Abstract
STUDY QUESTION
Is high adiposity in childhood associated with menstrual irregularity and polycystic ovary syndrome (PCOS) in later life?
SUMMARY ANSWER
Overall, greater childhood BMI was associated with menstrual irregularity, and greater childhood BMI and waist/height ratio (WHtR) in white but not black participants were associated with PCOS in adulthood.
WHAT IS KNOWN ALREADY
Increased childhood BMI has been associated with irregular menstrual cycles and PCOS symptoms in adulthood in two longitudinal population-based studies, but no study has reported on associations with childhood abdominal obesity. Few studies have investigated whether there are racial differences in the associations of adiposity with PCOS though there has been some suggestion that associations with high BMI may be stronger in white girls than in black girls.
STUDY DESIGN, SIZE, DURATION
The study included 1516 participants (aged 26–41 years) from the Australian Childhood Determinants of Adult Health study (CDAH) and 1247 participants (aged 26–57 years) from the biracial USA Babies substudy of the Bogalusa Heart Study (BBS) who were aged 7–15 years at baseline. At follow-up, questions were asked about menstruation (current for CDAH or before age 40 years for BBS), ever having had a diagnosis of PCOS and symptoms of PCOS.
PARTICIPANTS/MATERIALS, SETTING, METHODS
In CDAH, a single childhood visit was conducted in 1985. In BBS, multiple childhood visits occurred from 1973 to 2000 and race was reported (59% white; 41% black). In childhood, overweight and obesity were defined by international age–sex-specific standards for BMI and WHtR was considered as an indicator of abdominal obesity. Multilevel mixed-effects Poisson regression estimated relative risks (RRs) adjusting for childhood age, highest parental and own education and age at menarche.
MAIN RESULTS AND THE ROLE OF CHANCE
The prevalence of childhood obesity was 1.1% in CDAH and 7.5% in BBS. At follow-up, menstrual irregularity was reported by 16.7% of CDAH and 24.5% of BBS participants. The prevalence of PCOS was 7.4% in CDAH and 8.0% in BBS participants. In CDAH, childhood obesity was associated with menstrual irregularity (RR = 2.84, 95% CI: 1.63–4.96) and PCOS (RR = 4.05, 95% CI: 1.10–14.83) in adulthood. With each 0.01 unit increase in childhood WHtR there was a 6% (95% CI: 1–11%) greater likelihood of PCOS. Overall, in BBS, childhood obesity was associated with increased risk of menstrual irregularity (RR = 1.44, 95% CI: 1.08–1.92) in adulthood. Significant interaction effects between race and childhood adiposity were detected in associations with PCOS. In BBS white participants, childhood obesity was associated with PCOS (RR = 2.93, 95% CI: 1.65–5.22) and a 0.01 unit increase in childhood WHtR was associated with an 11% (95% CI: 5–17%) greater likelihood of PCOS in adulthood. In BBS black participants, no statistically significant associations of childhood adiposity measures with PCOS were observed.
LIMITATIONS, REASONS FOR CAUTION
The classification of menstrual irregularity and PCOS was based on self-report by questionnaire, which may have led to misclassification of these outcomes. However, despite the limitations of the study, the prevalence of menstrual irregularity and PCOS in the two cohorts was consistent with the literature. While the study samples at baseline were population-based, loss to follow-up means the generalizability of the findings is uncertain.
WIDER IMPLICATIONS OF THE FINDINGS
Greater childhood adiposity indicates a higher risk of menstrual irregularity and PCOS in adulthood. Whether this is causal or an early indicator of underlying hormonal or metabolic disorders needs clarification. The stronger associations of adiposity with PCOS in white than black participants suggest that there are racial differences in childhood adiposity predisposing to the development of PCOS and other environmental or genetic factors are also important.
STUDY FUNDING/COMPETING INTEREST(S)
The CDAH study was supported by grants from the Australian National Health and Medical Research Council (grants 211316, 544923 and 1128373). The Bogalusa Heart Study is supported by US National Institutes of Health grants R01HD069587, AG16592, HL121230, HD032194 and P50HL015103. No competing interests existed.
Keywords: BMI, waist/height ratio, childhood, menstrual irregularity, polycystic ovary syndrome
Introduction
Menstrual irregularity and polycystic ovary syndrome (PCOS) have been associated with higher risk of lower fecundity and cardiovascular diseases (Solomon et al., 2002; West et al., 2014) as well as some cancers (Harris et al., 2017; Harris et al., 2018). PCOS is recognized as the most common heterogeneous endocrine disorder, affecting 8–13% of women of reproductive age (March et al., 2010). Irregular menstrual cycles are part of the three diagnostic criteria (National Institutes of Health, Rotterdam and Androgen Excess Society diagnostic criteria) for PCOS in addition to hyperandrogenism and polycystic ovarian morphology (Teede et al., 2018).
General and abdominal obesity are associated with a greater risk of menstrual irregularity in adult women (Douchi et al., 2002; Wei et al., 2009; Jacobsen et al., 2012; Hahn et al., 2013). Our previous cross-sectional study suggested that obese women, defined by either BMI or waist circumference, were twice as likely to have irregular menstruation, compared with normal weight women (Wei et al., 2009). However, the association between adult obesity and PCOS is inconclusive. Although obesity, particularly abdominal obesity, is a common trait in women with PCOS, it is not part of the diagnostic criteria. The prevalence of obesity varies among different populations and races, but the prevalence of PCOS is relatively uniform (Legro, 2012). This implies that obesity might not cause PCOS or there could be geographic/ethnic differences affecting the relationship between obesity and PCOS.
Only two previous population-based longitudinal studies (Lake et al., 1997; Laitinen et al., 2003) have investigated the associations between childhood obesity, adult menstrual irregularity and PCOS, in which childhood BMI was the only indicator of obesity. The 1958 British birth cohort study of 5770 girls reported that overweight and obesity at 7 years of age increased the risk of menstrual irregularity before age 33 years (Lake et al., 1997). The Northern Finland 1966 birth cohort of 2007 girls suggested that overweight and obesity at age 14 years were associated with self-reported PCOS at age 31 years (Laitinen et al., 2003). Two further papers based on the same Northern Finland cohort reported associations of weight gain (Ollila et al., 2016) and age at adiposity rebound with PCOS (Koivuaho et al., 2019). Another study based on clinical study samples suggested that change in z-score from weight at birth to weight in adolescence may be greater in girls with PCOS than in healthy controls (de Zegher et al., 2017).
In this study, we used two cohorts with different racial characteristics who were followed through childhood to adulthood. We aimed first to investigate the associations of obesity (including abdominal obesity) in childhood with menstrual irregularity and PCOS in adulthood and, second, to determine whether these associations differed by country (Australia and USA) and race (white and black).
Materials and Methods
The Childhood Determinants of Adult Health Study: a cohort from Australia
Participants
The Childhood Determinants of Adult Health Study (CDAH) study is a follow-up of participants in the 1985 Australian Schools Health and Fitness Survey (ASHFS), a nationally representative sample of 8498 school children (4191 girls) aged 7–15 years (Gall et al., 2009) (Fig. 1). During 2004–2006, the first follow-up (CDAH-1) was conducted when participants were 26–36 years and 1598 female participants responded to questions on their menstrual cycle characteristics and PCOS. Among them 652 participants attended a study clinic and had plasma hormone measurements including total testosterone concentrations and sex hormone-binding globulin (SHBG) (Wei et al., 2009). The second follow-up (CDAH-2) was conducted during 2009–2011 when participants were aged 31–41 years and 1123 participants completed the same questions about menstrual cycles and PCOS. The current study included 1516 women who completed questions on menstrual cycles and/or PCOS in CDAH-1 and/or CDAH-2.
Figure 1.
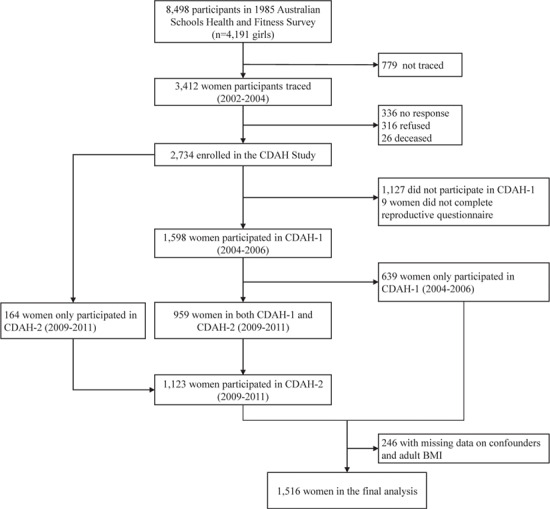
Flow chart of the study population for the Childhood Determinants of Adult Health Study in Australia, 1985–2011. CDAH: Childhood Determinants of Adult Health.
The study was approved by the Southern Tasmania Health and Medical Human Research Ethics Committee. Written informed consent was obtained during childhood from parents and at each follow-up from participants.
Childhood anthropometric measurements
BMI, calculated as weight (kg)/height (m)2, was derived from measured weight and height. BMI was classified as normal, overweight or obese according to the international age-sex-specific cut-points (Cole et al., 2000). BMI z-score was calculated based on age–sex-specific World Health Organization Child Growth standards (World Health Organization, 2006). Waist circumference was taken at the level of the umbilicus to the nearest 0.1 cm. Waist/height ratio (WHtR), calculated as waist circumference divided by height (cm), was the indicator of abdominal obesity when WHtR ≥ 0.5 (Brambilla et al., 2013).
Adult anthropometric measurements
Participants who attended CDAH-1 clinics (n = 2329) had weight, height and waist circumference measured. Participants who did not visit clinics (n = 1556) self-reported their weight and height, and a correction factor was applied to adjust for error, as described previously (Venn et al., 2007). BMI (kg/m2) was calculated from height and weight. Weight and height were self-reported at CDAH-2 and adjusted for error as described above. Adult BMI was categorized as normal (BMI < 25 kg/m2), overweight (25.0 ≤ BMI ≤ 29.9 kg/m2) or obese (BMI ≥ 30 kg/m2) (World Health Organization, 2000).
Adult menstrual irregularity and PCOS
We defined menstrual cycle length as the time from the first day of one period to the first day of the next and participants were questioned on the length of their usual menstrual cycle. Menstrual irregularity was defined as menstrual cycles ≥35 days or <25 days or reported as extremely irregular in CDAH-1 and/or CDAH-2. Women who were currently pregnant (n = 31), using hormonal contraceptives (n = 411) or had a hysterectomy (n = 1) were excluded.
Women were defined as having PCOS if they self-reported in CDAH-1 and/or CDAH-2 that they had ever been told by a doctor or they reported two symptoms of PCOS. The symptoms were menstrual cycle ≥35 days or totally variable and hirsutism. The validity of identifying women with PCOS by way of similar questions has been reported previously as moderately high (Taponen et al., 2004). The presence of hirsutism was defined as ever having seen a doctor because of concern about the amount of hair on their face.
Covariates
Age at menarche was self-reported in adulthood. Smoking history in childhood and adulthood were coded as ever or never smoked. Ever smoked in childhood was defined as having ≥10 cigarettes in their life. Former and current smokers in adulthood were defined as ever smoked. Highest parental education and own-education were classified as high school only, vocational training and any university education. Childhood alcohol consumption was classified as none (never consume alcohol), light (consume alcohol less than once/week), moderate (consume alcohol 1–2 days/week), heavy (consume alcohol 3–4 days/week) and very heavy (consume alcohol ≥5 days/week). Alcohol consumption in adulthood was classified according to daily alcohol intake: none (0 alcoholic drinks/day), light (0–1 alcoholic drinks/day), moderate (1–2 alcoholic drinks/day), heavy (>2–3 alcoholic drinks/day) and very heavy intake (>3 alcoholic drinks/day) based on Australian guidelines (Australian Government, 2009).
The Bogalusa Heart Study: a cohort from the USA
Participants
The Bogalusa Heart Study (BHS) is a biracial (65% white and 35% black) prospective cohort study of cardiovascular risk factors among children and young adults from Bogalusa, LA, USA (Brook, 1981). Initial study participants aged 3–18 years were enrolled from schools in 1973, and additional participants were recruited over time. Data collection occurred approximately every 2 years for children and 5 years for adults. These cross-sectional studies of children or adults were combined to create the overall BHS population.
The Bogalusa Babies substudy (BBS) began in May 2013 to examine the role of cardiovascular risk factors in childhood on reproductive outcomes. Women with at least one BHS visit (n = 5914) were eligible to participate. We included 1247 female participants who were aged 7–15 years during childhood visits (to align with the CDAH study), who participated in BBS when they were aged 26–57 years, and had height and weight reported between ages 26 and 40 years to align with their report of their menstrual cycle characteristics prior to age 40 years (Fig. 2).
Figure 2.
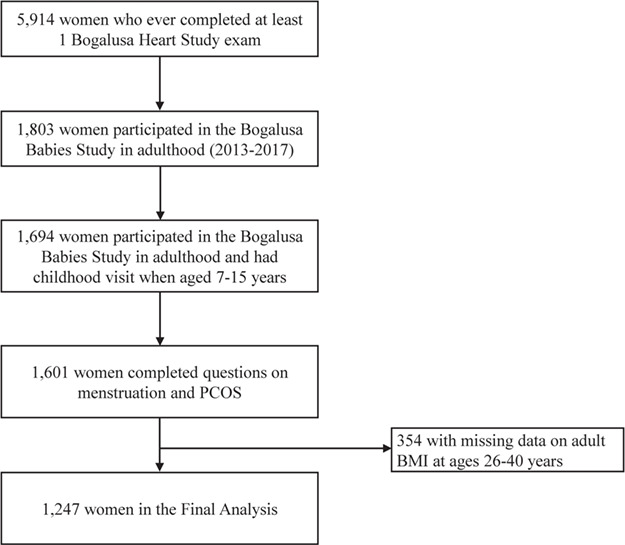
Flow chart of the study population for the Bogalusa Heart Study in the USA, 1973–2017. PCOS: polycystic ovary syndrome.
For child participants, parental permission and consent of the child were obtained and written informed consent was obtained from adult participants. All study procedures were approved by the Institutional Review Board of Tulane University.
Childhood anthropometric measurements
All BHS surveys followed an identical protocol for anthropometric measurements. In the subsample of BBS used in the current study, a total of 298 participants had childhood waist and hip circumference measured. Height, weight and waist circumference were measured twice to within 0.1 cm or 0.1 kg and mean values obtained. BMI, BMI z-score, WHtR, obesity and abdominal obesity were calculated or classified using the same criteria as described in the CDAH study.
Adult anthropometric measurements
Adult height and weight were recorded in the BBS (Paley et al., 2004). Where necessary, height and weight before age 40 years were extracted from records of the BHS (Berkey et al., 1993). BMI, overweight and obesity were calculated or classified using the same criteria as described in the CDAH study.
Adult menstrual irregularity and PCOS
Data on menstrual cycle characteristics were collected by questioning participants on the length of the average menstrual cycle between age 16 and 40 years (excluding any time spent pregnant, receiving birth control pills or injections, after menopause, or after having both ovaries or the uterus surgically removed). Participants reporting an average menstrual cycle of ≥35 days, <25 days, or totally variable were considered to have menstrual irregularity.
The classification of PCOS was based on the presence of both menstrual cycle ≥35 days or totally variable and hirsutism, or self-reported ever having been told by a doctor that she had PCOS. Hirsutism was determined by a series questions asking about the tendency to grow dark, coarse hair on eight body sites including upper lip, chin, breast, chest between the breasts, back, belly, upper arms and upper thighs. Those who indicated three or more sites were considered as having clinical hirsutism.
Covariates
Race (white/black) was recorded at the initial BHS visit. As previously described (Wattigney et al., 1999), information on age at menarche was obtained by a registered nurse. Smoking history in childhood and adulthood were coded as ever (currently or formerly at any visit) and never smoked. Highest parental and own-education were classified as high school only, vocational training and college or more (any university). Childhood and adulthood alcohol consumption were classified as none (tried or never drink), light (drink less than once/week), moderate (drink once or twice/week), heavy (drink three to four times/week) and very heavy drinker (drink daily or almost every day).
Statistical analyses
Means with SDs and numbers with proportions were used to describe participants’ sociodemographic characteristics, menstrual irregularity and PCOS in each cohort from baseline to follow-up. Taking into account the multiple adult visits conducted in CDAH and multiple childhood visits in BBS, multi-level generalized linear mixed effects models with Poisson regression were employed to estimate the relative risks (RRs) and 95% CIs.
In BBS, ~50% of participants had missing data on age at menarche and more than 20% of participants had missing data on highest parental education. Multiple imputation by chained equations was used to impute the missing data (Azur et al., 2011).
Covariates remaining in the final models were variables, which were causally related to the outcome, imbalanced between the exposure groups and resulted in more than 10% change in the coefficient of the principal study factor when added to the model. In analyses of the BBS, the models were additionally adjusted for race as appropriate.
Interactions between race and childhood adiposity on menstrual irregularity and PCOS in BBS were investigated in the regression model. There was no interaction between race and obesity on menstrual irregularity (P = 0.362); however, a statistically significant race interaction was present for PCOS (P = 0.042). Therefore, PCOS analyses in BBS were further stratified by race.
The following sensitivity analyses were conducted. First, we repeated the analysis by using the United States Centres for Disease Control and Prevention (CDC) growth reference to calculate BMI z-score and to classify childhood weight status (Harris et al., 2018). Second, the analysis was repeated after excluding persons who may have been of black (n = 8) or other non-white race (n = 35) in CDAH (race was inferred from the childhood questionnaire including the information on father’s and mother’s country of birth and language spoken at home) to compare with the results in BBS white participants. Third, associations were examined with the change between birth weight z-score and BMI z-score in childhood in a subsample of BBS (n = 788) with the relevant information on birth weight and gestational age available from birth certificates (Chen et al., 2012). Fourth, as hyperandrogenism is also a key diagnostic feature for PCOS, the association of childhood adiposity with biochemical hyperandrogenism was analysed in a subsample of CDAH (n = 652) who attended CDAH-1 clinics and were not using hormonal contraceptives. Biochemical hyperandrogenism was assessed by calculated free testosterone (cFT) levels (Vermeulen et al., 1999). The association of childhood adiposity with hirsutism was also analysed in CDAH and BBS. Fifth, we restricted our sample in BBS to women who were aged under 40 years at follow-up to ensure reporting of current menstrual characteristics and excluding retrospective reports from women aged 41–57 years. Last, a subgroup of underweight children was classified to investigate the associations of underweight in childhood with menstrual irregularity and PCOS in adulthood.
All analyses were performed using STATA software, version 15.0 (Stata Corp., College Station, TX, USA); a P value of <0.05 was considered statistically significant.
Results
Participant characteristics
Our sample included 1516 participants from the CDAH study and 1247 (white: 730; black: 517) participants from the BBS. Anthropometric and sociodemographic characteristics of participants in the two cohorts are shown in Table I. On average, BBS participants had a higher childhood BMI z-score and WHtR than CDAH participants. The prevalence of childhood obesity and abdominal obesity was 1.1 and 5.3% in CDAH and 7.5% (white: 5.2%; black: 10.8%) and 22.5% (white: 20.2%; black: 23.8%) in BBS. At follow-up, the mean age in CDAH-1 was 31.5 years, sand 36.4 years at CDAH-2. In BBS, the mean age was 44.1 years. The prevalence of menstrual irregularity was 16.7% in CDAH and 24.5% in BBS (white: 25.4%; black: 23.2%). The prevalence of PCOS was 7.4% in CDAH (the average of CDAH-1 and CDAH-2) and 8.0% (white: 10.7%; black: 4.3%) in BBS. Identification of PCOS by menstrual characteristics and hirsutism alone classified seven more participants with PCOS in CDAH and 16 more participants in BBS.
Table I.
Participants’ characteristics in the Childhood Determinants of Adult Health Study and the Babies substudy of the Bogalusa Heart Studya.
| Variable | CDAH (n = 1516) | BBS (n = 1247) | |||
|---|---|---|---|---|---|
| Childhood | Adulthood | Childhood | Adulthood | ||
| CDAH-1 | CDAH-2 | BBS | |||
| Race, % (n) | |||||
| White | 58.5(730) | ||||
| Black | 41.5(517) | ||||
| Age, years, mean (SD)b | 11.0 (2.5) | 31.5 (2.6) | 36.4 (2.6) | 11.6(2.0) | 44.1(7.9) |
| BMI, kg/m2, mean (SD)b | 18.2 (2.8) | 24.9 (5.2) | 25.4 (5.5) | 19.5(4.0) | 29.2(7.8) |
| BMI z-score, mean (SD)b | 0.16 (0.90) | 0.36(1.24) | |||
| BMI category, % (n)b | |||||
| Normal | 91.2 (1383) | 62.4 (943) | 58.7 (505) | 74.9(934) | 36.2(451) |
| Overweight | 7.7 (116) | 23.7 (358) | 24.5 (211) | 17.6(219) | 24.9(310) |
| Obese | 1.1 (17) | 14.0 (211) | 16.7 (144) | 7.5(94) | 39.0(486) |
| Waist/height ratio, mean (SD)b | 0.43 (0.04) | 0.46(0.07) | |||
| WHtR categoryb | |||||
| < 0.5 | 94.7 (1436) | 77.6(228) | |||
| ≥ 0.5 | 5.3 (80) | 22.5(66) | |||
| Highest parental education, % (n) | |||||
| University education | 28.0 (425) | 29.8(334) | |||
| Vocational training | 33.5 (508) | 21.7(234) | |||
| High school | 38.5 (583) | 48.5(543) | |||
| Highest own-education, % (n) | |||||
| University education | 46.6 (704) | 54.0 (464) | 28.1(350) | ||
| Vocational training | 25.9 (392) | 25.4 (218) | 33.4(416) | ||
| High school | 27.5 (416) | 20.7 (178) | 38.6(481) | ||
| Smoking, % (n) | |||||
| Never smoked | 88.2 (1035) | 54.7 (827) | 59.2 (507) | 76.8(763) | 53.3(471) |
| Ever smoked | 11.8 (1439) | 45.3 (684) | 40.8 (350) | 23.2(230) | 46.7(412) |
| Alcohol consumption, % (n) | |||||
| None, light, moderate drinker | 99.1 (1166) | 93.8 (1398) | 95.8 (800) | 98.5(400) | 82.9(707) |
| Heavy and very heavy drinker | 0.9 (11) | 6.2 (93) | 4.2 (35) | 1.5(6) | 17.1(146) |
| Age at menarche, years, mean (SD) | 13.1 (1.3) | 12.6(1.5) | |||
| SHBG, nmol/l, mean (SD)c | 52.1 (27.4) | ||||
| Testosterone, nmol/l, mean (SD)c | 1.5 (0.6) | ||||
| Free testosterone, nmol/l, mean (SD)c | 23.9 (14.0) | ||||
| Hirsutism, % (n) | |||||
| Yes | 3.5 (52) | 4.0 (34) | 6.5(81) | ||
| No | 96.5 (1439) | 96.0 (820) | 93.5(1160) | ||
| Menstrual irregularity, % (n) | |||||
| Yes | 16.6 (139) | 16.7 (87) | 24.5(303) | ||
| No | 83.4 (699) | 83.3 (434) | 75.5(935) | ||
| PCOS (menstrual irregularity+hirsutism), % (n)d | |||||
| Yes | 1.5 (12) | 1.4 (7) | 2.2(27) | ||
| No | 98.6 (817) | 98.7 (513) | 97.8(1211) | ||
| Self-reported doctor diagnosed PCOS, % (n) | |||||
| Yes | 5.8 (88) | 8.2 (70) | 6.9(84) | ||
| No | 94.2 (1423) | 91.8 (785) | 93.2(1142) | ||
| PCOS, % (n) | |||||
| Yes | 6.2 (93) | 8.6 (74) | 8.0(100) | ||
| No | 93.9 (1419) | 91.4 (786) | 92.0(1147) | ||
aSample size varied [range from 652–1512 for Childhood Determinants of Adult Health (CDAH) and range from 294–1241 for Babies substudy of the Bogalusa Heart Study (BBS)] because of the missing data
bThe variables were calculated using the mean values in multiple childhood visits in BBS
cAvailable in a subsample of 622 participants who attended the first follow-up clinic in CDAH
dDefined as reporting menstrual cycle ≥ 35 days/totally variable and presenting hirsutism
PCOS, polycystic ovary syndrome; SHBG, sex hormone-binding globulin; WHtR, waist/height ratio
Childhood adiposity and menstrual irregularity
Table II shows the associations of childhood adiposity with menstrual irregularity in CDAH and in the overall BBS. In CDAH (after adjusting for childhood age, age at menarche, highest parental and their own education), compared with normal-weight girls, the risk of reporting menstrual irregularity was almost 3-fold in those who were obese in childhood. Similarly, in the BBS, when further adjusted for race, childhood obesity was associated with nearly twice the risk of having menstrual irregularity.
Table II.
Associations of adiposity in childhood with menstrual irregularity in adulthood in CDAH and BBS.
| Childhood adiposity | CDAH | BBS | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted model (n = 1010) | Model 1 (n = 1010) | Unadjusted model (n = 1238) | Model 1a (n = 1238) | |||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | |
| BMI z-score | 1.11 | 0.94–1.31 | 1.13 | 0.96–1.34 | 1.09 | 1.00–1.18 | 1.09 | 1.00–1.19 |
| BMI category | ||||||||
| Normal | Ref. | — | Ref. | — | Ref. | — | Ref. | — |
| Overweight | 1.50 | 0.99–2.28 | 1.62 | 1.06–2.48 | 1.07 | 0.88–1.29 | 1.07 | 0.88–1.30 |
| Obese | 2.72 | 1.60–4.64 | 2.84 | 1.63–4.96 | 1.43 | 1.08–1.89 | 1.44 | 1.08–1.92 |
| WHtR, per 0.01 unitb | 1.03 | 1.00–1.06 | 1.03 | 0.99–1.06 | 1.03 | 1.00–1.06 | 1.03 | 0.99–1.06 |
| WHtR categoryb | ||||||||
| < 0.5 | Ref. | — | Ref. | — | Ref. | — | Ref. | — |
| ≥ 0.5 | 1.44 | 0.87–2.39 | 1.47 | 0.89–2.45 | 1.62 | 1.06–2.46 | 1.56 | 1.00–2.45 |
Model 1: adjust for childhood age, age at menarche, highest parental education and own-education
aModel 1 further adjust for race in the BBS
b n = 293 in BBS
RR, relative risk
Childhood adiposity and self-reported PCOS
In CDAH, childhood obesity defined by BMI and childhood abdominal obesity defined by WHtR were significantly associated with an increased risk of self-reported PCOS (Table III). A 0.01 unit increase in childhood WHtR was associated with a 5% increased likelihood of self-reported PCOS. In the BBS sample overall, results were consistent with CDAH: childhood obesity was associated with a higher risk of self-reported PCOS and every 0.01 unit increase in WHtR was associated with 8% greater likelihood of PCOS (Table III).
Table III.
Associations of adiposity in childhood with PCOS in adulthood in CDAH and BBS.
| Childhood adiposity | CDAH | BBS | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted model (n = 1516) | Model 1 (n = 1516) | Unadjusted model (n = 1247) | Model 1a (n = 1247) | |||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | |
| BMI z-score | 1.25 | 0.98–1.58 | 1.26 | 0.98–1.62 | 1.31 | 1.10–1.56 | 1.42 | 1.19–1.69 |
| BMI category | ||||||||
| Normal | Ref. | — | Ref. | — | Ref. | — | Ref. | — |
| Overweight | 2.33 | 1.30–4.15 | 2.28 | 1.25–4.16 | 1.73 | 1.21–2.46 | 1.96 | 1.35–2.83 |
| Obese | 3.08 | 0.85–11.21 | 4.05 | 1.10–14.83 | 1.68 | 1.00–2.83 | 1.95 | 1.19–3.29 |
| WHtR, per 0.01 unitb | 1.06 | 1.01–1.10 | 1.06 | 1.01–1.11 | 1.05 | 0.98–1.11 | 1.06 | 1.01–1.11 |
| WHtR categoryb | ||||||||
| <0.5 | Ref. | — | Ref. | — | Ref. | — | Ref. | — |
| ≥0.5 | 2.22 | 1.11–4.42 | 2.26 | 1.16–4.42 | 0.99 | 0.34–2.91 | 1.09 | 0.39–3.07 |
Model 1: adjust for childhood age, age at menarche, highest parental education and own-education
aModel 1 further adjust for race in BBS
b n = 294 in BBS
Racial differences in the associations of self-reported PCOS in BBS
Significant racial differences were observed in the associations of childhood adiposity with self-reported PCOS, but not with menstrual irregularity, in BBS white and black participants (Table IV). Childhood obesity and a 0.01 unit increase in WHtR were both associated with an increased risk of PCOS in BBS white participants, but no significant associations of childhood obesity or WHtR with PCOS were found in BBS black participants.
Table IV.
Associations of adiposity in childhood with PCOS in adulthood in BBS, by race.
| Race and childhood adiposity | PCOS | ||||
|---|---|---|---|---|---|
| n | Unadjusted model | Model 1 | |||
| RR | 95% CI | RR | 95% CI | ||
| White | 730 | ||||
| BMI z-score | 1.50 | 1.25–1.79 | 1.54 | 1.28–1.87 | |
| BMI category | |||||
| Normal | Ref. | — | Ref. | — | |
| Overweight | 2.07 | 1.38–3.11 | 2.19 | 1.42–3.35 | |
| Obese | 2.82 | 1.63–4.86 | 2.93 | 1.65–5.22 | |
| WHtR, per 0.01 unita | 1.08 | 1.04–1.11 | 1.11 | 1.05–1.17 | |
| WHtR categorya | |||||
| <0.5 | Ref. | — | Ref. | — | |
| ≥0.5 | 2.00 | 0.66–6.07 | 2.00 | 0.64–6.27 | |
| Black | 517 | ||||
| BMI z-score | 1.00 | 0.70–1.43 | 1.03 | 0.72–1.47 | |
| BMI category | |||||
| Normal | Ref. | — | Ref. | — | |
| Overweight | 1.36 | 0.59–3.13 | 1.43 | 0.64–8.26 | |
| Obese | 0.29 | 0.04–2.35 | 0.19 | 0.03–2.78 | |
| WHtR, per 0.01 unita | 0.89 | 0.76–1.04 | 0.88 | 0.76–1.02 | |
| WHtR categorya | |||||
| <0.5 | Ref. | — | Ref. | — | |
| ≥0.5 | N/A | N/A | |||
Model 1: adjust for childhood age, age at menarche, highest parental education and own-education
a n = 109 in white race and n = 185 in black race in BBS
Influence of weight status from childhood into adulthood
The RR of menstrual irregularity by change of weight status from childhood to adulthood is displayed in Table V. Compared with participants who had persistently normal BMI in childhood and adulthood, those who became overweight or obese in adulthood reported a higher risk of menstrual irregularity in BBS. Participants who were persistently overweight/obese since childhood had significantly higher risks of menstrual irregularity in both CDAH and BBS.
Table V.
Associations of weight status change from childhood to adulthood with menstrual irregularity in CDAH and the BBS.
| Weight status from childhood to adulthood | CDAH | BBS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Cases (%)a | Unadjusted model | Model 1 | n | Cases (%)a | Unadjusted model | Model 1a | |||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | |||||
| 1010 | 1238 | |||||||||||
| Persistently normal | 786 (57.9) | Ref. | — | Ref. | — | 850 (36.4) | Ref. | — | Ref. | — | ||
| Normal to overweight/obese | 447 (32.9) | 0.97 | 0.93–1.30 | 1.04 | 0.77–1.39 | 889 (38.0) | 1.26 | 1.01–1.57 | 1.29 | 1.03–1.61 | ||
| Overweight/obese to normal | 16 (1.2) | 1.57 | 0.65–3.81 | 1.65 | 0.70–3.89 | 44 (1.9) | 1.47 | 0.89–2.43 | 1.47 | 0.88–2.46 | ||
| Persistently overweight/obese | 108 (8.0) | 1.63 | 1.09–2.45 | 1.76 | 1.15–2.71 | 554 (23.7) | 1.36 | 1.01–1.83 | 1.39 | 1.01–1.90 | ||
Model 1: adjust for age at menarche, highest parental education and own-education
aThe total number of observations in each weight status category from childhood to adulthood
No significant association of any weight status category from childhood to adulthood with PCOS was found in BBS black participants (Table VI). In white participants, those who were overweight or obese in childhood only, or persistently overweight or obese from childhood to adulthood, had a significantly increased risk of PCOS (Table VI).
Table VI.
Associations of weight status change from childhood to adulthood with PCOS in CDAH and BBS.
| Weight status from childhood to adulthood | CDAH | BBS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Cases (%)a | Unadjusted model | Model 1 | n | Cases (%)a | Unadjusted model | Model 1b | |||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | |||||
| Overall | 1516 | 1247 | ||||||||||
| Persistently normal | 1414 (59.7) | Ref. | — | Ref. | — | 855 (36.2) | Ref. | — | Ref. | — | ||
| Normal to overweight/obese | 455 (31.9) | 1.19 | 0.81–1.73 | 1.34 | 0.93–1.94 | 900 (38.1) | 0.80 | 0.52–1.22 | 1.00 | 0.65–1.53 | ||
| Overweight/obese to normal | 31 (1.3) | 0.92 | 0.13–6.40 | 1.02 | 0.15–6.93 | 46 (2.0) | 1.99 | 0.78–5.05 | 2.69 | 1.10–6.62 | ||
| Persistently overweight/obese | 169 (7.1) | 2.93 | 1.65–5.18 | 3.66 | 2.05–6.56 | 560 (23.7) | 2.55 | 1.47–4.43 | 3.72 | 2.12–6.54 | ||
| White | 730 | |||||||||||
| Persistently normal | 651 (44.9) | Ref. | — | Ref. | — | |||||||
| Normal to overweight/obese | 489 (33.7) | 1.03 | 0.67–1.60 | 1.06 | 0.69–1.63 | |||||||
| Overweight/obese to normal | 25 (1.7) | 4.00 | 1.66–9.62 | 4.70 | 1.93–11.43 | |||||||
| Persistently overweight/obese | 286 (19.7) | 4.66 | 2.62–8.28 | 5.41 | 2.98–9.83 | |||||||
| Black | 517 | |||||||||||
| Persistently normal | 204 (22.4) | Ref. | — | Ref. | — | |||||||
| Normal to overweight/obese | 411 (45.2) | 0.43 | 0.13–1.42 | 0.46 | 0.15–1.43 | |||||||
| Overweight/obese to normal | 21 (2.3) | N/A | N/A | |||||||||
| Persistently overweight/obese | 274 (30.1) | 0.75 | 0.21–2.65 | 0.88 | 0.28–2.80 | |||||||
Model 1: adjust for age at menarche, highest parental education and own-education
aThe total number of observations in each BMI category from childhood to adulthood
bModel 1 further adjust for race in the overall BBS
Sensitivity analyses
Similar estimates were found in sensitivity analyses, in which the US CDC standards were used to calculate childhood BMI z-score and classify childhood obesity according to BMI (Supplementary Table SI, Tables SII and SIII). When women of non-white races (n = 43) were excluded in CDAH, the associations between increased childhood BMI and menstrual irregularity remained statistically significant. The associations between increased childhood BMI, WHtR and PCOS also remained statistically significant with only small changes in the mean coefficients (−2.5–11.20%). In a subsample of participants who had birth weight and gestational age in BBS (n = 788), we found the z-score increment between weight at birth and BMI in childhood was associated with increased risk of menstrual irregularity and PCOS in white participants but no statistically significant association was found in black participants (Supplementary Table SIV).
In a subsample of participants who attended CDAH-1 clinics (n = 652), childhood BMI z-score (β = 2.82 pmol/l, 95% CI: 1.67–3.98) and childhood WHtR (β = 0.59 pmol/l, 95% CI: 0.33–0.86) were positively associated with cFT in adulthood. In CDAH, childhood BMI z-score (RR = 1.50, 95% CI: 1.30–2.00) and WHtR (RR = 1.07, 95% CI: 1.01–1.12) were positively associated with hirsutism at follow-up; similar associations of childhood BMI z-score (RR = 1.61 95% CI: 1.25–2.09) and WHtR (RR = 1.13, 95% CI: 1.05–1.21) with hirsutism were found in BBS white but not black participants.
When restricting the sample to women who were aged under 40 years in the analysis of menstrual irregularity in BBS (n = 431) (Supplementary Table SV), the risks of menstrual irregularity remained elevated for participants with high childhood adiposity, although less so, and achieved borderline significance for childhood obesity (RR = 1.50, 95% CI 0.94–2.41, P = 0.090) and childhood abdominal obesity (RR = 1.55, 95% CI 0.99–2.41, P = 0.055). No significant associations of childhood underweight in CDAH (n = 14) and BBS (n = 22) with menstrual irregularity and PCOS in adulthood were found.
Discussion
This study is the first to report the association of childhood abdominal obesity with menstrual irregularity and PCOS in adulthood, using data from two independent large prospective cohorts in two countries. Overall, in both cohorts, childhood obesity but not abdominal obesity was associated with greater risks of menstrual irregularity. A significant racial difference was observed in the associations of childhood obesity and abdominal obesity with PCOS, with significant associations found in white participants, but not in black participants. The risks of menstrual irregularity and PCOS were consistently significantly higher in participants with persistent overweight/obesity since childhood.
The positive association between childhood obesity and adulthood menstrual irregularity is consistent with prior findings from the 1958 British birth cohort (Lake et al., 1997). Though some studies have suggested that the distribution of body fat in adult women may be a risk factor of menstrual irregularity cross-sectionally (Douchi et al., 2002; Wei et al., 2009), no statistically significant association of childhood abdominal obesity with menstrual irregularity was found in CDAH and BBS. The mechanisms underlying the associations of greater childhood BMI with menstrual irregularity in adulthood may include a series of hormonal factors. Childhood obesity is a risk factor for increased concentrations of testosterone, LH, insulin and reduced concentrations of SHBG in adulthood (Marcovecchio and Chiarelli, 2013; Elizondo-Montemayor et al., 2017). These changes may cause a disruption of normal ovulation and menstrual irregularity.
It is known that PCOS and menstrual irregularity are strongly correlated. We found that the positive associations of childhood BMI and WHtR with self-reported PCOS in adulthood were strong in CDAH and BBS white participants. Menstrual irregularity is part of the diagnostic criteria for PCOS (Teede et al., 2018), and childhood obesity was correlated with menstrual irregularity in the current study, therefore, this may explain the observed associations. Phenotypic features (including menstrual irregularity and hyperandrogenism) of PCOS are known to be regulated by obesity cross-sectionally, typically involving a distribution of central fat (Legro, 2012; de Zegher et al., 2018). Our finding of the positive associations between childhood BMI, childhood WHtR and cFT in adulthood in a subsample of participants in CDAH suggested that higher childhood adiposity increased the risk of hyperandrogenism. Childhood obesity as well as abdominal obesity may act to promote menstrual irregularity and hyperandrogenism in those at higher risk of PCOS.
No significant association of adiposity with PCOS was found in BBS black girls. A previous cross-sectional study by Christensen et al., (2013) also reported that the association between BMI and PCOS was weaker in black girls than white girls. The literature has indicated that although there are substantial racial differences in the prevalence of obesity, the prevalence of PCOS is similar in different races (Knochenhauer et al., 1998; Azziz et al., 2004; Wolf et al., 2018). In our study, BBS black participants had a higher prevalence of childhood obesity than white participants (10.8 versus 5.2%, respectively), but their prevalence of PCOS was lower than white participants (4.3 versus 10.7%, respectively). The explanations for this racial difference are unclear. It is possible that lower socioeconomic status and poorer health service access and utilisation among black women may result in a lower rate of diagnosis (Merkin et al., 2016). These factors may thereby dilute the associations observed in black participants. However, in BBS, a stronger association of childhood adiposity with hirsutism was still observed among white compared to black participants. While previous studies have suggested that black women with PCOS have increased risk of metabolic syndrome and cardiovascular disease compared with white women with PCOS (Hillman et al., 2014; Chan et al., 2017), the associations of adiposity with PCOS between races have not been clearly defined. We are the first to report in longitudinal studies that there are racial differences in how childhood adiposity associates with the development of PCOS.
The lack of association of childhood adiposity with PCOS in black participants also suggests that high childhood adiposity is not the only driver of adult PCOS and many other factors may play a role in PCOS development and progression. Prenatal androgen exposure has been proposed as a cause of PCOS although the evidence from human studies is inconsistent (Hickey et al., 2009). Familial trends in PCOS are reported, but no specific genetic association has been reported and more research is necessary to define the genetic basis (Crespo et al., 2018). Environmental factors, including health-related behaviours or lifestyles and economic disadvantage, are potentially involved in the aetiology, prevalence and modulation of PCOS (Merkin et al., 2016). It is likely that there are genetic, molecular and environmental factors that contribute to the racial differences in childhood adiposity-related PCOS.
The risks of menstrual irregularity and PCOS were significantly higher in women with persistent overweight/obesity since childhood in both CDAH and BBS, consistent with findings from the Northern Finland 1966 birth cohort study (Laitinen et al., 2003). Furthermore, in our study, for white participants in BBS, we found for the first time that women who were overweight/obese in childhood but not in adulthood also reported a significantly higher risk of PCOS, suggesting independent effects of childhood adiposity that need to be confirmed in larger studies.
There are several limitations in our study. First, menstrual cycle characteristics and PCOS were self-reported by questionnaire. Previous studies have suggested that women’s retrospective self-report of menstrual length can be prone to error (Small et al., 2007) and the agreement between diary records and retrospectively recalled menstrual cycle length was moderate (Jukic et al., 2008). Self-reported PCOS likely tends to underestimate prevalence (Varanasi et al., 2018; Wang et al., 2018). Also, if the accuracy of self-reported menstrual cycle length and PCOS differed by obesity status, then our effect estimates might have been biased. However, previous studies have shown no evidence of this (Laitinen et al., 2003; Small et al., 2007; Jukic et al., 2008).
A second potential limitation of this study was the exclusion of women using hormonal contraception (28.4%) in the analysis of menstrual irregularity in CDAH. Since hormonal contraception is commonly prescribed for menstrual irregularity (Bulletins—Gynecology, 2013), we may have under-estimated the prevalence of menstrual irregularity. Third, we have limited information on the age at which PCOS was diagnosed in the two cohorts. Only in the second follow-up in CDAH were participants asked to report the age when their PCOS was diagnosed (ages ranged from 14–36 years with only four participants reporting the diagnosis of PCOS before age 18 years). It has been suggested that adolescents with characteristics of PCOS should be reassessed at or before full reproductive maturity, at 8 years post menarche (Teede et al., 2018) to confirm a diagnosis. In this study, participants reporting a diagnosis of PCOS during adolescence may have been misclassified. Fourth, the diagnostic criteria for PCOS have recently changed (Teede et al., 2018) and there may have been differences in how PCOS was diagnosed in Australia compared to the USA. Despite all of these limitations, we showed that the prevalence of menstrual irregularity and PCOS in the two cohorts was consistent with the literature (Weller and Weller, 1998; March et al., 2010).
Finally, some characteristics of those continuing in the study differed from those lost to follow-up, and this might limit the generalizability of the findings. In CDAH, non-participants had higher BMI and WHtR values, on average, in childhood than the participants, indicating the current sample may have comprised healthier participants. However, if non-participants were also more likely to have menstrual irregularity and PCOS in adulthood than participants, the effect of this bias would be to underestimate the magnitude of the associations observed. Participants in the BBS were more likely to be black (41 versus 34%) compared with the rest of the study cohort, but childhood BMI was similar among participants and non-participants (Wang et al., 2018).
Strengths of our study include that this is the first prospective study to investigate the long-term associations between childhood abdominal obesity measures and menstrual irregularity and PCOS. Second, we used two independent cohorts from two countries and reported consistent findings. Third, we were able to consider associations by race in BBS.
In conclusion, greater childhood BMI was associated with an increased risk of menstrual irregularity in adulthood in both CDAH and BBS. Greater childhood BMI and WHtR were associated with an increased risk of PCOS in adulthood in CDAH, and in BBS white participants. These risks were significantly higher in women with persistent overweight/obesity since childhood. No significant association of adiposity with PCOS was found in BBS black participants, suggesting there are racial differences in childhood adiposity associating with the development of PCOS, and other environmental or genetic factors are important. Whether high childhood adiposity is causal or an early independent indicator of underlying hormonal or metabolic disorders related to PCOS needs further clarification.
Supplementary Material
Acknowledgements
We gratefully acknowledge the contributions of the CDAH project manager, Marita Dalton, and BHS project manager, Dr Shu Tian, CDAH and BHS participants and all other project staff.
Authors’ roles
Y.H. performed the statistical analysis and drafted the manuscript. J.T. provided analytical and interpretive advice and helped draft the manuscript. L.B. assisted with the data analysis and provided interpretive advice. W.H.O. provided interpretive advice and helped draft the manuscript. T.D. was involved in conceptualization of the study and provided interpretive advice. L.A.B. helped with acquisition of data and provided interpretive advice. M.H. provided interpretive advice and provided critical revision of the manuscript. E.W.H helped with acquisition of data, provided interpretive advice and critical revision of the manuscript. A.J.V. was involved in the conceptualization of the study, acquisition of data, and helped draft the manuscript. All authors have reviewed and approved the final manuscript.
Funding
The CDAH study was supported by grants from the National Health and Medical Research Council (grants 211316, 544923 and 1128373). The Bogalusa Heart Study is supported by National Institutes of Health grants R01HD069587, AG16592, HL121230, HD032194 and P50HL015103.
Conflict of interest
There is no conflict of interest.
References
- Australian Government NHMRC Australian Guidelines to Reduce Health Risks from Drinking Alcohol. Canberra: National Health and Medical Research Council, 2009 [Google Scholar]
- Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res 2011;20:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 2004;89:2745–2749. [DOI] [PubMed] [Google Scholar]
- Berkey CS, Dockery DW, Wang X, Wypij D, Ferris B Jr. Longitudinal height velocity standards for U.S. adolescents. Stat Med 1993;12:403–414. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Bedogni G, Heo M, Pietrobelli A. Waist circumference-to-height ratio predicts adiposity better than body mass index in children and adolescents. Int J Obes (Lond) 2013;37:943–946. [DOI] [PubMed] [Google Scholar]
- Brook CGD. Cardiovascular risk factors in children: the early natural history of atherosclerosis and essential hypertension. Arch Dis Child 1981;56:488. [Google Scholar]
- Bulletins—Gynecology CoP Practice bulletin no. 136: management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet Gynecol 2013;122:176–185. [DOI] [PubMed] [Google Scholar]
- Chan JL, Kar S, Vanky E, Morin-Papunen L, Piltonen T, Puurunen J, Tapanainen JS, Maciel GAR, Hayashida SAY, Soares JM Jr et al. . Racial and ethnic differences in the prevalence of metabolic syndrome and its components of metabolic syndrome in women with polycystic ovary syndrome: a regional cross-sectional study. Am J Obstet Gynecol 2017;217:189.e1–189.e8. [DOI] [PubMed] [Google Scholar]
- Chen W, Srinivasan SR, Yao L, Li S, Dasmahapatra P, Fernandez C, Xu J, Berenson GS. Low birth weight is associated with higher blood pressure variability from childhood to young adulthood: the Bogalusa Heart Study. Am J Epidemiol 2012;176Suppl 7:S99–S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen SB, Black MH, Smith N, Martinez MM, Jacobsen SJ, Porter AH, Koebnick C. Prevalence of polycystic ovary syndrome in adolescents. Fertil Steril 2013;100:470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000;320:1240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo RP, Bachega T, Mendonca BB, Gomes LG. An update of genetic basis of PCOS pathogenesis. Arch Endocrinol Metab 2018;62:352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegher F, Lopez-Bermejo A, Ibanez L. Central obesity, faster maturation, and ‘PCOS’ in girls. Trends Endocrinol Metab 2018;29:815–818. [DOI] [PubMed] [Google Scholar]
- Zegher F, Reinehr T, Malpique R, Darendeliler F, Lopez-Bermejo A, Ibanez L. Reduced prenatal weight gain and/or augmented postnatal weight gain precedes polycystic ovary syndrome in adolescent girls. Obesity (Silver Spring) 2017;25:1486–1489. [DOI] [PubMed] [Google Scholar]
- Douchi T, Kuwahata R, Yamamoto S, Oki T, Yamasaki H, Nagata Y. Relationship of upper body obesity to menstrual disorders. Acta Obstet Gynecol Scand 2002;81:147–150. [DOI] [PubMed] [Google Scholar]
- Elizondo-Montemayor L, Hernandez-Escobar C, Lara-Torre E, Nieblas B, Gomez-Carmona M. Gynecologic and obstetric consequences of obesity in adolescent girls. J Pediatr Adolesc Gynecol 2017;30:156–168. [DOI] [PubMed] [Google Scholar]
- Gall SL, Jamrozik K, Blizzard L, Dwyer T, Venn A. Healthy lifestyles and cardiovascular risk profiles in young Australian adults: the Childhood Determinants of Adult Health Study. Eur J Cardiovasc Prev Rehabil 2009;16:684–689. [DOI] [PubMed] [Google Scholar]
- Hahn KA, Wise LA, Riis AH, Mikkelsen EM, Rothman KJ, Banholzer K, Hatch EE. Correlates of menstrual cycle characteristics among nulliparous Danish women. Clin Epidemiol 2013;5:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HR, Babic A, Webb PM, Nagle CM, Jordan SJ, Risch HA, Rossing MA, Doherty JA, Goodman MT, Modugno F et al. . Polycystic ovary syndrome, oligomenorrhea, and risk of ovarian cancer histotypes: evidence from the Ovarian Cancer Association Consortium. Cancer Epidemiol Biomarkers Prev 2018;27:174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HR, Titus LJ, Cramer DW, Terry KL. Long and irregular menstrual cycles, polycystic ovary syndrome, and ovarian cancer risk in a population-based case-control study. Int J Cancer 2017;140:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey M, Sloboda DM, Atkinson HC, Doherty DA, Franks S, Norman RJ, Newnham JP, Hart R. The relationship between maternal and umbilical cord androgen levels and polycystic ovary syndrome in adolescence: a prospective cohort study. J Clin Endocrinol Metab 2009;94:3714–3720. [DOI] [PubMed] [Google Scholar]
- Hillman JK, Johnson LN, Limaye M, Feldman RA, Sammel M, Dokras A. Black women with polycystic ovary syndrome (PCOS) have increased risk for metabolic syndrome and cardiovascular disease compared with white women with PCOS [corrected]. Fertil Steril 2014;101:530–535. [DOI] [PubMed] [Google Scholar]
- Jacobsen BK, Knutsen SF, Oda K, Fraser GE. Obesity at age 20 and the risk of miscarriages, irregular periods and reported problems of becoming pregnant: the Adventist Health Study-2. Eur J Epidemiol 2012;27:923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukic AM, Weinberg CR, Wilcox AJ, McConnaughey DR, Hornsby P, Baird DD. Accuracy of reporting of menstrual cycle length. Am J Epidemiol 2008;167:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab 1998;83:3078–3082. [DOI] [PubMed] [Google Scholar]
- Koivuaho E, Laru J, Ojaniemi M, Puukka K, Kettunen J, Tapanainen JS, Franks S, Jarvelin MR, Morin-Papunen L, Sebert S et al. . Age at adiposity rebound in childhood is associated with PCOS diagnosis and obesity in adulthood-longitudinal analysis of BMI data from birth to age 46 in cases of PCOS. Int J Obes (Lond) 2019;43:1370–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen J, Taponen S, Martikainen H, Pouta A, Millwood I, Hartikainen AL, Ruokonen A, Sovio U, McCarthy MI, Franks S et al. . Body size from birth to adulthood as a predictor of self-reported polycystic ovary syndrome symptoms. Int J Obes Relat Metab Disord 2003;27:710–715. [DOI] [PubMed] [Google Scholar]
- Lake JK, Power C, Cole TJ. Women’s reproductive health: the role of body mass index in early and adult life. Int J Obes Relat Metab Disord 1997;21:432–438. [DOI] [PubMed] [Google Scholar]
- Legro RS. Obesity and PCOS: implications for diagnosis and treatment. Semin Reprod Med 2012;30:496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod 2010;25:544–551. [DOI] [PubMed] [Google Scholar]
- Marcovecchio ML, Chiarelli F. Obesity and growth during childhood and puberty. World Rev Nutr Diet 2013;106:135–141. [DOI] [PubMed] [Google Scholar]
- Merkin SS, Phy JL, Sites CK, Yang D. Environmental determinants of polycystic ovary syndrome. Fertil Steril 2016;106:16–24. [DOI] [PubMed] [Google Scholar]
- Obesity: preventing and managing the global epidemic. Report of a WHO consultation World Health Organ Tech Rep Ser 2000;894:i–253. [PubMed] [Google Scholar]
- Ollila MM, Piltonen T, Puukka K, Ruokonen A, Jarvelin MR, Tapanainen JS, Franks S, Morin-Papunen L. Weight gain and dyslipidemia in early adulthood associate with polycystic ovary syndrome: prospective cohort study. J Clin Endocrinol Metab 2016;101:739–747. [DOI] [PubMed] [Google Scholar]
- Paley J, Talor J, Levin A, Bhave A, Paley D, Herzenberg JE. The multiplier method for prediction of adult height. J Pediatr Orthop 2004;24:732–737. [DOI] [PubMed] [Google Scholar]
- Small CM, Manatunga AK, Marcus M. Validity of self-reported menstrual cycle length. Ann Epidemiol 2007;17:163–170. [DOI] [PubMed] [Google Scholar]
- Solomon CG, Hu FB, Dunaif A, Rich-Edwards JE, Stampfer MJ, Willett WC, Speizer FE, Manson JE. Menstrual cycle irregularity and risk for future cardiovascular disease. J Clin Endocrinol Metab 2002;87:2013–2017. [DOI] [PubMed] [Google Scholar]
- Taponen S, Ahonkallio S, Martikainen H, Koivunen R, Ruokonen A, Sovio U, Hartikainen AL, Pouta A, Laitinen J, King V et al. . Prevalence of polycystic ovaries in women with self-reported symptoms of oligomenorrhoea and/or hirsutism: Northern Finland Birth Cohort 1966 Study. Hum Reprod 2004;19:1083–1088. [DOI] [PubMed] [Google Scholar]
- Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ, International PN . Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod 2018;33:1602–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varanasi LC, Subasinghe A, Jayasinghe YL, Callegari ET, Garland SM, Gorelik A, Wark JD. Polycystic ovarian syndrome: prevalence and impact on the wellbeing of Australian women aged 16-29 years. Aust N Z J Obstet Gynaecol 2018;58:222–233. [DOI] [PubMed] [Google Scholar]
- Venn AJ, Thomson RJ, Schmidt MD, Cleland VJ, Curry BA, Gennat HC, Dwyer T. Overweight and obesity from childhood to adulthood: a follow-up of participants in the 1985 Australian Schools Health and Fitness Survey. Med J Aust 2007;186:458–460. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–3672. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xiong X, Bazzano L, Harville EW. Childhood cardiovascular health and subfertility: the Bogalusa Heart Study. Pediatr Res 2018;84:625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattigney WA, Srinivasan SR, Chen W, Greenlund KJ, Berenson GS. Secular trend of earlier onset of menarche with increasing obesity in black and white girls: the Bogalusa Heart Study. Ethn Dis 1999;9:181–189. [PubMed] [Google Scholar]
- Wei S, Schmidt MD, Dwyer T, Norman RJ, Venn AJ. Obesity and menstrual irregularity: associations with SHBG, testosterone, and insulin. Obesity (Silver Spring) 2009;17:1070–1076. [DOI] [PubMed] [Google Scholar]
- Weller A, Weller L. Assessment of menstrual regularity and irregularity using self-reports and objective criteria. J Psychosom Obstet Gynaecol 1998;19:111–116. [DOI] [PubMed] [Google Scholar]
- West S, Lashen H, Bloigu A, Franks S, Puukka K, Ruokonen A, Jarvelin MR, Tapanainen JS, Morin-Papunen L. Irregular menstruation and hyperandrogenaemia in adolescence are associated with polycystic ovary syndrome and infertility in later life: Northern Finland Birth Cohort 1986 study. Hum Reprod 2014;29:2339–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Obesity: preventing and managing the global epidemic. World Health Organ Tech Rep Ser 2000;894:i-253. [PubMed] [Google Scholar]
- WHO child growth standards WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. 2006. World Health Organization, Geneva. [Google Scholar]
- Wolf WM, Wattick RA, Kinkade ON, Olfert MD. Geographical prevalence of polycystic ovary syndrome as determined by region and race/ethnicity. Int J Environ Res Public Health 2018;15,2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


