Social media quote
Studies of prenatal acetaminophen exposure and neurodevelopment are subject to multiple biases.
What are we to make of a recent flood of studies suggesting that prenatal exposure to acetaminophen is associated with neurodevelopmental problems in toddlers and young children? Neurodevelopment is a notoriously slippery outcome to define, as it encompasses multiple phenotypes, including cognition and intelligence, psychiatric diagnoses, developmental milestone attainment, behavioral problems, motor and communication skills, and emotional regulation. Others have noted that many of the survey-based instruments used to measure neurodevelopment have uncertain predictive value for clinically-relevant developmental problems.1 Even assuming that studies have selected outcome measures that capture important neurodevelopmental phenotypes, studying the effect of acetaminophen on these outcomes is extraordinarily challenging. Nonetheless, acetaminophen is one of the most commonly used medications among pregnant women, and if prenatal exposure truly has a causal effect on neurodevelopment, even if this effect is small, it is important to understand it.
Consider the hypothetical case where acetaminophen has a true causal effect on neurodevelopment, but affects only impulsivity, and only for exposures occurring between gestational weeks 17 and 20. Acetaminophen is highly subject to exposure misclassification, both in terms of confusion with other drugs and with respect to timing of use. Many studies rely on maternal report for both the exposure and the outcome, which can introduce dependent measurement error. Acetaminophen is taken for a wide range of reasons and severity, including pain, inflammation, and fever, making confounding by indication very difficult to understand and control, either through design or analysis. Ascertainment of neurodevelopmental problems often requires follow-up over the course of years, leaving the sample vulnerable to bias from selection, especially if parents of children with more behavioral problems are less likely to sustain their participation in a long-term study, if participation is also associated with exposure. Considered singly, any one of these sources of bias should worry conscientious researchers, but taken together, a picture of multiple competing biases emerges, making the task of identifying a true causal effect very challenging. The figure depicts the classic threats to validity—information, selection, and confounding bias—in terms of how they are likely to move effect estimates in studies of prenatal exposure to acetaminophen and neurodevelopmental outcomes in children (panel A), and a possible directed acyclic graph (panel B) suggests a possible causal mechanism through which these biases may act.
Figure.
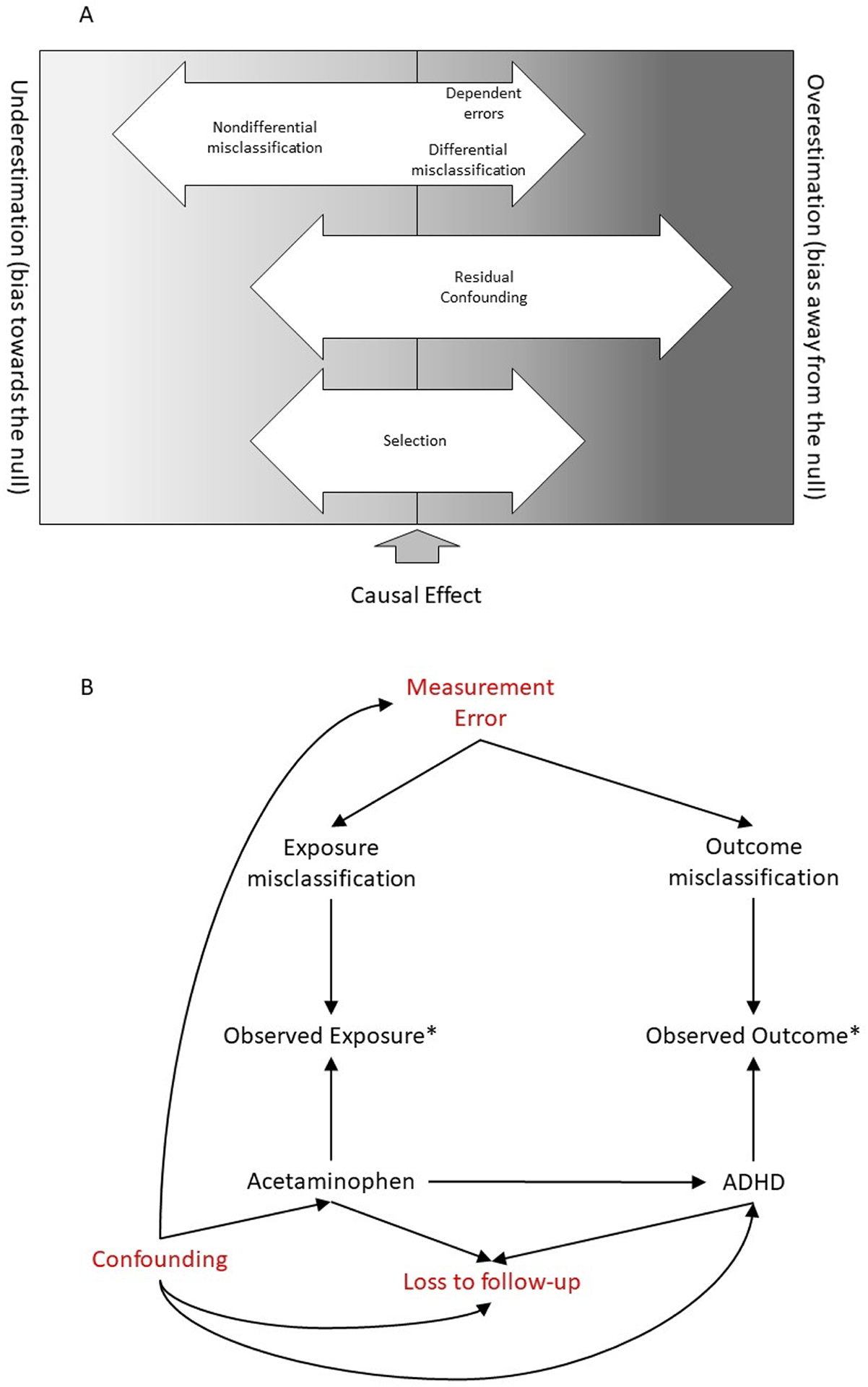
The potential for bias in studies of prenatal acetaminophen exposure and child neurodevelopment, including (A) the possible magnitude and direction of these biases, and (B) a directed acyclic graph depicting sources of bias (measurement error, confounding, loss to follow-up) as well as the true causal effect of exposure on outcome.
Information bias in the form of both exposure misclassification and outcome misclassification are not just possible but likely in this type of study, where the exposure of interest is an intermittently-used medication perceived to be of low risk, and where the outcomes are often reported by parents using sub-optimal screening tools. Panel B of the Figure shows measurement error simultaneously affecting exposure and outcome misclassification and providing an opportunity for bias to act through either path singly, or both together. Studies included in this issue uniformly used designs in which exposure information was collected prior to measurement of the outcome, meaning that misclassification of exposure is likely to be nondifferential with respect to outcome. Given that all exposure collected in the included studies involved some degree of recall, with latency ranging from weeks to years, some degree of misclassification is inevitable. Further, several studies included self-reported exposure and outcome data from the same source (i.e., maternal report),2–5 which may leave them vulnerable to dependent measurement error. If over-reporting of exposures co-occurs with over-reporting of outcomes, for example, this could produce the appearance of a strong effect of exposure on outcome. Several studies addressed this by using multi-informant ascertainment, such as including reports from both the parent and a teacher,5,6 or including a clinical evaluation of neurodevelopment.3,4,6 In two studies, parent and teacher reports diverged,5,6 but in a third they did not3, making this difficult to interpret. One study used probabilistic bias analysis to quantify the potential impact of exposure misclassification,2 noting that realistic levels of misclassification could have substantially attenuated the observed effect estimates. On balance, the information bias in these studies should move estimates towards the null: this is a reasonable expectation with dichotomous exposures. However, we cannot rule out the possibility of bias in the opposite direction.
Selection is also a plausible source of bias. Most the studies included in this special issue of Paediatric and Perinatal Epidemiology had lost more than half of their original sample to follow up between birth and the time of neurodevelopmental assessment,2–4,6 shown in panel B of the figure as loss to follow-up. If children not available for assessment have different neurodevelopmental profiles then those present at follow-up, this could be a source of bias if loss to follow-up is also associated with exposure, either directly or through a backdoor path. For example, if mothers of children with more severe attention problems less often responded to requests for follow-up, and if they themselves used more acetaminophen during pregnancy, we could expect to see bias towards the null, but cannot rule out bias in the opposite direction, especially in the presence of confounders of the exposure-selection or selection-outcome paths. Conversely, mothers using less acetaminophen might participate less often, which could produce bias away from the null. Several of the studies in this issue used weighting methods to account for measured predictors of loss to follow up,2–5,7 but noted minimal differences between weighted and unweighted estimates, suggesting that selection bias may not be a major threat to validity in this instance. Alternately, there may be common causes of exposure and selection that were not included in the weights.
Residual confounding is a third source of bias, and in our representation (panel A, Figure), the most likely to cause bias away from the null, or an overestimation of the true effect. Acetaminophen presents a difficult confounding problem, since it is used to treat a wide range of conditions, including pain and fever, but as observed in several studies, it is also used in greater amounts by pregnant women who report more psychiatric diagnoses,8 or more depressive or anxiety symptoms.3,4 This suggests that the underlying indications and causes of exposure are complex, and points to a potential for heritable or familial confounding by susceptibility to psychiatric comorbidities. All of the included studies used an analytic method for control of measured confounders, including multivariable adjustment3,5–7 or inverse probability (of treatment) weighting.2 In addition, some used a negative control2,5 or other sensitivity analyses2 to understand the possible impact of unmeasured confounding. Notably, several studies did not adjust for underlying pain conditions, due to either lack of confounder data3,4 or analytic choice.6 Further, different researchers relied on different procedures for confounder selection in outcome models, including a priori selection of potential confounders2,5,7 with or without assessment of material impact on effect estimates,3,4 as well as methods relying on statistical significance testing.6 Given the heterogeneity of the outcomes studied and the adjustment strategies employed, it is difficult to ascertain the global impact of confounder adjustment; however, we note that studies reporting elevated unadjusted effect estimates for acetaminophen exposure, also reported estimates that were substantially reduced by including indication for use (e.g., fever, migraine) in the set of confounders. Some robustness checks suggested the potential for residual unmeasured confounding, as in the Trønnes analysis of observations in the tails of the propensity score.2 However, use of ibuprofen as a negative control produced conflicting information, with some studies showing no association of neurodevelopmental problems with ibuprofen exposure (consistent with a conclusion of no confounding by indication)5, while others showed the opposite.3
It is important to note that these sources of bias may not be independent of one another. Confounders, such as depression or anxiety symptoms, may also affect measurement error, and participation in the study, as suggested in Panel B of the Figure. Although not depicted in the Figure, confounders can also be misclassified, and misclassification could suffer from similar dependent misclassification as discussed for the exposure and outcome. In addition to the individual level studies included in this issue of Paediatric and Perinatal Epidemiology, a systematic review combined with quantitative bias analysis noted that most published effect estimates for prenatal acetaminophen exposure and neurodevelopment can be largely explained by realistic confounding, and are additionally subject to information and selection biases,9 as observed previously. Many studies included in this issue have carefully addressed one set of biases, and several have addressed more than one. However, no study has addressed all three, and none has used techniques to consider biases simultaneously, such as multiple bias analysis. This means that readers must combine these quantitative estimates of bias with qualitative weighing of their relative importance and impact. Further, this weighing must occur concurrently with consideration of a wide range of neurodevelopmental phenotypes, varying definitions of exposure, and different populations.
In sum, both carrying out and interpreting studies of prenatal acetaminophen exposure and child neurodevelopment are extremely challenging. The research landscape on this topic continues to show a picture of a modest association between prenatal acetaminophen exposure and neurodevelopment in children, with a suggestion that more intense and prolonged exposure is associated with larger effect sizes, but that very little residual confounding would be sufficient to explain observed effects. This is of particular interest in studies where the effect estimate is reported as an odds ratio, which systematically overestimates risks, especially when the outcome is not rare. While methods to address biases singly often resulted in modest changes in effect estimates, it is unclear what impact, if any, multiple co-occurring biases may have. Future studies should avoid self-inflicted threats to validity by employing multiple-informant methods, negative controls, and careful follow-up of all participants. Importantly, further work should directly address the joint biases resulting from residual confounding inflating observed associations, and exposure misclassification reducing them.
Acknowledgements
I would like to thank Sonia Hernandez-Diaz for her helpful comments on an early version of this commentary. The National Institutes of Health (T32HL098048–11) supported this work.
About the author
Mollie Wood is a reproductive and perinatal epidemiologist, with a particular interest in studying the use of medications during pregnancy. Her research focuses on the treatment of chronic pain and psychiatric illness during pregnancy and subsequent effects on both maternal and child health. She is currently a postdoctoral research fellow at the Harvard TH Chan School of Public Health (USA) and a guest researcher at the University of Oslo (Norway).
References
- 1.Damkier P, Pottegård A, dePont Christensen R, Hallas J. Annotations and Reflections. Basic Clin Pharmacol Toxicol. 2015;116(1):2–5. doi: 10.1111/bcpt.12322 [DOI] [PubMed] [Google Scholar]
- 2.Trønnes JN, Wood M, Lupattelli A, Ystrom E, Nordeng H. Prenatal paracetamol exposure and neurodevelopmental outcomes in preschool‐aged children. Paediatr Perinat Epidemiol. 2019;(May):1–10. doi: 10.1111/ppe.12568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rifas‐Shiman SL, Cardenas A, Hivert M, Tiemeier H, Bertoldi AD, Oken E. Associations of prenatal or infant exposure to acetaminophen or ibuprofen with mid‐childhood executive function and behaviour. Paediatr Perinat Epidemiol. 2019;(May):ppe.12596. doi: 10.1111/ppe.12596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertoldi AD, Rifas-Shiman S, Boing A, et al. Associations of acetaminophen use during pregnancy and the first year of life with neurodevelopment in early childhood. Paediatr Perinat Epidemiol. 2019. doi: 10.1017/S0007114518003884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parker SE, Collett BR, Werler MM. Maternal Acetaminophen Use during Pregnancy and Childhood Behavioral Problems: Discrepancies between Mother and Teacher Reported Outcomes. Paediatr Perinat Epidemiol. 2020. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golding J, Gregory S, Clark R, Ellis G, Iles‐Caven Y, Northstone K. Associations between paracetamol (acetaminophen) intake between 18 and 32 weeks gestation and neurocognitive outcomes in the child: A longitudinal cohort study. Paediatr Perinat Epidemiol. 2020. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tovo-Rodrigues L, Carpena M, Martins-Silva T, et al. Low neurodevelopmental performance and behavioural/emotional problems at 24 and 48 months in Brazilian children exposed to acetaminophen during foetal development. Paediatr Perinat Epidemiol. 2020. (In press). [DOI] [PubMed] [Google Scholar]
- 8.Bandoli G, Palmsten K, Chambers CD. Acetaminophen use in pregnancy: examining prevalence, timing and indication of use in a prospective birth cohort. Paediatr Perinat Epidemiol. 2020. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masarwa R, Platt RW, Fillion K. Acetaminophen use during pregnancy and the risk of attention deficit hyperactivity disorder: A causal association or bias? Paediatr Perinat Epidemiol. 2020. (In press). [DOI] [PubMed] [Google Scholar]


