Abstract
Introduction:
Menthol cigarette use remains a serious public health problem, prompting the consideration of tobacco regulatory efforts to ban menthol cigarettes. The current study uses a novel empirical design to model the potential effects of a ban of menthol cigarettes on smoking behavior among current menthol smokers.
Methods:
29 non-treatment-seeking adults who smoked menthol cigarettes were recruited in Connecticut in 2017–18 (n=15 female; n=17 Black, n=10 White, n=5 Hispanic). Repeated-measures analyses examined within-person changes in smoking behavior when participants were switched from smoking their usual brand menthol cigarettes to a matched-brand non-menthol cigarette for 2 weeks to model a potential ban of menthol cigarettes.
Results:
Participants smoked significantly fewer non-menthol (vs. menthol) cigarettes per day (Mean decrease=2.2 cigarettes, SD=3.2, p<.001), confirmed by significant reductions in urine cotinine levels (p=.013). After switching to non-menthol cigarettes, participants had significantly lower nicotine dependence scores (reduced by >18%, p<.001) and greater increases in quitting motivation and confidence (rated 1–10) (motivation: Mean increase=2.1, SD=2.8, p<.001; confidence: Mean increase=1.3, SD=3.3, p=.04). Exploratory analyses indicated significant interactions by race (p=.004); Black smokers had greater reductions in cigarettes per day (Mean decrease=3.5 cigarettes, SD=2.8) vs. non-Black smokers (Mean decrease=0.2, SD=2.6).
Conclusions:
Banning menthol as a characterizing flavor in cigarettes may decrease smoking and reduce the addictive potential of cigarettes among current smokers. Results provide additional support for tobacco regulatory policies banning menthol flavor in an effort to improve public health.
Trial Registration:
INTRODUCTION
Although the Tobacco Control Act banned the sale of cigarettes with characterizing flavors, menthol is currently exempt from this ban, and menthol cigarette use remains a serious public health problem. While the overall rate of cigarette smoking has declined, menthol cigarette use has not declined at the same rate1, and the prevalence of menthol cigarette use among current smokers has increased over time (2008–2014)2. Although menthol smokers try to quit at similar or higher rates compared to non-menthol smokers3, they are less likely to quit successfully3–6, and use of menthol cigarettes is associated with higher rates of smoking and dependence7,8. Menthol flavor additives may influence the appeal and addictive potential of these cigarettes by reducing the aversive effects of cigarette smoke9–12 and/or inhibiting nicotine metabolism, resulting in greater nicotine exposure9,13.
Experts assert that banning characterizing menthol flavors in tobacco products may have a positive benefit on public health14, and empirical evidence regarding the potential impact of a ban of menthol cigarettes on smoking behavior is important for informing tobacco regulatory efforts. Many menthol smokers believe they would smoke less or try to quit smoking if menthol cigarettes were no longer available15,16, which is supported by results from population-level simulation modeling that predict a substantial decrease in smoking prevalence if menthol cigarettes were banned17. Additionally, research from Canada indicated significant increases in quit attempts among menthol smokers after menthol cigarettes were banned16,18.
The current study further informs this important research area using a novel empirical design to investigate within-person changes in smoking behavior after current menthol smokers were switched to using non-menthol cigarettes to model the potential impact of a menthol ban. The primary outcome examined changes in cigarettes smoked per day, and secondary outcomes included changes in nicotine dependence and quitting motivation and confidence after switching to non-menthol cigarettes. We expected that switching to non-menthol cigarettes may also decrease cigarette satisfaction and reinforcement, so changes in withdrawal and subjective ratings of cigarettes were explored. Additionally, since menthol smoking rates are highest nationally among specific subgroups of smokers, including a higher proportion of menthol cigarette use among those who identify as Black compared to any other race2, and women compared to men2,19, we explored whether the primary outcome of changes in cigarettes per day differed by race or gender.
METHODS
Procedures
All procedures were approved by the University Institutional Review Board. Interested participants were recruited from the community and screened by telephone. Those meeting initial eligibility criteria attended an in-person appointment to provide written informed consent and complete further assessments. Eligible participants then began the 4-week study and returned weekly for visits. During the first week, participants smoked their own cigarettes which they purchased on their own. Next, participants were provided with their own usual menthol brand cigarettes, at no cost, to smoke for 1 week (Phase I, week 2). Lastly, participants were provided with non-menthol cigarettes to smoke for 2 weeks (Phase II, week 3–4). This time frame was based on evidence from other studies that changes in smoking behavior can be seen within 2 weeks of switching to alternative cigarettes20,21. We chose a replacement non-menthol cigarette of the same brand (e.g., switching from Marlboro menthol to Marlboro non-menthol) based on evidence from the Canadian menthol ban where the tobacco industry encouraged consumers to switch to their same-brand non-menthol products after the ban22,23. Available data indicates comparable nicotine content between these matched-brand menthol and non-menthol cigarettes24–26 with minimal detectable levels of menthol in the non-menthol cigarettes (e.g., Marlboro menthol > 4.3 mg/cig vs. Marlboro non-menthol 0.0129 mg/cig27,28). In Phase I and II, cigarettes were provided for free to encourage adherence to smoking the specified cigarettes and to control for the possibility of increased consumption due to free cigarettes (as seen in other studies21). This way, cost of cigarettes was removed as a factor that could potentially influence differences in cigarette consumption when switching to non-menthol cigarettes. Participants were compensated for visits and could earn bonuses (e.g., $10/week for returning spent cigarette filters, see below for details) for a total of up to $300.
Participants
Inclusion Criteria were: age (18 or older), English literate, smoking ≥5 cigarettes per day for ≥6 months, expired breath carbon monoxide (CO) level ≥ 6 parts per million (ppm) at baseline, and currently smoking menthol cigarettes; current cigarette brand was confirmed in-person at the intake appointment. Exclusion Criteria were: current use of cessation treatments or trying to quit smoking, reporting a history of a serious psychiatric condition (i.e., bipolar disorder, schizophrenia), current drug use (excluding caffeine) or a positive urine toxicology screen at intake. We excluded participants who reported living with a menthol smoker to limit access to menthol cigarettes during the non-menthol phase of the trial. Female participants were excluded if they were currently pregnant, breastfeeding, or unwilling to use effective birth control for the duration of the study.
Measures
Baseline measures included self-reported demographics and smoking history. Participants reported their age, race/ethnicity, gender, marital status, income, employment, and education. Participants also provided information about their smoking behavior such as smoking heaviness, brand of cigarettes they currently smoke, and quitting history.
Several measures were collected at baseline and repeated within-person during the study to evaluate responses after use of the menthol and non-menthol cigarettes. Repeated measures are described below.
Timeline Follow-Back Interview (TLFB)29:
a reliable and valid way of collecting information about substance use quantity and frequency. TLFB was administered at each visit to assess daily cigarette consumption and use of other tobacco products.
Menthol product use:
Access to other mentholated products (e.g., gum, toothpaste) was not restricted to maximize the real-world applicability of findings. Use of other mentholated products was assessed at weekly visits.
Spent cigarette filters:
Participants were given dated bags to collect all the spent filters after the cigarettes were smoked each day. Bags were returned at each visit and used to confirm daily cigarette quantity (i.e., the count of spent filters in each bag) as well as protocol adherence (i.e., the number of menthol and non-menthol filters in each bag). Participants received a weekly bonus for returning cigarette filters, and earnings were not tied to protocol adherence (i.e., participants were paid even if they returned menthol filters during the non-menthol phase) to increase honest reporting. Trained research staff measured the spent filter length to further quantify smoking heaviness between the smoked menthol and non-menthol cigarettes. A random sample of 6 filters was used to determine the average cigarette length for each week, as there was high agreement (ICC=.95) in initial analyses when compared to the entire week of cigarette filters.
Biochemical measures:
Expired breath CO was collected at each visit (CO Check +, MD Diagnostics Ltd). Urine samples were collected at baseline and week 2, 3, and 4 to assess urinary cotinine levels and menthol glucuronide (MG) as a marker of recent menthol exposure30,31. Menthol glucuronide values were corrected for creatinine concentration (ng/mg creatinine).
Nicotine Dependence:
The 37-item Wisconsin Inventory of Smoking Dependence Motives (WISDM-37)32,33 assessed multiple dimensions of tobacco dependence. Items were rated from 1 (not at all true of me) to 7 (extremely true of me). Nicotine dependence was measured with the total WISDM score as well as the average score on the Primary Dependence Motives (i.e., Automaticity, Loss of Control, Craving, and Tolerance).
Quitting motivation and confidence:
Motivation to quit smoking and confidence quitting smoking were measured with the items “How important is quitting smoking to you?” and “How confident are you that you could quit smoking if you tried?” rated from 1 (not at all) to 10 (extremely).
Withdrawal:
The Wisconsin Smoking Withdrawal Scale (WSWS)34, assessed 7 constructs of nicotine withdrawal: anger, anxiety, concentration, craving, hunger, sadness, and sleep. Items were rated from 1 (strongly disagree) to 5 (strongly agree) and scores were averaged for each construct.
Modified Cigarette Evaluation Scale (MCEQ):
The 12-item MCEQ measured subjective effects of the smoked cigarettes35. Items were rated from 1 (not at all) to 7 (extremely) and assessed 5 subscales: satisfaction (e.g., cigarettes tasted good and were enjoyable), psychological reward (e.g., smoking helped with feeling calm, less irritable), enjoyment of respiratory tract sensations (e.g., sensations in the throat and chest), craving reduction (e.g., smoking alleviated craving), and aversion (e.g., nausea, dizziness).
Cigarette Purchase Task:
The cigarette purchase task (CPT) is a validated self-report measure that asks participants to indicate how many cigarettes they would smoke if each cigarette cost a specific price, with values ranging from free ($0) to $1,12036,37. Responses were used to generate a demand curve, reflecting the relationship between escalating price and demand for the menthol and non-menthol cigarettes. Additionally, we examined differences between menthol and non-menthol cigarettes in the intensity of demand (i.e., consumption at the lowest price), peak expenditure per cigarette (i.e., maximum expenditure divided by the demand at that price), and the breakpoint (i.e., the first price where reported consumption is zero), as these indices have been shown to relate to nicotine dependence and smoking behavior36.
End of Study Assessments
Two items at the end of the study assessed “If menthol cigarettes were no longer available, how likely is it that you would continue to use the non-menthol cigarettes you tried in this study?” and “If menthol cigarettes were no longer available, how likely is it that you would decide to stop smoking completely?” rated from 1 (not at all) to 10 (extremely).
Data Analysis
Descriptive statistics were used to summarize baseline characteristics and outcomes over time. Paired sample t-tests evaluated within-person changes in outcomes between the menthol and non-menthol phases of the study. The primary outcome evaluated changes in smoking heaviness after a 2-week period switching to non-menthol cigarettes. Planned comparisons for changes in smoking heaviness and associated biomarkers were between Phase I (the end of the period of free menthol cigarettes that were the participant’s usual brand) and Phase II (the end of the study after switching to the free matched-brand non-menthol cigarette). To evaluate adherence to the switching protocol, we compared consistency across multiple measures of smoking behavior, including self-reported cigarette consumption and spent cigarette filters. We also compared the length of the spent cigarette filters between the menthol and non-menthol phases to further quantify changes in smoking heaviness.
Secondary outcomes examined changes in nicotine dependence and quitting motivation and confidence. We also evaluated changes in withdrawal and subjective ratings of cigarettes. Lastly, we conducted exploratory analyses to examine whether the primary outcome of changes in cigarettes per day differed by race or gender. Repeated measures general linear models were used with time entered as a within-subject factor to account for observations over time within individuals and race (Black vs. non-Black) or gender (male vs. female) entered as a between-subject factor. Cohen’s d values were calculated to estimate effect sizes (reported as dz for repeated measures and d for between-subjects comparisons by race and gender)38.
Results
Participants
Of the 39 participants who completed eligibility screening and provided written informed consent, 6 were determined not eligible at intake: n=4 positive urine drug screen or positive blood alcohol reading, n=1 positive urine pregnancy screen, n=1 CO reading too low to confirm smoking status. Of the 33 eligible, 4 dropped out prior to switching from menthol to non-menthol cigarettes (analysis sample size n=29). See Table 1 for sample description.
Table 1.
Demographic and Smoking History Characteristics N=29
| Variable | Mean (SD) or N (%) |
|---|---|
| Age, M (SD) | 34.8 (11.6) |
| Female, N (%) | 15 (51.7%) |
| Racea/Ethnicityb, N (%) | |
| Hispanic | 5 (17.2%) |
| Black | 17 (58.6%) |
| White | 10 (34.5%) |
| Other | 3 (10.3%) |
| Marital Status, N (%) | |
| Single | 24 (82.8%) |
| Married | 2 (6.9%) |
| Other | 3 (10.3%) |
| Employment status, N (%) | |
| Unemployed | 13 (44.8%) |
| Employed Part or Full Time | 13 (44.8%) |
| Other | 3 (10.3%) |
| Education, N (%) | |
| Less than high school (HS) | 6 (20.7%) |
| HS or GED | 19 (65.5%) |
| Some college or more | 4 (13.8%) |
| Cigarettes per day, M (SD) | 12.3 (6.0) |
| Years Smoked, M (SD) | 15.6 (9.6) |
| Ever tried quitting, N (%) | 14 (48.3%) |
| Menthol cigarette brandc | |
| Newport | 25 (86.2%) |
| Marlboro | 3 (10.3%) |
| Natural American Spirits | 1 (3.5%) |
| Typical cigarette purchasing quantity | |
| Cartons, N (%) | 0 (0.0%) |
| Packs, N (%) | 24 (82.8%) |
| Single loose cigarettes, N (%) | 5 (17.2%) |
Note:
Participants could select all that apply.
Ethnicity defined as Hispanic yes/no.
Indicates the menthol cigarette brands used by participants at baseline. Participants received these same menthol cigarettes during Phase I of the study and received matched-brand non-menthol cigarettes during Phase II (i.e., those smoking Newport menthol received replacement Newport non-menthol)
Smoking Heaviness
Participants smoked significantly fewer cigarettes per day after switching from menthol (M=12.2, SD=5.6) to non-menthol cigarettes (M=10.0, SD=5.6), t(28)=3.68, p=.001, Cohen’s dz=.68 (see Figure 1). This reduction in smoking heaviness was corroborated by a significant decrease in urinary cotinine (available for a subsample of participants, n=26); menthol M=1785.6 ng/ml, SD=1159.5 vs. non-menthol M=1440.3 ng/ml, SD=1007.4, t(25)=2.69, p=.013, Cohen’s dz=.53, and a non-significant decrease in expired breath CO for the entire sample (n=29) (menthol M=32.0, SD=20.3 ppm vs. non-menthol M=28.2, SD=19.4 ppm), t(28)=1.92, p=.06, Cohen’s dz=.36. We received filter bags from n=28/29 participants (96.6%), and comparisons of the length of the spent cigarette filters indicated that participants also smoked significantly less of the non-menthol cigarettes (i.e., longer filter lengths on average for non-menthol cigarettes: M=35.0mm, SD=3.3, vs. menthol cigarettes: M=33.6mm, SD=4.3, t(27)=−2.12, p=.04, Cohen’s dz=.40).
Figure 1. Within-subject comparison of the average cigarettes smoked per day by study visit (N=29).
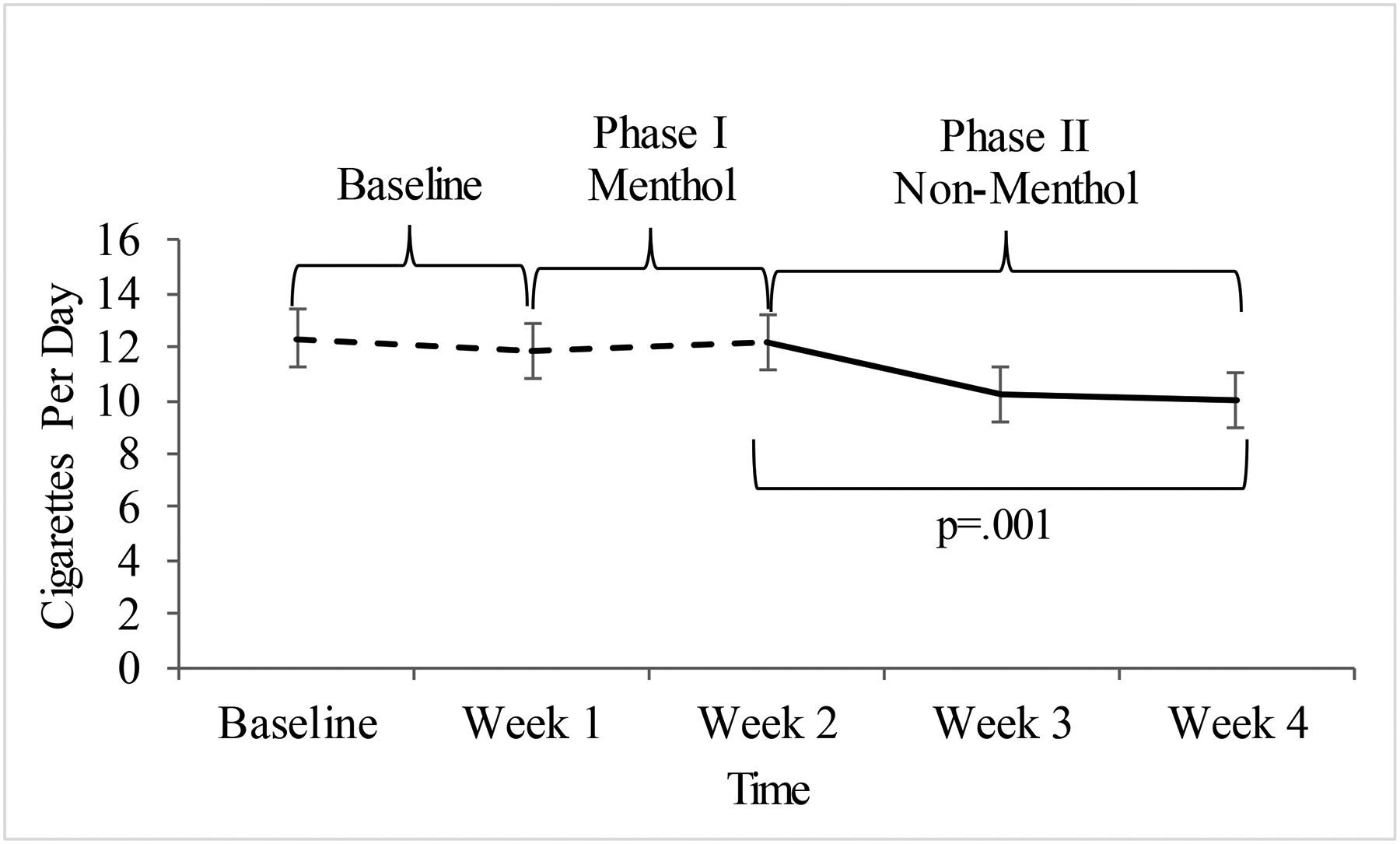
Values represent the mean and standard error of cigarettes smoked per day at each week by cigarette type. Planned comparisons evaluated changes in cigarettes per day between Phase I (the end of the period of free menthol cigarettes that were the participant’s usual brand) and Phase II (the end of the study after switching to the free matched-brand non-menthol cigarette).
Adherence
There was high agreement between cigarette consumption measured by self-report and objective filter count (ICC=.89). Analyses of urinary MG concentrations indicated that while there was a significant reduction at the beginning of the switching phase (week 2–3), t(28)=2.84, p=.008, there was wide between-subject variability in MG at the end of the study and a non-significant change overall during this period (week 2–4), t(28)=0.38, p=.70, Cohen’s dz=.07 (see Figure 2).
Figure 2. Within-person comparison of average creatinine-corrected menthol glucuronide concentrations by study visit (N=29).
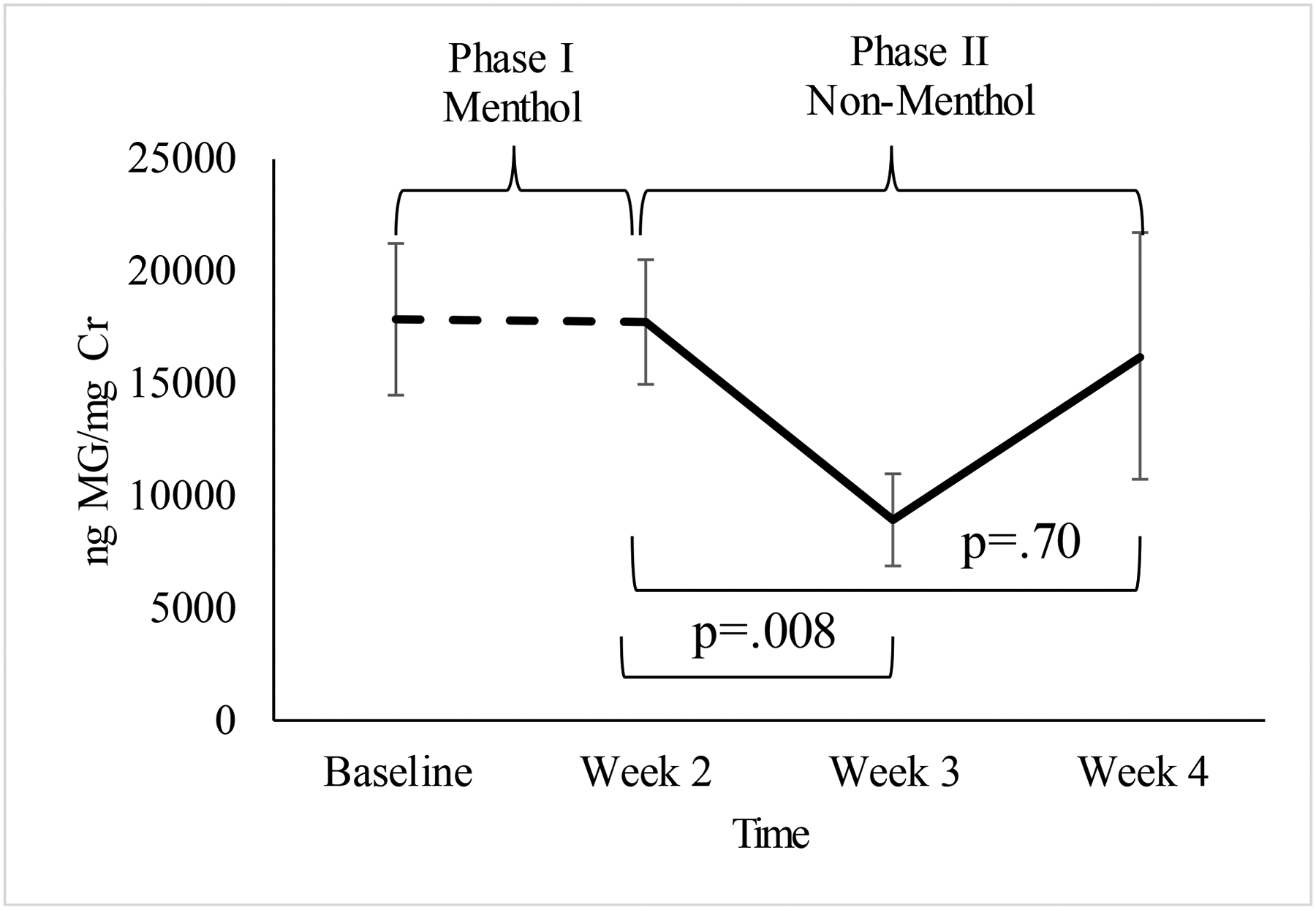
Access to other mentholated products (e.g., gum, toothpaste) was not restricted during the study to maximize the real-world applicability of findings. Values reflect the mean and standard errors of concentrations of menthol glucuronide detected in urine samples, corrected for creatinine (ng MG/mg Cr), and compared between the menthol cigarette (Phase I) and non-menthol cigarette (Phase II) phases.
Over half of the sample (n=16) reported smoking a menthol cigarette at some point during the non-menthol phase, and adherence varied widely; across participants, 1.4%−51.8% of the total number of cigarettes smoked during the switching phase were menthol cigarettes instead of non-menthol cigarettes. Returned cigarette filters were visually coded as menthol or non-menthol to further evaluate adherence during the switching phase. In total, n=15 participants were completely adherent at the end of the study, based on both self-report and cigarette filters. Analyses based on the adherent subsample (n=15) were consistent with the results found for the entire subsample (n=29) for the primary outcome.
Eleven participants (37.9%) reported compensatory menthol product use during the non-menthol phase, such as chewing gum or brushing their teeth more often. Only 1 participant reported other tobacco product use: increased use of their fruit-flavored e-cigarette during the non-menthol period.
Nicotine Dependence and Quitting Motivation and Confidence
Table 2 summarizes differences from baseline to the study endpoint. Participants reported lower nicotine dependence after switching from menthol to non-menthol cigarettes on the primary dependence motives of the WISDM, t(28)=4.46, p<.001, and the WISDM total dependence score, t(28)=5.07, p<.001. Participants reported greater motivation to quit smoking, t(28)=−4.07, p<.001 and more confidence in their ability to quit smoking, t(28)=−2.11, p=.04, after switching to non-menthol cigarettes
Table 2.
Changes in smoking outcomes from baseline to the end of the study after switching to non-menthol cigarettes (N=29)
| Measure | Baseline: Menthol Cigarettes | End of Study: After Switching to Non-Menthol Cigarettes | p-value | Cohen’s dz |
|---|---|---|---|---|
| Nicotine Dependence: WISDM | ||||
| Total (range 11–77) | 45.0 (10.7) | 36.8 (10.9) | <.001 | 0.94 |
| Primary dependence motives (range 1–7) | 4.3 (1.1) | 3.6 (1.2) | <.001 | 0.83 |
| Quitting Smoking | ||||
| Importance of quitting (range 1–10) | 3.3 (2.4) | 5.4 (2.5) | <.001 | 0.76 |
| Confidence quitting smoking (range 1–10) | 4.2 (2.9) | 5.5 (2.7) | 0.04 | 0.39 |
| Withdrawal: WSWS | ||||
| Anger (range 1–5) | 2.4 (1.2) | 2.3 (1.1) | 0.56 | 0.11 |
| Anxiety (range 1–5) | 2.4 (0.7) | 2.1 (0.8) | 0.02 | 0.46 |
| Concentration (range 1–5) | 1.8 (0.7) | 1.7 (0.7) | 0.51 | 0.13 |
| Craving (range 1–5) | 3.3 (0.6) | 2.8 (0.8) | <.001 | 0.91 |
| Hunger (range 1–5) | 2.6 (0.9) | 2.5 (0.9) | 0.73 | 0.06 |
| Sadness (range 1–5) | 1.5 (0.7) | 1.4 (0.7) | 0.30 | 0.20 |
| Sleep (range 1–5) | 1.6 (0.7) | 1.5 (0.7) | 0.33 | 0.19 |
| Cigarette Subjective Effects: MCEQ | ||||
| Smoking satisfaction (range 1–7) | 5.0 (1.3) | 3.0 (1.5) | <.001 | 1.06 |
| Psychological reward (range 1–7) | 4.2 (1.2) | 2.8 (1.4) | <.001 | 0.84 |
| Enjoyment of respiratory tract sensations (range 1–7) | 3.8 (1.4) | 2.2 (1.4) | <.001 | 0.98 |
| Craving reduction (range 1–7) | 5.1 (1.6) | 3.6 (1.8) | 0.002 | 0.61 |
| Aversion (range 1–7) | 1.9 (1.0) | 2.1 (1.4) | 0.23 | 0.23 |
Note: WISDM=Wisconsin Smoking Dependence Motives (Piper et al., 2004). WSWS=Wisconsin Smoking Withdrawal Scale (Welsch et al., 1999) measures constructs of nicotine withdrawal (e.g., “craving” or having frequent urges to smoke). MCEQ=Modified Cigarette Evaluation Scale (Cappelleri et al., 2007) measures the subjective effects of cigarettes (e.g., “craving reduction” or how much smoking alleviates craving). Cohen’s dz=effect size estimate for repeated measures (Cohen, 1988).
Withdrawal
There were no significant changes in most withdrawal measures (anger, concentration, hunger, sadness, and sleep) between baseline and the study endpoint (ps>.30). However, there were reductions in reported anxiety (e.g., lower ratings of being tense or anxious, impatient), t(28)=2.47, p=.02, and craving (e.g., lower ratings of frequent urges to smoke, being bothered by the desire to smoke), t(28)=4.90, p<.001.
Cigarette Subjective Effects
Compared to their usual-brand menthol cigarettes, participants reported significant reductions in the subjective ratings of the non-menthol cigarettes: lower ratings of smoking satisfaction, psychological reward, enjoyment of respiratory tract sensations, and craving reduction, ps≤.002. There were no significant differences in the ratings of aversion (e.g., nausea and dizziness) between the menthol and non-menthol phases, t(28)=−1.22, p=.23.
Cigarette Purchase Task
Figure 3 displays the demand curves by cigarette type comparing the reinforcing value of the menthol and non-menthol cigarettes. Paired sample t-tests indicated significant differences in intensity of demand, t(28)=2.45, p=.02, Cohen’s dz=.45, and peak expenditure per cigarette, t(27)=2.06, p=.05, Cohen’s dz=.39, indicating greater demand and expenditure for menthol compared to non-menthol cigarettes. There was a non-significant difference in average breakpoint (i.e., price at which self-reported cigarette consumption was zero, menthol M=14.8, SD=27.4 vs. non-menthol M=4.8, SD=8.8, t(27)=2.0, p=.06, Cohen’s dz=.38).
Figure 3. Cigarette Purchase Task Demand Curve by Cigarette Type.
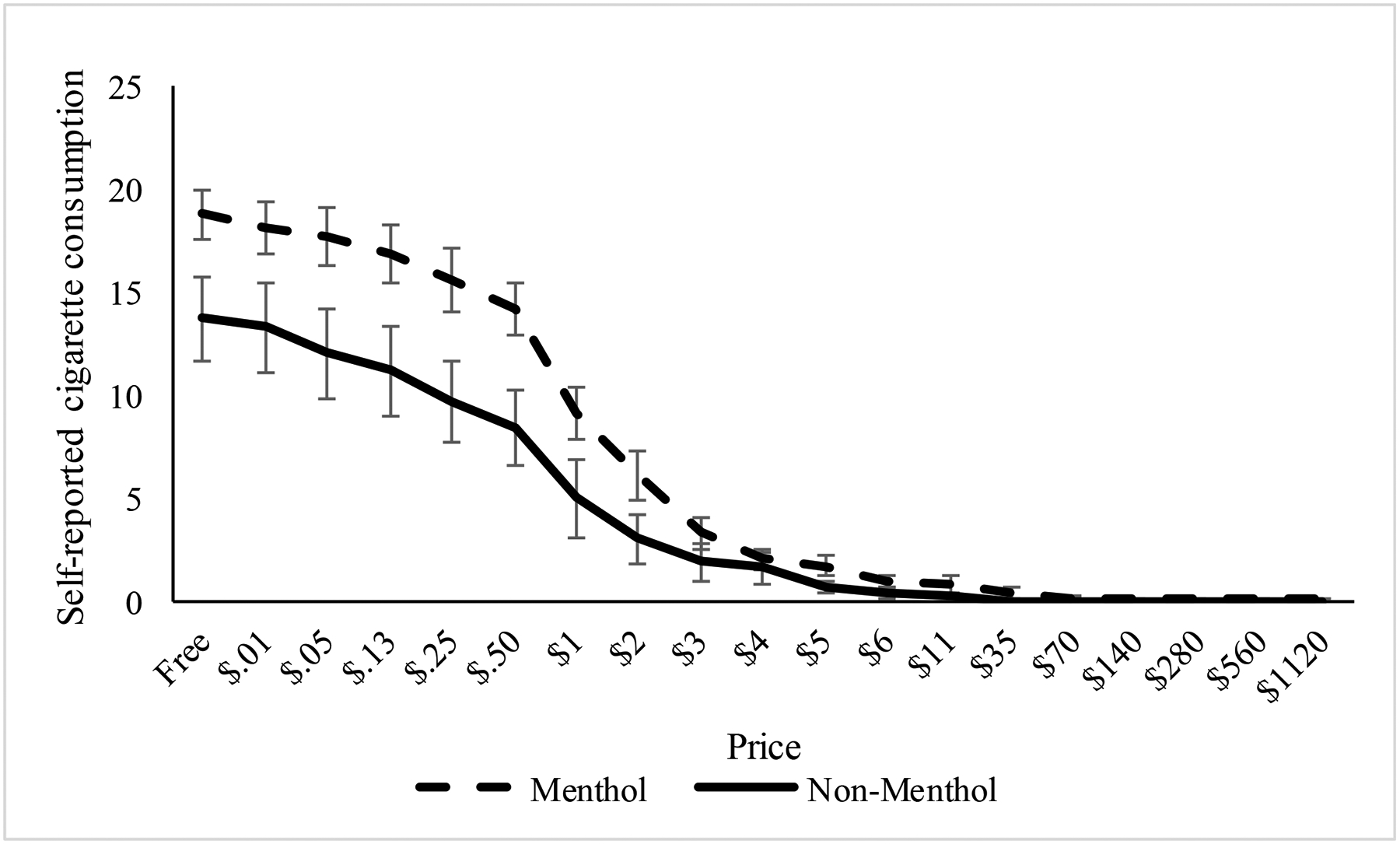
Values reflect the hypothetical daily cigarette consumption reported by participants at varying prices per cigarette. This measure was repeated within-person and assessed after the menthol and non-menthol cigarette phases of the study.
End of Study Assessments
At the end of the study, participants were responded about their expected behavior if menthol cigarettes were no longer available. Participants reported they were significantly more likely to quit smoking (M=6.5 out of 10, SD=3.0) than to continue smoking the non-menthol cigarettes they tried (M=4.1 out of 10, SD=2.6), t(28)=2.52, p=.02, Cohen’s dz=.47, if menthol cigarettes were no longer available.
Exploratory Outcomes by Race and Gender
Baseline smoking characteristics (i.e., cigarettes per day, number of years smoked, expired breath CO, nicotine dependence, prior quit attempts) did not differ significantly by race (Black N=17 vs. non-Black N=12) or gender (male N=14 vs. female N=15), with the exception that males reported smoking for more years on average (M=20.6, SD=9.0 vs. females M=10.9, SD=7.8, t(27)=3.11, p=.004). Repeated measures general linear models examined whether changes in cigarettes smoked per day following the switching period differed by race or gender. There was a significant interaction between race and cigarettes per day, F(1,27)=10.15, p=.004, Cohen’s d=1.21, indicating a greater reduction in cigarettes per day among Black smokers (M=−3.5, SD=2.8) than non-Black smokers (M=−0.2, SD=2.6). There was not a significant interaction between gender and changes in cigarettes per day: F(1,27)=0.04, p=.84, Cohen’s d=.07, indicating similar reductions in cigarettes per day among males (M=−2.3, SD=3.6) and females (M=−2.1, SD=2.8).
Discussion
This study provides new information about the potential impact of banning characterizing menthol flavors in cigarettes and indicates this regulatory policy may reduce the appeal and addictive potential of cigarettes among current menthol smokers. Using a novel procedure designed to model a ban on menthol cigarettes by switching current adult menthol smokers to non-menthol cigarettes, we found that participants smoked significantly fewer cigarettes per day; less of each cigarette on average; and reported lower ratings of nicotine dependence, satisfaction, and reinforcement from the cigarettes after switching to non-menthol cigarettes. Additionally, participants reported greater motivation and confidence quitting smoking. Exploratory analyses also indicated there are important differences by race. These findings provide additional data to support regulatory policies banning characterizing menthol flavors in cigarettes.
Our results build on earlier survey research and population modeling that predict a decrease in smoking prevalence if menthol cigarettes were banned15,17, and provide empirical evidence of changes in smoking behavior. These findings have important public health implications. Menthol cigarette use has not decreased at the same rate as non-menthol cigarette use1,2, indicating this group of smokers is particularly vulnerable to the health consequences of smoking39 and may be less helped by existing tobacco control policies40. Consistent with predictions from simulation models17, our results indicate that banning menthol cigarettes may be especially beneficial for Black smokers, a population that uses menthol cigarettes at higher rates2 and is targeted by tobacco industry marketing41–43. Although our subgroup analyses by race are preliminary due to sample size, the results indicate that Black smokers had greater reductions in smoking heaviness. Future research should investigate how these changes relate to quitting success. If a menthol ban reduces smoking heaviness and reinforcement and increases quitting motivation or quit attempts (as seen in Canada16,18) this policy could benefit current menthol smokers and help reduce tobacco-related health disparities specifically among Black smokers4,5,44.
This study design has several important strengths, including the use of multiple measures to corroborate smoking behavior (such as collecting spent cigarette filters) and testing the effect of switching to a matched-brand non-menthol cigarette, as this models what the tobacco companies encouraged smokers to do in the context of an actual menthol ban22,23. However, findings should be interpreted in light of study limitations. First, analyses may be underpowered, especially to detect subgroup differences, and the sample does not represent the population who smoke menthol cigarettes. A larger replication trial is needed to better understand the potential impact of banning menthol cigarettes for the broader population, including vulnerable subgroups who use menthol cigarettes at elevated rates (e.g., adolescent smokers2, those with serious mental illness45,46). Second, participants could still access other menthol products, and some participants continued smoking menthol cigarettes during the study, although the primary results were consistent in the subgroup of participants who were completely adherent and only smoked non-menthol cigarettes. Additionally, cigarettes were provided to encourage protocol adherence, so smoking behavior may differ in the context of an actual menthol ban when people would have to purchase their own cigarettes. Given the study design, we cannot rule out other possible factors that may have influenced study findings. For example, smoking may be influenced by completing repeated assessments asking about smoking behavior or switching from a preferred to a non-preferred cigarette. Furthermore, the specific menthol and nicotine levels were not verified quantitatively for the menthol and matched-brand non-menthol cigarettes, although available data indicates comparable nicotine concentrations between these matched-brand menthol and non-menthol cigarettes24–26 with minimal detectable menthol levels in the non-menthol cigarettes27,28. Most participants smoked Newport menthol cigarettes (86.2%), so additional research is needed to examine the effects across users of other cigarette brands. Lastly, in the context of a regulatory policy banning characterizing menthol flavor in cigarettes, it would be important to also study how the availability of characterizing flavors in other tobacco products may influence cigarette smoking behavior and other outcomes examined here.
Conclusions
The current study provides preliminary information to support regulatory efforts to ban characterizing menthol flavors in cigarettes. Our findings suggest that such a policy might have a meaningful benefit for current menthol smokers by reducing smoking heaviness, nicotine dependence, smoking reinforcement, and increasing quitting motivation and confidence. These findings provide additional empirical evidence to further support the Tobacco Products Scientific Advisory Committee recommendations to ban menthol as a characterizing flavor in cigarettes in the US14. Results from this empirical study converge with evidence from simulation models17 and studies of smoking behavior after a menthol cigarette ban in Canada16,18 to suggest that banning menthol cigarettes has the potential to benefit public health.
What this paper adds.
What is already known on this subject
Menthol cigarette use remains a large public health problem.
Tobacco control efforts that ban menthol flavor in cigarettes may reduce smoking rates or promote quitting.
What this paper adds
We evaluated the potential impact of a ban of menthol cigarettes on smoking behavior by substituting current menthol smokers’ cigarettes with non-menthol cigarettes.
Banning menthol in cigarettes may decrease smoking and reduce the addictive potential of cigarettes among current menthol smokers.
Acknowledgements:
We would like to acknowledge and thank Haleh Nadim, Annabel Burnley, and Sera-Maren Wiechert for their assistance with data collection and laboratory analysis.
Funding: Research reported in this publication was supported by grant numbers P50DA036151 and U54DA036151 from the NIDA and FDA Center for Tobacco Products (CTP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration.
Footnotes
Conflicts of interest: Dr. O’Malley reported having been a consultant or an advisory board member for Alkermes, Amygdala, Arkeo, Cerecor, Mitsubishi Tanabe, Opiant, Pfizer; honoraria from the American Society of Clinical Psychopharmacology Alcohol Clinical Trials Initiative supported by Abbott, Amygdala, Ethylpharm, Lilly, Lundbeck, Otsuka, Pfizer, Arbor Pharmaceuticals, and Indivior and from the Emmes Corporation as a DSMB member for the NIDA Clinical Trials Network; a coinvestigator on studies receiving donated medications from Astra Zeneca, Norvatis; a contract from Lilly; and a scientific panel member for Hazelden Foundation. No other authors have conflicts to report.
REFERENCES
- 1.SAMHSA. The NSDUH Report: Recent Trends in Menthol Cigarette Use. Center for Behavioral Health Statistics and Quality; 2011. [Google Scholar]
- 2.Villanti AC, Mowery PD, Delnevo CD, Niaura RS, Abrams DB, Giovino GA. Changes in the prevalence and correlates of menthol cigarette use in the USA, 2004–2014. Tob Control. 2016;25(Suppl 2):ii14–ii20. [DOI] [PubMed] [Google Scholar]
- 3.Levy DT, Blackman K, Tauras J, et al. Quit attempts and quit rates among menthol and nonmenthol smokers in the United States. Am J Public Health. 2011;101(7):1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith SS, Fiore MC, Baker TB. Smoking cessation in smokers who smoke menthol and non‐menthol cigarettes. Addiction. 2014;109(12):2107–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delnevo CD, Gundersen DA, Hrywna M, Echeverria SE, Steinberg MB. Smoking-cessation prevalence among US smokers of menthol versus non-menthol cigarettes. Am J Prev Med. 2011;41(4):357–365. [DOI] [PubMed] [Google Scholar]
- 6.Foulds J, Hooper MW, Pletcher MJ, Okuyemi KS. Do smokers of menthol cigarettes find it harder to quit smoking? Nic Tob Res. 2010;12(suppl_2):S102–S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahijevych K, Garrett BE. The role of menthol in cigarettes as a reinforcer of smoking behavior. Nic Tob Res. 2010;12:S110–S116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azagba S, Minaker LM, Sharaf MF, Hammond D, Manske S. Smoking intensity and intent to continue smoking among menthol and non-menthol adolescent smokers in Canada. Cancer Causes & Control. 2014;25(9):1093–1099. [DOI] [PubMed] [Google Scholar]
- 9.Ha MA, Smith GJ, Cichocki JA, et al. Menthol attenuates respiratory irritation and elevates blood cotinine in cigarette smoke exposed mice. PloS one. 2015;10(2):e0117128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willis DN, Liu B, Ha MA, Jordt S-E, Morris JB. Menthol attenuates respiratory irritation responses to multiple cigarette smoke irritants. The FASEB Journal. 2011;25(12):4434–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wayne GF, Connolly GN. Application, function, and effects of menthol in cigarettes: a survey of tobacco industry documents. Nic Tob Res. 2004;6(Suppl 1):S43–S54. [DOI] [PubMed] [Google Scholar]
- 12.Yerger VB, McCandless PM. Menthol sensory qualities and smoking topography: a review of tobacco industry documents. Tob Control. 2011;20(Suppl 2):ii37–ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benowitz NL, Herrera B, Jacob B. Mentholated cigarette smoking inhibits nicotine metabolism. Journal of Pharmacology and Experimental Therapeutics 2004;310(3):1208–1215. [DOI] [PubMed] [Google Scholar]
- 14.Tobacco Products Scientific Advisory Committee. Menthol cigarettes and public health: review of the scientific evidence and recommendations. Washington DC: US Food and Drug Administration; 2011. [Google Scholar]
- 15.O’Connor RJ, Bansal‐Travers M, Carter LP, Cummings KM. What would menthol smokers do if menthol in cigarettes were banned? Behavioral intentions and simulated demand. Addiction. 2012;107(7):1330–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaiton M, Schwartz R, Cohen JE, Soule E, Eissenberg T. Association of Ontario’s ban on menthol cigarettes with smoking behavior 1 month after implementation. JAMA internal medicine. 2018;178(5):710–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy DT, Pearson JL, Villanti AC, et al. Modeling the future effects of a menthol ban on smoking prevalence and smoking-attributable deaths in the United States. Am J Public Health. 2011;101(7):1236–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaiton MO, Nicolau I, Schwartz R, et al. Ban on menthol-flavoured tobacco products predicts cigarette cessation at 1 year: a population cohort study. Tob Control. 2019:tobaccocontrol-2018–054841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith PH, Akpara E, Haq R, El-Miniawi M, Thompson AB. Gender and Menthol Cigarette Use in the United States: A Systematic Review of the Recent Literature (2011 – May 2017). Current Addiction Reports. 2017;4(4):431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatsukami DK, Kotlyar M, Hertsgaard LA, et al. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction. 2010;105(2):343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donny EC, Denlinger RL, Tidey JW, et al. Randomized trial of reduced-nicotine standards for cigarettes. N Engl J Med. 2015;373(14):1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown J, DeAtley T, Welding K, et al. Tobacco industry response to menthol cigarette bans in Alberta and Nova Scotia, Canada. Tob Control. 2017;26(e1):e71–e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz R, Chaiton M, Borland T, Diemert L. Tobacco industry tactics in preparing for menthol ban. Tob Control. 2018;27(5):577–577. [DOI] [PubMed] [Google Scholar]
- 24.Federal Trade Commission. “Tar,” Nicotine, and Carbon Monoxide of the Smoke of 1294 Varieties of Domestic Cigarettes for the Year 1998 2000.
- 25.Nicotine, tar, and CO content of domestic cigarettes in 2007 (Regular brands). 2007; http://www.econdataus.com/cigrs.html.
- 26.Nicotine, Tar, and CO content of domestic cigarettes in 2007 (Menthol brands). 2007; http://www.econdataus.com/cigms.html.
- 27.Ai J, Taylor KM, Lisko JG, Tran H, Watson CH, Holman MR. Menthol levels in cigarettes from eight manufacturers. Tob Control. 2018;27(3):335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ai J, Taylor KM, Lisko JG, Tran H, Watson CH, Holman MR. Menthol content in US marketed cigarettes. Nic Tob Res. 2015;18(7):1575–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors. 1998;12(2):101. [Google Scholar]
- 30.Tanner J-A, Novalen M, Jatlow P, et al. Nicotine metabolite ratio (3-hydroxycotinine/cotinine) in plasma and urine by different analytical methods and laboratories: implications for clinical implementation. Cancer Epidemiology and Prevention Biomarkers. 2015;24(8):1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benowitz NL, Dains KM, Dempsey D, Havel C, Wilson M, Jacob P. Urine menthol as a biomarker of mentholated cigarette smoking. Cancer Epidemiology and Prevention Biomarkers. 2010;19(12):3013–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piper ME, Piasecki TM, Federman EB, et al. A multiple motives approach to tobacco dependence: the Wisconsin Inventory of Smoking Dependence Motives (WISDM-68). Journal of consulting and clinical psychology. 2004;72(2):139. [DOI] [PubMed] [Google Scholar]
- 33.Smith SS, Piper ME, Bolt DM, et al. Development of the brief Wisconsin inventory of smoking dependence motives. Nic Tob Res. 2010;12(5):489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Exp Clin Psychopharmacol. 1999;7(4):354. [DOI] [PubMed] [Google Scholar]
- 35.Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addict Behav. 2007;32(5):912–923. [DOI] [PubMed] [Google Scholar]
- 36.MacKillop J, Murphy JG, Ray LA, et al. Further validation of a cigarette purchase task for assessing the relative reinforcing efficacy of nicotine in college smokers. Exp Clin Psychopharmacol. 2008;16(1):57. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs EA, Bickel WK. Modeling drug consumption in the clinic using simulation procedures: demand for heroin and cigarettes in opioid-dependent outpatients. Exp Clin Psychopharmacol. 1999;7(4):412. [DOI] [PubMed] [Google Scholar]
- 38.Cohen J. Statistical power analysis for the behavioral sciences. New York, NY: Routledge Academic; 1988. [Google Scholar]
- 39.USDHHS. The health consequences of smoking—50 years of progress: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014;17. [Google Scholar]
- 40.Tauras JA, Levy D, Chaloupka FJ, et al. Menthol and non‐menthol smoking: the impact of prices and smoke‐free air laws. Addiction. 2010;105:115–123. [DOI] [PubMed] [Google Scholar]
- 41.Richardson A, Ganz O, Pearson J, Celcis N, Vallone D, Villanti AC. How the industry is marketing menthol cigarettes: the audience, the message and the medium. Tob Control. 2015;24(6):594–600. [DOI] [PubMed] [Google Scholar]
- 42.Moran MB, Heley K, Pierce JP, Niaura R, Strong D, Abrams D. Ethnic and socioeconomic disparities in recalled exposure to and self-reported impact of tobacco marketing and promotions. Health Commun. 2019;34(3):280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cruz TB, Wright LT, Crawford G. The menthol marketing mix: targeted promotions for focus communities in the United States. Nic Tob Res. 2010;12(suppl_2):S147–S153. [DOI] [PubMed] [Google Scholar]
- 44.Stahre M, Okuyemi KS, Joseph AM, Fu SS. Racial/ethnic differences in menthol cigarette smoking, population quit ratios and utilization of evidence‐based tobacco cessation treatments. Addiction. 2010;105:75–83. [DOI] [PubMed] [Google Scholar]
- 45.Young-Wolff KC, Hickman NJ, Kim R, Gali K, Prochaska JJ. Correlates and Prevalence of Menthol Cigarette Use Among Adults With Serious Mental Illness. Nic Tob Res. 2015;17(3):285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hickman NJ, Delucchi KL, Prochaska JJ. Menthol use among smokers with psychological distress: findings from the 2008 and 2009 National Survey on Drug Use and Health. Tob Control. 2014;23(1):7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]


