Supplemental Digital Content is available in the text.
Keywords: artificial respiration, coronavirus disease 2019, mechanical ventilation, ventilator
Abstract
Objectives:
To design and test a ventilator circuit that can be used for ventilation of two or more patients with a single ventilator, while allowing individualization of tidal volume, fractional concentration of oxygen, and positive end-expiratory pressure to each patient, irrespective of the other patient’s respiratory system mechanics.
Design:
Description and proof of concept studies.
Settings:
Respiratory therapy laboratory.
Subjects:
Ventilation of mechanical test lungs.
Interventions:
Following a previously advocated design, we used components readily available in our hospital to assemble two “bag-in-a-box” breathing circuits. Each patient circuit consisted of a flexible bag in a rigid container connected via one-way valve to a test lung, along with an inline positive end-expiratory pressure valve, connected to the ventilator’s expiratory limb. Compressed gas fills the bags during “patient” exhalation. During inspiration, gas from the ventilator, in pressure control mode, enters the containers and displaces gas from the bags to the test lungs. We varied tidal volume, “respiratory system” compliance, and positive end-expiratory pressure in one lung and observed the effect on the tidal volume of the other.
Measurements and Main Results:
We were able to obtain different tidal volume, dynamic driving pressure, and positive end-expiratory pressure in the two lungs under widely different compliances in both lungs. Complete obstruction, or disconnection at the circuit connection to one test lung, had minimal effect (< 5% on average) on the ventilation to the co-ventilated lung.
Conclusions:
A secondary circuit “bag-in-the-box” system enables individualized ventilation of two lungs overcoming many of the concerns of ventilating more than one patient with a single ventilator.
The current coronavirus disease 2019 pandemic has led to a demand for mechanical ventilators that has outstripped supply in a number of jurisdictions and threatens to do so in more hospitals in the very near future. To address the terrible dilemma that this may raise, clinicians have proposed splitting the tidal volume (Vt) delivered by a ventilator between two or more patients (1, 2) and thoughtful guidelines to support its implementation have been developed (3). The Food and Drug Administration considered the need to expand ventilator capacity sufficiently urgent that it very rapidly provided Emergency Use Authorization for a device that facilitated ventilator sharing (4).
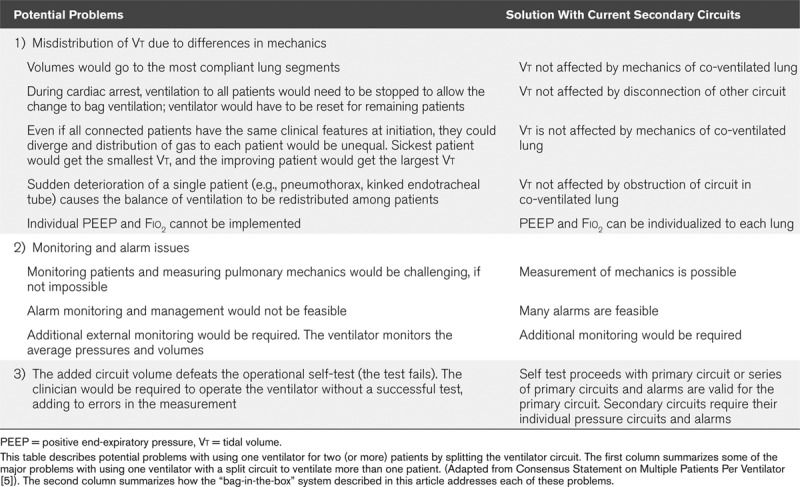
However, the splitting of ventilator output has received widespread criticism. On March 26, 2020, a number of medical societies, including the Society for Critical Care Medicine, published a “Consensus Statement on Multiple Patients Per Ventilator” (5) advising against the use of the technique, citing inherent risks which are summarized in Table 1. The major disadvantages they considered can be summarized as follows: 1) inability to match ventilatory variables such as Vt, Fio2, and positive end-expiratory pressure (PEEP) to individual patient needs, and 2) a change in respiratory mechanics in one patient would adversely affect ventilation to the co-ventilated patient(s).
TABLE 1.
Ventilator Splitting Circuit: Potential Problems and Solutions
To address these issues, we designed a patient ventilation circuit based on the work of Sommer et al (6). The circuit is centered on the principle that the interdependence between patients could be minimized if each patient was ventilated by their own secondary circuit (i.e., “a bag-in-the-box”), with no direct contact between the individual inspiratory circuits (Fig. 1). Each secondary circuit would have its own PEEP valve and its own blend of oxygen and air to provide a fresh gas flow (FGF) and Fio2. Ventilation to all patients would be driven by a single ventilator in pressure control mode (PCV); during inspiration, gas from the ventilator flows into the “boxes” increasing the pressure therein and displacing gas from the flexible “bags” into the patients.
Figure 1.
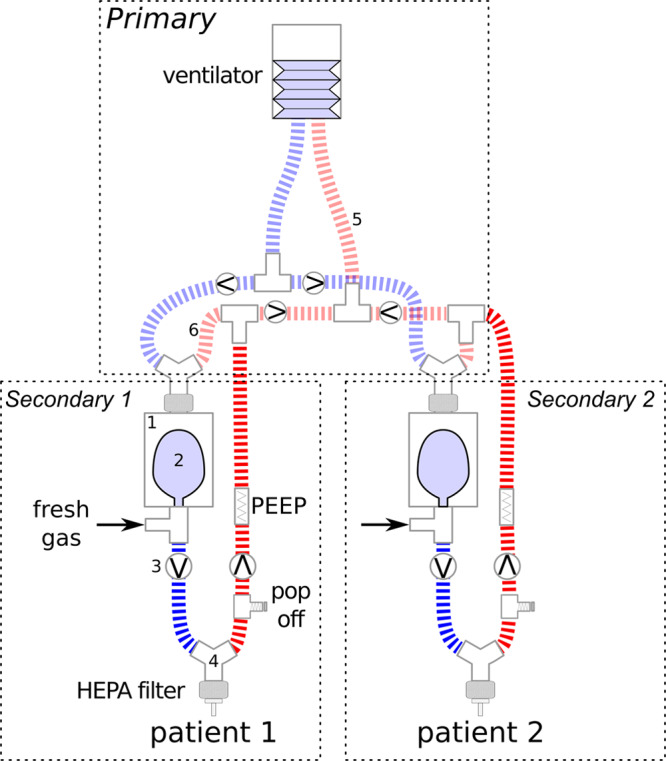
Schematic diagram of two secondary circuits being driven by a single ventilator. A secondary circuit consists of a “bag-in-the-box” configuration. The “box” in our circuit consisted of a suction canister with the lid glued to the rim to prevent it dislodging with positive pressure (fully described in online supplement, Supplemental Digital Content 1, http://links.lww.com/CCX/A173). This “box” (1) contains two ports. A 2-L anesthetic bag (2) opens to one port, which leads to the patient inspiratory tube. The inspiratory tube also contains a port for fresh gas flow (FGF) consisting of oxygen or blend of oxygen and air, and a one-way valve (3). The inspiratory tube is connected to the patient via a three-way wye connector (4) and a high-efficiency particle absorbing (HEPA) filter. The expiratory tube has a one-way valve, a pressure relief (“pop off”) valve at 40 cm H2O, and may contain flow-through mechanical positive end-expiratory pressure (PEEP) valves which also function to assure the “bag” in the box inflates during exhalation; it connects to the expiratory limb of the ventilator (5). The primary driving circuit from the ventilator: The inspiratory limb of the ventilator is split such that there is one branch for each secondary circuit. Each branch consists of an inspiratory and expiratory tube, connected to a wye piece, which is connected to the second (driving) port of the “box” (1). The expiratory limb from the wye (6) connects back to the expiratory limb of the ventilator (5). Additional secondary circuits may be added by connecting additional branches of the ventilator primary circuit. Circuit caveats: 1) For the PEEP valves in the expiratory limb of the secondary circuit, one should not use a valve that vents to air as it will decrease the flow of gas returning to the ventilator and may cause the ventilator to alarm. This PEEP valve also ensures that the FGF fills the “bag” rather than flowing out of the circuit with exhaled gas. 2) Higher cumulative FGF will increase the pressure at the ventilator expiratory valve which will be additive to the PEEP applied with the PEEP valves. 3) Note the inspiratory limb of the secondary circuit needs to contain a one-way valve to prevent backflow of expired gas into the “bag.” 4) Although it would seem to be expedient, single duck-billed (nonrebreathing) valves should not be used in the inspiratory and expiratory limbs of the secondary circuit. This would be “very dangerous,” since the duck-billed valves may become stuck in the inspiratory position with high FGF, or with PEEP and will result in breath stacking.
On expiration, gas flows through the expiratory circuit from each patient, mixing in the tubing just before the ventilator’s exhalation valve. During this exhalation phase, gas continues to flow from the blend of air and oxygen to inflate the “bag” for the next inspiration. Using this configuration, co-ventilated patients would share the same respiratory rate (RR) and inspiratory:expiratory (I:E) ratio, but there would be no cross-contamination of inspiratory airway gases. Individual values of Vt, Fio2, and PEEP, would remain substantially constant for each patient, independent of a change in the mechanics or ventilator settings of a co-ventilated patient.
Herein we describe this system and provide detailed instructions on how to assemble it from standard parts that can be found in most hospitals. We document the results of bench testing confirming that Vt and PEEP in one lung are substantially maintained in light of changes in mechanics and PEEP in the other.
MATERIALS AND METHODS
A schematic of the circuit is shown in Figure 1. A detailed description of the parts required and how to assemble the circuit are given in the online supplement (Supplemental Digital Content 1, http://links.lww.com/CCX/A173). We used two test lungs (QuickLung; InGMAR Medical, Pittsburgh, PA), representing patient lungs.
An ICU ventilator (Nellcor Puritan Bennet 840 Ventilator; Covidien, Mansfield, MA) in PCV was used with inspiratory pressure set at 60 cm H2O. We recorded airway pressure and Vt in both lungs, using the FluxMed Gr (MBMed, Buenos Aires, Argentina) under the following conditions:
-
1)
We examined the effect of different respiratory system compliances (Crs) for the two lungs (10 or 50 mL/cm H2O), different PEEP values (≈5 or ≈20 cm H2O), and RR (10 or 30 breaths/min) to determine if we could independently apply different ventilatory strategies for each lung, despite differences in respiratory system mechanics.
-
2)
We assessed whether we could provide a lung protective ventilation strategy to each lung with different PEEP values, Vt, and different dynamic driving pressures (ΔPs) (peak inspiratory pressure [PIP]–PEEP). PIP was used to compute ΔP as the constant FGF results in increasing airway pressure, albeit minimal, even in the absence of ventilator-delivered flow.
-
3)
We also examined the effects of a disconnection from the circuit, or of a sudden occlusion of the inspiratory tube of one lung on the Vt of the nonaffected lung.
For each combination of tests, we increased the FGF to each lung separately until the first occurrence of either Vt of 400 mL (roughly corresponding to 6 mL/kg in a patient with a predicted body weight of ≈70 kg) or to a PIP maximum of 40 cm H2O. FGF was read from the rotameter.
-
4)
Finally, we assessed whether this system would allow for delivery of reasonable-sized Vt in lungs with extremely low Crs.
When the two lungs were being ventilated with their individually allocated strategy, we used the exhaled Vt as measured by the ventilator, and then set an alarm limit equal to the total exhaled volume minus the smaller Vt of the two test lungs which would alarm if either circuit became disconnected at the endotracheal tube (ETT).
RESULTS
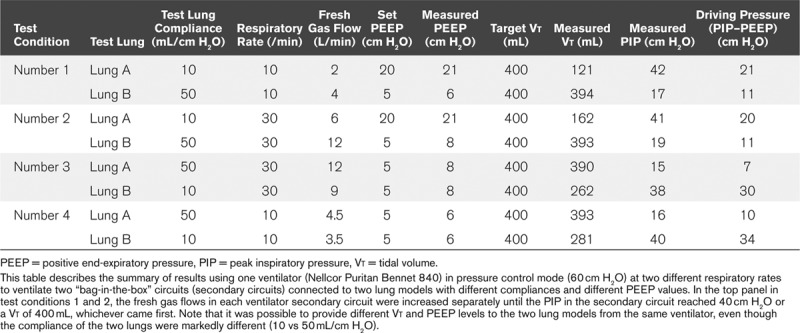
Table 2 shows that it is possible to independently set Vt and PEEP in lungs with substantially different Crs (10 and 50 mL/cm H2O). In extreme conditions, with a Crs of 10 mL/cm H2O, PEEP of 20 and a RR 30, a Vt of 162 mL was delivered. However, this Vt was only limited by our predetermined PIP cutoff of ~40 cm H2O (test condition number 2).
TABLE 2.
Effect of Fresh Gas Flow, Respiratory Rate, and Positive End-Expiratory Pressure on Tidal Volume of Two Co-Ventilated Lung Models With Disparate Compliance
Tables 3 and 4 present the results of the disconnection of the system and the occlusion of the ETT. The impact on the ventilation to the second test lung was relatively small with an average of less than 5% change in Vt. The same results were similar when different Crs were set (10 and 50 mL/cm H2O), demonstrating the relative independence of the ventilation pattern of one lung from the other. When the test lung was disconnected from the circuit, the ventilator’s alarm triggered within two breaths.
TABLE 3.
Effect of Disconnection of the Inspiratory Limb of One Co-Ventilated Lung on the Remaining Lung With Disparate Compliances, Respiratory Rates, and Positive End-Expiratory Pressure Settings
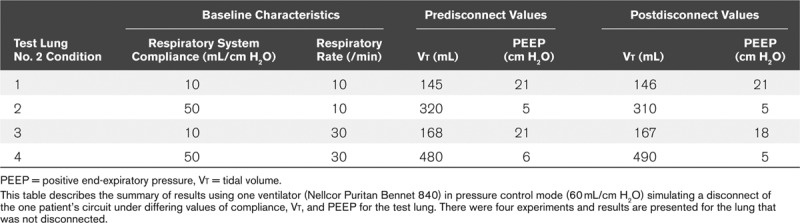
TABLE 4.
Effect of Occlusion of the Inspiratory Limb of One Co-Ventilated Lung on the Remaining Lung With Disparate Compliances, Respiratory Rates, and Positive End-Expiratory Pressure Settings
DISCUSSION
We describe a novel system that can reliably ventilate two patients with different respiratory system mechanics and ventilation requirements using a single ventilator. We showed that changes in Crs, Vt, and PEEP of one co-ventilated lung minimally affect the ventilation delivered to the other lung.
Current approaches for ventilating two or more patients by splitting the flow of a single ventilator can lead to problems (2, 7) as summarized in Table 1. Using the ventilator in the pressure-cycled mode in a split circuit without secondary circuits would provide a consistent Vt to one patient despite changes in resistance and compliance in other co-ventilated patients. However, it is not possible to individualize Vt, Fio2, and PEEP for each of the patients. Furthermore, if one patient becomes disconnected from the circuit, Vt will be lost to the other patient. To address these problems, clinicians have developed a detailed protocol and risk mitigation strategy as recently proposed by a group out of New York (3). This approach can decrease the risks, but it is still not possible to individualize PEEP or Fio2, or to optimize Vt in each patient independently.
Our approach of using a secondary circuit for each patient overcomes these problems and addresses the concerns raised by the Societies’ joint statement (5) (summarized in Table 1). The Vt each patient receives is determined by the FGF to that patient’s circuit, providing complete independence in terms of Vt delivery, even in situations where patients with significant differences in respiratory system mechanics are placed on the system. We also found that ventilation was essentially unaffected by extreme changes of a co-ventilated patient’s respiratory mechanics, as demonstrated when we disconnected or clamped the circuit at the lung entrance. Although we performed experiments with two test lungs, this approach is applicable for ventilating three, four, or more subjects assuming the ventilator has the flow capacity to generate sufficient pressure in the “bag-in-the-box” systems to collapse all of the “bags.” PEEP can also be individually set with inline PEEP valves.
Our approach has a number of important limitations. First, patients must be sedated and paralyzed, and RR and I:E ratios are identical for all patients. Second, PEEP levels may be impacted in a manner that is dependent on the FGF to all secondary circuits and the characteristics of the ventilator’s expiratory valve. Third, the setup is not optimized for weaning, and patients would have to be transferred to a separate ventilator for weaning. Fourth, as this is improvised emergency ventilatory support, clinical vigilance is mandatory. The traditional monitored variables of the ventilator are not able to monitor each secondary circuit but can be used in some ventilators to monitor disconnections at the ETT of either patient. Spirometry equipment is not freely available even in well-stocked hospitals, requiring Vts to be assessed by portable devices or clinical signs such as chest excursions. As such, individualized monitoring for each circuit should be performed using stand-alone devices for Fio2, capnography, and airway pressure and flow, as available.
A pressure relief valve in the inspiratory limb of the secondary circuit is required for patient safety. Placing a one-way valve in the wrong direction in either limb of the patient circuit will increase the airway pressure leaving the pressure relief valve as the mitigation of last resort to prevent barotrauma. Our data were gathered without the relief valve because some of these valves began to leak gas at pressures below the set threshold pressure and interfered with our proof of concept measurements.
CONCLUSIONS
This shared ventilator function is proposed as a “last ditch” ventilatory assist device and not as a preferred ventilation mode. In a time of crisis where resources are limited, we introduce a system of multiple secondary breathing circuits driven by a ventilator in preference to that of simply splitting the breathing circuits, which have been shown to raise multiple risks for patients. It is our hope that neither approach will be needed.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Dr. Mashari is a Senior Scientific Advisor (pro bono) for Glia, nonprofit company making regulatory-approved open-source medical devices. Dr. Goligher has received personal fees and research equipment and from Getinge. Dr. Kacmarek is a consultant for Medtronic on Airway Care and a consultant for Orange Med on mechanical ventilators; he received a research grant from Orange Medical for ventilator comparison. Dr. Slutsky is a consultant for Baxter and Xenios. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Neyman G, Irvin CB. A single ventilator for multiple simulated patients to meet disaster surge. Acad Emerg Med. 2006; 13:1246–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paladino L, Silverberg M, Charchaflieh JG, et al. Increasing ventilator surge capacity in disasters: Ventilation of four adult-human-sized sheep on a single ventilator with a modified circuit. Resuscitation. 2008; 77:121–126 [DOI] [PubMed] [Google Scholar]
- 3.Beitler JR, Kallet R, Kacmarek R, et al. Columbia University College of Physicians & Surgeons and New York-Presbyterian Hospital.; 2020. Ventilator sharing protocol: Dual-patient ventilation with a single mechanical ventilator for use during critical ventilator shortages. Available at: https://www.gnyha.org/wp-content/uploads/2020/03/Ventilator-Sharing-Protocol-Dual-Patient-Ventilation-with-a-Single-Mechanical-Ventilator-for-Use-during-Critical-Ventilator-Shortages.pdf. Accessed April 7, 2020. [Google Scholar]
- 4.U.S. Food and Drug Administration: Appendix B. Authorized Ventilators, Ventilator Tubing Connectors, and Ventilator Accessories. 2020. Available at: https://www.fda.gov/media/136528/download. Accessed April 7, 2020.
- 5.Society of Critical Care Medicine: Society of Critical Care Medicine, American Association for Respiratory Care, American Society of Anesthesiologists, Anesthesia Patient Safety Foundation, American Association of Critical-Care Nurses, and American College of Chest Physicians: Joint Statement on Multiple Patients Per Ventilator. 2020. Available at: https://www.sccm.org/getattachment/Disaster/Joint-Statement-on-Multiple-Patients-Per-Ventilato/Joint-Statement-Patients-Single-Ventilator.pdf?lang=en-US. Accessed April 7, 2020.
- 6.Sommer DD, Fisher JA, Ramcharan V, et al. Improvised automatic lung ventilation for unanticipated emergencies. Crit Care Med. 1994; 22:705–709 [PubMed] [Google Scholar]
- 7.Branson RD, Blakeman TC, Robinson BR, et al. Use of a single ventilator to support 4 patients: Laboratory evaluation of a limited concept. Respir Care. 2012; 57:399–403 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


