Abstract
BACKGROUND
While saturated fat intake leads to insulin resistance and nonalcoholic fatty liver, Mediterranean-like diets enriched in monounsaturated fatty acids (MUFA) may have beneficial effects. This study examined effects of MUFA on tissue-specific insulin sensitivity and energy metabolism.
METHODS
A randomized placebo-controlled cross-over study enrolled 16 glucose-tolerant volunteers to receive either oil (OIL, ~1.18 g/kg), rich in MUFA, or vehicle (VCL, water) on 2 occasions. Insulin sensitivity was assessed during preclamp and hyperinsulinemic-euglycemic clamp conditions. Ingestion of 2H2O/acetaminophen was combined with [6,6-2H2]glucose infusion and in vivo 13C/31P/1H/ex vivo 2H-magnet resonance spectroscopy to quantify hepatic glucose and energy fluxes.
RESULTS
OIL increased plasma triglycerides and oleic acid concentrations by 44% and 66% compared with VCL. Upon OIL intervention, preclamp hepatic and whole-body insulin sensitivity markedly decreased by 28% and 27%, respectively, along with 61% higher rates of hepatic gluconeogenesis and 32% lower rates of net glycogenolysis, while hepatic triglyceride and ATP concentrations did not differ from VCL. During insulin stimulation hepatic and whole-body insulin sensitivity were reduced by 21% and 25%, respectively, after OIL ingestion compared with that in controls.
CONCLUSION
A single MUFA-load suffices to induce insulin resistance but affects neither hepatic triglycerides nor energy-rich phosphates. These data indicate that amount of ingested fat, rather than its composition, primarily determines the development of acute insulin resistance.
TRIAL REGISTRATION
ClinicalTrials.gov NCT01736202.
FUNDING
German Diabetes Center, German Federal Ministry of Health, Ministry of Culture and Science of the state of North Rhine-Westphalia, German Federal Ministry of Education and Research, German Diabetes Association, German Center for Diabetes Research, Portugal Foundation for Science and Technology, European Regional Development Fund, and Rede Nacional de Ressonancia Magnética Nuclear.
Keywords: Endocrinology, Metabolism
Keywords: Diabetes, Glucose metabolism, Insulin signaling
A single monounsaturated fatty acid-enriched lipid load rapidly induces insulin resistance but does not affect hepatic triglycerides or energy-rich phosphates.
Introduction
There is an ongoing debate as to whether nutrient quality or quantity is mainly responsible for the effects on health or disease (1). Currently, energy-dense foods, rich in saturated fatty acids (SAFA), are considered the main culprits of the epidemic rise of obesity, type 2 diabetes mellitus (T2D), and nonalcoholic fatty liver disease (NAFLD) (1). In contrast, Mediterranean diet, which is rich in monounsaturated fatty acids (MUFA), may lower the risk of T2D, NAFLD (2) and cardiovascular disease (3).
Chronic high-fat diets cause hepatic triglyceride (TG) deposition and insulin resistance in humans (4). Of interest, fatty acid composition appears to play an important role for lipid-induced metabolic alterations, which is supported by the finding of higher liver TG content and insulin resistance with diets enriched in SAFA compared with those with MUFA, polyunsaturated fatty acids (PUFA), or simple sugars (4).
We recently demonstrated that a single oral SAFA-rich lipid load initiates hepatic insulin resistance (HEP-IR) and fat accumulation in healthy lean men (5), likely resulting from lipid-mediated inhibition of insulin signaling (6). This lipid load also raised hepatic gluconeogenesis (GNG), which is possibly due to lipid-induced allosteric stimulation of hepatic mitochondrial activity, as reported in rodent models (6). However, the acute effects of an identical amount of a MUFA-rich lipid load on hepatic glucose and energy metabolism still remain unclear (7). Moreover, the susceptibility to exogenous lipid-induced insulin resistance may differ between men and women, suggesting sex-specific metabolic differences upon unsaturated lipid administration (8).
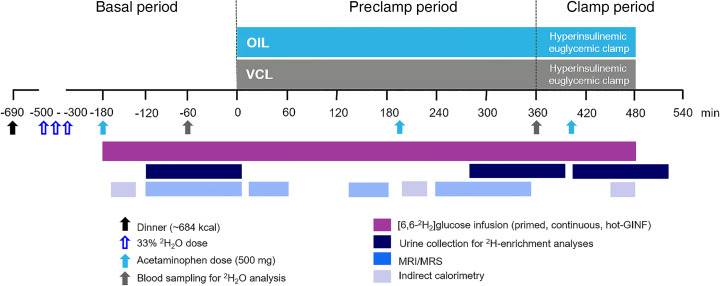
Here, we therefore tested whether a MUFA-rich lipid load acutely induces insulin resistance in females and males by comparing the effects of a single oral dose of canola oil (OIL) versus placebo (vehicle [VCL]) on (a) tissue-specific insulin sensitivity during endogenous (preclamp) insulinemia and hyperinsulinemic-euglycemic clamp conditions as well as on (b) hepatic energy and glucose fluxes in healthy lean humans. To this end, we performed comprehensive real-time metabolic monitoring using stable isotopes and in vivo multinuclear and ex vivo 2H-magnetic resonance (MR) techniques (Figure 1).
Figure 1. Study design.
Participants randomly received either an oral dose of canola oil (OIL, blue) or an identical volume of water (vehicle [VCL]) on 2 occasions spaced by an 8-week period. Hepatic glucose and energy metabolism was measured by in vivo 13C/31P/1H and ex vivo 2H-magnetic resonance spectroscopy (MRS) combined with 2H2O and acetaminophen ingestion before and during hyperinsulinemic-euglycemic clamps, for which a “hot” glucose infusion (hot-GINF) protocol with [6,6-2H2]glucose was used.
Results
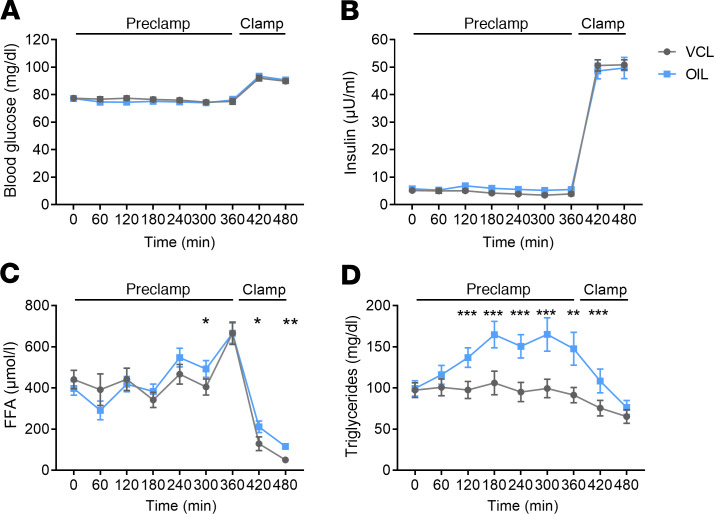
OIL raises plasma TG and oleic acid concentrations during the preclamp period.
Blood glucose and plasma insulin concentrations did not differ between OIL and VCL during both preclamp and clamp periods (Figure 1 and Figure 2, A and B). Total free fatty acids (FFA) were higher at +420 and +480 minutes (Figure 2C). During OIL intervention, plasma TG were higher compared with those during VCL from +120 minutes to +420 minutes (incremental area under the curve [iAUC] for TG, OIL vs. VCL, P < 0.0001; Figure 2D). At +360 minutes, plasma oleic acid was increased by 66% upon OIL intervention (+0 minutes to +360 minutes; P = 0.017), while other FFA concentrations did not change (Supplemental Table 1; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.134520DS1).
Figure 2. Time course of circulating metabolites and hormones.
Blood glucose (A), plasma insulin (B), free fatty acids (FFA) (C), and TG (D) in healthy humans after canola oil (OIL, blue) or water (vehicle [VCL], gray) administration at 0 minutes. Data are shown as mean ± SEM. ANOVA was adjusted for repeated measures with Bonferroni’s testing. n = 16; *P < 0.05 vs. CON; **P < 0.005 vs. CON; ***P < 0.001 vs. CON.
OIL does not acutely affect circulating hormones and cytokines.
During the preclamp period, release of IL-18, calculated as iAUC for serum IL-18, was 54% higher after intervention with OIL compared with VCL (P < 0.05, Supplemental Table 2). The iAUCs of leptin, high-molecular-weight (HMW) adiponectin, IL-1ra, FABP4, FGF21, and cortisol were not different between interventions (Supplemental Table 2).
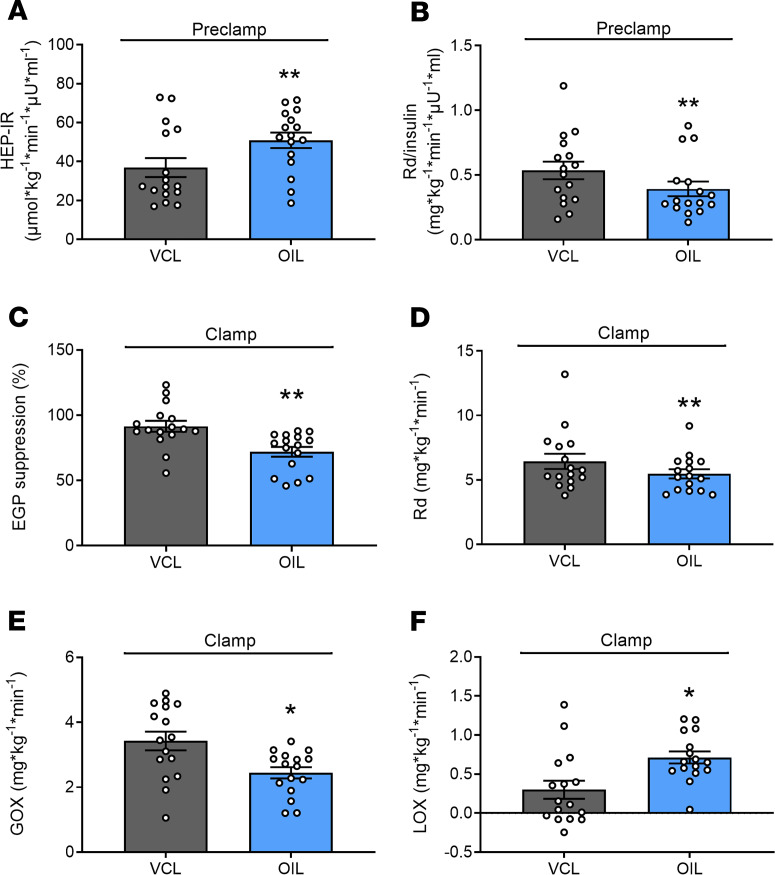
OIL results in whole-body and HEP-IR during preclamp and clamp conditions.
During the preclamp period, whole-body resting energy expenditure (REE) was higher with OIL than with VCL (1657 ± 223 kcal/d vs. 1509 ± 203 kcal/d, P = 0.0018). Rates of whole-body lipid oxidation (LOX; OIL 0.9 ± 0.4 mg/kg/min vs. VCL 0.8 ± 0.4 mg/kg/min; P = 0.306) and glucose oxidation (GOX; OIL 1.8 ± 1.0 mg/kg/min vs. VCL 1.9 ± 0.9 mg/kg/min; P = 0.899) were not different between the interventions. HEP-IR was 28% higher after OIL intervention than after VCL (P = 0.0037; Figure 3A).Whole-body glucose disposal (Rd), related to the ambient serum insulin concentrations, was 27% lower after OIL intervention than after VCL (P = 0.0043; Figure 3B).
Figure 3. Whole-body glucose disposal and hepatic insulin sensitivity of the preclamp and clamp period.
Insulin resistance of the liver (HEP-IR; A); rate of glucose disappearance (Rd) per serum insulin concentration (Rd/insulin; B) between +300 minutes and +360 minutes of the preclamp period; hepatic insulin sensitivity (EGP suppression; C); Rd (D); GOX (E); and LOX (F) between +420 minutes and +480 minutes of the clamp period in healthy humans after canola oil (OIL, blue) and water (vehicle [VCL], gray) ingestion at 0 minutes. Data are shown as mean ± SEM; cross-over test, n = 16. **P < 0.005 vs. VCL; +P < 0.05 GOX OIL vs. GOX VCL; ##P < 0.005 NOXGD OIL vs. NOXGD VCL. GOX, glucose oxidation; LOX, lipid oxidation; EGP, endogenous glucose production.
During the clamp period, REE was comparable between OIL and VCL (1680 ± 244 kcal/d vs. 1608 ± 233 kcal/d, P = 0.697). Insulin-mediated EGP suppression was 21% (P = 0.0011) lower with OIL than with VCL (Figure 3C). Insulin-stimulated Rd was 18% lower after intervention with OIL compared with that after VCL (P = 0.011, Figure 3D). The reduction of Rd was mainly due to the 25% decrease in rates of GOX (OIL vs. VCL, P = 0.0072, Figure 3E) but not nonoxidative Rd (NOXGD; OIL 3.0 ± 0.3 mg/kg/min vs. VCL 3.0 ± 0.6 mg/kg/min; P = 0.139). LOX was 137% higher after OIL intervention (P = 0.022, Figure 3F).
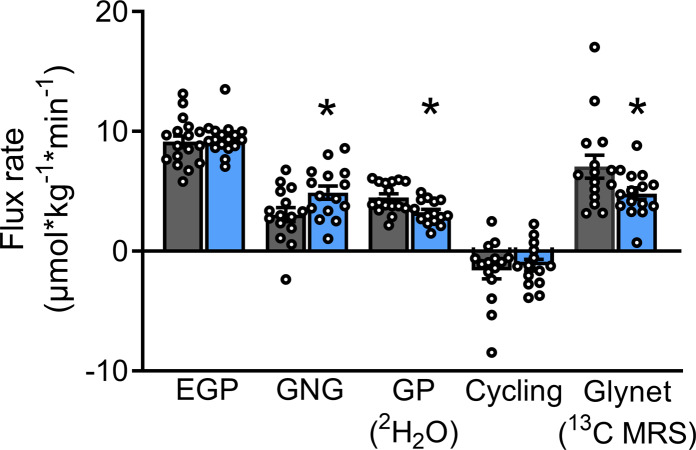
OIL increases hepatic GNG but does not affect ATP nor the TG content.
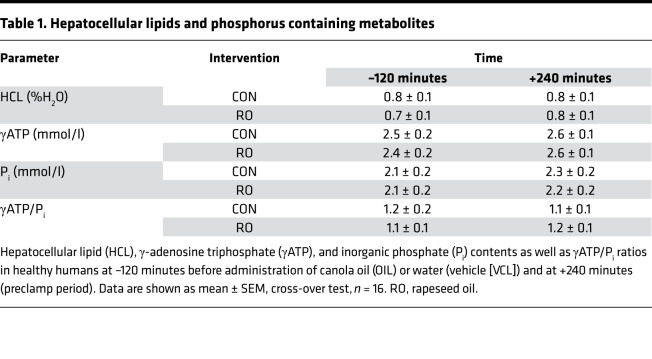
During the preclamp period, endogenous glucose production (EGP) was not different between the interventions (OIL vs. VCL, P = 0.585). However, the rate of GNG was 60% higher (OIL vs. VCL, P = 0.022), and the rates of net glycogenolysis (GLYnet) and glycogen phosphorylase (GP) flux were 47% (P = 0.0201) and 38% (P = 0.0082) lower, after OIL intervention than after VCL (Figure 4). Hepatic glycogen cycling was negligible under both conditions (P = 0.576; Figure 4). Hepatocellular lipid content was unchanged at preclamp conditions and comparable between groups (Table 1).
Figure 4. Hepatic glucose and glycogen fluxes between +15 minutes and +360 minutes of the preclamp period.
Rates of gluconeogenesis (GNG), glycogen phosphorylase flux (GP), glycogen cycling (Cycling), and net glycogenolysis (GLYnet) were assessed using in vivo 13C/31P/1H and ex vivo 2H-MRS combined with 2H2O/acetaminophen ingestion in humans after canola oil (OIL, blue) or water (vehicle, [VCL], gray) administration. Data are shown as mean ± SEM; cross-over test, n = 16; GP and cycling n = 15. *P < 0.05 vs. CON.
Table 1. Hepatocellular lipids and phosphorus containing metabolites.
γ-Adenosine triphosphate (γATP) and inorganic phosphate (Pi) concentrations as well as γATP/Pi ratios were not different at –120 minutes (basal period) or at +240 (preclamp period) minutes between OIL and VCL (Table 1).
Effect of sex on the metabolic effects of OIL.
A subanalysis of a possible interaction of sex with the effects of OIL during the preclamp and clamp period revealed no such effects on LOX (P = 0.156) nor GOX (P > 0.999) or on whole-body (preclamp period, Rd/insulin, P = 0.094; clamp period, Rd, P = 0.125) and hepatic (preclamp, P = 0.156 for HEP-IR; clamp period, P = 0.072 for percentage EGP suppression) insulin sensitivity.
Discussion
This study demonstrates that a single oral dose of oleic acid–rich OIL induces insulin resistance in skeletal muscle and liver during hyperinsulinemia but also already under preclamp insulinemia in whole body and liver. Additionally, OIL increased the rate of hepatic GNG and its contribution to EGP, but — in contrast to a former study on saturated fat intake — affected neither hepatic energy metabolism nor lipid deposition (5).
Effect of OIL on tissue-specific insulin resistance.
The present study shows that a single MUFA-rich lipid drink reduces hepatic insulin sensitivity during preclamp and clamp conditions, as assessed from Hep-IR and EGP suppression, to a similar degree as a SAFA-rich lipid drink (5), whereas PUFA-enriched soy bean oil has no such effect (9). The impairment of hepatic insulin sensitivity during the preclamp period is reflected by the 61% higher rates of GNG, which is nominally similar to the 70% higher GNG observed upon SAFA-rich lipid loading (5). During clamp, reduced insulin action is presumed by the lower insulin-stimulated EGP suppression also reported for SAFA but not PUFA (5, 9). In the present study, the rise in GNG occurred along with reduced rates of glycogenolysis resulting in unchanged EGP. Likewise, in insulin-resistant states, such as obesity, increased rates of fasting GNG without changes in total EGP due to decreased hepatic glycogenosis were reported (10), along with impaired insulin-stimulated EGP suppression during clamp in nondiabetic humans (11). This indicates the operation of an autoregulatory mechanism limiting EGP in response to elevated GNG, previously demonstrated for other metabolic conditions (12).
Whole-body insulin sensitivity, which mainly reflects skeletal muscle insulin action, was equally impaired by 25% with OIL and an identical dose of SAFA-rich palm oil and PUFA-rich soy oil (5, 9). Moreover, Rd adjusted from prevailing insulin plasma levels was reduced during the preclamp period. Of note, a previous study found an increased insulin-to-glucose ratio after ingestion of SAFA-rich oil for 24 hours but not with unsaturated fatty acids compared with water control (13). However, this ratio was obtained from overweight and obese humans during hyperglycemic clamps, a method not considered a gold standard for measuring whole-body insulin sensitivity. In general, palmitate but not oleate is known to induce skeletal muscle insulin resistance (14). In this context, increasing MUFA and decreasing SAFA intake has been shown to improve insulin sensitivity measured by the insulin sensitivity index; however, this beneficial effect disappears at high-fat intake of >37% of daily energy intake (15). In addition, both oleate and palmitate are able to increase the serine phosphorylation of insulin receptor substrate-1 (16), suggesting activation of the diacylglycerol/protein kinase C pathway promoting insulin resistance. FFA may also act through c-jun N-terminal kinase and S6 kinase p70 (16) and oleate through increased incomplete β-oxidation (17). Taken together, these findings suggest that differential effects of fatty acid saturation critically depend on the ingested lipid quantity.
Effects of OIL on inflammatory markers and adipokines.
The present study found no changes in circulating antiinflammatory cytokines IL-1ra and FGF21, in line with our previous studies on acute effects on SAFA (5) and PUFA, reporting constant levels of the classical inflammatory markers TNF-α and IL-6 after oil ingestion. Only, IL-18 levels (iAUC) were moderately increased after OIL, possibly reflecting metabolic adaptation rather than a proinflammatory response, as IL-18 has been shown to increase skeletal muscle LOX (18). The MUFA load did not effect circulating concentrations of leptin in accordance with unchanged plasma leptin concentrations after intravenous or oral lipid challenges in humans (19). In addition, HMW adiponectin and cortisol levels were not affected by the MUFA load, in line with unchanged levels after oral lipid challenge and during a PUFA-enriched infusion (5, 9).
Sex-specific differences in lipid handling.
The present study found no effect of sex on the effects of OIL ingestion on rates of substrate oxidation or tissue-specific insulin sensitivity during the preclamp and clamp periods. Of note, a previous study observed sex-specific differences in muscle insulin sensitivity but not in EGP and HEP-IR (8). While the present data suggest no major sex-dependent difference in the susceptibility to acute lipid ingestion, they cannot exclude any such effects after long-term high-fat diets — maybe mediated by inflammatory pathways — as proposed for mice (20).
Metabolic and clinical relevance of MUFA in NAFLD and T2D.
Current guidelines of the American Diabetes Association, European Association for Study of the Liver, European Association for Study of Diabetes, and European Association for Study of Obesity recommend a diet enriched in MUFA and a reduced intake of SAFA below 10% of total caloric intake as a treatment for T2D and NAFLD (21, 22). In the PREDIMED trial, which reported reduced cardiovascular and T2D risk with Mediterranean diet (3), daily fat intake was about 40% of total calorie intake, derived from sources rich in unsaturated fatty acids, mostly olive oil (50 g/d) and equivalent to 86 g daily fat at a total calorie intake of 2000 kcal/d. The amount of OIL of the study (~1.2 g/kg BW) resembles 1 meal rich in monounsaturated fat, such as 381 g pasta with pesto sauce containing about 80.1 g fat (59% MUFA; 24% PUFA, 17% SAFA) (https://www.fatsecret.com/calories-nutrition/generic/pasta-with-pesto-sauce?portionid=53757&portionamount=100.000). Our data suggest that the amount of fat intake per meal mainly determines acute insulin resistance, while its degree of saturation may be more relevant for hepatic energy metabolism and ectopic lipid storage. We did not detect higher hepatic concentrations of TG and phosphorus metabolites after OIL, whereas an acute rise in both TGs and ATP levels was found upon palm oil ingestion (5). Accordingly, a high-MUFA isocaloric diet for 8 weeks reduced liver fat content by 30% in patients with T2D, which was at least in part attributed to higher postprandial fatty acid β-oxidation, as measured from circulating β-hydroxybutyrate levels (23). In contrast to our previous study on the effects of SAFA (5), the present study found no differences in preclamp LOX but increased REE with OIL, which may contribute to favorable metabolic control and reduced NAFLD risk after MUFA intake (24). Furthermore, studies in mouse models with restricted MUFA supply and synthesis showed that oleate prevents from hepatic endoplasmic reticulum stress and inflammation (25). Nevertheless, randomized long-term controlled intervention studies are needed to clarify the effect of MUFA-enriched diets specifically on NAFLD.
Strengths and limitations.
This study benefits from its design, which allows for direct real-time monitoring of hepatic metabolic fluxes in vivo by using 2 independent state-of-the-art techniques, as well as from the comparability with our previous study on saturated fat ingestion, due to an identical study design (5).
Limitations of this study include the application of a pure fat load, which allows for examination of lipid-mediated effects per se but does not necessarily reflect ingestion of mixed meals also containing carbohydrates and proteins. In addition, OIL does not exclusively contain MUFA but also contains about 5% SAFA and approximately 25% PUFA (26). Of note, only plasma concentrations of oleic acid, but not those of other fatty acid species, increased with OIL, pointing to the major contribution of oleic acid to the observed metabolic effects (Supplemental Table 1). While it is generally assumed that the prevailing fatty acid represents the fatty acid of relevance for the observed results (27), we cannot rule out contributions of the other fatty acids to the net metabolic effect. In addition, using water as the control intervention induces a difference in the total caloric load and can lead to other metabolic and endocrine alterations during fasting, which may result in effects independent of those of the ingested oil (28). Nevertheless, this approach has been used before in intervention studies on the metabolic effects of different dietary fats (5, 13). Finally, this study cannot provide data on the molecular mechanisms of action, as no liver biopsies were available due to ethical reasons.
In conclusion, the acute effects of OIL ingestion comprise (a) early decrease in hepatic insulin sensitivity but increased whole-body energy expenditure at fasting insulinemia, (b) increased hepatic gluconeogenic and lower glycogenolytic flux rates but unchanged hepatic TGs and energy-rich phosphates, and (c) hepatic and muscle insulin resistance during hyperinsulinemia. One may therefore speculate that a high-MUFA load may induce acute insulin resistance but without deleterious effects on hepatic lipid and energy metabolism, in contrast to the reported effects of SAFA.
Methods
Volunteers.
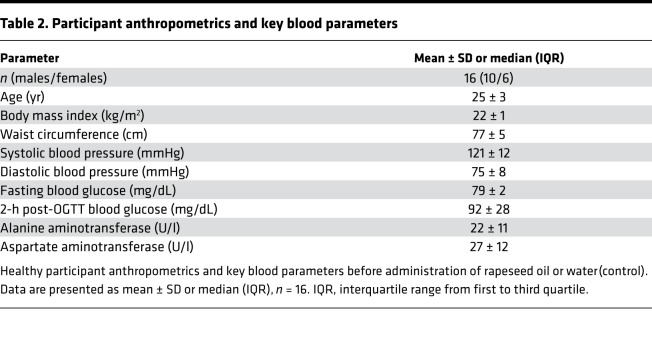
Sixteen (10 male, 6 female) glucose-tolerant, lean, young volunteers were included into this randomized, cross-over, placebo-controlled study (ClinicalTrials.gov NCT01736202) (Table 2 and Supplemental Figure 1).
Table 2. Participant anthropometrics and key blood parameters.
Experimental protocol.
All volunteers underwent screening, including medical history and clinical examination, lean body mass assessment, routine laboratory tests, and a 75-g oral glucose tolerance test. This study follows up a previous study with identical design, except for the use of OIL instead of palm oil; the previous study reported data on the control intervention from 7 of 10 male participants (5). The inclusion and exclusion criteria were described in detail before (5). Females were examined between days 5 and 9 of their menstrual cycle. Briefly, all participants arrived at the study center at 5:30 pm, received a standardized dinner (~684 kcal) at 6:00 pm, and drank 3 portions of 2H2O (99.9%, MilliporeSigma), diluted to 33% with mineral water at 8:00 pm, 10:00 pm, and 12:00 am to yield a total dose of 5 g 2H2O per kg body water. On the next day, at 5:00 am (defined as time point –180 minutes; basal period, –180 minutes to 0 minutes), 2 intravenous catheters were inserted to contralateral forearm veins. Participants drank 200 mL 2H2O (0.5% in water) every 60 minutes to maintain isotopic equilibrium in body water. At –180, +200, and +400 minutes, participants ingested 500 mg acetaminophen. From –180 minutes, a bolus-continuous (0.036 mg/kg BW/min) infusion of [6,6-2H2]glucose (99% enriched in 2H; Cambridge Isotope Laboratories) was administered. At 0 minutes, with start of the preclamp period (0 minutes to +360 minutes), participants received either OIL (63% oleic acid, 30% linoleic acid, 7% SAFA; Rapso, VOG AG, and Mazola, Peter Kölln GmbH & Co. KGaA), rich in MUFA, or VCL (water). Patients with more than 70 kg BW drank 92 g, and those with <70 kg BW drank 80 g OIL (~1.18 g/kg BW OIL) within 5–10 minutes (5). To yield a homogenous drink, OIL was heated to 60°C, mixed with 1.8 g or 1.6 g emulsifier (Glice, Texturas, Albert y Ferran Adria), 9 or 8 g sugar-free vanilla syrup (Torani), and 81.2 or 70.4 mL bottled still water, for 92 g and 80 g MUFA mix, respectively. To guarantee a stable emulsion, oil drinks were stirred constantly and served warm (40°C–45°C). The VCL drink was of equal composition but instead of OIL 173.2 mL or 150.4 mL bottled still water was used, respectively. The clamp period started at +360 minutes and continued until +480 minutes with a hyperinsulinemic-euglycemic clamp (40 mU/m2 body surface area/min; human regular insulin, Insuman Rapid, Sanofi). Blood glucose concentration was maintained at 90 mg/dL by adapting the glucose infusion rate using 20% glucose (B. Braun AG) enriched with 2% [6,6-2H2]glucose. Blood and urine samples were collected at timed intervals (Figure 1).
Indirect calorimetry.
Indirect calorimetry (IC) was performed in the canopy mode using Vmax Encore 29n (CareFusion) during the basal (–180 to 0 minutes), preclamp (0 to +360 minutes), and steady-state clamp periods (+460 to +480 minutes; Figure 1) as described previously (5, 9) (Supplemental Table 3).
Analyses of metabolites and hormones.
Whole-blood glucose, HbA1c, serum TG, plasma FFA, plasma insulin, and cortisol were measured as previously described (5). Serum concentrations of IL-1 receptor antagonist (IL-1ra), leptin, FGF21, and fatty acid–binding protein 4 (FABP4) were determined using Quantikine ELISA kits from R&D Systems/Bio-Techne. Serum IL-18 was measured using the Human IL-18 ELISA kit from MBL. Serum concentrations of HMW adiponectin were assessed with the HMW adiponectin ELISA kit (ALPCO).
Gas chromatography–mass spectrometry.
Measurement of atom percentage enrichment of [6,6-2H2]glucose in the blood glucose pool was performed on a Hewlett-Packard 6890 gas chromatograph equipped with a 25-m CPSil5CB capillary column (0.2 mm i.d., 0.12-μm film thickness; Chrompack/Varian) interfaced to a Hewlett Packard 5975 mass selective detector (5). Fatty acid spectra were analyzed as fatty acid methyl esters (FAMEs) using gas chromatography–mass spectrometry as previously described, with minor changes (29). Lipids were extracted from plasma after addition of internal standard using isopropyl alcohol/heptane/sulfuric acid (40:10:1). After separation by thin layer chromatography, extraction and derivatization to their corresponding methyl esters, FAMEs were analyzed on a Hewlett Packard 6890 gas chromatograph interfaced to a Hewlett Packard 5975 mass selective detector. Calibration curves of reference fatty acids were processed in parallel to tissue samples and were used for quantification of analytes.
Ex vivo 2H nuclear MR spectroscopy.
Positional 2H enrichments in 5-O-acetyl monoacetone glucuronic lactone (MAGLA) derivatized from urinary acetaminophen glucuronide and in monoacetone glucose (MAG) derivatized from plasma glucose (30) were obtained with a Bruker Avance III HD 500 spectrometer equipped with a 2H-selective 5-mm probe incorporating a 19F lock channel and analyzed using the NUTS PC-based NMR spectral analysis program (Acorn NMR) as described previously (5, 31). Samples from all 16 participants yielded sufficient data for nuclear MR spectroscopy analysis.
In vivo 13C/31P/1H MRS.
Examinations were performed on participants in the supine position within a whole-body 3.0-T Achieva X-Series Philips scanner (Philips Healthcare) (5). Liver 1H-decoupled 13C spectra of glycogen were obtained with a 7-cm dual-tuned 13C/1H coil (PulseTeq Ltd.) at –120, +15, +130, and +300 minutes. Liver 31P and 1H MRS were performed at the basal (–120 minutes) and preclamp period (+240 minutes). For liver ATP and Pi concentrations, 31P spectra were acquired with a 14-cm circular surface coil (Philips Healthcare) (5). 1H spectra were obtained using the Q-body coil with a single-voxel stimulated echo acquisition mode sequence with and without water suppression by the chemical shift saturation technique and analyzed by jMRUI 4.0 and the AMARES algorithm (32, 33). Liver volumes were assessed from transverse T2-weighted turbo spin-echo images (5).
Calculations.
During preclamp and clamp periods, whole-body Rd was obtained by the rate of glucose disappearance (Rd) from [6,6-2H2]glucose enrichments using Steele’s steady-state equations (34) and divided by plasma insulin levels at the respective time points during the preclamp and clamp period (+300 to +360 minutes and +450 to +480 minutes). From indirect calorimetry, GOX rates (mg/kg/min) were calculated as follows: ([4.55 × VCO2] – [3.21 × VO2] × 1.44) – 0.459(0.15 × REE/16.74) × 1000/(BW × 1440), where VCO2 and VO2 are in ml/min, REE is in kJ/d, and BW is in kg. LOX (mg/kg/min) was calculated by the formula ([(1.67 × VO2) – (1.67 × VCO2) × 1.44] – 0.307 × POX) × 1000/(BW × 24 × 60) (35), where VCO2 and VO2 are in ml/min, POX is in g/d, and BW is in kg (35). NOXGD was calculated as the difference between Rd and GOX. At between +300 minutes and +360 minutes in the preclamp period, HEP-IR was calculated as follows: EGP × mean insulin concentration (36). During insulin-stimulated conditions (clamp period), hepatic insulin sensitivity was assessed from EGP suppression, calculated as 100 – (mean clamp steady-state EGP concentrations × 100)/(basal EGP concentrations at 0 minutes) (Supplemental Table 3) (5).
The rate of GNG was calculated as the difference between EGP and GLYnet. GLYnet was derived from linear regression of hepatic glycogen concentrations — from 13C MRS — over time using the least mean square method (5, 37). Fractional GP flux was calculated as 1-(H5)/(H2), where H5/H2 is the ratio of 1H enrichment at carbon position 5 of glucuronide to that at position 2 after 2H2O ingestion (38). Absolute GP flux was calculated by multiplying fractional GP flux by EGP during the respective time period (38). Glycogen cycling, i.e., simultaneous fluxes through glycogen synthase and GP, was assessed by calculating the difference between GP and GLYnet (5, 39).
Total iAUCs for glucose, insulin, TG, FFA, and individual fatty acids, as well as for TG, IL-18, IL-1ra, FABP4, HMW adiponectin, and FGF21 during the preclamp period were calculated using the trapezoidal rule corrected for the respective AUC during the basal period (40).
Statistics.
The power calculation was based on a 2-tailed t test, assuming a mean difference in EGP (clamp period) of 0.1 and a SD of 0.11 resulting in a sample size of n = 16 to reach a power of 92%. Results are presented as mean ± SEM or percentages. In crossover studies, differences between treatment effects were tested using the classical crossover test, which compares the outcome of intraindividual period differences between the sequence groups (41). For statistical analysis of time courses of distinct parameters, a mixed-model repeated-measures ANOVA was used, adjusted for basal values with Bonferroni’s correction on PROC MIXED of SAS 9.3. Variables with skewed distributions were ln transformed before analysis. Statistical significance of differences was defined at P < 0.05. Calculations were performed using SAS version 9.4 (SAS Institute).
Study approval.
All volunteers gave their written informed consent before inclusion into this study (ClinicalTrials.gov NCT01736202), which was performed according to the 2013 version of the Declaration of Helsinki and approved by the ethics committee of the medical faculty at Heinrich Heine University Düsseldorf.
Author contributions
MR initiated the investigation, lead the clinical experiments, and wrote, reviewed, and edited the manuscript. TS led the clinical experiments, obtained and analyzed the data, and wrote, edited, and reviewed the manuscript. SK obtained/analyzed data and aided in devising the clinical study design and wrote, edited, and reviewed the manuscript. JS and OPZ helped to conduct the study and reviewed the manuscript. FW conducted the MRS study and reviewed and edited the manuscript. CB performed derivatization experiments and enrichment analysis for 2H-MRS and reviewed and edited the manuscript. JGJ performed derivatization experiments and enrichment analysis for 2H-MRS and reviewed and edited the manuscript. DFM and CH conducted laboratory analyses and reviewed and edited the manuscript. PB performed statistical analysis and reviewed the manuscript. JHH supervised the MRS study and reviewed and edited the manuscript. All authors have given final approval of the version to be published.
Supplementary Material
Acknowledgments
We would like to thank Paul Begovatz, Myrko Eßer, David Höhn, Bettina Nowotny, Peter Nowotny, Ulrike Partke, Andrea Sparla, and Kai Tinnes for their excellent support. This study was supported by grants to the German Diabetes Center, which is funded by the German Federal Ministry of Health and the Ministry of Culture and Science of the State North Rhine-Westphalia, and to the German Center for Diabetes Research by the German Federal Ministry of Education and Research, the European Regional Development Fund (KomIT, EFRE-0400191), the German Research Foundation (DFG; SFB 1116/2), and the Portuguese Foundation for Science and Technology (FCT-FEDER-02/SAICT/2017/028147). This study was also supported by structural funding for the Centre for Neurosciences and Cell Biology and the UC-NMR facility, supported in part by European Regional Development Fund (FEDER) — through the COMPETE Programme and the Portuguese Foundation for Science and Technology (POCI-01-0145-FEDER-007440, REEQ/481/QUI/2006, RECI/QEQ-QFI/0168/2012, CENTRO-07-CT62-FEDER-002012), and Rede Nacional de Ressonancia Magnética Nuclear. The funding sources had no role in the design, execution, analyses, and interpretation of the data of this study or decision to submit results. MR is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Version 1. 05/21/2020
Electronic publication
Footnotes
Conflict of interest: MR received personal fees from Boehringer Ingelheim, Eli Lilly, Fishawack Group, Novo Nordisk, ProSciento, Sanofi, Servier Laboratories, Target NASH, and Terra Firma and investigator-initiated research support from Boehringer Ingelheim, Danone Nutricia, and Sanofi-Aventis. CH received research support from Sanofi-Aventis.
Copyright: © 2020, American Society for Clinical Investigation.
Reference information: JCI Insight. 2020;5(10):e134520.https://doi.org/10.1172/jci.insight.134520.
Contributor Information
Theresia Sarabhai, Email: Theresia.Sarabhai@ddz.de.
Sabine Kahl, Email: sabine.kahl@ddz.de.
Julia Szendroedi, Email: julia.szendroedi@ddz.uni-duesseldorf.de.
Daniel F. Markgraf, Email: Daniel.Markgraf@DDZ.UNI-DUESSELDORF.DE.
Cristina Barosa, Email: c.barosa@hotmail.com.
Christian Herder, Email: Christian.herder@ddz.de.
Frithjof Wickrath, Email: frithjof.wickrath@hhu.de.
Pavel Bobrov, Email: pavel.bobrov@ddz.uni-duesseldorf.de.
Michael Roden, Email: Michael.Roden@ddz.uni-duesseldorf.de.
References
- 1.Echouffo-Tcheugui JB, Ahima RS. Does diet quality or nutrient quantity contribute more to health? J Clin Invest. 2019;129(10):3969–3970. doi: 10.1172/JCI131449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baratta F, et al. Adherence to Mediterranean diet and non-alcoholic fatty liver disease: effect on insulin resistance. Am J Gastroenterol. 2017;112(12):1832–1839. doi: 10.1038/ajg.2017.371. [DOI] [PubMed] [Google Scholar]
- 3.Estruch R, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378(25):e34. doi: 10.1056/NEJMoa1800389. [DOI] [PubMed] [Google Scholar]
- 4.Luukkonen PK, et al. Saturated fat is more metabolically harmful for the human liver than unsaturated fat or simple sugars. Diabetes Care. 2018;41(8):1732–1739. doi: 10.2337/dc18-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernández EÁ, et al. Acute dietary fat intake initiates alterations in energy metabolism and insulin resistance. J Clin Invest. 2017;127(2):695–708. doi: 10.1172/JCI89444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roden M, Shulman GI. The integrative biology of type 2 diabetes. Nature. 2019;576(7785):51–60. doi: 10.1038/s41586-019-1797-8. [DOI] [PubMed] [Google Scholar]
- 7.Parks E, Yki-Järvinen H, Hawkins M. Out of the frying pan: dietary saturated fat influences nonalcoholic fatty liver disease. J Clin Invest. 2017;127(2):454–456. doi: 10.1172/JCI92407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frias JP, Macaraeg GB, Ofrecio J, Yu JG, Olefsky JM, Kruszynska YT. Decreased susceptibility to fatty acid-induced peripheral tissue insulin resistance in women. Diabetes. 2001;50(6):1344–1350. doi: 10.2337/diabetes.50.6.1344. [DOI] [PubMed] [Google Scholar]
- 9.Nowotny B, et al. Mechanisms underlying the onset of oral lipid-induced skeletal muscle insulin resistance in humans. Diabetes. 2013;62(7):2240–2248. doi: 10.2337/db12-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Müller C, et al. Endogenous glucose production, gluconeogenesis and liver glycogen concentration in obese non-diabetic patients. Diabetologia. 1997;40(4):463–468. doi: 10.1007/s001250050701. [DOI] [PubMed] [Google Scholar]
- 11.Ter Horst KW, et al. Impaired insulin action in the liver, but not in adipose tissue or muscle, is a distinct metabolic feature of impaired fasting glucose in obese humans. Metab Clin Exp. 2016;65(5):757–763. doi: 10.1016/j.metabol.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Clore JN, Glickman PS, Nestler JE, Blackard WG. In vivo evidence for hepatic autoregulation during FFA-stimulated gluconeogenesis in normal humans. Am J Physiol. 1991;261(4 Pt 1):E425–E429. doi: 10.1152/ajpendo.1991.261.4.E425. [DOI] [PubMed] [Google Scholar]
- 13.Xiao C, Giacca A, Carpentier A, Lewis GF. Differential effects of monounsaturated, polyunsaturated and saturated fat ingestion on glucose-stimulated insulin secretion, sensitivity and clearance in overweight and obese, non-diabetic humans. Diabetologia. 2006;49(6):1371–1379. doi: 10.1007/s00125-006-0211-x. [DOI] [PubMed] [Google Scholar]
- 14.Gancheva S, Jelenik T, Álvarez-Hernández E, Roden M. Interorgan metabolic crosstalk in human insulin resistance. Physiol Rev. 2018;98(3):1371–1415. doi: 10.1152/physrev.00015.2017. [DOI] [PubMed] [Google Scholar]
- 15.Vessby B, et al. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The KANWU Study. Diabetologia. 2001;44(3):312–319. doi: 10.1007/s001250051620. [DOI] [PubMed] [Google Scholar]
- 16.Ragheb R, Shanab GM, Medhat AM, Seoudi DM, Adeli K, Fantus IG. Free fatty acid-induced muscle insulin resistance and glucose uptake dysfunction: evidence for PKC activation and oxidative stress-activated signaling pathways. Biochem Biophys Res Commun. 2009;389(2):211–216. doi: 10.1016/j.bbrc.2009.08.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capel F, et al. Oleate dose-dependently regulates palmitate metabolism and insulin signaling in C2C12 myotubes. Biochim Biophys Acta. 2016;1861(12 Pt A):2000–2010. doi: 10.1016/j.bbalip.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Lindegaard B, et al. Interleukin-18 activates skeletal muscle AMPK and reduces weight gain and insulin resistance in mice. Diabetes. 2013;62(9):3064–3074. doi: 10.2337/db12-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stingl H, Raffesberg W, Nowotny P, Waldhäusl W, Roden M. Reduction of plasma leptin concentrations by arginine but not lipid infusion in humans. Obes Res. 2002;10(11):1111–1119. doi: 10.1038/oby.2002.151. [DOI] [PubMed] [Google Scholar]
- 20.Camporez JP, et al. Anti-inflammatory effects of oestrogen mediate the sexual dimorphic response to lipid-induced insulin resistance. J Physiol (Lond) 2019;597(15):3885–3903. doi: 10.1113/JP277270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.European Association for the Study of the Liver (EASL) European Association for the Study of Diabetes (EASD) European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association. 4. Lifestyle Management: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S38–S50. doi: 10.2337/dc18-S004. [DOI] [PubMed] [Google Scholar]
- 23.Bozzetto L, et al. Reduction in liver fat by dietary MUFA in type 2 diabetes is helped by enhanced hepatic fat oxidation. Diabetologia. 2016;59(12):2697–2701. doi: 10.1007/s00125-016-4110-5. [DOI] [PubMed] [Google Scholar]
- 24.Jones PJ, Jew S, AbuMweis S. The effect of dietary oleic, linoleic, and linolenic acids on fat oxidation and energy expenditure in healthy men. Metab Clin Exp. 2008;57(9):1198–1203. doi: 10.1016/j.metabol.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Burhans MS, Flowers MT, Ntambi JM. Hepatic oleate regulates liver stress response partially through PGC-1α during high-carbohydrate feeding. J Hepatol. 2016;65(1):103–112. doi: 10.1016/j.jhep.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dupont J, et al. Food safety and health effects of canola oil. J Am Coll Nutr. 1989;8(5):360–375. doi: 10.1080/07315724.1989.10720311. [DOI] [PubMed] [Google Scholar]
- 27.Palomer X, Pizarro-Delgado J, Barroso E, Vázquez-Carrera M. Palmitic and oleic acid: the yin and yang of fatty acids in type 2 diabetes mellitus. Trends Endocrinol Metab. 2018;29(3):178–190. doi: 10.1016/j.tem.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Grey NJ, Karl I, Kipnis DM. Physiologic mechanisms in the development of starvation ketosis in man. Diabetes. 1975;24(1):10–16. doi: 10.2337/diab.24.1.10. [DOI] [PubMed] [Google Scholar]
- 29.Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res. 1986;27(1):114–120. [PubMed] [Google Scholar]
- 30.Jones J, Kahl S, Carvalho F, Barosa C, Roden M. Simplified analysis of acetaminophen glucuronide for quantifying gluconeogenesis and glycogenolysis using deuterated water. Anal Biochem. 2015;479:37–39. doi: 10.1016/j.ab.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Jones JG, Merritt M, Malloy C. Quantifying tracer levels of (2)H(2)O enrichment from microliter amounts of plasma and urine by (2)H NMR. Magn Reson Med. 2001;45(1):156–158. doi: 10.1002/1522-2594(200101)45:1<156::AID-MRM1020>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 32.Vanhamme L, Van Huffel S, Van Hecke P, van Ormondt D. Time-domain quantification of series of biomedical magnetic resonance spectroscopy signals. J Magn Reson. 1999;140(1):120–130. doi: 10.1006/jmre.1999.1835. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton G, et al. In vivo characterization of the liver fat ¹H MR spectrum. NMR Biomed. 2011;24(7):784–790. doi: 10.1002/nbm.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- 35.Schadewaldt P, Nowotny B, Strassburger K, Kotzka J, Roden M. Indirect calorimetry in humans: a postcalorimetric evaluation procedure for correction of metabolic monitor variability. Am J Clin Nutr. 2013;97(4):763–773. doi: 10.3945/ajcn.112.035014. [DOI] [PubMed] [Google Scholar]
- 36.Hattersley JG, et al. Quantifying the improvement of surrogate indices of hepatic insulin resistance using complex measurement techniques. PLoS One. 2012;7(6):e39029. doi: 10.1371/journal.pone.0039029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krssak M, et al. Alterations in postprandial hepatic glycogen metabolism in type 2 diabetes. Diabetes. 2004;53(12):3048–3056. doi: 10.2337/diabetes.53.12.3048. [DOI] [PubMed] [Google Scholar]
- 38.Kraegen EW, James DE, Jenkins AB, Chisholm DJ. Dose-response curves for in vivo insulin sensitivity in individual tissues in rats. Am J Physiol. 1985;248(3 Pt 1):E353–E362. doi: 10.1152/ajpendo.1985.248.3.E353. [DOI] [PubMed] [Google Scholar]
- 39.Kacerovsky M, et al. Postprandial and fasting hepatic glucose fluxes in long-standing type 1 diabetes. Diabetes. 2011;60(6):1752–1758. doi: 10.2337/db10-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rathmann W, et al. A variant of the glucose transporter gene SLC2A2 modifies the glycaemic response to metformin therapy in recently diagnosed type 2 diabetes. Diabetologia. 2019;62(2):286–291. doi: 10.1007/s00125-018-4759-z. [DOI] [PubMed] [Google Scholar]
- 41.Wellek S, Blettner M. On the proper use of the crossover design in clinical trials: part 18 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2012;109(15):276–281. doi: 10.3238/arztebl.2012.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.