Abstract
Objective
Identify blood pressure (BP) response to spironolactone in patients with apparent therapy-resistant hypertension (aTRH) using electronic medical records (EMRs) in order to estimate response in a real-world clinical setting.
Design
Developed an algorithm to determine BP and electrolyte response to spironolactone for use in a retrospective cohort study.
Setting
An academic medical centre in Nashville, Tennessee.
Population
Patients with aTRH prescribed spironolactone.
Main outcome measures
Baseline BP and BP response, determined as the change in mean systolic BP (SBP) and diastolic BP (DBP) following spironolactone initiation. Additional response measures were serum sodium, potassium and creatinine, estimated glomerular filtration rate, haemoglobin A1c (HbA1c), glucose, high-density lipoprotein, low-density lipoprotein and triglycerides. Demographic characteristics included race, age, gender, body mass index (BMI), diabetes mellitus, chronic kidney disease stage 3, ischaemic heart disease and smoking.
Results
The mean decreases in SBP and DBP were 8.1 and 3.4 mm Hg, consistent with clinical trial data. Using a mean decrease in SBP of 5 mm Hg or in DBP of 2 mm Hg to define ‘responders’, 30.3% of patients did not respond. In univariable analyses, responders had higher BMI, baseline SBP, DBP, sodium and HbA1c, and lower creatinine. In multivariable analysis, responders were older and had significantly higher BMI and baseline SBP and DBP, and lower potassium. Increases in potassium and creatinine following spironolactone were larger in responders. When BP was evaluated as a continuous variable, decreases in SBP and DBP correlated with baseline BP, decrease in sodium and increases in potassium and creatinine following spironolactone. The decrease in SBP was associated with decreasing glucose in European Americans.
Conclusions
We developed an algorithm to assess BP response to a commonly prescribed medication for aTRH using EMRs. Electrolyte changes associated with the BP response to spironolactone are consistent with its mechanism of action of blocking the mineralocorticoid receptor and decreasing epithelial sodium channel activity.
Keywords: spironolactone, blood pressure response, mineralocorticoid receptor antagonist, electronic medical records, hypertension
Strengths and limitations of this study.
A strength of this study is the accuracy of the algorithm developed to determine blood pressure response to spironolactone in a large number of patients with apparent therapy-resistant hypertension.
The availability of clinical electrolyte measurements, in addition to blood pressure measurements, is another strength as it provides data supporting the mechanism of mineralocorticoid receptor antagonism in responders to spironolactone.
Additional strengths are that this study provides methodology which can be applied in future pharmacogenetic studies using electronic health records, and that the algorithm can be adapted for use with other medications or in other large-scale electronic medical record systems with linked genetic data.
Limitations of this study include the inability to confirm medication adherence, a lack of ambulatory blood pressure measurements and a lack of some laboratory measures, such haemoglobin A1c and lipids, for the entire population.
Introduction
The mineralocorticoid receptor (MR) antagonist spironolactone has been identified as effective add-on therapy for blood pressure (BP) control in patients with apparent therapy-resistant hypertension (aTRH) in clinical trials, including the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm and the Prevention and Treatment of Hypertension with Algorithm Based Therapy-2 (PATHWAY-2).1–4 In the PATHWAY-2 trial, addition of spironolactone at a dose of 25–50 mg/day to a therapeutic regimen containing an ACE inhibitor or angiotensin receptor blocker, calcium channel blocker (CCB) and thiazide-like diuretic significantly decreased home systolic blood pressure (SBP) by a mean of 8.7 mm Hg.1 In prior clinical studies, the SBP/diastolic blood pressure (DBP) responses to spironolactone have ranged from a mean decrease of 4.6/1.8 mm Hg to a mean decrease of 25/12 mm Hg.1–5
Clinical trials often assess homogenous groups of patients limiting the applicability in a real-world clinical setting. We hypothesised that we could use electronic medical records (EMR) to assess the BP response to the addition of spironolactone in patients with resistant hypertension who were on a stable antihypertensive regimen of at least three medications including a thiazide diuretic or dihydropyridine CCB. We used a previously published algorithm for the identification of patients with aTRH.6 To evaluate the BP response to spironolactone, we developed an additional algorithm to identify patients who were prescribed spironolactone during a period of stable medication use from up to 6 months before the start of spironolactone to 6 months after. The accuracy of the algorithm was validated by electronic record review.
We collected all outpatient BPs and laboratory measurements during the period of stable medication use before and after initial spironolactone prescription. We assessed BP response as a continuous variable and as a dichotomised variable (responders vs non-responders). We also assessed electrolyte measurements relevant to the mechanism of action of spironolactone, MR antagonism.
Methods
Electronic medical record
The Synthetic Derivative (SD) is a deidentified copy of the Vanderbilt University Medical Center (VUMC) EMR with Health Insurance Portability and Accountability Act of 1996 identifiers removed by established deidentification software as well as custom techniques.7 The SD contains almost all available clinical data including basic demographics, such as race and sex; text from clinical care notes; laboratory values; inpatient and outpatient medication data; international classification of disease (ICD) and current procedural terminology (CPT) codes; and other diagnostic reports.7 To date, the SD contains approximately 2.8 million records, approximately 1 million of which contain detailed longitudinal data.
Spironolactone response algorithm development
Patients within the SD were identified as having aTRH using a previously published algorithm.6 Patients were defined as having aTRH if their BP was greater than or equal to 140/90 mm Hg, despite concurrent use of three antihypertensives including a thiazide diuretic or dihydropyridine CCB, or if they were taking four or more antihypertensive medications, including a thiazide diuretic or dihydropyridine CCB. Patients with secondary hypertension, chronic kidney disease (CKD) stages 4 and 5, heart failure with reduced ejection fraction less than 35%, thyroid and parathyroid disorders, nephrotic syndrome, chronic glomerulonephritis, anomalies of the bulbus cordis, coarctation of the aorta, adrenal gland neoplasms and disorders (excluding adrenal insufficiencies), chronic pulmonary heart disease, thyrotoxicosis, disorders of thyrocalcitonin secretion and obstructive uropathy were excluded by the aTRH algorithm (see online supplementary table 1).
bmjopen-2019-033100supp001.pdf (106KB, pdf)
All drug exposures to antihypertensive medications including spironolactone were identified from the SD by electronic-prescribing tools and MedEx.8 The utility of these tools for extracting medication data from the EMR has been shown previously.9 10 For a medication exposure to be considered valid, at least one of the following identifiers—dose, route, frequency or duration—was required.
We developed an algorithm to identify patients in the SD who were prescribed spironolactone and in whom BP was measured within a stable window of time before and after initiation of spironolactone. Using electronic prescribing tools and MedEx,8 the novel start date (NSD) for a spironolactone prescription was determined by the earliest mention of spironolactone, aldactone or aldactazide in a patient’s record after the patient met the aTRH case definition. In addition, spironolactone, aldactone or aldactazide prescription had to have been listed at least twice, at least 1 month apart, during the subsequent 6-month period. Patients who were initiated on aldactazide (aldactone/hydrochlorothiazide (HCTZ) combination therapy) were required to have been using a thiazide diuretic immediately prior to the NSD for aldactazide. Patients who were not prescribed a thiazide prior to the start of aldactazide were excluded as having been started on spironolactone and HCTZ concurrently. Use of the MR antagonist eplerenone was not included in the study as it was prescribed to only 2% of the resistant hypertensive population.
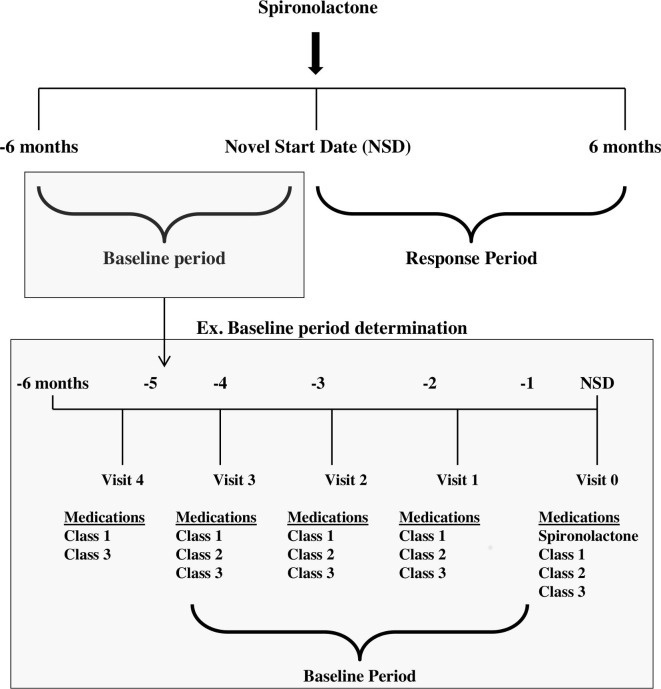
Patients prescribed spironolactone without an outpatient BP measurement in the 6 months before or after the NSD were excluded. Patients who were diagnosed with other indications for spironolactone including heart failure, hepatic cirrhosis, hyperandrogenism, acne or polycystic ovarian syndrome within a year of the NSD were also excluded. Using the algorithm, we identified sliding time windows up to 6 months before (baseline) and 6 months after the NSD (response) when there were no changes to each patient’s prescribed antihypertensive medications, including dose (when available), the number of medications and class type (figure 1).
Figure 1.
Schematic of the spironolactone response algorithm and identification of the baseline and response periods for patients. The earliest date a patient was prescribed spironolactone is indicated by the NSD. The baseline period is determined by identifying all visits in which same three medication classes as prescribed to the patient as on the NSD. If the visit date for the start of the baseline period occurred more than 6 months before the NSD, a baseline period of 6 months was used. The determination of the response period is not shown in the schematic above. Similar logic was applied to the selection of this period.
Once the baseline and response periods were defined, all outpatient BP measurements taken during these periods were identified. Any patient without at least two BP measurements during the baseline and response periods was excluded. The SBP and DBP responses were calculated as the difference between the mean SBP or DBP in the stable post-treatment window minus the mean SBP or DBP in the pre-treatment window (eg, ). Any SBP and DBP responses that were more than 2 standard deviations from the mean were reviewed manually to confirm accuracy.
We also defined patients as responders versus non-responders based on a review of BP responses reported in clinical trials of spironolactone in European (EA) and African Americans (AA).1–5 We defined responders as those who had a decrease in mean SBP of at least 5 mm Hg or a decrease in mean DBP of at least 2 mm Hg, corresponding to the smallest SBP and DBP responses to spironolactone reported among the studies reviewed.5
All patient characteristics, including age, gender, race, body mass index (BMI), outpatient BP measurements, serum potassium, creatinine, and sodium, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, glucose, haemoglobin A1C (HbA1c) and history of CKD stage 3, ischaemic heart disease (IHD), type two diabetes mellitus and smoking, were extracted from the SD using a combination of ICD-9 and ICD-10 codes, CPT codes, laboratory measurements and natural-language processing (see online supplementary table 2). Aldosterone, renin, renin activity and aldosterone–renin ratio (ARR) were not evaluated due to the small numbers of patients with data. For each patient, age and BMI at NSD or the date closest to NSD was used. The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease formula.11 CKD stage 3 was defined as an eGFR >30 mL/min/1.73 m2 and <60 mL/min/1.73 m2 or equivalent ICD-9 or ICD-10 code at any point before NSD. Patient race was administratively assigned in the SD based on either physician or patient report. Previous work has shown that self-identified race is highly correlated with genetic ancestry12 13 and administratively assigned race in the VUMC SD is sufficient for genetic association analyses14 and correlates tightly with genetic ancestry.13
After the algorithms were iteratively refined, blinded chart reviews of randomly chosen, never overlapping, charts were performed to determine algorithm efficacy. Based on a population size of 17 082, review of 138 charts would allow detection of a misclassification rate of 10% with a margin of error of 5%. We therefore reviewed 150 charts to determine algorithm efficacy. The review consisted of 75 charts from patients with resistant hypertension that were included by the algorithm and 75 that were excluded. The algorithm was refined until a negative predictive value (NPV), positive predictive value (PPV), sensitivity and specificity greater than 90% were achieved based on the review of 150 charts. The final version of the algorithm will be made available at Phenotype KnowledgeBase.15
Statistical methods
Data are presented as frequencies for categorical variables and mean±standard deviations for continuous variables. Univariable analysis for binary BP response was performed using Pearson’s χ2 test for categorical variables and the Wilcoxon rank-sum test for continuous variables. A multivariable logistic regression model or multivariable linear regression model was fitted for binary BP response and continuous BP change on all variables available in more than 70% of the population. Missing values were not imputed. HDL cholesterol, LDL cholesterol, triglycerides and HbA1c were excluded from multivariable regression models due to large amounts of missing values. The method of complete-case analysis was used and a linear relationship was assumed for all the continuous variables. All statistical analyses were conducted using the SPSS software V.24 (SPSS) or R V.3.3.0.16
Patient and public involvement
Because this study involved the use of the VUMC SD, patients were not recruited and there was no intervention. While patients were not involved in the development of the specific research question or study design, there has been extensive patient and community engagement in the establishment of the SD. Further, a community advisory board within the Vanderbilt Institute for Clinical and Translational Research reviews programmes including the SD. Dissemination of study results will occur through local reporting of study results.
Results
Algorithm validation
NPV, PPV, sensitivity and specificity of the algorithm for spironolactone response were determined after the blinded review of 150 electronic records. All but one excluded record were excluded appropriately. That record was excluded by the algorithm due to an erroneous ascertainment that a medication other than spironolactone had been added during the response periods. Of the 75 records, 5 included in the algorithm should have been excluded. Four of these patients had inconsistencies in their medication history during the baseline or response period. In another patient, spironolactone was listed as a potential therapy in several notes but was not apparently prescribed. Based on this review, the NPV was 98.7%, PPV was 93.3%, sensitivity was 98.6% and specificity was 93.7%.
Identification of spironolactone response population
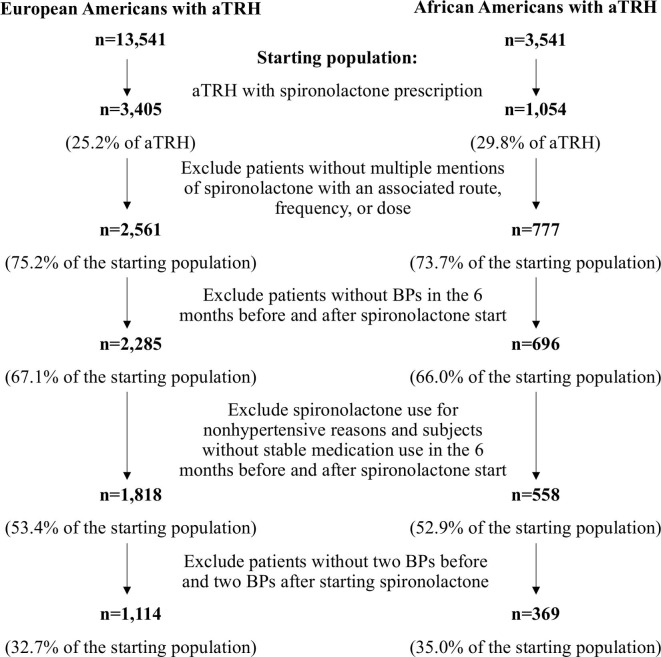
Among EA and AA patients with aTRH in the SD 3405 EA and 1054 AA were prescribed spironolactone. Consistent with the aTRH definition, in addition to other antihypertensive medications, patients were prescribed a thiazide diuretic or a dihydropyridine CCB prior to spironolactone initiation. The median daily dose of thiazide diuretic was 25 mg with a range from 12.5 mg to 50 mg (see online supplementary table 3). The predominant dihydropyridine CCBs prescribed were amlodipine and nifedipine. The median daily dose of amlodipine and nifedipine was 10 mg with a range from 2.5 mg to 10 mg and 90 mg with a range from 30 mg to 120 mg, respectively (see online supplementary table 3). For a subset of these patients, the thiazide or dihydropyridine CCB dose at spironolactone initiation, for example, dose of the medication identified in the month preceding or following spironolactone prescription, could not be determined. From the patients with confirmed doses, 566 (80.6%) were prescribed a 25 mg thiazide, 312 (73.9%) were prescribed 10 mg amlodipine and 110 (40.3%) were prescribed 90 mg nifedipine.
After applying exclusion and inclusion criteria, 1114 EA patients and 369 AA patients were included in the study for evaluation of spironolactone response, and 32.7% and 35.0% of the original number of patients prescribed spironolactone, respectively (figure 2). The median dose of spironolactone was 25 mg and ranged from 12.5 to 200 mg. The majority of patients included in the study, 1050 (70.8%), were prescribed 25 mg of spironolactone. One hundred seven patients were prescribed spironolactone at a dose of 12.5 mg and one at a dose of 200 mg. In total, 261 patients (17.6%) patients were prescribed a 50 mg or greater dose of spironolactone. The average number of outpatient BPs measured during the baseline and response periods was 4 and 4.8, respectively.
Figure 2.
Diagram of the algorithm for the identification of patients with apparent therapy-resistant hypertension (aTRH) in the Vanderbilt University Medical Center Synthetic Derivative prescribed spironolactone during a period of stable medication use for the evaluation of blood pressure (BP) response.
Characteristics of spironolactone responders and non-responders
Defining spironolactone response as a reduction in SBP of at least 5 mm Hg or in DBP of at least 2 mm Hg, we identified 1034 responders (69.7%) and 449 non-responders (30.3%) in the total population. Of the responders, 15.8% met the criteria for a DBP response, 19.4% for an SBP response and 64.8% for both a DBP and SBP response. Patient characteristics appear in table 1. In univariable analyses, patients who responded to spironolactone were heavier and less likely to have a history of IHD. Responders had significantly higher baseline SBP, DBP, serum sodium and HbA1c, and significantly lower baseline creatinine than non-responders (table 1). Responders also had greater decreases in serum sodium and eGFR as well as greater increases in serum potassium and creatinine after starting spironolactone than non-responders (table 1). HbA1c did not change in responders whereas it declined over time in non-responders. In a multivariable model adjusting for all variables present in at least 1000 patients, responders were older and heavier, had higher baseline SBP and DBP, had lower baseline serum potassium and had a greater increase in serum potassium and creatinine after starting spironolactone than non-responders (table 2).
Table 1.
Characteristics of spironolactone responders and non-responders in the total population
| Variable | N | Non-responders (n=449) |
Responders (n=1034) |
P value |
| Age, years | 1483 | 63.67±13.18 | 64.28±12.63 | 0.33 |
| Female, n (%) | 1483 | 215 (47.9%) | 532 (51.5%) | 0.21 |
| Black race, n (%) | 1483 | 106 (23.6%) | 263 (25.4%) | 0.46 |
| BMI, kg/m2 | 1358 | 32.20±8.00 | 33.50±8.30 | 0.004 |
| Diagnostic history | ||||
| T2DM, n (%) | 1483 | 236 (52.6%) | 566 (54.7%) | 0.44 |
| CKD3, n (%) | 1483 | 163 (36.3%) | 398 (38.5%) | 0.43 |
| IHD, n (%) | 1483 | 179 (39.9%) | 351 (33.9%) | 0.03 |
| Smoking, n (%) | 1483 | 96 (21.4%) | 211 (20.4%) | 0.67 |
| Baseline measurements | ||||
| SBP, mm Hg | 1483 | 133.20±17.40 | 147.59±18.70 | <0.001 |
| DBP, mm Hg | 1483 | 71.66±11.74 | 79.00±13.08 | <0.001 |
| Serum sodium, mmol/L | 1176 | 138.67±3.22 | 139.25±2.98 | 0.004 |
| Serum potassium, mmol/L | 1177 | 3.95±0.47 | 3.91±0.43 | 0.15 |
| Creatinine, mg/dL | 1184 | 1.12±0.36 | 1.09±0.42 | 0.03 |
| eGFR, mL/min/1.73 m2 | 1382 | 76.73±23.78 | 77.38±20.95 | 0.51 |
| Glucose, mg/dL | 1179 | 125.56±58.56 | 126.98±50.61 | 0.80 |
| HbA1c, % | 538 | 6.91±1.81 | 7.23±3.06 | 0.02 |
| HDL cholesterol, mg/dL | 575 | 47.48±17.97 | 45.53±15.31 | 0.44 |
| LDL cholesterol, mg/dL | 537 | 95.65±39.74 | 98.54±34.72 | 0.41 |
| Triglycerides, mg/dL | 578 | 163.98±111.71 | 169.39±121.20 | 0.85 |
| Difference from baseline following initial spironolactone prescription | ||||
| Serum sodium, mmol/L | 1101 | −0.49±2.63 | −0.95±2.70 | 0.006 |
| Serum potassium, mmol/L | 1104 | 0.13±0.48 | 0.25±0.42 | <0.001 |
| Creatinine, mg/dL | 1108 | 0.02±0.23 | 0.134±0.30 | <0.001 |
| eGFR, mL/min/1.73 m2 | 1254 | −5.22±15.73 | −9.09±15.10 | <0.001 |
| Glucose, mg/dL | 1101 | −0.27±47.82 | −0.37±40.62 | 0.88 |
| HbA1c, % | 284 | −0.25±1.23 | 0.02±1.05 | 0.02 |
| HDL cholesterol, mg/dL | 214 | 0.70±11.51 | −1.36±9.35 | 0.23 |
| LDL cholesterol, mg/dL | 197 | −5.74±32.54 | −7.48±34.94 | 0.77 |
| Triglycerides, mg/dL | 220 | −17.86±107.95 | −19.43±107.16 | 0.91 |
Data are presented as mean±standard deviation for continuous variables and count (%) for categorical variables.
BMI, body mass index; CKD3, chronic kidney disease stage 3; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; IHD, ischaemic heart disease; LDL, low-density lipoprotein; N, number of individuals with the specific measure; SBP, systolic blood pressure; T2DM, type 2 diabetes mellitus.
Table 2.
Multivariable logistic regression model for responder versus non-responder in the total population without missing data (n=1019)
| ORs* | 95% CI | P value | |
| Age, years | 1.034 | 1.017 to 1.052 | <0.001 |
| Gender, male:female | 0.897 | 0.603 to 1.323 | 0.59 |
| Race, white:black | 1.217 | 0.800 to 1.849 | 0.36 |
| Mean BMI, kg/m2 | 1.030 | 1.008 to 1.054 | 0.01 |
| Diagnostic history | |||
| T2DM, Yes:No | 1.132 | 0.794 to 1.615 | 0.49 |
| CKD3, Yes:No | 0.932 | 0.654 to 1.330 | 0.70 |
| IHD, Yes:No | 1.256 | 0.905 to 1.749 | 0.18 |
| Smoking, Yes:No | 1.074 | 0.745 to 1.559 | 0.71 |
| Baseline measurements | |||
| SBP, mm Hg | 1.034 | 1.023 to 1.046 | <0.001 |
| DBP, mm Hg | 1.043 | 1.025 to 1.062 | <0.001 |
| Serum sodium, mmol/L | 1.013 | 0.957 to 1.071 | 0.66 |
| Serum potassium, mmol/L | 1.686 | 1.106 to 2.588 | 0.02 |
| Creatinine, mg/dL | 1.266 | 0.603 to 2.995 | 0.57 |
| eGFR, mL/min/1.73 m2 | 1.007 | 0.993 to 1.021 | 0.32 |
| Glucose, mg/dL | 0.999 | 0.996 to 1.003 | 0.80 |
| Difference from baseline following initial spironolactone prescription | |||
| Serum sodium, mmol/L | 0.946 | 0.888 to 1.007 | 0.08 |
| Serum potassium, mmol/L | 1.968 | 1.290 to 3.030 | 0.002 |
| Creatinine, mg/dL | 3.570 | 1.610 to 9.131 | 0.004 |
| eGFR, mL/min/1.73 m2 | 1.001 | 0.987 to 1.015 | 0.92 |
| Glucose, mg/dL | 0.997 | 0.992 to 1.001 | 0.12 |
*For continuous variables, the ORs reflects the effect of a one-unit increase. For example, a 1 mm Hg increase in baseline SBP increases the ORs for responder versus non-responder 0.03.
BMI, body mass index; CKD3, chronic kidney disease stage 3; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; IHD, ischaemic heart disease; N, number of individuals included in the analysis; SBP, systolic blood pressure.
Among EA patients, 343 (30.8%) did not respond to spironolactone. EA responders were heavier and less likely to have IHD. They had higher baseline SBP, DBP and serum sodium than non-responders (see online supplementary table 4). EA responders also had a greater decrease in serum sodium and eGFR and a greater increase in serum potassium and creatinine after starting spironolactone (see online supplementary table 4).
Among AA patients, 106 (28.7%) did not respond to spironolactone. Like EA responders, AA responders had significantly higher baseline SBP and DBP and a greater decrease in serum sodium and increase in creatinine after starting spironolactone than non-responders (see online supplementary table 5).
BP response to spironolactone as a continuous variable
For the entire group, the mean decrease in SBP following initiation of spironolactone was 8.1 mm Hg and the mean decrease in DBP was 3.4 mm Hg following initial spironolactone prescription. In total, 933 patients (62.9%) achieved a decrease in BP to <140/90 mm Hg, the pressure goal recommended in guidelines at the time.17 An additional 23 patients achieved a decrease in SBP but not DBP to guideline recommendation and 262 patients achieved DBP but not SBP control. Analyses of BP change as a continuous variable were performed using a multivariable model that included all variables except for HbA1c, HDL cholesterol, LDL cholesterol and triglycerides, as data were not available in the majority of patients.
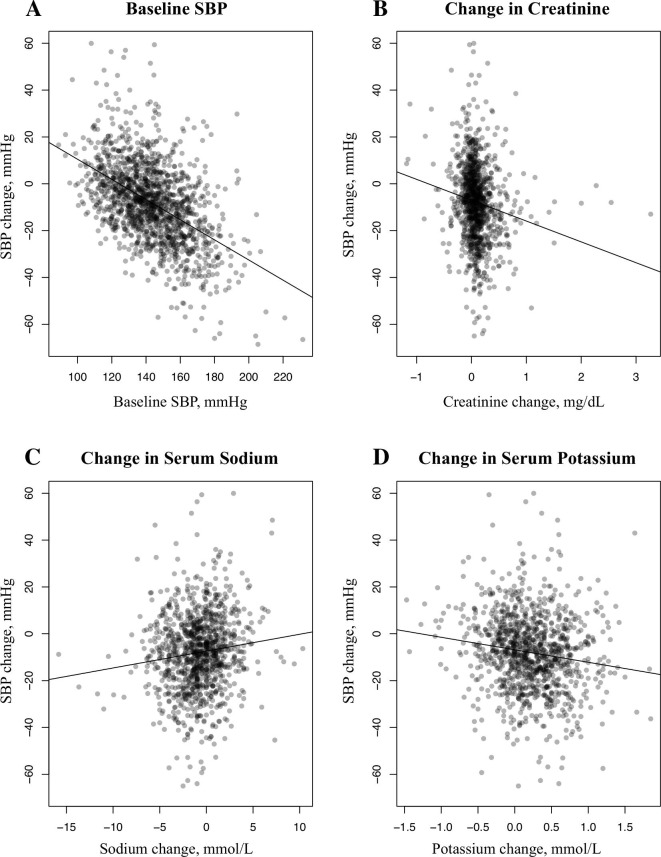
In the total population, patients with higher baseline SBP, lower baseline creatinine and a greater decrease in serum sodium, increase in serum potassium, or increase in creatinine after starting spironolactone had greater reductions in SBP (table 3 and figure 3). Patients with higher baseline SBP and DBP and a greater decrease in serum sodium, increase in serum potassium or increase in creatinine after spironolactone had greater reductions in DBP (table 3). Older patients and female patients also had significantly greater reductions in DBP (table 3).
Table 3.
Significant coefficients in multivariable models for change in systolic and diastolic blood pressures (SBP/DBP)
| Associated variable | Coefficient | 95% CI | P value | |
| Total population (n=1019) | ||||
| Change in SBP | Baseline SBP | −0.415 | −0.475 to 0.356 | <0.001 |
| Baseline creatinine | 3.628 | 0.202 to 7.054 | 0.04 | |
| Change in serum sodium | 0.701 | 0.338 to 1.064 | <0.001 | |
| Change in serum potassium | −3.483 | −5.834 to 1.131 | 0.004 | |
| Change in creatinine | −5.039 | −8.704 to 1.374 | 0.01 | |
| Change in DBP | Age | −0.141 | −0.196 to 0.086 | <0.001 |
| Gender—male:female | 1.390 | 0.170 to 2.610 | 0.03 | |
| Baseline SBP | −0.045 | −0.080 to 0.011 | 0.01 | |
| Baseline DBP | −0.379 | −0.436 to 0.321 | <0.001 | |
| Change in serum sodium | 0.274 | 0.063 to 0.485 | 0.01 | |
| Change in serum potassium | −2.042 | −3.413 to 0.671 | 0.004 | |
| Change in creatinine | −3.979 | −6.116 to 1.841 | <0.001 | |
| European Americans (n=753) | ||||
| Change in SBP | Baseline SBP | −0.401 | −0.470 to 0.333 | <0.001 |
| Change in serum sodium | 0.525 | 0.117 to 0.933 | 0.01 | |
| Change in serum potassium | −3.074 | −5.766 to 0.381 | 0.03 | |
| Change in glucose | 0.031 | 0.003 to 0.059 | 0.03 | |
| Change in DBP | Age | −0.135 | −0.200 to 0.070 | <0.001 |
| Gender, Male:Female | 1.669 | 0.230 to 3.108 | 0.02 | |
| Baseline SBP | −0.045 | −0.084 to 0.005 | 0.03 | |
| Baseline DBP | −0.370 | −0.436 to 0.303 | <0.001 | |
| Change in serum potassium | −1.809 | −3.385 to 0.232 | 0.03 | |
| Change in creatinine | −3.328 | −5.772 to 0.884 | 0.01 | |
| African Americans (n=266) | ||||
| Change in SBP | Baseline SBP | −0.445 | −0.571 to 0.319 | <0.001 |
| Baseline creatinine | 5.572 | 0.017 to 11.127 | 0.05 | |
| Change in serum sodium | 1.338 | 0.517 to 2.159 | 0.002 | |
| Change in potassium | −5.206 | −10.205 to 0.207 | 0.04 | |
| Change in creatinine | −10.923 | −19.456 to 2.391 | 0.01 | |
| Change in DBP | Age | −0.156 | −0.265 to 0.046 | 0.01 |
| Baseline DBP | −0.407 | −0.512 to 0.278 | <0.001 | |
| Change in sodium | 0.530 | 0.053 to 1.007 | 0.03 | |
| Change in potassium | −2.864 | −5.768 to 0.039 | 0.05 | |
| Change in creatinine | −7.370 | −12.326 to 2.413 | 0.004 | |
N, number of individuals included in the analysis.
Figure 3.
The significant correlations with systolic blood pressure (SBP) change in the total population. Correlation between the change in SBP after starting spironolactone and baseline. (A) SBP (correlation coefficient (CC)=−0.415, p<0.001), and the change in (B) creatinine (CC=−5.039, p=0.01), (C) serum sodium (CC=0.701, p<0.001) and (D) serum potassium (CC=−3.483, p=0.004).
In EA alone, a greater decrease in either SBP or DBP was significantly associated with baseline SBP, as well as increase in serum potassium (table 3). A greater reduction in SBP was further associated with greater decreases in serum sodium and glucose (table 3). The change in DBP was also significantly associated with age, sex and creatinine (table 3).
In AA, greater reductions in SBP and DBP were significantly associated with greater decreases in serum sodium and increases in serum potassium and creatinine (table 3). Patients with higher baseline SBP and baseline creatinine had a greater reduction in SBP, while patients with higher baseline DBP and older age had a greater reduction in DBP (table 3).
Discussion
We developed a highly accurate algorithm to define the BP response to spironolactone in patients with aTRH using the EMR. The mean decreases in SBP and DBP for the total study population, 8.1 mm Hg and 3.4 mm Hg, respectively, are consistent with responses reported in prior clinical trials, such as the PATHWAY-2 trial.1 In total, 30.3% of patients prescribed spironolactone did not achieve a 5 mm Hg decrease in SBP or 2 mm Hg decrease in DBP in the 6 months following spironolactone initiation. Higher pretreatment SBP and DBP predicted a greater therapeutic response to spironolactone, measured either as a dichotomous or continuous variable.
In addition, using the electronic algorithm, we were able to detect not only the BP response, but also changes in serum sodium, potassium and creatinine after spironolactone initiation. Regardless of race, the response to spironolactone was associated with a greater decrease in serum sodium and greater increase in serum potassium, consistent with MR antagonism. These changes are also consistent with a decrease in epithelial sodium channel (ENaC) activity. In the present study, however, we are unable to determine if this inadequate BP response is due to inadequate spironolactone dose, spironolactone noncompliance or non-MR mediated ENaC activation. Further, we found a significant correlation between decreasing BP and increasing creatinine after starting spironolactone in all groups. This correlation is likely a result from haemodynamic effects of BP medications and especially from blocking the renin–angiotensin–aldosterone system.1
Race was not significantly associated with BP response to spironolactone nor electrolyte changes which suggests spironolactone exerts a consistent BP lowering effect regardless of race. These findings are supported by those of Nishizaka et al who determined that low-dose spironolactone provided a significant decrease in BP in AA and EA with resistant hypertension with and without primary aldosteronism.4
In EA, we also observed a significant correlation between the SBP response and decreasing glucose; whereas among those in whom HbA1c was measured before and after therapy, HbA1c declined in non-responders but not responders. The latter observation may reflect the fact that responders had a higher baseline HbA1c than non-responders. While these findings may suggest a potential benefit to glucose management from MR antagonism, it is important to note that the exact relationship between MR antagonism and glucose is not perfectly understood. Because our findings, as well as others, suggest an association between spironolactone use and glucose levels, additional studies to better characterise this relationship are warranted.
A limitation of this study and many other studies of aTRH is the inability to measure adherence directly in the patients prescribed spironolactone without measuring drug levels, which is not routinely done in clinical practice. Non-adherence alone does not likely explain the lack of BP response in non-responders, however. First, patients non-adherent to spironolactone would likely be non-adherent to other medications. Non-adherent patients, therefore, would be expected to have higher baseline BPs than adherent patients. To the contrary, we found that non-responders had lower baseline SBP and DBP than responders and baseline SBP and DBP significantly predicted BP response. In addition, initiation of spironolactone resulted in an increase in serum potassium and decrease in serum sodium in non-responders as well as responders, although to a lesser degree. Taken together, these findings suggest that non-adherence is not the predominant driver of the lack of BP response in non-responders.
Another way to assess adequacy of spironolactone dose is to assess whether renin activity remains suppressed. Unfortunately, an insufficient number of patients had renin measured during the baseline and response periods to assess change in renin concentration or activity. The limited number of renin measurements in the EMR is reflective of poor screening rates for primary aldosteronism.18 Previous studies have reported limited value in the addition of aldosterone levels or the ARR to prediction models of BP response to MR antagonism in aTRH, however.19 20
Other limitations of the study include the exclusion of a significant number of patients with aTRH due to inadequate documentation of pre-treatment and post-treatment BPs, which limits our power of detection for some responses. The relatively small number of AA, for example, limits the power to detect predictors of response to spironolactone in this group.
The advantage of this approach is that it is an accurate, rapid, high throughput and inexpensive approach for quantifying clinical response to medications and determine responders and non-responders. The identified population can then be used as a research cohort to investigate other relevant topics including pharmacogenetic inquiries, long-term outcome and event studies, as well as evaluate medication levels to determine compliance or the presence of rapid or insufficient metabolizers. Further, the method can easily be adapted for use with other medication types and in other EMR systems. These adaptations could also allow for inquires related to personnel and infrastructure performance, for example, when the algorithm is adapted for evaluation of a response for a medication used exclusively in hospital.
In conclusion, we have developed a highly accurate algorithm for the assessment of BP response to spironolactone, a commonly prescribed medication, in patients with aTRH using EMR. Electrolyte changes associated with the BP response to spironolactone are consistent with its mechanism of action to block the MR and decrease activity of ENaC. A strength of this algorithm is its applicability to evaluate BP and electrolyte responses to medications other than spironolactone as well as its utility to evaluate the long-term clinical consequences of medication use. Further, this electronic algorithm could be amended for use in other EMR systems.
Supplementary Material
Footnotes
Contributors: MS and NB contributed to the design of the study and the drafting of the manuscript. All authors contributed to the analysis and interpretation of the results and editing of the manuscript and final approval for submission.
Funding: This study was supported by the National Institutes of Health (DK108444-01A1). The dataset used for the analyses described were obtained from the Vanderbilt University Medical Center Synthetic Derivative, which is supported by institutional funding, the 1S10RR025141-01 instrumentation award, and by the CTSA grant UL1TR000445 from National Center for Advancing Translational Sciences/National Institutes of Health.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The authors obtained Institutional Review Board approval to access the Vanderbilt University Medical Center Synthetic Derivative.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: The full data set is available upon request to the corresponding author.
References
- 1.Williams B, MacDonald TM, Morant S, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet 2015;386:2059–68. 10.1016/S0140-6736(15)00257-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapman N, Dobson J, Wilson S, et al. Anglo-Scandinavian cardiac outcomes trial I. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension 2007;49:839–45. [DOI] [PubMed] [Google Scholar]
- 3.de Souza F, Muxfeldt E, Fiszman R, et al. Efficacy of spironolactone therapy in patients with true resistant hypertension. Hypertension 2010;55:147–52. 10.1161/HYPERTENSIONAHA.109.140988 [DOI] [PubMed] [Google Scholar]
- 4.Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens 2003;16:925–30. 10.1016/S0895-7061(03)01032-X [DOI] [PubMed] [Google Scholar]
- 5.Saha C, Eckert GJ, Ambrosius WT, et al. Improvement in blood pressure with inhibition of the epithelial sodium channel in blacks with hypertension. Hypertension 2005;46:481–7. 10.1161/01.HYP.0000179582.42830.1d [DOI] [PubMed] [Google Scholar]
- 6.Shuey MM, Gandelman JS, Chung CP, et al. Characteristics and treatment of African-American and European-American patients with resistant hypertension identified using the electronic health record in an academic health centre: a case−control study. BMJ Open 2018;8:e021640 10.1136/bmjopen-2018-021640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roden DM, Pulley JM, Basford MA, et al. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther 2008;84:362–9. 10.1038/clpt.2008.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu H, Stenner SP, Doan S, et al. Medex: a medication information extraction system for clinical narratives. J Am Med Inform Assoc 2010;17:19–24. 10.1197/jamia.M3378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu H, Doan S, Birdwell KA, et al. An automated approach to calculating the daily dose of tacrolimus in electronic health records. Summit Transl Bioinform 2010;2010:71–5. [PMC free article] [PubMed] [Google Scholar]
- 10.Xu H, Jiang M, Oetjens M, et al. Facilitating pharmacogenetic studies using electronic health records and natural-language processing: a case study of warfarin. J Am Med Inform Assoc 2011;18:387–91. 10.1136/amiajnl-2011-000208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. modification of diet in renal disease Study Group. Ann Intern Med 1999;130:461–70. 10.7326/0003-4819-130-6-199903160-00002 [DOI] [PubMed] [Google Scholar]
- 12.Tang H, Quertermous T, Rodriguez B, et al. Genetic structure, self-identified race/ethnicity, and confounding in case-control association studies. Am J Hum Genet 2005;76:268–75. 10.1086/427888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumitrescu L, Ritchie MD, Brown-Gentry K, et al. Assessing the accuracy of observer-reported ancestry in a biorepository linked to electronic medical records. Genet Med 2010;12:648–50. 10.1097/GIM.0b013e3181efe2df [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birdwell KA, Grady B, Choi L, et al. The use of a DNA biobank linked to electronic medical records to characterize pharmacogenomic predictors of tacrolimus dose requirement in kidney transplant recipients. Pharmacogenet Genomics 2012;22:32–42. 10.1097/FPC.0b013e32834e1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirby JC, Speltz P, Rasmussen LV, et al. PheKB: a catalog and workflow for creating electronic phenotype algorithms for transportability. J Am Med Inform Assoc 2016;23:1046–52. 10.1093/jamia/ocv202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Team RC R: A language and environment for statistical computing [internet]. 2014 Vienna, austria, 2017. [Google Scholar]
- 17.Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003;289:2560–71. 10.1001/jama.289.19.2560 [DOI] [PubMed] [Google Scholar]
- 18.Ruhle BC, White MG, Alsafran S, et al. Keeping primary aldosteronism in mind: deficiencies in screening at-risk hypertensives. Surgery 2019;165:221–7. 10.1016/j.surg.2018.05.085 [DOI] [PubMed] [Google Scholar]
- 19.Parthasarathy HK, Alhashmi K, McMahon AD, et al. Does the ratio of serum aldosterone to plasma renin activity predict the efficacy of diuretics in hypertension? results of RENALDO. J Hypertens 2010;28:170–7. 10.1097/HJH.0b013e328332b79b [DOI] [PubMed] [Google Scholar]
- 20.Václavík J, Sedlák R, Jarkovský J, et al. Effect of spironolactone in resistant arterial hypertension: a randomized, double-blind, placebo-controlled trial (ASPIRANT-EXT). Medicine 2014;93:e162. 10.1097/MD.0000000000000162 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-033100supp001.pdf (106KB, pdf)