Abstract
Background
Cognitive function is an important factor for secondary prevention in elderly patients with cardiovascular diseases. The objective of this study was to evaluate the impact of cardiac rehabilitation (CR) on the improvement of cognitive function.
Methods
A total of 66 consecutive elderly patients (≥70 years old) with cardiovascular diseases were prospectively enrolled. The change in cognitive function during 6 months was compared between the patients with monthly CR (at least once per month; n = 27) and those without monthly CR (n = 39). Cognitive function was evaluated using the Mini-mental State Examination (MMSE) and Frontal Assessment Battery (FAB).
Results
There was no significant difference in baseline characteristics between the 2 groups. The change in the MMSE score was significantly greater in patients with monthly CR than in those without monthly CR (2.3 ± 0.4 vs. −0.1 ± 0.3 points; p <0.001). Among the MMSE items, the change in temporal orientation and attention and calculation was significantly greater in the monthly CR group than in the non-monthly CR group (0.8 ± 0.7 vs. −0.1 ± 0.8 points [p <0.001] and 1.0 ± 1.5 vs. −0.1 ± 0.1 points [p <0.001], respectively). The general linear model revealed that monthly CR (effect estimate, 1.455; 95% confidence interval, 0.747–2.163; p <0.001) was independently associated with the change in the MMSE score.
Conclusions
Cognitive function may improve with regular CR. These results might partly explain the efficacy of CR for secondary prevention.
Introduction
An association between the onset of cardiovascular disease (CVD) and cognitive impairment in elderly patients through the so-called heart–brain continuum hypothesis has been proposed. [1] The presence or onset of CVD impairs cognitive function through the activation of a thrombotic state, cardiac dysfunction, and vascular endothelial dysfunction. [2] Conversely, the presence of cognitive impairment may worsen the status of CVD through insufficient medicine compliance, poor nutritional status, and limited physical activities. [3] Thus, the maintenance of cognitive function is an important factor for secondary prevention, and can be a therapeutic and interventional target in elderly patients with CVD. Previous studies have reported the efficacy of comprehensive rehabilitation including dietary therapy, exercise, and cognitive training on the improvement of cognitive function in patients with cognitive impairment. [4, 5] However, the efficacy of cardiac rehabilitation (CR) in improving cognitive function remains unclear. Therefore, in the present study, we investigated the change in cognitive function during the CR period and evaluated the efficacy according to the frequency of CR in elderly patients with CVD.
Materials and methods
Study population
This study was a prospective observational study conducted from October 2015 to June 2016. A total of 84 patients with CVD, who were enrolled in the CR program of Kitasato University East Hospital, were identified. CVDs included ischemic heart disease, atrial fibrillation, and chronic heart failure. A total of 18 patients were excluded because of age (<70 years), use of medications for dementia, refusal to participate in the study, or loss to follow-up. Finally, we included 66 patients in the analysis (S1 Fig). The study protocol was approved by the Kitasato University Medical Ethics Organization (KMEO B15-68) and conformed to the ethical guidelines of the Declaration of Helsinki. All patients signed the written informed consent form before participation.
Assessment of cognitive function
Cognitive function was assessed using the Mini-mental State Examination (MMSE) and Frontal Assessment Battery (FAB) at baseline and at 6 months follow-up. The MMSE is a test evaluating orientation, registration, recording, writing, visual construction, and other items related to cognitive function, based on a 30-point scale. [6] The cognitive status were evaluated validated criteria. [7, 8] Cognitive improvement was defined as ΔMMSE > 0 point. [9] The FAB is a cognitive function test designed to evaluate the function of the prefrontal cortex based on an 18-point scale. [10] The investigators for MMSE and FAB measurements were blinded to the patients’ baseline clinical characteristics.
Comprehensive CR program
The comprehensive CR program at the Center for Cardiovascular Disease Prevention, Kitasato University East Hospital included exercise and education, as follows: combined exercise with stretching, resistance, and aerobic training; education to increase physical activities; dietary therapy to improve coronary risk factors; and disease management to prevent atherosclerosis progression and cardiac events. [11, 12] The patients were encouraged to join an on-site supervised aerobic exercise session for 30 min at least once per month according to international guidelines. [13]
Measurement of endothelial function
The reactive hyperemia peripheral arterial tonometry (RH-PAT) index was measured as a surrogate for endothelial function, by using a finger plethysmograph (EndoPAT2000; Itamar Medical, Caesarea, Israel). We measured the digital pulse amplitudes in patients while they were in the supine position for 5 min at baseline and after the induction of reactive hyperemia by means of forearm cuff occlusion for 5 min. [2] Data were digitized and computed automatically using the EndoPAT2000 software. The RH-PAT index was defined as the ratio of the mean post-deflation signal (in the 90–120 s post-deflation interval) to the baseline signal in the hyperemic finger, and was normalized using the same ratio in the contralateral finger. This value was multiplied by a baseline correction factor calculated using the EndoPAT2000 software. The detailed methods and definitions of the present study are described in Supplementary methods (S1 File).
Measurement of physical function
Physical functions were measured at baseline and at 6 months follow-up. Quadriceps isometric strength was measured with a hand-held dynamometer (μ-Tas; ANIMA, Tokyo, Japan). With the patient in sitting position, 5-s maximal isometric voluntary contractions of the quadriceps were collected for both legs. The strength values on the both sides were averaged and expressed as absolute value (kg). [14] The 6-min walk distance was measured according to standard guidelines established by the American Thoracic Society. [15] Further details are described in Supplementary methods (S1 File).
Definition
Ischemic heart disease included myocardial infarction, angina pectoris and vasospastic angina. Atrial fibrillation included both paroxysmal and chronic. Heart failure included both systolic dysfunction and diastolic dysfunction. Hypertension was defined as arterial blood pressure > 140/90 mmHg or taking antihypertensive medication. Other definitions are described in Supplementary methods.
Statistical analysis
Normally distributed continuous variables were presented as means ± standard deviations, and continuous variables deviating from the normal distribution were presented as means and interquartile ranges. Comparison of variables between baseline and 6 months after treatment was done using the paired t-test or Wilcoxon signed-rank test. Student’s t-tests or Mann–Whitney U-tests were used to examine variables between the monthly CR group and the non-monthly CR group. Binary variables were presented as percentages and examined using chi-square tests. A general linear model with multiple predictor variables was used to determine independent clinical predictors of absolute changes in the MMSE and FAB scores. The receiver-operating characteristic (ROC) curve was constructed to assess the number of CR session needed to detect cognitive improvement evaluated using MMSE and FAB. A statistically significant difference was considered when the p values were ≤0.05. Statistical analyses were performed using JMP 9.0 version (SAS Institute, Cary, NC, USA).
Results
Comparison of baseline characteristics between monthly CR and non-monthly CR
Of 66 patients, 27 (41%) were allocated to the monthly CR group and 39 (59%) were allocated to the non-monthly CR group. There was no significant difference in the MMSE and FAB scores at baseline between the 2 groups. There was also no significant difference in other baseline clinical characteristics and physical function parameters between the 2 groups (Table 1).
Table 1. Baseline patient characteristics.
| Variables | All patients n = 66 | Monthly CR n = 27 | Non-monthly CR n = 39 | p value |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, years | 77.1 ± 4.7 | 78.2 ± 4.5 | 76.3 ± 4.8 | 0.105 |
| Male, n (%) | 37 (56) | 17 (62) | 20 (51) | 0.345 |
| Body mass index, kg/m2 | 23.6 ± 3.4 | 22.9 ± 3.7 | 24.0 ± 3.2 | 0.229 |
| Education, years | 12.5 ± 1.6 | 12.5 ± 1.8 | 12.5 ± 1.5 | 0.962 |
| Mean Blood pressure, mmHg | 89.3 ± 11.0 | 91.4 ± 13.8 | 87.8 ± 8.7 | 0.196 |
| Myocardial infarction, n (%) | 19 (29) | 8 (29) | 11 (28) | 0.900 |
| Atrial fibrillation, n (%) | 22 (33) | 6 (22) | 16 (41) | 0.106 |
| Paroxysmal, n (%) | 2 (3) | 0 (0) | 2 (5) | 0.232 |
| Chronic, n (%) | 20 (30) | 6 (22) | 14 (35) | 0.234 |
| Heart failure, n (%) | 22 (33) | 9 (33) | 13 (33) | 1.000 |
| Systolic, n (%) | 8 (12) | 2 (7) | 6 (15) | 0.328 |
| Diastolic, n (%) | 14 (21) | 7 (25) | 7 (25) | 0.435 |
| Hypertension, n (%) | 59 (89) | 25 (92) | 34 (87) | 0.474 |
| Hyperlipidemia, n (%) | 55 (83) | 20 (74) | 35 (89) | 0.095 |
| Diabetes mellitus, n (%) | 23 (34) | 11 (40) | 12 (30) | 0.404 |
| Blood glucose, mg/dL | 115 ± 18.4 | 111 ± 12.6 | 118 ± 21.6 | 0.156 |
| Hemoglobin A1c, % | 6.39 ± 1.00 | 6.64 ± 1.25 | 6.22 ± 0.77 | 0.093 |
| LDL-cholesterol, mg/dL | 97.5 ± 23.3 | 97.8 ± 21.9 | 97.3 ± 24.6 | 0.935 |
| HDL-cholesterol, mg/dL | 59.7 ± 14.1 | 59.5 ± 16.5 | 59.8 ± 12.5 | 0.932 |
| Triglyceride, mg/dL | 106.5 [76.5–140.0] | 119.0 [77.0–199.0] | 100.0 [75.0–134.0] | 0.113 |
| Brain natriuretic peptide, pg/mL | 73.5 [39.1–123.7] | 99.1 [46.1–140.0] | 58.5 [35.5–108.7] | 0.116 |
| Left ventricular ejection fraction, % | 61.0 ± 8.5 | 61.2 ± 8.9 | 60.8 ± 8.3 | 0.880 |
| E/e′ | 13.1 ± 4.0 | 13.6 ± 4.5 | 12.8 ± 3.4 | 0.470 |
| Intima media thickness, mm | 2.3 ± 0.8 | 2.3 ± 0.7 | 2.2 ± 0.7 | 0.628 |
| Pulse wave velocity, cm/s | 1750 ± 319 | 1818 ± 301 | 1703 ± 326 | 0.152 |
| RH-PAT index | 1.74 ± 0.52 | 1.85 ± 0.65 | 1.67 ±0.41 | 0.160 |
| MMSE, points | 25.3 ± 2.8 | 25.2 ± 2.9 | 25.3 ± 2.7 | 0.929 |
| Normal (MMSE >27), n (%) | 15 (22) | 7 (25) | 8 (20) | 0.792 |
| Cognitive impairment without dementia (23< MMSE ≤27), n (%) | 35 (53) | 13 (48) | 22 (56) | |
| Mild degree dementia (MMSE ≤23), n (%) | 16 (24) | 7 (25) | 9 (23) | |
| FAB, points | 13.5 ± 2.2 | 13.9 ± 1.9 | 13.2 ± 2.3 | 0.223 |
| Physical functions | ||||
| Grip, kg | 24.1 ± 9.9 | 24.1 ± 9.9 | 25.1 ± 9.3 | 0.695 |
| Quadriceps isometric strength, kg | 30.3 ± 9.9 | 30.3 ± 9.9 | 30.8 ± 9.4 | 0.849 |
| Walking speed, m/s | 1.60 ± 0.34 | 1.60 ± 0.34 | 1.69 ± 0.37 | 0.353 |
| One-leg standing, s | 38.2 ± 23.5 | 38.2 ± 23.5 | 32.2 ± 23.5 | 0.286 |
| Functional reach, cm | 34.6 ± 5.8 | 34.6 ± 5.8 | 37.0 ± 4.6 | 0.062 |
| Six-minute walking distance, m | 465 ± 97.5 | 465 ± 97.5 | 456 ± 85.1 | 0.679 |
Values are presented as mean ± standard deviation, or n (%). CR, cardiac rehabilitation; LDL, low-density lipoprotein; HDL, high-density lipoprotein; E/e′, peak early diastolic velocity/basal septal diastolic velocity ratio; RH-PAT, reactive hyperemia peripheral arterial tonometry; MMSE, Mini-mental State Examination; FAB, Frontal Assessment Battery.
Comparison of the changes in laboratory findings and physical function between the monthly CR and non-monthly CR groups
The changes in laboratory findings and physical function are shown in Table 2. There was no cardiovascular re-hospitalizations during the study period in both groups. The decrease in hemoglobin A1c, triglyceride, and brain natriuretic peptide level was greater in the monthly CR group than in the non-monthly CR group. The change in physical function parameters, including knee extension strength, maximum walking speed, and other physical function measures, was greater in the monthly CR group than in the non-monthly CR group.
Table 2. Changes in laboratory findings and physical function.
| Variables | All patients n = 66 | Monthly CR n = 27 | Non-monthly CR n = 39 | p value |
|---|---|---|---|---|
| Clinical characteristics | ||||
| ΔBody mass index, kg/m2 | 0.83 ± 3.7 | 1.4 ± 5.7 | 0.77 ± 1.2 | 0.244 |
| ΔMean blood pressure, mmHg | −2.3 ± 10.2 | −4.9 ± 12.0 | −0.6 ± 8.8 | 0.095 |
| ΔBlood glucose, mg/dl | 0.1 ± 19.8 | 1.7 ± 11.6 | −1.1 ± 23.8 | 0.558 |
| ΔHemoglobin A1c, % | −0.11 ± 0.27 | −0.21 ± 0.31 | −0.04 ± 0.22 | 0.014* |
| ΔLDL-cholesterol, mg/dL | 1.0 ± 7.3 | −0.7 ± 6.8 | 2.3 ± 7.5 | 0.103 |
| ΔHDL-cholesterol, mg/dL | 0.24 ± 6.6 | −0.3 ± 8.7 | 0.6 ± 4.8 | 0.563 |
| ΔTriglyceride, mg/dL | −6.0 [−26.2−7.0] | −12.0 [−3.0−2.0] | −3.0 [−18.0−8.0] | 0.027 * |
| ΔBrain natriuretic peptide, pg/mL | −5.7 [−38.1−22.9] | −30.6 [−61.4−6.0] | 15.6 [−13.8−33.9] | 0.015 * |
| ΔLeft ventricular ejection fraction, % | 0.1 ± 1.7 | 0.3 ± 1.8 | 0.0 ± 1.7 | 0.462 |
| ΔE/e′ | −0.5 ± 3.4 | −1.4 ± 0.7 | 1.0 ± 0.6 | 0.007 * |
| ΔIntima media thickness, mm | −0.1 ± 0.2 | −0.1 ± 0.2 | 0.0 ± 0.2 | 0.054 |
| ΔPulse wave velocity, cm/s | 7.5 ± 134 | −22.2 ± 145 | 28.1 ± 123 | 0.133 |
| ΔRH-PAT index | 0.01 ± 0.25 | 0.14 ± 0.27 | −0.08 ± 0.18 | <0.001 * |
| Physical functions | ||||
| ΔGrip, kg | −0.2 ± 3.6 | −0.2 ± 3.7 | −0.2 ± 3.6 | 0.985 |
| ΔQuadriceps isometric strength, kg | 0.0 ± 3.4 | 2.3 ± 2.8 | −1.6 ± 2.7 | <0.001 * |
| ΔWalking speed, m/s | 0.0 ± 0.1 | 20.5 ± 4.1 | −8.4 ± 3.3 | <0.001 * |
| ΔOne-leg standing, s | −0.3 ± 13.3 | 6.1 ± 13.3 | −4.7 ± 11.5 | <0.001 * |
| ΔFunctional reach, cm | −0.1 ± 2.8 | 2.2 ± 0.4 | −1.1 ± 0.3 | <0.001 * |
| ΔSix-minute walking distance, m | 2.8 ± 17.2 | 40.0 ± 7.1 | −14.1 ± 6.5 | <0.001 * |
Values are presented as mean ± standard deviation.
* p <0.05. CR, cardiac rehabilitation; LDL, low-density lipoprotein; HDL, high-density lipoprotein; E/e′, peak early diastolic velocity/basal septal diastolic velocity ratio; RH-PAT, reactive hyperemia peripheral arterial tonometry.
Comparison of cognitive function change between the monthly CR and non-monthly CR groups
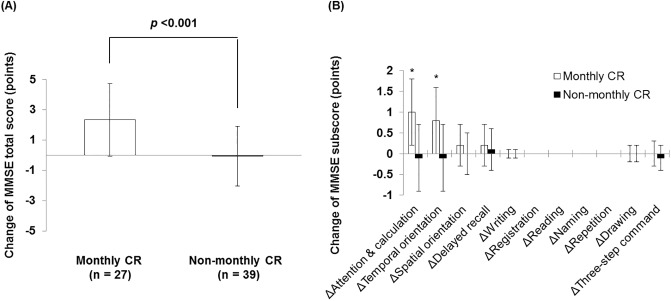
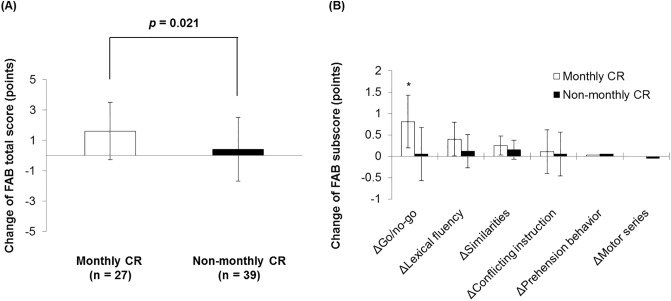
The change in the MMSE score for 6 months was greater in the monthly CR group than in the non-monthly CR group (−0.1 ± 0.3 vs. 2.3 ± 0.4 points; p <0.001; Fig 1A). The change in orientation for time and computing power was also greater in the monthly CR group than in the non-monthly CR group (Fig 1B). The change in the FAB score was also greater in the monthly CR group than in the non-monthly CR group (0.4 ± 2.1 vs. 1.6 ± 1.9 points; p = 0.021; Fig 2A). The change in the Go/No-Go task of the FAB was also greater in the monthly CR group than in the non-monthly CR group (Fig 2B). The comparisons of change in MMSE and FAB between the monthly CR group and the non-monthly CR group according to the baseline MMSE categories are shown in S2 Fig (S2 Fig, S1 Table in S1 File).
Fig 1. Change in the MMSE score during 6 months.
(A) Change in the MMSE total score for 6 months between the monthly CR group and the non-monthly CR group. (B) Changes in the MMSE items for 6 months between the monthly CR group and the non-monthly CR group. CR, cardiac rehabilitation; MMSE, Mini-mental State Examination.
Fig 2. Change in the FAB score during 6 months.
(A) Change in the FAB total score during 6 months between the monthly CR group and the non-monthly CR group. (B) Changes in FAB items during 6 months between the monthly CR group and the non-monthly CR group. CR, cardiac rehabilitation; FAB, Frontal Assessment Battery.
Independent predictors for changes in cognitive function
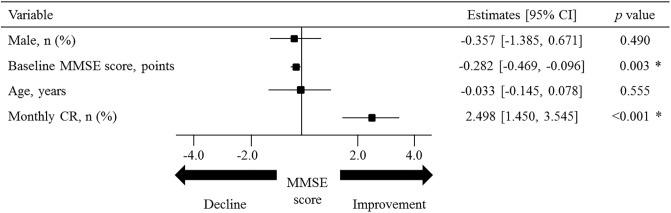
The general linear model revealed that monthly CR (effect estimate, 1.455; 95% confidence interval [CI], 0.747–2.163; p <0.001) was an independent predictor for the absolute change in the MMSE score (Fig 3). Similar results were obtained in the analysis for the FAB score (S3 Fig).
Fig 3. Clinical factors for the response of the MMSE score.
General linear modeling analysis for the absolute change in the MMSE score shows that the baseline MMSE score and monthly CR are predictors of unfavorable and favorable response, respectively. CI, confidence interval; CR, cardiac rehabilitation; MMSE, Mini-mental State Examination.
Correlation between the number of CR and changes in cognitive function
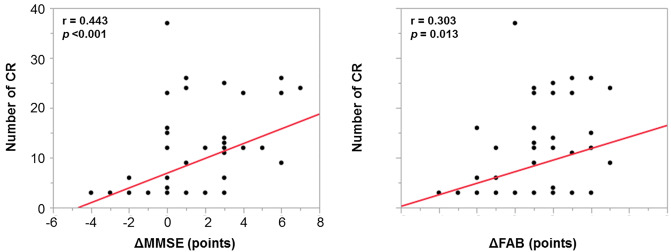
Significant correlations were observed between the number of CR and the change in the MMSE score (Pearson r = 0.443, p <0.001) and the FAB score (Pearson r = 0.303, p = 0.013) (Fig 4).
Fig 4. Correlation between the number of CR and change in cognitive function.
A significant linear correlation was observed between the number of CR and the change in cognitive function assessed using MMSE and FAB. CR, cardiac rehabilitation; FAB, Frontal Assessment Battery; MMSE, Mini-mental State Examination.
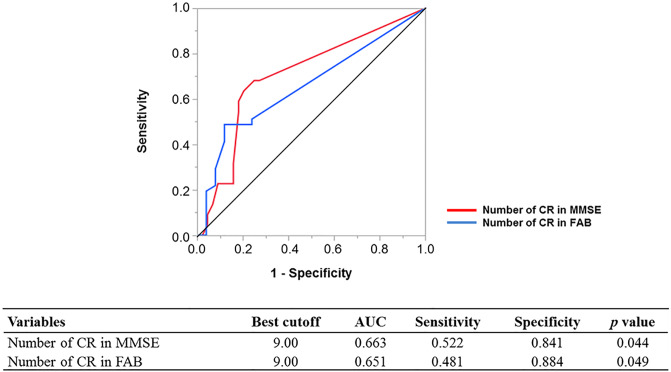
ROC analysis for the number of CR needed to detect cognitive improvement
The area under the ROC curve for the detection of cognitive improvement assessed using MMSE and FAB was 0.66 (95% CI, 0.42–0.89; p = 0.044) and 0.65 (95% CI, 0.53–0.95; p = 0.049), respectively (Fig 5). The best cutoff value of the number of CR for the detection of cognitive improvement assessed using the MMSE and FAB was 9.0 and 9.0, respectively.
Fig 5. Receiver-operating characteristic curve to detect cognitive improvement.
AUC, area under curve; CR, cardiac rehabilitation; FAB, Frontal Assessment Battery; MMSE, Mini-mental State Examination.
Discussion
The main findings of the present study were as follows: 1) the improvement of cognitive function was significantly greater in the monthly CR group than in the non-monthly CR group; 2) among cognitive functions, the improvement of frontal cortical function was significantly greater in the monthly CR group than in the non-monthly CR group; and 3) the improvement of laboratory parameters related to arteriosclerotic risks, cardiac function, and physical abilities were also greater in the monthly CR group than in the non-monthly CR group. The results of the present study suggest that regular CR may help improve cognitive function in addition to physical function in elderly patients with CVD.
Efficacy of CR in improving cognitive function
Previous studies have demonstrated the efficacy of comprehensive rehabilitation including cognitive function training on the maintenance and improvement of cognitive function. [16, 17] In a randomized controlled trial, Kwak et al. demonstrated that program-based regular exercise (30–60 min/day, 2–3 times per week for 12 months) improved the MMSE score from 14.5 ± 5.3 to 17.5 ± 6.9 points in patients with severe cognitive impairment. [18] Ngandu et al. demonstrated the efficacy of 2-year multidomain intervention including regular exercise and cognitive training on the improvement of cognitive function evaluated using a neuropsychological test battery in elderly participants with dementia in a randomized trial. [4] On the other hand, the present study demonstrated the efficacy of a CR program without cognitive training on the improvement of MMSE and FAB scores. Although the exact causal mechanisms of the efficacy of CR in improving cognitive function are still undetermined, we believe that 3 factors potentially contributed to the improvement of cognitive function by CR.
First, the improvement of endothelial function might contribute to the improvement of cognitive function. Our group recently reported a significant association between the presence of endothelial dysfunction evaluated using RH-PAT and impaired cognitive function. [2] In the present study, we further reported that the change in the RH-PAT index was greater in the monthly CR group than in the non-monthly CR group. The improvement of endothelial function may contribute to the recovery or maintenance of cognitive function through improvements in the cerebral microcirculation via enhanced nitric oxide synthesis, prostacyclin, tissue plasminogen activator, and shear stress. [19]
Second, the improvement of cardiac function might also contribute to the improvement of cognitive function. [20, 21] Although the direct influence of cardiac function on the improvement of cognitive function is still unclear, the significant correlations between the 2 factors were demonstrated in several previous studies. Jefferson et al. investigated a cohort in the Framingham Heart Study and reported that low left ventricular ejection fraction (<62.0%) was associated with low cognitive performance in patients with CVD. [22] Sauvé et al. also reported that the odds of cognitive impairment were 4.47 greater in elderly patients with heart failure than in healthy controls. [23] In the present study, the monthly CR group showed greater reduction in brain natriuretic peptide levels and greater recovery of left ventricular ejection fraction, in addition to the significant improvement of cognitive function, than the non-monthly CR group.
Third, the improvement of physical function may also contribute to the improvement of cognitive function. Verghese et al. reported that the gait velocity was significantly lower in elderly persons with cognitive impairment than in those with healthy cognitive function. [24] Another study reported that gait abnormalities including slow gait confer a greater risk for the development of dementia (hazard ratio, 1.96) in the elderly. [25] In the present study, improvements in maximum walking speed, knee extension strength, 1-leg standing, and functional reach, which are related to skeletal muscle mass and balance function, were observed. This resulted in an improvement in the 6-min walking distance, which is related to exercise tolerance. [26] Presumably, these comprehensive effects of CR may improve cognitive function without cognitive training.
Efficacy of CR in improving frontal cortical function
A previous study reported that a comprehensive rehabilitation program including cognitive training improved the frontal cortical function including executive function and processing speed. [4, 27] The present study also demonstrated that monthly CR significantly improved the frontal cortical function. However, the reason for the improvement in specific functions after rehabilitation programs remains unclear. Although increase in whole cerebral blood flow after exercise therapy was reported, [28] the increase of cerebral flow in a specific cortex after rehabilitation programs has not been demonstrated thus far. In addition to the exercise itself, scheduled visits to the rehabilitation site and conversations with rehabilitation staff might have stimulatory effects on the functions of the frontal cortex. [29]
Limitations
Several limitations of this study need to be acknowledged. First, this was an observational study with a small-sized specific cohort from a single center. Thus, it might be difficult to generalize the results. Further studies with a randomized design are required to further clarify the impact of CR on cognitive functions. Second, the type of CVD in the present cohort varied. The impact of CR on each disease remains undetermined. Third, the study period was only 6 months. A longer follow-up may further clarify the relevance of CR in changing the cognitive functions. Fourth, the causal relationship between CR and cognitive improvement was not clarified by the findings of this observational study.
Conclusions
In elderly patients with CVDs, regular cardiac rehabilitation had a potential to improve cognitive function. The present results may partly explain the efficacy of cardiac rehabilitation for secondary prevention. Further studies with a randomized design are required to further clarify the impact of CR on the improvement of cognitive functions.
Supporting information
CR, cardiac rehabilitation.
(TIF)
MMSE, Mini-mental State Examination; FAB, Frontal assessment battery; CR, cardiac rehabilitation.
(TIF)
General linear modeling analysis for the absolute change in the FAB score shows that the baseline FAB score and monthly CR are predictors of unfavorable and favorable response, respectively. CI, confidence interval; CR, cardiac rehabilitation; FAB, Frontal assessment battery.
(TIF)
Assessment of endothelial function, Measurement of physical functions, Definition; Supplementary table. S1 Table. Comparisons of baseline and change in cognitive functions according to the baseline MMSE categories; Supplementary references.
(DOCX)
(XLSX)
Acknowledgments
The authors thank Dr. Chiharu Noda, Ms. Emiko Sekine, Ms. Yumi Takahashi, and other staff of the Center for Cardiovascular Disease Prevention, Kitasato University East Hospital for their sincere support.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by MEXT KAKENHI (Grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology) grant number JP15K01389.
References
- 1.Abete P, Della-morte D, Gargiulo G, et al. Cognitive impairment and cardiovascular diseases in the elderly. A heart–brain continuum hypothesis. Ageing Res Rev 2014; 18: 41–52. [DOI] [PubMed] [Google Scholar]
- 2.Fujiyoshi K, Yamaoka-Tojo M, Minami Y, et al. Endothelial Dysfunction Is Associated with Cognitive Impairment of Elderly Cardiovascular Disease Patients. Int Heart J 2018; 59: 1034–1040. [DOI] [PubMed] [Google Scholar]
- 3.Rich MW, Maurer MS, Mcclurken JB, et al. Knowledge Gaps in Cardiovascular Care of the Older Adult Population. J Am Coll Cardiol 2016; 67: 2419–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 2015; 385: 2255–2263. [DOI] [PubMed] [Google Scholar]
- 5.Mavros Y, Gates N, Wilson GC, et al. Mediation of Cognitive Function Improvements by Strength Gains After Resistance Training in Older Adults with Mild Cognitive Impairment: Outcomes of the Study of Mental and Resistance Training. J Am Geriatr Soc 2017; 65: 550–559. [DOI] [PubMed] [Google Scholar]
- 6.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”A practical method for grading the cognitive state of patients for the clinician. J Psychiat Res 1975; 12: 189–193. [DOI] [PubMed] [Google Scholar]
- 7.Kaufer DI, Williams CS, Braaten AJ, et al. Cognitive screening for dementia and mild cognitive impairment in assisted living: comparison of 3 tests. J Am Med Dir Assoc 2008; 9(8): 586–593. [DOI] [PubMed] [Google Scholar]
- 8.Tsoi KK, Chan JY, Hirai HW, et al. Cognitive Tests to Detect Dementia: A Systematic Review and Meta-analysis. JAMA Intern Med 2015; 175(9): 1450–1458. [DOI] [PubMed] [Google Scholar]
- 9.Shi J, Ni J, Lu T, et al. Adding Chinese herbal medicine to conventional therapy brings cognitive benefits to patients with Alzheimer’s disease: a retrospective analysis. BMC Complement Altern Med 2017; 17(1): 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubois B, Slachevsky A, Litvan I, et al. The FAB: a Frontal Assessment Battery at bedside. Neurology 2000; 55: 1621–6. [DOI] [PubMed] [Google Scholar]
- 11.Thomas RJ, Balady G, Banka G, et al. 2018 ACC/AHA Clinical Performance and Quality Measures for Cardiac Rehabilitation: A Report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol 2018; 71: 1814–1837. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher GF, Landolfo C, Niebauer J, et al. Promoting Physical Activity and Exercise: JACC Health Promotion Series. J Am Coll Cardiol 2018; 72: 1622–1639. [DOI] [PubMed] [Google Scholar]
- 13.JCS Joint Working Group. Guidelines for Rehabilitation in Patients With Cardiovascular Disease (JCS 2012). Circ J 2014; 78: 2022–2093. [DOI] [PubMed] [Google Scholar]
- 14.Kamiya K, Masuda T, Tanaka S, et al. Quadriceps Strength as a Predictor of Mortality in Coronary Artery Disease. Am J Med 2015; 128(11): 1212–1219. [DOI] [PubMed] [Google Scholar]
- 15.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166(1): 111–117. [DOI] [PubMed] [Google Scholar]
- 16.Groot C, Hooghiemstra AM, Raijmakers PG, et al. The effect of physical activity on cognitive function in patients with dementia: A meta-analysis of randomized control trials. Ageing Res Rev 2016; 25: 13–23. [DOI] [PubMed] [Google Scholar]
- 17.Salzwedel A, Heidler MD, Meng K, et al. Impact of cognitive performance on disease-related knowledge six months after multi-component rehabilitation in patients after an acute cardiac event. Eur J Prev Cardiol 2019; 26: 46–55. [DOI] [PubMed] [Google Scholar]
- 18.Kwak YS, Um SY, Son TG, et al. Effect of regular exercise on senile dementia patients. Int J Sport Med 2008; 29: 2008. [DOI] [PubMed] [Google Scholar]
- 19.Verma S, Wang CH, Li SH, et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation 2002; 106: 913–9. [DOI] [PubMed] [Google Scholar]
- 20.Leto L, Feola M. Cognitive impairment in heart failure patients. J Geriatr Cardiol 2014; 11: 316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugie M, Harada K, Takahashi T, et al. Peak exercise stroke volume effects on cognitive impairment in community-dwelling people with preserved ejection fraction. ESC Hear Fail 2018; 5: 876–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jefferson AL, Himali JJ, Au R, et al. Relation of left ventricular ejection fraction to cognitive aging. Am J Cardiol 2012; 108: 1346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sauvé MJ, Lewis WR, Blankenbiller M, et al. Cognitive impairments in chronic heart failure: A case controlled study. J Card Fail 2009; 15: 1–10. [DOI] [PubMed] [Google Scholar]
- 24.Verghese J, Robbins M, Holtzer R, et al. Gait Dysfunction in Mild Cognitive Impairment Syndromes. J Am Geriatr Soc 2008; 56: 1244–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verghese J, Lipton RB, Hall CB, et al. Abnormality of gait as a predictor of non-Alzheimer’s dementia. N Engl J Med 2002; 347: 1761–8. [DOI] [PubMed] [Google Scholar]
- 26.Kamiya K, Hamazaki N, Matsue Y, et al. Gait speed has comparable prognostic capability to six-minute walk distance in older patients with cardiovascular disease. Eur J Prev Cardiol 2018; 25: 212–219. [DOI] [PubMed] [Google Scholar]
- 27.Blumenthal JA, Sherwood A, Smith PJ, et al. Enhancing cardiac rehabilitation with stress management training : a randomized, clinical efficacy trial. Circulation 2016; 133: 1341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alfini AJ, Weiss LR, Nielson KA, et al. Resting Cerebral Blood Flow After Exercise Training in Mild Cognitive Impairment. J Alzheimer’s Dis 2019; 67: 671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aguirre E, Woods RT, Spector A, et al. Cognitive stimulation for dementia: A systematic review of the evidence of effectiveness from randomised controlled trials. Ageing Res Rev 2013; 12: 253–262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CR, cardiac rehabilitation.
(TIF)
MMSE, Mini-mental State Examination; FAB, Frontal assessment battery; CR, cardiac rehabilitation.
(TIF)
General linear modeling analysis for the absolute change in the FAB score shows that the baseline FAB score and monthly CR are predictors of unfavorable and favorable response, respectively. CI, confidence interval; CR, cardiac rehabilitation; FAB, Frontal assessment battery.
(TIF)
Assessment of endothelial function, Measurement of physical functions, Definition; Supplementary table. S1 Table. Comparisons of baseline and change in cognitive functions according to the baseline MMSE categories; Supplementary references.
(DOCX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.