To the Editor:
In helminth-endemic areas, elevated levels of cross-reactive IgE to environmental and food allergens are often seen that do not translate into positive skin prick test results or allergy symptoms.1 Among Ghanaian children, such cross-reactivity was shown to be associated with Schistosoma haematobium infection and dominated by high IgE against cross-reactive carbohydrate determinants (CCDs).2 The specific carbohydrate motifs involved in this IgE recognition were not determined.2
Recently, glycan microarrays have been developed that allow detailed characterization of IgE binding to glycan motifs.3,4 Here, we report the use of a microarray with 126 synthetic N-glycans and short oligosaccharides to identify specific glycan motifs associated with IgE cross-reactivity among urban and rural Ghanaian children.
Study methodology details are provided in this article’s Online Repository at www.jacionline.org. Sera from children attending schools we classified as rural (n = 20), urban low socioeconomic status (SES) (n = 20), and urban high SES (n = 20) were assessed. We also included sera from Italian pollen-allergic controls with anti-CCD IgE (n = 5) and meat-allergic controls from the United States with anti–galactose-α−1,3-galactose (α−1,3-gal) IgE (n = 4).
The characteristics of the Ghanaian subjects are presented in Table E1 in this article’s Online Repository at www.jacionline. org. Rural participants had high burdens of intestinal helminthiasis (50%) and malaria parasitemia (40%) compared with urban subjects. S haematobium infection was found in 26.3% of urban low SES children compared with 5% in the other schools. ImmunoCAP-determined IgE sensitization (≥0.35 kU/L) to all allergens was most prevalent in the rural group and lowest in the urban high SES school.
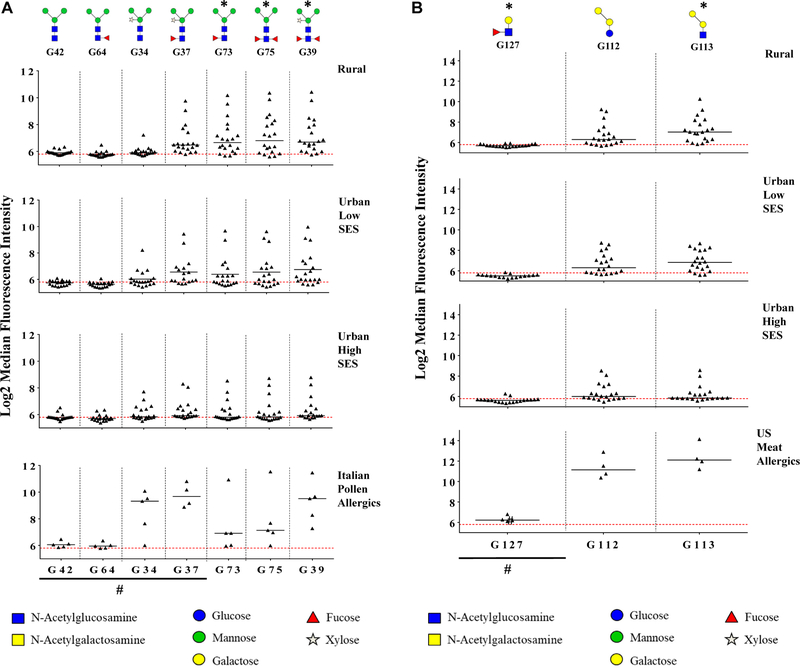
A heatmap identified glycan motifs preferentially boundbyIgE in each area (see Fig E2 in this article’s Online Repository at www.jacionline.org). High responses to structures with core α−1,3-fucose without core xylose (G73 and G75) were observed in rural children compared with urban high SES children (Fig 1, A; see Table E2 in this article’s Online Repository at www.jacionline.org). Although rural children had low responses to core xylose alone (structure G34), a few individuals in both urban groups had elevated IgE to this structure. Responses to structures that had both core xylose and core α−1,3-fucose (G37 and G39) were similar to those seen to core α−1,3-fucose alone (G73). Interestingly, in Italian controls, elevated responses were seen to structures with core xylose (G34, G37, and G39) and less to structures with core α−1,3-fucose without core xylose (G73 and G75).
FIG 1.
A, IgE responses to selected N-glycan motifs. N-glycan structures eliciting the greatest IgE binding among Ghanaian children were identified by heatmap. Responses to these structures are shown among Ghanaian children (stratified by school category) and Italian pollen-allergic controls. Four additional structures with key variants were included for comparison and are indicated on the figure. *Kruskal-Wallis test of between-area differences in IgE response among Ghanaian subjects only (P < .05). #Structures included for comparison. **Response missing for 1 Italian pollen-allergic control for structure G37 due to smear on the specific microarray slide that obstructed the reading of microarray spots for this structure only. B, IgE responses to α-1,3-gal motifs. IgE reactivities to α-1,3-gal motifs are shown among Ghanaian children (stratified by school category) and US meat-allergic controls. Lewis X saccharide (G127) was included as a control for small synthetic oligosaccharides. *Kruskal-Wallis test of between-area differences in IgE response among Ghanaian subjects only (P < .05). #Structure included for comparison.
IgE responses to structure G113 containing the α−1,3-gal motif were most elevated in rural children and lowest in urban high SES children (Fig 1, B). Weaker responses to structure G112 (α−1,3-gal attached to glucose instead of N-acetylglucosamine) were observed among rural children. For US meat-allergic patients, median fluorescence intensities for structures G112 and G113 were higher than those seen for rural Ghanaian children.
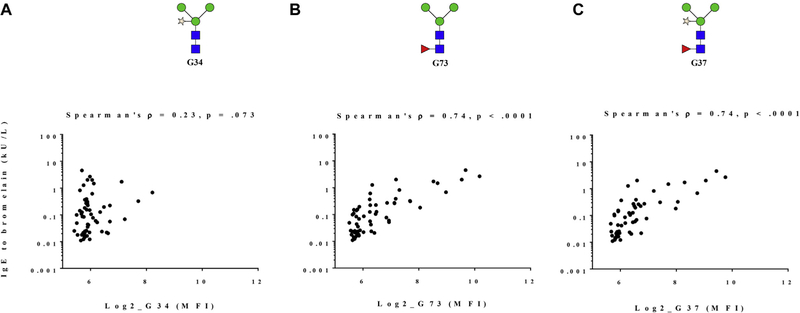
IgE reactivities to core xylose and to core α−1,3-fucose found in bromelain (measured by ImmunoCAP) were compared to the same motifs on the microarray (Fig 2). Although a weak correlation was observed between IgE to bromelain and to core xylose alone (structure G34), there were strong correlations between IgE to bromelain and to core α-1,3-fucose alone (structure G73, Spearman ρ = 0.74; P < .001) as well as to structure G37 with both core xylose and α−1,3-fucose (Spearman ρ = 0.74; P < .001). Correlations between IgE to α−1,3-gal (measured by ImmunoCAP) and IgE to the 2 α−1,3-gal motifs on the array were relatively weak (for G113, Spearman ρ = 0.52; P <.001, and for G112, Spearman ρ = 0.29; P =.03).
FIG 2.
Correlations between IgE to bromelain measured by ImmunoCAP and IgE to N-glycans measured by glycan microarray in Ghanaian children. Correlationbetween IgE to bromelain andcore xylose only (structure G34) (A), core α−1,3 fucose only (structure G73) (B) and core α−1,3 fucose 1 core xylose (structure G37) (C).
To examine whether S haematobium infection played a role in anti-glycan IgE responses, motifs preferentially bound by IgE among S haematobium positives were identified by heatmap (see Fig E3 in this article’s Online Repository at www.jacionline.org). IgE responses to core xylose alone were elevated in most S haematobium positives but not in negatives (see Fig E4, A, in this article’s Online Repository at www.jacionline.org). In contrast, responses to structures that included core α−1,3-fucose were elevated in both S haematobium positives and negatives (see Fig E4, B-D). No significant associations were found between intestinal helminthiasis or malaria parasitemia and IgE responses to microarray glycans.
Our study is the first to use a synthetic glycan microarray to elucidate specific motifs associated with carbohydrate-related IgE cross-reactivity (CCDs as well as α−1,3-gal). Overall, higher anti-glycan IgE responses were observed in the rural area compared with both urban groups. Strikingly, the prevalence of IgE sensitization to α−1,3-gal among rural subjects as determined by ImmunoCAP and glycan microarray was very high. The relatively poor correlation between IgE titers in both assays may be explained by the use of different antigen sources representing α−1,3-gal: bovine thyroglobulin with a heterogeneous glycan composition5 on ImmunoCAP versus synthetic glycans on the microarray.
Relatively high percentages of IgE sensitization to α−1,3-gal have also been observed in rural areas of Kenya (76%) and Ecuador (37%).6 It has been proposed (but yet to be demonstrated) that besides ticks, other ectoparasites or helminths may be involved.6 In our small study, we observed no association between helminth infection and α−1,3-gal sensitization. Although α-galactobiose has been found in some helminths, this is mostly in α−1,4 or β−1,6 linkages. Only for Fasciola hepatica has α−1,3-gal been demonstrated to be expressed on glycolipids.7 In-depth studies are needed to explore whether other helminths express α−1,3-gal and associations with IgE sensitization.
The N-linked glycans containing core α−1,3-fucose and β−1,2-linked core xylose are the main plant and insect glycoprotein residues associated with carbohydrate-related IgE cross-reactivity.8 IgE reactivity in Ghanaian children was higher to structures with core α−1,3-fucose compared with core xylose alone although a few individuals in the urban groups had elevated IgE to the latter. This observation may reflect the source of primary sensitization. N-glycans with core xylose together with core α−1,3-fucose and modifications have been identified in the egg stage of schistosomes.9 In our study, S haematobium infection, mainly in urban low SES subjects, was significantly associated with raised IgE to core xylose. Although some S haematobium positives had elevated IgE to structures with core α−1,3-fucose only, similarly raised levels to these structures were observed among S haematobium negatives, suggesting that these may not have been driven by schistosome infection. Therefore, our observations link schistosome infection in IgE cross-reactivity involving core xylose.
Overall, our findings suggest that cross-reactive IgE among Ghanaian children may be directed against both core xylose and core α−1,3-fucose independently. Further investigations are needed to explore factors aside from helminths such as insects that may drive reactivity to these motifs. Recently, protein microarray technology has revolutionized allergy diagnostics by allowing the simultaneous assessment of specific IgE to multiple allergens with a small amount of serum. Our investigation illustrates how glycan microarrays can further improve molecular diagnosis of specific IgE to allergenic motifs by providing additional information on IgE profiles of patients.
Supplementary Material
Acknowledgments
We thank Yvonne Kruize-Hoeksma for technical expertise, Dziedzom DeSouza for the design of the database, Richard A. Akuffo for data entry, and Linda Hevor for technical assistance in parasitology. We are most grateful to the study participants, their families, and teachers for their time as well as commitment.
This study was supported by the European Academy of Allergy and Clinical Immunology Long-term Research Fellowship (E.K.A.-B.) for the project titled “Molecular Understanding of IgE cross-reactivity in Africa,” EuroPrevall (grant no. FOOD-CT2005–514000), Global View of Food Allergy-GLOFAL (grant no. FOOD-CT2005–517812), Wellcome Trust (grant no. 075791/Z/04/Z), and the Spanish Ministry of Economy and Competitiveness-MINECO (grant no. CTQ2014–58779-R). Funding bodies played no role in the design, collection, analysis, and interpretation of data or in the writing of the manuscript or the decision to submit it for publication.
Disclosure: A. S. Amoah has received a grant from the Wellcome Trust (grant no. 075791/Z/04/Z). E. K. Asuming-Brepong has received a grant from the European Academy of Allergy and Clinical Immunology. T. A. E. Platts-Mills has received a grant from the National Institute of Allergy and Infectious Disease and Phadia/Thermo Fisher, has received travel support from Phadia/Thermo Fisher, receives payment for a patent from the US Patent and Trademark Office, and has received royalties from IBT/Viracor. K. Brzezicka has received a grant from the Spanish Ministry of Economy and Competitiveness (MINECO, CTQ2011–27874 grant fellowship). D. A. Boakye has received grants from EuroPrevall (grant no. FOOD-CT-2005–514000) and GLOFAL (grant no. FOOD-CT-2005–517812). R. van Ree has consultant arrangements with HAL Allergy BV and Citeq BV; has received grants from the European Commission and the Dutch Science Foundation; and has received payment for lectures from HAL Allergy and ThermoFisher Scientific. M. Yazdanbakhsh has received grants from EuroPrevall (grant no. FOOD-CT2005–514000) and GLOFAL (grant no. FOOD-CT-2005–517812) and has a board membership with the European Journal of Immunology.
Footnotes
potential conflict of interest:
The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Hamid F, Amoah AS, van Ree R, Yazdanbakhsh M. Helminth-induced IgE and protection against allergic disorders. Curr Top Microbiol Immunol 2015;388: 91–108. [DOI] [PubMed] [Google Scholar]
- 2.Amoah AS, Obeng BB, Larbi IA, Versteeg SA, Aryeetey Y, Akkerdaas JH, et al. Peanut-specific IgE antibodies in asymptomatic Ghanaian children possibly caused by carbohydrate determinant cross-reactivity. J Allergy Clin Immunol 2013;132: 639–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brzezicka K, Echeverria B, Serna S, van Diepen A, Hokke CH, Reichardt NC. Synthesis and microarray-assisted binding studies of core xylose and fucose containing N-glycans. ACS Chem Biol 2015;10:1290–302. [DOI] [PubMed] [Google Scholar]
- 4.Yang YYM, Li XH, Brzezicka K, Reichardt N-C, Wilson RA, van Diepen A, et al. Specific anti-glycan antibodies are sustained during and after parasite clearance in Schistosoma japonicum-infected rhesus macaques. PLoS Negl Trop Dis 2017;11: e0005339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macher BA, Galili U. The gala1,3galb1,4GlcNAc-R (α-gal) epitope: a carbohydrate of unique evolution and clinical relevance. Biochim Biophys Acta 2008; 1780:75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-a1,3 galactose. J Allergy Clin Immunol 2011;127: 1286–93.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wuhrer M, Grimm C, Zahringer U, Dennis RD, Berkefeld CM, Idris MA, et al. A novel GlcNAcalpha1-HPO3–6Gal(1–1)ceramide antigen and alkylated inositolphosphoglycerolipids expressed by the liver fluke Fasciola hepatica. Glycobiology 2003;13:129–37. [DOI] [PubMed] [Google Scholar]
- 8.Altmann F. The role of protein glycosylation in allergy. Int Arch Allergy Immunol 2007;142:99–115. [DOI] [PubMed] [Google Scholar]
- 9.Smit CH, van Diepen A, Nguyen DL, Wuhrer M, Hoffmann KF, Deelder AM, et al. Glycomic analysis of life stages of the human parasite Schistosoma mansoni reveals developmental expression profiles of functional and antigenic glycan motifs. Mol Cell Proteomics 2015;14:1750–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.