Abstract
BACKGROUND
Rapid sequence induction (RSI) is a standard procedure, which should be implemented in all patients with a risk of aspiration/regurgitation during anaesthesia induction.
OBJECTIVE
The primary aim was to evaluate clinical practice in RSI, both in adult and paediatric populations.
DESIGN
Online survey.
SETTINGS
A total of 56 countries.
PARTICIPANTS
Members of the European Society of Anaesthesiology.
MAIN OUTCOME MEASURES
The aim was to identify and describe the actual clinical practice of RSI related to general anaesthesia.
RESULTS
From the 1921 respondents, 76.5% (n=1469) were qualified anaesthesiologists. When anaesthetising adults, the majority (61.7%, n=1081) of the respondents preoxygenated patients with 100% O2 for 3 min and 65.9% (n=1155) administered opioids during RSI. The Sellick manoeuvre was used by 38.5% (n=675) and was not used by 37.4% (n=656) of respondents. First-line medications for a haemodynamically stable adult patient were propofol (90.6%, n=1571) and suxamethonium (56.0%, n=932). Manual ventilation (inspiratory pressure <12 cmH2O) was used in 35.5% (n=622) of respondents. In the majority of paediatric patients, 3 min of preoxygenation (56.6%, n=817) and opioids (54.9%, n=797) were administered. The Sellick manoeuvre and manual ventilation (inspiratory pressure <12 cmH2O) in children were used by 23.5% (n=340) and 35.9% (n=517) of respondents, respectively. First-line induction drugs for a haemodynamically stable child were propofol (82.8%, n=1153) and rocuronium (54.7%, n=741).
CONCLUSION
We found significant heterogeneity in the daily clinical practice of RSI. For patient safety, our findings emphasise the need for international RSI guidelines.
TRIAL REGISTRATION
ClinicalTrials.gov identifier: NCT03694860
Introduction
Rapid sequence induction (RSI) is a set of actions during induction of anaesthesia in unfasted patients or patients at risk of aspiration/regurgitation of gastric contents. The purpose of RSI is to secure the airway quickly and safely while actively reducing the risk of aspiration. RSI was first described in 1970.1 Although its safety and efficiency were not (and still are not) based on high-quality evidence-based data, RSI has gradually been implemented into anaesthesiology clinical practice.2 ‘Classical RSI’ consists of several steps, such as: monitoring of vital signs, intravenous line insertion, position of the patient before anaesthesia induction, functional and switched on suction with a wide bore suction catheter in place, preoxygenation, intravenous anaesthetic induction, an intravenous muscle relaxant drug with a rapid onset, the Sellick manoeuvre, intubation with the cuffed tracheal tube. Although there is an empiric recommendation for the use of RSI in high-risk patients, there are currently no clear recommendations as to whether RSI can be considered effective and well tolerated. ‘Classical RSI’ is associated with risks of hypoxaemia and cardiovascular complications,3,4 raising concerns about the efficiency and safety of the current method of RSI.
Another problem is the heterogeneity of RSI techniques in the published data.5–7 Such a variation in clinical practice can be dangerous for both patients (safety aspects) and physicians (legal consequences). The lack of internationally accepted RSI guidelines stimulated us to create an electronic survey with the aim of evaluating the actual RSI clinical practice internationally. The survey was designed to capture the clinical practice of RSI in both adult and paediatric patients.
Materials and methods
The survey was designed using the SurveyMonkey platform, registered on clinicaltrials.gov (NCT03694860) and conducted over 5 months (February 2019 to June 2019). The questionnaire consisted of 25 questions (two general pieces of information about the respondent, 11 questions about the RSI practice in adults and 12 questions about the RSI practice in paediatric patients) and could be completed within 5–7 min. The survey was supported by the European Society of Anaesthesiology (ESA, endorsed by Scientific Subcommittee 5 of ESA, 6 February 2019) and by the Czech Society of Anaesthesiology and Intensive Care (ČSARIM). Omitting answering questions was allowed. There were two multiple-choice questions (highlighted in brackets). An e-mail with the survey link (https://www.surveymonkey.com/r/CTGQWBB) was sent to all active ESA members by the ESA secretary. The survey electronic link was also accessible from ESA Facebook during the study period. The complete questionnaire is shown in Table 1. The data were assessed and prepared by a statistician to enable a comparison between adult and paediatric RSI practices. The statistical analysis was undertaken in cooperation with the Institute of Biostatistics and Analyses, Faculty of Medicine, Masaryk University, Czech Republic. The STATISTICA application was used for statistical analysis. Pearson's χ2 tests and Fischer's exact test were used for the comparison of adult and paediatric sections of RSI. The statistical significance was set to P = 0.05.
Table 1.
The questionnaire
| Question 1 | ‘Your official working position’ |
| Question 2 | ‘Your anaesthesiology practice is located?’ |
| Questions 3 and 14a | ‘In what situations do you indicate RSI?’ |
| Questions 4 and 16a | ‘What kind of patient's position do you prefer for Rapid-sequence induction?’ |
| Question 5 | ‘What's your gastric tube management for RSI in adults?’ |
| Questions 6 and 17a | ‘Do you use Sellick manoeuvre in RSI?’ |
| Questions 7 and 18a | ‘How do you preoxygenate the patient before RSI?’ |
| Questions 8 and 19a | ‘Do you use opioids for anaesthesia induction during RSI?’ |
| Questions 9 and 21a | ‘What kind of induction agent do you use for haemodynamically unstable patient for RSI?’ |
| Questions 10 and 20a | ‘What kind of induction agent do you use for haemodynamically stable patient for RSI?’ |
| Questions 11 and 22a | ‘What kind of muscle relaxant do you prefer for anaesthesia induction during RSI?’ |
| Questions 12 and 23a | ‘Do you ventilate the patient via face mask before anaesthesia induction?’ |
| Questions 13 and 24a | ‘Do you monitor the onset of neuromuscular blockade before intubation attempt?’ |
| Question 15 | ‘Do you perform RSI in younger children with risk of regurgitation or aspiration?’ |
| Question 25 | ‘Do you intubate the paediatric patients indicated for RSI with a cuffed endotracheal tube?’ |
RSI, rapid sequence induction.
aSame question for paediatric and adult questionnaire.
Results
Overall, 1921 colleagues completed the survey, of whom 1469 were anaesthesiologists who had completed training (76.6%), and 424 (22.1%) were anaesthesiology trainees. The invitation to take part in the survey was sent to 9097 members of the ESA, and 4475 of ESA members opened the e-mail. Of those who opened the e-mail, the response rate was 42.9%, thus only 21% of the total membership responded. We received responses from more than 58 countries (Supplemental Digital Content 1). The majority of respondents were European (85%, n=1615), most of them being from Germany (9.0%, n=171), the Czech Republic (8.1%, n=154), Spain (5.0%, n=95) and the United Kingdom (4.8%, n=92).
In adult practice, the majority of respondents used RSI in high-risk patients: acute abdomen (88.6%, n=1546), a risk of regurgitation or aspiration (94.6%, n=1651), trauma (less than 6 h between last meal and trauma) (92.8%, n=1621). Head-up was the preferred patient position for anaesthesia induction in adults (60.1%, n=1052). The majority of respondents considered that a gastric tube for RSI in adults was unnecessary (38.3%, n=669). The Sellick manoeuvre was used by 38.5% (n=675) and never used by 37.4% (n=656) of respondents. The preferred type of preoxygenation during adult RSI was a tight-fitting face mask with 100% O2 for 3 min (61.7%, n=1081). Opioids were used during anaesthesia induction by 65.9% (n=1155) of respondents. The preferred choice of RSI induction agents for haemodynamically stable patients were propofol (90.6%, n=1571) or thiopentone (11.2%, n=158), in combination with suxamethonium (56.0%, n=932). For haemodynamically unstable adult patients, ketamine (42.3%, n=664) or etomidate (37.9%, n=560) were the drugs of choice. Monitoring the onset of neuromuscular blockade was considered unnecessary by 33.2% (n=580) of respondents. In high-risk patients, ventilation with limited inspiratory pressure (<12 cmH2O) was used by 35.5% (n=622) of respondents.
In paediatric practice, RSI was chosen for the majority of high-risk patients: acute abdomen 88.6% (n=1304), a risk of regurgitation or aspiration (91.0%, n=1339), trauma (less than 6 h between last meal and trauma) 88.7% (n=1305). The majority of respondents used RSI in older children only (44.1%, n=630), with 9.7% (n=138) never using RSI at all in children. The preferred position was supine (50.9%, n=736). A cuffed endotracheal tube was used for all children by 40.8% (n=585) of respondents, and by 30.6% (n=438) of respondents for all children except neonates. The majority of respondents used preoxygenation for 3 min (56.6%, n=817) and opioids (54.3%, n=797) without the Sellick manoeuvre (54.2%, n=784) during RSI in paediatric patients. The drugs of choice for RSI induction in haemodynamically stable paediatric patients were propofol (82.8%, n=1153) and rocuronium (54.7%, n=741). In the case of haemodynamic instability, the preferred drugs were ketamine (58.4%, n=748) and propofol (29.7%, n=348). Monitoring the onset of neuromuscular blockade was considered unnecessary by 37.2% (n=536) of respondents. Manual ventilation via face mask before anaesthesia induction was not performed by 41.4% of respondents in paediatric RSI, but in high-risk patients, manual ventilation with limited inspiratory pressure (<12 cmH2O) was used by 35.9% (n=517) of respondents.
The differences between adult and paediatric RSI for the Sellick manoeuvre are presented in Fig. 1, the first-choice induction agent in haemodynamically stable patients for RSI in Fig. 2, and mask ventilation before the first intubation attempt in Fig. 3. Detailed comparisons between adult and paediatric practice are presented in Table 2. Detailed results are accessible in Supplemental Digital Content 1.
Fig. 1.
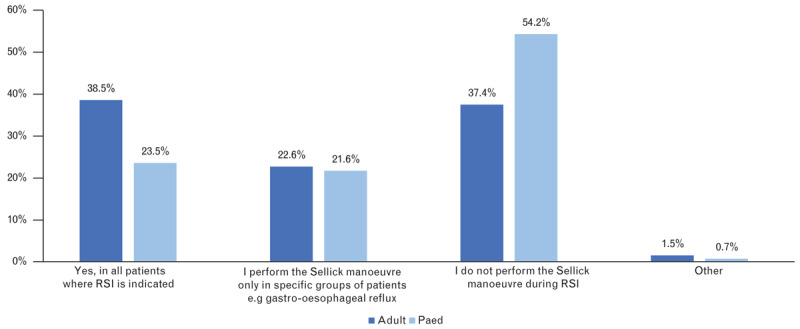
Do you use Sellick manoeuvre in rapid sequence induction?
Fig. 2.
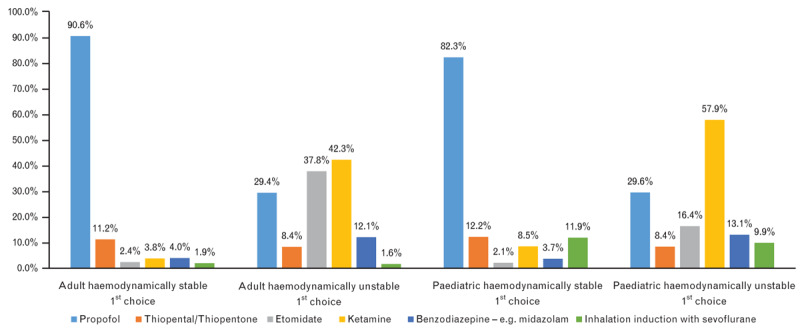
What kind of induction agent do you use for rapid sequence induction?
Fig. 3.
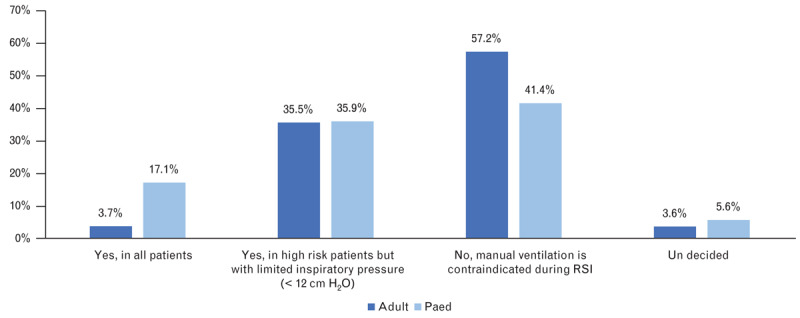
Do you ventilate the patient via face mask before anaesthesia induction (before first intubation attempt)?
Table 2.
Adult and paediatric rapid sequence induction questions
| Question | Options | Adult RSI | Paediatric RSI | P value (Pearson χ2 test) |
| In what situations do you indicate RSI? | Acute abdomen | 88.6% (n=1546) | 88.6% (n=1304) | 0.97045 |
| Risk of regurgitation or aspiration (hiatus hernia, gastro-oesophageal reflux, nonfasted patient) | 94.6% (n=1651) | 91.00% (n=1339) | 0.00008 | |
| Trauma patient (with less than 6 h between last meal and injury) | 92.8% (n=1621) | 88.7% (n=1305) | 0.00004 | |
| Other (please specify) | 26.6% (n=464) | 6.3% (n=92) | 0.0000 | |
| What kind of patient position do you prefer for rapid-sequence induction? | Trendelenburg position (head down by 15 to 30°) | 4.9% (n=86) | 5.1% (n=74) | 0.0000 |
| Anti-Trendelenburg position (head up by 15 to 30°) | 60.1% (n=1052) | 44.0% (n=637) | 0.0000 | |
| Supine position | 35.0% (n=613) | 50.9% (n=736) | 0.79370 | |
| Do you use the Sellick manoeuvre in RSI? | Yes, in all patients indicated for RSI | 38.5% (n=675) | 23.5% (n=340) | 0.0000 |
| I perform the Sellick manoeuvre only in specific groups of patients (e.g. gastro-oesophageal reflux) | 22.6% (n=397) | 21.6% (n=313) | 0.49663 | |
| I don’t perform the Sellick manoeuvre in RSI | 37.4% (n=656) | 54.2% (n=784) | 0.0000 | |
| Other | 1.48% (n=26) | 0.69% (n=10) | 0.03462 | |
| How do you preoxygenate the patient before RSI? | 100% O2 with tight-fitting face mask for 3 min | 61.7% (n=1081) | 56.6% (n=817) | 0.00360 |
| 100% O2 with tight-fitting face mask for 5 min | 19.9% (n=348) | 19.1% (n=275) | 0.56739 | |
| 80% O2 with tight-fitting face mask for 3 min | 4.1% (n=72) | 7.2% (n=104) | 0.00013 | |
| 80% O2 with tight-fitting face mask for 5 min | 2.2% (n=38) | 2.3% (n=33) | 0.82191 | |
| I don’t routinely preoxygenate patients during RSI | 1.5% (n=27) | 4.2% (n=61) | 0.00000 | |
| Other | 10.6% (n=186) | 10.6% (n=153) | 0.99014 | |
| Do you use opioids for anaesthesia induction during RSI? | Yes, in all patients | 65.9% (n=1156) | 54.9% (n=792) | 0.00000 |
| In minority of patients, for example, with severe pain | 19.6% (n=344) | 25.7% (n=371) | 0.00004 | |
| No, never during RSI | 9.3% (n=163) | 17.3% (n=250) | 0.00000 | |
| Other | 5.1% (n=90) | 2.2% (n=31) | 0.00001 | |
| Do you ventilate the adult patient via face mask before anaesthesia induction? | Yes, in all patients | 3.7% (n=65) | 17.1% (n=246) | 0.0000 |
| Yes, in high-risk patients, with limited inspiratory pressure (<12 cmH2O) | 35.5% (n=622) | 35.9% (n=517) | 0.81623 | |
| No, manual ventilation is contraindicated in RSI | 57.2% (n=1003) | 41.4% (n=597) | 0.0000 | |
| Not decided | 3.6% (n=63) | 5.6% (n=81) | 0.00600 | |
| Do you monitor the onset of neuromuscular blockade before intubation attempt? | Yes, in all patients | 14.0% (n=244) | 11.2% (n=162) | 0.02157 |
| Yes, sometimes | 32.9% (n=575) | 28.6% (n=413) | 0.00971 | |
| No, it is not needed | 33.2% (n=580) | 37.2% (n=536) | 0.01869 | |
| No, I don’t have a monitor available | 20.0% (n=349) | 23.0% (n=331) | 0.04024 |
RSI, rapid sequence induction. Bold - P value < 0.05.
Discussion
The primary aim of the survey was to evaluate the actual clinical practice of RSI in adult and paediatric patients. Our results confirmed the significant heterogeneity both in the components of RSI and in RSI practice between adults and paediatric patients. Although the majority of respondents used RSI in patients with a risk of aspiration, the number of respondents who did not do so in different situations varied from 5.4 to 11.4% in adult RSI and from 9.0 to 11.4% in paediatric RSI, respectively. This could be considered a dangerous practice as pulmonary aspiration remains the most common cause of death associated with anaesthesia.7 In the majority of these reported cases, risk factors for pulmonary aspiration were not identified, and therefore, RSI was not performed.8 Currently, RSI is indicated in patients who have any of the following conditions, nonfasted, active vomiting, subileus, ileus, limited protective laryngeal reflexes and gastrointestinal obstruction. In addition, RSI should be performed in pregnant women after the third trimester and during labour.9 On the basis of previously published data, point-of-care gastric ultrasound for the residual gastric volume (the antral area) measurement could be promising for further identification of patients at risk.10,11The head-up position is associated with an increase of functional residual capacity, improved preoxygenation and a longer time to desaturation.12–14 This adjustment can easily decrease morbidity and incidence of desaturation. In our survey, the head-up position was preferred by 60.1% of respondents in adults and by 44.0% in paediatric patients. In previously published studies, the head-up position was preferred by 76 to 84% of respondents.15–17Although high-quality evidence-based data are still lacking, the head-up position for RSI should be recommended.9
Preoxygenation with 100% O2 using a tight-fitting face mask for 3 to 5 min with or without continuous positive airway pressure can significantly increase the oxygen reserve. The risk of atelectasis formation is outweighed by the increase in patient safety. The majority of respondents preoxygenated patients with a tight-fitting mask with 100% O2 for 3 min (adults 61.7%/paediatric 56.6%, or for 5 min 19.9%/19.1%). Preoxygenation with a tight-fitting face mask is considered a standard part of anaesthesia induction. In recent years, high-flow nasal oxygen cannulae (HFNC) have been tested as a potential upgrade of standard preoxygenation (to prolong the time to desaturation during apnoea), but the results are conflicting.17–19
There are only limited data about the requirement for a gastric tube before anaesthesia induction. Gastric tube insertion before induction can allow the evacuation of gastric contents, and therefore, lead to the reduction of regurgitation/aspiration risk. However, leaving the gastric tube in situ during the induction of anaesthesia compromises the lower oesophageal sphincter creating a risk of regurgitation. In another survey, 65% of respondents inserted a gastric tube before anaesthesia induction in patients with small bowel obstruction and left it in place during induction.16 The majority of respondents in our survey inserted the gastric tube for RSI in adults (inserted and left in place, 27.7%; inserted with gastric content evacuation and removal before RSI in 20.8%). Evidence-based data does not specify the correct gastric tube management in RSI. Still, it should always be considered whether the gastric tube can reduce the associated risk. Overall, 38.3% of respondents indicated there was no need for a gastric tube for RSI, but this may be a risk in patients with bowel obstruction (e.g. ileus).
Nowadays, one of the most controversial parts of RSI is the Sellick manoeuvre (cricoid pressure). The correct technique for the Sellick manoeuvre is the application of a 10 N pressure on the cricoid cartilage before anaesthesia induction and further increase of the pressure to 30 N after the induction.12 The published data shows a wide variation of practice when using the Sellick manoeuvre. Sellick manoeuvre is used during RSI in 70 to 100% of patients.15,16,20 Considering the paediatric population, Sellick manoeuvre is used less often (58.6% in infants compared with 95.3% in schoolchildren).6 The controversy of Sellick manoeuvre is also reflected in our results: 38.5%/23.5% always performed Sellick manoeuvre during RSI whereas 37.4%/54.2% never performed Sellick manoeuvre during RSI (adults/children). In addition, the Sellick manoeuvre is frequently used incorrectly, for example, in 71% it is only applied after anaesthesia induction.20 On the other hand, Sellick manoeuvre can worsen the laryngoscopy view and make intubation difficult or impossible. The effectiveness and safety of Sellick manoeuvre has never been proven in a well designed, adequately powered, randomised controlled trial. The recently published IRIS trial21 was the first-ever randomised, double-blind noninferiority trial to compare a sham Sellick manoeuvre with cricoid pressure. The results failed to prove the noninferiority between sham Sellick manoeuvre and cricoid pressure, but the study was underpowered. Pulmonary aspiration was comparable between the groups (0.6% in Sellick manoeuvre versus 0.5% in the sham group), but with higher incidence of Cormack Lehane grade 3 and 4 (10 versus 5%, P < 0.001) and a longer intubation time (intubation time >30 s, 47 versus 40%, P < 0.001) in the Sellick manoeuvre group.21 The results of the IRIS trial raised further concerns about the safety and efficacy of Sellick manoeuvre in clinical practice. Although the debate about Sellick manoeuvre in the anaesthesiology community is ongoing, there are several national guidelines no longer recommending Sellick manoeuvre as a part of RSI in clinical practice.22
Opioids were not considered as a part of classical RSI. However, opioids during RSI reduce the cardiovascular response to laryngoscopy and can reduce the dose of the induction agent.12 Currently, up to 92% of physicians use opioids during RSI.23 This is in concordance with the results of our survey, where opioids were administered during RSI in 66.0%/54.9% of cases and sometimes administered in 19.6%/25.7% of cases (adult/paediatric). Overall use of opioids in RSI was 85.6% in adults and 80.5% in paediatric patients.
In classical RSI, the drug of choice for anaesthesia induction was thiopentone in combination with suxamethonium. This has changed over the past two decades. In 2001, thiopentone was still used in 88% of RSI4 but now propofol is the drug of choice for induction of anaesthesia for RSI.15 This change is also seen in our survey: propofol was the drug of choice for RSI in haemodynamically stable patients in 90.6%/82.8% of cases (adult/paediatric).
Currently, there are two drugs (ketamine, etomidate) that are considered safer during the induction of anaesthesia in haemodynamically unstable patients, or in patients with a high risk of hypotension. Etomidate is linked with the suppression of corticosteroid synthesis after administration and could be dangerous in patients with sepsis or septic shock. Unlike other intravenous anaesthetics, ketamine can even lead to the elevation of blood pressure and heart rate. The majority of respondents selected ketamine 42.3%/58.4% (adult/paediatric) and etomidate 37.9%/16.8% in haemodynamically unstable patients for RSI. Propofol or thiopentone were selected in 29.5%/29.7% and 8.4% /8.5% (adult/paediatric) of cases. However, as these drugs can lead to a further deterioration of the circulatory status, many consider them to be dangerous in haemodynamically unstable patients.
Suxamethonium is a part of the classical RSI technique but rocuronium (1.2 mg kg−1)24 provides comparable intubation conditions. Worldwide, suxamethonium remains the first-choice drug for neuromuscular blockade induction during RSI. However, the availability of suggamadex, a selective antidote for rocuronium, has increased the use of rocuronium for RSI over the past few years. In our survey, suxamethonium remained the drug of choice for RSI in adults, with 56% of respondents favouring it compared with 49.3% using rocuronium. This situation was reversed in paediatric patients, where 54.7% of respondents used rocuronium and 48.3% used suxamethonium. This could be explained by the fear of malignant hyperthermia in children, or by the availability of sugammadex for rapid reversal of rocuronium and the additional possibility of minimising the incidence of residual neuromuscular blockade postoperatively. In 1992, the Food and Drug Administration (FDA) published a warning regarding serious adverse effects and fatal cases of malignant hyperthermia after suxamethonium administration.25 Suxamethonium in children should be reserved for the treatment of laryngospasm and for RSI. Considering the serious side effects, the role of suxamethonium in paediatric RSI is debatable. Only a minority of respondents monitored the onset of neuromuscular blockade (adult/paediatric, sometimes 32.9%/28.6%, always 14.0%/11.2%). This could be a possible area for improvement. The majority of aspiration episodes during RSI have been linked to attempts to intubate the trachea during light anaesthesia before the onset of neuromuscular blockade.26
The basic principle of the classical RSI is the avoidance of manual ventilation, thus minimising the risk of air insufflation into the stomach and reducing the risk of aspiration/regurgitation. However, this practice is associated with a risk of hypoxaemia and cardiovascular complications.3,4 Manual or mechanical controlled ventilation with a limited inspiratory pressure (≤12–15 cmH2O) can lead to effective ventilation and oxygenation without insufflating the stomach, and according to the published data, it can be considered well tolerated.27–29 The inclusion of such pressure limited ventilation in the RSI algorithm is described as ‘controlled RSI’ or ‘modified RSI’. Such ‘controlled RSI’ has been used by 67 to 85% of physicians (mainly in patients with respiratory insufficiency and in paediatric patients).8,30
The standard care for patients at risk of aspiration is to secure the airway for general anaesthesia with a cuffed tracheal tube. Historically, in children under 8 years of age, an uncuffed tube has been used because of the fear of post-extubation stridor.31 However, the modern paediatric cuffed tracheal tubes (e.g. MicroCuff) are considered well tolerated and they can significantly reduce the need for tube exchange. In addition, the ideal seal reduces the risk of regurgitation/aspiration.32,33 By improving the airway seal, cuffed tubes facilitate measurement of end-tidal CO2 and enable positive pressure ventilation with higher inspiratory pressures. The new cuffed tracheal tubes can be used safely in all paediatric patients, including in the intensive care setting. The only safety prerequisite is the need to measure and limit the intracuff pressure to 20 cmH2O or less; this will minimise the risk of mucosal damage and postextubation airway-related complications.34 The majority of respondents (40.8%) in our survey used cuffed tracheal tubes for paediatric RSI, although 30.6% did not use cuffed tubes in neonates. This latter practice could be explained by a lack of supportive data and the low internal diameter of the cuffed tubes for neonates.
Several surveys in recent decades have evaluated the practice of RSI, revealing a wide variation in clinical practice.6,8,15,16,20,23,30 However, only a minority of these surveys compared adult and paediatric RSI practice, and none of them was followed by the formation of any guidelines. The results of our survey confirmed the continuation of a wide variation in clinical practice when performing RSI, revealing several potentially dangerous aspects.
A possible limitation of the survey is the low response rate, with only 21% of the ESA members responding to the survey, thus the practice of the remaining 79% is unknown. Another possible limitation is the composition of respondents, with 26.9% of respondents from five European countries). On the other hand, we consider the data obtained from 56 countries around the world as a strong point of the survey.
In conclusion, the results of the survey confirmed wide variations of RSI in clinical practice. After 50 years of RSI with no evidence-based proven benefit (or harm, there is an urgent need for international RSI guideline formation to improve the safety of our patients).
Supplementary Material
Acknowledgements relating to this article
Assistance with the study: special thanks to Dr Nicola Disma and Ms. Jennifer Rose from the European Society of Anaesthesiology and Prof. Vladimir Cerny from the Czech Society of Anaesthesiology and Intensive Care (CSARIM) for their support and cooperation.
Financial support and sponsorship: the study was supported by the Specific University Research provided by MŠMT (MUNI/A/1111/2018 and MUNI/A/0943/2019), supported by MH CZ – DRO (FNBr, 65269705) and supported by the funds from the Faculty of Medicine MU to junior researchers (JK, MK).
Conflicts of interest: none.
Presentation: preliminary results of the survey were presented on the National Congress of Czech Society of Anaesthesiology and Intensive Care in Brno, 2019 in the form of an oral presentation.
Jozef Klucka and Martina Kosinova contributed equally to this manuscript.
Published online 28 March 2020
References
- 1.Bhananker SM, Ramamoorthy C, Geiduschek JM, et al. Anesthesia-related cardiac arrest in children: update from the Pediatric Perioperative Cardiac Arrest Registry. Anesth Analg 2007; 105:344–350. [DOI] [PubMed] [Google Scholar]
- 2.Neilipovitz DT, Crosby ET. No evidence for decreased incidence of aspiration after rapid sequence induction. Can J Anaesth 2007; 54:748–764. [DOI] [PubMed] [Google Scholar]
- 3.Hayes AH, Breslin DS, Mirakhur RK, et al. Frequency of haemoglobin desaturation with the use of succinylcholine during rapid sequence induction of anaesthesia. Acta Anaesthesiol Scand 2001; 45:746–749. [DOI] [PubMed] [Google Scholar]
- 4.Heier T, Feiner JR, Lin J, et al. Hemoglobin desaturation after succinylcholine-induced apnea: a study of the recovery of spontaneous ventilation in healthy volunteers. Anesthesiology 2001; 94:754–759. [DOI] [PubMed] [Google Scholar]
- 5.Zelicof-Paul A, Smith-Lockridge A, Schnadower D, et al. Controversies in rapid sequence intubation in children. Curr Opin Pediatr 2005; 17:355–362. [DOI] [PubMed] [Google Scholar]
- 6.Stedeford J, Stoddart P. RSI in pediatric anesthesia - is it used by nonpediatric anesthetists? A survey from south-west England. Paediatr Anaesth 2007; 17:235–242. [DOI] [PubMed] [Google Scholar]
- 7.Cook T, Woodall N, Frerk C. The Fourth National Audit Project. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: anaesthesia. Br J Anaesth 2011; 106:617–631. [DOI] [PubMed] [Google Scholar]
- 8.Brown JP, Werrett GC. Bag-mask ventilation in rapid sequence induction: a survey of current practice among members of the UK Difficult Airway Society. Eur J Anaesthesiol 2015; 32:446–448. [DOI] [PubMed] [Google Scholar]
- 9.Eichelsbacher C, Ilper H, Noppens R, et al. “Rapid sequence induction and intubation” beim aspirationsgefährdeten Patienten Handlungsempfehlungen für das praktische anästhesiologische Management. Anaesthesist 2018; 67:568–583. [Article in German]. [DOI] [PubMed] [Google Scholar]
- 10.Perlas A, Arzola C, Van de Putte P. Point-of-care gastric ultrasound and aspiration risk assessment: a narrative review. Can J Anaesth 2018; 65:437–448. [DOI] [PubMed] [Google Scholar]
- 11.Gola W, Domagała M, Cugowski A. Ultrasound assessment of gastric emptying and the risk of aspiration of gastric contents in the perioperative period. Anaesthesiol Intensive Ther 2018; 50:297–302. [DOI] [PubMed] [Google Scholar]
- 12.Wallace C, McGuire B. Rapid sequence induction: its place in modern anaesthesia. Continuing Educ Anaesth Crit Care Pain 2014; 14:130–135. [Google Scholar]
- 13.Langeron O, Birenbaum A, Le Saché F, et al. Airway management in obese patient. Minerva Anestesiol 2014; 80:382–392. [PubMed] [Google Scholar]
- 14.Lane S, Saunders D, Schofield A, et al. A prospective, randomised controlled trial comparing the efficacy of preoxygenation in the 20 degrees headup vs supine position. Anaesthesia 2005; 60:1064–1067. [DOI] [PubMed] [Google Scholar]
- 15.Sajayan A, Wicker J, Ungureanu N, et al. Current practice of rapid sequence induction of anaesthesia in the UK - a national survey. Br J Anaesth 2016; 117: Suppl 1: i69–i74. [DOI] [PubMed] [Google Scholar]
- 16.Rohsbach C1, Wirth S, Lenz K, et al. Survey on the current management of rapid sequence induction in Germany. Minerva Anestesiol 2013; 79:716–726. [PubMed] [Google Scholar]
- 17.Rajan S, Joseph N, Tosh P, et al. Effectiveness of transnasal humidified rapid-insufflation ventilatory exchange versus traditional preoxygenation followed by apnoeic oxygenation in delaying desaturation during apnoea: a preliminary study. Indian J Anaesth 2018; 62:202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fong KM, Au SY, Ng GWY. Preoxygenation before intubation in adult patients with acute hypoxemic respiratory failure: a network meta-analysis of randomized trials. Crit Care 2019; 23:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frat JP, Ricard JD, Quenot JP. Noninvasive ventilation versus high-flow nasal cannula oxygen therapy with apnoeic oxygenation for preoxygenation before intubation of patients with acute hypoxaemic respiratory failure: a randomised, multicentre, open-label trial. Lancet Respir Med 2019; 4:303–312. [DOI] [PubMed] [Google Scholar]
- 20.Morris J1, Cook TM. Rapid sequence induction: a national survey of practice. Anaesthesia 2001; 56:1090–1097. [DOI] [PubMed] [Google Scholar]
- 21.Birenbaum A, Hajage D, Roche S, et al. Effect of cricoid pressure compared with a sham procedure in the rapid sequence induction of anesthesia: the IRIS Randomized Clinical Trial. JAMA Surg 2019; 154:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piepho T1, Cavus E, Noppens R, et al. S1 guidelines on airway management: guideline of the German Society of Anesthesiology and Intensive Care Medicine. Anaesthesist 2015; 64: Suppl 1: 27–40. [DOI] [PubMed] [Google Scholar]
- 23.Koerber JP, Roberts GEW, Whitaker R, et al. Variation in rapid induction techniques: current practice in Wales. Anaesthesia 2009; 64:54–59. [DOI] [PubMed] [Google Scholar]
- 24.Tran DT, Newton EK, Mount VA, et al. Rocuronium versus succinylcholine for rapid sequence induction intubation. Cochrane Database Syst Rev 2015; 10:CD002788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg H, Gronet GA. Intractable cardiac arrests in children given scoline. Anesthesiology 1992; 77:1054–1056. [DOI] [PubMed] [Google Scholar]
- 26.Matthew R, Mittiga, Andrea S, et al. A modern and practical review of rapid-sequence intubation in pediatric emergencies. Clin Pediatr Emerg Med 2015; 16:172–185. [Google Scholar]
- 27.Qian X, Hu Q, Zhao H, et al. Determination of the optimal inspiratory pressure providing adequate ventilation while minimizing gastric insufflation using real-time ultrasonography in Chinese children: a prospective, randomized, double-blind study. BMC Anesthesiol 2017; 17:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JH, Jung H, Jang YE, et al. Manual vs pressure-controlled facemask ventilation during the induction of general anesthesia in children: A prospective randomized controlled study. Paediatr Anaesth 2019; 29:331–337. [DOI] [PubMed] [Google Scholar]
- 29.Bouvet L, Albert ML, Augris C, et al. Real-time detection of gastric insufflation related to facemask pressure-controlled ventilation using ultrasonography of the antrum and epigastric auscultation in nonparalyzed patients: a prospective, randomized, double-blind study. Anesthesiology 2014; 120:326–334. [DOI] [PubMed] [Google Scholar]
- 30.Ehrenfeld JM, Cassedy EA, Forbes VE, et al. Modified rapid sequence induction and intubation: a survey of United States current practice. Anesth Analg 2012; 115:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brambrink AM, Meyer RR. Management of the paediatric airway: new developments. Curr Opin Anaesthesiol 2002; 15:329–337. [DOI] [PubMed] [Google Scholar]
- 32.Klučka J, Štourač P, Štoudek R, et al. Controversies in pediatric perioperative airways. Biomed Res Int 2015; 2015:368761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Ungern-Sternberg BS, Boda K, Chambers NA, et al. Risk assessment for respiratory complications in paediatric anaesthesia: a prospective cohort study. Lancet 2010; 376:773–783. [DOI] [PubMed] [Google Scholar]
- 34.Kneyber MCJ, de Luca D, Calderini E, et al. Section Respiratory Failure of the European Society for Paediatric and Neonatal Intensive Care. Recommendations for mechanical ventilation of critically ill children from the Paediatric Mechanical Ventilation Consensus Conference (PEMVECC). Intensive Care Med 2017; 43:1764–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


