Purpose of review
Neuropsychiatric lupus (NPSLE) comprises a disparate collection of syndromes affecting the central and peripheral nervous systems. Progress in the attribution of neuropsychiatric syndromes to SLE-related mechanisms and development of targeted treatment strategies has been impeded by a lack of objective imaging biomarkers that reflect specific neuropsychiatric syndromes and/or pathologic mechanisms. The present review addresses recent publications of neuroimaging techniques in NPSLE.
Recent findings
Imaging studies grouping all NPSLE syndromes together are unable to differentiate between NPSLE and non-NPSLE. In contrast, diffusion tensor imaging, FDG-PET, resting, and functional MRI techniques in patients with stable non-NPSLE demonstrate abnormal network structural and functional connectivity and regional brain activity in multiple cortical areas involving the limbic system, hippocampus, frontal, parietal, and temporal lobes. Some of these changes associate with impaired cognitive performance or mood disturbance, autoantibodies or inflammatory proteins. Longitudinal data suggest progression over time. DCE-MRI demonstrates increased Blood–brain barrier permeability.
Summary
Study design issues related to patient selection (non-NPSLE vs. NPSLE syndromes, SLE disease activity, medications) are critical for biomarker development. Regional and network structural and functional changes identified with advanced brain imaging techniques in patients with non-NPSLE may be further developed as biomarkers for cognitive and mood disorders attributable to SLE-related mechanisms.
Keywords: biomarkers, cognitive dysfunction, neuroimaging, neuropsychiatric lupus
INTRODUCTION
Neuropsychiatric systemic lupus erythematosus (SLE; NPSLE) is a general descriptor referring to the psychiatric and neurologic manifestations of SLE present in 15–75% of lupus patients [1,2]. The scope of NPSLE encompasses at least 19 clinical neuropsychiatric syndromes defined by the American College of Rheumatology (ACR) [3], affecting the central nervous systems (CNS) and peripheral nervous systems (PNS; Table 1). Central diffuse neuropsychiatric manifestations of cognitive dysfunction and mood disorder were recently cited among the top symptoms negatively affecting quality of life (Lupus: Patient Voices 2018) and in some cases NPSLE outcomes are severe, even fatal [4]. The most challenging clinical issue related to NPSLE is attribution of clinical neuropsychiatric syndromes to SLE-related mechanisms and not separate comorbid disorders or medication effects. Attribution is critical as treatment strategies reflect attribution. Different attribution models have been developed [5], however, our ability to correctly ascribe neuropsychiatric events to SLE-related mechanisms remains compromised by a lack of objective and specific biomarkers, reflecting in part our insufficient understanding of pathophysiologic mechanisms responsible for these syndromes. Diagnosis and attribution of central focal manifestations (Table 1), which frequently arise from ischemic events reflecting vasculopathy or thrombosis related to clotting antibodies or atherosclerotic disease, is generally straightforward using structural MRI. In contrast, proposed pathologic mechanisms for central diffuse NPSLE syndromes; vasculitis, vasculopathy, immune complexes, brain-reactive autoantibodies, microglial cell activation, cytokine-induced, and/or cell-mediated inflammation and thrombosis (reviewed in [6]) can lead to perfusion abnormalities, neuronal dysfunction, axonal damage and microstructural damage that are not detectable on conventional MRI. This review will focus on recent studies using brain imaging techniques to evaluate NPSLE manifestations and underlying pathophysiologic mechanisms.
Table 1.
ACR classification of neuropsychiatric manifestations of SLE
| Central nervous system | ||
| Diffuse manifestations | Focal manifestations | Peripheral nervous system |
| Mood disorder | Cerebrovascular disease | Cranial neuropathy |
| Cognitive dysfunction | Seizures | Autonomic neuropathy |
| Anxiety disorder | Aseptic meningitis | Mononeuropathy |
| Psychosis | Movement disorder | Polyneuropathy |
| Acute confusional state | Myelopathy | Myasthenia gravis |
| Headache | Demyelinating syndrome | Acute inflammatory Demyelinating Polyradiculopathy (Guillain–Barre syndrome) |
| Plexopathy | ||
Box 1.
no caption available
Neuroanatomical imaging: MRI
Structural and volumetric MRI
Common findings on conventional brain MRI in SLE include white matter hyperintense lesions (WMHs) and brain atrophy but these are not specific for NPSLE [7,8]. As previously reported [9,10], increased frequency or volume of WMHs in combined groups of patients with non-NPSLE and NPSLE compared to healthy controls has been recently reported in three studies [11,12▪,13▪]. None detected differences in WMHs between the non-NPSLE and NPSLE subgroups, despite use of the more stringent Systemic Lupus International Collaborating Clinics (SLICC) attribution model for NPSLE [14] compared to the ACR model [3] by Cannerfelt et al.[11]. Number of WMHs did not correlate with cognitive test scores or other clinical parameters [11,12▪,13▪] and smaller hippocampal volumes in the NPSLE subgroup reported by Cannerfelt et al.[11] also did not correlate with neuropsychiatric test scores. Moreover, in a longitudinal study of 15 patients with non-NPSLE, 38% demonstrated WMHs on conventional MRI that either stayed the same or resolved over time [15]. White matter lesion volumes did correlate with impaired memory testing in a combined NPSLE/non-NPSLE group [11], suggesting perhaps that evaluation of associations between WMHs and an individual neuropsychiatric syndrome, cognitive dysfunction, may be more informative than associations with the diverse collection of NPSLE syndromes. Magro-Checa et al.[16] employed a more mechanistic approach seeking to identify associations between serum autoantibodies and structural lesions including WMHs, ischemic lesions, inflammatory-like lesions and cerebral atrophy in a cohort of 325 patients with active NPSLE. Of these, 24% were attributed to inflammatory NPSLE mechanisms, 13.2% to ischemic NPSLE events, and 62.8% to non-NSPLE events. Although none of the autoantibodies were associated with inflammatory-type lesions or WMHs, the lupus anticoagulant was significantly associated with ischemic lesions and cerebral atrophy, consistent with previous reports [10,17–19]. Thus, although MRI remains the neuroimaging technique of choice in clinical practice, structural MRI lesions do not reliably distinguish NPSLE from non-NPSLE, regardless of the attribution model used.
Diffusion tensor MRI
Diffusion tensor MRI (DTI) provides assessments of white matter microstructural changes using measures of fractional anisotropy in normal appearing areas on conventional MRI. Mackay et al. studied 37 stable, patients with non-NPSLE and 25 healthy controls and identified regions with significantly reduced fractional anisotropy in SLE in the parietal, occipital, and frontal lobes, cingulum, hippocampus, uncinate fasciculus and corpus callosum [20▪]. The visualized and reconstructed tracts from these seed regions revealed significant underlying fiber pathway abnormalities as shown in Fig. 1. Decreased parahippocampal fractional anisotropy correlated with increased serum levels of a neurotoxic autoantibody (anti-N-methyl D-aspartate receptor antibody, anti-NMDAR ab) and poor performance on a spatial memory task; suggesting a potential imaging biomarker for autoantibody-mediated damage with cognitive consequences.
FIGURE 1.
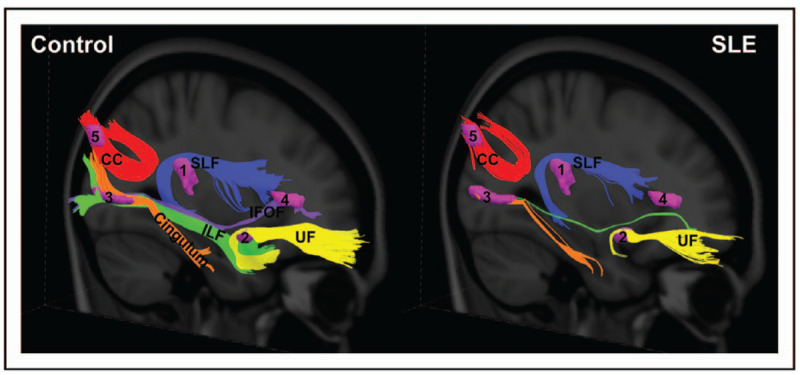
White matter pathways associated with the abnormal SLE-related regions visualized with group tractography. The superior longitudinal fasciculus (temporal part; SLF; noted as 1), uncinate fasciculus (UF; noted as 2), cingulum (hippocampus part) and inferior longitudinal fasciculus (ILF; noted as 3), inferior frontal occipital fasciculus (IFOF; noted as 4), and the splenium of the corpus callosum (CC; noted as 5) pathways reconstructed in the healthy control (left) and SLE (right) groups. Fewer tracts were visualized in the SLE group relative to the controls in the SLF (temporal part; −74%), UF (−86%), cingulum (hippocampus part; −82%), ILF (–99.5%), IFOF (–100%), and splenium CC (−48%) [20▪].
Similarly, decreased fractional anisotropy values in the parietal and frontal lobes, uncinate fasciculus, the inferior frontal occipital fasciculus (IFOF), anterior thalamic radiation and corpus callosum were also reported in 67 SLE patients (20 with memory deficits and 47 without) relative to 22 healthy controls by Corrêa et al.[21]. No microstructural differences were found between the SLE patients grouped by memory deficits. Kozora et al. reported decreased FA in the parahippocampal gyrus, thalamus, precentral gyrus, postcentral gyrus, angular gyrus, parietal lobe, and cerebellum over a period of 18 months in 15 patients with non-NPSLE with stable disease activity and medications [15]. This is the first published longitudinal study of DTI in SLE and importantly it demonstrates microstructural brain changes in patients with non-NPSLE in the absence of changes in cognitive testing.
Wiseman et al. applied graph theoretical analysis to DTI to investigate relationships between brain network structural connectivity metrics, cognitive ability and systemic organ damage in 47 SLE patients with variable disease activity including 3 patients with active NPSLE and stroke [22]. Cognitive abilities associated positively with network connectivity measures of density and strength and with greater nodal strength in multiple cortical regions including the frontal lobe, putamen, caudate and pallidum. Conversely, systemic damage was associated with reduced network connectivity measures of strength, global efficiency and clustering coefficient and with decreased nodal strength in the frontal, temporal, occipital and parietal lobes and caudate. These data suggest that patterns of structural brain network connections and node properties might be useful for monitoring cognitive function. Preziosa et al. also used DTI graph theoretical analysis in 32 SLE patients, including 12 with NPSLE, compared to 32 healthy controls [13▪]. Structural global network metrics; strength, transitivity, and efficiency were lower and path length were higher in SLE compared to healthy controls , especially in patients with elevated serum anti-dsDNA autoantibodies. Structural hubs (nodes with above average numbers of connections) were the same in SLE and healthy controls but hub metrics (strength, clustering coefficient) were significantly abnormal in SLE patients. Abnormal structural network and node properties were not associated with NPSLE. Neither paper reported correlations between SLE disease activity and structural network or node metrics, suggesting that DTI findings are unrelated to peripheral inflammation and perhaps more reflective of chronic damage.
Blood brain barrier (BBB) imaging with dynamic contrast enhanced MRI (DCE-MRI)
Reports of increased albumin, immunoglobulin and neurotoxic autoantibody levels in the CSF provide indirect evidence of a compromised BBB in acute central NPSLE [23–27]. Chi et al. are the first to evaluate BBB permeability (BBBP) in SLE using DCE-MRI in a small cohort of 6 patients with non-NPSLE and 5 healthy controls [28▪]. They reported abnormal BBBP parameters in SLE indicating increased flow from the intravascular to the extravascular/extracellular space (Ktrans) coupled with accumulation of fluid in the extra-cellular, extravascular space (Ve). Significant positive correlations between Ktrans and Ve and cerebral blood flow (CBF) suggested abnormally increased leakage across the BBB as CBF is increased. Comparison of mean DCE curves, representing regional signal intensity over time, also demonstrated significantly increased hippocampal BBBP in SLE patients, a region known to modulate cognition and that is targeted by a neurotoxic autoantibody identified in SLE [29].
Cerebral perfusion
While brain histopathological studies reveal evidence of extensive vasculopathy in SLE [30,31], measures of regional CBF and perfusion have previously failed to distinguish non-NPSLE and NPSLE [32–36]. Papadaki et al. measured CBF using dynamic susceptibility contrast MRI (DSC-MRI) in 31 NPSLE, 19 non-NPSLE and 23 healthy controls in brain regions governing emotional response. Within the NPSLE group, a specific hypoperfusion pattern was identified in the frontostriatal and limbic structures (hippocampus, cingulate) that correlated specifically with anxiety and was unrelated to non-CNS disease activity, age or depression [37].
Jia et al. used 3D arterial spin labeling MRI (ASL-MRI), a contrast-free perfusion imaging technique, to evaluate CBF in 16 NPSLE, 19 non-NPSLE and 30 healthy controls and reported asymmetric reduced perfusion in the frontal, temporal, parietal and occipital lobes in the combined SLE group compared to healthy controls that was verified in the quantitative CBF analyses [38]. Although 100% of those with reduced frontal lobe perfusion had acute NPSLE, approximately 40% of the hypoperfusion in the other regions was seen in the patients with non-NPSLE, suggesting a subclinical process in patients with no overt NPSLE manifestations. Difficulties distinguishing CBF in NPSLE from non-NPSLE may be related to the diversity of neuropsychiatric syndromes included; in this case, peripheral neuropathy, headache, stroke and raised intracranial pressure.
Zhuo et al.[39] used voxel based analysis of ASL-MRI to evaluate CBF in 24 non-NPSLE, 31 NPSLE and 32 healthy controls. Compared to healthy controls, white matter CBF was increased in both SLE groups whereas gray matter CBF was decreased in NPSLE. Compared to non-NPSLE, NPSLE CBF was significantly reduced in the frontal lobe, cerebellum and corpus callosum. Support vector machine modeling (SVM) modeling found that reduced CBF in the corpus callosum distinguished NPSLE with an accuracy of 83.6% and specificity of 87.5%. The authors suggest that increased regional CBF may be an initial compensatory response that diminishes in NPSLE and that the different perfusion patterns, particularly in the frontal lobe, cerebellum and corpus callosum, can be used to distinguish NPSLE.
The current studies suggest, perhaps, a threshold effect of vascular disease that occurs in virtually all SLE patients and support clinical interventions to preserve vascular function in order to avoid CNS effects. However, as all NPSLE were grouped together and no studies reported effects of parameters that impact CBF such as medications, arterial blood pressure, intracranial pressure, hematocrit, carotid atherosclerotic disease and autoregulatory mechanisms [37–39], these studies provide little insight into the role of CBF in individual neuropsychiatric syndromes.
Functional imaging: PET and functional MRI
Fluorine-18 fluorodeoxyglucose-PET
FDG-PET provides an objective tool for assessments of regional brain metabolism and evaluation of correlations with clinical parameters. In contrast to prior studies that employed a prespecified, regions-of-interest (ROIs) method to investigate patients with non-NPSLE and NPSLE and reported decreased metabolism in the frontal, temporal, parietal, and occipital regions [40–44], Mackay et al.[29] recruited patients with stable non-NPSLE and used an unbiased, voxel-wise search of the whole brain to investigate a mechanism for autoantibody-mediated cognitive dysfunction. They report abnormal resting hypermetabolism in the hippocampus, orbitofrontal cortex and posterior putamen/Globus Pallidus (GP)/thalamus of patients with non-NPSLE relative to healthy controls [45], validating similar results from a previous cohort [46]. Analysis of the larger, combined non-NPSLE cohort additionally revealed hypermetabolism in the sensorimotor cortex, occipital lobe, and temporal lobe [45]. Interregional correlation analysis among these hypermetabolic regions, which also correlated with poor performance on a working memory test, revealed a striking change from the ‘hippocampal-putamen/GP/thalamic-temporal lobe’ pattern in healthy controls, to a disease pattern of ‘hippocampal-putamen/GP/thalamic-sensorimotor cortex’ in non-NPSLE. Longitudinal data showed persistent regional hypermetabolism in patients with SLE over time [45]. Concurrent DTI imaging in this same cohort (see DTI section) demonstrated areas of reduced microstructural integrity adjacent to the hypermetabolic regions, suggesting that cognitive dysfunction in SLE is characterized by a diffuse, subclinical process resulting in reproducible, resting regional gray matter hypermetabolism and disruption of normal resting metabolic patterns that associate with poor performance on cognitive testing and clusters of decreased microstructural integrity connected by diminished white matter tracts.
Ploran et al.[47▪] used resting FDG-PET to explore changes in regional metabolism associated with performance on a spatial navigation task (SNT) and the neurotoxic anti-NMDAR ab in 19 patients with non-NPSLE. Significantly fewer patients with non-NPSLE with high serum anti-NMDAR antibody titers were able to complete the SNT compared to healthy controls. Patients with non-NPSLE who successfully completed the SNT exhibited higher metabolism in the caudate/anterior putamen and the prefrontal/frontal cortical areas than those unable to complete the task. This network is different from the abnormal ‘hippocampal-posterior putamen/GP/thalamic-sensorimotor cortex’ functional pathway, which does not differentiate patients based on SNT performance [20▪]. These suggest that the anterior and posterior portions of the putamen are associated with distinct cognitive abilities in SLE; the anterior putamen is part of the hyperactive SNT-related neuronal loop recruited to successfully complete the SNT [47▪], whereas the posterior putamen is part of the hypermetabolic SLE-associated pathway that is correlated with poor performance on a nonspatial, working memory task [45,46]. These two studies suggest that regional resting metabolism may potentially be used as neuroimaging markers for objective assessments of cognitive dysfunction and treatment responses in clinical trials.
Functional MRI
Resting state functional MRI
Resting state functional MRI (Rs-fMRI) is used to assess regional brain activity and resting state networks. Importantly, it avoids performance confounds inherent in task-fMRI studies. Measures of resting brain activity include regional homogeneity (ReHo) and regional changes in the amplitude of low-frequency fluctuations (ALFF) in blood-oxygen-level dependent (BOLD) signal. Resting networks are assessed with resting state functional connectivity (rsFC) measures. In a large cohort of 114 patients with non-NPSLE compared to 75 healthy controls, Liu et al.[48▪] report significantly increased resting ReHo values in the limbic lobe (parahippocampal gyrus and uncus) and decreased values in the fusiform gyrus and left thalamus. Anxiety and depression scores correlated inversely with ReHo values in the paracentral lobule, postcentral gyrus, precuneus, cuneus, fusiform, and superior temporal gyrus; regions associated with motor and sensory function, memory, visuospatial processing, hearing, and language comprehension and facial recognition. Additionally, disease activity correlated positively with ReHo values in the cerebellum and negatively with ReHo values in the frontal gyrus. Similarly, in two separate cohorts of patients with non-NPSLE compared to healthy controls, increased ALFF was reported in the inferior temporal gyrus, putamen, cuneus, and right calcarine fissure surrounding cortex and decreased ALFF was reported in the precentral gyrus, postcentral gyrus, and precuneus [49,50].
Niu et al.[51] employed a ROI-based approach using abnormal cortical thickness regions as seeds to detect disrupted brain rsFC in 33 patients with non-NPSLE and 32 healthy controls. Cortical thickness was reduced in the fusiform gyrus and lingual gyrus (areas involved in emotional face processing, visual object recognition and attention) and the superior frontal cortex (SFC) (involved in cognition and executive function). Reduced thickness in the lingual gyrus associated with increased rsFC between it and the posterior cingulate cortex, a hub region of the Default Modal Network (DMN). Reduced thickness in the SFC associated with reduced frontal cortex between it and the cerebellum. A similar ROI-based approach in 36 patients with non-NPSLE and 30 healthy controls using abnormal ALFF-based regions as seeds [50] also demonstrated increased rsFC between the precuneus and occipital gyrus plus frontal gyrus, and between the cuneus and precuneus plus posterior cingulate gyrus. Both studies show abnormalities in the DMN and suggest that cortical structural abnormalities in SLE may disrupt normal resting functional connectivity in the brain.
RsFC in the DMN, the Central Executive Network, and between these two networks correlated inversely with cognitive performance in multiple domains in 61 SLE (25 non-NPSLE, 36 NPSLE) compared to 20 healthy controls [12▪]. These changes were present in both SLE subgroups, although the NPSLE group tended to display higher rsFC than non-NPSLE on the network correlating with the cognitive test of interest, suggesting that abnormal rsFC may represent suboptimal compensatory mechanisms present in all SLE.
Cao et al.[52▪] used rs-fMRI and graph theory approach to evaluate topological characteristics of functional networks and correlations with clinical parameters in 41 SLE and 35 healthy control. Patients with SLE demonstrated significantly reduced nodal efficiency in the insula (pain matrix), putamen (dorsal striatum, learning), and transverse temporal gyrus and decreased numbers of nodal connections (degree centrality) in the amygdala and transverse temporal gyrus. They also showed decreased network frontal cortex between the transverse temporal gyrus and vermis and between the putamen and cerebellum that correlated positively with cognitive ability. Both nodal efficiency and degree centrality in the transverse temporal gyrus correlated positively with disease duration.
Task-based functional MRI
Previous task-fMRI studies in SLE, recording BOLD signals as a measure of neuronal metabolism during task performance, have suggested activation of compensatory mechanisms to complete tasks [53]. A task-fMRI study with the computer-based Iowa gambling task (IGT), a decision making task [54▪], demonstrated impaired decision making abilities in 16 patients with non-NPSLE compared to 16 healthy controls that associated with lower activation in the anterior and posterior cingulate (limbic system), the orbitofrontal cortex, ventromedial prefrontal cortex (vmPFC), and occipital cortex, and increased activation in the dorsolateral prefrontal cortex, insula and striatum (associated with memory, emotion, and behavior). Another task-based fMRI study revealed a reduced ability to suppress the transverse and superior temporal gyrus (regions in the DMN, a network that normally is suppressed during goal-oriented tasks) and increased attenuation of the lingual gyrus and caudate during a working memory task of sustained attention (N-back task) in 23 patients with non-NPSLE and 29 healthy control [55▪▪]. Higher peripheral organ damage and vascular cell adhesion molecule-1 (VCAM-1) levels were associated with diminished attenuation of the DMN and lower BOLD signal in the caudate. Increased interleukin 6 (IL-6) was also associated with lower BOLD signal in the caudate.
CONCLUSION
Challenging clinical dilemmas in the central, diffuse manifestations of NPSLE relate to attribution and treatment and these are highly dependent on the development of unbiased imaging biomarkers for specific neuropsychiatric syndromes and pathologic mechanisms. Basic science research in the last 20 years has yielded multiple proposed pathogenic mechanisms resulting in CNS dysfunction and structural damage [6,56], many of which may be blocked with novel therapeutics or other methods. In this review we have highlighted neuroimaging techniques, combined with appropriate methodology, that are demonstrating promising results in some of the central neuropsychiatric manifestations.
Conventional MRI, as reported previously (reviewed in [8]) and supported by recent studies reviewed here [11,12▪,13▪,15] does not reliably distinguish between non-NPSLE and NPSLE much less between different neuropsychiatric syndromes and the WMHs remain nondiagnostic. It therefore remains most useful for diagnosis of structural focal lesions.
Given the immense heterogeneity of NPSLE, studies using imaging techniques to identify biomarkers and/or investigate pathologic mechanisms of individual neuropsychiatric syndromes are more likely to be informative than those that combine multiple NPSLE syndromes with disparate pathophysiologic mechanisms. Several of the recent papers assessing structural MRI, perfusion imaging, rs-fMRI, and DTI have included patients with NPSLE as a comparator group [11,12▪,13▪,16,37–39]. Interpretation of their results is limited by the inclusion of such disparate neuropsychiatric syndromes as mood and cognitive disorders, headache, psychosis, cranial neuropathy, and peripheral neuropathy [37] as each of these likely has a different underlying mechanism and the goal is to associate imaging results with specific syndromes and mechanisms.
In contrast, studies focused on assessments of patients with non-NPSLE with stable disease activity and medication use and no history of CNS events ensure fewer potentially confounding variables, thus allowing assessments of imaging biomarkers for subclinical cognitive dysfunction and pathologic mechanisms. Recent studies using DTI, FDG-PET, and rs-fMRI all recruited this patient population to assess regional changes in microstructural integrity, metabolism, brain activity, and network rsFC [15,20▪,21,47▪,48▪,49–51]. Collectively, they demonstrate significant resting changes in similar brain regions (the hippocampus/parahippocampus, frontal, parietal and temporal lobes, putamen, thalamus, and cingulate gyrus are identified repeatedly in the included studies) and in network connectivity, suggesting ongoing inflammatory or neurotoxic mechanisms unrelated to acute or chronic NPSLE syndromes, age, comorbid disease, or disease activity or duration. Some of these regional or network frontal cortex changes were associated with impaired performance on specific cognitive tasks [12▪,20▪,22,47▪,52▪,54▪,55▪▪] or mood disorders [37,48▪], suggesting a possible use as markers of progressive cognitive or behavioral decline. Additionally, associations identified between regional metabolic and structural alterations with a neurotoxic antibody [20▪,47▪], between altered frontal cortex in the DMN and VCAM-1 levels and between caudate activation and IL-6 levels [55▪▪] demonstrate the potential of these neuroimaging techniques for eliciting pathogenic mechanisms. Importantly, these studies indicate that subclinical changes, possibly augmented by a leaky BBB [28▪], are occurring in SLE that associate with impaired cognitive abilities and one longitudinal study demonstrates further decline in microstructural integrity over time [15].
In summary, advanced imaging techniques are identifying changes in regional brain microstructure (DTI-MRI) and function (FDG-PET, rs-fMRI), resting state network and node functional connectivity (rs-fMRI) and neuronal activity associated with task performance (task-fMRI) in SLE. The evolving challenge is to design studies that use these techniques effectively to develop objective biomarkers for the diagnosis and attribution of specific neuropsychiatric syndromes and response to targeted therapy. Design considerations of particular importance in SLE include variables with potential to impact brain function such as; history of CNS disease, SLE disease activity, comorbid disease, and medications. Use of multiple imaging techniques to simultaneously ascertain structure and function as they relate to clinical parameters provides valuable information, however, longitudinal studies are necessary to determine prognostic value of abnormalities and associations with specific neuropsychiatric manifestations or biologic markers. Cognitive dysfunction and mood disorders are common in SLE and imaging suggests that changes associated with these neuropsychiatric syndromes are occurring sub-clinically and have potential for use as biomarkers. In contrast, imaging has not yet provided significant insight into other diffuse NPSLE syndromes, because of the rarity of individual syndromes and logistics of studying patients during acute illness.
Acknowledgements
The authors would like to thank Drs. Erik Anderson and Cynthia Aranow for their contributions to the literature review.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Hanly JG, Urowitz MB, Su L, et al. Prospective analysis of neuropsychiatric events in an international disease inception cohort of patients with systemic lupus erythematosus. Ann Rheum Dis 2010; 69:529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeltsch-David H, Muller S. Neuropsychiatric systemic lupus erythematosus: pathogenesis and biomarkers. Nat Rev Neurol 2014; 10:579–596. [DOI] [PubMed] [Google Scholar]
- 3.The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes Arthritis Rheum 1999; 42:599–608. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Xiang X, Sun J, Feng L, et al. Prevalence, outcome and prognostic factors of neuropsychiatric systemic lupus erythematosus: a real world single center study. Mod Rheumatol 2019; 30:321–326. [DOI] [PubMed] [Google Scholar]
- 5.Bortoluzzi A, Scire CA, Govoni M. Attribution of neuropsychiatric manifestations to systemic lupus erythematosus. Front Med (Lausanne) 2018; 5:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz N, Stock AD, Putterman C. Neuropsychiatric lupus: new mechanistic insights and future treatment directions. Nat Rev Rheumatol 2019; 15:137–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luyendijk J, Steens SC, Ouwendijk WJ, et al. Neuropsychiatric systemic lupus erythematosus: lessons learned from magnetic resonance imaging. Arthritis Rheum 2011; 63:722–732. [DOI] [PubMed] [Google Scholar]
- 8.Postal M, Lapa AT, Reis F, et al. Magnetic resonance imaging in neuropsychiatric systemic lupus erythematosus: current state of the art and novel approaches. Lupus 2017; 26:517–521. [DOI] [PubMed] [Google Scholar]
- 9.Ainiala H, Dastidar P, Loukkola J, et al. Cerebral MRI abnormalities and their association with neuropsychiatric manifestations in SLE: a population-based study. Scand J Rheumatol 2005; 34:376–382. [DOI] [PubMed] [Google Scholar]
- 10.Sarbu N, Alobeidi F, Toledano P, et al. Brain abnormalities in newly diagnosed neuropsychiatric lupus: systematic MRI approach and correlation with clinical and laboratory data in a large multicenter cohort. Autoimmun Rev 2015; 14:153–159. [DOI] [PubMed] [Google Scholar]
- 11.Cannerfelt B, Nystedt J, Jonsen A, et al. White matter lesions and brain atrophy in systemic lupus erythematosus patients: correlation to cognitive dysfunction in a cohort of systemic lupus erythematosus patients using different definition models for neuropsychiatric systemic lupus erythematosus. Lupus 2018; 27:1140–1149. [DOI] [PubMed] [Google Scholar]
- 12▪.Nystedt J, Mannfolk P, Jonsen A, et al. Functional connectivity changes in core resting state networks are associated with cognitive performance in systemic lupus erythematosus. J Comp Neurol 2019; 527:1837–1856. [DOI] [PubMed] [Google Scholar]; This study identified many SLE-specific changes in regional functional connectivity in SLE (both NPSLE and non-NPSLE) compared to healthy control that correlated with cognitive testing. It highlights abnormal hyperconnectivity within the Default Mode Network and extending to other resting networks (the Central Executive Network in particular), that may represent suboptimal compensatory mechanisms present in all SLE.
- 13▪.Preziosa P, Rocca MA, Ramirez GA, et al. Structural and functional brain connectomes in patients with systemic lupus erythematosus. Eur J Neurol 2020; 27:113-e2. [DOI] [PubMed] [Google Scholar]; This study shows that structural global network metrics including strength, transitivity, efficiency and path length, and the nodal network metrics including strength and clustering coefficient are abnormal in patients with SLE with a diffuse disruption of structural integrity. The findings of structural network metrics in this article may be useful for monitoring white matter damage in SLE.
- 14.Hanly JG, Urowitz MB, Sanchez-Guerrero J, et al. Neuropsychiatric events at the time of diagnosis of systemic lupus erythematosus: an international inception cohort study. Arthritis Rheum 2007; 56:265–273. [DOI] [PubMed] [Google Scholar]
- 15.Kozora E, Filley CM, Erkan D, et al. Longitudinal evaluation of diffusion tensor imaging and cognition in systemic lupus erythematosus. Lupus 2018; 27:1810–1818. [DOI] [PubMed] [Google Scholar]
- 16.Magro-Checa C, Kumar S, Ramiro S, et al. Are serum autoantibodies associated with brain changes in systemic lupus erythematosus? MRI data from the Leiden NP-SLE cohort. Lupus 2019; 28:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaichi Y, Kakeda S, Moriya J, et al. Brain MR findings in patients with systemic lupus erythematosus with and without antiphospholipid antibody syndrome. AJNR 2014; 35:100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valdes-Ferrer SI, Vega F, Cantu-Brito C, et al. Cerebral changes in SLE with or without antiphospholipid syndrome. a case-control MRI study. J Neuroimaging 2008; 18:62–65. [DOI] [PubMed] [Google Scholar]
- 19.Hachulla E, Michon-Pasturel U, Leys D, et al. Cerebral magnetic resonance imaging in patients with or without antiphospholipid antibodies. Lupus 1998; 7:124–131. [DOI] [PubMed] [Google Scholar]
- 20▪.Mackay M, Vo A, Tang CC, et al. Metabolic and microstructural alterations in the SLE brain correlate with cognitive impairment. JCI Insight 2019; 4: pii: 124002. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates and validates the presence of abnormally elevated metabolism in multiple brain regions (e.g. hippocampus), associated with decreased microstructural integrity and impaired cognitive performance, in patients with SLE without active or prior neuropsychiatric symptoms. The findings have important implications on the future application of these FDG-PET and MRI-DTI imaging markers to improve the accuracy of early diagnosis, evaluation of treatment response, and assessment of disease progression in SLE.
- 21.Correa DG, Zimmermann N, Borges RS, et al. White-matter integrity in patients with systemic lupus erythematosus and memory deficits. Neuroradiol J 2018; 31:587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiseman SJ, Bastin ME, Amft EN, et al. Cognitive function, disease burden and the structural connectome in systemic lupus erythematosus. Lupus 2018; 27:1329–1337. [DOI] [PubMed] [Google Scholar]
- 23.Yoshio T, Okamoto H, Kurasawa K, et al. IL-6, IL-8, IP-10, MCP-1 and G-CSF are significantly increased in cerebrospinal fluid but not in sera of patients with central neuropsychiatric lupus erythematosus. Lupus 2016; 25:997–1003. [DOI] [PubMed] [Google Scholar]
- 24.Ho RC, Thiaghu C, Ong H, et al. A meta-analysis of serum and cerebrospinal fluid autoantibodies in neuropsychiatric systemic lupus erythematosus. Autoimmun Rev 2016; 15:124–138. [DOI] [PubMed] [Google Scholar]
- 25.Arinuma Y, Yanagida T, Hirohata S. Association of cerebrospinal fluid anti-NR2 glutamate receptor antibodies with diffuse neuropsychiatric systemic lupus erythematosus. Arthritis Rheum 2008; 58:1130–1135. [DOI] [PubMed] [Google Scholar]
- 26.Fragoso-Loyo H, Cabiedes J, Orozco-Narvaez A, et al. Serum and cerebrospinal fluid autoantibodies in patients with neuropsychiatric lupus erythematosus. Implications for diagnosis and pathogenesis. PLoS One 2008; 3:e3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshio T, Hirata D, Onda K, et al. Antiribosomal P protein antibodies in cerebrospinal fluid are associated with neuropsychiatric systemic lupus erythematosus. J Rheumatol 2005; 32:34–39. [PubMed] [Google Scholar]
- 28▪.Chi JM, Mackay M, Hoang A, et al. Alterations in blood-brain barrier permeability in patients with systemic lupus erythematosus. AJNR 2019; 40:470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study is the first to evaluate BBB permeability in a small cohort. It identifies increased BBB permeability in the hippocampus, an area known to orchestrate cognition and is susceptible to autoantibody-mediated attack.
- 29.Mader S, Brimberg L, Diamond B. The role of brain-reactive autoantibodies in brain pathology and cognitive impairment. Front Immunol 2017; 8:1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen D, Rijnink EC, Nabuurs RJ, et al. Brain histopathology in patients with systemic lupus erythematosus: identification of lesions associated with clinical neuropsychiatric lupus syndromes and the role of complement. Rheumatology (Oxford) 2017; 56:77–86. [DOI] [PubMed] [Google Scholar]
- 31.Sibbitt WL, Jr, Brooks WM, Kornfeld M, et al. Magnetic resonance imaging and brain histopathology in neuropsychiatric systemic lupus erythematosus. Semin Arthritis Rheum 2010; 40:32–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zimny A, Szmyrka-Kaczmarek M, Szewczyk P, et al. In vivo evaluation of brain damage in the course of systemic lupus erythematosus using magnetic resonance spectroscopy, perfusion-weighted and diffusion-tensor imaging. Lupus 2014; 23:10–19. [DOI] [PubMed] [Google Scholar]
- 33.Gasparovic CM, Roldan CA, Sibbitt WL, Jr, et al. Elevated cerebral blood flow and volume in systemic lupus measured by dynamic susceptibility contrast magnetic resonance imaging. J Rheumatol 2010; 37:1834–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang PI, Cagnoli PC, McCune WJ, et al. Perfusion-weighted MR imaging in cerebral lupus erythematosus. Acad Radiol 2012; 19:965–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papadaki E, Fanouriakis A, Kavroulakis E, et al. Neuropsychiatric lupus or not? Cerebral hypoperfusion by perfusion-weighted MRI in normal-appearing white matter in primary neuropsychiatric lupus erythematosus. Ann Rheum Dis 2018; 77:441–448. [DOI] [PubMed] [Google Scholar]
- 36.Emmer BJ, van Osch MJ, Wu O, et al. Perfusion MRI in neuro-psychiatric systemic lupus erthemathosus. J Magn Reson Imaging 2010; 32:283–288. [DOI] [PubMed] [Google Scholar]
- 37.Papadaki E, Kavroulakis E, Bertsias G, et al. Regional cerebral perfusion correlates with anxiety in neuropsychiatric SLE: evidence for a mechanism distinct from depression. Lupus 2019; 28:1678–1689. [DOI] [PubMed] [Google Scholar]
- 38.Jia J, Xie J, Li H, et al. Cerebral blood flow abnormalities in neuropsychiatric systemic lupus erythematosus. Lupus 2019; 28:1128–1133. [DOI] [PubMed] [Google Scholar]
- 39.Zhuo Z, Su L, Duan Y, et al. Different patterns of cerebral perfusion in SLE patients with and without neuropsychiatric manifestations. Hum Brain Mapp 2020; 41:755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Curiel R, Akin EA, Beaulieu G, et al. PET/CT imaging in systemic lupus erythematosus. Ann NY Acad Sci 2011; 1228:71–80. [DOI] [PubMed] [Google Scholar]
- 41.Komatsu N, Kodama K, Yamanouchi N, et al. Decreased regional cerebral metabolic rate for glucose in systemic lupus erythematosus patients with psychiatric symptoms. Eur Neurol 1999; 42:41–48. [DOI] [PubMed] [Google Scholar]
- 42.Lee SW, Park MC, Lee SK, Park YB. The efficacy of brain (18)F-fluorodeoxyglucose positron emission tomography in neuropsychiatric lupus patients with normal brain magnetic resonance imaging findings. Lupus 2012; 21:1531–1537. [DOI] [PubMed] [Google Scholar]
- 43.Saito T, Tamura M, Chiba Y, et al. Regional cerebral glucose metabolism in systemic lupus erythematosus patients with major depressive disorder. J Neurologic Sci 2017; 379:127–130. [DOI] [PubMed] [Google Scholar]
- 44.Weiner SM, Otte A, Schumacher M, et al. Diagnosis and monitoring of central nervous system involvement in systemic lupus erythematosus: value of F-18 fluorodeoxyglucose PET. Ann Rheum Dis 2000; 59:377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mackay M, Bussa MP, Aranow C, et al. Differences in regional brain activation patterns assessed by functional magnetic resonance imaging in patients with systemic lupus erythematosus stratified by disease duration. Mol Med 2011; 17 (11–12):1349–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mackay M, Tang CC, Volpe BT, et al. Brain metabolism and autoantibody titres predict functional impairment in systemic lupus erythematosus. Lupus Sci Med 2015; 2:e000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47▪.Ploran E, Tang C, Mackay M, et al. Assessing cognitive impairment in SLE: examining relationships between resting glucose metabolism and anti-NMDAR antibodies with navigational performance. Lupus Sci Med 2019; 6:e000327. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that performance of an SNT is associated with serum anti-NMDAR ab titres and regional hypermetabolism in the anterior putamen/caudate and frontal cortex in patients with SLE with no history of CNS disease. It demonstrates, for the first time, the ability of patients with SLE to recruit a compensatory neural circuit comprised of the SNT-associated regions for successful completion of the task, in contrast to those patients who are unable to activate this circuit and thus fail to perform the task.
- 48▪.Liu S, Cheng Y, Xie Z, et al. A conscious resting state fMRI study in SLE patients without major neuropsychiatric manifestations. Front Psychiatry 2018; 9:677. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates a negative correlation between anxiety status and functional connectivity in brain regions including paracentral lobule, postcentral gyrus, precuneus, and superior temporal gyrus in a large cohort on patients with non-NPSLE and healthy control. These changes of functional connectivity in response to neuronal damage may be the foundation of anxiety and depression in SLE.
- 49.Yu Y, Chen L, Wang Q, et al. Altered amplitude of low-frequency fluctuations in inactive patients with nonneuropsychiatric systemic lupus erythematosus. Neural Plast 2019; 2019:9408612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu H, Qiu X, Zhang YQ, et al. Abnormal amplitude of low frequency fluctuation and functional connectivity in nonneuropsychiatric systemic lupus erythematosus: a resting-state fMRI study. Neuroradiology 2019; 61:331–340. [DOI] [PubMed] [Google Scholar]
- 51.Niu C, Tan X, Liu X, et al. Cortical thickness reductions associate with abnormal resting-state functional connectivity in nonneuropsychiatric systemic lupus erythematosus. Brain Imaging Behav 2018; 12:674–684. [DOI] [PubMed] [Google Scholar]
- 52▪.Cao ZY, Wang N, Jia JT, et al. Abnormal topological organization in systemic lupus erythematosus: a resting-state functional magnetic resonance imaging analysis. Brain Imaging Behav 2020; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]; This study demonstrates the abnormal topological structure of the functional network and the correlations between the topological characteristics of functional network and clinical information and the correlation between functional connectivity and cognitive status in patients with SLE. This finding may be useful for evaluating the progressive cognitive decline in patients with SLE.
- 53.Barraclough M, Elliott R, McKie S, et al. Cognitive dysfunction and functional magnetic resonance imaging in systemic lupus erythematosus. Lupus 2015; 24:1239–1247. [DOI] [PubMed] [Google Scholar]
- 54▪.Wu BB, Ma Y, Xie L, et al. Impaired decision-making and functional neuronal network activity in systemic lupus erythematosus. J Magn Reson Imaging 2018; 48:1508–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that patients with non-NPSLE exhibit impaired decision making abilities that are associated with decreased activation in the limbic system, but increased activation in memory, emotion, and behavior systems. These systems may be key factors in the pathologic mechanisms underlying SLE.
- 55▪▪.Barraclough M, McKie S, Parker B, et al. Altered cognitive function in systemic lupus erythematosus and associations with inflammation and functional and structural brain changes. Ann Rheum Dis 2019; 78:934–940. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study suggests that poor attenuation brain responses in default mode network regions during a working memory task, which are associated with organ damage, VCAM-1, and IL-6 may contribute to cognitive impairments in patients with SLE. This article highlights the involvement of default mode network in pathologic mechanisms underlying cognitive dysfunction in SLE.
- 56.Bendorius M, Po C, Muller S, Jeltsch-David H. From systemic inflammation to neuroinflammation: the case of neurolupus. Int J Mol Sci 2018; 19: pii: E3588. [DOI] [PMC free article] [PubMed] [Google Scholar]


