Abstract
Background
This study aims to evaluate the pharmacokinetics of an increased dose of darunavir (800 mg twice daily) with 100 mg ritonavir during pregnancy and postpartum.
Methods
Darunavir (DRV) and ritonavir (RTV; r) intensive pharmacokinetic evaluations were performed at steady state during the second and third trimesters of pregnancy (DRV/r 800/100 mg bid) and 2–3 weeks postpartum (DRV/r 600/100 mg twice daily). Plasma concentrations of darunavir and ritonavir were measured using high-performance liquid chromatography (HPLC). Target darunavir area under the concentration time curve (AUC) was >70% (43.6 mcg*hr/mL) of median AUC (62.3 mcg*hr/mL) in non-pregnant adults on twice daily darunavir-ritonavir 600/100 mg.
Results
Twenty-four women were included in the analysis. Darunavir AUC0–12 was lower with the increased dose during the second [(geometric mean ratio (GMR) of 0.62 (IQR 0.44–0.88; p=0.055)] and third trimesters (GMR 0.64 (IQR 0.55–0.73; p=<0.001) compared to postpartum. Darunavir apparent clearance was higher in during the second (GMR 1.77 (IQR 1.24–2.51; p=0.039) and third trimesters (GMR 2.01 (IQR 1.17–2.35; p=<0.001) compared to postpartum. Similarly, ritonavir AUC0–12 was lower during the third trimester (GMR 0.65 (IQR 0.52–0.82; p=0.007) compared to postpartum, while its apparent clearance was higher during the third trimester (GMR 1.53 (IQR 1.22–1.92; p=0.008) compared to postpartum. No major drug-related safety concerns were noted.
Conclusion
Increasing darunavir dose to 800 mg BID failed to significantly increase darunavir exposure compared to 600 mg BID. Other strategies, such as increasing the ritonavir dose should be investigated.
Keywords: Darunavir, ritonavir, pregnancy, HIV AIDS, pharmacokinetics, postpartum
INTRODUCTION
Darunavir (DRV), in combination with low-dose ritonavir, is one of the two protease inhibitors (PIs) currently recommended by the US Perinatal Guidelines Panel for use in pregnant women living with HIV for treatment of HIV infection and for prevention of perinatal transmission.1 In most countries, darunavir is available as 600 mg and 800 mg tablets, and dosed as darunavir/ritonavir (DRV/RTV) 800mg/100 mg daily for darunavir naïve patients and 600mg/100mg twice daily for treatment of antiretroviral experienced patients. Due to physiological changes that occur during pregnancy, there is decreased exposure to many protease inhibitors during the 3rd trimester of pregnancy.2,3
The clinical relevance of these changes during pregnancy were described in prior PK studies of DRV/RTV during pregnancy and postpartum.4–7 In PK studies of 600mg/100mg DRV/RTV twice daily and 800mg/100mg DRV/RTV, darunavir and ritonavir exposures (area under the concentration time curve and plasma trough concentrations) were lower during the third trimester of pregnancy compared to postpartum.4–7 For pregnant women living with HIV, these lower antiretroviral drug exposures during pregnancy can increase the risk of maternal viremia, and, in turn, increase the potential for drug resistance and perinatal transmission.8 Although plasma concentrations of RTV boosted DRV were lower during pregnancy compared to postpartum in these prior studies, the reduced DRV concentrations were still above the exposures needed for viral suppression.
Examining known pharmacokinetic-pharmacodynamic (PKPD) relationships of darunavir (AUC, viral response and protein-adjusted IC50/IC90) in the context of lower exposures and what a clinically relevant decrease means in relation to these targets is critical during pregnancy. Three darunavir/ritonavir randomized clinical trials - POWER I,9 POWER II10 and POWER III11 demonstrated a dose-response relationship between darunavir plasma trough concentrations (Cmin) and HIV antiviral response.12,13 However, this PKPD relationship between Cmin and viral response were not observed in two other darunavir randomized clinical trials - ODIN14 and ARTEMIS.15,16 Hence, darunavir exposure-response data from these five trials were not sufficient to recommend a minimum trough concentration.17 Therefore, darunavir Cmin might not be the most appropriate PK parameter to evaluate DRV/RTV antiviral response. The established darunavir EC50 for wild-type virus and resistant-type virus are 0.055 μg/mL17,18 and 0.55 μg/mL respectively,19 while darunavir EC90 for wide type virus is 0.2. These parameters are frequently used for monitoring response of darunavir in both treatment naïve and treatment experienced patients in pregnant and non-pregnant adults.
Due to low darunavir/ritonavir concentrations with 800 mg once-daily dosing of darunavir in these studies, only the 600 mg twice-daily dosing is currently recommended by the US Perinatal Guidelines Panel for use in pregnancy.1 The objective of the current study was to evaluate the hypothesis that an increased dose darunavir (800/100 twice daily) during pregnancy would increase darunavir plasma exposure to levels similar to those seen in non-pregnant women.
METHODS
The study protocol, the informed consent documents, and all subsequent modifications were reviewed and approved by the local institutional review board (IRB)/Ethics Committee responsible for oversight of the study. The study followed all relevant human subject research guidelines. All participants provided signed informed consent before participation, and the study was registered in ClinicalTrials.gov [ NCT00042289]. Data were collected as part of International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) protocol P1026s, an ongoing, multicenter, non-blinded, prospective Phase IV study of the pharmacokinetics and safety of selected antiretrovirals (ARVs) in HIV infected pregnant women that included an arm for pregnant women receiving darunavir 800 mg with ritonavir 100 mg twice daily.
Pregnant women living with HIV were eligible for enrollment in the second and third trimesters if they were receiving darunavir as part of clinical care according to the following dosing schedule: darunavir/ritonavir 800/100 mg twice daily during pregnancy and decreased to darunavir/ritonavir 600/100 mg twice daily within a week after delivery, and postpartum PK was performed up to 6 weeks after delivery. All antiretroviral medications were prescribed by the participants’ clinical care providers and dispensed by local pharmacies, as per local standard of care. Maternal exclusion criteria were current use of medications known to interfere with darunavir metabolism (including amiodarone, atazanavir and boceprevir), presence of hemophilia, liver disease, hyperlipidemia, phenylketonuria, and other clinical or laboratory toxicity that, per site investigators, would require a change in the antiretroviral regimen. Mothers and their infants continued in the study for safety evaluations until 6 months after delivery. Infant HIV status was evaluated during the first 6 months of life by standard laboratory tests. To be definitively diagnosed as uninfected, an infant needed to have at least two negative HIV nucleic acid tests with one after 1 month and the other after 4 months of age. Infants were classified as indeterminate if their available HIV nucleic acid test results were negative but did not include 2 negative tests with one after 1 month and another after 4 months of age.
Clinical and laboratory data
Maternal demographic and clinical information were abstracted from the medical record, including maternal HIV-1 RNA, CD4+ lymphocyte count, maternal age, ethnicity, weight and concomitant medications. Plasma HIV-1 RNA assays were performed locally. Study mothers and infants were followed through six months after delivery. Neonatal gestational age at the time of delivery, birth weight and HIV infection status data were collected from the infant’s medical record. Physical examinations were performed on neonates after delivery, and infant laboratory evaluations were performed as clinically indicated, and darunavir wash-out pharmacokinetic sampling was performed on the neonates. Maternal clinical and laboratory toxicities were assessed through clinical and laboratory evaluations on each pharmacokinetic sampling day, at delivery, and at 24 weeks postpartum. Any additional toxicities noted as part of clinical care were also recorded. The study team reviewed toxicity reports on monthly conference calls, although each participant’s physician was responsible for toxicity management. The Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 2.0, dated November 2014, was used to grade adverse events for study participants.20 All toxicities were followed through resolution or 24 weeks postpartum.
Sample collection and drug assays
Plasma darunavir and ritonavir samples for intensive PK sampling were drawn immediately prior to an observed dose and at 1, 2, 4, 6, 8, and 12 hours post-dose. Samples were collected at 20–26 weeks gestation for 2nd trimester PK evaluation; at 30–36 weeks gestation for 3rd trimester PK evaluation and between the time of delivery up to 6 weeks after delivery for postpartum evaluation. Paired maternal and cord blood samples were collected at delivery and infant washout PK samples were collected at 2–10, 18–28, 36–72 hours after birth, and at 5–9 days of life. Plasma darunavir and ritonavir concentrations were determined by high-performance liquid chromatography (HPLC) with ultraviolet detection at the University of California, San Diego Pediatric Pharmacology Laboratory. Briefly, plasma proteins were precipitated using acetonitrile (ACN) and supernatant injected directly onto a LUNA C-18 reversed phase HPLC column (Phenomenex Inc., Torrance, CA, USA). Drugs were separated isocratically using a mobile phase consisting of 10mM potassium phosphate buffer pH 4.2: ACN (62:38 v/v). The flow rate was 1.2 mL/min and ultraviolet (UV) detection was at 206 nm. The detection limit for both darunavir and ritonavir was 0.09mcg/mL (1/2 limit = 0.045 mcg/mL). The mean inter and intra-assay coefficients of variation based on validation data (quality control samples run at multiple concentrations over the range of 0.092–20 ug/mL) were 5.1% and 3.8%, respectively. Darunavir and ritonavir were stable in plasma stored at −20°C. Darunavir and ritonavir were stable in plasma for over 60 days (long-term stability) at −20C, and plasma samples of both darunavir and ritonavir were stable over at least six freeze/thaw cycles. Concentrations below the detection limit were treated as half this limit for analysis.
Pharmacokinetic and statistical analysis
Darunavir and ritonavir plasma concentrations were analyzed using standard descriptive statistics and are presented as medians with interquartile ranges. Areas under the concentration time curve (AUC) for plasma from pre-dose concentration (C0) to 12 hours post dose (AUC0–12) were estimated using the trapezoidal rule, with apparent clearance as dose/AUC0–12. Target darunavir AUC was >70% (43.6 mcg*hr/mL) of median AUC (62.3 mcg*hr/mL) in non-pregnant adults on darunavir-ritonavir 600/100 mg twice daily. The P1026s protocol has an early stopping provision allowing an arm to be closed at any time after a minimum of 12 participants have been enrolled in an arm if six or more pregnant women fail to meet the PK exposure target for that arm.21 PK parameters were calculated with standard non-compartmental methods. Within-participant comparisons (second or third trimester versus postpartum) were performed for continuous outcome measures using the Wilcoxon signed-rank test and for dichotomous outcome measures using McNemar’s test. Between-participant comparisons were performed for continuous outcome measures using the Wilcoxon rank-sum test and for dichotomous outcome measures using the chi-square or Fisher exact test. A two-sided p-value <0.1 was considered statistically significant. 90% confidence limits for the geometric mean of the within-person ratios of the PK exposure parameters were calculated to describe the range of values that were consistent with the observed data, to assess whether there was a clinically important difference in exposure. Data analysis was done using WinNonlin (version 7.0; Pharsight Corporation, Mountain View, CA, USA) and SAS (version 9.4, SAS Institute, Cary NC).
RESULTS
Demographic characteristics and clinical outcomes for the 24 study mother-infant pairs are shown in Table 1. Plasma concentration data were available for 9 (37.5%) women in the second trimester, 24 (100%) women in the third trimester, and 24 (100%) postpartum. The median age of the mothers participating in this study was 26.9 years (IQR 21.4 to 34.4). Twelve (50%) of the 24 mothers were black, eleven were Hispanic (46%) and one (4%) was Asian. The median gestational age at the time of sampling was 23.9 weeks (IQR: 23.1 to 24.7) in the 2nd trimester, 33.5 weeks (32.5 to 34.4 weeks) in the 3rd trimester, and median postpartum sampling time was 2.8 weeks after delivery (IQR: 2 to 3 weeks postpartum), Table 1.
Table 1:
Increased dose darunavir/ritonavir subjects: demographic characteristics and outcomes (n=24).
| Maternal characteristics | N(%) or median (IQR) |
|---|---|
| Age at delivery (years) | 26.9 (21.4, 34.4) |
| Weight at delivery (kg) | 86.2 (68.4, 95.7) |
| Race/Ethnicity | |
| Asian, Pacific Islander | 1 (4%) |
| Black Non-Hispanic | 12 (50%) |
| Hispanic (Regardless of Race) | 11 (46%) |
| Duration of darunavir before PK evaluations (weeks) | |
| Before 2nd trimester PK evaluations | 149 (64.9, 262.1) |
| Before 3rd trimester PK evaluations | 102.1 (26.0, 190.4) |
| Number of mothers taking concomitant ARVS at the time of 3rd pharmacokinetic evaluations | *FTC 15; TDF 15; ZDV 4; 3TC 3; RAL 5; RPV 2; DTG 3; ATV 2; ENF 1. |
| Second trimester | |
| Gestational age (weeks) | 23.9 (23.1, 24.7 ) |
| Number of mothers with viral load ≤50 copies/mL | 6 (66.7%) |
| CD4 (cells/mm3) | 682 (300, 761) |
| Third trimester | |
| Gestational age (weeks) | 33.5 (32.5, 34.4) |
| Number of mothers with viral load ≤50 copies/mL | 21 (87.5%) |
| CD4 (cells/mm3) | 537.5 (303, 910.5) |
| Delivery | |
| Number of mothers with viral load ≤50 copies/mL | 20 (80%) |
| CD4 (cells/mm3) | 506 (338, 786) |
| Postpartum | |
| Weeks post-delivery (weeks) | 2.8 (2.4, 3.2) |
| Number of mothers with viral load ≤50 copies/mL | 17 (70.8%) |
| CD4 (cells/mm3) | 652.5 (395, 910.5) |
| Pregnancy outcomes | |
| Gestational age (weeks) | 39 (38.1, 39.6) |
| Birth weight (grams) | 3118 (2770, 3405) |
| Infection status | 20 uninfected/4 indeterminate |
ARVs (Antiretrovirals), FTC (emtricitabine), TDF (tenofovir disoproxil fumarate), ZDV (zidovudine), 3TC (lamivudine), RAL (raltegravir), RPV (rilpivirine), ATV (atazanavir); ENF (enfuvirtide) and DTG (dolutegravir).
Interquartile ranges (IQR) are in brackets.
Six women (66.7 %) had plasma HIV-1 RNA ≤50 copies/mL during the second trimester, twenty-one women (87.5%) had plasma HIV-1 RNA ≤50 copies/mL during the third trimester, twenty women (80%) had plasma HIV-1 RNA ≤50 at delivery, and seventeen women (70.8%) had plasma HIV-1 RNA ≤50 copies/mL in the postpartum period. The median CD4 count (cells/mL) was 682 (IQR, 300–761) in the second trimester, 538 (303–911) in the third trimester, and 653 (IQR 395–911) postpartum. The median gestational age at delivery was 39.0 weeks (range 38.1–39.6), with an average birth weight of 3118 grams (range 2770 to 3405).
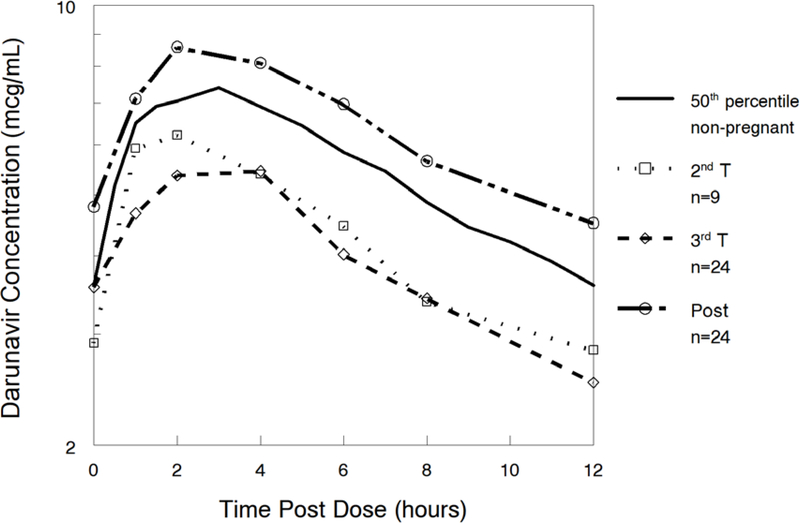
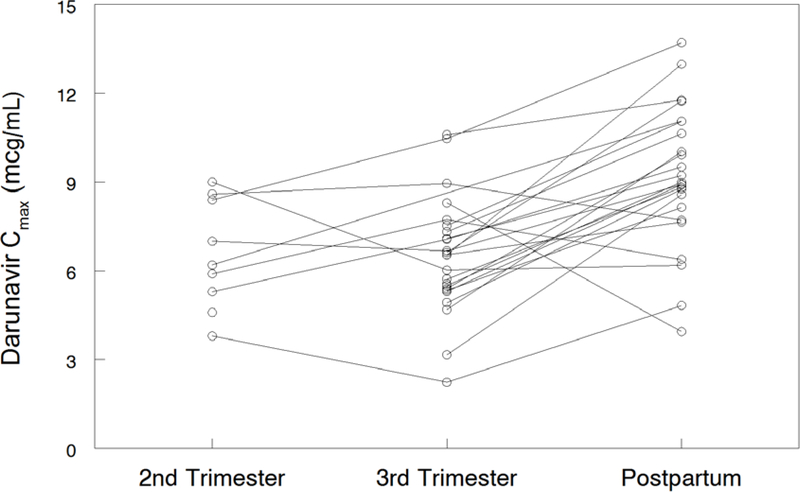
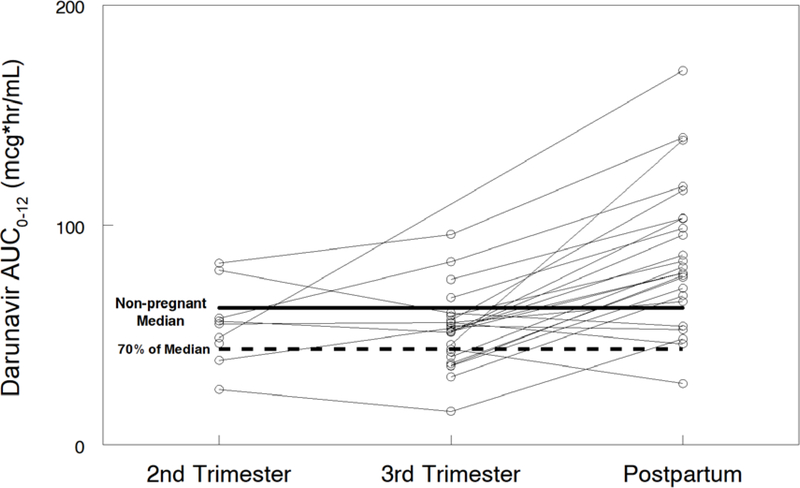
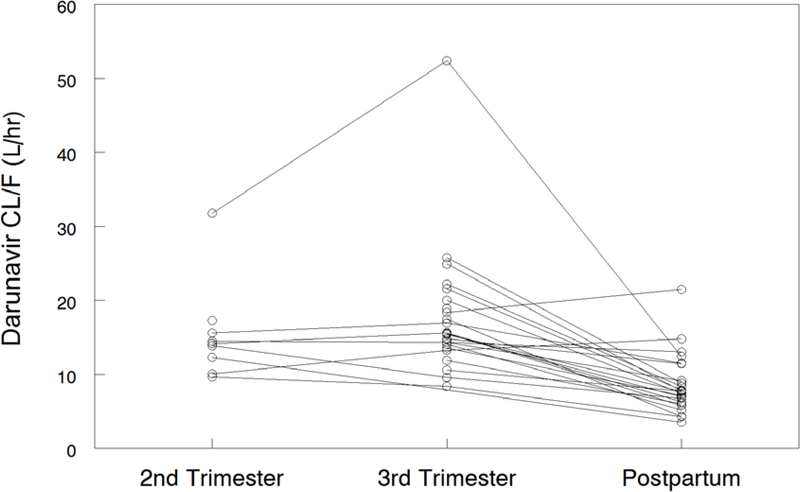
Darunavir pharmacokinetic data are shown in Table 2. Darunavir AUC0–12 was lower in the 2nd trimester (geometric mean ratio, GMR 0.62 (CI 0.44–0.88; p=0.055) and 3rd trimester (GMR 0.64 (CI 0.55–0.73; p<0.001) compared to postpartum. Darunavir apparent clearance (CL/F) was higher in the 2nd trimester (GMR 1.77 (CI 1.24–2.51; p=0.039) and 3rd trimester (GMR 2.01 (IQR 1.17–2.35) compared to postpartum (P<0.001). Darunavir maximum plasma concentration (Cmax) [(GMR 0.71 (CI 0.62–0.81); p<0.001) and the last observed quantifiable darunavir concentration (Clast) [(GMR 0.59 (CI 0.50–0.69); p<0.001) were lower in the 3rd trimester compared to postpartum. Darunavir apparent volume of distribution (V/F) was higher in the second trimester [(GMR 1.58 (CI 1.23–2.04); p=0.016)] and third trimester [(GMR 2.01 (CI 1.53–2.65); p<0.001)] compared to postpartum. Figures 1A–D show mean darunavir concentrations (1A); darunavir area under the curve (1B); darunavir apparent clearance (1C); and darunavir maximum concentration (1D) in the 2nd trimester, 3rd trimester and postpartum respectively.
Table 2:
Darunavir Pharmacokinetics Comparison of 2nd Trimester (N=9) versus postpartum (N=24); and 3rd Trimester (N=24) versus postpartum (N=24)
| PK Parameter | #Second trimester (2T); (n=9) | #Third trimester (3T); (n=24) | #Postpartum (PP); (n=24) | Geometric mean Ratio, GMR (90% CI); 2T/PP, n=8 | p-value | Geometric mean Ratio, GMR (90% CI); 3T/PP, n=23 | p-value |
|---|---|---|---|---|---|---|---|
| DRV AUC0–12 (μg*hr/mL) | 55.1 (46.4, 57.7) | 51.8 (41.2, 57.7) | 79.6 (66.6, 103.0) | 0.62 [0.44, 0.88] | 0.055 | 0.64 [0.55, 0.73] | <0.001 |
| DRV CL/F (L/hr) | 14.2 (12.3, 15.6) | 15.6 (13.9, 19.4) | 7.72 (6.19, 10.32) | 1.77 [1.24, 2.51] | 0.039 | 2.01 [1.17, 2.35] | <0.001 |
| DRV V/F (Liters) | 152.7 (137.0, 259.2, | 174.6 (143.3, 233.7) | 101.2 (62.1, 133.6) | 1.58 [1.23, 2.04] | 0.016 | 2.01 [1.53, 2.65] | <0.001 |
| DRV T1/2 (hours) | 7.95 (6.21, 9.82) | 7.65 (6.57, 9.04) | 8.66 (6.72, 10.38) | 0.90 [0.74, 1.08] | 0.313 | 1.01 [0.83, 1.22] | 0.637 |
| DRV Cmin (μg/mL) | 2.84 (1.21, 3.23) | 2.39 (1.72, 2.82) | 1.12 (0.52, 5.01) | 2.52 [0.68, 9.35] | 0.578 | 0.90 [0.52, 1.58] | 0.011 |
| DRV Clast (μg/mL) | 2.84 (2.24, 3.23) | 2.52 (2.06, 2.90) | 4.51 (3.72, 5.28) | 0.66 [0.39, 1.12] | 0.109 | 0.59 [0.50, 0.69] | <0.001 |
| DRV Cmax (μg/mL) | 6.22 (5.30, 8.42) | 6.55 (5.38, 7.43) | 8.96 (7.93, 10.85) | 0.81 [0.64, 1.01] | 0.148 | 0.71 [0.62, 0.81] | <0.001 |
| DRV C0 (μg/mL) | 2.91 (1.55, 5.61) | 3.57 (2.79, 4.09) | 3.56 (2.14, 6.42) | 2.97 [0.75, 11.8] | 0.469 | 1.00 [0.56, 1.81] | 0.045 |
| DRV C12 (μg/mL) | 2.84 (2.24, 3.23) | 2.52 (2.06, 2.91) | 4.51 (3.72, 5.30) | 0.66 [0.39, 1.12] | 0.109 | 0.58 [0.49, 0.69] | <0.001 |
p-value for Wilcoxon rank-sum test; AUC0–12 = area under concentration (AUC) vs time curve (0 to 12 hours post-dose), Cl/F = apparent oral clearance, V/F = apparent volume of distribution; T1/2 = elimination half-life; Clast = last observed quantifiable concentration; C0 = initial concentration at time zero; C12 = concentration at 12 hours post-dose; Cmin= minimum concentration, Cmax= maximum concentration; CI = confidence interval.
Values are medians (interquartile ranges).
Figure 1A:
Median darunavir concentrations.
The 50th percentile data in this figure represents DRV/RTV 600 mg/100 mg BID in non-pregnant adults.
Figure 1D:
Darunavir Cmax.
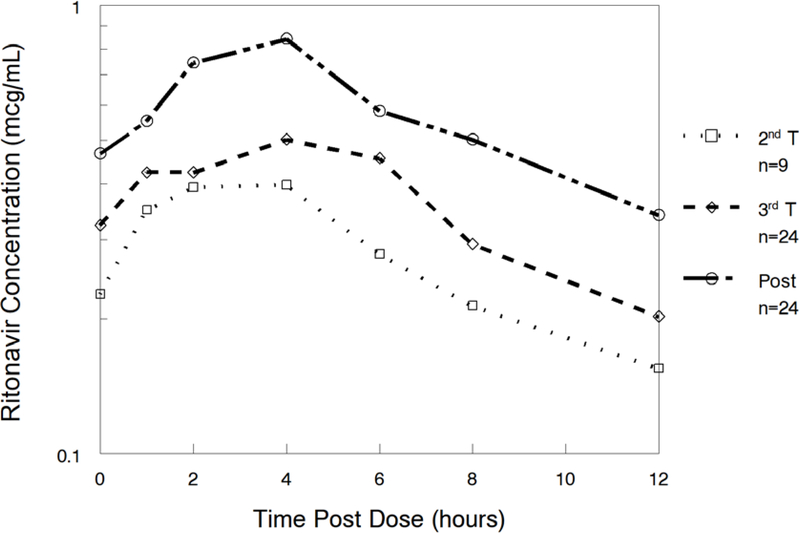
Ritonavir pharmacokinetic data are shown in Table 3. Ritonavir AUC0–12 was lower in the 3rd trimester (geometric mean ratio 0.65 (CI 0.52–0.82; p=0.007) compared to postpartum. Ritonavir apparent clearance (CL/F) was higher in the 3rd trimester (geometric mean ratio 1.53 (CI 1.22–1.92; p=0.008) compared to postpartum. Ritonavir last observed quantifiable concentration (Clast) [(geometric mean ratio 0.64 (CI 0.40–1.04); p=0.065)] and maximum serum concentration (Cmax) [(geometric mean ratio 0.67 (CI 0.54–0.83); p=0.004)] were lower in the 3rd trimester compared to postpartum. Ritonavir apparent volume of distribution (V/F) was higher in the second trimester [(GMR 2.12 (CI 1.42–3.16); p=0.012)] compared to postpartum. Figure 2 shows mean ritonavir concentrations in the 2nd trimester, 3rd trimester and postpartum.
Table 3:
Ritonavir Pharmacokinetics Comparison of 2nd Trimester (N=9) versus postpartum (N=24); and 3rd Trimester (N=24) versus postpartum (N=24)
| PK Parameter |
#Second trimester (2T); (n=9) |
#Third trimester (3T); (n=24) |
#Postpartum (PP); (n=24) |
Geometric mean Ratio, GMR (90% CI); 2T/PP, n=8 |
p-value | Geometric mean Ratio, GMR (90% CI); 3T/PP, n=23 |
p-value |
|---|---|---|---|---|---|---|---|
| RTV AUC0–12 (μg*hr/mL) | 3.19 (2.67, 6.10) | 4.83 (3.23, 7.00) | 6.68 (5.07, 11.2) | 0.88 [0.61, 1.27] | 0.547 | 0.65 [0.52, 0.82] | 0.007 |
| RTV CL/F (L/hr) | 14.2 (12.3, 15.6) | 15.6 (13.9, 19.4) | 7.72 (6.20, 10.3) | 1.14 [0.79, 1.64] | 0.383 | 1.53 [1.22, 1.92] | 0.008 |
| RTV V/F (Liters) | 245.0 (194.4, 389.3) | 210.7 (131.0, 328.9) | 89.6 (48.3, 163.5) | 1.41 [0.73, 2.72] | 0.547 | 2.12 [1.42, 3.16] | 0.012 |
| RTV T1/2 (hours) | 7.21 (5.16, 17.5) | 6.51 (3.67, 12.33) | 4.73 (3.85, 6.35) | 1.24 [0.78, 1.97] | 0.383 | 1.50 [0.95, 2.37] | 0.226 |
| RTV Cmin (μg/mL) | 0.12 (0.05, 0.34) | 0.20 (0.08, 0.29) | 0.29 (0.05, 0.44) | 1.26 [0.76, 2.11] | 0.313 | 0.90 [0.48, 1.69] | 0.164 |
| RTV Clast (μg/mL) | 0.16 (0.12, 0.40) | 0.20 (0.11, 0.39) | 0.35 (0.19, 0.64) | 0.85 [0.67, 1.08] | 0.469 | 0.64 [0.40, 1.04] | 0.065 |
| RTV Cmax (μg/mL) | 0.40 (0.35, 0.66) | 0.62 (0.45, 0.89) | 0.92 (0.65, 1.43) | 0.92 [0.68, 1.23] | 0.547 | 0.67 [0.54, 0.83] | 0.004 |
| RTV C0 (μg/mL) | 0.23 (0.11, 0.34) | 0.32 (0.16, 0.51) | 0.47 (0.08, 0.71) | 1.80 [1.02, 3.18] | 0.078 | 0.88 [0.56, 1.37] | 0.143 |
| RTV C12 (μ/mL) | 0.16 (0.12, 0.40) | 0.20 (0.11, 0.39) | 0.34 (0.18, 0.60) | 0.85 [0.67, 1.08] | 0.469 | 0.67 [0.41, 1.11] | 0.116 |
p-value for Wilcoxon rank-sum test; AUC0–12 = area under concentration (AUC) vs time curve (0 to 12 hours post-dose); Cl/F = apparent oral clearance; Cmin= minimum concentration; V/F = apparent volume of distribution; T1/2 = elimination half-life; Clast = last observed quantifiable concentration; C0 = initial concentration at time zero; C12 = concentration at 12 hours post-dose; Cmin= minimum concentration, Cmax= maximum concentration; CI = confidence interval.
Values are medians (interquartile ranges).
Figure 2:
Median Ritonavir Concentrations.
Darunavir cord blood median (IQR) was 0.27 (0.14 – 0.55) mcg/mL in 20 samples. Darunavir maternal delivery sample median (IQR) was 2.33 (1.07 – 3.21) mcg/mL in 21 samples. Median (IQR) ratio of cord/maternal darunavir concentrations was 0.15 (0.12 – 0.17) in 16 paired measurable concentrations. Darunavir was below the quantitation limit in 4 of the 20 cord blood samples, but was measurable in all maternal samples. For ritonavir, two cord blood samples were measured using an older assay method with a quantitation limit of 0.094 mcg/mL, and both were below quantitation. Eighteen cord blood samples were measured using a newer assay with a quantitation limit of 0.01 mcg/mL and 7 had measurable ritonavir concentrations, ranging from 0.013 – 0.035 mcg/mL. Combining cord blood results for ritonavir concentration from both assays, 13 of 20 samples were below quantitation. In the maternal delivery samples, 1 was below quantitation, and 20 of 21 had measurable ritonavir concentrations. The median (IQR) was 0.154 (0.106 – 0.279) mcg/mL. In 7 pairs of samples with measurable ritonavir concentrations in both sample types, the median (IQR) ratio of cord/maternal ritonavir concentrations was 0.07 (0.05 – 0.10).
All the 24 women enrolled in the cohort were on other antiretrovirals in addition to darunavir/ritonavir, as listed in Table 1. Four women (16.7 %) experienced adverse events that were possibly treatment related, including moderately increased alanine aminotransferase (ALT), proteinuria, oligohydramnios and intrauterine growth restriction (IUGR). Three infants had birth abnormalities, including a short frenulum and sacral Mongolian spots. None of these birth abnormalities were thought to be related to darunavir or ritonavir exposure. One infant had an adverse event, hyperbilirubinemia, which was thought to be unrelated to darunavir or ritonavir exposure.
DISCUSSION
Pregnancy is known to modify the activity of some drug metabolizing enzymes, impacting drug exposure.22 Previous pharmacokinetic data from the IMPAACT P1026s and the Pediatric AIDS Clinical Trials Group (PACTG) 353 studies demonstrated decreases in exposure during pregnancy with standard doses of other CYP3A4 metabolized antiretrovirals, including lopinavir, atazanavir, and nelfinavir.23–27 In subsequent trials, these decreased drug exposures were overcome with increased doses of lopinavir, atazanavir, and nelfinavir during the third trimester of pregnancy, to achieve drug exposures during pregnancy equivalent to those seen in nonpregnant adults.25,28
In prior studies of the pharmacokinetics of darunavir during pregnancy, darunavir AUC and Cmax were substantially decreased in pregnancy with standard darunavir/ritonavir once and twice daily dosing. Darunavir plasma area under the curve (AUC) during the second and third trimester compared with postpartum was reduced by 26% with darunavir/ritonavir 600 mg/100 mg twice daily and by 38–39% with 800 mg/100 mg once a day dosing. Darunavir trough concentrations with twice daily dosing were not different from postpartum but with once daily dosing they were reduced by 63% during the second trimester and 57% during the third trimester compared to postpartum.4–7 Therefore, the US Panel on Treatment of Pregnant Women with HIV Infection and Prevention of Perinatal Transmission recommends use of darunavir 600 mg twice daily and not 800 mg once daily during pregnancy because of the reductions in trough darunavir concentrations seen with once-daily dosing during pregnancy. Given the experience with use of increased doses of other protease inhibitors during pregnancy, we postulated that increasing the dose of darunavir during pregnancy would increase maternal darunavir drug exposure. However, in the current study, use of an increased dose of 800/100mg DRV/RTV BID during pregnancy resulted in larger differences between darunavir exposure during pregnancy and postpartum, with mean darunavir AUC 38% lower in the second trimester and 36% lower in the third trimester compared to postpartum darunavir AUC with the use of 600/100 mg DRV/RTV BID in the same women.
Darunavir is a substrate and inhibitor of cytochrome P450 (CYP3A) enzymes, and is almost exclusively metabolized by these CYP3A isoforms,27 while ritonavir, an inhibitor of CYP3A4, is administered as a booster to increase the plasma concentration of darunavir. Darunavir/ritonavir combinations may induce CYP2C9 and CYP2C19 enzymes. Ritonavir inhibition of darunavir metabolism occurs in the liver, and the reduction in plasma ritonavir concentration seen in pregnancy may lead to reduced ritonavir inhibition of darunavir metabolism and lower darunavir exposure. The increased dose of darunavir we used during pregnancy may have been inadequate to overcome the effect of the reduction in plasma ritonavir exposure. P-gp inhibition by ritonavir may also explain some of the failure of the increased pregnancy dose to result in increased darunavir exposure. Ritonavir can cause mixed inhibition/induction of P-gp, and ritonavir in the gut may lead to reduced darunavir absorption, which could not be overcome by the increased darunavir dose.
Darunavir is known to be highly protein-bound, with about 95% bound to plasma proteins (mainly alpha 1-acid glycoprotein).27 Plasma protein binding of drugs to albumin and alpha 1-acid glycoprotein decreases during pregnancy due to reduced concentrations of both binding proteins.27 Previous studies on darunavir protein binding during pregnancy suggest that while there is a marked reduction in total serum concentrations of darunavir in pregnancy, the reduction in protein binding may allow the concentration of unbound darunavir and antiviral activity to be maintained during pregnancy.4–6
Examining known PKPD relationships of darunavir (AUC, viral response and protein-adjusted IC50/IC90) in the context of lower exposures and what a clinically relevant decrease in relation to these targets, is critical during pregnancy. Steady state PKPD and efficacy relationships show that trough concentrations (Cmin) of darunavir are not a good predictor of decrease in viral load, as darunavir exposure-response data were not sufficient to recommend a minimum trough concentration.17,19 However, the darunavir trough concentrations (Cmin) during the second and third trimesters, including postpartum (Table 2), were all greater than 10-fold above the mean darunavir protein-adjusted IC50 of 0.055μg/mL (55 ng/mL), 5-fold above the mean darunavir protein-adjusted IC50 of 0.55 μg/L for resistant virus,5 and greater than 10-fold above the mean darunavir protein-adjusted EC90 of 0.2 μg/L for wild-type virus. Although lower during pregnancy compared to postpartum, protein bound darunavir concentrations remained well above the viral activity of HIV as shown by its effect on the EC50 and EC90, and there were no recorded cases of perinatal transmission of HIV.
Pharmacogenomic drug-drug interactions could also contribute to reduced darunavir concentrations during pregnancy. CYP3A5 polymorphisms have been demonstrated to lower darunavir plasma exposure in participants who express CYP3A5 compared to non-expressors.29 CYP3A5 activity is extremely dependent on the genetic status of participants due to various genetic polymorphisms related to CYP3A5 activity, leading to either loss or gain of function variants. The most prevalent loss-of-function variant of CYP3A5 in pregnant and non-pregnant adults is CYP3A5*3.29 This single nucleotide polymorphism (SNP) comprising of a change within intron 3, affects messenger RNA splicing, resulting in a truncated non-functional protein.29,30 As a result, only participants carrying at least one CYP3A5*1 (wild-type) allele in pregnancy express functional CYP3A5 activity, while participants who are homozygous for the loss-of-function allele (CYP3A5*3/*3) are non expressors of CYP3A5. The impact of pregnancy on these genetic differences in darunavir metabolism are unknown.
Our study has strengths. To our knowledge, this is the first pharmacokinetic study to evaluate the use of an increased darunavir dose (800mg twice daily) during pregnancy. The pregnant patients in the darunavir arm of the IMPAACT 1026s study were followed in a longitudinal pattern throughout pregnancy and postpartum, during which evaluation of clinical findings related to darunavir exposure occurred at regular time intervals. Because this was a prospective cohort study, confounding, recall and selection biases were minimized. In addition, any random error (misclassifications) in darunavir plasma measurements that arose from the study would tend to be conservative by the prospective nature of this study. The collection of darunavir plasma samples followed a rigorous and stringent protocol, with directly observed dosing aimed at minimizing systematic errors during sample collection. Another strength of this study is that all 24 women (100%) that were studied during the third trimester of pregnancy had complete pharmacokinetic data during the postpartum period.
This study had its limitations. First, this is an observational pharmacokinetic/safety study of a heterogeneous group of pregnant women receiving darunavir for clinical care. There was variation in their background characteristics, and pregnant women who began darunavir/ritonavir but did not tolerate it or demonstrate adequate initial efficacy would be taken off drug and not be eligible for the study. Second, we did not assess the relationship between increased darunavir dosing and genetic resistance to HIV virus in pregnancy. Third, we did not study the precise pharmacokinetic mechanism(s) associated with reduced darunavir concentrations during pregnancy, as this was not part of the study design, although prior pharmacokinetic studies of protease inhibitors in pregnant women show that increased darunavir protein-binding, increased volume of distribution during pregnancy, and increased renal clearance of drugs are likely reasons for lower exposures of darunavir during the 3rd trimester compared to the postpartum period.31–33
In conclusion, our findings confirm that darunavir exposure is decreased during pregnancy, and increasing the darunavir/ritonavir dose to 800mg/100 mg twice daily during pregnancy and continuing 600mg/100mg twice daily in the postpartum period failed to significantly increase darunavir exposure compared to 600 mg twice daily throughout pregnancy and postpartum. This is in contrast to our findings with the other protease inhibitors atazanavir, lopinavir and nelfinavir, where increased dosing during pregnancy did improve drug exposure.23–25 While viral suppression was fairly good in the participants, if achieving darunavir exposure during pregnancy equivalent to that in non-pregnant adults is desired, other strategies, such as increasing the ritonavir dose should be investigated.32
Figure 1B –
Darunavir area under the curve (AUC0–12)
Figure 1C:
Darunavir Apparent Clearance (CL/F).
ACKNOWLEDGEMENTS
We would like to thank all the women who participated in the darunavir/ritonavir arm of the P1026s protocol, the sites that participated in this study, all the Principal Investigators and Staff, and all the members of the P1026s protocol team: 2802 New Jersey Medical School CRS (Linda Bettica, RN; Charmane Calilap-Bernardo, MA, PNPC; Arlene Bardeguez, MD, MPH); 3801 Texas Children’s Hospital CRS (Shelley Buschur, RN, CNM; Chivon Jackson, RN, BSN, ADN; Mary Paul, MD); 4101 Columbia CRS (Philip La Russa, MD); 4201 University of Miami Pediatric Perinatal HIV/AIDS CRS (Claudia Florez, MD; Patricia Bryan, BSN, MPH; Monica Stone, MD); 4601 University of California San Diego Mother-Child-Adolescent Program CRS (Andrew D. Hull, MD; Mary Caffery, RN, MSN; Stephen A. Spector, MD); 4701 Duke University Medical Center CRS (Joan Wilson, RN, BSN, MPH; Julieta Giner, RN, ACRN; Margaret A. Donnelly, PA-C); 5012 New York University, New York NICHD CRS (Nagamah Deygoo, MD;Adita Kaul, MD;William Borkowsky M.D.); 5013 Jacobi Medical Center Bronx NICHD CRS (Mindy Katz, MD; Raphaelle Auguste, RN; Andrew Wiznia, MD); 5018 University of South Florida - Tampa NICHD CRS (Karen L. Bruder, MD; Gail Lewis, RN; Denise Casey, RN); 5048 University of Southern California School of Medicine– Los Angeles County NICHD CRS (Françoise Kamer, MD; LaShonda Spencer, MD;James Homans, MD);5052 University of Colorado Denver NICHD CRS (Torri Metz, MD; Jenna Wallace, MSW; Alisa Katai, MHA); 5072 Hospital dos Servidores Rio de Janeiro NICHD CRS (Esau C. Joao MD, PhD; Plinio Tostes Berardo Carneiro da Cunha, MD, PhD;); Maria Isabel Fragoso da Silveira Gouvêa, MD); 5082 Hospital General de Agudos Buenos Aires NICHD CRS (Marcelo H. Losso, MD; Silvina A. Ivalo, MD; Alejandro Hakim, MD); 5093 Miller Children’s Hospital NICHD CRS (Audra Deveikis MD; Jagmohan Batra MD; Janielle Jackson Alvarez RN); 5098 Hopsital Santa Casa Porto Alegre Brazil NICHD CRS (Regis Kreitchmann, PhD, MD; Debora Fernandes Coelho, MN, PhD; Marcelo Comerlato Scotta, MSc, MD); 6501 St Jude CRS (Katherine M. Knapp, MD; Nina Sublette, FNP, PhD; Thomas Wride, MS); 6601 University of Puerto Rico Pediatric HIV/AIDS Research Program CRS (Irma L. Febo MD; Ruth Santos RN, MPH; Vivian Tamayo MD); 6701 The Children’s Hospital of Philadelphia (Steven D. Douglas, MD; Carol A. Vincent, PhD, CRNP; Samuel Parry, MD); 6901 Bronx-Lebanon Hospital CRS (Jenny Gutierrez, MD; Mary Elizabeth Vachon, MPH; Murli Purswani, MD); 8251 Siriraj Hospital Mahidol University, Bangkok, Thailand CRS (Thanomsak Anekthananon, MD; Amphan Chalermchokcharoenkit, MD; Kulkanya Chokephaibulkit, MD).
Financial disclosures/Conflicts of interest: “Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.”
REFERENCES
- 1.Panel on Treatment of Pregnant Women with HIV Infection and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Transmission in the United States. 2018; Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf Accessed Accessed 1/11/19.
- 2.Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Seminars in perinatology. 2015;39(7):512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eke AC, Dooley KE, Sheffield J. Pharmacologic Research in Pregnant Women – Time to Get it Right. The New England journal of medicine. 2019;380(14):1293–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crauwels HM, Kakuda TN, Ryan B, et al. Pharmacokinetics of once-daily darunavir/ritonavir in HIV-1-infected pregnant women. HIV Med. 2016;17(9):643–652. [DOI] [PubMed] [Google Scholar]
- 5.Colbers A, Molto J, Ivanovic J, et al. Pharmacokinetics of total and unbound darunavir in HIV-1-infected pregnant women. J Antimicrob Chemother. 2015;70(2):534–542. [DOI] [PubMed] [Google Scholar]
- 6.Stek A, Best BM, Wang J, et al. Pharmacokinetics of Once Versus Twice Daily Darunavir in Pregnant HIV-Infected Women. J Acquir Immune Defic Syndr. 2015;70(1):33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zorrilla CD, Wright R, Osiyemi OO, et al. Total and unbound darunavir pharmacokinetics in pregnant women infected with HIV-1: results of a study of darunavir/ritonavir 600/100 mg administered twice daily. HIV Med. 2014;15(1):50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slogrove AL, Clayden P, Abrams EJ. Toward a universal antiretroviral regimen: special considerations of pregnancy and breast feeding. Current opinion in HIV and AIDS. 2017;12(4):359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katlama C, Esposito R, Gatell JM, et al. Efficacy and safety of TMC114/ritonavir in treatment-experienced HIV patients: 24-week results of POWER 1. AIDS (London, England). 2007;21(4):395–402. [DOI] [PubMed] [Google Scholar]
- 10.Haubrich R, Berger D, Chiliade P, et al. Week 24 efficacy and safety of TMC114/ritonavir in treatment-experienced HIV patients. AIDS (London, England). 2007;21(6):F11–18. [DOI] [PubMed] [Google Scholar]
- 11.Arasteh K, Yeni P, Pozniak A, et al. Efficacy and safety of darunavir/ritonavir in treatment-experienced HIV type-1 patients in the POWER 1, 2 and 3 trials at week 96. Antiviral therapy. 2009;14(6):859–864. [DOI] [PubMed] [Google Scholar]
- 12.Pozniak A, Opravil M, Beatty G, Hill A, de Bethune MP, Lefebvre E. Effect of baseline viral susceptibility on response to darunavir/ritonavir versus control protease inhibitors in treatment-experienced HIV type 1-infected patients: POWER 1 and 2. AIDS research and human retroviruses. 2008;24(10):1275–1280. [DOI] [PubMed] [Google Scholar]
- 13.Clotet B, Bellos N, Molina JM, et al. Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet (London, England). 2007;369(9568):1169–1178. [DOI] [PubMed] [Google Scholar]
- 14.Lathouwers E, De La Rosa G, Van de Casteele T, et al. Virological analysis of once-daily and twice-daily darunavir/ritonavir in the ODIN trial of treatment-experienced patients. Antiviral therapy. 2013;18(3):289–300. [DOI] [PubMed] [Google Scholar]
- 15.Estrada V, Fuster M. [Darunavir in treatment-naive patients. The ARTEMIS study]. Enfermedades infecciosas y microbiologia clinica. 2008;26 Suppl 10:10–13. [DOI] [PubMed] [Google Scholar]
- 16.Lathouwers E, De Meyer S, Dierynck I, et al. Virological characterization of patients failing darunavir/ritonavir or lopinavir/ritonavir treatment in the ARTEMIS study: 96-week analysis. Antiviral therapy. 2011;16(1):99–108. [DOI] [PubMed] [Google Scholar]
- 17.Gutierrez-Valencia A, Torres-Cornejo A, BenMarzouk-Hidalgo OJ, et al. Darunavir minimum plasma concentration and ritonavir-boosted darunavir monotherapy outcome in HIV-infected patients. Antiviral therapy. 2014;19(5):443–447. [DOI] [PubMed] [Google Scholar]
- 18.Boffito M, Miralles D, Hill A. Pharmacokinetics, efficacy, and safety of darunavir/ritonavir 800/100 mg once-daily in treatment-naive and -experienced patients. HIV clinical trials. 2008;9(6):418–427. [DOI] [PubMed] [Google Scholar]
- 19.Prezista (darunavir) oral suspension, for oral use. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021976s034,202895s011lbl.pdf. Accessed October 20th, 2019.
- 20.Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events. 2014; Available at https://rsc.niaid.nih.gov/sites/default/files/daids-ae-grading-table-v2-nov2014.pdf. Accessed 1/31/2019.
- 21.IMPAACT P1026s. Pharmacokinetics properties of antiretroviral and related drugs during pregnancy and postpartum: A Multi-center Trial of the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT). https://impaactnetwork.org/DocFiles/P1026s/P1026SF8_17Jan13.pdf. Accessed October 20th, 2019.
- 22.Jeong H Altered drug metabolism during pregnancy: hormonal regulation of drug-metabolizing enzymes. Expert opinion on drug metabolism & toxicology. 2010;6(6):689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Best BM, Stek AM, Mirochnick M, et al. Lopinavir tablet pharmacokinetics with an increased dose during pregnancy. J Acquir Immune Defic Syndr. 2010;54(4):381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stek AM, Mirochnick M, Capparelli E, et al. Reduced lopinavir exposure during pregnancy. AIDS (London, England). 2006;20(15):1931–1939. [DOI] [PubMed] [Google Scholar]
- 25.Kreitchmann R, Best BM, Wang J, et al. Pharmacokinetics of an increased atazanavir dose with and without tenofovir during the third trimester of pregnancy. J Acquir Immune Defic Syndr. 2013;63(1):59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mirochnick M, Best BM, Stek AM, et al. Atazanavir pharmacokinetics with and without tenofovir during pregnancy. J Acquir Immune Defic Syndr. 2011;56(5):412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rittweger M, Arasteh K. Clinical pharmacokinetics of darunavir. Clinical pharmacokinetics. 2007;46(9):739–756. [DOI] [PubMed] [Google Scholar]
- 28.Eke AC, McCormack SA, Best BM, et al. Pharmacokinetics of Increased Nelfinavir Plasma Concentrations in Women During Pregnancy and Postpartum. Journal of clinical pharmacology. 2019;59(3):386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hustert E, Haberl M, Burk O, et al. The genetic determinants of the CYP3A5 polymorphism. Pharmacogenetics. 2001;11(9):773–779. [DOI] [PubMed] [Google Scholar]
- 30.Kuehl P, Zhang J, Lin Y, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nature genetics. 2001;27(4):383–391. [DOI] [PubMed] [Google Scholar]
- 31.Eke AC, Mirochnick M. Ritonavir and cobicistat as pharmacokinetic enhancers in pregnant women. Expert opinion on drug metabolism & toxicology. 2019;15(7):523–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eke AC, Mirochnick MH. Cobicistat as a Pharmacoenhancer in Pregnancy and Postpartum: Progress to Date and Next Steps. Journal of clinical pharmacology. 2019;59(6):779–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eke AC, Chakhtoura N, Kashuba A, et al. Rilpivirine Plasma and Cervicovaginal Concentrations in Women During Pregnancy and Postpartum. Journal of acquired immune deficiency syndromes (1999). 2018;78(3):308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]