Abstract
The genus Tubakia is revised on the basis of morphological and phylogenetic data. The phylogenetic affinity of Tubakia to the family Melanconiellaceae (Diaporthales) was recently postulated, but new analyses based on sequences retrieved from material of the type species of Tubakia, T. dryina, support a family of its own, viz. Tubakiaceae fam. nov. Our phylogenetic analyses revealed the heterogeneity of Tubakia s. lat. which is divided into several genera, viz., Tubakia s. str., Apiognomonioides gen. nov. (type species: Apiognomonioides supraseptata), Involutiscutellula gen. nov. (type species: Involutiscutellula rubra), Oblongisporothyrium gen. nov. (type species: Oblongisporothyrium castanopsidis), Paratubakia gen. nov. (type species: Paratubakia subglobosa), Racheliella gen. nov. (type species: Racheliella wingfieldiana sp. nov.), Saprothyrium gen. nov. (type species: Saprothyrium thailandense) and Sphaerosporithyrium gen. nov. (type species: Sphaerosporithyrium mexicanum sp. nov.). Greeneria saprophytica is phylogenetically closely allied to Racheliella wingfieldiana and is therefore reallocated to Racheliella. Particular emphasis is laid on a revision and phylogenetic analyses of Tubakia species described from Japan and North America. Almost all North American collections of this genus were previously referred to as T. dryina s. lat., which is, however, a heterogeneous complex. Several new North American species have recently been described. The new species Sphaerosporithyrium mexicanum, Tubakia melnikiana and T. sierrafriensis, causing leaf spots on several oak species found in the North-Central Mexican state Aguascalientes and the North-Eastern Mexican state Nuevo León, are described, illustrated, and compared with similar species. Several additional new species are introduced, including Tubakia californica based on Californian collections on various species of the genera Chrysolepis, Notholithocarpus and Quercus, and T. dryinoides, T. oblongispora, T. paradryinoides, and Paratubakia subglobosoides described on the basis of Japanese collections. Tubakia suttoniana nom. nov., based on Dicarpella dryina, is a species closely allied to T. californica and currently only known from Europe. Tubakia dryina, type species of Tubakia, is epitypified, and the phylogenetic position and circumscription of Tubakia are clarified. A revised, supplemented key to the species of Tubakia and allied genera on the basis of conidiomata is provided.
Keywords: Ascomycota, DNA phylogeny, epitypification, key, systematics, Tubakiaceae, 24 new taxa
INTRODUCTION
Tubakia species are endophytes in leaves and twigs of many tree species, but can also cause conspicuous leaf symptoms as plant pathogens (Cohen 1999, Gonthier et al. 2006, Hashizume et al. 2008, Sieber 2007). Obvious leaf spots are dotted with minute punctiform conidiomata (pycnothyria) composed of convex scutella with radiating threads of cells fixed to the substratum by a central columella, mostly surrounded by a sheath of small fertile cells that give rise to one-celled, phialidic conidiogenous cells. The conidia are globose, subglobose, ellipsoid, broad ellipsoid-obovoid to subcylindrical or somewhat irregular in shape, aseptate, hyaline, subhyaline to pigmented. The conidiomata and the conidia are distinct and readily allow identification to generic level. Other fungal fruiting structures including crustose-pycnidial conidiomata on overwintering twigs that open by lateral to irregular dehiscence or sporodochia formed on leaf veins (Holdenrieder & Kowalski 1989, Harrington et al. 2012) may also be formed and have been described for T. dryina and T. iowensis. Some species produce a second type of much smaller conidia (microconidia), either in “normal” pycnothyria or in separate, mostly smaller pycnothyria. Reports of the occurrence and sporulation of Tubakia dryina as a saprobe on necrotic tissue caused by other fungi (Munkvold & Neely 1990) are unconfirmed and need further study and clarification.
Tubakia was previously assigned to the Diaporthales within Ascomycota (Yokoyama & Tubaki 1971, Yun & Rossman 2011). In a more detailed study on its phylogenetic affinity and position in the hierarchical system of the Ascomycota, Senanayake et al. (2017) placed Tubakia in the newly introduced family Melanconiellaceae. The sexual morph of “T. dryina” has been referred to as Dicarpella dryina (Belisario 1991). However, D. dryina is not the type species of Dicarpella, and at least one species, Dicarpella quercifolia was linked to Mastigosporella hyalina (≡ Harknessia hyalina; Barr 1979, Nag Raj & Di Cosmo 1981, Rossman et al. 2015). Dicarpella quercifolia, together with D. georgiana, were subsequently reallocated to the genus Wuestneiopsis (Reid & Dawsett 1990). The relation and synonymy of Dicarpella and Tubakia are, however, unclear. Clarification requires phylogenetic data for D. bina, the type species of this genus, which is only known from the type collection.
Saccardo (1913) introduced the genus Actinopelte for A. japonica, a scutellate fungus found in Japan on Castanea crenata (= C. pubinervis). Saccardo (l.c.) confused the large conidia of this species with asci, which was clarified and corrected by Theissen (1913: 509) who provided a detailed discussion, description, and illustration (Theissen 1913: 508, fig. VI) of A. japonica. Höhnel (1925) revisited Actinopelte, added a new species, A. americana, and introduced the new combination A. dryina, based on Leptothyrium dryinum. Yokoyama & Tubaki (1971) discussed the history of this genus in detail, published results of comprehensive examinations of Japanese collections in vivo and in vitro, and described A. castanopsidis, A. rubra, and A. subglobosa based on Japanese collections. Since Saccardo’s Actinopelte turned out to be illegitimate (Art. 53.1, later homonym of Actinopelte Stitzenb. 1861), Sutton (1973) introduced the replacement name Tubakia and reallocated all species recognised and treated in Yokoyama & Tubaki (1971) to this genus. Three additional Tubakia species have subsequently been described: T. seoraksanensis (Yun & Rossman 2011), T. iowensis (Harrington et al. 2012), and T. chinensis (Braun et al. 2014). So far, all European collections in the genus Tubakia have been assigned to a single species, T. dryina, whereas in Asia this genus has a much higher degree of species diversity. Additional undescribed species from Asia were predicted, and were detected in the course of the present studies. Braun et al. (2014) described and illustrated T. chinensis on Castanea henryi, a species with very large pycnothyria (135–200 μm diam), and globose to subglobose conidia. Based on its very small scutella, Braun et al. (2014) described and illustrated a second Tubakia species on Castanea henryi in China, tentatively referred to as Tubakia sp. The recently introduced Tubakia thailandensis (Senanayake et al. 2017) is morphologically very close to the Chinese collection on Castanea henryi and could be conspecific. According to Harrington et al. (2012), Japanese specimens referred to as T. dryina in Yokoyama & Tubaki (1971) do not belong to T. dryina s. str. The phylogeny of the Asian species of Tubakia has not yet been determined. Boroń & Grad (2017) examined numerous Polish strains referred to as T. dryina and found two different ITS groups designated as haplotypes. The diversity of Tubakia species in North America is not well known, although it is likely to contain more than just the single species listed in various publications as T. dryina. North American collections assigned to T. dryina are undoubtedly heterogeneous, i.e., T. dryina in North America is a complex of cryptic species (Harrington et al. 2012). An important contribution to resolve problems around the diversity of the T. dryina complex in North America has recently been published by Harrington & McNew (2018), including several new species based on US collections. Several Tubakia samples recently collected in Mexico on endemic oaks appear to be morphologically similar to T. dryina but are sufficiently distinct to suggest that they are undescribed species. A Tubakia causing serious oak diseases in California also appears to represent an additional undescribed species. Therefore, the present study presents a comprehensive examination and revision of Tubakia spp. in North America and worldwide, based on in vivo and in vitro morphological analyses as well as phylogenetic data. This work has been accomplished in collaboration by an international group of mycologists and phytopathologists from Asia, Europe and North America.
MATERIALS AND METHODS
Isolates
Isolates included in this study were obtained from symptomatic leaves of diverse hosts, and identified as species of Tubakia based on primarily the presence of pycnothyrial conidiomata and aseptate conidia. In addition, several isolates were obtained from the culture collection of the Westerdijk Fungal Biodiversity Institute (CBS culture collection), in Utrecht, The Netherlands, the CDFA Plant Pathogen Collections (CDFA), Sacramento, USA, and from the working collection of Pedro Crous (CPC), housed at the Westerdijk Institute. Japanese isolates were obtained from NBRC (National Institute of Technology and Evaluation, Culture Collection Division, Chiba, Japan) and examined by C. Nakashima. Single conidial colonies were established from sporulating conidiomata on Petri dishes containing pine needle agar (PNA) (Smith et al. 1996), 2 % malt extract agar (MEA), potato-dextrose agar (PDA), and oatmeal agar (OA) (Crous et al. 2009b), and incubated at 22 °C under continuous near-ultraviolet light to promote sporulation. Descriptions of culture characteristics on Czapek agar and potato sucrose agar refer to Yokoyama & Tubaki (1971). The cultures included in this study are listed in Table 1.
Table 1.
Collection details and GenBank accession numbers of Tubakia and tubakia-like isolates considered in this study.
| Species name | Culture accession number(s)1 | Collector and Collection date | Substrate (including host) | Country |
GenBank accession number2 |
||||
|---|---|---|---|---|---|---|---|---|---|
| ITS | LSU | tef1 | tub2 | rpb2 | |||||
| Apiognomonioides supraseptata | CBS 632.92Ex-type of Apiognomonia supraseptata = ATCC 58737 = TMI 70024 | S. Kaneko | Quercus glauca | Japan | MG976447 | MG976448 | – | – | – |
| Involutiscutellula rubra | CBS 192.71Ex-isotype of Actinopelte rubra = A684 = IFO 9271 = MUCC2302 = NBRC 9271 | T. Yokoyama, 7 Jun. 1969 | Quercus phillyraeoides, dead leaf | Japan | MG591899 | MG591993 | MG592086 | MG592180 | MG976476 |
| MUCC2303 = NBRC 9272 | T. Yokoyama, 7 Jun. 1969 | Quercus phillyraeoides, dead leaf | Japan | MG591900 | MG591994 | MG592087 | MG592181 | MG976477 | |
| MUCC2304Ex-type of Tubakia rubra = NBRC 9273 = ATCC 22473 = IMI 157597 | T. Yokoyama, 28 Oct. 1969 | Quercus phillyraeoides, living leaf | Japan | MG591901 | MG591995 | MG592088 | – | MG976478 | |
| MUCC2305 = NBRC 9274 | T. Yokoyama, 27 Jan. 1970 | Quercus phillyraeoides | Japan | MG591902 | MG591996 | MG592089 | MG592182 | MG976479 | |
| MUCC2306 = NBRC 9275 | T. Yokoyama, 27 Jan. 1970 | Quercus phillyraeoides | Japan | MG591903 | MG591997 | MG592090 | – | MG976480 | |
| MUCC2307 = NBRC 9276 | T. Yokoyama, 14 Apr. 1970 | Quercus phillyraeoides | Japan | MG591904 | MG591998 | MG592091 | MG592183 | MG976481 | |
| MUCC2308 = NBRC 9277 | T. Yokoyama, 29 May 1970 | Quercus phillyraeoides | Japan | MG591905 | MG591999 | MG592092 | MG592184 | MG976482 | |
| MUCC2309 = NBRC 9371 | T. Yokoyama, 2 Aug. 1970 | Quercus phillyraeoides | Japan | MG591906 | MG592000 | MG592093 | MG592185 | MG976483 | |
| Melanconis groenlandica | CBS 116540Ex-type of Myrothecium groenlandicum = UPSC 3407 | M. Bohn, Jul. 1991 | Betula nana, twigs | Greenland | KU878552 | KU878553 | KU878554 | KU878555 | – |
| Oblongisporothyrium castanopsidis | CBS 124732 = MUCC2288 = NBRC 9262 | T. Yokoyama, 26 Mar. 1970 | Castanopsis cuspidata | Japan | MG591849 | MG591942 | MG592037 | MG592131 | MG976453 |
| CBS 189.71Ex-type of Actinopelte castanopsidis = A686 = ATCC 22470 = IFO 9263 = IMI 157598 = MUCC2289 = NBRC 9263 | T. Yokoyama, 27 Jan. 1970 | Castanopsis cuspidata, dead leaf | Japan | MG591850 | MG591943 | MG592038 | MG592132 | MG976454 | |
| Paratubakia subglobosa | CBS 124733 = MUCC2311 = NBRC 9344 | T. Yokoyama, 27 Feb. 1970 | Quercus glauca | Japan | MG591913 | MG592008 | MG592102 | MG592194 | MG976489 |
| CBS 193.71Ex-type of Actinopelte subglobosa = A685 = ATCC 22474 = IFO 8931 = IMI 157596 = MUCC2310 = NBRC 8931 | T. Yokoyama, 30 Jan. 1968 | Quercus glauca, dead leaf | Japan | MG591914 | MG592009 | MG592103 | MG592195 | MG976490 | |
| Paratubakia subglobosoides | MUCC2293Ex-type of Paratubakia subglobosoides = NBRC 9343 | T. Yokoyama, 27 Feb. 1970 | Quercus glauca | Japan | MG591915 | MG592010 | MG592104 | MG592196 | MG976491 |
| Racheliella saprophytica | MFLUCC 12–0298Ex-type of Greeneria saprophytica = NTCL052-1 | N. Tangthirasunun, 21 Mar. 2012 | Syzygium cumini, dead leaves | Thailand | KJ021933 | KJ021935 | – | – | – |
| Racheliella wingfieldiana | CBS 143669Ex-type of Racheliella wingfieldiana = CPC 13806 | P.W. Crous, 6 Mar. 2007 | Syzigium guineense | South Africa | MG591911 | MG592006 | MG592100 | MG592192 | MG976487 |
| Saprothyrium thailandense | MFLUCC 12–0303Ex-type of Tubakia thailandensis | K. Wisitrassameewong, 2 May 2012 | Decaying leaf | Thailand | MF190163 | MF190110 | – | – | – |
| Sphaerosporithyrium mexicanum | CPC 31361 | O. Moreno-Rico, Apr. 2016 | Quercus eduardi | Mexico | MG591894 | MG591988 | MG592081 | MG592175 | – |
| CPC 32258 = CFNL 2941 = id 11 | J. G. Marmolejo, 9 Nov. 2012 | Quercus eduardi | Mexico | MG591895 | MG591989 | MG592082 | MG592176 | – | |
| CPC 33021Ex-type of Tubakia mexicana = CFNL 2945 | O. Moreno-Rico, 19 Jan. 2017 | Quercus eduardi | Mexico | MG591896 | MG591990 | MG592083 | MG592177 | MG976473 | |
| Tubakia americana | A1105 | M. Guthmiller, 23 Sep. 2011 | Quercus macrocarpa, leaf | USA: Wisconsin | TCH | – | TCH | – | – |
| A1190 | – | Quercus sp. | USA: Missouri | TCH | – | – | – | – | |
| A1201 | D. McNew, 5 Aug. 2012 | Quercus bicolor, leaf | USA: Missouri | – | – | TCH | – | – | |
| A693 | D. McNew, 19 May 2009 | Quercus macrocarpa, acorn cup | USA: Iowa | TCH | – | TCH | – | – | |
| A791 | – | Quercus robur | USA: Iowa | TCH | – | TCH | – | – | |
| A822 | – | Quercus macrocarpa, acorn | USA: Iowa | TCH | – | – | – | – | |
| A829 | – | Quercus macrocarpa | USA: Iowa | TCH | – | – | – | – | |
| A844 | – | Quercus bicolor | USA: Iowa | TCH | – | – | – | – | |
| A845 | – | Quercus bicolor | USA: Iowa | TCH | – | TCH | – | – | |
| CBS 129014 = A749 = BH3-L2 | D. McNew, 10 Jun. 2009 | Quercus macrocarpa, leaf spot | USA: Iowa | MG591873 | MG591966 | MG592058 | MG592152 | MG976449 | |
| CBS 137350 = A1007 | D. McNew, 6 Apr. 2011 | Quercus rubra, twig | USA: Iowa | TCH | – | TCH | – | – | |
| CBS 137353 = A1158 | D. McNew, 12 Oct. 2011 | Quercus macrocarpa, leaf spot | USA: Iowa | MG605064 | – | MG603571 | – | – | |
| no culture (NY E&E FC286 ex McClatchieLectotype of Tubakia americana) | J.B. Ellis & B.M. Everhart, Aug. 1883 | Quercus coccinea, leaf spot | USA: New Jersey | MG605063 | – | – | – | – | |
| Tubakia californica | CPC 31496 = CDFA#993 | S. Rooney-Latham, 3 Apr. 2012 | Quercus agrifolia | USA: California | MG591829 | MG591922 | MG592017 | MG592111 | MG976450 |
| CPC 31497 = CDFA#1007 | S. Rooney-Latham, 27 Apr. 2012 | Quercus agrifolia | USA: California | MG591830 | MG591923 | MG592018 | MG592112 | – | |
| CPC 31498 = CDFA#1029 | S. Rooney-Latham, 23 May 2012 | Quercus agrifolia | USA: California | MG591831 | MG591924 | MG592019 | MG592113 | – | |
| CPC 31499 = CDFA#1076 | S. Rooney-Latham, 18 Jul. 2012 | Quercus wislizeni | USA: California | MG591832 | MG591925 | MG592020 | MG592114 | – | |
| CPC 31502 = CDFA#1103 | S. Rooney-Latham, 9 Aug. 2012 | Quercus wislizeni | USA: California | MG591833 | MG591926 | MG592021 | MG592115 | – | |
| CPC 31504 = CDFA#1105 | S. Rooney-Latham, 9 Aug. 2012 | Quercus kelloggii | USA: California | MG591834 | MG591927 | MG592022 | MG592116 | – | |
| CBS 143670Ex-type of Tubakia californica = CPC 31505 = CDFA#1428 | S. Rooney-Latham, 19 Sep. 2014 | Quercus kelloggii | USA: California | MG591835 | MG591928 | MG592023 | MG592117 | MG976451 | |
| CPC 31506 = CDFA#1430 | S. Rooney-Latham, 19 Sep. 2014 | Quercus kelloggii | USA: California | MG591836 | MG591929 | MG592024 | MG592118 | – | |
| CPC 31507 = CDFA#1431 | S. Rooney-Latham, 19 Sep. 2014 | Quercus kelloggii | USA: California | MG591837 | MG591930 | MG592025 | MG592119 | – | |
| CPC 31508 = CDFA#1434 | S. Rooney-Latham, 19 Sep. 2014 | Quercus kelloggii | USA: California | MG591838 | MG591931 | MG592026 | MG592120 | – | |
| CPC 31509 = CDFA#1407 | S. Rooney-Latham, 28 Sep. 2014 | Quercus kelloggii | USA: California | MG591839 | MG591932 | MG592027 | MG592121 | – | |
| CPC 31510 = CDFA#1408 | S. Rooney-Latham, 28 Sep. 2014 | Quercus kelloggii | USA: California | MG591840 | MG591933 | MG592028 | MG592122 | – | |
| CPC 31512 = CDFA#1448 | S. Rooney-Latham, 12 Oct. 2014 | Quercus wislizeni | USA: California | MG591841 | MG591934 | MG592029 | MG592123 | – | |
| CPC 31513 = CDFA#1455 | S. Rooney-Latham, 12 Oct. 2014 | Quercus kelloggii | USA: California | MG591842 | MG591935 | MG592030 | MG592124 | – | |
| CPC 31514 = CDFA#1477 | S. Rooney-Latham, 30 Oct. 2014 | Lithocarpus densiflorus | USA: California | MG591843 | MG591936 | MG592031 | MG592125 | – | |
| CPC 31515 = CDFA#1542 | S. Rooney-Latham, 26 Feb. 2015 | Lithocarpus densiflorus | USA: California | MG591844 | MG591937 | MG592032 | MG592126 | – | |
| CPC 31516 = CDFA#1800 | S. Rooney-Latham, 10 Dec. 2015 | Quercus agrifolia | USA: California | MG591845 | MG591938 | MG592033 | MG592127 | – | |
| CPC 31517 = CDFA#1782 | S. Rooney-Latham, 12 Jan. 2016 | Chrysolepis chrysophylla | USA: California | MG591846 | MG591939 | MG592034 | MG592128 | – | |
| CPC 32250 = CFNL 2934 = id 02 | J. G. Marmolejo, 6 Oct. 2012 | Quercus canbyi | Mexico | MG591847 | MG591940 | MG592035 | MG592129 | MG976452 | |
| CPC 32251 = CFNL 2935 = id 03 | J. G. Marmolejo, 6 Oct. 2012 | Quercus canbyi | Mexico | MG591848 | MG591941 | MG592036 | MG592130 | – | |
| Tubakia dryina | A658 | – | Quercus alba | USA: Iowa | TCH | TCH | – | – | – |
| A789 | – | Quercus alba | USA: Iowa | TCH | TCH | – | – | – | |
| A841 | – | Quercus robur | Germany | TCH | – | – | – | – | |
| CBS 112097Ex-epitype of Tubakia dryina = A680 | S. Mutto-Accardi, – | Quercus robur | Italy | MG591851 | MG591944 | MG592039 | MG592133 | MG976455 | |
| CBS 114386 = A681 | R. Afford, 11 Nov. 2003 | Quercus robur, leaves with lesions | New Zealand | MG591852 | MG591945 | MG592040 | MG592134 | – | |
| CBS 114912 | – | Quercus sp., green leaf | Netherlands | MG591853 | MG591946 | MG592041 | MG592135 | MG976456 | |
| CBS 114915 | – | Quercus sp., green leaf | Netherlands | MG591854 | MG591947 | MG592042 | MG592136 | – | |
| CBS 114919 | – | Quercus sp., green leaf | Netherlands | MG591855 | MG591948 | MG592043 | MG592137 | – | |
| CBS 114920 | – | Quercus sp., green leaf | Netherlands | MG591856 | MG591949 | MG592044 | MG592138 | – | |
| CBS 115007 | – | Quercus robur, green leaf | Netherlands | MG591857 | MG591950 | MG592045 | MG592139 | – | |
| CBS 115009 | – | Quercus robur, green leaf | Netherlands | MG591858 | MG591951 | MG592046 | MG592140 | – | |
| CBS 115012 | – | Quercus robur, green leaf | Netherlands | MG591859 | MG591952 | MG592047 | MG592141 | – | |
| CBS 115014 | – | Quercus robur, green leaf | Netherlands | MG591860 | MG591953 | MG592048 | MG592142 | – | |
| CBS 115096 | – | Quercus robur, green leaf | Netherlands | MG591861 | MG591954 | MG592049 | MG592143 | – | |
| CBS 115097 | – | Quercus robur, green leaf | Netherlands | MG591862 | MG591955 | – | – | – | |
| CBS 115098 | – | Quercus sp., dead leaf | Netherlands | MG591863 | MG591956 | – | – | – | |
| CBS 115100 | – | Quercus sp., dead leaf | Netherlands | MG591864 | MG591957 | MG592050 | MG592144 | – | |
| CBS 115102 | – | Quercus robur, green leaf | Netherlands | MG591865 | MG591958 | MG592051 | MG592145 | – | |
| CBS 115306 | – | Quercus robur, dead leaf | Netherlands | MG591866 | MG591959 | MG592052 | MG592146 | – | |
| CBS 115308 | – | Quercus robur, dead leaf | Netherlands | MG591867 | MG591960 | MG592053 | MG592147 | – | |
| CBS 115971 | – | – | Netherlands | MG591868 | MG591961 | MG592054 | MG592148 | – | |
| CBS 116070 | – | – | Netherlands | MG591869 | MG591962 | MG592055 | MG592149 | – | |
| CBS 129016 = A876 = WO 60 | D. McNew, 23 Mar. 2010 | Quercus alba, twig | USA: Iowa | MG591870 | MG591963 | MG592056 | MG592150 | MG976457 | |
| CBS 129018 = A996 = WO 165 | D. McNew, 2 Aug. 2010 | Quercus macrocarpa, leaf spots | USA: Iowa | MG591871 | MG591964 | MG592057 | MG592151 | – | |
| CBS 213.66 = IFO 9101 | H.A. van der Aa, 26 Jun. 1966 | Quercus robur, leaf spot in young leaf | Netherlands | MG591872 | MG591965 | – | – | – | |
| EMS9 | – | Pinus tabulaeformis, endophyte | China | AY546028 | – | – | – | – | |
| mh1548.5 | – | Liquidambar styraciflua, seedling upper stem | USA: North Carolina | GQ996086 | – | – | – | – | |
| Tubakia dryinoides | 1-52 | X.H. Wu, – | Lindera glauca, endophyte | China | JF502454 | – | – | – | – |
| 2007LN001 | – | Quercus sp. | China | FJ598616 | – | – | – | – | |
| CBS 115970 | – | – | Netherlands | AY853242 | – | – | – | – | |
| CBS 329.75 = 847 B | M. Morelet, 14 May 1974 | Quercus sp., fruits | France | MG591874 | MG591967 | MG592059 | MG592153 | MG976458 | |
| CBS 335.86 = A682 | H.A. van der Aa, – | Cynips kollari, gall on Quercus pedunculata | France | MG591875 | – | MG592060 | MG592154 | – | |
| MUCC2290 = NBRC 9265 = ATCC 22471 = CBS 190.71 | T. Yokoyama, 11 Nov. 1968 | Castanea crenata, leaf | Japan | MG591876 | MG591968 | MG592061 | MG592155 | MG976459 | |
| MUCC2291 = NBRC 9266 | T. Yokoyama, 8 Oct. 1969 | Castanea crenata | Japan | MG591877 | MG591969 | MG592062 | MG592156 | MG976460 | |
| MUCC2292Ex-type of Tubakia dryinoides = NBRC 9267 | T. Yokoyama, 8 Oct. 1969 | Quercus phillyraeoides | Japan | MG591878 | MG591970 | MG592063 | MG592157 | MG976461 | |
| O7-1141 | – | Endophyte | China | FJ025349 | – | – | – | – | |
| Tubakia hallii | A663 | – | Quercus stellata | USA: Missouri | TCH | TCH | – | – | – |
| A664 | – | Quercus stellata | USA: Missouri | TCH | TCH | – | – | – | |
| A906 | – | Quercus macrocarpa | USA: Iowa | TCH | – | TCH | – | – | |
| A940 | – | Quercus macrocarpa | USA: Missouri | TCH | – | TCH | – | – | |
| A969 | – | Quercus stellata | USA: Missouri | TCH | – | TCH | – | – | |
| A1000 | – | Quercus stellata | USA: Kansas | TCH | – | TCH | – | – | |
| A1102 | M. Guthmiller, 23 Sep. 2011 | Quercus macrocarpa | USA: Wisconsin | TCH | – | TCH | – | – | |
| A1188 | – | Quercus macrocarpa | USA: Minnesota | TCH | – | – | – | – | |
| A1197 | – | Quercus macrocarpa | USA: Wisconsin | TCH | – | – | – | – | |
| CBS 129013Ex-type of Tubakia hallii = A666 = MDC 144-FB1 | S. Kim, 2 Sep. 2009 | Quercus stellata, leaf spot | USA: Missouri | MG591880 | MG591972 | MG592065 | MG592159 | MG976462 | |
| CBS 129015 = A762 = W014 | –, 27 Aug. 2009 | Quercus stellata, leaf spot and vein | USA: Arkansas | MG591881 | MG591973 | MG592066 | MG592160 | – | |
| CBS 137348 = A949 | D. McNew, 25 Aug. 2010 | Quercus macrocarpa | USA: Iowa | TCH | – | – | – | – | |
| CPC 23753 | – | Quercus sp., dead leaf | Iran | MG591884 | MG591976 | MG592069 | MG592163 | MG976463 | |
| Tubakia iowensis | A569 | – | Quercus macrocarpa | USA: Iowa | TCH | TCH | – | – | – |
| A573 | – | Quercus macrocarpa, petiole | USA: Iowa | JF704196 | JF704185 | – | – | – | |
| A668 | – | Quercus macrocarpa, endophyte | USA: Iowa | TCH | TCH | – | – | – | |
| A947 | – | Quercus macrocarpa | USA: Nebraska | TCH | – | – | – | – | |
| A999 | – | Quercus macrocarpa | USA: Kansas | – | – | TCH | – | – | |
| A1086 | – | Quercus macrocarpa | USA: Minnesota | – | – | TCH | – | – | |
| A1088 | – | Quercus macrocarpa | USA: Minnesota | TCH | – | TCH | – | – | |
| A1089 | – | Quercus macrocarpa | USA: Wisconsin | TCH | – | TCH | – | – | |
| A1104 | – | Quercus macrocarpa | USA: Wisconsin | TCH | – | TCH | – | – | |
| A1200 | – | Quercus bicolor | USA: Iowa | TCH | – | – | – | – | |
| A1217 | – | Quercus macrocarpa | USA: South Dakota | TCH | – | – | – | – | |
| A1219 | – | Quercus macrocarpa | USA: Minnesota | TCH | – | – | – | – | |
| CBS 129012Ex-type of Tubakia iowensis = A607 = BH2-L(2) | T. Harrington, 21 Aug. 2008 | Quercus macrocarpa, hanging leaf tissue | USA: Iowa | MG591879 | MG591971 | MG592064 | MG592158 | – | |
| CBS 129017 = A995 = WO164 | K. Scanlon, Oct. 2010 | Quercus macrocarpa, leaves | USA: Wisconsin | MG591882 | MG591974 | MG592067 | MG592161 | – | |
| CBS 129019 = A1003 = WO169 = BH2 | D. McNew, 16 Nov. 2010 | Quercus macrocarpa, petioles | USA: Iowa | MG591883 | MG591975 | MG592068 | MG592162 | – | |
| Tubakia japonica | CBS 191.71 = A872 = IFO 9340 = MUCC2299 = NBRC 9340 | T. Yokoyama, 2 Sep. 1970 | Castanea crenata, leaf | Japan | MG591885 | MG591977 | MG592070 | MG592164 | MG976464 |
| MUCC2296Ex-epitype of Tubakia japonica = NBRC 9268 = ATCC 22472 | K. Uchida, 13 Aug. 1969 | Castanea crenata | Japan | MG591886 | MG591978 | MG592071 | MG592165 | MG976465 | |
| MUCC2297 = NBRC 9269 | Y. Kobayashi, 17 Sep. 1969 | Castanea crenata | Japan | – | MG591979 | MG592072 | MG592166 | MG976466 | |
| MUCC2298 = NBRC 9270 | Y. Kobayashi, 17 Sep. 1969 | Castanea crenata | Japan | – | MG591980 | MG592073 | MG592167 | MG976467 | |
| MUCC2300 = NBRC 9341 | T. Yokoyama, 2 Sep. 1970 | Castanea crenata | Japan | MG591887 | MG591981 | MG592074 | MG592168 | MG976468 | |
| MUCC2301 = NBRC 9342 | T. Yokoyama, 30 Oct. 1970 | Castanea crenata | Japan | MG591888 | MG591982 | MG592075 | MG592169 | MG976469 | |
| Tubakia liquidambaris | CBS 139744 = A771 | D. McNew, 27 Aug. 2009 | Liquidambar styraciflua, leaf spot | USA: Arkansas | MG605068 | MG605077 | MG603578 | – | – |
| CBS 139745 = A830 | D. McNew, 27 Aug. 2009 | Liquidambar styraciflua | USA: Arkansas | TCH | – | TCH | – | – | |
| Tubakia macnabbii | A1001 | – | Quercus sp. | USA: Kansas | TCH | – | TCH | – | – |
| A1039 | – | Quercus sp. | – | TCH | – | TCH | – | – | |
| A1177 | S. Latham, 27 Apr. 2012 | Quercus agrifolia, twig | USA: California | TCH | – | – | – | – | |
| A1192 | – | Quercus rubra | USA: New Hampshire | TCH | – | TCH | – | – | |
| A1193 | – | Quercus rubra | USA: New Hampshire | TCH | – | – | – | – | |
| A608 | – | Quercus rubra | USA: Iowa | TCH | TCH | – | – | – | |
| A655 | – | Quercus macrocarpa | USA: Iowa | TCH | TCH | – | – | – | |
| A766 | – | Castanea dentata | USA: Iowa | TCH | TCH | – | – | – | |
| A805 | – | Quercus velutina | USA: Arkansas | TCH | – | TCH | – | – | |
| A806 | – | Quercus marilandica | USA: Arkansas | TCH | – | TCH | – | – | |
| A809 | – | Quercus sp. | USA: Arkansas | TCH | – | TCH | – | – | |
| A853 | – | Quercus nigra | USA: Florida | TCH | – | TCH | – | – | |
| A859 | – | Quercus imbricaria | USA: Missouri | TCH | – | TCH | – | – | |
| A929 | D. McNew, 28 Jul. 2010 | Quercus rubra, leaf | USA: Illinois | TCH | – | TCH | – | – | |
| A954 | T. Harrington, 27 Aug. 2010 | Quercus macrocarpa, leaf | USA: Iowa | TCH | – | TCH | – | – | |
| CBS 137346 = A810 | D. McNew, 27 Aug. 2009 | Quercus muehlenbergii, leaf vein | USA: Arkansas | MG605071 | – | TCH | – | – | |
| CBS 137347 = A852 | T. Schubert, Nov. 2009 | Castanea dentata x mollissima (Dunstan), leaf spot | USA: Florida | MG605070 | – | TCH | – | – | |
| CBS 137349 = A989Ex-type of Tubakia macnabbii | D. Brandt, Sep. 2010 | Quercus palustris, leaf spot | USA: Missouri | MG605069 | – | MG603579 | – | – | |
| Tubakia melnikiana | CPC 32249 = CFNL 2933 = id 01 | J. G. Marmolejo, 6 Oct. 2012 | Quercus canbyi | Mexico | MG591889 | MG591983 | MG592076 | MG592170 | MG976470 |
| CPC 32252 = CFNL 2936 = id 04 | J. G. Marmolejo, 29 Oct. 2012 | Quercus canbyi | Mexico | MG591890 | MG591984 | MG592077 | MG592171 | MG976471 | |
| CPC 32253 = id 05 | J. G. Marmolejo, 29 Oct. 2012 | Quercus canbyi | Mexico | MG591891 | MG591985 | MG592078 | MG592172 | – | |
| CPC 32254 = CFNL 2938 = id 06 | J. G. Marmolejo, 29 Oct. 2012 | Quercus laeta (= Q. prinopsis) | Mexico | MG591892 | MG591986 | MG592079 | MG592173 | – | |
| CPC 32255Ex-type of Tubakia melnikiana = CFNL 2939 = id 07 | J. G. Marmolejo, 29 Oct. 2012 | Quercus canbyi | Mexico | MG591893 | MG591987 | MG592080 | MG592174 | MG976472 | |
| Tubakia oblongispora | MUCC2295Ex-type of Tubakia oblongispora = NBRC 9885 | T. Yokoyama, 11 Jun. 1972 | Quercus serrata | Japan | MG591897 | MG591991 | MG592084 | MG592178 | MG976474 |
| Tubakia paradryinoides | MUCC2294Ex-type of Tubakia paradryinoides = NBRC 9884 | T. Kobayashi & K. Sasaki, 12 Oct. 1972 | Quercus acutissima | Japan | MG591898 | MG591992 | MG592085 | MG592179 | MG976475 |
| Tubakia seoraksanensis | CBS 127490Ex-type of Tubakia seoraksanensis = SA3V = A785 | Hye Young Yun, 31 Aug. 2009 | Quercus mongolica, living leaves | South Korea | MG591907 | KP260499 | MG592094 | MG592186 | – |
| CBS 127491Ex-isotype of Tubakia seoraksanensis = SA3S = A786 | Hye Young Yun, 31 Aug. 2009 | Quercus mongolica, living leaves | South Korea | HM991735 | KP260500 | MG592095 | MG592187 | MG976484 | |
| CBS 127492Ex-isotype of Tubakia seoraksanensis = SA4S = A787 | Hye Young Yun, 31 Aug. 2009 | Quercus mongolica, living leaves | South Korea | MG591908 | MG592001 | MG592096 | MG592188 | MG976485 | |
| CBS 127493Ex-isotype of Tubakia seoraksanensis = SA4V = A788 | Hye Young Yun, 31 Aug. 2009 | Quercus mongolica, living leaves | South Korea | HM991737 | MG592002 | – | – | – | |
| CPC 26552 | Ying Zhang, 23 Jul. 2014 | Quercus mongolica | China | MG591909 | MG592003 | MG592097 | MG592189 | – | |
| CPC 26553 | Ying Zhang, 23 Jul. 2014 | Quercus mongolica | China | – | MG592004 | MG592098 | MG592190 | – | |
| Tubakia sierrafriensis | CPC 33020Ex-type of Tubakia sierrafriensis = CFNL 2944 | O. Moreno-Rico, 19 Jan. 2017 | Quercus eduardi | Mexico | MG591910 | MG592005 | MG592099 | MG592191 | MG976486 |
| Tubakia sp. 1 | CBS 115011 | – | Quercus robur, green leaf | Netherlands | MG591912 | MG592007 | MG592101 | MG592193 | MG976488 |
| Tubakia sp. E | A901 | – | Quercus sp. | USA: Iowa | TCH | – | – | – | – |
| NY7452a | K.S. Jefferson-George, – | Quercus rubra | USA: New York | HM855225 | – | – | – | – | |
| NY7452b | K.S. Jefferson-George, – | Quercus rubra | USA: New York | HM855226 | – | – | – | – | |
| Tubakia suttoniana | CBS 114911 | – | Quercus sp., green leaf | Netherlands | MG591916 | MG592011 | MG592105 | MG592197 | – |
| CBS 115006 | – | Quercus robur, green leaf | Netherlands | MG591917 | MG592012 | MG592106 | MG592198 | – | |
| CBS 115300 | – | Quercus robur, green leaf | Netherlands | MG591918 | MG592013 | MG592107 | MG592199 | – | |
| CBS 229.77 | H.J. Boesewinkel, Nov. 1975 | Quercus cerris, branch and trunk canker | New Zealand | MG591919 | MG592014 | MG592108 | MG592200 | MG976492 | |
| CBS 387.89 | Centro di Sperimentazione Agr. Forest., Roma, Italia, – | Quercus rubra, dead leaf | Italy | MG591920 | MG592015 | MG592109 | MG592201 | – | |
| CBS 639.93Ex-isotype of Dicarpella dryina | A. Belisario, Feb. 1989 | Quercus rubra, overwintered fallen leaves | Italy | MG591921 | MG592016 | MG592110 | MG592202 | MG976493 | |
| Tubakia tiffanyae | A1035 | – | Quercus rubra | USA: Iowa | TCH | – | TCH | – | – |
| A1108 | – | Quercus rubra | USA: Iowa | TCH | – | TCH | – | – | |
| A627 | – | Quercus rubra | USA: Iowa | TCH | TCH | – | – | – | |
| A792 | – | Quercus imbricaria | USA: Iowa | TCH | – | TCH | – | – | |
| A800 | – | Quercus rubra | USA: Iowa | TCH | TCH | TCH | – | – | |
| A802 | – | Quercus rubra | USA: Iowa | TCH | – | TCH | – | – | |
| A808 | – | Quercus rubra | USA: Iowa | TCH | – | TCH | – | – | |
| CBS 137345Ex-type of Tubakia tiffanyae = A803 | T. Harrington, 5 Sep. 2009 | Quercus rubra, leaf spot | USA: Iowa | MG605081 | – | MG603581 | – | – | |
| CBS 137351 = A1042 | –, 29 Jul. 2011 | Quercus rubra, leaf spot | USA: Iowa | MG605082 | – | TCH | – | – | |
| CBS 137352 = A1052 | D. McNew, 27 Jul. 2011 | Quercus imbricaria, leaf | USA: Iowa | TCH | – | TCH | – | – | |
1ATCC: American Type Culture Collection, Virginia, USA; CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands; CDFA: CDFA Plant Pathogen Collections, 3294 Meadowview Road, Sacramento, CA 95832, USA; CFNL: Herbarium and culture collection at the Faculty of Forestry Sciences, University of Nuevo León, México; CPC: Culture collection of Pedro Crous, housed at CBS; IFO: Institute for Fermentation, Osaka, Yodogawa-ku, Osaka, Japan (collection transferred to NBRC); IMI: International Mycological Institute, CABI-Bioscience, Egham, Bakeham Lane, United Kingdom; MFLUCC: Mae Fah Luang University Culture Collection, Chiang Rai, Thailand; MUCC (Japan): Culture Collection, Laboratory of Plant Pathology, Mie University, Tsu, Mie Prefecture, Japan; NBRC: NITE Biological Resource Center, Department of Biotechnology, National Institute of Technology and Evaluation, Kisarazu, Chiba, Japan, TMI: Tottori Mycological Institute, Japan Kinoko Research Center Foundation, Tottori, Japan.
2ITS: internal transcribed spacers and intervening 5.8S nrDNA; LSU: large subunit (28S) of the nrRNA gene operon; tef1: partial translation elongation factor 1-alpha gene; tub2: partial beta-tubulin gene; rpb2: partial DNA-directed RNA polymerase II second largest subunit gene. TCH refers to sequences obtained directly from Tom C. Harrington.
DNA extraction, amplification (PCR) and phylogeny
Fungal mycelium of strains (Table 1) was harvested with a sterile scalpel and the genomic DNA was isolated using the Wizard® Genomic DNA Purification Kit (Promega Corporation, WI, USA) following the manufacturers’ protocols. Four partial nuclear genes were subjected to PCR amplification and sequencing: 28S nrRNA gene (LSU), internal transcribed spacer regions and intervening 5.8S nrRNA gene (ITS) of the nrDNA operon, beta-tubulin (tub2) and translation elongation factor 1-alpha (tef1) using the primers listed in Table 2. The PCR amplifications were performed on a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA, USA). The PCR mixtures consisted of 1 μL genomic DNA, 1× NH4 reaction buffer (Bioline, Luckenwalde, Germany), 2–5 mM MgCl2 (ITS: 2.5 mM, LSU: 5 mM, rpb2 and tef1: 2 mM, tub2: 3 mM), 40–60 μM of dNTPs (ITS: 56 μM, LSU: 60 μM, rpb2, tef1 and tub2: 40 μM), 5–5.5 % dimethyl sulfoxide (DMSO; ITS, LSU, and tub2: 5 %, rpb2 and tef1: 5.5 %), 0.2 μM of each primer and 0.5 U Taq DNA polymerase (Bioline) in a total volume of 12.5 μL. The PCR cycling conditions for ITS, LSU, tef1 and tub2 were: initial denaturation (94 °C, 5 min); 35 cycles amplification (denaturation 94 °C for 30 s; annealing 48 °C for 50 s; extension 72 °C for 90 s), and final extension (72 °C, 7 min). The PCR cycling conditions for rpb2 were: initial denaturation (95 °C, 5 min); 5 cycles amplification (denaturation 94 °C for 45 s, annealing 60 °C for 45 s, extension 72 °C for 2 min); 30 cycles amplification (denaturation 94 °C for 45 s, annealing 54 °C for 45 s, extension 72 °C for 2 min), and final extension (72 °C, 10 min). The resulting fragments were sequenced in both directions using the respective PCR primers and the BigDye Terminator Cycle Sequencing Kit v. 3.1 (Applied Biosystems Life Technologies, Carlsbad, CA, USA). DNA sequencing amplicons were purified through Sephadex G-50 Superfine columns (Sigma-Aldrich, St. Louis, MO) in MultiScreen HV plates (Millipore, Billerica, MA). Purified sequence reactions were analysed on an Applied Biosystems 3730xl DNA Analyzer (Life Technologies, Carlsbad, CA, USA). The DNA sequences generated were analysed and consensus sequences were computed using SeqMan Pro v. 13 (DNASTAR, Madison, WI, USA). All novel sequences generated in this study were deposited in GenBank (Table 1).
Table 2.
List of primers used for amplification and sequencing in this study.
| Name1 | Sequence (5’ – 3’) | Orientation | Reference |
|---|---|---|---|
| ITS | |||
| ITS4 | TCC TCC GCT TAT TGA TAT GC | Reverse | White et al. (1990) |
| ITS5 | GGA AGT AAA AGT CGT AAC AAG G | Forward | White et al. (1990) |
| LSU | |||
| LR0R | GTA CCC GCT GAA CTT AAG C | Forward | Rehner & Samuels (1994) |
| LR5 | TCC TGA GGG AAA CTT CG | Reverse | Vilgalys & Hester (1990) |
| rpb2 | |||
| RPB2-5F2 | GGG GWG AYC AGA AGA AGG C | Forward | Sung et al. (2007) |
| RPB2-7cR | CCC ATR GCT TGY TTR CCC AT | Reverse | Liu et al. (1999) |
| tef1 | |||
| EF-2 | GGA RGT ACC AGT SAT CAT GTT | Reverse | O’Donnell et al. (1998) |
| EF1-728F | CAT CGA GAA GTT CGA GAA GG | Forward | Carbone & Kohn (1999) |
| tub2 | |||
| Bt2a | GGT AAC CAA ATC GGT GCT GCT TTC | Forward | Glass & Donaldson (1995) |
| Bt2b | ACC CTC AGT GTA GTG ACC CTT GGC | Reverse | Glass & Donaldson (1995) |
1ITS: internal transcribed spacers and intervening 5.8S nrDNA; LSU: large subunit (28S) of the nrRNA gene operon; rpb2: partial DNA-directed RNA polymerase II second largest subunit gene; tef1: partial translation elongation factor 1-alpha gene; tub2: partial beta-tubulin gene.
The generated sequences for each gene were subjected to megablast searches (Zhang et al. 2000) to identify closely related sequences in the NCBIs GenBank nucleotide database. For the LSU and rpb2 alignments, subsets of sequences from the alignments of Senanayake et al. (2017) and Fan et al. (2018) were used as backbones. For the tef1 alignment, tef1 sequences from Harrington & McNew (2018) were downloaded from GenBank or kindly supplied by the authors when not available in a public database. Loci were aligned with the online version of MAFFT v. 7 (Katoh & Standley 2013) after which the alignments were manually checked and improved where necessary using MEGA v. 7 (Kumar et al. 2016). Isolates missing both tef1 and tub2 sequences were excluded from the concatenated ITS/tef1/tub2 alignments.
The phylogenetic methods used in this study included Bayesian analyses performed with MrBayes v. 3.2.6 (Ronquist et al. 2012) and Maximum Parsimony analyses performed with PAUP v. 4.0b10 (Swofford 2003) as explained in Videira et al. (2017). For the Bayesian analyses, trees were sampled every 100 generations, the temperature parameter was set to 0.25 and the stop value to 0.01. The Bayesian analyses were applied to the separate overview LSU and rpb2 alignments as well as the concatenated ITS/tef1/tub2 alignments and the expanded tef1 alignment after MrModelTest v. 2.2 (Nylander 2004) was used to determine the best nucleotide substitution model settings for each data partition.
All resulting trees were printed with Geneious v. 11.0.3 (http://www.geneious.com, Kearse et al. 2012) and the layout of the trees was done in Adobe Illustrator v. CC 2017. The alignments and respective phylogenetic trees were deposited in TreeBASE, study number 21897.
Morphology
All fungal structures were examined by means of light microscopy, using an Olympus BX50 or Zeiss Axio imager A1 microscope. Shear’s liquid or distilled water and lactic acid were used as mounting media, and aniline blue (cotton blue) was used to stain colourless structures (columella, conidiogenous structures, and conidia). If possible, measurements of 30 conidia and other structures were made at a magnification of ×1000, and the 95 % confidence intervals were determined (extreme values in parentheses). Cultures were studied on MEA, OA, PDA, and PNA. Colony colours were rated using the charts of Rayner (1970).
RESULTS
Phylogeny
LSU phylogeny
The alignment contained 127 isolates representing a large majority of families known from sequence data belonging to Diaporthales. A strain of Phialemonium atrogriseum (CBS 981.70, GenBank HQ231984.1; Sordariales) was used as outgroup. The final alignment contained a total of 704 characters used for the phylogenetic analyses, including alignment gaps. MrModelTest recommended that the Bayesian analysis should use dirichlet base frequencies and the GTR+I+G model. The Bayesian analyses generated 153 402 trees from which 115 052 trees were sampled after 25 % of the trees were discarded as burn-in. The posterior probability values (PP) were calculated from the 115 052 trees (Fig. 1; first value: PP >0.74 shown). The alignment contained a total of 208 unique site patterns. The Maximum Parsimony (MP) analyses generated the maximum of 1 000 equally most parsimonious trees and the bootstrap support values (MP-BS) were mapped on the Bayesian tree as the second value (Fig. 1; MP-BS >74 % shown). From the analysed characters, 481 were constant, 63 were variable and parsimony-uninformative and 160 were parsimony-informative. A strict consensus tree was calculated from the equally most parsimonious trees and the branches were mapped with a thicker line on the Bayesian tree (Fig. 1; Length = 790, CI = 0.376, RI = 0.841, RC = 0.316). The parsimony phylogeny showed the same terminal family clades as those presented in the Bayesian phylogeny (Fig. 1); however, the order of the clades was different, for example, in the parsimony analysis Apoharknessiaceae was the sister clade to Tubakia and not Melanconiellaceae (data not shown, available in TreeBASE).
Fig. 1.
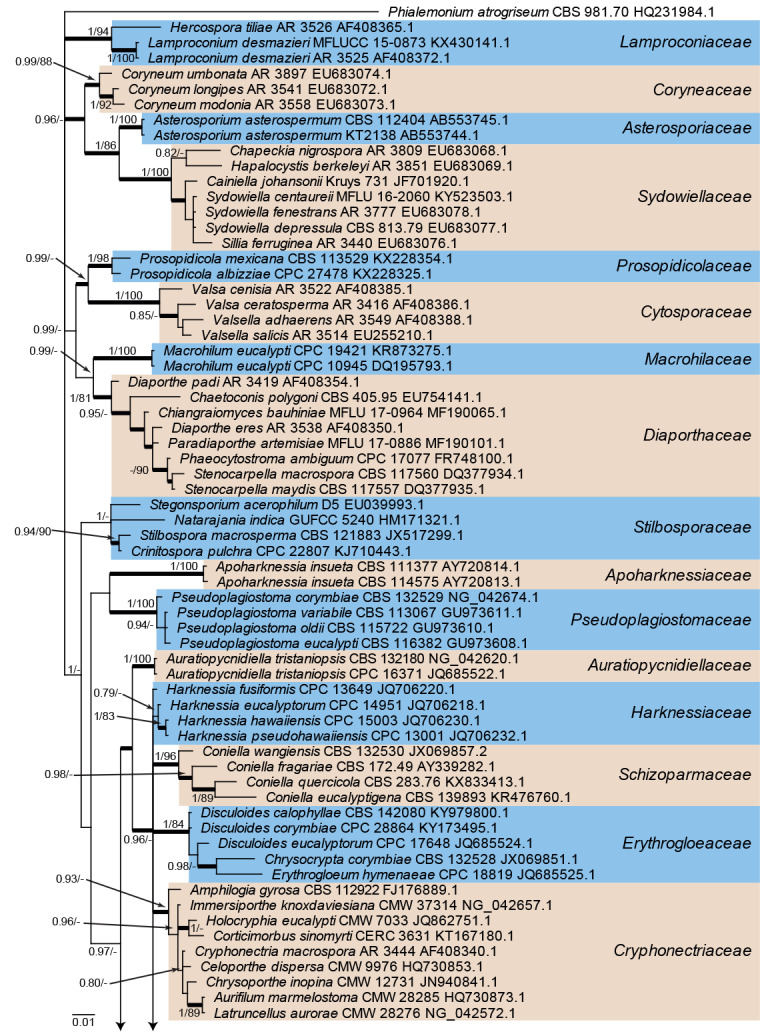
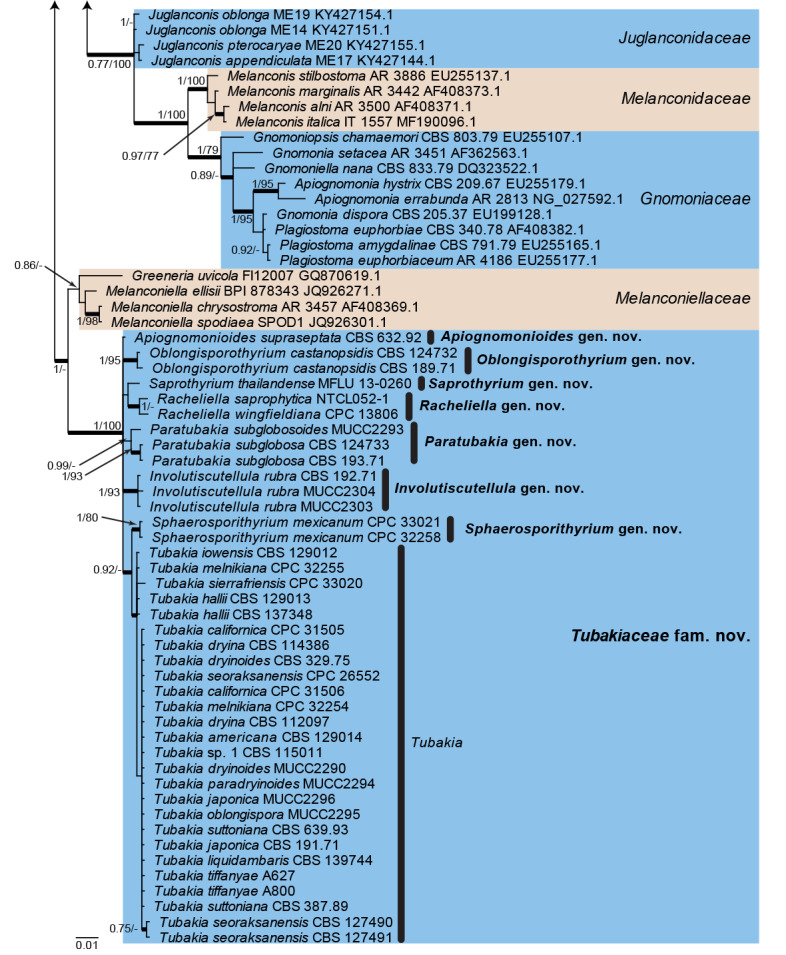
Consensus phylogram (50 % majority rule) from a Bayesian analysis of the LSU sequence alignment. Bayesian posterior probabilities (PP) >0.74 and maximum parsimony bootstrap support values (MP-BS) >74 % are shown at the nodes (PP/MP-BS) and thickened lines represent those branches present in the strict consensus maximum parsimony tree. The scale bar represents the expected changes per site. Families are indicated with coloured blocks to the right of the tree and the genera in Tubakiaceae are highlighted on the tree. Culture/specimen and GenBank accession numbers are indicated behind the species names. The tree is rooted to Phialemonium atrogriseum (GenBank HQ231984.1) and the novel family and genera are indicated in bold face.
Twenty-one families are represented in the phylogenetic tree (Fig. 1). The majority of families were supported by high posterior probability or bootstrap support values. However, Stilbosporaceae was only fully supported by the Bayesian analysis whilst Harknessiaceae did not receive significant support from either the Bayesian or the maximum parsimony analysis. Cryphonectriaceae and Melanconiellaceae each received moderate Bayesian posterior probability support but no maximum parsimony bootstrap support. The clade containing the Tubakia sequences was fully supported in both the Bayesian and maximum parsimony analyses and a novel family is proposed for this genus below. The node connecting Melanconiellaceae and Tubakiaceae was only fully supported in the Bayesian analysis but not supported in the maximum parsimony analysis. A sequence of the ex-type culture of “Greeneria” saprophytica is also included in this clade; this species was described by Tangthirasunun et al. (2014) but no Tubakia sequences were included in their phylogenetic tree. Our phylogenetic tree shows that this species has to be excluded from Greeneria and is better accommodated in Tubakiaceae. Supported by the isolated position in the LSU tree within the Tubakiaceae but outside of Tubakia s. str. and distant from all other genera of the family, this species warrants a genus of its own. Phylogenetic signals of LSU are low within Diaporthales in general and its families. Therefore, phylogenetic analyses using rpb2 sequences were also performed to distinguish whether the family Tubakiaceae contains a single genus, Tubakia, or several genera, but even the overall topology of the LSU tree suggests the heterogeneity of Tubakia s. lat.
rpb2 phylogeny
The alignment contained 130 isolates representing a large majority of families known from sequence data belonging to Diaporthales. A strain of Sordaria fimicola (CBS 723.96, GenBank DQ368647.1; Sordariales) was used as outgroup. The final alignment contained a total of 785 characters used for the phylogenetic analyses, including alignment gaps. MrModelTest recommended that the Bayesian analysis should use dirichlet base frequencies and the GTR+I+G model. The Bayesian analyses generated 6 742 trees from which 5 058 trees were sampled after 25 % of the trees were discarded as burn-in. The posterior probability values (PP) were calculated from the 5 058 trees (Fig. 2; first value: PP >0.74 shown). The alignment contained a total of 473 unique site patterns. The Maximum Parsimony (MP) analyses generated the maximum of 20 equally most parsimonious trees and the bootstrap support values (MP-BS) were mapped on the Bayesian tree as the second value (Fig. 2; MP-BS >74 % shown). From the analysed characters, 313 were constant, 28 were variable and parsimony-uninformative and 444 were parsimony-informative. A strict consensus tree was calculated from the equally most parsimonious trees and the branches were mapped with a thicker line on the Bayesian tree (Fig. 2; Length = 3 934, CI = 0.237, RI = 0.792, RC = 0.188). The parsimony phylogeny showed the same overall topology as that presented in the Bayesian phylogeny (Fig. 2); however, the order of the genera in Gnomoniaceae differed slightly between the analyses (data not shown, available in TreeBASE).
Fig. 2.
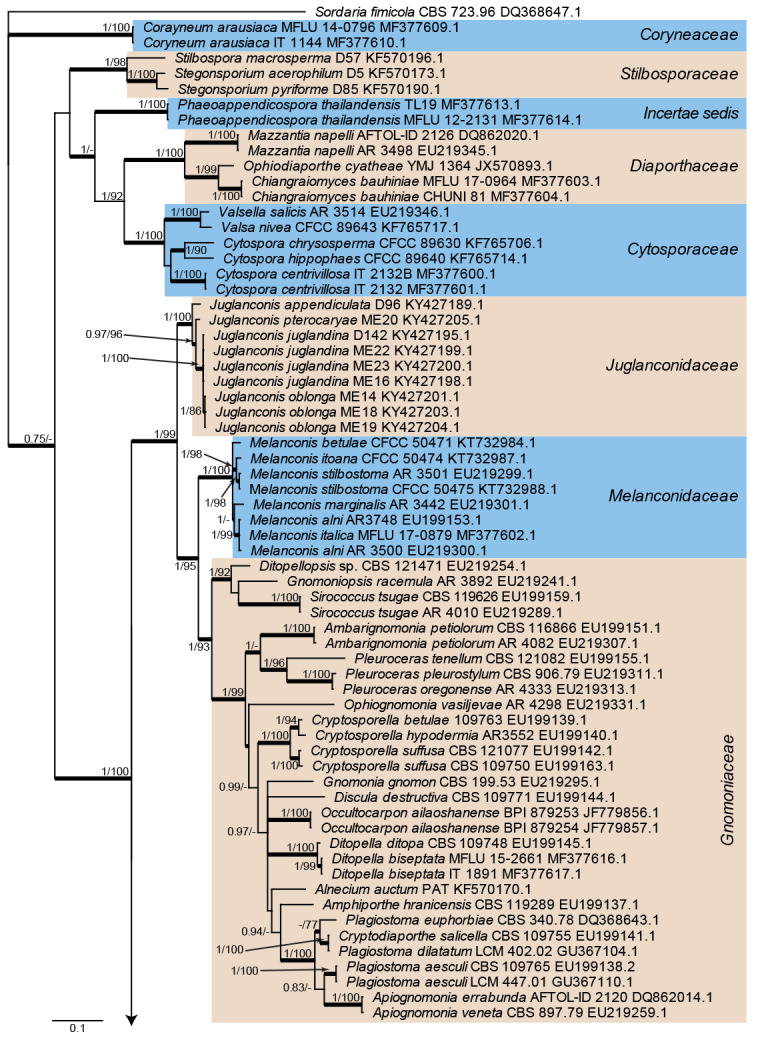
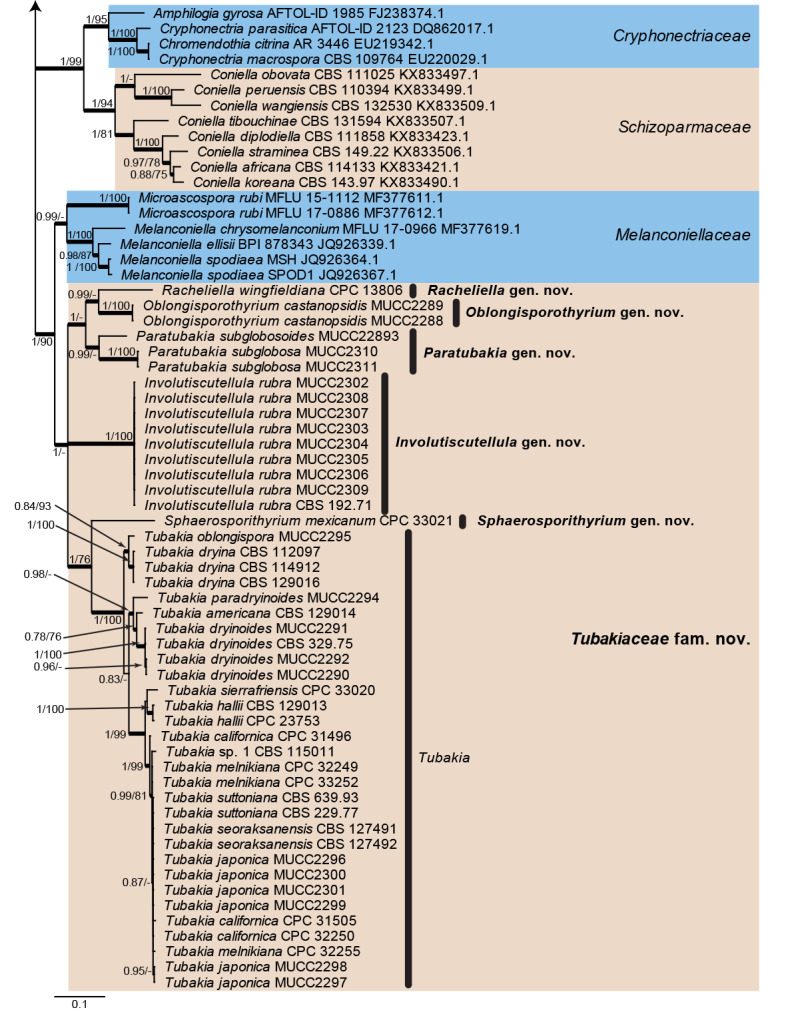
Consensus phylogram (50 % majority rule) from a Bayesian analysis of the rpb2 sequence alignment. Bayesian posterior probabilities (PP) >0.74 and maximum parsimony bootstrap support values (MP-BS) >74 % are shown at the nodes (PP/MP-BS) and thickened lines represent those branches present in the strict consensus maximum parsimony tree. The scale bar represents the expected changes per site. Families are indicated with coloured blocks to the right of the tree and the genera in Tubakiaceae are highlighted on the tree. Culture/specimen and GenBank accession numbers are indicated behind the species names. The tree is rooted to Sordaria fimicola (GenBank DQ368647.1) and the novel family and genera are indicated in bold face.
Twelve families are represented in the phylogenetic tree (Fig. 2). The majority of families was supported by high posterior probability and bootstrap support values. However, Melanconiellaceae and Tubakiaceae were both only fully or highly supported by the Bayesian analysis while the node connecting Melanconiellaceae and Tubakiaceae was only fully supported in the Bayesian analysis and supported with 90 % in the maximum parsimony analysis. The rpb2 sequences demonstrate a clear substructure in Tubakia s. lat. with highly supported lineages, and therefore several novel genera are introduced in the Taxonomy section below to accommodate those lineages.
Combined ITS/tef1/tub2 phylogeny – all species
It was not possible to generate all loci for all of the included isolates, mainly due to the fact that some loci failed to amplify for some isolates, even though several attempts were made to obtain a product suitable for sequencing. To reduce the amount of missing data in the alignment, such isolates were excluded from the analyses. The alignment contained 95 isolates representing Tubakia and allied taxa, and a strain of Melanconis groenlandica (CBS 116540; GenBank KU878552.1, KU878554.1 and KU878555.1, respectively ITS/tef1/tub2) was used as outgroup. The final alignment contained a total of 1 740 characters used for the phylogenetic analyses, including alignment gaps. The Maximum Parsimony (MP) analyses generated the maximum of 1 000 equally most parsimonious trees, the first of which is shown in Fig. 3 (Length = 2 660, CI = 0.665, RI = 0.916, RC = 0.609), and the bootstrap support values (MP-BS) were mapped on the tree as the second value (MP-BS >74 % shown). From the analysed characters, 734 were constant, 184 were variable and parsimony-uninformative and 822 were parsimony-informative. A strict consensus tree was calculated from the equally most parsimonious trees and the strict consensus branches were mapped with a thicker line on the presented phylogenetic tree (Fig. 3). MrModelTest recommended that the Bayesian analysis should use dirichlet base frequencies for the tef1 and tub2 data partitions and equal base frequencies for the ITS partition. The SYM+I+G model was proposed for ITS and HKY+I+G for tef1 and tub2. The Bayesian analyses generated 12 702 trees from which 9 528 trees were sampled after 25 % of the trees were discarded as burn-in. The posterior probability values (PP) were calculated from the 9 528 trees (Fig. 3; first value: PP >0.74 shown). The alignment contained a total of 925 unique site patterns (ITS: 229, tef1: 423, tub2: 273). The Bayesian phylogeny supported the same terminal clades as those presented in the parsimony phylogeny (Fig. 3), with some rearrangements in the order of the clades (data not shown, available in TreeBASE).
Fig. 3.
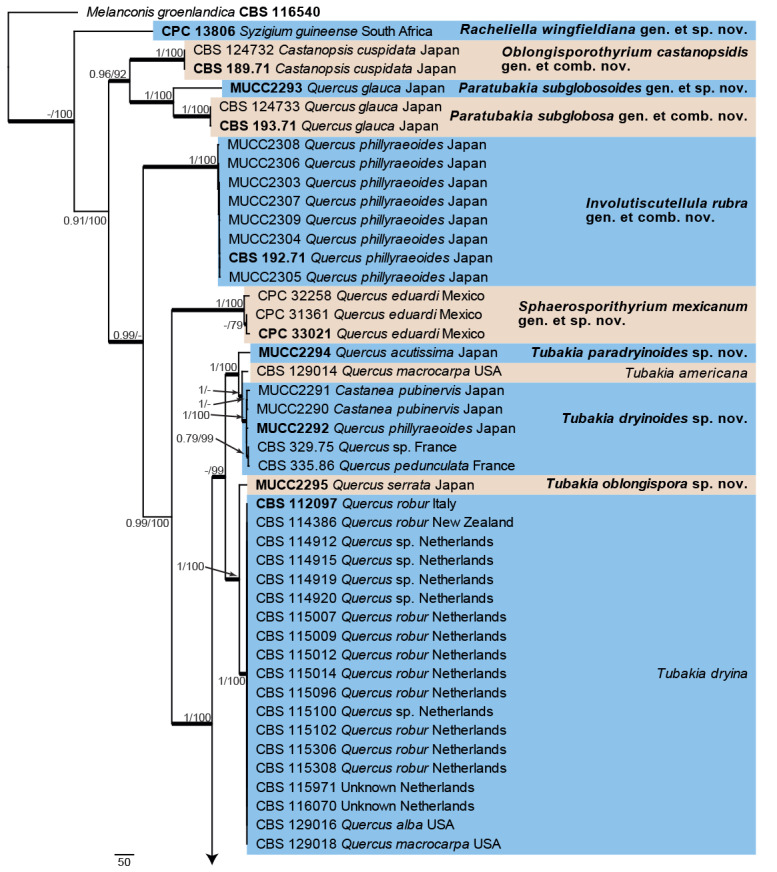
The first of 1 000 equally most parsimonious trees obtained from the combined ITS/tef1/tub2 alignment. Bayesian posterior probabilities (PP) >0.74 and maximum parsimony bootstrap support values (MP-BS) >74 % are shown at the nodes (PP/MP-BS) and thickened lines represent those branches present in the strict consensus maximum parsimony tree. The scale bar represents the number of changes per site. Species and species complexes are indicated with coloured blocks to the right of the tree. Culture numbers, host and country of origin are indicated for each strain. The tree is rooted to Melanconis groenlandica (culture CBS 116540) and taxonomic novelties and ex-type cultures are indicated in bold face.
Eighteen species lineages/clades are represented in the phylogenetic tree (Fig. 3). The majority of species were supported by high posterior probability or bootstrap support values, especially in the basal part of the tree (Fig. 3, part 1). However, the bottom half of the second part of the phylogeny was not as well resolved as the basal part of the phylogeny, with most of the phylogenetic signal coming from the tub2 sequences.
Overall, the same species clades/lineages are observed in the individual gene trees, although the order or basal organisation sometimes changed between the different loci (data not shown). ITS was the least successful in resolving species clades (for example T. paradryinoides/dryinoides and the T. suttoniana complex). In addition, two isolates of T. japonica (MUCC2297, MUCC2298) and one of T. seoraksanensis (CPC 26553) clustered in the T. dryina clade. The tef1 phylogeny could not resolve T. paradryinoides/dryinoides and isolates of T. suttoniana did not cluster together in a monophyletic lineage. The tub2 phylogeny provided the best resolution for T. oblongispora and T. paradryinoides. However, two isolates of T. rubra (MUCC2304, MUCC2306) clustered in the T. dryinoides clade and one isolate of T. californica (CPC 32250) clustered in the T. melnikiana clade. Given the overlap in hosts and geographical distribution between the different species, exchange of genetic material between the different species cannot be ruled out.
Combined ITS/tef1/tub2 phylogeny – T. suttoniana complex
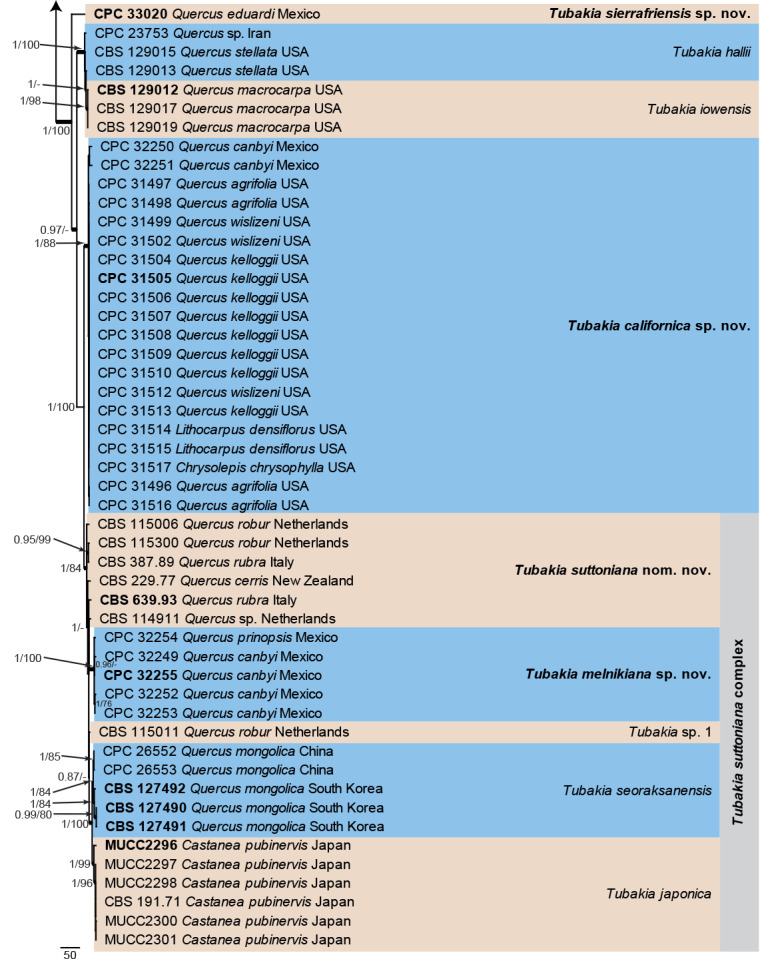
A subset combined alignment was subjected to Bayesian and parsimony analyses to better resolve the species in the T. suttoniana complex. The alignment contained 48 isolates and a strain of Tubakia castanopsidis (CBS 189.71; GenBank accession numbers listed in Table 1) was used as outgroup. The final alignment contained a total of 1 542 characters used for the phylogenetic analyses, including alignment gaps. The Maximum Parsimony (MP) analyses generated four equally most parsimonious trees, the first of which is shown in Fig. 4 (Length = 553, CI = 0.915, RI = 0.947, RC = 0.867), and the bootstrap support values (MP-BS) were mapped on the tree as the second value (MP-BS >74 % shown). From the analysed characters, 1 097 were constant, 322 were variable and parsimony-uninformative and 123 were parsimony-informative. A strict consensus tree was calculated from the equally most parsimonious trees and the strict consensus branches were mapped with a thicker line on the presented phylogenetic tree (Fig. 4). MrModelTest recommended that the Bayesian analysis should use dirichlet base frequencies for the tef1 and tub2 data partitions and equal base frequencies for the ITS partition. The SYM+I+G model was proposed for ITS and HKY+I+G for tef1 and tub2. The Bayesian analyses generated 2 602 trees from which 1 952 trees were sampled after 25 % of the trees were discarded as burn-in. The posterior probability values (PP) were calculated from the 1 952 trees (Fig. 4; first value: PP >0.74 shown). The alignment contained a total of 310 unique site patterns (ITS: 63, tef1: 149, tub2: 98). The Bayesian phylogeny supported the same terminal clades as those presented in the parsimony phylogeny (Fig. 4; data not shown, available in TreeBASE) and the same species as described above for Fig. 3.
Fig. 4.

The first of four equally most parsimonious trees obtained from the combined ITS/tef1/tub2 alignment focused on the T. suttoniana complex and closely related species. Bayesian posterior probabilities (PP) >0.74 and maximum parsimony bootstrap support values (MP-BS) >74 % are shown at the nodes (PP/MP-BS) and thickened lines represent those branches present in the strict consensus maximum parsimony tree. The scale bar represents the number of changes per site. Species and species complexes are indicated with coloured blocks to the right of the tree. Culture numbers, host and country of origin are indicated for each strain. The tree is rooted to Oblongisporothyrium castanopsidis (culture CBS 189.71) and taxonomic novelties and ex-type cultures are indicated in bold face. The length of the most basal branch was halfed to facilitate layout.
tef1 phylogeny
A tef1 phylogeny was also generated (Fig. 5) to enable a more direct comparison of the data in the present paper with the recently published data from Harrington & McNew (2018). The alignment contained 136 isolates and a strain of Melanconis groenlandica (CBS 116540; GenBank accession numbers listed in Table 1) was used as outgroup. The final alignment contained a total of 592 characters used for the phylogenetic analyses, including alignment gaps. The Maximum Parsimony (MP) analyses generated the maximum of 1 000 equally most parsimonious trees, the first of which is shown in Fig. 5 (Length = 1 356, CI = 0.620, RI = 0.919, RC = 0.570), and the bootstrap support values (MP-BS) were mapped on the tree as the second value (MP-BS >74 % shown). From the analysed characters, 179 were constant, 39 were variable and parsimony-uninformative and 374 were parsimony-informative. A strict consensus tree was calculated from the equally most parsimonious trees and the strict consensus branches were mapped with a thicker line on the presented phylogenetic tree (Fig. 5). MrModelTest recommended that the Bayesian analysis should use dirichlet base frequencies and the HKY+I+G model. The Bayesian analyses generated 19 002 trees from which 14 252 trees were sampled after 25 % of the trees were discarded as burn-in. The posterior probability values (PP) were calculated from the 14 252 trees (Fig. 5; first value: PP >0.74 shown). The alignment contained 400 unique site patterns. The Bayesian phylogeny showed the same terminal clades as those presented in the parsimony phylogeny with some rearrangements in the backbone of the tree (Fig. 5; data not shown, available in TreeBASE). This tef1 phylogeny highlights the close relation between T. hallii and T. iowensis and the broad concept currently being applied to T. macnabbii and T. suttoniana.
Fig. 5.
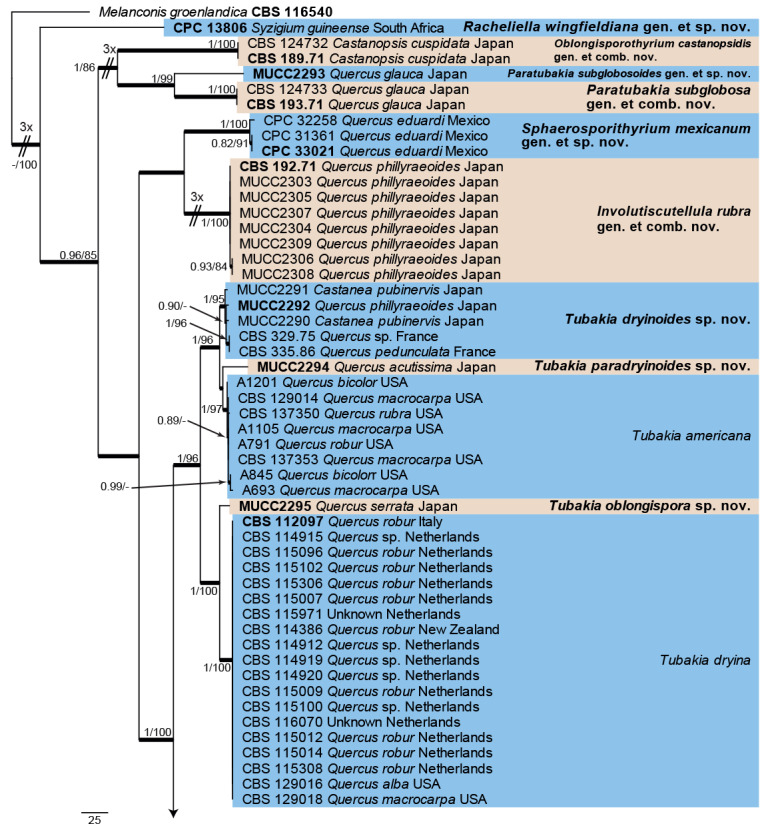

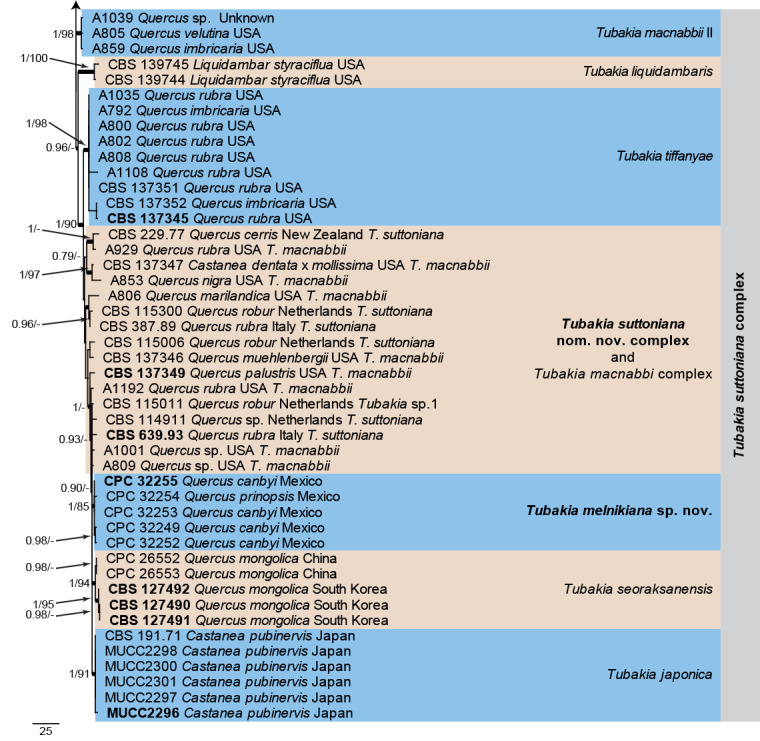
The first of 1 000 equally most parsimonious trees obtained from the expanded tef1 alignment of species in the Tubakiaceae. Bayesian posterior probabilities (PP) >0.74 and maximum parsimony bootstrap support values (MP-BS) >74 % are shown at the nodes (PP/MP-BS) and thickened lines represent those branches present in the strict consensus maximum parsimony tree. The scale bar represents the number of changes per site. Species and species complexes are indicated with coloured blocks to the right of the tree. Culture numbers, host and country of origin are indicated for each strain. The tree is rooted to Melanconis groenlandica (culture CBS 116450) and taxonomic novelties and ex-type cultures are indicated in bold face. The lengths of some of the most basal branches were shortened to facilitate layout.
TAXONOMY
Tubakiaceae U. Braun, J.Z. Groenew. & Crous, fam. nov. MycoBank MB823660.
Type genus: Tubakia B. Sutton.
Classification: Ascomycota, Pezizomycotina, Sordariomycetes, Sordariomycetidae, Diaporthales.
Saprobic, endophytes in leaves and twigs and plant pathogens causing leaf spots and twig dieback. Asexual morphs: sporodochia, crustose to pustulate pycnidioid stromatic conidiomata and superficial scutellate pycnothyria. Conidiogenous cells monophialidic, colourless, often with collarettes. Conidia formed singly, mostly globose to broad ellipsoid-obovoid, aseptate, hyaline to pigmented, often with basal frill or truncate peg-like hilum. Sexual morphs: apiognomonia- and dicarpellum-like, diaporthaloid, dark, rostrate, ostiolate perithecial ascomata, with dark stromatic layers, polyascal; asci unitunicate, 8-spored; ascospores aseptate or with a single septum near the apex, hyaline.
Notes: The clade containing all former Tubakia species was highly supported in both Bayesian and maximum parsimony analyses (Fig. 1; PP = 1, MP-BS = 99 %). In addition, its sister relation changed between the two analyses; in the Bayesian analysis, it was sister to Melanconiellaceae and in the maximum parsimony analysis it was sister to Apoharknessiaceae. The branch separating the Tubakiaceae clade from its closest sister clade was longer than the branches separating several other families in the phylogeny, e.g. Melanconidaceae / Gnomoniaceae and Harknessiaceae / Schizoparmaceae (Fig. 1). Analyses of LSU and above all rpb2 data and corresponding trees clearly indicate the heterogeneity of the genus Tubakia s. lat. Some lineages are included that warrant the division of Tubakia s. lat. into several genera.
Apiognomonioides U. Braun, J.Z. Groenew. & Crous, gen. nov. MycoBank MB824479.
Etymology: Composed of the name of the genus Apiognomonia and -oides (resembling), referring to the similarity between the new genus and the latter one.
Type species: Apiognomonioides supraseptata (Kaneko & Kobayashi) U. Braun, J.Z. Groenew. & Crous (≡ Apiognomonia supraseptata Kaneko & Kobayashi).
Genus of Tubakiaceae. Sexual morph reseming Apiognomonia, but ascospores with a single septum near the apex. Perithecia immersed, globose to depressed, with a central to rarely eccentric beak, erumpent, perithecial wall 2–3 cell layers thick, composed of dark brown, flattened cells and a hyaline innermost layer; asci numerous, clavate to cylindrical-clavate, with an apical ring at the thickened apex, unitunicate, 8-spored; ascospores irregularly biseriate, ellipsoid, 1-septate near the apex, slightly constricted at the septum, hyaline, thin-walled.
Notes: Kaneko & Kobayashi (1984) introduced the name Apiognomonia supraseptata on the basis of ascomata formed on leaves of Quercus glauca incubated in a humid petri dish. Conidia were not formed. An ex-type strain (ATTC 58737) was used to retrieve a LSU rDNA sequence (GenBank AF277127, Zhang & Blackwell 2001) which was used by Harrington & McNew (2018) as basis to transfer A. supraseptata to Tubakia. They classified the mycelial colonies in culture to be tubakia-like. According to Kaneko & Kobayashi (1984), Apiognomonia has two-celled ascospores in which the top cell is larger than the bottom cell, but in A. supraseptata the top cell is smaller. Harrington & McNew (2018) postulated that A. supraseptata represents the only clearly demonstrated sexual morph of Tubakia. The allocation of Apiognomonia supraseptata to Tubakiaceae is reasonable and could be confirmed in our own analyses (Fig. 1). Apiognomonia supraseptata is not included in the rpb2 tree due to lack of sequence data, but in the LSU (Fig. 1) and ITS trees (not shown) this species clusters distantly from all other lineages outside of the Tubakia s. str. clade, i.e., this species cannot be maintained in Tubakia s. str. Owing to its isolated position in the LSU tree within the Tubakiaceae, but distant from all other genera of this family, Apiognomonia supraseptata is best accommodated in a genus of its own. A. supraseptata and the sexual morph of Tubakia suttoniana have various morphological characters in common (rostrate perithecia, unitunicate 8-spored asci, colourless conidia), but A. supraseptata differs in forming uniseptate ascospores with a septum near the apex.
Apiognomonioides supraseptata (Kaneko & Kobayashi) U. Braun, J.Z. Groenew. & Crous, comb. nov. MycoBank MB824480.
Basionym: Apiognomonia supraseptata Kaneko & Kobayashi, Trans. Mycol. Soc. Japan 25: 11. 1984.
Synonym: Tubakia supraseptata (Kaneko & Kobayashi) T.C. Harr. & McNew, Antonie van Leeuwenhoek J. Microbiol. Serol. doi.org/10.1007/s10482-017-1001-9 [16]. 2017.
Illustrations: Kaneko & Kobayashi (1984: 13, figs 1–10).
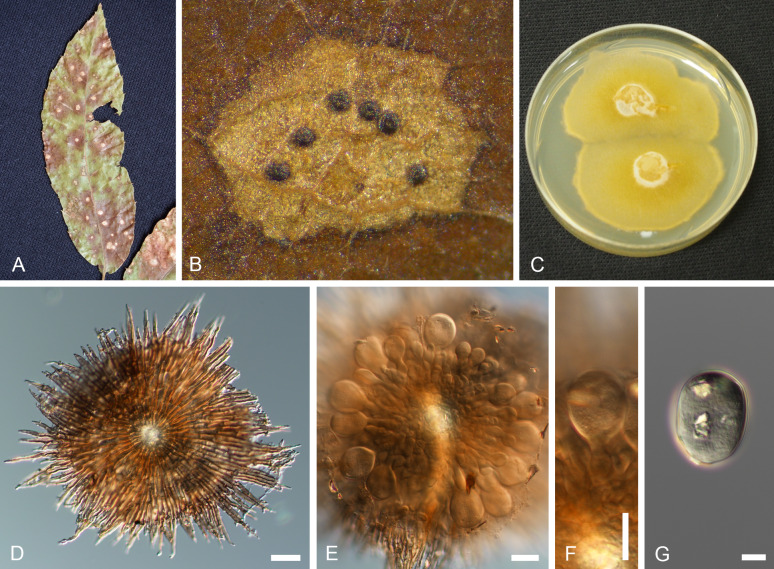
Fig. 10.
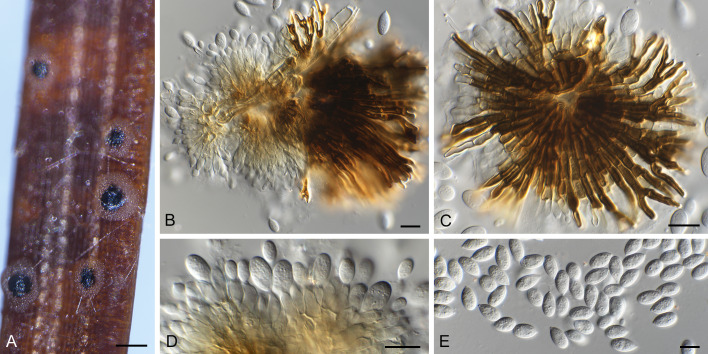
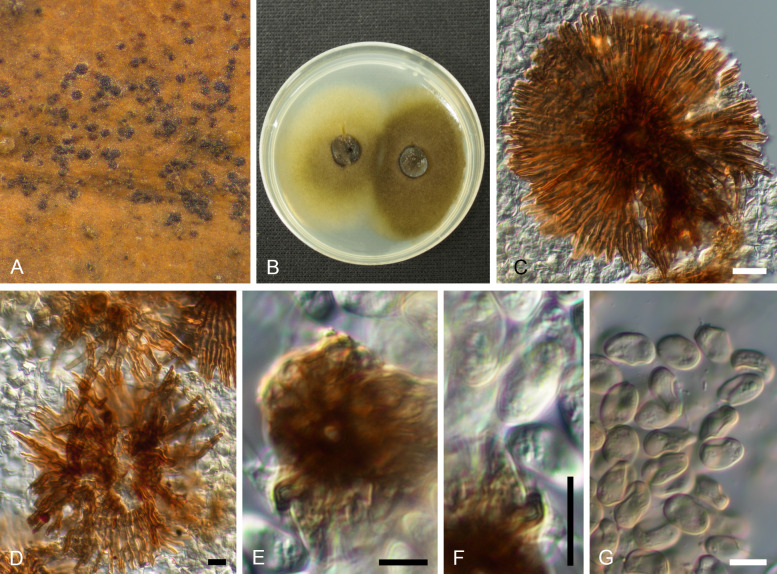
Racheliella wingfieldiana (CBS H-23399 – holotype). A. Pycnothyria forming in culture on PNA. B, C. Pycnothyria in culture (from PNA). D. Conidiogenous cells giving rise to conidia. E. Conidia. Bars = 150 mm (A), 10 mm (B–E).
Description in vivo: Perithecia formed in lesions on leaves after incubation in a humid petri dish, black, immersed, globose or depressed at the base, 130–220 μm diam, 80–180 μm high, with a central to rarely eccentric beak, erumpent, mostly hypophyllous, to 300 μm long and 50–75 μm wide at the base, perithecial wall 2–3 cell layers thick, composed of dark brown, flattened cells and a hyaline innermost layer. Asci numerous, clavate to cylindrical-clavate, tapering towards the base, with an apical ring at the thickened apex, unitunicate, 50–70 × 10–12.5 μm, 8-spored. Ascospores irregularly biseriate, ellipsoid, 1-septate near the apex, slightly constricted at the septum, 11–15 × 4.5–6 μm, hyaline, thin-walled.
In vitro: On PSA rapidly growing at 25 °C, attaining 35–45 mm diam after 10 d, more or less zonate, wooly, white, reverse white to pale luteous, colony colour deeper at temperatures higher than 27 °C; aerial mycelium composed of branched, hyaline, septate hyphae, 1.5–2 μm wide; immersed hyphae 2–5 μm wide; development of perithecia in culture 14 d after incubation at 20 °C with continuous fluorescent light, scattered or in concentric circles, somewhat immersed (ascomata formed in culture 250–280 μm diam; asci 65–78 × 10–12.5 μm, basal part more elongated than on the host, ascospores 11.5–15 × 4.5–6.5 μm).
Type: Japan, Tottori Pref., Tottori City, Ohchidani Park, on leaves of Quercus glauca [incubated in a humid petri dish for 28 d after collection], 11 Feb. 1982, S. Kaneko (TMI 7647 – holotype; TFM : FPT5447; ATTC 58737 = CBS 632.92 = TMI cult. 70024 – ex-type strains).
Involutiscutellula U. Braun & C. Nakash., gen. nov. MycoBank MB824481.
Etymology: Composed of “involutus” (involute), “scutellulum” (referring to the pycnothyrial scutella), and -ula (diminutive) = minute scutellum with involute margin.
Type species: Actinopelte rubra T. Yokoy. & Tubaki [≡ Involutiscutellula rubra (T. Yokoy. & Tubaki) U. Braun & C. Nakash.].
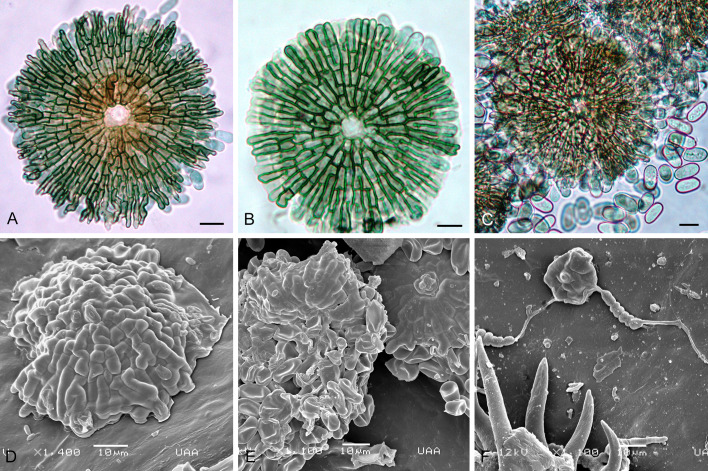
Genus of Tubakiaceae. Living as endophytes in leaves. Colonies in vitro finally turning reddish brown. Mycelium internal, hyaline, and external, pigmented; hyphae in culture finally turning reddish brown. Asexual morphs forming crustose conidiomata on shed leaves (litter) and superficial pycnothyria on symptomless leaves, occasionally on reddish discolorations. Pycnothyria subcircular when viewed from above, superficial, easily removable, scutellate, fixed to the leaf by a central columella; scutellum convex to flattened, membranous, compact, neither loose nor splitting, outline regular, circular-subcircular, margin continuous, more or less undulate, distinctly involute; conidiophores reduced to conidiogenous cells, conical, cylindrical, ampulliform, pale orange yellowish to yellowish brown, arising from the underside of the scutella, around the columella, conidiogenous cells phialidic; conidia formed singly, globose, subglobose to broad ellipsoid-obovoid, smooth; microconidia oblong-bacilliform, cylindrical, straight to curved-sigmoid, very narrow, only 1 μm wide.
Notes: In all of the phylogenetic trees, especially the LSU and rpb2 phylogenies, Actinopelte rubra takes an isolated basal position and forms a single species lineage that warrants a classification as a genus of its own (Figs 1, 2). A. rubra is morphologically readily distinguishable from all other tubakia-like species by colonies and hyphae turning reddish brown with age and distinctive pycnothyria with continuous, more or less undulate, distinctly involute margin and very narrow oblong-bacilliform to cylindrical microconidia.
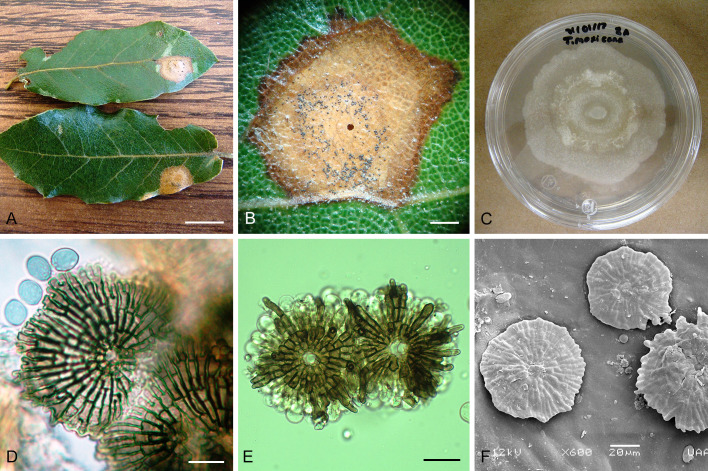
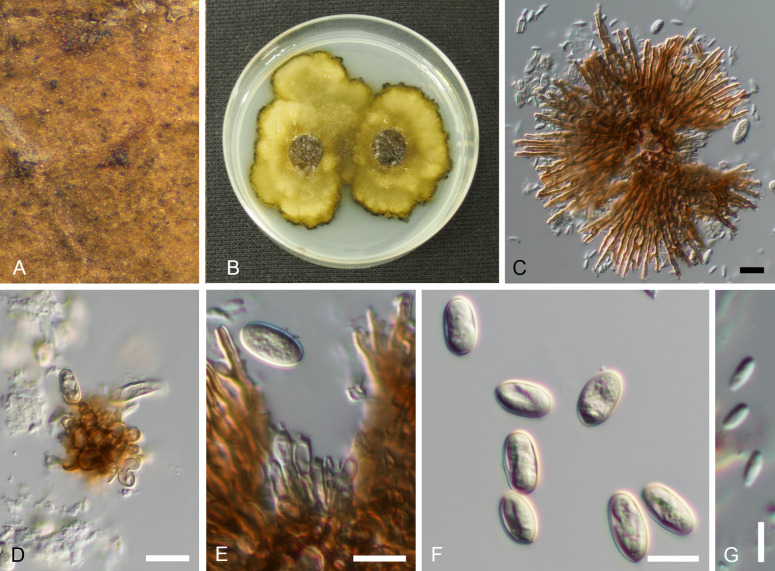
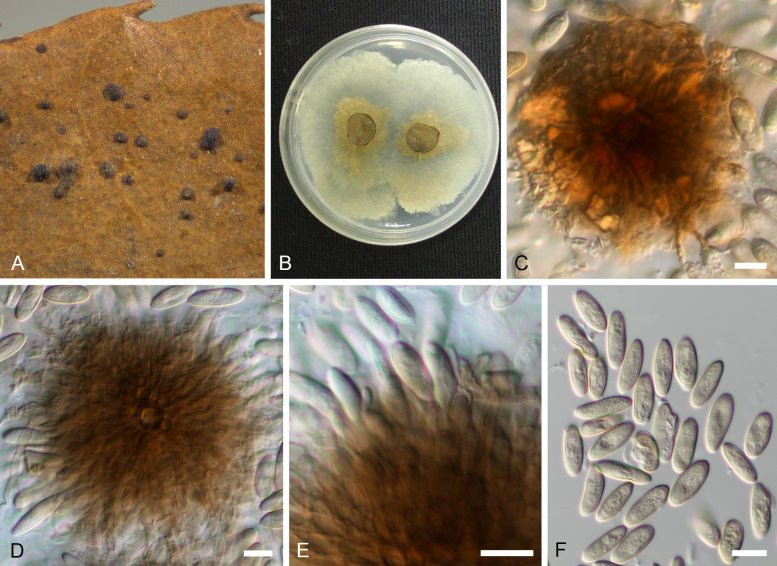
Involutiscutellula rubra (T. Yokoy. & Tubaki) U. Braun & C. Nakash., comb. nov. MycoBank MB824482. Fig. 6.
Fig. 6.
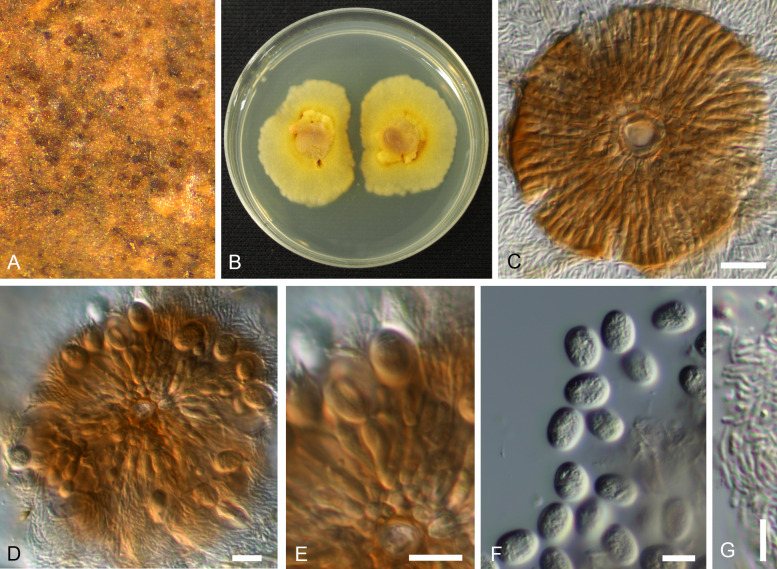
Involutiscutellula rubra (NBRC H-11622 – holotype). A. Pycnothyria on the leaf surface. B. Culture on MEA (NBRC 9273 – ex-type culture). C. Scutellum. D. Central columella. E. Conidiophores. F. Conidia. G. Microconidia. Bars = 10 μm.
Basionym: Actinopelte rubra T. Yokoy. & Tubaki, Res. Commun. Inst. Ferment. Osaka 5: 47. 1971.
Synonym: Tubakia rubra (T. Yokoy. & Tubaki) B. Sutton, Trans. Brit. Mycol. Soc. 60: 165. 1973.
Illustrations: Yokoyama & Tubaki (1971: 65, pl. 1F, 67, pl. 2D; 69, pl. 3D; 72, pl. 6A–G; 76, pl. 10A–H).
Description in vivo: Living as endophyte in leaves, forming crustose conidiomata on the surface of shed leaves (litter), usually without distinct symptoms (lesions), sometimes causing a reddish tinge on the leaf surface. Mycelium internal and external, forming hyaline, branched intra- and intercellular external hyphae, external hyphae observed on the upper leaf surface, pale brown, branched. Conidiomata (pycnothyria) amphigenous, scattered to gregarious, occasionally confluent, punctiform, superficial, easily removable, subcircular in outline, 60–120 μm diam, ochraceous to orange brown (stereomicroscopy), scutellate, fixed to the leaf surface by a central columella. Scutella convex to flat, membranous, dense, compact, neither loose nor splitting, outline regular, circular-subcircular, margin continuous, more or less undulate, distinctly involute, with a central hyaline disc, 6–12 μm diam, surrounded by oblong hyphal cells, rarely branched, 2–3 μm wide, giving rise to radiating hyphal strands, cells 5–10 × 2.5–3 μm, pale brown to medium dark brown, thick-walled (–1 μm), smooth, rarely bifurcating, ultimate branchlets with rounded tips. Central columella composed of a central cell surrounded by pseudoparenchymatous cells or distinct large or small cells, 12–30 μm diam. Conidiophores reduced to conidiogenous cells, arising from the underside of the scutella, around the columella, radiating, orientation outward and downward, conical, cylindrical, ampulliform, larger at the base and attenuated towards a narrow tip, delicate, about 7–20 × 2–4.5 μm, neck about 1 μm wide, hyaline to pale brown, thin-walled, smooth. Conidia solitary, globose, subglobose to broad ellipsoid-obovoid, 10–14 × 6–10 μm, length/width ratio 1–1.9, conidiogenesis phialidic, apex and base rounded, wall thin, at first hyaline, later pale orange yellowish to yellowish brown, with inconspicuous to conspicuous basal hilum, up to 1 μm, occasionally truncate when conspicuous. Microconidia oblong-bacilliform, cylindrical, straight to curved-sigmoid, 6–10 × 1 μm, formed in common pycnothyria or in separate conidiomata.
In vitro: On MEA with optimal growth at 20 °C, attaining 20–25 mm after 14 d, margin scalloped, creamy yellow, ochraceous, yellowish brown, finally reddish brown; aerial mycelium poorly developed, concolorous; immersed hyphae rapidly growing, white, creamy to ochraceous, later reddish [sporulation on MEA not observed after 14 d; according to Yokoyama & Tubaki (1971) sporulation at the centre or spread, forming pale yellowish brown, viscid, yeast-like masses]. On potato sucrose agar rapidly growing, ochraceous to yellowish brown, later reddish brown to deep reddish fuscous; aerial mycelium moderately developed, compact, viscid; immersed hyphae rapidly growing, pale ochraceous to reddish brown; reverse concolorous; sporulation abundant, above all in the centre, forming yeast-like conidial masses. On OA moderately growing, creamy to pale ochraceous, later reddish brown, smooth, viscid; aerial mycelium poorly developed; immersed hyphae moderately developed, ochraceous to reddish brown; reverse concolorous; sporulation abundant, evenly scattered, forming yeast-like conidial masses. On Czapek agar growth lacking or only restricted, reddish brown (from Yokoyama & Tubaki 1971).
Type: Japan, Kyoto Pref., Kyoto, on Quercus phillyraeoides, 28 Oct. 1969, T. Yokoyama 44102801 (NBRC H-11622 – holotype; NBRC 9273 = ATCC 22473 = IMI 157597 = MUCC2304 – ex-type cultures).
Additional collections examined: Japan, Kyoto Pref., Kyoto, on Quercus phillyraeoides, 7 Jun. 1969, T. Yokoyama, NBRC H-11620; NBRC 9271 = MUCC2302 = CBS 192.71 and NBRC H-11621; NBRC 9272 = MUCC2303; Shiga Pref., Ootsu, on Quercus phillyraeoides, 27 Jan. 1970, T. Yokoyama, NBRC H-11623; NBRC 9274 = MUCC2305 and NBRC H-1624; NBRC 9275 = MUCC2306; Ootsu, on Quercus phillyraeoides, 14 Apr. 1970, T. Yokoyama, NBRC H-11625; NBRC 9276 = MUCC2307 and NBRC H-1624; NBRC 9275 = MUCC2306; Kagoshima Pref., Yaku Is., on Quercus phillyraeoides, 29 May 1970, T. Yokoyama, NBRC 9277 = MUCC2308; Mie Pref.; Miyagawa, on Quercus phillyraeoides, 2 Aug. 1970, T. Yokoyama, NBRC 9371 = MUCC2309.
Host range and distribution: On Quercus (phillyraeoides, serrata), Fagaceae, Asia (Japan, Korea).
Notes: Records from Korea on Quercus serrata date from Lee et al. (1991) and Cho & Shin (2004).
Oblongisporothyrium U. Braun & C. Naksh., gen. nov. MycoBank MB824483.
Etymology: Composed of “oblongisporo-“ (oblong spores) and “-thyrium” (referring to the conidioma, i.e., pycnothyrium).
Type species: Actinopelte castanopsidis T. Yokoy. & Tubaki [≡ Oblongisporothyrium castanopsidis (T. Yokoy. & Tubaki) U. Braun & C. Nakash.].
Genus of Tubakiaceae. Living as endophyte in leaves. Mycelium internal, hyaline, and external, pigmented. Asexual morphs forming crustose conidiomata on the surface of leaf litter and superficial pycnothyria on brown, necrotic areas on leaves. Pycnothyria usually circular or subcircular when viewed from above, superficial, easily removable, scutellate, fixed to the leaf by a central columella; scutellum convex to flattened, often recurved at the edge, membranous, dense, compact when young, later loose at the margin, outline regular, circular to subcircular, composed of hyphal strands, mostly branched, thick-walled, pigmented, margin often recurved at the edge, ultimate tips of the hyphal strands obtuse to rounded; conidiophores reduced to conidiogenous cells, obclavate, hyaline to pigmented, arising from small, colourless fertile cells around the central pycnothyrial columella; conidiogenous cells phialidic, sometimes forming indistinct periclinal thickenings or annellations; conidia formed singly, oblong to oblong-ellipsoid, wall thin to somewhat thickened, hyaline, smooth; microconidia not observed.
Notes: In all of the phylogenetic trees, especially the LSU and rpb2 phylogenies (Figs 1, 2), Actinopelte castanopsidis clusters outside and basal of the Tubakia s. str. clade, i.e., this species does not belong to the Tubakia core species. Hence, this species has to be excluded from Tubakia s. str. Actinopelte castanopsidis is allied to Paratubakia species [on Quercus (Cyclobalanopsis) glauca] (Figs 2, 3, 5), but forms a separate single species lineage, suggesting a genus of its own. This species shares scutella with margins somewhat curved inwardly, hyaline to pigmented conidiogenous cells, and colourless conidia with Paratubakia subglobosa, type species of Paratubakia, but differs in having oblong conidia (vs. globose to subglobose in T. subglobosa).
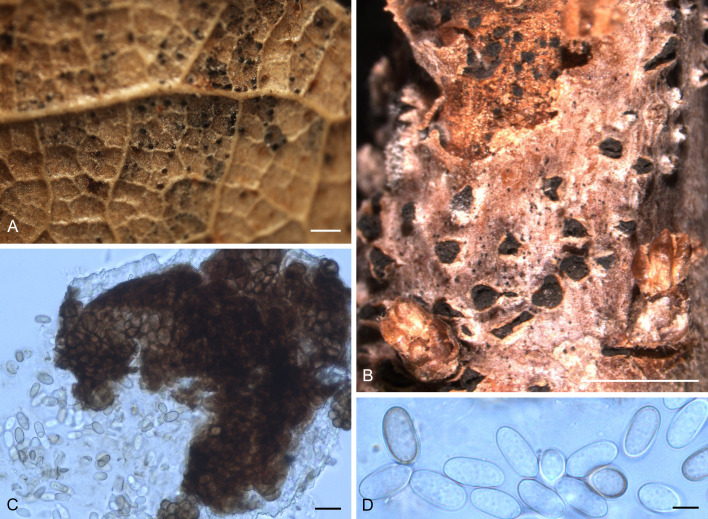
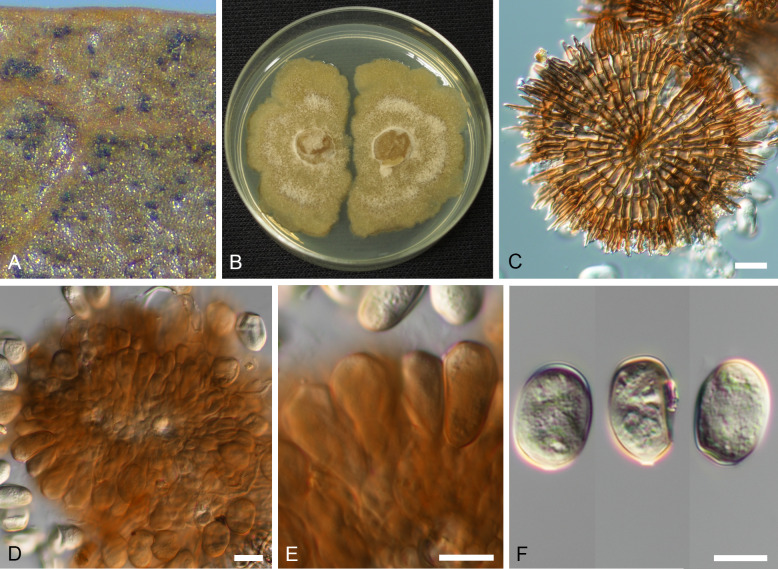
Oblongisporothyrium castanopsidis (T. Yokoy. & Tubaki) U. Braun & C. Nakash., comb. nov. MycoBank MB824484. Fig. 7.
Fig. 7.
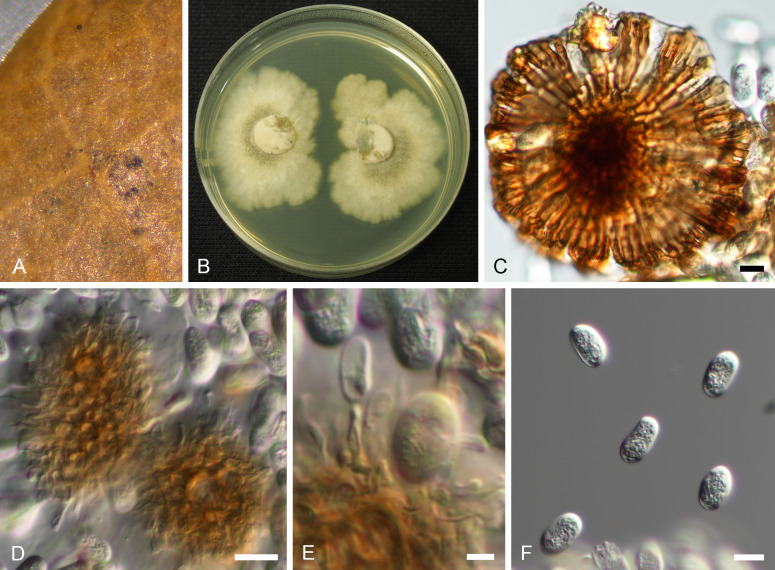
Oblongisporothyrium castanopsidis (NBRC H-11631 – holotype). A. Pycnothyria on the surface of leaf litter. B. Culture on MEA (NBRC 9263 – ex-type culture). C. Scutellum. D. Central columellas. E. Conidiophores. F. Conidia. Bars = 10 μm.
Basionym: Actinopelte castanopsidis T. Yokoy. & Tubaki, Res. Commun. Inst. Ferment. Osaka 5: 50. 1971.
Synonym: Tubakia castanopsidis (T. Yokoy. & Tubaki) B. Sutton, Trans. Brit. Mycol. Soc. 60: 165. 1973.
Illustrations: Yokoyama & Tubaki (1971: 65, pl. 1H, 67, pl. 2F; 69, pl. 3F; 73, pl. 7A–D).
Description in vivo: Living as endophyte in leaves, forming crustose conidiomata on the surface of leaf litter. Mycelium internal and external, forming hyaline, branched intra- and intercellular hyphae, external hyphae observed on the lower leaf surface, pale brown, branched. Conidiomata (pycnothyria) epiphyllous, rarely hypophyllous, on brown, necrotic areas, scattered, sometimes gregarious, occasionally confluent, punctiform, dark brown to blackish, superficial, easily removable, circular to subcircular when view from above, 100–170 μm diam, scutellate, fixed to the leaf surface by a central columella. Scutella convex to flattened, often recurved at the edge, membranous, dense, compact when young, later loose at the margin, outline regular, circular to subcircular, with a central hyaline to subhyaline disc, 4–8 μm diam, scutellum more or less uniformly pigmented, pale brown to brown, central cells subcircular or angular-irregular in outline, giving rise to radiating strands of oblong hyphal cells, 1–3 times bifurcating, 8–12 × 3–5 μm, smooth, septate, walls thickened, ultimate branchlets with obtuse to rounded tips. Central columella below the scutellum composed of a central cell surrounded by small, hyaline, fertile cells, forming a pseudoparenchymatous sheath, 25–50 μm diam. Conidiophores reduced to conidiogenous cells, arising from the underside of the scutella, from parenchymatous cells around the upper part of the columella, radiating, orientation outward and downward, obclavate, enlarged at the base and attenuated towards a narrow tip, 1 μm wide, delicate, 11–20 × 2.5–6 μm, hyaline to pale brown, thin-walled, smooth, apex obtuse to truncate, conidiogenesis phialidic, sometimes forming indistinct periclinal thickenings or annellations. Conidia solitary, oblong to oblong-ellipsoid, 14–17 × 7–9.5 μm, length/width ratio 1.6–2.2, apex rounded, base rounded, often with distinct frill, wall thin to somewhat thickened, hyaline, smooth. Microconidia not observed.
In vitro: On MEA with optimal growth at 20 °C, attaining 25–30 mm after 14 d, margin scalloped, dingy white to clay-coloured, more or less viscid; aerial mycelium effuse, floccose, white; immersed hyphae rapidly growing, moderately sporulating after 14 d, sporodochial conidiomata [abundantly formed in concentric ring] scattered, blackish brown, forming white to creamy mucous masses of released conidia. Conidia originating from sporodochia ellipsoid-ovoid, 10–15 × 7–10 μm, smooth, hyaline, later pale olivaceous brown. On potato sucrose agar moderately growing, white to clay-coloured, zonate, cartilaginous; aerial mycelium poorly developed, white; immersed hyphae moderately growing; reverse concolorous; sporulation abundant, creamy-white, forming yeast-like masses. On OA very rapidly growing, white to pale clay-coloured; aerial mycelium lacking or poorly developed, white; immersed hyphae abundant, pale ochraceous to clay-coloured; reverse concolorous; sporulation abundant, forming numerous punctiform, blackish brown conidiomata that release creamy, viscid, mucous masses of conidia. On Czapek agar without any colonies or, if any, strongly restricted (from Yokoyama & Tubaki 1971).
Type: Japan, Shiga Pref., Ootsu, on Castanopsis cuspidata, 27 Jan. 1970, T. Yokoyama (NBRC H-11631 – holotype; NBRC 9263 = ATCC 22470 = CBS 189.71 = IMI 157598 = MUCC2289 – ex-type cultures).
Additional collection examined: Japan, Ootsu, on Castanopsis cuspidata, 26 Mar. 1970, T. Yokoyama, NBRC 9262 = CBS 124732 = MUCC2288.
Hosts range and distribution: on Castanopsis cuspidata, Fagaceae, Asia (Japan).
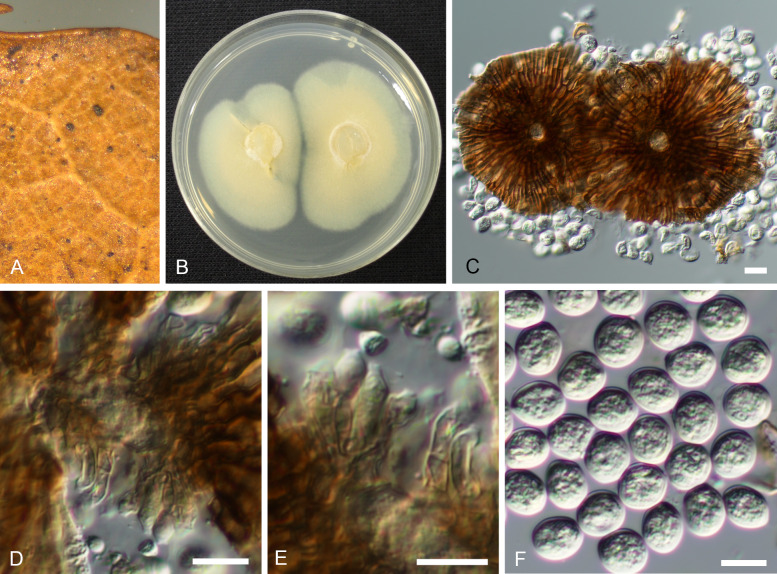
Notes: Morphologically, Actinopelte castanopsidis belongs to a group of tubakia-like species characterised by having obtuse to rounded outer tips of the scutellum strands, and resembles T. sierrafriensis from which it is readily distinguishable by its uniformly oblong-ellipsoid, colourless conidia. Tubakia oblongispora is another morphologically similar species but differs in having narrower conidia, 12–20 × 4.5–7.5 μm, with a length/width ratio of 1.8–3.8, and much longer conidiogenous cells, 13–35 × 2–5.5 μm. Phylogenetically, Actinopelte castanopsidis (≡ Tubakia castanopsidis) is quite distantly related to the two morphologically similar species (Fig. 2).
Paratubakia U. Braun & C. Nakash., gen. nov. MycoBank MB824485.
Etymology: Composed of “para-“ (next to, near) and Tubakia (referring to the similarity with the latter genus).
Type species: Actinopelte subglobosa T. Yokoy. & Tubaki [≡ Paratubakia subglobosa (T. Yokoy. & Tubaki) U. Braun & C. Nakash.].
Genus of Tubakiaceae. Living as endophyte in leaves. Mycelium internal, hyaline, and external, pigmented. Asexual morphs forming crustose conidiomata on shed leaves (litter) and superficial pycnothyria on leaf spots of living leaves. Pycnothyria usually circular or subcircular when viewed from above, superficial, easily removable, scutellate, fixed to the leaf by a central columella; scutellum convex to flattened, often recurved at the edge, membranous, dense to looser towards the periphery, outline irregular, sometimes splitting with age, subcircular, composed of hyphal strands, mostly branched, thick-walled, pigmented, ultimate tips of the hyphal strands obtuse to pointed; conidiophores reduced to conidiogenous cells, subcylindrical, oblong-obclavate, hyaline to pale olivaceous brown or pale brown, arising from small, colourless fertile cells around the central pycnothyrial columella, conidiogenous cells phialidic; conidia formed singly, globose, subglobose to broad ellipsoid, wall thin, hyaline to pale yellowish ochraceous, smooth to faintly rough; microconidia not observed.
Notes: Actinopelte subglobosa (≡ Tubakia subglobosa) and a new closely allied Japanese species cluster together, but outside of the Tubakia s. str. clade, i.e., these species do not belong to the Tubakia core species, so that they have to be excluded from Tubakia s. str. This lineage is fully supported in the Bayesian trees based on rpb2 and LSU (Figs 1, 2) and requires a genus of its own, which is described as Paratubakia. Actinopelte castanopsidis is allied to Paratubakia, but forms a separate single species lineage, treated as separate genus, Oblongisporothyrium gen. nov. The type species of the latter genus shares scutella with margins somewhat curved inwardly and colourless conidia with Paratubakia subglobosa, type species of Paratubakia, but differs in having oblong conidia (vs. globose to subglobose in T. subglobosa).
Paratubakia subglobosa (T. Yokoy. & Tubaki) U. Braun & C. Nakash. comb. nov. MycoBank MB824486. Fig. 8.
Fig. 8.
Paratubakia subglobosa (NBRC H-11629 – holotype). A. Pycnothyria on the surface of leaf litter. B. Culture on MEA (NBRC 8931 – ex-type culture). C. Scutella. D. Central columella. E. Conidiophores. F. Conidia. Bars = 10 μm.
Basionym: Actinopelte subglobosa T. Yokoy. & Tubaki, Res. Commun. Inst. Ferment. Osaka 5: 49. 1971.
Synonym: Tubakia subglobosa (T. Yokoy. & Tubaki) B. Sutton, Trans. Brit. Mycol. Soc. 60: 165. 1973.
Illustrations: Yokoyama & Tubaki (1971: 65, pl. 1G, 67, pl. 2E; 69, pl. 3E; 73, pl. 7E–H; 76, pl. 10A–H).
Description in vivo: Living as endophyte in leaves, forming crustose conidiomata on the surface of shed leaves (litter); leaf spots formed on living and fallen leaves, irregularly shaped, with distinct margin. Mycelium internal and external, forming hyaline, branched intra- and intercellular hyphae, external hyphae observed on the lower leaf surface, pale brown, branched. Conidiomata (pycnothyria) epiphyllous, rarely hypophyllous, scattered to more or less gregarious, punctiform, superficial, easily removable, subcircular in outline when viewed from above, 50–150 μm diam, almost black, brown to dark brown (stereomicroscopy), scutellate, fixed to the leaf surface by a central columella. Scutella convex to flattened, margin often recurved, membranous, dense to loser around the periphery, sometimes splitting with age, outline irregular, subcircular, with a central hyaline disc, 6–10 μm diam, surrounded by small cells, subcircular to angular in outline, 3–6 μm diam, composed of radiating threads of oblong hyphal strands, cells 7–22 × 3.5–6.5 μm, pale to medium brown, fuscous, thick walled (–1 μm), smooth, simple or 1–3 times bifurcating, septate, ultimate branchlets with obtuse to pointed tips. Central columella below the scutellum delicate, easily collapsing and loose, ephemeral, about 25–30 μm wide, surrounded by small, thin walled, pseudoparenchymatous cells [according to Yokoyama & Tubaki (1971) composed of a single cell, 8–10 μm wide, usually surrounded by smaller, spherical, hyaline, fertile cells, 5–10 μm diam, that form a pseudoparenchymatous sheath]. Conidiophores reduced to conidiogenous cells, delicate, arising from the underside of the scutella, from parenchymatous cells around the columella, radiating, orientation outward and downward, 8–12 × 2–3 μm, subcylindrical, oblong-obclavate, attenuated towards a narrow tip, 0.5–1 μm diam, hyaline to pale olivaceous brown, thin-walled, smooth. Conidia solitary, globose to subglobose, 10–13 × 8–11 μm, length/width ratio 0.9–1.4, wall thin, up to 1 μm wide, hyaline to pale yellowish ochraceous, conidiogenesis phialidic, smooth, with inconspicuous to conspicuous basal hilum, occasionally somewhat peg-like and truncate when conspicuous. Microconidia not observed.
In vitro: On MEA with optimal growth at 20 °C, attaining 30–35 mm after 14 d, margin smooth, creamy white, pale ochraceous to pale tan, later cinnamon with reddish tint in zonate circles, viscid; aerial mycelium effuse, finely floccose, varying from thin to thick within concentric zonation, whitish to pale greyish brown; immersed hyphae rapidly growing, well-developed, pale ochraceous to pale greyish brown [sporulation not observed]. On potato sucrose agar moderately growing, white, clay-coloured, blackish brown, viscous, zonate; aerial mycelium effuse, finely floccose; immersed hyphae moderately developed, concolorous; reverse greyish fuscous. On OA rapidly growing, clay-coloured, olive brown to dark brown, with reddish tint, viscid, zonate; aerial mycelium effuse, finely floccose, concolorous; immersed hyphae rapidly growing, concolorous; reverse concolorous, sporulation abundant, evenly scattered. On Czapek agar growth lacking or very reduced (from Yokoyama & Tubaki 1971).
Type: Japan, Kyoto Pref., Kyoto, on Quercus glauca, 30 Jan. 1968, T. Yokoyama (NBRC H-11629 – holotype; NBRC 8931 = ATCC 22474 = CBS 193.71 = IMI 157596 = MUCC2310 – ex-type cultures).
Additional collection examined: Japan, Shiga Pref., Ootsu, on Quercus glauca, 27 Feb. 1970, T. Yokoyama, NBRC 9344 = MUCC2311 = CBS 124733.
Hosts range and distribution: On Quercus glauca, Fagaceae, Asia (Japan).
Notes: Paratubakia subglobosa and P. subglobosoides sp. nov. are characterised by having obtuse to acute scutellum tips, resembling scutella of Tubakia dryina and T. iowensis, but they are morphologically readily distinguishable from the latter two species by their globose, subglobose to broad ellipsoid conidia. Furthermore, they are phylogenetically clearly distinct from and not closely allied to T. dryina (Figs 1–3, 5), i.e., they cluster outside of the Tubakia s. str. clade.
Paratubakia subglobosoides C. Nakash., sp. nov. MycoBank MB823668. Fig. 9.
Fig. 9.
Paratubakia subglobosoides (NBRC H-11619 – holotype). A. Pycnothyria on the surface of leaf litter. B. Culture on MEA (NBRC 8931 – ex-type culture). C. Scutellum. D. Hyphal strands uprising radially from scutellum. E. Central columella. F. Conidiophore. G. Conidia. Bars = 10 μm.
Etymology: Composed of the epithet of the comparable species, Paratubakia subglobosa, and -oides (similar to).
Description in vivo: Living as endophyte in leaves, forming crustose conidiomata on the surface of leaf litter. Mycelium internal and external, forming hyaline, branched intra- and intercellular hyphae, external hyphae observed on the lower leaf surface, pale brown, branched. Conidiomata (pycnothyria) epiphyllous, scattered to gregarious, punctiform, superficial, easily removable, subcircular in outline when viewed from above, 80–130 μm diam, blackish (stereomicroscopy), scutellate, fixed to the leaf surface by a central columella. Scutella convex to flattened, membranous, dense to looser around the periphery, sometimes splitting with age, outline irregular, subcircular, with a central hyaline disc, 5–8 μm diam, surrounded by small cells, subcircular to angular in outline, 2–3 μm diam, composed of radiating threads of oblong hyphal strands, often uprising radially from scutella, cells 5–25 × 2–5 μm, pale to medium brown, fuscous, thick-walled (–1 μm), smooth, simple or 1–3 times bifurcating, septate, ultimate branchlets with pointed tips. Central columella below the scutellum delicate, easily collapsing and loose, ephemeral, about 25–33 μm wide, surrounded by large, thin walled, brown cells, 4–8 μm diam. Conidiophores reduced to conidiogenous cells, delicate, arising from the underside of the scutella, from large brown cells around the columella, radiating, orientation outward and downward, subcylindrical, oblong-obclavate, attenuated towards a narrow tip, 0.5–1 μm diam, 8–18 × 2–4 μm, pale olivaceous brown to pale brown, thin-walled, smooth. Conidia solitary, subglobose to ellipsoid, 10–12.5 × 5.5–10 μm, length/width ratio 1.27–2, wall thin, to 1 μm wide, hyaline to pale yellowish ochraceous, smooth to faintly rough, conidiogenesis phialidic, with inconspicuous to conspicuous basal hilum, occasionally somewhat peg-like and truncate when conspicuous. Microconidia not observed.
In vitro: On MEA with optimal growth at 20 °C, attaining 38–40 mm after 14 d, margin indefinite, at first pale olivaceous, reverse pale olivaceous brown. On the dried culture prepared from the culture NBRC 9343, abundant sporulation was observed.
Type: Japan, Shiga Pref., Ootsu, on Quercus glauca, 27 Feb. 1970, T. Yokoyama (NBRC H-11619 – holotype; NBRC 9343 = MUCC2293 – ex-type culture).
Hosts range and distribution: On Quercus glauca, Fagaceae, Asia (Japan).
Notes: Paratubakia subglobosoides is phylogenetically (Figs 1–3, 5) closely allied to P. subglobosa and the pycnothyria formed by the two species are morphologically similar. However, the conidia in P. subglobosa are globose-subglobose, with a smaller length/width ratio of 0.9–1.4, and this species forms distinct leaf spots. Furthermore, the culture characteristics of the two species on MEA are quite different, and they are genetically distinct (Figs 1–3, 5).
Racheliella Crous & U. Braun,gen. nov. MycoBank MB824487.
Etymology: Named for Rachel Wingfield, the first grandchild of Michael J. Wingfield and Brenda D. Wingfield, born 27 January 2018.
Genus of Tubakiaceae. Saprobic and plant pathogenic (possibly endophytic) on Syzygium spp. (Myrtaceae). Mycelium internal, forming branched intra- and intercellular hyphae. Asexual morphs forming stromatic (crustose) conidiomata on shed leaves (litter) or superficial pycnothyria on leaf spots of living leaves. Pycnothyria amphigenous, scattered, separate to gregarious, punctiform, blackish, circular or subcircular, superficial; scutella convex to flattened, membranous, dense at the centre, looser at the edge, with radiating hyphal strands, bifurcating, ultimate branchlets subcylindrical to attenuated towards the tips, obtuse to pointed; central columella below the scutellum delicate, easily collapsing and loose; conidiophores reduced to conidiogenous cells or with a supporting cell, orientation outward-downward, subcylindrical to ampulliform, hyaline, thin-walled, smooth, apex truncate, phialidic, with distinct periclinal thickenings or minute percurrent proliferation; conidia solitary, ellipsoid to obovoid, hyaline to subhyaline, guttulate, smooth, apex obtuse, base with conspicuous basal hilum. Stromatic (crustose) conidiomata on lead leaves, superficial to semi-immersed, pigmented, wall pseudoparenchymatous, cells of textura angularis; conidiophores compactly arranged above the pseudoparenchymatous tissue layer, relatively long, branched, septate, hyaline, phialidic with a flared, conspicuous collarette with a serrate margin; conidia solitary, fusiform to oval or obovoid, aseptate, pale brown to olivaceous brown.
Type species: Racheliella wingfieldiana Crous & U. Braun.
Notes: A new tubakia-like species found in South Africa on Syzygium guineense clusters together with Greeneria saprophytica, described from Thailand on old shed leaves of Syzygium cumini (Fig. 1). The two taxa are undoubtedly closely allied but unfortunately no culture or DNA of Greeneria saprophytica was available to generate more sequence data for comparison. However, based on ITS, these two species are 464/553 (84 %) similar. They cluster outside of Tubakia s. str. and distant from all other recognised genera of Tubakiaceae, suggesting that they pertain to a genus of their own. The pycnothyria of the type species are reminiscent of conidiomata of other genera of Tubakiaceae. Colourless conidia are in line with other tubakioid genera excluded from Tubakia s. str. The stromatic conidiomata formed by Greeneria saprophytica clearly differ from crustose conidiomata of all other tubakioid genera in having branched, septate conidiophores with phialidic conidiogenous cells provided with flared, conspicuous collarettes with serrate margin.
Racheliella saprophytica (N. Tangthirasunun et al.) Crous & U. Braun, comb. nov. MycoBank MB824498.
Basionym: Greeneria saprophytica N. Tangthirasunun et al., Phytotaxa 184(5): 277. 2014.
Illustrations: Tangthirasunun et al. (2014: 279, fig. 2, 280, fig. 3).
Description in vivo: Saprobic on dead leaves. Conidiomata stromatic (crustose), 140–175 μm diam, superficial to semi-immersed, separate, dark olivaceous to black; wall 3–7 layered, 30–44 μm wide, pale to moderately dark brown, with a well-developed pseudoparenchymatous layer of cells of textura angularis at the base that becomes thinner at the margin of conidiomata. Conidiophores 24–44 × 3–4 μm, septate, branched, compactly arranged above the pseudoparenchymatous tissue layer, hyaline, cylindrical, tapered towards the apex, smooth. Conidiogenous cells enteroblastic, phialidic with a flared, conspicuous collarette with a serrate margin, rarely percurrently proliferating, discrete or integrated, cylindrical to vase-like, tapered towards the apex. Conidia fusiform to oval or obovoid, with a rounded apex and narrowly truncate to pointed base, 9–15 × 5–6 μm, length/width ratio 2.2:1, aseptate, pale brown to olivaceous brown, thick-walled, smooth-walled, with one to many oil droplets.
In vitro: Colonies on PDA medium to dark brown from above and reverse, with medium to dense surface mycelium, flat, irregular to rhizoidal, with undulate to lobate margin, attaining 25 mm diam in 7 d at 27 °C; sporulating after 14 d.
Types: Thailand, Chiang Rai, Mae Fah Luang University campus, on dead leaves of Syzygium cumini, 21 Mar. 2012, N. Tangthirasunun (MFLU 13-0255 – holotype; PDD 105206 – isotype; MFLUCC 12-0298 = NTCL 052-1 = ICMP 20057 – ex-type cultures).
Host range and distribution: Only known from the type collection.
Notes: The position of Greeneria saprophytica in the consensus tree published in Tangthirasunun et al. (2014) clearly suggested that this species and G. uvicola, the type species of Greeneria, are not congeneric. Tubakia species were not included in the phylogenetic analyses which led to an allocation of the new species collected in Thailand on dead leaves of Syzygium cumini to Greeneria. In the current analyses, the sequences retrieved from the ex-type culture of G. saprophytica cluster within the Tubakiaceae, but outside of the Tubakia s. str. clade, i.e., this species does not belong to the latter genus (Fig. 1). A collection of “Tubakia sp.” found in South Africa on Syzygium guineensis is closely allied and clusters together with G. saprophytica and the two species require a genus of their own. At first glance, Greeneria saprophytica seems to be morphologically quite distinct from species of Tubakia s. lat. However, the conidiomata of the latter species are reminiscent of those of Tubakia californica, a species that does not form any pycnothyria but only stromatic conidiomata, usually (50–)80–200(–220) μm diam, composed of basal stromatic layers and phialidic conidiogenous cells. The size of the conidiomata in Greeneria saprophytica and Tubakia californica is comparable. Aseptate pigmented conidia are common in Tubakia s. lat. A clear difference between Greeneria saprophytica and all species hitherto assigned to Tubakia lies in the characters of the conidiophores, which are longer, septate and sometimes branched in G. saprophytica [vs. shorter, unbranched, usually aseptate (conidiophores reduced to conidiogenous cells) in species of all other genera of Tubakiaceae]. In addition, the conidiogenous cells have flared collarettes with serrate margins. G. saprophytica is phylogenetically allied to Tubakia thailandensis, which differs in forming small pycnothyria, aseptate, unbranched conidiophores, and subglobose, colourless conidia. Stromatic conidiomata are not developed. G. saprophytica found in Thailand on Syzygium cumini was assigned to Greeneria (Melanconiellaceae) owing to morphological similarities of the conidiomata, but since this species pertains to the Tubakiaceae it has to be excluded from Greeneria and is reallocated together with a new species on Syzygium from South Africa to the new genus Racheliella.
Racheliella wingfieldiana Crous & U. Braun, sp. nov. MycoBank MB824490. Fig. 10.
Etymology: Named for the Wingfield family, Michael J. Wingfield (South African mycologist and forest pathologist), his wife Brenda D. Wingfield (South African fungal geneticist), Beverley Wingfield (daughter and fanatical cyclist), and Anthony and Jane Wingfield, the parents of Rachel Wingfield.
Description in vivo: Occurring on leaves, forming distinct leaf lesions, amphigenous, shape and size variable. Mycelium internal, forming branched intra- and intercellular hyphae. Conidiomata (pycnothyria) amphigenous, scattered on unaffected portions of leaves, separate to gregarious on leaf spots, punctiform, blackish, circular or subcircular when viewed from above, superficial, easily removable, scutellate, fixed to the leaf by a central columella, 80–130 μm diam. Scutella convex sometimes more flattened, membranous, dense at the centre, looser at the edge, with a central hyaline or pale disc, giving rise to radiating hyphal strands, cells 5–10 × 2–5 μm, dark brown, thick-walled (–1 μm), smooth, simple or 1–3 times bifurcating, ultimate branchlets subcylindrical to attenuated towards the tips, obtuse to pointed. Central columella below the scutellum delicate, easily collapsing and loose, ephemeral, about 20–30 μm wide, surrounded by large brown cells. Conidiophores reduced to conidiogenous cells or with a supporting cell, arising from the underside of the scutella, around the columella, radiating, orientation outward-downward, subcylindrical to ampulliform, 6–10 × 3–4 μm, hyaline, thin-walled, smooth, apex truncate, phialidic, with distinct periclinal thickenings or minute percurrent proliferation. Conidia solitary, ellipsoid to obovoid, (11–)12–14(–15) × (6.5–)7(–7.5) μm, hyaline to subhyaline, guttulate, smooth, apex obtuse, base with conspicuous hilum (frill), peg-like and truncate, 1 μm diam.
In vitro: Colonies flat, spreading, with sparse aerial mycelium and feathery, lobed margins, reaching 60 mm diam after 2 wk. On MEA, PDA and OA surface isabelline with cinnamon spore masses, reverse isabelline. On PDA, surface olivaceous to isabelline, reverse honey to isabelline.
Type: South Africa, Eastern Cape Province, Haga Haga, on leaves of Syzygium guineense, 6 Mar. 2007, M.J. Wingfield (CBS H-23399 – holotype; CBS 143669 = CPC 13806 – ex-holotype culture).
Host range and distribution: Only known from the type collection.
Saprothyrium U. Braun, Crous & J.Z. Groenew., gen. nov. MycoBank MB824491.
Etymology: Composed of “sapro-“ (saprobic) and -thyrium (referring to the conidiomata [pycnothyrium]).
Type species: Tubakia thailandensis Senan. et al. [≡ Saprothyrium thailandense (Senan. et al.) U. Braun, Crous & J.Z. Groenew.].
Genus of Tubakiaceae. Foliicolous, saprobic (perhaps also living as endophyte). Mycelium internal. Asexual morph forming superficial pycnothyria on dead leaves; pycnothyria usually circular or subcircular when viewed from above, superficial, easily removable, scutellate, fixed to the leaf by a central columella; scutellum convex, membranous, dense, outline irregular, composed of hyphal strands, arising from a small central disc, cells thick-walled, pigmented, ultimate tips of the hyphal strands obtuse; conidiophores reduced to conidiogenous cells, small, arising below the scutellum, phialidic, with a minute collarette and wide periclinal thickening; conidia formed singly, globose, subglobose, wall thickened, hyaline, smooth; microconidia not observed.
Notes: Tubakia thailandensis was not included in the phylogenetic analyses used to generate Fig. 2 as only ITS and LSU sequence data are available for it. Based on a megablast search against the ITS sequences used in the combined alignment, the closest match was T. dryinoides with 89 % (404/452). It is also not included in the RPB2 tree, but its position in the LSU tree and a comparision with other species included in the LSU as well as the RPB2 tree clearly indicate that T. thailandensis clusters within the Tubakiaceae, but outside of the Tubakia clade, i.e., this species has to be excluded from Tubakia s. str. It forms a separate lineage in the LSU tree close to Racheliella, which justifies the classification as genus of it own. Pycnothyria of Saprothyrium are well characterised by having obtuse outer tips of the scutellum strands combined with globose-subglobose colourless conidia, and shares these traits with the genus Sphaerosporithyrium, which is, however, phylogenetically distant, clustering in basal position to Tubakia s. str. Colourless globose-subglobose conidia formed in pycnothyria distinguish Saprothyrium from all species of Tubakia s. str. in terms of morphology.
Saprothyrium thailandense (Senan. et al.) U. Braun, Crous & J.Z. Groenew., comb. nov. MycoBank MB824499.
Basionym: Tubakia thailandensis Senan. et al., Stud. Mycol. 86: 281. 2017.
Illustration: Senanayake et al. (2017: 280, fig. 34).
Description in vivo: Saprobic on dead leaves. Conidiomata 40–50 μm high, 50–75 μm wide, pycnothyria with radiate scutella, scattered to gregarious, superficial on the substratum. Scutella convex, brown to dark brown, thick-walled cells, radiating from a central point, ultimate tips obtuse, not pointed. Conidiophores short, forming under the developing scutella. Conidiogenous cells 5–10 μm high, 2–4 μm wide, phialidic, with a minute collarette and wide periclinal thickening. Conidia globose to subglobose, 10–12.5 × 7.5–8.5 μm (on average 11.3 × 8.1 μm, n = 20), smooth, hyaline, thick-walled.
In vitro: Mycelium on PDA white when young, dark green, light grey to black from above and reverse when aged, with medium mycelium, flat, rhizoid to irregular form, lobate margin, and attaining a diam of 46 mm in 7 d at 27 °C.
Type: Thailand: Chiang Rai: Doi Mae Salong, on dead leaf, K. Wisitrassameewong, 2 May 2012, NTCL059 (MFLU 13–0260 – holotype; MFLUCC 12–0303 – ex-type culture).
Notes: Saprothyrium thailandense is morphologically close to “Tubakia sp.” on Castanea henryi in China described and illustrated in Braun et al. (2014). The relation between S. thailandense, collected as saprobic fungus on dead leaves in Thailand, and Tubakia sp., found in China on distinct leaf spots on Castanea henryi, is unclear. It is also unclear if the leaf spots associated with “Tubakia sp.” in China are truly caused by this tubakia-like fungus. The leaves were covered with lesions of several other fungi, including Tubakia chinensis, i.e., it is possible that it represents an endophyte or saprobic species forming conidiomata on necrotic tissue caused by other fungi. Culture and sequence data are not available for the Chinese Tubakia, although urgently necessary for a comparison of the two taxa.
Sphaerosporithyrium U. Braun, Crous, O. Moreno-Rico & Marm., gen. nov. MycoBank MB824492.
Etymology: Composed of “sphaero-“ (globose), “spori-“ (spore) and “-thyrium (referring to pycnothyrium, the special conidioma type).
Type species: Sphaerosporithyrium mexicanum O. Moreno-Rico, U. Braun & Marm.
Genus of Tubakiaceae. Living as endophyte in leaves and causing leaf spots. Mycelium internal. Asexual morph forming superficial pycnothyria on leaf spots of living leaves. Pycnothyria usually circular or subcircular when viewed from above, superficial, easily removable, scutellate, fixed to the leaf by a central columella; scutellum flattened-convex, membranous, compact, dense, uniformly pigmented, pale to medium dark brown, or with a darker central zone, outline regular, subcircular, composed of hyphal strands, mostly branched, thick-walled, pigmented, ultimate tips of the hyphal strands obtuse or even truncate; conidiophores usually reduced to conidiogenous cells, conical, ampulliform-cuspidate, subcylindrical, delicate, not very conspicuous, arising from the underside of the scutella, around the upper portion of the columella, radiating, conidiogenous cells phialidic; conidia formed singly, globose, subglobose to broad ellipsoid-ovoid, thin-walled, hyaline or with a very pale greenish to olivaceous tinge, smooth; microconidia not observed.
Notes: A new undescribed tubakia-like species was collected in Mexico on Quercus eduardi. Sequences retrieved from the new Mexican species cluster in all phylogenetic trees close to but always outside of the Tubakia s. str. clade (Figs 1–3, 5), suggesting that this species cannot be assigned to the latter genus, i.e., it warrants a genus of its own. Owing to the conidial shape and colour, the new genus Sphaerosporithyrium is morphologically comparable with Paratubakia. Pycnothyria formed by species of the latter genus differ, however, in having pointed tips of hyphal scutellum strands. Colourless globose-subglobose conidia clearly distinguish Sphaerosporithyrium from all species of Tubakia s. str.
Sphaerosporithyrium mexicanum O. Moreno-Rico, U. Braun & Marm., sp. nov. MycoBank MB823664. Fig. 11.
Fig. 11.
Sphaerosporithyrium mexicanum (CFNL 2945 – holotype). A. Leaf spots on Quercus eduardi. B. Close-up of a leaf spot. C. Culture on MEA. D, E. Two pycnothyria and conidia. F. Three pycnothyria (SEM picture). Bars = 1 cm (A), 0.5 cm (B), 20 μm (D, E).
Etymology: Named after Mexico, the country where this species was collected.
Description in vivo: Leaf spots amphigenous, subcircular to angular-irregular, 2–10 mm diam or confluent and larger, developing to large blotches, 10–40 mm diam, pale to medium dark brown, centre sometimes paler, dingy greyish white, margin indefinite or with darker brown to reddish brown margin or marginal line, sometimes zonate. Conidiomata (pycnothyria) amphigenous, more abundant on the upper leaf surface, scattered to gregarious, punctiform, superficial, easily removable, circular to subcircular in outline, 80–120 μm diam, dark brown to blackish (stereomicroscopy), scutellate, fixed to the leaf surface by a central columella. Scutella convex to flattened-convex, membranous, compact, dense, outline regular, with a central colourless or pale disc, 8–20 μm diam, scutellum uniformly pigmented (microscopy) pale to medium dark brown, or with a darker central zone, central cells subcircular or angular-irregular in outline, 3–8 μm diam, giving rise to radiating threads of hyphal cells, 1–3 times bifurcating, cells 6–30 × 2–6 μm, walls to 1.5 μm thick, tips of the threads simple or 1–2 times forked, branchlets short, ultimate tips consistently obtuse or even truncate; central columella below the scutellum delicate, easily collapsing and loose, short, composed of thin-walled, colourless or pale fertile cells, circular to somewhat angular-irregular in outline, 2–6 μm diam. Conidiophores reduced to conidiogenous cells, rarely 1–2-septate, arising from the underside of the scutella, around the upper portion of the columella, radiating, conical, ampulliform-cuspidate, subcylindrical, delicate, not very conspicuous, about 6–14 × 3–5 μm, hyaline, thin-walled, smooth. Conidia solitary, globose, subglobose to broad ellipsoid-ovoid, small conidia 7–10 × 5–8 μm, larger fully developed conidia 9–16(–19) × (7–)9–12 μm, length width ratio 1.0–1.5(–1.6), aseptate, apex rounded, base rounded or with a minute truncate hilum or even with a small peg-like base, thin-walled, hyaline or with a very pale greenish to olivaceous tinge, smooth. Microconidia not observed in vivo.
In vitro: On MEA at 22 °C colonies attaining 67–69 mm diam after 16 d, margin undulate, with concentric rings of aerial mycelium, center white, reverse colourless, without sporulation.
Type: Mexico, Aguascalientes, San José de Gracia, Las Manzanitas, 22°11’31.3”N, 102°36’50.4”W, 2 612 m alt, on Quercus eduardi, 19 Jan. 2017, O. Moreno-Rico (CFNL 2945 – holotype; CFNL 2945 = CPC 33021 – ex-type cultures).
Additional collections examined: Mexico, Nuevo León, Iturbide, Bosque Escuela, 24°42’23.9”N, 99°51’44.2”W, 1 618 m alt, on Quercus eduardi, 4 Nov. 2016, J. Marmolejo, HAL 3180 F; CFNL 2942; ibid., 24°42’23.9”N, 99°51’44”W, 1 618 m alt, on Q. eduardi, 10 Nov. 2016, J. Marmolejo, HAL 3183 F; CFNL 2941 = CPC 32258.
Notes: Sphaerosporithyrium mexicanum is well-characterised by a combination of globose, subglobose to broad ellipsoid-ovoid conidia and pycnothyria with obtuse to truncate tips of the radiating scutellum threads. The conidial shape is reminiscent of Paratubakia subglobosa conidia, but the latter species, known from Asia on Quercus glauca (≡ Cyclobalanopsis glauca), differs in having scutella with pointed tips of radiating hyphal threads. The status of S. mexicana as species in its own right is fully supported in the phylogenetic trees (Figs 1–3, 5).
Tubakia B. Sutton, Trans. Brit. Mycol. Soc. 60: 164. 1973, emend.
Basionym: Actinopelte Sacc., Ann. Mycol. 11: 315. 1913, non Sitzenb., 1861.
Type species: Actinopelte japonica Sacc. [≡ Tubakia japonica (Sacc.) B. Sutton].
Genus of Tubakiaceae. Living as endophytes in leaves and twigs, and pathogenic, forming distinct leaf lesions. Asexual morphs forming sporodochial or crustose to pustulate, pycnidioid, stromatic, unilocal conidiomata on petioles and twigs, erumpent, dehiscing by irregular fissures, and superficial pycnothyria on living, faded or shed leaves, without distinct symptoms on living leaves or forming distinct leaf spots. Pycnidia usually circular or subcircular when viewed from above, superficial, easily removable, scutellate, fixed to the leaf by a central columella; scutellum composed of loose to dense hyphal strands, mostly branched, thick-walled, pigmented, margin compact or outer portions of the radiating hyphal strands looser to free, tips rounded, truncate or pointed, margin usually not recurved; conidiophores reduced to conidiogenous cells, usually subcylindrical-conical, lageniform, hyaline to pale brown, arising from small, colourless fertile cells around the upper part of the central pycnothyrial columella, conidiogenous cells phialidic, percurrently proliferating, sometimes forming indistinct periclinal thickenings or annellations (collarettes); conidia formed singly, globose to broad ellipsoid-obovoid, sometimes subcylindrical or somewhat irregular, wall thin to somewhat thickened, smooth to faintly rough, hyaline to pigmented, apex rounded, base rounded to attenuated, sometimes with distinct frill or peg-like basal hilum. Sexual morph: Diaporthaloid, dicarpella-like, forming dark stromatic pseudoparenchymatic layers with pigmented, dark perithecia on fallen overwintered leaves, rostrate, beak short, usually lateral-eccentric, slightly protuberant, ostiolate, ostiole periphysate, peridium variable in thickness, paler than stromatic layers, polyascal; asci unitunicate, 8-spored, oblong-ellipsoid, stalk short to oblong, ascal apex with two refractive conoid structures, asci deliquescing at maturity; paraphyses lacking; ascospores more or less uniseriate, becoming irregularly biseriate, one-celled, hyaline, ellipsoid to fusiform, often inequilateral or slightly curved, wall finely ornamented, content granular-guttulate. Putative sexual morph: Dicarpella dryina (see Tubakia suttoniana).
Notes: In its traditional circumscription, Tubakia s. lat. comprises a wide range of species with characteristic superficial conidiomata composed of radiating scutella, a basal columella fixing the scutellum to the leaf surface, and colourless phialidic conidiogenous cells arising from fertile cells around the upper part of the columella bearing solitary, one-celled, colourless to pigmented conidia. However, results of phylogenetic analyses revealed the heterogeneity of Tubakia s. lat. and led to a new circumscription of Tubakia s. str. (emend.) which forms a separate well-supported clade within the family Tubakiaceae evident in the rpb2 and LSU trees (Figs 1, 2). Species clustering outside of the Tubakia clade, belonging to other lineages, are excluded and assigned to other (new) genera. Tubakia species may form different types of asexual morphs. Pycnothyria with typical scutella are characteristic, diagnostic and commonly formed. In addition, sporodochial conidiomata composed of clusters of conidiogenous cells may be developed, e.g. on leaf veins formed by T. iowensis. Crustose or pustulate pycnidioid conidiomata may be formed by several Tubakia species, including T. californica, T. dryina, and T. iowensis. In T. californica they are the only hitherto known fructification. They are mostly formed on petioles and leaf blades (often on and close to veins) of old necrotic leaves, either shed (litter) or still attached as in the case of T. californica. The classification of these conidiomata caused some uncertainty and confusion. Holdenrieder & Kowalski (1989) designed these conidiomata as “pycnidial” although they clearly described non-ostiolate conidiomata dehiscing by irregular rupture. Harrington et al. (2012) used the general term “conidioma” and added “pycnothyrium” in brackets. These conidiomata cannot be referred to as pycnidia owing to lacking ostioles, but due to stromatic wall layers and a dehiscence by irregular fissures they should rather be classified as stromatic.
Tubakia americana(Höhn.) T.C. Harr. & McNew, Antonie van Leeuwenhoek J. Microbiol. Serol. doi.org/10.1007/s10482-017-1001-9 [14]. 2017.
Basionym: Actinopelte americana Höhn., Mitt. Bot. Inst. Techn. Hochsch. Wien 2(3): 68. 1925.
Illustrations: Harrington & McNew (2018, figs 2, 4).
Description in vivo: Leaf and twig endophyte, also causing leaf spots, amphigenous, subcircular to angular-irregular, 1–18 mm diam, brown, with narrow darker margin, dark violet, brown to blackish or along necrotic leaf veins. Conidiomata (pycnothyria) amphigenous, mainly hypophyllous, superficial, scattered to gregarious, dark brown to blackish, 40–115 μm diam, about 30 μm high, circular in outline, scutellate, fixed to the leaf surface by a central columella. Scutella somewhat convex, centre with a colourless or pale disc, about 5–30 μm diam, surrounding small cells, about 2.5–3 μm diam, giving rise to radiating threads of hyphal cells, simple to often 1–4 times bifurcating, cells 5–20 μm long and 2–5 μm wide, wall about 1 μm thick, ends of the radiating threads simple or furcate, tips obtuse; central columella below the scutellum parenchymatic, colourless or pale, about 20 μm high and 25–30 μm broad; Conidiophores reduced to conidiogenous cells, delicate, formed around the upper part of the columella. Conidia solitary, broad ellipsoid(-ovoid), 9–13(–14) × 6–8.5 μm, aseptate, subhyaline or pale to medium olivaceous brown, wall thin (0.5–1 μm), smooth or almost so. Microconidia sometimes produced from same pycnothyrium as macroconidia or from smaller pycnothyria, hyaline, fusiform, curved, aseptate, (3.5–)4–7 × 2–3 μm. Crustose conidiomata forming in winter or spring, erumpent on twigs or inside of acorn caps, black, irregular in shape, 150–500 μm diam, covering layer composed of thick-walled cells, textura angularis, dehiscing marginally or by irregular fissures; conidiophores lining inside of crustose conidiomata; conidia thick-walled, hyaline to light brown, obovoid to ellipsoidal, aseptate, 9.5–14 × 5.5–7.5 μm.
In vitro: On MYEA with optimal growth at 25 °C, attaining a diam of 56 to 64 mm after 7 d, initially with a distinct ring of dense aerial mycelium, later developing concentric rings of fluffy white to grey aerial mycelium with wet conidial masses that are initially hyaline, becoming olive green then black, coalescing into large areas. Conidia in culture abundant, thick-walled, smooth to finely varicose, hyaline to dark brown, obovoid to ellipsoidal, aseptate, 9–15(–16.5) X (4–)4.5–7.5(–8) μm.
Type: USA, New Jersey, Newfield, on Quercus coccinea, Aug. 1883 [Ellis & Everh., Fungi Columb. 286] (NY – lectotype, designated by Harrington & McNew 2017; isolectotypes – Ellis & Everh., Fungi Columb. 286 [e.g. BPI 390073, 390075, BRU 1082, CUP, ISC 453285, NY, WIS-F-120]; topotypes [from 1881 and 1894] – Ellis & Everh., N. Amer. Fungi 732, 3168 [e.g., CUP-A-33522, 33530; ILLS 45680, 45682, 73344; MU 246655]).
Host range and distribution: on Quercus (bicolor, coccinea, macrocarpa, robur, rubra), Fagaceae, North America (USA, Illinois, Iowa, Missouri, Wisconsin).
Notes: Höhnel (1925) described Actinopelte americana based on a North American collection on Quercus coccinea and distinguished his new species from T. dryina by having smaller conidia. Type material of this species has been examined and shown to be morphologically rather similar to T. dryina, but distinguishable by having obtuse (non-pointed) ultimate tips of radiating hyphal scutellum threads. Attempts to locate the particular specimen of Fungi Columb. 286 that Höhnel had examined failed. Therefore, Harrington & McNew (2018) designated a duplicate from the McClatchie Herbarium in NY as lectotype, which, however, contains pycnothyria of two Tubakia spp. associated with different necrotic spots. One of these Tubakia species has relatively small conidia, as described by Höhnel (1925) for A. americana, and the tips of the radiating scutellum strands are blunt (Harrington & McNew 2018, fig 2a, b). The second type of pycnothyria on the lectotype material has scutella with acute tips and larger conidia (Harrington & McNew 2018, fig 2c), rather matching the wide concept of T. macnabbii (Harrington & McNew 2018). According to Harrington & McNew (2018), other duplicates of Fungi Columb. 286 in NY and ISC each contained leaves with two Tubakia species. In other duplicates, e.g., BPI 390073, 390075, only the small-spored Tubakia with obtuse tips of scutellum strands has been observed. The lectotypification proposed by Harrington & McNew (2018) is reasonable, follows and maintains Höhnel’s original description.
Harrington & McNew (2018) managed to extract DNA form the lectotype of A. americana and a generated sequence matched that of Tubakia sp. A (Harrington & McNew 2016; Harrington et al. 2012), which they have commonly isolated as a twig and leaf endophyte in Q. macrocarpa and other Quercus spp. in Iowa, often also associated with leaf spots and necrotic veins (Harrington & McNew 2018, fig 4).
Tubakia americana is phylogenetically (Figs 3, 5) closely related to T. dryina, and both species produce crustose pycnothyria on twigs (Harrington et al. 2012), but the two species are readily distinguishable by differences in the pycnothyrial scutella (tips of the scutellum strands obtuse in T. americana and pointed in T. dryina). In culture, the mycelium of T. americana is much lighter in colour than the dark mycelium of T. dryina (Harrington et al. 2012). Species of Quercus sect. Quercus (Q. bicolor, Q. macrocarpa and Q. robur) seem to be the primary hosts of T. americana in Iowa (Harrington et al. 2012). The “T. americana” ITS cluster published in Harrington et al. (2012) is divided in two groups – the upper one composed of North American sequences represents T. americana, and the lower one is composed of allied cryptic taxa from Eurasia. The Asian taxon in this group is described herein as T. dryinoides. The European sequences based on isolates from France might represent another cryptic species, but due to insufficient sampling and lacking ecological and morphological data we prefer to maintain them in T. dryinoides, at least tentatively. ITS sequences retrieved from leaves of Quercus robur and Fagus sylvatica in Poland (haplotype 2 in Boroń & Grad 2017) seem to belong to this cryptic European taxon and suggest that it might be rather common.
Tubakia californica S. Rooney-Latham & U. Braun, sp. nov. MycoBank MB823661. Figs 12–14.
Fig. 12.
Tubakia californica (on Quercus kellogii, non-preserved sample). A. Conidiomata on a necrotic leaf (close-up). B. Old conidiomata on a petiole (close-up). C. Crushed conidiomata and conidia. D. Conidia. Bars = 1 mm (A, B), 20 μm (C), 5 μm (D).
Fig. 14.
Tubakia californica, symptoms on other Fagaceae hosts. A. Tanoak (Notholithocarpus densiflorus) tree in Humboldt Co., CA with significant dieback of the lower two-thirds of the crown foliage. B. Canopy loss and dieback on an interior live oak (Quercus wislizeni) tree in Shasta Co., CA. C. Tanoak leaf with marginal necrosis. D. Tanoak leaf with necrotic spots and dark midrib. E. Interior live oak leaf with necrotic veins. Bars (C–E) = 1 cm.
Etymology: Named after California, the origin of the type collection and numerous additional isolates.
Description in vivo: Living as endophyte in leaves and twigs, causing necrotic leaf lesions and twig dieback. Symptoms and development on Quercus kelloggii: Deciduous leaves of affected trees do not fully defoliate in the fall. Symptomatic trees are primarily mature trees, approximately 25 years and older; dry, brown leaves from the previous seasons’ growth remain attached on branches in the spring; symptom progression in the late summer and fall is variable depending on location; in the California foothills where the disease is prominent, the new distal growth appears healthy until late August when small, brown lesions develop on the leaves, predominantly on the abaxial side; irregular, brown lesions with chlorotic borders may also occur near the leaf margins; lesions enlarge over time, and by early September, the associated lateral leaf veins become brownish black, by late September, more than half of the leaf area on affected leaves is brown and discoloration of the veins extends to the midrib; in October, many of the affected leaves become dry and uniformly brown and most leaf veins turn black; small, black, embedded crustose Tubakia conidiomata with conidia develop in late September on symptomatic petioles and leaves, often associated with the veins, but also on the leaf blade. Conidiomata crustose, pycnidioid, stromatic, scattered to loosely aggregated, subepidermal, erumpent, at first “closed” (covered by a black stromatic layer), protruding portion hemispherical to depressed-convex (hourglass-shaped), dehiscing by irregular fissures, finally exposed, widely opened, subcircular to somewhat irregular in outline, usually (50–)80–200(–220) μm diam, wall about 10–20 μm thick, black, crustose covering layer composed of large cells, mostly 4–10 μm diam, with thin to thickened walls, 0.5–1 μm wide, pale to medium dark brown, dark brown in mass, rounded, oblong to angular-irregular in outline (textura globulosa to angularis), basal layer paler; conidiogenous cell lining the base of the conidiomata, colourless, thin-walled, attenuated towards the tip, about 10 × 3–4 μm, but early collapsing and lapsing (hence detailed observations and measurements not possible); conidia solitary, broad ellipsoid, ellipsoid-ovoid to short and broad subcylindrical, straight, rarely slightly curved or somewhat irregular in shape, both ends rounded to subtruncate, basal frills or truncate peg-like bases not observed, 8–15 × 4.5–7 μm, length/width ratio 1.4–2.3 (on average 1.8), at first subhyaline to pale greenish, later greenish, pale olivaceous to brownish, wall thin, to about 0.5 μm, smooth or almost so (light microscopy), microconidia not observed (pycnothyria with free radiating scutella not developed).
In vitro: Optimal growth at 25–30°C on MEA, colonies attaining 60 mm after 7 d, dingy white to pale yellow with regular margin, becoming yellowish grey with concentric rings in reverse, conidial formation not observed. On PDA, colonies initially creamy white, becoming greyish beige and forming olivaceous brown concentric rings in reverse, margin irregular to scalloped. Conidiomata brownish black, forming in concentric rings; conidia broadly ellipsoid, ellipsoid-ovoid, straight, initially subhyaline, later becoming olivaceous brown, 10–14 × 7–9 μm (average 11.9 × 8.0), length/width ratio 1.2–1.9 (average 1.5).
Type: USA, California, Tuolumne County, Groveland, 956 m alt., on Quercus kelloggii, 19 Sep. 2014, J. Haas [dried culture, plated and dried 2017] (BPI 910537 – holotype; CBS 143670 = CPC 31505 = CDFA#1428, ex-holotype culture).
Additional cultures examined: See Table 1.
Additional material examined: USA, California, Tuolumne County, Groveland, on Quercus kelloggii, 5 May 2017, J. Haas (HAL 3212 F – paratype).
Hosts range and distribution: On Chrysolepis chrysophylla (≡ Castanopsis chrysophylla), Notholithocarpus densiflorus (≡ Lithocarpus densiflorus), Quercus (agrifolia, kelloggii, wislizeni), Fagaceae, North America (USA, California).
Notes: Tubakia californica is an endophyte found in California on several oak species and two additional fagaceous host species. It causes symptoms and lesions on the affected tree species, crustose, stromatic, pycnidioid conidiomata may be formed, but characteristic leaf spots spread over the whole leaf blade (comparable with those formed by T. dryina and most other Tubakia spp.) and pycnothyria are not developed or have at least not yet been observed. The leaf lesions caused by T. californica are rather reminiscent of those formed by T. iowensis. In October 2014, Tubakia was cultured from Q. kelloggii trees in California, El Dorado Co., with foliar symptoms similar to those seen in Groveland. Between 2012 and 2017, this fungus was also isolated from similar leaf lesions and twig dieback of other Fagaceae species throughout California including the counties of Contra Costa, Del Norte, Humboldt, Marin, San Luis Obispo, and Shasta. Overall, symptoms seem to develop later in the season in the Sierra Nevada foothill locations (Tuolumne Co. and El Dorado Co.) than in the other California counties. The elevations of the Groveland and the El Dorado Co. sites are 956 m and about 850 m respectively, while most of the other locations were at or near sea level. In addition to colder winters, both foothill locations leaf out later in the season, which may explain the difference in the onset of disease symptoms to the valley and coastal locations. Overall, disease severity was greater on trees in low areas and on branches in the lower portion of the canopy (unpublished observations).
Owing to lacking pycnothyria, a phylogenetic approach was required to evaluate the Californian taxon. Analyses of the sequence data and their position in the phylogenetic trees clearly favour a separate species. The T. californica clade is well supported in both combined analyses (Fig. 3: PP = 1, MP-BS 88 %; Fig. 4: PP = 1, MP-BS 99 %). Two cultures isolated from Tubakia on Quercus canbyi in Mexico (CPC 32250 and 32251) belong to this clade and are currently only tentatively assigned to T. californica since they are not identical to Californian strains. Whether they are conspecific with T. californica or if they represent a closely allied Mexican species cannot yet be determined without a larger sampling of Mexican collections. Conidiomata of T. californica are macroscopically not easily discernible on old necrotic leaves since several macroscopically similar coelomycetes may develop together with the crustose conidiomata of the new species. In an examined collection on Quercus kelloggii (HAL 3212 F), conidiomata of T. californica developed on leaves together with acervular conidiomata of Apiognomonia errabunda as dominating fungus. The conidia of the latter species are similar in length but narrower (about 6–17 × 3–5 μm), and consistently colourless.
Tubakia chinensis U. Braun, S. Bien & Hantsch, Schlechtendalia 28: 23. 2014.
Illustration: Braun et al. (2014: 24, fig. 1).
Description in vivo: Leaf spots amphigenous, subcircular to angular-irregular, 0.5–5 mm diam, at first dingy greenish to greyish green, later brownish to dingy greyish brown, finally grey to greyish white, with narrow darker margin, brown to reddish brown, finally darker, often slightly raised or limited by veins. Conidiomata (pycnothyria) epiphyllous, small lesions with a single pycnothyrium, larger ones with up to 18 pycnothyria, punctiform, black, circular or subcircular when viewed from above, superficial, easily removable, scutellate, fixed to the leaf by a central columella. Scutella convex, 135–200 μm diam, membranous, somewhat translucent (conidia more or less visible beneath the scutellum when viewed from above), with a central hyaline or pale disc, 15–20 μm diam, giving rise to radiating hyphae, cells (5–)8–15(–20) × 3–8 μm, medium brown, thick-walled (–1 μm), smooth, usually 1–3 times bifurcating, either only at the periphery or deeply cleft, ultimate branchlets with obtuse or often pointed tips. Conidiophores reduced to conidiogenous cells, arising from the underside of scutella around the upper part of the columella, radiating downward and towards margin, subcylindrical, subclavate, ampulliform, mostly attenuated towards the tip, straight to slightly curved, 10–20 × 4–8 μm, hyaline or subhyaline, thin-walled, smooth, with a single terminal locus, monophialidic. Conidia solitary, globose, subglobose or broad ellipsoid-obovoid, (20–)25–40 × 20–30 μm, length/width ratio 1.1–1.4, wall 0.7–1.2 μm wide, hyaline or pale, smooth, cell content pale brownish, sometimes somewhat granular, apex and base broadly rounded, with inconspicuous to conspicuous basal hilum, somewhat peg-like when conspicuous, about 4 μm wide and 1 μm high, with delicate frill. Microconidia not observed.
Type: China, Jiangxi Province, Xingangshan, subtropical forest site of the BEF-China Project, 29.1250°N, 117.9085°E, on living leaves of Castanea henryi, 8 Sep. 2013, S. Bien (HAL 2674 F – holotype).
Host range and distribution: on Castanea henryi, Fagaceae, Asia (China, Jiangxi Province).
Notes: Tubakia japonica (Yokoyama & Tubaki 1971) and T. seoraksanensis (Yun & Rossman 2011) form a group of Tubakia species with rather large conidia (length on average > 15 μm) to which T. chinensis belongs, but it can be easily differentiated by traits of the scutella and conidia. Tubakia japonica has much larger, colourless conidia, 40–55 × 35–45 μm, and forms microconidia, 5–7 × 1.5–2 μm (Yokoyama & Tubaki 1971), and T. seoraksanensis differs in having smaller scutella, 90–160 × 90–130 μm, narrower conidiogenous cells, 14–22 × 3–5 μm, and much smaller conidia, 13–25 × 10–15 μm. The size of mature conidia of T. chinensis does not overlap with those of T. japonica and T. seoraksanensis. Cultures and data of sequence analyses are not yet available for T. chinensis, i.e., the phylogenetic affinity of this species is still unknown. However, T. japonica and T. seoraksanensis are phylogenetically very closely related (Figs 3–5).
Tubakia dryina (Sacc.) B. Sutton, Trans. Brit. Mycol. Soc. 60: 165. 1973, emend. (s. str.).
Basionym: Leptothyrium dryinum Sacc., Michelia 1: 200. 1878.
Synonym: Actinopelte dryina (Sacc.) Höhn., Mitt. Bot. Inst. Techn. Hochsch. Wien 2(3): 69. 1925.
Literature: Jones & Holcomb (1978 – cytology and ontogeny), Glawe & Crane (1987 – comprehensive treatment), Holdenrieder & Kowalski (1989 – pathogenicity and fructification), Taylor & Clark (1996 – infection and development of pycnothyria), Taylor (2001 – ultrastructure of pycnothyria), Harrington et al. (2012: 88–89 – comprehensive survey, taxonomy).
Illustrations: Glawe & Crane (1987: 106, figs 1–3, 107, figs 4–11, 108, figs 12–17), El-Gholl et al. (1996: 2, fig. 2), Harrington et al. (2012: 88, fig. 3).
Description in vivo: Living as endophyte in leaves and twigs, forming crustose to pustulate pycnidial conidiomata on twigs, erumpent, and pathogenic, forming distinct leaf lesions, amphigenous, shape and size variable, subcircular to angular-irregular, 2–15 mm diam, yellowish ochraceous, straw-coloured to brown, finally sometimes dingy greyish brown, margin indefinite or with narrow darker border, dark purplish violet, brown, reddish brown to blackish, occasionally with a diffuse halo, sometimes forming large ochraceous to brown necroses, to 60 mm diam. Mycelium internal, forming branched intra- and intercellular hyphae. Conidiomata (pycnothyria) amphigenous, scattered to gregarious, punctiform, blackish, circular or subcircular when viewed from above, superficial, easily removable, scutellate, fixed to the leaf by a central columella, (40–)60–120(–140) μm diam. Scutella convex, sometimes more flattened, membranous, dense, compact, later sometimes less compact, looser, with a central hyaline or pale disc, 5–20 μm diam, surrounded by small cells, subcircular to angular in outline, 3–8 μm diam, giving rise to radiating hyphal strands, cells 6–30(–35) × 2–6(–7) μm, pale to medium dark brown, thick-walled (–1 μm), smooth, simple or 1–3 times bifurcating, ultimate branchlets with obtuse to mostly pointed tips (uniformly pointed or at least a large portion of tips being acute). Central columella below the scutellum delicate, easily collapsing and loose, ephemeral, about 10–20 μm wide, surrounded by small, thin-walled, colourless or pale (pseudoparenchymatous) cells. Conidiophores reduced to conidiogenous cells, arising from the underside of the scutella, around the columella, radiating, orientation outward-downward, conical, ampulliform-cuspidate, delicate, about 8–18 × 2–5(–6) μm, hyaline, thin-walled, smooth, apex obtuse to truncate, conidiogenesis phialidic, percurrently proliferating, sometimes forming indistinct periclinal thickenings or annellations (collarettes). Conidia solitary, broad ellipsoid-obovoid, (7–)9–16(–18) × (5–)6–10(–10.5) μm, length/width ratio 1.1–1.6(–2.2), wall thin, to 1 μm wide, at first hyaline, subhyaline, later pale olivaceous, olivaceous brown to brownish, smooth, old conidia occasionally faintly rough-walled, apex and base broadly rounded, with inconspicuous to conspicuous basal hilum (frill), occasionally somewhat peg-like and truncate when conspicuous. Microconidia formed in some collections, fusiform, mostly straight, 4–9 × 1.5–4 μm, hyaline, thin-walled, smooth. Crustose to pustulate pycnidioid conidiomata may be formed on twigs, black, circular, oblong to somewhat irregular in outline; pustulate conidiomata 0.2–1 mm diam, scattered to gregarious, wall to about 20 μm thick, composed of textura angularis, cells about 3–4 μm diam, at the base textura angularis or globosa, cells paler and thinner, forming a pulvinate conidiogenous layer, non-ostiolate, opening by irregular dehiscence; conidiogenous cells usually arising from the basal pulvinate layer, lageniform, 8–34 × 4–6 μm, straight to curved, tips of the neck 1.5–2(–2.5) μm wide, conidia developed in crustose conidiomata ellipsoid-obovoid, oblong or even slightly asymmetrical, 10–16 × 6–7.5(–8.5) μm, wall somewhat thickened, smooth to faintly rough, at first colourless, later somewhat pigmented, pale brown.
In vitro: on MEA with optimal growth at 25 °C, attaining 60–66 mm diam after 7 d, margin scalloped, at first creamy white, forming concentric rings of aerial hyphae, reverse in the middle dark grey, yellow to medium brown towards the rim at 10 d; sporodochial conidiomata abundantly formed at 10 d, in concentric rings, conidial mass dark grey to blackish, sporodochial conidia formed in culture, ellipsoid-ovoid, 10–16 × 5.5–8.5 μm, with somewhat thickened walls, finely rough, at first colourless, later somewhat pigmented.
Type: Italy, Veneto, Treviso, Bosco Montello, on Quercus robur, Sep. 1875 [Sacc., Mycoth. Ven. 555] (PAD, MBT379573 – lectotype designated here). Isolectotypes: Sacc., Mycoth. Ven. 555 (e.g. BPI 391920, CUP, HAL, ILLS 420). Italy, Veneto, Treviso, Fagaré Forest, on Quercus robur, undated, S. Mutto-Accardi (CBS H-23357, MBT379616 – epitype designated here; CBS 112097 – ex-epitype culture).
Host range and distribution: on Fagus sylvatica, Quercus (alba, macrocarpa, robur), Fagaceae, Europe (Germany, Italy, Netherlands, Poland, Romania, Russia, UK), North America (USA, Iowa, Louisiana), and New Zealand.
Notes: Tubakia dryina, originally described as leaf-spotting fungus on Quercus robur in Italy, was previously circumscribed and applied in a very broad sense comprising collections from Asia, Europe, and North America mainly on Quercus spp., but also on some additional fagaceous genera, e.g. on Castanea and Fagus spp., and in North America on host plants belonging to numerous other plant families (Glawe & Crane 1987, Yokoyama & Tubaki 1971, etc.). Numerous collections with leaf spots and pycnothyria morphologically indistinguishable from T. dryina have been examined: Mexico, Nuevo León, Linares, Vivero Facultad de Ciencias Forestales, 24°47’47.9”N, 99°32’30.9”W. 380 m alt, on Quercus shumardii, 9 Nov. 2016, J. Marmolejo, HAL 3181 F, 3182 F; CFNL 2940. USA (collections arranged according to host names and with herbarium accession numbers of BPI and ILLS – details of the particular collection available via: http://mycoportal.org/portal/collections/index.php): Acer saccharum, ILLS 29734, Acer sp., BPI 391880, Arbutus menziesii, BPI 391882, Castanea dentata, BPI 391884, C. sativa, BPI 390033, 390034, 391883, 391885, Cercis canadensis, ILLS 25737, Eucalyptus sp., BPI 391886, Fraxinus americana, ILLS 29744, F. nigra, ILLS 5562, F. pennsylvanica, BPI 391887, F. profunda, ILLS 29738, Nyssa sylvatica, BPI 391889–391891, Quercus alba, BPI 863075, Q. bicolor, ILLS 16125, Q. borealis, BPI 391905, 391906, Q. coccinea, BPI 390074, 391907, Q. ellipsoidalis, BPI 391908–391912, Q. falcata, ILLS 32849, Q. macrocarpa, BPI 291913–391914, Q. marilandica, ILLS 29755, 30075, 32836, 32841, Q. nigra, BPI 391918, 840864, Q. phelos, BPI 391921, 391922, Q. rubra, BPI 391917, 391928, 391929, 390077–390081, 390084, 390085, 391925–391927, 803135, 863076, Q. shumardii, ILLS 117552, Q. stellata, BPI 391930, Q. velutina, BPI 390087, 390088, 391931, 391933–391936, 863077, Q. virginiana, BPI 391932, Toxicodendron radicans [≡ Rhus radicans], ILLS 30072, Ulmus alata, ILLS 29740. First molecular examinations and analyses of T. dryina s. lat. carried out by Harrington et al. (2012) suggested that this species represents a strongly heterogeneous complex comprising several cryptic species. Some of them have recently been described by Harrington & McNew (2018), and additional ones are introduced in the present work. Host range and distribution of T. dryina s. str. is insufficiently known and probably largely unrevealed due to its basically endophytic life strategy. Documented collections usually refer to material with distinct leaf spots and developed pycnothyria. Nevertheless, T. dryina seems to be a primarily European species with Quercus robur as principal host plant on which this fungus has probably been introduced to other regions of the world, including proven cases in New Zealand and North America (USA, Harrington & McNew 2018). In the eastern USA, T. dryina s. str. occurs also on Quercus alba and Q. macrocarpa (Harrington et al. 2012, Harrington & McNew 2018). Therefore, it is currently not possible to finally decide if T. dryina being a native North American species or an introduced neomycete. However, most North American Tubakia collections previously referred to as T. dryina pertain to other species, including T. americana, T. hallii, T. iowensis, T. liquidambaris, T. macnabbii, and T. tiffanyae, but Tubakia on numerous Quercus spp. and on numerous non-fagaceous hosts in North America has not yet been properly examined, i.e., identifications based on cultures and sequence analyses are still lacking. Heredia (1993) reported “T. dryina” from Mexico on Quercus germana and Q. sartorii. These records are doubtful and belong very probably to other Tubakia species (current analyses of Tubakia in Mexico revealed several endemic species – see Sphaerosporithyrium mexicanum, Tubakia melnikiana and T. sierrafriensis).
Collections of “T. dryina” from Asia do not belong to this species. Harrington et al. (2012) emphasised that Japanese specimens referred to as T. dryina in Yokoyama & Tubaki (1971) do not agree with the concept of T. dryina s. str., which could be confirmed in the course of our own re-examinations of the collections concerned (see T. dryinoides). Zahedi et al. (2011) reported and illustrated “Tubakia dryina” on Quercus castaneifolia from North Iran (Guilan Province), which is, however, quite distinct from true T. dryina s. str. (see notes under Tubakia sp. [Excluded, doubtful and insufficiently known species]). The identity of “T. dryina s. lat.” on Quercus hartwissiana in Turkey (Huseyinov & Selçuk 2001) is unclear as well.
Boroń & Grad (2017) examined T. dryina in Poland and analysed the variation of ITS data of numerous isolates. Haplotype 1 in Boroń & Grad (2017) is representative for T. dryina s. str. The published results and analyses confirm T. dryina as common and widespread species on Quercus robur and Fagus sylvatica, at least in Poland. In a single case, they found this species on Tilia cordata (Tiliaceae), which needs, however, further research and confirmation. Haplotype 2 probably belongs to European sequences of T. dryinoides (s. lat.).
The conidiogenesis of Tubakia dryina was examined in detail by Jones & Holcomb (1978) and described to be phialidic, which was later confirmed in Glawe & Crane (1987). Taylor & Clarke (1996) described the formation of internal mycelium and conidial germination, and Taylor (2001) dealt with the ultrastructure of pycnothyria. Holdenrieder & Kowalski (1989) emphasized that T. dryina may live as endophyte in healthy leaves and twigs, and Boddy & Rayner (1984) isolated this fungus from living and dead twigs in the UK.
Tubakia dryinoides C. Nakash., sp. nov. MycoBank MB823662. Fig. 15.
Fig. 15.
Tubakia dryinoides (NBRC H-11618 – holotype). A. Pycnothyria on the surface of leaf litter. B. Culture on MEA (NBRC 9267 – ex-type culture). C. Scutellum. D. Central columella. E. Conidiophores. F. Conidia. G. Microconidia. Bars = 10 μm.
Etymology: Named after the morphological and phylogenetic affinity to Tubakia dryina.
Description in vivo: Living as endophyte in leaves, forming distinct leaf lesions, amphigenous, shape and size variable, subcircular to angular-irregular, 2–5 mm diam, purplish brown to dingy greyish brown, margin indefinite or with narrow darker border, dark purplish violet, reddish brown to blackish, occasionally with a diffuse halo. Mycelium internal, forming branched intra- and intercellular hyphae. Conidiomata (pycnothyria) amphigenous, scattered on unaffected portions of leaves, gregarious on leaf spots, punctiform, greyish to blackish, circular or subcircular when viewed from above, superficial, easily removable, scutellate, fixed to the leaf by a central columella, 50–145 μm diam. Scutella convex sometimes more flattened, membranous, dense at the centre, looser at the edge, with a central hyaline or pale disc, 4–6 μm diam, surrounded by cells subcircular to angular in outline and 2–6 μm diam, giving rise to radiating hyphal strands, cells 7–25 × 2–5 μm, pale to dark brown, thick-walled (–1 μm), smooth, simple or 1–3 times bifurcating, ultimate branchlets with obtuse to mostly pointed tips. Central columella below the scutellum delicate, easily collapsing and loose, ephemeral, about 13–37 μm wide, surrounded by large brown cells. Conidiophores reduced to conidiogenous cells, arising from the underside of the scutella, around the columella, radiating, orientation outward-downward, cylindrical, conical, delicate, about 8–10 × 2–7 μm, hyaline, thin-walled, smooth, apex obtuse to truncate, conidiogenesis phialidic, sometimes forming indistinct periclinal thickenings. Conidia solitary, ellipsoid to obovoid, 8.5–14.5 × 5.5–8.5(–10) μm, length/width ratio 1.3–2.3, wall thin, up to 1 μm, hyaline to subhyaline, smooth, apex and base broadly rounded, with inconspicuous to conspicuous basal hilum (frill), occasionally somewhat peg-like and truncate when conspicuous. Microconidia narrowly ellipsoid-ovoid, fusiform, mostly straight, 3–10 × 1.5–2.5 μm, hyaline, thin-walled, smooth.
In vitro: on MEA with optimal growth at 20° C, attaining 30–40 mm diam after 14 d, margin scalloped, at first creamy white, forming concentric rings of olivaceous mycelium, reverse greyish white, with olivaceous edge. Conidial formation not observed.
Type: Japan, Osaka, Suita, on Quercus phillyraeoides, 8 Oct. 1969, T. Yokoyama (NBRC H-11618 – holotype; NBRC 9267 = MUCC2292 – ex-type cultures).
Hosts range and distribution: On Castanea crenata and Quercus phillyraeoides, Fagaceae, Asia (Japan) [(?) on Fagus sylvatica and Quercus robur, Europe (France, Poland)].
Additional collections examined: Japan, Osaka, Ikeda, on Castanea crenata, 11 Nov. 1968, T. Yokoyama, NBRC H-11616; NBRC 9265 = MUCC2290; Minoo, on Castanea crenata, 8 Oct. 1969, T. Yokoyama, NBRC H-11617; cultures NBRC 9266 = MUCC2291.
Notes: Tubakia dryinoides is morphologically similar to T. dryina, at least in terms of characters of the pycnothyria, and was referred to as T. dryina in Yokoyama & Tubaki (1971). In contrast to genuine T. dryina collections, the conidia in T. dryinoides remain hyaline or subhyaline, at least until germination (see Yokoyama & Tubaki 1971). The phylogenetic analyses corroborated a close affinity of the Japanese collections to T. dryina but clearly suggested a separate species (Figs 3–5), which confirms the doubts upon the correct assignment of Japanese collections to T. dryina by Harrington et al. (2012). Tubakia dryinoides belongs to a cluster undoubtedly composed of several species, including T. paradryinoides, which is genetically clearly distinct from T. dryinoides and morphologically easily distinguishable by its much larger conidia, 14–21 × 10–15 μm. Tubakia americana (Figs 3–5) is another closely allied species. Harrington & McNew (2018) included ITS sequences belonging to T. dryinoides in their T. americana “clade”, which is, however, heterogeneous and divided into at least two clades representing T. americana and T. dryinoides. Several Chinese strains, isolated from Quercus sp. (GenBank FJ598616), Linderia glauca (GenBank JF502454) and from an unknown host as endophyte (GenBank FJ025349), all unpublished, belong to this clade and may be T. dryinoides. The identity of European strains (CBS 329.75 and CPC 33586, Quercus spp., France) belonging to this cluster is still unclear, but may indicate the presence of an additional cryptic European species. The current sampling is, however, not sufficient for a final conclusion. Above all, the morphology of pycnothyria and the colour of conidia of the European taxon are unknown (conidia colourless in T. dryinoides). Therefore, the collections concerned are tentatively maintained in T. dryinoides. ITS sequences retrieved from leaves of Quercus robur and Fagus sylvatica in Poland (“T. dryina” haplotype 2 in Boroń & Grad 2016) seem to belong to this cryptic European taxon and suggest that it might be rather common, but pycnothyria and the conidial colour of the Polish collections were not described.
Tubakia hallii T.C. Harr. & McNew, Antonie van Leeuwenhoek J. Microbiol. Serol. doi.org/10.1007/s10482-017-1001-9 [10]. 2017.
Illustrations: Harrington & McNew (2018, figs 1a–c).
Description in vivo: Conidiomata (pycnothyria) superficial, hypophyllous or epiphyllous on necrotic interveinal spots and along necrotic mid and lateral leaf veins. Scutella 45–155 μm diam, radiate, composed of a series of dark brown, thick-walled cells originating from a central cell, ending in blunt to acute tips. Sporodochia superficial with only a few radiating dark hyphae, mainly hypophyllous, on necrotic interveinal tissues and along leaf veins. Conidiophores reduced to conidiogenous cells, on underside of scutella or on top of sporodochia. Conidia hyaline, turning light brown with age, smooth to slightly varicose, aseptate, obovoid to ovoid, 9.5–14.5(–16) × 7.5–10(–11) μm (mean 12.4 × 8.7 μm). Microconidia not seen on leaves.
In vitro: On MYEA with optimal growth between 25–30 °C, attaining 50–70 mm diam after 7 d, initially white with dense aerial mycelium, smooth to scalloped at edge, turning cream to light grey at 10 d, developing concentric rings of dense mycelium, underside yellow, becoming golden yellow to brown at 10 d, sometimes with dark cell masses on surface or subsurface. Conidiophores rare to abundant, short, hyaline, aggregated (sporodochia) on agar surface, producing conidia in dark brown to black, wet masses. Conidia hyaline, becoming light brown, thick-walled, aseptate, ellipsoidal to obovate, 12.5–16.5(–17) × 5.3–7.5 μm (mean 14.5 × 6.2 μm). Microconidia not seen on agar media but produced from scutella developing on autoclaved pieces of leaves of Q. macrocarpa placed on MEA, hyaline, aseptate, fusiform, 3.5–7.5 × 1.0–2.5 μm.
Type: USA, Missouri, Kirbyville, on leaf of Quercus stellata, 2 Sep 2008, D. Brandt (ISC 453286 – holotype; CBS 129013 = A666 – ex-type strains).
Host range and distribution: On Quercus (alba, bicolor, macrocarpa, muehlenbergii, stellata), Fagaceae, North America (USA, Arkansas, Iowa, Kansas, Minnesota, Missouri, Wisconsin).
Notes: Tubakia hallii has recently been introduced by Harrington & McNew (2018) for collections previously referred to as Tubakia sp. B (Harrington et al. 2012, Harrington & McNew 2016). Tubakia hallii is morphologically and genetically close to T. iowensis (Figs 3, 5), but the analysis of a combined dataset of ITS and tef1 sequences (Harrington & McNew 2018) showed that the two species form monophyletic sister groups, and they are morphologically differentiated. Tubakia iowensis causes characteristic oak blight characterised by forming necroses of leaf veins (Harrington et al. 2012) and has a restricted host range and distribution, whereas T. hallii is associated with leaf spots and necrotic veins, appears to have a broader host range (Quercus alba, Q. bicolor, Q. macrocarpa, Q. muehlenbergii, and Q. stellata) and geographic distribution, and crustose pycnothyria have not been observed on the petioles of overwintering leaves. Pycnothyria may be larger in T. hallii (up to 155 μm) compared to T. iowensis (up to 110 μm), but the sizes are variable and overlapping. In culture on MYEA, the production of conidia in T. hallii is not uncommon, but rare in T. iowensis (Harrington & McNew 2016).
Tubakia iowensis T.C. Harr. & D. McNew, Mycologia 104: 86. 2012.
Illustrations: Harrington et al. (2012: 82, fig. 1A–N, 87, fig. 2A–L).
Description in vivo: Causing a late-season disease on bur oak (Quercus macrocarpa), named bur oak blight, first symptom on leaves visible as small purple to brown spots on veins, hypophyllous, occasionally forming small necrotic spots on the leaf blade, lesions later expanding along the veins and coalescing, portions of the leaf blade sometimes becoming chlorotic-necrotic and die off; severe infections may cause early leaf dieback and defoliation as well as branch dieback in later stages; some leaves with black pustulate conidiomata at the base of the petioles, remaining green or becoming necrotic, but usually remaining attached to the twigs. Mycelium internal, forming branched intra- and intercellular hyphae. Conidiomata (pycnothyria) amphigenous, scattered to gregarious on and along leaf veins (midveins and major lateral veins), punctiform, blackish, circular or subcircular when viewed from above, superficial, easily removable, scutellate, fixed to the leaf by a central columella, 40–120(–160) μm diam. Scutella convex, membranous, dense to less compact, looser, with a small central pale disc, 5–15(–20) μm diam, surrounded by small cells, subcircular to angular in outline, (3–)4–8(–10) μm diam, giving rise to radiating hyphal strands, cells 5–20(–30) × 2–8 μm, pale to medium dark brown, thick-walled (–1 μm), smooth, simple or 1–3 times bifurcating, ultimate branchlets with obtuse to often pointed tips. Central columella below the scutellum delicate, easily collapsing and loose, ephemeral, about 10–20 μm wide, surrounded by small, thin-walled, colourless or pale (pseudoparenchymatous) fertile cells. Conidiophores reduced to conidiogenous cells, arising from the underside of the scutella, around the columella, radiating, subcylindrical to usually conical-ampulliform, delicate, 5–10(–15) × 2–5 μm, hyaline, thin-walled, smooth, apex obtuse to truncate, conidiogenesis phialidic. Conidia solitary, broad ellipsoid-obovoid to subglobose, 9–17 × 6.5–10.5 μm, length/width ratio 1.1–2.4, wall thin, to about 1 μm wide, at first hyaline, subhyaline, later somewhat pigmented, olivaceous to brown, smooth, older conidia may be faintly rough-walled, apex and base broadly rounded, with inconspicuous to conspicuous basal hilum (frill), occasionally somewhat peg-like and truncate when conspicuous, about 1 μm wide. Microconidia formed in some collections, narrowly fusiform, often curved, 4–8.5 × 1–2 μm, hyaline, thin-walled, smooth. Sporodochial conidiomata, composed of conidiophore clusters, hypophyllous, on veins, irregular, occasionally with small, poorly developed scutella. Crustose conidiomata may be formed on overwintered leaves, at the base of petioles, and twigs, subepidermal, black, irregular, 15–500 μm diam, occasionally confluent, opening by irregular dehiscence; conidia developed in crustose conidiomata ellipsoid-obovoid, 9.5–14 × 6.5–8.5 μm, wall somewhat thickened, smooth to faintly roughened, at first colourless, later pigmented.
In vitro: On MEA with optimal growth at 25 °C, attaining 50–56 mm diam after 7 d, margin scalloped, at first white, felt-like, light grey at 10 d, forming concentric rings of aerial hyphae, reverse yellow at 10 d, with dark grey, dense “tissue” in concentric rings; conidia formed in culture ellipsoid-obovoid, 9–15.5(–18.5) × 5–8(–8.5) μm, with somewhat thickened walls, smooth to finely rough, at first colourless, later somewhat pigmented.
Type: USA, Iowa, Ames, Brookside Park, on Quercus macrocarpa, 21 Aug. 2008, T. Harrington (ISC 448599 – holotype); BPI 881219 – isotype.
Additional collection examined: USA, Iowa, Ames, Brookside Park, on Quercus macrocarpa, 21 Aug. 2008, T. Harrington, BPI 881221.
Additional cultures examined: See Table 1.
Host range and distribution: On Quercus (macrocarpa, stellata), Fagaceae, North America (USA, Arkansas, Iowa, Missouri, Wisconsin) [? Quercus sp., Asia, Iran].
Notes: Tubakia iowensis is a species belonging to the T. dryina complex and is morphologically similar to T. dryina s. str. Harrington et al. (2012) published results of detailed examinations of this species, described it as new species and pointed out core differences between the new species and T. dryina s. str. Although the pycnothyria of T. iowensis are difficult to differentiate from T. dryina conidiomata, the symptoms of bur oak blight are quite different. Characteristic circular to angular-irregular leaf spots dispersed on the whole leaf blade are not formed with T. iowensis, as they are for T. dryina; the lesions are rather confined to and spread along the veins. Severe infections may lead to early leaf dieback and defoliation, and, finally, branch dieback. Fusiform microconidia formed in pycnothyria are narrower, only 1–2 μm wide (vs. 1.5–2.5 μm wide in T. dryina). The status of T. iowensis as a separate species has been confirmed by sequence analyses; it is fully to highly supported in both the Bayesian and maximum parsimony analyses (Fig. 3). The Mexican isolate presenting T. sierrafriensis on Quercus eduardi is related to T. iowensis/californica/suttoniana complex as well, but these species are genetically separated (Figs 3–5) and morphologically quite different: they have pycnothyrial scutella with obtuse to sometimes even truncate tips of radiating cell threads and clear differences in conidial shape. A sequence retrieved from a Tubakia culture (CPC 23753) isolated from dead leaves of Quercus sp. in Iran clusters in the T. iowensis clade. This unusual finding requires further research based on a broader sampling of Iranian collections.
Tubakia japonica (Sacc.) B. Sutton, Trans. Brit. Mycol. Soc. 60: 165. 1973. Fig. 16.
Fig. 16.
Tubakia japonica (NBRC H-11611 – epitype). A. Symptoms on Castanea crenata. B. Pycnothyria on the surface of leaf spot. C. Culture on MEA (NBRC 9268 – ex-epitype culture). D. Scutellum. E. Central columella. F. Conidiophore. G. Conidium. Bars = 10 μm.
Basionym: Actinopelte japonica Sacc., Ann. Mycol. 11: 312. 1913.
Illustrations: Yokoyama & Tubaki (1971: 65, pl. 1A–D, 67, pl. 2A; 69, pl. 3A; 70, pl. 4A–H; 74, pl. 8A–B).
Description in vivo: Living as endophytes in leaves, forming crustose to pustulate conidiomata on the surface of shed leaves (litter), and plant pathogenic, forming distinct leaf lesions. Leaf spots amphigenous, circular, subcircular to angular-irregular, 2–6 mm diam or oblong, ochraceous to pale brown, finally greyish white, margin distinct, reddish brown to fuscous. Conidiomata (pycnothyria) amphigenous, scattered to gregarious, occasionally confluent, punctiform, superficial, easily removable, circular to subcircular in outline, 155–260(–310) μm diam when mature, yellowish brown to blackish brown or almost black (stereomicroscopy), scutellate, fixed to the leaf surface by a central columella. Scutella convex to flattened, membranous, dense when young, later loose at the margin, outline regular, circular-subcircular, with a central colourless to pale brown disc, 8–15 μm diam, scutellum more or less uniformly pigmented, brown, central cells subcircular or angular-irregular in outline, 3–10 μm diam, giving rise to radiating threads of oblong hyphal cells, 7–35(–120) × 2.5–6 μm, septate, pale brown to brown, thick-walled (–1 μm), smooth, simple or one to two times bifurcating, tips of the threads simple to forked, ultimate tips obtuse to pointed. Central columella delicate, easily collapsing, loose, ephemeral, cylindrical, 15–60(–80) μm wide (in culture to 200 μm diam), central cells surrounded by smaller, hyaline to pale brown fertile cells that form a pseudoparenchymatous sheath. Conidiophores reduced to conidiogenous cells, arising from the underside of the scutella, from parenchymatous cells around the upper part of the columella, radiating, orientation outward and downward, delicate, enlarged at the base and attenuated towards a narrow tip, cylindrical, conical to ampulliform, 7–28 × 4–12 μm, neck about 2–3 μm wide, hyaline to pale brown, thin-walled, smooth, apex obtuse to truncate, conidiogenesis phialidic, forming indistinct periclinal thickenings, sometimes percurrently proliferating, forming annellations. Conidia solitary, globose, subglobose to broad ellipsoid, large, 30–55 × 21–43 μm, length/width ratio 0.8–1.5, apex rounded, base rounded, often with distinct frill, wall somewhat thickened, hyaline or with a pale ochraceous tinge, smooth, wall 1 μm thick. Microconidia bacilliform, botuliform or narrowly navicular, 5–10 × 1–2 μm, formed in smaller conidiomata, 60–80 μm diam and 15–30 μm high.
In vitro: On MEA with optimal growth at 20 °C, attaining 35–40 mm after 14 d, margin scalloped, ivory white, velvety and flattened on the surface of colony, reverse pale greyish (conidial formation not observed). On potato sucrose agar rapidly growing, pale yellowish brown to chestnut-brown; aerial mycelium compact, silky, white to pale yellow; immersed hyphae rapidly growing; reverse yellowish brown to blackish brown; sporulation rare. On OA rapidly growing, ochraceous to pale yellowish brown; aerial mycelium compact, finely floccose, white to cream; immersed hyphae rapidly growing, reverse concolorous. On Czapek agar growth only restricted, greyish brown to pale blackish brown; aerial mycelium very poorly developed; immersed hyphae scarcely developed, reverse concolorous.
Types: Japan, Gifu Pref. (Prov. Mino), Kawauye-mura, on Castanea crenata (= C. vesca var. japonica), Oct. 1910, K. Hara (PAD – holotype; FH 888612 – isotype). Topotypes: Japan, Gifu Pref., Kawauye-mura, on Castanea crenata, Aug. 1920, K. Hara [Syd., Fungi Exot. Exs. 526] (e.g. BPI 391948, CUP, FH, HBG, K, MICH, MIN, PH, S). Epitype (designated here, MycoBank, MBT379574): Japan, Ibaraki Pref., Chiyoda, on Castanea crenata, 13 Aug. 1969, K. Uchida (NBRC H-11611; NBRC 9268 = MUCC2296 = ATCC 22472 – ex-epitype cultures).
Additional collection examined: Japan, Ibaraki Pref., Kasama, on Castanea crenata (= C. pubinervis), 17 Sep. 1969, Y. Kobayashi, NBRC H-11612; NBRC 9269 = MUCC2297; Kasama, on Castanea crenata, 17 Sep. 1969, Y. Kobayashi, NBRC H-11883; NBRC 9270 = MUCC2298; Shiga Pref., Ootsu, on Castanea crenata, 2 Sep. 1970, T. Yokoyama, NBRC H-11613; NBRC 9340 = MUCC2299 = CBS 191.71; Ootsu, on Castanea crenata, 30 Oct. 1970, T. Yokoyama, NBRC H-11615; NBRC 9342 = MUCC2301; Shiga Pref., Ootsu, on Castanea crenata, 2 Sep. 1970, T. Yokoyama, NBRC9341 = MUCC2300.
Host range and distribution: On Castanea (crenata, mollissima, Castanea sp.), Quercus (acutissima, aliena), Fagaceae, Asia (China, Japan, Korea).
Notes: Tubakia japonica is characterised by very large conidia, larger than those of any other Tubakia spp., and differs from any other species in forming narrow, mostly bacilliform microconidia (1–2 μm wide) that develop in special small conidiomata. The Chinese report of T. japonica on Castanea mollissima goes back to Chen (2002), and Korean records of this species on Castanea crenata, Quercus acutissima and Q. aliena refer to Lee et al. (1991) and Cho & Shin (2004). Phylogenetically T. japonica is closely allied to T. seoraksanensis (Figs 3–5), but differs from this species in having much smaller conidia and host range.
Tubakia liquidambaris (Tehon & Stout) T.C. Harr. & McNew, Antonie van Leeuwenhoek J. Microbiol. Serol. doi.org/10.1007/s10482-017-1001-9 [16]. 2017.
Basionym: Leptothyriella liquidambaris Tehon, Mycologia 21: 192. 1929.
Description in vivo: Leaf spots amphigenous, subcircular, 1–6 mm diam, brown. Pycnothyria mainly epiphyllous, scattered to gregarious, 60–140 μm diam, morphologically barely distinguishable from pycnothyria of T. dryina. Conidia 8.5–14 × 6–9 μm, hyaline, subhyaline, later slightly pigmented.
In vitro: On MYEA similar to cultures of T. macnabbii, but with slower growth, a flat surface, and abundant production of conidia formed from sporodochia in concentric rings (CBS 139744 = A771 and CBS 139745 = A830, both from ISC 453303).
Type: USA, Illinois, Pulaski County, Olmsteadt, necrotic leaf spots on Liquidambar styraciflua, 9 Aug. 1922, P. A. Young 4985 (ILLS 1445 – holotype).
Host range and distribution: on Liquidambar styraciflua, Altingiaceae, North America (USA, Arkansas, Florida, Illinois, Maryland, Mississippi, North Carolina, Oklahoma).
Notes: Pycnothyria of Leptothyriella liquidambaris are morphologically indistinguishable from those of Tubakia dryina. Therefore, it is not surprising that L. liquidambaris was previously usually considered a synonym of the latter species. However, based on culture characteristics and results of molecular sequence analyses, Harrington & McNew (2018) demonstrated that Tubakia on Liquidambar styraciflua represents a species of its own (Fig. 5).
Tubakia macnabbii T.C. Harr. & McNew, Antonie van Leeuwenhoek J. Microbiol. Serol. doi.org/10.1007/s10482-017-1001-9 [12]. 2017.
Illustrations: Harrington & McNew (2018, figs 1d–g).
Description in vivo: Conidiomata (pycnothyria) superficial, hypophyllous or epiphyllous, on necrotic spots and along mid and lateral leaf veins, scutella radiate, (45–)65–135(–178) μm diam, composed of a series of dark brown, thick-walled cells radiating from a central cell, ending in a blunt to acute point. Sporodochia with no scutella or poorly developed scutella, epiphyllous or hypophyllous on necrotic veins and tissue, light to dark brown due to mass of conidia. Conidiophores on underside of scutella or on sporodochia. Conidia from radiate pycnothyria and sporodochia hyaline, turning light brown with age, smooth to slightly varicose, aseptate, obovoid to ellipsoidal, 9.5–14.5 × (6–)7–9(–10) μm (mean 11.8 × 8.2 μm). Microconidia sterile, hyaline, aseptate, fusiform, 3.5–9(–11.5) × 1–3 μm, from small, radiate pycnothyria, alone or along with macroconidia. Crustose conidiomata hypophyllous or epiphyllous on necrotic mid and lateral veins of late-season leaves, leaves of the current year and overwintering leaves still hanging from trees, black, pulvinate, irregularly shaped, 0.7–1.5 mm diam, single or grouped, covered with thick-walled cells, breaking open with swelling when wet. Conidia hyaline to light brown, aseptate, ellipsoidal to obovate or irregular in shape, (8–)10–15(–19) × (6–) 6.5–8(–9) μm (mean 13 × 7.1).
In vitro: On MYEA with optimal growth at 25 °C, diam. 50–65 mm after 7 d, creamy-white aerial mycelium, smooth to scalloped at edge, may develop concentric rings of dense mycelium, underside golden yellow to slightly darker with age, sometimes with dark cell masses on surface or subsurface. Conidiophores rare to abundant, short, hyaline, sometimes aggregated (sporodochia) on agar surface. Conidia hyaline to dark brown, thick-walled, smooth to slightly varicose, aseptate, obovoid to ellipsoidal, 8.5–15 × 5.5–10 μm.
Type: USA, Missouri, Jackson County, on leaves of Quercus palustris, Sep 2010, D. Brandt (ISC 453290 – holotype; CBS 137349 = A989 – ex-type strains).
Host range and distribution: On Castanea sp., Quercus (alba, hemisphaerica, imbricaria, kelloggii, laurifolia, macrocarpa, marilandica, muehlenbergii, nigra, palustris, rubra, stellata, velutina, virginiana), North America (USA, Arkansas, Florida, Illinois, Iowa, Kansas, Louisiana, Maryland, Minnesota, Missouri, New Hampshire, Ohio, Oklahoma, Wisconsin).
Notes: According to Harrington & McNew (2018), Tubakia macnabbii, previously referred to as Tubakia sp. D (Harrington and McNew 2016), was the most commonly encountered Tubakia species in the eastern USA, where it appears to be indigenous and widespread on oaks belonging to sect. Lobatae, but this species was also found on Castanea spp. in Florida and Iowa. Harrington & McNew (2018) assigned Tubakia specimens from California on leaves and twigs of Quercus agrifolia, Q. wislizeni, Q. kelloggii, and Lithocarpus densiflorus, collected by S. Latham, to T. macnabbii, which belong, however, to T. californica (see discussion under the latter species). CBS 639.93, ex-type strain of Dicarpella dryina, isolated from leaves of Quercus rubra collected in a nursery in Italy, was also included in T. macnabbii, but has to be excluded (see discussion under T. suttoniana). Harrington & McNew (2018) questioned that the sexual morph described by Belisario (1991) actually pertains to Tubakia, although the ex-type strain of D. dryina clusters in the Tubakia clade and was assigned by them to T. macnabbii.
Harrington & McNew (2018) distinguished Tubakia macnabbii from T. dryina by the production of crustose conidiomata on vein and leaf tissue, rather than twigs (Harrington et al. 2012), larger conidia formed in crustose conidiomata of T. macnabbii, and cultures lacking the concentric rings typical of T. dryina. The conidia of T. macnabbii from radiate scutella are slightly smaller than those of T. hallii and T. iowensis, which usually occur on oaks of Quercus sect. Quercus. Unlike T. iowensis, T. macnabbii readily produces conidia in culture. Tubakia californica, another species belonging to the T. macnabbii complex, forms quite distinct symptoms, and radiate pycnothyria are lacking. Tubakia tiffanyae is another closely allied species on red oaks, which differs from T. macnabbii by somewhat larger conidia and characteristically circular leaf spots. Tubakia suttoniana, although insufficiently known, seems to be morphologically distinguished from T. macnabbii by having obtuse tips of radiating scutellum strands and short cylindrical to subclavate microconidia with rounded apex and broadly truncate base.
Tubakia macnabbii, as currently circumscribed and confined to North American collections, represents a genetically heterogeneous complex of cryptic taxa that needs further research (Fig. 5).
Tubakia melnikiana Marm. & U. Braun, sp. nov. MycoBank MB823663. Fig. 17.
Etymology: Dedicated to the recently deceased Russian mycologist, Vadim A. Mel’nik (*1937, †2017).
Description in vivo: Leaf spots amphigenous, subcircular to angular-irregular, often spread along veins, 2–20 mm diam, sometimes expanded, occupying large leaf portions, to 40 mm diam, pale to medium dark brown, finally sometimes paler brown, ochraceous, straw-coloured or greyish, margin indefinite or with a narrow darker border, dark purplish violet, brown to blackish. Mycelium internal, forming branched intra- and intercellular hyphae. Conidiomata (pycnothyria) amphigenous, scattered to gregarious, punctiform, blackish, circular or subcircular when viewed from above, superficial, easily removable, scutellate, fixed to the leaf by a central columella, 80–100(–140) μm diam. Scutella convex, sometimes more flattened, membranous, dense, compact, later sometimes less compact, looser, with a central hyaline or pale disc, 5–15 μm diam, surrounded by small cells, subcircular to angular in outline, 3–8 μm diam, giving rise to radiating hyphal strands, cells oblong, 6–30(–35) × 2–6 μm, pale to medium dark brown, thick-walled (–1 μm), smooth, simple or 1–2(–3) times bifurcating, ultimate branchlets with obtuse to pointed tips. Central columella below the scutellum delicate, easily collapsing and loose, ephemeral, surrounded by small, thin-walled, colourless (pseudoparenchymatous) cells. Conidiophores reduced to conidiogenous cells, arising from the underside of the scutella, around the columella, radiating, orientation outward-downward, conical, ampulliform-cuspidate, delicate, about 10–18 × 2–5(–6) μm, hyaline, thin-walled, smooth, apex obtuse to truncate, conidiogenesis phialidic, percurrent proliferations not observed. Conidia solitary, subglobose, broad ellipsoid-obovoid, occasionally subcylindrical, (7–)9–16 × 5–9 μm, length/width ratio 1.2–2.2, wall thin, up to 1 μm wide, at first hyaline, subhyaline, later pale olivaceous to olivaceous brown, smooth or almost so, apex rounded, base rounded or with truncate basal hilum, occasionally somewhat peg-like. Microconidia occasionally formed narrowly ellipsoid(-subcylindrical) to broad ellipsoid-ovoid, apex obtuse, rounded, base round to short obconically truncate, straight, 4–8 × 2–4 μm, hyaline, thin-walled, smooth.
In vitro: On MEA at 22°C colonies attaining 60–70 mm diam after 12 d, margin scalloped, at first white, felted, later cream, forming concentric rings, older cultures becoming light brown. Conidia formed in older cultures ovoid to broad ellipsoid, 10–16 × 7–10 μm, with slightly thickened walls, at first colourless, later becoming light brown.
Type: Mexico, Nuevo León, Iturbide, Bosque Escuela, 24°42’30”N, 99°51’44.6”W, 1 620 m alt, on Quercus canbyi, 29 Oct. 2016, J. Marmolejo (HAL 3179 F – holotype; CFNL 2939 = CPC 32255 – ex-type cultures).
Additional collections examined: Mexico, Nuevo León, Iturbide, Bosque Escuela, 24°42’30.1”N, 99°51’48.2”W, 1 620 m alt, on Quercus canbyi, 6 Oct. 2016, J. Marmolejo, HAL 3174 F, 3175 F; CFNL 2933 = CPC 32249, CFNL 2934 = CPC 32250; ibid., 24°42’30.1”N, 99°51’44.7”W, 1 617 m alt, on Quercus canbyi, 6 Oct. 2016, J. Marmolejo, HAL 3176 F; CFNL 2935 = CPC 32251; ibid., 24°42’30.7”N, 99°47.3”W, 1614 m alt, on Quercus canbyi, 29 Oct. 2016, J. Marmolejo, HAL 3177 F; CFNL 2936 = CPC 32252; ibid., 24°42’30.2”N, 99°51’47.8”W, 1 619 m alt, on Quercus laeta (= Q. prinopsis), 29 Oct. 2016, J. Marmolejo, HAL 3178 F; CFNL 2938 = CPC 32254; ibid., 24°42’23.9”N, 99°51’44”W, 1 620 m alt, on Quercus eduardi, 10 Nov. 2016, J. Marmolejo, HAL 3184 F; CFNL 2943.
Host range and distribution: On Quercus canbyi, Q. eduardi, and Q. laeta (= Q. prinopsis), Fagaceae, North America, Mexico.
Notes: Tubakia melnikiana is morphologically close to and confusable with T. dryina and only distinguishable from typical collections of the latter species by having obtuse to acute ultimate tips of the radiating pycnothyrial hyphal strands and narrowly ellipsoid (-subcylindrical) to broad ellipsoid-ovoid microconidia with obtuse to rounded apex and round to short obconically truncate base. The differences in the scutella of the two species are only gradual, and microconidia are only occasionally formed. However, T. melnikiana and T. dryina are genetically clearly distinct and not closely allied (Figs 3–5). Tubakia melnikiana is closer to T. suttoniana. The T. melnikiana clade is fully supported by both the Bayesian and maximum parsimony analyses (Figs 3, 4).
Tubakia oblongispora C. Nakash.,sp. nov. MycoBank MB623665. Fig. 18.
Etymology: Epithet referring to the more oblong conidia in comparison with T. dryina.
Description in vivo: Living as endophyte in leaves, forming crustose conidiomata on the surface of shed leaves (litter). Mycelium internal and external, forming hyaline, branched intra- and intercellular hyphae, external hyphae observed on the lower leaf surface, pale brown, branched. Conidiomata (pycnothyria) amphigenous, scattered to gregarious, punctiform, blackish grey to blackish, circular or subcircular when viewed from above, superficial, easily removable, scutellate, fixed to the leaf by a central columella, 63–150 μm diam. Scutella convex to campanulate, sometimes more flattened, membranous, dense, compact, later sometimes less compact, with a central hyaline or pale brown disc, 5–10 μm diam, surrounded by small cells, subcircular to angular in outline, 3–5 μm diam, giving rise to radiating hyphal strands, cells 16–40 × 2–5.5 μm, subhyaline to brown, thick-walled (–1 μm), smooth, simple or 1–2 times bifurcating, ultimate branchlets with obtuse tips. Central columella below the scutellum delicate, easily collapsing and loose, ephemeral, about 10–20 μm wide, surrounded by small, thin-walled, pale to brown cells. Conidiophores reduced to conidiogenous cells, arising from the underside of the scutella, around the columella, radiating, orientation outward-downward, conical, ampulliform-cuspidate, delicate, 13–35 × 2–5.5 μm, subhyaline to brown, thin-walled, smooth, apex obtuse to conically truncate, conidiogenesis phialidic, proliferating percurrently, forming periclinal thickenings or collarettes. Conidia solitary, ellipsoid-obovoid, fusiform, oblong, straight to slightly curved, 12–20 × 4.5–7.5 μm, length/width ratio 1.8–3.8, wall thin, to 1 μm wide, subhyaline to pale brown, smooth, apex broadly rounded, base obconically truncate, with inconspicuous to conspicuous basal hilum. Microconidia not observed.
In vitro: On MEA with optimal growth at 20 °C, attaining 20–25 mm diam after 14 d, margin scalloped, at first ivory white, velvety and flattened on the surface of colony, reverse pale grey. Conidial formation not observed in vitro.
Type: Japan, Osaka, Mt. Yamato-Katsuragi San, on Quercus serrata, 11 Jun. 1972, T. Yokoyama (NBRC H-11881 – holotype; NBRC 9885 = MUCC2295 – ex-type cultures).
Hosts range and distribution: On Quercus serrata, Fagaceae, Asia (Japan).
Notes: Tubakia oblongispora is phylogenetically closely allied to T. dryina but morphologically quite distinct (see diagnosis; Figs 3, 5). It belongs to a group of Tubakia species with obtuse, non-acute tips of the ultimate branchlets of radiating scutellum strands. Among species of this morphological group, T. oblongispora is comparable with Oblongisporothyrium castanopsidis, which differs, however, in having hyaline, broader conidia, 11–20 × 7–9.5 μm, with a length/width ratio of 1.6–2.2.
Tubakia paradryinoides C. Nakash., sp. nov. MycoBank MB823666. Fig. 19.
Etymology: Composed of para- (similar to) and the name of the comparable species, Tubakia dryinoides.
Description in vivo: Living as endophyte in leaves, forming crustose conidiomata on the surface of leaves, and pathogenic, causing leaf spots, amphigenous, subcircular to irregular, 5–25 mm diam, yellowish ochraceous, straw-coloured to greyish brown, margin distinct. Mycelium internal, forming hyaline, branched intra- and intercellular hyphae, external hyphae not observed. Conidiomata (pycnothyria) amphigenous, mainly epiphyllous, scattered to gregarious, punctiform, yellowish brown to blackish, circular or subcircular when viewed from above, superficial, easily removable, scutellate, fixed to the leaf by a central columella, 50–155 μm diam. Scutella convex, sometimes more flattened, membranous, dense at centre, looser towards the margin, with a central hyaline or pale brown disc, 5–12 μm diam, surrounded by small pale brown cells, subcircular to angular in outline, 3–6 μm diam, giving rise to radiating hyphal strands, cells 7–30(–37) × 3–6 μm, pale brown to medium dark brown, thick-walled (–1 μm), smooth, simple or 1–3 times bifurcating, ultimate branchlets with pointed tips. Central columella below the scutellum delicate, easily collapsing and loose, ephemeral, about 18–27 μm wide, surrounded by small, thin-walled, colourless or pale pseudoparenchymatous cells. Conidiophores reduced to conidiogenous cells, arising from the underside of the scutella, around the columella, radiating, orientation outward-downward, conical to ampulliform, 10–20 × 4–8 μm, subhyaline to pale brown, thin-walled, smooth, apex obtuse to truncate, conidiogenesis phialidic, sometimes forming indistinct periclinal thickenings. Conidia solitary, broad ellipsoid-obovoid, 14–21 × 10–15 μm, length/width ratio 1.1–1.8, wall thin, up to 1 μm wide, hyaline to subhyaline, faintly rough-walled, apex and base broadly rounded, with inconspicuous to conspicuous basal hilum, 2–2.5 μm diam. Microconidia not observed.
In vitro: On MEA with optimal growth at 20 °C, attaining 35–40 mm after 14 d, margin scalloped, straw coloured, forming concentric ring of aerial hyphae, reverse in straw coloured, forming a dark brown concentric ring. Conidial formation not observed.
Type: Japan, Ibaraki Pref., Hokota, on Quercus acutissima, 12 Oct. 1972, T. Kobayashi & K. Sasaki (TFM:FPH 3923 – holotype; NBRC 9884 = MUCC2294 – ex-type cultures).
Hosts range and distribution: On Quercus acutissima, Fagaceae, Asia (Japan).
Notes: Tubakia paradryinoides is phylogenetically very closely allied to T. dryina and more distant from T. dryinoides and T. oblongispora (Figs 3, 5), which is also reflected in the morphological characters of pycnothyria of the species involved. The pycnothyrial scutella of these species are characterised by having hyphal strands with acute ultimate tips. However, T. paradryinoides is easily distinguishable from T. dryina and T. dryinoides by its hyaline to subhyaline, much larger conidia, 14–21 × 10–15 μm (vs. at first hyaline, subhyaline, but later pale olivaceous, olivaceous brown to brownish and (7–)9–16(–18) × (5–)6–10(–10.5) μm in T. dryina, and 8.6–14.7 × 5.5–8.5 (–10) μm in T. dryinoides). The independence of T. paradryinoides as a distinct species is mainly supported by its unique tub2 sequence which influences its position in the phylogenetic tree (data not shown, see Fig. 3). Also see the notes under T. dryinoides; this assemblage of sequences could comprise several closely allied lineages that might represent additional cryptic species.
Tubakia seoraksanensis H.Y. Yun, Mycotaxon 115: 371. 2011.
Illustrations: Yun & Rossman (2011: 372, fig. 1A–F).
Description in vivo: Causing leaf spots on Mongolian oak (Quercus mongolica), amphigenous, mainly epiphyllous, circular to broad elliptical, 2–10 mm diam, sometimes confluent, spread over the leaf blade, or forming necrotic lesions along the midrib or veins, pale brown, with regular to irregular margin, darker brown. Conidiomata (pycnothyria) amphigenous, mainly epiphyllous, mostly associated with leaf spots, superficial, scattered to gregarious, sometimes confluent, above all at veins, brown to dark brown, 90–160 μm diam, circular in outline, scutellate, fixed to the leaf surface by a central columella, easily removable. Scutella somewhat convex, membranous, dense to less compact, looser, centre with a colourless or pale disc, consisting of a single hyaline cell, surrounded by small cells, subcircular to angular in outline, giving rise to radiating hyphal strands, cells 4–6 μm wide, brown to dark brown, thick-walled (to about 1 μm), smooth, simple or 1–3 times bifurcating, ultimate branchlets with acute, cornuted tips. Central columella below the scutellum delicate, easily collapsing and ephemeral. Conidiophores reduced to conidiogenous cells, arising from the underside of the scutella, around the upper part of the columella, radiating downward and towards the margin, cylindrical, conical-clavate, fusiform, delicate, 14–22 × 3–5 μm, thin-walled, smooth, hyaline to pigmented, narrowed towards a thin point at neck, conidiogenesis phialidic. Conidia solitary, subglobose, ellipsoid to broad ellipsoid, 13–25 × 10–15 μm, length/width ratio 1.15–1.56, wall thin or finally slightly thickened, hyaline, later sometimes pale yellowish brown, smooth, apex broadly rounded, with a conspicuous basal hilum and prominent frill. Microconidia not observed.
In vitro: On MEA reaching 32–44 mm diam after 10 d at 25 °C in the dark, low velutinous to fuzzy, margin uneven, whitish to pale yellow, centre darker, becoming paler towards the margin, olive brown, light olive brown to yellow, margin white, reverse wrinkled, non-sporulating; hyphae branched, septate, 3.2–4.8 μm wide, hyaline to slightly brownish in mass, some hyphae with short coils on side branches.
Type: South Korea, Gangwon, Seorakasan National Park, Seorak-dong, Sokcho-si, Gangwon-do, on Quercus mongolica, Fagaceae, 31 Aug. 2009, H.Y. Yun (BPI 880799 – holotype; CBS 127490–127493 – ex-type cultures).
Host range and distribution: Only known from the type collection.
Notes: This species belongs to a group of relatively large-spored Tubakia spp., but differs from T. japonica and T. chinensis in having smaller conidia, not overlapping in size. T. seoraksanensis belongs to the T. suttoniana complex and is closely related to T. japonica, but well supported as a separate species (Fig. 3: PP = 1.0, MP-BS = 84 %; Figs 4, 5: PP = 1.0, MP-BS = 94 %).
Tubakia sierrafriensis O. Moreno-Rico & U. Braun, sp. nov. MycoBank MB823667. Figs 20, 21.
Etymology: Named after Sierra Fría, mountain in Aguascalientes, México, the origin of the type collection.
Description in vivo: Leaf spots amphigenous, usually 1–3 per leaf, subcircular to angular-irregular, 2–15 × 2–10 mm, usually ochraceous brown to medium dark brown, margin indefinite or with narrow darker brown margin or marginal line, occasionally somewhat raised, sometimes zonate, with slightly paler centre surrounded by a darker marginal line and broad brown halo. Conidiomata (pycnothyria) amphigenous, effuse, loose to aggregated, dense, sometimes in concentric rings, punctiform, superficial, easily removable, circular to subcircular in outline, (40–)50–120(–135) μm diam, dark brown to blackish (stereomicroscopy), scutellate, fixed to the leaf surface by a central columella. Scutella convex, membranous, loose to compact, outline regular to somewhat irregular, medium to medium dark brown (stereomicroscopy), with a central colourless or pale disc, 5–15 μm diam or sometimes even lacking, scutellum uniformly pigmented (microscopy), olivaceous to medium brown, or with a darker central zone, 20–60 μm diam, and paler periphery, central cells angular-irregular to almost rounded in outline, 2–10 μm diam, or oblong, 10–15 × 2–7 μm, giving rise to radiating threads of hyphal cells, simple to often 1–3 times bifurcating, cells 4–25 × 2–5 μm, walls about 1 μm thick, tips of the threads simple or once to two times forked, branchlets short, ultimate tips consistently obtuse or sometimes even truncate; central columella below the scutellum delicate, easily collapsing and loose, short and about 25–30 μm wide, composed of thin-walled, colourless or pale fertile cells. Conidiophores reduced to conidiogenous cells, arising from the underside of the scutella, from fertile cells around the upper part of the columella, radiating, conical, ampulliform-cuspidate, delicate, not very conspicuous, about 5–15 × 2–4 μm, hyaline, thin-walled, smooth. Conidia solitary, polymorphous, broad ellipsoid-obovoid, short subcylindrical, some conidia even subglobose or irregular in shape, occasionally with oblique base, straight to occasionally somewhat curved, 9–18 × 5–9 μm (length/width ratio 1.3–2.7, average 1.95), aseptate, apex rounded, base rounded to attenuated and then with a truncate hilum or even with a small peg-like base, thin-walled, hyaline, finally very pale olivaceous, smooth. Microconidia occasionally present in vivo, ellipsoid-fusiform, straight, rarely slightly curved, 5–10 × 2–3.5 μm, thin-walled, hyaline, smooth.
In vitro: On MEA at 22 °C colonies attaining 80–82 mm diam after 20 d, margin undulate, at first white, with concentric rings of aerial mycelium, center green olive with brown hyphal stripes, reverse still colourless after 20 d, without sporulation.
Type: México, Aguascalientes, San José de Gracia, Laguna Seca, 22°10’40.61”N, 102°38’36.48”W, 2.662 m alt, on Quercus eduardi, 19 Jan. 2017, O. Moreno-Rico (CFNL 2944 – holotype; CFNL 2944 = CPC 33020 – ex-type culture).
Additional collections examined: ibid., on Quercus eduardi, 21 Apr. 2016, O. Moreno-Rico, HAL 3163 F; 22 Nov. 2016, O. Moreno-Rico, HAL 3173 F.
Hosts range and distribution: On Quercus eduardi, Fagaceae, North America (Mexico).
Notes: Tubakia sierrafriensis belongs to a group of Tubakia species that are morphologically distinguished from T. dryina by having rounded-obtuse to truncate tips of the outer ends of the radiating scutellum threads, and is comparable to Oblongisporothyrium castanopsidis from which it differs in having smaller scutella [(40–)50–120(–135) μm diam, vs. 100–150 μm diam in O. castanopsidis] and polymorphous conidia, broad ellipsoid-obovoid, short subcylindrical, some conidia even subglobose or irregular in shape, occasionally with oblique base, at first hyaline, finally very pale olivaceous (vs. uniformly oblong-ellipsoid, 12–13 × 7–8 μm, and colourless). Tubakia sierrafriensis is phylogenetically distinct from the other species included in the phylogenetic analyses (Figs 3–5).
Tubakia suttoniana U. Braun & Crous, nom. nov. MycoBank MB823669.
Basionym: Dicarpella dryina Belisario & M.E. Barr, Mycotaxon 41: 154. 1991, non Tubakia dryina (Sacc.) B. Sutton, 1973.
Etymology: Named after the British mycologist B.C. Sutton, who introduced the name Tubakia.
Illustrations: Belisario (1990: 54–56, figs 1–3, 5–8; 1991: 149, figs 1–3, 151, figs 4–9).
Description in vivo: Leaf spots on living green leaves, numerous, scattered over the entire leaf blade, relatively small, angular-irregular, brown. Conidiomata (pycnothyria) on necrotic spots, morphologically close to pycnothyria of T. dryina but outer tips of radiating scutellum strands obtuse, not acute, and microconidia short cylindrical to subclavate, apex rounded, but base broadly truncate, mature macroconidia broad ellipsoid-obovoid, brown, 10.5–16 × 7.5–10 μm, on average 12.8 × 8.3 μm. Sexual morph: Formed on fallen overwintered leaves; perithecia scattered to gregarious, brownish to black, immersed, together with a stroma occupying the entire leaf thickness when mature, globose to slightly flattened, about 190–260 μm broad (diam) and 220–290 μm deep, sometimes oblique or horizontal; rostrate, beak short, usually lateral-eccentric, slightly protuberant through the upper leaf surface, rarely hypophyllous, 80–90 μm long and 15–20 μm wide at the base, apex rounded; ostiolate, ostiole periphysate, communicating with the cavity at maturity; stromatic pseudoparenchymatic layers centrally 30–40 μm thick, around the beak 40–50 μm thick, composed of several layers of dark, compact cells, rounded to ellipsoid, 8.5–14.5 μm diam; peridium variable in thickness, composed of a few layers of thin-walled, paler, compressed cells, tightly connected with the stroma; asci unitunicate, 8-spored, oblong-ellipsoid, 29–52.5 × 5.5–10.5 μm, formed in a spore-bearing part, peripheral, often with short stalk, or oblong, to 45 μm, ascal apex with two refractive conoid structures, asci deliquescing at maturity; paraphyses lacking; ascospores more or less uniseriate, becoming irregularly biseriate, 10–16 × 4.5–6.5 μm, one-celled, hyaline, ellipsoid to fusiform, often inequilateral or slightly curved, wall finely ornamented, content granular-guttulate.
In vitro: Colony covering dish in 2 wk at 25 °C with moderate aerial mycelium and feathery margins. On MEA surface smoke grey, reverse smoke grey with concentric circles of olivaceous grey. On PDA surface smoke grey, reverse smoke grey with olivaceous grey margin. On OA surface smoke grey with patches of olivaceous grey.
Types: Italy, Tuscany, Grosseto, farm nursery, on overwintered leaves of Quercus rubra, Feb. 1989, A. Belisario (ROHB – holotype; ROPV – isotype; ROPV, CBS 639.93 – ex-isotype cultures).
Hosts range and distribution: On Quercus rubra, Europe (Italy).
Notes: Dicarpella dryina was introduced as sexual morph of “Tubakia dryina” (Belisario 1991). Harrington et al. (2012) questioned that the two morphs belong together and speculated that D. dryina might instead be the sexual morph of Actinopelte americana or another related cryptic American Tubakia species. However, sequences retrieved from the ex-type strain of D. dryina cluster in the Tubakia clade and were assigned to T. macnabbii in Harrington & McNew (2018). Belisario (1990) emphasised that a comparison of cultures derived from single ascospores and the original cultures obtained from naturally infected tissue and conidia did not show any differences, i.e., the ex-type cultures represent single ascospore cultures. Taking this into account, there are currently no objective reasons to doubt that D. dryina represents a sexual morph belonging to Tubakia. Unfortunately, Belisario (1989, 1990) did not describe the pycnothyria associated with D. dryina in detail, but her figures (Belisario 1991: 149, figs 1–3) suggest that the terminal tips of radiating scutellum strands are obtuse-rounded and not acute as in T. dryina. The microconidia are cylindrical to short clavate with rounded apex and broadly truncate base vs. ellipsoid-ovoid to fusiform, attenuated towards a narrow base as those occurring in T. dryina. This confirms that the asexual morph of D. dryina does not coincide with T. dryina, and these differences do not support the conspecificity of D. dryina and T. dryina as discussed in Harrington et al. (2012). Furthermore, D. dryina (as T. suttoniana) and T. dryina are clearly confirmed as separate species in the phylogenetic trees (Figs 3, 5).
Harrington & McNew (2018) de facto reduced Dicarpella dryina to synonymy with the new species Tubakia macnabbii since the ex-type culture of D. dryina (CBS 639.93) was cited and included in the protologue of T. macnabbii. The latter species, based on type material from Missouri on Quercus palustris, is undoubtedly a common North American species with wide host range (Harrington & McNew 2018). The taxonomic conclusion to include Tubakia collections from Italy on Quercus rubra in T. macnabbii was just based on phylogenetic analyses. However, the resolution of the “T. macnabbii clade” in their ITS tree is insufficient, and their tef1 tree reflects a higher degree of variation, suggesting a complex of several cryptic taxa (also see Fig. 5 in the present study). Harrington & McNew (2018) supposed that the North America T. macnabbi occurring on various hosts of Quercus sect. Lobatae had been introduced to Europe (Italy), but the cluster including the sequence retrieved from the ex-type strain of Dicarpella dryina encompasses several sequences obtained from strains isolated from European collections (Netherlands) on the European oak Quercus robur and one strain on Q. cerris in New Zealand. Furthermore, Belisario’s (1990) micrographs of “T. dryina” on Q. rubra show pycnothyria with obtuse tips of radiating scutellum strands and short cylindrical to subclavate microconidia with rounded apex but broadly truncate base, which is not in agreement with morphological characters of T. macnabbii. Therefore, we prefer to keep D. dryina as separate species.
Although the ITS sequence is not distinct from sequences of the newly described Tubakia from California (T. californica sp. nov.), T. suttoniana clusters separately from T. californica in the tef1 (Fig. 5), tub2 (not shown, see TreeBASE) and combined trees (Figs 3, 4). Based on their ITS tree, Harrington & McNew (2018) assigned a strain isolated from twigs of Quercus agrifolia in California, 27 April 2012, S. Latham (A1177) to T. macnabbii. However, this strain was also included in our own analyses (CPC 31497 = CDFA#1007) and clearly belongs to T. californica, which further demonstrates the heterogeneity of T. macnabbii.
As outlined above, in the phylogenetic trees, T. suttoniana belongs to an assemblage of poorly resolved Tubakia strains from Europe (Italy, Netherlands, isolated from Quercus robur, Q, rubra and Quercus sp.) and New Zealand (isolated from Quercus cerris) that neither form a distinct cluster nor a clade, but rather reflect different lineages which might be different species. “Tubakia dryina” formed on leaf spots on Quercus cerris (Belisario 1993) and isolated as endophyte from buds and shoots of Q. cerris (Gennario et al. 2001) was also reported from Italy. Tubakia macnabbii is also part of this complex. The sample numbers are currently too small to resolve this assemblage of some taxa and more isolates are needed. Tubakia suttoniana represents a cryptic species morphologically and is phylogenetically distinct from T. dryina, but currently only known with certainty from its type material. In addition, it forms a complex of closely related species which also includes T. japonica, T. melnikiana, T. seoraksanensis and “Tubakia sp. nov. II” (Figs 3–5).
According to Art. 55.1, the name D. dryina is legitimate and applicable as basionym although published under the illegitimate genus name Dicarpella Syd. & P. Syd. (younger homonym, Art. 53.1). In the type paragraph, Belisario (1990) cited two different collections, one from Grosseto, Tuscany, the other one from Rome, and specified ROHB as herbarium in which the holotype was deposited [as “Belisario (RHOB)”], but without clear indication which of the two cited collections represents the genuine holotype. Due to this imprecise, confusing citation, the name D. dryina would be invalid according to Art. 40.1, 40.6. However, on page 148 it was mentioned that she (Belisario) first found this fungus in the Grosseto farm nursery in February 1989. Later the same fungus was collected on dead leaves in a nursery in Rome. The reference to “Belisario” may be accepted as reference to the first collection from “Grosseto” as holotype so that one can consider D. dryina to be validly published.
Tubakia tiffanyae T.C. Harr. & McNew, Antonie van Leeuwenhoek J. Microbiol. Serol. doi.org/10.1007/s10482-017-1001-9 [13]. 2017.
Illustrations: Harrington & McNew (2018, figs 1h–m).
Description in vivo: Conidiomata (pycnothyria) superficial, hypophyllous or epiphyllous, on circular leaf spots or along necrotic leaf veins. Scutella radiate, (40–)60–150 μm diam, composed of a series of dark brown, thick-walled cells originating from a central cell, ending in blunt to acute tips. Sporodochia with no scutella or poorly developed scutella epiphyllous or hypophyllous on necrotic vein tissue, light to dark brown due to masses of conidia. Conidiophores on underside of scutella or on sporodochia. Conidia hyaline, turning light brown with age, smooth to slightly varicose, aseptate, obovoid to ovoid (9–)10–15.5 × (8–)8.5–11.5(–12) μm (mean 12.9 × 9.6 μm). Microconidia sterile, hyaline, aseptate, fusiform 3–8 × 1–2.5 μm may develop from small pycnothyria, alone or along with macroconidia. Crustose conidiomata developing in late summer to fall on underside of necrotic veins and found on overwintering leaves still hanging from twigs, erumpent, brown to black, irregularly shaped, 80–580 μm diam, single to grouped, covered with dark, thick-walled cells that break open in fissures due to swelling when wet. Conidia hyaline to brown, ellipsoidal to obovate to irregularly shaped, aseptate, (11–)13–18(–20) × (6–)7–9.5 μm (mean 15.2 × 7.9 μm).
In vitro: On MYEA with optimal growth at 25 C, 40–58 mm diam after 7 d, creamy white aerial mycelium, smooth to scalloped at edge, developing concentric rings of dense mycelium, underside golden yellow to slightly darker with age, sometimes with dark cell masses on surface or subsurface. Conidiophores rare to abundant, short, hyaline, sometimes aggregated (sporodochia) on agar surface. Conidia hyaline to dark brown, thick-walled, smooth to slightly varicose, aseptate, obovoid to ellipsoidal, 10–16 × 7–11 μm.
Type: USA, Iowa, Ames, N42° 04’ 4” W93° 65’ 22”, on leaf of Quercus rubra, 5 Sep. 2009, T. Harrington (ISC 453296 – holotype; ex-type strains CBS 137345 = A803).
Host range and distribution: On Quercus (ellipsoidalis, imbricaria, rubra), Fagaceae, North America (USA, Iowa, Minnesota).
Notes: This species was recently introduced by Harrington & McNew (2018) for Tubakia sp. C (Harrington & McNew 2016) which is closely allied to T. macnabbii but genetically separated (Fig. 5) and morphologically distinguished by having wider conidia and causing characteristic circular leaf spots with light-coloured centres (see Harrington & McNew 2018: fig 1h) in addition to the vein necrosis.
Excluded, doubtful and insufficiently known species
Actinopelte acnisti (Syd.) Toro, Bol. Acad. Ci. Fís. 2(7): 217. 1935, nom. inval. (Art. 36.1, c).
Basionym: Calopeltis acnisti Syd., Ann. Mycol. 23: 393. 1915.
Notes: Calopeltis is a recognised genus belonging to the Microthyriaceae. Syntypes of this species are distributed in several herbaria and include “Syd., Fungi Exot. Exs. 689” (BPI 645025–6425029, CUP, ILL 10525–10528, LSU 156932, MICH 13880, S-F10919,10920, 194870, 194872, 194873).
Actinopelte psychotriae I. Hino & Katum., Bull. Fac. Agric. Yamaguchi Univ. 15: 506. 1964.
Notes: This species, described by Hino & Katumoto (1964) on leaves of Psychotria serpens from Japan, is unrelated to Tubakia. In the original description and illustration (Hino & Katumoto 1964: 506, fig. 1), this species was described to form pycnothyria with scattered globose ostiolate locules and small “pycnospores” (6–8 × 2.5–3 μm), which is quite distinct from pycnothyria of Tubakia.
Actinopelte stellata M.L. Farr, Mycopathol. Mycol. Appl. 31: 63. 1967.
Synonym: Tubakia stellata (M.L. Farr) T.C. Harr. & McNew, Antonie van Leeuwenhoek J. Microbiol. Serol. doi.org/10.1007/s10482-017-1001-9 [16]. 2017.
Notes: The generic affinity of this species is unclear. Cultures and results of molecular sequence analyses are not yet available. This species is only known from its type collection (Brazil, Para, Belem, on leaves of Byrsonima coriacea, 3 Feb. 1963, F.C. Albuquerque, BPI 391941) and has not yet been revised and reassessed. However, the described and illustrated characteristics of A. stellata do not fit into the generic concept of Tubakia. Farr (1967) described a basal membrane, unknown in true Tubakia species, a one-celled columella, and conidiogenous cells arising from the underside of the scutellum. In Tubakia spp., the columella is composed of several cells or a single cell surrounded by small, fertile, parenchymatous cells giving rise to conidiogenous cells, above all around the point of attachment of the columella at the scutellum and peripherally. Tubakia species are usually confined to fagaceous hosts in the northern hemisphere. The reallocation of A. stellata to Tubakia by Harrington & McNew (2018) was just a formal act, neither supported by culture and sequence data nor accompanied by a critical discussion of the morphology of this species.
Actinothyrium gloeosporioides Tehon, Mycologia 16: 136. 1924.
Synonym: Tubakia gloeosporioides (Tehon) T.C. Harr. & McNew, Antonie van Leeuwenhoek J. Microbiol. Serol. doi.org/10.1007/s10482-017-1001-9 [15]. 2017.
Holotype: USA: Illinois, Franklin County, Christopher, on Sassafras albidum, Lauraceae, 20 Jul. 1922, P. A. Young 3311 (ILLS 2972).
Notes: Harrington & McNew (2018) examined type material of A. gloeosporioides and additional specimens on Sassafras albidum from Illinois (ILLS 2972, 3671, 29748, 17547) and New Jersey (as Leptothyrium dryinum f. sassafras). The pycnothyria were 45–132 μm diam (reported as 50–95 μm diam by Tehon 1924) with conidia 9.5–12.5 × 7–8.5 μm (reported as 11–12 × 6–7.5 μm in Tehon 1924), which are similar to pycnothyria and conidia of T. dryina s. lat. Harrington & McNew (2018) compared it with T. macnabbii and commented that the two species are indistinguishable without cultures or DNA. They introduced the new combination T. gloeosporioides just based on the assumption that the latter taxon represents a species of its own since it was described on a lauraceous host. However, it should be noted that an ITS sequence retrieved from leaves of Lindera glauca (Lauraceae) in China clusters with T. dryinoides. Hence, the status and true affinity of A. gloeosporioides remain unclear and unresolved pending cultures and results of molecular sequence analyses.
Leptothyrium castaneicola Ellis & Everh., J. Mycol. 4: 137. 1888.
Type: USA, New Jersey, on Castanea sativa [vesca], 20 Oct. 1888, without collector (NY 927812 – holotype).
Notes: This species has usually been considered a synonym of Tubakia dryina. Type material has been re-examined. Pycnothyria formed on leaf spots on Castanea sativa are morphologically barely distinguishable from those of T. dryina and allied North American species with pointed tips of radiating scutellum strands. This species may be a synonym of one of the North American Tubakia species, but it can also not be excluded that an additional cryptic species on sweet chestnut being involved, but cultures and sequence data are not yet available to be able to answer this question.
Pirostoma nyssae Tehon, Mycologia 16: 137. 1924.
Synonym: Tubakia nyssae (Tehon) T.C. Harr. & McNew, Antonie van Leeuwenhoek J. Microbiol. Serol. doi.org/10.1007/s10482-017-1001-9 [16]. 2017.
Holotype: USA, Illinois, Johnson County, Tunnel Hill, on Nyssa sylvatica, 25 Jul. 1922, P. A. Young 3665 (ILLS 2940).
Notes: Collections of Pirostoma nyssae have been examined (Nyssa sylvatica, BPI 391889–391891). Pycnothyria (65–110 μm diam, tips of the radiating scutellum strands pointed) are barely distinguishable from those of T. dryina, so that it is not surprising that P. nyssae has previously usually been considered a synonym of the latter species. Harrington & McNew (2018) compared P. nyssae rather with T. macnabbii, which is, however, genetically totally heterogeneous and undoubtedly composed of several cryptic taxa. Without any cultures and sequence data, the status and affinity of P. nyssae remain quite unclear. The reallocation to Tubakia was just based on the occurrence of this species on Nyssa sylvatica (Nyssaceae), i.e., on a non-fagaceous host, which is, however, insufficient for a final conclusion and reassessment.
Tubakia sp. (see Braun et al. 2014: 25)
Leaf spots amphigenous, subcircular to angular-irregular, 1–7 mm diam, sometimes oblong, up to 10 μm, medium brown on the upper leaf surface, paler below, finally with pale centre, pale brownish to ochraceous, margin indefinite or narrow and somewhat darker, occasionally surrounded by a narrow diffuse halo, yellowish to yellow-green. Conidiomata (pycnothyria) epiphyllous, up to about 15 per leaf spot, punctiform, scutellate, blackish. Scutella convex, 40–80 μm diam, membranous, barely translucent, with a central paler disc, 8–15 μm diam, giving rise to radiating hyphae, cells 4–15 × 2–5 μm, peripheral cells mostly somewhat broadening towards the margin, medium brown, thick-walled (–1 μm), smooth, up to three times bifurcating, either only at the periphery or deeply cleft, peripheral bifurcations mostly shallow, branchlets with obtuse to truncate tips. Central columella below the scutellum delicate, easily collapsing and ephemeral. Conidiophores reduced to conidiogenous cells, arising from the underside of scutella around the columella, radiating downward and towards margin. Conidia solitary, globose to subglobose, 9–11 × 7–9 μm, length/width ratio 1–1.2, wall thin, 0.3–0.8 μm wide, hyaline or subhyaline, very pale greenish or faintly olivaceous, smooth, apex and base broadly rounded, basal hilum inconspicuous or with minute, not very conspicuous, delicate frill or peg. Microconidia not observed.
Material examined: China, Jiangxi Province, Xingangshan, subtropical forest site of the BEF-China Project, 29.1250° N, 117.9085° E, on living leaves of Castanea henryi, Fagaceae, 8 Sep. 2013, S. Bien, HAL 2675 F.
Notes: Tubakia sp. from Castanea henryi in China belongs to an undescribed species. Cultures and results of sequence analyses are not available, and the material deposited at HAL is too meagre for a formal description of a new species. The Chinese fungus on Castanea henryi is morphologically close to, and possibly identical with, Saprothyrium thailandensis, but unproven due to lack of cultures and sequence data for comparison. The ecology of the Chinese collection on Castanea henryi is unclear. The pycnothyria were found on leaf spots of living leaves, but lesions of several fungi, including Tubakia chinensis, were developed. Thus, it cannot be excluded that the Chinese Tubakia on Castanea henryi with small pycnothyria was formed on necrotic spots caused by other fungi.
Tubakia sp. (see Zahedi et al. 2011: 67, fig. 3).
Notes: Zahedi et al. (2011) reported and illustrated “Tubakia dryina” on Quercus castaneifolia from North Iran (Guilan Province). This fungus is quite distinct from true T. dryina (s. str.) and readily distinguishable by its small pycnothyria, about 60 μm diam, blunt tips of radiating scutellum strands, and broad ellipsoid-ovoid, colourless or pale conidia, about 7–10 × 5–7 μm. Shape and size of the pycnothyria are reminiscent of those of Tubakia sp. on Castanea henryi in China (Braun et al. 2014: 25) and Saprothyrium thailandense, which differ, however, in having globose to subglobose conidia. There is no Tubakia species with comparable pycnothyria. Therefore, the collection on Q. castaneifolia from Iran represents probably an undescribed species, but without cultures and sequence data the generic affinity remains unclear.
Key to species of Tubakia and allied genera (Tubakiaceae) based on conidiomatal characters
1. Pycnothyria with characteristically radiating scutella not developed; only acervuloid, crustose, pycnidioid, stromatic black conidiomata formed, (50–)80–200(–220) μm diam; on fagaceous hosts in California or Syzygium cumini (Myrtaceae) in Thailand ..................................................................................................................................................................................... 2
1* Pycnothyria developed; sporodochial and crustose, pycnidioid, stromatic conidiomata may be developed in addition to pycnothyria or may be absent .................................................................................................................................................. 3
2. Conidiomata crustose, pycnidioid, stromatic, black, dehiscing by irregular fissures, formed on petioles and leaf blades of necrotic, brown leaves, often close to veins, dry, brown leaves with conidioma from the previous seasons’ growth remain attached to many branches of affected trees until spring; conidiophores aseptate, unbranched; conidia broad ellipsoid, ellipsoid-ovoid to short and broad subcylindrical, rarely irregular in shape, 8–15 × 4.5–7 μm, length/width ratio 1.4–2.3 (on average 1.8), thin-walled, at first subhyaline to pale greenish, later greenish, pale olivaceous to brownish; on Chrysolepis chrysophylla (≡ Castanopsis chrysophylla), Notholithocarpus densiflorus (≡ Lithocarpus densiflorus), and various Quercus spp., North America, California (and perhaps Mexico) ........................................................................................... Tubakia californica
2* Conidiomata acervuloid, basistromatic; conidiophores septate, branched; 9–15 × 5–6 μm, light brown to olivaceous brown, thick-walled; on dead leaves of Syzygium cumini, Thailand .................................................................. Racheliella saprophytica
3. Conidia large, length on average > 25 μm .................................................................................................................................. 4
3* Conidia smaller, length on average < 25 μm ............................................................................................................................... 5
4. Conidia very large, 40–55 × 35–45 μm; microconidia present, 5–10 × 1–2 μm, formed in smaller conidiomata; on Castanea crenata, C. mollissima and Quercus acutissima, Q. aliena, Asia (China, Japan, Korea) ...................................... Tubakia japonica
4* Conidia (20–)25–40 × 20–30 μm; microconidia not formed on Castanea henryi ......................................Tubakia chinensis
5(3) Scutella relatively small, 60–100 μm diam, margin continuous, compact, more or less undulate and distinctly involute; conidia broad ellipsoid-ovoid, 12–15 × 10–13 μm, at first hyaline, later pale yellowish brown to light orange yellow; microconidia formed, bacilliform, 8–10 × 1 μm; on Quercus phillyreoides, Q. serrata, Japan, Korea ........................... Involutiscutellula rubra
5* Scutella larger, up to 160 μm diam and/or conidia narrower (width < 10 μm), margin of the scutellum different, loose, not distinctly involved, tips either pointed or obtuse-truncate; microconidia either much broader or not developed ................... 6
6. Outer tips of the bifurcating hyphal scutellum strands obtuse, rounded or even more or less truncate, but not pointed ........ 7
6* Outer tips of the bifurcating hyphal scutellum strands always or predominantly pointed (or at least all or most ultimate branchlets distinctly attenuated towards an obtuse to pointed tip) ......................................................................................... 15
7. Conidia globose or subglobose to broad ellipsoid-obovoid ........................................................................................................ 8
7* Conidia not consistently globose-subglobose, either consistently broad ellipsoid-obovoid or oblong to oblong-ellipsoid or polymorphous, broad ellipsoid-obovoid, short subcylindrical, some conidia even subglobose or irregular in shape, occasionally with oblique base ..................................................................................................................................................................... 10
8. Scutella larger, 80–120 μm diam; conidia globose, subglobose to broad ellipsoid-obovoid, small conidia 7–10 × 5–8 μm, larger fully developed conidia 9–16(–19) × (7–)9–12 μm, length/width ratio 1.0–1.5(–1.6), on average 1.27, hyaline to pale greenish or olivaceous; on Quercus eduardi, Mexico ............................................................................. Sphaerosporithyrium mexicanum
8* Scutella small, 40–80 μm diam; conidia subglobose, mature conidia narrower, 7–9 μm, hyaline or subhyaline ....................... 9
9. Isolated as saprobic fungus from an unidentified leaf in Thailand ................................................... Saprothyrium thailandense
9* On leaf spots on Castanea henryi, China .................................................................................................................... Tubakia sp.
10(7*) Conidia polymorphous, broad ellipsoid-obovoid, short subcylindrical, some conidia even subglobose or irregular in shape, occasionally with oblique base, 9–18 × 5–9 μm, at first hyaline, finally very pale olivaceous; on Quercus eduardi, Mexico .................................................................................................................................................................... Tubakia sierrafriensis
10* Conidia uniform, not polymorphous, not or barely curved; in Africa, Asia or Europe .............................................................. 11
11. Conidia hyaline ......................................................................................................................................................................... 12
11* Conidia pigmented, at least when mature ............................................................................................................................... 13
12. Pycnothyria large, 100–170 μm diam; conidiophores 11–20 μm long; conidia 7–9.5 μm wide; on Castanopsis cuspidata (Fagaceae), Japan ............................................................................................................... Oblongisporothyrium castanopsidis
12* Pyconothyria smaller, 80–130 μm diam; conidiophores 6–10 μm long; conidia (6.5–)7(–7.5) μm wide; on Syzygium guineense (Myrtaceae) ......................................................................................................................................... Racheliella wingfieldiana
13(11*) Conidia long and narrow, 12–20 × 4.5–7.5 μm, length/width ratio 1.8–3.8, subhyaline to pale brown; conidiogenous cells long, 13–35 × 2–5.5 μm; on Quercus serrata, Japan .......................................................................................... Tubakia oblongispora
13* Conidia broader, 9–16 × 6–10 μm, length/width ratio below 2.3 ............................................................................................ 14
14. Conidia 9–14 × 6–8.5 μm, on average 11.1 × 6.9 μm; microconidia fusiform, attenuated towards apex and base; on oaks in North America ............................................................................................................................................... Tubakia americana
14* Conidia somewhat larger, 10.5–16 × 7.5–10 μm, on average 12.8 × 8.3 μm; microconidia short cylindrical to subclavate, apex rounded, but base broadly truncate; on Quercus rubra, Europe (Italy) ......................................................... Tubakia suttoniana
15(6*) Conidia large, 13–25 × 10–15 μm ............................................................................................................................................ 16
15* Conidia narrower, (7–)9–16(–18) × (5–)6–10(–10.5) μm, or subglobose, 10–13 × 9–11 μm .................................................... 17
16. Scutella 90–160 × 90–130 μm; conidiogenous cells 3–5 μm wide; on Quercus mongolica, Korea ......... Tubakia seoraksanensis
16* Scutella 55–155 μm diam; conidiogenous cells 4–8 μm wide; on Quercus acutissima, Japan ............... Tubakia paradryinoides
17(15*) Conidia globose or subglobose, 10–13 × 9–11 μm (width on average > 9 μm), length/width ratio 0.9–1.4, hyaline to pale yellowish ochraceous; on leaf spots on Quercus glauca, Japan ............................................................. Paratubakia subglobosa
17* Conidia mostly broad ellipsoid-obovoid, (7–)9–16(–18) × 5.5–10(–11) μm (width on average < 9 μm), length/width ratio (1.1–)1.2–2.3 ............................................................................................................................................................................ 18
18. Ends of the radiating hyphal strands of the scutella often attenuated towards the tips, which are obtuse to pointed ........... 19
18* Ends of the radiating hyphal strands of the scutella usually with pointed ultimate tips .......................................................... 20
19. Conidia hyaline to yellowish ochraceous, 10–12.5 × 5.5–10 μm; on leaf litter of Quercus glauca, Japan, leaf spots lacking ......................................................................................................................................................... Paratubakia subglobosoides
19* Conidia hyaline or subhyaline, (11–)12–14(–15) × (6.5–)7(–7.5) μm; on Syzygium guineense, South Africa, causing leaf spots ............................................................................................................................................................. Racheliella wingfieldiana
20(18*) Conidia hyaline to subhyaline, not pigmented; on Quercus phillyreoides and Castanea pubinervis, Japan ........................................................................................................................................................................ Tubakia dryinoides
20* Conidia at first hyaline to subhyaline, later distinctly pigmented ............................................................................................. 21
21. Microconidia narrowly fusiform, often curved, 4–8.5 × 1–2 μm wide; causing bur oak blight characterised by forming lesions rather confined to and spread along veins, severe infections may lead to early leaf dieback and defoliation, and, finally, branch dieback; on Quercus macrocarpa and Q. stellata, North America, USA ............................................................ Tubakia iowensis
21* Microconidia wider, 4–9 × 1.5–4 μm, usually straight, or not formed on leaves; causing definite leaf spots spread over the entire leaf surface, pronounced dieback symptoms and premature defoliation not developed (T. dryina complex in terms of morphology) ............................................................................................................................................................................ 22
22. Leaf spots formed on Liquidambar styraciflua in North America; pycnothyria as in T. dryina ................. Tubakia liquidambaris
22* On fagaceous hosts ................................................................................................................................................................. 23
23. On Quercus canbyi, Mexico; ultimate tips of the hyphal strands of the scutella obtuse to pointed when mature; microconidia narrowly ellipsoid (-subcylindrical) to broad ellipsoid-ovoid, apex obtuse, rounded, base round to short obconically truncate; sporodochia and crustose conidiomata not observed ................................................................................... Tubakia melnikiana
23* On fagaceous hosts, Europe and North America; ultimate tips of the hyphal strands of the scutella consistently or predominantly pointed; microconidia narrowly ellipsoid-ovoid, fusiform, attenuated towards both ends; forming sporodochia and/or crustose conidiomata ............................................................................................................................................................................. 24
24. Sporodochia not formed; crustose conidiomata formed on twigs; on Fagus and Quercus spp., Europe, North America, and introduced in New Zealand .................................................................................................................................. Tubakia dryina
24* Sporodochia and/or crustose conidiomata formed on leaves (on leaf spots or along veins), North American species ........... 25
25. Leaf spots characteristically circular with light-coloured centre; sporodochia formed on necrotic veins; crustose conidiomatain late summer to fall on underside of necrotic veins and found on overwintering leaves still hanging from twigs ..........................................................................................................................................................................Tubakia tiffanyae
25* Leaf spots variable, not characteristically circular; sporodochia formed on leaf spots and along veins; crustose conidiomata either lacking or formed on leaf tissue before overwintering ............................................................................................... 26
26. Sporodochia formed on leaf lesions and along leaf veins; crustose conidiomata lacking; microconidia not developed on leaves (microconidia formed on autoclaved pieces of leaves of Q. macrocarpa placed on MEA) ...................................... Tubakia hallii
26* Sporodochia and crustose conidiomata formed on leaves (fusiform microconidia, 3.5–9(–11.5) × 1–3 μm, formed in small, radiate pycnothyria, alone or along with macroconidia) ................................................................................ Tubakia macnabbii
Discussion
The genus Tubakia was assigned to the Diaporthales (Yokoyama & Tubaki 1971, Yun & Rossman 2011, http://www.mycobank.org/ and http://www.indexfungorum.org/names/names.asp) primarily based on the description of Dicarpella dryina as the putative sexual morph of Tubakia dryina. However, the latter species is not the type species of Tubakia, and D. dryina is also not the type species of Dicarpella, which requires a more detailed discussion. Phylogenetic analyses of these species were previously not available. During the course of the present studies on Tubakia spp., ex-type cultures of Tubakia japonica, type species of Tubakia, and Dicarpella dryina could be included in phylogenetic analyses and proved to be congeneric. However, Dicarpella dryina is not conspecific with Tubakia dryina, as previously assumed and postulated in Harrington et al. (2012), and phylogenetically represents a separate species belonging to an assemblage of related Tubakia species, including T. californica, T. dryinoides, T. japonica, T. macnabbii, T. melnikiana, and T. seoraksanensis. All recognised species of Tubakia, except for T. chinensis, are known in vitro and have been included in the present molecular sequence analyses, which revealed that Tubakia s. lat., as previously circumscribed, represents a complex of cryptic genera. The question that remains is whether the genera Dicarpella and Tubakia s. str. are congeneric. A simple answer to this question is not yet possible since it requires phylogenetic data for Dicarpella bina, the type species of Dicarpella, which is hitherto only known from its type collection (Sognov et al. 2008). Based on the morphological similarity between D. bina and D. dryina, it might be possible that the two genera are, indeed, congeneric. Dicarpella bina was originally described from living leaves of Quercus agrifolia in California. Quercus agrifolia is also a common host of Tubakia californica. On the other hand, it can also not be excluded that the type species of Dicarpella might be more distantly related to Tubakia as currently suspected or even unrelated. A final conclusion is still pending and further cultures and sequence data for D. bina are needed. In any case, the older name Dicarpella would not threaten the younger name Tubakia in case that the two genera were congeneric, since Dicarpella Syd. & P. Syd., 1913 (nom. illeg.) being a younger homonym of Dicarpella Sitzenb., 1861, i.e., Tubakia would be the correct name in any case.
Barr (1978) assigned Dicarpella to the Pseudovalsaceae subfam. Pseudovalsoideae tribe Ditopelleae, and later included Dicarpella quercifolia with Mastigosporella hyalina as putative asexual morph (Barr 1979). A similar unnamed asexual morph was found in D. georgiana, which was later described as M. nyssae (Nag Raj & Di Cosmo 1981). Mastigosporella hyalina (≡ Harknessia hyalina) was the reason for previous reports that Dicarpella spp. might be associated with Harknessia spp. as asexual morphs (Cannon 2001). Monod (1983) retained Dicarpella georgiana in Gnomoniella, recognised D. bina and D. quercifolia in Dicarpella, and added D. liquidambaris-styracifluae and D. orientalis. Rossman et al. (2007) cited Harknessia spp. under diaporthalean fungi of uncertain position. However, true Harknessia spp. are not closely allied to former Dicarpella spp. with Mastigosporella asexual morphs and have been placed in a family of its own, viz. Harknessiaceae (Crous et al. 2012). Reid & Dowsett (1990) examined Dicarpella in detail, excluded D. georgiana and D. quercifolia, both associated with asexual morphs belong to Mastigosporella, and reallocated them to the new genus, Wuestneiopsis. Rossman et al. (2015) recommended to protect the name Mastigosporella and to reduce Wuestneiopsis to its synonymy. Thus, the illegitimate name Dicarpella currently comprises D. bina, D. liquidambaris-styracifluae, and D. orientalis, but to date none of them has been phylogenetically clarified. The illegitimate name Dicarpella was in need of a new genus name, but since the relation between Dicarpella and Tubakia remains unproven and unclear, any corresponding nomenclatural change would be premature.
Another question concerns the affiliation of Tubakia in the hierarchical system of the Ascomycota. The placement within the Diaporthales is clearly resolved. Tubakia is currently usually listed as diaporthalean genus of unclear family affinity (Yokoyama & Tubaki 1971, Yun & Rossman 2011, http://www.mycobank.org/ and http://www.indexfungorum.org/names/names.asp). Dicarpella was usually assigned to the Melanconidaceae s. lat., comprising up to almost 30 genera (Eriksson et al. 2001, Lumbsch & Huhndorf 2007, Maharachchikumbura et al. 2015, Maharachchikumbura et al. 2016). This affiliation is, however, disputable (see discussion above). Most genera allocated to the Melanconidaceae s. lat. have not yet been phylogenetically examined, i.e., the affinity of the genera concerned remains unclear and unproven. Melanconis, the type genus of the family Melanconidaceae, has been phylogenetically examined and the independent status of the latter family has been confirmed (Castlebury et al. 2002, Du et al. 2017). Castlebury et al. (2002) emphasised that the family Melanconidaceae should rather be confined to the genus Melanconis, and recommended to exclude the numerous other previously included genera, including Melanconiella (Voglmayr et al. 2012). Voglmayr et al. (2017) introduced the new family Juglanconidaceae for the new genus Juglanconis, which splits the genus Melanconis and Melanconidaceae s. lat. The present phylogenetic analyses indicate a relation of Tubakia to Melanconiella spp., possibly in sister positions, depending on the kind of analyses employed. Voglmayr et al. (2012) confirmed Melanconiella as a separate genus, clearly distinct from the morphologically similar genus Melanconis and not affiliated to the Melanconidaceae, and stressed that this genus does not belong to any established dothidealean family. Morphological traits of Melanconiella spp., including the characters of associated asexual morphs, do not favour Melanconiella spp. and Tubakia being placed in the same family. In contrast to Tubakia, the bark-inhabiting Melanconiella spp. are characterised by forming ectostromatic discs with a central column, perithecia with lateral non-rostrate ostioles, broad band-like paraphyses, consistently bicellular ascospores, sometimes with blunt appendages or a thin gelatinous sheath, and they are associated with acervular discosporina- and melanconium-like asexual morphs (Voglmayr et al. 2012). On the basis of phylogenetic analyses of dothidealean genera and their relations to putative families, Senanayake et al. (2017) validated the invalidly published family Melanconiellaceae and assigned Tubakia to this family, although it was not based on sequence data of the type species. The present phylogenetic analyses, including sequence data of the type species of Tubakia confirm that Tubakia warrants a family of its own, viz., Tubakiaceae fam. nov.
On the basis of ITS and LSU sequences retrieved from an ex-type strain, Harrington & McNew (2018) reallocated Apiognomonia supraseptata (Kaneko & Kobayashi 1984), a sexual morph described from Japan without any asexual morphs, to Tubakia. Apiognomonia supraseptata differs from true Apiognomonia spp. in having ascospores with a septum near the apex. Apiognomonia supraseptata and D. dryina, the only two sexual morphs associated with Tubakia, have various characters in common, viz., rostrate perithecia of similar size, unitunicate 8-spored asci, and colourless ascospores of similar size. The only basic difference is in the septation of the ascospores which are aseptate in D. dryina and 1-septate near the apex in A. supraseptata. Our own phylogenetic analyses confirm the inclusion of A. supraseptata in the Tubakiaceae. However, this allocation raises the question whether the inclusion of the latter species in Tubakia, as proposed by Harrington & McNew (2018), was reasonable, or if this result might be an indication of a separation of the Tubakiaceae cluster into two or several genera as the LSU phylogeny (Fig. 1) already indicated some heterogeneity. Therefore, the LSU phylogeny was also supplemented with rpb2 sequence data (Fig. 2) and proved that the Tubakiaceae cluster represents an assemblage of several cryptic genera, including Tubakia s. str. that forms a separate, well-supported clade. Tubakia castanopsidis, T. rubra, T. subglobosa, and T. thailandensis do not belong in the Tubakia s. str. clade and cluster outside, i.e., they have to be excluded from Tubakia s. str. and reallocated to other genera. The Tubakia clade is large, comprising numerous species, and is based on multigene sequence data. Several lineages, often only represented by a single species, are evident in the portions of excluded taxa in the phylogenetic trees, and led to the introduction of the new genera Apiognomonioides (type species: Apiognomonia supraseptata), Involutiscutellula (type species: Actinopelte rubra), Oblongisporothyrium (type species: Actinopelte castanopsidis), Paratubakia (type species: Actinopelte subglobosa), Racheliella (type species: R. wingfieldiana), Saprothyrium (type species: Tubakia thailandensis) and Sphaerosporithyrium (type species S. mexicanum). Racheliella wingfieldiana and Greeneria saprophytica share Syzygium (Myrtaceae) as host genus and are phylogenetically closely allied, suggesting the allocation of the latter species to the new genus Racheliella. The available phylogenetic data for Apiognomonia supraseptata and Tubakia thailandensis, belonging in Tubakiaceae, suggest that the two species require separate (new) genera. Interestingly, almost all excluded taxa and new genera are confined to east and southeast Asia (Japan, Thailand). This region seems to be a hot spot of the generic diversity of Tubakiaceae, whereas Europe and above all North America exhibit a higher diversity of species of the genus Tubakia s. str. Although the new genera segregated from Tubakia s. lat. are basically phylogenetically established, there are also morphological peculiarities that distinguish these genera from Tubakia s. str., e.g., Involutiscutellula differs from all other species of tubakia-like genera in having small pycnothyria with compact scutella provided with continuous, more or less undulate and distinctly involute margin. Paratubakia spp., confined to Quercus (Cyclobalanopsis) glauca, are characterised by having more or less globose-subglobose and usually hyaline conidia.
Within Tubakia s. str., T. dryina and T. iowensis, are clearly confirmed as species in the phylogenetic trees, although the two species possess very similar pycnothyria. On the other hand, there are differences in the habit and biology of these species. Although T. japonica and T. seoraksanensis seem to be phylogenetically closely allied, and morphologically similar with larger spores and similar pycnothyrial scutella, they are morphologically and genetically distinct and represent two distinct species. The new Mexican species Sphaerosporithyrium mexicanum and T. sierrafriensis are also phylogenetically supported as new, separate species and are clearly distinct in the trees. The position of Californian Tubakia sequences supports a separate, undescribed species belonging to the T. suttoniana complex in the phylogenetic trees. This species, described as T. californica, is an endophyte, forming conidia in pustulate conidiomata, but pycnothyria are lacking. Two strains isolated from leaves of Quercus canbyi in Mexico are close and tentatively included. Another Tubakia on Quercus canbyi and Q. laeta in Mexico, described as T. melnikiana, is morphologically only gradually distinguished from T. dryina, but phylogenetically clearly separated and belongs to the T. suttoniana complex. The inference by Harrington et al. (2012) that Japanese collections referred to as T. dryina s. lat. are not conspecific with the genuine T. dryina s. str. was confirmed in the course of the present examinations. Tubakia dryinoides, T. paradryinoides, T. oblongispora, and Paratubakia subglobosoides were proven to belong to genetically and morphologically distinct new species by analysing herbarium material, including corresponding cultures, and phylogenetic data of the collections concerned. Greeneria saprophytica, described from Thailand on Syzygium cumini, turned out to cluster within the Tubakiaceae, close to a new South African species. The morphology of this species is not in conflict with the current concept of taxa of the Tubakiaceae, but it clustered outside of the Tubakia s. str. clade. Owing to its phylogenetic position and morphological peculiarities, G. saprophytica cannot be assigned to Tubakia, but rather requires a genus of its own, viz., Racheliella, with R. wingfieldiana sp. nov. as type species.
Harrington & McNew (2018) recently revised the North American T. dryina complex, described several new species previously classified as Tubakia sp. (Harrington et al. 2012, Harrington & McNew 2016), and introduced several new combinations, including T. americana and T. liquidambaris. Tubakia hallii, T. liquidambaris, T. tiffanyae are well-supported recognised species confirmed in our own analyses. Tubakia americana is, however, heterogeneous in the circumscription of Harrington & McNew (2018) and comprises T. americana s. str. (on American oaks) and T. dryinoides. The clade representing the latter species contains Asian and European sequences. The European ones might reflect an additional cryptic species, but the current sampling is not sufficient for a final conclusion, and morphological characters of conidiomata of the European taxon are not yet known. Tubakia macnabbii turned out to be genetically quite heterogeneous and can currently only be considered as a complex compound species (s. lat.) that we currently confine to North American collections on oaks, but excluding Californian collections that form a clade of its own described herein as T. californica. Tubakia gloeosporioides (≡ Actinothyrium gloeosporioides) and T. nyssae (≡ Pirostoma nyssae) are two additional combinations introduced by Harrington & McNew (2018), but cultures and sequence data are not yet available for the two taxa, and the morphological characters of the pycnothyria formed on leaves of Nyssa and Sassafras are not distinguishable from those of T. dryina s. lat. Therefore, we prefer to assign these species to the list of “Excluded, doubtful and insufficiently known species”, at least tentatively until the affinity of the two taxa can be elucidated by means of cultures and sequence data.
Thus, the number of species pertaining to the family Tubakiaceae (Tubakia s. lat. in its previous circumscription) increased from eight in 2016 to the current 26 accepted here. However, examination of the individual gene trees has shown that there is some movement of isolates between species clades for ITS and tub2 (see results above), indicating some degree of gene transfer between species, which could be explained by the overlap in host species. More isolates need to be subjected to multi-gene phylogenies or other molecular analyses to understand the underlying processes and frequency at which these transfers happen.
Fig. 13.
Tubakia californica, symptoms on infected California black oak (Quercus kelloggii) trees in Tuolumne County, CA. A. Early spring growth showing healthy new leaves and infected brown leaves still hanging from the previous season. B. Current season foliage with angular lesions developing near the leaf margin and progressing along the leaf veins. C. Symptomatic branch showing necrotic lesions on the new growth as well as old, infected brown leaves that did not abscise and presumably served as the primary inoculum source. D. Progression of foliar symptoms from healthy leaves (top left) to leaves with small black veins, to leaves with more prominent black veins along the midrib and larger necrotic areas to completely brown leaves. Bar (C, D) = 10 cm.
Fig. 17.

Tubakia melnikiana (HAL 3179 F – holotype). A. Necrotic leaf lesion with pycnothyria. B. Pycnothyrium. C. Conidia. Bars = 1 cm (A), 20 μm (B), 5 μm (C).
Fig. 18.
Tubakia oblongispora (NBRC H-11881 – holotype). A. Pycnothyria on the surface of leaf litter. B. Colonies on MEA (NBRC 9885 – ex-type culture). C. Scutellum. D. Central columella. E. Conidiophores. F. Conidia. Bars = 10 μm.
Fig. 19.
Tubakia paradryinoides (TFM:FPH 3923 – holotype). A. Pycnothyria on the surface of leaf spot. B. Culture on MEA (NBRC 9884 – ex-type culture). C. Scutellum. D. Central columella. E. Conidiophores. F. Conidia. Bars = 10 μm.
Fig. 20.
Tubakia sierrafriensis (CFNL 2944 – holotype). A, B. Pycnothyria. C. Pycnothyria (crushing preparation) with conidia. D. Pycnothyria (SEM picture). E. Pycnothyria and conidia (SEM picture). F. Two pycnothyria with superficial hyphae (SEM picture). Bars = 10 μm.
Fig. 21.
Tubakia sierrafriensis (CFNL 2944 – holotype). A. Affected oak tree (Q. eduardi). B. Symptoms (leaf spots) on leaves of Quercus eduardi. C. Leaf with leaf spot. D. Close-up of an epiphyllous leaf spot with pycnothyria. E. Close-up of a hypophyllous leaf spot with pycnothyria. F. Culture on MEA. Bars = 0.5 cm (C), 1 cm (D, E), 20 μm.
Acknowledgements
We are very grateful to the directors and curators of BPI, ILLS, ISC, NBRC and TFM for loaning type material and other collections in their keeping during the course of monographic studies of Tubakia spp. This work was partially supported by grants to Chiharu Nakasima by JSPS KAKENHI (grant #17K07837).
REFERENCES
- Barr ME. (1978). The Diaporthales in North America with emphasis on Gnomonia and its segregates. Mycologia Memoirs 7: 1–232. [Google Scholar]
- Barr ME. (1979). Additions to the Diaporthales. Mycotaxon 10: 213–216. [Google Scholar]
- Belisario A. (1990). Tubakia dryina (Sacc.) Sutton segnalato per la prima volta su Quercus rubra L. in Italia (Nota preliminare). Informatore Fitopatologico 12: 54–56. [Google Scholar]
- Belisario A. (1991). Dicarpella dryina sp. nov., teleomorph of Tubakia dryina. Mycotaxon 41: 147–155. [Google Scholar]
- Belisario A. (1993). First report of Tubakia dryina on Quercus cerris. Plant Disease 77: 647. [DOI] [PubMed] [Google Scholar]
- Boddy L, Rayner ADM. (1984). Fungi inhabiting oak twigs before and at fall. Transactions of the British Mycological Society 82: 501–505. [Google Scholar]
- Boroń P, Grad B. (2017). The occurrence of Tubakia dryina in Poland – new hosts and ITS variation. Forest Pathology 47: e12294 (1–6). [Google Scholar]
- Braun U, Bien S, Hantsch L, et al. (2014). Tubakia chinensis sp. nov. and a key to the species of the genus Tubakia. Schlechtendalia 28: 23–28. [Google Scholar]
- Cannon PF. (2001). Rediscovery and redescription of the genus Uleoporthe (Melanconidaceae). Fungal Diversity 7: 17–25. [Google Scholar]
- Cannon PF, Kirk PM. (2007). Fungal Families of the World. CAB International. [Google Scholar]
- Castlebury LA, Rossman AY, Jaklitsch WJ, et al. (2002). A preliminary overview of the Diaporthales based on large subunit nuclear ribosomal DNA sequences. Mycologia 94: 1017–1031. [PubMed] [Google Scholar]
- Chen MM. (2002). Forest fungi phytogeography: Forest fungi phyto-geography of China, North America, and Siberia and international quarantine of tree pathogens. Pacific Mushroom Research and Education Center, Sacramento, California. [Google Scholar]
- Cho WD, Shin HD, eds. (2004). List of plant diseases in Korea. Fourth edition Korean Society of Plant Pathology. [Google Scholar]
- Cohen SD. (1999). Technique for large scale isolation of Discula umbrinella and other foliar endophytic fungi from Quercus species. Mycologia 91: 917–922. [Google Scholar]
- Crous PW, Summerell BA, Shivas RG, et al. (2012). A re-appraisal of Harknessia (Diaporthales), and the introduction of Harknessiaceae fam. nov. Persoonia 28: 49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z, Hyde KD, Yang Q, et al. (2017). Melansporellaceae: a novel family of Diaporthales (Ascomycota). Phytotaxa 305(9): 191–200. [Google Scholar]
- El-Gholl NE, Schubert TS, Peacock ME. (1996). Tubakia leaf spot on chestnut. Plant Pathology Circular 375: 1–3. [Google Scholar]
- Eriksson OE, Baral H-O, Currah RS, et al. (2001). Outline of Ascomycota—2001. Myconet 7: 1–88. [Google Scholar]
- Fan XL, Bezerra JDP, Tian CM, Crous PW. (2018). Families and genera of diaporthalean fungi associated with canker and dieback of tree hosts. Persoonia 40: 119–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr ML. (1967). A new species of Actinopelte from Brazil. Mycopathologia et Mycologia Applicata 31: 63–66. [Google Scholar]
- Gennaro M, Gonthier P, Nicolotti G, Cellerino GP. (2001). First report of Tubakia dryina in buds and shoots of Quercus cerris and Quercus robur. Plant Disease 85: 1289. [DOI] [PubMed] [Google Scholar]
- Glawe DA, Crane JL. (1987). Illinois fungi XIII. Tubakia dryina. Mycotaxon 29: 101–112. [Google Scholar]
- Gonthier P, Gennaro M, Nicolotti G. (2006). Effects of water stress on the endophytic mycota of Quercus robur. Acta Mycologica 41: 69–80. [Google Scholar]
- Harrington TC, McNew DL. (2016). Distribution and intensification of bur oak blight in Iowa and the Midwest. In: Porter KM, Conkling BL. (eds): Forest health monitoring: national status, trends, and analysis 2015. Forest Service & Research Station, Southern Research Station, General Technical Report SRS-213: 105–110. [Google Scholar]
- Harrington TC, McNew DM. (2018). A re-evaluation of Tubakia, including three new species on Quercus and six new combinations. Antonie van Leeuwenhoek doi.org/10.1007/s10482-017-1001-9. [DOI] [PubMed] [Google Scholar]
- Harrington TC, McNew D, Yun HY. (2012). Bur oak blight, a new disease on Quercus macrocarpa caused by Tubakia iowensis sp. nov. Mycologia 104: 79–92. [DOI] [PubMed] [Google Scholar]
- Hashizume Y, Sahashi N, Fukuda K. (2008). The influence of altitude on endophytic mycobiota in Quercus acuta leaves collected in two areas 1000 km apart. Forest Pathology 38: 1–9. [Google Scholar]
- Hino I, Katumoto K. (1964). Notes on some new species of fungi collected in the Ryukyu Archipelago. Bulletin of the Faculty of Agriculture, Yamaguchi University 15: 505–516. [Google Scholar]
- Holdenrieder O, Kowalski T. (1989). Pycnidial formation and pathogenicity in Tubakia dryina. Mycological Research 92: 166–169. [Google Scholar]
- Holcomb GE, Jones JP. (1984). Hosts of the leaf spot fungus Tubakia dryina in Louisiana. Plant Disease 68: 251. [Google Scholar]
- Huseyinov E, Selçuk F. (2001). Contribution to study of mycoflora of Turkey I. Coelomycetes of orders Melanconiales and Sphaeropsidales on forest trees and shrubs in the Black Sea coast (Rize and Trabzon Provinces). Mikologiya i Fitopatologiya 35: 28–33. [Google Scholar]
- Jones JP, Holcomb GE. (1978). Conidium ontogeny and cytology of Tubakia dryina from Louisiana Hardwoods. Mycologia 70: 1212–1216. [Google Scholar]
- Kaneko S, Kobayashi T. (1984). Fungi inhabiting fagaceous trees V. Three species of Diaporthaceae on evergreen oak leaves. Transactions of the Mycological Society of Japan 25: 11–19. [Google Scholar]
- Katoh K, Standley DM. (2013). MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M, Moir R, Wilson A, et al. (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T. (2007). Index of fungi inhabiting woody plants in Japan. Host, Distribution and Literature. Zenkoku-Noson-Kyoiku Kyokai Publishing Co., Ltd. [Google Scholar]
- Kumar S, Stecher G, Tamura K. (2016). MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Molecular Biology and Evolution 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Cho WD, Kim WK, et al. (1991). Report on host-unrecorded diseases identified from economical crops in Korea. Research Report to Rural Development Administration (Suwon, Korea) 33: 15–19 [Google Scholar]
- Limber DP, Cash EK. (1945). Actinopelte dryina. Mycologia 37: 129–137. [Google Scholar]
- Liu YJ, Whelen S, Hall BD. (1999). Phylogenetic relationships among ascomycetes: evidence from an RNA Polymerase II subunit. Molecular Biology and Evolution 16: 1799–1808. [DOI] [PubMed] [Google Scholar]
- Lumbsch HT, Huhndorf SM. eds (2007). Outline of Ascomycota – 2007. Myconet 13: 1–58. [Google Scholar]
- Maharachchikumbura SSN, Hyde KD, Jones EGB, et al. (2015). Towards a natural classification and backbone tree for Sordariomycetes. Fungal Diversity 72: 199–301. [Google Scholar]
- Maharachchikumbura SSN, Hyde KD, Gareth Jones EB, et al. (2016). Families of Sordariomyctes. Fungal Diversity 79: 1–317. [Google Scholar]
- Monod M. (1983). Monographie taxonomique des Gnomoniaceae. Sydowia, Beiheft IX: 1–315. [Google Scholar]
- Munkvold GP, Neely D. (1990). Pathogenicity of Tubakia dryina. Plant Disease 74: 518–522 [Google Scholar]
- Nag Raj TR, DiCosmo F. (1981). A monograph of Harknessia and Mastigosporella, with notes on associated teleomorphs. Bibliotheca Mycologica 80: 1–62. [Google Scholar]
- Nylander JAA. (2004). MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University, Sweden. [Google Scholar]
- Reid J, Dowsett JA. (1990). On Dicarpella, Sphaerognomonia, and Apiosporopsis. Canadian Journal of Botany 68: 2398–2407. [Google Scholar]
- Ronquist F, Teslenko M, Van der Mark P, et al. (2012). MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman AY, Farr DF, Castlebury LA. (2007). A review of the phylogeny and biology of the Diaporthales. Mycoscience 48: 135–144. [Google Scholar]
- Saccardo PA. (1913). Notae mycologicae. Series XVI. Annales Mycologici 11: 493–511. [Google Scholar]
- Senanayake IC, Crous PW, Groenewald JZ, et al. (2017). Families of Diaporthales based on morphological and phylogenetic evidence. Studies in Mycology 86: 217–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber TN. (2007). Endophytic fungi in forest trees: Are they mutualists? Fungal Biology Reviews 21(2-3): 75–89. [Google Scholar]
- Sognov MV, Castlebury LA, Meija LC, et al. (2008). Leaf-inhabiting genera of the Gnomoniaceae, Diaporthales. Studies in Mycology 62: 1–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung G-H, Sung J-M, Hywel-Jones NL, et al. (2007). A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): identification of localized incongruence using a combinational bootstrap approach. Molecular Phylogenetics and Evolution 44: 1204–1223. [DOI] [PubMed] [Google Scholar]
- Sutton BC. (1973). Tubakia nom. nov. Transactions of the British Mycological Society 60: 164–165. [Google Scholar]
- Swofford DL. (2003). PAUP*: phylogenetic analysis using parsimony. (*and other methods). Version 4.0b10. Sinauer Associates, Sunderland. [Google Scholar]
- Tangthirasunun N, Silar P, Jayarama Bhat D, et al. (2014). Greeneria saprophytica sp. nov. on dead leaves of Syzygium cumini from Chiang Rai, Thailand. Phytotaxa 184: 275–282. [Google Scholar]
- Taylor J. (2001). Pycnothyrium ultrastucture in Tubakia dryina. Mycological Research 105: 119–121. [Google Scholar]
- Taylor J, Clark S. (1996). Infection and fungal development of Tubakia dryina on Sweet Gum (Liquidambar styraciflua). Mycologia 88: 613–618. [Google Scholar]
- Tehon LR. (1924). Notes on the parasitic fungi of Illinois. Mycologia 16: 135–142. [Google Scholar]
- Tehon LR, Stout GL. (1929). Notes on the parasitic fungi of Illinois: IV. Mycologia 21: 180–196. [Google Scholar]
- Theissen F. (1913). Ueber einige Mikrothyriaceen. Annales Mycologici 11: 493–511. [Google Scholar]
- Videira SIR, Groenewald JZ, Nakashima C, et al. (2017). Mycosphaerellaceae – Chaos or clarity? Studies in Mycology 87: 257–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Rossman AY, Castlebury LA, et al. (2012). Multigene phylogeny and taxonomy of the genus Melanconiella (Diaporthales). Fungal Diversity 57: 1–44. [Google Scholar]
- Voglmayr H, Castlebury LA, Jaklitsch WM. (2017). Juglanconis gen. nov. on Juglandaceae, and the new family Juglanconidaceae (Diaporthales). Persoonia 38: 136–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Höhnel F. (1925). Neue Fungi imperfecti. 5. Mitteilung. Mitteilungen des Botanischen Instituts der Technischen Hochschule Wien 2: 65–73. [Google Scholar]
- Yokoyama T, Tubaki K. (1971). Cultural and taxonomical studies on the genus Actinopelte. Institute of Fermentation, Osaka, Research Communication 5: 43–77. [Google Scholar]
- Yun HY, Rossman AY. (2011). Tubakia seoraksanensis, a new species from Korea. Mycotaxon 115: 369–373. [Google Scholar]
- Zahedi M, Elahinia SA, Khodaparast SA, Bujari J. (2011). Introduction of some new mitosporic fungi causing leaf spot on broad leaf trees in Guilan province (N Iran). Rostaniha 12: 63–71. [Google Scholar]
- Zhang Z, Schwartz S, Wagner L, et al. (2000). A greedy algorithm for aligning DNA sequences. Journal of Computational Biology 7: 203–214. [DOI] [PubMed] [Google Scholar]