Abstract
Background:
The opioid crisis has led to an increase in hepatitis C virus positive donors in the past decade. Whereas historically hepatitis C seropositive organs were routinely discarded, the advent of direct-acting antiviral agents has notably expanded the utilization of organs from donors with hepatitis C. There has been growing experience with liver transplantation from hepatitis C seropositive donors to hepatitis C seropositive recipients. However, data remain limited on liver transplantation from hepatitis C seropositive or hepatitis C ribonucleic acid positive donors to hepatitis C seronegative recipients.
Methods:
We performed a retrospective study of 26 hepatitis C seronegative recipients who received hepatitis C seropositive donor livers followed by preemptive antiviral therapy with direct-acting antiviral treatment at the Johns Hopkins Hospital Comprehensive Transplant Center from January 1, 2017 to August 31, 2019.
Results:
Twenty-five of the 26 recipients are alive with proper graft function; 20 of them received livers from hepatitis C nucleic acid testing positive donors. All 12 recipients who completed their direct-acting antiviral courses and have reached sufficient follow-up for sustained virologic response have achieved sustained virologic response. Nine of our recipients have either completed direct-acting antiviral treatment without sufficient follow-up time for sustained virologic response or are undergoing direct-acting antiviral treatment. One patient is awaiting antiviral treatment initiation pending insurance approval. Of note, 11 of 12 patients with sustained virologic response, received a hepatitis C nucleic acid testing positive donor liver.
Conclusion:
Hepatitis C seronegative patients who receive a hepatitis C seropositive or hepatitis C nucleic acid testing positive liver allograft can enjoy good short-term outcomes with hepatitis C cure following direct-acting antiviral treatment.
Keywords: hepatitis C virus positive donor liver, hepatitis C virus negative recipient, liver transplantation, direct acting antiviral, preemptive antiviral therapy
Introduction
Liver transplantation (LT) is the only curative and lifesaving treatment for end-stage liver disease and acute liver failure. The median wait time in the United States for an LT is 11.3 months; accordingly, waitlist mortality remains considerably high.1 The recent decline in wait times was in large part related to the increase of deceased-donor livers, which coincided with the opioid crisis and deaths from narcotic overdose.1
Amidst this opioid epidemic, there has been a rise in the number of increased-risk donors due to intravenous drug abuse.2 Increased-risk donors have a higher than average risk of transmitting human immunodeficiency virus, hepatitis B virus, or hepatitis C virus (HCV) infection to their organ recipients due to behavioral factors, but they do not necessarily affect the quality of the organ or graft survival.3,4 At times, donor risk index can be lower in increased-risk liver donors than in standard donors.5 Since the approval and the remarkable clinical success of anti-HCV direct-acting antiviral (DAA) medications, the nationwide acceptance of potentially HCV positive liver allografts has increased.6,7
Between 1995 and 2016, 4.1% of deceased donors in the United States were HCV seropositive (HCV Ab+) (Box 1).8 Prior to the introduction of DAAs, livers from HCV Ab+ donors were discarded at a higher rate than livers from HCV seronegative (HCV Ab−) donors, leading to the loss of precious transplant resources.9 While HCV Ab+ recipients of HCV Ab+ livers have demonstrated acceptable patient and graft survivals up to 5 years post-transplant, outcomes data for transplantation of HCV Ab+ livers to HCV Ab− recipients remain scarce.10,11 HCV Ab+ donors can be further classified by the detectability of HCV ribonucleic acid (RNA) through serum nucleic acid amplification testing: i.e., HCV nucleic acid testing (NAT) positive (HCV NAT+) or HCV NAT negative (HCV NAT−). HCV NAT+ donor livers universally transmit HCV infection to their recipients; in contrast, HCV Ab+/NAT− donor livers result in HCV transmission up to 16% of the time.12 The aim of the present study was to describe our institutional experience with 27 HCV Ab− patients who received HCV Ab+ donor livers.
Box 1: Terminology of Hepatitis C Virus (HCV) Infection Status used in this Manuscript.
| Term | Abbreviation | Definition |
|---|---|---|
| HCV seropositive | HCV Ab+ | Detectable anti-HCV antibody in the serum. Can be either HCV Ab+/NAT+ or HCV Ab+/NAT−. |
| HCV seronegative | HCV Ab− | Undetectable anti-HCV antibody in the serum. |
| HCV NAT positive | HCV NAT+ | Detectable HCV RNA through nucleic acid amplification test in the serum. |
| HCV NAT negative | HCV NAT− | Undetectable HCV RNA through nucleic acid amplification test in the serum. |
Materials and Methods
We performed a retrospective study of 268 adult liver transplant recipients at the Johns Hopkins Hospital Comprehensive Transplant Center (CTC) from January 1, 2017 to August 31, 2019. The electronic medical record was reviewed for recipient data: age at transplant, sex, race, etiology of liver failure, blood type, DAA regimen selected, date of DAA initiation, date of liver transplant, HCV antibody status prior to transplant, HCV RNA viral load, liver-associated enzyme (LAE), biologic model of end-stage liver disease (MELD) score at transplant, and donor liver biopsy pathology. We obtained donor information from DonorNet, including age, sex, race, HCV antibody status, HCV NAT, and Public Health Services (PHS) increased risk status.
Before transplantation, patients who were willing to accept HCV Ab+ donor organs initially provided consent to their primary hepatologist. If offered an HCV Ab+ organ, patients then additionally consented to the operating surgeon. The Johns Hopkins clinical consent form for HCV Ab+ LT delineated the risks and benefits of receiving an HCV Ab+ liver. Specifically, the form detailed the efficacy of DAAs, their potential side effects, and the expectation that treatment will be started within 3 months of LT. Our approach to HCV treatment was preemptive antiviral therapy with DAA, defined as DAA initiated in the early post‐LT period prior to clinical evidence of HCV. The specific DAA selected was based on the HCV treatment guidelines published by the American Association for the Study of Liver Disease. We did not communicate with insurance companies prior to transplant, but insurance companies have not denied coverage in our experience. All transplants were performed with the knowledge of our CTC administrator. In the event the patient’s insurance does not cover DAA, we verbally informed our patients that our CTC would cover the cost of the medications. We informed patients of a small possibility that DAA would not lead to HCV cure. We stated that the alternative would be to remain on the transplant waitlist with a potentially longer wait time and that there is a risk of death on the waitlist.
The Johns Hopkins Institutional Review Board approved the present study protocol (IRB00201219).
Results
During the study period, there were 191 liver recipients who were HCV Ab− pre-transplant; 26 (13.6%) received an HCV Ab+ donor organ. There were also 77 recipients who had pre-transplant HCV Ab+; among them, 33 (42.9%) received an HCV Ab+ liver.
The present analysis focused on the 26 HCV Ab− recipients who received an HCV Ab+ donor liver. Their median biologic MELD was 21.5 prior to transplant; the median allocation MELD was 29.5. Table 1 provides the baseline characteristics for each recipient in our cohort.
Table 1:
HCV Seronegative Liver Transplant Recipients of HCV Seropositive Livers between January 2014 and August 2019 at Johns Hopkins Hospital
| Recipient number | Age† - years/sex | Etiology of cirrhosis | Race | Blood type | Biologic MELD at transplant | Allocation MELD at time of transplant (reason for exception points) |
|---|---|---|---|---|---|---|
| 1 | 24/M | Chronic rejection‡ | Caucasian | O+ | 15 | 28 (encephalopathy) |
| 2 | 53/M | Alcohol | Caucasian | O+ | 17 | 17 |
| 3 | 60/M | Alcohol | Caucasian | O+ | 21 | 28 (hydrothorax) |
| 4 | 59/M | Cryptogenic | Caucasian | O− | 22 | 22 |
| 5 | 66/M | NASH | Caucasian | A− | 17 | 17 |
| 6 | 53/M | Alcohol | Caucasian | O+ | 30 | 30 |
| 7 | 71/M | PBC | Caucasian | A+ | 19 | 19 |
| 8 | 60/F | HCC | Caucasian | A+ | 6 | 28 (HCC) |
| 9 | 57/F | Alcohol | Caucasian | O+ | 17 | 25 (encephalopathy) |
| 10 | 66/M | NASH | Caucasian | O+ | 30 | 30 |
| 11 | 44/M | Alcohol | Caucasian | O− | 37 | 37 |
| 12 | 25/F | Dyskeratosis congenita | Caucasian | O+ | 7 | 29 (hepatopulmonary syndrome) |
| 13 | 71/M | PBC | Hispanic | O+ | 25 | 25 |
| 14 | 60/M | NASH | Caucasian | O+ | 27 | 27 |
| 15 | 70/F | NASH | Caucasian | A+ | 33 | 33 |
| 16 | 57/M | NASH | Caucasian | O+ | 26 | 26 |
| 17 | 57/M | NASH | Caucasian | B+ | 19 | 19 |
| 18 | 55/M | A1AT deficiency | Caucasian | B+ | 11 | 11 |
| 19 | 56/M | NASH | Caucasian | O− | 18 | 18 |
| 20 | 67/F | NASH | Caucasian | O+ | 29 | 29 |
| 21 | 21/F | Wilson disease | Black | O+ | 40 | Status 1A |
| 22 | 21/M | PSC | Caucasian | O+ | 27 | 27 |
| 23 | 54/M | Alcohol | Caucasian | O+ | 26 | 26 |
| 24 | 71/F | NASH | Caucasian | A+ | 31 | 31 |
| 25 | 28/M | Oxaluria | Caucasian | A+ | 20 | 30 (oxaluria) |
| 26 | 65/M | HBV | Caucasian | A+ | 7 | 27 (HCC) |
Age at the time of transplantation
original indication for transplant was biliary atresia in 24 years prior to current transplant, with an episode of possible autoimmune hepatitis in 2015 (antinuclear antibody titer 1:160, anti-smooth muscle antibody titer 1:80, immunoglobulin G level normal) thought to be leading to graft failure, but explant pathology ultimately was most consistent with chronic rejection
Abbreviations: alpha-1-antitrypsin (A1AT), female (F), hepatitis B virus (HBV), hepatitis C virus (HCV), hepatocellular carcinoma (HCC), male (M), model for end-stage liver disease (MELD), nonalcoholic steatohepatitis (NASH), primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC)
The liver donors in our cohort were all HCV Ab+. Importantly, 20 of the 26 donors were HCV NAT+ before graft harvest. PHS increased risk status was elevated in 19 out of 26 donors. Table 2 summarizes the donor demographic data and liver biopsy pathologies. For clarity, we have numbered the donors to correspond to their respective recipients.
Table 2:
HCV Seropositive Donors Corresponding to Recipients in Table 1
| Donor number | Donor age/sex | Donor race | Donor blood type | Donor HCV NAT | PHS increased risk organ | Donor liver pathology | |||
|---|---|---|---|---|---|---|---|---|---|
| Inflammation | Fibrosis | Steatosis | Iron | ||||||
| 1 | 37/M | Caucasian | O | positive | Yes | Mild, portal, chronic | None significant | None significant | None significant |
| 2† | 40/M | Caucasian | O | positive | Yes | N/A | N/A | N/A | N/A |
| 3 | 57/F | Caucasian | O | positive | No | Mild, portal, chronic | None significant | Mild macrovesicular (<5%) | Patchy, mild, hepatocellular distribution |
| 4 | 46/M | Latino | O | positive | Yes | None significant | None significant | None significant | None significant |
| 5 | 42/F | Black | A | positive | Yes | Moderate, portal, chronic. Multiple portal non-caseating granulomata with giant cells. | Moderate, periportal | Moderate macrovesicular (40%) | Mild increase in reticuloendothelial cells and hepatocytes |
| 6 | 57/M | Black | O | positive | No | Moderate, chronic, portal. | Moderate, portal | Large droplet macrovesicular (<5%), small droplet | Increased iron stores noted in reticuloendothelial cells |
| 7 | 33/M | Caucasian | A | positive | Yes | Mild, portal, chronic | None significant | Large droplet (25%) and small droplet (30%) macrovesicular | Mild hepatocellular and reticuloendothelial stainable iron. |
| 8 | 20/F | Caucasian | A | positive | Yes | Moderate, portal, chronic | Moderate, periportal | None significant | None significant |
| 9 | 49/F | Hispanic | O | positive | Yes | Mild, portal, chronic | Mild, portal | Severe small droplet (90%), mild large droplet (<5%) | None significant |
| 10 | 20/F | Caucasian | O | positive | Yes | N/A | N/A | N/A | N/A |
| 11 | 22/M | Caucasian | O | positive | Yes | None significant | None significant | Small droplet macrovesicular (70%), minimal large droplet macrovesicular (<5%) | None significant |
| 12 | 23/F | Caucasian | O | positive | Yes | Mild, portal, chronic | None significant | Small droplet macrovesicular (20%), minimal large droplet macrovesicular (<5%) | None significant |
| 13 | 56/M | Hispanic | O | positive | No | Mild to moderate, portal, chronic | Minimal fibrosis | None significant | None significant |
| 14 | 49/M | Caucasian | O | positive | Yes | Mild, portal, chronic | Mild, periportal | Small droplet microvesicular (5%) | None significant |
| 15 | 50/F | Black | A | positive | No | Mild, portal, chronic. Minimal interface activity. | None significant | None significant | None significant |
| 16 | 28/M | Caucasian | O | positive | Yes | Mild, portal, chronic | Mild, periportal | Small droplet macrovesicular (10%) | None significant |
| 17 | 32/F | Caucasian | B | positive | Yes | Mild, portal, chronic | None significant | Moderate small droplet macrovesicular (60%) | None significant |
| 18 | 34/F | Caucasian | B | positive | Yes | Mild, portal, chronic | Mild to focal moderate periportal | Small droplet macrovesicular (80%) | None significant |
| 19 | 60/M | Black | O | positive | Yes | Mild, chronic, portal | Moderate portal fibrosis. Rare bridging fibrosis. | Minimal (<5%) | None significant |
| 20 | 30/M | Hispanic | O | positive | Yes | Mild, lobular and portal | None significant | None significant | N/A |
| 21‡ | 55/F | Caucasian | O | negative | No | N/A | N/A | N/A | N/A |
| 22 | 27/F | Caucasian | O | negative | Yes | Mild, portal, chronic | None significant | Severe, diffuse, small droplet (100%) | None significant |
| 23 | 41/M | Caucasian | A | negative | Yes | None significant | Mild, pericellular | Mild | None significant |
| 24 | 68/F | Caucasian | A | negative | No | Moderate lobular and mild portal, mixed acute and chronic | None significant | Large droplet (10%) and small droplet (10%) macrovesicular | None significant |
| 25 | 22/M | Black | A | negative | Yes | Mild, portal, mixed acute and chronic | None significant | Small droplet macrovascular (60%) | Mild increase in hepatocytes and Kupffer cells |
| 26 | 58/M | Caucasian | A | negative | No | None significant | None significant | None significant (<5%) | Mild, intrahepatocyte |
Donor liver was sampled but there was no liver tissue identified on pathology. The sample obtained only revealed fibroadipose tissue and peribiliary glands.
Recipient died a week after transplant and biopsy was not obtained intraoperatively during transplant.
Abbreviations: female (F), hepatitis C virus (HCV), male (M), not available (N/A), nucleic acid testing (NAT), Periodic Acid-Schiff/Diastase (PAS/D), Public Health Service (PHS)
All but one of our HCV Ab− recipients are still alive. Recipient #21, the most critically ill patient in our cohort, had a biologic MELD score of 40 prior to transplant and was listed as status 1A. The indication for liver transplantation was Wilson disease with fulminant liver failure. The post-transplant course was complicated by ischemic bowel, multiorgan failure, and acute respiratory distress syndrome. Unfortunately, the recipient succumbed to these complications within a week after transplant. The corresponding donor was HCV Ab+/NAT−, but we did not measure a post-transplant HCV viral load before the recipient’s death.
The 20 HCV Ab− recipients of HCV NAT+ donor liver all acquired active HCV infection post-LT. In contrast, only 2 out of 5 HCV Ab− recipients of HCV Ab+/NAT− donor liver acquired active HCV infection. We planned for all recipients who developed active HCV infection to receive a treatment course of DAAs to be initiated after HCV RNA became detectable in the recipient’s blood. To date, 12 of the 22 recipients who developed active HCV infection post-LT is in SVR12, another 3 completed DAA course awaiting sufficient follow-up to SVR12, 5 are still undergoing DAA treatment, and 1 is awaiting insurance approval for DAA. The number of days from LT to the initiation of DAAs ranged from 9 to 74 (median of 37). The most common reason for the lag period was the insurance approval process. For recipients #5 and #22, our CTC paid for the initial portion of their DAA course while we awaited insurance approval because liver biopsy findings could not exclude HCV as a cause of LAE elevations. Recipient #6 suffered massive blood loss during transplant that required delayed closure of the abdomen and bile duct reconstruction, which led to prolonged intubation, subglottic stenosis, and subsequent dysphagia. There was a 56-day gap until DAA initiation because of the lack of a liquid or crushed formulation of DAA amenable to nasogastric tube administration.
All 25 surviving recipients still enjoy normal graft functions after a median follow-up time of 8 months as of September 2019. Six of the recipients required liver biopsy post-LT. The biopsy of recipient #1 indicated mild acute cellular rejection (ACR), and his LAEs normalized after increasing the dose of corticosteroids. Recipient #3 had a liver biopsy for refractory ascites and biopsy showed nodular regenerative hyperplasia, which was also present on explant of pre-transplant liver; no predisposing factor was identified for nodular regenerative hyperplasia. Recipient #5 had a gradual uptrend of LAEs up to 3 to 5 times the upper limit of normal (ULN) in the 4 weeks between the LT and a liver biopsy that showed mild ACR with concurrent HCV infection. We increased the steroid dose and started DAA immediately after the biopsy. The recipient’s aspartate transaminase (AST) and alanine transaminase (ALT) continued to rise to a peak of 8 times the ULN at 8 weeks after DAA initiation, but a repeat biopsy 3 weeks after the first biopsy showed interval improvement. The LAEs normalized over the subsequent 12 weeks. Recipient #5 did eventually have another repeat biopsy 42 weeks after transplant for persistent alkaline phosphatase up to 6 times the ULN; biopsy result was consistent with mild to moderate ACR, and LAEs normalized with a steroid pulse and taper. Recipient #6 had cholestatic elevations; liver biopsy was consistent with mild to moderate ACR without features of HCV activity. The abnormal LAEs normalized after high dose corticosteroids. Recipient #6 subsequently had a repeat biopsy 38 weeks after transplant for LAE elevations and was found to have mild ACR; we increased steroid dose and started mycophenolate mofetil, and LAEs normalized within one week. Recipient #9 had a liver biopsy in the setting of persistent hepatic encephalopathy with elevated ammonia, but biopsy was not consistent with graft dysfunction and she improved clinically after withdrawal of narcotics. Recipient #22 had an initial decrease of LAEs post-LT, but LAEs rapidly rose during the subsequent hospital-acquired pneumonia. Liver biopsy showed changes consistent with mild ACR, but it could not exclude concurrent HCV infection. We initiated both DAA and high dose corticosteroids immediately after biopsy, and LAEs normalized within 2 weeks. Table 3 presents additional details on these liver biopsies.
Table 3:
Liver Transplant Recipients Requiring Liver Biopsy Post-transplant
| Recipient number | Indication for liver biopsy | Time between transplant and biopsy - weeks | Liver biopsy pathology |
|---|---|---|---|
| 1 | LAE elevations | 69 | Bile duct injury, mild endothelitis, and moderate eosinophil-rich mixed portal inflammation with scattered lobular eosinophils noted. Findings were consistent with mild ACR, with possible superimposed drug reaction. |
| 3 | Ascites refractory to transjugular intrahepatic portosystemic shunt | 43 | Vascular abnormality characterized by focal sinusoidal dilatation and features suggestive of nodular regenerative hyperplasia. Mild portal chronic inflammation. Reticulin stain highlights focal vague nodularity. No bile duct injury or endothelitis. No significant steatosis or fibrosis or stainable iron. |
| 5 | LAE elevations | 4 | Focal endothelitis and bile duct injury associated with moderate chronic portal inflammation with rare eosinophils and lymphoid aggregates found. Findings were consistent with mild ACR and concurrent recurrent HCV. |
| 6 | Scattered zone 3 hepatocyte necrosis. Bile duct injury with intraepithelial lymphocytes and scattered endothelitis. In keeping with moderate cellular rejection. | ||
| 7 | Persistent cholestasis associated with focal hepatocyte injury noted, but interval improvement in endothelitis and inflammation. Findings were consistent with interval improvement in chronic HCV and resolving acute process. | ||
| 42 | Bile duct injury with mild ductular reaction. Moderate portal inflammation, focal endothelitis, and mild lobular inflammation with spotty necrosis. Mild portal fibrosis with rare bridging fibrosis. No steatosis or stainable iron. Findings are consistent with mild to moderate acute cellular rejection. No features of chronic rejection. | ||
| 6 | LAE elevations | 3 | Moderate chronic portal inflammation with associated eosinophils, bile duct injury, and endothelitis noted. Mild lobular chronic inflammation noted. Findings were consistent with mild to moderate ACR, without strong evidence for HCV. |
| 38 | Moderate mixed portal inflammation with focal bile duct injury and focal endothelitis. Mild chronic lobular inflammation. Focal bile ductular proliferation. Moderate periportal fibrosis. Mild increased iron storage in reticuloendothelial cells. No significant steatosis. The findings are consistent with mild acute cellular rejection. No strong features to suggest active hepatitis C infection. | ||
| One-year post-transplant liver biopsy | 52 | Mild lobular inflammation (lymphocytes) and mild portal inflammation. Focal bile duct loss and mild macrovesicular steatosis (10%); no evidence of acute cellular rejection. No significant fibrosis and minimal stainable iron. | |
| 9 | Hepatic encephalopathy with persistent ammonia elevation | 35 | Mild bile ductular reaction and minimal bile duct injury. Focal lobular inflammation with few apoptotic bodies. Mild portal chronic inflammation (lymphocytes, plasma cells, neutrophils). Mild to focally moderate portal fibrosis (trichrome and reticulin). Stainable iron in reticuloendothelial cells. No steatosis. |
| 22 | LAE elevations | 2 | Few endothelitis and mild bile duct injury noted. Mild chronic portal inflammation admixed with eosinophils and neutrophils with mild lobular inflammation and few apoptotic bodies observed. Findings were consistent with mild ACR, but HCV infection could not be excluded. |
Abbreviations: acute cellular rejection (ACR), hepatitis C virus (HCV), liver-associated enzyme (LAE)
The viral loads for all recipients started on DAA decreased rapidly from their baseline pre-DAA viral load. Figure 1 depicts each recipient’s viral load as a function of time for the first 90 days post-DAA, highlighting the rapid response of every surviving recipient’s viral load after DAA initiation. SVR12 was achieved in 12 out of 12 patients who completed DAA with sufficient follow-up; eleven of these patients received an HCV NAT+ liver. Nine patients either have completed DAA course or are still on DAA treatment, but their viral loads are all undetectable. Although protease inhibitors are not recommended for use for Child-Pugh B, glecaprevir/pibrentasvir was used for recipients #5 and #6 who both reached Child-Pugh B at one point during their treatment course. We did not use protease inhibitor containing therapy on any patient with Child-Pugh C classification, for which protease inhibitors would be contraindicated. In general, the selection of DAA regimen used was per HCV treatment guidelines published by the American Association for the Study of Liver Disease (AASLD) and this was also explicitly stated in our consent form. One exception was for recipient #22 who received a total 24-week course of therapy with 8 days of sofosbuvir/velpatasvir followed by ledipasvir/sofosbuvir, which was due to insurance company preference for a 24-week course of treatment. The initial choice of sofosbuvir/velpatasvir was per our transplant team prior to genotyping results and our CTC paid for the medication prior to insurance approval. The DAA regimen selected, the timing of initiation relative to transplant, and the current HCV treatment status for each recipient are summarized in Table 4.
Figure 1: Viral Load for Each Patient Versus Time After Direct Antiviral Initiation.
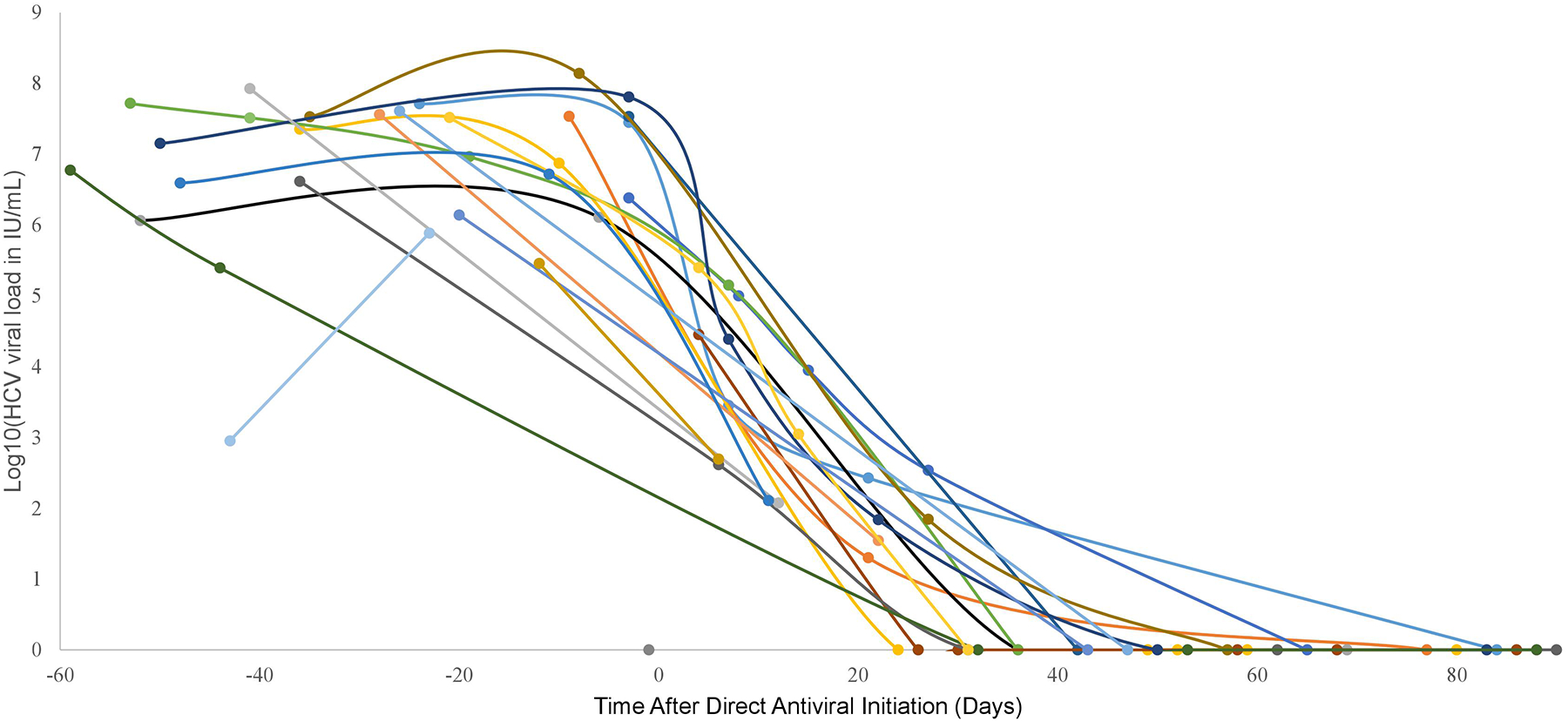
Only the first 90 days after initiation of antiviral therapy is shown for each patient. Each series of dots connected by lines represents the viral load trend for a given patient treated with DAA. The above graph contains the 21 patients treated with DAA; 4 patients were not infected post-transplant thus never started DAA and 1 patient died prior to DAA treatment. Abbreviations: hepatitis C virus (HCV), international units per milliliter (lll/mL), direct-acting antiviral (DAA)
Table 4:
Pre-transplant HCV Viral Loads of Liver Transplant Recipients and Current Treatment Status
| Recipient number | Post-transplant HCV genotype | Time between transplant and DAA initiation - days | DAA selected (duration of treatment) | Viral load before DAA initiation -IU/mL (days before DAA initiation) | Current treatment status |
|---|---|---|---|---|---|
| 1 | 1A | 25 | Ledipasvir/sofosbuvir (12 weeks) | 28,200,000 (3) | SVR12 |
| 2 | 3A | 12 | Glecaprevir/pibrentasvir (12 weeks) | 34,400,000 (9) | SVR12 |
| 3 | 2B | 54 | Glecaprevir/pibrentasvir (12 weeks) | 1,290,000 (6) | SVR12 |
| 4 | 1A | 39 | Glecaprevir/pibrentasvir (12 weeks) | 7,440,000 (10) | SVR12 |
| 5 | 1B | 30 | Glecaprevir/pibrentasvir (12 weeks) | 2,410,000 (3) | SVR12 |
| 6 | 1A | 56 | Glecaprevir/pibrentasvir (12 weeks) | 9,250,000 (19) | SVR12 |
| 7 | 1A | 9 | Glecaprevir/pibrentasvir (12 weeks) | 33,900,000 (3) | SVR12 |
| 8 | 3A | 17 | Glecaprevir/pibrentasvir (12 weeks) | 829 (12) | SVR12 |
| 9 | 3A | 49 | Glecaprevir/pibrentasvir (12 weeks) | 4,140,000 (36) | SVR12 |
| 10 | 3A | 37 | Glecaprevir/pibrentasvir (12 weeks) | 138,000,000 (8) | SVR12 |
| 11 | 3A | 52 | Glecaprevir/pibrentasvir (12 weeks) | 64,300,000 (3) | SVR12 |
| 12 | 3A | 74 | Glecaprevir/pibrentasvir (12 weeks) | 248,000 (44) | completed DAA |
| 13 | 1A | 28 | Glecaprevir/pibrentasvir (12 weeks) | 40,700,000 (26) | completed DAA |
| 14 | 1A | 31 | Glecaprevir/pibrentasvir (12 weeks) | 36,500,000 (28) | completed DAA |
| 15 | 1A | 44 | Glecaprevir/pibrentasvir (12 weeks) | 84,400,000 (41) | DAA ongoing |
| 16 | 1A | 24 | Glecaprevir/pibrentasvir (12 weeks) | 33,100,000 (21) | DAA ongoing |
| 17 | 2B | 22 | Glecaprevir/pibrentasvir (12 weeks) | 1,380,000 (20) | DAA ongoing |
| 18 | 3A | 46 | Glecaprevir/pibrentasvir (12 weeks) | 32,500,000 (41) | DAA ongoing |
| 19 | 1A | 54 | Glecaprevir/pibrentasvir (12 weeks) | 5,230,000 (11) | DAA ongoing |
| 20 | 1B | DAA not initiated | DAA not initiated | DAA not initiated | DAA not initiated† |
| 21 | N/A | DAA not initiated | DAA not initiated | DAA not initiated | Deceased‡ |
| 22 | 1A | 13 | Sofosbuvir/velpatasvir (8 days) then ledipasvir/sofosbuvir (23 weeks)§ | 287,000 (12) | SVR12 |
| 23 | N/A | DAA not initiated | DAA not initiated | DAA not initiated | Not infected, VL undetectable at 37 weeks after liver transplant |
| 24 | N/A | DAA not initiated | N/A | DAA not initiated | Not infected, VL undetectable at 4 weeks after liver transplant |
| 25 | 2B | 55 | Glecaprevir/pibrentasvir (12 weeks) | 769,000 (23) | DAA ongoing |
| 26 | N/A | DAA not initiated | NA | DAA not initiated | Not infected, VL undetectable at 4 weeks after liver transplant |
Currently pending insurance approval for DAA.
recipient #1 died of bowel ischemia and subsequent multi-organ failure within a week of liver transplant
sofosbuvir/velpatasvir was used as a pan-genotypic agent prior to the genotyping result, then transitioned to ledipasvir/sofosbuvir after insurance approval. Insurance approved a total 24-week treatment course which we delivered.
Abbreviations: direct antiviral agent (DAA), hepatitis C virus (HCV), international units per milliliter (IU/mL), not available (N/A), sustained virologic response at 12 weeks after treatment (SVR12), viral load (VL)
To summarize, all except one patient in our series currently possess functioning liver grafts. All 12 recipients who completed their DAA courses and have reached sufficient follow-up for SVR12 have achieved SVR12. Nine of our recipients have either completed DAA treatment without sufficient follow-up time for SVR12 or are undergoing DAA treatment. One patient is awaiting DAA initiation pending insurance approval. Of note, 11 out of our 12 patients in SVR12 received an HCV NAT+ donor liver and have normal graft function.
Discussion
Our results are consistent with a prior case series of 10 HCV NAT− LT recipients of HCV NAT+ donors, with all of the patients achieving SVR12 post-LT. However, 7 out of 10 recipients in the prior series were HCV Ab+ before transplant whereas all recipients in our case series were HCV Ab−.13 More recently, Luckett et al. described 55 LT recipients of HCV Ab+ donor livers of whom 49 recipients were HCV Ab−.14 None of the donors were HCV NAT+, which differed notably from our present series.
Transplantation of organs from HCV NAT+ donors to HCV Ab− patients is not an entirely novel concept. Goldberg et al. evaluated kidney transplants from HCV NAT+ donors to 10 HCV Ab− recipients, and they treated recipients with DAA at the first elevated HCV RNA level in the Transplanting Hepatitis C Kidneys Into Negative Kidney Recipients (THINKER) trial.15 Our group also previously described 10 HCV Ab− recipients of HCV Ab+ donor kidneys who received DAA as pre- and post-transplant prophylaxis with treatment regimen modified according to genotype results.16 All patients in the two abovementioned trials reached SVR12. Transplantation of heart and lungs from HCV NAT+ donors to HCV Ab− recipients have similarly been reported with acceptable short-term outcomes and HCV cure with DAA treatment.17–20 Despite these reports, liver transplantation with an HCV Ab+ donor into an HCV Ab− recipient is fundamentally and conceptually different as HCV infects hepatocytes; thus, the liver is the largest reservoir of HCV in the body. Little is understood about the impact of immunosuppression in HCV NAT− transplant recipients receiving an HCV NAT+ liver and, by extension, a large HCV load. Acute HCV infections do not typically cause significant liver injury in the non-transplant setting. However, the clinical course of transmitting new HCV infection to liver transplant recipients on active immunosuppression has the potential to progress differently. Our series helps to address this knowledge gap since we administered standard immunosuppression regimens without dose reduction despite HCV infection.
Despite enthusiasm for using HCV Ab+ donors for LT, the long-term outcomes of donor-derived HCV infection in HCV Ab− patients are yet to be determined.14 Some possible risks of donor-derived HCV infection include fibrosing cholestatic hepatitis and graft rejection following HCV cure.21 Furthermore, our series observed a 16% rate of ACR (4 out of 25 surviving patients), albeit 2 out of 4 of these cases were complicated by concurrent post-transplant HCV infection. This raises the possibility that ACR may occur more rapidly for HCV Ab− patients who receive HCV NAT+ liver transplantation, since prior experience with immune-mediated graft dysfunction primarily occurs after completion of DAA treatment post-LT.22 Transplant providers must weigh these possibilities against the mortality risk while on the waitlist. For instance, Croome et al. showed a 136% increase in mortality for waitlisted patients who declined an increased risk donor liver relative to those who accepted one.5 A recent mathematical model using UNOS data also suggested improved life expectancy for patients with MELD 20+ should they be willing to accept HCV Ab+ organs.23
Our study demonstrates the paradigm shift brought by DAA agents with high rates of treatment response for donor-derived HCV infections. Nonetheless, until long-term data become available, we recommend reserving the transplantation of HCV NAT+ donor livers to HCV Ab− recipients for specific subsets of LT candidates. Patients who may benefit the most include those whose MELD scores underrepresent the clinical severity of disease (e.g., recurrent cholangitis episodes), as well as those in whom prolonged waiting risks eventual transplant ineligibility (e.g., progressing hepatopulmonary syndrome) or other calamities (e.g., acute liver failure).24
All patients in our series started DAA within 12 weeks of LT. Awaiting insurance approval was the most common source of the lag time between LT and DAA initiation. In one patient, an inability to swallow led to the delay in DAA initiation. Additional studies should focus on the optimal treatment regimen and timing, specifically whether prophylactic treatment at the time of liver transplant would promote even better outcomes. While the availability of pan-genotypic DAA improves the theoretical feasibility of prophylactic treatments, administration through a nasogastric tube immediately post-transplant may encounter poor drug absorption. A recent study of the pan-genotypic DAA glecaprevir/pibrentasvir in healthy subjects showed variable drug plasma levels when the medication was crushed or ground, precluding it from reliable delivery through nasogastric tubes.25 We did note that, in the Using Hepatitis C positive hearts for Negative Recipients (USHER) trial, Mclean et al. reported HCV cure in an undisclosed number of HCV Ab− orthotopic heart transplant recipients who received crushed elbasvir/grazoprevir through nasogastric tube administration.26 However, elbasvir/grazoprevir is not pan-genotypic. Its role may be limited to treatments after the HCV genotype result is confirmed, when the immediate post-operative need for crushed administration has often resolved.
Given the rise of HCV Ab+ donors in the United States based on available national data, the addition of DAA agents to our therapeutic arsenal has allowed us to explore the utilization of these increased-risk organs.27 The transplant community underwent a similar transformation for hepatitis B core antibody positive liver grafts in the past decade. Despite the initial uncertainty, literature now supports the routine use of hepatitis B core antibody positive livers for transplantation.28,29 In time, we may also view HCV Ab+ liver grafts in the same light. In conclusion, our case series presented the successful transplant of HCV Ab+ livers in HCV Ab− recipients when HCV receives treatment after transplant. The practice should be considered after a careful risk and benefit discussion with the potential candidate. Further discussions at the national level are warranted to reach a consensus within the transplant community regarding the use of HCV Ab+ donor livers for HCV Ab− recipients.
Acknowledgements/Funding:
This work was supported by grant numbers K23DK115908 (Garonzik-Wang) from the National Institute of Diabetes and Digestive and Kidney Diseases, K23CA177321 (Durand) from the National Cancer Institute, KL2TR001077 (Chen) from the Johns Hopkins Institute for Clinical and Translational Research (ICTR) and the National Center for Advancing Translational Sciences, and K24DA034621 (Sulkowski) from the National Institute on Drug Abuse. Dr. Ting was supported by the Johns Hopkins Osler Medical Housestaff Training Program Osler Fund. The analyses described here are solely the responsibility of the authors and do not necessarily represent the official view of the National Institutes of Health, Johns Hopkins ICTR, or Osler Medical Housestaff Training Program.
Abbreviations:
- ACR
acute cellular rejection
- ALT
alanine transaminase
- Ab
antibody
- AST
aspartate transaminase
- CTC
Comprehensive Transplant Center
- DAA
direct-acting antiviral
- HCV
hepatitis C virus
- LAE
liver-associated enzyme
- LT
liver transplantation
- MELD
model of end-stage liver disease
- NAT
nucleic acid testing
- PHS
Public Health Services
- RNA
ribonucleic acid
- SVR12
sustained virologic response at 12 weeks after treatment
- ULN
upper limit of normal
References
- 1.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2016 Annual Data Report: Liver. Am J Transplant. 2018;18 Suppl 1:172–253. [DOI] [PubMed] [Google Scholar]
- 2.Durand CM, Bowring MG, Thomas AG, et al. The Drug Overdose Epidemic and Deceased-Donor Transplantation in the United States: A National Registry Study. Ann Intern Med. 2018;168(10):702–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wholley CL. Guidance on Explaining Risk Related to Use of U.S. PHS Increased Risk Donor Organs When Considering Organ Offers. OPTN/UNOS Briefing Paper. 2017. [Google Scholar]
- 4.Seem DL, Lee I, Umscheid CA, Kuehnert MJ, United States Public Health S. PHS guideline for reducing human immunodeficiency virus, hepatitis B virus, and hepatitis C virus transmission through organ transplantation. Public Health Rep. 2013;128(4):247–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croome KP, Lee DD, Pungpapong S, Keaveny AP, Taner CB. What are the outcomes of declining a public health service increased risk liver donor for patients on the liver transplant waiting list? Liver Transpl. 2018;24(4):497–504. [DOI] [PubMed] [Google Scholar]
- 6.Shaffer AA, Thomas AG, Bowring MG, et al. Changes in practice and perception of hepatitis C and liver transplantation: Results of a national survey. Transpl Infect Dis. 2018;20(6):e12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciftcibasi Ormeci A, Yildiz C, Seberi B, Gurakar M, Simsek C, Gurakar A. Usage of HCV viremic organs in liver transplantation to anti-HCV negative recipients: The current status and review of literature. Turk J Gastroenterol. 2019;30(9):771–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levitsky J, Formica RN, Bloom RD, et al. The American Society of Transplantation Consensus Conference on the Use of Hepatitis C Viremic Donors in Solid Organ Transplantation. Am J Transplant. 2017;17(11):2790–2802. [DOI] [PubMed] [Google Scholar]
- 9.Bowring MG, Kucirka LM, Massie AB, et al. Changes in Utilization and Discard of Hepatitis C-Infected Donor Livers in the Recent Era. Am J Transplant. 2017;17(2):519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballarin R, Cucchetti A, Spaggiari M, et al. Long-term follow-up and outcome of liver transplantation from anti-hepatitis C virus-positive donors: a European multicentric case-control study. Transplantation. 2011;91(11):1265–1272. [DOI] [PubMed] [Google Scholar]
- 11.Saab S, Ghobrial RM, Ibrahim AB, et al. Hepatitis C positive grafts may be used in orthotopic liver transplantation: a matched analysis. Am J Transplant. 2003;3(9):1167–1172. [DOI] [PubMed] [Google Scholar]
- 12.Bari K, Luckett K, Kaiser T, et al. Hepatitis C transmission from seropositive, nonviremic donors to non-hepatitis C liver transplant recipients. Hepatology. 2018;67(5):1673–1682. [DOI] [PubMed] [Google Scholar]
- 13.Kwong AJ, Wall A, Melcher M, et al. Liver Transplantation for HCV Non-Viremic Recipients with HCV Viremic Donors. Am J Transplant. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luckett K, Kaiser TE, Bari K, et al. Use of Hepatitis C Virus Antibody-Positive Donor Livers in Hepatitis C Nonviremic Liver Transplant Recipients. J Am Coll Surg. 2018. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg DS, Abt PL, Blumberg EA, et al. Trial of Transplantation of HCV-Infected Kidneys into Uninfected Recipients. N Engl J Med. 2017;376(24):2394–2395. [DOI] [PubMed] [Google Scholar]
- 16.Durand CM, Bowring MG, Brown DM, et al. Direct-Acting Antiviral Prophylaxis in Kidney Transplantation From Hepatitis C Virus-Infected Donors to Noninfected Recipients: An Open-Label Nonrandomized Trial. Ann Intern Med. 2018;168(8):533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moayedi Y, Gulamhusein AF, Ross HJ, Teuteberg JJ, Khush KK. Accepting hepatitis C virus-infected donor hearts for transplantation: Multistep consent, unrealized opportunity, and the Stanford experience. Clin Transplant. 2018;32(7):e13308. [DOI] [PubMed] [Google Scholar]
- 18.Martins PN, Movahedi B, Bozorgzadeh A. Transplanting HCV-Infected Kidneys into Uninfected Recipients. N Engl J Med. 2017;377(11):1104–1105. [DOI] [PubMed] [Google Scholar]
- 19.Schlendorf KH, Zalawadiya S, Shah AS, et al. Early outcomes using hepatitis C-positive donors for cardiac transplantation in the era of effective direct-acting anti-viral therapies. J Heart Lung Transplant. 2018;37(6):763–769. [DOI] [PubMed] [Google Scholar]
- 20.Theodoropoulos N, Whitson BA, Martin SI, Pouch S, Pope-Harman A. Successful treatment of donor-derived hepatitis C infection in a lung transplant recipient. Transpl Infect Dis. 2017;19(2). [DOI] [PubMed] [Google Scholar]
- 21.Somerville L, Doucette K. Hepatitis C: Current Controversies and Future Potential in Solid Organ Transplantation. Curr Infect Dis Rep. 2018;20(7):18. [DOI] [PubMed] [Google Scholar]
- 22.Chan C, Schiano T, Agudelo E, et al. Immune-mediated graft dysfunction in liver transplant recipients with hepatitis C virus treated with direct-acting antiviral therapy. Am J Transplant. 2018;18(10):2506–2512. [DOI] [PubMed] [Google Scholar]
- 23.Chhatwal J, Samur S, Kues B, et al. Optimal timing of hepatitis C treatment for patients on the liver transplant waiting list. Hepatology. 2017;65(3):777–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saberi B, Hamilton JP, Durand CM, et al. Utilization of hepatitis C virus RNA-positive donor liver for transplant to hepatitis C virus RNA-negative recipient. Liver Transpl. 2018;24(1):140–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oberoi RK, Zhao W, Sidhu DS, Viani RM, Trinh R, Liu W. A Phase 1 Study to Evaluate the Effect of Crushing, Cutting Into Half, or Grinding of Glecaprevir/Pibrentasvir Tablets on Exposures in Healthy Subjects. J Pharm Sci. 2018;107(6):1724–1730. [DOI] [PubMed] [Google Scholar]
- 26.McLean RC, Reese PP, Acker M, et al. Transplanting hepatitis C virus-infected hearts into uninfected recipients: A single-arm trial. Am J Transplant. 2019. [DOI] [PubMed] [Google Scholar]
- 27.@CDCgov. Characteristics of Deceased Solid Organ Donors and Screening Results for Hepatitis B, C, and Human Immunodeficiency Viruses — United States, 2010–2017 | MMWR. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen PH, Limketkai BN, Trilianos P, et al. Effect of prior hepatitis B virus exposure on long-term risk of liver-related events after liver transplantation. Clin Transplant. 2016;30(5):579–588. [DOI] [PubMed] [Google Scholar]
- 29.Lei M, Yan LN, Yang JY, et al. Safety of hepatitis B virus core antibody-positive grafts in liver transplantation: A single-center experience in China. World J Gastroenterol. 2018;24(48):5525–5536. [DOI] [PMC free article] [PubMed] [Google Scholar]


