During the coronavirus disease 2019 (COVID-19) pandemic, many of us have faced new challenges as we manage some of the country’s most vulnerable citizens, our patients with cancer. We look to informatics tools to inform our decisions and manage our patient population during this time of escalating risk and complexity.
One of the most prominent changes is the accelerated expansion or launching of telemedicine for widespread use across the United States. This is a transformation of our clinics from physical locations that manage a high volume of patient encounters, to physical locations with dramatically reduced volumes of in-person patient visits in clinics, to seeing more patients virtually face to face through a telemedicine platform. Acute care visits, established patient visits, new patient consultations, genetic counselor visits, and even support groups managed by social workers are transitioning to virtual locations where patients, clinicians, and staff interact through bimodal synchronous and Health Insurance Portability and Accountability Act (HIPAA)–compliant platforms.
Digital health applications have always been an important part of clinical cancer informatics and have been featured in JCO Clinical Cancer Informatics in a series compiled by one of our associate editors, Adam Dicker, MD, PhD.1,2 While effective telemedicine technology platforms have existed for some time, laws with regard to compliance and payment parity have been persistent challenges to robust adoption of telemedicine in cancer care. During the COVID-19 pandemic, laws and regulations that govern compliance and payment parity have changed overnight. In recognition of the challenge of managing the dichotomy of minimizing person-to-person transmission of the contagion and having patients who are infected seeking medical care in proximity with unaffected individuals, state and federal governments have taken unprecedented steps to liberalize telemedicine policy and payment parity to allow this tool to be maximally effective at minimizing the impact of COVID-19 on Americans.
The United States reported its first case of COVID-19 in January 2020, and by late January, the Centers for Disease Control and Prevention was issuing regular updates.3 While the first cases seemed to be travel related, by late February it became evident that there were cities in the United States that had community spread of the disease.
On March 6, 2020, the bipartisan Coronavirus Preparedness and Response Supplemental Appropriations Act of 2020 was passed, which authorizes the US Department of Health and Human Services to temporarily waive certain Medicare requirements for telehealth services. Historically, Medicare has been limited in implementation of telehealth for cancer care because patients must interact with a platform at a clinical originating site. The expansion of telehealth through the 1135 waiver includes permitting telehealth broadly in a patient’s residence. On March 13, 2020, President Trump announced further liberalization of Medicare’s policies that govern telehealth. The most important is that Medicare would both allow and pay for patients to originate a telemedicine visit from their homes. The administration also articulated a set of visits that would be paid for by Medicare. To further expand access, the administration stipulated that temporarily private and non–HIPAA-compliant platforms were permissible to conduct telehealth services.4,5 Many commercial plans that fall under Employee Retirement Income Security Act of 1974 (ERISA) jurisdiction followed the administration’s lead and elected to liberalize the ability for patients who have their insurance products to use telemedicine services more robustly.
Many state governments also followed the administration’s lead by liberalizing telemedicine regulations, with some states offering payment parity for commercial and public plans that fall under state jurisdiction. In Texas, approximately 74% of insured patients have plans that are regulated solely by federal policy and ERISA guidance; the remainder fall under state jurisdiction. Texas governor Greg Abbott immediately followed the federal administration’s lead the evening of March 13, 2020, by allowing patients to initiate visits in their homes and mandating broad payment coverage for these visits.6 Many states have acted similarly.
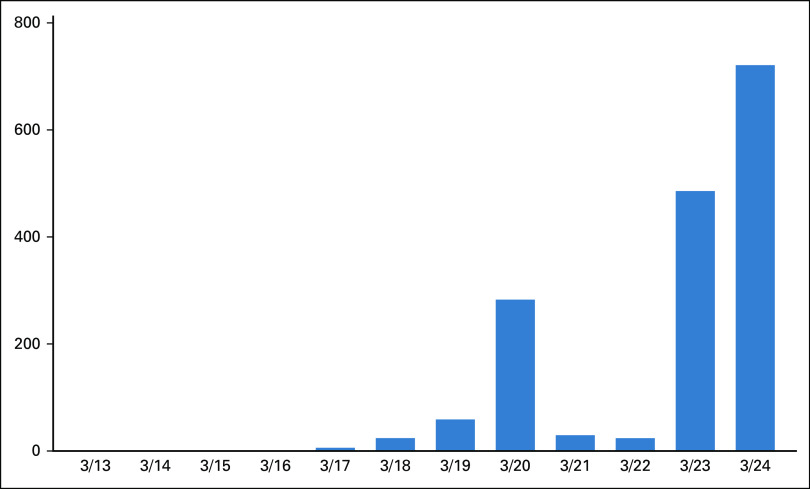
In my own large private practice that cares for approximately half of the patients with cancer across Texas, we rapidly expanded our telemedicine capabilities. Within 1 week, 15% of our providers were trained and ready to use telehealth services. Within 2 weeks of the policy, 90% of our providers were using the telehealth platform. Use has increased daily, with upwards of 500 telemedicine visits per day and growing (Fig 1).
FIG 1.
Telehealth visits in the practice 1 week after going live.
Challenges remain. In some rural sites of service, patients may not have bandwidth to permit effective bimodal synchronous telemedicine services. In addition, some of our patients with cancer do not have smartphones or home computers. Finally, some services that are provided by cancer specialists and other clinicians are not yet covered. For the majority, however, these policy changes and the technology that exists have allowed providers to swiftly act to continue care for our vulnerable patient population, reduce the risk of social interaction, evaluate sick patients effectively without universally requiring them to physically enter the clinic or go to a hospital, and continue to manage their patients’ cancer care.
Telemedicine is just one of the many clinical informatics solutions at the epicenter of managing this crisis. We are crowdsourcing resources around triage and operations. Professional organizations like ASCO, the Community Oncology Alliance, and the National Comprehensive Cancer Network are crowdsourcing information and making it available to the masses through web-based communication and informational webinars. We are sharing information on the supply chain to acquire personal protection equipment and to better understand the availability of testing where we have all had limitations. The telemedicine policy changes and the resultant implementation empowers cancer providers to better serve our patients. More patients are likely to survive this pandemic because we are newly capable of further reducing their risk and managing their care.
Footnotes
Dr Patt is the Editor In Chief of JCO Clinical Cancer Informatics. She is the Executive Vice President of Policy and Strategy for Texas Oncology, a member of The US Oncology Network, the President-elect of the Texas Society of Clinical Oncology, Chair of the Council on Legislation for the Texas Medical Association, and a Clinical Professor at Dell Medical School at The University of Texas at Austin.
AUTHOR’S DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by the author of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/cci/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Debra Patt
Employment: Texas Oncology, McKesson, MEDNAX (I)
Leadership: McKesson, MEDNAX (I), Texas Oncology
Stock and Other Ownership Interests: MEDNAX (I)
Speakers’ Bureau: Pfizer
Research Funding: Merck (Inst), Eisai (Inst), Seattle Genetics (Inst), Eli Lilly (Inst)
Travel, Accommodations, Expenses: McKesson
No other potential conflicts of interest were reported.
REFERENCES
- 1.Rising KL, Ward MM, Goldwater JC, et al. Framework to advance oncology-related telehealth. JCO Clinical Cancer Informatics 1031200/CCI.17.00156. [DOI] [PubMed]
- 2.Devine KA, Viola AS, Coups EJ, et al. Digital health interventions for adolescent and young adult cancer survivors. JCO Clinical Cancer Informatics 10.1200/CCI.17.00138. [DOI] [PMC free article] [PubMed]
- 3.Centers for Disease Control and Prevention: Coronavirus disease 2019 (COVID-19): Cases in U.S., 2020 https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html
- 4.Centers for Medicare & Medicaid Services: Medicare telemedicine health care provider fact sheet 2020. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet.
- 5.Center for Connected Health Policy: Telehealth coverage policies in the time of COVID-19 to date 2020. https://www.cchpca.org/sites/default/files/2020-03/CORONAVIRUS%20TELEHEALTH%20POLICY%20FACT%20SHEET%20MAR%2017%202020%203%20PM.pdf.
- 6.Walters E. Texas is urging patients to seek remote health care. Some insurance plans won’t pay for it, 2020. https://www.texastribune.org/2020/03/19/texas-telemedicine-patients-during-coronavirus-face-insurance-roadbloc.