Abstract
Introduction
Bangladesh has a history of endemic malaria transmission, with 17.5 million people at risk. The objective of this study was to assess the cost-effectiveness of universal childhood malaria vaccination in Chittagong Hill Tracts (CHT) of Bangladesh with newly developed RTS,S/AS01 malaria vaccines.
Methods
A decision model was been developed using Microsoft® Excel to examine the potential impact of future vaccination in Bangladesh. We estimated the economic and health burden due to malaria and the cost-effectiveness of malaria vaccination from the health system and societal perspective. The primary outcomes include the incremental cost per Disability-Adjusted Life Year (DALY) averted, incremental cost per case averted, and the incremental cost per death averted.
Results
Introducing childhood malaria vaccination in CHT in Bangladesh for a single birth cohort could prevent approximately 500 malaria cases and at least 30 deaths from malaria during the first year of vaccination. The cost per DALY averted of introducing the malaria vaccine compared to status quo is US$ 2,629 and US$ 2,583 from the health system and societal perspective, respectively.
Conclusions
Introduction of malaria vaccination in CHT region is estimated to be a cost-effective preventive intervention and would offer substantial future benefits particularly for young children vaccinated today. Policies should, thus, consider the operational advantages of targeting these populations, particularly in the CHT area, with the vaccine along with other malaria control initiatives.
Background
Malaria, an infectious disease, is still prevalent throughout tropical and subtropical regions of the world. According to the World Health Organization (WHO), approximately 212 million new cases and 0.42 million estimated deaths occurred globally due to malaria in 2015 [1]. The latest Global Burden of Disease 2017 data showed that neglected tropical diseases including malaria are responsible for approximately 62,300 thousands of global disability adjusted life years (DALYs), of which malaria alone contributed 72% of the total DALYs [2]. Malaria is a type of vector-borne disease transmitted through the bites of Anopheles mosquitoes; a tiny fraction of infections are transmitted via transfusion or congenital transmission routes [3]. Almost all malaria deaths are caused by Plasmodium falciparum and most of these deaths occur in African children less than 5 years old [3].
Bangladesh has a history of endemic malaria transmission in 13 out of its 64 districts, and approximately 17.5 million people are at risk, although only 27,737 cases were reported in 2016 [4]. However, it has been assumed that unreported cases might have been as high as 250,000 each year, highlighting the true burden of malaria disease in Bangladesh [5]. The number of malaria cases in these areas fluctuates seasonally, and the highest malaria incidence occurs during the rainy season, from April to October each year in Bangladesh whereas Plasmodium falciparum is the main malaria parasite in Bangladesh [4,6]. Despite apparent declines in prevalence, it still remains as a significant public health problem and a leading cause of hospital admissions during the rainy season in Bangladesh [7]. Among all cases, approximately 80% belong to the 3 most malarial-prone districts (Bandarban, Khagrachari and Rangamati), which are collectively known as Chittagong Hill Tracts (CHT); it has the highest malarial incidence reported within the country [7]. A number of other relevant factors, such as proximity to forest, household density and culture, and elevation made the CHT regions more vulnerable for malaria cases and CHT has been declared as a “malaria prone endemic area” [8], and known as malaria hotspots in Bangladesh. Malaria affects all of the population irrespective of age, sex, and occupations, though children are the most vulnerable [9]. In terms of health and economic burden, the costs of treating malaria cases vary amongst different socio-economic strata and geographical locations. Furthermore, due to the absence of financial security, expenses for treating malaria cases came exclusively from out-of-pocket payments [10,11]. Although malaria diagnosis and treatment is free in Bangladesh, however, many malaria patients spent for treatment care, including transport costs, diagnostic costs, and antimalarials [4,12]. The treatment costs have been shown to be proportionately higher for poor households, and are catastrophic to poorer households and rural dwellers [11].
According to World Health Organization, RTS,S/AS01 is the most advanced malaria vaccine candidate, which is a complementary malaria control tool that could potentially be added to-and not replace-the core package of proven malaria preventive, diagnostic, and treatment measures [13]. The prevention of disease burden and death through vaccination is one of the most cost-effective and public health achievements in developing world [14–16]. However, the vaccine is not the single important issue. the efficacy or effectiveness of the vaccine, disease burden, and financial issues are the common concerns of vaccine introduction [17, 18]. A vaccine introduction decision-making study in Bangladesh demonstrated that the Government and other technical personnel would only be convinced if the information on the burden of disease comes from the real setting [19]. Value for money has become an increasingly necessary criterion for vaccine introduction and, therefore, the cost-effectiveness analysis could contribute to guide decision making about introduction of vaccines versus other health interventions [20]. There are various cost-effectiveness studies available on different infection preventive vaccines in Bangladesh [21,22] but none of them are pertinent to malaria. Cost-effectiveness analysis study is, thus, required to implement sustainable control programs and to assess the future needs for malaria elimination. To address the literature gap, our intention was to conduct a cost-effectiveness analysis of malaria vaccination in Bangladesh and to highlight to policy makers whether or not there is a potential need for malaria vaccination introduction as a part of the malaria control initiatives. As a consequence, the objective of this study was to assess the cost-effectiveness of universal childhood malaria vaccination in CHT regions in Bangladesh with the newly developed RTS,S/AS01 malaria vaccines. In the light of other malaria vaccination studies [23–26], this analysis is limited to young children aged 3 years or less, as immunity tends to increase with increasing age [27].
Materials and methods
Model
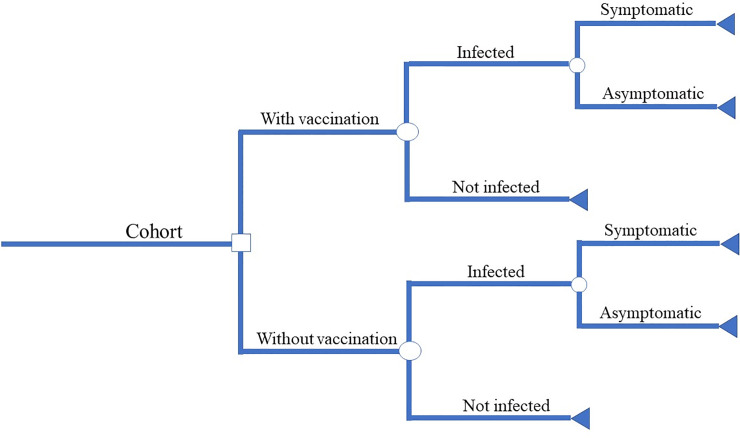
A decision model was developed using Microsoft ® Excel to examine the potential impact of future vaccination in high risk, malaria-prone areas in Bangladesh. We estimated the economic and health burden due to malaria and the cost-effectiveness of malaria vaccination from the health system and societal perspective. The decision tree of cost-effectiveness analysis is shown in Fig 1.
Fig 1. Decision tree for cost-effectiveness analysis of malaria vaccination.
The primary outcomes include the incremental cost per Disability-Adjusted Life Year (DALY) averted, incremental cost per case averted, and the incremental cost per death averted. Incremental cost-effectiveness ratios (ICERs) are calculated by dividing the cost difference with and without the universal childhood malaria vaccination program by the difference in health outcomes with and without the intervention. For these perspectives, several parameters were taken from various published papers and regional data sources. The various health associated outcomes, health care costs averted and the reduction of disease burden after the introduction of childhood malaria vaccines in regional settings were estimated in this model. Principal model parameters are described in Table 1. For comparing the pre- and post-malaria vaccination scenario, we estimated the potential events and possible costs to capture the baseline disease burden, and then assessed the number of malaria disease-associated events and possible costs that would occur after the introduction of the vaccination program. The model was applied to the 2016 annual static birth cohort (N = 146,255) based on national district web portal and the population projection of the Government of the People’s Republic of Bangladesh [28], and analysis was based on one year period. In addition, sensitivity analysis was carried out from 0.6 years up to 5 year time horizon [24,29,30]. The cost-effectiveness analysis was reported based on the health system and societal perspective according to the Panel on Cost-Effectiveness in Health in Medicine [31]. For the health system perspective, we included the medical care costs related to malarial episodes and the cost of vaccination program; whereas in the societal perspective, both direct medical (e.g., medicine, diagnostic), direct non-medical cost (e.g., transportation, lodging), and indirect cost (e.g., income loss) were included. However, the intangible costs, such as pain and discomfort were excluded from the estimation. In the current analysis, the costs averted by vaccination were subtracted from the costs invested in malaria vaccination program, divided by the DALYs or the number of deaths and cases averted. All future costs and benefits were discounted at a rate of 3% annually [32]. For reporting the cost-effectiveness scenario, we used the common cost-effectiveness threshold level proposed by the World Health Organization: an intervention is considered cost-effective if cost per DALY averted is less than three times of the national annual per capita GDP, whereas costs less than the GDP per capita are considered as highly cost-effective [33]. The concept of DALYs was used to quantify the disease burden incorporating life lost due to premature death and the time spent in unhealthy states [34]. The DALY is a time-based measure which combines years of life lost (YLL) due to premature mortality and the years of healthy life lost to living in a state of less than perfect health (years lost to disability or YLD) in a country-specific context [35]. Therefore, DALY is the summation of YLL+YLD, and 1 DALY can be considered as equivalent to one lost year of healthy life. Using DALYs, it is possible to measure the gap between current health status and an ideal situation, where everyone lives according to their life expectancy without disease and disability [36].The detailed understanding, theory, methodology, and limitations of the DALY estimation have been described elsewhere [34,37]. Like previous studies [38,39], the following four equations were applied to estimate the total DALYs avoided due to introduction of malaria vaccination in CHT regions. The four Eqs (1–4) are written as:
Table 1. Cost-effectiveness model parameters, with uncertainty ranges.
| Input parameter | Value | Sensitivity |
|---|---|---|
| Fully vaccinate population | 146,255 [28] | - |
| Vaccination campaign coverage (%) | 65% [47] | 40 [63] to 80% [49] |
| Overall protective effectiveness (%) | 39% [23] | 34.3 to 43.3 [23–25] |
| Duration of vaccine protection (years) | 1 [29] | 0.6 to 5 [Assumption] |
| Incidences (cases/1000) | 4.3 [40] | 2 to 11.34 [7] |
| Case Fatality Ratio (cases/1000) | 0.23[5] | 0.09–0.41[5] |
| Duration of illness (days) | 07 [41] | 3–10 [Assumption] |
| Daly weight | 0.2 [41] | 0.061 to 0.281[42] |
| Life Expectancy, (years) | 70 [64] | 65 to75 [Assumptions] |
| Outpatient cost for provider (US $) | 5.48 [44] | 2.26 to 23.65 [44] |
| Outpatient cost for households (US $) | 16.64 [44] | 9.14 to 37.99 [44] |
| Inpatient cost for provider (US $) | 30.26 [44] | 15.64 to137.87 [44] |
| Inpatient cost for households (US $) | 32.24 [44] | 26.99 to 288.79[44] |
| Vaccine purchasing cost per dose (US $) | 0.16 [21] | 0.1 to 5 [23,24,26] |
| Vaccine delivery cost per dose (US $) | 0.835 [43] | 0.5 to 1 [Assumption] |
| Vaccine recipient cost | 0.02 [43] | 0.01 to 0.1 [Assumption] |
| Discount rate (%) | 3% [65,66] | 1 to 10% [63] |
| GDP Threshold for DALYs * | ||
| Very cost effective (per capita GDP**) | (US $) 1,466 | |
| Cost effective (3* per capita GDP) | (US $) 4,398 |
(*World Health Organization guideline
** Bangladesh Bank 2016)
| (1) |
| (2) |
| (3) |
| (4) |
In the above equations, Eff t is the effectiveness of the malaria vaccine in year t, Cover is the percentage of young children that would be vaccinated if the vaccine were provided for free, CFR, I, and N are the case fatality rate, incidence of malaria, and number of young children, respectively; Length is average duration of illness (i.e., number of days sick with malaria), DW is the disability weight, LE is the life expectancy and Durr is the duration of the vaccine effectiveness. The incremental cost-effectiveness ratio (ICER) defined as the ratio of the change in costs of malaria vaccination program (compared to doing nothing) to the change in effects of the vaccination in terms cases, deaths and DALYs averted. The following equation is used for ICER:
Incremental cost-effectiveness ratio (ICER) = (C1 –C0) / (E1 –E0)
Where C1 and E1 are the cost and effect of the malaria vaccination program while C0 and E0 are the cost and effect of the comparator respectively. Principal model parameters are described in Table 1.
Incidence and case fatality rate
Among the ten malaria-endemic countries of the WHO Southeast Asian region, Bangladesh is considered as hypo-endemic for malaria transmission, where 90% of malaria is caused by Plasmodium falciparum [40]. The prevalence of malaria is 0.92 per 1000 population in these endemic areas, however, children under five years of age were found to have a higher prevalence, i.e., 4.37 per 1000 young children [40]. The overall malaria related mortality was 0.09 per 1000 population which was higher for adult people (>14 years), followed by the younger group ages (4.1 to 14.0 years old), i.e., 0.40 vs 0.32 per 1000 population respectively [5].
Duration of illness and DALY weights
The frequency of episodes of malaria and the characteristics of malaria disease varies depending on the infected individual’s age, genetics, type of parasite, and immune response from previous malaria infections, and the intensity and seasonality of malaria transmission [3]. Due to limitation of data, like earlier study, we assume that in moderate or high transmission areas, children commonly experience 4–6 febrile illnesses per year attributable to malaria [3]. Consistent with an earlier study, we assumed that without effective malarial treatment, the average duration of a malaria episode is 7 days [41]. We used the DALY weights as 0.20 to measure the pain, suffering, and discomfort associated with malaria diseases [41]. To observe the possible effects of the universal malaria vaccination, the sensitivity range goes from 0.12 to 0.281 [41,42].
Malaria vaccination and cost
RTS, S/AS01 Plasmodium falciparum is a safe and moderately efficacious vaccine which is closer to domestic licensure and has already been implemented in endemic Africa [23–25]. With its safety and efficacy upheld in the phase 3 trial, the vaccine has received a positive regulatory assessment, however, further evaluation of RTS,S/AS01 is required for vaccine introduction at wider level in different countries [3]. On the light of earlier study, we assumed four doses schedules, first dose will be administered at six months, second and third before 9 months, and fourth dose at 27 months with overall vaccine efficacy will 39.0% (95% CI, 34.3–43.3) [23]. The cost-effectiveness of the vaccination was evaluated over 1 year time horizon with sensitivity analysis from 0.6 years up to 5 years [29,30]. As per vaccination study regarding infectious diseases, due to absence of data, we assumed that the Global Alliance for Vaccines and Immunisation (GAVI) will subsidize the malaria vaccine prices for Bangladesh, which will be approximately US 0.16 [21] and will vary from US$ 0.1 up to US$ 5.0, and additionally, vaccine delivery cost will be incurred accordingly [23–26]. Based on an earlier vaccination trial, it was assumed that an additional US$ 0.5 to US$ 1 will be incurred per person for delivery-related activities [43]. Furthermore, in case of societal perspective, additional vaccine recipient cost (e.g., travel cost, time cost) will be incurred.
Cost of illness due to malaria infections
A systematic review of the published literature on the costs and cost-effectiveness of malaria interventions was carried our earlier [44], and the findings of the review revealed that the average outpatient cost per treating malaria was US$ 5.84 from health system perspective, while approximately US$ 22.48 was spent from societal perspective. For inpatients, i.e. those who had severe episodes of malaria, approximately US$ 30.26 was incurred from the perspective of health system while the societal average cost was estimated at US$ 64.50 [44]. Due to limited information, in light of the earlier study, we assumed that approximately 24% of the malarial cases sought treatment from hospital care and the remaining patients utilized the hospital outpatient services [45].
Coverage of the vaccine
The Expanded Program on Immunization (EPI) having the highest priority in Bangladesh recommended that every child should complete the scheduled immunization within their first year of life [46]. Recently, a large oral cholera vaccine trial was conducted in urban Bangladesh where the migration rate is higher than in other parts of the country and found that the two dose vaccine coverage was at least 65% in that area [47]. In this analysis, we assumed that the malaria vaccination will have a moderate coverage. Indeed, the overall immunization coverage is about 86% in Bangladesh [48]. Similar to earlier studies, we used a vaccine coverage from 40% to 80% for uncertainty analysis in this model [49].
Sensitivity analysis
One-way sensitivity analyses were conducted based on the published and unpublished values of each parameter, in order to ascertain the impact of uncertainty in input values on the cost-effectiveness ratio. In scenario analyses, the cost-effectiveness ratios were estimated using low or high values of selected parameters, and compared with the base-case scenario, i.e., no-vaccination strategy.
Results
Input parameters, vaccination cost, health burden, and decision criteria of future universal malaria vaccination program are summarized in Table 1. Introducing childhood malaria vaccination in CHT in Bangladesh for a single birth cohort of 146,255 young children, is projected to prevent approximately 500 cases and at least 30 deaths from malaria during the 1st year of vaccination period. By implementing the vaccination program, a total of 206.64 Disability Adjusted Life Years (DALYs) could then be averted (Table 2). The results also demonstrated that approximately US$ 5,697 could be saved within the shorter time horizon whereas US$ 3,620 could be saved as a consequence of avoiding the inpatient visits from the health system perspective. Furthermore, considering the societal perspective, about US$ 15,858 in the form of indirect costs (e.g. time cost) and out-of-pocket cost (e.g. transportation) involved for seeking care could be saved by investing in the universal vaccination (Table 2).
Table 2. Key vaccination program outcomes over 1 year’s period.
| Parameters | Health system perspective | Societal perspective |
|---|---|---|
| Number of vaccinations ('000) | 146.26 | 146.26 |
| Average costs per vaccine | 0.16 | 0.16 |
| Average delivery costs per fully vaccinated child | 0.84 | 0.84 |
| Total inpatients cost averted ('000) | 3.62 | 7.48 |
| Total outpatient cost averted ('000) | 2.08 | 8.38 |
| Total costs averted ('000) | 5.70 | 15.86 |
| Net discounted costs of the vaccination ('000) | 543.31 | 533.73 |
| Total number of cases averted ('000) | 0.50 | 0.50 |
| Total number of deaths averted ('000) | 0.03 | 0.03 |
| Total number of DALYs averted | 206.64 | 206.64 |
| Incremental cost- effectiveness ratio (ICER) per | ||
| Case averted | 1,089.84 | 1,070.62 |
| Life saved | 20,706.89 | 20,341.83 |
| DALY averted | 2,629.27 | 2,582.91 |
| GDP Thresholds (for references) | ||
| Cost-effective (3* GDP/capita) | US $ 1,466 | |
| Very cost-effective (GDP/capita) | US $ 4,398 |
(*World Health Organization guideline
** Bangladesh Bank 2016)
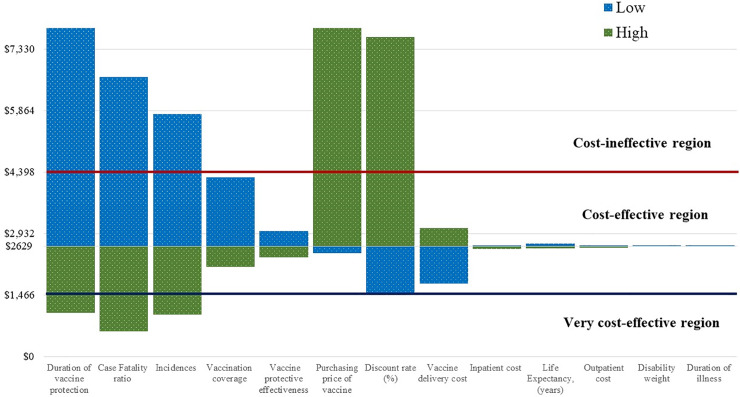
The cost-effectiveness estimates of this study demonstrated that the universal childhood malaria vaccination appeared to be a cost-effective investment both for health system and societal perspective, even with the lower efficacy of the current vaccine. By introducing the malaria vaccine compared to status quo, the cost per DALY averted is US$ 2,629 and US$ 2,583 from the health and societal perspective, respectively. Incremental cost-effectiveness ratios (ICERs) for both perspectives fell below the GDP per capita in Bangladesh (US$ 1,466) of 2015–2016 fiscal year, which was used as a threshold for determining the cost-effectiveness of any intervention. Therefore, these results demonstrate that cost-effectiveness of universal malaria vaccination program in CHT, Bangladesh laid between “very cost-effective” and “cost-ineffective region”, according to WHO criteria (Fig 2).
Fig 2. Cost per DALY averted: Health system perspective.
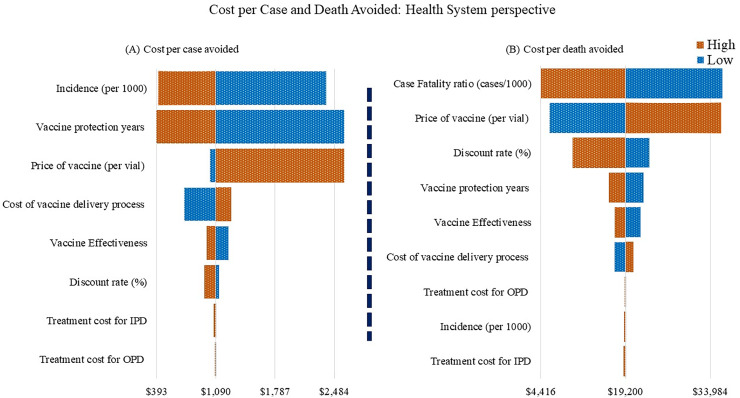
We assessed the robustness of the impact predictions by varying key model inputs (Table 1). Our scenario analysis found that the most influential parameters were duration of vaccine protection years, case fatality ratio, incidence, price of the vaccine, discount rate, vaccination coverage, and the cost of vaccine delivery-related activities from the health system perspective (Fig 2). The same parameters were also considered influential for societal perspective. However, vaccination becomes attractive if the longer time horizon is considered, and it is measured as a very cost-effective investment. Further, if the price would reduce to US$ 0.1, the vaccination program would be a very cost-effective investment, while at the highest price (US$ 5), it became a cost-ineffective option (Fig 2). Whilst the higher incidence and higher mortality rate of malaria will make the immunization program attractive and cost saving option from the health system perspective, a similar scenario is also observed from the societal perspective. Vaccine coverage and duration of protection years are other influential parameters for vaccine introduction into the CHT regions. Most of the above parameters (e.g. duration of vaccine protection years, case fatality ratio, incidence, and price of the vaccine) also appeared as influential parameters in this regard, considering cost per case and death averted due to vaccination (Fig 3).
Fig 3. Cost per case and death averted: Health system perspective.
Discussion
Bangladesh has made significant progress towards malaria control, and from 2000 to 2014, malaria incidence has been reduced approximately 75% by introducing vector control interventions [50]. However, malaria is still a devastating health problem in endemic regions of the country, where approximately 156.6 million people are at risk of malaria infections [51]. Globally, there are various malaria control initiatives, such as insecticide-treated mosquito nets (ITNs) including long-lasting insecticidal nets (LLINs), indoor residual spraying (IRS), artemisinin combination therapy (ACT), seasonal malaria chemoprevention (SMS), rapid diagnostic tests (RDTs), intermittent preventive treatment (IPT), whose efficacy and effectiveness have been repeatedly demonstrated over many years [52–56]. Since malaria prevalence is highest in CHT region than in other endemic districts of the country, resources should be engaged in such a way that it could optimize the benefit of investment and reduce the malaria burden from this malaria hotspot area. Community participation is vital for controlling and preventing malaria disease, however, as a component of prevention strategies, malaria vaccination might be another alternative option, since benefits of vaccines are well known as they avert infections directly (immunization) and indirectly (via herd immunity) [15].
In Low- and Middle-Income Countries like Bangladesh, the introduction of new health technology such as new vaccines are expected to implement with delays. For instance, it takes years or even decades after the invention [57,58]. We have estimated the potential cost-effectiveness of malaria vaccination in terms of cost per life saved, death and DALYs averted which might be capable to guide upcoming malaria vaccine policy formulation and nationwide vaccine implementation. In a resource poor country like Bangladesh, precise information, when there are competing health priorities and other national and local priorities, is a prerequisite for policy makers [58]. Therefore, this study could be referenced as a starting point for such discussion. Our estimation demonstrated that introducing the universal childhood malaria vaccination in CHT region could avert approximately 500 cases and approximately 30 deaths during the first vaccination year, using direct protection of vaccines. The cost per case and death averted are US$ 1,090 and US$ 20,707, respectively, for health system perspective, which are slightly higher than societal perspective.
Again, the incremental cost per DALY averted is also within the ranges of cost-effectiveness analysis using WHO GDP criteria. Once the vaccination is fully implemented in the endemic districts, the vaccine effectiveness will be higher, incorporating distinct characteristics of herd immunity of infectious vaccines [39]. Compared to the other malaria prevention strategies, the effectiveness of RTS,S/AS01 is less attractive intervention than other preventive control measure even in African regions. A systematic literature review reported that the incremental cost-effectiveness ratio per DALY averted was $27 (range $8.15-$110) for ITNs, $ 143 (range $ 135-$ 150) for IRS, and $ 24 (range $ 1.08-$ 44.24) for IPT [44]. A mathematical model for an African setting predicted that by introducing RTS,S in every 100,000 fully vaccinated children, approximately 116,480 clinical cases and 484 deaths could be averted in a 15 year time horizon, where the cost per DALY averted was US$ 87 [59]. Another Sub-Saharan African study estimated the cost per DALY averted ranges between US$ 44-US$ 279 and considered as a ‘secondary intervention’ and not prioritized due to their high cost than WHO recommended other preventive interventions like SMS, IRS and even LLINs [26]. In a recent study, Galactionova and colleagues reported that RTS,S/AS01 could reduce malaria burden substantially in 43 endemic Sub-Saharan African regions and the median cost per DALY averted would be US$ 188, which is a highly cost-effective intervention in those contexts [24]. However, most of the cost-effectiveness studies have been conducted in African regions where the malaria prevalence is higher than in Asian region, and are, thus, not comparable, although the context-specific cost-effectiveness study is crucial for such decision making process [26].
In scenario analysis, we observed that incidence, case fatality rate, price of the vaccines, coverage, and protective effectiveness are the major influencing parameters in the analysis, which make the vaccination program attractive. Due to limited information about malaria related clinical data, we have adopted the parameter values from various sources which was reflected in the sensitivity analysis. Introduction of malaria vaccine in this context requires significant financial resources. For introduction of malaria vaccination program, price of the vaccine is critical. Although the price reported here was based on GAVI subsidization, additional vaccine-related costs will be incurred (i.e., cost of vaccine delivery). Therefore, in the future, locally produced vaccine would be highly recommended, which would reduce the price of the vaccines and make vaccination more financially affordable. RTS,S vaccination trial has not been administered yet in CHT region or in any endemic region of the country; therefore, the country’s real vaccine efficacy-related data are missing, however, such studies have been conducted in African regions [23–26,60]. Cost-effectiveness analysis presented in this study relies on generic assumptions of various parameters which will be different across regions, particularly where the prevalence is low and the labor cost is high.
Although this analysis concludes that the vaccination would be highly cost-effective investment in CHT region, however, a number of limitations should be taken into account as we made several assumptions which could affect the cost-effectiveness ratio. Since the malaria vaccine protection years figure is very low compared to other infectious vaccines; WHO does not yet recommend the inclusion of RTS,S/AS01 in the Expanded Programme of Immunisation due to this lower efficacy of vaccine [61]. Therefore, future development of vaccines considering the higher protective efficacy is prerequisite for such decision [61]. Again, due to unavailability of nationwide vaccine effectiveness and epidemiological data, we used various sources of data. Further, CHT had some distinct characteristics such as healthcare seeking behaviour, vaccine uptake, and lifestyle. Therefore, the actual scenario might be different than we found in our study. The country representative data (e.g., incidence rate, vaccination coverage) might be higher or lower, which can be handled via adequate sensitivity analysis. The other important limitation was that, we used static model rather than dynamic model. The dynamic model is most useful for predicting the cost-effectiveness of infectious disease control program as the indirect effect of reduced transmission is a significant public health gain. However, the dynamic model provided the higher cost-effectiveness ratio than the static model as higher coverage rate reduce the transmission of the disease, therefore, the result could be underestimating the actual benefit of the malaria vaccination program [59,62]. However, the advantage of static model is that it requires minimum data, can be built and understood easily and has a low cost. It was not possible to collect rigorous information that are essential for the dynamic model. It is often laborious to capture the seasonal factors even the probability that an unvaccinated susceptible is infected either from the environment or from direct contact due to presence of a single unvaccinated infective in such distinct area in CHT region. Although we did not compare the vaccination with other existing malaria control initiatives, RTS,S/AS01 has the potentiality for substantial reduction of malaria-related mortalities and morbidities, which suggests that malaria vaccination could potentially be a complementary intervention with other malaria control initiatives, especially in high malaria endemic districts of Bangladesh.
Conclusions
Introduction of malaria vaccination in CHT region is estimated to be a cost-effective preventive intervention and would offer substantial future benefits particularly for young children vaccinated today. Policies should, thus, consider the operational advantages of targeting these populations, particularly in the CHT area, with the vaccine along with other malaria control initiatives. A malaria surveillance targeting endemic is also recommended for addressing the data related gap which would enable to implement an effective malaria control program in high endemic districts of Bangladesh.
Acknowledgments
We would like to thank Bangladesh Institute of Development Studies (BIDS) for providing research support. The authors would also like to the thank Raisul Akram, a health economist researcher, for his comments on an earlier draft of the manuscript.
Data Availability
All relevant data are within the manuscript.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.WHO. Malaria [Internet]. Fact Sheet: World Malaria Report 2016. Geneva Switzerland; 2016. Available: http://www.who.int/malaria/media/world-malaria-report-2016/en/
- 2.Kyu HH, Degu Abate, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392: 1859–922. 10.1016/S0140-6736(17)32130-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Malaria vaccine: WHO position paper-January 2016. Wkly Epidemiol Rec. 2016;4: 33–52. 10.1371/jour [DOI] [PubMed] [Google Scholar]
- 4.UCSF Global Health Group. An Investment Case for Eliminating Malaria in Bangladesh [Internet]. San Francisco, CA; 2017. Available: http://www.shrinkingthemalariamap.org/sites/www.shrinkingthemalariamap.org/files/content/page/an-investment-case-for-eliminating-malaria-in-indonesia.pdf
- 5.Haque U, Overgaard HJ, Clements ACA, Norris DE, Islam N, Karim J, et al. Malaria burden and control in Bangladesh and prospects for elimination: An epidemiological and economic assessment. Lancet Glob Heal. Haque et al. Open Access article distributed under the terms of CC BY-NC-ND; 2014;2: e98–e105. 10.1016/S2214-109X(13)70176-1 [DOI] [PubMed] [Google Scholar]
- 6.Haque U, Sunahara T, Hashizume M, Shields T, Yamamoto T, Haque R, et al. Malaria prevalence, risk factors and spatial distribution in a Hilly forest area of Bangladesh. PLoS One. 2011;6 10.1371/journal.pone.0018908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haque U, Ahmed SM, Hossain S, Huda M, Hossain A, Alam MS, et al. Malaria prevalence in endemic districts of Bangladesh. PLoS One. 2009;4: 1–9. 10.1371/journal.pone.0006737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haque U, Soares Magalhães RJ, Mitra D, Kolivras KN, Schmidt W-P, Haque R, et al. The role of age, ethnicity and environmental factors in modulating malaria risk in Rajasthali, Bangladesh. Malar J. 2011;10: 367 10.1186/1475-2875-10-367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starzengruber P, Fuehrer H-P, Ley B, Thriemer K, Swoboda P, Habler V, et al. High prevalence of asymptomatic malaria in south-eastern Bangladesh. Malar J. 2014;13: 16 10.1186/1475-2875-13-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ezeoke OP, Onwujekwe OE, Uzochukwu BS. Towards universal coverage: Examining costs of illness, payment, and coping strategies to different population groups in Southeast Nigeria. Am J Trop Med Hyg. 2012;86: 52–57. 10.4269/ajtmh.2012.11-0090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onwujekwe O, Hanson K, Uzochukwu B, Ichoku H, Ike E, Onwughalu B. Are malaria treatment expenditures catastrophic to different socio-economic and geographic groups and how do they cope with payment? A study in southeast Nigeria. Trop Med Int Heal. 2010;15: 18–25. 10.1111/j.1365-3156.2009.02418.x [DOI] [PubMed] [Google Scholar]
- 12.Haque U, Glass GE, Bomblies A, Hashizume M, Mitra D, Noman N, et al. Risk factors associated with clinical malaria episodes in Bangladesh: A longitudinal study. American Journal of Tropical Medicine and Hygiene. 2013. 10.4269/ajtmh.12-0456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. Malaria vaccine development. In: Malaria [Internet]. 2016 [cited 22 Aug 2016]. Available: http://www.who.int/malaria/areas/vaccine/en/
- 14.Greenwood B. The contribution of vaccination to global health: Past, present and future. Philos Trans R Soc B Biol Sci. 2014;369 10.1098/rstb.2013.0433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozawa S, Mirelman A, Stack ML, Walker DG, Levine OS. Cost-effectiveness and economic benefits of vaccines in low- and middle-income countries: A systematic review. Vaccine. Elsevier Ltd; 2012;31: 96–108. 10.1016/j.vaccine.2012.10.103 [DOI] [PubMed] [Google Scholar]
- 16.Stack ML, Ozawa S, Bishai DM, Mirelman A, Tam Y, Niessen L, et al. Estimated economic benefits during the “decade of vaccines” include treatment savings, gains in labor productivity. Health Aff. 2011;30: 1021–1028. 10.1377/hlthaff.2011.0382 [DOI] [PubMed] [Google Scholar]
- 17.Burchett HED, Mounier-Jack S, Griffiths UK, Mills AJ. National decision-making on adopting new vaccines: A systematic review. Health Policy Plan. 2012;27 10.1093/heapol/czr049 [DOI] [PubMed] [Google Scholar]
- 18.Mahoney RT, Krattiger A, Clemens JD, Curtiss R. The introduction of new vaccines into developing countries. IV: Global Access Strategies. Vaccine. 2007;25: 4003–4011. 10.1016/j.vaccine.2007.02.047 [DOI] [PubMed] [Google Scholar]
- 19.Uddin J, Sarma H, Bari TI, Koehlmoos TP. Introduction of New Vaccines: Decision-making Process in Bangladesh. J Heal Popul Nutr. 2013;31: 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer TK, Anh DD, Antil L, Cat NDL, Kilgore PE, Thiem VD, et al. Health care costs of diarrheal disease and estimates of the cost-effectiveness of rotavirus vaccination in Vietnam. J Infect Dis. 2005;192: 1720–1726. 10.1086/497339 [DOI] [PubMed] [Google Scholar]
- 21.Pecenka C, Parashar U, Tate JE, Khan JAM, Groman D, Chacko S, et al. Impact and cost-effectiveness of rotavirus vaccination in Bangladesh. Vaccine. 2017;35: 3982–3987. 10.1016/j.vaccine.2017.05.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Troeger C, Sack DA, Chao DL. Evaluation of Targeted Mass Cholera Vaccination Strategies in Bangladesh: A Demonstration of a New Cost-Effectiveness Calculator. Am J Trop Med Hyg. 2014;91: 1181–1189. 10.4269/ajtmh.14-0159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.RTSS Clinical Trials Partnership. Effi cacy and safety of RTS, S / AS01 malaria vaccine with or without a booster dose in infants and children in Africa: fi nal results of a phase 3, individually randomised, controlled trial. Lancet. Elsevier Ltd; 2015;6736: 31–45. 10.1016/S0140-6736(15)60721-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galactionova K, Tediosi F, Camponovo F, Smith TA, Gething PW, Penny MA. Country specific predictions of the cost-effectiveness of malaria vaccine RTS,S/AS01 in endemic Africa. Vaccine. The Author(s); 2017;35: 53–60. 10.1016/j.vaccine.2016.11.042 [DOI] [PubMed] [Google Scholar]
- 25.The RTS SCTP. The Phase 3 Trial of RTS,S/AS01 Malaria Vaccine in African Infants. N Engl J Med. 2012; 367:2284–2295. 10.1056/NEJMoa1208394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winskill P, Walker PG, Griffin JT, Ghani AC. Modelling the cost-effectiveness of introducing the RTS,S malaria vaccine relative to scaling up other malaria interventions in sub-Saharan Africa. BMJ Glob Heal. 2017;2: e000090 10.1136/bmjgh-2016-000090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Longini IM, Nizam A, Ali M, Yunus M, Shenvi N, Clemens JD. Controlling endemic cholera with oral vaccines. PLoS Med. 2007;4: 1776–1783. 10.1371/journal.pmed.0040336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.BBS. Population projection of Bangladesh- Dynamics and Trends 2011–2061 [Internet]. Dhaka, Bangladesh: Bangladesh Bureau of Statistics, Ministry of Planning, Government of The People’s Republic Of Bangladesh; 2015. Available: www.bbs.gov.bd [Google Scholar]
- 29.Neafsey DE, Juraska M, Bedford T, Benkeser D, Valim C, Griggs A, et al. Genetic Diversity and Protective Efficacy of the RTS,S/AS01 Malaria Vaccine. N Engl J Med. 2015;373: 2025–2037. 10.1056/NEJMoa1505819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han L, Hudgens MG, Emch ME, Juliano JJ, Keeler C, Martinson F, et al. RTS,S/AS01 Malaria Vaccine Efficacy is Not Modified by Seasonal Precipitation: Results from a Phase 3 Randomized Controlled Trial in Malawi. Sci Rep. 2017;7: 7200 10.1038/s41598-017-07533-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost- Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [DOI] [Google Scholar]
- 32.Atherly D, Dreibelbis R, Parashar UD, Levin C, Wecker J, Rheingans RD. Rotavirus vaccination: cost-effectiveness and impact on child mortality in developing countries. J Infect Dis. 2009;200 Suppl: S28–38. 10.1086/605033 [DOI] [PubMed] [Google Scholar]
- 33.Tan-Torres E, Bultussen R, Adam T, Hutubessy R, Acharya A, Evans D, et al. Making choices in health: WHO guide to costeffectiveness analysis. Edejer T, Bultussen R, Adam T, Hutubessy R, Acharya A, Evans D, et al. , editors. World Health Organization. Geneva, Switzerland:World Health Organization. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 34.Murray CJL, Lopez AD. The global burden of disease: a comprehensive assessment of mortality and disability from disease,injuries and risk factors in 1990 and projected to 2020. Cambridge:Harvard University Press. Cambridge: Harvard University Press; 1996. [Google Scholar]
- 35.McKenna MT, Michaud CM, Murray CJL, Marks JS. Assessing the burden of disease in the United States using disability-adjusted life years. Am J Prev Med. 2005;28: 415–423. [DOI] [PubMed] [Google Scholar]
- 36.Megiddo I, Colson AR, Nandi A, Chatterjee S, Prinja S, Khera A, et al. Analysis of the Universal Immunization Programme and introduction of a rotavirus vaccine in India with IndiaSim. Vaccine. Elsevier Ltd; 2014;32: A151–A161. 10.1016/j.vaccine.2014.04.080 [DOI] [PubMed] [Google Scholar]
- 37.Lindstrand A, Bergstrom S, Rosling H, Rubenson B, Stenson B, Tylleskar T. Global Health: An Introductory Textbook. Copenhagen: Narayana Press; 2008. [Google Scholar]
- 38.Cook J, Jeuland M, Whittington D, Poulos C, Clemens J, Sur D, et al. The cost-effectiveness of typhoid Vi vaccination programs: Calculations for four urban sites in four Asian countries. Vaccine. 2008;26: 6305–6316. [DOI] [PubMed] [Google Scholar]
- 39.Jeuland M, Cook J, Poulos C, Clemens J, Whittington D. Cost-effectiveness of new-generation oral cholera vaccines: A multisite analysis. Value Heal. 2009;12: 899–908. 10.1111/j.1524-4733.2009.00562.x [DOI] [PubMed] [Google Scholar]
- 40.Alam MS, Kabir MM, Hossain MS, Naher S, Ahmed BN, Islam A, et al. Reduction in malaria prevalence and increase in malaria awareness in endemic districts of Bangladesh. Malar J. BioMed Central; 2016; 1–7. 10.1186/s12936-016-1603-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen IT, Aung T, Thant HNN, Sudhinaraset M, Kahn JG. Cost-effectiveness analysis of malaria rapid diagnostic test incentive schemes for informal private healthcare providers in Myanmar. Malar J. 2015;14: 55 10.1186/s12936-015-0569-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salomon JA, Vos T, Hogan DR, Gagnon M, Naghavi M, Mokdad A, et al. Common values in assessing health outcomes from disease and injury: Disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380: 2129–2143. 10.1016/S0140-6736(12)61680-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarker AR, Islam Z, Khan IA, Saha A, Chowdhury F, Khan AI, et al. Estimating the cost of cholera-vaccine delivery from the societal point of view: A case of introduction of cholera vaccine in Bangladesh. Vaccine. Elsevier Ltd; 2015;33: 4916–4921. 10.1016/j.vaccine.2015.07.042 [DOI] [PubMed] [Google Scholar]
- 44.White MT, Conteh L, Cibulskis R, Ghani AC. Costs and cost-effectiveness of malaria control interventions—a systematic review. Malar J. 2011;10: 337 10.1186/1475-2875-10-337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Etiaba E, Onwujekwe O, Uzochukwu B, Uguru N, Okoronkwo I, Adjagba A. What co-morbidities do people with malaria have and what are their patterns of health seeking in Nigeria? Niger J Clin Pract. 2015;18: 22–26. 10.4103/1119-3077.146974 [DOI] [PubMed] [Google Scholar]
- 46.National Institute of Population Research and Training, Mitra and Associates, ICF International. Bangladesh Demographic and Health Survey 2014: Key Indicators. 2015; 34. [Google Scholar]
- 47.Qadri F, Ali M, Chowdhury F, Khan A. Feasibility and eff ectiveness of oral cholera vaccine in an urban endemic setting in Bangladesh: a cluster randomised open-label trial. Lancet. 2015;386: 1362–71. [DOI] [PubMed] [Google Scholar]
- 48.Sarker AR, Akram R, Ali N, Sultana M. Coverage and factors associated with full immunisation among children aged 12–59 months in Bangladesh: Insights from the nationwide cross-sectional demographic and health survey. BMJ Open. 2019. 10.1136/bmjopen-2018-028020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murray J, McFarland DA, Waldman RJ. Cost-effectiveness of oral cholera vaccine in a stable refugee population at risk for epidemic cholera and in a population with endemic cholera. Bull World Health Organ. 1998;76: 343–352. [PMC free article] [PubMed] [Google Scholar]
- 50.World Health Organization. The World Malaria Report 2015. Geneva, Switzerland:; 2015.
- 51.WHO. World malaria report 2014. [Internet]. WHO GLOBAL MALARIA PROGRAMME. Geneva Switzerland; 2014. Available: http://www.who.int/malaria/publications/world_malaria_report_2014/wmr-2014-no-profiles.pdf?ua=1%5Cnhttp://doi.wiley.com/10.1002/(SICI)1096-987X(19981115)19:14%3C1639::AID-JCC10%3E3.0.CO;2-B%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3907
- 52.Greenwood B. Review: Intermittent preventive treatment—A new approach to the prevention of malaria in children in areas with seasonal malaria transmission. Trop Med Int Heal. 2006;11: 983–991. 10.1111/j.1365-3156.2006.01657.x [DOI] [PubMed] [Google Scholar]
- 53.Wongsrichanalai C, Barcus MJ, Muth S, Sutamihardja A, Wernsdorfer WH. A review of malaria diagnostic tools: Microscopy and rapid diagnostic test (RDT). Am J Trop Med Hyg. 2007;77: 119–127. 10.3126/ajms.v1i2.2965 [DOI] [PubMed] [Google Scholar]
- 54.Sinclair D, Zani B, Donegan S, Olliaro P, Garner P. Artemisinin-based combination therapy for treating uncomplicated malaria. Cochrane Database Syst Rev. 2009; CD007483 10.1002/14651858.CD007483.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanser FC, Pluess B, Lengeler C, Sharp BL. Indoor residual spraying for preventing malaria. Cochrane Database Syst Rev. 2007; 10.1002/14651858.CD006657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004; CD000363 10.1002/14651858.CD000363.pub2 [DOI] [PubMed] [Google Scholar]
- 57.MOHFW. Comprehensive Multi-year Plan: 2011–2016 Expanded Programme on Immunization (EPI) Bangladesh [Internet]. Dhaka, Bangladesh; 2010. Available: http://www.nationalplanningcycles.org/sites/default/files/country_docs/Bangladesh/bangladesh_cmyp2011-2016.pdf
- 58.Romore I, Njau RJA, Semali I, Mwisongo A, Ba Nguz A, Mshinda H, et al. Policy analysis for deciding on a malaria vaccine RTS,S in Tanzania. Malar J. BioMed Central; 2016;15: 143 10.1186/s12936-016-1197-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Penny MA, Verity R, Bever CA, Sauboin C, Galactionova K, Flasche S, et al. Public health impact and cost-effectiveness of the RTS,S/AS01 malaria vaccine: A systematic comparison of predictions from four mathematical models. Lancet. Penny et al. Open Access article distributed under the terms of CC BY; 2016;387: 367–375. 10.1016/S0140-6736(15)00725-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jin H, Wang B, Fang Z, Duan Z, Gao Q, Liu N, et al. Hospital-based study of the economic burden associated with rotavirus diarrhea in eastern China. Vaccine. Elsevier Ltd; 2011;29: 7801–6. 10.1016/j.vaccine.2011.07.104 [DOI] [PubMed] [Google Scholar]
- 61.Gosling R, von Seidlein L. The Future of the RTS,S/AS01 Malaria Vaccine: An Alternative Development Plan. PLoS Med. 2016;13: 1–6. 10.1371/journal.pmed.1001994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lugner AK, Myliusa SD, Wallinga J. Dynamic versus static models in cost-effectiveness analyses of anti-viral drug therapy to mitigate an influenza pandemic. Health Econ. 2010;19: 518–531. 10.1002/hec.1485 [DOI] [PubMed] [Google Scholar]
- 63.Jeuland M, Lucas M, Clemens J, Whittington D. A Cost–Benefit Analysis of Cholera Vaccination Programs in Beira, Mozambique. World Bank Econ Rev. 2009;23: 1–33. [Google Scholar]
- 64.BBS. Report on Bangladesh Sample Vital Statistics 2014 [Internet]. Reproduction Documentation & Publication Section Bangladesh Bureau of Statistics, editor. Dhaka, Bangladesh: Monitoring the Situation of Vital Statistics of Bangladesh (MSVSB) Project Bangladesh Bureau of Statistics; 2015. Available: www.bbs.gov.bd
- 65.Jamison DT, Breman JG, Anthony R. Measham AR, Alleyne G, Claeson, Evans D V, et al. Disease control priorities in developing countries. Second TheWorld Bank and Oxford University Press. Washington DC: OXFORD UNIVERSITY PRESS; 2006. [PubMed] [Google Scholar]
- 66.Drummond M, Sculpher MJ, Laxton KC, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Third edit Oxford University Press; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.