Abstract
Background
Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of multiple cancers. However, these promising therapies may also cause immune-related adverse events (irAEs) in a substantial proportion of patients. These autoimmune phenomena may affect almost any organ system and may occur at almost any point in therapy. In some instances, these toxicities are life-threatening and potentially permanent. Diverse clinical presentation and unpredictable timing further complicate their anticipation and diagnosis.
Content
To improve patient safety and selection for ICI use, biomarkers for irAE diagnosis and prediction are under development. Clinicians may use traditional laboratory markers such as routine chemistries, creatinine clearance, thyroid function tests, and serum cortisol/adrenocorticotrophic hormone to monitor for specific irAEs, but noted aberrations may not necessarily represent an immune-mediated etiology. Novel biomarkers have the potential to be more specific to assist in the diagnosis of irAEs. The prediction of irAEs is more challenging. Apart from a history of autoimmune disease, no other clinical parameters are routinely used to project risk. Biomarker candidates under investigation for irAE diagnosis and prediction include blood cell analysis, chemokines/cytokines, autoantibodies, and genetic predisposition, such as human leukocyte antigen haplotype. Among other emerging candidates are immune-cell subsets, T-cell repertoire, fecal microbiome, tumor genomics, and radiomic characterization.
Summary
Several conventional laboratory indexes of end-organ dysfunction are currently in routine clinical use for irAE monitoring and diagnosis. Novel biomarkers for the prediction and diagnosis of these irAEs, which primarily characterize patient immune function, represent an area of active investigation.
The advent of immune checkpoint inhibitor (ICI) therapy has resulted in a paradigm shift in the treatment of cancer. Approved agents include anti–cytotoxic T-lymphocyte antigen 4 (anti-CTLA-4) antibody (ipilimumab) and anti–programmed death 1 (anti-PD-1) antibodies (nivolumab, pembrolizumab, cemiplimab), and anti–programmed death 1 ligand (anti-PD-L1) antibodies (atezolizumab, avelumab, durvalumab). They exert anticancer effects by inhibiting the negative regulation of T cells that cancers can co-opt to evade T-cell mediated death. Although the numerous PD-1/PD-L1 therapies differ in IgG antibody subtype, species, and ligand target, they are generally considered to have comparable intraclass therapeutic benefits and adverse events. Nevertheless, a recent meta-analysis indicates a potential survival advantage for PD-1 inhibitors compared with PD-L1 inhibitors (1). ICIs were initially approved for the treatment of melanoma, non–small cell lung cancer, and renal cell cancer (2). More recently, these agents have been approved to treat Hodgkin lymphoma, urothelial cancer, hepatocellular carcinoma, small cell lung cancer, gastric cancer, head and neck squamous cell carcinoma, primary mediastinal large B-cell lymphoma, cervical cancer, endometrial cancer, Merkel cell cancer, esophageal squamous cell carcinoma, triple-negative breast cancer, cutaneous squamous cell carcinoma, and microsatellite instability–high cancer (2). An estimated 43% of metastatic cancers diagnosed in the United States are eligible for ICI therapy (3). Although these therapies can provide patients with meaningful clinical benefit and—in a minority of cases—truly durable responses, they may also cause distinct autoimmune toxicities termed “immune-related adverse events” (irAEs).
The phenomenon of irAE can be defined broadly as immune-mediated host organ dysfunction secondary to aberrant immune system activity secondary to treatment with immunotherapy. Although irAEs may occur in up to three-quarters of patients treated with combination ICI (Table 1) (6), most of these diverse events are readily managed and may not require treatment discontinuation. Nevertheless, in some cases, irAEs may cause substantial or even permanent morbidity and may be fatal in approximately 1% of patients (24). Common irAEs include dermatitis, hepatitis, and thyroiditis. Less common but clinically important irAEs include hypophysitis, myocarditis, pneumonitis, and colitis. Anti-CTLA-4 antibodies are more likely to cause colitis and hypophysitis, whereas anti-PD-1 and anti-PD-L1 have stronger associations with pneumonitis and thyroiditis (4). Table 1 lists irAE characteristics.
Table 1.
Incidence, clinical manifestations and diagnostic criteria for common and clinically important ICI induced irAEs (modified from Brahmer et al. 2018) (4).
| irAE | Incidence, % |
Clinical manifestations | Median time to onset | Diagnostic criteria/workup | Screening recommendations | ||
|---|---|---|---|---|---|---|---|
| Anti- CTLA-4 | Anti- PD-1/L1 | Combination | |||||
| Cardiac | |||||||
| Myocarditis | 3.3 (5) | 0.5–2.4 (5) | 1-2.4 (5) | Chest pain, palpitations, dyspnea, peripheral edema, fatigue | 34 days (5) | Elevated troponins, NT-proBNP, ECG, CXR TTE, enhancement on cardiac MRI, endomyocardial biopsy | None established but some have suggested baseline and before each ICI dose troponin (5) |
| Dermatologic | 37–70 (4) | 17–37 (4) | 48 (6) | Pruritus, vitiligo, morbilliform/ eczematous/lichenoid rash, bullae, SJS | 25 days (7) | History of pruritus and/or physical examination evidence of rash, skin biopsy | None established |
| Endocrine | |||||||
| Hypothyroidism | 4 (8) | 6 (8) | 13 (8) | Constipation, cold intolerance, weight gain, hair loss | 73 days (9) | TSH above ULN with normal or low FT4 | TSH/FT4 at baseline and every 4-6 weeks OR At baseline and before each ICI dose (10) |
| Hyperthyroidism | 2 (8) | 2–3 (8) | 8 (8) | Palpitations, diaphoresis, diarrhea, anxiety | 37 days (14 days with combination ICI) (9) | TSH below LLN with normal high or elevated FT4 | TSH/FT4 at baseline and every 4-6 weeks OR At baseline and before each ICI dose (10) |
| Hypophysitis/ hypopituitarisma | 4 (8) | 1 (8) | 8 (8) | Fatigue, N/V, visual disturbance, headacheb | 59 days (11) | AM ACTH and cortisol below LLN, MRI brainb | ACTH/cortisol at baseline and before each ICI dose (10) |
| Type 1 diabetes mellitus | 0.1 (8) | 1 (12) | 2.7 (10) | Polyuria, polydipsia, polyphagia, fatigue | 49 days (12) | Serum glucose, HbA1c %, increased anion gap, urine ketones | Serum glucose at baseline and before each ICI dose (10) |
| Gastrointestinal | |||||||
| Colitis | 13–54 (13) | <19 (4) | 29 (6) | Mild diarrhea to fulminant colitis; abdominal pain, fever, blood/mucus in stool | 38 days (14) | Increase in stool frequency or ostomy output over baseline, lactoferrin/calprotectin, colonoscopy with biopsy | None established |
| Hepatitis | 2–10 (4) | 2–10 (4) | 25-30 (4) | Asymptomatic to jaundice, RUQ pain, N/V | 6-14 weeks (15) | AST/ALT/total bilirubin >1× ULN, biopsy | AST/ALT/total bilirubin at baseline and before each ICI dose |
| Neurologic | |||||||
| Myasthenia gravis | 0.15 (16) | 0.57 (16) | 0.36 (16) | Fatigable weakness (proximal greater than distal), ptosis, dysphagia, dysarthria | 29 days (16) | Antiacetylcholinesterase, muscle specific kinase, and lipoprotein-related 4 antibodies, PFTs | None established |
| Meningoencephalitis | 0.43 (16) | 0.64 (16) | 1.26 (16) | Headache, photophobia, neck stiffness, confusion | 61 days (16) | MRI brain, lumbar puncture, EEG | None established |
| Ophthalmologic | |||||||
| Uveitis | <1 (4) | <1 (4) | 1-6 (17) | Blurred vision, photophobia, eye pain | Not determined (17) | Complete ophthalmologic examination | None established |
| Pulmonary | |||||||
| Pneumonitis | <1 (4) | 2.7 (4) | 10 (4) | Asymptomatic to severe dyspnea, cough, wheezing, fever, chest pain, fatigue | 3 months (4) | Radiographic focal or diffuse ground-glass opacities, patchy peripheral infiltrates, interstitial markings, interlobular thickening on CT chest, bronchoscopy, lung biopsy (18–20) | None established |
| Renal | |||||||
| Nephritis | 1-2 (4) | 1-2 (4) | 4.5 (4) | Usually asymptomatic | 91 days (4) | Serum creatinine, urinalysis | Creatinine at baseline and before each ICI dose (10) |
| Rheumatologic | |||||||
| Arthralgia/arthritis | 5 (21) | 6-12 (21) | 11 (21) | Joint pain, swelling, stiffness with inactivity | 3 months (22) | Rheumatologic history and synovitis on exam, X-ray | None established |
| Myositis | <1 (10) | <1 (23) | <1 (23) | Diffuse weakness, muscle pain, can involve ocular muscles, diaphragm | 1 month (22) | Increased creatine kinase, AST, ALT, lactate dehydrogenase, aldolase, electromyogram, MRI, muscle biopsy | None established |
ACTH, adrenocorticotropic hormone; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CT, computed tomography; CXR, chest X-ray; ECG, electrocardiogram; EEG, electroencephalogram; FT4, free thyroxine; LLN, lower limit of normal; MRI, magnetic resonance imaging; NT-pro BNP; N-terminal probrain natriuretic peptide; N/V, nausea and/or vomiting; PFT, pulmonary function test; RUQ, right upper quadrant; SJS, Stevens-Johnson syndrome; TSH, thyroid stimulating hormone; TTE, transthoracic echocardiogram; ULN, upper limit of normal.
In the rare instance of adrenalitis causing primary adrenal insufficiency, AM serum ACTH is increased and cortisol is low.
Describing central adrenal insufficiency, the most common manifestation of hypophysitis (less commonly other pituitary axes can be involved causing secondary hypothyroidism and/or secondary hypogonadism).
The prediction, diagnosis, and characterization of irAEs are particularly challenging. Current diagnostic guidelines use conventional laboratory indexes of end-organ dysfunction, imaging, and, in some cases, tissue analysis (Table 1) (4). These toxicities may affect almost any organ, including complex regulatory systems such as the hypothalamic-pituitary-adrenal axis, which may require urgent multidisciplinary consultation. Common adverse events of other cancer treatments generally occur at predictable intervals. Myelosuppression from conventional cytotoxic chemotherapy, for example, is usually greatest 10 to 15 days after treatment administration. Acneiform rash from epidermal growth factor receptor inhibitors generally occurs within the first 3 weeks of treatment. Although characteristic temporal windows of occurrence exist, in reality, irAE can occur at any point throughout ICI therapy, including up to months after treatment discontinuation (25).
The ICI mechanism of effect contributes to this complexity. With cytotoxic agents, the drug itself, or a metabolite, is directly responsible for efficacy and toxicity. Consequently, metabolic variations such as dihydropyrimidine dehydrogenase deficiency (for 5-fluorouracil) or UDP glucuronosyltransferase family 1 member A1 polymorphisms (for irinotecan) may identify heightened risk in population subsets. For molecularly targeted therapies, pharmacodynamic effects predict toxicity. For example, antagonistic effects on target expressed in normal tissues explain the dermatologic and gastrointestinal effects of epidermal growth factor receptor inhibitors. Disruption of the physiologic role of vascular endothelial growth factor account for the thrombosis, bleeding, hypertension, and proteinuria observed with vascular endothelial growth factor or vascular endothelial growth factor receptor inhibitors. In contrast, the detrimental effects of ICI therapy are mediated via modulation of patient’s immune system, a far more complex consideration than plasma drug levels or target distribution.
It follows that irAEs represent major diagnostic challenges. In a recent study examining concordance in the occurrence, timing, and severity of 8 characteristic irAEs, interobserver agreement achieved acceptable levels (κ > 0.7) only for hypothyroidism (26). In addition, single clinical categories may represent diverse disease processes. For instance, the definition of colitis is based on stool frequency, but there may be a marked difference between the immune pathophysiology underlying mild diarrhea compared with fulminant colitis (4). These uncertainties have major clinical implications. Underdiagnosis of irAEs could result in inappropriate continuation of a toxic therapy and worsening of adverse effects. Conversely, overdiagnosis could result in inappropriate withholding or permanent discontinuation of ICI, as well as inappropriate administration of glucocorticoids or other immunosuppressants.
Broadly, 2 types of biomarkers are useful in clinical medicine: those used to predict risk of disease and those used to screen and diagnose disease. The ability to predict irAEs could help personalize treatment selection and customize monitoring. Furthermore, given the current unreliability of irAE clinical recognition and characterization, biomarkers could potentially be incorporated into diagnostic pathways. We review the clinical predictors of irAE risk; laboratory indexes currently used for irAE diagnosis; and emerging blood-, tissue-, and imaging-based biomarkers under investigation for ICI irAE prediction and diagnosis.
For this review, we queried PubMed for MeSH terms “biomarkers” and “immunotherapy” and other search terms “irAE,” “immune-related adverse events,” and “immune checkpoint inhibitors” combined with Boolean operators AND and OR. All 3 authors selected articles for inclusion based on clinical and scientific importance.
Mechanisms of irAE
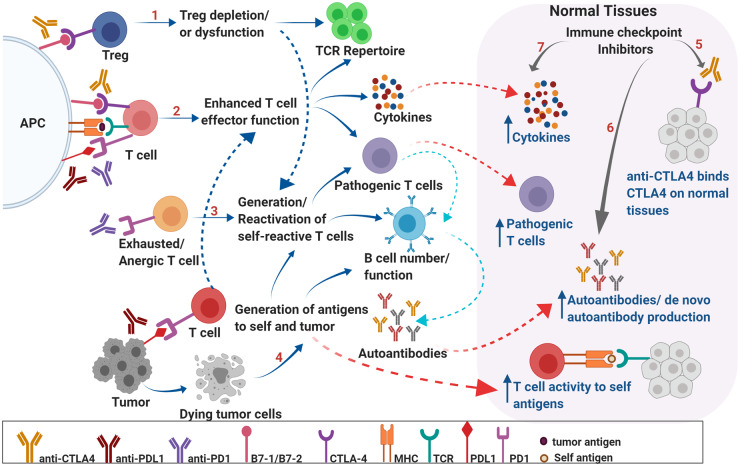
A central challenge in the development of irAE biomarkers is that despite extensive research efforts, the mechanisms underlying irAEs are not well understood. Recent studies indicate that irAEs may occur through a variety of mechanisms, including self-reactive T cells, decreased immune tolerance, molecular mimicry, and antigen spread. Fig. 1 displays the role of antigen-specific T-cell stimulation and response, B-cell stimulation, autoantibody production, and inflammatory cytokines in these events (27). Normally, T-cell surface CTLA-4 prevents the costimulation and activation of novel self-reactive T cells (27). Anti-CTLA-4 therapy disrupts this homeostatic peripheral negative selection, thereby promoting development of new self-reactive T cells (27). Anti-PD-1 therapy activates transcription factors, altering the epigenome of exhausted T cells, resulting in reactivation and potential for attack against self-tissue (27). In addition, ICI therapy interferes with peripheral tolerance by depleting regulatory T cells, thereby activating previously anergic self-reactive T cells and resulting in cell-mediated tissue damage (27). ICIs with anti-CTLA-4 and anti-PD-1 may stimulate T cells targeting tumor proteins that cross-react with host tissue (molecular mimicry) (23). Immune-mediated tumor cell destruction can lead to cellular debris otherwise hidden from immune detection, with subsequent generation of new tumor- and self-reactive T cells (antigen spread) (28). Furthermore, some irAEs may be the result of direct antibody effects on tissue. For example, pituitary CTLA-4 expression is thought to result in the antibody-dependent, cell-mediated cytotoxicity after anti-CTLA-4 administration, resulting in hypophysitis (pituitary dysfunction) (29). Specific irAEs likely involve different underlying pathophysiology and the role of genetics, underlying immune system homeostasis, and other environmental factors such as host microbiome have yet to be understood.
Fig. 1.
Mechanisms underlying irAEs. Schematic representation of potential mechanisms underlying irAEs associated with ICI therapy. CTLA-4 expression on regulatory T cells is critical in maintaining peripheral tolerance by preventing activation of self-reactive T cells. CTLA-4 blockade may result in depletion of Tregs, may alter Treg function, and may modulate T-cell repertoire, resulting in autoreactive T cells that can, in turn, affect B-cell function and increased autoantibody production (A). PD-1- and CTLA-4-mediated negative regulation of T cells is critical in maintaining self-tolerance via suppressing costimulation. PD-1 and CTLA-4 blockade results in enhanced effector function of T cells and may lead to generation of pathogenic T cells, overproduction of cytokines, alteration of B cell numbers/function and increased production of autoantibodies leading to inflammation and autoimmunity (B). PD-1 blockade may result in reactivation of exhausted/anergic T cells resulting in pathogenic/self-reactive T cells (C). Epitope spreading can lead to breakdown of tolerance. Tumor cell death results in production of self and tumor antigens that are ingested by APCs, which migrate to lymph nodes and prime T cells. These self-reactive T cells can reenter normal tissues, recognize self-antigens, and result in increased cytokine production and autoantibodies leading to breakdown of tolerance and tissue destruction (D). ICI therapy may directly result in breakdown of tolerance in organs via binding to CTLA-4 expressed on normal tissues (E); production of de novo autoantibodies and/or increased levels of preexisting autoantibodies (F); and increased levels of pro inflammatory cytokines locally, leading to infiltration of pathogenic immune cells (G). These factors can generate a local inflammatory milieu resulting in organ damage. APC, antigen-presenting cell; MHC, major histocompatibility complex; Treg, regulatory T cell; TCR, T-cell receptor.
Clinical Predictors of irAE Risk
In addition to laboratory biomarkers, the immune-oncology field has also evaluated clinical features that confer heightened risk of irAEs. However, many studies present conflicting findings. For instance, increased incidence of irAEs was observed in patients who were female, had lower performance status, and were sarcopenic (30, 31). In another study, older and male patients had increased incidence of hypophysitis with anti-CTLA-4 therapy (11). NSAID use increased incidence of anti-CTLA-4–induced colitis (32).
To date, the history of autoimmune disease represents the most commonly used clinical criterion for ICI patient selection. Hypothetically, prior or active autoimmune disease could affect both safety and efficacy of ICIs. Autoimmune disease could convey a preexisting state of heightened antiself immune activity, which might result in flare of that condition or increased risk of a de novo irAE. Separately, if autoimmune disease requires active immunosuppression, those medical therapies could hamper anticancer effects of ICIs.
Although many ICI clinical trials exclude patients with autoimmune disease, this practice is applied heterogeneously, suggesting inadequate understanding of clinical implications. Some trials exclude patients with any history of autoimmune disease (33). Others exclude only those patients with “active” autoimmunity (requiring corticosteroids equivalent to prednisone >10 mg daily) (34). Still others make exceptions for conditions that may pose a lower risk of organ dysfunction if exacerbated, such as psoriasis and vitiligo (35). Furthermore, analogous to the diagnosis of irAEs, the diagnosis of autoimmune disease is fraught with challenges. In contrast to a diagnosis of cancer, which generally relies on pathologic confirmation, a diagnosis of autoimmune disease may incorporate clinical, radiographic, serologic, and histologic data. For example, although only 0.25% of the North American population is estimated to have systemic lupus erythematosus, more than a quarter of clinically healthy adults have positive antinuclear antibody titers, and 2.5% have high titers (36). Reflecting these nuances, the estimated prevalence of autoimmune disease among individuals with lung cancer ranges from 14% to 25%, depending on whether a conservative (≥2 outpatient diagnostic codes at least 30 days apart or any inpatient diagnostic code) or more liberal (any diagnostic code) approach is taken (37).
The tolerability of ICIs among individuals with preexisting autoimmune disease differs widely across reports. Incidence of autoimmune disease flare ranges from 25% to 75% (38). One study found that irAE risk was not increased among individuals with prior autoimmune disease (38), whereas another identified heightened incidence of grade 1 and 2 events in this population (31).
Taken together, difficulties in identifying and characterizing both preexisting autoimmune disease and ICI-associated irAEs demonstrate the importance of predictive and diagnostic biomarkers to assist with patient selection, to improve diagnosis and management, and to provide insight into the irAE pathophysiology.
Clinical Laboratory Monitoring for End-Organ Dysfunction
Routine clinical laboratory assays for monitoring and diagnosing irAEs include serum creatinine (for nephritis); aspartate aminotransferase, alanine aminotransferase, and bilirubin (for hepatitis); troponin and creatine kinase (for myocarditis/myositis); lipase (for pancreatitis); and adrenocorticotrophic hormone, cortisol, luteinizing hormone, follicle-stimulating hormone, insulin-like growth factor 1, growth hormone, thyroid-stimulating hormone, and free thyroxine (for endocrinopathies such as hypophysitis and thyroiditis) (4). These parameters reflect end-organ toxicity from ICI therapy; however, they generally do not provide meaningful insight into toxicity pathophysiology or provide predictive value. Furthermore, particularly for clinical events that otherwise occur frequently in cancer populations undergoing active therapy (e.g., renal or hepatic dysfunction), test result abnormalities may not be specific for an ICI-induced, immune-mediated etiology. Although evidence supporting the optimal screening frequency for these parameters remains scarce, general recommendations exist and are summarized in Table 1.
Blood-Based Predictive Biomarkers
Baseline Organ Function
Two cohort studies found that an increased baseline level of thyroid-stimulating hormone is associated with increased risk of anti-PD-1–induced thyroid dysfunction (39, 40). One study reported the correlation in males but not in females (Table 2) (40). Other hormone measurements have not been found to be predictive of irAE. It may be conceivable that baseline thyroid dysfunction demonstrated by increased thyroid-stimulating hormone indicates underlying predisposition to develop an inflammatory response to thyroid tissue after ICI initiation. Other endocrine markers such as adrenocorticotrophic hormone and cortisol have not been evaluated as biomarkers for irAEs.
Table 2.
Investigational biomarkers for irAE prediction and diagnosis.
| Biomarker | Association | Pros/cons of biomarker | Study type | Strength/limitation of study a |
|---|---|---|---|---|
| Cellular | ||||
| Absolute lymphocyte count | Increased baseline and 1-month levels may indicate increased irAE risk | Readily available and inexpensive but appears to be inadequately sensitive and specific for utility | Retrospective cohort (41) | Moderate study size but small effect size for association with irAE, would require validation in prospective trials |
| Neutrophil/ lymphocyte ratio | Increased ratio at time of toxicity may indicate increased grade 3/4, lung and GI irAE | Readily available and inexpensive but appears to be inadequately sensitive and specific for utility | Retrospective cohort (42) | Moderate study size, moderate effect size without predictive value, requires validation in prospective trials |
| Absolute eosinophil count | Increased baseline and 1-month levels may indicate increased overall, grade 2, endocrine, dermatologic irAE | Readily available and inexpensive but appears to be inadequately sensitive and specific for utility | Retrospective/ prospective cohorts (41, 43, 44) | Small to moderate study sizes, multiple studies, requires validation in prospective trials |
| CD4+ | Increased baseline levels may indicate increased risk of colitis | Limited availability, more expensive, may provide insight into individual irAEs; may not differentiate irAE from ICI efficacy | Prospective cohort (45) | Small study limited to predicting colitis, requires validation in larger trials |
| Regulatory T cells | Lower baseline levels may indicate increased risk of colitis | Limited availability, more expensive, may provide insight into individual irAEs; may not differentiate irAE from ICI efficacy | Prospective cohort (45) | Small study limited to predicting colitis, requires validation in larger trials |
| T-cell repertoire | Increased T cell diversity may indicate increased risk of irAE | Limited availability, more expensive, unclear whether provides insight into individual irAEs | Retrospective cohort (46) | Small retrospective study, requires validation in larger prospective trials |
| CD8+ cells | Increased clonal expansion >55 clones may indicate increased risk of irAE | Limited availability, more expensive, unclear whether provides insight into individual irAEs | Retrospective cohort (47) | Small retrospective study, requires validation in larger prospective trials |
| Endocrine | ||||
| TSH | Increased baseline levels may indicate increased risk of thyroid dysfunction | Readily available and inexpensive, appears to be specific for thyroiditis | Retrospective cohorts (39, 40) | Moderate size but retrospective studies, requires validation in larger prospective trials |
| Antibody | ||||
| Antithyroglobulin antibody | Detected at baseline and predicted thyroid irAE | Readily available and inexpensive, appears to be specific for thyroiditis | Retrospective cohort (39) | Moderate size study, requires validation in larger prospective trials |
| Anti-GAD65, IA-2, ZnT8, islet cell antibodies | Associated with development of immune-related diabetes mellitus at time of diagnosis | Expensive, unclear if antibody present before development of irAE, may have limited utility | Retrospective cohort (48) | Small study, not predictive, requires prospective validation in larger studies |
| ANA | May be associated with development of irAE | Readily available and inexpensive but appears to be nonspecific for individual irAEs | Retrospective cohort (49) | Small limited study with no comparison of patients that did not develop irAE, unclear utility |
| Cytokines/chemokines | ||||
| IFN-γ | Lower levels posttreatment may indicate development of pneumonitis | May be specific for pneumonitis | Retrospective cohort (50) | Small study in 1 cancer type, only 1 cytokine measured, requires larger studies and comparison with multiple cytokines |
| CXCL9, CXCL10, CXCL11, CXCL19 | Lower baseline levels may indicate increased irAE risk | May predict pretreatment risk of irAE but limited current availability of clinical cytokine testing, high expense | Prospective cohort (51) | Small study but with control group negative for irAE, requires validation in larger trials |
| CXCL9, CXCL10, CCL5, G-CSF | Increased posttreatment levels may indicate increased irAE risk | May predict posttreatment risk of irAE but limited current availability of clinical cytokine testing, high expense | Prospective cohorts (51, 52) | Small studies, require validation in larger trials |
| G-CSF, GM-CSF, fractalkine, FGF-2, IFN-α2, IL-12p70, IL-1a, IL-1b, IL-RA, IL-2, IL-13 | Increased baseline and posttreatment levels in composite score may indicate increased irAE | May predict pretreatment risk of irAE but limited current availability of clinical cytokine testing, high expense | Prospective cohort with validation cohort (53) | Small but well-designed study including a validation cohort, requires larger prospective validation |
| IL-6 | Lower baseline levels may indicate increased irAE and colitis. Increased levels at toxicity time may indicate increased psoriasis | May predict pretreatment risk of irAE but limited current availability of clinical cytokine testing, high expense | Prospective cohorts (30, 45) Case control (54) | Multiple studies, 1 of moderate size but requires validation in larger trials in wider variety of cancer types and inclusion of anti-PD1/anti-PDL1 ICI |
| IL-8, soluble CD25 | Lower baseline levels may indicate increased risk of colitis | May predict pretreatment risk of irAE but limited current availability of clinical cytokine testing, high expense | Prospective cohort (45) | Small study, requires validation in larger trials |
| IL-17 | Increased baseline levels may indicate increased G3 colitis | May predict pretreatment risk of irAE but limited current availability of clinical cytokine testing, high expense | Prospective cohort (55) | Small but well-designed study, requires validation in larger trials in wider variety of cancer types and inclusion of anti-PD1/anti-PDL1 ICI |
| Leptin | Lower levels posttreatment may indicate increased irAE risk | Expensive, unclear if can predict pretreatment irAE risk or specific irAE risk | Prospective cohort (52) | Small study requires validation in larger trials in variety of cancer types and inclusion of anti-CTLA-4 ICI |
| Soluble CD163 | Increased levels posttreatment may indicate increased irAE risk | Expensive, unclear if can predict pretreatment irAE risk or specific irAE risk | Prospective cohort (56) | Small study requires validation in larger trials in variety of cancer types and inclusion of anti-CTLA-4 ICI |
| TNF-α and IFN-α2 | Increased levels may correlate to high-grade irAE | Limited clinical availability, unclear if specific for individual irAE | Retrospective cohort (49) | Small limited study with no comparison of patients that did not develop irAE, unclear utility |
| CRP | Increased levels after baseline may indicate increased irAE risk | Readily available and inexpensive but appears to be nonspecific for individual irAEs | Retrospective cohort (57) | Single small retrospective study that needs validation in larger prospective studies |
| Tissue | ||||
| Tumor gene mutations | Mutations in 7 tumor genes may be associated with increased risk of irAE | Expensive and often limited availability of tumor tissue | Retrospective cohort (58) | Small study that requires validation in larger prospective trials |
| Microbiome | ||||
| Firmicutes/Faecalibacterium | Increased baseline representation may indicate increased irAE risk | Expensive, limited clinical experience in microbiome analysis, may be specific for colitis | Prospective cohort (45) | Small study, requires validation in larger trials in wider variety of cancer types and inclusion of anti-PD1/anti-PDL1 ICI |
| Bacteroidetes | Increased baseline representation may indicate reduced risk of colitis | Expensive, limited clinical experience in microbiome analysis, may be specific for colitis | Prospective cohort (59) | Small but well conducted study, requires validation in larger trials in wider variety of cancer types and inclusion of anti-PD1/anti-PDL1 ICI |
| Metabolic products | Increased polyamine transport system, synthesis of thiamine, riboflavin, pantothenate; may indicate increased risk of colitis | Expensive, limited clinical experience in microbiome metabolic products analysis | Prospective cohort (59) | Small but well conducted study, requires validation in larger trials in wider variety of cancer types and inclusion of anti-PD1/anti-PDL1 ICI |
| Genetics | ||||
| HLA-DR4 | Associated with increased risk of immune-related diabetes mellitus | Expensive but appears to be relatively sensitive for predicting risk of type 1 diabetes mellitus | Retrospective cohort (48) | Small size study requires validation in larger prospective cohorts |
| Gene expression panel | Composite signature score associated with increased risk of irAE (but low sensitivity) | Expensive and appears to be inadequately sensitive for clinical utility | Retrospective cohort (60) | Moderate size study requires validation in larger prospective cohorts |
| CD177 and CEACAM1 | Increased expression post-treatment may indicate increased risk of irAE | Expensive and appears to be inadequately sensitive for clinical utility | Retrospective cohort (60) | Moderate size study requires validation in larger prospective cohorts |
| Imaging | ||||
| CT chest radiomics | Increased score associated with pneumonitis | Expensive but appears to be sensitive and specific | Case control (61) | Small case control study, requires validation in larger prospective studies |
Abbreviations: ANA, antinuclear antibody; CCL5, chemokine ligand 5; CEACAM1, carcinoembryonic antigen-related cell adhesion molecule 1; CRP, C-reactive protein; CT, computed tomography; FGF-2, fibroblast growth factor 2; G-CSF, granulocyte colony-stimulating factor; GI, gastrointestinal; GM-CSF, granulocyte-macrophage colony-stimulating factor; HLA, human leukocyte antigen; IA-2, islet antigen 2; TSH, thyroid stimulating hormone; ZnT8, zinc transporter 8.
Small study <100 patients, moderate >100 patients.
Cellular Biomarkers
Several studies have assessed complete blood count parameters for associations with irAEs. Higher baseline and posttreatment absolute lymphocyte counts (>2000/µL) were associated with irAEs (41). Increased neutrophil/lymphocyte ratio at time of toxicity correlated to increased risk of grades 3 and 4 lung and gastrointestinal irAEs with anti-PD-1 therapy (42). Increased baseline and 1-month eosinophil cell count correlated with increased overall risk of grade >2 irAEs (41), risk of endocrine irAEs with anti-PD1 therapy (62), and risk of dermatologic irAEs (43, 44).
Studies of T-cell subtypes in individuals treated with anti-CLTA-4 antibodies demonstrated associations between future development of colitis and higher baseline levels of cluster of differentiation 4–positive (CD4+) and lower baseline regulatory-T-cell populations (45). Such observations are consistent with mouse xenograft models, in which prolonged depletion of regulatory T cells causes expansion of effector T cells, increase in interferon-γ (IFN-γ) and tumor necrosis factor, and development of irAEs (63). T-cell repertoire studies indicate that increased T-cell diversity correlated with and preceded the development of irAEs (46). Another study found that expansion of ≥55 CD8+ T-cell clones after ICI initiation correlated to irAEs (47). Taken together, these observations suggest that increased T-cell diversity confers a heightened risk of T-cell–mediated attack on host tissue, resulting in increased likelihood of irAEs.
Humoral Biomarkers
Relatively little is known about the associations between autoantibodies, B-cell function, and irAEs. Increased baseline antithyroglobulin antibody titers, but not anti–thyroid peroxidase antibody titers, correlate with future development of thyroid irAEs among individuals treated with anti-PD-1 ICIs (39). Among patients who develop immune-related type 1 diabetes mellitus at diagnosis, 40% were found to have an associated autoantibody (anti–glutamate decarboxylase 2, islet antigen 2, zinc transporter8, islet cell antibodies) (48). Antinuclear antibody positivity, a nonspecific marker associated with a range of autoimmune diseases, was found in 11% of patients who developed irAEs, and borderline increased antinuclear antibody was detected in 27% (49). Given the relative ease with which antibody titers can be longitudinally collected and measured, this area of research remains active. The close temporal relationship between some autoantibodies and autoimmune disease and potential involvement in the pathophysiology suggests that humoral biomarkers could potentially also have utility in the diagnosis of irAEs.
Cytokines/Chemokines
Cytokines/chemokines are critical regulators of immune activity. Longitudinal analysis of a cohort of patients who developed irAEs revealed lower baseline levels of the IFN-γ–inducible chemokines C-X-C motif chemokine ligand 9 (CXCL9), CXCL10, CXCL11, and CXCL19 but higher posttreatment levels of CXCL9 and CXCL10, which are involved in T-cell activation and recruitment, compared with patients without irAEs (51). This correlation indicates a potential underlying mechanism of irAEs, whereby a robust cytokine storm of T-cell–activating cytokines induces broad overactivity of T cells targeting self-tissues. However, another small cohort study found that IFN-γ levels were decreased compared with baseline in patients who developed irAEs, particularly immune-related pneumonitis (50). Therefore, other factors than IFN-γ levels may drive cytokine changes. In a separate longitudinal study, higher posttreatment levels of chemokine ligand 5 and granulocyte colony stimulating factor were found in patients who developed irAE (52). In a cohort of patients with melanoma who developed severe irAE, at baseline and early during treatment, levels of 11 cytokines (granulocyte colony stimulating factor, granulocyte-macrophage colony stimulating factor, fractalkine, fibroblast growth factor 2, IFN-α2, interleukin-12p70 [IL-12p70], IL-1a, IL-1b, IL-1 receptor antagonist [IL-1RA], IL-2, IL-13) were increased and, in a composite score, were predictive of irAEs in a validation cohort with areas under the curve of 0.68 to 0.7 (53). Similarly, lower baseline and subsequently increased levels of IL-6 have been observed in irAEs such as colitis and psoriasis (30, 45, 54), an observation consistent with this proinflammatory cytokine’s association with autoimmune diseases (64). Indeed, IL-6 blocking therapy is being investigated as a strategy to maintain ICI activity while preventing immune-related colitis (65). Overall, in these studies it appears that lower baseline levels and higher posttreatment levels of key cytokines correlate with irAEs.
Case series and reports have identified numerous—and occasionally conflicting—cytokine associations that require confirmatory testing. Among others, these include increased levels of IL-8 receptor β and IL-1RA and IL-2RA at time of diagnosis of ICI pneumonitis (66); lower posttreatment levels of leptin, IL-10, and soluble CD163 in irAE cases (52, 56, 67); increased C-reactive protein shortly before clinical irAE onset (57); increased tumor necrosis factor α (60%), and IFN-α2 (44%) in irAE cases, with further correlation with high-grade irAEs (49); lower baseline IL-8 and soluble CD25 levels in anti-CTLA-4–mediated colitis (45); and higher baseline levels of IL-17 in grade 3 anti-CTLA-4–mediated colitis (55).
From a technical standpoint, a high degree of analyte stability renders cytokine analysis feasible for clinical laboratory testing. One study revealed that 9 of 10 studied cytokines (with the exception of IL-1RA) were stable in unprocessed EDTA blood stored at 4 to 8 °C for 24 hours (68). Nevertheless, a number of considerations affect widespread implementation. There appears to be large interpatient variation in baseline and dynamic cytokine levels (53), suggesting that related biomarkers may need to be individualized (51, 53). Other factors include timing of serum collection relative to ICI exposure, circadian variation, effects of corticosteroid exposure, and impact of other stresses such as fasting and physical activity (68).
DNA and Gene Expression Biomarkers
Genetic variants, most notably in human leukocyte antigen locus, have long been known to influence autoimmunity. An emerging literature has reported genetic variants and gene expression signatures associations with irAE. For instance, among patients who developed immune-related diabetes mellitus, 76% were found to be human leukocyte antigen–DR4 positive (48). An expression panel of 27 genes in peripheral blood at baseline correlated to an increased rate of development of irAE colitis but had insufficient sensitivity for clinical use (60). In addition, gene expression profiling identified that expression of neutrophil-activating factors, CD177, and carcinoembryonic antigen-related cell adhesion molecule 1 were specific but not sensitive indicators of anti-CTLA-4–mediated colitis, raising the possibility that neutrophilic activity or infiltration mediates this irAE (60).
Tissue Biomarkers
Although tissue-based biomarkers, including tumor PD-L1 expression, mutational burden, and microsatellite stability, correlate with ICI efficacy, associations with toxicity are less apparent. In 1 study, tumor type correlated with irAE patterns, with higher frequency of dermatologic and gastrointestinal irAEs in melanoma, versus higher frequency of pneumonitis in lung and kidney cancer (69). However, these associations did not persist in multivariate analysis and thus may have represented other factors, such as category of checkpoint inhibitor administered. Nevertheless, it seems conceivable that tumors may express varying potential neoantigens that create differing T-cell and humoral responses to ICI therapy, which in turn would correlate to differing irAE risk profiles. In a separate study, tumors from patients who developed irAEs had distinct mutations compared with those without irAE, but overall tumor mutational burden did not correlate to irAEs (58).
Analysis of affected end organs in irAE cases also suggests cross-reactivity between tumor and host tissue. Myocardial histologic analysis of immune-related myocarditis identified clonal T-cell infiltrates identical to the T-cell infiltrates in the primary tumor (23). Analysis of the primary tumor in this case showed expression of the muscle antigens desmin and troponin, which may have triggered the fatal irAE through immune reaction against normal cardiac tissue (23).
Analysis of brain tissue in a patient who developed anti-PD-1–induced encephalitis revealed CD4+ and CD8+ cell infiltration (70). Interestingly, there were also infiltrates of CD68+ cells (macrophage marker) and high expression of PD-1 and PD-L1 on cells with macrophage morphology in tissue analysis, suggesting the possibility of an attempt at homeostatic attenuation of cellular inflammation after irAE occurrence. Muscle biopsies in patients with myositis also revealed infiltrates of CD68+ cells expressing PD-L1 and CD8+ T cells expressing PD-1 (71). For practical reasons, end-organ histologic analysis is not feasible in all cases of irAEs and is not tenable as a predictive biomarker. Nevertheless, tissue biopsy may provide insight into irAE pathophysiology and inform the development of more clinically practical biomarkers.
Microbiome Biomarkers
A growing body of evidence has emerged describing the importance of the microbiome to immune function and response to ICI. In particular, the fecal microbiome in the gastrointestinal tract exerts effects on regulatory T cell function (72). Some microbiome–irAE associations have been reported, such as increased anti-CTLA-4–induced colitis in the setting of increased baseline representation of Faecalibacterium and other Firmicutes (45). By contrast, increased baseline representation of Bacteroidetes phylum was associated with reduced incidence of anti-CTLA-4 colitis (59). Bifidobacterium was found to abrogate disease in a mouse model of ICI-induced colitis (72). Metabolic products of the fecal microbiome including a polyamine transport system, and synthesis of thiamine, riboflavin, and pantothenate were computed in a machine learning algorithm that predicted development of anti-CTLA-4 colitis with sensitivity of 70% and specificity of 100% (59). No specific studies have evaluated overall diversity of the gastrointestinal microbiome and irAEs.
Given the exceptional diversity of the host microbiome, biomarker development will need to focus on replicable and potentially generalizable findings. Promising approaches include genetic analysis of 1 or multiple microbes, measurement of secondary metabolic compounds or enzymes, and measurement of interactions between the microbiome, and immune, endocrine, and nervous systems (72). Inherent challenges with microbiome analysis include identifying important variables and assays and inconvenience and patient acceptance of stool sample collection.
Imaging Biomarkers
To date, imaging biomarkers have been investigated most extensively for the evaluation of pneumonitis, a condition lacking routine serologic markers and for which tissue analysis may often be impractical. In addition, imaging changes may occur with thyroiditis, hepatitis, pancreatitis, myocarditis, and hypophysitis. However, for some irAEs, radiographic findings may be neither necessary nor sufficient to render a diagnosis (4).
To incorporate radiographic data into irAE prediction, some investigators have used a radiomics approach. In a small study, pretreatment radiographic features identified patients at risk of developing pneumonitis with 100% sensitivity and specificity, but these features have not been validated in a larger cohort (61). Interestingly, a small cohort study investigating fluorodeoxyglucose positron emission tomography/computed tomography found that detecting imaging evidence of irAEs, specifically thyroiditis, was associated with increased ICI efficacy (73).
Discussion
Biomarkers for the diagnosis and prediction of irAEs represent a nascent but promising field. Advancing technology now permits detailed analysis of complex biological data, such as T-cell repertoires, autoantibodies, genetic factors, and cytokines/chemokines, relatively quickly and with relatively small biological samples. Despite this methodologic progress, these analyses may be limited by clinical knowledge. Challenges in the accurate characterization of irAEs remain a concern. For many irAEs, there is no gold standard to render a diagnosis. Laboratory anomalies might reflect ICI toxicity or, alternatively, effects of other cancer therapies, nononcologic medications, or the underlying cancer. Some concurrent or sequential treatments, such as radiation therapy, may cause inflammatory changes that are clinically, radiographically, and histologically indistinguishable from ICI-mediated effects. Furthermore, the current classification of irAEs may not reflect underlying biology. If achievable, categorization of irAEs based of pathophysiologic mechanism may provide a framework for targeted and more effective biomarker development and treatment.
A growing body of evidence supports the association between development of irAEs and ICI therapy efficacy (74). Although a mechanistic understanding of this effect is not known, there may be common immune system changes that both cause irAEs and mediate ICI anticancer effects. Therefore, in the exploration of biomarkers, the extent to which biomarkers predicting irAEs overlap with efficacy biomarkers remains to be determined.
The technical measurement of biomarkers deserves further discussion. Biomarkers in advanced investigational or clinical stages for ICI efficacy (PD-L1, microsatellite instability–high, tumor mutational burden) still raise critical questions. Notable mentions include the lack of a standardized assay for detecting tumor PD-L1, polymerase chain reaction versus immunohistochemical analysis for detecting microsatellite instability–high or deficient mismatch repair enzyme status, and agreement on optimal cutoff points (75). The field of biomarkers for irAEs is at an even earlier stage of exploration; therefore, no standardized methodologies for measurement of samples exist. Several strategies are currently being investigated, from single-biomarker measurements in single-case reports to large cohorts reporting numerous concurrent biomarkers using multiplex analytical assays. Given the number of potential parameters to measure, it seems most feasible to perform multiplex analyses of samples to screen a larger number of potential biomarkers and obtain data more efficiently. Another consideration is the complexity of the biomarker measurement. Some potential biomarkers such as autoantibodies and cytokines are relatively simple to measure and have been routinely analyzed for decades. Others, such as T-cell repertoire, are relatively novel and may pose significant cost and replication challenges. With these considerations in mind, as further irAE-biomarker associations are discovered and reported, analytical technique is likely to emerge as an area of controversy and debate.
What characteristics are desired for a clinically useful biomarker? For prediction of irAE risk, the ideal biomarker would be measurable before initiation of ICI therapy to assist in assessment of risk–benefit calculations. This could conceivably take the form of tissue or genetic analysis, measurement of cytokines and autoantibodies in peripheral blood, or microbiome analysis. To date, potential biomarkers found to predict baseline irAE risk include tumor histology and IFN-γ–inducible cytokines. However, these biomarkers do not yet have sufficient discriminating power for clinical implementation, indicating that more sensitive and specific biomarker discoveries are required. Biomarkers for screening of irAEs should be detectable before the development of clinical symptoms and signs, whereas biomarkers for diagnosis should be rapidly detectable on development of clinical symptoms and signs and able to differentiate irAEs from non-ICI–induced end-organ toxicity (currently used laboratory indexes are unable to achieve this). The most promising biomarkers for screening and diagnosis may be cytokines and autoantibodies, as these biomarkers have the potential to be measured frequently and have results determined rapidly. Screening biomarkers may be most useful if detectible at least 2 to 3 weeks before the onset of clinical signs and symptoms (the typical dosing frequency of ICI administration and the frequency of routine peripheral blood draws).
Finally, any efforts to predict irAEs must account for clinical context. Metastatic cancer has historically been considered an incurable condition, with survival often measured in months rather than years. Furthermore, conventional therapies such as cytotoxic chemotherapy may convey substantial toxicity. Accordingly, the notion of withholding potentially effective immunotherapy because toxicity risk appears elevated is not clinically acceptable. Instead, toxicity biomarkers may be more appropriate for the tailoring of specific regimens and monitoring parameters.
In conclusion, given the frequency and potential morbidity of irAEs, the availability of predictive biomarkers would clearly benefit oncologists seeking to provide safe and effective patient care. Continuous advances in the field and multiple investigational immunotherapies in development mean that ongoing biomarker development will likely be required. Current and future biomarkers may one day culminate in a combined immune genotype and phenotype for each patient—a complete immune repertoire—to inform treatment selection, duration of therapy, and toxicity and efficacy monitoring. Until that time, clinicians will need to remain consistently vigilant and have access to multidisciplinary input for the timely recognition, diagnosis, and response to irAEs.
Nonstandard Abbreviations
ICI, immune checkpoint inhibitor; CTLA4, cytotoxic T-lymphocyte antigen 4; PD-1, programmed death 1; PD-L1, programmed death 1 ligand; irAEs, immune-related adverse events; CD, cluster of differentiation; IFN, interferon; CXCL, C-X-C motif chemokine ligand; IL, interleukin; RA, receptor antagonist.
Author Contributions
All authors confirmed they have contributed to the intellectual content of this paper and have met the following 4 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
M.S. von Itzstein, administrative support; D.E. Gerber, financial support, administrative support.
Authors’ Disclosures or Potential Conflicts of Interest
Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership
None declared.
Consultant or Advisory Role
D.E. Gerber, Bristol-Myers Squibb.
Stock Ownership
None declared.
Honoraria
None declared.
Research Funding
Supported in part by a National Cancer Institute (NCI) Midcareer Investigator Award in Patient-Oriented Research (K24 CA201543-01, to D.E. Gerber), a V Foundation Robin Roberts Cancer Survivorship Award (DT2019-007 to D.E. Gerber and S. Khan), an American Cancer Society–Melanoma Research Alliance Multidisciplinary Team Award (MRAT-18-114-01-LIB to D.E. Gerber and S. Khan), a Melanoma Research Alliance–Society for Immunotherapy of Cancer Young Investigator Award in immune-related adverse events (to S. Khan), and the University of Texas Lung Cancer Specialized Program in Research Excellence (SPORE, P50-CA-070907-08S1, to D.E. Gerber).
Expert Testimony
None declared.
Patents
D.E. Gerber, PCT/US2018/018594, US patent pending (62/654,025); S. Khan, US patent pending (62/654,025).
Role of Sponsor
The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, preparation of manuscript, or final approval of manuscript.
Acknowledgments
The authors thank Dru Gray for assistance with article preparation.
References
- 1. Duan J, Cui L, Zhao X, Bai H, Cai S, Wang G, et al. Use of immunotherapy with programmed cell death 1 vs programmed cell death ligand 1 inhibitors in patients with cancer: a systematic review and meta-analysis. JAMA Oncol 2019;6:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Food and Drug Administration. Drugs@FDA: FDA-approved drug products. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm (Accessed March 2020).
- 3. Haslam A, Prasad V.. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open 2019;2:e192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clinc Oncol 2018;36:1714–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 2018;71:1755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gu L, Khadaroo PA, Su H, Kong L, Chen L, Wang X, et al. The safety and tolerability of combined immune checkpoint inhibitors (anti-PD-1/PD-L1 plus anti-CTLA-4): a systematic review and meta-analysis. BMC Cancer 2019;19:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weber JS, Kahler KC, Hauschild A.. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clinc Oncol 2012;30:2691–7. [DOI] [PubMed] [Google Scholar]
- 8. Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol 2018;4:173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iyer PC, Cabanillas ME, Waguespack SG, Hu MI, Thosani S, Lavis VR, et al. Immune-related thyroiditis with immune checkpoint inhibitors. Thyroid 2018;28:1243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yervoy. Prescribing information. Princeton, NJ: Bristol-Myers Squibb; 2019. https://packageinserts.bms.com/pi/pi_yervoy.pdf (Accessed February 2020).
- 11. Faje AT, Sullivan R, Lawrence D, Tritos NA, Fadden R, Klibanski A, et al. Ipilimumab-induced hypophysitis: a detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J Clin Endocrinol Metab 2014;99:4078–85. [DOI] [PubMed] [Google Scholar]
- 12. Akturk HK, Kahramangil D, Sarwal A, Hoffecker L, Murad MH, Michels AW.. Immune checkpoint inhibitor-induced type 1 diabetes: a systematic review and meta-analysis. Diabet Med 2019;36:1075–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gupta A, De Felice KM, Loftus EV Jr, Khanna S.. Systematic review: colitis associated with anti-CTLA-4 therapy. Aliment Pharmacol Ther 2015;42:406–17. [DOI] [PubMed] [Google Scholar]
- 14. Geukes Foppen MH, Rozeman EA, van Wilpe S, Postma C, Snaebjornsson P, van Thienen JV, et al. Immune checkpoint inhibition-related colitis: symptoms, endoscopic features, histology and response to management. ESMO Open 2018;3:e000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reynolds K, Thomas M, Dougan M.. Diagnosis and management of hepatitis in patients on checkpoint blockade. Oncologist 2018;23:991–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnson DB, Manouchehri A, Haugh AM, Quach HT, Balko JM, Lebrun-Vignes B, et al. Neurologic toxicity associated with immune checkpoint inhibitors: a pharmacovigilance study. J Immunother Cancer 2019;7:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abdel-Rahman O, Oweira H, Petrausch U, Helbling D, Schmidt J, Mannhart M, et al. Immune-related ocular toxicities in solid tumor patients treated with immune checkpoint inhibitors: a systematic review. Expert Rev Anticancer Ther 2017;17:387–94. [DOI] [PubMed] [Google Scholar]
- 18. Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clinc Oncol 2017;35:709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Delaunay M, Cadranel J, Lusque A, Meyer N, Gounaut V, Moro-Sibilot D, et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J 2017;50:1700050. [DOI] [PubMed] [Google Scholar]
- 20. Ueno R, Nemoto M, Uegami W, Fukuoka J, Misawa M.. Pembrolizumab-induced pneumonitis with a perilymphatic nodular pattern in a lung cancer patient: a radio-pathologic correlation. Respir Med Case Rep 2019;26:168–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Benfaremo D, Manfredi L, Luchetti MM, Gabrielli A.. Musculoskeletal and rheumatic diseases induced by immune checkpoint inhibitors: a review of the literature. Curr Drug Saf 2018;13:150–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abdel-Wahab N, Suarez-Almazor ME.. Musculoskeletal and rheumatic diseases induced by immune checkpoint inhibitors: a review of the literature. Rheumatology (Oxford) 2019;58:vii40–vii8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016;375:1749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 2018;4:1721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Couey MA, Bell RB, Patel AA, Romba MC, Crittenden MR, Curti BD, et al. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: diagnostic hazard of autoimmunity at a distance. J Immunother Cancer 2019;7:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hsieh D, Watters MK, Lu R, Xie Y, Gerber DE.. Variation in the assessment of immune-related adverse event occurrence, grade, and timing. J Clinc Oncol 2019;37:9047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. June CH, Warshauer JT, Bluestone JA.. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat Med 2017;23:540–7. [DOI] [PubMed] [Google Scholar]
- 28. Gulley JL, Madan RA, Pachynski R, Mulders P, Sheikh NA, Trager J, et al. Role of antigen spread and distinctive characteristics of immunotherapy in cancer treatment. J Natl Cancer Inst 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P.. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med 2014;6:230ra45. [DOI] [PubMed] [Google Scholar]
- 30. Valpione S, Pasquali S, Campana LG, Piccin L, Mocellin S, Pigozzo J, et al. Sex and interleukin-6 are prognostic factors for autoimmune toxicity following treatment with anti-CTLA4 blockade. J Transl Med 2018;16:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cortellini A, Buti S, Santini D, Perrone F, Giusti R, Tiseo M, et al. Clinical outcomes of patients with advanced cancer and pre-existing autoimmune diseases treated with anti-programmed death-1 immunotherapy: a real-world transverse study. Oncologist 2019;24:e327–e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marthey L, Mateus C, Mussini C, Nachury M, Nancey S, Grange F, et al. Cancer immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J Crohns Colitis 2016;10:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clinc Oncol 2017;35:3924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hammers HJ, Plimack ER, Infante JR, Rini BI, McDermott DF, Lewis LD, et al. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: the CheckMate 016 Study. J Clinc Oncol 2017;35:3851–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017;18:312–22. [DOI] [PubMed] [Google Scholar]
- 36. Wandstrat AE, Carr-Johnson F, Branch V, Gray H, Fairhurst AM, Reimold A, et al. Autoantibody profiling to identify individuals at risk for systemic lupus erythematosus. J Autoimmun 2006;27:153–60. [DOI] [PubMed] [Google Scholar]
- 37. Khan SA, Pruitt SL, Xuan L, Gerber DE.. Prevalence of autoimmune disease among patients with lung cancer: implications for immunotherapy treatment options. JAMA Oncol 2016;2:1507–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leonardi GC, Gainor JF, Altan M, Kravets S, Dahlberg SE, Gedmintas L, et al. Safety of programmed death-1 pathway inhibitors among patients with non-small-cell lung cancer and preexisting autoimmune disorders. J Clinc Oncol 2018;36:1905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kimbara S, Fujiwara Y, Iwama S, Ohashi K, Kuchiba A, Arima H, et al. Association of antithyroglobulin antibodies with the development of thyroid dysfunction induced by nivolumab. Cancer Sci 2018;109:3583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ma C, Hodi FS, Giobbie-Hurder A, Wang X, Zhou J, Zhang A, et al. The impact of high-dose glucocorticoids on the outcome of immune-checkpoint inhibitor-related thyroid disorders. Cancer Immunol Res 2019;7:1214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Diehl A, Yarchoan M, Hopkins A, Jaffee E, Grossman SA.. Relationships between lymphocyte counts and treatment-related toxicities and clinical responses in patients with solid tumors treated with PD-1 checkpoint inhibitors. Oncotarget 2017;8:114268–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fujisawa Y, Yoshino K, Otsuka A, Funakoshi T, Fujimura T, Yamamoto Y, et al. Fluctuations in routine blood count might signal severe immune-related adverse events in melanoma patients treated with nivolumab. J Dermatol Sci 2017;88:225–31. [DOI] [PubMed] [Google Scholar]
- 43. Jaber SH, Cowen EW, Haworth LR, Booher SL, Berman DM, Rosenberg SA, et al. Skin reactions in a subset of patients with stage IV melanoma treated with anti-cytotoxic T-lymphocyte antigen 4 monoclonal antibody as a single agent. Arch Dermatol 2006;142:166–72. [DOI] [PubMed] [Google Scholar]
- 44. Shi VJ, Rodic N, Gettinger S, Leventhal JS, Neckman JP, Girardi M, et al. Clinical and histologic features of lichenoid mucocutaneous eruptions due to anti-programmed cell death 1 and anti-programmed cell death ligand 1 immunotherapy. JAMA Dermatol 2016;152:1128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol 2017;28:1368–79. [DOI] [PubMed] [Google Scholar]
- 46. Oh DY, Cham J, Zhang L, Fong G, Kwek SS, Klinger M, et al. Immune toxicities elicted by CTLA-4 blockade in cancer patients are associated with early diversification of the T-cell repertoire. Cancer Res 2017;77:1322–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Subudhi SK, Aparicio A, Gao J, Zurita AJ, Araujo JC, Logothetis CJ, et al. Clonal expansion of CD8 T cells in the systemic circulation precedes development of ipilimumab-induced toxicities. Proc Natl Acad Sci USA 2016;113:11919–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stamatouli AM, Quandt Z, Perdigoto AL, Clark PL, Kluger H, Weiss SA, et al. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes 2018;67:1471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Head L, Gorden N, Van Gulick R, Amato CM, Frazer-Abel A, Robinson W, et al. Biomarkers to predict immune-related adverse events with checkpoint inhibitors [Abstract]. J Clinc Oncol 2019;37:131–131. [Google Scholar]
- 50. Hirashima T, Kanai T, Suzuki H, Yoshida H, Matsushita A, Kawasumi H, et al. The levels of interferon-gamma release as a biomarker for non-small-cell lung cancer patients receiving immune checkpoint inhibitors. Anticancer Res 2019;39:6231–40. [DOI] [PubMed] [Google Scholar]
- 51. Khan S, Khan SA, Luo X, Fattah FJ, Saltarski J, Gloria-McCutchen Y, et al. Immune dysregulation in cancer patients developing immune-related adverse events. Br J Cancer 2019;120:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Oyanagi J, Koh Y, Sato K, Mori K, Teraoka S, Akamatsu H, et al. Predictive value of serum protein levels in patients with advanced non-small cell lung cancer treated with nivolumab. Lung Cancer 2019;132:107–13. [DOI] [PubMed] [Google Scholar]
- 53. Lim SY, Lee JH, Gide TN, Menzies AM, Guminski A, Carlino MS, et al. Circulating cytokines predict immune-related toxicity in melanoma patients receiving anti-PD-1-based immunotherapy. Clin Cancer Res 2019;25:1557–63. [DOI] [PubMed] [Google Scholar]
- 54. Tanaka R, Okiyama N, Okune M, Ishitsuka Y, Watanabe R, Furuta J, et al. Serum level of interleukin-6 is increased in nivolumab-associated psoriasiform dermatitis and tumor necrosis factor-alpha is a biomarker of nivolumab recativity. J Dermatol Sci 2017;86:71–3. [DOI] [PubMed] [Google Scholar]
- 55. Tarhini AA, Zahoor H, Lin Y, Malhotra U, Sander C, Butterfield LH.. Baseline circulating IL-17 predicts toxicity while TGF-beta1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J Immunother Cancer 2015;3:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fujimura T, Sato Y, Tanita K, Kambayashi Y, Otsuka A, Fujisawa Y, et al. Serum levels of soluble CD163 and CXCL5 may be predictive markers for immune-related adverse events in patients with advanced melanoma treated with nivolumab: a pilot study. Oncotarget 2018;9:15542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Abolhassani A-R, Schuler G, Kirchberger MC, Heinzerling L. C-reactive protein as an early marker of immune-related adverse events. J Cancer Res Clin Oncol 2019;145:2625–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wells KR, Amato CM, Hintzsche J, Robinson W.. Identification of somatic mutations to predict development of autoimmune adverse events to immune therapy in melanoma [Abstract]. J Clinc Oncol 2016;34:3041–3041. [Google Scholar]
- 59. Dubin K, Callahan MK, Ren B, Khanin R, Viale A, Ling L, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun 2016;7:10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shahabi V, Berman D, Chasalow SD, Wang L, Tsuchihashi Z, Hu B, et al. Gene expression profiling of whole blood in ipilimumab-treated patients for identification of potential biomarkers of immune-related gastrointestinal adverse events. J Transl Med 2013;11:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Colen RR, Fujii T, Bilen MA, Kotrotsou A, Abrol S, Hess KR, et al. Radiomics to predict immunotherapy-induced pneumonitis: proof of concept. Invest New Drugs 2018;36:601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nakamura Y, Tanaka R, Maruyama H, Ishitsuka Y, Okiyama N, Watanabe R, et al. Correlation between blood cell count and outcome of melanoma patients treated with anti-PD-1 antibodies. Jpn J Clin Oncol 2019;49:431–7. [DOI] [PubMed] [Google Scholar]
- 63. Liu J, Blake SJ, Harjunpaa H, Fairfax KA, Yong MC, Allen S, et al. Assessing immune-related adverse events of efficacious combination immunotherapies in preclinical models of cancer. Cancer Res 2016;76:5288–301. [DOI] [PubMed] [Google Scholar]
- 64. Yoshida Y, Tanaka T.. Interleukin 6 and rheumatoid arthritis. Biomed Res Int 2014;2014:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Johnson DH, Hailemichael Y, Foo WC, Hess KR, Haymaker CL, Wani KM, et al. Interleukin-6 is potential target to de-couple checkpoint inhibitor-induced colitis from antitumor immunity [Abstract]. J Clinc Oncol 2019;37:2616–2616. [Google Scholar]
- 66. Schoenfeld JD, Nishino M, Severgnini M, Manos M, Mak RH, Hodi FS.. Pneumonitis resulting from radiation and immune checkpoint blockade illustrates characteristic clinical, radiologic and circulating biomarker features. J Immunother Cancer 2019;7:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sun J, Schiffman J, Raghunath A, Ng Tang D, Chen H, Sharma P.. Concurrent decrease in IL-10 with development of immune-related adverse events in a patient treated with anti-CTLA-4 therapy. Cancer Immun 2008;8:9. [PMC free article] [PubMed] [Google Scholar]
- 68. Aziz N, Detels R, Quint JJ, Li Q, Gjertson D, Butch AW.. Stability of cytokines, chemokines and soluble activation markers in unprocessed blood stored under different conditions. Cytokine 2016;84:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR.. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol 2017;28:2377–85. [DOI] [PubMed] [Google Scholar]
- 70. Johnson DB, McDonnell WJ, Gonzalez-Ericsson PI, Al-Rohil RN, Mobley BC, Salem JE, et al. A case report of clonal EBV-like memory CD4(+) T cell activation in fatal checkpoint inhibitor-induced encephalitis. Nat Med 2019;25:1243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Touat M, Maisonobe T, Knauss S, Ben Hadj Salem O, Hervier B, Aure K, et al. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology 2018;91:e985–e94. [DOI] [PubMed] [Google Scholar]
- 72. Helmink BA, Khan MAW, Hermann A, Gopalakrishnan V, Wargo JA.. The microbiome, cancer, and cancer therapy. Nat Med 2019;25:377–88. [DOI] [PubMed] [Google Scholar]
- 73. Nobashi T, Baratto L, Reddy SA, Srinivas S, Toriihara A, Hatami N, et al. Predicting response to immunotherapy by evaluating tumors, lymphoid cell-rich organs, and immune-related adverse events using FDG-PET/CT. Clin Nucl Med 2019;44:e272–e9. [DOI] [PubMed] [Google Scholar]
- 74. Indini A, Di Guardo L, Cimminiello C, Prisciandaro M, Randon G, De Braud F, et al. Immune-related adverse events correlate with improved survival in patients undergoing anti-PD1 immunotherapy for metastatic melanoma. J Cancer Res Clin Oncol 2019;145:511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Duffy MJ, Crown J.. Biomarkers for predicting response to immunotherapy with immune checkpoint inhibitors in cancer patients. Clin Chem 2019;65:1228–38. [DOI] [PubMed] [Google Scholar]