Abstract
Constitutive loss of the type 3 deiodinase (DIO3) causes abnormally increased levels of thyroid hormone action in the developing and adult brain, leading to an array of behavioral abnormalities. To determine to what extent those phenotypes derive from a lack of DIO3 in the adult brain, versus developmental consequences, we created a mouse model of conditional DIO3 inactivation. Mice carrying “floxed” Dio3 alleles and a tamoxifen-inducible cre transgene were injected with tamoxifen at two months of age. Compared to oil-injected controls, the brain tissue of these mice showed a 75–80% decrease in DIO3 activity and 85–95% Dio3 mRNA was expressed from recombinant alleles. Mice with adult DIO3 deficiency did not show significant differences in growth, serum thyroid hormone parameters or behaviors related to anxiety and depression. However, female mice exhibited elevated locomotor activity and increased marble-burying behavior. They also manifested relatively modest alterations in the expression of T3-dependent genes and genes related to hyperactivity in a sex- and region-specific manner. Upon thyroid hormone treatment, the expression response of T3-regulated genes was generally more pronounced in DIO3-deficient female mice than in female controls, while the opposite effect of altered genotype was noticed in males. The extent of the molecular and behavioral phenotypes of adult-onset DIO3 deficiency suggests that a substantial proportion of the neurological abnormalities caused by constitutive DIO3 deficiency has a developmental origin. However, our results show that DIO3 in the adult brain also influences behavior and sensitivity to thyroid hormone action in a sexually dimorphic fashion.
1. Introduction
Thyroid hormones (THs) are crucial for brain development. They regulate broad gene expression programs in the central nervous system (CNS), influencing the proliferation, differentiation and migration of neural cells and ultimately adult brain function and behavior (Bernal, 2005; Flamant et al., 2017). In humans, developmental deficits of THs cause cretinism and mental retardation (Legrand, 1984), and alterations in TH status during development and adulthood are also associated with neurological conditions including schizophrenia (Gyllenberg et al., 2016), autism (Andersen et al., 2014; Khan et al., 2014; Molloy et al., 2006)), attention-deficit and hyperactive disorder (ADHD) (Andersen et al., 2014; Modesto et al., 2015) and depression (Kim et al., 2015; Medici et al., 2014).
Under the control of the hypothalamic-pituitary axis, the thyroid gland secretes thyroxine (T4) and 3,5,3′- triiodothyronine (T3). The latter exerts most of the biological actions of THs due to its higher affinity for TH receptors, which are transcription factors capable of binding DNA (Brent, 2012). Tissue T3 availability and action, especially in the CNS, is also influenced by cell transporters and deiodinase enzymes (Richard and Flamant, 2018). In the brain, T4 is the major source of T3 due to the presence in glial cells of the type II deiodinase (DIO2), which is responsible for T4 to T3 conversion (Arrojo et al., 2013). Lack of DIO2 function in glial cells leads to reduced brain T3-signaling and abnormal behaviors in mice (Bocco et al., 2016). In humans, loss of T3 availability in the CNS due to deficiency in the MCT8 transporter leads to severe mental retardation and motor function impairment (Kersseboom et al., 2013; Schwartz et al., 2005). In contrast, brain T3 action is limited by the type 3 deiodinase (DIO3) (Hernandez and Stohn, 2018), which converts both T4 and T3, respectively, to reverse T3 (rT3) and 3,3′-diiodothyronine (3,3′-T2), which have poor affinity for thyroid hormone receptors.
Although the importance of THs for brain development and function is well established, the neurological consequences of developmental excess of THs are not well understood. In neonatal rodents, the administration of pharmacological doses of THs leads to adult brain abnormalities affecting neuroendocrine function (Bakke et al., 1974; Walker and Courtin, 1985), behavior (Chen and Fuller, 1975; Davenport and Gonzalez, 1973; Stone and Greenough, 1975) and density of receptors for neurotransmitters in selected brain regions (Roskoden et al., 2002). In addition, the prominent expression of DIO3 in the fetal and neonatal brain and, to a significant extent in the adult CNS (Kaplan et al., 1981; Kaplan and Yaskoski, 1980), suggests that DIO3-mediated modulation of T3 action is also an important regulatory process for the normal CNS maturation and function. Mice constitutively lacking a functional DIO3 exhibit systemic and brain thyrotoxicosis during development, and excessive T3 action in the adult CNS despite a hypothyroid state in the circulation (Hernandez et al., 2007, 2006). These alterations in brain T3 status lead to abnormal patterns of gene expression and brain cytoarchitecture (Hernandez et al., 2012, 2010), disrupted physiology of social neuropeptides (Stohn et al., 2018) and aberrant behavior including reduced anxiety- and depression-related behavior, increased locomotor activity, elevated aggression and poor maternal behavior (Stohn et al., 2016, 2018). Furthermore, a reduction in brain DIO3 have also been identified in adult rats subject to fetal alcohol syndrome and in different rat strain in association with behavioral and cognitive abnormalities (Sittig et al., 2011; Tunc-Ozcan et al., 2018).
Considering the presence of active DIO3 in the CNS both in development and in adult age, it is not possible to discern whether the broad neurological phenotypes of global DIO3 deficiency result from developmental effects or from the lack of DIO3 in the adult CNS. To delineate the developmental and adult-onset origin of the broad neurological phenotypes associated with global DIO3 deficiency, we created a transgenic mouse model with a conditional Dio3 allele and examined the neurobehavioral consequences of adult-onset DIO3 deficiency. We observed that these mice exhibit sexually dimorphic changes in brain gene expression, increased sensitivity to T3 and increased locomotor activity, but no abnormalities in anxiety- and depression-related behaviors, suggesting significant roles for DIO3 in the adult CNS as well as the developing mammalian brain.
2. Material & methods
2.1. Animals
To generate a conditional Dio3 allele, we constructed a targeting vector containing loxP sites flanking the selenocysteine insertion sequence (SECIS) of the Dio3 gene. We then used homologous recombination in mouse embryonic stem cells, as described previously (Hernandez et al., 2002). Targeted clones were identified by restriction enzyme digestion, injected into C57BL/6 blastocysts, and re-implanted in CD1 foster mothers. Chimeric males that showed germ-line transmission were mated to C57BL/6 females expressing flp DNA recombinase to remove the neomycin cassette from the locus. The heterozygous male offspring were mated with C57Bl/6 females for 4 more generations to establish a colony on a C57Bl/6 genetic background. Primers used for genotyping were: Forward 5′-GGAGTCCTGCTGCTTTTGTG-3′, Reverse 5′-CGAGCCTCTCTGCAATTCAG-3′. We then crossed the resulting mutant mice with mice carrying a transgene expressing cre DNA recombinase under the control of the tamoxifen-inducible β-actin promoter (Actin-cre or B6.Cg-Tg(CAG-cre/Esr1*)5Amc/J, Stock No: 004682, Jackson Lab). These animals were also on a C57Bl/6 genetic background. Animals positive for cre were mated again with the newly generated mutant mouse to obtain mice that were homozygous for the floxed allele and cre positive (KO/Act+). At two months of age these animals were injected intraperitoneal either two times with 2 mg tamoxifen in sesame oil or vehicle only. At four and a half months all animals underwent behavioral testing as follows: Day 1: elevated plus maze, day 4: elevated zero maze, day 5; light/dark box, day 6: open field trial, day 7: tail suspension test, day 8: marble burying test. At five months of age, 3–5 animals received 0.25ug/ml T3 in their drinking water for three weeks before tissue collection. All mice were kept in a 12 h light/dark cycle and fed regular chow ad libitum. Adult animals (6 months old) were sacrificed by CO2 asphyxiation. The Institutional Animal Care and Use Committee of Maine Medical Center Research Institute approved all procedures and behavioral tests.
2.2. Quantitative real time RT-PCR (qRT-PCR)
Tissues were harvested and frozen on dry ice, and total RNA was extracted using the RNeasy kit from Qiagen (Valencia, CA). Total RNA (1 µg) was reverse transcribed with M-MLV reverse transcriptase in the presence of random decamers (both from Thermo Fisher Scientific, Waltham, MA) at 25 C for 10 min, then 37 °C for 50 min. Reverse transcription reactions were diluted by to a total volume of 250 μl with DNase and RNase free water. An aliquot of each sample was mixed together for an internal standard and diluted fourfold. Real-time PCR reactions were set up in duplicate with gene-specific primers and SYBR Select Master Mix (Thermo Fisher Scientific, Waltham, MA) and run on the CFX384 from Bio-Rad (Hercules, CA). Real time PCR reactions underwent an initial 10 min denaturing step, followed by 36 cycles of a denaturing step (94 °C for 30 s) and an annealing/extension step (60 °C for 1 min). The sequences of the primers used were (5′ to 3′): Rn18s, GGAGTATGGTTGCAAAGCTG and TCGCTCCACCAACTAAGAAC; Aldh1a1, CCTTGCATTGTGTTTGCAGATG and GCTCGCTCAACACTCCTTTTC; Bdnf, TGCAGGGGCATAGACAAAAGG and CTTATGAATCGCCAGCCAATTCTC; Cnr1, GGGCAAATTTCCTTGTAGCA and GGCTCAACGTGACTGAGAAA; Dio2, CCTCCTAGATGCCTACAAACAGG and CATTCGGCCCCATCAGCGGTC; Hr, AGCACTGTGTGGCATGTGTT and AACCCTGCATCCAAGTAGCA; Itih3, GCACGTTCAGTTGGCTAGAC and CCATCTCCAAAGGACACCAC; Klf9, GGCTGTGGGAAAGTCTATGG and AAGGGCCGTTCACCTGTATG; Sema7a, AAGTGGTCGTTCACCGCATG and CCACCACCTTGTGAATGGTG; Shh, TTCTGTGAAAGCAGAGAACTCC and GGACGTAAGTCCTTCACCAG; Slc17a7, CCAACGTGCGAAAGCTCATG and CGATGTCCAAGTGGTTCACG; Snap25, GGGCAATAATCAGGATGGAG and GCTCCAGGTTCTCATCCATC; Trh, GGCTCAGCATCTTGGAAAGCTCTGC and CAAGGCGCAGGATTTGGGGATACCA; Ugt8, ACTCCATATTTCATGCTCCTGTG and AGGCCGATGCTAGTGTCTTGA. Expression was normalized using Rn18s as a reference gene. Data are shown in arbitrary units relative to the level of expression observed in control females (control females were assigned an arbitrary average expression of 1 unit). In data representing response to T3, the data represented is relative to baseline expression levels in the corresponding untreated animals (untreated groups for all genotypes and sexes were assigned an arbitrary expression value of 1 and the gene expression values in T3-treated mice were adjusted accordingly). To appreciate baseline differences in gene expression across brain regions, gene expression data for the most common genes used, relative to the cortex is represented in Supplementary Fig. 3 for control tissues.
2.3. Hormone determinations
Blood was collected from the descending vena cava and allowed to coagulate at room temperature for 30 min. Serum was obtained by centrifugation and stored at −70 °C until further analysis. Serum thyroid hormone levels were determined with the T3 AccuBind and T4 AccuBind ELISA kits from Monobind Inc (Lake Forest, CA). Serum TSH determinations were performed by Samuel Refetoff at the University of Chicago using a sensitive, heterologous, disequilibrium, double-antibody precipitation RIA as previously described (Pohlenz et al., 1999).
2.4. Behavioral tests
Behavioral tests were conducted in a specifically equipped room following standard procedures as described (Stohn et al., 2016, 2018). The test apparatuses were purchased from Stoelting (Wood Dale, IL) and experiments were recorded with the ANY-maze™ video tracking system v5.14 (Stoelting, Wood Dale, IL) or a camcorder (Samsung HMX-F90). Videos were analyzed offline with The Observer XT v12.5 from Noldus (Leesburg, VA). Mice were generally group-housed (2–4 animals per cage) after weaning. The experimental animals were placed in the testing room for two to three hours prior to the test and left undisturbed during this period to acclimate.
2.4.1. Elevated plus maze test
The test was performed using the elevated plus maze (EPM), which has a cross shape. Opposing arms of the EPM were either walled or open. Mice were placed either in the center (neutral square) to start the test and were recorded for 5 min, while exploring the maze freely. Mice falling of the maze were excluded from the tests and analysis.
2.4.2. Light/dark box test
The light-dark box (LDB) has a square shape and is divided in half, using clear acrylic glass walls for the light area and black acrylic glass for the dark area including a top cover. The two areas are connected through an opening in the middle of the dividing wall allowing the animal to move freely from one half into the other. At the beginning of the test the animal is placed in the light area with its back against the wall facing the opening in the dividing wall. The animal is allowed to explore the arena freely for 10 min.
2.4.3. Open field test
We used the square setup with opaque walls for the open field test (OFT). The center area was defined as a 20 cm by 20 cm, while the remaining area was referred to as periphery. At the beginning of the test the animal was placed in the periphery facing the center and allowed to explore the maze freely for 5 min.
2.4.4. Marble burying test
A cage with the dimensions 45 cm by 23 cm was used and filled with a 5–6 cm deep layer of corncob bedding (Envigo, South Easton, MA). 28 black marbles were spaced out equally on top of the bedding in five alternating rows of six and five. The mouse was placed in the cage and allowed to explore it freely for 30 min under low lighting. The mouse was removed gently from the cage at the end of the test to avoid disturbing the bedding and marbles buried by at least two thirds of their diameter or more were counted as buried.
2.4.5. Tail suspension test
Mice were suspended and by their tail and fixed with adhesive tape to a shelf, so their heads would be located approximately 8 cm above a flat surface. Animals caged together were tested in parallel. Mice were video recorded for 5 min. An investigator blind to the genotype of the mice analyzed the recordings and scored the latency to become immobile, the episodes of immobility, the duration of these episodes and the total time that the mice spent immobile during the test.
2.5. DIO3 enzymatic activity
D3 enzymatic activities were determined as previously described (Hernandez et al., 2006). In brief, tissues were homogenized in a 10-mM Tris−HCl, 0.25 sucrose buffer (pH 7.4). A suitable volume of tissue homogenate was used in the enzymatic reaction to ensure that deiodination do not exceed 40% and was proportional to the amount of protein content. Tissue homogenates were incubated at 37 °C for an hour with 2 nM1250049-labeled T3 (PerkinElmer) in the presence of 25 mM dithiothreitol. Deiodination was determined based on the percentage of 125I-3,3_-diiodothyronine produced. The latter was determined by measuring the amount of radioactivity associated with the reaction products after separation by paper chromatography as described (Galton and Hiebert, 1987).
2.6. Statistical analysis
Statistical analysis was performed using Student’s t-test (paired or unpaired), one-way ANOVA or two-way ANOVA followed by Tukey’s test, which corrects for multiple comparisons between experimental groups using the statistical tools of GraphPad Prism 6 (GraphPad Software, Inc.). Statistical significance was defined as P<0.05. *, **, *** indicate P<0.05, 0.01 and 0.001 statistical differences, respectively, between genotypes (black asterisks) or sexes (red asterisks). #, ##, ### indicate P<0.05, P<0.01, P<0.001 statistical differences, respectively, between T3-treated and untreated mice within a given genotype and sex.
3. Results
3.1. Generation of a mouse model for conditional DIO3 deficiency
To investigate whether the previously reported changes of behavior in Dio3−/− mice are due to developmental effects or increased thyroid hormone action in the brain of adult animals, we established an experimental model of conditional DIO3 deficiency by generating a mouse carrying a “floxed” Dio3 allele. The Dio3 gene was targeted by homologous recombination in ES cells with a Dio3 mutant whereby the selenocysteine insertion sequences (SECIS) in the 3′-untranslated region of the mRNA is flanked by loxP sites and followed by a Neomycin cassette flanked by frt sequences (Supplementary Fig. 1A). The SECIS stem-loop structure in the Dio3 mRNA is essential for the insertion of selenocysteine in the polypeptide chain, and this aminoacid is critical for deiodinase activity (Low et al., 1995). Selenocysteine is encoded by a UGA codon, which functions as a stop codon in the absence of the SECIS.
ES cells showing successful targeting (Supplementary Fig. 1B) were used to generate mutant mice with a floxed SECIS in the Dio3 gene. Postnatal day 1 tissues of mice homozygous for the SECIS-floxed allele exhibited a decreased in DIO3 enzymatic activity compared to tissues of wild type mice (Fig. 1C). This reduction in enzymatic activity did not affect all tissues equally. After the neomycin cassette was excised by breeding these mice with transgenic mice expressing frt DNA recombinase, DIO3 enzymatic activity in mice homozygous for the floxed-SECIS allele were fully normalized in most tissues, with the exception of the brain and the testis, that showed a 20 and 30% reduction in DIO3 enzymatic activity, respectively, when compared to that in WT mice (Supplementary Fig. 1C). These results suggest that the neomycin cassette was interfering with the mechanisms of SECIS-mediated selenocysteine translation. They also demonstrate that the newly created mutant mice are largely DIO3-sufficient in the native state.
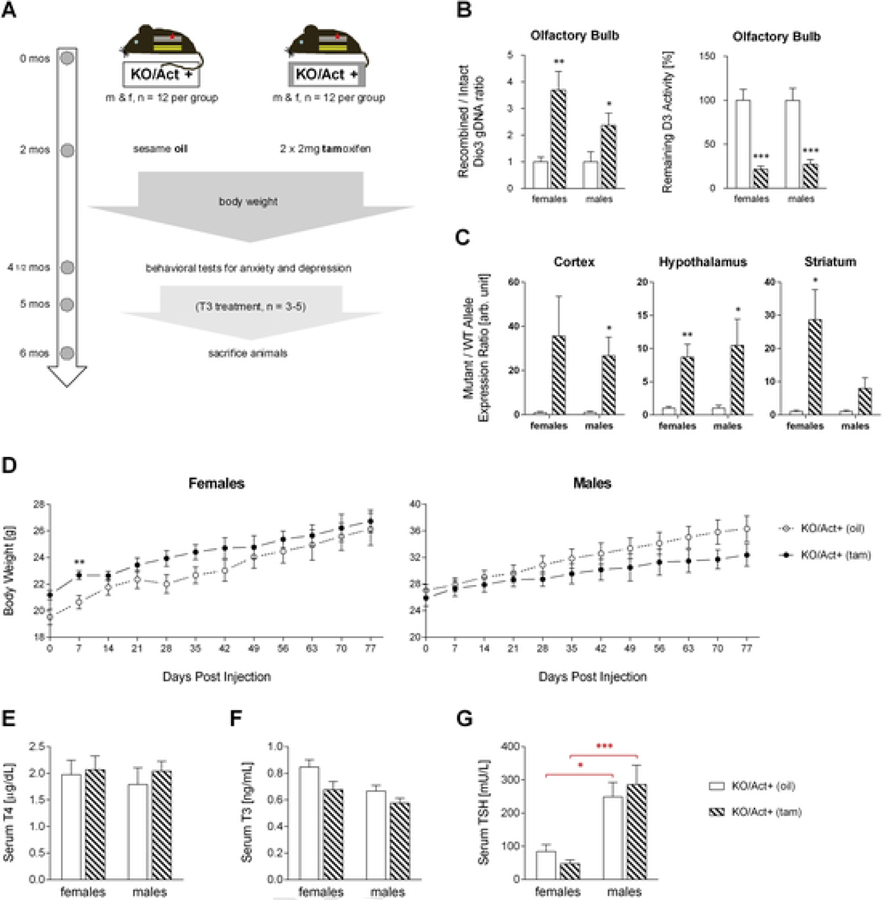
Fig. 1.
Effects of adult onset DIO3-deficiency on body weight and the thyroid status.Timeline and overview of study (A). Genomic DNA recombination efficiency and remaining DIO3 activity in the olfactory bulb four months after tamoxifen (tam) or vehicle (oil) injection (B). Recombined vs intact mRNA allele expression ration in brain regions of experimental animals (C). Bodyweight was measured for two and a half month after injection (D). Serum T4 (E), T3 (F), and TSH (G) level of six month old mice Data are shown as mean±SEM with n=6–8 mice per experimental group. *, **, *** indicate P<0.05, P<0.01 and P<0.001 statistical differences, respectively, between genotypes (black asterisks) or sexes (red asterisks), as determined by Student’s t-test for each gender.
3.2. Effects of adult onset DIO3 deficiency on the thyroid status
To inactivate DIO3 in adulthood, we crossed our conditional Dio3SECIS f/f mouse with tamoxifen-inducible actin-cre mice to generate Actin-cre/Dio3SECIS f/f (KO/Act+) experimental animals (see methods for more details). The experimental timeline is outlined in Fig. 1A. The animals were randomly divided in two groups for both males and females and allowed to develop normally. At two months of age mice were injected with tamoxifen (tam) to initiate recombination and abolish DIO3 activity or with sesame oil (oil) as a control. Bodyweight was monitored for two and a half months before the behavioral tests for anxiety and depression were conducted. At 5 months of age some of the animals of both groups were treated with T3 in the drinking water until all animals were sacrificed at 6 months of age.
We analyzed the olfactory bulb and the adrenal gland (not shown) to assess the level of recombination and found that the remaining DIO3 activity in KO/Act+ (tam) mice was significantly reduced by 64–83% compared to the control animals (Fig. 1B). The mutant versus wild type allele expression ratio in brain tissue of KO/Act+ (tam) mice of both sexes was approximately 8–30 times higher than in KO/Act+ (oil) mice (Fig. 1C). This indicates that more than 88% of the Dio3 mRNA expressed in the brain of KO/Act+ (tam) mice is generated by recombined DNA and produces an inactive enzyme.
Dio3−/− mice show reduced growth post weaning, which persists throughout their life (Hernandez et al., 2006). In contrast, adult onset of DIO3 deficiency in adulthood had no significant effect on the bodyweight in females or males during adulthood; however, there was a trend in the male KO/Act+ (tam) mice to gain less weight than the control animals as they age (Fig. 1D). The thyroid status in the KO/Act+ (tam) animals of both genders was not significantly different from the KO/Act+ (oil) control mice. Serum T4, T3 and TSH level were not significantly different from the controls in KO/Act+ (tam) mice of either sex (Fig. 1E–G), indicating normal thyroid hormone status. Serum TSH trended lower in the KO/Act+ (tam) females and higher in the males compared to control animals (Fig. 1G), but no statistical significance was achieved.
To determine if leaking of the cre-transgene in the absence of tamoxifen was causing a baseline change in the neurological phenotype of our control group (KO/Act+ (oil) mice), we determined a set of physiological endpoints to compare KO/Act+ (oil) mice with tamoxifen-treated mice homozygous for the floxed Dio3 allele but not carrying the inducible cre transgene (KO/Act− (tam) mice). We observed no significant changes in growth, thyroid hormone parameters or behavior (Supplementary Fig. 2A, 2B and 2C). Gene expression profile in brain regions was largely unchanged except for modest changes in the striatal expression of Itih3 and Dio2 (Supplementary Fig. 2D).
3.3. Loss of Dio3 activity during adulthood increases hyperactivity in females
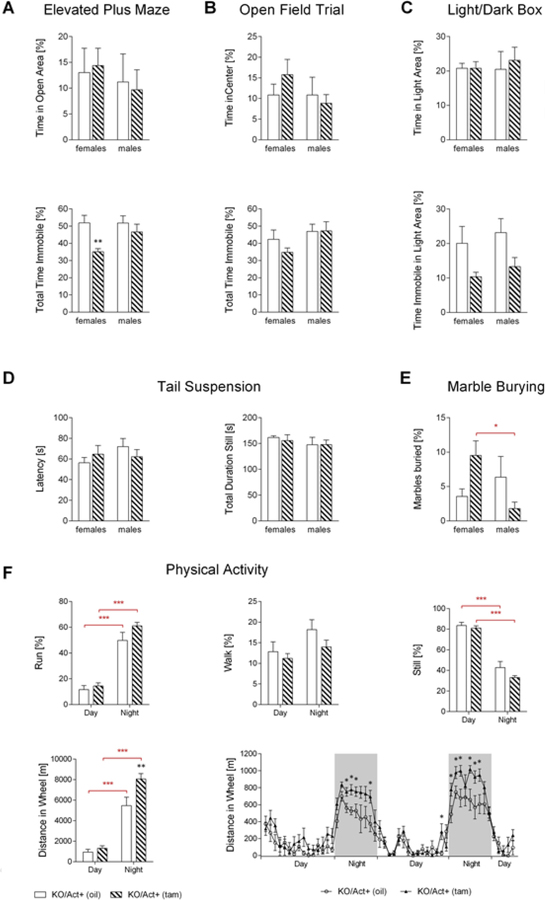
We have shown that the lack of active DIO3 in mice results in T3 excess in the brain, both during development and in adulthood (Hernandez et al., 2006, 2010). This is associated with decreased anxiety- and depression-like behaviors, and increased hyperactivity (Stohn et al., 2016). To test whether these abnormal behavioral phenotypes are of developmental origin or derived from increased thyroid hormone levels in the adult brain, experimental animals underwent behavioral testing for anxiety and depression-like behaviors two and a half months after tamoxifen or vehicle injection. We did not observe any significant changes for the time spent in the open arms of the elevated plus maze (EPM), time spent in the center of the open field trial (OFT), time spent in the light area in the light/dark box (LDB), latency to being still, or total time being still in the tail suspension test in either the female or male KO/Act+ (tam) mice (Fig. 2A–D). This indicates that the loss of DIO3 activity in the adult mouse had no effect on anxiety- or depression-like behaviors. However, the overall time spent immobile in the EPM was significantly reduced in the KO/Act+ (tam) females (Fig. 2A), suggesting increased locomotor activity. In addition, the total time immobile trended lower in the OFT (Fig. 2B) and in the LDB, and the percentage time spent immobile in the light area was almost significantly reduced (p = 0.068) (Fig. 2C), suggesting hyperactivity in female mice with adult onset DIO3 deficiency. In the marble burying test, the female KO/Act+ (tam) mice buried significant more marbles than the control animals. In the males, this behavior appeared to be reversed, but did not reach any statistical significance (Fig. 2E).
Fig. 2.
Anxiety- and depression-like behavior in mice with adult onset DIO3-deficiency.KO/Act+ (oil) and KO/Act+ (tam) mice underwent behavioral testing at 4 ½ months of age. Shown here are the percentages of the time spent in the maze’s open arms and the total time immobile in the elevated plus maze (A), time spent in the center and total time immobile in the open field trial (B), total time and time immobile spent in the light area of the light/dark box (C) as well as the latency to stop moving and total time still in the tail suspension test (D), the percentage of marbles buried in the marble burying tests (E) and the physical activity in female mice (F). Data represent the mean±SEM of results from 8 to 12 mice (per genotype and sex). *, **, *** indicate P<0.05, 0.01 and 0.001 statistical differences, respectively, between genotypes (black asterisks) or sexes (red asterisks), as determined by two-way ANOVA and Tukey’s test for multiple comparisons or Student’s t-test (last panel).
Studies in metabolic cages indicated no differences in distance run (Fig. 2F), but DIO3-deficient females consistently showed a marked increase in voluntary running in the wheel during the night period (Fig. 2F). This phenotype could not be observed in the males available for the study.
3.4. Gene expression in the cortex, hypothalamus, and striatum
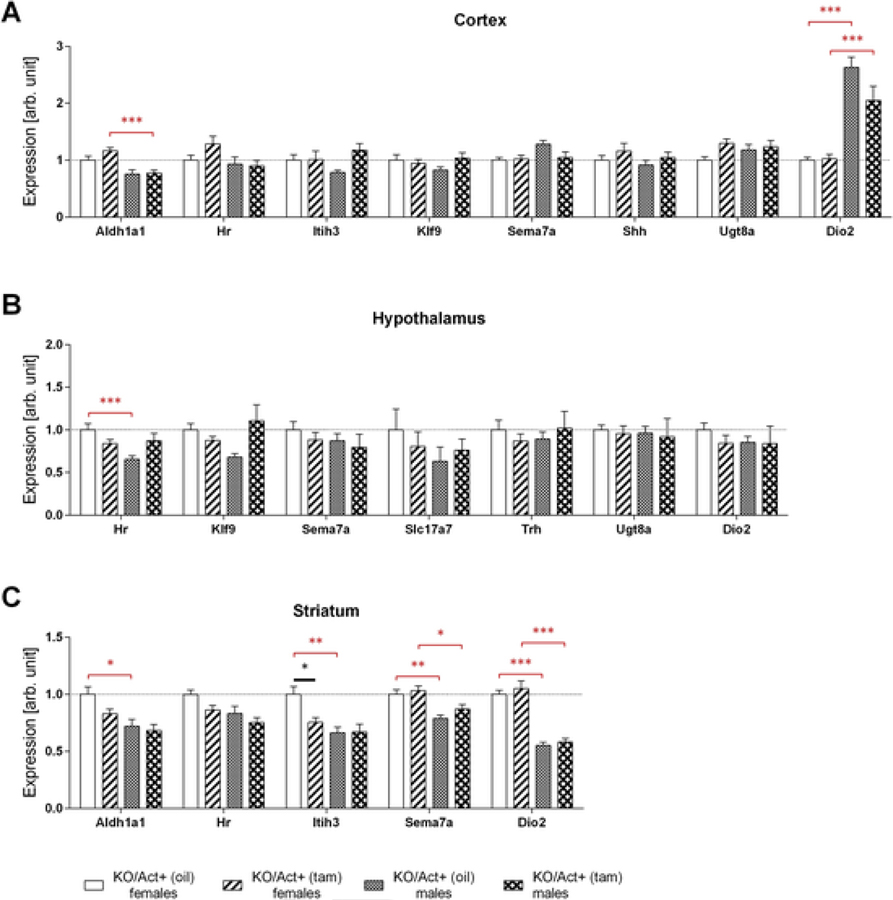
To evaluate the degree of thyroid hormone signaling in the brain, we determined the expression of several T3-dependent genes in the cortex, hypothalamus, and striatum, including hairless (Hr), inter-alpha-trypsin inhibitor heavy chain 3 (Itih3), kruppel-like factor 9 (Klf9), sonic hedgehog (Shh), semaphoring 7a (Sema7a), aldehyde dehydrogenase 1 family member a1 (Aldh1a1), solute carrier family 17 member 7 (Slc17a7), UDP glycosyltransferase 8 (Ugt8a), and thyrotropin-releasing hormone (Trh). Adult-onset DIO3 deficiency had little effect on the expression of these genes in the different brain regions (Fig. 3A–C). Only the striatal expression of Itih3 was modestly reduced as a result of DIO3 inactivation (Fig. 3C). However, we observed several instances of sexually dimorphic gene expression, both in DIO3-sufficient and insufficient animals. These genes included the cortical expression of Aldh1a1 and Dio2, the hypothalamic expression of Hr (Fig. 3B), and the striatal expression of Aldh1a1, Itih3, Sema7a and Dio2 (Fig. 3C), an important determinant of brain T3 action (Bocco et al., 2016; Dietz et al., 2012). DIO2 enzymatic activity, which is post-transcriptionally regulated by its own substrate T4, was not determined as serum levels of T4 were unchanged.
Fig. 3.
Gene expression in the cortex, hypothalamus, and striatum.Mice were injected with tamoxifen (KO/Act+ (tam)) or vehicle (KO/Act+ (oil)) at two months of age, and tissues were collected at six months of age. Cortical (A), hypothalamic (B), striatal (C) expression of the genes indicated in male and female mice as determined by qPCR. Data were normalized to values in KO/Act+ (oil) females and are shown as mean ± SEM with n=6–8 mice per experimental group. *, **, *** indicate P<0.05, P<0.01, P<0.001 statistical differences, respectively, between genotypes (black asterisks) or sexes (red asterisks), as determined by two-way ANOVA and Tukey’s post hoc test for multiple comparisons for the effect of sex and genotype.
3.5. Response to T3 treatment
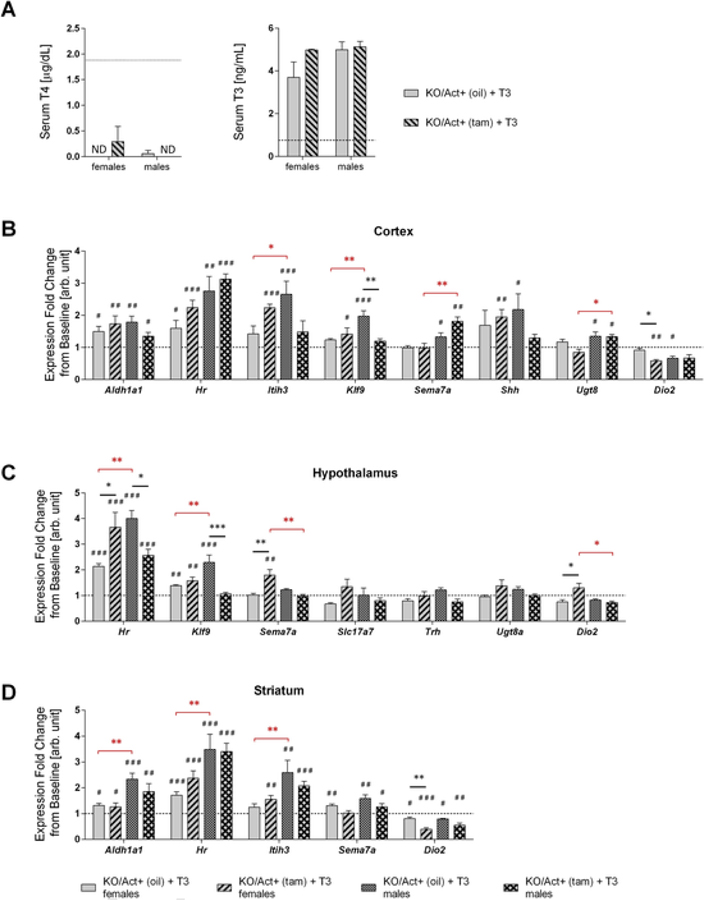
To test whether the brain of mice with adult onset DIO3 deficiency is more sensitive to a hyperthyroid status than the brain of control mice, we added T3 to the drinking water to cause hyperthyroidism in the mice. Consistent with previous findings serum T4 level in all T3-treated experimental mice were markedly reduced or not detectable compared to untreated animals due to suppression of the thyroid axis. T3 treatment markedly increased serum levels of T3 to a similar extent in KO/Act+ (tam) and KO/Act+ (oil) animals (Fig. 4A). TSH level were not detectable in any of the T3-treated experimental groups (data not shown). For a majority of the genes tested, T3-treatment led to expression changes in one or more brain areas of both experimental groups of mice (Fig. 4B–D). Some of the gene responses to T3 treatment were sex-specific, region-specific or specific to DIO3 inactivation. Changes specific to Dio3 inactivation included a Kfl9 expression decrease in the cortex and hypothalamus of males; a reduction in cortical and striatal Dio2 expression in females (Fig. 4A and 4C), while the hypothalamus showed an increase in Dio2 (Fig. 4B); and increase in Sema7a expression in the hypothalamus of females; and changes in the hypothalamic expression of Hr (Fig. 4C), which increased in females and decreased in males. Additional sexually dimorphic patterns of gene expression were noted. These findings reveal a sexual dimorphism in how mice with adult-onset DIO3 deficiency respond to T3.
Fig. 4.
Changes in brain gene expression in response to T3-treatment.Serum T4 and T3 level in treated animals compared with non-treated ones (dotted line) (A), dotted line indicates level of untreated animals. Cortical (B), hypothalamic (C), striatal (D) expression of selected genes in female or male of either genotype expressed as a fold increase over the levels found in the corresponding group of untreated animals (dotted line). All data are shown as mean ± SEM with n=3–5 mice per experimental group. *, **, *** indicate P<0.05, P<0.01, P<0.001 statistical differences, respectively, between genotypes (black asterisks) or sexes (red asterisks). #, ##, ### indicate P<0.05, P<0.01, P<0.001 statistical differences, respectively, between T3-treated and untreated mice within a given genotype and sex. Statistical significance was determined by two-way ANOVA followed by Tukey’s test for multiple comparisons.
3.6. Expression of genes related to hyperactivity
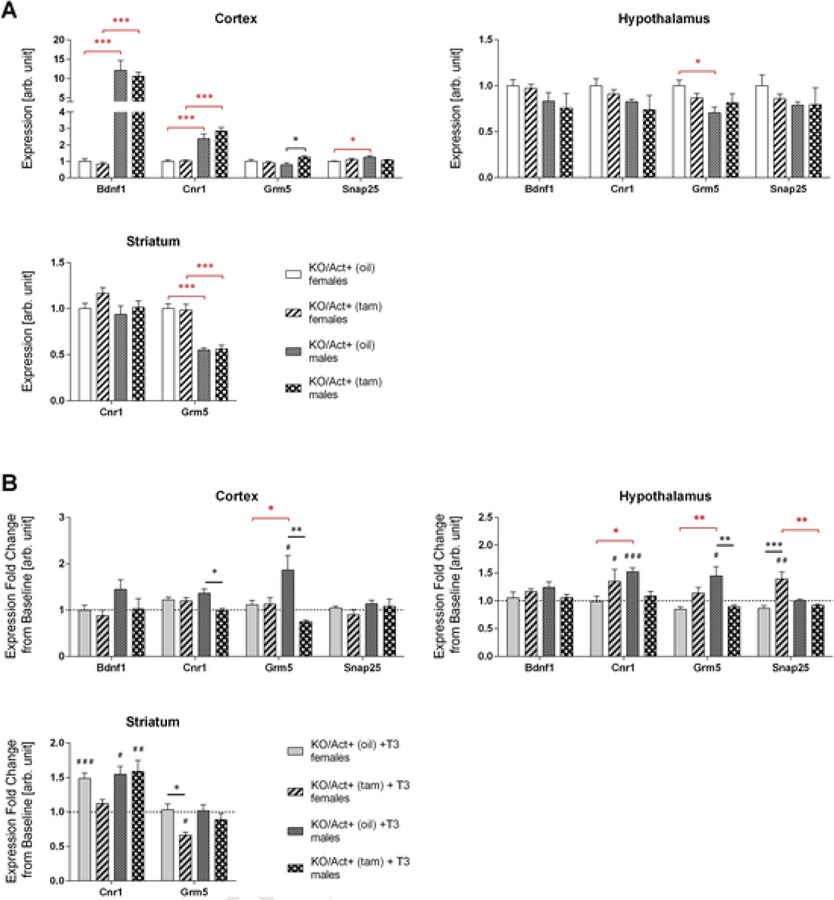
The behavioral tests showed that females exhibit hyperactivity when DIO3 deficiency was achieved during adulthood. We determined the activity of some genes that have been described as related to hyperactivity or ADHD including Brain-derived neurotrophic factor 1 (Bdnf1) (Rios et al., 2001), cannabinoid receptor 1 (Cnr1) (Franke et al., 2009), glutamate metabotropic receptor 5 (Grm5) (Elia et al., 2011), and synaptosomal-associated protein 25 (Snap25) (Grunblatt et al., 2012). At baseline, we found no gene expression differences in females (Fig. 5A), but we observed a significant increase in Grm5 expression in the cortex of KO/Act+ (tam) male mice (Fig. 5A). In addition, we noted several sexual dimorphisms in gene expression that were independent of DIO3 inactivation and affecting Bdnf, Cnr1, Snap25 and Grm5.
Fig. 5.
Expression of hyperactivity-related genes.Mice were injected with tamoxifen (KO/Act+ (tam)) or vehicle (KO/Act+ (oil)) at two months of age, and tissues were collected at six months of age. Baseline cortical, hypothalamic and striatal expression of Bdnf1, Cnr1, Grm5, and Snap25 in untreated females or male mice with or without Dio3 inactivation (A) or in animals treated with T3 (B). Data were normalized to values in KO/Act+ (oil) female mice. Data in (B) are represented as a fold change over the corresponding group of untreated animals (dotted line). Data are shown as mean ± SEM with n=6–8 mice per experimental group (A) or 3–5 mice (B) per experimental group. *, **, *** indicate P<0.05, P<0.01, P<0.001 statistical differences, respectively, between genotypes (black asterisks) or sexes (red asterisks). #, ##, ### indicate P<0.05, P<0.01, P<0.001 statistical differences, respectively, between T3-treated and untreated mice within a given genotype and sex. Statistical significance was determined by two-way ANOVA followed by Tukey’s multiple comparison test for multiple comparisons.
To test whether hyperthyroidism has an effect on these genes, we also measured their expression levels after T3-treatment. T3-treatment induced hypothalamic Cnr1 and Snap25 expression in KO/Act+ (tam) females but not in KO/Act+ (oil) females (Fig. 5B), suggesting increased sensitivity to T3 in DIO3-deficiency. In contrast, the opposite was observed in the female striatum, where Cnr1 and Grm5 expression was induced or not changed, respectively, in KO/Act+ (oil) females, but was unaltered or reduced in KO/Act+ (tam) females (Fig. 5B). We observed no changes in gene expression in the cortex of females of any genotype (Fig. 5B). Concerning the expression of these genes, KO/Act+ (oil) males were overall more responsive to T3 than KO/Act+ (tam) males (Fig. 5B), showing expression increased cortical Bdnf and Grm5, and hypothalamic and striatal Cnr1, while only striatal Cnr1 was induced in KO/Act+ (tam) males.
3.7. Brain expression of Dio3os
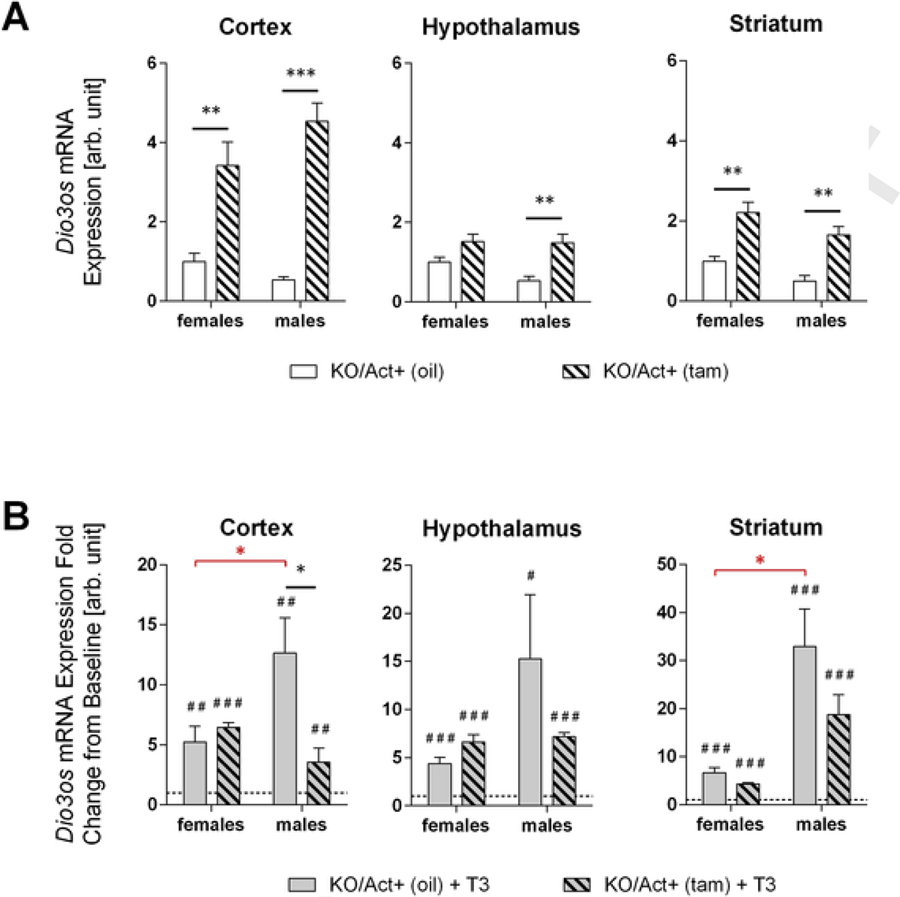
Dio3os is a long non-coding RNA expressed from the DNA strand opposite to Dio3 (Hernandez et al., 2002). Dio3os expression is highly sensitive to T3 and strongly correlates with that of Dio3 in rodent brain tissue and brain human cell lines (Dietz et al., 2012; Kester et al., 2006). We examined Dio3os expression in experimental animals, treated or not with T3, to further assess T3 signaling and sensitivity in brain tissue. Compared to untreated KO/Act+ (oil) male mice, untreated KO/Act+ (tam) male mice exhibited a 6-, 2- and 3-fold increase in Dio3os expression in cortex, hypothalamus and striatum, respectively (Fig. 6A). In KO/Act+ (tam) female mice, Dio3os expression was increased only 3- and 2-fold in cortex and striatum, but not significantly different in the hypothalamus (Fig. 6A). When animals were treated with T3, Dio3os expression was markedly increased in all brain tissues of KO/Act+ (tam) and KO/Act+ (oil) mice of both sexes. However, T3-response in Dio3os expression relative to the corresponding baseline level differed between sexes, genotypes and brain regions. The magnitude of the response to T3 varied within 4 to 6-fold in female brain tissues and was similar between KO/Act+ (tam) and KO/Act+ (oil) female mice (Fig. 6B). In KO/Act+ (oil) male mice, the magnitude of the T3 response was markedly higher than in KO/Act+ (oil) females in all tissues examined (12–30-fold increases 4–6-fold increases in Dio3os expression), while T3 responses in KO/Act+ (tam) mice were comparable between males and females, except in the striatum, where the male response was higher (Fig. 6B). T3 responses were similar between genotypes for all female issues, but were higher in KO/Act+ (oil) male tissues that in KO/Act+ (tam) ones, suggesting that the dynamic range of response to T3 is reduced in males with adult onset DIO3 deficiency.
Fig. 6.
Brain expression of Dio3os.Cortical, hypothalamic and striatal expression of Dio3os in untreated (A) or T3-treated mice of either sex and Dio3 inactivation status (B). Data in (B) are represented as a fold change over the corresponding group of untreated animals (dotted line). Data are shown as mean ± SEM with n=6–8 mice per experimental group (A) or 3–5 mice (B) per experimental group. *, **, *** indicate P<0.05, P<0.01, P<0.001 statistical differences, respectively, between genotypes (black asterisks) or sexes (red asterisks). #, ##, ### indicate P<0.05, P<0.01, P<0.001 statistical differences, respectively, between T3-treated and untreated mice within a given genotype and sex. Statistical significance was determined by two-way ANOVA followed by Tukey’s test for multiple comparisons.
4. Discussion
Timely regulation of T3 action in different regions of the CNS is critical to ensure normal brain function (Bernal, 2005). A global deficit in the principal enzyme that limits T3 action, DIO3, leads to an excess of T3 signaling in the brain during development and in adulthood (Hernandez et al., 2010) that is associated with altered brain gene expression and behaviors (Stohn et al., 2016, 2018). However, it is unclear to what extent these abnormalities have a developmental origin and result from thyrotoxicosis in early life, or are the consequence of increased T3 action in the adult brain. To address this question, we have initiated studies in a mouse model of conditional inactivation of DIO3 in adulthood.
Mice homozygous for the floxed Dio3 allele exhibit normal DIO3 activity in most tissues testes, but a 20% decrease in expression was noted in the brain. This modest decrease is unlikely to exert significant neurological effects, especially most tissues contributing to systemic thyroid hormone clearance show normal DIO3 activity. Thus, our control mice do not exhibit any significant phenotype. In this regard, we show that these animals, when carrying the inducible cre transgene, exhibit no alterations in behavior and minimal changes in brain gene expression compared to animals lacking the cre transgene. This indicates negligible leaking of cre expression and a comparably normal phenotype in the control animals used in our studies. The marked reduction in DIO3 activity and in the relative abundance of the functional Dio3 mRNA upon tamoxifen injection demonstrate a large degree of DIO3 deficiency in the brain of these mice.
Adult-onset DIO3 deficiency did not significantly affect body weights, although a sexually dimorphic tendency for a leaner phenotype was noted in DIO3-deficient males. This is consistent with observations in mice with constitutive DIO3 deficiency showing that the brain T3 excess exerts effects on energy balance that could be sexually dimorphic and partially compensate each other in the regulation of body weight (Wu et al., 2017). Mice with adult DIO3 deficiency did not show any abnormalities in thyroid function tests. Since mice with constitutive DIO3 deficiency exhibit marked abnormalities in the serum thyroid hormone parameters and in the function of the hypothalamic-pituitary-thyroid axis (Hernandez et al., 2007, 2006), our present results suggest that the thyroid axis phenotype resulting from constitutive DIO3 deficiency is primarily the consequence of abnormal programming during development and does not result from altered factors in adult life. Alternatively the absence of a thyroid axis phenotype may be due to the fact that DIO3 is not a 100% inactivated. Also, preliminary results from our laboratory indicate a partial suppression of serum T3 and T4 in adult animals two weeks Dio3 inactivation. As we have currently studied animals several months after tamoxifen injection, this observation suggests that DIO3 inactivation may cause a short term change in the thyroid axis that is later compensated by other mechanisms. This intriguing possibility warrants further studies.
DIO3 inactivation during adulthood did not lead to major alterations in behavior. However, KO/Act+ (tam) female – but not male - mice showed increased physical activity, as determined by several behavioral tests. These results suggest that adult onset DIO3 deficiency increases locomotor activity, which is consistent with the hyperactivity in systemic hyperthyroidism (Levine et al., 2003) and models of brain T3 excess (Stohn et al., 2016; Wu et al., 2017). The lack of marked behavioral phenotypes associated with anxiety and depression, as those observed in mice with constitutive DIO3 deficiency (Stohn et al., 2016, 2018), suggests these behaviors are more susceptible to be influenced by T3 excess during development than in adulthood.
The relatively modest behavioral phenotype cause by adult-onset of DIO3 deficiency correlated with few significant changes in the expression of T3-dependent genes across brain regions, consistent with observations that many genes regulated by T3 in the brain exhibit a developmental window of maximal sensitivity that is later reduced in adulthood (Bernal et al., 2003). However, the response of these genes to T3 treatment was generally enhanced in the brain tissue of mice with DIO3 deficiency, suggesting that adult inactivation of DIO3 does increase brain sensitivity to T3. The increased sensitivity may be partly due to the well-established observation that Dio3 expression is greatly induced by an excess of T3 (Hernandez et al., 2012; Tu et al., 1999). Thus, upon T3 treatment, the DIO3 status of control animals will be elevated, while that of experimental animals will largely remain the same. Interestingly, the increased sensitivity to T3 of animals with adult Dio3 inactivation was observed in females, not in males, in which no effect or the opposite effect was observed. Suppression of the brain expression of Dio2, the gene that locally generates T3 from T4, may also have mitigated, in part, the effects of T3 treatment. Thus, local changes in Dio2 expression may be a general compensatory mechanism for the lack of Dio3 in this animal model.
We observed similar profiles of expression in a subset of genes associated with hyperactivity, with only cortical expression of Cnr1 and Grm5 being affected in males with adult-onset DIO3 deficiency. Again, T3 treatment regulated the expression of this gene subset in a genotype-, tissue- and sex-dependent manner. This is best illustrated by the varied response to T3 of Cnr1 expression across female brain tissues with or without DIO3 inactivation. The observation of differential regulation between brain regions of T3-regulated genes is well established, for example by the striatal-specific T3-regulation of Nrgn (Iñiguez et al., 1996).
The observed sexual dimorphisms in gene expression are best illustrated by the results of the expression and T3-response of Dio3os, which is very sensitive to T3 regulation (Dietz et al., 2012). These results suggest that not only KO/Act+ (tam) mice are more sensitive to DIO3 deficiency, but also that the dynamic range of Dio3os response to T3 is sexually dimorphic in both DIO3 sufficiency and deficiency. The mechanisms underlying sex-specific differences in the context of brain T3 action remain to be elucidated. However, we speculate that thyroid hormone may have a role in the sexual differentiation of the brain. This is supported by the observation that during a transient neonatal period Dio3 is very highly expressed in the amygdala and hypothalamic and preoptic areas (Escamez et al., 1999), which are well known for their ultimate sexual dimorphism and role in brain sexual differentiation. In addition, unpublished results from our laboratory suggest that Dio3 expression is sexually dimorphic in many areas of the adult brain. Thus, Dio3 may be involved in both brain sexual differentiation and in the sex-specific regulation of brain T3 availability.
Were our findings fully applicable to humans, they would suggest that in the absence of overt thyroid hormone pathologies the lack of DIO3 activity in the adult brain has limited impact on behavior and brain gene expression and potentially on neurological outcomes. However, our findings suggest that development is the most critical period during which an excess of thyroid hormone, due to maternal pathology or epigenetic dysregulation of human DIO3, may lead to neurodevelopmental disorders. A large body of literature in humans and rodents has also defined the developmental period as critically period during which the central nervous system is most sensitive to a deficit of thyroid hormone (Gyllenberg et al., 2016; Lavado-Autric et al., 2003; Roman, 2007; Rovet, 2014). Taken together, these observations support the idea that timely balanced thyroid hormone action is required for normal brain development.
It is interesting to note the more severe consequences of the T3 excess derived from a developmental deficit in DIO3, compared with the reported lack of effect of exogenously administered T3 in rodent fetuses (Grijota-Martinez et al., 2011; Schwartz et al., 1997). This suggests an important role for DIO3 during this critical period for the preventing neurodevelopmental disorders. Since the prevalence of these exhibits a marked sex bias, our sexually dimorphic results suggest that alterations in thyroid hormone action may contribute to the etiology of these disorders.
In summary, our results show that adult-onset DIO3 deficiency leads to increased locomotor activity and influences brain gene expression, rendering the CNS more sensitive to T3 action. These behavioral and molecular effects demonstrate the functional relevance of DIO3 in the adult brain and indicate that an important component of the neurological phenotypes associated with constitutive DIO3 deficiency has a developmental origin. Additional studies are needed to further define the role of DIO3 in different brain regions and across developmental stages.
Supplementary Material
Acknowledgments
Role of funding sources
This work was supported by grants DK54716, MH083220, DK095908 and MH096050 from the National Institute of Mental Health and from the National Institute of Diabetes, Digestive and Kidney Disease. Our studies used Molecular Phenotyping and Physiology Core Facilities at Maine Medical Center Research Institute that are partially supported by grants P30GM106391, P20GM121301 and U54GM115516 from the National Institute of General Medical Sciences.
Footnotes
Declaration of Competing Interest
The authors declare no conflict of interest
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.psyneuen.2019.104439.
References
- Andersen SL, Laurberg P, Wu CS, Olsen J, 2014. Attention deficit hyperactivity disorder and autism spectrum disorder in children born to mothers with thyroid dysfunction: a Danish nationwide cohort study. BJOG 121, 1365–1374. [DOI] [PubMed] [Google Scholar]
- Arrojo EDR, Fonseca TL, Werneck-de-Castro JP, Bianco AC, 2013. Role of the type 2 iodothyronine deiodinase (D2) in the control of thyroid hormone signaling. Biochim. Biophys. Acta 1830, 3956–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakke JL, Lawrence N, Wilber JF, 1974. The late effects of neonatal hyperthyoridism upon the hypothalamic-pituitary-thyroid axis in the rat. Endocrinology 95, 406–411. [DOI] [PubMed] [Google Scholar]
- Bernal J, 2005. Thyroid hormones and brain development. Vitam. Horm 71, 95–122. [DOI] [PubMed] [Google Scholar]
- Bernal J, Guadano-Ferraz A, Morte B, 2003. Perspectives in the study of thyroid hormone action on brain development and function. Thyroid 13, 1005–1012. [DOI] [PubMed] [Google Scholar]
- Bocco BM, Werneck-de-Castro JP, Oliveira KC, Fernandes GW, Fonseca TL, Nascimento BP, McAninch EA, Ricci E, Kvarta-Papp Z, Fekete C, Bernardi MM, Gereben B, Bianco AC, Ribeiro MO, 2016. Type 2 deiodinase disruption in astrocytes results in anxiety-depressive-Like behavior in male mice. Endocrinology 157, 3682–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent GA, 2012. Mechanisms of thyroid hormone action. J. Clin. Invest 122, 3035–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Fuller JL, 1975. Neonatal thyroxine administration, behavioral maturation, and brain growth in mice of different brain weight. Dev. Psychobiol 8, 355–361. [DOI] [PubMed] [Google Scholar]
- Davenport JW, Gonzalez LM, 1973. Neonatal thyroxine stimulation in rats: accelerated behavioral maturation and subsequent learning deficit. J. Comp. Physiol. Psychol 85, 397–408. [DOI] [PubMed] [Google Scholar]
- Dietz WH, Masterson K, Sittig LJ, Redei EE, Herzing LB, 2012. Imprinting and expression of Dio3os mirrors Dio3 in rat. Front. Genet 3, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia J, Glessner JT, Wang K, Takahashi N, Shtir CJ, Hadley D, Sleiman PM, Zhang H, Kim CE, Robison R, Lyon GJ, Flory JH, Bradfield JP, Imielinski M, Hou C, Frackelton EC, Chiavacci RM, Sakurai T, Rabin C, Middleton FA, Thomas KA, Garris M, Mentch F, Freitag CM, Steinhausen HC, Todorov AA, Reif A, Rothenberger A, Franke B, Mick EO, Roeyers H, Buitelaar J, Lesch KP, Banaschewski T, Ebstein RP, Mulas F, Oades RD, Sergeant J, Sonuga-Barke E, Renner TJ, Romanos M, Romanos J, Warnke A, Walitza S, Meyer J, Palmason H, Seitz C, Loo SK, Smalley SL, Biederman J, Kent L, Asherson P, Anney RJ, Gaynor JW, Shaw P, Devoto M, White PS, Grant SF, Buxbaum JD, Rapoport JL, Williams NM, Nelson SF, Faraone SV, Hakonarson H, 2011. Genome-wide copy number variation study associates metabotropic glutamate receptor gene networks with attention deficit hyperactivity disorder. Nat. Genet 44, 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escamez MJ, Guadano-Ferraz A, Cuadrado A, Bernal J, 1999. Type 3 iodothyronine deiodinase is selectively expressed in areas related to sexual differentiation in the newborn rat brain. Endocrinology 140, 5443–5446. [DOI] [PubMed] [Google Scholar]
- Flamant F, Gauthier K, Richard S, 2017. Genetic investigation of thyroid hormone receptor function in the developing and adult brain. Curr. Top. Dev. Biol 125, 303–335. [DOI] [PubMed] [Google Scholar]
- Franke B, Neale BM, Faraone SV, 2009. Genome-wide association studies in ADHD. Hum. Genet 126, 13–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galton VA, Hiebert A, 1987. Hepatic iodothyronine 5-deiodinase activity in Rana cates-beiana tadpoles at different stages of the life cycle. Endocrinology 121, 42–47. [DOI] [PubMed] [Google Scholar]
- Grijota-Martinez C, Diez D, Morreale de Escobar G, Bernal J, Morte B, 2011. Lack of action of exogenously administered T3 on the fetal rat brain despite expression of the monocarboxylate transporter 8. Endocrinology 152, 1713–1721. [DOI] [PubMed] [Google Scholar]
- Grunblatt E, Geissler J, Jacob CP, Renner T, Muller M, Bartl J, Gross-Lesch S, Riederer P, Lesch KP, Walitza S, Gerlach M, Schmitt A, 2012. Pilot study: potential transcription markers for adult attention-deficit hyperactivity disorder in whole blood. Atten. Defic. Hyperact. Disord 4, 77–84. [DOI] [PubMed] [Google Scholar]
- Gyllenberg D, Sourander A, Surcel HM, Hinkka-Yli-Salomaki S, McKeague IW, Brown AS, 2016. Hypothyroxinemia during gestation and offspring schizophrenia in a national birth cohort. Biol. Psychiatry 79, 962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A, Fiering S, Martinez E, Galton VA, St Germain D, 2002. The gene locus encoding iodothyronine deiodinase type 3 (Dio3) is imprinted in the fetus and expresses antisense transcripts. Endocrinology. 143, 4483–4486. [DOI] [PubMed] [Google Scholar]
- Hernandez A, Martinez E, Liao X, Van Sande J, Refetoff S, Galton VA, St Germain D, 2007. Type 3 deiodinase deficiency results in functional abnormalities at mutiple levels of the thyroid axis. Endocrinology 148, 5680–5687. [DOI] [PubMed] [Google Scholar]
- Hernandez A, Martinez ME, Fiering S, Galton VA, St Germain D, 2006. Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J. Clin. Invest 116, 476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A, Morte B, Belinchon MM, Ceballos A, Bernal J, 2012. Critical role of types 2 and 3 deiodinases in the negative regulation of gene expression by T(3)in the mouse cerebral cortex. Endocrinology 153, 2919–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A, Quignodon L, Martinez ME, Flamant F, St. Germain DL, 2010. Type 3 deiodinase deficiency causes spatial an temporal alterations in brain T3 signaling that are dissociated from serum thyroid hormone levels. Endocrinology 151, 5550–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A, Stohn JP, 2018. The type 3 deiodinase: epigenetic control of brain thyroid hormone action and neurological function. Int. J. Mol. Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez MA, De Lecea L, Guadaño-Ferraz A, Morte B, Gerendasy D, Sutcliff JG, Bernal J, 1996. Cell-specific effects of thyroid hormone on RC3/neurogranin expression in rat brain. Endocrinology 137, 1032–1041. [DOI] [PubMed] [Google Scholar]
- Kaplan MM, McCann UD, Yaskoski KA, Larsen PR, Leonard JL, 1981. Anatomical distribution of phenolic and tyrosyl ring iodothyronine deiodinases in the nervous system of normal and hypothyroid rats. Endocrinology 109, 397–402. [DOI] [PubMed] [Google Scholar]
- Kaplan MM, Yaskoski KA, 1980. Phenolic and tyrosyl ring deiodination of iodothyronines in rat brain homogenates. J. Clin. Invest 66, 551–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersseboom S, Kremers GJ, Friesema EC, Visser WE, Klootwijk W, Peeters RP, Visser TJ, 2013. Mutations in MCT8 in patients with Allan-Herndon-Dudley-syndrome affecting its cellular distribution. Mol. Endocrinol 27, 801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kester MH, Kuiper GG, Versteeg R, Visser TJ, 2006. Regulation of type III iodothyronine deiodinase expression in human cell lines. Endocrinology 147, 5845–5854. [DOI] [PubMed] [Google Scholar]
- Khan A, Harney JW, Zavacki AM, Sajdel-Sulkowska EM, 2014. Disrupted brain thyroid hormone homeostasis and altered thyroid hormone-dependent brain gene expression in autism spectrum disorders. J. Physiol. Pharmacol 65, 257–272. [PubMed] [Google Scholar]
- Kim EY, Kim SH, Rhee SJ, Huh I, Ha K, Kim J, Chang JS, Yoon DH, Park T, Ahn YM, 2015. Relationship between thyroid-stimulating hormone levels and risk of depression among the general population with normal free T4 levels. Psychoneuroendocrinology 58, 114–119. [DOI] [PubMed] [Google Scholar]
- Lavado-Autric R, Auso E, Garcia-Velasco JV, Arufe MC, Escobar del Rey F, Berbel P, Morreale de Escobar G, 2003. Early maternal hypothyroxinemia alters histogenesis and cerebral cortex cytoarchitecture of the progeny. J. Clin. Invest 111, 1073–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand J, 1984. Effects of thyroid hormones on central nervous system development In: Yanai J (Ed.), Neurobehavioral Teratology. Elsevier, New York, pp. 331–363. [Google Scholar]
- Levine JA, Nygren J, Short KR, Nair KS, 2003. Effect of hyperthyroidism on spontaneous physical activity and energy expenditure in rats. J. Appl. Physiol 94, 165–170. [DOI] [PubMed] [Google Scholar]
- Low SC, Berry M, Maia AL, Harney JW, Croteau W, St Germain DL, Larsen PR, 1995. Type 3 lodothyronine deiodinase: cloning, in vitro expression, and functional analysis of the placental selenoenzyme. J. Clin. Invest 96, 2421–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici M, Direk N, Visser WE, Korevaar TI, Hofman A, Visser TJ, Tiemeier H, Peeters RP, 2014. Thyroid function within the normal range and the risk of depression: a population-based cohort study. J. Clin. Endocrinol. Metab 99, 1213–1219. [DOI] [PubMed] [Google Scholar]
- Modesto T, Tiemeier H, Peeters RP, Jaddoe VW, Hofman A, Verhulst FC, Ghassabian A, 2015. Maternal mild thyroid hormone insufficiency in early pregnancy and Attention-Deficit/Hyperactivity disorder symptoms in children. JAMA Pediatr 169, 838–845. [DOI] [PubMed] [Google Scholar]
- Molloy CA, Morrow AL, Meinzen-Derr J, Dawson G, Bernier R, Dunn M, Hyman SL, McMahon WM, Goudie-Nice J, Hepburn S, Minshew N, Rogers S, Sigman M, Spence MA, Tager-Flusberg H, Volkmar FR, Lord C, 2006. Familial autoimmune thyroid disease as a risk factor for regression in children with Autism Spectrum disorder: a CPEA Study. J. Autism Dev. Disord 36, 317–324. [DOI] [PubMed] [Google Scholar]
- Pohlenz J, Maqueem A, Cua K, Weiss RE, Van Sande J, Refetoff S, 1999. Improved radioimmunoassay for measurement of mouse thyrotropin in serum: strain differences in thyrotropin concentration and thyrotroph sensitivity to thyroid hormone. Thyroid 9, 1265–1271. [DOI] [PubMed] [Google Scholar]
- Richard S, Flamant F, 2018. Regulation of T3 availability in the developing brain: the mouse genetics contribution. Front. Endocrinol 9, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, Lechan RM, Jaenisch R, 2001. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol. Endocrinol. (Baltimore, Md.) 15, 1748–1757. [DOI] [PubMed] [Google Scholar]
- Roman GC, 2007. Autism: transient in utero hypothyroxinemia related to maternal flavonoid ingestion during pregnancy and to other environmental antithyroid agents. J. Neurol. Sci 262, 15–26. [DOI] [PubMed] [Google Scholar]
- Roskoden T, Zilles K, Schleicher A, Schwegler H, 2002. Transient postnatal thyroxine treatment leads to variation in transmitter binding site densities in the hippocampus of rats. Neurosci. Lett 333, 21–24. [DOI] [PubMed] [Google Scholar]
- Rovet JF, 2014. The role of thyroid hormones for brain development and cognitive function. Endocr. Dev 26, 26–43. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, May MM, Carpenter NJ, Rogers RC, Martin J, Bialer MG, Ward J, Sanabria J, Marsa S, Lewis JA, Echeverri R, Lubs HA, Voeller K, Simensen RJ, Stevenson RE, 2005. Allan-Herndon-Dudley syndrome and the monocarboxylate transporter 8 (MCT8) gene. Am. J. Hum. Genet 77, 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz HL, Ross ME, Oppenheimer JH, 1997. Lack of effect of thyroid hormone on late fetal rat brain development. Endocrinology 139, 3119–3124. [DOI] [PubMed] [Google Scholar]
- Sittig LJ, Shukla PK, Herzing LB, Redei EE, 2011. Strain-specific vulnerability to alcohol exposure in utero via hippocampal parent-of-origin expression of deiodinase-III. FASEB J 25, 2313–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohn JP, Martinez ME, Hernandez A, 2016. Decreased anxiety- and depression-like behaviors and hyperactivity in a type 3 deiodinase-deficient mouse showing brain thyrotoxicosis and peripheral hypothyroidism. Psychoneuroendocrinology 74, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohn JP, Martinez ME, Zafer M, Lopez-Espindola D, Keyes LM, Hernandez A, 2018. Increased aggression and lack of maternal behavior in Dio3-deficient mice are associated with abnormalities in oxytocin and vasopressin systems. Genes Brain Behav 17, 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JM, Greenough WT, 1975. Excess neonatal thyroxine: effects on learning in infant and adolescent rats. Dev. Psychobiol 8, 479–488. [DOI] [PubMed] [Google Scholar]
- Tu HM, Legradi G, Bartha T, Salvatore D, Lechan RM, Larsen PR, 1999. Regional expression of the type 3 iodothyronine deiodinase messenger ribonucleic acid in the rat central nervous system and its regulation by thyroid hormone. Endocrinology 140, 784–790. [DOI] [PubMed] [Google Scholar]
- Tunc-Ozcan E, Wert SL, Lim PH, Ferreira A, Redei EE, 2018. Hippocampus-dependent memory and allele-specific gene expression in adult offspring of alcohol-consuming dams after neonatal treatment with thyroxin or metformin. Mol. Psychiatry 23, 1643–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P, Courtin F, 1985. Transient neonatal hyperthyroidism results in hypothyroidism in the adult rat. Endocrinology 116, 2246–2250. [DOI] [PubMed] [Google Scholar]
- Wu Z, Martinez ME, St Germain DL, Hernandez A, 2017. Type 3 deiodinase role on central thyroid hormone action affects the leptin-melanocortin system and circadian activity. Endocrinology 158, 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.