Abstract
Taxonomy of the Postia caesia complex is revised based on morphology and two genetic markers, ITS and tef1. In total, we recognize 24 species, multiplying the known species diversity in the complex. We provide descriptions for 20 temperate Northern Hemisphere taxa. Identity of the core species, P. caesia, is re-established, and a neotype from the type locality is selected. Four new combinations are proposed, and 10 new species are described: P. arbuti, P. auricoma, P. bifaria, P. comata, P. cyanescens, P. glauca, P. livens, P. magna, P. populi, and P. yanae.
Keywords: allopatric speciation, sympatric speciation, host specificity, type studies, systematics, 14 new taxa, Dacryobolaceae
INTRODUCTION
The Postia caesia species complex contains closely related brown-rot polypore species with blue-tinted basidiocarps making them easy to recognize. Distinct blue colors are rather rare among fungi, and among polypores only species of Skeletocutis nivea coll. develop a similar blue-tinted pore surface. For a long time, all blue-tinted Postia spp. went under the name Postia caesia (=Oligoporus caesius), described from conifers in Europe.
David (1974, 1980) first showed through mating tests and morphological analyses that two other species are present in Europe besides P. caesia, describing P. luteocaesia and P. subcaesia as new species. Jahn (1979) noted that David’s P. subcaesia comes in many forms. He then introduced P. subcaesia “f. minor”, which Niemelä and Vampola later described as Postia alni (Niemelä et al. 2001). Lastly, Pieri & Rivoire (2005) introduced the fifth European species, P. mediterraneocaesia.
Outside the Northern temperate area, a few further species have been included in the complex. Ryvarden (1983) noted that Patouillard’s Polyporus caesioflavus from Ecuador is closely related but separate from P. caesia. Ryvarden (1988a) described Oligoporus africanus from Burundi. Corner (1989) introduced two new species from Malaysian Borneo, Tyromyces amyloideus and T. coeruleivirens, confirmed by Hattori (2005) to belong to the Postia caesia species complex. Papp (2015) provided combinations of the above-mentioned species to Postia. In New Zealand, Rajchenberg (1995) noted that Postia atrostrigosa is a relative of Postia caesia.
The above-mentioned authors, however, never reviewed the availability of older names for European taxa, and no revision of the species complex in Europe or elsewhere has been attempted. Yao et al. (2005) showed that three ITS-sequence-based groups were present in England, but they could not connect those groups to existing names. Ortiz-Santana et al. (2013) and Pildain & Rajchenberg (2013) presented genus-level phylogenies that included representatives of the Postia caesia complex. Both concluded that the Postia caesia complex belongs to the genus Postia, but did not touch upon species concepts.
In this study we have sampled specimens of the Postia caesia species complex originating from Europe, Siberia, East Asia and North America from a molecular and morphological perspective. The material is extensive, covering 146 localities from 20 countries. Our aim is to revise the species concepts within the northern temperate area. To establish a firm nomenclatural basis for species concepts in this group, we have conducted type studies of all known species names from the temperate Northern Hemisphere.
MATERIAL AND METHODS
Morphology
We studied types and specimens from the herbaria BPI, CFMR, CUP, H, K, LE, LY, MJ and O as well as from private herbarium of the author JV. Herbarium acronyms are given according to Index Herbariorum (2017). Sequenced specimens are marked with an asterisk (*).
Due to small morphological differences between P. caesia and its relatives, all specimens were examined following the same routine. Number of pores per mm was measured with a stereomicroscope targeting areas with regular pore form. When studying hyphal structure and measuring hyphae, the part of the basidiome cut may influence the outcome. Thus we studied context cut from its lower or middle part, and trama from the middle (Fig. 1).
Fig. 1.
Cross-section of a Postia livens basidiome (Miettinen 16714) showing which parts should be studied under microscope for comparable results.
All microscopical structures were measured with Leica microscopes using Cotton Blue in lactic acid (CB, Merck 1275), with ×1250 magnification and phase contrast illumination. At least 20 hyphae from the context and hymenophoral trama, as well as 10 basidia and 30 basidiospores were measured per each specimen reported in Supplements 2 and 3. For presenting variation of hyphal width and basidiospores, the 20 % and 5 % extreme tails are given in parentheses, respectively (hyphal width variation is larger than spore size variation). Additionally, Melzer’s reagent (IKI) and 5 % KOH were used for microscopy. In KOH and to lesser extent also in Melzer’s reagent the hyphal walls swell inward, and our descriptions of hyphal wall thickness and width are not valid for these reagents.
Sketches were made using a drawing tube with the exception of spores that were drawn with free hand after real measured spores. The sketches were then imported to CorelDRAW 2017 and redrawn to vector graphics on Wacom DTK-2700 drawing board. Spore statistics were produced with R v. 3.2.2 (R Core Team 2013).
Variation between juvenile, well-developed and senescent specimens may be significant and should be taken into account when reading the descriptions. Young basidiomes have typically thinner-walled hyphae, while senescent and overwintered specimens tend to produce longer basidia and more thick-walled, larger and sometimes slightly sigmoid spores. Our descriptions have generally excluded such variation and refer to normal, well-developed specimens.
We define matt here as a surface which is felt-like or finely hairy under the dissecting microscope (i.e. hyphae are not agglutinated). What matters is the distinction of hairy or pubescent (visible hairs of about a millimeter or longer as in P. subcaesia) versus glabrous (no hairs at all) or matt (projecting hairs visible only with a lens, as in P. populi). When describing basidiome size, small refers to about 1–3 cm wide caps, and medium about 4–8 cm). When describing basidiome thickness, we define thin as 2–5 mm and thick as 15 mm and above.
DNA extraction
DNA extractions and PCR products were prepared and sequencing undertaken with one of the following methods:
-
1.
Essentially as described in Ortiz-Santana et al. (2013). For tef1 primers 983F and 1567R were used (Matheny et al. 2007). In the case of critical samples, the concentration of NaCl in the extraction buffer was lowered from 0.7 M to 0.5 M which reduced the extraction of contaminating polysaccharides from fungal material. Also, the weight of processed tissue was reduced from 20–200 mg to 1–2 mg which enabled in many cases to obtain acceptable DNA also from tissues with moderate yeast contamination. 35 cycles PCR was then used for both ITS and TEF amplification.
-
2.
With Phire Animal Tissue Direct PCR Kit (ThermoFisher), using the following PCR protocols: ITS primers ITS5-LR22 98 °C 5 min, (98 °C 5 s, 50 °C 30 s, 72 °C 20 s) ×40, 72 °C 1 min, 4 °C forever; TEF primers 983.2F-1567R 98 °C 5 min, (98 °C 5 s, 66 °C 20 s, 72 °C 20 s) ×8, (98 °C 5 s, 53 °C 40 s, 72 °C 20 s) ×36, 72 °C 1 min, 4 °C forever. Primer sequence 983.2F (modified after Matheny et al. 2007): GCHYCHGGNCAYCGTGAYTTYAT. PCR products were sent to Finnish Institute for Molecular Medicine (FIMM) or Macrogen for sequencing.
Phylogenetic analyses
We sequenced nuclear ribosomal DNA internal transcribed spacer (ITS) from 100 samples, large subunit (nLSU, 28S) from 9 samples and translation elongation factor 1-ɑ (tef1) from 44 samples. The resulting sequences are available in INSDC under the accession numbers MG137026–MG137169. We also utilized three ITS sequences provided by Viktor Papp as well as 63 ITS and 28S sequences from the INSDC (Suppl. 1), based on BLAST searches and Ortiz-Santana et al. (2013), Pildain & Rajchenberg (2013), Shen et al. (2014), and Justo et al. (2017).
We constructed four datasets for analyses:
-
1.
The 28S-ITS-dataset includes sequences of 34 species in Dacryobolaceae combining 34 ITS and 26 28S sequences. The purpose of this dataset is to assess whether Postia caesia coll. form a monophyletic group within Postia. Total alignment length is 1393 bp (454 bp ITS, 939 28S) with 205 (113 and 93) parsimony informative characters. The tree was rooted with Dacryobolus karstenii following Justo et al. (2017).
-
2.
The ITS-dataset includes all available ITS sequences of the Postia caesia complex (139), excluding bad-quality sequences. The purpose of this dataset was to assess species number and limits. Alignment length is 572 bp with 66 parsimony informative characters. Rooted with Postia auricoma based on the 28S-ITS-dataset analysis.
-
3.
The tef1-dataset includes 44 tef1 sequences of the Postia caesia complex, to complement the ITS-dataset in species delimitation. Alignment length is 583 bp with 112 parsimony informative characters, midpoint rooting.
-
4.
The ITS-tef1 dataset combines sequences from the ITS and tef1 datasets in a joint analysis for the 41 specimens with both ITS and tef1 sequences. Total alignment length is 1154 bp with 150 parsimony informative characters
MAFFT online v. 7.310 was used for aligning sequences with the strategy E-INS-I (http://mafft.cbrc.jp, Katoh & Standley 2013). Resulting alignments were refined and characters with unclear homology excluded manually using PhyDE v. 0.9971 (Müller et al. 2010). Numbers of characters were calculated in MEGA6 (Tamura et al. 2013).
Phylogenies were constructed with MrBayes v. 3.2.3 and 3.2.4 (Ronquist et al. 2012). The 28S-ITS-dataset was partitioned to 28S and ITS. Nucleotide substitution models were chosen with jmodeltest v. 2.1.10 based on AIC (Darriba et al. 2012): GTR+I+G for the full ITS-dataset, for the ITS partition of the ITS-tef1-dataset, and 28S partition of the 28S-ITS-dataset; SYM+I+G for the tef1-datasets and for the ITS partition of the 28S-ITS-dataset. Analyses were run with eight chains in three parallel runs, temp=0.1 for 10 million generations sampling a tree every 2000 generations (5 million generations for tef1-dataset). All runs had converged to below 0.01 average standard deviation of split frequencies by the end of the run. A burn-in of 25 % was used before computing the consensus tree.
Also maximum likelihood analyses were conducted for each of the datasets with RAxML v. 8.1.3 (Stamatakis 2014), using similar partitioning and GTR+G for all partitions. A hundred parallel analyses were run to find the highest likelihood tree, and 1000 bootstrap replicates to calculate bootstrap support values. Trees of the Bayesian and maximum likelihood analyses were identical in all well-supported nodes, so below we only report bootstrap support values of the maximum likelihood analyses mapped to Bayesian consensus trees.
The phylogenetic analyses were conducted at the CSC – IT Center for Science (https://www.csc.fi) multi-core computing environment. The alignments and phylograms have been deposited in TreeBASE (S22087).
Maps and distribution terms
Distribution maps for each species (Suppl. 4) were drawn with R v. 3.2.2 with the help of package sp (Pebesma & Bivand 2005) and Natural Earth (http://www.naturalearthdata.com). We use definitions of Hämet-Ahti (1984) for boreal zone subdivision when discussing species ranges.
RESULTS
Phylogenetic analysis of the 28S-ITS-dataset confirms that the Postia caesia complex forms a distinct lineage of closely related species within the genus Postia (Fig. 2). The closest, but still distant, relatives of the P. caesia complex in our analysis were P. lactea (the type species of Postia) and P. venata. In the absence of close relatives and small interspecific variation, no firm conclusions can be reached about the earliest diverging species and branching order within the P. caesia complex.
Fig. 2.

Phylogeny of the Dacryobolaceae around Postia caesia complex. Bayesian consensus tree based on 28S and ITS sequences. Numbers up to one denote posterior probabilities, above one bootstrap support values of a maximum likelihood analysis. Genus types are marked with stars. Two-letter codes after species names denote country of origin (ISO 3166).
Our ITS-dataset separates 19 species with good support (clades with posterior probability≥0.9): P. auricoma, P. subvirids, P. arbuti, P. magna, P. coeruleivirens, P. subcasesia, P. bifaria, P. glauca, P. caesia, P. livens, P. yanae, P. cyanescens, P. alni, and P. populi (Fig. 3). In addition, one variable clade that includes P. luteocaesia and P. simulans, receives fair support (PP=0.85), and one morphologically defined species, P. caesiosimulans, isn’t supported against its closest relatives. These two difficult-to-reconcile cases are discussed below.
Fig. 3.

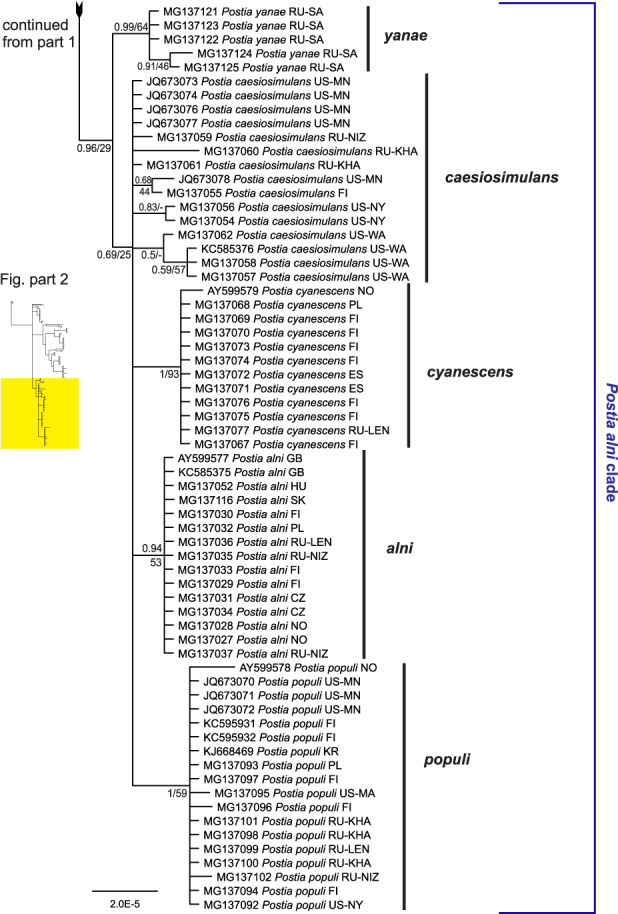
Postia caesia complex ITS phylogeny. Bayesian consensus tree, where numbers up to one denote posterior probabilities and above one bootstrap support values of a maximum likelihood analysis. Two- and three-letter codes after species names denote country and province of origin (ISO 3166, IATA for Argentina, GB/T 2260 for China).
The tef1-dataset, with a more limited sampling, shows excellent support (PP≥0.95) for 14 species/clades: P. bifaria, P. magna, P. livens-subcaesia, P. caesia, P. mediterraneocaesia, P. glauca, P. gossypina, P. arbuti, P. simulans, P. yanae, P. subviridis, P. populi, P. alni, and P. cyanescens (Fig. 4). In addition, P. caesiosimulans receives lower support (PP=0.73). Two species, P. mediterraneocaesia and P. gossypina, are only represented by a tef1-sequence in this paper.
Fig. 4.
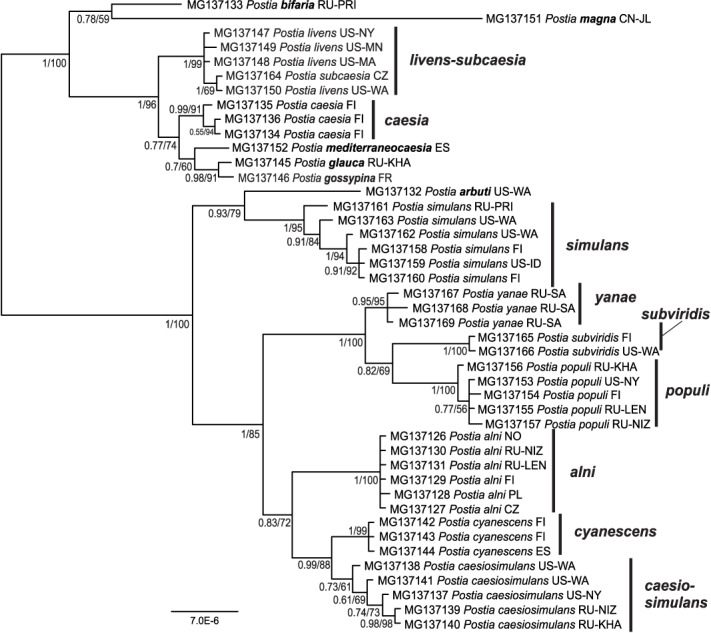
Postia caesia complex tef1 phylogeny. Bayesian consensus tree, where numbers up to one denote posterior probabilities and above one bootstrap support values of a maximum likelihood analysis. Two- and three-letter codes after species names denote country and province of origin (ISO 3166, IATA for Argentina, GB/T 2260 for China).
The delimitation of terminal clades or species is congruent in the ITS- and tef1-analysis except for two cases: First, the tef1-dataset does not separate between P. livens and P. subcaesia (Fig. 4), while the ITS-dataset clearly does (Fig. 3). Second, sequences of P. caesiosimulans form as a clade only in the tef1-analysis (Fig. 4).
The concatenated ITS-tef1-datset includes only specimens, for which both ITS and tef1 were sequenced (Fig. 5). It resolves 13 species with excellent support (PP≥0.99), while failing to treat P. caesiosimulans as a distinct clade from the well-supported P. cyanescens. The ITS-tef1-analysis supports distinction of P. livens from P. subcaesia, a conflict between analysis of ITS (separate clades) and tef1 (one clade).
Fig. 5.

Postia caesia complex tef1-ITS phylogeny for a concatenated alignment. Bayesian consensus tree, where numbers up to one denote posterior probabilities and above one bootstrap support values of a maximum likelihood analysis. Two- and three-letter codes after species names denote country and province of origin (ISO 3166, IATA for Argentina, GB/T 2260 for China).
Combining results of these three datasets, we have sequence data available for altogether 24 species in the Postia caesia complex, including 2–3 Southern Hemisphere species we will not treat further here. Also one putative species represented by two ITS sequences from England (AY599575, AY599576) belonging to the difficult P. alni clade (Fig. 3) had to be left out of this treatment due to absence of tef1-sequences. Combined with morphological evidence, this allows us to treat 20 species in detail below. Our concepts of two of these species, P. caesiosimulans and P. simulans, may represent species complexes, i.e. contain further species than we are able to uncover here. Although genetic differences are small in many cases, all the 20 species we treat are supported phylogenetically with one exception: P. luteocaesia, an already existing, morphologically distinct species discussed below.
Comparing genetic markers, tef1 shows generally larger differences than ITS between species. Without tef1-sequences revision of the P. alni clade (Fig. 3) would have been difficult: ITS sequences vary only between 1 and 6 bp between species in this clade, whereas tef1 variation is between 9 and 32 bp. An extreme case is P. yanae, which differs from P. caesiosimulans by only 1 bp in ITS but by 25 bp in tef1. Combining the two genetic markers with ecological and morphological characters we have reached a reasonable solution for splitting the P. alni clade into four homogenous (P. alni, P. cyanescens, P. populi and P. yanae) and one variable species (P. caesiosimulans), while leaving the above-mentioned English ITS-sequenced material untreated. It is evident that more work has to be done around P. caesiosimulans to fully understand species limits. As this species is already described, the best interim solution is to apply the name to a wide set of specimens while considering that we have only limited phylogenetic support for this wide concept.
Another puzzle lies in the P. simulans clade, which includes two previously described species, yellow-colored P. luteocaesia and white P. simulans. Here we have a situation, in which ITS and tef1 data show enough variation (up to 3 and 6 bp respectively) that the clade probably contains several species. It would appear that East Asian P. simulans is distinct from European and North American material (1 bp ITS, 6 bp tef1 difference), but its ITS sequence is identical with P. luteocaesia (no tef1 available for the latter). East Asian specimens are white in color in contrast to the European P. luteocaesia. One Western North American specimen is also distinct both in ITS and tef1 (differing by 2 and 4 bp respectively against P. simulans). Microscopically spore variation within this group is negligible but tramal hyphae vary more, potentially offering useful characters for future species delimitation. For now our data does not allow dividing this clade to phylogenetically well-defined species, and our pragmatic solution is to recognize two morphological species, P. luteocaesia for yellow European specimens and P. simulans for all the white-colored specimens.
Morphological differences between species in the P. caesia complex are generally small, but we have managed to find reliable characters to separate nearly all species and relate them to the phylogenetic results. Of importance are basidiome color and size, pore size, upper surface hairiness, hyphal width and wall thickness, and spore size. In addition, the host species greatly helps in identifying many (but not all) species. The differences are too small to construct any sensible dichotomous identification key, but we have constructed comparative tables that summarize the main characters between species for identification purposes (Tables 1–3).
TAXONOMY
Postia caesia complex
Basidiocarps pileate to effused-reflexed, white, whitish or rarely yellow, with bluish tints at least deep in tubes, small to medium sized, caps projecting up to 40 mm, 5–100 mm wide, 3–40 mm thick. Margin sharp to blunt. Consistency soft, fragile when dry. Pores rounded angular, regular but when old merging together, more rarely sinuous, mouths even to serrate, 4–8 per mm. Section: context white (cream-colored in old herbarium specimens), tube layer white or yellow, discoloring bluish gray upon drying or when old. Hyphal system monomitic, clamps always present. Hyphae thin- to thick-walled, CB– to CB(+), weakly amyloid in masses. Context hyphae loosely arranged, some of them in bundles, thin- to slightly thick-walled, often developing refractive (sclerified) content leaving visible only a capillary, irregular cytoplasm. Tramal hyphae loosely to rather tightly packed, narrower than in context, subparallel to interwoven (particularly older parts). Free-floating oily matter present in slides. Amorphous aggregates (Fig. 7) develop slowly in CB, and upon long exposure form needle-like crystals. Hyphal walls in all parts swell strongly inwards in KOH and to lesser degree in IKI. Hymenium: Cystidioles sometimes present, poorly differentiated. Basidia shortly clavate, slightly tapering to the apical part, often with a slight medial constriction, mostly terminal but occasionally pleural. Basidiospores allantoid, usually with slightly thickened walls, 4–7×1–2 μm, CB(+), hyaline to greyish, inamyloid to weakly amyloid.
Fig. 7.


Tramal hyphae and hymenial cells in the Postia caesia complex. All drawings are from holo-, iso-, lecto- or neotypes, except P. caesiosimulans and P. simulans from epitypes, P. coeruleivirens from Spirin 5301 and P. subcaesia from Legon 14 Oct. 1995. Drawings of P. caesia and P. populi depict amorphous aggregates that are characteristic to the complex.
Ecology: Brown-rotters on conifers and deciduous trees.
Distribution: Found in all forested continents. In the tropics mostly found in the mountains. Holarctic taxa: P. caesiosimulans, P. populi, P. simulans, P. subviridis. European taxa: P. alni, P. auricoma, P. caesia, P. cyanescens, P. gossypina, P. luteocaesia sensu typi, P. mediterraneocaesia, P. subcaesia. Temperate Asian taxa: P. auricoma, P. bifaria, P. coeruleivirens, P. glauca, P. magna, P. yanae. North American taxa: P. arbuti, P. comata, P. livens. Tropical and Southern Hemisphere taxa not treated here: P. africana, P. amyloidea, P. atrostrigosa, and P. caesioflava.
Remarks: The blue color, slightly thick-walled, curved, weakly cyanophilous and greyish spores and amorphous aggregates (Fig. 7) characterize the complex within Postia. Similar amorphous bodies are also found in slides of Rhodonia placenta (=Postia placenta) and to lesser extent in Postia balsamea, but we know of no other polypore species with this character. We have noticed that KOH stains the bottom of the tubes bluish or greenish in fresh specimens even when blue color is absent otherwise. Other than this use, we strongly recommend against using KOH when identifying species in this group, since hyphae and their walls swell rendering important characters useless.
Context hyphae are often sclerified i.e. their walls appear to thicken inwards, but this takes place so that the original wall is still distinct while the refracting, irregular “inner wall” dominates hyphal content; these hyphae appear to collapse just as easily as non-sclerified ones, so it is unclear if this phenomenon is really caused by growth of hyphal walls inwards. In any case it looks like the space for cytoplasm shrinks considerably (Fig. 6 under P. populi). In want of a better term and deeper understanding we use the term “sclerified” here.
Fig. 6.

Context hyphae in the Postia caesia complex. All drawings are from holo-, iso- or neotypes, except P. caesiosimulans from epitype and P. subcaesia from Legon 14 Oct. 1995.
The genus name Cyanosporus McGinty (=Postia subg. Cyanosporus (McGinty) Papp) has sometimes been used for Postia caesia complex (Papp 2015; Pieri and Rivoire 1998). McGinty is a pseudonym of C. G. Lloyd, who used it to ridicule his fellow mycologists who in his opinion were creating too many names. Cyanosporus is such a case, never intended to be taken seriously, as stated by Stevenson & Cash (1936) in their compendium of new fungal names published by Lloyd. Also Donk (1960) rejected the name for this reason. The code states that a name is valid only if it is accepted by the author, and thus Cyanosporus should be considered invalid (ICN art. 36.1). If Cyanosporus is deemed invalid, as we do, also Postia subg. Cyanosporus should be viewed invalid. Furthermore, Cyanosporus cannot be resurrected either due to Cyanospora Heald & F.A. Wolf 1910.
Quite aside the nomenclatural situation around Cyanosporus we see no reason to split the Postia caesia complex to a separate genus from Postia, either on morphological or phylogenetic grounds. The complex is distinct morphologically, but nevertheless very similar to other Postia spp. Separating the P. caesia complex to its own genus would create a cascade of splitting that would end up with many morphologically unrecognizable Postia-like genera.
The type species of Postia, P. lactea (=P. tephroleuca sensu auct.), and its close relative, P. grata, can look confusingly similar to the P. caesia complex when the blue color hasn’t emerged yet. In such a case the formation of amorphous aggregates in microscopical slides of Cotton Blue is the best separating character.
Postia alni Niemelä & Vampola, Karstenia 41: 7. 2001. Figs 6–9.
Fig. 9.
Postia alni. A. Basidiomes with the distinct brownish upper surface (Miettinen 15830). B. Paler basidiomes (Niemelä 9233).
Holotype: Slovakia, Bratislava, Svätý Jur, Alnus glutinosa, 12 Oct. 1995, Vampola* (H 7019137, studied).
Basidiocarps conchate to flabelliform, rarely effused-reflexed, small or rarely medium-sized polypores, mostly thin; margin sharp. Upper surface first cream colored, almost glabrous to matt, then pubescent, radially striate, ochraceous to brownish, often with bluish-greyish hues. Tubes white to cream-colored, in older and dry specimens with light bluish-greyish tint; pores 4–6(–7) per mm. Section: Context 1–4 mm thick, tubes 1–6 mm long. Context hyphae thin- or only slightly thick-walled, (2.4–)3.9–5.5(–7.4) μm in diam. Tramal hyphae with slightly to distinctly thickened walls (0.2–0.8 μm thick), (2.0–)2.9–3.6(–4.3) μm in diam. Basidia 10–14.8(–16) × 3.3–4.2 μm. Basidiospores (4.1–)4.3–6.1(–6.8) × (1.0–)1.1–1.3(–1.5) μm, L=5.05 μm, W=1.20 μm, Q=4.22.
Distribution and ecology: Europe, temperate to southern boreal, common; hardwood logs and thick fallen branches (Acer, Alnus, Betula, Carpinus, Corylus, Fagus, Prunus, Quercus, Ulmus), most common in riversides and coastal areas, herb-rich forests in the north.
Specimens examined: Czech Republic, Jihočeský kraj, Chlum u Třeboně, Bukové Kopce Nat. Res., Fagus sylvatica, 18 Sep. 2010, Vampola* (MJ 27/10); Jihočeský kraj, Český Krumlov, Žofín Nat. Res., F. sylvatica, 16.IX.2012 Vampola* (MJ 17/12). Denmark, Lolland, Faursted, F. sylvatica, 4 Oct. 2007 Schigel 5425 (H). Finland, Uusimaa, Helsinki, Veräjämäki, Alnus incana, 19 Oct. 2011, Miettinen 14918.2* (H), Sorbus aucuparia, 12 Dec. 2015, Miettinen 19883 (H); Uusimaa, Helsinki, Pornaistenniemi, A. incana, 24 Sep. 2012, Miettinen 15741 (H); Uusimaa, Kirkkonummi, Sundsberget, Prunus padus, 24 Oct. 2012, Miettinen 15830* (H); Etelä-Häme, Hämeenlinna, Lammi, Betula sp., 21 Sep. 2016, Niemelä 9233* (H); S. aucuparia, 14 Sep. 2015, Miettinen 19386 (H); Satakunta, Ylöjärvi, Viljakkala, Alnus glutinosa, 2 Oct. 2011, Niemelä 8843 (H). Germany, Schleswig-Holstein, Sachsenwald, F. sylvatica, 13 Oct. 1907, Jaap (Fungi Selecti Exsiccati 927, H). Norway, Akershus, Enebakk, Omberg, Corylus avellana, 1 Sep. 2016, Nordén* (H); Akershus, Nesodden, Roerskogen, Quercus robur, 18 Sep. 2014, Larsson* (O 248173). Poland, Podlasie, Hajnówka, Białowieża, Carpinus betulus, 16 Sep. 2012, Niemelä 8933* (H). Russia, Leningrad Reg., Boksitogorsk Dist., Goryun, Salix sp., 23 Sep. 2011, Spirin 4602* (H); Leningrad Reg., Volkhov Dist., Zagubie, Acer platanoides, 3 Oct. 2010, Spirin 3640 (H); Nizhny Novgorod Reg., Lukoyanov Dist., Razino, Ulmus glabra, 18. Aug. 2015, Spirin 9502* (H); Nizhny Novgorod Reg., Panzelka, Betula pubescens (?), 15 Aug. 2006, Spirin 2548* (H); Sverdlovsk Reg., Pervouralsk, Khomutovka, P. padus, 26 Aug. 2002, Kotiranta 19807 (H). Slovakia, Bratislava (holotype, see above).
Remarks: Postia alni belongs to the narrow-spored group within the P. caesia complex (Table 1). In North Europe it has been mixed with P. populi, and indeed some of the paratypes of P. alni actually represent P. populi. Postia populi differs in having tightly arranged, slightly narrower and more thick-walled tramal hyphae. Useful aids in identification are also the undulating margin of many P. populi specimens, absent in P. alni, and the deep brown cap color of mature specimens of P. alni. Postia populi occurs almost exclusively on Populus tremula in Europe, while P. alni prefers other deciduous trees. Postia populi is a northerly species in Europe; however, their distribution areas overlap, and the two species can be found growing side by side in the same forest.
Another very similar though much rarer species in Europe is P. caesiosimulans, which has been detected so far only a few times growing on Corylus in the hemiboreal zone. We have not been able to find consistent microscopical differences between the two species, although spore Q-values are generally higher in P. alni. Cap surface of P. alni is typically pubescent and turns brown when old, whereas cap of P. caesiosimulans is matt to glabrous and remains light-colored even when old. When young, specimens of the two species are indistinguishable morphologically.
Temperate P. subcaesia produces thicker and softer basidiocarps than P. alni, its hyphae are wider and tramal hyphae possess thinner walls. Papp (2014) provided an illustrated comparison of P. alni and P. subcaesia.
Postia arbuti Spirin, sp. nov. MycoBank MB823896. Figs 7, 8.
Fig. 8.

Basidiospores of species in the Postia caesia complex. All drawings are from holo-, iso-, lecto- or neotypes, except P. caesiosimulans and P. simulans from epitypes, P. coeruleivirens from Spirin 5301 and P. subcaesia from Legon 14 Oct. 1995; in addition two rightmost spores were drawn from Miettinen 14828 for P. livens and from Rivoire 1903 for P. mediterraneocaesia.
Holotype: USA, Washington: Jefferson Co., Port Townsend, Fort Worden, 48.13778° N 122.7673° W, alt. 74 m, Arbutus menziesii, 9 Oct. 2014, Spirin 8327* (H 7008651).
Etymology: After the host, Arbutus menziesii.
Basidiocarps conchate, pendant to effused-reflexed, small, thin; margin sharp. Upper surface white to pale cream colored, almost glabrous to matt. Tubes white to cream-colored, in older and dry specimens with light bluish-greyish tint; pores 6–8 per mm, angular to sinuous. Section: context 0.5–2 mm thick, tubes 0.5–2 mm long. Context hyphae thin-walled but sclerified more distinctly than in other species, (2.3–)3.2–4.6(–5.4) μm. Tramal hyphae with slightly to distinctly thickened (up to 1 μm thick) walls, densely packed, (1.8–)2.4–3.1(4.0) μm. Basidia (9.7–)11–17(–19.2) × 3.3–4.2 μm. Basidiospores (4.0–)4.1–5.1(–5.2) × 1.0–1.2(–1.3) μm, L=4.56 μm, W=1.14 μm, Q=4.00.
Distribution and ecology: North America (North-West), temperate; so far collected only from fallen dry branches of madrona (Arbutus menziesii).
Specimens examined: USA, Washington, Jefferson Co., Port Townsend, Fort Worden, Arbutus menziesii, 9 Oct. 2014, Spirin 8318 (H), 8327* (holotype, see above).
Remarks: Postia arbuti is morphologically virtually indistinguishable from P. populi. Genetically it is clearly a separate species. For now the only reliable non-molecular characters we can point to are the host species in Ericaceae and slightly smaller pores.
Postia auricoma Spirin & Niemelä, sp. nov. MycoBank MB823897. Figs 7, 8, 10.
Fig. 10.
Postia auricoma. A. Niemelä 8315. B. Spirin 4598.
Holotype: Finland, Pohjois-Savo, Enonkoski, Kolovesi National Park, Vaajasalo, 62.23° N 28.87° E, alt. 90 m, old-growth pine forest, rocky hilltop, on a fallen Pinus sylvestris, wood still hard, 26 Sep. 2006, Niemelä 8310* (H 6014002).
Etymology: Auricoma (Lat.), with golden yellow head, refers to the yellow surface.
Basidiocarps conchate, small. Upper surface first while to cream colored, then yellowish to bright yellow, in older basidiocarps pale to dark ochraceous, matt to pubescent. Pore surface first bright yellow, quickly turning green when bruised, then with ochraceous tints; pores 4–6 per mm. Section: Context 2–3 mm thick, white to pale cream-colored, tubes 2–4 mm long, concolorous with or slightly paler than pore surface. Context hyphae slightly thick-walled, (3.8–)4.2–5.2(–6.2) μm. Tramal hyphae slightly to distinctly thick-walled (walls 0.2–1 μm thick), some hyphal segments with strongly amyloid (greenish in IKI) and cyanophilous content, (2.0–)3.1–4.0(–4.5) μm. Basidia (11.8–)14–20(–24) × 3.8–5.3 μm. Basidiospores (4.2–)4.4–5.6(–6.0)×(1.4–)1.5–1.8(–2.0) μm, L=5.04 μm, W=1.65 μm, Q=3.06.
Distribution and ecology: Eurasia, temperate to boreal, rare; fallen logs of Pinus sylvestris and Larix gmelinii.
Specimens examined: Finland, Etelä-Savo, Kouvola, Repovesi NP, 16 Sep. 2004, Niemelä 7887 (H); Pohjois-Savo, Enonkoski, Kolovesi NP, Pinus sylvestris, 26 Sep. 2006, Niemelä 8310 (holotype, see above), 8315 (H). Poland, Podlasie, Hajnówka, Białowieża, P. sylvestris, 4 Oct. 2010, Niemelä 8760 (H). Russia, Irkutsk Reg., Irkutsk, Talzi, 20 Aug. 2000, Kotiranta 17047 (H); Leningrad Reg., Boksitogorsk Dist., Vozhani, P. sylvestris, 22 Sep. 2011, Spirin 4586* (H), Goryun, P. sylvestris, 23 Sep. 2011, Spirin 4598, 4608 (H).
Remarks: Postia auricoma is a bright yellow species restricted to Pinus sylvestris and Larix gmelinii in the north temperate and boreal zones of Eurasia. Up to now, it has been mixed up with the morphologically similar P. luteocaesia, occurring in the Mediterranean on southern pine species. However, DNA data show that these species are not closely related. One more yellow-colored species in the Postia caesia complex is the South American P. caesioflava.
European collections derive from old-growth forests or their vicinity. The Siberian collection came from a young manage forest. Three of the five European known localities are well-preserved old-growth forests. The fourth locality (Spirin 4586) is a recent clear-cut but with old, rotten pine logs persisting from the time before the forest was logged, indicating that even that site had an old-growth forest history; also some indicator species of high conservation value such as Crustoderma dryinum were present in the site, and proportion of old-growth forests in the surrounding landscape was relatively high. The fifth (Niemelä 7887) was collected in a trivial site but in a national park where old-growth forest species are present. Thus it may be that P. auricoma is dependent on old-growth forests in Europe.
Postia bifaria Spirin, sp. nov. MycoBank MB823898. Figs 7, 8.
Holotype: Russia, Primorie: Krasnoarmeiskii Dist., Valinku, 48.2159° N 136.6902° E, alt. 1420 m, Picea ajanensis, 27 Aug. 2013, Spirin 6402* (H 7008646).
Etymology: Bifarius (Lat.), two-faced, refers to color contrast between upper and lower surfaces of the basidiocarp.
Basidiocarps conchate, small or medium-sized. Upper surface first light grey, then with ochraceous hues, strigose. Tubes white to cream-colored, with light ochraceous tings in older and dry specimens; pores 6–8 per mm. Section: Context 2–4 mm thick, tubes 2–3 mm long. Context hyphae thin- to slightly (up to 0.5 μm) thick-walled, (3.0–)4.0–7.0(–8.0) μm. Tramal hyphae thin- or slightly thick-walled, easily collapsing, (2.0–)2.5–3.8(–4.2) μm. Basidia 9.8–14.8×3.4–4.5 μm. Basidiospores (3.6–)3.7–4.4(–5.2) × 1.0–1.2(–1.3) μm, L=4.1 μm, W=1.14 μm, Q=3.60.
Distribution and ecology: East Asia, cold temperate mountains, rather rare; fallen conifer logs (Pinus, Picea, Larix).
Specimens examined: China, Jilin, Antu, Changbaishan Nat. Res., Pinus sp., 5 Sep. 1993, Dai 1059 (H). Japan, Hokkaido, Akan, 21 Sep. 1994, Nuñez 602 (H, O). Russia, Khabarovsk Reg., Khabarovsk Dist., Bolshoi Khekhtsir Nat. Res., Picea ajanensis, 2 Sep. 2013, Spirin 6532 (H); Malyi Niran, P. ajanensis, 5 Aug. 2012, Spirin 4850* (H); 6 Aug. 2012, Spirin 4987 (H), Malyi Kukachan, Larix gmelinii, 20 Aug. 2012, Spirin 5461 (H); Primorie, Krasnoarmeiskii Dist., Valinku (holotype, see above).
Remarks: Postia bifaria is most similar to P. glauca. Both species inhabit coniferous hosts and sometimes occur side by side in the same habitats. Basidiocarps of P. bifaria discolor when bruised, as fresh specimens of P. glauca do. Smaller spore size (P. bifaria: 4.1 × 1.14 μm in average, P. glauca: 4.64 × 1.27 μm) is the best character for identification.
Postia caesia (Schrad.) P. Karst. Revue mycol., Toulouse 3(no. 9): 19. 1881. Figs 6–8, 11.
Fig. 11.
Postia caesia. A. Basidiome in situ (Niemelä 7798). B. Microscopical slide of the neotype tube trama showing characteristic green hyphal segments in Melzer.
Basionym: Boletus caesius Schrad., Spicilegium Florae Germaniae: 167. 1794.
Neotype: Germany, Niedersachsen: Göttingen, Staufenberg, 51.58800° N 9.98217° E, alt. 390 m, Picea abies, 27 Sep. 2012, Schuster* LY BR-6776, (selected here, MBT380822, duplicate H 7008647).
Synonyms: Boletus coeruleus A. Schumach., Enumeratio Plantarum, in partibus Saellandiae septentrionalis et orientalis crescentium 2: 387. 1803.
Neotype: Denmark, Lolland, Krenkerup, Haveskov, 54.773° N 11.666° E, alt. 15 m, Fagus sylvatica, 4 Oct. 2007, Schigel 5436 (H 7034978 selected here, MBT380823).
Polyporus caesiocoloratus Britzelmayr, Bot. Centralblatt 54: 10. 1893.
Neotype: Czech Republic, Vysočina, Zbilidy, Panský les, alt. 650 m, Picea abies, 7 Aug. 1993 Vampola*, Polyporales Exsiccati Čechoslovaciae 121, (H 7034977 selected here, MBT380824).
Basidiocarps conchate, often effused-reflexed, small or medium-sized, caps usually triquetrous (relatively thick at base). Upper surface first cream colored, matt, as a rule with bluish flecks, then plumbeous to bluish grey or greyish-brown, often distinctly pubescent. Tubes white to cream-colored, in older and dry specimens with light bluish-greyish tint, in vigorously growing specimens with bluish stains when bruised; pores 4–5(–6) per mm. Section: context 1–8 mm thick, tubes 2–6 mm thick. Context hyphae thin or only slightly thick-walled (walls 0.1–0.3 μm thick), (2.6–)3.7–5.2(–6.1) μm. Tramal hyphae thin- or only slightly thick-walled, clearly parallel, easily collapsing, (1.9–)2.8–3.6(–4.5) μm, hyphal segments with strongly amyloid (greenish-black in IKI) and cyanophilous/golden yellow content (in CB). Basidia (9.3–)10–15(–16.7) × 3.7–4.5 μm. Basidiospores (3.9–) 4.1–5.3(–6.0) × (1.2–)1.3–1.7(–1.9) μm, L=4.64 μm, W=1.48 μm, Q=3.13.
Distribution and ecology: Europe, common in temperate to hemiboreal, rare in south boreal zone; mostly Picea but also other coniferous (Abies, Pinus) and more rarely also deciduous trees (Fagus, Salix, Syringa).
Specimens examined: Czech Republic, Vysočina, Zbilidy (neotype of P. caesiocoloratus, see above). Denmark, Lolland, Krenkerup, Fagus sylvatica, 4 Oct. 2007, Schigel 5436 (H, neotype of Boletus coeruleus). Finland, Uusimaa, Helsinki, Toukola, Betula sp., 25 Oct. 2008, Miettinen 13610* (H); Uusimaa, Helsinki, Veräjämäki, Pinus sylvestris, 22 Sep. 2010, Miettinen 14156.2* (H); Etelä-Häme, Hämeenlinna, Lammi, Picea abies, 17 Sep. 2015, Miettinen 19424 (H); Satakunta, Ylöjärvi, Viljakkala, P. abies, 8 Sep. 2013, Niemelä 9086* (H). France, Ardèche, St. Cirgues en Montagne, P. abies, 26 Sep. 2004, Rivoire 2486 (LY, H); Aveyron, Salles-Curan, P. abies, 28 Oct. 2004, Rivoire 2566 (LY, H); Haute Savoie, Sallanches, Abies alba, 13 Sep. 2010, Rivoire 3859 (LY, H); Rhône, Courzieu, P. abies, 12 Sep. 2004, Rivoire 2444 (LY, H); Rhône, Yzeron, Bois de Malval, A. alba, 20 Oct. 2006, Rivoire 2985 (LY, H); Savoie, Doucy, A. alba, 19 Sep. 2005, Rivoire 2756 (LY, H). Germany, Baden-Würtemberg, Freiburg, Triberg, 2 Sep. 1996, Kytövuori 96–887 (H); Niedersachsen, Göttingen (neotype, see above). Russia, Leningrad Reg., Podporozhie Dist., Chogozero, P. sylvestris, 22 Sep. 2009, Spirin 3307 (H); Vazhiny, P. abies, 29 Sep. 2010, Spirin 3529 (H); Leningrad Reg., Volkhov Dist., Zagubie, P. abies, 18 Sep. 2011, Spirin 4576 (H); Nizhny Novgorod Reg., Lukoyanov Dist., Razino, P. abies, 21 Aug. 2015, Spirin 9787* (H); Panzelka, P. sylvestris, 3 Aug. 2004 Spirin 2075 (H); Nizhny Novgorod Reg., Sharanga Dist., Kilemary Nat. Res., Salix caprea, 16 Aug. 2004, Spirin 2102 (H), P. abies, 17 Aug. 2004, Spirin 2143, 2157 (H); St. Petersburg, Volkovka, Syringa vulgaris, 16 Sep. 2003, Spirin 1991 (H). Slovakia, Banská Bystrica, Dobroč, A. alba, Sep. 2009, Vlasák 0909/23 (H, JV). UK, Scotland, South Lanarkshire, Cleghorn Glen, P. sylvestris, 6 Aug. 2010, Miettinen 14133* (H).
Additional specimen examined: Postia aff. caesia. Australia, Tasmania, Mt Field NP, Russell Falls, 31 May 2003, Gates (H 7036111*, O 918359).
Remarks: Morphologically P. caesia is easy to recognize due to its hairy, often distinctly bluish basidiocarps and microscopically by its amyloid/cyanophilous hyphal segments in trama. It is most similar to P. simulans, which however does not produces pigmented, strongly amyloid hyphae in tube trama, and has longer basidiospores and smaller pores as a rule.
Boletus caesius was described based on material from Germany (Schrader 1794), and this name was subsequently sanctioned by Fries (1821). Fries’s concept of the species and its current synonymy are unclear. No type material survives from Schrader or Fries. Fries (1821) referred directly to two illustrations and indirectly to a third one in his description:
1. Schaeffer’s plate 124 (1763) depicting a pure white semistipitate polypore like Postia floriformis or P. stiptica;
2. Sowerby’s plate 226 (1799), which is a pure white polypore like Postia caesia s.l., P. lactea or P. stiptica;
3. Fries cites Schrader (1794), who in turn cites Schaeffer’s plate 314 (1774). The fungus in that figure is something strange, an unidentifiable, non-poroid species (possibly a young Fomitopsis pinicola). It is in direct conflict with Schrader’s description stating that the species has a blue upper surface.
All of the above-mentioned illustrations are technically suitable as lectotypes, but are in conflict with Schrader’s and Fries’s description referring to a blue or bluish fungus and help in no way to anchor the name Postia caesia to modern species concepts. We therefore designate a sequenced neotype from locus classicus for P. caesia.
Fries (1821) considered Boletus coeruleus as a synonyms of Polyporus caesius, and this opinion persists in current literature and mycological databases. The protologue of B. coeruleus (Schumacher 1803) refers to a robust, bluish species occurring on oak in Denmark, and the two species that may come into question are P. caesia and P. subcaesia. In the name of nomenclatural stability we choose here a neotype for B. coeruleus among specimens of P. caesia - otherwise the name B. coeruleus might replace the much younger name P. subcaesia.
Identity of Polyporus caesiocoloratus described from Bavaria has been unclear. In the protologue, Britzelmayr (1893) stated that his species has basidiospores 5–6 × 0.75–1 μm. There are no polypore species known to us which possess spores of this size, and considering the quality of microscopes at the time these measurements are best viewed as an approximation. Otherwise the description by referring to bluish upper surface and steel blue pores fits only to the Postia caesia complex. Other hints in the description fit best P. caesia sensu stricto (brownish upper surface, pores turning blue, growth on spruce). No type material exists in M (Dagmar Triebel, pers. comm.), and Killermann (1922) did not report any specimens although he studied nearly all of Britzelmayr’s material. To settle its identity we designate here a neotype from Czech Republic, in effect reducing Polyporus caesiocoloratus to a synonym of Postia caesia.
Postia caesiosimulans (G.F. Atk.) Spirin & Miettinen, comb. nov. MycoBank MB823899. Figs 6–8, 12.
Fig. 12.
Postia caesiosimulans, Miettinen 15489.1, basidiomes from above (A) and below (B).
Basionym: Tyromyces caesiosimulans G.F. Atk., Ann. Mycol. 6: 61. 1908.
Lectotype: USA, New York, Tompkins Co., Ithaca, ‘on rotten wood’, 12 Oct. 1907, Humphrey (CUP A-022240 selected here, MBT380825).
Epitype: USA, New York, Essex Co., Catlin Lake, 44.06869° N 74.25795° W, alt. 560 m, Fagus grandifolia (?), 18 Sep. 2013, Miettinen 16976.1* CUP (selected here, MBT380826, duplicate H 7008645).
Basidiocarps conchate, sometimes effused-reflexed, mostly thin, small or medium-sized. Upper surface first white to cream colored, matt, then greyish to pale ochraceous, very rarely with bluish flecks or faint zones, more or less glabrous. Tubes white to cream-colored, in older and dry specimens with light bluish-greyish tint; pores 5–7 per mm. Section: Context 1–3 mm thick, tubes 1–3 mm long. Context hyphae thin- to slightly thick-walled (rarely distinctly thick-walled), often sclerified, (2.2–)3.4–5.2(–6.4) μm. Tramal hyphae for the most part slightly to distinctly thick-walled (walls up to 1 μm thick), mainly interwoven, sometimes partly glued together, (2.0–)2.9–3.8(–4.8) μm. Basidia (9–)10.5–15.5(–19) × 3.2–5.2(–6.5) μm. Basidiospores (4.1–)4.2–5.5(–7.0) × (1.0–)1.1–1.4(–1.6) μm, L=4.80 μm, W=1.22 μm, Q=3.93.
Distribution and ecology: Holarctic, temperate, common in North America; on fallen logs and branches of deciduous trees (in Europe preferably on Corylus, in North America on Fagus), in East Asia and North America also on Abies and Picea spp.
Specimens examined: Finland, Ahvenanmaa, Finström, Mangelbo Almskogen Nat. Res., C. avellana, 13 Oct. 2006, Schigel 4798* (H). Russia, Khabarovsk Reg., Solnechnyi Dist., Suluk-Makit, Abies nephrolepis, 17 Aug. 2011, Spirin 4199* (H); Gorin, A. nephrolepis, 13 Aug. 2011, Spirin 4125* (H); Nizhny Novgorod Reg., Lukoyanov Dist., Sanki, Corylus avellana, 18 Aug. 2006, Spirin 2610* (H). USA, Massachusetts, Worcester, Hadwen Arboretum, hardwood (branch), 20 Oct. 2013, Miettinen 17340.1 (H); Minnesota, St. Louis Co., Independence, Populus tremuloides, 27 Sep. 2010, Lindner 2010–062 (CFMR); 29 Sep. 2010, Lindner 2010–081 (CFMR); Melrude, P. tremuloides, 29 Sep. 2010, Lindner 2010–106, 2010–111 (CFMR); Minnesota, Orr, P. tremuloides, 28 Sep. 2010, Lindner 2010–136 (CFMR); New York, Essex Co., Catlin Lake, Acer (?), 14 Aug. 2012, Miettinen 15489.1 (H), Fagus grandifolia (?), 18 Sep. 2013, Miettinen 16976.1* (CUP epitype, H isoepitype), New York, Essex Co., Wolf Lake, F. grandifolia, 20 Sep. 2013, Miettinen 17075* (H); New York, Tompkins Co., Ithaca (lectotype, see above); North Carolina, Swain Co., Bryson City, Picea sp., Sep. 2005, Vlasák 0509/24 (H, JV); Washington, Pend Oreille Co., Gypsy Meadows, Abies lasiocarpa, 17 Oct. 2014, Spirin 8717* (H); Picea/Abies, 17 Oct. 2014, Miettinen 18924 (H); Picea sp., 17 Oct. 2014, Miettinen 18927 (H); Washington, Thurston Co., Nisqually Land Trust, Abies procera, 11 Oct. 2014, Miettinen 18663*, 18665* (H).
Remarks: This species was introduced primarily on the basis of its globose basidiospores (Atkinson 1908). However, as Lowe (1974) stated, these spores belong to Tremella polyporina. We disagree with Lowe’s opinion that alien spores in Atkinson’s description are a reason to reject T. caesiosimulans. The protologue certainly refers to the polypore, not to a species of Tremella, and we select the lectotype here according to ICN Art. 9.14.
Mature basidiocarps with an ochraceous, glabrous upper surface, collected on deciduous hosts in Northeastern United States, are rather easy to recognize. However, in other cases morphological identification may be difficult, particularly without material of comparison at hand. Young, small-sized or half-resupinate specimens of P. caesiosimulans can be mistaken for P. populi. Both species have similar basidiospores, and hence attention should be paid to tramal hyphae: they are not so thick-walled and densely arranged in P. caesiosimulans as in P. populi. When small-sized, P. livens can be quite similar as well. Pubescent upper surface and in relative terms thick context should aid in correct identification. When well-developed, basidiocarps of P. livens are much larger in size than those of P. caesiosimulans.
In Europe, P. caesiosimulans can be mixed up with P. alni as well (see discussion under the latter name). Postia simulans is yet another potential look-alike, but wider basidiospores and collapsing, thin-walled tramal hyphae tell it apart.
Our picture of variation within and around P. caesiosimulans is still incomplete. Collections from Northwestern USA have wider basidiospores (W=1.46 μm) than rest of the material; and they have been excluded from the statistics. These basidiospores are similar to those of P. simulans (Suppl. 2). Minor intraspecific variation in ITS and tef1 suggests that the Northwest population on conifers might be differentiated from the rest of P. caesiosimulans, but more extensive sampling and further markers would be needed to ascertain this. Also excluded from the description above are P. caesiosimulans material from Minnesota from the study by Brazee et al. (2012). These specimens are very similar to Postia populi, with narrower than average spores and thick-walled tramal hyphae; yet the ITS data places them in the P. caesiosimulans complex (no tef1 data available).
Postia coeruleivirens (Corner) V. Papp, Mycotaxon 129: 411. 2015. [2014]. Figs 7, 8.
Basionym: Tyromyces coeruleivirens Corner, Beih. Nova Hedwigia 96: 163. 1989.
Basidiocarps conchate, small or medium-sized. Upper surface first cream-colored, then pale ochraceous, sometimes with bluish flecks or indistinct; pubescent. Pore surface white to cream-colored, developing a bluish-greyish tint when aging; pores 6–8 per mm. Section: Context 3–5 mm thick, tubes 3–4 mm long. Context hyphae thin-walled to slightly thick-walled, sometimes sclerified, (3.0–)3.6–6.0(–7.8) μm, with frequent finger-like outgrowths. Tramal hyphae thin- or moderately thick-walled (walls up to 0.8 μm thick), (1.8–)2.4–3.4(–4.0) μm. Basidia 8.8–13.5 × 3.3–4.3 μm. Basidiospores (3.6–)3.8–4.8(–5.2) × 1.0–1.3 μm, L=4.23 μm, W=1.16 μm, Q=3.64.
Distribution and ecology: East and Southeast Asia, warm temperate to tropical; fallen logs and branches of deciduous trees (Populus, Tilia, and Ulmus in East Asia).
Specimens examined: China, Jilin, Antu, Baoma, Ulmus sp., 7 Sep. 1993, Dai 1134 (H); Wangqing, Lanjia, Tilia sp., 11 Sep. 1993, Dai 1198 (H). Indonesia, Bali, Bedugul, Mt. Tapak, 24 Jul. 2007, Miettinen 12214* (H). Russia, Khabarovsk Reg., Khabarovsk Dist., Ulika, Tilia amurensis, 15 Aug. 2012, Spirin 5301* (H); Solnechnyi Dist., Elga, Populus maximowiczii, 22 Aug. 2011, Spirin 4245* (H); Primorie, Ternei Dist., Maisa, T. amurensis, 15 Sep. 1990, Parmasto (H, O ex TAAM 151125).
Remarks: Corner (1989) described Tyromyces coeruleivirens and T. amyloideus from Borneo, and both are considered members of the P. caesia group (Hattori 2002). Appropriate combinations to Postia were made by Papp (2014). The three Asian collections we have studied possess mutually identical ITS sequences and are tentatively labelled as P. coeruleivirens sensu auct. Two of them, from Russian Far East, fit well with the protologue of T. coeruleivirens, and the description above is based on these Northeast Asian specimens. The third specimen collected in Bali (Miettinen 12214) is morphologically different, and is excluded from the description above. It has the smallest basidiospores in the whole group, L=3.69, W=1.00 (Suppl. 2). More material (especially from the type locality) and tef1 sequences are needed to settle proper identities in this group.
Postia coeruleivirens, as understood here, is morphologically most similar to P. bifaria, and these species can be separated by their preferred host (angiosperms versus gymnosperms). Tramal hyphae in P. coeruleivirens are predominately thick-walled (up to 0.8 μm) while in P. bifaria they are almost thin-walled (<0.5μm).
Postia comata Miettinen, sp. nov. MycoBank MB823900. Figs 7, 8.
Holotype: USA, Massachusetts, Petersham, Tom Swamp, 42.5121° N 72.2112° E, alt. 230 m, Tsuga canadensis, 19 Sep. 2011, Miettinen 14755.1* (H 7005691).
Etymology: Comatus (Lat.), hairy.
Basidiocarps conchate or effused-reflexed, medium-sized. Upper surface cream colored to pale ochraceous, pubescent. Tubes cream-colored, in older specimens with bluish-greyish tint; pores 4–6 per mm, angular to sinuous. Section: Context 5–10 mm thick, tubes 3–6 mm long. Context hyphae slightly thick-walled, sclerified, (3.2–)4.2–5.3(–7.1) μm. Tramal hyphae predominantly thick-walled (walls up to 0.8–1 μm thick), (2.2–) 2.8–3.8(–4.5) μm. Basidia 8.8–14.2(–15.2)×(3.4–)3.7–4.9 μm. Basidiospores (3.8–)4.1–4.9(–5.1) × 1.1–1.3 μm, L=4.36 μm, W=1.21 μm, Q=3.62.
Distribution and ecology: Northeastern United States, temperate, evidently uncommon; fallen logs and branches of conifers (Tsuga canadensis) and deciduous trees (Acer).
Specimens examined: USA, Massachusetts, Worcester Co., Petersham (holotype, see above); New York, Essex Co., Harris Lake, Acer sp., 23 Sep. 2013, Miettinen 17180 (H); New York, Warren Co., Pack Demonstration Forest, Tsuga canadensis, 24 Sep. 2013, Miettinen 17197 (H).
Remarks: Postia comata is morphologically similar to P. livens but it differs in having mostly thick-walled tramal hyphae and slightly smaller basidiospores. Its closest relative is East Asian P. bifaria, which has smaller pores and spores, and collapsing, thing-walled tramal hyphae (Table 3).
Postia cyanescens Miettinen, sp. nov. MycoBank MB823901. Figs 7, 8, 13A.
Fig. 13.
A. Postia cyanescens (Niemelä 8844). B. Postia glauca (Miettinen 10567).
Holotype: Finland, Uusimaa, Helsinki, Veräjämäki, 60.222° N 24.973° E, alt. 40 m, on a cut stump of Picea abies, 19 Oct. 2008, Miettinen 13602 (H 6014001, culture HAMBI/FBCC 2205*).
Etymology: Cyanescens (Lat.), turning bluish.
Basidiocarps conchate to flabelliform, rarely effused-reflexed, thin. Upper surface first white to cream colored, matt, then pale ochraceous, rarely with bluish-greyish hues. Tubes white to cream-colored, in older and dry specimens with light bluish-greyish tint, unchanged when bruised; pores (3–)5–6 per mm. Section: Context 0.5–3 mm thick, tubes 1–2 mm long. Context hyphae thin- to slightly thick-walled, sclerified, (2.6–)4.1–5.2(–6.8) μm. Tramal hyphae thin-walled or with distinctly thickened (0.2–0.8 μm thick) walls, more or less parallel, (2.0–)2.9–3.7(–4.4) μm. Basidia 11.4–19.8 × 3.7–5.4 μm. Basidiospores (4.2–) 4.7–6.1(–6.8) × (1.0–)1.1–1.6(–1.9) μm, L=5.22 μm, W=1.33 μm, Q=3.92.
Distribution and ecology: Europe, temperate to boreal, rather common on fallen conifer logs. The Spanish records are from mountains and derive from Abies alba (?) and A. pinsapo. All other records derive from Picea abies, except one Finnish record on Pinus sylvestris.
Specimens examined: Estonia, Jõgevamaa, Umbusi, Picea abies, 19 Sep. 1993, Kinnunen 35 (H); Tartumaa, Võnnu, Järvselja, P. abies, 6 Oct. 2001, Niemelä 7224 (H). Finland, Uusimaa, Helsinki, Lammassaari, P. abies, 15 Nov. 2010, Miettinen 14425* (H); Uusimaa, Helsinki, Veräjämäki (holotype, see above); Uusimaa, Kirkkonummi, P. abies, 24 Oct. 2012, Miettinen 15815.1 (H); Satakunta, Ylöjärvi, Viljakkala, P. abies, 2 Oct. 2011, Niemelä 8844* (H); Etelä-Häme, Padasjoki, Koivukannonsuo, P. abies, 6 Jul. 2003, Miettinen 7511 (H); Pohjois-Karjala, Ilomantsi, Kaitavaara, P. abies, 15 Sep. 2003, Penttilä 14560* (H); Pohjois-Karjala, Ilomantsi, Petkeljärvi, Pinus sylvestris, 6 Oct. 2012, Niemelä 8998* (H); Etelä-Savo, Lappeenranta, Ihalinen, P. abies (?), 23 Sep. 2003, Salo 9196* (H); Satakunta, Ylöjärvi, Viljakkala, P. abies, 2 Oct. 2011, Niemelä 8844* (H); Kittilän Lappi, Kittilä, Linkukero, P. abies, 22 Sep. 2001, Niemelä 7135 (H). France, Drôme, St. Agnan en Vercors, P. abies, 6 Oct. 2001, Rivoire 2051 (LY, H), St. Martin en Vercors, P. abies, 6 Oct. 2001, Rivoire 2052 (LY, H). Poland, Podlasie, Hajnówka, Białowieża, P. abies, 14 Oct. 2008, Kinnunen 5087* (H). Russia, Leningrad Reg., Podporozhie Dist., Tokari, P. abies, 28 Sep. 2007, Spirin 2752, 2756* (H); Nizhny Novgorod Reg., Sharanga Dist., Kilemary Nat. Res., P. abies, 26 Sep. 1999, Spirin (LE 211334). Spain, Huesca, Hecho, Abies sp., 10 Nov. 1977, Ryvarden 15129 (O, H); Málaga, Estepona, Los Reales de Sierra Bermeja, Abies pinsapo, 21 Nov. 2012, Miettinen 15919.2* (H); 23 Nov. 2012, Miettinen 15988 (H); 15989* (H 7008640). Sweden, Halland, Halmstad, Biskopstorp Nat. Res., P. abies, 28 Sep. 2012, Schigel 7436 (H).
Remarks: In most cases, P. cyanescens is easy to recognize. Thin, flabelliform basidiocarps on spruce and long, narrow basidiospores clearly separate it from P. caesia. Postia simulans has on average wider spores and its tramal hyphae are more loosely arranged and easily collapsing.
The Spanish specimens from Málaga have slightly larger spores than typical North European material and have more interwoven hyphae. Their ITS and tef1 sequences are identical with those of North European material, however.
Phylogenetic analysis utilizing ITS and tef1 are not able to separate P. cyanescens specimens unequivocally to a separate clade from P. caesiosimulans (Fig. 3–5). European material of the two species differs ecologically (angiosperms vs. gymnosperms) as well as morphologically (basidiome shape, arrangement of tramal hyphae, basidial length, spore size). We have utilized a wide, pragmatic concept of P. caesiosimulans here, and American material from conifers is morphologically closer, yet still separable, from P. cyanescens. Our extensive P. cyanescens material has uniform and unique ITS- and tef1-sequences, so separation of the two is justified even in the face of only limited phylogenetic support. Adding further informative markers to phylogenetic analyses would no doubt increase the phylogenetic support for P. cyanescens.
Postia glauca Spirin & Miettinen, sp. nov. MycoBank MB823902. Figs 7, 8, 13B.
Holotype: Russia, Khabarovsk Reg.: Khabarovsk Dist., Hologu, 50.0677° N 134.4297° E, alt. 449 m, Abies nephrolepis, 17 Aug. 2012, Spirin 5317* (H 7008648).
Etymology: Glaucus (Lat.), greyish bluish.
Basidiocarps conchate, small, thin to rather thick. Upper surface first greyish, matt to strigose, as a rule with bluish flecks, then plumbeous to bluish grey or greyish-brown. Tubes white to cream-colored, with light bluish-greyish tint in older and dry specimens, fresh specimens discoloring bluish when bruised; pores 5–8 per mm. Section: Context 1–3 mm thick, tubes 2–4 mm long. Context hyphae thin- to slightly thick-walled, some hyphae sclerified and appearing nearly solid, (2.5–)3.4–5.1(–6.3) μm. Tramal hyphae thin- or slightly thick-walled, (1.8–)2.6–3.3(–3.8) μm, amyloid (greenish in IKI) and cyanophilous hyphal segments common. Basidia 9.8–14.8(–17.0) × 3.1–4.3 μm. Basidiospores (4.0–)4.1–5.4(–6.2) × 1.1–1.5(–1.6) μm, L=4.64 μm, W=1.27 μm, Q=3.64.
Distribution and ecology: East Asia, cold temperate mountains, common; fallen conifer logs (Abies, Picea).
Specimens examined: China, Jilin, Antu Co., Changbaishan Nat. Res., Picea sp., 27 Aug. 2005, Miettinen 10567* (H). Russia, Khabarovsk Reg., Khabarovsk Dist., Bolshoi Khekhtsir Nat. Res., Picea ajanensis, 2–3 Sep. 2013, Spirin 6548, 6580* (H); Hologu (holotype, see above), Malyi Kukachan, P. ajanensis, 19 Aug. 2012, Spirin 5424 (H); Levyi Ulun, P. ajanensis, 24 Aug. 2012, Spirin 5577* (H); Solnechnyi Dist., Igdomi, P. ajanensis, 3 Sep. 2016, Spirin 10884, 10892 (H); Verkhnebureinskii Dist., Dublikan, P. ajanensis, 20 Aug. 2014, Spirin 7627 (H).
Remarks: Postia glauca is a common species, occurring mainly in spruce-dominated forests. It is most similar to P. bifaria, but their spore mean values differ a little (4.64 × 1.27 μm and 4.1 × 1.14 μm respectively), and only the specimens of P. glauca stain blue when bruised.
Postia gossypina (Moug. & Lév.) Spirin & Rivoire, comb. nov. MycoBank MB823903. Figs 7, 8.
Basionym: Polyporus gossypinus Moug. & Lév., Ann. Sci. Nat. Bot. 9: 124. 1848.
Lectotype: France, “Vosges”, fallen log, Mougeot (PC, isolectotype BPI US0209624, selected by Ryvarden 1981: 181, studied).
Basidiocarps conchate to flabelliform, small to medium-sized, thin. Upper surface first cream colored, then light grey, matt. Tubes cream-colored to bluish-greyish; pores 4–6 per mm. Section: Context 1–2 mm thick, tubes 2–3 mm long. Context hyphae thin- or slightly thick-walled, no sclerified hyphae seen, (2.9–)3.6–4.8(–5.4) μm. Tramal hyphae thin- to slightly thick-walled, (1.9–)2.3–3.0(–3.8) μm. Basidia 8.7–16.8 × 3.8–5.0 μm Basidiospores (4.0–)4.1–5.1(–5.2) × 1.2–1.7 μm, L=4.47 μm, W=1.44 μm, Q=3.11.
Distribution and ecology: Europe, temperate; logs of an unidentified fallen tree and Cedrus atlantica.
Specimens examined: France, “Vosges” (lectotype, see above); Vaucluse, Bonnieux, Cédraie du Luberon, Cedrus atlantica, 13 Oct. 2002, Rivoire 6658* (LY, H).
Remarks: Our concept of P. gossypina derives from the lectotype and one recent collection from France. We did not find essential morphological differences between them, and therefore we consider these specimens conspecific. Only tef1 sequence has been successfully produced from the newly collected specimen, and it places P. gossypina in the close vicinity of the East Asian P. glauca. The latter species possesses smaller pores (5–8 vs. 4–6 per mm) and on average narrower basidiospores (W=1.27 μm vs. W=1.44 μm), as well as partly amyloid tramal hyphae. Despite tef1 similarity (3 bp) these morphological differences are significant enough to treat them as separate species.
Postia simulans is morphologically very similar, but has usually longer spores and wider tramal hyphae. More material is needed to confirm differences between these species, but after comparing them side by side, we think it is likely they are not conspecific and treat them as separate species. Tef1 difference between our sequenced specimen and P. simulans material is very clear, over 50 bp.
Postia livens Miettinen & Vlasák, sp. nov. MycoBank MB823904. Figs 1, 6, 7, 14.
Fig. 14.
Postia livens. A. Miettinen 16714. B. Holotype.
Holotype: USA, New York, Essex Co., Harris Lake, 43.9790° N 74.1472° W, alt. 500 m, Larix laricina (?), 23 Sep. 2013, Miettinen 17177* (H 7008642, isotype BPI).
Etymology: Livens (Lat.), greyish bluish.
Basidiocarps conchate, small to medium-sized, rarely large, thin to thick. Upper surface first cream colored, matt to distinctly pubescent, then plumbeous to bluish grey to ochraceous, often with bluish tints. Tubes cream-colored, in older and dry specimens with light bluish-greyish tint; pores 4–6 per mm. Section: Context 1–15 mm thick, tubes 1–6 mm long. Context hyphae slightly thick-walled, often sclerified (2.5–)3.7–5.3(–7.2) μm. Tramal hyphae thin- to moderately thick-walled (walls up to 0.5–0.8 μm thick), (1.9–)2.9–4.0(–4.7) μm. Basidia 9.3–14.3(–15.7) × 4.0–5.3 μm. Basidiospores 4.1–5.7(–7.0) × 1.1–1.5(–1.7) μm, L=4.78 μm, W=1.28 μm, Q=3.74, variable within and between specimens.
Distribution and ecology: North America (from East coast to Rocky Mts.), temperate, common; fallen logs and branches of conifers (Abies, Picea, Larix, Tsuga) and deciduous trees (Acer, Betula, Fagus).
Specimens examined: Canada, Québec, Bas-Saint-Laurent, Notre-Dame-du-Portage, Betula papyrifera, 21 Aug. 2004, Pieri (LY-BR 2468); Québec, Estrie: Sherbrooke, Acer rubrum, 4 Sep. 2004, Pieri (LY-BR 2466, H). USA, Maine, Portland, Crescent Beech State Park, Abies sp., Sep. 2008, Vlasák 0809/118 (H, JV); Massachusetts, Worcester Co., Worcester, deciduous tree (?), 20 Sep. 2011, Miettinen 14775* (H); 8 Oct. 2011, Miettinen 14878 (H); Holden, Tsuga canadensis (?), 26 Sep. 2011, Miettinen 14828* (H); 6 Sep. 2013, Miettinen 16816, 16819, 16825 (H); T. canadensis, 14 Apr. 2013, Miettinen 16056* (H); New York, Essex Co., Catlin Lake, rotten wood, 14 Aug. 2012, Ortiz-Santana (H); Harris Lake (holotype, see above); Arbutus Lake, T. canadensis, 16 Sep. 2013, Miettinen 16899 (H); Minnesota, Waseca Co., Janesville, hardwood, 21 Aug. 2013, Miettinen 16714* (H); North Carolina, Buncombe Co., Blue Ridge Assembly, Tsuga (?), 24 Sep. 2015, Miettinen 19439* (H); Betula sp., 24 Sep. 2015, Miettinen 19446 (H); North Carolina, Swain Co., Clingmans Dome, Abies fraseri (?), 1 Oct. 2015, Miettinen 19644 (H); Pennsylvania, Montgomery Co., Schwenksville, Gossenhoppen Creek, hardwood, Sep. 2008, Vlasák 0809/121 (H, JV); Pennsylvania, Wayne Co., Tobyhanna State Park, Fagus grandifolia, Sep. 2010, Vlasák 1009/57* (H, JV); Tennessee, Cocke Co., Cosby Creek, T. canadensis (?), 2 Oct. 2015, Miettinen 19666.2* (H); Washington, Pend Oreille Co., Gypsy Meadows, Picea engelmannii, 17 Oct. 2014, Spirin 8728* (H).
Remarks: Postia livens is the most common representative of the P. caesia complex in North America. Its fully developed basidiocarps can be identified easily by their size and hairy upper surface. Identification of younger or dwarf-sized collections is more difficult, however. Postia caesiosimulans and P. populi have in general thicker-walled tramal hyphae, narrower context hyphae and spores, and their pores are slightly smaller. Postia simulans produces in average longer and wider basidiospores, and its tramal hyphae collapse commonly unlike those of P. livens.
One specimen from Washington (Spirin 8728) is excluded from the description above, having a slightly deviating ITS (3 bp) and larger spores than typical P. livens. Even so the intraspecific morphological variation (spore size, hyphal wall thickness) is among the highest in Postia caesia complex.
Postia luteocaesia (A. David) Jülich, Persoonia 11(4): 423. 1982. Figs 7, 8.
Basionym: Spongiporus luteocaesius A. David, Bull. Soc. Linn. Lyon 49: 29. 1980.
Holotype: France, Var, Massif des Maures, Pinus sp., 26 Dec. 1970, David 929 (LY, studied).
Basidiocarps conchate, medium-sized. Upper surface first cream colored, then yellowish to bright yellow, pale to dark ochraceous on aging, often with brownish flecks, matt to pubescent. Pore surface first bright yellow, then with ochraceous tints; pores 3–5 per mm. Section: Context 2–5 mm thick, white to pale cream-colored, tubes 2–5 mm long. Context hyphae thin-walled, mostly sclerified, often with several, randomly oriented side branches, (3.3–)4.1–5.8(–6.7) μm. Tramal hyphae thin- to moderately thick-walled (walls up to 0.8 μm thick), partly glued together, (2.1–)2.7–3.2(–4.2) μm, partly with amyloid (greenish in IKI) and strongly cyanophilous content. Basidia 11.1–16.2(–18.2) × 4.0–5.2 μm. Basidiospores (4.2–)4.3–6.1(–7.1) × (1.4–)1.5–1.9(–2.0) μm, L=5.06 μm, W=1.68 μm, Q=3.02.
Distribution and ecology: Europe, Mediterranean France, rare; so far only on Pinus halepensis and Pinus sp.
Specimens examined: France, Var, Massif des Maures (holotype, see above); Port-Cros, Pinus halepensis, 12 Dec. 1992, Rivoire 733 (LY, H); Porquerolles, P. halepensis, 13 Nov. 2004, Rivoire 2605* (LY, H).
Remarks: Collections from Pinus sylvestris in Central and North Europe earlier addressed to P. luteocaesia belong to P. auricoma. The two species are very similar morphologically and grow on pine. The only constant morphological difference we can point to are the tramal hyphae, which are wider in P. auricoma (Table 1). According to current knowledge, their distribution areas do not overlap. Phylogenetically the closest relative of P. luteocaesia is P. simulans, a species without yellow coloration.
Postia magna Miettinen, sp. nov. MycoBank MB823905. Figs 7, 8, 15.
Fig. 15.
Postia magna holotype photographed in the field.
Holotype: China, Jilin, Antu Co., Changbaishan Nat. Res., Erdao Bai He, 42.3994° N 128.1015° E, alt. 730 m, Populus koreana, 28 Aug. 2005, Miettinen 10634* (H 7008643, isotype BJFC).
Etymology: Magnus (Lat.), big.
Basidiocarps conchate, medium-sized (7×4 cm), thick, margin sharp. Upper surface white, drying cream colored to light greyish and ochraceous, distinctly pubescent. Tubes white, drying ochraceous with bluish tint, pores (3–)4–5 per mm. Section: Context 3–10 mm thick, white, tubes 3–6 mm long. Context hyphae thin-walled (to slightly thick-walled), (3.4–)4.2–6.0(–6.6) μm. Tramal hyphae slightly thick-walled, regularly branched, (1.5–)2.2–3.3(–3.8) μm. Basidia 10–12.5×3.2–4 μm. Basidiospores 3.6–4.4(–4.5) × 1.0–1.2 μm, L=3.97 μm, W=1.13 μm, Q=3.51.
Distribution and ecology: Temperate China and South Korea, so far known from hardwoods only.
Specimen examined: Postia magna. China, Jilin, Antu (holotype, see above).
Remarks: This species reminds P. subcaesia and P. livens with its large, hairy basidiocarps. Microscopically P. magna seems to differ from them by its smaller spores. As far as we know their distribution areas do not overlap. Although we have examined only one collection from Northeast China, available ITS sequences indicate that the species is widespread in China and Korea (Shen et al. 2014, Kim et al. 2015, Jang et al. 2016).
Postia mediterraneocaesia M. Pieri & B. Rivoire, Bull. Semestriel Féd. Assoc. Mycol. Méditerranéennes 28: 34. 2005. Figs 7, 8.
Holotype: France, Bouches du Rhône, St. Rémy de Provence, Pinus halepensis, 11 Nov. 2000, Pieri & Rivoire 1946 (LY, studied).
Basidiocarps effused-reflexed or resupinate with detaching or adnate margin, thin. Upper surface white to cream colored or pale ochraceous, matt or almost glabrous. Tubes white to cream-colored, in older and dry specimens pale ochraceous, with light bluish-greyish tint; pores (4)5–6 per mm. Section: Context 0.2–1 mm thick, tubes 0.5–2 mm long. Context hyphae thin- to slightly thick-walled, often sclerified, (2.4–)3.1–4.0(–4.8) μm. Tramal hyphae thin to distinctly thick-walled (walls 0.2–1 μm thick), densely interwoven, collapsing easily, twisted, branching at random directions, (1.8–)2.3–3.2(–4.2) μm. Basidia (8.8–)12–18.5(–21.8) × 3.5–4.6(–5.1) μm. Basidiospores (3.9–)4.2–5.8(–6.3) × (1.2–)1.3–1.7(–1.9) μm, L=4.83 μm, W=1.48 μm, Q=3.26.
Distribution and ecology: Europe, warm temperate to Mediterranean (France, Spain), rather common; on fallen logs and dry branches of deciduous trees (Buxus, Erica, Populus, Quercus) and conifers (Cedrus, Juniperus, Pinus).
Specimens examined: France, Alpes-Maritimes, Lac de St. Cassien, Juniperus oxycedrus, 31 Oct. 1996 Rivoire 1356 (LY, H); 19. Nov. 2000, Rivoire 1903 (LY, H); Bouches du Rhône, St. Rémy de Provence (holotype, see above); Vaucluse, Goult, Quercus pubescens, 7 Nov. 2007, Rivoire 3278 (LY, H); Vaucluse, Bédouin, Pinus nigra, 22 Oct. 2000, Rivoire 1834 (LY, H). Spain, Navarra, Abaurea Alta, Juniperus communis, 28 Oct. 2001, Rivoire 2083* (LY, H); P. sylvestris, 31 Oct. 2001, Rivoire 2107 (LY, H); Garayoa, Buxus sempervirens, 30 Oct. 2001, Rivoire 2100 (LY, H).
Remarks: Postia mediterraneocaesia produces small-sized, often resupinate or pendant basidiocarps, and it usually occurs on dry branches in xerophilic habitats. Its spores are similar to those of P. caesia, P. cyanescens and P. simulans, but tramal hyphae of P. mediterraneocaesia are strikingly different, unevenly thick-walled and irregularly branched. For a photo and illustration see Pieri & Rivoire (2005).
Postia populi Miettinen, sp. nov. MycoBank MB823906. Figs 6–8, 16.
Fig. 16.
Postia populi. A. Young, typically light-colored basidiome (Miettinen 14790.3). B. Old basidiomes with a characteristic wrinkled margin (Miettinen 15827.2).
Holotype: USA, New York, Essex Co., Wolf Lake, 44.0336° N 74.2262° W, alt. 560 m, Populus tremuloides, 20 Sep. 2013, Miettinen 17043* (H 7008644, isotype BPI).
Etymology: After Populus spp., the most common host species.
Basidiocarps conchate to flabelliform, sometimes effused-reflexed, mostly thin, later fusing together, small to medium-sized; margin often undulating. Upper surface first white to cream colored, matt, then pale ochraceous to greyish, rarely with bluish flecks or indistinct zones, more or less glabrous. Tubes white to cream-colored, in older and dry specimens with light bluish-greyish tint; pores 5–7(–8) per mm, dissepiments first uneven, then strongly serrate. Section: Context 1–3 mm thick, tubes 1–5 mm long. Context hyphae slightly thick-walled, sclerified, tightly arranged, firm, (2.6–)3.2–4.8(–5.6) μm. Tramal hyphae predominantly thick- or very thick-walled (walls regularly over 1 μm thick), tightly arranged, (2.0–)2.7–3.3(–4.2) μm. Basidia (9–)10–16(–18.5) × (3.2–)3.5–4.2(–4.8) μm. Basidiospores (4.0–)4.2–5.6(–6.1) × 1.0–1.3(–1.6) μm, L=4.84 μm, W=1.17 μm, Q=4.14.
Distribution and ecology: Holarctic, boreal to temperate, common; on fallen logs and large branches of Populus spp., rarely on other deciduous trees (Acer, Alnus, Betula, Salix).
Specimens examined: China, Jilin, Antu Co., Changbaishan Nat. Res., Populus sp., 1 Sep. 1993, Dai 960 (H); Acer sp., 2 Sep. 1993, Dai 1001 (H). Finland, Uusimaa, Helsinki, Veräjämäki, P. tremula, 8 Nov. 2015, Miettinen 19821 (H); Uusimaa, Kirkkonummi, Betula sp., 24 Oct. 2012, Miettinen 15827.2* (H); Etelä-Häme, Hämeenlinna, Musta-Kotinen, Populus/Salix, 18 Sep. 2007, Niemelä 8379* (H 6007874); Pohjois-Häme, Jyväskylä, Tourujoki, hardwood, 10 Sep. 2011, Miettinen 14701* (H); Pohjois-Savo, Pieksämäki, Sorsasalo, Populus tremula, 16 Sep. 1996, Haikonen 18147 (H); Kainuu, Hyrynsalmi, Paljakka, P. tremula, 27 Sep. 2010, Miettinen 14211* (H); Kittilän Lappi, Kittilä, Aakenus, Vasalaki SW, 1 Sep. 2000, Kinnunen 1155 (H). Norway, Østfold, Trøgstad, Håkås, P. tremula, 8 Sep. 2016, Nordén (H, NINA). Poland, Podlasie, Hajnówka, Białowieża, fallen deciduous log, 11 Oct. 2008, Kinnunen 4938* (H). Russia, Chukchi Reg., Anadyr, Alnus fruticosa, 20 Aug. 2009, Kotiranta 27132 (H 7033597); Kamtchatka, Bering Island, Alnus (?), 4 Sep. 2015, Kotiranta 27600 (H 7033451); Khabarovsk Reg., Khabarovsk Dist., Bolshoi Khekhtsir Nat. Res., Acer ukurunduense, 3 Sep. 2013, Spirin 6598* (H); Ulun, P. maximowiczii, 28 Aug. 2012, Spirin 5771* (H); Solnechnyi Dist., Suluk-Makit, Salix sp., 19 Aug. 2011, Spirin 4194* (H); Leningrad Reg., Boksitogorsk Dist., Vozhani, fallen log, 22 Sep. 2011, Spirin 4587* (H); Shidrozero, P. tremula, 28 Sep. 2012, Spirin 5869 (H); Podporozhie Dist., Nemzha, P. tremula, 20 Sep. 2009, Spirin 3224 (H); Volkhov Dist., Chernetskoe, P. tremula, 17 Sep. 2009, Spirin 3194 (H); Nizhny Novgorod Reg., Lukoyanov Dist., Razino, P. tremula, 4 Aug. 2004, Spirin 2092 (H); 16 Aug. 2015, Spirin 9353* (H). USA, Massachusetts, Worchester, Acer sp. (?), 25 Sep. 2011, Miettinen 14790.3*, 14794 (H); hardwood, 8 Oct. 2011, Miettinen 14876 (H); New York, Essex Co., Wolf Lake, Populus tremuloides, 20 Sep. 2013, Miettinen 17043* (holotype, see above), Miettinen 17048.3, 17052.1 (H).
Remarks: Postia populi is a narrow-spored species morpho-logically and phylogenetically very close to P. alni and P. caesiosimulans. In Eurasia narrow-spored specimens growing on Populus tremula belong almost always to P. populi, at least in cold temperate and boreal areas. However, records exist from other hosts, and in this case thick-walled, tightly arranged hyphae in trama and narrow context hyphae help in morphology-based identification. In North America growth on aspen is a less reliable character since Populus tremuloides is only one of many hosts of P. populi, and P. caesiosimulans is apparently common on aspen in the Midwest. In problematic cases, ITS or tef1 sequences are required for identification.
Postia simulans (P. Karst.) Spirin & Rivoire, comb. nov. MycoBank MB823907. Figs 7, 8, 17.
Fig. 17.
Postia simulans. A. A half-resupinate, young basidiome with no blue color (Spirin 4689). B. Pileate basidiome with a characteristic blue upper surface (Miettinen 20422).
Basionym: Bjerkandera simulans P. Karst., Rev. Mycol. 10: 73. 1888.
Synonym: Polyporus karstenii Sacc., Sylloge Fungorum (Abellini) 9: 170. 1891. (nomen novum)
Lectotype: Finland, Etelӓ-Pohjanmaa, Vaasa, Picea abies, 19 May 1864, Karsten, BPI 871870 (selected here, MBT380827, duplicate H 6060173). Both specimens were studied.
Epitype: Finland, Satakunta, Ylöjärvi, Viljakkala, Inkula, 61.72° N 23.25° E, P. abies, 2 Oct. 2011, Niemelä 8846* (H 6034704 selected here, MBT380828).
Basidiocarps conchate, effused-reflexed or resupinate with detaching or adnate margin, thin to rather thick. Upper surface first white to cream colored, matt or almost glabrous, more rarely pubescent, occasionally turning blue, greyish or pale ochraceous. Tubes white to cream-colored, in older and dry specimens with light bluish-greyish tint and rarely with bluish dots; pores (4–)5–7 per mm. Section: Context 0.5–3 mm thick, tubes 0.5–4 mm long. Context hyphae thin to slightly thick-walled (walls 0.1–0.5 μm thick), rarely sclerified, easily collapsing, (2.9–)3.9–5.0(–6.4) μm. Tramal hyphae thin-walled to slightly thick-walled (walls up to 0.6 μm thick) and easily collapsing, often parallel, (1.7–)2.8–3.6(–4.8) μm, very rarely with strongly amyloid (greenish-black in IKI) and cyanophilous content; in East Asian material tramal hyphae sometimes thick-walled (up to 1 μm) and then not so easily collapsing. Basidia 10–14.8 × 3.7–5.2 μm. Basidiospores (4.1–)4.4–6.3(–8.1) × (1.2–)1.3–1.8(–1.9) μm, L=5.24 μm, W=1.46 μm, Q=3.6.
Distribution and ecology: Holarctic, warm temperate to boreal, common; mostly on conifers (Abies, Cedrus, Juniperus, Picea, Pinus, Thuja, Tsuga) but regularly also on deciduous trees (Corylus, Fagus, Populus, Sorbus, Ulmus).
Specimens examined: Canada, Québec, Saguenay – Lac-Saint-Jean, Alma, conifer, 31 Aug. 2004, Pieri (LY-BR 2467, H). China, Jilin, Antu Co., Changbaishan Nat. Res., Pinus sp., 12 Nov. 1995, Dai 2054b (H). Estonia, Hiiumaa, Käina, Nasva, Juniperus communis, 27 Sep. 2007, Kotiranta 22018 (H); Valgamaa, Palupera, Käpa, P. abies, 13 Sep. 2012, Spirin 5803 (H). Finland, Varsinais-Suomi, Kustavi, Kaurissalo, Picea abies, 2 Oct. 1979, Alava 19067 (H), Varsinais-Suomi, Karjalohja, Karkali Nat. Res., Corylus avellana, 5.X.2006 Niemi 183b* (H); Satakunta, Ylöjärvi (epitype, see above); Etelӓ-Pohjanmaa, Vaasa (lectotype, see above); Etelä-Häme, Padasjoki, Vesijako, Pinus sylvestris, 23 Sep. 2016, Miettinen 20422* (H); Kainuu, Hyrynsalmi, Paljakka, P. abies, 24 Sep. 2010, Miettinen 14167.2* (H); Betula, 26 Sep. 2010, Miettinen 14190 (H). France, Loire, Bessat, P. abies, 5 Oct. 2002, Rivoire 2201 (LY, H); Loire, Chalmazel, Fagus sylvatica, 1 Sep. 2006, Rivoire 2936 (LY, H); Rhône, Larajasse, P. sylvestris, 30 Sep. 2002, Rivoire 2195 (LY, H); Rhône, Sérézin, Populus nigra, 30 Sep. 1995, Rivoire 1166 (LY, H); Vaucluse, Bonnieux, Cedrus atlantica, 12 Nov. 1994, Rivoire 1046 (LY, H); Vosges, La Bresse, A. alba, 22 Sep. 2002, Rivoire 2177 (LY, H). Germany, Hesse, Alsfeld, Kestrich, Fagus sylvatica, 3 Sep. 1972, Hupke (H). Norway, Akershus, Ski, Svartoren, 12 Sep. 2014, Ursin* (O 75832). Russia, Leningrad Reg., Boksitogorsk Dist., Perelesok, P. abies, 27 Sep. 2011, Spirin 4689 (H); Podporozhie Dist., Chogozero, P. abies, 22 Sep. 2009, Spirin 3407 (H); Kurba, P. sylvestris, 19 Sep. 2009, Spirin 3239 (H); Ostrechiny, P. abies, 28 Sep. 2008, Spirin 2834, 2841 (H); Tokari, P. abies, 28 Sep. 2007, Spirin 2746 (H); Tikhvin Dist., Urya, Ulmus glabra, 25 Sep. 2011, Spirin 4629 (H); Khabarovsk Reg., Khabarovsk, Voronezhskoe, Quercus mongolica, 19 Aug. 2013, Spirin 6177 (H); Khabarovsk Dist., Malyi Niran, Picea ajanensis, 6–8 Aug. 2012, Spirin 4983, 5062 (H); Malyi Kukachan, P. ajanensis, 19 Aug. 2012, Spirin 5418 (H); Solnechnyi Dist., Igdomi, Pinus pumila, 2 Sep. 2016, Spirin 10810 (H); Suluk-Makit, P. ajanensis, 19 Aug. 2011, Spirin 4217 (H); Razlivnoi, P. ajanensis, 22–24 Aug. 2011, Spirin 4271*, 4273, 4386* (H); Evoron, P. ajanensis, 26–27 Aug. 2011, Spirin 4397, 4424 (H); Nizhny Novgorod Reg., Lukoyanov Dist., P. sylvestris, 15 Aug. 2006, Spirin 2546 (H); Primorie, Krasnoarmeiskii Dist., Valinku, Betula ermanii, 25 Aug. 2013, Spirin 6306 (H); P. ajanensis, 25 Aug. 2013, Spirin 6310 (H); Spirin 6328* (H 7008649); Sverdlovsk Reg., Sysert’ Dist., Dvurechensk, P. sylvestris, 25 Aug. 2002, Kotiranta 19754 (H). USA, Idaho: Bonner Co., Trapper Creek, Tsuga heterophylla, 14 Oct. 2014, Miettinen 18735* (H); Idaho, Boundary Co., Upper Priest River, Thuja/Tsuga, 16 Oct. 2014, Miettinen 18882* (H); New York, Essex Co., Catlin Lake, Fagus grandifolia, 19 Sep. 2013, Miettinen 17015 (H); New York, Warren Co., Pack Demonstration Forest, Acer, 24 Sep. 2013, Miettinen 17197 (H); North Carolina, Swain Co., Clingmans Dome, Sorbus americana, 1 Oct. 2015, Miettinen 19612* (H); Abies fraseri/Picea rubens, 1 Oct. 2015, Miettinen 19632.3 (H); Washington, Pend Oreille Co., Slate Creek, Pinus ponderosa, 15 Oct. 2014, Spirin 8542* (H); 8587* (H 7008650); Thuja plicata, 15 Oct. 2014. Miettinen 18817 (H).
Remarks: This species was described by Karsten (1888) as Bjerkandera simulans and later tentatively placed in the synonyms of Postia lactea (as Polyporus tephroleucus) (Lowe 1956, Ryvarden 1991). However, the presence of characteristic amorphous aggregates developing in CB as well as spore dimensions in the type material preclude this synonymy. The most similar species is P. yanae. For an overview of differences, see remarks under that species and Tables 1–3.
In herbaria, Postia simulans has been mixed up mainly with P. caesia in the strict sense, but also mislabeled as other Postia species. This is not surprising due to its wide morphological variation. Young basidiocarps of P. simulans are white and lack bluish tints, and only microscopical study can reveal their identity. Senescent, distinctly bluish basidiocarps can be mistaken for other species of the complex, in Europe P. caesia in particular. In this case, rather long basidiospores (regularly reaching 6 μm) and almost total absence of colored hyphae in tube trama are the best features to recognize P. simulans. Most of the European, distinctly pubescent, blue specimens on conifers belong to P. caesia.
The East Asian material differs from other Eurasian material we have studied in having often more thick-walled and slightly narrower tramal hyphae (Suppl. 3). This population seems to be genetically slightly different as well - the ITS sequences differ only by 1 bp between European and North American P. simulans while tef1 differs by 5–7 bp, albeit with only one East Asian specimen sequenced for tef1. However, the ITS sequences of this East Asian material are identical with the Mediterranean P. luteocaesia. Although otherwise morphologically very similar, P. luteocaesia sensu typi differs from P. simulans in having yellow basidiocarps and wider basidiospores. In the light of these differences and the absence of tef1 sequences from P. luteocaesia we have kept P. simulans and P. luteocaesia separate. We are also refraining from split the P. simulans complex further. Extensive sampling of material and sequencing tef1, and possibly other markers, are needed to clarify whether these taxa and populations represent more than one species.
Postia subcaesia (A. David) Jülich, Persoonia 11(4): 424. 1982. Figs 6–8, 18.
Fig. 18.
Postia subcaesia, Rivoire 1370.
Basionym: Tyromyces subcaesius A. David, Bull. Soc. Linn. Lyon 15: 120. 1974.
Holotype: France, Isère, Crémieu, Malus domestica, Oct.1968, David 652 (LY, isotype H 7034976* studied).
Basidiocarps conchate to triquetrous, small to medium-sized. Upper surface first cream colored, then pale to dark ochraceous, first matt to later distinctly pubescent, rarely with bluish flecks or faint zones. Pore surface white to cream-colored, bluish-greyish on aging; pores 4–6(–7) per mm. Section: Context 3–17 mm thick, tubes 2–10 mm long. Context hyphae thin- to slightly thick-walled, (2.3–)4.2–6.6(–7.8). Tramal hyphae thin- or only slightly thick-walled, (2.2–)3.1–4.1(–5.8) μm. Basidia 10.3–17.8 × 3.3–4.6 μm. Basidiospores (3.8–)4.0–5.3(–6.1) × 1.0–1.4(–1.5) μm, L=4.60 μm, W=1.21 μm, Q=3.80.
Distribution and ecology: Europe, temperate, rather rare; fallen logs of deciduous trees (Alnus, Carpinus, Crataegus, Corylus, Fagus, Fraxinus, Malus, Populus, Quercus, Salix, Ulmus).
Specimens examined: Czech Republic, Jihomoravský Reg., Břeclav, Lanžhot, Carpinus betulus, 31 Aug. 1989, Vampola (H, MJ 1563); Crataegus sp., Oct. 2001, Vlasák 0110/24* (H, JV). France, Allier, Tronçais, Quercus sp., 19 Oct. 1969, David 785 (LY, H); Alpes Maritimes: Montauroux, Salix sp., 2 Nov. 1996, Rivoire 1370 (LY, H); Isère (holotype, see above); Loire, St. Foy St. Sulpice, Populus tremula, 31 Oct. 2000, Rivoire 1868 (LY, H); Saône-et-Loire: Tramayes, Quercus sp., 29 Aug. 1965, David 150 (LY, H). Russia, Nizhny Novgorod Reg., Lukoyanov Dist., Sanki, Corylus avellana, 12 Aug. 2005, Spirin 2390 (H), 10 Aug. 2013, Spirin 6083* (H). UK, England, Hampshire, New Forest, Alnus glutinosa, 14 Oct. 1995. Legon* (K(M) 31967, O, H).
Remarks: In most cases, P. subcaesia is easily recognized by its large, soft basidiocarps with a matt or pubescent upper surface. The distinct bluish color only appears in senescent specimens. Microscopically, it differs from other European narrow-spored species (P. alni, P. caesiosimulans, P. populi) in having thin-walled, wide and loosely arranged hyphae both in context and tubes. Papp (2014) provides a detailed comparison with P. alni.
Postia subviridis (Ryvarden & Guzmán) Spirin, comb. nov. MycoBank MB823908. Figs 7, 8.
Basionym: Tyromyces subviridis Ryvarden & Guzmán, Mycotaxon 78: 252. 2001.
Holotype: Mexico, Veracruz, Cofre de Perote, fallen log, 8 Jan. 1991, Tapia 468 (XAL, isotypes O, H studied).
Basidiocarps conchate, small to medium-sized, thin. Upper surface first pale ochraceous, then ochraceous to greyish, glabrous to matt. Tubes white to cream-colored, with light bluish-greyish ting upon aging and drying; pores 6–8 per mm, angular to sinuous. Section: Context 1–2 mm thick, tubes 1–4 mm long. Context hyphae slightly thick-walled, often sclerified, (2.8)4.8–5.8(7.8) μm. Tramal hyphae predominantly thick-walled (walls up to 1 μm thick) to slightly thick-walled, (2.2–)2.5–3.2(–3.7) μm. Basidia (9–)10–13(–14) × 3.2–4.4 μm. Basidiospores (3.5–) 3.8–4.5(–4.6) × 1.0–1.3 μm, L=4.11 μm, W=1.15 μm, Q=3.58.
Distribution and ecology: North America and Europe, temperate to boreal. Only three records so far (mountains in Mexico, western US, Finland); conifer logs (Picea, Pinus; the type probably from Abies or Picea).
Specimens examined: Finland, Pohjois-Karjala, Ilomantsi, Lahnavaara, Pinus sylvestris, 5 Sep. 2003, Penttilä 14376* (H). Mexico, Veracruz, Cofre de Perote (holotype, see above). USA, Washington, Jefferson Co., Hoh River, Picea sitchensis, 20 Oct. 2014, Spirin 8774a* (H).
Remarks: Our concept of the species may still include several species. Tyromyces subviridis was described from a highland conifer forest in Mexico (Guzmán & Ryvarden 2001). We studied the type specimen, as well as another collection from Washington, USA. The morphological description above is based on these two specimens. A third collection from Finland has a tef1 sequence identical to the Washington specimen, but morphologically it is deviating, being pure white and having nearly thin-walled tramal hyphae and longer spores (L=4.92 μm, Tables 1, Suppl. 2).
Both morphological and DNA data show that P. subviridis is a member of the difficult narrow-spored complex which also includes P. alni, P. populi, and P. caesiosimulans. Of them, P. subviridis has the smallest pores and the shortest basidiospores, and it might be restricted to conifers.
Postia yanae Miettinen & Kotiranta, sp. nov. MycoBank MB823909. Figs 7, 8.
Holotype: Russia, Sakha, Verkhoyansk Reg., Verkhoyansk-Batagai road, 67.5° N 133.67° E, alt. 370 m, old larch dominated forest, decorticated Larix gmelinii branch (3 cm diam, decay stage 2/5), 10 Aug. 2016, Kotiranta 27454* (H 7034942).
Etymology: After river Yana, the type locality.
Basidiocarps small to medium-sized, effused-reflexed or resupinate with detaching or adnate margin. Upper surface white to cream colored, pale ochraceous or bluish, deep brown in large specimens, matt to glabrous. Pore surface white, with light to strong bluish-greyish tint; pores 5–7 per mm, angular to sinuous. Section: Context 0.5–2 mm thick, white, tubes 1–3 mm long, concolorous with pore surface. Context hyphae thin- to slightly thick-walled, (2.0–)3.0–4.0(–5.2) μm. Tramal hyphae thin- to slightly thick-walled (walls 0.2–0.5 μm thick), winding, collapsing easily, (1.5–)2.2–2.9(–4.3) μm. Basidia 9–14 × 3.5–4.2 μm. Basidiospores (4.0–)4.3–5.8(–7.5) × 1.2–1.6(1.7) μm, L=5.02 μm, W=1.41 μm, Q=3.56.
Distribution and ecology: Known from four localities in Eastern Siberia, on branches, cut wood and small diameter trunks of Pinus and Larix in dry environments.
Specimens examined: Russia, Sakha, Churapacha Dist., Telei, Larix gmelinii, 22 Aug. 2016, Kotiranta 27772* (H 7036326); Verkhoyansk Dist. (holotype, see above); Sakha, Xañalas Dist., Buotama, L. gmelinii, 17 Aug. 2016, Kotiranta 27606* (H); P. sylvestris, 18 Aug. 2016, Kotiranta 27677* (H); Nizhny Bestjah, Pinus sylvestris, 23 Aug. 2016, Kotiranta 27879* (H 7036282).
Remarks: Postia yanae belongs to the wide-spored group of conifer-dwelling species within the P. caesia complex. It is morphologically very similar to P. simulans. Postia simulans shares with P. yanae collapsing, thin-walled tramal hyphae, but its tramal and context hyphae are usually wider (Suppl. 3, context: 3.9–5.0 vs. 3.0–4.0 μm, trama: 2.8–3.6 vs. 2.2–2.9 μm). Spores of P. simulans are often longer in average (L=5.24 μm vs. 5.02 μm), but some specimens deviate from this trend (Suppl. 2). Possibly of importance is autecology: P. simulans inhabits various tree species (rarely Pinus) in mesic forests, whereas P. yanae collections derive from dry continental forests from Larix and Pinus branches. Postia yanae basidiomes are mostly very small while P. simulans usually makes sturdier basidiomes. In borderline cases sequencing is needed for definite identification. Also the basidiocarps and spores of P. mediterraneocaesia are similar, but beside the widely different distribution area, the richly branched tramal hyphae tell this species apart.
FURTHER SPECIES ASSOCIATED WITH POSTIA CAESIA complex
Bjerkandera ciliatula Karst., Medd. Soc. Fauna Flora Fennica 14: 80. 1887.
Remark: We have studied the type and it represents Tyromyces chioneus.
Boletus candidus Roth, Catalecta botanica quibus plantae novae et minus cognitae describuntur atque illustrantur 1: 244. 1797.
Remarks: Fries (1821) treated this species as a synonym of Postia caesia, but he later (Fries 1836–1838) considered it a form of Bjerkandera adusta. No type is known to exist and the description is vague: all we can say is that it is a light-colored wood-inhabiting polypore. The name should be treated as nomen dubium.
Postia africana (Ryvarden) V. Papp, Mycotaxon 129: 411. 2015. [2014]
Basionym: Oligoporus africanus Ryvarden, Mycotaxon 31: 407. 1988.
Remarks: The species has been described based on the single collection from highlands of Burundi (Ryvarden 1988a). Large and thick basidiocarps, wide tramal hyphae and short and narrow basidiospores of P. africana point towards P. subcaesia and related species. The identity of this species should be reestablished after sequencing newly collected specimens from Central Africa.
Postia amyloidea (Corner) V. Papp, Mycotaxon 129: 411. 2015. [2014]
Basionym: Tyromyces amyloideus Corner, Beih. Nova Hedwigia 96: 160. 1989.
Remarks: Corner (1989) described this species alongside with P. coeruleivirens, and the only reliable characters in the protologue to distinguish these species are amyloid tramal hyphae and slightly smaller basidiospores of P. amyloidea. However, the taxonomic value of these features, as well as identity of both aforementioned species, must be revised after collecting new material in the type locality, Mount Kinabalu.
Postia atrostrigosa (Cooke) Rajchenb., N.Z. Jl Bot. 33: 104. 1995.
Basionym: Polyporus atrostrigosus Cooke, Grevillea 19 (89): 2. 1890.
Remarks: Rajchenberg (1995) re-introduced this species after morphological and cultural studies of specimens from New Zealand. We sequenced a Tasmanian specimen, which differs by just 1 bp from two Argentinian ITS sequences available in GenBank (JX090109 and JX090110); they represent a unique species that may well be P. atrostrigosa.
Postia caesioflava (Pat.) V. Papp, Mycotaxon 129: 411. 2015. [2014]
Basionym: Polyporus caesioflavus Pat., Bull. Soc. Mycol. France 8: 114. 1892.
Remarks: Patouillard described this species from Ecuador. It is the only yellow-colored representative of the P. caesia complex known so far from the tropics. It differs from similarly colored European species, P. auricoma and P. luteocaesia, in having larger basidiocarps, not turning green when bruised, and smaller pores, 8–10 per mm (Ryvarden 2016).
Postia wakefieldiae (Kotl. & Pouzar) Pegler & E.M. Saunders, Mycologist 8: 28. 1994.
Basionym: Tyromyces wakefieldiae Kotl. & Pouzar, Česká Mykol. 43: 39. 1989.
Specimens examined: Germany, Schleswig-Hollstein, Travemünde, Hermannshöhe, dicot, 24 Jun. 2007, Miettinen 11703 (H). UK, England, Hampshire, New Forest, Quercus sp., 4 Nov. 1995, Legon (O, H). Suffolk, Brettenham, Nov. 1935, Pearson (PRM 611292, holotype).
Remarks: Pegler & Saunders (1994) transferred this species to Postia due to “close affinity of this species to the Postia caesia complex”. They emphasize a context “bruising blue” as a key character. When Kotlaba & Pouzar (1989) described Tyromyces wakefieldiae, they cited a letter from D.A. Reid stating that the species sometimes shows blue coloration. Also Wakefield stated, according to Kotlaba and Pouzar, that “the flesh” stained blue when fresh. Ryvarden & Melo (2014) note that the blue discoloration sometimes persists even in dry specimens.
Despite mentioning the blue color, neither Kotlaba & Pouzar (1989) nor Ryvarden & Melo (2014) suggest that the species would belong in the vicinity of P. caesia. Pieri & Rivoire (1998) rejected affinity to P. caesia due to the lack of amyloid reaction and blue spore color. We have seen the type and other material from England without any blue coloration and conclude that P. wakefieldiae does not belong to the P. caesia complex, but is a good member of the genus Postia.
Tyromyces setiger (Cooke) G. Cunn., Bull. N.Z. Dept. Sci. Industr. Res., Pl. Dis. Div.: 763. 1963.
Basionym: Polyporus setiger Cooke, Grevillea 19(89): 1. 1890.
Remarks: Cunningham (1965) mentions that he reported this species previously under Postia caesia, so there is some resemblance. Another Austro-American species, P. atrostrigosa, differs in having wider basidiospores. Ryvarden (1988b) also treated this species under Tyromyces. New collections are needed to settle its proper taxonomic position.
DISCUSSION
Our study has raised the species number in the Postia caesia complex from 10 to 24. The most important contribution of this paper are the rigorous type studies that allowed us to combine existing temperate names to phylogenetic species concepts and previously unaccounted diversity.
Considering that we did not revise tropical or Southern Hemisphere material, the number of species known from Northern Hemisphere more than tripled, from 6 to 20 species. Why this diversity has gone unnoticed or at least undescribed for so long is due to the combination of minute morphological differences and small interspecific differences in the most commonly used genetic marker ITS (in some cases below 1% between species). Obviously, the insight provided by DNA sequences proved fundamental in revising species concepts accurately.
ITS sequences were commonly polymorphic, exhibiting double bases and even length variation. Considering that ITS sequence differences between some species are very low (< 1 %) they may fall within error margins of current mass sequencing methods used in environmental studies. Tef1 sequences are in general more variable between species and in this sense more reliable for molecular identification, but reference sequences are available for much fewer species.
Our sampling and analyses allowed distinguishing 20 species in the temperate Northern Hemisphere. However, even in the best sampled area, Europe, we have indications that still more species are present: Postia cf. subviridis from Finland does not agree well the North American material though their tef1 sequence are identical; two English specimens collected on Acer and Fraxinus from the study of Yao et al. (2005) have distinct ITS sequences. Variation within the wide-spread P. caesiosimulans leaves open the possibility that our concept of the species encompasses more than one taxon (species or subspecies). We can also expect a good number of new species from the Southern Hemisphere, the Himalayas as well as subtropical and tropical areas.
While species differences at the extremes of the morphological variations within the complex are very clear (e.g. P. alni vs. P. auricoma), some species are very difficult to separate, even to the extent that DNA sequences are required for a reliable identification of some individuals. Such examples include P. alni vs. P. caesiosimulans in Europe, P. arbuti vs. P. populi in North America and P. simulans vs. P. yanae in Asia. Young or senescent specimens may also have to be left unnamed even after a careful microscopical examination. However, the great majority of specimens from the Postia caesia complex can be identified without DNA sequences, often in the field.
Most species of the P. caesia complex are restricted geographically to either western or eastern part of Eurasia or to North America, indicating that geographic isolation has probably played a role in speciation. Only three species (P. caesiosimulans, P. populi, P. simulans) and possibly a fourth (P. subviridis) are distributed in both Eurasia and North America. In addition, one further species (P. auricoma) is found in Europe and East Asia. Within North America some species have been recorded only from the east (P. comata) or from the west coast (P. arbuti), but collection intensity is too low to judge whether this is just accidental or a true pattern of distribution. Continental divide and possibly ice ages - we expect that some of the species divisions such as P. alni – P. cyanescens – P. caesiosimulans – P. populi are relatively young - have thus shaped the species diversity.
Host tree, particularly division between angiosperms and gymnosperms, is an important character for identification but also when contemplating speciation patterns in this complex. Six out of 20 north temperate species grow only on angiosperms (P. arbuti, P. alni, P. coeruleivirens, P. magna, P. populi, P. subcaesia), and eight only on gymnosperms (P. auricoma, P. bifaria, P. cyanescens, P. glauca, P. gossypina, P. luteocaesia, P. subviridis, P. yanae). Six species (P. caesia, P. caesiosimulans, P. comata, P. livens, P. mediterraneocaesia, P. simulans) grow both on gymno- and angiosperms, although in the case of P. caesiosimulans the conifer records from North America may represent distinct, possibly differentiating populations.
In a few cases Postia species strongly prefer a particular tree species, at least regionally. Thus Postia cyanescens is almost always found on Picea abies in the North, while Postia populi prefers Populus tremula in Europe. P. luteocaesia have been recorded only from Pinus spp., and P. auricoma only from Pinus sylvestris in Europe.
Although host specialization clearly plays a role in the evolution of the P. caesia complex, it is an open question whether sympatric speciation has significance. We can point to one case where sympatric speciation seems likely: the spruce-inhabiting species P. cyanescens is distributed in Europe, where its closest relatives are P. alni, P. caesiosimulans and P. populi, all angiosperm-inhabiting species. Tef1 sequence places P. cyanescens in the vicinity of P. caesiosimulans, which occurs on conifers in East Asia and North America. In this case host jump as a primary speciation process would appear to be a plausible option.
Despite small ITS differences, overlapping distribution areas and similar ecology we did not observe clear cases of hybridization between species with the exception of a single individual: Collection Spirin 9353 from Nizhny Novgorod produced polymorphic ITS sequence that can be interpreted as having ITS copies from two parental species, P. alni and P. populi. Since the tef1 sequence is a typical P. populi sequence this specimen is not a first generation hybrid. It nevertheless indicates that limited gene flow may be taking place between closely related species.
A number of Postia species have been red-listed or are considered indicators of old-growth forests in Northern Europe. Most species of the P. caesia complex do not seem to be associated with endangered habitats at the continental level. It is worth mentioning that P. auricoma records derive from forests with a continuum of big pine logs, so this species may well be old-growth-forest dependent. Also all the records of P. bifaria derive from virgin or near virgin conifer forests. The number of records is so low for many species that it is premature to assess how common and ecologically specialized they are.
We hope to see other researchers continue where we have left in studying this fascinating, easily recognizable species group. While doing a decent job at delineating species, our study did not fare as well in establishing a robust phylogeny for the Postia caesia complex. For this, we need further genetic markers, whose sequencing will require cultures or recently collected specimens. Fresh collections are really needed since even ITS sequence production in this group is difficult from specimens older than a few years, and other markers are likely to be more difficult to sequence.
Table 1.
Morphological comparison of European members of the Postia caesia complex. Data for P. subviridis are based on European material only, and P. simulans on European and North American material (excluding East Asia).
| Species | Distribution/ host | Upper surface | Pores per mm | Tramal hyphae (measurements include 60 % of variation around median diameter) | Basidiospores (variation of specimen means) | Other | |
|---|---|---|---|---|---|---|---|
| Narrow-spored species (W<1.4 μm) | alni | Europe; temperate / hemiboreal; deciduous trees (but not Populus) | smooth to pubescent, brown when old | 5–6 | slightly to moderately thick-walled, 2.9–3.6 μm (walls 0.2–0.8 μm) | L=4.85-5.3 μm, W=1.16-1.22 μm, Q=3.98-4.34 | |
| caesiosimulans | holarctic; temperate; known from Corylus in Europe | smooth | 5–7 | slightly to distinctly thick-walled, often densely arranged, 2.9–3.8 μm (walls up to 1 μm) | L=4.47-5.14 μm, W=1.14-1.27 μm, Q=3.79-4.05 | Q mostly <4, context hyphae wide | |
| cyanescens | Europe; boreal, temperate to mediterranean mountains; Picea, Abies | smooth to matt | 5–6 | thin- to moderately thick-walled, +-parallel, 2.9–3.7 μm | L=4.89-5.64 μm, W=1.23-1.45 μm, Q=3.38-4.49 | rarely W>1.4 μm, hyphae not collapsing or colored | |
| populi | holarctic; temperate / boreal; almost exclusively Populus | smooth | 5–7 | thick-walled, densely arranged, 2.7–3.3 μm (walls regularly >1 μm) | L=4.58-5.07 μm, W=1.12-1.24 μm, Q=3.86-4.37 | undulating cap margin, Q mostly >4, context hyphae narrow | |
| subcaesia | Europe; temperate; deciduous trees | pubescent | 4–6 | thin- to slightly thick-walled, 3.1–4.1 μm | L=4.29-5.08 μm, W=1.18-1.29 μm, Q=3.61-3.94 | big basidiome, thick context, wide context hyphae (4.2–6.6 μm) | |
| subviridis | Holarctic; temperate / boreal, conifers | smooth | 6–8 | thin- to slightly thick-walled, 2.5–3.0 μm | L=4.92 μm, W=1.10 μm, Q=4.46 | ||
| Wide-spored species (W>1.4 μm) | caesia | Europe; temperate / hemiboreal; prefers conifers | pubescent, often blue | 4–5 | thin- to slightly thick-walled, loosely arranged, 2.8–3.6 μm | L=4.45-4.94 μm, W=1.42-1.64 μm, Q=2.95-3.36 | greenish-bluish when bruised, colored (amyloid) hyphae |
| gossypina | Europe; temperate | smooth to matt | 4–6 | thin- to slightly thick-walled, 2.3–3.0 μm | L=4.41-4.52 μm, W=1.41-1.46 μm, Q=3.1-3.13 | ||
| mediterraneocaesia | Europe; warm temperate - mediterranean; various hosts | smooth | 5–6 | thick-walled, twisted, 2.3–3.2 μm | L=4.45-5.47 μm, W=1.43-1.55 μm, Q=3.11-3.53 | dry habitat, thin branches, basidiocarps pendant | |
| simulans | holarctic; temperate / boreal; preferrably conifers | smooth to pubescent, often blue | 5–7 | thin- to slightly thick-walled, collapsing, 2.8–3.8 μm | L=4.89-5.93 μm, W=1.44-1.55 μm, Q=3.17-4.12 | ||
| Yellow species | auricoma | Eurasia; boreal / temperate; Larix and Pinus | matt to pubescent, sometimes blue | 4–6 | slightly to distinctly thick-walled, loosely arranged, 3.1–4.1 μm | L=5.03-5.05 μm, W=1.62-1.69 μm, Q=2.98-3.12 | deep green when bruised |
| luteocaesia | Europe; mediterranean, Pinus | matt to pubescent | 3–5 | thin- to moderately thick-walled, 2.7–3.2 μm | L=4.89-5.26 μm, W=1.65-1.7 μm, Q=2.96-3.13 | deep green when bruised |
Table 2.
Morphological comparison of East Asian members of the Postia caesia complex. Description of P. simulans is based on East Asian material only.
| Species | Distribution/ host | Upper surface | Pores per mm | Tramal hyphae (measurements include 60 % of variation around median diameter) | Basidiospores (variation of specimen means) | Other | |
|---|---|---|---|---|---|---|---|
| Narrow-spored species (W<1.3 μm) | bifaria | East Asia; cold temperate; conifers | pubescent | 6–8 | thin- to slightly thick-walled, collapsing, 2.5–3.8 μm | L=4.09-4.1 μm, W=1.11-1.16 μm, Q=3.53-3.69 | |
| caesiosimulans | holarctic; temperate; Abies in East Asia, various hosts elsewhere | smooth | 5–7 | slightly to distinctly thick-walled, often densely arranged, 2.9–3.8 μm (walls up to 1 μm) | L=4.47-5.14 μm, W=1.14-1.27 μm, Q=3.79-4.05 | Q mostly <4 | |
| coeruleivirens | East Asia; warm temperate - tropical; deciduous trees | pubescent | 6–8 | thin- to moderately thick-walled, 2.4–3.4 μm (walls up to 0.8 μm) | L=3.69-4.32 μm, W=1-1.16 μm, Q=3.59-3.72 | ||
| glauca | East Asia; cold temperate; conifers | smooth to pubescent | 5–8 | thin- to slightly thick-walled, loosely arranged, 2.6–3.3 μm | L=4.45-4.9 μm, W=1.27-1.28 μm, Q=3.48-3.86 | colored (amyloid) hyphae | |
| magna | East Asia; temperate; deciduous trees | pubescent | 4–5 | slightly thick-walled, 2.2–3.3 μm | L=3.97 μm, W=1.13 μm, Q=3.51 | big basidiomes, thick context, wide context hyphae (4.2–6.0 μm) | |
| populi | holarctic; temperate / boreal; deciduous trees, prefers Populus | smooth | 5–7 | thick-walled, densely arranged, 2.7–3.3 μm (walls regularly >1 μm) | L=4.58-5.07 μm, W=1.12-1.24 μm, Q=3.86-4.37 | undulating cap margin, Q mostly >4 | |
| Wide-spored species (W>1.3 μm) | simulans | holarctic; temperate / boreal; prefers conifers | smooth, brown when old | 5–7 | slightly to distinctly thick-walled, 2.7–3.2 μm (walls 0.2-1 μm thick) | L=5.17-5.65 μm, W=1.34-1.53 μm, Q=3.69-4 | spores often slightly fusiform |
| yanae | Siberia; boreal; Larix and Pinus | smooth, white to ochraceous | 5–7 | thin- to slightly thick-walled, winding, collapsing, 2.2–2.9 μm | L=4.74-5.36 μm, W=1.35-1.45 μm, Q=3.39-3.81 | small basidiomes on branches, dry habitat | |
| Yellow species | auricoma | Eurasia; boreal / temperate; Larix and Pinus | matt to pubescent, sometimes blue | 4–6 | slightly to distinctly thick-walled, loosely arranged, 3.1–4.1 μm | L=5.03-5.05 μm, W=1.62-1.69 μm, Q=2.98-3.12 | deep green when bruised |
Table 3.
Morphological comparison of North American members of the Postia caesia complex. Description of P. simulans is based on European and North American material (excluding East Asia).
| Species | Distribution/ host | Upper surface | Pores per mm | Tramal hyphae (measurements include 60 % of variation around median diameter) | Basidiospores (variation of specimen means) | Other | |
|---|---|---|---|---|---|---|---|
| \ | arbuti | North America (NW); temperate; Arbutus | smooth | 6–8 | thick-walled, densely arranged, 2.4–3.1 μm (walls up to 1 μm) | L=4.54-4.58 μm, W=1.13-1.15 μm, Q=3.95-4.05 | |
| caesiosimulans | holarctic; temperate; Fagus, deciduous trees, rarely conifers | smooth | 5–7 | slightly to distinctly thick-walled, often densely arranged, 2.9–3.8 μm (walls up to 1 μm) | L=4.47-5.14 μm, W=1.14-1.27 μm, Q=3.79-4.05 | Q mostly <4; few western conifer specimens W>1.4 μm; context usually thin | |
| comata | North America (NE); temperate; conifers | pubescent | 4–6 | thick-walled, 2.8–3.8 μm (walls up to 1 μm) | L=4.34-4.39 μm, W=1.2-1.21 μm, Q=3.59-3.66 | ||
| livens | North America; temperate; conifers and deciduous trees | matt to pubescent | 4–6 | thin- to moderately thick-walled, 2.9–4.0 μm (walls up to 0.5–0.8 μm) | L=4.30-5.25 μm, W=1.2-1.37 μm, Q=3.31-4.17 | thick context, often big basidiomes | |
| populi | holarctic; temperate / boreal; deciduous trees, prefers Populus | smooth | 5–7 | thick-walled, densely arranged, 2.7–3.3 μm (walls regularly >1 μm) | L=4.58-5.07 μm, W=1.12-1.24 μm, Q=3.86-4.37 | undulating cap margin, Q mostly >4 | |
| subviridis | North America (W); temperate; conifers | smooth | 6–8 | thick-walled, 2.5–3.1 μm (walls up to 1 μm) | L=4.07-4.92 μm, W=1.1-1.16 μm, Q=3.51-4.47 | ||
| Wide-spored species(W≥1.4 μm) | simulans | holarctic; temperate / boreal; prefers conifers | smooth to pubescent, often blue | 5–7 | thin- to slightly thick-walled, rarely thick-walled, often collapsing, (2.2)2.8–3.8 μm | L=4.87-5.93 μm, W=1.4-1.55 μm, Q=3.17-4.12 |
ACKNOWLEDGEMENTS
We are indebted to Frank Dämmrich, Ellen Larsson, Karl-Henrik Larsson, Tuomo Niemelä, Beatriz Ortiz-Santana, Viktor Papp, Dmitry Schigel, Gerhard Schuster and Petr Vampola for valuable specimens and sequences. Tuomo Niemelä also commented the manuscript. Sampsa Lommi prepared the distribution maps. Alix David provided valuable ideas for species delimitation in this complex. We are ever grateful for Yu-Cheng Dai and his students for arranging a collecting trip in China resulting in valuable collections. Part of the sequences were produced by the Finnish Barcode of Life (FinBOL) initiative. CSC – IT Center for Science (Espoo, Finland) provided computational resources. This research was made possible by the PolyPEET project led by David Hibbett (NSF grant DEB0933081) and the European Commission Marie Curie grant PIOF-GA-2011–302349.
Supplement 1 - INSDC accession numbers
INSDC accession numbers for DNA sequences used in this study. Specimens provided with collector and collection number information have been sequenced for this study, the rest retrieved from the INSDC database.
| Species | Collector, coll. no. (herbarium) | Country (province/state) | ITS | LSU | TEF1 |
|---|---|---|---|---|---|
| Postia alni | Viktor Papp 188 (BP 106943) | Hungary | MG137038 | ||
| Postia alni | (K 41769) | United Kingdom | AY599577 | ||
| Postia alni | FP-135373-Sp (CFMR) | United Kingdom | KC585375 | ||
| Postia alni | Petr Vampola 12.10.1995 (H 7019137), isotype | Slovakia | MG137026 | ||
| Postia alni | Otto Miettinen 15830 (H 6013476) | Finland | MG137030 | ||
| Postia alni | Tuomo Niemelä 8933 (H) | Poland | MG137032 | MG137128 | |
| Postia alni | Viacheslav Spirin 4602 (H) | Russia (LEN) | MG137036 | MG137131 | |
| Postia alni | Viacheslav Spirin 2548 (H) | Russia (NIZ) | MG137035 | MG137130 | |
| Postia alni | Tuomo Niemelä 9233 (H) | Finland | MG137033 | MG137129 | |
| Postia alni | Otto Miettinen 14918,2 (H 6013089) | Finland | MG137029 | ||
| Postia alni | P. Vampola 16.9.2012 (MJ 17/12) | Czech Republic | MG137031 | MG137127 | |
| Postia alni | Vampola 12.10.2010 (MJ 27/10) | Czech Republic | MG137034 | ||
| Postia alni | Karl-Henrik Larsson 18.9.2014 (O F-248173) | Norway | MG137028 | ||
| Postia alni | Björn Nordén 1.9.2016 (H) | Norway | MG137027 | MG137126 | |
| Postia alni | Viacheslav Spirin 9502 (H) | Russia (NIZ) | MG137037 | ||
| Postia arbuti | Viacheslav Spirin 8327 (H 7008651), holotype | United States (WA) | MG137039 | MG137132 | |
| Postia auricoma | Tuomo Niemelä 8310 (H 6014002), holotype | Finland | MG137040 | ||
| Postia auricoma | Viacheslav Spirin 4586 (H) | Russia (LEN) | MG137042 | ||
| Postia auricoma | Heikki Kotiranta 17047 (H) | Russia (KDA) | MG137041 | ||
| Postia bifaria | Viacheslav Spirin 4850 (H) | Russia (KHA) | MG137044 | ||
| Postia bifaria | Viacheslav Spirin 6402 (H 7008646), holotype | Russia (PRI) | MG137043 | MG137133 | |
| Postia caesia | Viktor Papp 1107 (BP 106944) | Hungary | MG137052 | ||
| Postia caesia | Petr Vampola, Exsicc. Cech. #121 (H 7034977) | Czech Republic | MG137046 | ||
| Postia caesia | Otto Miettinen 14156,2 (H) | Finland | MG137048 | MG137134 | |
| Postia caesia | Otto Miettinen 13610 (H) | Finland | KC595935 | KC595935 | |
| Postia caesia | Otto Miettinen 14133 (H) | United Kingdom | MG137047 | ||
| Postia caesia | Gerhard Schuster 51 (LY BR-6776), neotype | Germany | MG137045 | ||
| Postia caesia | Tuomo Niemelä 9086 (H) | Finland | MG137050 | MG137136 | |
| Postia caesia | Viacheslav Spirin 9787 (H) | Russia (NIZ) | MG137051 | ||
| Postia caesia | Otto Miettinen 19424 (H) | Finland | MG137049 | MG137135 | |
| Postia caesia aff | G. Gates & D. Ratkowsky 31.5.2003 (H 7036111) | Australia | MG137053 | ||
| Postia caesia aff AR | CIEFAP 350 | Argentina (CH) | JX090110 | JX090130 | |
| Postia caesia aff AR | CIEFAP 174 | Argentina (CH) | JX090109 | JX090129 | |
| Postia caesia aff GB | (K 32713) | United Kingdom | AY599576 | ||
| Postia caesia aff GB | (K 32425) | United Kingdom | AY599575 | ||
| Postia caesia aff NZ | (PDD 95774) | New Zealand | HQ533007 | ||
| Postia caesiosimulans | HHB-14891 (CFMR) | United States (WA) | KC585376 | ||
| Postia caesiosimulans | Daniel Lindner DLL2010-136 (CFMR) | United States (MN) | JQ673078 | ||
| Postia caesiosimulans | Daniel Lindner DLL2010-111 (CFMR) | United States (MN) | JQ673077 | ||
| Postia caesiosimulans | Daniel Lindner DLL2010-106 (CFMR) | United States (MN) | JQ673076 | ||
| Postia caesiosimulans | Daniel Lindner DLL2010-081 (CFMR) | United States (MN) | JQ673073 | ||
| Postia caesiosimulans | Daniel Lindner DLL2010-062 (CFMR) | United States (MN) | JQ673074 | ||
| Postia caesiosimulans | Viacheslav Spirin 2610 (H) | Russia (NIZ) | MG137059 | MG137139 | |
| Postia caesiosimulans | Viacheslav Spirin 8717 (H) | United States (WA) | MG137062 | MG137141 | |
| Postia caesiosimulans | Otto Miettinen 17075 (H) | United States (NY) | MG137056 | ||
| Postia caesiosimulans | Viacheslav Spirin 4125 (H) | Russia (KHA) | MG137060 | ||
| Postia caesiosimulans | Viacheslav Spirin 4199 (H) | Russia (KHA) | MG137061 | MG137140 | |
| Postia caesiosimulans | Otto Miettinen 18665 (H) | United States (WA) | MG137058 | MG137138 | |
| Postia caesiosimulans | Otto Miettinen 16976,1 (H 7008645), epitype | United States (NY) | MG137054 | MG137137 | |
| Postia caesiosimulans | Dmitry Schigel 4798 (H) | Finland | MG137055 | ||
| Postia caesiosimulans | Otto Miettinen 18663 (H) | United States (WA) | MG137057 | ||
| Postia coeruleivirens | Dai 11834 | China (ZJ) | KF699119 | ||
| Postia coeruleivirens | Otto Miettinen 12214 (H) | Indonesia (BA) | MG137063 | ||
| Postia coeruleivirens | Viacheslav Spirin 4245 (H) | Russia (KHA) | MG137064 | ||
| Postia coeruleivirens | Viacheslav Spirin 5301 (H) | Russia (KHA) | MG137065 | ||
| Postia comata | Otto Miettinen 14755,1 (H 7005691), holotype | United States (MA) | MG137066 | ||
| Postia cyanescens | (K 56144) | Norway | AY599579 | ||
| Postia cyanescens | Juha Kinnunen 5087 (H) | Poland | MG137068 | ||
| Postia cyanescens | Mari Niemi 183b (H), strain FBCC 2025 | Finland | MG137069 | ||
| Postia cyanescens | Otto Miettinen 14425 (H), strain FBCC 2063 | Finland | MG137070 | MG137143 | |
| Postia cyanescens | Pertti Salo, Ulla Nummela-Salo 9196 (H 6002543) | Finland | MG137073 | ||
| Postia cyanescens | Reijo Penttilä 14560 (H 6032360) | Finland | MG137074 | ||
| Postia cyanescens | Otto Miettinen 15989 (H 7008640) | Spain | MG137072 | ||
| Postia cyanescens | Otto Miettinen 15919,2 (H) | Spain | MG137071 | MG137144 | |
| Postia cyanescens | Tuomo Niemelä 8998 (H 6034674) | Finland | MG137076 | ||
| Postia cyanescens | Tuomo Niemelä 8844 (H) | Finland | MG137075 | ||
| Postia cyanescens | Viacheslav Spirin 2756 (H) | Russia (LEN) | MG137077 | ||
| Postia cyanescens | Otto Miettinen 13602 (H 6014001), holotype | Finland | MG137067 | MG137142 | |
| Postia glauca | Otto Miettinen 10567 (H) | China (JL) | MG137079 | ||
| Postia glauca | Viacheslav Spirin 5317 (H 7008648), holotype | Russia (KHA) | MG137078 | ||
| Postia glauca | Viacheslav Spirin 6580 (H) | Russia (KHA) | MG137081 | MG137145 | |
| Postia glauca | Viacheslav Spirin 5577 (H) | Russia (KHA) | MG137080 | ||
| Postia gossypina | Bernard Rivoire 6658 (LY) | France | MG137146 | ||
| Postia livens | CFMR DLL2011-237 () | United States (WI) | KJ140724 | ||
| Postia livens | CFMR DLL2011-158 () | United States (WI) | KJ140657 | ||
| Postia livens | CFMR DLL2011-055 () | United States (WI) | KJ140576 | ||
| Postia livens | Otto Miettinen 14828 (H) | United States (MA) | MG137085 | ||
| Postia livens | Otto Miettinen 14775 (H) | United States (MA) | MG137084 | ||
| Postia livens | Otto Miettinen 16056 (H) | United States (MA) | MG137086 | MG137148 | |
| Postia livens | Otto Miettinen 19439 (H) | United States (NC) | MG137088 | ||
| Postia livens | Viacheslav Spirin 8728 (H) | United States (WA) | MG137090 | MG137150 | |
| Postia livens | Otto Miettinen 17177 (H 7008642), holotype | United States (NY) | MG137082 | MG137147 | |
| Postia livens | Otto Miettinen 19666,2 (H) | United States (TN) | MG137089 | ||
| Postia livens | Otto Miettinen 16714 (H) | United States (MN) | MG137087 | MG137149 | |
| Postia livens | Josef Vlasák 1009/57 (JV) | United States (PA) | MG137083 | ||
| Postia luteocaesia | Bernard Rivoire 2605 (LY) | France | MG137091 | ||
| Postia magna | KUC20130808-39 | South Korea | KJ668471 | ||
| Postia magna | KA12-1375 | South Korea | KR673585 | ||
| Postia magna | Yu-Cheng Dai 10854 | China (HI) | KF699117 | ||
| Postia magna | Bao-Kai Cui 10094 | China (JL) | KF699116 | ||
| Postia magna | Otto Miettinen 10634 (H 7008643), holotype | China (JL) | KC595944 | KC595944 | MG137151 |
| Postia mediterraneocaesia | Bernard Rivoire 2083 (LY) | Spain | MG137152 | ||
| Postia populi | (K 56143) | Norway | AY599578 | ||
| Postia populi | Daniel Lindner DLL2010-074 (CFMR) | United States (MN) | JQ673071 | ||
| Postia populi | Daniel Lindner DLL2010-047 (CFMR) | United States (MN) | JQ673072 | ||
| Postia populi | Daniel Lindner DLL2010-043 (CFMR) | United States (MN) | JQ673070 | ||
| Postia populi | KUC20131001-22 | South Korea | KJ668469 | ||
| Postia populi | Juha Kinnunen 4938 (H) | Poland | MG137093 | ||
| Postia populi | Otto Miettinen 14211 (H) | Finland | KC595931 | KC595931 | |
| Postia populi | Mari E. Niemi 2008 (H), strain FBCC 2022 | Finland | KC595932 | KC595932 | |
| Postia populi | Tuomo Niemelä 8379 (H 6007874) | Finland | MG137097 | MG137154 | |
| Postia populi | Otto Miettinen 14790,3 (H) | United States (MA) | MG137095 | ||
| Postia populi | Otto Miettinen 15827,2 (H 6013475) | Finland | MG137096 | ||
| Postia populi | Viacheslav Spirin 6598 (H) | Russia (KHA) | MG137101 | MG137156 | |
| Postia populi | Viacheslav Spirin 4194 (H) | Russia (KHA) | MG137098 | ||
| Postia populi | Viacheslav Spirin 4587 (H) | Russia (LEN) | MG137099 | MG137155 | |
| Postia populi | Viacheslav Spirin 5771 (H) | Russia (KHA) | MG137100 | ||
| Postia populi | Viacheslav Spirin 9353 (H) | Russia (NIZ) | MG137102 | MG137157 | |
| Postia populi | Otto Miettinen 14701 (H 6012900) | Finland | MG137094 | ||
| Postia populi | Otto Miettinen 17043 (H 7008644), holotype | United States (NY) | MG137092 | MG137153 | |
| Postia simulans | (UBC F19741) | Canada (BC) | HQ604800 | ||
| Postia simulans | Mari E. Niemi 183b (H 6032361) | Finland | MG137104 | ||
| Postia simulans | Otto Miettinen 20422 (H) | Finland | MG137110 | MG137160 | |
| Postia simulans | Viacheslav Spirin 6328 (H 7008649) | Russia (PRI) | MG137113 | MG137161 | |
| Postia simulans | Tuomo Niemelä 8846 (H 6034704), epitype | Finland | MG137103 | ||
| Postia simulans | Viacheslav Spirin 4271 (H) | Russia (KHA) | MG137111 | ||
| Postia simulans | Viacheslav Spirin 4386 (H) | Russia (KHA) | MG137112 | ||
| Postia simulans | Viacheslav Spirin 8587 (H 7008650) | United States (WA) | MG137115 | MG137163 | |
| Postia simulans | Otto Miettinen 18882 (H) | United States (ID) | MG137108 | ||
| Postia simulans | Otto Miettinen 18735 (H) | United States (ID) | MG137107 | MG137159 | |
| Postia simulans | Otto Miettinen 19612 (H) | United States (NC) | MG137109 | ||
| Postia simulans | Otto Miettinen 14167,2 (H) | Finland | MG137106 | MG137158 | |
| Postia simulans | Viacheslav Spirin 8542 (H) | United States (WA) | MG137114 | MG137162 | |
| Postia simulans | Mette Ursin 12.9.2014 (O F-75832) | Norway | MG137105 | ||
| Postia subcaesia | Viktor Papp 43 (BP 106945) | Hungary | MG137119 | ||
| Postia subcaesia | (K 32115) | United Kingdom | AY599574 | ||
| Postia subcaesia | (K 31711) | United Kingdom | AY599573 | ||
| Postia subcaesia | (K 32116) | United Kingdom | AY599571 | ||
| Postia subcaesia | (K 24318) | United Kingdom | AY599570 | ||
| Postia subcaesia | (K 17744) | United Kingdom | AY599569 | ||
| Postia subcaesia | (K 31967) | United Kingdom | AY599568 | ||
| Postia subcaesia | (K 31967) | United Kingdom | AY599567 | ||
| Postia subcaesia | Alix David 652 (H 7034976), isotype | France | MG137116 | ||
| Postia subcaesia | Viacheslav Spirin 6083 (H) | Russia (NIZ) | MG137118 | ||
| Postia subcaesia | Josef Vlasák 0110/24 (JV) | Czech Republic | MG137117 | MG137164 | |
| Postia subviridis | Viacheslav Spirin 8774a (H) | United States (WA) | MG137120 | MG137166 | |
| Postia subviridis | Reijo Penttilä 14376 (H) | Finland | MG137165 | ||
| Postia yanae | Heikki Kotiranta 27454 (H 7034942), holotype | Russia (SA) | MG137121 | MG137167 | |
| Postia yanae | Heikki Kotiranta 27677 (H) | Russia (SA) | MG137123 | ||
| Postia yanae | Heikki Kotiranta 27606 (H) | Russia (SA) | MG137122 | MG137168 | |
| Postia yanae | Heikki Kotiranta 27772 (H 7036326) | Russia (SA) | MG137124 | MG137169 | |
| Postia yanae | Heikki Kotiranta 27879 (H 7036282) | Russia (SA) | MG137125 |
Supplement 2 - Spore measurements in the Postia caesia complex
Bold-face values represent composite statistics for species. L = average of spore length, W = average of spore width, Q = L/W, and n = number of spores measured. The whole range is given in parentheses; 90% range excluding 5% extreme values from both ends of variation is given without parentheses; in case the values are identical, parentheses are omitted. Specimens marked with asterisk (*) have been excluded from the combined statistics for that species.
| species/specimen | Length | L | Width | W | Q | n |
|---|---|---|---|---|---|---|
| Postia alni | (4.1–)4.3–6.1(–6.8) | 5.05 | (1.0–)1.1–1.3(–1.5) | 1.20 | 4.22 | 192/5 |
| holotype | (4.3–)4.6–6.2(–6.8) | 5.30 | (1.0–)1.1–1.4 | 1.22 | 4.35 | 72 |
| Miettinen 15830 | (4.1–)4.2–6.0(–6.8) | 4.88 | (1.0–)1.1–1.3 | 1.19 | 4.10 | 30 |
| Spirin 2548 | (4.2–)4.3–5.5(–6.1) | 4.85 | (1.0–)1.1–1.4(–1.5) | 1.22 | 3.98 | 30 |
| Spirin 3640 | 4.2–5.7(–5.8) | 4.91 | 1.0–1.2(–1.3) | 1.16 | 4.24 | 30 |
| Vampola 27/10 | (4.3–)4.5–6.2(–6.3) | 5.00 | 1.0–1.3 | 1.18 | 4.22 | 30 |
| Postia arbuti | (4.0–)4.1–5.1(–5.2) | 4.56 | 1.0–1.2(–1.3) | 1.14 | 4.00 | 70/2 |
| holotype | 4.1–5.1(–5.2) | 4.54 | 1.0–1.2(–1.3) | 1.15 | 3.96 | 40 |
| Spirin 8318 | (4.0–)4.1–5.1(–5.2) | 4.58 | 1.0–1.2(–1.3) | 1.13 | 4.06 | 30 |
| Postia auricoma | (4.2–)4.4–5.6(–6.0) | 5.04 | (1.4–)1.5–1.8(–2.0) | 1.65 | 3.06 | 72/2 |
| holotype | (4.2–)4.4–5.6(–6.0) | 5.05 | (1.4–)1.5–1.8(–1.9) | 1.62 | 3.11 | 42 |
| Spirin 4586 | (4.4–)4.7–5.6(–5.8) | 5.03 | 1.5–1.8(–2.0) | 1.69 | 2.98 | 30 |
| Postia bifaria | (3.6–)3.7–4.4(–5.2) | 4.10 | 1.0–1.2(–1.3) | 1.14 | 3.60 | 70/2 |
| holotype | (3.6–)3.7–4.4(–5.2) | 4.09 | 1.0–1.2(–1.3) | 1.16 | 3.54 | 40 |
| Spirin 4850 | (3.7–)3.8–4.3(–5.0) | 4.10 | 1.0–1.2 | 1.11 | 3.70 | 30 |
| Postia caesia | (3.9–)4.1–5.3(–6.0) | 4.64 | (1.2–)1.3–1.7(–1.9) | 1.48 | 3.13 | 297/10 |
| neotype | (4.0–)4.1–5.2 | 4.46 | 1.3–1.7 | 1.45 | 3.08 | 30 |
| Miettinen 14133 | (4.1–)4.2–5.6(–6.0) | 4.74 | (1.2–)1.3–1.7(–1.8) | 1.49 | 3.19 | 30 |
| Miettinen 14146.2 | (4.1–)4.2–5.2 | 4.45 | (1.2–)1.3–1.7 | 1.43 | 3.11 | 30 |
| Spirin 1991 | (3.9–)4.0–5.7(–5.9) | 4.94 | 1.3–1.8(–1.9) | 1.64 | 3.02 | 30 |
| Spirin 2075 | 4.0–5.2 | 4.58 | 1.3–1.8(–1.9) | 1.54 | 2.97 | 30 |
| Spirin 2102 | (4.0–)4.1–5.3(–5.5) | 4.77 | 1.2–1.7 | 1.42 | 3.36 | 30 |
| Spirin 2157 | 4.1–5.2(–5.4) | 4.73 | 1.3–1.7 | 1.45 | 3.27 | 27 |
| Spirin 3529 | (3.9–)4.0–5.2(–5.3) | 4.48 | 1.3–1.7(–1.8) | 1.52 | 2.95 | 30 |
| Spirin 9787 | (4.2–)4.3–5.2(–5.3) | 4.74 | 1.3–1.7(–1.9) | 1.44 | 3.30 | 30 |
| Vampola 7.VIII.1993 | 4.2–4.9(–5.1) | 4.55 | 1.3–1.7 | 1.46 | 3.11 | 30 |
| Postia caesiosimulans | (4.1–)4.2–5.5(–7.0) | 4.80 | (1.0–)1.1–1.4(–1.6) | 1.22 | 3.93 | 190/6 |
| epitype | (4.1–)4.2–5.3(–5.8) | 4.77 | 1.1–1.3 | 1.22 | 3.92 | 40 |
| holotype | (4.1–)4.2–5.0(–5.1) | 4.47 | 1.0–1.2(–1.3) | 1.14 | 3.93 | 30 |
| Miettinen 17075 | (4.3–)4.4–6.2(–7.0) | 5.14 | (1.1–)1.2–1.5(–1.6) | 1.27 | 4.06 | 30 |
| Miettinen 18663* | 4.6–5.3 | 5.02 | 1.3–1.5 | 1.41 | 3.56 | 10 |
| Miettinen 18665* | 4.2–5.2 | 4.67 | 1.4–1.7 | 1.51 | 3.09 | 30 |
| Spirin 2610 | 4.2–5.2(–5.6) | 4.69 | 1.1–1.3(–1.4) | 1.19 | 3.94 | 30 |
| Spirin 4199 | 4.3–5.0(–5.2) | 4.70 | 1.1–1.3(–1.5) | 1.24 | 3.80 | 30 |
| Spirin 8717 | (4.3–)4.4–5.5(–6.1) | 5.02 | (1.1–)1.2–1.5(–1.6) | 1.27 | 3.95 | 30 |
| Postia coeruleivirens | (3.6–)3.8–4.8(–5.2) | 4.23 | 1.0–1.3 | 1.16 | 3.64 | 72/2 |
| Miettinen 12214 | (3.3–)3.4–4.1 | 3.69 | 0.9–1.1(–1.2) | 1.00 | 3.68 | 30 |
| Spirin 4252 | 3.9–4.9(–5.0) | 4.32 | 1.0–1.3 | 1.16 | 3.74 | 30 |
| Spirin 5301 | (3.6–)3.7–4.7(–5.2) | 4.16 | 1.0–1.2(–1.3) | 1.16 | 3.57 | 42 |
| Postia comata | (3.8–)4.1–4.9(–5.1) | 4.36 | 1.1–1.3 | 1.21 | 3.62 | 70/2 |
| holotype | (3.8–)4.1–4.8(–5.1) | 4.34 | 1.1–1.3 | 1.21 | 3.59 | 40 |
| Miettinen 17180 | 4.0–4.9(–5.1) | 4.39 | 1.1–1.3 | 1.20 | 3.66 | 30 |
| Postia cyanescens | (4.2–)4.7–6.1(–6.8) | 5.22 | (1.0–)1.1–1.6(–1.9) | 1.33 | 3.92 | 296/9 |
| holotype | (4.8–)4.9–5.5(–5.6) | 5.21 | 1.3–1.6(–1.7) | 1.42 | 3.67 | 30 |
| Kinnunen 5087 | (4.8–)5.1–6.1(–6.2) | 5.43 | (1.1–)1.2–1.6 | 1.29 | 4.20 | 30 |
| Miettinen 15919.2 | (4.2–)4.3–6.2(–6.3) | 5.17 | 1.2–1.7(–1.8) | 1.43 | 3.61 | 30 |
| Miettinen 15989 | (4.2–)4.3–5.3(–6.0) | 4.89 | 1.2–1.8(–1.9) | 1.45 | 3.38 | 46 |
| Niemelä 8844 | 4.8–5.6(–5.8) | 5.10 | (1.1–)1.2–1.5(–1.6) | 1.28 | 3.98 | 40 |
| Penttilä 14560 | 4.7–6.2(–6.7) | 5.15 | (1.1–)1.2–1.6 | 1.30 | 3.97 | 30 |
| Salo 9196 | (4.8–)5.1–6.2(–6.3) | 5.41 | (1.1–)1.2–1.5(–1.6) | 1.28 | 4.23 | 30 |
| Spirin 211334 | (4.5–)4.6–6.0 | 5.21 | 1.0–1.5 | 1.23 | 4.23 | 30 |
| Spirin 2756 | (4.7–)5.1–6.2(–6.8) | 5.64 | 1.1–1.5 | 1.26 | 4.49 | 30 |
| Postia glauca | (4.0–)4.1–5.4(–6.2) | 4.64 | 1.1–1.5(–1.6) | 1.27 | 3.64 | 72/2 |
| Miettinen 10567 | (4.1–)4.2–5.4(–6.2) | 4.90 | 1.1–1.5 | 1.27 | 3.87 | 30 |
| Spirin 5317 | (4.0–)4.1–4.9(–5.8) | 4.45 | 1.1–1.5(–1.6) | 1.28 | 3.47 | 42 |
| Postia gossypina | (4.0–)4.1–5.1(–5.2) | 4.47 | 1.2–1.7 | 1.44 | 3.11 | 66/2 |
| isotype | (4.0–)4.1–5.2 | 4.52 | 1.2–1.7 | 1.46 | 3.09 | 32 |
| Rivoire 6658 | (4.0–)4.1–5.0 | 4.41 | 1.2–1.7 | 1.41 | 3.12 | 34 |
| Postia livens | 4.1–5.7(–7.0) | 4.78 | 1.1–1.5(–1.7) | 1.28 | 3.74 | 132/5 |
| holotype | (4.1–)4.2–5.2(–5.4) | 4.69 | (1.1–)1.2–1.6(–1.7) | 1.37 | 3.43 | 40 |
| Miettinen 14775 | (4.2–)4.3–5.6(–6.1) | 4.88 | 1.1–1.3 | 1.20 | 4.06 | 30 |
| Miettinen 14828 | 4.1–4.5 | 4.30 | 1.3–1.3 | 1.30 | 3.31 | 2 |
| Miettinen 16056 | 4.1–5.2(–5.3) | 4.35 | 1.1–1.4(–1.6) | 1.24 | 3.52 | 30 |
| Vlasák 1009/57 | (4.3–)4.4–5.9(–7.0) | 5.25 | 1.2–1.4(–1.5) | 1.26 | 4.15 | 30 |
| Postia luteocaesia | (4.2–)4.3–6.1(–7.1) | 5.06 | (1.4–)1.5–1.9(–2.0) | 1.68 | 3.02 | 101/3 |
| holotype | 4.2–5.8(–6.1) | 4.89 | (1.4–)1.5–1.8(–1.9) | 1.65 | 2.96 | 41 |
| Rivoire 2605 | 4.3–6.1(–7.1) | 5.08 | (1.4–)1.5–1.9(–2.0) | 1.70 | 3.00 | 30 |
| Rivoire 733 | (4.4–)4.6–6.2(–6.6) | 5.26 | (1.4–)1.5–2.0 | 1.68 | 3.12 | 30 |
| Postia magna | 3.6–4.4(–4.5) | 3.97 | 1.0–1.2 | 1.13 | 3.51 | 30 |
| Postia mediterraneocaesia | (3.9–)4.2–5.8(–6.3) | 4.83 | (1.2–)1.3–1.7(–1.9) | 1.48 | 3.26 | 134/4 |
| isotype | (3.9–)4.0–5.1(–5.3) | 4.45 | (1.2–)1.3–1.6(–1.8) | 1.43 | 3.12 | 40 |
| Rivoire 1356 | (4.1–)4.2–5.4(–6.1) | 4.78 | (1.2–)1.3–1.7(–1.8) | 1.46 | 3.27 | 30 |
| Rivoire 1903 | 4.2–5.4(–5.8) | 4.76 | 1.3–1.7(–1.8) | 1.50 | 3.16 | 34 |
| Rivoire 2083 | (4.8–)5.0–6.2(–6.3) | 5.47 | 1.3–1.8(–1.9) | 1.55 | 3.52 | 30 |
| Postia populi | (4.0–)4.2–5.6(–6.1) | 4.84 | 1.0–1.3(–1.6) | 1.17 | 4.14 | 245/8 |
| holotype | (4.0–)4.1–5.3(–5.8) | 4.58 | 1.1–1.3(–1.4) | 1.18 | 3.89 | 30 |
| Kinnunen 4938 | (4.1–)4.2–5.1 | 4.73 | 1.0–1.2 | 1.12 | 4.24 | 30 |
| Miettinen 14211 | (4.1–)4.3–5.2(–5.3) | 4.85 | 1.0–1.2(–1.3) | 1.15 | 4.23 | 30 |
| Miettinen 15827.2 | 4.4–5.3 | 4.89 | 1.0–1.2(–1.3) | 1.13 | 4.32 | 35 |
| Spirin 3194 | 4.3–6.1 | 5.07 | 1.0–1.3(–1.4) | 1.16 | 4.37 | 30 |
| Spirin 3224 | 4.3–5.8(–6.1) | 4.99 | 1.0–1.4(–1.5) | 1.18 | 4.23 | 30 |
| Spirin 4587 | 4.2–5.4(–6.0) | 4.79 | (1.0–)1.1–1.4(–1.6) | 1.24 | 3.87 | 30 |
| Spirin 5771 | 4.1–6.0(–6.1) | 4.81 | 1.1–1.4(–1.5) | 1.21 | 3.98 | 30 |
| Postia simulans | (4.1–)4.4–6.3(–8.1) | 5.24 | 1.2–1.8(–1.9) | 1.46 | 3.60 | 403/12 |
| holotype | 4.3–5.7(–6.8) | 4.92 | 1.3–1.8(–1.9) | 1.55 | 3.17 | 40 |
| epitype | 4.5–5.2(–5.7) | 4.95 | 1.3–1.7(–1.9) | 1.50 | 3.29 | 40 |
| Alava 19067 | (4.1–)4.3–5.6 | 4.89 | (1.2–)1.3–1.8 | 1.52 | 3.21 | 30 |
| Miettinen 20422 | 4.8–6.8(–7.1) | 5.70 | 1.3–1.8 | 1.54 | 3.71 | 30 |
| Niemi 183b | (4.8–)4.9–6.3(–6.5) | 5.42 | (1.2–)1.3–1.8 | 1.51 | 3.59 | 30 |
| Rivoire 2936 | (4.7–)4.8–7.1(–7.3) | 5.93 | (1.2–)1.3–1.7 | 1.44 | 4.12 | 30 |
| Spirin 4217 | (4.3–)4.8–7.3(–8.1) | 5.65 | (1.2–)1.3–1.8(–1.9) | 1.53 | 3.70 | 30 |
| Spirin 4271 | (4.7–)4.8–6.2(–6.3) | 5.35 | 1.2–1.7(–1.8) | 1.39 | 3.86 | 30 |
| Spirin 4386 | (4.2–)4.3–5.9(–6.0) | 5.06 | 1.2–1.6(–1.7) | 1.33 | 3.80 | 38 |
| Spirin 5803 | (4.1–)4.3–6.1 | 5.14 | 1.2–1.8(–1.9) | 1.44 | 3.58 | 30 |
| Spirin 6328 | (4.6–)4.7–6.2(–6.8) | 5.34 | 1.2–1.7(–1.8) | 1.35 | 3.96 | 35 |
| Spirin 8587 | 4.2–5.4(–5.8) | 4.87 | 1.2–1.6(–1.7) | 1.40 | 3.46 | 40 |
| Postia subcaesia | (3.8–)4.0–5.3(–6.1) | 4.60 | 1.0–1.4(–1.5) | 1.21 | 3.80 | 201/6 |
| isotype | 4.0–5.2(–5.8) | 4.59 | 1.0–1.4(–1.5) | 1.18 | 3.89 | 40 |
| Legon 14.X.1995 | (4.0–)4.1–5.4(–6.1) | 4.65 | (1.0–)1.1–1.4(–1.5) | 1.20 | 3.87 | 41 |
| Rivoire 1868 | (3.9–)4.0–5.0(–5.4) | 4.33 | 1.0–1.3 | 1.18 | 3.66 | 30 |
| Spirin 2390 | (4.5–)4.6–5.4(–5.6) | 5.08 | (1.1–)1.2–1.5 | 1.29 | 3.93 | 30 |
| Spirin 6083 | 4.2–5.1(–5.3) | 4.65 | 1.1–1.4(–1.5) | 1.22 | 3.82 | 30 |
| Vampola 1563 | (3.8–)3.9–4.9(–5.0) | 4.29 | 1.0–1.3(–1.4) | 1.19 | 3.60 | 30 |
| Postia subviridis | (3.5–)3.8–4.5(–4.6) | 4.11 | 1.0–1.3 | 1.15 | 3.58 | 62/2 |
| isotype | (3.5–)3.7–4.3(–4.5) | 4.07 | 1.0–1.3 | 1.16 | 3.51 | 40 |
| Penttilä 14376* | (4.3–)4.5–5.3(–5.4) | 4.92 | 1.0–1.2 | 1.10 | 4.46 | 30 |
| Spirin 8774a | 4.0–4.5(–4.6) | 4.20 | 1.0–1.3 | 1.14 | 3.70 | 22 |
| Postia yanae | (4.0–)4.3–5.8(–7.5) | 5.02 | 1.2–1.6(–1.7) | 1.41 | 3.56 | 102/3 |
| holotype | (4.0–)4.3–5.8(–7.5) | 4.91 | (1.2–)1.3–1.6 | 1.45 | 3.38 | 42 |
| Kotiranta 27677 | 4.2–5.8(–6.0) | 4.74 | 1.3–1.5 | 1.39 | 3.41 | 30 |
| Kotiranta 27606 | (4.5–)4.9–6.2 | 5.36 | 1.3–1.6(–1.7) | 1.43 | 3.75 | 30 |
| Kotiranta 27772 | (4.5–)4.6–5.7(–5.9) | 5.13 | 1.2–1.6 | 1.35 | 3.81 | 30 |
Supplement 3 - Hyphal measurements in the Postia caesia complex
Bold-face values represent composite statistics for species, n = number of spores measured. The whole range is given in parentheses; 60% range excluding 20% extreme values from both ends of variation is given without parentheses; in case the values are identical, parentheses are omitted.
| species | specimen | tramal hyphal width | tramal hyphal mean | tramal hyphal median | tramal hyphal n | context hyphal width | context hyphal mean | context hyphal median | context hyphal n |
|---|---|---|---|---|---|---|---|---|---|
| Postia alni | compound stats | (2,0–)2,9–3,6(–4,3) | 3,22 | 3,2 | 144 | (2,4–)3,9–5,5(–7,4) | 4,67 | 4,7 | 104 |
| Postia alni | holotype | (2,0–)2,9–3,4(–4,3) | 3,11 | 3,2 | 72 | (2,4–)3,5–5,2(–7,4) | 4,38 | 4,2 | 64 |
| Postia alni | Miettinen 14909 | (2,3–)2,7–3,2 | 2,93 | 3 | 10 | ||||
| Postia alni | Miettinen 15830 | (2,7–)3,0–3,8(–4,1) | 3,38 | 3,2 | 20 | (3,9–)4,8–5,7(–6,2) | 5,22 | 5,15 | 20 |
| Postia alni | Spirin 3640 | (2,7–)3,3–3,9(–4,3) | 3,6 | 3,6 | 22 | (4,0–)4,2–5,8(–6,2) | 5,08 | 5,1 | 20 |
| Postia alni | Vampola 27/10 | (2,3–)2,8–3,7(–4,2) | 3,18 | 3,05 | 20 | ||||
| Postia arbuti | compound stats | (1,8–)2,4–3,1(–4,0) | 2,78 | 2,8 | 60 | (2,3–)3,2–4,6(–5,4) | 3,96 | 4,2 | 47 |
| Postia arbuti | holotype | (1,8–)2,4–3,1(–3,3) | 2,76 | 2,8 | 40 | (2,3–)3,0–4,6(–5,4) | 3,82 | 4 | 27 |
| Postia arbuti | Spirin 8318 | (2,2–)2,3–3,1(–4,0) | 2,82 | 2,85 | 20 | (2,7–)3,3–4,7(–5,4) | 4,14 | 4,25 | 20 |
| Postia auricoma | compound stats | (2,0–)3,1–4,0(–4,5) | 3,5 | 3,5 | 64 | (3,8–)4,2–5,2(–6,2) | 4,8 | 4,8 | 40 |
| Postia auricoma | holotype | (2,0–)3,0–4,0(–4,5) | 3,49 | 3,45 | 44 | (4,0–)4,2–5,3(–6,2) | 4,82 | 4,8 | 20 |
| Postia auricoma | Spirin 4586 | (2,9–)3,1–4,0(–4,2) | 3,52 | 3,5 | 20 | (3,8–)4,2–5,2(–5,9) | 4,78 | 4,85 | 20 |
| Postia bifaria | compound stats | (2,0–)2,5–3,8(–4,2) | 3,17 | 3,2 | 44 | (3,0–)4,0–7,0(–8,0) | 5,26 | 5,1 | 33 |
| Postia bifaria | holotype | (2,0–)2,3–3,3(–4,0) | 2,83 | 2,85 | 24 | (3,0–)3,5–5,0(–5,8) | 4,31 | 4,3 | 13 |
| Postia bifaria | Spirin 4850 | (3,1–)3,2–3,9(–4,2) | 3,56 | 3,5 | 20 | (3,5–)4,2–7,1(–8,0) | 5,88 | 5,8 | 20 |
| Postia caesia | compound stats | (1,9–)2,8–3,6(–4,5) | 3,23 | 3,2 | 48 | (2,6–)3,7–5,2(–6,1) | 4,38 | 4,3 | 40 |
| Postia caesia | neotype | (1,9–)2,8–3,6(–4,5) | 3,21 | 3,2 | 28 | (2,6–)2,8–4,2(–5,0) | 3,73 | 3,8 | 20 |
| Postia caesia | Miettinen 14133 | (2,6–)2,8–3,6(–4,2) | 3,25 | 3,15 | 20 | (4,0–)4,3–5,6(–6,1) | 5,04 | 5,1 | 20 |
| Postia caesiosimulans | compound stats | (2,0–)2,9–3,8(–4,8) | 3,31 | 3,2 | 184 | (2,2–)3,4–5,2(–6,4) | 4,3 | 4,2 | 163 |
| Postia caesiosimulans | holotype | (2,3–)3,0–3,9(–4,3) | 3,39 | 3,3 | 22 | (3,2–)3,4–4,9(–5,6) | 4,25 | 4,15 | 20 |
| Postia caesiosimulans | epitype | (2,4–)3,0–3,9(–4,8) | 3,39 | 3,3 | 47 | (2,9–)3,9–5,3(–6,3) | 4,63 | 4,6 | 35 |
| Postia caesiosimulans | Miettinen 14156,2 | (2,6–)3,1–4,0(–4,3) | 3,54 | 3,7 | 20 | (4,0–)4,6–5,4(–6,4) | 5,08 | 5,05 | 20 |
| Postia caesiosimulans | Miettinen 18663 | 3,2–3,4(–4,0) | 3,44 | 3,3 | 5 | (3,3–)4,8–5,8(–6,0) | 4,98 | 5 | 6 |
| Postia caesiosimulans | Miettinen 18665 | (2,0–)2,3–3,3(–4,0) | 2,82 | 2,8 | 31 | (2,2–)2,8–3,7(–5,0) | 3,34 | 3,3 | 42 |
| Postia caesiosimulans | Spirin 2610 | (2,8–)3,1–3,9(–4,1) | 3,48 | 3,45 | 20 | (3,9–)4,4–5,8(–6,3) | 5,19 | 5,2 | 20 |
| Postia caesiosimulans | Spirin 4798 | (2,0–)2,8–3,3(–3,7) | 2,96 | 3 | 19 | (3,2–)3,7–4,2(–4,8) | 3,93 | 3,9 | 20 |
| Postia caesiosimulans | Spirin 8717 | (2,9–)3,2–4,1(–4,4) | 3,65 | 3,5 | 20 | ||||
| Postia coeruleivirens | compound stats | (1,8–)2,4–3,4(–4,0) | 2,95 | 3 | 43 | (3,0–)3,6–6,0(–7,8) | 4,92 | 4,9 | 34 |
| Postia coeruleivirens | Spirin 4252 | (2,7–)3,0–3,6(–4,0) | 3,31 | 3,2 | 20 | (3,5–)3,8–5,3(–6,2) | 4,88 | 4,95 | 20 |
| Postia coeruleivirens | Spirin 5301 | (1,8–)2,2–3,1(–3,6) | 2,64 | 2,6 | 23 | (3,0–)3,5–6,5(–7,8) | 4,96 | 4,7 | 14 |
| Postia comata | compound stats | (2,2–)2,8–3,8(–4,5) | 3,27 | 3,2 | 75 | (3,2–)4,2–5,3(–7,1) | 4,88 | 4,95 | 20 |
| Postia comata | holotype | (2,2–)3,0–4,0(–4,5) | 3,4 | 3,3 | 55 | (3,2–)4,2–5,3(–7,1) | 4,88 | 4,95 | 20 |
| Postia comata | Miettinen 17180 | (2,2–)2,7–3,2 | 2,91 | 3 | 20 | ||||
| Postia cyanescens | compound stats | (2,0–)2,9–3,7(–4,4) | 3,25 | 3,2 | 185 | (2,6–)4,1–5,2(–6,8) | 4,61 | 4,6 | 116 |
| Postia cyanescens | holotype | (2,2–)2,9–3,5(–4,0) | 3,12 | 3,05 | 24 | ||||
| Postia cyanescens | Kinnunen 5087 | (2,6–)2,8–3,5(–4,1) | 3,25 | 3,2 | 20 | (2,6–)3,3–4,6(–5,3) | 4,09 | 4,2 | 20 |
| Postia cyanescens | Miettinen 15989 | (2,3–)2,9–3,7(–4,4) | 3,27 | 3,2 | 41 | (3,4–)4,2–5,3(–6,8) | 4,87 | 5 | 33 |
| Postia cyanescens | Miettinen 15919,2 | (2,8–)3,1–3,9(–4,4) | 3,42 | 3,3 | 20 | ||||
| Postia cyanescens | Niemelä 8844 | (2,0–)2,5–3,6(–4,0) | 3,07 | 3,05 | 20 | ||||
| Postia cyanescens | Penttilä 14560 | (2,8–)3,0–3,9(–4,3) | 3,49 | 3,45 | 20 | (3,4–)4,0–5,1(–5,7) | 4,55 | 4,45 | 20 |
| Postia cyanescens | Salo 9196 | (2,6–)3,0–3,4(–4,2) | 3,29 | 3,2 | 20 | (3,5–)3,8–5,2(–6,0) | 4,47 | 4,2 | 23 |
| Postia cyanescens | Spirin 2756 | (2,7–)3,0–3,3(–3,8) | 3,15 | 3,1 | 20 | (3,8–)4,3–5,2(–6,2) | 4,9 | 4,95 | 20 |
| Postia glauca | compound stats | (1,8–)2,6–3,3(–3,8) | 2,96 | 3 | 44 | (2,5–)3,4–5,1(–6,3) | 4,26 | 4,2 | 31 |
| Postia glauca | holotype | (1,8–)2,7–3,3(–3,8) | 3 | 3 | 24 | (2,5–)2,8–3,7(–4,2) | 3,31 | 3,3 | 11 |
| Postia glauca | Miettinen 10567 | (2,2–)2,5–3,2(–3,7) | 2,92 | 3 | 20 | (3,4–)4,0–5,3(–6,3) | 4,79 | 4,85 | 20 |
| Postia gossypina | compound stats | (1,9–)2,3–3,0(–3,8) | 2,71 | 2,8 | 64 | (2,9–)3,6–4,8(–5,4) | 4,21 | 4,2 | 40 |
| Postia gossypina | isotype | (1,9–)2,3–3,0(–3,3) | 2,67 | 2,8 | 44 | (3,2–)4,0–5,1(–5,4) | 4,41 | 4,2 | 20 |
| Postia gossypina | Rivoire 6658 | (2,1–)2,3–3,1(–3,8) | 2,79 | 2,75 | 20 | (2,9–)3,4–4,3(–5,3) | 4,03 | 4,05 | 20 |
| Postia livens | compound stats | (1,9–)2,9–4,0(–4,7) | 3,4 | 3,3 | 136 | (2,5–)3,7–5,3(–7,2) | 4,56 | 4,4 | 133 |
| Postia livens | holotype | (2,3–)2,8–3,8(–4,7) | 3,36 | 3,35 | 48 | (3,3–)4,3–5,7(–7,0) | 4,99 | 5,1 | 41 |
| Postia livens | Miettinen 14775 | (2,7–)3,0–4,0(–4,6) | 3,59 | 3,4 | 20 | (3,4–)4,4–6,2(–7,2) | 5,3 | 5,15 | 20 |
| Postia livens | Miettinen 14828 | (1,9–)2,5–3,2(–3,4) | 2,87 | 3 | 25 | (2,6–)3,2–4,0(–4,9) | 3,67 | 3,7 | 32 |
| Postia livens | Miettinen 16056 | (2,4–)3,0–3,8(–4,7) | 3,33 | 3,2 | 23 | (3,8–)4,3–5,6(–6,7) | 5,08 | 5,05 | 20 |
| Postia livens | Vlasák 1009/57 | (3,3–)3,6–4,2(–4,7) | 4,01 | 4,1 | 20 | ||||
| Postia luteocaesia | compound stats | (2,1–)2,7–3,2(–4,2) | 3 | 3 | 91 | (3,3–)4,1–5,8(–6,7) | 5 | 5,1 | 40 |
| Postia luteocaesia | holotype | (2,3–)3,0–3,3(–4,2) | 3,17 | 3,2 | 51 | (4,0–)4,3–5,7(–6,7) | 5,18 | 5,2 | 20 |
| Postia luteocaesia | Rivoire 2605 | (2,1–)2,4–2,9(–3,2) | 2,74 | 2,8 | 20 | ||||
| Postia luteocaesia | Rivoire 733 | (2,2–)2,3–3,1(–3,3) | 2,82 | 2,9 | 20 | (3,3–)3,8–5,9(–6,7) | 4,81 | 4,75 | 20 |
| Postia magna | compound stats | (1,5–)2,2–3,3(–3,8) | 2,79 | 2,9 | 43 | (3,4–)4,2–6,0(–6,6) | 5,17 | 5,25 | 32 |
| Postia magna | holotype | (1,5–)2,2–3,3(–3,8) | 2,79 | 2,9 | 43 | (3,4–)4,2–6,0(–6,6) | 5,17 | 5,25 | 32 |
| Postia mediterraneocaesia | compound stats | (1,8–)2,3–3,2(–4,2) | 2,89 | 2,9 | 65 | (2,4–)3,1–4,0(–4,8) | 3,61 | 3,7 | 37 |
| Postia mediterraneocaesia | isotype | (1,9–)2,2–3,0(–4,0) | 2,73 | 2,8 | 24 | (2,9–)3,2–3,8(–4,8) | 3,59 | 3,5 | 17 |
| Postia mediterraneocaesia | Rivoire 1903 | (1,8–)2,3–3,2(–3,9) | 2,76 | 2,8 | 21 | (2,4–)3,0–4,1(–4,8) | 3,63 | 3,7 | 20 |
| Postia mediterraneocaesia | Rivoire 2083 | (2,2–)2,9–3,8(–4,2) | 3,23 | 3 | 20 | ||||
| Postia populi | compound stats | (2,0–)2,7–3,3(–4,2) | 3,02 | 3 | 143 | (2,6–)3,2–4,8(–5,6) | 4 | 4 | 120 |
| Postia populi | holotype | (2,0–)2,5–3,0(–3,5) | 2,72 | 2,7 | 25 | (2,6–)2,9–3,2(–4,0) | 3,09 | 3,05 | 20 |
| Postia populi | Kinnunen 4938 | (2,4–)2,7–3,1(–3,8) | 2,94 | 3 | 20 | (3,1–)3,6–4,9(–5,6) | 4,37 | 4,55 | 20 |
| Postia populi | Miettinen 14211 | (2,4–)2,8–3,6(–4,1) | 3,18 | 3,1 | 20 | (3,0–)3,3–4,3(–5,2) | 4,08 | 4,15 | 20 |
| Postia populi | Miettinen 15827,2 | (2,1–)2,8–3,5(–4,2) | 3,15 | 3,2 | 39 | (3,1–)3,3–4,7(–5,2) | 4,06 | 4 | 20 |
| Postia populi | Spirin 3224 | (2,3–)2,4–3,2(–3,3) | 2,8 | 2,8 | 19 | (3,4–)3,9–5,0(–5,2) | 4,43 | 4,25 | 20 |
| Postia populi | Spirin 4587 | (2,8–)3,0–3,5(–3,9) | 3,27 | 3,2 | 20 | (3,2–)3,3–4,3(–4,9) | 3,99 | 4,05 | 20 |
| Postia simulans | compound stats | (1,7–)2,8–3,6(–4,8) | 3,11 | 3,1 | 279 | (2,9–)3,9–5,0(–6,4) | 4,43 | 4,3 | 227 |
| Postia simulans | isotype | (2,8–)3,1–3,9(–4,8) | 3,65 | 3,75 | 30 | (4,0–)4,1–5,1(–5,2) | 4,64 | 4,65 | 10 |
| Postia simulans | epitype | (2,2–)3,0–3,8(–4,8) | 3,35 | 3,3 | 44 | (3,4–)4,0–5,1(–6,0) | 4,58 | 4,6 | 46 |
| Postia simulans | Rivoire 2936 | (2,8–)3,1–3,8(–4,3) | 3,45 | 3,25 | 20 | (4,0–)4,2–5,4(–6,2) | 4,93 | 4,8 | 20 |
| Postia simulans | Spirin 5803 | (2,3–)2,8–3,2(–4,0) | 3,06 | 3,1 | 20 | (3,6–)4,2–5,1(–5,3) | 4,65 | 4,8 | 20 |
| Postia simulans | Spirin 4271 | (2,0–)2,3–3,2(–3,3) | 2,81 | 2,9 | 20 | (2,9–)3,5–4,6(–5,1) | 4,1 | 4,1 | 20 |
| Postia simulans | Spirin 4386 | (2,1–)2,6–3,1(–3,8) | 2,88 | 2,85 | 30 | (3,4–)4,0–4,8(–5,3) | 4,42 | 4,35 | 20 |
| Postia simulans | Spirin 6328 | (2,7–)3,0–3,8(–4,0) | 3,32 | 3,2 | 31 | (4,0–)4,2–5,3(–6,4) | 4,95 | 4,9 | 20 |
| Postia simulans | Spirin 8542 | (2,2–)2,5–3,1(–3,6) | 2,83 | 2,8 | 20 | (3,1–)3,2–4,4(–4,8) | 3,96 | 3,95 | 20 |
| Postia simulans | Spirin 8587 | (1,7–)2,2–3,2(–4,1) | 2,82 | 2,9 | 64 | (3,0–)3,8–4,4(–5,0) | 4,07 | 4 | 51 |
| Postia subcaesia | compound stats | (2,2–)3,1–4,1(–5,8) | 3,45 | 3,3 | 60 | (2,3–)4,2–6,6(–7,8) | 5,42 | 5,3 | 60 |
| Postia subcaesia | isotype | (3,2–)3,3–4,1(–4,8) | 3,8 | 3,85 | 20 | (3,4–)4,0–5,3(–6,4) | 4,79 | 4,7 | 20 |
| Postia subcaesia | Legon K(M)31967 | (2,3–)3,2–4,4(–5,8) | 3,83 | 3,8 | 46 | (2,3–)3,8–6,8(–7,8) | 5,3 | 5,9 | 35 |
| Postia subcaesia | Spirin 2390 | (2,5–)2,8–3,4(–4,3) | 3,17 | 3,2 | 20 | (4,1–)4,2–5,7(–6,4) | 5,08 | 5,1 | 20 |
| Postia subcaesia | Spirin 6083 | (2,2–)2,9–4,0(–4,7) | 3,37 | 3,15 | 20 | (4,8–)5,3–7,0(–7,3) | 6,41 | 6,7 | 20 |
| Postia subviridis | compound stats | (2,2–)2,5–3,1(–4,0) | 2,85 | 2,8 | 83 | (2,8–)4,2–5,8(–7,8) | 5,05 | 5,1 | 61 |
| Postia subviridis | isotype | (2,2–)2,5–3,0(–3,4) | 2,77 | 2,8 | 42 | (2,8–)4,6–5,8(–7,0) | 5,15 | 5,2 | 26 |
| Postia subviridis | Penttilä 14376 | (2,2–)2,5–3,0(–4,0) | 2,83 | 2,8 | 21 | (3,1–)3,5–4,5(–5,5) | 4,13 | 4 | 15 |
| Postia subviridis | Spirin 8774a | (2,3–)2,7–3,3(–3,7) | 3,04 | 3,05 | 20 | (4,2–)4,8–6,2(–7,8) | 5,6 | 5,6 | 20 |
| Postia yanae | compound stats | (1,5–)2,2–2,9(–4,3) | 2,62 | 2,6 | 93 | (2,0–)3,0–4,0(–5,2) | 3,5 | 3,5 | 80 |
| Postia yanae | holotype | (1,8–)2,2–2,7(–3,0) | 2,45 | 2,4 | 32 | (2,0–)3,0–3,8(–4,5) | 3,31 | 3,3 | 32 |
| Postia yanae | Kotiranta 27606 | (2,2–)2,6–3,2(–4,2) | 2,98 | 2,9 | 20 | (2,8–)3,3–4,1(–4,9) | 3,75 | 3,8 | 20 |
| Postia yanae | Kotiranta 27677 | (1,5–)2,3–3,0(–4,3) | 2,75 | 2,8 | 20 | (2,3–)2,8–3,6(–4,0) | 3,21 | 3,2 | 20 |
| Postia yanae | Kotiranta 27772 | (1,9–)2,0–2,8(–3,5) | 2,43 | 2,2 | 21 | (3,2–)3,8–5,0(–5,2) | 4,35 | 4,35 | 8 |
Supplement 4 – Distribution maps of north temperate Postia caesia species





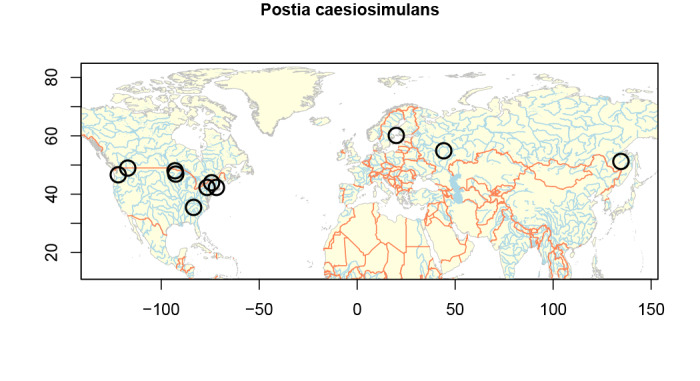












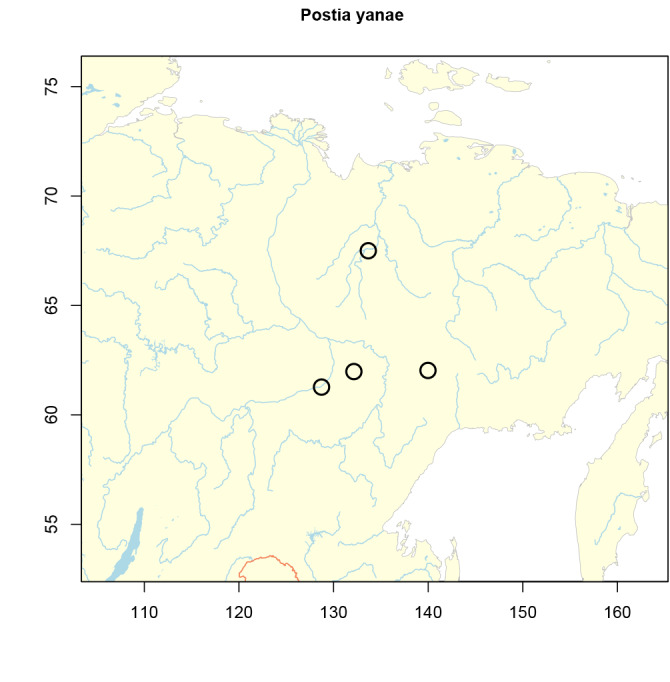
REFERENCES
- Atkinson GF. (1908). Notes on some new species of fungi from the United States. Annales Mycologici 6: 54–62. [Google Scholar]
- Brazee NJ, Lindner DL, Fraver S, et al. (2012). Wood-inhabiting, polyporoid fungi in aspen-dominated forests managed for biomass in the U.S. Lake States. Fungal Ecology 5: 600–609. [Google Scholar]
- Britzelmayr M. (1893). Materialien zur Beschreibung der Hymeno-myceten 3. Botanisches Zentralblatt 54: 97–105. [Google Scholar]
- Corner EJH. (1989). Ad polyporaceas V. Beihefte zur Nova Hedwigia 96: 1–218. [Google Scholar]
- Cunningham GH. (1965). Polyporaceae of New Zealand. Bulletin of the New Zealand Department of Industrial Research 164: 1–304. [Google Scholar]
- Darriba D, Taboada GL, Doallo R, et al. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David A. (1974). Une nouvelle espèce de Polyporaceae: Tyromyces subcaesius. Bulletin de la Société Linnéenne de Lyon 43: 119–126. [Google Scholar]
- David A. (1980). Etude du genre Tyromyces sensu lato: repartition dans le genres Leptoporus, Spongiporus et Tyromyces sensu stricto. Bulletin mensuel de la Société linnéenne de Lyon 49: 6–56. [Google Scholar]
- Donk MA. (1960). The generic names proposed for Polyporaceae. Persoonia 1: 173–302. [Google Scholar]
- Fries EM. (1821). Systema mycologicum 1. Ex officina Berlingiana, Lund. [Google Scholar]
- Fries EM. (1836–1838). Epicrisis systematis mycologici. Typographia Academica, Uppsala. [Google Scholar]
- Guzmán G, Ryvarden L. (2001). Studies in neotropical polypores 12. New and noteworthy polypores from Mexico. Mycotaxon 78: 245–256. [Google Scholar]
- Hattori T. (2002). Type studies of the polypores described by E.J.H. Corner from Asia and West Pacific Areas. IV. Species described in Tyromyces (1). Mycoscience 43: 307–315. [Google Scholar]
- Hattori T. (2005). Type studies of the polypores described by E.J.H. Corner from Asia and West Pacific Areas. VII. Species described in Trametes (1). Mycoscience 46: 303–312. [Google Scholar]
- Hämet-Ahti L. (1984). The boreal zone and its biotic subdivision. Fennia 159: 69–75. [Google Scholar]
- Index Herbariorum (2017). A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium; http://sweetgum.nybg.org/science/ih [accessed 10.3.2017]. [Google Scholar]
- Jahn H. (1979). Pilze die an Holz wachsen. Busse, Herford. [Google Scholar]
- Jang Y, Jang S, Lee J, et al. (2016). Diversity of Wood-Inhabiting Polyporoid and Corticioid Fungi in Odaesan National Park, Korea. Mycobiology 44: 217–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justo A, Miettinen O, Floudas D, et al. (2017). A revised family-level classification of the Polyporales (Basidiomycota). Fungal Biology 121: 798–824. [DOI] [PubMed] [Google Scholar]
- Karsten PA. (1888). Diagnoses fungorum nonnullorum novorum, in Fennia detectorum. Revue Mycologique Toulouse 10: 73–75. [Google Scholar]
- Katoh K, Standley DM. (2013). MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killermann S. (1922). Pilze aus Bayern. Kritische Studien besonders zu M. Britzelmayr; Standortsangaben u. (kurtze) Bestimmunstabellen. 1. Teil: Thelephoraceen, Hydnaceen, Polyporaceen, Clariaceen un Tremellaceen. Denkschriften der Bayerischen Botanischen Gesellschaft in Regensburg 15: 1–134. [Google Scholar]
- Kim CS, Jo JW, Kwag Y-N, et al. (2015). Mushroom flora of Ulleung-gun and a newly recorded Bovista species in the Republic of Korea. Mycobiology 43: 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlaba F, Pouzar Z. (1989). Type studies of polypores described by A. Pilát 2. Česká Mykologie 43: 36–44. [Google Scholar]
- Lowe JL. (1956). Type studies of the polypores described by Karsten. Mycologia 1: 99–125. [Google Scholar]
- Lowe JL. (1974) Solution to a mycological mystery. Bulletin de la Société Linnéenne de Lyon 43: 239–240. [Google Scholar]
- Matheny PB, Wang Z, Binder M, et al. (2007). Contributions of rpb2 and tef1 to the phylogeny of mushrooms and allies (Basidiomycota, Fungi). Molecular Phylogenetics and Evolution 43: 430–451. [DOI] [PubMed] [Google Scholar]
- Niemelä T, Kinnunen J, Lindgren M, et al. (2001). Novelties and records of poroid Basidiomycetes in Finland and adjacent Russia. Karstenia 41: 1–21. [Google Scholar]
- Ortiz-Santana B, Lindner DL, Miettinen O, et al. (2013). A phylogenetic overview of the antrodia clade (Basidiomycota, Polyporales). Mycologia 105: 1391–1411. [DOI] [PubMed] [Google Scholar]
- Papp V. (2014). Postia alni Niemela & Vampola (Basidiomycota, Polyporales) - member of the problematic Postia caesia complex - has been found for the first time in Hungary. Biodiversity Data Journal 2: e1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp V. (2015). Nomenclatural novelties in the Postia caesia complex. Mycotaxon 129: 407–413. [Google Scholar]
- Pebesma EJ, Bivand RS. (2005). Classes and methods for spatial data in R. R News 5: 9–13. [Google Scholar]
- Pegler DN, Saunders EM. (1994). British poroid species formerly placed in the genus Tyromyces (Coriolaceae). Mycologist 8: 24–31. [Google Scholar]
- Pieri M, Rivoire B. (1998). Postia inocybe (David & Malençon) Jülich f. inocybe et f. pileatus, f. nov. Notes nomenclaturales sur le genre Postia. Bulletin trimestriel de la Société mycologique de France 114: 19–33. [Google Scholar]
- Pieri M, Rivoire B. (2005). Postia mediterraneocaesia, une nouvelle espèce de polypore découverte dans le sud de l’Europe. Bulletin Semestriel de la Fédération des Associations Mycologiques Méditerranéennes 28: 33–38. [Google Scholar]
- Pildain MB, Rajchenberg M. (2013). The phylogenetic position of Postia s.l. (Polyporales, Basidiomycota) from Patagonia, Argentina. Mycologia 105: 357–367. [DOI] [PubMed] [Google Scholar]
- Rajchenberg M. (1995). Notes on New Zealand polypores (Basidiomycetes) 2. Cultural and morphological studies of selected species. New Zealand Journal of Botany 33: 99–109. [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, et al. (2012). MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryvarden L. (1983). Type studies in the Polyporaceae 14. species described by N. Patouillard, either alone or with other mycologists. Occasional papers of the Farlow Herbarium of Cryptogamic Botany. Harvard University Herbaria, Cambridge, MA. [Google Scholar]
- Ryvarden L. (1988a). Two new polypores from Burundi in Africa. Mycotaxon 31: 407–409. [Google Scholar]
- Ryvarden L. (1988b). Type studies in the Polyporaceae 19. Species described by M.C. Cooke. Mycotaxon 31:45–58 [Google Scholar]
- Ryvarden L. (1991). Genera of polypores. Nomenclature and taxonomy. Synopsis Fungorum 5: 1–363. [Google Scholar]
- Ryvarden L. (2016). Neotropical polypores 3. Polyporaceae, Obba-Wrightoporia. Synopsis Fungorum 36: 447–613. [Google Scholar]
- Ryvarden L, Melo I. (2014). Poroid fungi of Europe. Fungiflora, Oslo. [Google Scholar]
- Schaeffer JC. (1763). Fungorum qui in Bavaria et Palatinatu circa Ratisbonam nascuntur Icones, Regensburg. [Google Scholar]
- Schaeffer JC. (1774). Fungorum qui in Bavaria et Palatinatu circa Ratisbonam nascuntur Icones, Regensburg. [Google Scholar]
- Schrader HA. (1794). Spicilegium Florae Germanicae. Ritscher, Hannover. [Google Scholar]
- Schumacher CF. (1803). Enumeratio plantarum in partibus Saellandiae septentrionalis et orientalis, pars posterior. Friedrich Brummer, Hafniae. [Google Scholar]
- Shen L, Cui B, Dai Y. (2014). A new species of Postia (Polyporales, Basidiomycota) from China based on morphological and molecular evidence. Phytotaxa 162: 147. [Google Scholar]
- Sowerby J. (1799). Coloured Figures of English fungi or mushrooms. J. Davis, London. [Google Scholar]
- Stamatakis A. (2014). RAxML Version 8: A tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson JA, Cash EK. (1936) The new fungus names proposed by C. G. Lloyd. Bulletin of the Lloyd library and Museum 35: 1–209. [Google Scholar]
- Tamura K, Stecher G, Peterson D, et al. (2013). MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Molecular Biology and Evolution 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao YJ, Pegler DN, Chase MW. (2005). Molecular variation in the Postia caesia complex. FEMS Microbiology Letters 242: 109–116. [DOI] [PubMed] [Google Scholar]













