Abstract
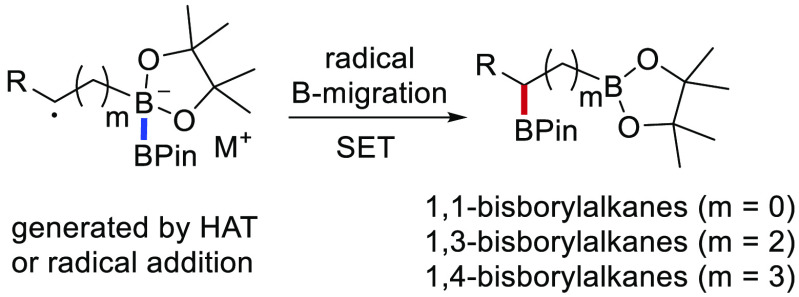
A systematic study of radical boron migration in diboronate complexes to form synthetically valuable 1,n-bisborylalkanes is reported. The boronate complexes are readily generated by reaction of commercial bis(pinacolato)diboron with alkyl Grignard compounds. C-radical generation at a defined position with respect to the diboron moiety is achieved either via intermolecular H-abstraction with a CF3-radical or via alkene perfluoroalkyl radical addition. It is shown that radical 1,2- and 1,4-boron migrations to provide geminal and 1,3-bisborylalkanes are efficient transformations. The 1,5-boron migration in the homologous series leading to 1,4-bisborylalkanes is also occurring, albeit with lower efficiency. Experimental results are supported by DFT calculations which also reveal the corresponding 1,3-boron migration in such diboronate complexes to be feasible.
Bisborylalkanes are functionalized and versatile building blocks in organic synthesis. Such B-compounds can act as coupling partners in transition-metal-catalyzed cross-coupling reactions or as radical precursors, and both boryl moieties can, in principle, be selectively converted to different functionalities.1 Boronate complexes are reactive intermediates that are readily generated by the reaction of organoboronic esters with organometallic reagents. These B-ate complexes are reducing species that also undergo facile hydrolysis.2 Recently, radical chemistry on boronate complexes has emerged.3 It was found that C-radicals of type I derived from boronate complexes are readily oxidized by single electron transfer (SET) to give zwitterions of type II in a radical/polar crossover step (Scheme 1A). Intermediates II in turn undergo a Matteson-type 1,2-alkyl/aryl shift to afford α-functionalized boronic esters III.4 Although such transformations on boronate complexes generated from alkyl and aryl boronic esters are meanwhile well investigated, the corresponding radical reactions on diboronate complexes derived from diborons (see IV) are underdeveloped. Along these lines, Shi recently reported the construction of 1,1-bisborylalkanes enabled by radical addition/1,2-boron migration. As mechanism of the boron migration, it was proposed that diboronate complexes IV (m = 0) are SET-oxidized to intermediates of type II (R′ = BPin), which rearrange to geminal bisborylalkanes via an ionic process.5 Herein, we disclose our results on the systematic study of radical boron migration in radical anions of type IV (m = 0–4) to give intermediates V, where the two B-atoms can interact. SET oxidation finally leads to 1,n-bisborylalkanes VI (Scheme 1B). Considering the 1,2-boron shift, the radicals VIII are generated from ate complexes VII via intermolecular hydrogen atom transfer (HAT)4d (Scheme 1C). Further, we will provide mechanistic insights into the reported5 1,2-boron migration. In all other cases, site-selective C-radical generation (see XI) is achieved via radical addition to B-ate complexes of type X (Scheme 1D).
Scheme 1. Synthesis of 1,n-Bisborylalkanes via Radical Boron Migration.
The 1,2-boron shift to access geminal bisborylalkanes was studied first. Notably, 1,1-bisborylalkanes are important building blocks to access multifunctionalized compounds.6,7 The current methods to prepare these compounds use gem-dihalides,8 diazo compounds,9 alkynes,10 alkenes, etc.11 as substrates and mostly require a transition metal to catalyze or mediate the transformation.12 It is of interest to develop a convenient method to access 1,1-bisborylalkanes from simple starting materials under transition-metal-free conditions.
In 2019, our group achieved transition-metal-free cross-coupling of organometallic reagents and organoboronic esters by intermolecular α-HAT on the corresponding boronate complexes with the trifluoromethyl radical followed by SET oxidation and ionic 1,2-alkyl/aryl migration.4d Encouraged by this study, we decided to apply this strategy to prepare 1,1-bisborylalkanes via diboronate complexes (see Scheme 1C). The reaction of bis(pinacolato)diboron (B2Pin2) with cyclopentylmagnesium bromide (1.2 equiv) targeting gem-bisborylalkane 2a was selected for optimization (Table 1).
Table 1. Reaction Optimization for the 1,1-Diborylation of Cyclopentylmagnesium Bromide with B2Pin2a.
| yield (%) |
||||
|---|---|---|---|---|
| entry | PC | 2a | Cp-Bpin | conv (%) |
| 1 | Ir(ppy)3 | 59 | 8 | 86 |
| 2 | Ir(ppy)2(dtbbpy)PF6 | 62 | 7 | 85 |
| 3 | Ru(bpy)3(PF6)2 | 63 | 9 | 82 |
| 4 | Eosin Y | 56 | 8 | 83 |
| 5 | Rose Bengal | 35 | 20 | 82 |
| 6 | Rhodamine B base | 49 | 5 | 74 |
| 7b | Ru(bpy)3(PF6)2 | 52 | 7 | 82 |
| 8c | Ru(bpy)3(PF6)2 | 18 | 28 | 100 |
| 9 | – | 30 | 20 | 65 |
| 10d | Ru(bpy)3(PF6)2 | 68 | <1 | 82 |
| 11e | – | 76 (74f) | <1 | 91 |
| 12g | – | 16 | 17 | 61 |
Reactions were conducted on a 0.2 mmol scale in CH3CN (2 mL), conversion (conv) was determined based on the recovered bisboryl reagent, and yields were determined by GC analysis with n-tetradecane as internal standard on the crude reaction mixture.
Cyclopentylmagnesium chloride used instead of cyclopentylmagnesium bromide.
Bis(neopentyl glycolato)diboron (B2(neop)2) used instead of bis(pinacolato)diboron (B2Pin2).
Reaction conducted at −20 °C.
365 nm (3 W) at −20 °C.
Isolated yield.
Cyclopentyllithium used instead of cyclopentylmagnesium bromide.
The diboronate complex 1a was generated in 1,2-dimethoxyethane (DME) at 0 °C. The solvent was exchanged by acetonitrile, and CF3I was chosen as the terminal oxidant, with the CF3-radical engaging in selective HAT abstraction at the α-position to the B-atom in B-ate complexes.4a Pleasingly, with tris[2-phenylpyridinato-C2,N]iridium(III) (Ir(ppy)3, 1 mol%) as a smart initiator13 at room temperature, 2a was formed in 59% yield (Table 1, entry 1). Besides 2a, we detected 8% cyclopentylboronic acid pinacol ester (Cp-BPin) and 14% B2Pin2. Other metal-based and organic initiators gave similar results (entries 2–6). The complex derived from cyclopentylmagnesium chloride provided a slightly lower yield (52%, entry 7, compare with entry 3), but the yield significantly dropped to 18% with bis(neopentyl glycolato)diboron (B2(neop)2) in place of B2Pin2 (entry 8). Without redox initiator, the reaction also proceeded, albeit with lower efficiency (entry 9). Upon lowering the temperature, formation of cyclopentylboronic acid pinacol ester was suppressed and the yield of 2a improved to 68% (entry 10). Initiation by simple UV (365 nm) irradiation at −20 °C led to a further improvement, providing 2a in 76% yield (entry 11). The boronate complex derived from cyclopentyllithium gave a poor yield under the optimized conditions (16%, entry 12).
With optimized conditions in hand, we prepared a series of 1,1-bisborylalkanes from different alkyl Grignard reagents (Table 2). Both primary (2c–d) and secondary (2a,b,e–o) alkylmagnesium bromides engaged in the transformation to afford 1,1-bisborylalkanes in moderate to good yields (28–80%). Some functionalities such as phenyl, trifluoromethyl, and alkoxy moieties were tolerated. The reaction was found to be sensitive to sterics. For example, ate complex 1b derived from 3-phenyl cyclopentylmagnesium bromide delivered the 1,1-bisboron compound 2b with a decreased yield (44%) as compared to its less bulky congener 1a (74%). Considering diboronate complexes derived from primary alkylmagnesium bromides, the less bulky ethyl derivative (2c) gave a slightly better yield than the corresponding butyl-ate complex (2d). For complexes generated from secondary alkylmagnesium bromides, the least bulky isopropyl system provided the highest yield (80%, 2e). Due to the higher steric demand of an ethyl over a methyl group, the yield of 2o was lower than the yields obtained for 1,1-bisborylalkanes 2e–n. Of note, as Grignard reagents can be easily accessed from commercial alkyl bromides, the introduced method offers a cheap and convenient approach to 1,1-bisborylalkanes.
Table 2. Geminal Diborylation of Alkylmagnesium Bromides—Scopea.
Conducted at 0.2 mmol scale. Isolated yields provided in all cases.
We noted that there is currently no general method available for the synthesis of 1,n-bisborylalkanes (n > 1),8a,14 and we assumed the unprecedented remote radical B-migration to offer a new approach to access such compounds. Along these lines, we first addressed the 1,4-boron migration and selected 3a, generated by reacting B2Pin2 with but-3-enylmagnesium bromide, as model substrate. To our delight, visible light irradiation (465 nm) of 3a in the presence of C4F9I (1.5 equiv) and Ir(ppy)3 (1 mol%) as an initiator in CH3CN provided the 1,3-bisborylalkane 4a in 79% yield besides the iodine atom transfer product 4a-I (4%) and recovered B2Pin2 (13%) (Table 3, entry 1). Solvent screening revealed that better yields were obtained in CH3CN than in other polar solvents like DMSO, DMF, and DMA (entries 1–4). Ir(ppy)3 could be replaced by organic dyes such as Eosin Y, Rose Bengal, and Rhodamine B base without diminishing the yield (76–79%, entries 5–7). Without redox initiator, the reaction also worked, but with lower efficiency (44%, entry 8), and with the cheap organic Rhodamine B base as smart initiator, the yield further increased to 87% upon lowering the temperature to −20 °C (entry 9). Notably, simple UV irradiation (365 nm) at −20 °C in the absence of any initiator provided a similar yield (entry 10). The boronate complex derived from but-3-enyllithium gave a poor yield under the optimized conditions (5%, entry 11).
Table 3. Reaction Optimization for 1,3,4-Trifunctionalization of Homoallylmagnesium Bromide—1,4-Boron Migrationa.
| yield (%) |
|||||
|---|---|---|---|---|---|
| entry | PC | solvent | 4a | 4a-I | conv (%) |
| 1 | Ir(ppy)3 | CH3CN | 79 | 4 | 87 |
| 2 | Ir(ppy)3 | DMSO | <1 | 33 | 85 |
| 3 | Ir(ppy)3 | DMF | 5 | 11 | 91 |
| 4 | Ir(ppy)3 | DMA | 2 | 2 | 93 |
| 5 | Eosin Y | CH3CN | 76 | 3 | 77 |
| 6 | Rose Bengal | CH3CN | 75 | 4 | 80 |
| 7 | Rhodamine B base | CH3CN | 79 | 3 | 80 |
| 8 | – | CH3CN | 44 | 16 | 94 |
| 9b | Rhodamine B base | CH3CN | 87 (83c) | 3 | 90 |
| 10d | – | CH3CN | 87 | 3 | 95 |
| 11e | Rhodamine B base | CH3CN | 5 | – | 57 |
Reactions were conducted on a 0.2 mmol scale in the specified solvent (2 mL), conversion (conv) was determined based on recovered bisboryl reagent, and yields were determined by crude GC analysis with n-tetradecane as internal standard.
Conducted at −20 °C.
Isolated yield.
365 nm (3 W) at −20 °C.
But-3-enyllithium, in situ generated by lithium/iodine exchange reaction of t-BuLi and 4-iodo-1-butene, used instead of but-3-enylmagnesium bromide, and THF instead of DME as solvent.
A scope study of the trifunctionalization of homoallyl Grignard reagents was conducted by applying the visible light/Rhodamine B base initiation protocol (Table 3, entry 9) that proved to be more general than the UV-initiation protocol. The perfluoroalkyl radical precursor was varied first, keeping complex 3a as the acceptor (Table 4). Linear n-perfluoroalkyl iodides provided the trifunctionalized 1,3-diboranes 4b–e in excellent yields (82–93%). The less reactive n-perfluoroalkyl bromide also worked as C-radical precursor, but as compared to the iodides, the yield dropped slightly (62%, 4f). With perfluoroisopropyl iodide, a 64% yield of 4g was obtained. 1-Chloro-2-iodotetrafluoroethane reacted chemoselectively at the I-bearing C-atom to give 4h (61%). Iodoacetonitrile and ethyl iodoacetate gave only trace amounts of the targeted products (not shown). Unfortunately, diastereoselectivity for the 1,4-boron shift in open-chain systems was very low (see 4i,j). However, for the cyclic rigid diboronate complex 3k, 1,4-syn-boron-migration selectivity was complete, and also the initial CF3-radical addition occurred with excellent stereocontrol (trans-addition) to provide product 4k as a single diastereoisomer (64%).
Table 4. 1,4- and 1,5-Boron Migration Reactionsa.
Conducted on a 0.2 mmol scale.
n-C6F13Br was used.
Reaction conducted at room temperature.
With 4-pentenylmagnesium bromide as starting material, we next addressed the 1,5-boron migration and noted that, with perfluoroalkyl iodides as C-radical precursors, the I-atom transfer compounds 5-I (not shown) were formed as major products and targets 5 were obtained in low yields. Therefore, we had to switch to the less reactive bromides. A moderate 31% yield of 5a was achieved with n-perfluorohexyl bromide under the conditions optimized for the 1,4-boron shift. The yield could be slightly improved to 44% (see 5b) by installing a 3,3-dimethyl substitution pattern, benefiting from the Thorpe–Ingold effect.15
We also attempted the 1,6-boron migration on the homologous diboronate complex derived from 5-hexenylmagnesium bromide with CF3I as the radical precursor. However, the targeted 1,5 bisborylalkane was not identified, and the reaction provided the corresponding I-atom transfer product as the major product. Switching to n-perfluorohexyl bromide, the 1,6-boron migration product 6 could not be identified, indicating that this migration cannot compete with other processes.
Finally, to complete the series, we tackled the 1,3-boron migration. The required diboronate complex was formed by the reaction of allylmagnesium bromide with B2Pin2. However, neither with perfluorobutyl iodide nor with its bromide was any 1,2-bisborylalkane identified, and B2Pin2 was formed in a large amount (85%) as major product. Hence, SET oxidation of allyl-B2Pin2MgX under all tested conditions generating the stabilized allyl radical was too fast, and therefore this alkene could not act as a radical acceptor under the applied conditions. Since we did not find any suitable system to experimentally investigate the 1,3-boron migration, we decided to approach that problem by using computational chemistry. DFT calculations16 were performed on a series of pent-(m+1)-yl-substituted radical anions 7a–d (m = 0–3, Scheme 2) to get a full picture on the boron migration aptitude in these diboronate radical anions.
Scheme 2. DFT Model Calculations of Diboronate Radical Anion Rearrangements.
In the study of the reactions, we have found bisborane radical anion intermediates 8 with the spin localized in a B–B single electron bond, similar to those found in the 1,2-carboboration of alkenes with B2Cat2.17 In the case of the 1,2-boron migration (m = 0),18 the initial radical 7a exhibits this structure already, which means that 1,2-boron radical migration is a spontaneous and barrierless process. In the case of the distonic (m > 0) radical anions 7b–d, a cyclization occurs with low free energy barriers (7–12 kcal/mol) to form the analogous intermediates 8b–d exergonically. These will readily transfer—likely assisted by the MgBr countercation17c—one electron to the iodo reagent and regenerate the trifluoromethyl radical, forming the 1,(m+1)-bisborylalkanes 9a–d. Compared to the 1,4-boron migration, the barrier for the radical 1,3-boron migration increases (from 7.1 to 11.8 kcal/mol). The 1,5-boron migration (8.1 kcal/mol) showed a slightly higher barrier than the 1,4-shift. In the case of radical anion 7b (1,3-boron migration), in the computation we did not find any indication for facile β-fragmentation leading to the B2Pin2-radical anion along with 1-pentene. This supports our suggestion that the observed formation of B2Pin2 in the reaction with allyl-B2Pin2MgX is likely caused by initial SET oxidation of allyl-B2Pin2MgX rather than β-fragmentation of the corresponding distonic radical anion of type 7b.
In summary, radical 1,2- and 1,4-boron migration reactions in diboronate complexes derived from B2Pin2 are useful preparative processes to access synthetically valuable 1,1- and 1,3-bisborylalkanes. Considering the 1,3-functionalized compounds, high selectivity in the boron migration can be achieved in cyclic systems. The 1,5-boron migration leading to 1,4-bisborylalkanes also occurs, albeit with lower efficiency. The experimental findings on the B-shift were supported by DFT calculations, which further revealed the currently experimentally inaccessible 1,3-boron migration to be feasible. Since B2Pin2 is commercially available and the Grignard reagents are readily prepared from the corresponding alkyl bromides, the introduced methods offer a straightforward approach to 1,n-bisborylalkanes.
Acknowledgments
We thank the Alexander von Humboldt foundation (postdoctoral fellowship to D.W.) and the European Research Council ERC (advanced grant agreement No. 692640) for supporting this work.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.0c03058.
Experimental details and characterization data, DFT calculations, and NMR spectra of new compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Duret G.; Quinlan R.; Bisseret P.; Blanchard N. Boron chemistry in a new light. Chem. Sci. 2015, 6, 5366–5382. 10.1039/C5SC02207J. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ollivier C.; Renaud P. Organoboranes as a Source of Radicals. Chem. Rev. 2001, 101, 3415–3434. 10.1021/cr010001p. [DOI] [PubMed] [Google Scholar]
- a Namirembe S.; Morken J. P. Reactions of organoboron compounds enabled by catalyst-promoted metalate shifts. Chem. Soc. Rev. 2019, 48, 3464–3474. 10.1039/C9CS00180H. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Leonori D.; Aggarwal V. K. Lithiation–Borylation Methodology and Its Application in Synthesis. Acc. Chem. Res. 2014, 47, 3174–3183. 10.1021/ar5002473. [DOI] [PubMed] [Google Scholar]
- a Kumar N.; Reddy R. R.; Eghbarieh N.; Masarwa A. α-Borylalkyl radicals: their distinctive reactivity in modern organic synthesis. Chem. Commun. 2020, 56, 13–25. 10.1039/C9CC08027A. [DOI] [PubMed] [Google Scholar]; b Kischkewitz M.; Friese F. W.; Studer A. Radical-induced 1,2-Migrations of Boron Ate Complexes. Adv. Synth. Catal. 2020, 10.1002/adsc.201901503. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Tappin N. D. C.; Renaud P. Radical Reactions of Boron-Ate Complexes Promoting a 1,2-Metaallate Rearrangement. Chimia 2020, 74, 33–38. 10.2533/chimia.2020.33. [DOI] [PubMed] [Google Scholar]
- a Kischkewitz M.; Okamoto K.; Mück-Lichtenfeld C.; Studer A. Radical-polar crossover reactions of vinylboron ate complexes. Science 2017, 355, 936–938. 10.1126/science.aal3803. [DOI] [PubMed] [Google Scholar]; b Gerleve C.; Kischkewitz M.; Studer A. Synthesis of alpha-Chiral Ketones and Chiral Alkanes Using Radical Polar Crossover Reactions of Vinyl Boron Ate Complexes. Angew. Chem., Int. Ed. 2018, 57, 2441–2444. 10.1002/anie.201711390. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Kischkewitz M.; Gerleve C.; Studer A. Radical-Polar Crossover Reactions of Dienylboronate Complexes: Synthesis of Functionalized Allylboronic Esters. Org. Lett. 2018, 20, 3666–3669. 10.1021/acs.orglett.8b01459. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Wang D.; Mück-Lichtenfeld C.; Studer A. Hydrogen Atom Transfer Induced Boron Retaining Coupling of Organoboronic Esters and Organolithium Reagents. J. Am. Chem. Soc. 2019, 141, 14126–14130. 10.1021/jacs.9b07960. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Silvi M.; Sandford C.; Aggarwal V. K. Merging Photoredox with 1,2-Metallate Rearrangements: The Photochemical Alkylation of Vinyl Boronate Complexes. J. Am. Chem. Soc. 2017, 139, 5736–5739. 10.1021/jacs.7b02569. [DOI] [PubMed] [Google Scholar]; f Lovinger G. J.; Morken J. P. Ni-Catalyzed Enantioselective Conjunctive Coupling with C(sp3) Electrophiles: A Radical-Ionic Mechanistic Dichotomy. J. Am. Chem. Soc. 2017, 139, 17293–17296. 10.1021/jacs.7b10519. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Tappin N. D. C.; Gnägi-Lux M.; Renaud P. Radical-Triggered Three-Component Coupling Reaction of Alkenylboronates, α-Halocarbonyl Compounds, and Organolithium Reagents: The Inverse Ylid Mechanism. Chem. - Eur. J. 2018, 24, 11498–11502. 10.1002/chem.201802384. [DOI] [PubMed] [Google Scholar]
- Zhao B.; Li Z.; Wu Y.; Wang Y.; Qian J.; Yuan Y.; Shi Z. An Olefinic 1,2-Boryl-Migration Enabled by Radical Addition: Construction of gem-Bis(boryl)alkanes. Angew. Chem., Int. Ed. 2019, 58, 9448–9452. 10.1002/anie.201903721. [DOI] [PubMed] [Google Scholar]
- a Wu C.; Wang J. Geminal bis(boron) compounds: Their preparation and synthetic applications. Tetrahedron Lett. 2018, 59, 2128–2140. 10.1016/j.tetlet.2018.04.061. [DOI] [Google Scholar]; b Nallagonda R.; Padala K.; Masarwa A. gem-Diborylalkanes: recent advances in their preparation, transformation and application. Org. Biomol. Chem. 2018, 16, 1050–1064. 10.1039/C7OB02978K. [DOI] [PubMed] [Google Scholar]; c Miralles N.; Maza R. J.; Fernández E. Synthesis and Reactivity of 1,1-Diborylalkanes towards C–C Bond Formation and Related Mechanisms. Adv. Synth. Catal. 2018, 360, 1306–1327. 10.1002/adsc.201701390. [DOI] [Google Scholar]
- a Zheng P.; Zhai Y.; Zhao X.; Xu T. Difunctionalization of ketones via gem-bis(boronates) to synthesize quaternary carbon with high selectivity. Chem. Commun. 2018, 54, 13375–13378. 10.1039/C8CC07781A. [DOI] [PubMed] [Google Scholar]; b Sun W.; Wang L.; Xia C.; Liu C. Dual Functionalization of α-Monoboryl Carbanions through Deoxygenative Enolization with Carboxylic Acids. Angew. Chem. 2018, 130, 5599–5603. 10.1002/ange.201801679. [DOI] [PubMed] [Google Scholar]; c Miura T.; Nakahashi J.; Zhou W.; Shiratori Y.; Stewart S. G.; Murakami M. Enantioselective Synthesis of anti-1,2-Oxaborinan-3-enes from Aldehydes and 1,1-Di(boryl)alk-3-enes Using Ruthenium and Chiral Phosphoric Acid Catalysts. J. Am. Chem. Soc. 2017, 139, 10903–10908. 10.1021/jacs.7b06408. [DOI] [PubMed] [Google Scholar]; d Miura T.; Nakahashi J.; Murakami M. Enantioselective Synthesis of (E)-δ-Boryl-Substituted anti-Homoallylic Alcohols Using Palladium and a Chiral Phosphoric Acid. Angew. Chem. 2017, 129, 7093–7097. 10.1002/ange.201702611. [DOI] [PubMed] [Google Scholar]; e Liu X.; Deaton T. M.; Haeffner F.; Morken J. P. A Boron Alkylidene–Alkene Cycloaddition Reaction: Application to the Synthesis of Aphanamal. Angew. Chem., Int. Ed. 2017, 56, 11485–11489. 10.1002/anie.201705720. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Lee Y.; Baek S.-y.; Park J.; Kim S.-T.; Tussupbayev S.; Kim J.; Baik M.-H.; Cho S. H. Chemoselective Coupling of 1,1-Bis[(pinacolato)boryl]alkanes for the Transition-Metal-Free Borylation of Aryl and Vinyl Halides: A Combined Experimental and Theoretical Investigation. J. Am. Chem. Soc. 2017, 139, 976–984. 10.1021/jacs.6b11757. [DOI] [PubMed] [Google Scholar]; g Murray S. A.; Green J. C.; Tailor S. B.; Meek S. J. Enantio- and Diastereoselective 1,2-Additions to α-Ketoesters with Diborylmethane and Substituted 1,1-Diborylalkanes. Angew. Chem., Int. Ed. 2016, 55, 9065–9069. 10.1002/anie.201603465. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Jo W.; Kim J.; Choi S.; Cho S. H. Transition-Metal-Free Regioselective Alkylation of Pyridine N-Oxides Using 1,1-Diborylalkanes as Alkylating Reagents. Angew. Chem. 2016, 128, 9842–9846. 10.1002/ange.201603329. [DOI] [PubMed] [Google Scholar]; i Joannou M. V.; Moyer B. S.; Meek S. J. Enantio- and Diastereoselective Synthesis of 1,2-Hydroxyboronates through Cu-Catalyzed Additions of Alkylboronates to Aldehydes. J. Am. Chem. Soc. 2015, 137, 6176–6179. 10.1021/jacs.5b03477. [DOI] [PubMed] [Google Scholar]; j Joannou M. V.; Moyer B. S.; Goldfogel M. J.; Meek S. J. Silver(I)-Catalyzed Diastereoselective Synthesis of anti-1,2-Hydroxyboronates. Angew. Chem., Int. Ed. 2015, 54, 14141–14145. 10.1002/anie.201507171. [DOI] [PubMed] [Google Scholar]; k Sun C.; Potter B.; Morken J. P. A Catalytic Enantiotopic-Group-Selective Suzuki Reaction for the Construction of Chiral Organoboronates. J. Am. Chem. Soc. 2014, 136, 6534–6537. 10.1021/ja500029w. [DOI] [PMC free article] [PubMed] [Google Scholar]; l Potter B.; Szymaniak A. A.; Edelstein E. K.; Morken J. P. Nonracemic Allylic Boronates through Enantiotopic-Group-Selective Cross-Coupling of Geminal Bis(boronates) and Vinyl Halides. J. Am. Chem. Soc. 2014, 136, 17918–17921. 10.1021/ja510266x. [DOI] [PMC free article] [PubMed] [Google Scholar]; m Hong K.; Liu X.; Morken J. P. Simple Access to Elusive α-Boryl Carbanions and Their Alkylation: An Umpolung Construction for Organic Synthesis. J. Am. Chem. Soc. 2014, 136, 10581–10584. 10.1021/ja505455z. [DOI] [PMC free article] [PubMed] [Google Scholar]; n Lee J. C. H.; McDonald R.; Hall D. G. Enantioselective preparation and chemoselective cross-coupling of 1,1-diboron compounds. Nat. Chem. 2011, 3, 894–899. 10.1038/nchem.1150. [DOI] [PubMed] [Google Scholar]; o Endo K.; Ohkubo T.; Hirokami M.; Shibata T. Chemoselective and Regiospecific Suzuki Coupling on a Multisubstituted sp3-Carbon in 1,1-Diborylalkanes at Room Temperature. J. Am. Chem. Soc. 2010, 132, 11033–11035. 10.1021/ja105176v. [DOI] [PubMed] [Google Scholar]
- a Ito H.; Kubota K. Copper(I)-Catalyzed Boryl Substitution of Unactivated Alkyl Halides. Org. Lett. 2012, 14, 890–893. 10.1021/ol203413w. [DOI] [PubMed] [Google Scholar]; b Hata T.; Kitagawa H.; Masai H.; Kurahashi T.; Shimizu M.; Hiyama T. Geminal Difunctionalization of Alkenylidene-Type Carbenoids by Using Interelement Compounds. Angew. Chem., Int. Ed. 2001, 40, 790–792. . [DOI] [PubMed] [Google Scholar]
- a Li H.; Shangguan X.; Zhang Z.; Huang S.; Zhang Y.; Wang J. Formal Carbon Insertion of N-Tosylhydrazone into B–B and B–Si Bonds: gem-Diborylation and gem-Silylborylation of sp3 Carbon. Org. Lett. 2014, 16, 448–451. 10.1021/ol403338s. [DOI] [PubMed] [Google Scholar]; b Li H.; Wang L.; Zhang Y.; Wang J. Transition-Metal-Free Synthesis of Pinacol Alkylboronates from Tosylhydrazones. Angew. Chem. 2012, 124, 2997–3000. 10.1002/ange.201108139. [DOI] [PubMed] [Google Scholar]; c Abu Ali H.; Goldberg I.; Srebnik M. Addition Reactions of Bis(pinacolato)diborane(4) to Carbonyl Enones and Synthesis of (pinacolato)2BCH2B and (pinacolato)2BCH2CH2B by Insertion and Coupling. Organometallics 2001, 20, 3962–3965. 10.1021/om010282r. [DOI] [Google Scholar]
- Endo K.; Hirokami M.; Shibata T. Synthesis of 1,1-Organodiboronates via Rh(I)Cl-Catalyzed Sequential Regioselective Hydroboration of 1-Alkynes. Synlett 2009, 2009, 1331–1335. 10.1055/s-0028-1088131. [DOI] [Google Scholar]
- a Teo W. J.; Ge S. Cobalt-Catalyzed Enantioselective Synthesis of Chiral gem-Bis(boryl)alkanes. Angew. Chem., Int. Ed. 2018, 57, 12935–12939. 10.1002/anie.201805705. [DOI] [PubMed] [Google Scholar]; b Feng X.; Jeon H.; Yun J. Regio- and Enantioselective Copper(I)-Catalyzed Hydroboration of Borylalkenes: Asymmetric Synthesis of 1,1-Diborylalkanes. Angew. Chem., Int. Ed. 2013, 52, 3989–3992. 10.1002/anie.201208610. [DOI] [PubMed] [Google Scholar]
- a Palmer W. N.; Obligacion J. V.; Pappas I.; Chirik P. J. Cobalt-Catalyzed Benzylic Borylation: Enabling Polyborylation and Functionalization of Remote, Unactivated C(sp3)–H Bonds. J. Am. Chem. Soc. 2016, 138, 766–769. 10.1021/jacs.5b12249. [DOI] [PubMed] [Google Scholar]; b Cho S. H.; Hartwig J. F. Iridium-catalyzed diborylation of benzylic C–H bonds directed by a hydrosilyl group: synthesis of 1,1-benzyldiboronate esters. Chem. Sci. 2014, 5, 694–698. 10.1039/C3SC52824C. [DOI] [Google Scholar]
- Studer A.; Curran D. P. Catalysis of Radical Reactions: A Radical Chemistry Perspective. Angew. Chem., Int. Ed. 2016, 55, 58–102. 10.1002/anie.201505090. [DOI] [PubMed] [Google Scholar]
- Blair D. J.; Tanini D.; Bateman J. M.; Scott H. K.; Myers E. L.; Aggarwal V. K. Selective uni- and bidirectional homologation of diborylmethane. Chem. Sci. 2017, 8, 2898–2903. 10.1039/C6SC05338F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M. E.; Piizzi G. gem-Disubstituent Effect: Theoretical Basis and Synthetic Applications. Chem. Rev. 2005, 105, 1735–1766. 10.1021/cr940337h. [DOI] [PubMed] [Google Scholar]
- a Perdew J. P.; Ernzerhof M.; Burke K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 1996, 105, 9982–9985. (PBE0) 10.1063/1.472933. [DOI] [Google Scholar]; b Goerigk L.; Grimme S. Efficient and accurate double-hybrid-meta-GGA density functionals—Evaluation with the extended GMTKN30 database for general main group thermo-chemistry, kinetics, and noncovalent interactions. J. Chem. Theory Comput. 2011, 7, 291–309. (PWPB5-D3) 10.1021/ct100466k. [DOI] [PubMed] [Google Scholar]; For more details of the DFT calculations, see the Supporting Information.
- a Cheng Y.; Mück-Lichtenfeld C.; Studer A. Transition Metal-Free 1,2-Carboboration of Unactivated Alkenes. J. Am. Chem. Soc. 2018, 140, 6221–6225. 10.1021/jacs.8b03333. [DOI] [PMC free article] [PubMed] [Google Scholar]; See also:; b Cheng Y.; Mück-Lichtenfeld C.; Studer A. Metal-Free Radical Borylation of Alkyl and Aryl Iodides. Angew. Chem., Int. Ed. 2018, 57, 16832–16836. 10.1002/anie.201810782. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Calculations in the presence of the MgBr countercation indicate that the additional electron in the bisborane radical anion intermediates 8 is transferred to the countercation (see the Supporting Information).
- 1,2-Boron migrations from carbon to C-centered radicals are known:; a Batey R. A.; Smil D. V. The First Boron-Tethered Radical Cyclizations and Intramolecular Homolytic Substitutions at Boron. Angew. Chem., Int. Ed. 1999, 38, 1798–1800. . [DOI] [PubMed] [Google Scholar]; b Kaiser D.; Noble A.; Fasano V.; Aggarwal V. K. 1,2-Boron Shifts of β-Boryl Radicals Generated from Bis-boronic Esters Using Photoredox Catalysis. J. Am. Chem. Soc. 2019, 141, 14104–14109. 10.1021/jacs.9b07564. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Jana K.; Bhunia A.; Studer A. Radical 1,3-Difunctionalization of Allylboronic Esters with Concomitant 1,2-Boron Shift. Chem. 2020, 6, 512–522. 10.1016/j.chempr.2019.12.022. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.