori G, Benfeitas R, Klevstig M, Zhang C, Nielsen J, Uhlen M, Boren J, Mardinoglu A. Metabolic network-based stratification of hepatocellular carcinoma reveals three distinct tumor subtypes. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(50):E11874–E11883. doi: 10.1073/pnas.1807305115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray et al. (2018).Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Cadenas et al. (2010).Cadenas C, Franckenstein D, Schmidt M, Gehrmann M, Hermes M, Geppert B, Schormann W, Maccoux LJ, Schug M, Schumann A, Wilhelm C, Freis E, Ickstadt K, Rahnenfuhrer J, Baumbach JI, Sickmann A, Hengstler JG. Role of thioredoxin reductase 1 and thioredoxin interacting protein in prognosis of breast cancer. Breast Cancer Research. 2010;12(3):R44. doi: 10.1186/bcr2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi et al. (2016).Caspi R, Billington R, Ferrer L, Foerster H, Fulcher CA, Keseler IM, Kothari A, Krummenacker M, Latendresse M, Mueller LA, Ong Q, Paley S, Subhraveti P, Weaver DS, Karp PD. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Research. 2016;44(D1):D471–480. doi: 10.1093/nar/gkv1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti (2015).Chakrabarti G. Mutant KRAS associated malic enzyme 1 expression is a predictive marker for radiation therapy response in non-small cell lung cancer. Radiation Oncology. 2015;10(1):145. doi: 10.1186/s13014-015-0457-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2019).Chen CW, Li Y, Hu S, Zhou W, Meng Y, Li Z, Zhang Y, Sun J, Bo Z, DePamphilis ML, Yen Y, Han Z, Zhu W. DHS (trans-4,4′-dihydroxystilbene) suppresses DNA replication and tumor growth by inhibiting RRM2 (ribonucleotide reductase regulatory subunit M2) Oncogene. 2019;38(13):2364–2379. doi: 10.1038/s41388-018-0584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2015).Chen MC, Zhou B, Zhang K, Yuan YC, Un F, Hu S, Chou CM, Chen CH, Wu J, Wang Y, Liu X, Smith DL, Li H, Liu Z, Warden CD, Su L, Malkas LH, Chung YM, Hu MC, Yen Y. The novel ribonucleotide reductase inhibitor COH29 inhibits DNA repair in vitro. Molecular Pharmacology. 2015;87(6):996–1005. doi: 10.1124/mol.114.094987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu et al. (2017).Chiu DK, Tse AP, Xu IM, Di Cui J, Lai RK, Li LL, Koh HY, Tsang FH, Wei LL, Wong CM, Ng IO, Wong CC. Hypoxia inducible factor HIF-1 promotes myeloid-derived suppressor cells accumulation through ENTPD2/CD39L1 in hepatocellular carcinoma. Nature Communications. 2017;8(1):517. doi: 10.1038/s41467-017-00530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho et al. (2019).Cho SY, Kim S, Son MJ, Rou WS, Kim SH, Eun HS, Lee BS. Clinical significance of the thioredoxin system and thioredoxin-domain-containing protein family in hepatocellular carcinoma. Digestive Diseases and Sciences. 2019;64(1):123–136. doi: 10.1007/s10620-018-5307-x. [DOI] [PubMed] [Google Scholar]
- Das et al. (2019).Das B, Roy J, Jain N, Mallick B. Tumor suppressive activity of PIWI-interacting RNA in human fibrosarcoma mediated through repression of RRM2. Molecular Carcinogenesis. 2019;58(3):344–357. doi: 10.1002/mc.22932. [DOI] [PubMed] [Google Scholar]
- Dawany, Dampier & Tozeren (2011).Dawany NB, Dampier WN, Tozeren A. Large-scale integration of microarray data reveals genes and pathways common to multiple cancer types. International Journal of Cancer. 2011;128(12):2881–2891. doi: 10.1002/ijc.25854. [DOI] [PubMed] [Google Scholar]
- Du et al. (2017).Du Y, Wang Q, Zhang X, Wang X, Qin C, Sheng Z, Yin H, Jiang C, Li J, Xu T. Lysophosphatidylcholine acyltransferase 1 upregulation and concomitant phospholipid alterations in clear cell renal cell carcinoma. Journal of Experimental & Clinical Cancer Research. 2017;36(1):66. doi: 10.1186/s13046-017-0525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks et al. (1995).Fairbanks LD, Bofill M, Ruckemann K, Simmonds HA. Importance of ribonucleotide availability to proliferating T-lymphocytes from healthy humans: disproportionate expansion of pyrimidine pools and contrasting effects of de novo synthesis inhibitors. Journal of Biological Chemistry. 1995;270(50):29682–29689. doi: 10.1074/jbc.270.50.29690. [DOI] [PubMed] [Google Scholar]
- Fu et al. (2017).Fu B, Meng W, Zeng X, Zhao H, Liu W, Zhang T. TXNRD1 is an unfavorable prognostic factor for patients with hepatocellular carcinoma. Biomed Research International. 2017;2017(6):4698167. doi: 10.1155/2017/4698167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao et al. (2013).Gao J, Chen H, Yu Y, Song J, Song H, Su X, Li W, Tong X, Qian W, Wang H, Dai J, Guo Y. Inhibition of hepatocellular carcinoma growth using immunoliposomes for co-delivery of adriamycin and ribonucleotide reductase M2 siRNA. Biomaterials. 2013;34(38):10084–10098. doi: 10.1016/j.biomaterials.2013.08.088. [DOI] [PubMed] [Google Scholar]
- Gao et al. (2017).Gao Y, Wang X, Sang Z, Li Z, Liu F, Mao J, Yan D, Zhao Y, Wang H, Li P, Ying X, Zhang X, He K, Wang H. Quantitative proteomics by SWATH-MS reveals sophisticated metabolic reprogramming in hepatocellular carcinoma tissues. Scientific Reports. 2017;7(1):45913. doi: 10.1038/srep45913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goan et al. (1999).Goan YG, Zhou B, Hu E, Mi S, Yen Y. Overexpression of ribonucleotide reductase as a mechanism of resistance to 2,2-difluorodeoxycytidine in the human KB cancer cell line. Cancer Research. 1999;59:4204–4207. [PubMed] [Google Scholar]
- Hanahan & Weinberg (2011).Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hanazaki et al. (2001).Hanazaki K, Kajikawa S, Koide N, Adachi W, Amano J. Prognostic factors after hepatic resection for hepatocellular carcinoma with hepatitis C viral infection: univariate and multivariate analysis. American Journal of Gastroenterology. 2001;96(4):1243–1250. doi: 10.1111/j.1572-0241.2001.03634.x. [DOI] [PubMed] [Google Scholar]
- He et al. (2017).He B, Yin J, Gong S, Gu J, Xiao J, Shi W, Ding W, He Y. Bioinformatics analysis of key genes and pathways for hepatocellular carcinoma transformed from cirrhosis. Medicine (Baltimore) 2017;96(25):e6938. doi: 10.1097/MD.0000000000006938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh et al. (2016).Hsieh YY, Chou CJ, Lo HL, Yang PM. Repositioning of a cyclin-dependent kinase inhibitor GW8510 as a ribonucleotide reductase M2 inhibitor to treat human colorectal cancer. Cell Death Discovery. 2016;2(1):16027. doi: 10.1038/cddiscovery.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang et al. (2019).Huang S, Li J, Tam NL, Sun C, Hou Y, Hughes B, Wang Z, Zhou Q, He X, Wu L. Uridine-cytidine kinase 2 upregulation predicts poor prognosis of hepatocellular carcinoma and is associated with cancer aggressiveness. Molecular Carcinogenesis. 2019;58(4):603–615. doi: 10.1002/mc.22954. [DOI] [PubMed] [Google Scholar]
- Hughes et al. (2018).Hughes DJ, Kunicka T, Schomburg L, Liska V, Swan N, Soucek P. Expression of selenoprotein genes and association with selenium status in colorectal adenoma and colorectal cancer. Nutrients. 2018;10(11):1812. doi: 10.3390/nu10111812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang et al. (2019).Jiang L, Zhao L, Bi J, Guan Q, Qi A, Wei Q, He M, Wei M, Zhao L. Glycolysis gene expression profilings screen for prognostic risk signature of hepatocellular carcinoma. Aging. 2019;11(23):10861–10882. doi: 10.18632/aging.102489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan et al. (2003).Khan S, Abdelrahim M, Samudio I, Safe S. Estrogen receptor/Sp1 complexes are required for induction of cad gene expression by 17beta-estradiol in breast cancer cells. Endocrinology. 2003;144(6):2325–2335. doi: 10.1210/en.2002-0149. [DOI] [PubMed] [Google Scholar]
- Kim, Kelly & Evans (1992).Kim H, Kelly RE, Evans DR. The structural organization of the hamster multifunctional protein CAD: controlled proteolysis, domains, and linkers. Journal of Biological Chemistry. 1992;267:7177–7184. [PubMed] [Google Scholar]
- Knoblich et al. (2014).Knoblich K, Wang HX, Sharma C, Fletcher AL, Turley SJ, Hemler ME. Tetraspanin TSPAN12 regulates tumor growth and metastasis and inhibits beta-catenin degradation. Cellular and Molecular Life Sciences. 2014;71(7):1305–1314. doi: 10.1007/s00018-013-1444-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolberg et al. (2017).Kolberg M, Bruun J, Murumagi A, Mpindi JP, Bergsland CH, Holand M, Eilertsen IA, Danielsen SA, Kallioniemi O, Lothe RA. Drug sensitivity and resistance testing identifies PLK1 inhibitors and gemcitabine as potent drugs for malignant peripheral nerve sheath tumors. Molecular Oncology. 2017;11(9):1156–1171. doi: 10.1002/1878-0261.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosakowska-Cholody et al. (2009).Kosakowska-Cholody T, Cholody WM, Hariprakasha HK, Monks A, Kar S, Wang M, Michejda CJ, Carr BI. Growth inhibition of hepatocellular carcinoma cells in vitro and in vivo by the 8-methoxy analog of WMC79. Cancer Chemotherapy and Pharmacology. 2009;63(5):769–778. doi: 10.1007/s00280-008-0801-z. [DOI] [PubMed] [Google Scholar]
- Lee et al. (2014).Lee B, Ha SY, Song DH, Lee HW, Cho SY, Park CK. High expression of ribonucleotide reductase subunit M2 correlates with poor prognosis of hepatocellular carcinoma. Gut and Liver. 2014;8(6):662–668. doi: 10.5009/gnl13392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al. (2019a).Lee D, Xu IM, Chiu DK, Leibold J, Tse AP, Bao MH, Yuen VW, Chan CY, Lai RK, Chin DW, Chan DF, Cheung TT, Chok SH, Wong CM, Lowe SW, Ng IO, Wong CC. Induction of oxidative stress through inhibition of thioredoxin reductase 1 is an effective therapeutic approach for hepatocellular carcinoma. Hepatology. 2019a;69(4):1768–1786. doi: 10.1002/hep.30467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al. (2019b).Lee WH, Chen LC, Lee CJ, Huang CC, Ho YS, Yang PS, Ho CT, Chang HL, Lin IH, Chang HW, Liu YR, Wu CH, Tu SH. DNA primase polypeptide 1 (PRIM1) involves in estrogen-induced breast cancer formation through activation of the G2/M cell cycle checkpoint. International Journal of Cancer. 2019b;144(3):615–630. doi: 10.1002/ijc.31788. [DOI] [PubMed] [Google Scholar]
- Leone et al. (2017).Leone A, Roca MS, Ciardiello C, Costantini S, Budillon A. Oxidative stress gene expression profile correlates with cancer patient poor prognosis: identification of crucial pathways might select novel therapeutic approaches. Oxidative Medicine and Cellular Longevity. 2017;2017:2597581. doi: 10.1155/2017/2597581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2017).Li B, Feng W, Luo O, Xu T, Cao Y, Wu H, Yu D, Ding Y. Development and validation of a three-gene prognostic signature for patients with hepatocellular carcinoma. Scientific Reports. 2017;7(1):5517. doi: 10.1038/s41598-017-04811-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2012).Li C, Ruan HQ, Liu YS, Xu MJ, Dai J, Sheng QH, Tan YX, Yao ZZ, Wang HY, Wu JR, Zeng R. Quantitative proteomics reveal up-regulated protein expression of the SET complex associated with hepatocellular carcinoma. Journal of Proteome Research. 2012;11(2):871–885. doi: 10.1021/pr2006999. [DOI] [PubMed] [Google Scholar]
- Liang et al. (2019).Liang WH, Li N, Yuan ZQ, Qian XL, Wang ZH. DSCAM-AS1 promotes tumor growth of breast cancer by reducing miR-204-5p and up-regulating RRM2. Molecular Carcinogenesis. 2019;58(4):461–473. doi: 10.1002/mc.22941. [DOI] [PubMed] [Google Scholar]
- Liao et al. (2018).Liao R, Ren G, Liu H, Chen X, Cao Q, Wu X, Li J, Dong C. ME1 promotes basal-like breast cancer progression and associates with poor prognosis. Scientific Reports. 2018;8(1):16743. doi: 10.1038/s41598-018-35106-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln et al. (2003).Lincoln DT, Ali Emadi EM, Tonissen KF, Clarke FM. The thioredoxin-thioredoxin reductase system: over-expression in human cancer. Anticancer Research. 2003;23:2425–2433. [PubMed] [Google Scholar]
- Liu et al. (2018).Liu M, Chen Y, Huang B, Mao S, Cai K, Wang L, Yao X. Tumor-suppressing effects of microRNA-612 in bladder cancer cells by targeting malic enzyme 1 expression. International Journal of Oncology. 2018;52:1923–1933. doi: 10.3892/ijo.2018.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2013a).Liu X, Zhang H, Lai L, Wang X, Loera S, Xue L, He H, Zhang K, Hu S, Huang Y, Nelson RA, Zhou B, Zhou L, Chu P, Zhang S, Zheng S, Yen Y. Ribonucleotide reductase small subunit M2 serves as a prognostic biomarker and predicts poor survival of colorectal cancers. Clinical Science. 2013a;124(9):567–578. doi: 10.1042/CS20120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2013b).Liu Y, Marks K, Cowley GS, Carretero J, Liu Q, Nieland TJ, Xu C, Cohoon TJ, Gao P, Zhang Y, Chen Z, Altabef AB, Tchaicha JH, Wang X, Choe S, Driggers EM, Zhang J, Bailey ST, Sharpless NE, Hayes DN, Patel NM, Janne PA, Bardeesy N, Engelman JA, Manning BD, Shaw RJ, Asara JM, Scully R, Kimmelman A, Byers LA, Gibbons DL, Wistuba II, Heymach JV, Kwiatkowski DJ, Kim WY, Kung AL, Gray NS, Root DE, Cantley LC, Wong KK. Metabolic and functional genomic studies identify deoxythymidylate kinase as a target in LKB1-mutant lung cancer. Cancer Discovery. 2013b;3(8):870–879. doi: 10.1158/2159-8290.CD-13-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long et al. (2018).Long J, Zhang L, Wan X, Lin J, Bai Y, Xu W, Xiong J, Zhao H. A four-gene-based prognostic model predicts overall survival in patients with hepatocellular carcinoma. Journal of Cellular and Molecular Medicine. 2018;22(12):5928–5938. doi: 10.1111/jcmm.13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu et al. (2012).Lu AG, Feng H, Wang PX, Han DP, Chen XH, Zheng MH. Emerging roles of the ribonucleotide reductase M2 in colorectal cancer and ultraviolet-induced DNA damage repair. World Journal of Gastroenterology. 2012;18(34):4704–4713. doi: 10.3748/wjg.v18.i34.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu et al. (2018).Lu YX, Ju HQ, Liu ZX, Chen DL, Wang Y, Zhao Q, Wu QN, Zeng ZL, Qiu HB, Hu PS, Wang ZQ, Zhang DS, Wang F, Xu RH. ME1 regulates NADPH homeostasis to promote gastric cancer growth and metastasis. Cancer Research. 2018;78(8):1972–1985. doi: 10.1158/0008-5472.CAN-17-3155. [DOI] [PubMed] [Google Scholar]
- Mann et al. (2007).Mann CD, Neal CP, Garcea G, Manson MM, Dennison AR, Berry DP. Prognostic molecular markers in hepatocellular carcinoma: a systematic review. European Journal of Cancer. 2007;43(6):979–992. doi: 10.1016/j.ejca.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Minami et al. (2015).Minami K, Shinsato Y, Yamamoto M, Takahashi H, Zhang S, Nishizawa Y, Tabata S, Ikeda R, Kawahara K, Tsujikawa K, Chijiiwa K, Yamada K, Akiyama S, Perez-Torras S, Pastor-Anglada M, Furukawa T, Yasuo T. Ribonucleotide reductase is an effective target to overcome gemcitabine resistance in gemcitabine-resistant pancreatic cancer cells with dual resistant factors. Journal of Pharmacological Sciences. 2015;127(3):319–325. doi: 10.1016/j.jphs.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Morikawa et al. (2010a).Morikawa T, Hino R, Uozaki H, Maeda D, Ushiku T, Shinozaki A, Sakatani T, Fukayama M. Expression of ribonucleotide reductase M2 subunit in gastric cancer and effects of RRM2 inhibition in vitro. Human Pathology. 2010a;41(12):1742–1748. doi: 10.1016/j.humpath.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Morikawa et al. (2010b).Morikawa T, Maeda D, Kume H, Homma Y, Fukayama M. Ribonucleotide reductase M2 subunit is a novel diagnostic marker and a potential therapeutic target in bladder cancer. Histopathology. 2010b;57(6):885–892. doi: 10.1111/j.1365-2559.2010.03725.x. [DOI] [PubMed] [Google Scholar]
- Morin et al. (2012).Morin A, Fritsch L, Mathieu JR, Gilbert C, Guarmit B, Firlej V, Gallou-Kabani C, Vieillefond A, Delongchamps NB, Cabon F. Identification of CAD as an androgen receptor interactant and an early marker of prostate tumor recurrence. FASEB Journal. 2012;26(1):460–467. doi: 10.1096/fj.11-191296. [DOI] [PubMed] [Google Scholar]
- Morita et al. (2013).Morita Y, Sakaguchi T, Ikegami K, Goto-Inoue N, Hayasaka T, Hang VT, Tanaka H, Harada T, Shibasaki Y, Suzuki A, Fukumoto K, Inaba K, Murakami M, Setou M, Konno H. Lysophosphatidylcholine acyltransferase 1 altered phospholipid composition and regulated hepatoma progression. Journal of Hepatology. 2013;59(2):292–299. doi: 10.1016/j.jhep.2013.02.030. [DOI] [PubMed] [Google Scholar]
- Munemoto et al. (2019).Munemoto M, Mukaisho KI, Miyashita T, Oyama K, Haba Y, Okamoto K, Kinoshita J, Ninomiya I, Fushida S, Taniura N, Sugihara H, Fujimura T. Roles of the hexosamine biosynthetic pathway and pentose phosphate pathway in bile acid-induced cancer development. Cancer Science. 2019;110:2408–2420. doi: 10.1111/cas.14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano et al. (2007).Nakano Y, Tanno S, Koizumi K, Nishikawa T, Nakamura K, Minoguchi M, Izawa T, Mizukami Y, Okumura T, Kohgo Y. Gemcitabine chemoresistance and molecular markers associated with gemcitabine transport and metabolism in human pancreatic cancer cells. British Journal of Cancer. 2007;96(3):457–463. doi: 10.1038/sj.bjc.6603559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohl et al. (2006).Ohl F, Jung M, Radonic A, Sachs M, Loening SA, Jung K. Identification and validation of suitable endogenous reference genes for gene expression studies of human bladder cancer. Journal of Urology. 2006;175(5):1915–1920. doi: 10.1016/S0022-5347(05)00919-5. [DOI] [PubMed] [Google Scholar]
- Pu et al. (2015).Pu H, Zhang Q, Zhao C, Shi L, Wang Y, Wang J, Zhang M. Overexpression of G6PD is associated with high risks of recurrent metastasis and poor progression-free survival in primary breast carcinoma. World Journal of Surgical Oncology. 2015;13(1):323. doi: 10.1186/s12957-015-0733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao et al. (2019).Qiao GJ, Chen L, Wu JC, Li ZR. Identification of an eight-gene signature for survival prediction for patients with hepatocellular carcinoma based on integrated bioinformatics analysis. PeerJ. 2019;7:e6548. doi: 10.7717/peerj.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacoman et al. (2012).Sacoman JL, Badish LN, Sharkey TD, Hollingsworth RI. The metabolic and biochemical impact of glucose 6-sulfonate (sulfoquinovose), a dietary sugar, on carbohydrate metabolism. Carbohydrate Research. 2012;362:21–29. doi: 10.1016/j.carres.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Schumacher et al. (2013).Schumacher FR, Wang Z, Skotheim RI, Koster R, Chung CC, Hildebrandt MA, Kratz CP, Bakken AC, Bishop DT, Cook MB, Erickson RL, Fossa SD, Greene MH, Jacobs KB, Kanetsky PA, Kolonel LN, Loud JT, Korde LA, Le Marchand L, Lewinger JP, Lothe RA, Pike MC, Rahman N, Rubertone MV, Schwartz SM, Siegmund KD, Skinner EC, Turnbull C, Van Den Berg DJ, Wu X, Yeager M, Nathanson KL, Chanock SJ, Cortessis VK, McGlynn KA. Testicular germ cell tumor susceptibility associated with the UCK2 locus on chromosome 1q23. Human Molecular Genetics. 2013;22(13):2748–2753. doi: 10.1093/hmg/ddt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah et al. (2014).Shah KN, Mehta KR, Peterson D, Evangelista M, Livesey JC, Faridi JS. AKT-induced tamoxifen resistance is overturned by RRM2 inhibition. Molecular Cancer Research. 2014;12(3):394–407. doi: 10.1158/1541-7786.MCR-13-0219. [DOI] [PubMed] [Google Scholar]
- Shida-Sakazume et al. (2015).Shida-Sakazume T, Endo-Sakamoto Y, Unozawa M, Fukumoto C, Shimada K, Kasamatsu A, Ogawara K, Yokoe H, Shiiba M, Tanzawa H, Uzawa K. Lysophosphatidylcholine acyltransferase1 overexpression promotes oral squamous cell carcinoma progression via enhanced biosynthesis of platelet-activating factor. PLOS ONE. 2015;10(3):e0120143. doi: 10.1371/journal.pone.0120143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindou & Shimizu (2009).Shindou H, Shimizu T. Acyl-CoA: lysophospholipid acyltransferases. Journal of Biological Chemistry. 2009;284(1):1–5. doi: 10.1074/jbc.R800046200. [DOI] [PubMed] [Google Scholar]
- Sigoillot, Sigoillot & Guy (2004).Sigoillot FD, Sigoillot SM, Guy HI. Breakdown of the regulatory control of pyrimidine biosynthesis in human breast cancer cells. International Journal of Cancer. 2004;109(4):491–498. doi: 10.1002/ijc.11717. [DOI] [PubMed] [Google Scholar]
- Singhal et al. (2012).Singhal A, Jayaraman M, Dhanasekaran DN, Kohli V. Molecular and serum markers in hepatocellular carcinoma: predictive tools for prognosis and recurrence. Critical Reviews in Oncology/Hematology. 2012;82(2):116–140. doi: 10.1016/j.critrevonc.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Souglakos et al. (2008).Souglakos J, Boukovinas I, Taron M, Mendez P, Mavroudis D, Tripaki M, Hatzidaki D, Koutsopoulos A, Stathopoulos E, Georgoulias V, Rosell R. Ribonucleotide reductase subunits M1 and M2 mRNA expression levels and clinical outcome of lung adenocarcinoma patients treated with docetaxel/gemcitabine. British Journal of Cancer. 2008;98(10):1710–1715. doi: 10.1038/sj.bjc.6604344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun et al. (2014).Sun Y, Gu X, Zhang E, Park MA, Pereira AM, Wang S, Morrison T, Li C, Blenis J, Gerbaudo VH, Henske EP, Yu JJ. Estradiol promotes pentose phosphate pathway addiction and cell survival via reactivation of Akt in mTORC1 hyperactive cells. Cell Death & Disease. 2014;5(5):e1231. doi: 10.1038/cddis.2014.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suner et al. (2019a).Suner A, Carr BI, Akkiz H, Karakulah G, Uskudar O, Yalcin K, Kuran S, Tokat Y, Yilmaz S, Ozakyol A, Tokmak S, Balli T, Yucesoy M, Bahceci HI, Ulku A, Akcam T, Polat KY, Ekinci N, Simsek H, Ormeci N, Sonsuz A, Demir M, Kilic M, Uygun A, Demir A, Delik A, Arslan B, Doran F, Altintas E, Temel T, Bektas A. C-reactive protein and platelet-lymphocyte ratio as potential tumor markers in low-alpha-fetoprotein hepatocellular carcinoma. Oncology. 2019a;96(1):25–32. doi: 10.1159/000492473. [DOI] [PubMed] [Google Scholar]
- Suner et al. (2019b).Suner A, Carr BI, Akkiz H, Uskudar O, Kuran S, Tokat Y, Tokmak S, Balli T, Ulku A, AkCam T, Delik A, Arslan B, Doran F, YalCin K, Ekinci N, Yilmaz S, Ozakyol A, Yucesoy M, BahCeci HI, Polat KY, Simsek H, Ormeci N, Sonsuz A, Demir M, Kili CM, Uygun A, Demir A, Altintas E, Karakulah G, Temel T, Bektas A. Inflammatory markers C-reactive protein and PLR in relation to HCC characteristics. Journal of Translational Science. 2019b;5(3):1–6. doi: 10.15761/JTS.1000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangkijvanich et al. (2000).Tangkijvanich P, Anukulkarnkusol N, Suwangool P, Lertmaharit S, Hanvivatvong O, Kullavanijaya P, Poovorawan Y. Clinical characteristics and prognosis of hepatocellular carcinoma: analysis based on serum alpha-fetoprotein levels. Journal of Clinical Gastroenterology. 2000;31(4):302–308. doi: 10.1097/00004836-200012000-00007. [DOI] [PubMed] [Google Scholar]
- Taraboletti et al. (1989).Taraboletti G, Perin L, Bottazzi B, Mantovani A, Giavazzi R, Salmona M. Membrane fluidity affects tumor-cell motility, invasion and lung-colonizing potential. International Journal of Cancer. 1989;44(4):707–713. doi: 10.1002/ijc.2910440426. [DOI] [PubMed] [Google Scholar]
- Tomoike et al. (2017).Tomoike F, Nakagawa N, Fukui K, Yano T, Kuramitsu S, Masui R. Indispensable residue for uridine binding in the uridine-cytidine kinase family. Biochemistry and Biophysics Reports. 2017;11:93–98. doi: 10.1016/j.bbrep.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara et al. (2016).Uehara T, Kikuchi H, Miyazaki S, Iino I, Setoguchi T, Hiramatsu Y, Ohta M, Kamiya K, Morita Y, Tanaka H, Baba S, Hayasaka T, Setou M, Konno H. Overexpression of lysophosphatidylcholine acyltransferase 1 and concomitant lipid alterations in gastric cancer. Annals of Surgical Oncology. 2016;23(S2):S206–S213. doi: 10.1245/s10434-015-4459-6. [DOI] [PubMed] [Google Scholar]
- Uhlen et al. (2015).Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Ponten F. Proteomics: tissue-based map of the human proteome. Science. 2015;347(6220):1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2012a).Wang J, Yuan W, Chen Z, Wu S, Chen J, Ge J, Hou F, Chen Z. Overexpression of G6PD is associated with poor clinical outcome in gastric cancer. Tumour Biology. 2012a;33(1):95–101. doi: 10.1007/s13277-011-0251-9. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2012b).Wang LM, Lu FF, Zhang SY, Yao RY, Xing XM, Wei ZM. Overexpression of catalytic subunit M2 in patients with ovarian cancer. Chinese Medical Journal. 2012b;125:2151–2156. [PubMed] [Google Scholar]
- Wang et al. (2016).Wang X, Liu H, Zhang X, Li X, Gu H, Zhang H, Fan R. G6PD downregulation triggered growth inhibition and induced apoptosis by regulating STAT3 signaling pathway in esophageal squamous cell carcinoma. Tumour Biology. 2016;37(1):781–789. doi: 10.1007/s13277-015-3861-9. [DOI] [PubMed] [Google Scholar]
- Wen et al. (2015).Wen D, Liu D, Tang J, Dong L, Liu Y, Tao Z, Wan J, Gao D, Wang L, Sun H, Fan J, Wu W. Malic enzyme 1 induces epithelial-mesenchymal transition and indicates poor prognosis in hepatocellular carcinoma. Tumour Biology. 2015;36(8):6211–6221. doi: 10.1007/s13277-015-3306-5. [DOI] [PubMed] [Google Scholar]
- Woo et al. (2016).Woo SH, Yang LP, Chuang HC, Fitzgerald A, Lee HY, Pickering C, Myers JN, Skinner HD. Down-regulation of malic enzyme 1 and 2: sensitizing head and neck squamous cell carcinoma cells to therapy-induced senescence. Head & Neck. 2016;38(S1):E934–E940. doi: 10.1002/hed.24129. [DOI] [PubMed] [Google Scholar]
- Wu et al. (2019).Wu M, Liu Z, Li X, Zhang A, Lin D, Li N. Analysis of potential key genes in very early hepatocellular carcinoma. World Journal of Surgical Oncology. 2019;17(1):77. doi: 10.1186/s12957-019-1616-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang et al. (2019).Xiang XH, Yang L, Zhang X, Ma XH, Miao RC, Gu JX, Fu YN, Yao Q, Zhang JY, Liu C, Lin T, Qu K. Seven-senescence-associated gene signature predicts overall survival for Asian patients with hepatocellular carcinoma. World Journal of Gastroenterology. 2019;25(14):1715–1728. doi: 10.3748/wjg.v25.i14.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. (2014).Xu B, Wang F, Song C, Sun Z, Cheng K, Tan Y, Wang H, Zou H. Large-scale proteome quantification of hepatocellular carcinoma tissues by a three-dimensional liquid chromatography strategy integrated with sample preparation. Journal of Proteome Research. 2014;13(8):3645–3654. doi: 10.1021/pr500200s. [DOI] [PubMed] [Google Scholar]
- Xu et al. (2001).Xu XR, Huang J, Xu ZG, Qian BZ, Zhu ZD, Yan Q, Cai T, Zhang X, Xiao HS, Qu J, Liu F, Huang QH, Cheng ZH, Li NG, Du JJ, Hu W, Shen KT, Lu G, Fu G, Zhong M, Xu SH, Gu WY, Huang W, Zhao XT, Hu GX, Gu JR, Chen Z, Han ZG. Insight into hepatocellular carcinogenesis at transcriptome level by comparing gene expression profiles of hepatocellular carcinoma with those of corresponding noncancerous liver. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(26):15089–15094. doi: 10.1073/pnas.241522398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan et al. (2019).Yan Y, Lu Y, Mao K, Zhang M, Liu H, Zhou Q, Lin J, Zhang J, Wang J, Xiao Z. Identification and validation of a prognostic four-genes signature for hepatocellular carcinoma: integrated ceRNA network analysis. Hepatology International. 2019;13(5):618–630. doi: 10.1007/s12072-019-09962-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh et al. (2017).Yeh HW, Lee SS, Chang CY, Hu CM, Jou YS. Pyrimidine metabolic rate limiting enzymes in poorly-differentiated hepatocellular carcinoma are signature genes of cancer stemness and associated with poor prognosis. Oncotarget. 2017;8(44):77734–77751. doi: 10.18632/oncotarget.20774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotov et al. (1999).Yotov WV, Hamel H, Rivard GE, Champagne MA, Russo PA, Leclerc JM, Bernstein ML, Levy E. Amplifications of DNA primase 1 (PRIM1) in human osteosarcoma. Genes Chromosomes Cancer. 1999;26:62–69. doi: 10.1002/(SICI)1098-2264(199909)26:1<62::AID-GCC9>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Yu et al. (2007).Yu D, Zhuang L, Sun X, Chen J, Yao Y, Meng K, Ding Y. Particular distribution and expression pattern of endoglin (CD105) in the liver of patients with hepatocellular carcinoma. BMC Cancer. 2007;7(1):122. doi: 10.1186/1471-2407-7-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Chen & Ding (2010).Yu DC, Chen J, Ding YT. Hypoxic and highly angiogenic non-tumor tissues surrounding hepatocellular carcinoma: the ‘niche’ of endothelial progenitor cells. International Journal of Molecular Sciences. 2010;11(8):2901–2909. doi: 10.3390/ijms11082901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu et al. (2017).Yu R, Tan Z, Xiang X, Dan Y, Deng G. Effectiveness of PIVKA-II in the detection of hepatocellular carcinoma based on real-world clinical data. BMC Cancer. 2017;17(1):608. doi: 10.1186/s12885-017-3609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu et al. (2019).Yu S, Li X, Guo X, Zhang H, Qin R, Wang M. UCK2 upregulation might serve as an indicator of unfavorable prognosis of hepatocellular carcinoma. IUBMB Life. 2019;71(1):105–112. doi: 10.1002/iub.1941. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2017).Zhang X, Zhang X, Li Y, Shao Y, Xiao J, Zhu G, Li F. PAK4 regulates G6PD activity by p53 degradation involving colon cancer cell growth. Cell Death & Disease. 2017;8(5):e2820. doi: 10.1038/cddis.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao et al. (2018).Zhao N, Cao J, Xu L, Tang Q, Dobrolecki LE, Lv X, Talukdar M, Lu Y, Wang X, Hu DZ, Shi Q, Xiang Y, Wang Y, Liu X, Bu W, Jiang Y, Li M, Gong Y, Sun Z, Ying H, Yuan B, Lin X, Feng XH, Hartig SM, Li F, Shen H, Chen Y, Han L, Zeng Q, Patterson JB, Kaipparettu BA, Putluri N, Sicheri F, Rosen JM, Lewis MT, Chen X. Pharmacological targeting of MYC-regulated IRE1/XBP1 pathway suppresses MYC-driven breast cancer. Journal of Clinical Investigation. 2018;128(4):1283–1299. doi: 10.1172/JCI95873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng et al. (2012).Zheng FJ, Ye HB, Wu MS, Lian YF, Qian CN, Zeng YX. Repressing malic enzyme 1 redirects glucose metabolism, unbalances the redox state, and attenuates migratory and invasive abilities in nasopharyngeal carcinoma cell lines. Chinese Journal of Cancer. 2012;31:519–531. doi: 10.5732/cjc.012.10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng et al. (2019).Zheng RS, Sun KX, Zhang SW, Zeng HM, Zou XN, Chen R, Gu XY, Wei WW, He J. Report of cancer epidemiology in China, 2015. Zhonghua Zhong Liu Za Zhi. 2019;41:19–28. doi: 10.3760/cma.j.issn.0253-3766.2019.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A-B) Age <60 and >=60 in training cohort (C-D) Male and female in training cohort (E-F) Grade 1-2 and grade 3-4 in training cohort (G-H) Stage I-II and stage III-IV in training cohort (I-J) Age <60 and >=60 in internal testing cohort (K-L) Male and female in internal testing cohort (M-N) Grade 1-2 and grade 3-4 in internal testing cohort (O-P) Stage I-II and stage III-IV in internal testing cohort
(A-B) Age <60 and >=60 in GSE14520 testing cohort (C-D) Male and female in GSE14520 testing cohort (E-F) Stage I-II and stage III-IV in GSE14520 testing cohort (G-H) Age <60 and >=60 in ICGC testing cohort (I-J) Male and female in ICGC testing cohort (K-L) Stage I-II and stage III-IV in ICGC testing cohort
(A-B) Age <60 and >=60 in entire testing cohort (C-D) Male and female in entire testing cohort (E-F) Stage I-II and stage III-IV in entire testing cohort (G-I) Stage I, stage II and stage III in entire testing cohort
The risk score was grouped by (A-C) Tissue type (H) Tumor grade (I-K) TNM stage. The distribution of high-risk and low-risk patients in different stages and grades (D) Tumor grade (E-G) TNM stage A, D, E, H, I were from training cohort. B, F, J were from ICGC testing cohort. C, G, K were from GSE14520 testing cohort
The capacity in differentiating between normal and HCC(A-C), different grade (D-E), different stage ( F-K). A, D, E, F, G were from training cohort. B,H,I were from ICGC testing cohort. C,J,K were from GSE14520 testing cohort
(A) Biological processes (B) KEGG signaling pathway
Data Availability Statement
The following information was supplied regarding data availability:
The data and code files are available as Supplemental Files.
The raw data is available from TCGA (search terms: HCC), NCBI GEO (GSE14520) and from the ICGC project (https://icgc.org/; specific datasets used: “Liver Cancer - NCC, JP”).