Abstract
Objective:
Summarise the published evidence on otitis media and associated hearing loss in low to middle-income countries (LMIC) and disadvantaged populations.
Data sources:
PubMed and other databases.
Review methods:
Firstly, sensitive search strategy using ‘otitis media’, combined with specific key words for each topic of the review, from January 2015 to June 2019. Then, restriction to LMIC and disadvantaged populations. Topics covered included prevention, epidemiology, risk factors, microbiology, prognosis, diagnosis, and treatment.
Conclusions:
There was a high degree of methodological heterogeneity and high risk of bias. The majority of studies were school-based. In Africa, Asia and Oceania (e.g., Australian Aboriginal populations) the prevalence of OM was respectively 8% (range 3–16%), 14% (range 7–22%) and 50% (4–95%). Prevalence of any hearing loss in these regions was 12% (range 8–17%), 12% (range 3–24%), and 26% (range 25–28%) respectively. Risk factors in LMIC and disadvantaged populations included age, gender, exposure to smoke and pollution. Microbiology was reported for otitis media with effusion at time of surgery or ear discharge (acute otitis media with perforation or chronic suppurative otitis media). Specimen handling and processing in hospital laboratories was associated with low detection of S. pneumoniae and H. influenzae. Case series described complicated cases of OM due to M. tuberculosis, multidrug resistance and HIV. QOL studies identified discrimination of persons with OM and hearing loss. Diagnostic methods varied greatly, from naked eye to tympanometry. Treatment interventions were reported from four RCTs. Non-RCTs included evaluations of guidelines, surgery outcomes, access to ENTs.
Implications for Clinical Practice:
Chronic suppurative otitis media, otitis media with effusion and conductive hearing loss are common in LMIC and disadvantaged populations. Paucity of research, poor regional representation, non-standardised methods and low-quality reporting preclude accurate assessment of disease burden in LMIC and disadvantaged populations. Awareness and adherence to reporting Guidelines should be promoted.
Keywords: Otitis media, Low to middle income countries, Disadvantaged populations, Epidemiology, Aetiology, Risk factors, Microbiology, Diagnosis, Prognosis, Treatment
1. Epidemiology
1.1. Introduction
PREVALENCE OF OM (Table 1) and HL (Table 2): In the previous ISOM Epidemiology Panel report, almost all new studies on OM prevalence were from developing countries Turkey, China, Bangladesh, Kenya, Nepal, and India. Prevalence of OME was 16% in Turkey ~4% in China, 12% in Egypt. The prevalence of CSOM in the entire population varies by geography as has been noted in past literature reviews and was reported among school children in Bangladesh (5.6%), and Kenya (3.3%) where prevalence was higher in rural (6%) than urban areas, and similar in boys and girls. Hearing loss was reported from Nepal (4%–27% among those with OME), Brazil (30%), Egypt (19%), Greenland (2.5–50%), Bangladesh (20%) and Columbia (2–6%). Prevalence was highly dependent on threshold for age.
Table 1.
Prevalence of OM.
| SI | Author (Year of publication) and date of study | Age | Study design; Sample (Country) | Methods; personnel performed examination | N | Any OM (n) | % | OME | % | CSOM | % | Other OME* | % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Adegbiji WA (2018), Feb 2016-Jan2018 | 1–5 years | Prospective; preschool children with complaints of hearing impairment (Nigeria) | Otoscope, tympanometry, otoacustic emission and auditory brain stem response audiometry; Not mentioned | 1726 | 13 | 0.75 | - | - | - | - | 13 | 0.75 |
| 2 | Basañez I (2015), NM | 5–14 years | Cross-sectional; school-aged children in Mbarara municipality (Uganda) | Otoscopy, Tuning fork testing, Battery powered, portable Earscan 3 screening audiometer; Principal investigator | 639 | 4 | 0.63 | - | - | 4 | 0.63 | - | - |
| 3 | Hunt L (2017), May-Jun 2016 | 4–6 years | Community-based cross-sectional; school-entry age children from 10 villages in Chikhwawa district; (Malawi) | Otoscopy; Clinical Health Officer and otolaryngologists | 281 | 23 | 8.19 | - | - | 15 | 5.34 | 8 | 2.85 |
| 4 | Ilechukwu GC (2016), Jun-Aug 2006 | 0–17 years | Cross-sectional; Children presenting at the University of Nigeria Teaching Hospital ear-related problems (Nigeria) | Auroscopy, Paediatricians and otolaryngologist | 248 | 151 | 60.89 | 2 | 0.81 | 75 | 30.24 | 74 | 29.84 |
| 5 | Libwea JN (2018), Mar-Jun 2013 | 2–3 years | Community-based cross-sectional; Randomly selected children form the pneumococcal disease sentinel surveillance sites at Yaounde (Cameroon) | Pneumatic otoscop, Tympanometry (Middle Ear Analyser Grason Stadler tympanometer), Study physician | 429 | 41 | 9.56 | - | - | 3 | 0.70 | 38 | 8.86 |
| 6 | Phanguphangu MC (2017), Mar-Jun 2015 | 5–7 years | Cross-sectional retrospective study; 11 primary schools in the Waterberg District of Limpopo, Mokopane and Mookgophong, were selected for the school health campaign (South Africa) | Otoscopic examinations on pupils; Audiologists | 1089 | 76 | 6.98 | - | - | - | - | 76 | 6.98 |
| 7 | Simoes EA (2016), Jun-Dec 2012 | 2–15 years | Prospective study; preschool, elementary, and secondary school (Kenyan) | Otoscopic, Interacoustics handheld tympanometers, pure-tone audiometry; Clinical officers | 13109 | 488 | 3.72 | 193 | 1.47 | 203 | 1.55 | 92 | 0.70 |
| 8 | Chadha SK (2015), 2010 to 2011 | 18 days and 15 | Pilot study, Children from non-slum urban areas, urban slums and rural area (India) | Otoscope, Oto-acoustic emission tests, loss; examining the external ear; Persons trained in ear examination and diagnoses were verified by an ENT specialist | 4626 | 279 | 6.03 | 93 | 2.01 | 168 | 3.63 | 18 | 0.39 |
| 9 | Jain R (2016), Jan-Jun 2013 | ≤18 years | Prospective study; Pediatric patients attending ENT OPD (India) | History, clinical examination and otoscopic examination | 1140 | 517 | 45.35 | 105 | 9.21 | 264 | 23.16 | 148 | 12.98 |
| 10 | Khan MA (2016), May–Jul 2014 | 5–16 years | Community based cross-sectional; 8 schools for the poor and most underdeveloped/deprived Shangla district (Pakistan) | History of ENT examination by Physicians and oto-rhino-laryngologists | 2882 | 186 | 6.45 | 54 | 1.87 | 93 | 3.23 | 39 | 1.35 |
| 11 | Muftah S (2015), Apr 2011–Jun 2011 | 6–16 years | Community-based survey cross-sectional survey; Healthy school-age children for selected 30 schools from urban and rural areas of Socotra Island (Yemen) | Clinical and otoscopic examinations, Tuning Fork examination, KAMPLEX diagnostic audiometer; NM | 686 | 64 | 9.33 | 13 | 1.90 | 51 | 7.43 | - | - |
| 12 | Parmar S (2018), NM | 5–15 years | Cross-sectional survey; Randomly selected students from primary schools in rural and urban areas of Muzaffarnagar (India)5.3% | Pure tone audiometry; Otoscopic examination; NM | 2158 | 78 | 3.61 | - | - | 78 | 3.61 | - | - |
| 13 | Riaz M (2018), Dec 2016-Nov 2017 | 4–14 years | Cross-sectional; Children visited outdoor of ENT Sir Ganga Ram Hospital Lahore with allergic rhinitis (Pakistan) | Tympanometry; NM | 220 | 42 | 19.09 | 42 | 19.09 | - | - | - | - |
| 14 | Rupa V (2016), Feb-Aug 2009 | 0–26 months | Cohort; Rural K.V Kuppam Vellore district of Tamil Nadu state (India) | Otoscopy; Doctor or nurse | 210 | 61 | 29.05 | - | - | - | - | 61 | 29.05 |
| 15 | Tarafder KH (2015), 2013 | ≥ 0–60 years | Nationally representative cross-sectional survey; 11 urban and 41 rural areas (Bangladesh) | Tuning fork tests, Pure tone audiometry, Tympanometry, Otoacoustic emissions testing, Research physician | 4260 | 495 | 11.62 | 226 | 5.31 | 264 | 6.20 | 5 | 0.12 |
| 16 | Kaspar A (2018), NM | 4–15 years | Cross-sectional; Primary school students in the capital city Honiara (Solomon Islands) | Tuning fork test, audiometry; Audiologist, Registered Nurses | 604 | 162 | 26.82 | 162 | 26.82 | - | - | - | - |
| 17 | Kaspar A (2018), Aug 2017 | ≥ 0–24 months | Cross-sectional; Eight Child Welfare Clinics (CWCs) in the capital city Honiara (Solomon Islands) | Vorotek-O-Scope equipment; Senior ENT Registered Nurse | 215 | 70 | 32.56 | 63 | 29.30 | - | - | 7 | 3.26 |
| 18 | Leach AJ (2016), Feb 2010-Aug 2013 | 0–6 years | Cross sectional survey; 25 Top End remote communities (Australia) | Tympanometer, pneumatic otoscopy, and a video-otoscope; Ear health research nurses | 651 | 561 | 86.18 | 327 | 50.23 | 45 | 6.91 | 189 | - |
NM: Not mentioned; Other OME: OM/AOM, AOM with/without perforation: OM: Otitis media; AOM: Acute Otitis media; CSOM: chronic supportive otitis media.
Table 2.
Prevalence of hearing loss.
| SI | Author (Year of publication) and date of study | Age | Study design; Sample (Country) | Methods; personnel performed examination | Hearing proof/treated; Other | Condition | N | n | % | Sensorineural | % | Conductive | % | Mixed | % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Adegbiji WA (2018), Febr 2016-Jan 2018 | 1–5 years | Prospective; preschool children with complaints of hearing impairment (Nigeria) | Otoscope, tympanometry, otoacustic emission and auditory brain stem response audiometry; Not mentioned | Not mentioned | Overall Moderate Severe Severe Profound |
1726 | 101 3 21 48 |
5.85 0.17 1.22 2.78 |
62 - |
3.59 | 27 | 1.56 | 12 | 0.70 |
| 2 | Basañez I (2015), Not mentioned | 5–14 years | Cross-sectional; school-aged children in Mbarara municipality (Uganda) | Otoscopy, Tuning fork testing, Battery powered, portable Earscan 3 screening audiometer; Principal investigator | The school library | 639 | 20 | 3.13 | 11 | 1.72 | 8 | 1.25 | 1 | 0.16 | |
| 3 | Ferrite S (2017), Aug-Oct 2013 | ≥ 0–80 years | Population-based survey (Cameroon) | Otoacoustic emission (OAE) test in both ears; manual pure-tone audiometry (PTA) screening, Ear-Nose-Throat nurses | Conducted in the field in the quietest space available; The degree of hearing impairment was graded as ‘moderate’ when 41–60 dB (18+ years) or 35–60 dB (4–17 years); ‘severe’ when 61–80 dB and ‘profound’ when ≥ 81 dB. | Overall Moderate (>4 Years) Moderate (>4 Years) Moderate (> 4 Years) |
3567 3092 3092 3092 |
127 76 15 9 |
3.56 2.46 0.49 0.29 |
- | |||||
| 4 | Hrapcak S (2016), Dec 2013-Mar 2014 | 4–14 years | Cross-sectional, HIV-infected children attending antiretroviral therapy clinic in Lilongwe between(Malawi) | Otoscopy, tympanometry, transient evoked otoacoustic emissions (TEOAE), and audiometry.; Trained audiology staff | Portable testing van containing two sound proof booths or at Largeer booths at African Bible College; Categories of hearing loss were defined as follows: normal (up to 20 dB), mild (21–40 dB), moderate (41–65 dB), severe (66–90 dB), and profound (≥91 dB) | Overall Mild Moderate Severe Profound |
380 | 126 84 26 9 7 |
33.16 22.11 6.84 2.37 1.84 |
17 6 1 3 7 |
4.47 1.58 0.26 0.79 1.84 |
103 77 24 2 0 |
27.11 20.26 6.32 0.53 0.00 |
6 1 1 4 0 |
1.58 0.26 0.26 1.05 0.00 |
| 5 | Hunt L (2017), May-Jun 2016 | 4–6 years | Community-based cross-sectional; school-entry age children from 10 villages in Chikhwawa district; (Malawi) | Otoscopy; Clinical Health Officer and otolaryngologists | Quietest available room in each community. Ambient noise levels were monitored and tests repeated if maximum permissible noise levels were exceeded | 279 | 64 | 22.94 | - | ||||||
| 6 | Ilechukwu GC (2016), Jun-Aug 2006 | 0–17 years | Cross-sectional; Children presenting at the University of Nigeria Teaching Hospital ear-related problems (Nigeria) | Auroscopy, Paediatricians and otolaryngologist | NM | 248 | 18 | 7.26 | - | ||||||
| 7 | Simoes EA (2016), Jun 2012-Dec 2012 | 2–15 years | Prospective study; preschool, elementary, and secondary school (Kenyan) | Otoscopic, Interacoustics handheld tympanometers, pure-tone audiometry; Clinical officers | Semi sound proof room | Overall Mild Moderate Severe |
12985 | 1243 952 243 48 |
9.57 7.33 1.87 0.37 |
- | |||||
| 8 | Smith AF (2017), Nov 2015-Apr 2016 | 7–20 years | Cross-sectional; school age children in Addis Ababa with HIV positive (107) and 147 HIV unknown (147) (Ethiopia) | Pure-tone audiometry | Circumaural headset; hearing loss greater than 25 dB | 254 | 59 | 23.23 | 25 | 9.84 | 25 | 9.84 | 8 | 3.15 | |
| 9 | Yousuf Hussein S (2018), NM | 3–6 years | Early childhood development (South Africa) | Innitial screening by HearScreen™ smartphone application, Handheld Welch Allyn or Heine mini 3000, Interacoustics Impedance Audiometer; Audiologist | NM | Overall Mild Mild to moderate Moderate Moderate to severe Severe Mild to sever |
245 | 46 25 5 5 4 1 2 |
18.78 10.20 2.04 2.04 1.63 0.41 0.82 |
12 - |
4.90 | 30 | 12.24 | 3 | 1.22 |
| 10 | Bright T (2019), 2014 | ≥ 6 months | Population-based cross-sectional survey; (India) | Otoacoustic emissions (OAE) test,.Audiologist | Aged 6 months to 3 years 11 months: participants who failed OAE in both ears; ≥4 years: pure-tone average of thresholds at 0.5, 1, 2, and 4 kHz in the better ear of ≥41 dB HL in adults (≥18 years of age) and ≥31 dB HL in children (4–17 years of age | Overall Mild Moderate Severe Profound |
3573 | 313 160 104 34 15 |
8.76 4.48 2.91 0.95 0.42 |
- | |||||
| 11 | Garg S (2018), Jan-Jun 2017 | ≥3 months (32.17 ± 20.85) | Community-based cross-sectional, rural and urban areas of Delhi (India) | Handheld oto-acoustic emission (OAE) in children <5 years of age and pure tone audiometry (PTA) in persons >5 years; Not mentioned | Mild (26–40 dB), Moderate (41–60 dB), Severe (61–80 dB), Profound (>81 dB) | Overall Mild Moderate Severe Profound |
595 | 160 48 22 82 5 |
26.89 8.07 3.70 13.78 0.84 |
94 - |
15.80 | 61 | 10.25 | 5 | 0.84 |
| 12 | Muftah S (2015), Apr-Jun 2011 | 6–16 years | Community-based survey cross-sectional survey; Healthy school-age children for selected 30 schools from urban and rural areas of Socotra Island (Yemen) | Clinical and otoscopic examinations, Tuning Fork examination, KAMPLEX diagnostic audiometer; Not mentioned | Soundproof headphones; normal (<30 dB), mild (31 dB-40 dB), moderate (41 dB-60 dB), severe (61 dB-80 dB), and profound (>80 dB), *Prevalence of Hearing loss among children with disabling hearing impairment |
Overall Mild Moderate Severe |
686 | 34 16 13 2 |
4.95 2.33 1.90 0.29 |
3* - |
0.44 | 26* | 3.79 | 5* | 0.73 |
| 13 | Tarafder KH (2015), 2013 | ≥ 0–60 years | Nationally representative cross-sectional survey; 11 urban and 41 rural areas (Bangladesh) | Tuning fork tests, Pure tone audiometry, Tympanometry, Otoacoustic emissions testing, Research physician | No impairment (0–25 dB), mild impairment (26–40 dB), moderate impairment (41–60 dB), severe impairment (61–80 dB) and profound impairment (greater than 80 dB) | Overall Mild Moderate Severe Profound | 4260 4260 |
1215 1112 258 53 49 |
28.52 26.10 6.06 1.24 1.15 |
178 - |
4.18 | 478 | 11.22 | 152 | 3.57 |
| 14 | Kaspar A (2018), NM | 4–15 years | Cross-sectional; Primary school students in the capital city Honiara (Solomon Islands) | Tuning fork test, audiometry; Audiologist, Registered Nurses | Non-sound-treated room | 604 | 89 | 14.74 | - | ||||||
| 15 | Sanders M (2015), 2008 | ≥5 years | Census data (Soma) | Not mentioned | Not mentioned | Overall 20–34 dBHL 35–49 dB 50–64 dB 65–79 dB 80–94 dB ≥95 dB |
160987 | 44754 29783 10303 3220 966 322 161 |
27.80 18.50 6.40 2.00 0.60 0.20 0.10 |
- | |||||
| ≥5 years | Census data (Tonga) | Not mentioned | Not mentioned | Overall 20–34 dBHL 35–49 dB 50–64 dB 65–79 dB 80–94 dB ≥95 dB |
87301 | 24182 15801 5675 1833 524 175 175 |
27.70 18.10 6.50 2.10 0.60 0.20 0.2 |
- |
1.2. Methods
Search strategy: Key words were conductive hearing loss, otitis media, epidemiology, prevalence, incidence, survey, and cross sectional. The search retrieved 2828 articles of which 2801 studies were rejected, and 27 studies from LMIC or disadvantaged populations (Fig. 1 were included. Of the 27 studies, 11 were from African, 12 from Asia and 4 from Oceania (including Australia). All but five prospective studies followed a cross-sectional design or survey. Ten studies reported only prevalence of otitis media (OM), seven studies reported only prevalence of hearing loss (HL), two studies reported incidence of OM, eight studies reported prevalence of both OM and HL, and one study reported both prevenance of HL and incidence of OM. Most of the study participants were children and adolescents (younger than 19 years); five studies included the general population (three from Asia, and one each from Africa and Oceania and Australia). All but one study reported the diagnostic technique and who performed the ear examination or hearing assessment, although sufficient details of hearing assessment were rarely provided. Nine studies were conducted in 2015 or later, fourteen studies were conducted earlier, and four studies did not report dates (see Fig. 2).
Fig. 1.
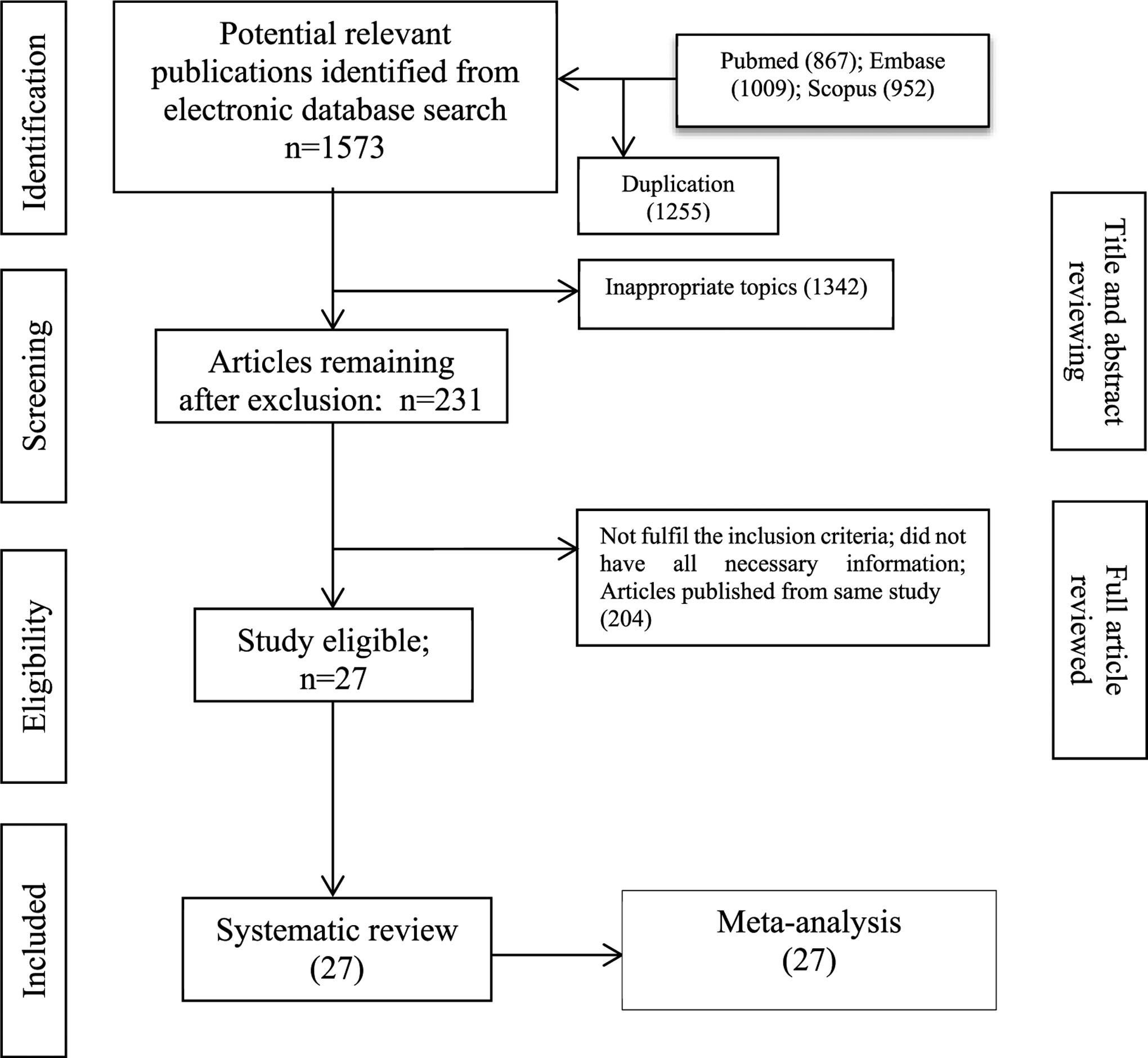
Selection framing of literature for systematic review and meta-analysis.
Fig. 2.
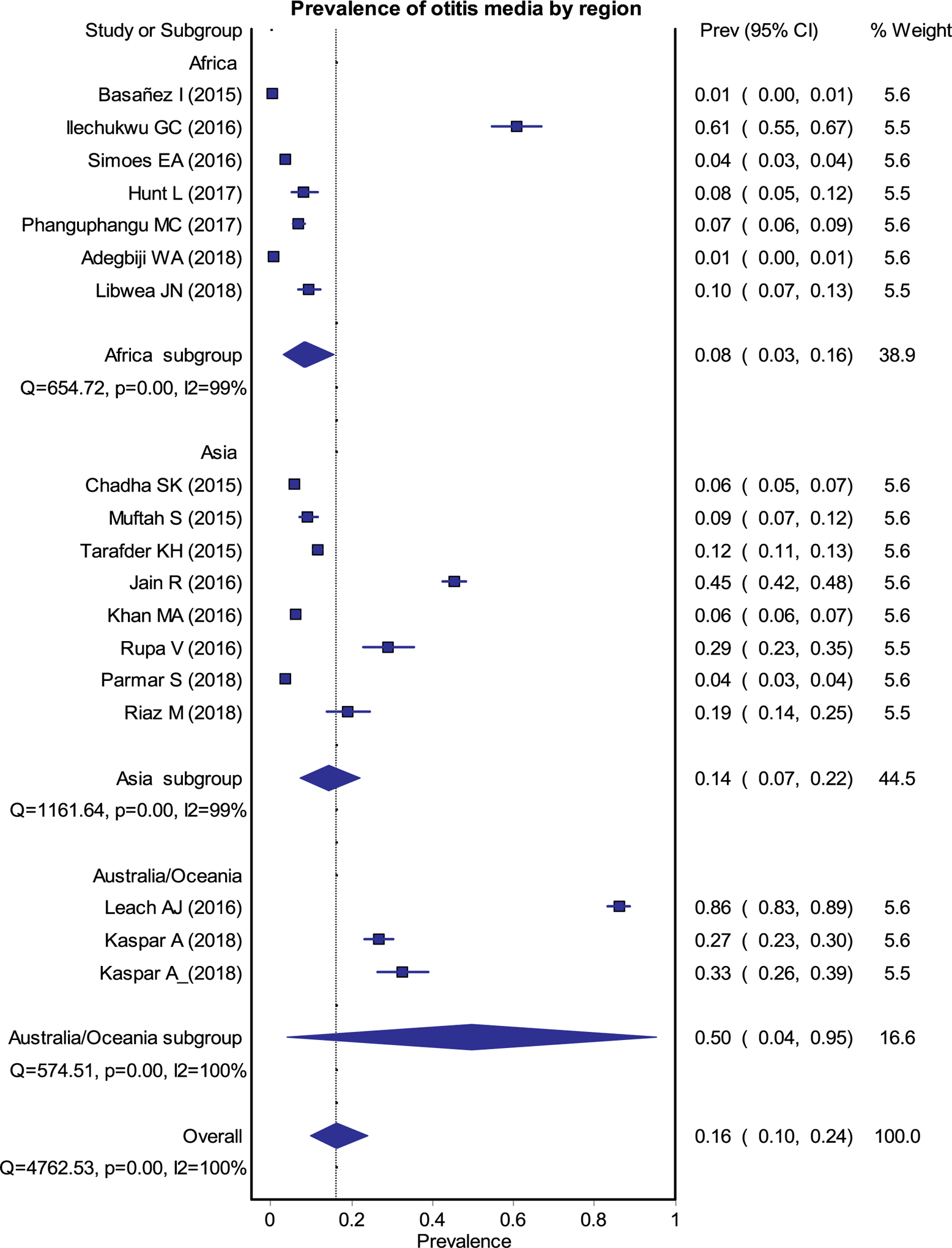
Forest plot for the pooled estimates of prevalence of any OM.
1.3. Discussion
A total of 18 studies (7 Africa, 8 Asia and 3 Oceania) representing 35,173 participants (17,521 from Africa, 16,182 from Asia and 1,470 from Oceania) provided a pooled prevalence of any type of OM of 16% (95% Confidence Interval (CI) 0.10–0.24) (Fig. 2). Region specific OM prevalence rates (Fig. 3) were 8% (0.03–0.16), 14% (0.07–0.22) and 50% (0.04–0.95) in Africa, Asia and Oceania respectively. The overall, pooled prevalence of OME was 10% (0.05–0.16), CSOM 6% (0.03–0.09), and other types of OM (OME with or without perforation and others) was 0.6% (0.03–0.10) (Fig. 4) [1–16].
Fig. 3.
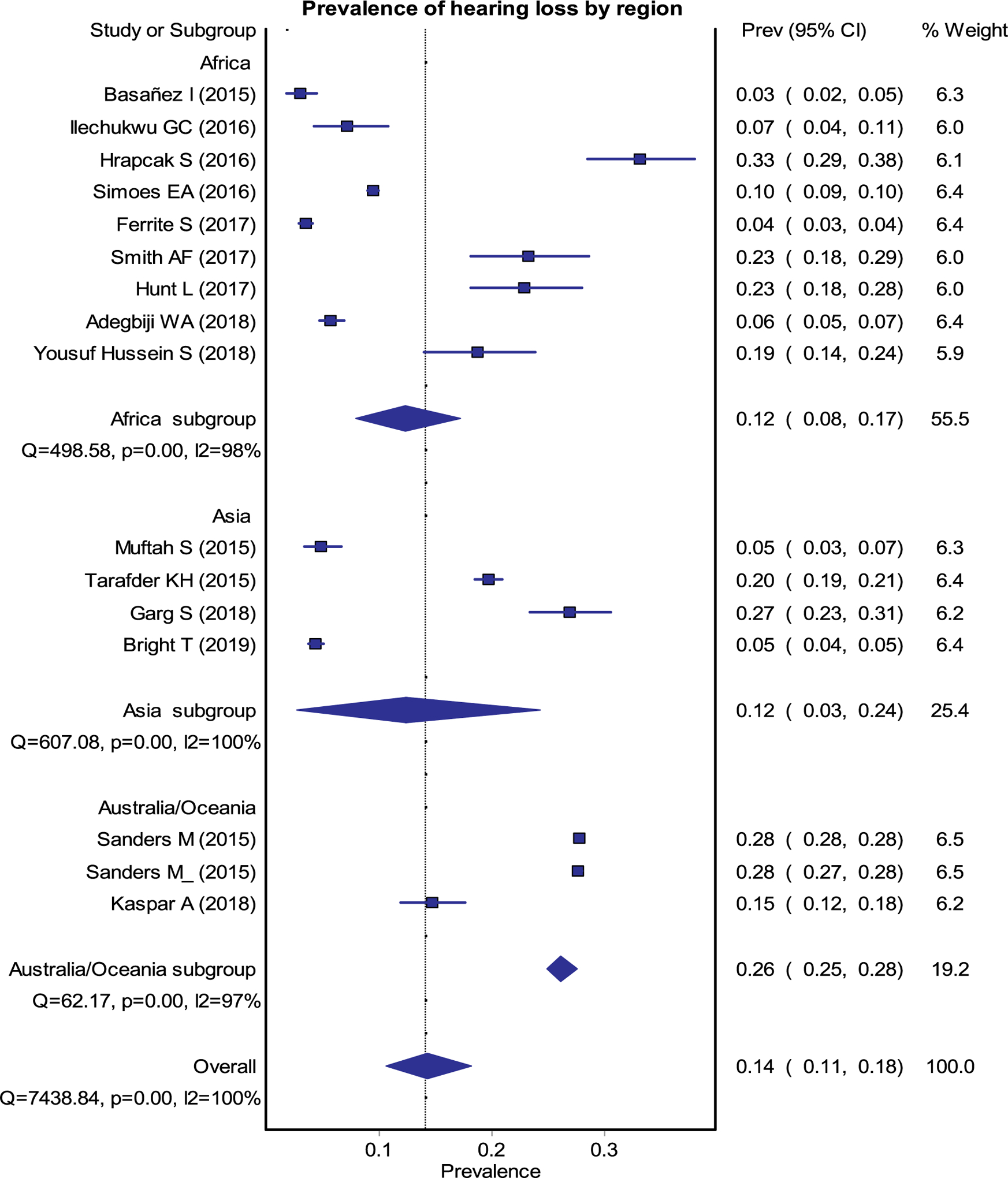
Forest plot for the pooled estimates of prevalence of any HL.
Fig. 4.
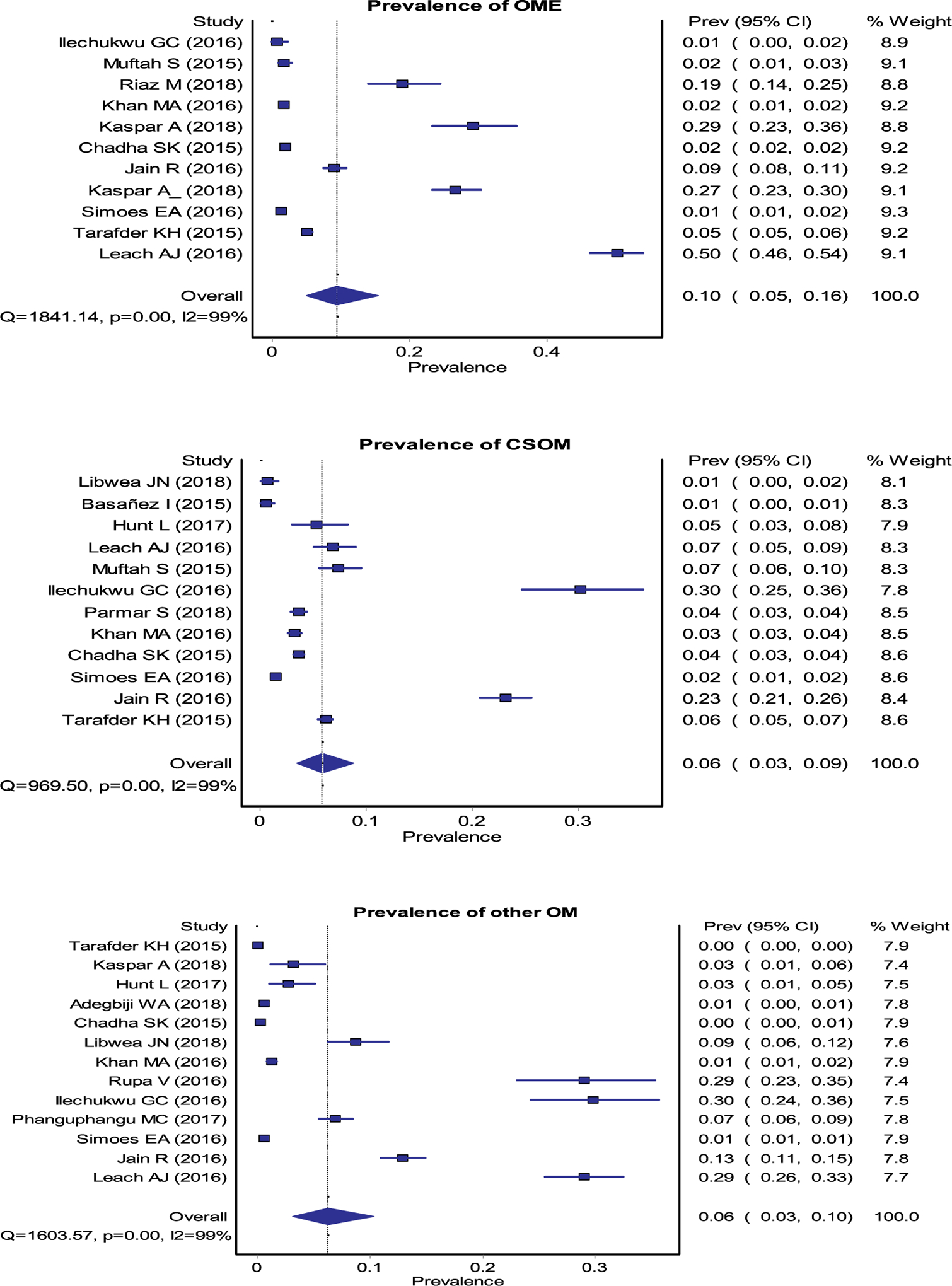
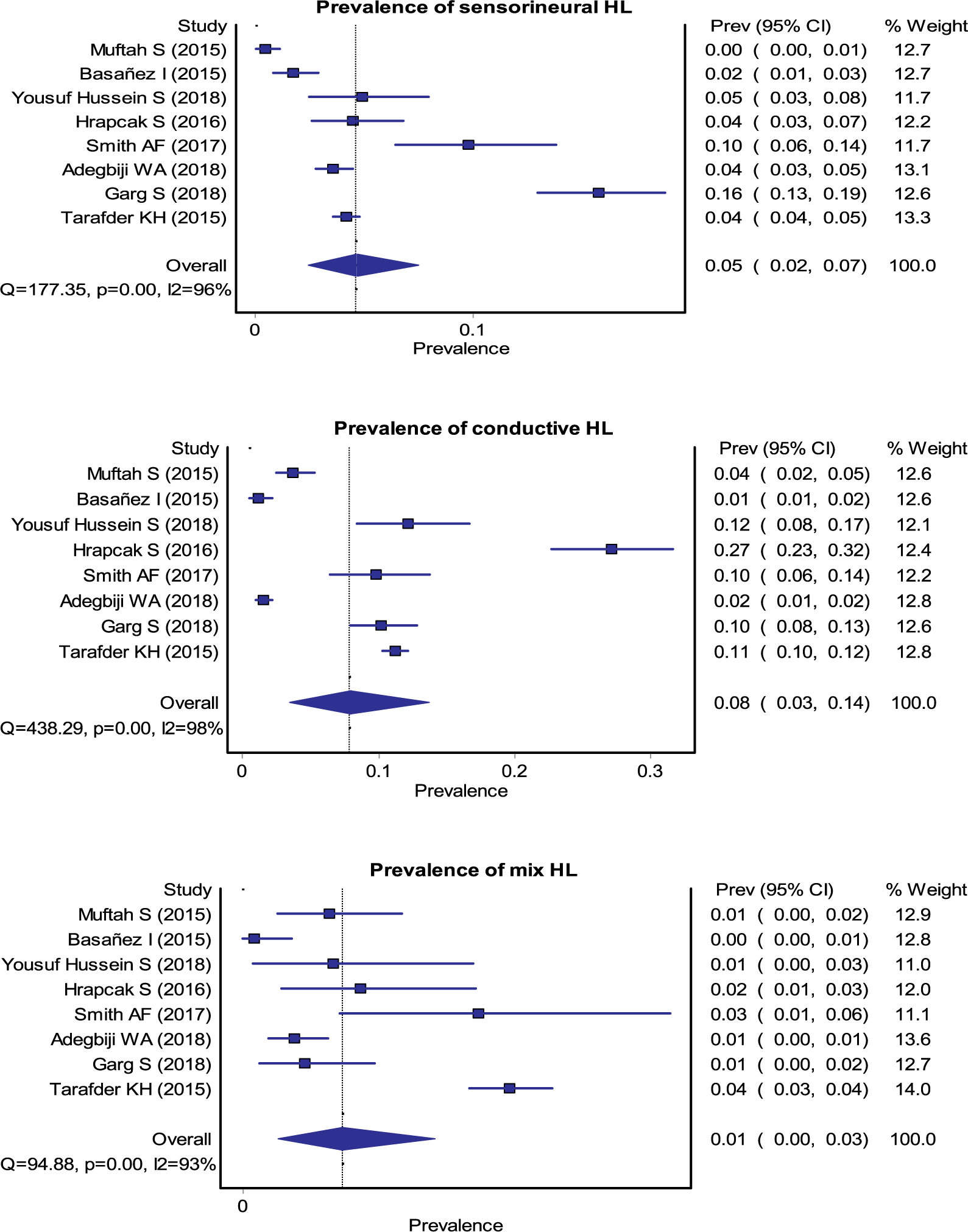
Forest plots for the pooled estimates of prevalence of OME, CSOM, SNHL, CHL and mixed HL.
Data from 15 studies (9 Africa, 4 Asia and 2 Oceania) representing 278,329 study participants (20,293 from Africa, 9,144 from Asia and 248,892 from Oceania) provided a pooled prevalence of any HL (conductive, sensorineural and mixed) was 14% (95% CI 0.11–0.18) (Fig. 3). Region specific prevalence rates for any level of HL were 12% (0.08–0.17), 12% (0.03–0.24), and 26% (0.25–0.28) in Africa, Asia, and Oceania, respectively (Fig. 3). The overall prevalence of sensorineural, conductive, or mixed HL was 5% (0.02–0.07), 8% (0.03–0.14) and 1% (0.0–0.03), respectively (Fig. 4) [1–4,7,10,13,14,17–23].
Studies which reported the incidence of OM estimated between 400 and 410 per 1000 population in Oceania and between 128 and 138 per 1000 population in Asia.
1.4. Implications for clinical practice and future research goals
High quality data are needed from LMICs (Lower to Middle Income Countries) and disadvantaged populations to determine disease burden at local, regional and country levels. These data would raise awareness and inform policy and practice augmentation in ear and hearing services for of children, particularly Indigenous and disadvantaged children.
2. Risk factors
2.1. Introduction
Our aim was to determine whether previously established risk factors for OM were consistent with those identified specifically among low to middle-income countries or disadvantaged populations.
2.2. Methods
The search string used was (“otitis media”[MeSH Terms] OR (“otitis”[All Fields] AND “media”[All Fields]) OR “otitis media”[All Fields]) AND (“humans”[MeSH Terms] OR “humans”[All Fields] OR “human”[All Fields]) AND English[All Fields] AND (aetiology[All Fields] OR (“risk factors”[MeSH Terms] OR (“risk”[All Fields] AND “factors”[All Fields]) OR “risk factors”[All Fields] OR (“risk”[All Fields] AND “factor”[All Fields]) OR “risk factor”[All Fields])) AND (has abstract[text] AND (“2015/06/01”[PDAT]: “2019/06/01”[PDAT]))
2.3. Discussion
There were 214 studies of which 60 remained after title screening, 16 after abstract reading and finally eight were included after text reading. These eight studies are shown in Table 3 with identified risk factor associations. The studies are from India [24], Brazil, Mozambique [25], Malawi [3], China [26], Philippines [27] and Fiji [28]. All studies are rated as having high risk of bias due to diagnostic methodology, lack of definitions, small sample sizes, lack of control groups, selection bias and use of univariate statistics etc.
Table 3.
Risk factors for OM.
| Author, Year of study, Year of publication, Journal/Conference | Country, Population | Study design | Population - risk group, age | Diagnostic method | Outcome | Study period | Risk of bias | Results and Conclusions |
|---|---|---|---|---|---|---|---|---|
| T. Bandyopadhyay; E. V. Raman 2018 Indian J Otolaryngol Head & Neck | Indian urban children | Case-control Selection method of controls not mentioned | Children 1–10 years old. 50 from tertiary care and 50 controls | Otoscopy, tymp, history recording, | Describe epidemiological characteristics and risk factors for OME | 2012–2014 | High Only univariate statistics |
Not significant: Birth order, maternal age >30, prematurity, perinatal complication, Nose block, allergy, siblings, family structure, accommodation pattern Significant: NICU attendance, Bottle feeding, passive smoking, daycare, |
| Bowatte et al., 2018 Int J Environment Research and Public Health | Mozambique Bhopal Sao Paulo |
Systematic review | Children various ages | Systematic review | Air pollution and OM | Older studies from 1990’s | High (rely on cross-sectional studies and case-control) |
Higher risk with charcoal or wood use in houses (OR 3.09–3.18), living close to coking works, and air polluted areas |
| Hunt et al., 2018 PlosOne | Malawi | Cross-sectional with clinical examination | 281 children aged 4–6 years | Video-otoscopy and hearing test | Chronic OM and risk factors | 2016 | High Cross-sectional |
No significant associations |
| Deng et al., 2017 Chemosphere | Changsha, China | Retrospective | 1617 children aged 3–4 years | Parental questionnaire | Lifetime prevalence of OM and pre- and postnatal pollution | 2011–12 | High Retrospective - parental questionnaire |
adjusted OR (95% CI) = 1.44 (1.09–1.88) for a 27 μg/m3 increase in SO2 and postnatal exposure to indoor renovations with OR (95% CI) = 1.62 (1.05–2.49) for new furniture and 1.81 (1.12–2.91) for redecoration |
| Santos-Cortez et al., 2016 Otolaryngol Head Neck Surg | Philippines, community | Cross-sectional clinical study | All ages (n = 187) | Otoscopy, interviews, genetic testing | Identification of genetic and environmental determinants for OM | ? | High Cross-sectional small survey |
no association between otitis media and age, gender, body mass index, breastfeeding, tobacco exposure or deep swimming Association with A2ML1 genotype (OR 3.7 (95%CI: 1.3, 10.8; p = 0.005) |
| Fang et al., 2016 Int J Ped Otorhinolaryngol | Fiji | Cross-sectional clinical study | Children 0–18 years (n = 467) | Otoscopy and tympanometry | Examine prevalence, clinical features and QoL | 2015 | High Cross-sectional, wide age-range |
Significant: Age (OR 0.53, 95% CI: 0.36–0.77) is a significant predictor of AOM, whereas male gender (OR 2.46, 95% CI: 1.13–5.37), smoke exposure (OR 2.81, 95% CI: 1.01–7.82), and concomitant chronic sinusitis (OR 6.05, 95% CI: 2.31–15.88) are significant predictors of OME. |
| Wang J et al., 2016 Acta Otolaryngol | China | Case-control Age-matched | Adults - Han Chinese - 206 cases and 210 controls | Otoscopy and standardized questionnaire | Risk factors for CSOM | ? | High Case-control. Controls not described. Not validated questionnaire |
male (OR1/40.42; 95% CI: 0.21–0.83), BMI increasing (OR1/40.85; 95% CI: 0.77–0.93), URTI (OR1/4152.85; 95% CI: 34.11–684.93), smoke/passive smoke (OR1/47.11; 95% CI: 3.36–15.07), residential location (urban area) (OR1/40.27; 95% CI: 0.13–0.56), serum calcium increasing (OR1/40.09; 95% CI: 0.01–0.71) were prime risk factors for CSOM |
| Orji et al., 2014 EAORL | Nigeria | Consecutive series of cases presenting at tertiary hospital | All ages 128 non-healing 58 healing | Not mentioned | Risk factor difference between healing (within 6 months) and non-healing (>24 months) CSOM | 2010–2012 | High Hospital selected cases Methods not mentioned |
Significant: by logistic regression analysis: rural residence, multidrug-resistant bacteria, and bilateral CSOM (P = 0.001, 0.001, and 0.008, respectively). Others were onset of ear discharge before the age of 10 years, diabetes mellitus, persistent rhinorrhoea, home [10 miles away from hospital, and [7 persons in a family (P = 0.012, 0.041, 0.013, 0.010, and 0.043, respectively |
2.4. Implications for clinical practice and future research goals
The methodological limitations described decrease the level of confidence in the findings, however, the risk factors identified are consistent with the evidence in other populations. We have also included some factors that are of local interest due to special local circumstances that cannot be generalized to other areas. Additional high-quality studies are needed to accurately identify risk factors associated with OM in LMIC populations.
3. Microbiology
3.1. Introduction
Previous ISOM reports have not specifically addressed aetiology, risk factors or microbiology in disadvantaged populations or LMIC. Whist there has been reports of studies of CSOM, these have not identified disadvantaged populations or LMIC separately. Microbiology panels have provided excellent reviews of laboratory and animal model studies of microbial pathogenesis, molecular epidemiology, genomics, virology, and polymicrobial interactions of the organisms known to cause OM. However, the microbiology of the middle ear was not included.
3.2. Methods
(“otitis”[MeSH Terms] OR “otitis”[All Fields] OR conductive hearing loss) AND microbiology Filters: Publication date from 2015/01/01 to 2019/06/01 AND Human AND English = = 597. Title, abstract and full text search for studies that included middle ear fluid or ear disharge microbiology. Studies known to the author included.
3.3. Discussion
Twenty-two studies met inclusion criteria (Table 4). Seven studies were reviews or retrospective analyses, and 14 were original studies. Studies published between 1/1/2015 and 1/6/2019 reported that data were originally collected over a long time-period (reviews) and often ten or more years ago. Data collection dates were reported in seven reviews and retrospective analyses [29–35] and 14 original studies [16,36–47]. 2016 was the most recent data collection. Twenty-one countries were included. Data from 9 countries or populations were reported in reviews only and include Alaska Natives [35], Greenlandic [35], Chile [29], Columbia [29], Mexico [29], Venezuela [29], Ethiopia [31], India [31], Turkey [31]). Original surveillance or case series studies were reported from 10 countries: Angola [36], Nigeria [48,49], Nepal [39], Pakistan [30], Thailand [40], Indigenous Australians [16,44,47], Israel Bedouins [32,34], South Africa [42], China [45] and Malawi [46]. Several reports focussed on single pathogens [38] or pneumococcal serotypes [41,43]. All studies included children. Some included all ages [36,38], the mean age being 12 years. Twelve original studies were case series in hospitals. Community-based surveillance was reported from the Australian Indigenous population [16,47] and Papua New Guinea [43]. Diagnoses included OM [31], CSOM [16,33,34,36,38,39, 43,46–49], AOMwiP [16,29,33,40,42,43,45,47,49], any TMP [30,34,35, 37], OME [33,44,50], and AOM [29,32,34,41,42]. Twenty studies reported ear discharge (ED) findings from CSOM, any tympanic membrane perforation (TMP) (not defined), or AOMwiP. Tympanocentesis (OME cases) was reported in the three reviews([29,33,35]) and three original studies of AOMwoP [41,42]or OME [44]. Few studies described method of canal cleaning. The World Health Organisation technical advisory group on pneumococcal nasopharyngeal carriage studies recommend methods for specimen collection (including ED and MEF) and laboratory processing [51]. Most studies failed to report methods used or reported other methods [29,32,41]. We assumed that WHO methods were followed if recommended methods for transport and storage were reported [16,35–38,47]. No details were provided in reviews. One microbiome study reported otopathogens [44] Almost all studies used hospital laboratory standard culture methods and non-selective culture media [30,32,35,37,39,40,46]. Few studies used selective media [34,36], qPCR for density [33,35], or PCR for detection of A.o [33] or other pathogens [29,35]. Where pneumococcal serotypes were reported, the Quellung method was used [29,34].
Table 4.
Microbiology of ear discharge (ED) or middle ear fluid (MEF).
| Author, Year of publication, Year of data, | Country, Population | Study design | Study Question Population - risk group, age |
Diagnostic method | Specimen collection and transport method | Culture conditions, PCR included, serotype method | Results |
|---|---|---|---|---|---|---|---|
| Coleman 2018 2 Data 1972 to 2016 Search to 15/8/17 |
Australian Aboriginal communities (22) Alaskan (1) Greenlandic (2) |
Review [PRISMA] See additional file for search strategy. Quality |
Microbiology of OM in Indigenous children < 18 yo | NP at time of OM ED of CSOM MEF if OME |
Table 2. Mostly culture. WHO methods not mentioned in review. |
Culture Pathogens, some PCR |
ED - Less otopathogens cultured cf molecular methods ED (CSOM): Ps.a and Sa MEF (OME): otopathogen < 20% NP: high otopathogen in both AOM & OME (no controls) = = 75% |
| Van Dyke 2017 3 GSK Data 2006 to 2011 |
Including (%PCV7): Chile (2%) Columbia (18%) Mexico (42%) Sth Africa (9%) Thailand (0%) Venezuela (74%) |
Pooled analysis of observational studies | Review of AOM Spn serotypes and NTHi. Children < 5yo with AOM |
AOM: otoscopy and tympanometry. OME: type B tymp CSOM > 2w or > 6w |
Tympanocentesis (AOMwoP) Ear discharge (AOMwiP) Amies <48hr. |
Selective agars. Culture ± PCR. Quellung |
Countries very similar. MEF or ED: Spn & NTHi ≫ S.pyogenes & Mcat 2006 to 2011: Spn: PCVVT+6A dec NTHi inc relative risk Sth Africa, Spain, Venezuela: NTHi < Spn |
| Lewnard 2017 4 (2004 to 2016) |
Bedouin population survey | Retrospective analysis of clinical and microbiology data | Rates of clinical progression from NP carriage to MEF culture + ve in PCV-vaccinated vs PCV-non-vaccinated | ED MEF if required Chronic and acute cases |
See Dagan 2000, Ben-Shimol 2016 | Quellung | Progression from NPcarr to ED/MEF < 12 mo old: dec by 92% (Bedouin), 80% (Jewish) > 12 mo old: dec by 32% (Bedouin) 61% (Jewish) |
| Jervis-Bardy 2017 5 2004 to 2016 |
Australian Indigenous Remote communities |
Review of OME surgeries in hospital and published data on ED microbiology. | ED from cases of AOMwiP CSOM MEF from OME |
Mainly WHO methods for culture | Selective and non-selective media. qPCR |
Culture: ED (AOMwiP): NTHi 31–57%, & Spn 4–35% ≫ Mcat 0–6%, Sa, Spy Bacterial Load predicts severity PCR: A. otitidis co-infection in OME (10/22 + ve for Aboriginal & non-Aboriginal children). PCR inc detection of NTHi, Spn & Mcat in ED. ED (CSOM): Ps.a 62%. NTHi (greater in << 6yo) MEF (OME in surgery canal sterilised): Ps.a, Sa 3 to 42% culture + ve. (1985, 2003, 2007) |
|
| Ilechukwu 2017 6 Data July & Sept 2007 |
Nigeria Hospital |
Consecutive case series | Children 1mo to 17yo. N = 100 Exclusion: foreign bodies, Antibiotics < 2 weeks, OE |
Any ED | Otoscopy, canal clean EtOH, swab. No STGG, no −70 °C. | Aerobic culture. Choc agar (candle jar). Cystine-Lactose-Electrolyte deficient (CLED) agar |
53% Low SES. ED: 91% culture + ve. Acute ED: Sa 31% Proteus 25% Ps.a 23% Chronic ED: Proteus 39% Sa 28%. No Spn or Hi |
| Filipe 2017 7 Data 2016 |
Angola Hospital |
Case series. Patient selection methods not specified |
Traditional use of bird faeces to prevent ear secretions caused by primary ear infection All ages N = 188 |
ED related to OM | ED: Clean canal w EtOH. Swab in STGG −70 °C to Sweden | Culture (online techappendix - NA) | ED (CSOM): Alcaligens faecalis co-colonisation in 11%, mostly with Ps.a (50%) Fluoroquinolone R? Recommendation - change to colistin±oral amoxi-clav |
| Basnet 2017 8 Data May 2015 to Jan 2016 |
Nepal Hospital ENT. |
Prospective case series. Patient selection not specified. | N = 263 pus samples from 240 patients | Pus present - 151 chronic 65 acute pus |
Hospital lab using standard protocol. Swab in peptone water. | BA, CA, Mannitol, MacConkey agars. | ED: 216 culture + ve Sa 36%, Ps.a 33%. All sens to Gent. All Ps.s sens to Imipenem. All Sa sens to Amikacin (co-trimox 55%) No Spn nor Hi -(even in acute type OM) |
| Sonsuwan 2016 9 Data 2008 |
Thailand Hospital Dept ORL |
Prospective = = consecutive AOMwiP cases | Age 3mo – 5yo (mean 24mo) with AOMwiP N = 40 |
AOMwiP = = fever, pain, non-chronic. Exclusion: antibiotic treatment, tympanostomy tubes, pneumococcal or NTHi vaccine |
ED swab | Culture & sensitivity Chocolate, Blood and MacConkey agars |
ED: 100% culture + ve 13 organisms Hi 36%, Sa 26%, Spn 9% (no serotypes), Ps.a 11% Hi sensitivity amp 74% co-tri 47% Excluded coag -ve Staph |
| Shakoor 2016 10 Data 2004 to 2013 |
Pakistan Hospital laboratory data |
Retrospective case series | Pre-Hib (2004 to 2008) versus post-Hib (2009 to 2013) vaccination. Children 0–24mo (n = 179) and 25–58mo (n = 98) |
None. Ear pus, aspirate, MEF ear fluid. | Ear pus. Methods not specified. | Standard methods across multiple labs. No Hib test! | ED: 277/352 (79%) children were pathogen + ve. AGE: 0–24mo (n = 179), 134 (75%) monomicrobial, Spn 56% Hi 38% 25–58mo (n = 98), 68 (69%) monomicrobial, Spn 18% Hi 17%. Sa, Ps.a most common in both age groups, esp older. Post-Hib: n = 159. 33% polymicrobial. Sa 38%, Psa. 26%. Spn 27%, Hi 25%, Spy 6% |
| Ofogbu 2016 11 Abstract only, no date of data collection |
SE Nigeria Hospital | Prospective. HIV+ vs age and sex-matched HIV-ve children mean age ~7yo & ~8yo |
HIV+ vs HIV-ve micro of CSOM | Chronically discharging ears | ED swabs culture and sensitivity. No detail in abstract |
NA | ED: Ps.a most prevalent in HIV + ve Fungal elements more common in HIV-ve |
| Leach 2016 12 Data Feb 2010 to Aug 2013 |
Northern Territory Aboriginal and Torres Strait Islander remote communities | Cross sectional comparison of vaccine groups. PHiD-CV10 vs PCV13 |
Children < 6yo | AOMwiP < 6w & perf < 6w CSOM > 6w & perf >2% Dry Perf Any TMP Otoscopy |
WHO methods STGGB −70 °C |
Selective and non-selective agar. Quellung | ED swabs collected from 51/511 children in PHiD-CV10 & 11/140 in PCV13 PHiD-CV10:PCV13 NTHi 36%:64% Spn 17%:43% Mc 8%:7% Sa 40%:7% Serotypes in ED: PHiD-CV10 = = 11A, 15A, 16F, 19F, 21, 22A, 35F PCV13 = = 33F, 1, 9N, 35B |
| Flasche 2016 13 Data 2009 |
Israel Bedouin. Hospital |
Prospective daily first 4 patients in paediatric emergency. < 2yo |
Value of NP carriage in < 5yo to monitor serotypes causing OM in < 2yo OM incidence. |
acute symptoms (<7 days) necessitating a visit to clinic or hospital and resulting in MEF culture | NP swab in < 5yo MEF in < 2 yo via tympanocentesis. Swabs of MEF into Amies processed within 16hrs |
Gent BA. Quellung |
NP: in < 5yo - inc non-vaccine types (non-VT replacement). ED: in < 2yo - smaller inc in non-VT OM Suggests non-VT are less virulent than VT. Bedouin and Jewish - same trend. No NTHi reported No individual serotypes reported. |
| DeAntonio 2016 14 Data 2002 to 2011 |
Developing and newly industrialised. Assume hospitals |
Systematic Review Epidemiology and aetiology. |
Children < 6yo | Any OM Studies reporting pathogens causing OM |
No details | No details |
TABLE 3 ED 2002 Nigeria: AOM n = 53. Spn 9%, Hi 7%, Sa 25% 2001 Ethiopia: CSOM n = 63 Sa 8% 2004 India: CSOM n = 278 Ps.a 10% (<2yo) 2006 Turkey; AOM n = 120 Spn 36–38%, Hi 16–24% |
| Ben-Shimol 2016 15 Data 2004 to 2015 |
Israel Bedouin Population based |
active surveillance | < 3 yo N = 7475 |
All OM episodes submitted for MEF. | See Ben-Shimol 2014, Dagan 2000. Amies processed within 12hrs |
Non-selective agar for Spn, NTHi, Mc, GAS, culture -ve. | MEF: 64% culture + ve. Spn 30%. NTHi 26%. Spn+NTHi 12%. Other 5% Spn-OM incidence dec in both Bedouin & Jewish children (−22%,−36%) NTHi-OM dec in Bedouins (−17%) OM-other inc (53%) OM-ve dec in Bedouin (−29%) |
| Madhi 2016 17 Data May 2009 to April 2010 |
Sth Africa Hospital |
Primary Health Care prospective | AOM in HIV ± in 3mo to 5yo, n = 260 episodes in 248 children | AOM symptoms + bulging… or otorrhoea | NPA - Viral PCR MEF - ED or tympanocentesis culture. Amies transport < 16hrs. |
Selective agar. Quellung. | ED or MEF: Bacteria 54%. Hi 31%. Spn 20%, Sa 16%, Mc 5%, GAS 1.5%. Spn non-sus 64% 19F 23%>19A 11% = 15B11%. Resp viruses 74% cases. Rhinovirus 38% ± bacteria HIV + ve = = HIV-ve |
| Aho 2016 18 Data 2005 to 2009 |
Papua New Guinea Community |
RCT of PCV7 in neonatal, infant and control groups. | Birth to 18 months of age Spn infection in ED of 49 episodes of AOMwiP or CSOM (n = 13, 20, 16) in 36 children (all fully vaccinated) | Purulent ear discharge on examination | WHO method in STGGB −70 °C | WHO methods. Selective agar. Semi-Q. Quellung |
ED: Spn isolates in ED 46% (6/13), 65% (13/20) and 50% (8/16) in neonatal, infant and control vaccine groups. 27 Spn isolates. VT = 1,1, & 3. Non-VT 6,10,4. 19A in 7/33 PCV7 gps vs 1/16 controls. |
| Nwokoye 2015 16 Data no date |
Nigeria Hospital (13mo) | Prospective case series | 212 children 6mo to 10y with OM requiring treatment. 130 AOM (61%) 82 CSOM | AOM acute pain +fluid+opaque+dec mobility. Probably AOMwiP and CSOM current and previous ED |
No details | ED aerobic + anaerobic culture. Refer to Nwokoye 2012 (not on Pubmed) | < 1 yo peak incidence of AOM & CSOM. ED: AOM = 55%, CSOM = 45% Aerobes in AOMwiP: Hi (12), Mc (8), Sa (66), Spn (32) GAS (6) |
| Leach 2015 19 Data 2008 to 2011 |
Northern Territory Aboriginal and Torres Strait Islander. Remote community. |
Cross-sectional survey. ED from AOMwiP or CSOM in PCV7 vs PHiDCV10 era |
PCV7: 60 children, 85 perforations. PHiD-CV10: 47 children, 59 perforations. |
Otoscopy and tympanometry | WHO method in STGGB | CNA, BVCCA. Filter if swarming Proteus spp. Spn, NTHi, Mcat, Sa |
ED: PCV7 : PHiD-CV10 Spn 25% : 18% NTHi 61% : 34% (p = 0.008) Serotypes in ED 12 serotypes in the PCV7 group: 10A (n = 4), 7F (n = 2), 11C (n = 2) and one each of 10F, 12F, 16F, 17F, 19A, 19F, 23F, 6A and 6C. The hierarchy of 7 serotypes in the PHiD-CV10 group: 11A(n = 2) and one each of 15A, 16F, 19F, 21, 22A and 35F. |
| Jervis-Bardy 2015 20 May-June 2014 |
Australian Aboriginal Alice Springs General Hospital ENT |
Baseline for OME RCT | Mean age 5.4 yrs | Bilateral OME + HL + type B tympanogram. NP, MEF, Adenoid N = 11 |
WHO method in STGGB −80 °C | 16S rRNA No culture |
3 sites. N = 8 with sufficient bacterial biomass for microbiome analysis. Si variation in microbial diversity by site. Common to all sites: Mc, Hi, Sp. MEF: less diversity Ao & HI. Ao not in NP or adenoid. |
| Ding 2015 21 Jan 2011 to Dec 2013 |
China, Suzhou Hospital | Prospective cases | All children < 18yrs with AOMwiP N = 229. CSOM excluded |
AOM confirmed by Otolaryngologist | MEF culture. Immediate plating. | Gentamicin BA, CA only | ED or MEF: 159(69%) + ve for bact pathogens. Spn 47%, Sa 19%, Hi 7% 19F>19A predominant = = 80% |
| Chirwa 2015 22 July-Sept 2013 |
Malawi Hospital (ENT) |
Cross sectional descriptive CSOM random sampling | Mean age 18 yrs. 2 to 64 yo. N = 104 |
CSOM > 2w mostly purulent, scant in 53% | ED. Transported in anaerobic jar | Aerobic - BA, MacConkey, CA. no details for Spn, NTHi or Mc Anaerobic |
ED: P. mirabilis 29%, Sa 20%, Ec 8% Anaerobes 35% |
Original studies: In Angola, CSOM was associated with ear discharge culture positive for Proteus (15%), Pseudomonas (13%), enterococci (9%), 87 species were identified, and pneumococcal serotype 19F was found in 4 cases, serotypes 6A and 17F were found in fewer than 4 cases each [36]. Also in Angola, 11% of 188 CSOM cases were co-colonised with Alcaligens faecalis, attributed to a cultural practice of using bird faeces to prevent ear secretions [38]. In Nepal, 240 patients with acute or chronic pus cultured Staphylococcus aureus (Sa)(36%), Pseudomonas aeruginosa (Ps.a)(33%) and no Streptococcus pneumoniae (Spn) nor non-typeable Haemophilus influenzae (NTHi) were found [39]. Sa (31%), Proteus spp. (25%) and Ps.a (23%) dominated in Nigerian children (53% of low SES) with ear discharge, 91% of whom were culture positive [37]. Among HIV-positive and HIV-negative Nigerian children with CSOM, Pseudomonas spp. was more prevalent among HIV-positive children. Cases of AOMwiP (n = 40) in Thailand were all culture positive for any of 13 organisms, with Hi (36%), Sa (26%), Spn (9%) and Ps.a (11%). Pneumococcal serotypes were not reported [40]. In 352 Pakistani children with ear pus, aspirate or MEF, 79% were culture positive. Of these around 70% were monomicrobial. Pneumococcus was present in 56% children <2 years of age, and 18% older children, and Hi was detected in 38% and 17%, respectively. Sa and pseudomonas spp. were predominant, especially in older children. Pneumococcal serotypes were not reported [52]. Australian Indigenous PHiD-CV10-vaccinated children with AOMwiP or CSOM were less likely to be culture positive for NTHi (36%) compared to PCV13-vaccinated children (64%). Proportions for Spn were 17% and 43%, Mc 8% and 7%, and Sa 40%–7%, respectively. Serotypes were reported [16]. Some studies in Israel reported findings for pneumococcal OM in the Bedouin population. One study focussed on vaccine-type and non-vaccine-type pneumococci in MEF obtained by tympanocentesis from children with AOM, but individual serotypes were not reported, nor NTHi [41].
3.4. Implications for clinical practice and future research goals
Overall, reports provided few details of microbiological methods or clinical diagnoses. The majority of studies were of ear discharge from children with CSOM presenting at hospitals. The microbiology of CSOM appears to be similar across countries and populations with a dominance of Pseudomonas and Staphylococcus, but up to 87 species have been identified. Ear discharge from AOM in younger children is more likely to identify classic otopathogens, but results are highly dependent on specimen handling and laboratory methods. If CSOM is to be prevented, a better understanding of the aetiology of AOM, particularly the role of pneumococcus and NTHi, and potential benefit of vaccines, is needed.
4. Diagnosis
4.1. Introduction
Our aim was to describe the studies of otitis media diagnostic methods that are currently used Table 5a or may be relevant to low and middle income countries (LMIC) and disadvantaged populations that live within middle and high income countries (Table 5b). We only included studies that have been published since the 2015 International Symposium for Otitis Media conference.
Table 5a.
Diagnostic studies conducted in LMIC or disadvantaged populations.
| Country | Study Population | Method of diagnosis | Reference |
|---|---|---|---|
| Australia (Aborigine populations) | 1699 children (aged 0–17years) | Age appropriate audiometry, tympanometry, standard and pneumatic video-otoscopy | Gunasekera 2018 |
| Philippines | 47 children (aged less than 18 years) | Operating otoscope, video otoscope, tympanometry, distortion product otoacoustic emission, audiometry screening using noise cancelling headphones and a handheld Android device | Chan 2019 |
| Ethiopia | 173 | Ear discharge, otoscope with headlights where available otherwise naked eye was employed | Gorems 2018 |
| Indonesia | 36 (17–50 years) | Persistent or recurrence of ear discharge for more than 2 months, perforated tympanic membrane and negative findings of cholesteatoma from physical examination or radiological examination | Darmawan 2018 |
| India | 3000 patients | (ENTraview A store-and-forward telemedicine device that integrates a camera-enabled smart phone with an otoscope) | Gupta 2017 |
| Commercial video otoscopes | Myburgh 2016 | ||
| Yemen | 150 children | Ear discharge | Bin Mohanna 2016 |
| India | 30 patients (15–45 years) | History taking, clinical examination, pure tone audiometry, X-ray examination of mastoids | Santesh 2016 |
| Nigeria | 3021 Children (less than 18 years) | Medical history and auroscopy | Ilechukwu 2016 |
| Malawi | 104 patients with mean age 17.8 | Detailed clinical history, including duration of discharge | Chirwa 2015 |
| Iran | 62 adults | Thorough medical history, physical examination including anterior rhinoscopy and otoscopy. History of chronic otorrhoea (persisting for at least 3 months), accumulation of mucopurulent exudates in the external canal or middle ear and/or perforated tympanic membrane on otoscopy. | Nemati 2015 |
| Tanzania | 301 adult patients | Medical history, otoscopy, Rinne’s test, Weber’s test for assessment of hearing loss. Discharge for more than 6 weeks, tympanic membrane perforation | Mushi 2016 |
| India | 502 patients | tympanomastoidectomy | Kameswaran 2017 |
Table 5b.
Evidence table of relevant diagnostic studies.
| Study | Methods | Results | Comments |
|---|---|---|---|
| Mulwafu et al., 2017 | Cluster RCT in Malawi comparing 3 days of training in primary ear and hearing care in 5 intervention health centres (29 CHWs) compared to 5 control health centres (28 CHWs). The primary outcome was knowledge of ear and hearing care. | The average overall correct answers increased from 55% to 68% (95% CI 65 to 71) in the intervention group (p < 0.001). | The study cannot determine whether the increase in knowledge was translated into an improvement in clinical assessment. Since a large proportion of people identified did not attend for formal assessment, the accuracy of the identification process is unclear. |
| Stepniak et al., 2017 | RCT in USA of standard otology lectures plus 1 week access to a web-based otitis media diagnosis simulator (OtoTrain) in 21 2nd year medical students compared to standard otology lectures alone in 20 2nd year medical students. | With the standard otology lectures, the control group had a 31% improvement in their post-test score (mean ± standard error of the mean, 30.4 ± 1.5) compared with their pretest score (23.3 ± 1.8) (P < 0.001). The simulator group had the addition of OtoTrain to the otology lectures, and their score improved by 71% on their post-test (37.8 ± 1.6) compared to their pretest (22.1 ± 1.9) (P < 0.001). Comparing the post-test results, the simulator group had a 24% higher score than the control group (P < 0.002). |
The study cannot determine whether the increase in knowledge was translated into an improvement in clinical assessment. It is currently unclear what level of ability in the correct recognition of OM images is needed to ensure competent clinical examination. Technical ability is not asses in this study. |
| Gunasekera et al., 2018 | A diagnostic agreement and accuracy study of 5 audiologists compared to 3 ENT specialists (reference standard). 1310 of 1669 SEARCH participants (78.5%; mean age, 7.0 years; SD, 4.4 years) were assessed. and received a diagnosis using video otoscopy and tympanometry. Test results (but not case histories) were forwarded to one of three otolaryngologists for blinded independent assessment. Paired diagnoses by audiologists and otolaryngologists were available for 863 children at the child level and 1775 ears (989 children) at the ear level. | Agreement between audiologists and otolaryngologists for OM at the ear level was 92.2% (κ = 0.78; 95% CI, 0.74–0.82), and at the child level 91.7% (κ = 0.81; 95% CI, 0.77–0.85). | The reference standard was ENT specialist diagnosis but this relied on information provided by the audiologist. The audiologist also had additional information available from the clinical assessment. While the high level of agreement is encouraging, diagnostic accuracy estimates may be biased. |
| Erkkola-Anttinen et al., 2017 | A diagnostic accuracy study of spectral gradient acoustic reflectometry (SG-AR) by parents in Finland. 185 children (age 6–35 months) whose parents were willing to use the SG-AR at home daily. Measurement pairs of parental home SG-AR examination results were generated and analyzed. We defined the SG-AR level as increasing when the difference between two measurements was ≥2 levels from a lower to a higher level, suggesting development of AOM. | 361 paired SG-AR home measurements were obtained. The reference measurement was related to a healthy middle ear as determined by pneumatic otoscopy. Increasing SG-AR levels (59/361), were 63% (95% CI 50%−74%) sensitive and 94% (91%−97%) specific for deterioration of a healthy middle ear to AOM. The positive predictive value was 71% (58%−82%) and the negative predictive value was 92% (88%−95%). | Diagnostic tools that suitable to be used by parents are likely to great potential if they are accurate. To date, the accuracy of SG-AR has been assessed for the detection of OME. The development of a new MEE does not always indicate AOM if you require otoscopic features of acute inflammation to make the diagnosis. |
| Erkkola-Anttinen et al., 2017 | 359 children (age 6–35 months) whose parents were willing to use SG-AR at home. The parents were asked to perform bilateral SG-AR daily on their child. In this study, we included children who had undergone successful parental home SG-AR examination performed on the same day that a physician had also performed successful SG-AR examination and pneumatic otoscopy at the study clinic. We compared the parental and study physician SG-AR examination results to the study physicians’ pneumatic otoscopy, which served as the diagnostic standard. | We analyzed 571 successful parental home SG-AR examinations performed on the same day that a study physician had performed a successful SG-AR examination and pneumatic otoscopy at the study clinic. None of the evaluated SG-AR level combinations resulted in both high sensitivity and specificity. For symptomatic visits, the negative predictive value of a parental SG-AR level 1 to detect MEE was 64%. For parental SG-AR levels 4–5, the positive predictive value to detect MEE was 88%. However, for asymptomatic visits, the negative predictive value of a parental SG-AR level 1 to detect MEE was 83%. | Diagnostic tools that suitable to be used by parents are likely to great potential if they are accurate. In this study, while parents were not as successful in achieving SG-AR reading as clinicians, their results were similar ie. the accuracy appears to be determined by the method itself rather than the user. |
| Chan et al., 2019 | The presence of middle ear fluid is a key diagnostic marker for two of the most common pediatric ear diseases: acute otitis media and otitis media with effusion. We present an accessible solution that uses speakers and microphones within existing smartphones to detect middle ear fluid by assessing eardrum mobility. We conducted a clinical study on 98 patient ears at a pediatric surgical center in the USA. | The abstract reports 85% sensitivity and 82% specificity, comparable to published performance measures for tympanometry and pneumatic otoscopy. Similar results were obtained when testing across multiple smartphone platforms. Parents of pediatric patients (n = 25 ears) demonstrated similar performance to trained clinicians when using the smartphone-based system. | This is interesting adaption of smart phones to detect the presence of MEE. Need to check the full methods as I only currently have access to the abstract. |
| Bathla et al 2017 |
The aim of this study was to evaluate the routine use of HRCT of temporal bone in such cases. This study was a prospective study done at LG hospital, AMC MET Medical College, Ahmedabad to evaluate and compare the temporal bone findings in HRCT and intraoperative findings in 100 patients with atticoantral CSOM. All patients underwent HRCT screening followed by surgical exploration of middle ear cleft. | HRCT showed very high sensitivity and specificity for epitympanum (100, 94%) and mesotympanum (98, 98%) areas. It gave valuable information of disease extent in hidden areas like sinus tympani and facial recess of mesotympanum. HRCT satisfactorily delineated malleus and incus erosion but had 75% sensitivity for detecting erosion of stapes suprastructure, though specificity was of 97%. For bony anatomical landmarks HRCT showed very high sensitivity and specificity for detecting erosion of lateral semicircular canal, tegmen tympani and sinus plate. Detection of facial canal erosion on HRCT had moderate sensitivity of 75%. | This study provides useful information about how HRCT can assist in planning surgery for CSOM. However, it does not assess whether this information results in any differences in the type of surgery performed or whether the outcomes are improved. |
| Karki et al., 2017 | To evaluate the role of high resolution computed tomography temporal bone in Chronic suppurative otitis media atticoantral disease and to compare preoperative computed tomographic findings with intra-operative findings. Method Prospective, analytical study conducted among 65 patients with chronic suppurative otitis media atticoantral disease in Department of Radiodiagnosis, Kathmandu University Dhulikhel Hospital between January 2015 to July 2016. The operative findings were compared with results of imaging. The parameters of comparison were erosion of ossicles, scutum, facial canal, lateral semicircular canal, sigmoid and tegmen plate along with extension of disease to sinus tympani and facial recess. | High resolution computed tomography temporal bone offered sensitivity (Se) and specificity (Sp) of 100% for visualization of sigmoid and tegmen plate erosion. The performance of HRCT in detecting malleus (Se = 100%, Sp = 95.23%), incus (Se = 100%,Sp = 80.48%) and stapes (Se = 96.55%, Sp = 71.42%) erosion was excellent. It offered precise information about facial canal erosion (Se = 100%, Sp = 75%), scutum erosion (Se = 100%, Sp = 96.87%) and extension of disease to facial recess and sinus tympani (Se = 83.33%,Sp = 100%). high resolution computed tomography showed specificity of 100% for lateral semicircular canal erosion (Sp = 100%) but with low sensitivity (Se = 53.84%). | See comments above. |
| Nash et al., 2017 | To determine the diagnostic performance of diffusion-weighted magnetic resonance imaging in the assessment of patients with suspected, but not clinically evident, cholesteatoma. A retrospective analysis of a prospectively collected database of non-echo-planar diffusion-weighted magnetic resonance imaging studies (using a half-Fourier single-shot turbo-spin echo sequence) was conducted. Clinical records were retrospectively reviewed to determine indications for imaging and operative findings. Seventy-eight investigations in 74 patients with suspected cholesteatoma aged 5.7–79.2 years (mean, 41.7 years) were identified. Operative confirmation was available in 44 ears. Diagnostic accuracy of the imaging technique was calculated using operative findings as a ‘gold standard’. | The accuracy of diffusion-weighted magnetic resonance imaging in assessment of suspected cholesteatoma was 63.6 per cent. The imaging technique was significantly less accurate in assessment of suspected cholesteatoma than clinically evident disease (p < 0.001). | This study highlights the limitations of MRI in patients who have suspected cholesteatoma that is not clinically evident. |
| Sarmento et al., 2017. | High-frequency conductive hearing loss (HfCHL) has been considered a hallmark of incomplete ossicular discontinuity. This study aims to evaluate the use of HfCHL as a preoperative predictor of IOD in patients with non-cholesteatomatous chronic suppurative otitis media. The HfCHL test was defined as the preoperative air-bone gap (ABG) at 4 kHz minus the average of the ABG at 0.25 and 0.5 kHz. The test was applied in 328 patients before surgery and compared to intraoperative findings as the gold standard. | At surgery, 201 (61.3%) patients had an intact ossicular chain, 44 (13.4%) had a complete ossicular discontinuity, and 83 (25.3%) exhibited an IOD. The best cutoff level was calculated as 10 dB. The HfCHL test to diagnose IOD had a sensitivity of 83% and a specificity of 92% with a post-test probability of 78% and a likelihood ratio of 10.2. | This is a useful study describing an additional role for hearing assessment prior to surgery for CSOM. This information may affect the use of additional imaging or assist in places that do not routinely have access to additional imaging. This study does not assess whether this information results in any differences in the type of surgery performed or whether the outcomes are improved. Further studies testing the usefulness of the proposed 10dB threshold are needed. |
| Anwar et al., 2016 | The diagnostic accuracy of tympanometry in detecting fluid in the middle ear space in children with otitis media with effusion by comparing its findings with those of myringotomies. This prospective study was conducted at the Department of ENT& Head and Neck Surgery, Postgraduate Medical Institute Hayatabad Medical complex, Peshawar from July 1, 2012 to April 30, 2015. Patients with suspicion of OME underwent tympanometry and later myringotomies. Using Jerger’s classification, Type B tympanogram with normal canal volume was considered as conclusive evidence of fluid in the middle ear space. Its findings were compared with those of the respective myringotomies. | A total 117 ears of 63 patients were operated. The age range was 3 to 12 years. The commonest age group (58.7%) affected by OME was 6–8 years. Type B tympanogram with flat curve and normal canal volume was obtained in 71.4% of the ears. Comparison with myringotomy findings showed TP 85, TN 13, FP 5 and FN 14. The diagnostic value of tympanometry was; Sensitivity 85.85%, Specificity 72.22%, PPV 94.44%, NPV 48.14% and Accuracy of 83.76%. | This study indicates that tympanometry in Pakistan has a similar accuracy to the studies conducted in other populations. The age of the children having surgery was older than the children in other studies and this may affect the results. |
| Tuzcu et al., 2015 | This study aims to report the significance of echo-planar diffusion-weighted imaging (EP-DWI) in preoperative magnetic resonance imaging of patients with surgically corrected cholesteatoma and granulation tissue according to DWI and apparent diffusion coefficient (ADC) values. Ninety-one patients (52 males, 39 females; mean age 40.7 ± 15.8 years; range 3–77 years) who admitted to radiology clinic of our hospital between December 2009 and May 2011 with a pre-diagnosis of chronic otitis media with primary acquired cholesteatoma and assessed preoperatively in our clinic by ear magnetic resonance imaging and DWI were included in the study. Diffusion-weighted imaging results were compared with operative findings and pathology results. |
According to the results of operations, 50 patients had cholesteatoma and 41 patients had granulation tissue. The mean DWI values of patients with cholesteatoma were significantly higher than patients with granulation tissue (p < 0.05). The mean ADC values of patients with cholesteatoma were significantly lower than patients with granulation tissue (p < 0.05). The sensitivity and specificity of EP-DWI in detection of cholesteatoma were 97.6% and 92.0%, respectively. | Currently only have access to abstract. The article is in Turkish. This study does not assess whether this information results in any differences in the type of surgery performed or whether the outcomes are improved. |
| Park et al., 2015. | This study validated the 1000-Hz probe tone and evaluated the age at which it should be used in Korean infants. METHODS: Data from 83 infants (43 males, 40 females; mean age 9.2 ± 6.2 (range 1–30) months, 165 ears) were analyzed. Tympanograms were classified according to Baldwin’s modification of the method of Marchant et al. and correlated with results based on combined diagnostic tests, including an endoscopic examination of the tympanic membrane, myringotomy findings, and the air and bone conduction auditory brainstem response (ABR) thresholds. Data were analyzed in five age groups, each covering a 3-month range. The traces were measured for both 226- and 1000-Hz probe tones. |
For the 226-Hz probe tone, the tympanograms showed normal traces for most ears with otitis media effusions in infants younger than 12 months. By contrast, the tympanograms using the 1000-Hz probe tone showed abnormal traces in most of the infants with otitis media effusions in all age groups. In infants with no otitis media effusion, the tympanograms using both 226- and 1000-Hz probe tones were interpreted as normal in most cases in all age groups. In infants younger than 12 months, the sensitivity of the 226-Hz probe tone was very low (0–6.6%), whereas that of the 1000-Hz probe tone was very high (90–100%). In infants older than 13 months, however, the sensitivities of the 226- and 1000-Hz probe tones were 76.2% and 85.7%, respectively. Regarding specificity, the difference between the two probe tones was not significant for any age group. | These results are interesting. The finding in children 4–12mo are not consistent with previous studies. Further research in this area is appropriate. Need to check of this study as there was ePub at end of 2014. |
| Fischer et al., 2019. | This study aimed to evaluate readout-segmented echo-planar DWI for the detection of cholesteatoma and compare the results with surgical validation. MATERIALS AND METHODS: Fifty patients with chronic otitis media (24 females and 26 males; range, 12–76 years of age; mean age, 41 years) who underwent MR imaging before an operation of the middle ear (1–169 days) were included. The MR imaging protocol consisted of axial and coronal readout-segmented echo-planar DWI with b-values of 0 and 1000 s/mm2 and 3-mm slice thickness. The readout-segmented echo-planar diffusion-weighted images were fused with standard T2-weighted sequences for better anatomic assignment. |
Readout-segmented echo-planar DWI detected 22 of the 25 cases of surgically proved cholesteatoma. It has an accuracy of 92% (95% confidence interval, 80.8%−97.8%), a sensitivity of 88%, a specificity of 96%, a positive predictive value of 96%, and a negative predictive value of 89%. In 1 case, a positive finding for cholesteatoma with readout-segmented echo-planar DWI could not be proved by histology, and in 3 cases, histology yielded a cholesteatoma that was not detected with MR imaging. | This study does not assess whether this information results in any differences in the type of surgery performed or whether the outcomes are improved. |
4.2. Methods
We searched the PubMed database using the following strategy: (“otitis”[MeSH Terms] OR “otitis”[All Fields]) AND Diagnosis/Broad [filter] AND (“2015/01/01”[PDat]:”2019/06/01”[PDat]). The Diagnosis/Broad[filter] uses the following terms: (sensitiv*[Title/Abstract] OR sensitivity and specificity[MeSH Terms] OR diagnose [Title/Abstract] OR diagnosed[Title/Abstract] OR diagnoses[Title/Abstract] OR diagnosing[Title/Abstract] OR diagnosis[Title/Abstract] OR diagnostic[Title/Abstract] OR diagnosis[MeSH:noexp] OR diagnostic * [MeSH:noexp] OR diagnosis, differential[MeSH:noexp] OR diagnosis[Subheading:noexp]).
The search was repeated on the June 8, 2019 with 1492 articles identified. The titles and abstracts were reviewed to identify studies that were either: i) systematic reviews of diagnostic tests; ii) randomized controlled trials of diagnostic tests; iii) diagnostic comparisons with a reference standard (reporting sensitivity and specificity); or iv) describing a new diagnostic method; or v) describing a diagnostic method being used in a low or lower middle income countries or disadvantaged populations (including studies of agreement and reliability). Studies of agreement and reliability that were conducted in upper middle income and high income countries were not included unless they targeted a recognised disadvantaged population.
4.3. Discussion
Using this approach, the following were identified: i) 0 systematic reviews of diagnostic tests; ii) 2 randomized controlled trials of diagnostic tests (diagnostic training) [53,54]; iii) 12 diagnostic comparisons with a reference standard (reporting sensitivity and specificity) [55–64]; iv) 16 studies describing a new diagnostic method [56,65,66]; and v) 7 studies describing a diagnostic method being used in a low or middle income country or disadvantaged population [53,55,58,61, 67,68,69].
The evidence table is limited to include the first 3 categories (systematic reviews of diagnostic tests, randomized controlled trials of diagnostic tests, and diagnostic comparisons with a reference standard (reporting sensitivity and specificity)-see Tables 5a and 5b. To be eligible for inclusion in the evidence table, diagnostic accuracy studies needed to recruit participants who were: i) undifferentiated prior to testing; and ii) typical of individuals at risk of otitis media. Other reports of diagnostic accuracy (eg. reports from case-control comparisons) may be included in the text.
Several new technologies to identify otitis media have been described in the last 4 years (Table 5b). These include quantitative pneumatic otoscopy using a light-based ranging technique [65], red-green-blue (RGB) values of tympanic membrane (TM) images [70], store and forward of video-otoscopy images (telemedicine) [55,68], smart phone otoscopy [56,71,72], automated image analysis [73,69], tympanic membrane temperature (for unilateral AOM) [74], mean platelet volume (for cholesteatoma) [75], ultrasound (for MEE) [76], wideband acoustic absorbance testing (for MEE) [77–79], shortwave infrared otoscopy [66,80], and optical detection using spectroscopic techniques [66,80].
Finally, there were several papers describing either a new condition associated with otitis media or conditions without an agreed diagnostic reference standard. These conditions included amblyaudia, Eustachian tube dysfunction, and auditory processing disorder. Further research is needed to establish the clinical importance of these conditions and whether improvement in health outcomes is possible after the condition has been identified. For systems or geopraghic areas where there are limited resources for healthcare services, efforts to increase diagnostic capacity for OM should consider the balance of the benefit of improved diagnostic accuracy with the likelihood of achieving widespread implementaton of such efforts.
4.4. Implications for clinical practice and future research goals
Further research is needed to establish the sensitivity and specificity of new diagnostic approaches. For low-income and low middle-income countries, studies assessing the sensitivity and specificity of simple otoscopy using the available equipment in these settings are needed. In many countries, the otoscopes used in middle-income and high-income countries are too expensive to be widely available to primary healthcare workers. A better understanding of the potential health benefits of all the equipment currently in use is long overdue (Table 5a).
5. Prognosis
5.1. Introduction
OM especially in the severe form as CSOM with or without cholesteatoma can lead to hearing loss (mainly conductive), intra- and extra-cranial complications and death. In this section, recent publications have been identified describing prognosis and specifically severe complications. It is acknowledged that the majority of this research is Level V research (Case reports and reviews) and that with the Level of research it is difficult to make substantive recommendations. With this acknowledgement, there is a clear need for those with adequate resources and data to construct, where possible, broader longitudinal studies to capture additional data and to develop Level III and Level IV evidence with additional systematic reviews and consensus panels.
5.2. Methods
(((((((((“language development disorders”[MeSH Terms] OR “speech disorders”[MeSH Terms]) OR “learning disorders”[MeSH Terms]) OR “hearing loss”[MeSH Terms]) OR “facial paralysis”[MeSH Terms]) OR “labyrinthitis”[MeSH Terms]) OR “meningitis”[MeSH Terms]) OR “mortality”[MeSH Terms]) OR “cholesteatoma”[MeSH Terms]) AND “otitis media”[MeSH Terms]) OR “mastoiditis”[MeSH Terms] AND ((“2015/01/01”[PDAT]: “2019/07/01”[PDAT]) AND “humans”[MeSH Terms]). Added to search after reading:2. Complications have been broadened to anything that can contribute to the understanding of burden of OM in developing countries and indigenous populations. All study designs have been included.
434 articles identified, 366 excluded on title, 68 abstracts screened and 19 studies included (Table 6). Two studies were after reading references added.
Table 6.
Prognostic studies conducted in LMIC or disadvantaged populations.
 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year of publication | Country region | Study design | Participants | Age | Diagnostic methods | Study question | Study period | Results | Comments |
| Yen | 2015 | Taiwan | Retrospective cohort, register study | 10248 diagnoses with COM | ICD codes in Taiwan’s national health insurance program (covers 98% of population) | what is the risk of developing sudden SNHL if you have COM | 2001–2008 | Incidence of sudden sensorineural HL (sSNHL) was 3 times higher among pt’ens with COM compared to age and sex matched control group. The risk of sSNHL was 101/10248 among COM = 0,01 and non-COM 103/30744 = 0,003 | Large population, Side of COM and later sSNHL was not included. The relative risk is higher but the absolute risk is still small. | |
| Yehudai | 2015 | Israel | Tertiary centre, retrospective, preoperative audiograms, for COM or cholesteatoma. normal ear BC vs COM ear BC. | 124 with unilateral COM | 7–18 | otomicroscopy, audiometry 500–4000 Hz | Is SNHL in COM ears clinically significant, and what are the risk factors | 1997–2012 | Difference in mean BC at 1000, 2000 and 4000 Hz, 1.3 dB 4.3 dB and 7.2 dB. Statistically significant meaning COM ear showed SNHL compared to the healthy ear. Age >10 and presence of cholesteatoma increased the risk of SNHL | Small difference within the insecurity of an audiogram, maybe not clinically significant finding. The postop BC could have been evaluated too. |
| Orji | 2015 | Nigeria | retrospective, register, tertiary centre | 203 CSOM cases from 2009 to 10 compared with 343 cases from 1990 to 91 | mean age 27 and 21 years | otoscopy | incidence of CSOM at the centre, complication rate | 1990–2010 | CSOM incidence lowered, cases tended to be older, fewer intracranial complications in newest time period, but overall complication rate unchanged | |
| Touati | 2015 | Morocco | retrospective case series, tertiary centre, | 30 | 9–15 years | otoscopy, otomicroscopy? | describe COM with cholesteatoma, clinical and presentations and surgical intervention | jan 2009 to 2013 | 86% had conductive HL, 60% had airbone gap over 30 dB | |
| Peñaranda | 2015 | Colombia different regions |
school based | 1526 | 5–14 | otoscopy, audiometry | prevalence of AOM, perforation, sequelae, HL | Perforation variation between regions and races from 0 to 1.4%. Variation in sequelae and conductive HL | Demonstrates challenges in OM prevalence in countries with large differences in access to healthcare and racial heterogeneity | |
| Maile | 2015 | Nepal | questionnaire | 242 | all ages | Glasgow health Status Inventory and Glasgow benefit inventory | Adapt QOL instruments to Nepal. Quantify burden of disease. Determine QoL alterations associated with surgery for CSOM or Cholesteatoma. | 2012–2013 | CSOM associated with reduced QoL, same degree as cholesteatoma. Surgical intervention is associated with increased QoL | |
| Penido | 2015 | Brazil | retrospective register, case series tertiary centre | 51 | mean 31 years | Unclear for ear examination, but imaging modalities were available. CT; MR, MRA | describe epidemiological aspects of OM related ICC (intracranial complication) | 80% of complications were due to COM while 20 % were due to AOM. 4 died to ICC. Mean age ICC from AOM 30 years, but bifasic (majority under 15 or over 60) mean age icc from CSOM was 26 years. Mean time of hospitalization was 34 days permanent neurological sequelae were seen in 29% of all ICC cases. 4 patients died |
||
| Avsntorp | 2016 | Greenland | follow-up on population based cohort | 223 children | 4–10 years | Video-otoscopy, tympanometry, audiometry | prevalence of CSOM and OM related hearing loss | 2010 | 5.8% CSOM, 13.9% OME, 55% sequelae. CSOM median PTA high/low 34/31 dB, OME 23/23, normal ears 12/13 dB | High prevalence of CSOM, 1 case meningitis, mental retardation and bilateral severe HL. |
| Maranhão | 2016 | brazil | retrospective case series, register based, tertiary centre | 14 pts labyrinthitis secondary to OM | mean 40 | clinical factors and hearing level among pt’ens with OM related labyrinthitis | 1987–2013 | 43% had cholesteatoma, 43% AOM 14% had CSOM associated with facial paralysis, meningitis. Intracranial abscess, one death | ||
| Poole | 2016 | Nepal | questionnaire, case-control | N = 153. 82 non-OM vs 71 CSOM/AOM/HL recruited from waiting room health care centre | mean 39 vs 48 years | history of OM | Investigate knowledge, beliefs, attitude and practice regarding CSOM and HL | Interestingly 70 % responded that people with HL were discriminated in society | ||
| Zaidi | 2016 | Pakistan | Case series (described as cross sectional?) tertiary centre | CSOM | 6–45 years | audiometry average of 3 speech frequencies, bone conduction? | What is the frequency of SNHL in CSOM | 2013–2014 | 52% had SNHL | frequencies not reported, use of bone conduction not reported |
| Mushi | 2016 | Tanzania | prospective cross sectional tertiary centre | 301 CSOM cases with 6 weeks otorrhoea | All over 1 year | otoscopy and tuning fork tests, cultures. HIV tests | find predictors of complications in CSOM, evaluate treatment outcome and anti-microbiological susceptibility | 2013–2014 | Majority of patients with prolonged illness duration, otalgia, infected with multi drug resistant bacteria and those with positive HIV status poorly respond to treatment and tend to present with disease complications | 632 attends the clinic annually. 48% of patients present with CSOM at the clinic |
| Mushi | 2016 | Tanzania | Case series, tertiary centre | 410 CSOM cases | Median age 29.5 in cases with fungi. Age not stated in overall group | Prevalence of fungal infection | 44 (11%) of cases had fungi | There was an association but not significant between co-infection with fungi and poor treatment outcome. Causality cannot be established. High risk of bias | ||
| Dobriansky | 2017 | Brazil | Cross-sectional, register. Tertiary centre | 158 patients. 98 CSOM with otorrhoea and contralateral normal ear. 60 CSOM with dry perforation | median 26 years in otorrhoea group, unknown range | otoscopy, audiometry | is otorrhoea associated with SNHL | otorrhoea is associated with SNHL | ||
| Jensen PV | 2017 | Zimbabwe | case report | 1 fatal | 8 | fatal complication to AOM Gradenigo syndrome. Lethal outcome due to inaccessibility diagnosis and treatment |
||||
| Toros | 2017 | Turkey | case | 1 | 45 years | otoscopic | mastoiditis, bezold, paraspinal abscess, sinus thrombosis. Survived with hemiparesis | first description of paraspinal abscess due to OM | ||
| Qin | 2017 | China | case report | 1 | 10 year | Zygomatic root abscess and fistula, after 6 months of low grade infection | ||||
| Singh | 2017 | India | prospective case series tertiary centre | 46 CSOM/cholesteatoma patients | 7–50 | otomicroscopy, surgery | Occurrence of fungal infection | All had otorrhoea, 43% had fungal co-infection, 15% extra-cranial complications, no intracranial complications | Causal relation between chronic otorrhoea and fungal infection cannot be established. Highlights the importance of testing for fungal infection in recalcitrant cases. Prevalence rates are very high, high risk of selection bias. Authors hypothesis that it could be due to increased availability of AB in India |
|
| Kameswaran | 2017 | India, Tamil nadu | case series, tertiary centre | 502 patients undergoing tympanoplasty with mastoidectomy | otomicroscopy, surgery, CT | 2011–2012 | 25 (5%) patients had M tuberculosis in ear samples. Authors suspect TB OM to be under reported in India |
tuberculous otitis media higher risk of complications due to delayed diagnosis as many patient dońt have coincident pulmonary symptoms. More severe hearing loss and presence of complication. | ||
| Cordeiro | 2018 | Brazil | Prospective case series with follow-up. Tertiary centre. | 82 with AOM at first visit .41 followed- up | 5–65, median 38 years | Extended high frequency audiometry 8 to 16 KHz. Otoscopy, tympanometry | impact of first episode of AOM on hearing after 14,28,49 and 180 days | 2015–2016 | First OM episode led to high frequency elevated thresholds at 6 months follow-up in the affected ear. At 14 days follow-up standard audiometry did not show any HL | |
| Singh | 2018 | India | case | description of case | tuberculosis middle ear, mastoid, zygomatic and Bezold abscess | tuberculosis OM pathogen in India | ||||
| Jones | 2018 | Australia | semi-structured interviews | 9 caregivers to 12 children with bilateral OM, some with CSOM, participating in a program speech, language, hearing loss and school readiness | Medical history | what is the parents view on the program. | Parents were positive towards the program for various reasons. Parents ranked OM as the highest health concern |
The more OM concerned parents more likely to participate in the program. May not be representative for the community. High risk of bias. But only study that asked parents to rank “burden of disease” | ||
| Singer | 2018 | Egypt | prospective, case series (patient own control) tertiary centre | 200 unilateral CSOM, using contralateral as control-ear | 10–60 | Otomicroscopy, tympanometry audiometry 500–4000. BC average 25 dB or |
occurrence of SNHL in CSOM | 2016–2017 | 10 % had SNHL, CSOM associated with SNHL but other predisposing factors e.g. | |
5.3. Discussion
Complications have been broadened to anything that can contribute to the understanding of burden of OM in developing countries and indigenous populations. All study designs have been included. OM, especially in the severe form as CSOM with or without cholesteatoma, can lead to hearing loss (mainly conductive), intra- and extra-cranial complications and death.
Several studies investigated sensorineural hearing loss (SNHL) as a sign of permanent damage to the cochlea after COM. A nationwide register based study in Taiwan identified 10248 cases with COM and found a 3 times increased risk of sudden sensorineural hearing loss compared with age and sex matched controls [81]. Cochlear infection, even after AOM was described in multiple case series [82–86].
An important documentation of the variation of burden of OM and HL within a country was done in a large school based study of 1526 children in different regions of Columbia [87]. It is the only study that demonstrated a variation of perforations, sequelae and conductive HL between regions and ethnic groups.
A population-based cohort study in Greenland found a high level of OM related hearing loss and out of the 223 participants one child had OM related meningitis leading to mental retardation and bilateral deafness underlining the risk of severe intracranial complications in high risk areas [88].
Limited access to treatment of OM complications was only described in one case report from Zimbabwe where a child diagnosed with CSOM and Gradenigo’s syndrome later developed meningitis with a fatal outcome [89]. Lack of funding on several steps of contact with the hospital delayed diagnosis and treatment.
Tuberculous OM, previously reported from South Africa [90] and China [91] More recently in India a case of tuberculous mastoiditis with Bezolds abscess was described [92] and a large case series with 502 patients undergoing mastoidectomy, found M. Tuberculosis in 5% of all cases [93]. The authors were concerned of a resurgence of tuberculous OM in India and made suggestions for diagnostic criteria based on clinical criteria and diagnostic tests. Tuberculous OM was associated with a higher level of severe complications than other CSOM forms.
In Nigeria a study on CSOM and its complications in a tertiary center over 20 year time period found a reduction in the prevalence of CSOM and a shift of more adults presenting in the resent years [94]. There was also a reduction in incidence of intracranial complications of CSOM, but complication rates remained largely unchanged. A new aspect of the long-term effects of untreated OM was shown in a Nepalese questionnaire based study, where 71% responded that people with OM and HL were discriminated in society [95]. A semi-structured interview among aboriginal Australians found that parents ranked OM and it’s complications as the highest health concern [96]. A case series from Tanzania highlighted the impact of multidrug resistance and HIV on OM in Africa [97]. The majority of patients with prolonged illness from OM were infected with multi drug resistant bacteria and those with positive HIV status poorly responded to treatment and tended to present with complications. Cases with unusual complications or lethal outcomes can be seen in the prognosis Table [98–103]. Death from OM occurs not infrequently in low resource settings, whether due to meningitis, brain abscess or other causes [101,104].
5.4. Implications for clinical practice and future research goals
As noted above, several studies point out that CSOM leads to sensorineural hearing loss as a sign of permanent cochlear damage. Investigators in India suspect a possible rise of tuberculous otitis media leading to more complications. Reports from Africa gave concern to the impact of multidrug resistance and HIV on complicated and recalcitrant OM. In Nepal QOL studies showed the negative impact of CSOM but also demonstrated problems with discrimination of persons with OM and hearing loss. Finally, in the study from Columbia great variation of OM complications was found between regions and ethnic groups within the country. Thus, demonstrating the importance of collecting data in several regions within a nation to estimate the burden of disease. Prognostic studies in LMIC or disadvantaged populations are needed.
6. Treatment
6.1. Introduction
Treatment of otitis media in developing countries or disadvantaged populations is subject to factors that may differ from developed countries. This includes higher burden and severity of disease, lack of access to healthcare, and variations in how healthcare is delivered. We were particularly interested in research that may help us understand the impact of such factors.
6.2. Methods
A systematic review of all published studies was performed in PubMed between 01 January 2015 and 01 June 2019 with the following key words used for searching were; treatment, therapy, surgery, antibiotic, surveillance, steroid, hearing aid, rehabilitation, clinical trial and otitis, otitis media or mastoiditis. We retrieved 2823 titles, of which 2695 were excluded because they were case reports, studies not related to treatment, or not relevant to low resource settings or indigenous populations. After screening of abstracts, a further 85 were excluded for the same reasons, and 6 were excluded as they were not in the English language (4 in Russian, 2 in Chinese) This left 37 studies for inclusion (Table 7)..
Table 7.
Treatment.
| Author, Year of study, Year of publication, Journal/Conference | Country, Population | Study design | Study Question Population - risk group, age | Treatment method/Study method | Study Question Outcome | Study period | Risk of bias |
|---|---|---|---|---|---|---|---|
| Dave et al., 2019 Indian J Otolaryngol Head Neck Surg. | Urban Indian | Case series | All ages (n = 90) Tertiary referral centre |
Tympanoplasty ± mastoidectomy for CSOM | Graft uptake rate in relation with ET function | 2016–2017 | High - tertiary hospital based |
| Miyake et al., 2019 Einstein (Sao Paulo) | Brazil | Controlled Open-label randomized | All ages (n = 80) | Incidence of post- MVTI otorrhoea in patients with no water precaution | Protection should be recommended during the first month after surgery but not further on | July 2013–May 2015 | Low - lost to follow up, selection |
| Toman et al., 2019 Trop Med Infect Dis. | Rural South Africa | Case series | Adults (n = 14) | Antibiotic prescription according to culture results | Culture oriented treatment for better disease control | 6 week | High -selection, low numbers |
| Ramkumar et al., 2018 Am J Audiol. | Rural India - cleft palate patients | Case series | Children and adults (3–35 years) | Identifying and managing middle ear disorders through video-otoscopy and Telemedicine | Directing patients to appropriate therapy using video otoscopy and Telemedicine | 13 months | High-selection, low numbers |
| Zahreddine et al., 2018 Pharm Pract (Granada) | Lebanon | Cross -sectional study | Pharmacists and parents | Assessing the correct use of antibiotics for AOM | Necessary to implement educational campaigns to increase awareness of antibiotic misuse and resistance among pharmacists and patients | June-August 2017 | High - selection, small numbers |
| Kouhi et al., 2018 Ear Nose Throat J | Iran | Randomized controlled | All (n = 59) | Assess the influence of corticosteroids on tympanoplasty outcomes | No effect was found on graft uptake following corticosteroid treatment | 2013–2014 | High - selection, size |
| Jacups et al., 2018 J Eval Clin Pract. | Australia Indigenous population | Costing Evaluation | Children | Identifying the least costly model of delivery of ENT surgery | Low-risk ENT surgery from a state funded hospital in remote setting with high use of videoconference technology it the most cost effective | 2017 | Low risk |
| Udden et al., 2018 Infect Dis Poverty | Angola | Case series | All (n = 152) | Identification of aerobic pathogens in CSOM | The most common pathogens were Enterococcus spp, Pseudomonas aeruginosa and Proteus spp. Resistance rates to quinolones ranged 6–30% and suggested topical therapy with quinolones should be the mainstay | - | High - selection |
| Nasrallah et al., 2018 Int J Pediatr Otorhinolarngol | Lebanon | Anonymous survey | Physicians (n = 75) | Assessing physician knowledge of AOM diagnosis, management and treatment | Interventions for improving awareness of clinical guidelines should be taken | 2017 | High - questionnaire |
| Johnston et al., 2018 ANZ J Surg | New Zealand | Case series | Maori and non-Maroi children (n = 11941) | Compare incidence and outcomes of children with MVTI | No difference in the post-operative course between Maroi and non-Maroi children | January 1996–June 2016 | Low - case series, high number |
| Yazici et al., 2018 Clin Otolaryngol | Turkey | Case series | Children (n = 50) | Compare QOL using OM6 post MVTI | Improvement in QOL following MVTI | December 2016–April 2017 | High - case series, small number |
| Nshimirimana et al., 2018 Int J Otolaryngol | Rawanda | Cross-sectional | All (n = 109) | Determine factors for delayed care seeking in CSOM treatment | Low knowledge of disease and use of traditional medicine | 2017 | High - tertiary hospital |
| Smith et al., 2018 J Laryngol Otol | Cambodia | Case series (n = 124) | All | Measuring tympanic membrane closure rate at six weeks and PTA at three months after local surgeon performed surgery | Local training of surgeons shows high success rate in tympanoplasty results | 2016–2017 | High - case series |
| Piltcher et al., 2018 Braz J Otorhinolaryngol | Brazil | Literature review | - | Medical management of AOM | Periodic revisions on guidelines and recommendations for treatment should be performed | - | Low |
| Durham et al., 2018 PLoS One | Australia - Aboriginal and Torres Strait Islander | Online survey and face to face or phone interview | Documents (n = 20) Interview (n = 27) |
Driving sustained improvement in OM care in children | A holistic systemic approach is needed for OM care improvement | - | High - interview, survey |
| Master et al., 2018 Otolaryngol Clin North Am. | Developing countries | Overview | - | Seeking the difficulties in treating CSOM | Surgical management may be warranted and training of local surgeons is required | - | High |
| Jones et al., 2018 BMC Pediatr | Australia - Indigenous population | In depth semi-structured interviews (n = 21) | Children 0–3 years | Assessing the LiTTLE Program effect on Aboriginal community health | Positive views about the LiTTLe Program possible areas of improvement | High - interviews | |
| Buyukcam et al., 2018 Int J Pediatr Otorhinolaryngol | Turkey | Questionnaire (n = 977) | Paediatricians | Assess paediatricians’ attitude toward AOM treatment and pain management | Educational interventional strategies are needed to improve compliance with EBG for AOM treatment and management, watchful waiting should be applied when appropriate | January 2015-December 2016 | High - interviews, university hospital and research hospital paediatricians |
| Kong et al., 2017 J Paediatr Child Health | Australia - Indigenous and non-Indigenous | Review | Children | Identifying children with OME who need simple ENT surgery | Advancing children who meet surgical criteria to surgery | High - review | |
| Hussein et al., 2017 J Laryngol Otol | Egypt | Prospective randomized study | Children 2–11 years of age (n = 290) | Evaluate the effects of oral steroids alone or followed by intranasal steroids versus WW for OM resolution | Oral steroids lead to a quick resolution of OM with no long term benefits. Intranasal steroids non useful | Low - no randomization, not blinded | |
| Kaya et al., 2017 Eur Arch Otorhinolarngol | Turkey | Prospective randomized controlled | All (n = 13) | Compare the audiologic outcomes of the patients who underwent endoscopy on one ear and microscopic tympanoplasty on the other, and to investigate the operative time, graft success, postoperative pain and health status | Endoscopic approach for type 1 tympanoplasty offers shorter surgery time, better health status and lower postoperative pain than microscopic surgery as well as comparable improvement in air-bone gap and graft success | February 2015-September 2016 | High -selection, size |
| Jacups et al., 2017 Int J Pediatr Otorhinolaryngol | Cape York Indigenous children | Case series | Children (n = 16) | Improving accessibility to ENT surgery through usage of private health care facilities | Private health care provided quicker surgical access and well as good post-operative hearing results | High - small case series | |
| Akhtar et al., 2017 Mymensingh Med J | Bangladesh - tertiary center | Cross sectional prospective study | All (n = 117) | Identify the common microorganism involved and the antibiogram of CSOM patients | Staphylococcus aureus and Pseudomonas aeruginosa most common with sensitivity to gentamycin and ciprofloxacin, respectively | High - selection | |
| Khreesha et al., 2017 Int J Pediatr Otorhinolaryngol | Jordan | Survey | Physicians (n = 71) | Assess AOM treatment trends as well as adherence to guidelines | Encouraging awareness of AOM guidelines | - | High - survey |
| Ababneh et al., 2017 Int Health | Jordan | Prospective cross-sectional | Children | Assessing antibiotic prescription rate for URTI | Broad spectrum antibiotics are prescribed often for AOM and health policy initiatives should be taken to minimize such prescripitions | - | High |
| Demant et al., 2017 Trials | Greenland | Investigator initiated multicenter, randomized, blinded superiority trial | Children 9–36 months | Detection of a decrease of 2 visits to a health clinic during 2 years | On going | - | Low - selection bias |
| Sibthorpe et al., 2017 Aust J Prim Health | Australia - Aboriginal and Torres Strait Islanders | Expert Consensus | Detecting evidence based indicators for continuous quality improvement in the prevention and management of OM and its sequelae | Seven evidence - based indicators were developed from electronic health records | - | High - consensus, selection | |
| Sharma et al., 2016 Indian J Otolaryngol Head Neck Surg | India | Case series | - | Evaluate the outcome of type I tympanoplasty ± mastoidectomy for CSOM | Type I tympanoplasty with cortical mastoidectomy had better graft uptake and audiological results then tympanoplasty alone | One and a half years | High - selection, small series |
| Yousaf et al., 2106 J Auyb Med Coll Abbottabad | Pakistan | Randomized controlled | Children ages 4–12 years (n = 82) | Evaluate laser myringotomy for the resolution of OME | Laser myringotomy is less effective in clearance of mucoid effusion in long standing and recurrent OME | February 2012-January 2015 | High - selection, statistics |
| Singh et al., 2016 J Laryngol Otol | India | Prospective randomised | Adults (n = 100) | Evaluate the success rate of dry and wet temporalis fascia grafts in type I underlay tympanoplasty. | A dry or wet temporalis fascia graft does not influence the outcome of tympanoplasty type I. | - | ? |
| Silveira et al., 2016 Braz J Otorhinolaryngol | Brazil | Randomized controlled | All (n = 40) | Effect on healing of direct application of a bacterial cellulose graft on the tympanic membrane compared to the conventional approach with autologous fascia | Bacterial cellulose grafts promoted the closure of the tympanic membrane perforations | - | ? |
| Amer et al., 2016 Int Arch Otorhinolaryngol | Egypt | Case series | Children (n = 42) | Determine the usefulness of adjuvant IT steroids of OME after MVTI | IT steroids is safe we lower incidence of recurrent OME, tympanosclerosis and otorrhea | High - selection, small series | |
| Bin Mohanna et al., 2016 J Ayub Med Coll Abbottabad | Yemen | Cross - sectional with parental questionnaires | Children 1–15 years of age (n = 150) | Identify the bacterial etiologic agents of OM and determine their sensitivity patterns | Bacterial cultures are essential for guiding antibiotic therapy. Bacterial isolates were Staphylococcus aureus and Pseudomonas aeruginosa most common with sensitivity to cefotaxime and azithromycin as well as amoxicillin - clavulanic acid. | January-October 2015 | High - selection, tertiary centre |
| Maile et al., 2015 Trop Med Int Health | Nepal | Case series | All (n = 242) | Assessing the impact of ear disease and the effect of ear surgery on QoL as well as adapt the GHSI and GBI into relevant questionnaires for Nepali patients | Ear disease is associated with reduced QoL while surgical intervention is associated with an improved QoL. It is essential to invest in measures of QoL in developing nations | - | High - selection |
| Elsayed et al., 2015 Int J Otolaryngol | Questionnaire | Case series | Children < 2 year old (n = 100) | Assess the impact of educational program on the management of children with CSOM | Education of parents leads to a higher percentage of cure in patients with CSOM | September 2013-May 2014 | High - recall |
AOM - Acute otitis media; ENT - Ear, nose and throat; CSOM - Chronic suppurative otitis media; ET - Eustachian tube; MVTI - myringotomy plus ventilation tube insertion; OM6 - Otitis media 6 -item questionnaire; QOL - Quality of life; PTA - Pure tone audiometry; OM - Otitis media; LiTTLE Program - Learning to Talk, Talking to Learn; EBG - Evidence based guidelines; CHW - Community health worker; URTI - Upper respiratory tract infection; OME - otitis media with effusion; IT - intratympanic; QoL - Quality of life; GHSI - Glasgow Health Status Inventory; GBI - Glasgow Benefit Inventory.
6.3. Discussion
There are concerns about under and over treatment of acute otitis media (AOM) in low resource settings. For example, easy availability of antibiotics in some settings, and the high cost or poor accessibility of doctors, may favour self-medication with antibiotics, with potential over-treatment and development of antibiotic resistance. Conversely, low socio-economic conditions and a lack of access to healthcare may mean that AOM presenting to medical services is more severe than that seen in high resource settings and so under-treated.
Guidelines can help to target treatment of AOM. A review by Ovnat Tamir et al. [105] found that whereas watchful waiting was the standard of care for mild to moderate OM in high resource countries, this was an option in only 3 of 7 guidelines from low resource countries. A recent guideline for AOM developed by an expert panel in Brazil (Level IV evidence) was based upon data and guidelines from the USA, but it is unclear if this is or is not appropriate. In Jordan, a questionnaire study found that most physicians had good diagnostic skills for AOM, and were aware of treatment guidelines, but only half of them said they regularly adhered to these guidelines [106]. A separate study from Jordan using data from a variety of health settings found that almost all patients diagnosed with AOM were prescribed antibiotics [107]. Similarly, in Lebanon, Nasrallah et al. [108] reported that the majority of physicians were able to diagnose AOM, and many knew the criteria for treatment, but a separate study found that parents in Lebanon would often buy antibiotics directly from a pharmacy without a prescription, and that pharmacists had variable knowledge of the indications for antibiotic treatment of AOM. A survey in Turkey by Buyukcam et al. [109] found that the majority of paediatricians did not adopt a policy of watchful waiting for AOM.
For chronic serous otitis media (SOM), or ‘glue ear’, three studies in high risk populations suggest the disease behaves similar to other settings. A study of 40 patients from Pakistan showed that in 2/3rds there was resolution on conservative management [110]. In Turkey ventilation tube insertion was found to improve quality of life [111]. In New Zealand no difference was found in clinical characteristics between Maori and non-Maori children undergoing ventilation tube surgery [112].
In chronic suppurative otitis media (CSOM), there have been several reports on bacteriology and potential therapy. Staphylococcus aureus, Pseudomonas aeruginosa and Proteus were the most commonly isolated pathogens in South Africa, Angola, Bangladesh, Nigeria, and Yemen [36,113–115]. Resistance to common topical antibiotics such as quinolones was 2–30% depending upon setting and bacterial strain. Bareega et al. argued that in most low resource settings, empirical rather than culture directed therapy is preferable [116]. Correct administration of treatment is also critical: a trial from Egypt showed that parental education on techniques for ear wicking, and indications for antibiotic use, led to greater resolution of disease [117]. Surgical treatment for CSOM can be challenging to deliver where human, physical or financial resources are constrained. Maile et al. showed that surgical treatment for CSOM in Nepal was associated with large improvements in quality of life [118]. Smith et al. described a program for training surgeons in Cambodia, using teaching fellows who were resident in country for 4–6 months, with good learner outcomes and clinical success [119].
In recent years there has been an increasing focus on health services research and in particular in programmes utilizing community health workers (CHWs) or telemedicine to deliver ear and hearing care. A report from Malawi showed that training CHWs in ear and hearing care was feasible and effective [120]. Another from India demonstrated that it was possible to train CHWs in a cleft palate program to capture otoscopy or audiology data with simultaneous expert assessment, although this model was not without challenges [120]. An analysis of services for OM for remote Aboriginal populations in Australia showed that using telemedicine for consultation and a visiting service for surgery is at a lower cost than using a visiting service for both diagnosis and surgery, or transporting patients to a distant centre for such services [121]. Other researchers have used stake-holder engagement to evaluate important components of the ideal model for service delivery for OM. In the Pacific region, advocacy, funding and a long-term vision were identified as important [122]. For the Aboriginal population of Queensland (Australia), appropriate governance structures, shared goals, and mechanisms for feedback were identified [123]. In 2017 an expert panel defined a set of indicators that could be used to evaluate primary care programmes for OM [124]. However, the lack of a service is not the only barrier to care: in a survey from Rwanda, patients treated for CSOM reported a lack of awareness or financial constraints as the most common barriers to access to ear and hearing care [125].
6.4. Implications for clinical practice and future research goals
Very few randomized controlled trials have been undertaken in LMIC or disadvantaged populations during this time period. Researchers in these settings should be encouraged to apply high quality methods to answer the clinical questions of importance to practice in their region.
7. ISOM 2019 recommendations for reporting cross-sectional epidemiological studies on otitis media and hearing loss
7.1. Introduction
Reporting on otitis media research is complicated due to a lack of consensus on diagnostic definitions of otitis media conditions, best practice diagnostic techniques and cut-off values for normal vs. disease, the definition of hearing loss (best ear vs. any ear, average loss and number of frequencies tested for average or other method, cut-off dB threshold), and unit of analysis (ear vs. child).
7.2. Implications for clinical practice and future research goals
The Panel recommends otitis media researchers should use the STROBE checklist [126] (https://www.strobe-statement.org/index.php?id=available-checklists) for reporting of cross-sectional studies (Table 8).
Table 8.
Recommended reporting items and justification for studies of otitis media and associated hearing loss in LMIC or disadvantaged populations.
| STROBE item | Recommendation | Justification |
|---|---|---|
| Setting | Specify: |
|
| Participants |
|
|
| Variables |
|
|
| Measurement |
|
|
| Descriptive data |
|
|
| Outcome data for OM |
|
|
| Outcome data for hearing |
|
|
| Generalisability |
|
|
report best available local data if you do not know the precise rates for your specific sample
Funding
For The Generation And Publication Of This Panel Report Was Made Possible In Part By 1 R13 Dc017389-01 From The National Institute On Deafness And Other Communication Disorders.
Publication of this supplement was supported by the International Society for Otitis Media (ISOM) and a grant from the National Institutes of Health National Institute on Deafness and Other Communication Disorders.
References
- [1].Adegbiji WA, Olajide GT, Olatoke F, Olajuyin AO, Olubi O, Ali A, et al. , Preschool children hearing impairment: prevalence, diagnosis and management in a developing country, Epub 2018/07/12, Int. Tinnitus J 22 (1) (2018) 60–65, 10.5935/0946-5448.20180010. [DOI] [PubMed] [Google Scholar]
- [2].Basanez I, Nakku D, Stangl S, Wanna GB, Prevalence of hearing loss among primary school children in Mbarara, Uganda, Epub 2015/11/28, Int. J. Pediatr. Otorhinolaryngol 79 (12) (2015) 2359–2363, 10.1016/j.ijporl.2015.10.044. [DOI] [PubMed] [Google Scholar]
- [3].Hunt L, Mulwafu W, Knott V, Ndamala CB, Naunje AW, Dewhurst S, et al. , Prevalence of paediatric chronic suppurative otitis media and hearing impairment in rural Malawi: a cross-sectional survey, Epub 2017/12/22, PloS One 12 (12) (2017) e0188950, , 10.1371/journal.pone.0188950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ilechukwu GC, Ilechukwu C, Ezeanolue BC, Okoroafor IJ, Ojinnaka NC, Ubesie AC, et al. , Ear-related problems among children attending the paediatric and otorhinolaryngology out-patients clinics of the University of Nigeria Teaching Hospital, Epub 2016/09/09, Enugu. Afr Health Sci 16 (2) (2016) 363–366, 10.4314/ahs.v16i2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Libwea JN, Kobela M, Ndombo PK, Syrjanen RK, Huhtala H, Fointama N, et al. , The prevalence of otitis media in 2–3 year old Cameroonian children estimated by tympanometry, Epub 2018/10/29, Int. J. Pediatr. Otorhinolaryngol 115 (2018) 181–187, 10.1016/j.ijporl.2018.10.007. [DOI] [PubMed] [Google Scholar]
- [6].Phanguphangu MC, Otoscopic examinations reveal high prevalence of outer and middle ear pathologies in paediatrics in Limpopo, South Africa, Epub 2016/10/27, Int. J. Audiol 56 (4) (2017) 215–218, 10.1080/14992027.2016.1244868. [DOI] [PubMed] [Google Scholar]
- [7].Simoes EA, Kiio F, Carosone-Link PJ, Ndegwa SN, Ayugi J, Macharia IM, Otitis media and its sequelae in Kenyan schoolchildren, Epub 2015/09/26, J. Pediatr. Infect. Dis. Soc (2015), 10.1093/jpids/piv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chadha SK, Gulati K, Garg S, Agarwal AK, Prevalence of ear diseases in the children of Delhi, Epub 2015/04/18, J. Laryngol. Otol 129 (5) (2015) 425–429, 10.1017/S002221511500081X. [DOI] [PubMed] [Google Scholar]
- [9].Jain A, Nimonkar P, Bhola N, Borle R, Jadhav A, Sharma S, et al. , Does hamulotomy during palatoplasty have any effect on hearing ability in nonsyndromic cleft palate patients? A prospective, single blind, comparative study, Epub 2016/03/24, Sci. Tech. Rep 2016 (2016) 9641303, , 10.1155/2016/9641303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Muftah S, Mackenzie I, Faragher B, Brabin B, Prevalence of chronic suppurative otitis media (CSOM) and associated hearing impairment among school-aged children in Yemen, Epub 2015/10/01, Oman Med. J 30 (5) (2015) 358–365, 10.5001/omj.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Parmar Sms A, Chakkal HS, Prevalence of chronic suppurative otitis media in schoolgoing children, Indian J. Otol 24 (2018) 223–226, 10.4103/indianjotol.INDIANJOTOL_152_17. [DOI] [Google Scholar]
- [12].Rupa V, Isaac R, Rebekah G, Manoharan A, Association of Streptococcus pneumoniae nasopharyngeal colonization and other risk factors with acute otitis media in an unvaccinated Indian birth cohort, Epub 2016/03/05, Epidemiol. Infect 144 (10) (2016) 2191–2199, 10.1017/S0950268816000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tarafder KH, Akhtar N, Zaman MM, Rasel MA, Bhuiyan MR, Datta PG, Disabling hearing impairment in the Bangladeshi population, Epub 2015/01/30, J. Laryngol. Otol 129 (2) (2015) 126–135, 10.1017/S002221511400348X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kaspar A, Newton O, Kei J, Driscoll C, Swanepoel W, Goulios H, Prevalence of ear disease and associated hearing loss among primary school students in the Solomon Islands: otitis media still a major public health issue, Epub 2018/09/04, Int. J. Pediatr. Otorhinolaryngol 113 (2018) 223–228, 10.1016/j.ijporl.2018.08.004. [DOI] [PubMed] [Google Scholar]
- [15].Kaspar A, Newton O, Kei J, Driscoll C, Swanepoel W, Goulios H, Prevalence of otitis media and risk-factors for sensorineural hearing loss among infants attending Child Welfare Clinics in the Solomon Islands, Epub 2018/07/01, Int. J. Pediatr. Otorhinolaryngol 111 (2018) 21–25, 10.1016/j.ijporl.2018.05.021. [DOI] [PubMed] [Google Scholar]
- [16].Leach AJ, Wigger C, Beissbarth J, Woltring D, Andrews R, Chatfield MD, et al. , General health, otitis media, nasopharyngeal carriage and middle ear microbiology in Northern Territory Aboriginal children vaccinated during consecutive periods of 10-valent or 13-valent pneumococcal conjugate vaccines, Epub 2016/06/05, Int. J. Pediatr. Otorhinolaryngol 86 (2016) 224–232, 10.1016/j.ijporl.2016.05.011. [DOI] [PubMed] [Google Scholar]
- [17].Ferrite S, Mactaggart I, Kuper H, Oye J, Polack S, Prevalence and causes of hearing impairment in fundong health district, north-west Cameroon, Epub 2017/01/20, Trop. Med. Int. Health : TM & IH 22 (4) (2017) 485–492, 10.1111/tmi.12840. [DOI] [PubMed] [Google Scholar]
- [18].Hrapcak S, Kuper H, Bartlett P, Devendra A, Makawa A, Kim M, et al. , Hearing loss in HIV-infected children in lilongwe, Malawi, Epub 2016/08/24, PloS One 11 (8) (2016) e0161421, , 10.1371/journal.pone.0161421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Smith AF, Ianacone DC, Ensink RJH, Melaku A, Casselbrant ML, Isaacson G, Prevalence of hearing-loss among HAART-treated children in the Horn of Africa, Epub 2017/06/07, Int. J. Pediatr. Otorhinolaryngol 98 (2017) 166–170, 10.1016/j.ijporl.2017.04.050. [DOI] [PubMed] [Google Scholar]
- [20].Yousuf Hussein S, Swanepoel W, Mahomed-Asmail F, de Jager LB, Hearing loss in preschool children from a low income South African community, Epub 2018/10/29, Int. J. Pediatr. Otorhinolaryngol 115 (2018) 145–148, 10.1016/j.ijporl.2018.09.032. [DOI] [PubMed] [Google Scholar]
- [21].Bright T, Mactaggart I, Kim M, Yip J, Kuper H, Polack S, Rationale for a rapid methodology to assess the prevalence of hearing loss in population-based surveys, Epub 2019/09/22, Int. J. Environ. Res. Publ. Health 16 (18) (2019), 10.3390/ijerph16183405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Garg S, Kohli C, Mangla V, Chadha S, Singh MM, Dahiya N, An epidemiological study on burden of hearing loss and its associated factors in Delhi, India, Epub 2018/06/26, Ann. Otol. Rhinol. Laryngol 127 (9) (2018) 614–619, 10.1177/0003489418781968. [DOI] [PubMed] [Google Scholar]
- [23].Sanders M, Houghton N, Dewes O, McCool J, Thorne PR, Estimated prevalence of hearing loss and provision of hearing services in Pacific Island nations, Epub 2015/03/17., J. Prim. Health Care 7 (1) (2015) 5–15. [PubMed] [Google Scholar]
- [24].Bandyopadhyay T, Raman EV, Otitis media with effusion (OME) in urban pediatric population in a tertiary care centre: a clinical study, Epub 2018/07/07, Indian J. Otolaryngol. Head Neck Surg 70 (2) (2018) 267–272, 10.1007/s12070-017-1178-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bowatte G, Tham R, Perret JL, Bloom MS, Dong G, Waidyatillake N, et al. , Air pollution and otitis media in children: a systematic review of literature, Epub 2018/02/07, Int. J. Environ. Res. Publ. Health 15 (2) (2018), 10.3390/ijerph15020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Deng Q, Lu C, Jiang W, Zhao J, Deng L, Xiang Y, Association of outdoor air pollution and indoor renovation with early childhood ear infection in China, Epub 2016/11/25, Chemosphere 169 (2017) 288–296, 10.1016/j.chemosphere.2016.11.079. [DOI] [PubMed] [Google Scholar]
- [27].Santos-Cortez RL, Reyes-Quintos MR, Tantoco ML, Abbe I, Llanes EG, Ajami NJ, et al. , Genetic and environmental determinants of otitis media in an indigenous Filipino population, Epub 2016/11/03, Otolaryngol. Head Neck Surg 155 (5) (2016) 856–862, 10.1177/0194599816661703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fang TY, Rafai E, Wang PC, Bai CH, Jiang PL, Huang SN, et al. , Pediatric otitis media in Fiji: survey findings 2015, Epub 2016/06/01, Int. J. Pediatr. Otorhinolaryngol 85 (2016) 50–55, 10.1016/j.ijporl.2016.04.001. [DOI] [PubMed] [Google Scholar]
- [29].Van Dyke MK, Pircon JY, Cohen R, Madhi SA, Rosenblut A, Macias Parra M, et al. , Etiology of acute otitis media in children less than 5 Years of age: a pooled analysis of 10 similarly designed observational studies, Epub 2016/12/06, Pediatr. Infect. Dis. J 36 (3) (2017) 274–281, 10.1097/inf.0000000000001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shakoor S, Malik FR, Khan E, Bacterial aetiology of otitis media in children in Pakistan aged 0–59 months; laboratory surveillance data from 2004 to 2013: comparison between before and after the introduction of Hib vaccination, Epub 2016/10/21, Paediatr. Int. Child Health 36 (1) (2016) 34–38, 10.1179/2046905514y.0000000170. [DOI] [PubMed] [Google Scholar]
- [31].DeAntonio R, Yarzabal JP, Cruz JP, Schmidt JE, Kleijnen J, Epidemiology of otitis media in children from developing countries: a systematic review, Epub 2016/06/01, Int. J. Pediatr. Otorhinolaryngol 85 (2016) 65–74, 10.1016/j.ijporl.2016.03.032. [DOI] [PubMed] [Google Scholar]
- [32].Ben-Shimol S, Givon-Lavi N, Leibovitz E, Raiz S, Greenberg D, Dagan R, Impact of widespread introduction of pneumococcal conjugate vaccines on pneumococcal and nonpneumococcal otitis media, Epub 2016/05/27, Clin. Infect. Dis 63 (5) (2016) 611–618, 10.1093/cid/ciw347. [DOI] [PubMed] [Google Scholar]
- [33].Jervis-Bardy J, Carney AS, Duguid R, Leach AJ, Microbiology of otitis media in Indigenous Australian children: review, Epub 2017/01/17, J. Laryngol. Otol (2017) 1–10, 10.1017/s0022215116009294. [DOI] [PubMed] [Google Scholar]
- [34].Lewnard JA, Givon-Lavi N, Weinberger DM, Lipsitch M, Dagan R, Pan-serotype reduction in progression of Streptococcus pneumoniae to otitis media after rollout of pneumococcal conjugate vaccines, Epub 2017/10/12, Clin. Infect. Dis (2017), 10.1093/cid/cix673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Coleman A, Wood A, Bialasiewicz S, Ware RS, Marsh RL, Cervin A, The un-solved problem of otitis media in indigenous populations: a systematic review of upper respiratory and middle ear microbiology in indigenous children with otitis media, , Microbiome 6 (1) (2018) 199, 10.1186/s40168-018-0577-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Udden F, Filipe M, Reimer A, Paul M, Matuschek E, Thegerstrom J, et al. , Aerobic bacteria associated with chronic suppurative otitis media in Angola, Epub 2018/05/04, Infect Dis Poverty 7 (1) (2018) 42, 10.1186/s40249-018-0422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ilechukwu GC, Ilechukwu CA, Ubesie AC, Okoroafor I, Ezeanolue BC, Ojinnaka NC, Bacterial agents of the discharging middle ear among children seen at the University of Nigeria Teaching Hospital, Epub 2017/05/12, Enugu. Pan Afr Med J 26 (2017) 87, 10.11604/pamj.2017.26.87.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Filipe M, Reimer A, Matuschek E, Paul M, Pelkonen T, Riesbeck K, Fluoroquinolone-resistant alcaligenes faecalis related to chronic suppurative otitis media, Angola, Epub 2017/09/21, Emerg. Infect. Dis 23 (10) (2017) 1740–1742, 10.3201/eid2310.170268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Basnet R, Sharma S, Rana JC, Shah PK, Bacteriological study of otitis media and its antibiotic susceptibility pattern, Epub 2017/10/11., J. Nepal. Health. Res. Counc 15 (2) (2017) 124–129. [DOI] [PubMed] [Google Scholar]
- [40].Sonsuwan N, Watcharinyanon P, Sawanyawisuth K, What are the leading cau-sative pathogens in acute otitis media with tympanic membrane perforation? Epub 2016/10/13, Int. J. Pediatr. Otorhinolaryngol 90 (2016) 20–22, 10.1016/j.ijporl.2016.08.021. [DOI] [PubMed] [Google Scholar]
- [41].Flasche S, Givon-Lavi N, Dagan R, Using pneumococcal carriage data to monitor postvaccination changes in the incidence of pneumococcal otitis media, Epub 2016/11/03, Am. J. Epidemiol 184 (9) (2016) 652–659, 10.1093/aje/kww012. [DOI] [PubMed] [Google Scholar]
- [42].Madhi SA, Govender N, Dayal K, Devadiga R, Van Dyke MK, van Niekerk N, et al. , Bacterial and respiratory viral interactions in the etiology of acute otitis media in HIV-infected and HIV-uninfected South African children, Epub 2015/04/30, Pediatr. Infect. Dis. J 34 (7) (2015) 753–760, 10.1097/inf.0000000000000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Aho C, Michael A, Yoannes M, Greenhill A, Jacoby P, Reeder J, et al. , Limited impact of neonatal or early infant schedules of 7-valent pneumococcal conjugate vaccination on nasopharyngeal carriage of Streptococcus pneumoniae in Papua New Guinean children: a randomized controlled trial, Epub 2017/06/06, Vaccine. Rep 6 (2016) 36–43, 10.1016/j.vacrep.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jervis-Bardy J, Rogers GB, Morris PS, Smith-Vaughan HC, Nosworthy E, Leong LE, et al. , The microbiome of otitis media with effusion in Indigenous Australian children, Epub 2015/08/01, Int. J. Pediatr. Otorhinolaryngol 79 (9) (2015) 1548–1555, 10.1016/j.ijporl.2015.07.013. [DOI] [PubMed] [Google Scholar]
- [45].Ding Y, Geng Q, Tao Y, Lin Y, Wang Y, Black S, et al. , Etiology and epidemiology of children with acute otitis media and spontaneous otorrhea in Suzhou, China, Epub 2014/11/08, Pediatr. Infect. Dis. J 34 (5) (2015) e102–e106, 10.1097/inf.0000000000000617. [DOI] [PubMed] [Google Scholar]
- [46].Chirwa M, Mulwafu W, Aswani JM, Masinde PW, Mkakosya R, Soko D, Microbiology of chronic suppurative otitis media at Queen Elizabeth Central Hospital, Blantyre, Malawi: a cross-sectional descriptive study, Epub 2016/03/10., Malawi Med. J. :J. Med. Assoc. Malawi 27 (4) (2015) 120–124. [PMC free article] [PubMed] [Google Scholar]
- [47].Leach AJ, Wigger C, Hare K, Hampton V, Beissbarth J, Andrews R, et al. , Reduced middle ear infection with non-typeable Haemophilus influenzae, but not Streptococcus pneumoniae, after transition to 10-valent pneumococcal non-typeable H. influenzae protein D conjugate vaccine, Epub 2015/10/21, BMC Pediatr 15 (1) (2015) 162, 10.1186/s12887-015-0483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ofogbu CV, Orji FT, Ezeanolue BC, Emodi I, Microbiological profile of chronic suppurative otits media among HIV infected children in South Eastern Nigeria, Epub 2016/01/01., Niger. J. Med. : J. Natl. Assoc. Resid. Dr. Niger 25 (1) (2016) 5–11. [PubMed] [Google Scholar]
- [49].Nwokoye NN, Egwari LO, Olubi OO, Occurrence of otitis media in children and assessment of treatment options, Epub 2015/06/16, J. Laryngol. Otol 129 (8) (2015) 779–783, 10.1017/s0022215115001127. [DOI] [PubMed] [Google Scholar]
- [50].Coleman A, Wood A, Bialasiewicz S, Ware RS, Marsh RL, Cervin A, The un-solved problem of otitis media in indigenous populations: a systematic review of upper respiratory and middle ear microbiology in indigenous children with otitis media, Epub 2018/11/07, Microbiome 6 (1) (2018) 199, 10.1186/s40168-018-0577-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Satzke C, Turner P, Virolainen-Julkunen A, Adrian PV, Antonio M, Hare KM, et al. , Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae: updated recommendations from the world health organization pneumococcal carriage working group, Epub 2013/12/18, Vaccine 32 (1) (2013) 165–179, 10.1016/j.vaccine.2013.08.062. [DOI] [PubMed] [Google Scholar]
- [52].Shakoor S, Kabir F, Khowaja AR, Qureshi SM, Jehan F, Qamar F, et al. , Pneumococcal serotypes and serogroups causing invasive disease in Pakistan, 2005–2013, Epub 2014/06/04, PloS One 9 (6) (2014) e98796, , 10.1371/journal.pone.0098796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Mulwafu W, Kuper H, Viste A, Goplen FK, Feasibility and acceptability of training community health workers in ear and hearing care in Malawi: a cluster randomised controlled trial, Epub 2017/10/14, BMJ open 7 (10) (2017) e016457, , 10.1136/bmjopen-2017-016457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Stepniak C, Wickens B, Husein M, Paradis J, Ladak HM, Fung K, et al. , Blinded randomized controlled study of a web-based otoscopy simulator in undergraduate medical education, Epub 2016/09/01, Laryngoscope 127 (6) (2017) 1306–1311, 10.1002/lary.26246. [DOI] [PubMed] [Google Scholar]
- [55].Gunasekera H, Miller HM, Burgess L, Chando S, Sheriff SL, Tsembis JD, et al. , Agreement between diagnoses of otitis media by audiologists and otolaryngologists in Aboriginal Australian children, Epub 2018/06/30., Med. J. Aust 209 (1) (2018) 29–35. [DOI] [PubMed] [Google Scholar]
- [56].Erkkola-Anttinen N, Irjala H, Laine MK, Tahtinen PA, Loyttyniemi E, Ruohola A, Smartphone otoscopy performed by parents, Epub 2018/07/25, Telemed. J. e Health 25 (6) (2019) 477–484, 10.1089/tmj.2018.0062. [DOI] [PubMed] [Google Scholar]
- [57].Chan KH, Dreith S, Uhler KM, Tallo V, Lucero M, De Jesus J, et al. , Large-scale otoscopic and audiometric population assessment: a pilot study, Epub 2018/12/24, Int. J. Pediatr. Otorhinolaryngol 117 (2019) 148–152, 10.1016/j.ijporl.2018.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bathla M, Doshi H, Kansara A, Is routine use of high resolution computerized tomography of temporal bone in patients of atticoantral chronic suppurative otitis media without intracranial complications justified? Epub 2018/02/20, Indian J. Otolaryngol. Head Neck Surg 70 (1) (2018) 79–86, 10.1007/s12070-017-1103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Nash R, Lingam RK, Chandrasekharan D, Singh A, Does non-echo-planar diffusion-weighted magnetic resonance imaging have a role in assisting the clinical diagnosis of cholesteatoma in selected cases? Epub 2018/01/23, J. Laryngol. Otol 132 (3) (2018) 207–213, 10.1017/S0022215118000087. [DOI] [PubMed] [Google Scholar]
- [60].Sarmento KMA Jr., Sampaio ALL, Santos TGT, Oliveira C, High-frequency conductive hearing loss as a diagnostic test for incomplete ossicular discontinuity in non-cholesteatomatous chronic suppurative otitis media, Epub 2017/12/22, PloS One 12 (12) (2017) e0189997, , 10.1371/journal.pone.0189997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Anwar K, Khan S, Rehman HU, Javaid M, Shahabi I, Otitis media with effusion: accuracy of tympanometry in detecting fluid in the middle ears of children at myringotomies, Epub 2016/05/18, Pak J Med Sci 32 (2) (2016) 466–470, 10.12669/pjms.322.9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Tuzcu G, Yardimci AH, Turna O, Goner RE, Acioglu E, [Importance of diffusion weighted magnetic resonance imaging at differentiation of cholesteatoma and granulation tissue in patients with chronic suppurative otitis media], Epub 2015/10/20, Kulak burun bogaz ihtisas dergisi : KBB = Journal of ear, nose, and throat 25 (5) (2015) 255–265, 10.5606/kbbihtisas.2015.25675. [DOI] [PubMed] [Google Scholar]
- [63].Park M, Han KH, Jung H, Kim MH, Chang HK, Kim SH, et al. , Usefulness of 1000-Hz probe tone in tympanometry according to age in Korean infants, Epub 2014/12/04, Int. J. Pediatr. Otorhinolaryngol 79 (1) (2015) 42–46, 10.1016/j.ijporl.2014.10.041. [DOI] [PubMed] [Google Scholar]
- [64].Fischer N, Schartinger VH, Dejaco D, Schmutzhard J, Riechelmann H, Plaikner M, et al. , Readout-segmented echo-planar DWI for the detection of cholesteatomas: correlation with surgical validation, Epub 2019/05/28, AJNR Am J Neuroradiol 40 (6) (2019) 1055–1059, 10.3174/ajnr.A6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Shelton RL, Nolan RM, Monroy GL, Pande P, Novak MA, Porter RG, Boppart SA, Quantitative pneumatic otoscopy using a light-based ranging technique, Epub 2017 Jun 26, J Assoc Res Otolaryngol 18 (4) (2017. August) 555–568, 10.1007/s10162-017-0629-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hu L, Li W, Lin H, Li Y, Zhang H, Svanberg K, Svanberg S, Towards an optical diagnostic system for otitis media using a combination of otoscopy and spectroscopy, Epub 2019 Feb 28, J. Biophot 12 (6) (2019. June) e201800305, , 10.1002/jbio.201800305. [DOI] [PubMed] [Google Scholar]
- [67].Karki S, Pokharel M, Suwal S, Poudel R, Correlation between preoperative high resolution computed tomography (CT) findings with surgical findings in chronic otitis media (COM) squamosal type, Epub 2018/02/16., Kathmandu Univ. Med. J 15 (57) (2017) 84–87. [PubMed] [Google Scholar]
- [68].Ramkumar V, Rajendran A, Nagarajan R, Balasubramaniyan S, Suresh DK, Identification and management of middle ear disorders in a rural cleft care program: a telemedicine approach, Am. J. Audiol 27 (3S) (2018. November 19) 455–461, 10.1044/2018_AJA-IMIA3-18-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Myburgh HC, van Zijl WH, Swanepoel D, Hellström S, Laurent C, Otitis media diagnosis for developing countries using tympanic membrane image-analysis, EBio. Med 5 (2016) 156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Tugrul S, Degirmenci N, Eren SB, Dogan R, Meric Hafiz A, Ozturan O, RGB measurements as a novel objective diagnostic test for otitis media with effusion, Acta Otolaryngol 135 (4) (2015. April) 342–347, 10.3109/00016489.2014.984877.Epub.2015.Mar.5. [DOI] [PubMed] [Google Scholar]
- [71].Chan KN, Silverstein A, Bryan LN, McCracken CE, Little WK, Shane AL, Comparison of a smartphone otoscope and conventional otoscope in the diagnosis and management of acute otitis media, 9922818812480, Clin. Pediatr (2018. November 21), 10.1177/0009922818812480 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [72].Chan J, Raju S, Nandakumar R, Bly R, Gollakota S, Detecting middle ear fluid using smartphones, pii: eaav1102, Sci. Transl. Med (492) (2019. May 15) 11, 10.1126/scitranslmed.aav1102. [DOI] [PubMed] [Google Scholar]
- [73].Shie Chuen-Kai, Chuang Chung-Hisang, Chou Chun-Nan, Wu Meng-Hsi, Chang EY, Transfer representation learning for medical image analysis, Conf Proc IEEE Eng Med Biol Soc 2015 (2015. August) 711–714, 10.1109/EMBC.2015.7318461. [DOI] [PubMed] [Google Scholar]
- [74].Doğan HH, Sezer RG, Kırkgöz T, Bozaykut A, Comparison of axillary and tympanic temperature measurements in children diagnosed with acute otitis media, Epub 2016 Aug 28, Int. J. Pediatr 2016 (2016) 1729218, , 10.1155/2016/1729218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Eryilmaz MA, Derin S, Mean platelet volume as a potential predictor of cholesteatoma in children, J. Craniofac. Surg 27 (6) (2016. September) e575–e578, 10.1097/SCS.0000000000002881. [DOI] [PubMed] [Google Scholar]
- [76].Chen CK, Fang J, Wan YL, Tsui PH, Ultrasound characterization of the mastoid for detecting middle ear effusion: a preliminary clinical validation, Sci. Rep 6 (2016. June 9) 27777, 10.1038/srep27777.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Terzi S, Özgür A, Erdivanli ÖÇ, Coşkun ZÖ, Ogurlu M, Demirci M, Dursun E, Diagnostic value of the wideband acoustic absorbance test in middle-ear effusion, 1078–84, J. Laryngol. Otol 129 (11) (2015. November), 10.1017/S0022215115002339.Epub.2015.Sep.22. [DOI] [PubMed] [Google Scholar]
- [78].Pan JL, Yang J, [The clinical value of wideband tympanometry in the diagnosis of otitis media with effusion], Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 32 (17) (2018. September) 1309–1315, 10.13201/j.issn.1001-1781.2018.17.005. [DOI] [PubMed] [Google Scholar]
- [79].Aithal S, Aithal V, Kei J, Anderson S, Liebenberg S, Eustachian tube dysfunction and wideband Absorbance measurements at tympanometric peak pressure and 0 daPa, [Epub ahead of print], J. Am. Acad. Audiol (2018. November 14), 10.3766/jaaa.18002. [DOI] [PubMed] [Google Scholar]
- [80].Valdez TA, Carr JA, Kavanagh KR, Schwartz M, Blake D, Bruns O, Bawendi M, Initial findings of shortwave infrared otoscopy in a pediatric population, Epub 2018 Aug 23, Int. J. Pediatr. Otorhinolaryngol 114 (2018. November) 15–19, 10.1016/j.ijporl.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Yen YC, Lin C, Weng SF, Lin YS, Higher risk of developing sudden sensorineural hearing loss in patients with chronic otitis media, Epub 2015/03/06, JAMA otolaryngology- head & neck surgery 141 (5) (2015) 429–435, 10.1001/jamaoto.2015.102. [DOI] [PubMed] [Google Scholar]
- [82].Singer AEA, Abdel-Naby Awad OG, El-Kader RMA, Mohamed AR, Risk factors of sensorineural hearing loss in patients with unilateral safe chronic suppurative otitis media, Epub 2018/01/15, Am. J. Otolaryngol 39 (2) (2018) 88–93, 10.1016/j.amjoto.2018.01.002. [DOI] [PubMed] [Google Scholar]
- [83].Dobrianskyj FM, Goncalves IR, Tamaoki Y, Mitre EI, Ribeiro FA, Correlation between sensorineural hearing loss and chronic otorrhea, E43–E6, Ear Nose Throat J 96 (2017). [DOI] [PubMed] [Google Scholar]
- [84].Yehudai N, Most T, Luntz M, Risk factors for sensorineural hearing loss in pediatric chronic otitis media, Epub 2014/12/09, Int. J. Pediatr. Otorhinolaryngol 79 (1) (2015) 26–30, 10.1016/j.ijporl.2014.10.025. [DOI] [PubMed] [Google Scholar]
- [85].Ali Zaidi SS, Pasha HA, Suhail A, Qureshi TA, Frequency of Sensorineural hearing loss in chronic suppurative otitis media, Suppl 3. S42–s4. Epub 2016/11/30., J. Pakistan Med. Assoc 66 (10) (2016). [PubMed] [Google Scholar]
- [86].Cordeiro FP, da Costa Monsanto R, Kasemodel ALP, de Almeida Gondra L, de Oliveira Penido N, Extended high-frequency hearing loss following the first episode of otitis media, Epub 2018/09/09, Laryngoscope 128 (12) (2018) 2879–2884, 10.1002/lary.27309. [DOI] [PubMed] [Google Scholar]
- [87].Penaranda A, Garcia JM, Otoscopic and audiological findings in different populations of 5–14 year-old schoolchildren in Colombia, Epub 2015/05/04, Int. J. Pediatr. Otorhinolaryngol 79 (7) (2015) 993–997, 10.1016/j.ijporl.2015.04.005. [DOI] [PubMed] [Google Scholar]
- [88].Avnstorp MB, Homoe P, Bjerregaard P, Jensen RG, Chronic suppurative otitis media, middle ear pathology and corresponding hearing loss in a cohort of Greenlandic children, Epub 2016/03/13, Int. J. Pediatr. Otorhinolaryngol 83 (2016) 148–153, 10.1016/j.ijporl.2016.01.017. [DOI] [PubMed] [Google Scholar]
- [89].Jensen PVF, Avnstorp MB, Dzongodza T, Chidziva C, von Buchwald C, A fatal case of Gradenigo’s syndrome in Zimbabwe and the Danish-Zimbabwean ENT collaboration, Epub 2017/05/10, Int. J. Pediatr. Otorhinolaryngol 97 (2017) 181–184, 10.1016/j.ijporl.2017.04.014. [DOI] [PubMed] [Google Scholar]
- [90].Yaniv E, Tuberculous otitis: an underdiagnosed disease, Epub 1987/11/01, Am. J. Otolaryngol 8 (6) (1987) 356–360, 10.1016/s0196-0709(87)80020-0. [DOI] [PubMed] [Google Scholar]
- [91].Hao XP, Gong SS, Li YX, Xia Y, Zhao SQ, Zheng J, et al. , [Analysis of clinical features and treatment outcomes of patients with tuberculous otitis media and mastoiditis], Epub 2011/01/11., Zhonghua er bi yan hou tou jing wai ke za zhi 45 (11) (2010) 912–915. [PubMed] [Google Scholar]
- [92].Singh A, Irugu DVK, Verma H, Thakar A, Atypical presentation of aural tuberculosis with complication, Epub 2018/03/11, BMJ Case Rep 2018 (2018), 10.1136/bcr-2017-222482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Kameswaran M, Natarajan K, Parthiban M, Krishnan PV, Raghunandhan S, Tuberculous otitis media: a resurgence? Epub 2017/07/21, J. Laryngol. Otol 131 (9) (2017) 785–792, 10.1017/S0022215117001281. [DOI] [PubMed] [Google Scholar]
- [94].Orji FT, Ukaegbe O, Alex-Okoro J, Ofoegbu VC, Okorafor IJ, The changing epidemiological and complications profile of chronic suppurative otitis media in a developing country after two decades, Epub 2015/11/28, Eur. Arch. Oto-Rhino-Laryngol 273 (9) (2016) 2461–2466, 10.1007/s00405-015-3840-1. [DOI] [PubMed] [Google Scholar]
- [95].Poole NF, Skilton MK, Martin TC, Smith MC, Knowledge, attitudes, beliefs and practices related to chronic suppurative otitis media and hearing impairment in Pokhara, Nepal, Epub 2015/11/17, J. Laryngol. Otol (2015) 1–10, 10.1017/s0022215115002996. [DOI] [PubMed] [Google Scholar]
- [96].Jones C, Sharma M, Harkus S, McMahon C, Taumoepeau M, Demuth K, et al. , A program to respond to otitis media in remote Australian Aboriginal communities: a qualitative investigation of parent perspectives, Epub 2018/03/08, BMC Pediatr 18 (1) (2018) 99, 10.1186/s12887-018-1081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Mushi MF, Mwalutende AE, Gilyoma JM, Chalya PL, Seni J, Mirambo MM, et al. , Predictors of disease complications and treatment outcome among patients with chronic suppurative otitis media attending a tertiary hospital, Mwanza Tanzania, Epub 2016/01/15, BMC Ear Nose Throat Disord 16 (2016) 1, 10.1186/s12901-015-0021-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Zer Toros S, Tepe Karaca C, Kalaycik Ertugay C, Senbayrak S, Ertugay OC, Seneldir L, Simultaneous coexistence of complications of chronic otitis media in the same case, Epub 2017/07/19, J Int Adv Otol 13 (2) (2017) 282–284, 10.5152/iao.2017.3486. [DOI] [PubMed] [Google Scholar]
- [99].Qin Y, Li TC, Cong TC, Liu YH, Abscess of zygomatic root: a rare otogenic complication, Epub 2017/03/18, Chin. Med. J 130 (6) (2017) 749–750, 10.4103/0366-6999.201610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Maranhao AS, Godofredo VR, Penido Nde O, Suppurative labyrinthitis associated with years’ experience, Epub 2016/01/01, Braz. J. Otorhinolaryngol 82 (1) (2016) 82–87, 10.1016/j.bjorl.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Penido Nde O, Chandrasekhar SS, Borin A, Maranhao AS, Gurgel Testa JR, Complications of otitis media - a potentially lethal problem still present, Epub 2015/10/01, Braz. J. Otorhinolaryngol 82 (3) (2016) 253–262, 10.1016/j.bjorl.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Singh GB, Solo M, Kaur R, Arora R, Kumar S, Mycology of chronic suppurative otitis media-cholesteatoma disease: an evaluative study, Epub 2018/01/08, Am. J. Otolaryngol 39 (2) (2018) 157–161, 10.1016/j.amjoto.2017.12.001. [DOI] [PubMed] [Google Scholar]
- [103].Mushi MF, Buname G, Bader O, Gross U, Mshana SE, Aspergillus fumigatus carrying TR34/L98H resistance allele causing complicated suppurative otitis media in Tanzania: call for improved diagnosis of fungi in sub-Saharan Africa, Epub 2016/09/04, BMC Infect. Dis 16 (2016) 464, 10.1186/s12879-016-1796-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Jain A, Arora N, Meher R, Passey JC, Bansal R, Intracranial complications of CSOM in pediatric patients: a persisting problem in developing countries, Epub 2017/08/15, Int. J. Pediatr. Otorhinolaryngol 100 (2017) 128–131, 10.1016/j.ijporl.2017.06.038. [DOI] [PubMed] [Google Scholar]
- [105].Ovnat Tamir S, Shemesh S, Oron Y, Marom T, Acute otitis media guidelines in selected developed and developing countries: uniformity and diversity, Epub 2016/09/08, Arch. Dis. Child 102 (5) (2017) 450–457, 10.1136/archdischild-2016-310729. [DOI] [PubMed] [Google Scholar]
- [106].Khreesha L, Bacharouch A, Blackwood RA, Alkhoujah M, Issa MR, The use of practice guidelines in the management of pediatric cases of Acute Otitis Media in Amman, Jordan, Epub 2017/04/10, Int. J. Pediatr. Otorhinolaryngol 96 (2017) 39–46, 10.1016/j.ijporl.2017.02.024. [DOI] [PubMed] [Google Scholar]
- [107].Ababneh MA, Al-Azzam SI, Ababneh R, Rababa’h AM, Demour SA, Antibiotic prescribing for acute respiratory infections in children in Jordan, Epub 2017/03/25, Int Health 9 (2) (2017) 124–130, 10.1093/inthealth/ihx003. [DOI] [PubMed] [Google Scholar]
- [108].Nasrallah A, Bacharouch A, Jaafar F, Ayyash M, Blackwood RA, Antibiotic prescription patterns for management of acute otitis media in Lebanon, Epub 2018/09/29, Int. J. Pediatr. Otorhinolaryngol 114 (2018) 44–50, 10.1016/j.ijporl.2018.08.014. [DOI] [PubMed] [Google Scholar]
- [109].Buyukcam A, Kara A, Bedir T, Gulhan B, Ozdemir H, Sutcu M, et al. , Pediatricians’ attitudes in management of acute otitis media and ear pain in Turkey, Epub 2018/03/05, Int. J. Pediatr. Otorhinolaryngol 107 (2018) 14–20, 10.1016/j.ijporl.2018.01.011. [DOI] [PubMed] [Google Scholar]
- [110].Malik SA, Muhammad R, Yousaf M, Shah I, Effectiveness of conservative treatment in the management of secretory otitis media, Epub 2015/02/13., J. Ayub Med. Coll. Abbottabad 26 (3) (2014) 337–340. [PubMed] [Google Scholar]
- [111].Yazici A, Coskun ME, The effect of ventilation tube insertion to the health-related quality of life in a group of children in Southeast Anatolia, Epub 2018/08/31, Clin. Otolaryngol 43 (6) (2018) 1578–1582, 10.1111/coa.13220. [DOI] [PubMed] [Google Scholar]
- [112].Johnston J, McLaren H, Mahadevan M, Douglas RG, Surgical treatment of otitis media with effusion in Maori children, Epub 2018/09/14, ANZ J. Surg 88 (11) (2018) 1141–1144, 10.1111/ans.14788. [DOI] [PubMed] [Google Scholar]
- [113].Toman J, Moll A, Barnes M, Shenoi S, Porterfield JZ, The role of routine culture in the treatment of chronic suppurative otitis media: implications for the standard of care in rural areas of South Africa, Epub 2019/01/11, Trop. Med. Infect. Dis 4 (1) (2019), 10.3390/tropicalmed4010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Bin Mohanna Mb AA, Bacterial profile and antibiogram of otitis media among children in Yemen, J. Ayub Med. Coll. Abbottabad 28 (3) (2016). [PubMed] [Google Scholar]
- [115].Akhtar N, Datta PG, Yasmin M, Naha A, Jewel AM, Hossen F, et al. , Microbiology of chronic suppurative otitis media in a tertiary care hospital in Bangladesh, Epub 2017/09/19., Mymensingh Med. J 26 (3) (2017) 592–599. [PubMed] [Google Scholar]
- [116].Bareeqa SB, Ahmed SI, Comment to empirical therapy for chronic suppurative otitis media, Epub 2018/11/28, Clin. Med. Insights Ear, Nose Throat 11 (2018), 10.1177/1179550618810226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Elsayed Y, et al. , Impact of educational program on the management of chronic suppurative otitis media among children, Int J Otolaryngol (2015) 25792984, , 10.1155/2015/624317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Maile EJ, Tharu PB, Blanchford HL, Edmiston R, Youngs R, Quality of life of Nepali patients with ear disease before and after corrective surgery, Epub 2015/04/17, Trop. Med. Int. Health : TM & IH 20 (8) (2015) 1041–1047, 10.1111/tmi.12516. [DOI] [PubMed] [Google Scholar]
- [119].Smith AKK, Sokdavy T, Sothea C, Pastrana MKR, Ali RF, Huins CT, et al. , Implementation and results of a surgical training programme for chronic suppurative otitis media in Cambodia, Epub 2018/07/13, J. Laryngol. Otol 132 (8) (2018) 711–717, 10.1017/S0022215118001056. [DOI] [PubMed] [Google Scholar]
- [120].Ramkumar V, Rajendran A, Nagarajan R, Balasubramaniyan S, Suresh DK, Identification and management of middle ear disorders in a rural cleft care program: a telemedicine approach, Epub 2018/11/20, Am. J. Audiol 27 (3s) (2018) 455–461, 10.1044/2018_aja-imia3-18-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Jacups SP, Kinchin I, McConnon KM, Ear, nose, and throat surgical access for remote living Indigenous children: what is the least costly model? Epub 2018/10/13, J. Eval. Clin. Pract 24 (6) (2018) 1330–1338, 10.1111/jep.13044. [DOI] [PubMed] [Google Scholar]
- [122].Holt E, McCool J, Nosa V, Thorne P, Development of an otitis media strategy in the Pacific: key informant perspectives, Epub 2018/05/18., N. Z. Med. J 131 (1475) (2018) 69–76. [PubMed] [Google Scholar]
- [123].Durham J, Schubert L, Vaughan L, Willis CD, Using systems thinking and the Intervention Level Framework to analyse public health planning for complex problems: otitis media in Aboriginal and Torres Strait Islander children, Epub 2018/03/22, PloS One 13 (3) (2018) e0194275, , 10.1371/journal.pone.0194275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Sibthorpe B, Agostino J, Coates H, Weeks S, Lehmann D, Wood M, et al. , Indicators for continuous quality improvement for otitis media in primary health care for Aboriginal and Torres Strait Islander children, Epub 2017/01/17, Aust. J. Prim. Health (2017), 10.1071/py16096. [DOI] [PubMed] [Google Scholar]
- [125].Nshimirimana JPD, Mukara KB, Causes of delayed care seeking for chronic suppurative otitis media at a Rwandan tertiary hospital, Epub 2018/07/31, Int J Otolaryngol 2018 (2018) 5386217, , 10.1155/2018/5386217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, et al. , Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration, Epub 2014/07/22, Int. J. Surg 12 (12) (2014) 1500–1524, 10.1016/j.ijsu.2014.07.014. [DOI] [PubMed] [Google Scholar]


