Abstract
Background.
Although mental illness accounts for only 4% of aggressive behavior in the general population, there remains a modest association between aggressive behavior and psychotic disorders, particularly in the early stages of the illness. However, little is known about the specific factors associated to this increased risk.
Aims.
The present study aims to assess the rates, characteristics and risk factors of aggressive behavior in first episode psychosis patients (FEP).
Method.
We conducted a retrospective chart review of 449 FEP patients recruited from an outpatient early psychosis clinic. Aggressive behavior and clinical information were rated based upon information gathered from the chart review of data collected at baseline and after 6 months of follow-up.
Results.
Rates of aggressive behavior were 54.3% in FEP patients. Aggressive behavior was significantly associated with higher rates of history of birth complications, neurodevelopmental delays, learning difficulties, alcohol use disorders, and the clinical domain of poverty symptoms. In addition to aggressive behavior, 16.7% of FEP patients exhibited suicidal ideation or behaviors and 11.4% exhibited non-suicidal self-injurious behavior (NSSIB). In contrast to baseline, aggressive behaviors at 6 months follow up were almost entirely absent.
Conclusions.
Patients at early stages of psychosis have high rates of aggressive and suicidal behavior prior to contact with clinical services. Neurodevelopmental adversities, alcohol use disorders and poverty symptoms are associated to higher risk of aggression in early psychosis. Participation in early psychosis specialty care resulted in a dramatic reduction in aggressive behavior.
Keywords: aggression, self-harm, first episode psychosis, early psychosis, neurodevelopment
1. INTRODUCTION
While mental illness accounts for only 4% of aggressive behavior in the general population (Swanson et al 1990), aggression has been shown to be more prevalent in patients with psychosis than in the general population, at rates of 10% versus 2% respectively (Elbogen et al 2009). While the majority of aggressive behaviors are associated with factors other than mental illness (e.g. substance use), evidence remains for a modest association between violent behavior and schizophrenia (Fazel et al 2009). However little is known about what clinical and early life factors are associated with aggression in individuals in the earliest stages of psychosis.
Some studies suggest that the pre-treatment phase of early psychosis is a time of increased risk for aggressive behavior (Humphreys et al 1992, Large and Nielssen 2011, Winsper et al 2013), likely related to younger age, prominence of positive symptoms, lack of insight and/or lack of engagement in treatment (Winsper et al 2013). A recent meta-analysis has shown that approximately one third of patients in the first episode of psychosis exhibit some form of aggressive behavior, typically minor forms of violence prior to initiating treatment (Large and Nielssen 2011).
Several studies have found similar risk factors associated with aggression in first episode psychosis, including younger age, male gender, longer duration of untreated psychosis, mania, substance abuse, antisocial personality traits, lower educational attainment and a history of violence (Arseneault et al., 2000; Large and Nielssen 2011). Most of these factors are similar to those associated with violence in patients with mental illness in general (Swanson et al 2006) and with aggression in the general population (Swanson et al 1990). However the increased risk of aggression during the early stages of psychosis suggests that the risk factors described above may interact with additional unknown factors specific to early psychosis that could explain this increased risk of aggressive behavior. A better understanding of the factors associated with aggression in early psychosis could have treatment implications for prevention of aggression in this at-risk group.
The present study aims to describe the rates and characteristics of aggressive behavior in a large cohort of first episode psychosis patients (FEP) presenting for outpatient care. This research also seeks to explore an array of clinical, neurodevelopmental and personal factors that may be associated with aggressive behavior in this population.
2. METHODS
2.1. Study population
We conducted a retrospective chart review of 449 first episode psychosis (FEP) patients who presented for care to the UC Davis Early Diagnosis and Preventive Treatment (EDAPT) Clinic, an outpatient early psychosis clinic established in Sacramento (California), between October 13, 2004 and July 9, 2013. All patients were recruited for and voluntarily participated in a larger study of cognition in psychotic disorders. Participants gave written informed consent (assent for age<18 years with guardian consent) for their data to be collected via chart review. All patients were between the ages of 12-40, comfortable using English in their daily activities, and were referred to participate in research if they were eligible for intake at the EDAPT clinic. Patients were assessed at intake using the Structured Clinical Interview for DSM-IV-TR (SCID-IV-TR) (First et al., 2002) and review of any available clinical records (e.g. hospital discharge records, school assessment records) to determine eligibility as FEP. FEP individuals had onset of psychosis in the past 2 years and received primary psychotic (schizophrenia, schizoaffective, schizophreniform disorders) or mood disorder with psychotic features (bipolar or major depression) diagnoses according to the SCID-IV-TR (First et al 2002) or the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS) (Chambers et al, 1985; Kaufman et al 1996). If eligible after the clinical interview, participants were invited to complete additional research appointments. Individuals with a Weshler Abbreviated Scale of Intelligence (WASI) IQ below 70 were excluded from the study. This study was reviewed and approved by the UC Davis Institutional Review Board (protocol # 226043).
2.2. Coding procedure
Researchers in the program developed a coding guide for a breadth of domains (e.g. education information, developmental history, clinical symptoms, aggressive behavior, etc.) based on the available clinical and research measures (i.e. interviews and self-report questionnaires), collateral caregiver questionnaires, and information typically recorded in clinical charts (including progress notes written by EDAPT clinicians and psychiatrists). Ten preliminary charts were coded and this data was reviewed by TN to resolve inconsistencies and incorporate new themes, which resulted in modification to the guide until all themes were identified and appropriate codes were developed, including codes to note where data was missing or not reported. Research staff trained on a practice data set to ensure consistency prior to coding the baseline and 6 month data. Aggression outcome data was coded for all individuals who participated in research at the 6 month follow-up.
Presence of aggression was coded retrospectively based on patient and/or collateral report of a lifetime history of aggressive behavior using an adaptation of the Modified Overt Aggression Scale (MOAS) (Kay et al 1988). The MOAS scale is a modified version of the Overt Aggression Scale developed by Yudofsky and colleagues (Yudofsky et al 1986) which allows for objective reporting of aggressive behavior across multiple domains based upon all available information rather than relying on self-report alone. The MOAS is comprised of four domains: verbal aggression, aggression against property, aggression against self and physical aggression against others. Domains are weighted to capture the severity of behavior, and items within each domain also indicate increasing severity. The severity of aggressiveness was calculated through the total weighted score from the 4 sections of the MAOS scale. To capture the most severe level of historical aggression and code for presence/absence of aggression categories, we developed a 10-item coding to capture the severity continuum of domains and items consistent with the standardized MOAS format:
No Aggression
Verbal Aggression: yelling, screaming, cussing, argumentative
Verbal Aggression with Threat: threatening harm towards self or others, but no weapon or action.
Verbal Aggression with Threat/weapon: verbally threatening other with weapon, but no action.
Aggression against Property: slamming doors, ripping clothing, throwing objects, breaking small objects, fire setting.
Aggression against Property Accompanied by Threat: destroying large items with threat to do more
Auto aggression: banging head, pounding walls, banging fists, pulling hair out,
Auto aggression: Self Harm with Injury: punching wall (e.g. breaking hand), cutting/burning self (NSSIB), and aborted suicide attempts.
Physical Aggression: pushing others, shaking others, hitting, kicking, scratching, and pinning down.
Physical Aggression with Threat or Injury: hitting and kicking people with threat to do more, suicide attempts; causing injury, potentially or actually lethal.
Participants were classified as “aggressive” if they demonstrated significant verbal aggression towards others (categorical code of 3 or higher), or any rating of physical aggression towards property, others, or self (including suicide attempts and non-suicidal self-injurious behavior, NSSIB). Information on suicidal ideation/behavior and NSSIB were obtained from the Columbia Scale for the Rating of Suicide Severity (C-SSRS; Posner et al 2011), the Brief Psychiatric Rating Scale (BPRS) (Ventura et al 1993), or notes in the clinical chart.
Clinical symptoms were assessed through the 24-items Brief Psychiatric Rating Scale (BPRS) (Ventura et al 1993), Scale for the Assessment of Negative Symptoms (SANS) (Andreasen 1984a), and Scale for the Assessment of Positive Symptoms (SAPS) (Andreasen 1984b). As described previously (Barch et al 2003), three core symptom dimensions were computed: Poverty, Disorganization, and Reality Distortion.
Reported history of birth complications, developmental delay, learning difficulties, attention deficit disorder (ADHD) or pervasive developmental disorder (PDD) symptoms and/or diagnosis, alcohol abuse/dependence, and cannabis abuse/dependence were rated based upon SCID-IV ratings and information gathered from the retrospective chart review.
2.3. Data analysis
Statistical analyses were performed using the IBM SPSS statistical software package, version 24 (SPSS Inc., Chicago, Ill.). Data were checked for outliers and violations of normality and, in those cases, non-parametric tests were used. T-tests examined group differences in dimensional variables, while Chi-square tests examined differences in categorical variable. Significance level was established at p<0.05, with notable trends reported for p<.10, for the purpose of this exploratory analysis.
3. RESULTS
The retrospective clinical chart review included 449 FEP individuals evaluated for treatment in the EDAPT Clinic.
3.1. Demographic, clinical and neurodevelopmental characteristics
Demographic and clinical and neurodevelopmental characteristics of the FEP patients are shown in Table 1.
Table 1.
Demographic and clinical characteristics in FEP patients.
| Demographics | N | Mean ± SD or % |
|---|---|---|
| Age (mean, SD) | 445 | 19.6 ± 4.2 |
| Gender (%male) | 449 | 73.5 |
| Ethnicity (% Caucasian) | 449 | 52.5 |
| Education, years (mean, SD) | 355 | 11.8 ± 2.3 |
| IQ (mean, SD) | 280 | 98.7 ± 15 |
| DUP, months (mean, SD) | 356 | 5.8 ± 8.3 |
| Aggression rates (%) | 449 | 54.3 |
| Clinical Symptoms | ||
| BPRS (mean, SD) | 340 | 42.7 ± 10.8 |
| SAPS (mean, SD) | 344 | 5.9 ± 3.7 |
| SANS (mean, SD) | 355 | 8.7 ± 4.1 |
| Reality distorsion (mean, SD) | 343 | 15.3 ± 7.5 |
| Disorganization (mean, SD) | 355 | 6.9 ± 3.5 |
| Poverty (mean, SD) | 356 | 13.1 ± 5.3 |
| Alcohol abuse/dependence diagnosis (%) | 438 | 7 |
| Cannabis abuse/dependence diagnosis (%) | 438 | 12.1 |
| Neurodevelopmental Events | ||
| Birth complications (%) | 436 | 15.1 |
| Developmental delay (%) | 438 | 12.1 |
| Learning disabilities (%) | 415 | 21.7 |
| Comorbid ADHD (%) | 414 | 11.8 |
| Comorbid PDD (%) | 417 | 4.1 |
Comparisons in demographic and clinical variables between FEP patients with aggressive behavior (FEP+A) and without aggressive behavior (FEP-) are shown in Table 2. No significant differences in demographic factors were noted between FEP+A and FEP-.
Table 2.
Demographic and clinical characteristics in FEP patients with severe aggressive behavior and without aggressive behavior.
| Aggressive N=244 |
Non aggressive N=205 |
Statistic test; p | |||
|---|---|---|---|---|---|
| Demographics | N | N | |||
| Age (mean, SD) | 238 | 19.4 ± 4 | 202 | 19.9 ± 4.4 | t= 1.31; p=0.19 |
| Gender (%male) | 244 | 75 | 205 | 71.7 | X2= 0.62; p=0.43 |
| Ethnicity (% Caucasian) | 244 | 52 | 205 | 51.2 | X2= 0.92; p=0.63 |
| Education, years (mean, SD) | 194 | 11.6 ± 2.1 | 160 | 11.9 ± 2.6 | t= 0.99; p=0.32 |
| IQ (mean, SD) | 148 | 98.3 ± 14.9 | 132 | 99.1 ± 15.2 | t= 0.43; p=0.67 |
| DUP, months (mean, SD) | 184 | 6 ± 9.1 | 170 | 5.5 ± 7.4 | t= −1.47; p=0.14 |
| Clinical Symptoms | |||||
| BPRS (mean, SD) | 179 | 43.5 ± 9.9 | 161 | 41.8 ± 11.6 | t= −1.5; p=0.14 |
| SAPS (mean, SD) | 181 | 6.1 ± 3.6 | 163 | 5.7 ± 3.8 | t= −1; p=0.33 |
| SANS (mean, SD) | 188 | 9.2 ± 3.8 | 167 | 8.1 ± 4.3 | t= −2.5; p=0.01 |
| Reality distorsion (mean, SD) | 181 | 15.6 ± 7.4 | 162 | 14.9 ± 7.7 | t= −0.8; p=0.4 |
| Disorganization (mean, SD) | 189 | 7.1 ± 3.2 | 166 | 6.7 ± 3.8 | t= −1.1; p=0.26 |
| Poverty (mean, SD) | 189 | 13.6 ± 5 | 167 | 12.5 ± 5.6 | t= −2; p=0.04 |
| Alcohol abuse/dependence diagnosis (%) | 236 | 10.2 | 199 | 3.5 | X2= 7.29; p=0.007 |
| Cannabis abuse/dependence diagnosis (%) | 236 | 14.8 | 199 | 9 | X2= 3.38; p=0.06 |
| Neurodevelopmental Events | |||||
| Birth complications (%) | 238 | 18.9 | 198 | 10.6 | X2= 5.8; p=0.016 |
| Developmental delay (%) | 237 | 15.2 | 201 | 8.5 | X2= 4.63; p=0.031 |
| Learning difficulties (%) | 231 | 26.8 | 184 | 15.2 | X2= 8.15; p=0.004 |
| Comorbid ADHD (%) | 231 | 13.9 | 183 | 9.3 | X2= 2.04; p=0.15 |
| Comorbid PDD (%) | 232 | 5.6 | 185 | 2.2 | X2= 3.12; p=0.07 |
Aggressive behavior in FEP patients was significantly associated with higher rates of alcohol use diagnosis, birth complications, neurodevelopmental delay and learning difficulties (p ≤ .05) and there was a non significant trend for an association with PDD (p=.07) and with cannabis use diagnosis (p=.06) in FEP.
3.2. Rates and characteristics of aggressive behavior
The rate of aggression in FEP patients was 54.3%. Table 3 shows the characteristics of the aggressive behavior reported by the patients. According to the type of aggression, the aggressive behavior reported was predominantly physical (54%), although some individuals also reported both physical and verbal aggression (29.4%). Aggression toward others (49.2%) was reported more frequently than aggression towards self (25.4%) or towards objects (9.4%).
Table 3.
Characteristics of aggressive behavior and suicide risk in FEP patients.
| N | % or mean ± SD | |
|---|---|---|
| Type of aggression (%) | ||
| Verbal vs physical | ||
| Verbal only | 41 | 16.6 |
| Physical only | 133 | 54 |
| Verbal and physical | 72 | 29.4 |
| Self vs others vs objects | ||
| Self only | 65 | 25.4 |
| Others only | 126 | 49.2 |
| Self and others | 41 | 16 |
| Objects only | 24 | 9.4 |
| Degree of aggression (%) | ||
| Ideation | 7 | 2.7 |
| Threat | 6 | 2.3 |
| Actual aggression | 244 | 95 |
| Severity of aggression1 | 448 | 11.6 ± 7.1 |
| Suicide risk (%) | ||
| Ideation only | 19 | 4.2 |
| Threat | 8 | 1.8 |
| Attempt | 48 | 10.7 |
| Non-suicidal Self Injury (NSSI) (%) | ||
| NSSI behaviors reported | 449 | 11.4 |
The severity of aggressiveness was calculated through the total weighted score from the MAOS scale (maximum weighted score would be 40).
Of the participants that reported some aggression, almost all (95%) demonstrated aggressive behavior versus expressed ideation about aggression (2.7%) or only threated aggression (2.3%). Rates of aggression by sex are shown in Table 1. Supplementary material.
For FEP+A, the historical total severity (rated according to the MOAS scale) was 11.56. ± 7.1. Across the categorical levels of the aggressive behavior (see Figure 1. Supplementary material), the most severe type of aggressive behavior reported was physical aggression towards others with threat or injury (36%), which included suicide attempts, followed by physical aggression towards others (24%).
Regarding non-suicidal self-injurious behavior (e.g. cutting, burning, or hitting oneself), 11.4% of FEP patients reported engaging in NSSIB. Regarding suicidal thoughts and acts, 16.7% of FEP patients reported a history of suicidal ideation and behavior, with 10.7% reporting a prior suicide attempt.
3.3. Association between aggression and clinical domains
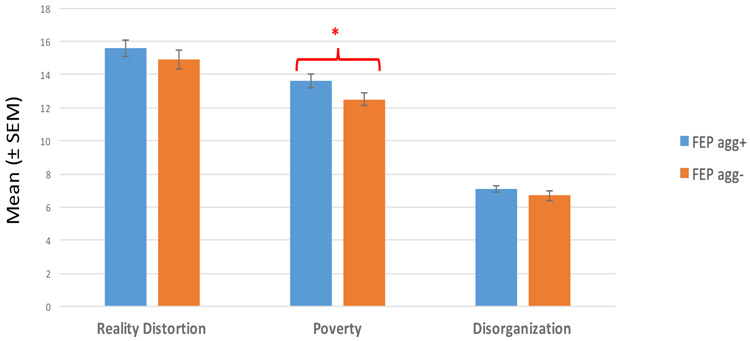
FEP+A had more severe poverty symptoms than FEP- individuals at baseline (p<0.05) (Figure 1); however no significant difference was observed between the groups on reality distortion or disorganization.
Figure 1.
Association between aggression and clinical domains
* Level of significance p<0.05
FEP agg+: First episode psychosis patients with aggressive behavior
FEP agg-: First episode psychosis patients without aggressive behavior
3.4. Aggression at 6 months follow up
Of the FEP patients that showed aggressive behavior at baseline assessment, 98.9% did not report any aggressive behavior in the past month when completing the 6 month follow up assessment.
4. DISCUSSION
This study reports results from a large cohort of subjects with FEP to determine the rates and characteristics of aggressive behavior and associated factors. Aggressive behavior was present in over half of FEP patients, consisting primarily of physical aggression toward others. We observed associations between neurodevelopmental adversities and negative symptoms and the presence of aggression. As previously described in the literature, we found an association between substance use (alcohol and cannabis) and aggressive behavior (Large et al 2011, Swanson 2006).
4.1. Rates, types and severity of aggression
The early stages of psychosis seem to be a period of heightened risk for aggression (Ballester et al 2012, Dack et al 2013, Fazel et al 2009). Recent meta-analysis have found aggression rates between 31% (Winsper et al 2013) and 35.4% (Large and Nielssen 2011) in FEP patients. We found fairly high rates of lifetime aggressive behavior in FEP patients (54.3%) reported prior to contact with the clinical services (EDAPT Clinic). Our results confirm the high rates of aggression across nonaffective and affective FEP in the largest early psychosis sample reported to date. In the present study we confirmed the common occurrence of aggressive behavior in this population. While the frequency of aggressive behavior was high, the severity of aggression in our sample, assessed through the Modified Overt Aggression Scale (MOAS), was low. Reports of physical aggression towards others was the most common. This result has also been found in some studies that suggest the presence of a gradient of violent behavior in FEP, in which minor violent occurrences are common, serious violent occurrences are less common, and severe violent occurrences are rare (Large and Nielssen 2011). Additional reports have suggested that minor forms of violence and assaults are often reported at the time of initial presentation of psychotic illness (Humphreys et al., 1992; Volavka et al., 1997).
4.2. Factors associated with aggression in FEP
An unexpected finding was the association between negative symptoms and aggression. Presence of psychotic symptoms has traditionally been considered as an important factor in increasing the risk of aggression (Khalid et al 2012, Link et al 1992, Taylor et al 1998). In contrast, this study found a significant association between poverty symptoms and aggression in FEP. Poverty reflects emotional withdrawal, affective flattening, anhedonia, apathy, asociality, alogia and motor retardation, therefore negative symptoms (Barch et al 2003). One possible explanation for this finding is that patients scoring high in the poverty domain have deficits in communication skills, which might limit their ability to resolve interpersonal conflict and lead to an increased risk of aggressive behavior. In general in psychotic disorders interpersonal skills, community activities and work skills are most strongly associated with performance on neuropsychological tests, negative symptoms and depression, and not with positive symptoms (Bowie et al 2006).
We did not find a significant difference in aggression between males and females. Our results are consistent with previous studies that have found that being male was not associated with serious violence in first episode psychosis (Large et al 2011). Male sex has been associated to violent behavior in the general population (Swanson et al 1990), however the clinical literature in FEP does not show not a clear sex effect associated to increased risk of aggressive behavior. Minor forms of violent behavior have been shown to be more likely among female sex FEP patients (Swanson 2006). As previously described in the literature, we have found an association between substance use (alcohol and cannabis) and aggressive behavior (Large et al 2011, Swanson 2006).
Increased duration of untreated psychosis (DUP), or time between the onset of psychotic symptoms and onset of appropriate treatment, has also been associated with aggressive behavior in schizophrenia (Latalova K 2014). Here we report slightly longer DUP among FEP with aggressive behavior, but we did not find significant differences compared to FEP without aggressive behavior. We conclude at this time that the available evidence is still inconclusive regarding the association between DUP and presence of aggression.
4.3. Neurodevelopmental adversity and aggression
One of the most interesting findings of our study is the significant association between birth complications, neurodevelopmental delay and learning difficulties and the presence of aggressive behavior in FEP. We also found a trend toward an association with a history of PDD and aggression in FEP. To our knowledge this is the first study to report an association between obstetric complications, atypical development and aggressive behavior in FEP. As for negative symptoms, poor social communication skills and an inability to resolve interpersonal conflict may be a mechanism by which neurodevelopmental factors might mediate increased aggressive behavior in our sample.
The nature of the relationship between aggressive behavior and psychosis is complex and multifactorial. Although several symptoms of psychosis, such as hallucinations or delusions, a number of social factors (e.g. trauma, child maltreatment, social deprivation) and individual factors (e.g. comorbid substance use), could explain the association between aggression and psychosis, distinct neurobiological and neurodevelopmental mechanisms may also play a role (Soyka 2011). In a large birth cohort followed for 30 years, complications in the neonatal period were found to be associated with an increased risk (odds ratio, OR: 2.79) for early onset violent behavior among persons with schizophrenia (Hodgins et al 2002). A recent meta-analysis (Fusar-Poli et al 2017) has found evidence for a significant association between unspecified obstetric complications during the prenatal/perinatal period and the ultra high risk (UHR) state (OR = 3.06). There is robust evidence that birth complications have a significant effect in increasing the risk of later schizophrenia (Hamlyn et al 2013).
High levels of birth complications, delayed attainment in neurodevelopment milestones and an increase in neurodevelopmental problems, support the neurodevelopment hypothesis of schizophrenia. Associations between early motor developmental milestones (Filatova et al 2017), speech problems (Jones et al 1994) and poorer receptive language skills (Cannon et al 2002) have previously been reported in schizophrenia. Our results show that FEP patients that exhibit aggressive behavior have a history of increased neurodevelopmental delay in comparison with non-aggressive FEP patients. Disturbances during early stages of brain development, probably interacting with genetic susceptibility factors, may increase the risk of later aggression in vulnerable subjects.
Children with learning difficulties have been found to be at increased risk of developing behavioral problems such as disruptive or aggressive behavior (Carroll et al 2005; Dalley et al 1992). Furthermore, learning difficulties have been proposed as a strong and early indicator of mental disorders (Zakopoulou et al 2014), probably interacting during the school age with other risk factors and impacting on the underlying predisposition for mental disorders. Deficits in premorbid school performance have previously been shown in FEP patients (Bilder et al 2006), worsening overtime.
These findings may have clinical implications, as they suggest that early detection and specific treatments to target learning difficulties and neurodevelopmental delay could prevent the development of aggressive behaviors later in life.
4.4. Suicide and NSSI behavior in early psychosis
In line with the literature on self-harm in first episode of psychosis, we found that around a fifth of FEP patients exhibited some kind of suicidal ideation and behavior, including suicide attempts. A recent meta-analysis (Challis et al 2013) has shown that 18.4% of patients with first episode psychosis attempt suicide at some point prior to treatment. A 3-year longitudinal study of FEP patients (Ayesa-Arriola et al 2015) found that 15.11% of patients made a suicide attempt. We also reported that 11.4% of FEP individuals in our sample engaged in NSSIB; this is lower than prior reports of NSSIB in schizophrenia (Monk et al 2013), which may be associated with the methodology used in the current analysis.
The risk of suicide and self-harm is higher earlier in the course of psychosis compared to later in the illness (Nordentoft et al 2011). It has been reported that the risk of self-harm behavior in FEP patients seems to be increased the month preceding to first contact with psychiatric services and the 2 months after the first contact (Ayesa-Arriola et al 2015).
4.5. Longitudinal course of aggression
We see a dramatic drop-off in the aggression rates of the subjects that have been followed for 6 months. Early detection and treatment programs of psychotic patients can reduce rates of aggressive behavior, including serious suicidal behavior, at the point of first contact. Our results emphasize the potential utility of first episode psychosis services in reducing harmful behaviors.
4.6. Limitations
Although we used a large database of subjects with FEP to determine the rates and characteristics of aggressive behavior and associated factors, there are some limitations that are intrinsic to the retrospective chart review methodology. Dependent variables were based on all available information, including behavioral and clinical data, collateral caregiver questionnaire data and clinical charts. Although this approach provides a wide range of information, missing information or a lack of detail may have led to biases in our results. We report data on individuals in the earliest stages of psychosis (within 2 years of psychosis onset), which may lead to clinical characteristics that cannot be generalized to broader psychosis populations. Another limitation of this cross-sectional design is that, while the reported associations are statistically robust, causality cannot be determined. Limitations from sampling and randomization as well as different statistical approaches should be addressed in future research. Prospective studies of large population cohorts would allow more definitive conclussions about the risk factors associated with aggressive behaviors in the early stages of psychosis. Such prospective approaches could also include qualitative analysis of triggers for FEP patient’s aggression, including contributions of substance use, social conflict and involuntary hospitalization, which could elucidate potential points of intervention to reduce risk for aggression. Despite these limitations, the findings are important as the identification of personal, clinical and neurodevelopmental factors associated to an increased risk of aggression in early psychosis can help to inform the prediction of and intervention for aggression in individuals with early psychosis.
5. CONCLUSIONS
Patients with first episode psychosis (FEP) have high rates of low intensity aggressive and suicidal behavior prior to contact with clinical services. Neurodevelopmental adversity, substance use and negative symptoms are associated with higher risk of aggression in early psychosis. Identification of these risk factors can inform risk assessment and the tailoring of prevention and early intervention strategies to reduce aggression among individuals with psychosis.
Supplementary Material
Footnotes
Conflict of interest
None of the authors have any conflict of interest.
REFERENCES
- Swanson JW, Holzer CE III, Ganju VK, Jono RT, 1990. Violence and psychiatric disorder in the community: evidence from the Epidemiologic Catchment Area surveys. Hosp Community Psychiatry 41, 761–70. [DOI] [PubMed] [Google Scholar]
- Elbogen EB, Johnson SC, 2009. The intricate link between violence and mental disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 66, 152–61. [DOI] [PubMed] [Google Scholar]
- Fazel S, Gulati G, Linsell L, Geddes JR, Grann M, 2009. Schizophrenia and violence: systematic review and meta-analysis. PLoS Med. 6(8), e1000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large MM and Nielssen O, 2011. Violence in first-episode psychosis: a systematic review and meta-analysis. Schizophr Res. 125, 209–220. [DOI] [PubMed] [Google Scholar]
- Humphreys MS, Johnstone EC, MacMillan JF, Taylor PJ, 1992. Dangerous behaviour preceding first admissions for schizophrenia. Br J Psychiatry 161:501–505. [DOI] [PubMed] [Google Scholar]
- Winsper C, Ganapathy R, Marwaha S, Large M, Birchwood M, Singh SP, 2013. A systematic review and meta-regression analysis of aggression during the First Episode of Psychosis. Acta Psychiatr Scand. 128, 413–421. [DOI] [PubMed] [Google Scholar]
- Arseneault L, Moffitt TE, Caspi A, Taylor PJ, Silva PA, 2000. Mental disorders and violence in a total birth cohort: results from the Dunedin Study. Arch Gen Psychiatry 57(10), 979–986. [DOI] [PubMed] [Google Scholar]
- Swanson JW, Swartz MS, Van Dorn RA, Elbogen EB, Wagner HR, Rosenheck RA, et al. , 2006. A national study of violent behavior in persons with schizophrenia. Arch Gen Psychiatry 63(5), 490–499. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M and Williams JB, 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P). Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Chambers WJ, Puig-Antich J, Hirsch M, Paez P, Ambrosini PJ, Tabrizi MA, et al. , 1985. The assessment of affective disorders in children and adolescents by semistructured interview. Arch Gen Psychiatry 42, 696–702. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U and Ryan N, 1996. Kiddie-SADS Present and Lifetime Version (K-SADS-PL). University of Pittsburgh, Department of Psychiatry. [Google Scholar]
- Kay SR, Wolkenfeld F, Murill LM, 1988. Profiles of aggression among psychiatric patients: I. Nature and prevalence. J Nerv Ment Dis. 176, 539–546. [DOI] [PubMed] [Google Scholar]
- Yudofsky SC, Silver JM, Jackson W, Endicott J and Williams D, 1986. The Overt Aggression Scale for the objective rating of verbal and physical aggression. Am J Psychiatry 143, 35–39. [DOI] [PubMed] [Google Scholar]
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. , 2011. The Columbia Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 168, 1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Lukoff D, Nuechterlein KH, Liberman RP, Green MF, Shaner A, 1993. Manual for the expanded brief psychiatric rating scale. Int J Methods Psychiatr Res. 3(3), 227–244. [Google Scholar]
- Andreasen NC, 1984a. Scale for the assessment of negative symptoms (SANS) Iowa City, IA: Department of Psychiatry, College of Medicine, The University of Iowa. [Google Scholar]
- Andreasen NC, 1984b. Scale for the Assessment of Positive Symptoms (SAPS) Iowa City, IA: Department of Psychiatry, College of Medicine, The University of Iowa. [Google Scholar]
- Barch DM, Carter CS, MacDonald AW 3rd, Braver TS and Cohen JD, 2003. Context-processing deficits in schizophrenia: diagnostic specificity, 4-week course, and relationships to clinical symptoms. J Abnorm Psychol. 112(1), 132–143. [PubMed] [Google Scholar]
- Ballester J, Goldstein T, Goldstein B, Obreja M, Axelson D, Monk K. et al. , 2012. Is bipolar disorder specifically associated with aggression? Bipolar Disord;14:283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dack C, Ross J, Papadopoulos C, Stewart D. and Bowers L, 2013. A review and meta-analysis of the patient factors associated with psychiatric in-patient aggression. Acta Psychiatr Scand. 127(4), 255–68. [DOI] [PubMed] [Google Scholar]
- Volavka J, Laska E, Baker S, Meisner M, Czobor P, Krivelevich I,1997. History of violent behaviour and schizophrenia in different cultures. Analyses based on the WHO study on Determinants of Outcome of Severe Mental Disorders. Br J Psychiatry 171, 9–14. [DOI] [PubMed] [Google Scholar]
- Khalid F, Ford T and Maughan B, 2012. Aggressive behaviour and psychosis in a clinically referred child and adolescent sample. Soc Psychiatry Psychiatr Epidemiol. 47(11), 1795–806. [DOI] [PubMed] [Google Scholar]
- Taylor PJ, Leese M, Williams D, Butwell M, Daly R and Larkin E, 1998. Mental disorder and violence: A special (high security) hospital study. Br J Psychiatry 172, 218–226. [DOI] [PubMed] [Google Scholar]
- Link BG, Andrews H and Cullen F, 1992. The violent and illegal behavior of mental patients reconsidered. Am Sociol Rev. 52, 275–292. [Google Scholar]
- Bowie CR, Reichenberg A, Patterson TL, Heaton RK and Harvey PD, 2006. Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. Am J Psychiatry 163, 418–25. [DOI] [PubMed] [Google Scholar]
- Látalová K, 2014. Violence and duration of untreated psychosis in first-episode patients. Int J Clin Pract. 68(3), 330–5. [DOI] [PubMed] [Google Scholar]
- Soyka M, 2011. Neurobiology of Aggression and Violence in Schizophrenia. Schizophr Bull. 37(5), 913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgins S, Kratzer L, McNeil TF, 2002. Obstetrical problems, parenting, and risk of criminal behaviour among persons who develop major mental disorders. Acta Psychiatr Scand.105, 179–188. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Tantardini M, De Simone S, Ramella-Cravaro V, Oliver D, Kingdon J, et al. , 2017. Deconstructing vulnerability for psychosis: Meta-analysis of environmental risk factors for psychosis in subjects at ultra high-risk. Eur Psychiatry 40, 65–75. [DOI] [PubMed] [Google Scholar]
- Hamlyn J, Duhig M, McGrath J, Scott J, 2013. Modifiable risk factors for schizophrenia and autism-shared risk factors impacting on brain development. Neurobiol Dis.53, 3–9. [DOI] [PubMed] [Google Scholar]
- Filatova S, Koivumaa-Honkanen H, Hirvonen N, Freeman A, Ivandic I, Hurtig T, et al. , 2017. Early motor developmental milestones and schizophrenia: A systematic review and meta-analysis. Schizophr Res. 188, 13–20. [DOI] [PubMed] [Google Scholar]
- Jones P, Rodgers B, Murray R, Marmot M, 1994. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet 344(8934), 1398–402. [DOI] [PubMed] [Google Scholar]
- Cannon M, Jones PB. and Murray RM, 2002. Obstetric complications and schizophrenia: historical and meta- analytic review. Am J Psychiatry 159, 1080–1092. [DOI] [PubMed] [Google Scholar]
- Dalley MB, Bolocofsky DN, Alcorn MB, and Baker C, 1992. Depressive symptomatology, attributional style, dysfunctional attitude, and social competency in adolescents with and without learning disabilities. School Psychology Review 21, 444–458. [Google Scholar]
- Carroll JM, Maughan B, Goodman R and Meltzer H, 2005. Literacy difficulties and psychiatric disorders: Evidence for comorbidity. J Child Psychol Psychiatry 46(5), 524–532. [DOI] [PubMed] [Google Scholar]
- Zakopoulou V, Mavreas V, Christodoulides P, Lavidas A, Fili E, Georgiou G, et al. , 2014. Specific learning difficulties: A retrospective study of their co morbidity and continuity as early indicators of mental disorders. Res Dev Disabil. 35, 3496–3507 [DOI] [PubMed] [Google Scholar]
- Bilder RM, Reiter G, Bates J, Lencz T, Szeszko P, Goldman RS, et al. , 2006. Cognitive development in schizophrenia: Follow-back from the first episode. J Clin Exp Neuropsychol. 28(2), 270–282. [DOI] [PubMed] [Google Scholar]
- Challis S, Nielssen O, Harris A, Large M, 2013. Systematic meta-analysis of the risk factors for deliberate self-harm before and after treatment for first-episode psychosis. Acta Psychiatr Scand. 127(6), 442–54. [DOI] [PubMed] [Google Scholar]
- Ayesa-Arriola R, Alcaraz EG, Hernández BV, Pérez-Iglesias R, López Moríñigo JD, Duta R, et al. , 2015. Suicidal behaviour in first-episode non-affective psychosis: Specific risk periods and stage-related factors. Eur Neuropsychopharmacol. 25(12), 2278–88. [DOI] [PubMed] [Google Scholar]
- Mork E, Walby F, Harkavy-Friedman J, Barrett E, Steen N, Lorentzen S, Andreassen O, Melle I and Mehlum L, 2013. Clinical characteristics in schizophrenia patients with or without suicide attempts and non-suicidal self-harm: A cross-sectional study. BMC Psychiatry 13, 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordentoft M, Mortensen PB, Pedersen CB, 2011. Absolute risk of suicide after first hospital contact in mental disorder. Arch Gen Psychiatry 68, 1058–1064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.