Abstract
Purpose of Review
Older adults currently represent the fastest growing demographic of cannabis users, yet few studies have investigated the effects of cannabis use on cognitive functioning in aging. We conducted a systematic review of the recent literature examining cognitive outcomes associated with cannabis use in older adults, with and without neurocognitive disorders, to clarify the potential neuroprotective benefits and risks of cognitive decline in this population.
Recent Findings
We identified 26 studies examining cognitive outcomes associated with medical and recreational use of cannabis in healthy aging, dementia, Parkinson’s disease, multiple sclerosis, HIV, and pain populations. Although variability in the cannabis products used, outcomes assessed, and study quality limits the conclusions that can be made, modest reductions in cognitive performance were generally detected with higher doses and heavier lifetime use.
Summary
This review highlights the need for additional high-quality research using standardized, validated assessments of cannabis exposure and cognitive outcomes. Reliable measures and longitudinal data are necessary to better characterize the effects of cannabis use on cognitive aging, as well as differential effects of recreational and medical cannabis.
Keywords: cannabis, marijuana, aging, cognition, neuropsychological, neurodegenerative disease
Introduction
The current number of older adults in the United States using cannabis is at an unprecedented high. Data from large epidemiological studies show a 250% increase in the number of adults aged 65+ reporting past-year cannabis use between 2006 and 2013 [⚫1–3]. Current estimates of past-year use range from 2.9% to 9.0% of adults aged 65+ and 50–64, respectively [3]. This increase has been attributed to unique characteristics of the aging Baby Boomers, who report higher rates of recreational drug use than previous older generations [4], as well as recent trends in research and marketing of the medical use of cannabis to alleviate many health conditions that are common in aging. According to a recent analysis of U.S. state registry data, the three most common qualifying conditions for medical cannabis are chronic pain, multiple sclerosis (MS), and cancer-associated symptoms [5]. Many of the other common qualifying conditions are known to disproportionately affect older adults, including dementia, Parkinson’s disease (PD), arthritis, and glaucoma, although unfortunately the evidence for the efficacy in treating these conditions is limited [5]. As more older adults turn to cannabis as a remedy for many age-related conditions, it is critical to better understand the effects in this population to weigh the potential benefits of symptom management against the risks that are relevant in aging.
Although the adverse neurocognitive effects of cannabis use in adolescents and younger adults are well documented [6-⚫8], these effects have been understudied in aging populations. The acute effects of cannabis on memory, processing speed, and executive functions [8, 9] could be particularly detrimental to older adults who may have already experienced some degree of decline in these abilities with normal aging [10]. Moreover, due to age-related changes in drug metabolism and neurotransmitter sensitivity that can alter drug pharmacokinetics and pharmacodynamics, older adults may be more likely to experience exacerbated or prolonged side effects of medications [11, 12]. A 2014 review of the safety and efficacy of medical cannabinoids (i.e., bioactive chemical compounds that act on cannabinoid receptors) in older adults found that sedation and lethargy were the most common adverse effects reported, although cannabinoids were otherwise fairly well tolerated [12].
In opposition to concerns of cannabis exacerbating age-related cognitive decline, preclinical evidence suggests that the main constituents of cannabis (i.e., delta-9-tetrahydrocannabinol [THC] and cannabidiol [CBD]) may have neuroprotective properties in normal and pathological aging, particularly in Alzheimer’s disease (AD). Recent in vivo and in vitro studies have shown that chronic low doses of THC paradoxically reverse age-related cognitive dysfunction in old mice, promote hippocampal neurogenesis, and prevent neurodegenerative and neuroinflammatory processes in animal models of AD [13–17]. Clinical evidence of the neuroprotective benefits of cannabinoids in older adults and in dementia, however, is lacking. Nonetheless, AD and associated symptoms of dementia (e.g., agitation) are an approved indication for medical cannabis in nearly half of the U.S. states with medical cannabis laws [18]. Several recent reviews summarize the results of a few small clinical trials examining the efficacy of cannabinoids for neuropsychiatric symptoms in dementia, including AD [19–⚫21], concluding that there may be possible benefit but that small sample sizes and lack of methodological control in most studies limits the confidence in this evidence. Surprisingly, the effects on cognitive symptoms were not reported in any review, despite cognitive decline being the primary symptom in AD dementia. The risks and benefits of cannabis on cognition in neurodegenerative diseases, and in aging more broadly, need to be better understood.
The purpose of this systematic review was to examine the current literature on the effects of cannabis on cognitive functioning in older adults aged 50+ with and without neurocognitive disorders. We use a broad definition of cannabis to include whole-plant, purified, and synthetic cannabis-based products, regardless of the indication (i.e., recreational or medical use). We focus our review on recent studies published in 2014 or later, in accordance with journal guidelines and evidence of changes in potency (i.e., from 8.9% to 17.1% mean THC concentration) that limits comparisons with studies published prior to that time [22]. Our aim was to identify whether the current literature answers the question of whether cannabis use affects cognition in older adulthood, specifically whether these effects are adverse or favorable depending on the condition in question and indication. We conclude with comments and recommendations regarding study methodology, outcome measurement, and research priorities to address this growing public health concern.
Methods
A protocol for this review was registered with the International Prospective Register of Systematic Reviews (PROSPERO; registration number CRD42019140625). This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Statement.
Search Strategy
A research informationist with expertise in conducing systematic reviews developed detailed search strategies for PubMed, Scopus, PsycINFO, and Cochrane Library databases. The search strategies used a combination of terms related to the concepts of cannabis, cognition, and older adults (see Supplementary Material for full search strategy and syntax). All databases were searched from inception through June 3, 2019 for the sake of comprehensiveness. To identify additional articles, the reference lists of relevant articles and citing articles were hand searched.
Study Selection and Appraisal
The first two authors independently screened all titles and abstracts for relevance, then independently reviewed the full texts of potentially relevant papers to determine eligibility according to pre-specified criteria shown in Table 1. Title/Abstract Screening criteria were developed to maximize sensitivity of the search, while the Full-Text Review criteria optimized specificity. Disagreements at either stage were resolved by consensus. The same two authors independently extracted key data from the final list of included studies, which are outlined in the review protocol. We provide a qualitative synthesis of the study findings and risk of bias specifically for cognitive outcomes rather than individual studies as a whole. We comment on methodological strengths and weaknesses, including design, handling of confounding variables, completeness of reporting, and quality of outcome measures.
Table 1.
Criteria Used for Screening and Selection of Studies
| Title/Abstract Screening Criteria | Full Text Review Criteria | |
|---|---|---|
| Sample: | Must include human subjects or biological samples obtained from humans | Must include human subjects or biological samples obtained from humans |
| Population: | Must either (a) include subjects with a majority or mean age of 50+ or (b) include separate analysis of an older subsample or of aging effects. Age can either be stated explicitly in the title/abstract or considered likely given the population (e.g., AD, PD) or methodology used (e.g. population-based survey). | Must either (a) include subjects with a majority or mean age of 50+ or (b) include separate analysis of an older subsample or of aging effects |
| Intervention/Exposure: | Must mention either cannabis (or synonym) of any kind, the endocannabinoid system, or if all other criteria are met, the title/abstract may simply mention substance use of any kind | Must study either phytocannabinoids (e.g., herbal cannabis), synthetic cannabinoids (including those used medically for any indication), or endocannabinoids (e.g. anandamide) |
| Outcome: | Must mention cognitive functions that relate to functional capacity or impairment (i.e., not beliefs or biases toward cannabis use), or if all other criteria are met, the title/abstract does not need to mention cognition | Quantitative assessment of cognitive functions that relate to functional capacity or impairment (i.e., not beliefs or biases toward cannabis use) using either performance-based test (e.g., neuropsychological or cognitive screening test) or rating scale/questionnaire that assesses cognition separately from other domains (e.g., psychiatric or motor functioning) |
| Design: | Original empirical research (not a review, case study/series, or qualitative study) | Original empirical research (not a review, case study/series, or qualitative study) |
| Language: | Available in English | Available in English |
Results
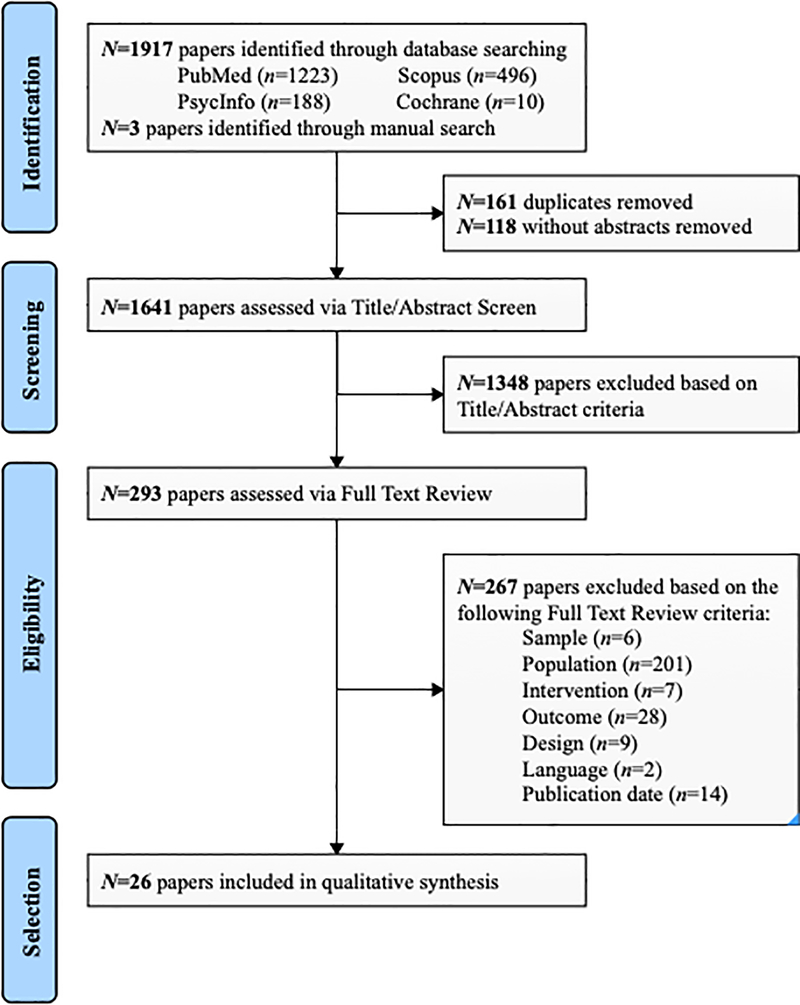
The study selection process and results of the search are presented in the PRISMA flowchart (Figure 1). We identified 1,641 unique papers with abstracts available. Of the 293 relevant papers that proceeded to full-text review, 26 were included in the final synthesis. Primary reasons for exclusion are shown in Figure 1. References for the 14 studies meeting all criteria but were published prior to 2014 are provided in the Supplementary Material.
Figure 1.
PRISMA flowchart
Table 2 presents key characteristics of the included studies, which are thematically grouped according to the population or condition under study: aging (n=7), dementia (n=6), Parkinson’s disease (PD; n=3), multiple sclerosis (MS; n=3), human immunodeficiency virus (HIV; n=4), and pain (n=3). Other than aging, all are considered qualifying conditions for medical cannabis in some U.S. states [5]. Thirteen studies examined cannabis used for medical purposes, two studied both recreational and medical use, and nine examined recreational use explicitly or did not specify. The specific cannabinoids assessed were herbal cannabis (Cannabis sativa or “marijuana”), natural isolated THC or THC/CBD (i.e., Namisol and nabiximols, respectively), and synthetic THC (i.e., dronabinol and nabilone). Two additional studies measured endogenous cannabinoids in vivo. Six studies were RCTs and the rest used a variety of observational or descriptive designs. Cognitive outcomes were assessed via a number of standardized neurocognitive tests (n=20) or patient-reported outcome measures (n=6). This heterogeneity precluded any meta-analysis.
Table 2.
Summary of Included Studies by Population or Condition (N=26)
| Author | Sample Size, Mean Age ± SD (range), % Male | Study Design | Cannabis Indication, Form, and Dose | Cognitive Outcome | Results / Cognitive Effects |
|---|---|---|---|---|---|
| Aging | |||||
| Sexton et al. (2019) [23] |
N=2,905 users; Older users (n=507): (50–80), 52.3% |
Cross-sectional survey | Recreational and medical Herbal cannabis (mixed THC and CBD), 91.3% inhaled |
Yes/no survey of “undesirable” acute cognitive effects | ↑ Older users reported fewer cognitive difficulties than younger users (p<0.001). However, this may be explained by medical use of cannabis, which was more common in older adults and associated with fewer subjective cognitive difficulties. |
| Kolla et al. (2016) [24] | N=5,080: 54.4 ± 16.5 (18–97); 53.4% | Cross-sectional survey (population-based sample) | Likely recreational (N/R) Herbal cannabis |
Adult ADHD Self-Report Version 1.1 Screener | ↓ effects for hyperactivity (OR=1.083) and impulsivity (OR=1.076) in men, and inattention (OR=1.129) in women |
| Auer et al. (2016) [25] |
N=3,499 at Year 25: Nonusers (n=533): 49.7 ± 3.9, 39.4% Ever users (n=2,852): 50.2 ± 3.6, 37.5% Current users (n=392) |
Longitudinal cohort Cognitive testing completed once at Year 25. |
N/R (likely recreational) Herbal cannabis (marijuana) |
1. Rey AVLT 2. DSST 3. Stroop-Interference (n=3,364 in cognitive analyses) |
1. ↓ verbal memory with current use and cumulative lifetime use. Every 5 years of use associated with 0.13 SD decrease in score 2. ↓ processing speed with current use, but not with cumulative use 3. ≈ executive functioning with current use or cumulative use |
| McKetin et al. (2016) [26] |
N=1,897 at baseline: Nonusers (n=4,986): 42.6 ± 1.5, 43% < weekly users (n=225): 42.3 ± 1.5, 58% ≥ weekly users (n=106): 42.7 ± 1.4, 70% |
Longitudinal cohort 4 and 8-year follow-up (n=1,653 at Year 8; mean age = 50) |
N/R (likely recreational) Herbal cannabis (marijuana/hash) |
1. CVLT 2. SDMT 3. Digit Backwards 4. Simple and choice reaction time tasks |
1. ↓ immediate memory for ≥ weekly users (−0.55 SD) 2. ≈ processing speed 3. ≈ working memory 4. ≈ reaction time No within-subject effects. Cannabis use was not associated with accelerated cognitive decline. |
| Burggren et al. (2018) [27] |
N=50: Former heavy users (n=24): 65.4 ± 7.2, 67.0% Nonusers (n=26): 67.7 ± 7.1, 54.0% |
Cross-sectional | Recreational Herbal cannabis (marijuana) |
1. WTAR 2. MMSE 3. SRT, WMS-II Logical Memory, Verbal Paired Associates, RCFT 4. TMT-A, Stroop-Word Reading, DSST 5. TMT-B, FAS, Animals, Stroop-Interference |
1. ↓ premorbid IQ 2. ≈ global cognition 3. ≈ immediate and delayed memory (d’s = −0.29 and −0.30) 4. ≈ processing speed (d =−0.36) 5. ≈ executive function (d =−0.07) Former heavy users < controls on all cognitive domains, but p’s > .05. |
| Thayer et al. (2019) [28] |
N=54; Users (n=28): 66.8 ± 5.3, 64% Nonusers (n=28): 69.8 ± 5.7, 39% |
Cross-sectional | Recreational Herbal cannabis (smoked and edibles) |
NIH Toolbox Cognition Battery (n=38; 28 users and 10 non-users) | ≈ global cognition. Users performed >0.5 SD below nonusers on working memory subtest, but this was non-significant after FDR correction (p=.05). |
| Ahmed et al. (2014) [29] | N=11; 72.1 ± 5.0, 55.0% | RCT (crossover; placebo-controlled) 8 weeks |
Medical Oral THC (Namisol) 3.0mg, 5.0mg, and 6.5mg |
Test for Attentional Performance – Alertness subtest | ≈ attention (p=.18); Effect size and raw data N/R |
| Dementia | |||||
| Ahmad et al. (2014) [30] |
N=18; AD (n=11): 71.8 ± 6.8, 27.3% Healthy (n=7): 68.0 ± 6.5, 42.9% |
Cross-sectional |
Endocannabinoids CB1 receptor availability via [18F]MK-9470 PET brain imaging |
1. MMSE 2. AVLT |
1. ≈ global cognition not associated with CB1 receptor availability 2. ≈ immediate and delayed memory not significantly associated with CB1 receptor availability (r2’s=.46 and .32, p’s>.05) |
| Altamura et al. (2015) [31] |
N=48; AD (n=37): 77.3 ± 6.4, 37.0% Healthy (n=11): 75.0 ± 3.6, 40.6% |
Cross-sectional |
Endocannabinoids AEA, 2-AG, PEA, and OEA in plasma |
1. Rey AVLT 2. Corsi’s test 3. Figure copy 4. Raven’s Progressive Matrices 5. Semantic fluency (n=37 AD) |
1. ↑ correlation between immediate memory and higher 2-AG (r=.334, p=.05) 2. ↑ correlation between attention and 2-AG (r=.423, p=.018) 3. ↓ correlation between constructional praxis and PEA (r=−.389, p=.019) 4. N/R 5. N/R Other results (e.g., AEA and OEA) were N/R. |
| Shelef et al. (2016) [32] | N=10 with AD: 73.2 ± 8.6, 60.0% | Single-group cohort; Open-label trial | Medical Cannabis oil (THC extract 1.65% potency) 2.5mg 2x/day titrated |
MMSE | ≈ global cognition; MMSE scores were stable from baseline (M=10.3) to Week 4 (M= 11.0) of treatment |
| van den Elsen et al. (2015) [33] | N=12 with AD, vascular, or mixed (selected from n=22 in RCT): 76.4 ± 5.3, 68%. | Single-group cohort; Open-label extension | Medical Oral THC (Namisol) mean 3.0mg/day; titrated |
1. MMSE (n=5 due to attrition [58.3%]) 2. Delirium Observation Scale |
1. ≈ global cognition over the 6-month duration of the extension study (p=.96); no comparison to baseline. 2. N/R |
| Herrmann et al. (2019) [34] | N=38 with AD: 87 ± 10, 77% | RCT (crossover; placebo-controlled) 14 weeks | Medical Synthetic oral THC (Nabilone) 1–2mg/day |
1. Standardized MMSE 2. SIB (n=25 with MMSE≤15) |
1. ↑ global cognition (b=1.1, 95% CI [0.1, 2.0], p=.026) 2. ↓ global cognition in the subgroup with severe dementia (b=−4.6, 95% CI [−7.3, −1.8], p=.003) |
| van den Elsen et al. (2015) [35] |
N=50 with AD, vascular, or mixed; THC (n=24): 79 ± 8; 45.8% Placebo (n=26): 78 ± 7, 53.8% |
RCT (parallel groups; placebo-controlled) 3 weeks |
Medical Oral THC (Namisol) 1.5mg 3x/day |
1. WMS-R Paired Associate Learning (n=18) | 1. ≈ episodic memory; THC and placebo groups showed similar decline from baseline (p=1.0). |
| Parkinson’s Disease (PD) | |||||
| Balash et al. (2017) [36] |
N=47: 64.2 ± 10.8, 85.1% 100% users |
Single-group retrospective survey | Medical Herbal cannabis (mixed THC and CBD; 81% smoked) mean 0.9g/day |
1. mCGI – Memory (n=40) 2. mCGI – Attention (n=42) |
1. ≈ self-reported memory (r2 = 0.04, p>0.05) 2. ↑ self-reported attention (r2 = 0.11, p=.01) |
| Kindred et al. (2017) [37] |
N=454i; Users (n=36.6%): 60 ± 9.2, 60.6% Nonusers (n=66.3%): 61.7 ± 9.5, 56.3% |
Cross-sectional survey | Medical and recreational Herbal cannabis (smoked and edibles) |
Guy’s Neurological Disability Scale | ↑ self-reported memory; Current users reported fewer problems with memory (F=4.717, p=.030). |
| Chagas et al. (2014) [38] |
N=21; CBD 75 (n=7): 65.9 ± 10.6, 71% CBD 300 (n=7): 63.4 ± 6.5; 71% Placebo (n=7): 67.3 ± 7.2, 71% |
RCTii (parallel groups; placebo-controlled) 6 weeks |
Medical CBD capsules 75mg or 300mg/day |
PDQ-39 – Cognition scale | ≈ self-reported cognitive functioning; No differences between placebo, 75mg, and 300mg (p=.332) |
| Multiple Sclerosis (MS) | |||||
| Kindred et al. (2018) [39] |
N=16; Users (n=8): 49.6 ± 15.0, 25% Nonusers (n=8): 50.8 ± 13.2, 25% |
Cross-sectional | Medical Herbal cannabis (mixed THC and CBD; smoked and edibles) |
PASAT, as part of the MSFC | ↓ working memory (p=.02) after an 8-hour abstinence period PASAT scores did not correlate with regional standardized uptake values from [18F]-FDG-PET. |
| Castelli et al. (2019) [40] |
N=22; Continuers (n=11): 49.4 ± 7.4, 45.5% Quitters (n=11iii): 50.1 ± 9.3, 36.4% |
Longitudinal cohort 0, 1, 3, and 12-month follow-ups |
Medical THC/CBD oral spray (nabiximols) median 6 puffs |
Stroop Color-Word Test under single and dual-task conditions | ↓ executive functioning in the dual-task condition (time × group η2=0.14) but ≈ in single-task condition (η2=0.06) |
| Ball et al. (2015) [41] |
N=493; THC (n=329): 52.3 ± 7.6, 40.4% Placebo (n=164): 51.8 ± 8.2, 41.5% |
RCT (parallel groups; placebo-controlled) 3 years |
Medical Synthetic oral THC (dronabinol) median 4 capsules/day, titrated |
PASAT, as part of the MSFC | ≈ working memory; mean annual change equivalent between groups (z-score diff. = −0.01, 95% CI [−0.01, 0.09], p=.92) |
| Human Immunodeficiency Virus (HIV) | |||||
| Schouten et al. (2016) [42] |
N=103: median 54.0 [49–62], 93% 16% used daily to monthly |
Cross-sectional | Likely recreational (N/R) Herbal cannabis (marijuana) |
Neuropsychological test battery of fluency, attention, processing speed, memory, motor, and executive functions | ↓ global cognition; Daily to monthly cannabis use was associated with increased risk of cognitive impairment (OR=27.76 [4.61, 167.18], p<0.001). |
| De Francesco et al. (2019) [43] |
N=1253; Older HIV+ (n=637): 56 [53–62], 88.5%; 14.3% users Younger HIV+ (n=340): 43 [37–47], 80.9% HIV− (n=276): 58 [53–63], 65.2% |
Cross-sectional | Recreational Herbal cannabis (marijuana and hashish) |
CogState computerized test battery | ↓ global cognition associated with use of hashish after adjusting for confounding variables (median z-score difference between users and non-users = −0.29, 95% CI [−0.49, −0.09], p=.005) ≈ association with marijuana (median z-score difference = 0.04, 95% CI [0.06, 0.14], p=.43) |
| Saloner et al. (2019) [44] |
N=734: 55.1 ± 4.0, 84.2%; 31.6% with lifetime cannabis use disorder |
Cross-sectional | Likely recreational (N/R) Herbal cannabis (marijuana) |
Neuropsychological test battery using Super Ager criteria based on global performance that is comparable to 25-year-olds | ↑ global cognition; Lifetime cannabis use disorder (in addition to younger age, higher verbal IQ, absence of diabetes, and fewer depression symptoms) increased the likelihood of being classified as a Super Ager vs. Cognitively Impaired (OR=0.46, 95% CI [0.28, 0.75], p=.002). |
| Okafor et al. (2019) [45] |
N=788 HIV+ men; Users (n=290): 41.6 ± 10.0, 100% Nonusers (n=498): 44.6 ± 9.7, 100% |
Longitudinal cohort Up to 17 years |
Likely recreational (N/R) Herbal cannabis (marijuana) |
1. TMT-A and SDMT 2. TMT-B |
1. ↓ processing speed; current daily use associated with 0.41%–0.70% annual decline (f2<0.002); Non-significant effect of cumulative use 2. ≈ cognitive flexibility for current and cumulative use |
| Pain | |||||
| Zaki et al. (2017) [46] | N=164 with cancer: 54.9 (70.7% age 50+), 56.1% | Single-group survey with 4-month follow-upiv | Medical N/R (supplied by a single provider) |
Adaptive survey designed by the cannabis supplier (n=24 who perceived concentration as relevant) | ≈ self-reported concentration |
| Bar-Sela et al. (2019) [47] |
N=34 with advanced cancer; Users (n=17): 63 (35–85), 31% Nonusers (n=17): 63 (40–85), 59% |
Longitudinal cohort 3-month follow-up |
Medical Herbal cannabis (mixed products; smoked, inhaled, or oil) |
1. MoCA 2. DSST |
1. ≈ global cognition; 35% cases vs. 18% controls had clinically significant decline (i.e., ≥0.5 SD) 2. ≈ processing speed |
| Wallace et al. (2015) [48] | N=16 with diabetic neuropathy: 56.9 ± 8.2, 56.0% | RCT (crossover; placebo-controlled) 8 weeks |
Medical Vaporized THC: low (1%), medium (4%), or high (7%) doses |
1. PASAT 2. TMT-B 3. TMT-A |
1. ↓ working memory at medium (d=−1.03) and high doses (d=−1.14) 2. ↓ executive functioning at high dose only (d=−1.15) 3. ≈ processing speed |
Notes: Ages are provided as mean ± standard deviation, median [interquartile range], or (range). ↑ = positive effect of cannabis on cognition; ↓ = negative effects of cannabis on cognition; ≈ = non-significant effect of cannabis on cognition; 2-AG = anandamide 2-arachidonoyl-sn-flycerol; AEA = anandamide; AD = Alzheimer’s disease; AVLT = Auditory Verbal Learning Test; CVLT = California Verbal Learning Test; mCGI = modified 5-point Clinical Global Impressions Scale; D-KEFS = Delis-Kaplan Executive Function System; DSST = Digit Symbol Substitution Test; MoCA = Montreal Cognitive Assessment; MSFC = Multiple Sclerosis Functional Composite; N/R = not reported; PASAT = Paced Auditory Serial Addition Test; PDQ-39 = Parkinson’s Disease Questionnaire; OEA = N-oleoyl-ethanolamide; PEA = N-palmitoyl-ethanolamide; RCFT = Rey Complex Figure Test; SDMT = Symbol Digit Modalities Test; SIB = Severe Impairment Battery; SRT = Buschke-Fuld Selective Reminding Test; TMT = Trail Making Test; WAIS = Wechsler Adult Intelligence Scale; WMS-R = Wechsler Memory Test-Revised; WTAR = Wechsler Test of Adult Reading.
We do not review results for the MS subsample from this study due to their mean age <50 years.
The authors referred to the design of this study as an “exploratory double-blind trial,” but stated that “patients were randomly assigned to three groups in accordance with the matching variables [gender, age and total UPDRS score].” No other details were provided on the randomization process.
The “quitters” group was made up of patients discontinuing Nabiximols for the following reasons: confusion (n=5), loss of efficacy (n=4), and drowsiness (n=2).
All participants had active cannabis prescriptions at baseline, although only 73.8% reported current use of cannabis at baseline (and most [56.3%] reported previous cannabis use. The authors note that it was unknown whether patients continued to fill cannabis prescriptions at the 4-month follow-up.
Aging
We identified seven studies focused on cannabis use in healthy aging, with varying designs and methodologies (i.e. 4 cross-sectional studies, 2 longitudinal cohort studies, and 1 RCT). In accordance with preclinical evidence suggesting that the endocannabinoid system plays a neuroprotective role in aging [49], findings from one large cross-sectional survey of recreational and medical cannabis users in Washington state suggest that cannabis’ adverse effects on cognition may be attenuated in older adults, who endorsed fewer undesirable acute cognitive effects than younger adults [23]. This finding, however, is likely mediated by the fact that older users were more likely to use cannabis for medical purposes, which was also associated with better cognitive outcomes, possibly related to alleviation of primary symptoms. Another large population-based survey of healthy adults ages 18–97 found that problematic cannabis use was associated with symptoms of attention-deficit hyperactivity disorder (ADHD), including impulsivity and hyperactivity in men and inattention in women [24], although no separate analysis of aging effects was conducted. Given the broad age range in this study, these findings may not be representative of older age groups.
Two cross-sectional neuroimaging studies failed to find statistically significant differences between older users (current or former) and nonusers on neurocognitive tests [27, 28]. In the study of current regular users, Thayer et al. [28] found no significant group differences in gray or white matter volumes or in any cognitive domains, despite users scoring lower than nonusers on some tests of fluid cognition (e.g., working memory) [50]. These cognitive analyses were also underpowered, as cognitive data was only available in a subsample (n=10) of nonusers. In a study by Burggren et al. [27], former heavy users were found to have thinner cortical hippocampal subfields than nonusers after adjusting for premorbid IQ, which was significantly lower among former users. Group differences on neurocognitive measures fell short of statistical significance, despite former users performing lower on average than nonusers on all domains. The authors interpreted these structural differences as possibly reflecting residual effects of prior cannabis use but acknowledged the possibility that other pre-existing factors may account for the observed differences between groups.
Two large longitudinal studies assessed the effects of recreational cannabis use on neurocognitive functioning using validated tests of memory, processing speed, and executive functioning into the fifth and sixth decades of life [25, 26]. In a cohort of over 3,000 young adults who were followed for 25 years, Aeur and colleagues [25] found that past exposure to cannabis was associated with worse verbal memory at the Year 25 visit in a dose-dependent manner, with every 5 years of use corresponding to a 0.13 SD decrement in verbal memory. Notably, neurocognitive testing was only completed once at Year 25, so changes over time could not be determined. The absence of serial neurocognitive data makes it impossible to determine whether these subtle cognitive differences existed before the onset of cannabis use. McKetin et al. (2016) [26] recruited a sample of middle-aged Australians (age 40–46 at baseline) who completed cognitive testing at three time points four years apart, allowing for examination of both between- and within-subject effects. Akin to findings from the study by Auer and colleagues, “at least weekly” users performed lower than nonusers on a test of verbal memory, although there were no significant within-person effects regarding changes in neurocognitive performance over time. Neither study found any effect processing speed or executive functioning after adjusting for relevant confounding variables. Together, these results suggest that heavy cannabis use is associated with modest deficits in verbal memory, but that this does not lead to accelerated cognitive decline.
One small crossover RCT assessing the safety and pharmacokinetics of single doses of oral THC in adults age 65+ with no psychiatric history found no effects on a test of attention [29], However, given the small sample (n=11), abbreviated cognitive assessment, and lack of quantitative data (i.e., only p value was reported), this study offers limited conclusions about the broader impact of cannabis use on cognitive function.
Dementia
Dementia is characterized by a decline in cognitive functioning that is severe enough to compromise one’s independence in activities of daily living. The risk of developing dementia doubles every 5 years after age 65 [51, 52]. AD is the most common cause of dementia, accounting for 60–80% of cases in older adulthood [53]. Cannabis has been explored at length in dementing diseases, particularly AD [54–56]. Preclinical AD studies exploring the polyvalent and synergistic effects activating pro-cognitive CB1 and anti-inflammatory CB2 receptors [57] in the endocannabinoid system, and indicate that cannabinoids may reduce excitotoxicity and formation of amyloid plaques and tau phosphorylation, which are the neuropathological hallmarks of AD [56, 58, 59]. Assaying this system clinically, however, remains a challenge. Of the six studies of dementia that we found, two studies examined associations between endogenous ligands and receptors and cognitive function in AD patients and controls [30, 31]. In a positron emission tomography (PET) study of CB1 receptor availability throughout the brain, Ahmad et al. [30] found no correlations between CB1 receptor availability and cognitive abilities or regional amyloid beta plaque density, and no differences between AD patients and controls, consistent with other studies of post-mortem AD brain tissue [60]. In contrast, Altamura et al. [31] found that endocannabinoids (e.g., anandamide [AEA], anandamide 2-arachidonoyl-sn-flycerol [2-AG]) assayed through plasma were differentially correlated with memory, attention, and praxis in AD patients, as well as indicators of cardio- and cerebrovascular disease. Although preliminary, these findings suggest that assaying circulating levels of endogenous ligands (e.g., AEA) may identify meaningful cognitive effects, but these analyses should take into account concurrent, systemic changes associated with aging including vascular disease and inflammation.
Although there are numerous clinical trials of cannabinoids in non-cognitive symptoms of dementia [20, 21], strikingly few studies have assessed their effects on cognition. Two small open-label studies without control groups reported stability in MMSE scores following a 4-week trial of medical cannabis oil [32] and a 6-month extension study of Namisol [33]. The lack of comparison groups, lack of adjustment for confounding variables (e.g., concurrent psychotropic medications), brevity of cognitive assessments, and attrition in the extension study limit the ability to detect possible subtle effects on cognition.
Two larger placebo-controlled RCTs of synthetic THC in patients with moderate to severe dementia (i.e., Nabilone [34] and Namisol [35]) identified similar non-clinically significant effects on cognition (i.e. MMSE or a more challenging verbal episodic memory test), although there was evidence for adverse effect of Nabilone on cognition in a subset of participants with severe dementia in the former trial. To echo a recent and broader review of the safety and effectiveness of cannabinoids in dementia, evidence from cross-sectional studies may provide continued impetus to pursue therapeutic cannabinoids but future studies must address the many limitations of the RCTs to date, including variability in trial design and outcome measures, underpowered analyses, and limited bioavailability of the formulations [21].
Parkinson’s Disease
PD is the second most common neurodegenerative disease after AD, affecting 1–2% of individuals over age 60 [61]. Although PD is primarily characterized by progressive motor symptoms (e.g., bradykinesia, rigidity, resting tremor), cognitive symptoms become more prevalent with disease progression, with up to 75% of PD patients developing dementia at later stages [62, 63]. Recently, cannabinoids have been investigated as neuroprotective agents for PD, in light of evidence that alterations in the endocannabinoid system may be implicated in the pathogenesis of PD [64]. However, clinical studies investigating cannabinoids for the treatment of motor and non-motor symptoms are sparse and evidence supporting their use is inconclusive [65].
We identified two surveys [36, 37] and one small clinical trial [38] that found mixed non-significant to small positive effects on patients’ subjective impressions of cognitive functioning. One large anonymous web-based survey posted on a national PD foundation webpage found that users reported fewer problems with memory than nonusers [37]. The users in this sample consisted of both recreational and medical users, although less than half of those reporting medical use possessed a medical marijuana card. Another retrospective telephone survey of PD patients who were prescribed medical cannabis from two clinics in Israel found that most patients reported no change in memory after 3 months of cannabis treatment [36]. Notably, this sample included patients at all stages of the disease (Hoehn & Yahr stages I-IV), 72% of whom reported memory impairment at baseline. In the absence of any corroborative reports from a spouse or caregiver, the reliability of these responses is seriously questioned. Finally, one exploratory, double-blind, placebo-controlled trial assessing the effects of CBD on motor and non-motor symptoms in PD found no subjective effect on cognition [38], as assessed by items on a self-report measure of quality of life. Data was collected at baseline and after 6 weeks of treatment with CBD at 75mg/day, 300mg/day, or placebo (n=7 in each group).
The reliance on subjective patient-reported surveys to assess cognitive functioning in PD studies is particularly problematic. Subjective reports of cognitive function are poorly correlated with performance-based testing in older adults [66, 67], particularly when it requires retrospective recall of cognitive functioning from months earlier. Thus, future work in PD could benefit from incorporating an objective measure of cognitive functioning that has been validated in this population. Additionally, corroborating reports from a spouse or caregiver would help to clarify effects on cognition.
Multiple Sclerosis
MS is a chronic demyelinating and neurodegenerative disease that has an extensive literature base on the symptom-modifying effects of cannabis use [68] to the extent that the American Academy of Neurology determined that there is moderate to high-quality evidence to support the use of cannabis for MS-associated spasticity and pain [69]. Nonetheless, MS is associated with mild cognitive impairments in processing speed and working memory, which may be worsened by cannabis use based on studies using small samples (n’s < 30) with mean ages below 50 years [70].
We identified three studies in which cognitive effects of medical cannabis were examined in MS patients with a mean age of 50 years. Two small observational studies identified small negative effects on cognitive performance [39, 40]. A cross-sectional study found that users (who were prescribed herbal cannabis products with varying amounts of THC and CBD) performed worse than nonusers on a test of working memory after an 8-hour abstinence period but showed no differences in resting brain glucose uptake using FDG-PET scan [39]. A prospective open-label study comparing patients who continued treatment with nabiximols (THC/CBD oromucosal spray) and those who discontinued due to side effects found that “continuers” performed worse on tests of balance and executive functioning under dual task conditions [40]. These effects were attributed to nabiximols, although the design of the study inherently raises the possibility that additional factors that differ systematically between groups may account for the observed differences. Finally, one large high-quality RCT studying the long-term effects of oral THC showed no effect on a test of working memory over the 3-year duration of the trial [41]. Strengths of the studies in MS include the use of objective neurocognitive tests that are validated and predictive of functional outcomes in this population [71].
Human Immunodeficiency Virus
Although the use of combination antiretroviral therapy has reduced many of the neurological comorbidities associated with HIV, nearly half of the HIV-positive population eventually develop HIV-associated neurocognitive disorders (HAND) [72]. However, the degree to which HAND in aging populations reflects disease progression as opposed to lifestyle factors is difficult to disentangle due to the high prevalence of concurrent substance use disorders in this population. As many as one quarter of patients report current cannabis use [73] for recreational and medical purposes to treat associated symptoms such as neuropathic pain, anorexia, nausea, and psychiatric symptoms.
We identified four large observational studies that sought to account for the variability in cognitive functioning in aging HIV samples [42–45]. Three cross-sectional studies examining the effects of cannabis or “marijuana” use on global neurocognitive performance yielded discrepant results. One study found daily to monthly cannabis use, along with cardiovascular disease, renal dysfunction, and diabetes, independently predicted mild cognitive impairment [42], while a larger study of 977 HIV+ and 276 HIV- controls [43] found that only use of hashish, but not marijuana, remained significantly associated with cognitive impairment after adjusting for multiple relevant factors (e.g., age, gender, ethnicity education, HIV status, depression, and alcohol use). In contrast, a third cross-sectional study found that diagnosis of lifetime cannabis use disorder was associated with better cognitive functioning on a standard neuropsychological test battery using criteria for “neurocognitive superaging” [44]. However, this association may be explained by differences in premorbid IQ, which was a stronger predictor of cognitive “superaging” (OR=0.95) than cannabis use (OR=0.46).
A recent longitudinal study by Okafor et al. [45] assessed current and cumulative effects of cannabis in a cohort of 788 HIV+ men over a 17-year period. In this sample, only current daily cannabis use, but not cumulative use, was associated with worse cognitive processing speed. No effects were found on a test of executive functioning. The authors indicated that the magnitude of this effect was very small and unlikely to be of clinical significance.
Strengths of the studies on cannabis use in the HIV population are the large sample sizes and use of validated neuropsychological tests to measure cognitive outcomes. Limitations involved the imprecise methods used to ascertain cannabis exposure, which generally relied on retrospective self-report. Furthermore, the discrepant findings across studies highlights the importance of adequately controlling for confounding variables, including premorbid factors that could account for any differences between cannabis users and nonusers.
Pain
Relief from chronic pain is by far the most common condition cited by patients for the medical use of cannabis [5]. A systematic review of cannabinoids for medical use found moderate-quality evidence supporting their use, particularly for neuropathic pain [74]. We identified two observational studies in patients experiencing pain and side effects from cancer, both finding no effects of medical cannabis on cognition. One survey included patients who were all prescribed medical cannabis by a single provider and found no change in subjective concentration ability after 4 months of treatment [46]. However, the risk of bias in this study was high for several reasons described in Table 2, including the selection of the subset of participants who were asked about concentration, the unclear ascertainment of cannabis exposure (e.g., with 74% already reporting cannabis use at baseline), and importantly, the fact that the cannabis supplier/provider designed the survey and selected the sample. Another small cohort study with advanced cancer patients undergoing chemotherapy who were “cases” taking medical cannabis and “controls” found no effects on neurocognitive performance at the 3-month follow-up [47]. Cannabis users abstained from use 12 hours prior to cognitive assessment, so it is unclear whether any acute effects of THC would be masked. Additionally, the generalizability of this study may be limited by the varying amounts of THC and CBD in the cannabis products, the high attrition rate (63% of cases and 37% of controls), and participants’ medical comorbidities and chemotherapy side effects.
A crossover RCT assessing the acute effects of vaporized THC in patients with painful diabetic neuropathy found modest negative effects on two tests of working memory and executive functioning at higher doses [48]. No effects were found on a test of processing speed. Participants repeated cognitive testing multiple times over a 3-hour period in four sessions that were two weeks apart, so it is unclear how fatigue or practice effects from the repeated administrations were considered, although the placebo control likely reduces this as a limitation.
Discussion
We reviewed the evidence from 26 recent studies (6 RCTs) examining cognitive outcomes associated with cannabis and cannabinoids in older adults, including those aging normally, with neurodegenerative diseases, and other common medical conditions. Across the different populations assessed, the quality of studies and their associated findings were quite mixed. Much of the variability in outcomes may be accounted for by the different designs, cannabinoids examined, outcome measures used, and sample characteristics. Cognitive analyses were underpowered in several studies, so it is possible that larger sample sizes would have detected small effects. Although this heterogeneity limits the overall confidence of this evidence base, it is clearer from RCTs and longitudinal cohort studies using objective tests of cognitive functioning, that higher doses and heavier use of cannabis are associated with modest negative effects. These findings are consistent with other systematic reviews and meta-analyses showing similar small adverse effects on cognition in younger populations [⚫8,9]. With that said, there was also evidence of modest improvements in subjective cognition on patient-reported outcome measures, particularly for medical cannabis users.
Importantly, cannabis is not one “thing.” The individual constituents of cannabis (e.g., whole plant or isolated compounds), route of administration, and indication of use (i.e., medical vs. recreational) all are important factors that can differentially influence cognitive outcomes. Furthermore, certain individuals or subgroups (e.g., with existing cognitive impairment) may be more vulnerable to these effects than others. In this review, we broadly defined cannabis to include any type of cannabinoid, including those produced synthetically for medical use. However, there is evidence to suggest that the cognitive deficits associated with heavy, recreational cannabis may not be applicable to medical cannabis users, who may use products with less THC and experience relief from other symptoms [75], which may contribute to improved cognitive functioning.
This review highlights the scarcity of high-quality studies examining cognitive effects of cannabis use in older adults. There are many political, ethical, and logistical challenges to conducting cannabis research, particularly for controlled trials. However, larger methodologically rigorous trials and longitudinal studies that assess cognition prior to the initiation of cannabis use are greatly needed to infer a causal relationship. This need is highlighted by the fact that many negative effects on cognition that were reported by studies in this review decreased after adjusting for confounding variables (e.g., differences in premorbid IQ for users and nonusers). Further research is needed to clarify the differential effects of cannabis used for medical and recreational purposes. Additionally, future studies should adopt standardized measures of cannabis exposure history (e.g., frequency, duration, magnitude) that take into account differences in route of administration, constituent composition, and purpose of use (i.e., medical vs. recreational) to enable better comparison across studies.
Although we used a systematic search process that optimized sensitivity, we may not have identified all relevant studies. Several limitations must be considered when interpreting the evidence reviewed in this paper. First and foremost, medical and recreational use of cannabis are not the same and their effects on cognition should not be assumed to be equivalent. Adding to this variability are the different constituents of cannabis studied, including whole plant cannabis and isolated compounds. Due to the small number of high-quality comparative studies available, we placed minimal restrictions on study design and included studies of varying designs and quality. This heterogeneity precluded any meta-analysis and subsequently limits the overall confidence we have in the cognitive effects of cannabis use in older populations. Furthermore, several studies included subjects that may be more appropriately described as middle aged, with a mean age of around 50 years, and these results may be less applicable to the oldest of older adults.
Conclusion
We conducted a comprehensive systematic search of the recent literature on cognitive outcomes associated with cannabis use and cannabinoids in adults age 50 and older. Although there is evidence of modest negative effects on cognition in this population, larger controlled trials using validated outcome measures are greatly needed to better understand the role of cannabinoids in cognitive aging, as small sample sizes and variability in study designs limit our ability to draw definitive conclusions at this time.
Supplementary Material
Footnotes
Conflict of Interest:
Andreana Benitez, Emmi Scott, and Emily Brennan declare that they have no conflicts of interest.
Studies With Human or Animals Subjects:
This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
Papers of particular interest, published recently, have been highlighted as:
⚫ Of importance
No studies of major importance were identified; the highest quality in this review either did not directly address cognitive outcomes or did not focus on older adults.
- 1.Lloyd SL, Striley CW (2018) Marijuana Use Among Adults 50 Years or Older in the 21st Century. Gerontol Geriatr Med 4:233372141878166.This paper reviews the epidemiological literature from 2000 to 2017 on marijuana use among older adults, and shows that adults ages 65 and older have had the greatest increase in marijuana use over time.
- 2.Han BH, Sherman S, Mauro PM, Martins SS, Rotenberg J, Palamar JJ (2017) Demographic trends among older cannabis users in the United States, 2006–13: Cannabis use among older adults. Addiction 112:516–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han BH, Palamar JJ (2018) Marijuana use by middle-aged and older adults in the United States, 2015–2016. Drug Alcohol Depend 191:374–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colliver JD, Compton WM, Gfroerer JC, Condon T (2006) Projecting drug use among aging baby boomers in 2020. Ann Epidemiol 16:257–265 [DOI] [PubMed] [Google Scholar]
- 5.Boehnke KF, Gangopadhyay S, Clauw DJ, Haffajee RL (2019) Qualifying Conditions of Medical Cannabis License Holders in the United States. Health Aff Proj Hope 38:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crane NA, Schuster RM, Fusar-Poli P, Gonzalez R (2013) Effects of Cannabis on Neurocognitive Functioning: Recent Advances, Neurodevelopmental Influences, and Sex Differences. Neuropsychol Rev 23:117–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorey C, Kuhns L, Smaragdi E, Kroon E, Cousijn J (2019) Age-related differences in the impact of cannabis use on the brain and cognition: a systematic review. Eur Arch Psychiatry Clin Neurosci 269:37–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nader DA, Sanchez ZM (2018) Effects of regular cannabis use on neurocognition, brain structure, and function: a systematic review of findings in adults. Am J Drug Alcohol Abuse 44:4–18This systematic review examines recent studies on the effects of regular cannabis use on cognition, brain structure, and function in adults, which show that regular cannabis use is associated with mild cognitive and brain changes in adults. This review highlights important questions that remain regarding whether or not these changes are consequent to or precede the onset of cannabis use.
- 9.Broyd SJ, van Hell HH, Beale C, Yücel M, Solowij N (2016) Acute and Chronic Effects of Cannabinoids on Human Cognition—A Systematic Review. Biol Psychiatry 79:557–567 [DOI] [PubMed] [Google Scholar]
- 10.Harada CN, Natelson Love MC, Triebel KL (2013) Normal cognitive aging. Clin Geriatr Med 29:737–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corsonello A, Pedone C, Incalzi RA (2010) Age-related pharmacokinetic and pharmacodynamic changes and related risk of adverse drug reactions. Curr Med Chem 17:571–584 [DOI] [PubMed] [Google Scholar]
- 12.van den Elsen G a. H, Ahmed AIA, Lammers M, Kramers C, Verkes RJ, van der Marck MA, Rikkert MGMO (2014) Efficacy and safety of medical cannabinoids in older subjects: a systematic review. Ageing Res Rev 14:56–64 [DOI] [PubMed] [Google Scholar]
- 13.Suliman NA, Taib CNM, Moklas MAM, Basir R (2018) Delta-9-Tetrahydrocannabinol (Δ9-THC) Induce Neurogenesis and Improve Cognitive Performances of Male Sprague Dawley Rats. Neurotox Res 33:402–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin-Moreno AM, Brera B, Spuch C, Carro E, Garcia-Garcia L, Delgado M, Pozo MA, Innamorato NG, Cuadrado A, de Ceballos ML (2012) Prolonged oral cannabinoid administration prevents neuroinflammation, lowers beta-amyloid levels and improves cognitive performance in Tg APP 2576 mice. J Neuroinflammation 9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramirez BG, Blazquez C, Gomez del Pulgar T, Guzman M, de Ceballos ML (2005) Prevention of Alzheimer’s disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci 25:1904–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bilkei-Gorzo A, Albayram O, Draffehn A, et al. (2017) A chronic low dose of Δ9-tetrahydrocannabinol (THC) restores cognitive function in old mice. Nat Med 23:782–787 [DOI] [PubMed] [Google Scholar]
- 17.Sarne Y, Toledano R, Rachmany L, Sasson E, Doron R (2018) Reversal of age-related cognitive impairments in mice by an extremely low dose of tetrahydrocannabinol. Neurobiol Aging 61:177–186 [DOI] [PubMed] [Google Scholar]
- 18.Maust DT, Bonar EE, Ilgen MA, Blow FC, Kales HC (2016) Agitation in Alzheimer Disease as a Qualifying Condition for Medical Marijuana in the United States. Am J Geriatr Psychiatry 24:1000–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu CS, Chau SA, Ruthirakuhan M, Lanctôt KL, Herrmann N (2015) Cannabinoids for the Treatment of Agitation and Aggression in Alzheimer’s Disease. CNS Drugs 29:615–623 [DOI] [PubMed] [Google Scholar]
- 20.Weier M, Hall W (2017) The Use of Cannabinoids in Treating Dementia. Curr Neurol Neurosci Rep. doi: 10.1007/s11910-017-0766-6 [DOI] [PubMed] [Google Scholar]
- 21.Hillen JB, Soulsby N, Alderman C, Caughey GE (2019) Safety and effectiveness of cannabinoids for the treatment of neuropsychiatric symptoms in dementia: a systematic review. Ther Adv Drug Saf 10:204209861984699.This systematic review comprehensively examines the evidence of the effectiveness and safety of cannabinoids in the treatment of neuropsychiatric symptoms of dementia. This paper highlights limitations in the current evidence base and provides recommendations for conducting future trials.
- 22.Chandra S, Radwan MM, Majumdar CG, Church JC, Freeman TP, ElSohly MA (2019) New trends in cannabis potency in USA and Europe during the last decade (2008–2017). Eur Arch Psychiatry Clin Neurosci 269:5–15 [DOI] [PubMed] [Google Scholar]
- 23.Sexton M, Cuttler C, Mischley LK (2019) A Survey of Cannabis Acute Effects and Withdrawal Symptoms: Differential Responses Across User Types and Age. J Altern Complement Med 25:326–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolla NJ, van der Maas M, Toplak ME, Erickson PG, Mann RE, Seeley J, Vingilis E (2016) Adult attention deficit hyperactivity disorder symptom profiles and concurrent problems with alcohol and cannabis: sex differences in a representative, population survey. BMC Psychiatry 16:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Auer R, Vittinghoff E, Yaffe K, et al. (2016) Association Between Lifetime Marijuana Use and Cognitive Function in Middle Age: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. JAMA Intern Med 176:352–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKetin R, Parasu P, Cherbuin N, Eramudugolla R, Anstey KJ (2016) A longitudinal examination of the relationship between cannabis use and cognitive function in mid-life adults. Drug Alcohol Depend 169:134–140 [DOI] [PubMed] [Google Scholar]
- 27.Burggren AC, Siddarth P, Mahmood Z, London ED, Harrison TM, Merrill DA, Small GW, Bookheimer SY (2018) Subregional Hippocampal Thickness Abnormalities in Older Adults with a History of Heavy Cannabis Use. Cannabis Cannabinoid Res 3:242–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thayer RE, YorkWilliams SL, Hutchison KE, Bryan AD (2019) Preliminary results from a pilot study examining brain structure in older adult cannabis users and nonusers. Psychiatry Res Neuroimaging 285:58–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed AI, van den Elsen GA, Colbers A, van der Marck MA, Burger DM, Feuth TB, Rikkert MG, Kramers C (2014) Safety and pharmacokinetics of oral delta-9-tetrahydrocannabinol in healthy older subjects: a randomized controlled trial. Eur Neuropsychopharmacol 24:1475–82 [DOI] [PubMed] [Google Scholar]
- 30.Ahmad R, Goffin K, Van den Stock J, De Winter FL, Cleeren E, Bormans G, Tournoy J, Persoons P, Van Laere K, Vandenbulcke M (2014) In vivo type 1 cannabinoid receptor availability in Alzheimer’s disease. Eur Neuropsychopharmacol 24:242–50 [DOI] [PubMed] [Google Scholar]
- 31.Altamura C, Ventriglia M, Martini MG, et al. (2015) Elevation of plasma 2-arachidonoylglycerol levels in Alzheimer’s disease patients as a potential protective mechanism against neurodegenerative decline. J Alzheimers Dis 46:497–506 [DOI] [PubMed] [Google Scholar]
- 32.Shelef A, Barak Y, Berger U, Paleacu D, Tadger S, Plopsky I, Baruch Y (2016) Safety and Efficacy of Medical Cannabis Oil for Behavioral and Psychological Symptoms of Dementia: An-Open Label, Add-On, Pilot Study. J Alzheimers Dis 51:15–9 [DOI] [PubMed] [Google Scholar]
- 33.van den Elsen GAH, Ahmed AIA, Verkes RJ, Feuth T, van der Marck MA, Olde Rikkert MGM (2015) Tetrahydrocannabinol in Behavioral Disturbances in Dementia: A Crossover Randomized Controlled Trial. Am J Geriatr Psychiatry 23:1214–1224 [DOI] [PubMed] [Google Scholar]
- 34.Herrmann N, Ruthirakuhan M, Gallagher D, Verhoeff NPLG, Kiss A, Black SE, Lanctôt KL (2019) Randomized Placebo-Controlled Trial of Nabilone for Agitation in Alzheimer’s Disease. Am J Geriatr Psychiatry S1064748119303550 [DOI] [PubMed] [Google Scholar]
- 35.van den Elsen GAH, Ahmed AIA, Verkes R-J, Kramers C, Feuth T, Rosenberg PB, van der Marck MA, Olde Rikkert MGM (2015) Tetrahydrocannabinol for neuropsychiatric symptoms in dementia: A randomized controlled trial. Neurology 84:2338–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balash Y, Bar-Lev Schleider L, Korczyn AD, Shabtai H, Knaani J, Rosenberg A, Baruch Y, Djaldetti R, Giladi N, Gurevich T (2017) Medical Cannabis in Parkinson Disease: Real-Life Patientsʼ Experience. Clin Neuropharmacol 40:268–272 [DOI] [PubMed] [Google Scholar]
- 37.Kindred JH, Li K, Ketelhut NB, Proessl F, Fling BW, Honce JM, Shaffer WR, Rudroff T (2017) Cannabis use in people with Parkinson’s disease and Multiple Sclerosis: A web-based investigation. Complement Ther Med 33:99–104 [DOI] [PubMed] [Google Scholar]
- 38.Chagas MH, Zuardi AW, Tumas V, Pena-Pereira MA, Sobreira ET, Bergamaschi MM, dos Santos AC, Teixeira AL, Hallak JE, Crippa JA (2014) Effects of cannabidiol in the treatment of patients with Parkinson’s disease: an exploratory double-blind trial. J Psychopharmacol 28:1088–98 [DOI] [PubMed] [Google Scholar]
- 39.Kindred JH, Honce JM, Kwak JJ, Rudroff T (2018) Multiple Sclerosis, Cannabis Use, and Clinical Disability: A Preliminary [ 18 F]-Fluorodeoxyglucose Positron Emission Tomography Study. Cannabis Cannabinoid Res 3:213–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castelli L, Prosperini L, Pozzilli C (2019) Balance worsening associated with nabiximols in multiple sclerosis. Mult Scler J 25:113–117 [DOI] [PubMed] [Google Scholar]
- 41.Ball S, Vickery J, Hobart J, et al. (2015) The Cannabinoid Use in Progressive Inflammatory brain Disease (CUPID) trial: a randomised double-blind placebo-controlled parallel-group multicentre trial and economic evaluation of cannabinoids to slow progression in multiple sclerosis. Health Technol Assess 19:1–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schouten J, Su T, Wit FW, et al. (2016) Determinants of reduced cognitive performance in HIV-1-infected middle-aged men on combination antiretroviral therapy. Aids 30:1027–38 [DOI] [PubMed] [Google Scholar]
- 43.De Francesco D, Underwood J, Bagkeris E, et al. (2019) Depression, lifestyle factors and cognitive function in people living with HIV and comparable HIV-negative controls. HIV Med 20:274–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saloner R, Campbell LM, Serrano V, et al. (2019) Neurocognitive SuperAging in Older Adults Living With HIV: Demographic, Neuromedical and Everyday Functioning Correlates. J Int Neuropsychol Soc 25:507–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okafor CN, Plankey MW, Li M, Chen X, Surkan PJ, Shoptaw S, Martin E, Cohen R, Sacktor N, Cook RL (2019) Association of Marijuana Use with Changes in Cognitive Processing Speed and Flexibility for 17 Years in HIV-Seropositive and HIV-Seronegative Men. Subst Use Misuse 54:525–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zaki P, Blake A, Wolt A, et al. (2017) The use of medical cannabis in cancer patients. J Pain Manag 10:353–362 [Google Scholar]
- 47.Bar-Sela G, Tauber D, Mitnik I, Sheinman-Yuffe H, Bishara-Frolova T, Aharon-Peretz J (2019) Cannabis-related cognitive impairment: a prospective evaluation of possible influences on patients with cancer during chemotherapy treatment as a pilot study. Anticancer Drugs 30:91–97 [DOI] [PubMed] [Google Scholar]
- 48.Wallace MS, Marcotte TD, Umlauf A, Gouaux B, Atkinson JH (2015) Efficacy of Inhaled Cannabis on Painful Diabetic Neuropathy. J Pain 16:616–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bilkei-Gorzo A (2012) The endocannabinoid system in normal and pathological brain ageing. Philos Trans R Soc Lond B Biol Sci 367:3326–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ (2013) NIH Toolbox for Assessment of Neurological and Behavioral Function. Neurology 80:S2–S6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corrada MM, Brookmeyer R, Paganini-Hill A, Berlau D, Kawas CH (2010) Dementia Incidence Continues to Increase with Age in the Oldest Old The 90+ Study. Ann Neurol 67:114–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jorm AF, Jolley D (1998) The incidence of dementia: a meta-analysis. Neurology 51:728–733 [DOI] [PubMed] [Google Scholar]
- 53.Hebert LE, Weuve J, Scherr PA, Evans DA (2013) Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 80:1778–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cassano T, Calcagnini S, Pace L, Marco FD, Romano A, Gaetani S (2017) Cannabinoid receptor 2 signaling in neurodegenerative disorders: From pathogenesis to a promising therapeutic target. Front Neurosci. doi: 10.3389/fnins.2017.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bedse G, Romano A, Lavecchia AM, Cassano T, Gaetani S (2015) The role of endocannabinoid signaling in the molecular mechanisms of neurodegeneration in Alzheimer’s disease. J Alzheimers Dis 43:1115–36 [DOI] [PubMed] [Google Scholar]
- 56.Aso E, Ferrer I (2014) Cannabinoids for treatment of alzheimer’s disease: Moving toward the clinic. Front Pharmacol. doi: 10.3389/fphar.2014.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Talarico G, Trebbastoni A, Bruno G, de Lena C (2019) Modulation of the Cannabinoid System: A New Perspective for the Treatment of the Alzheimer’s Disease. Curr Neuropharmacol 17:176–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aso E, Sánchez-Pla A, Vegas-Lozano E, Maldonado R, Ferrer I (2015) Cannabis-based medicine reduces multiple pathological processes in AβPP/PS1 mice. J Alzheimers Dis JAD 43:977–991 [DOI] [PubMed] [Google Scholar]
- 59.Casarejos MJ, Perucho J, Gomez A, Muñoz MP, Fernandez-Estevez M, Sagredo O, Fernandez Ruiz J, Guzman M, de Yebenes JG, Mena MA (2013) Natural cannabinoids improve dopamine neurotransmission and tau and amyloid pathology in a mouse model of tauopathy. J Alzheimers Dis JAD 35:525–539 [DOI] [PubMed] [Google Scholar]
- 60.Koppel J, Bradshaw H, Goldberg TE, Khalili H, Marambaud P, Walker MJ, Pazos M, Gordon ML, Christen E, Davies P (2009) Endocannabinoids in Alzheimer’s disease and their impact on normative cognitive performance: a case-control and cohort study. Lipids Health Dis 8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Lau LML, Breteler MMB (2006) Epidemiology of Parkinson’s disease. Lancet Neurol 5:525–535 [DOI] [PubMed] [Google Scholar]
- 62.Hely MA, Reid WGJ, Adena MA, Halliday GM, Morris JGL (2008) The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord Off J Mov Disord Soc 23:837–844 [DOI] [PubMed] [Google Scholar]
- 63.Buter TC, van den Hout A, Matthews FE, Larsen JP, Brayne C, Aarsland D (2008) Dementia and survival in Parkinson disease: a 12-year population study. Neurology 70:1017–1022 [DOI] [PubMed] [Google Scholar]
- 64.Babayeva M, Assefa H, Basu P, Chumki S, Loewy Z (2016) Marijuana Compounds: A Nonconventional Approach to Parkinson’s Disease Therapy. Park Dis. doi: 10.1155/2016/1279042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buhmann C, Mainka T, Ebersbach G, Gandor F. Evidence for the use of cannabinoids in Parkinson’s disease. Journal of Neural Transmission. 2019;126:913–24. [DOI] [PubMed] [Google Scholar]
- 66.Mendes T, Ginó S, Ribeiro F, Guerreiro M, de Sousa G, Ritchie K, de Mendonça A (2008) Memory complaints in healthy young and elderly adults: Reliability of memory reporting. Aging Ment Health 12:177–182 [DOI] [PubMed] [Google Scholar]
- 67.Reid LM, MacLullich AMJ (2006) Subjective Memory Complaints and Cognitive Impairment in Older People. Dement Geriatr Cogn Disord 22:471–485 [DOI] [PubMed] [Google Scholar]
- 68.Nielsen S, Germanos R, Weier M, Pollard J, Degenhardt L, Hall W, Buckley N, Farrell M (2018) The Use of Cannabis and Cannabinoids in Treating Symptoms of Multiple Sclerosis: a Systematic Review of Reviews. Curr Neurol Neurosci Rep 18:8. [DOI] [PubMed] [Google Scholar]
- 69.Yadav V, Bever C, Bowen J, Bowling A, Weinstock-Guttman B, Cameron M, Bourdette D, Gronseth GS, Narayanaswami P (2014) Summary of evidence-based guideline: complementary and alternative medicine in multiple sclerosis: report of the guideline development subcommittee of the American Academy of Neurology. Neurology 82:1083–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rice J, Cameron M (2017) Cannabinoids for Treatment of MS Symptoms: State of the Evidence. Curr Neurol Neurosci Rep. doi: 10.1007/s11910-018-0859-x [DOI] [PubMed] [Google Scholar]
- 71.Benedict RH, DeLuca J, Phillips G, LaRocca N, Hudson LD, Rudick R (2017) Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Mult Scler J 23:721–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alford K, Vera JH (2018) Cognitive Impairment in people living with HIV in the ART era: A Review. Br Med Bull 127:55–68 [DOI] [PubMed] [Google Scholar]
- 73.Skalski LM, Towe SL, Sikkema KJ, Meade CS (2016) The Impact of Marijuana Use on Memory in HIV-Infected Patients: A Comprehensive Review of the HIV and Marijuana Literatures. Curr Drug Abuse Rev 9:126–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Whiting PF, Wolff RF, Deshpande S, et al. (2015) Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA 313:2456. [DOI] [PubMed] [Google Scholar]
- 75.Colizzi M, Bhattacharyya S. Does Cannabis Composition Matter? Differential Effects of Delta-9-tetrahydrocannabinol and Cannabidiol on Human Cognition. Current Addiction Reports. 2017;4:62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.