Summary
Background
COVID-19 has spread globally. Epidemiological susceptibility to COVID-19 has been reported in patients with cancer. We aimed to systematically characterise clinical features and determine risk factors of COVID-19 disease severity for patients with cancer and COVID-19.
Methods
In this multicentre, retrospective, cohort study, we included all adult patients (aged ≥18 years) with any type of malignant solid tumours and haematological malignancy who were admitted to nine hospitals in Wuhan, China, with laboratory-confirmed COVID-19 between Jan 13 and March 18, 2020. Enrolled patients were statistically matched (2:1) with patients admitted with COVID-19 who did not have cancer with propensity score on the basis of age, sex, and comorbidities. Demographic characteristics, laboratory examinations, illness severity, and clinical interventions were compared between patients with COVID-19 with or without cancer as well as between patients with cancer with non-severe or severe COVID-19. COVID-19 disease severity was defined on admission on the basis of the WHO guidelines. Univariable and multivariable logistic regression, adjusted for age, sex, comorbidities, cancer type, tumour stage, and antitumour treatments, were used to explore risk factors associated with COVID-19 disease severity. This study was registered in the Chinese Clinical Trial Register, ChiCTR2000030807.
Findings
Between Jan 13 and March 18, 2020, 13 077 patients with COVID-19 were admitted to the nine hospitals in Wuhan and 232 patients with cancer and 519 statistically matched patients without cancer were enrolled. Median follow-up was 29 days (IQR 22–38) in patients with cancer and 27 days (20–35) in patients without cancer. Patients with cancer were more likely to have severe COVID-19 than patients without cancer (148 [64%] of 232 vs 166 [32%] of 519; odds ratio [OR] 3·61 [95% CI 2·59–5·04]; p<0·0001). Risk factors previously reported in patients without cancer, such as older age; elevated interleukin 6, procalcitonin, and D-dimer; and reduced lymphocytes were validated in patients with cancer. We also identified advanced tumour stage (OR 2·60, 95% CI 1·05–6·43; p=0·039), elevated tumour necrosis factor α (1·22, 1·01–1·47; p=0·037), elevated N-terminal pro-B-type natriuretic peptide (1·65, 1·03–2·78; p=0·032), reduced CD4+ T cells (0·84, 0·71–0·98; p=0·031), and reduced albumin–globulin ratio (0·12, 0·02–0·77; p=0·024) as risk factors of COVID-19 severity in patients with cancer.
Interpretation
Patients with cancer and COVID-19 were more likely to deteriorate into severe illness than those without cancer. The risk factors identified here could be helpful for early clinical surveillance of disease progression in patients with cancer who present with COVID-19.
Funding
China National Natural Science Foundation.
Introduction
The COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread globally. As of May 27, 2020, there were 5 491 678 confirmed cases and 349 190 deaths, with the numbers still surging across 217 countries worldwide.1 With the continued increase in cases and affected regions, extra preparations to protect high-risk populations should be made, especially for older patients and those with existing conditions.
Notably, initial analyses of patient characteristics from China have shown that diabetes, hypertension, and cardiovascular diseases are highly prevalent among patients with COVID-19, and patients with these comorbidities are predisposed to a poor clinical outcome.2, 3, 4 COVID-19 might act as a precipitating factor to worsen existing conditions, potentially leading to death.4 Cancer, which affects tens of millions of people each year worldwide, has been found to be a major risk factor in patients with COVID-19.5 Evidence suggests that patients with cancer might be more susceptible to COVID-19 than those without cancer.6 However, because of the small sample sizes in most of the reports, there are insufficient data to accurately describe or even to explore a possible association between cancer and COVID-19. Notably, detailed clinical symptoms, radiological findings, biochemical markers, clinical characteristics, and progression of patients with cancer and COVID-19 are scarce at this stage, especially for the risk factors affecting their COVID-19 progression or disease severity, such as inflammatory cytokines or immune cell changes. Therefore, a systemic understanding of the clinical features of patients with cancer and COVID-19 is urgently needed to protect these susceptible patients from SARS-CoV-2 infection, monitor reliable markers for disease course, and administer effective treatments for this particular population.
Research in context.
Evidence before this study
We searched PubMed and Preprint websites (medRxiv and bioRxiV) on Mar 18, 2020, for articles that documented the risk factors of COVID-19 illness severity of patients with cancer and COVID-19, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), using the search terms (“novel coronavirus” OR “SARS-CoV-2” OR “COVID-19”) AND (“cancer” OR “carcinoma” OR “tumor”) with no language or date restrictions. Epidemiological features and susceptibility of patients with cancer and COVID-19 have been reported. Among these studies, one study described baseline demographic data, such as age, sex, smoking status, and underlying comorbidities in 18 patients with cancer and COVID-19. The small sample sizes in most reports make it difficult to accurately describe or even to explore a possible association between cancer and COVID-19. Detailed clinical symptoms, radiological characteristics, biochemical markers, and progression of patients with cancer and COVID-19 are scarce at this stage, especially regarding risk factors influencing their progression or disease severity. Thus, a systematic understanding of the clinical features of patients with cancer and COVID-19 is urgently needed to protect susceptible patients with cancer from SARS-CoV-2 infection, monitor reliable markers for disease course, and administer effective treatment for this particular population.
Added value of this study
To our knowledge, this study is the largest multicentre cohort study published so far on the clinical features of patients with cancer and COVID-19. We found that the odds of COVID-19 progressing into severe illness was 3·611 times higher for patients with cancer than for those without cancer. Furthermore, we identified several novel risk factors for severity of COVID-19 in patients with cancer, such as advanced tumour stage and elevated tumour necrosis factor α (TNF-α) and N-terminal pro-B-type natriuretic peptide (NT-proBNP), as well as decreased CD4+ T cells and albumin–globulin ratio. These indices are helpful for assessing disease severity and should be extensively monitored during COVID-19 treatment in case of adverse status.
Implications of all the available evidence
Greater attention should be given to patients with COVID-19 who have cancer, as our findings suggest they are more likely to deteriorate into severe illness. The potential risk factors of elevated TNF-α and NT-proBNP and decreased CD4+ T cells or albumin–globulin ratio can be helpful for clinicians in terms of early surveillance of COVID-19 progression, alongside older age; elevated interleukin-6, procalcitonin, and D-dimer; and decreased lymphocytes.
Here, we report results from a cohort study from nine hospitals that were designated to treat patients with COVID-19 in Wuhan, China, the initial epicentre of the COVID-19 outbreak. We aimed to systematically characterise the clinical features and determine the risk factors of disease severity for patients with cancer and COVID-19.
Methods
Study design and participants
This multicentre, retrospective, cohort study was done in nine hospitals in Wuhan, China, that were designated centres for COVID-19 treatment: Tongji Hospital, Wuhan Union Hospital, Wuhan First Hospital, the Central Hospital of Wuhan, Wuhan Fourth Hospital and Puai Hospital, Fifth Hospital of Wuhan, Wuhan Pulmonary Hospital, Wuhan Jinyintan Hospital, and Wuhan Hankou Hospital. These hospitals are located across Wuhan and have admitted most of the patients with COVID-19 in Wuhan. We included all adult patients (aged ≥18 years) admitted between Jan 13 and March 18, 2020, with laboratory-confirmed SARS-CoV-2 infection by RT-PCR who had any type of malignant solid tumour and haematological malignancies. Enrolled patients were statistically matched with patients admitted with COVID-19 who did not have cancer by propensity score matching7 at an approximate ratio of 2:1 based on age, sex, and comorbidities. Hypertension, diabetes, coronary heart disease, chronic kidney damage, cerebrovascular disease, hepatitis, and chronic obstructive pulmonary disease (COPD) were used as matching factors in this study; these conditions have been reported to be risk factors for disease severity or death from COVID-19.2, 3
Because public health interventions in Wuhan changed over the enrolment period,8 we grouped patients into two groups according to time of admission: early stage (Jan 13 to Feb 16, 2020) or late stage (Feb 17 to March 18, 2020).
This study was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (TJ-IRB20200335) and granted a waiver of informed consent from study participants. Recruitment sites, principal investigators responsible for the sites, and the number of patients recruited are listed in the appendix (p 2).
Definitions
All enrolled patients were classified as having non-severe or severe COVID-19 on admission; disease classification was checked and revised if necessary during hospitalisation. We defined disease severity according to WHO guidelines,9 complemented by the Seventh Revised Trial Version of the COVID-19 Diagnosis and Treatment Guidance (2020) of China.10 Patients had severe disease if they had any of the following criteria: respiratory rate of at least 30 breaths per min, oxygen saturation of 93% or lower in a resting state, ratio of arterial partial pressure of oxygen and oxygen concentration no greater than 300 mm Hg, or more than 50% lesion progression in lung imaging within 24–48 h.
Time of follow-up was defined as the duration from hospital admission to outcomes (survivor or non-survivor) of patients; survivors were defined as patients who were discharged from hospital or still hospitalised at the end of the study.
Data collection
Epidemiological, clinical, laboratory, and radiological data of all patients with laboratory-confirmed SARS-CoV-2 were obtained with data collection forms from the electronic medical records of each designated hospital. Data were reviewed and verified by a team of physicians (XY, QZ, ZY, and BL). Any missing or uncertain records were collated and clarified through communication with involved health-care providers or patients and their families. Detailed demographics information, comorbidities, symptoms, and disease severity of all patients were recorded or diagnosed on admission. Laboratory examinations including routine blood tests; lymphocyte subsets; inflammatory or infection-related biomarkers; cardiac, renal, liver, and coagulation function tests; and chest CT scans were obtained at initial diagnosis. Clinical treatment details were also included. There were no cases lost to follow-up in this study.
Laboratory procedures
Concentrations of interleukin-1β (IL-1β), IL-2 receptor (IL-2R), IL-6, IL-8, IL-10, and tumour necrosis factor α (TNF-α) were assessed in serum samples and detected in most patients on admission and during hospitalisation, using a fully automated analyser (Cobas e602; Roche Diagnostics, Indianapolis, IN, USA) by chemiluminescence immunoassay method.11 Kits for IL-1β, IL-2R, IL-8, IL-10, and TNF-α were purchased from DiaSorin (Vercelli, Italy) and the IL-6 kit was purchased from Roche Diagnostics.
Statistical analysis
Continuous variables with a skewed distribution were described as median (IQR) using the empirical distribution function with averaging method. Categorical variables were expressed as frequencies. The Mann-Whitney U test was used to compare continuous variables. The χ2 test with Yates' correction was used for 2 × 2 contingency data,12 and Pearson's χ2 test was used for contingency data for variables with more than two categories. To explore risk factors, or their interactions, associated with severity of COVID-19 in patients with cancer, univariable and multivariable logistic regression models were used to estimate odds ratios (ORs) and 95% CIs, adjusting for age, sex, comorbidities, cancer type, tumour stage, and antitumour treatments. Cox proportional-hazards models were applied to determine hazard ratios (HRs) and 95% CIs for disease outcome, adjusted for the aforementioned confounders. Sensitivity analyses were done for all adjustment variables, comparing the results between univariable analysis without adjusting confounders and multivariable analysis with adjusting confounders for disease severity in patients with cancer and COVID-19. We did a power analysis for severity of COVID-19 in patients with or without cancer using the Pearson χ2 test for two proportions method, and the power was more than 0·95. A two-sided p<0·05 was considered statistically significant. All statistical analyses were done using SAS (version 9.4) and SPSS (version 22.0). This study was registered in the Chinese Clinical Trial Register, ChiCTR2000030807.
Role of the funding source
The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
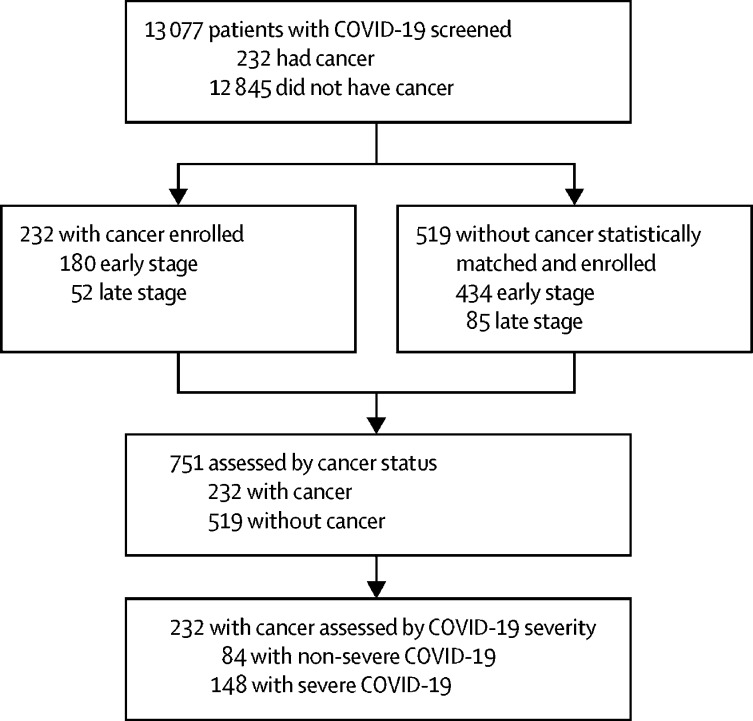
Between Jan 13 and March 18, 2020, 13 077 patients with confirmed SARS-CoV-2 infection were admitted to the nine hospitals in our study. Among these patients, 232 patients with cancer and 519 matched patients without cancer were enrolled in this study (figure ). Patients were followed up until March 26, 2020. 614 patients were included in the early stage group (ie, hospital admission between Jan 13 and Feb 16, 2020) and 137 in the late stage group (hospital admission between Feb 17 and March 18, 2020).
Figure.
Study profile
The patients with cancer had 24 different types of cancer; detailed distribution and baseline characteristics according to cancer type are presented in the appendix (pp 3–10, 17). Because medical care within different hospitals was done according to the Seventh Revised Trial Version of COVID-19 diagnosis and treatment guidance, the clinical outcomes (survivor and non-survivor) among different hospitals were not significantly different (p=0·30; appendix p 2). 92 (15%) of 614 patients admitted in the early stage died compared with ten (7%) of 137 patients admitted in the late stage.
When comparing symptoms at admission, patients with cancer were more likely to have dyspnoea and expectoration than patients without cancer, but less likely to have sore throat and coryza (table 1 ). Moreover, CT scans showed that ground-glass opacity and patchy shadows were more frequent in patients with cancer (table 1). Differences in some symptoms and CT findings were found also when comparing patients with cancer with severe and non-severe COVID-19 (table 2 ).
Table 1.
Demographic, clinical, radiographical, and laboratory findings of patients with COVID-19 with and without cancer
| All patients (n=751) | Patients without cancer (n=519) | Patients with cancer (n=232) | p value | ||
|---|---|---|---|---|---|
| Demographics | |||||
| Age, years | 64·0 (57·0–69·0) | 64·0 (56·0–70·0) | 64·0 (58·0–69·0) | 0·42 | |
| Sex | .. | 0·57 | |||
| Female | 379 (50%) | 266 (51%) | 113 (49%) | .. | |
| Male | 372 (50%) | 253 (49%) | 119 (51%) | .. | |
| Clinical characteristics and outcomes | |||||
| Current smoker | 45 (6%) | 31 (6%) | 14 (6%) | 0·89 | |
| Comorbidities | |||||
| Hypertension | 292 (39%) | 196 (38%) | 96 (41%) | 0·39 | |
| Diabetes | 198 (26%) | 143 (28%) | 55 (24%) | 0·31 | |
| Coronary heart disease | 74 (10%) | 52 (10%) | 22 (9%) | 0·92 | |
| Chronic kidney disease | 23 (3%) | 21 (4%) | 6 (3%) | 0·44 | |
| Cerebrovascular disease | 23 (3%) | 14 (3%) | 9 (4%) | 0·52 | |
| Hepatitis | 10 (1%) | 4 (1%) | 6 (3%) | 0·097 | |
| COPD | 4 (1%) | 1 (<1%) | 3 (1%) | 0·17 | |
| Symptoms at admission | |||||
| Fever | 514 (68%) | 364 (70%) | 150 (65%) | 0·16 | |
| Dry cough | 368 (49%) | 249 (48%) | 119 (51%) | 0·45 | |
| Fatigue | 153 (20%) | 101 (19%) | 52 (22%) | 0·41 | |
| Dyspnoea | 152 (20%) | 89 (17%) | 63 (27%) | 0·0022 | |
| Expectoration | 135 (18%) | 83 (16%) | 52 (22%) | 0·044 | |
| Chest tightness | 97 (13%) | 68 (13%) | 29 (13%) | 0·91 | |
| Diarrhoea | 89 (12%) | 63 (12%) | 26 (11%) | 0·81 | |
| Sore throat | 46 (6%) | 43 (8%) | 3 (1%) | 0·00042 | |
| Aversion to cold | 42 (6%) | 33 (6%) | 9 (4%) | 0·23 | |
| Coryza | 45 (6%) | 41 (8%) | 4 (2%) | 0·0018 | |
| Vomiting | 34 (5%) | 25 (5%) | 9 (4%) | 0·70 | |
| Headache | 37 (5%) | 29 (6%) | 8 (3%) | 0·29 | |
| COVID-19 severity | .. | .. | .. | <0·0001 | |
| Non-severe | 437 (58%) | 353 (68%) | 84 (36%) | .. | |
| Severe | 314 (42%) | 166 (32%) | 148 (64%) | .. | |
| Follow-up, days | 28 (20–36) | 27 (20–35) | 29 (22–38) | 0·0076 | |
| Clinical outcomes | .. | .. | .. | 0·0012 | |
| Survivor | 649 (86%) | 463 (89%) | 186 (80%) | .. | |
| Non-survivor | 102 (14%) | 56 (11%) | 46 (20%) | .. | |
| CT findings | |||||
| Ground-glass opacity | 331/496 (67%) | 183/301 (61%) | 148/195 (76%) | 0·00070 | |
| Patchy shadows | 278/496 (56%) | 152/301 (50%) | 126/195 (65%) | 0·0027 | |
| Fibrous stripes | 179/496 (36%) | 102/301 (34%) | 77/195 (39%) | 0·24 | |
| Pleural thickening | 145/496 (29%) | 85/301 (28%) | 60/195 (31%) | 0·61 | |
| Nodules | 74/496 (15%) | 46/301 (15%) | 28/195 (14%) | 0·88 | |
| Lymphadenopathy | 86/496 (17%) | 51/301 (17%) | 35/195 (18%) | 0·87 | |
| Bilateral pulmonary | 351/496 (71%) | 201/301 (67%) | 150/195 (77%) | 0·020 | |
| Right lung | 36/496 (7%) | 27/301 (9%) | 9/195 (5%) | 0·099 | |
| Left lung | 31/496 (6%) | 23/301 (8%) | 8/195 (4%) | 0·16 | |
| Laboratory examinations | |||||
| Cytokines and inflammatory factors | |||||
| TNF-α, pg/mL (n=425) | 7·1 (5·0–9·5) | 6·9 (4·8–9·3); n=336 | 8·7 (5·6–10·9); n=89 | 0·0040 | |
| IL-6, pg/mL (n=488) | 6·2 (2·2–21·8) | 4·9 (1·9–18·3); n=350 | 12·8 (3·7–32·5); n=138 | <0·0001 | |
| IL-10, pg/mL (n=426) | 5·0 (5·0–5·9) | 5·0 (5·0–5·5); n=338 | 5·0 (5·0–7·0); n=88 | 0·15 | |
| IL-2R, U/mL (n=419) | 555·0 (364·0–861·0) | 535·0 (348·5–839·0); n=340 | 615·0 (428·0–1028·0); n=79 | 0·012 | |
| Procalcitonin, ng/mL (n=412) | 0·1 (0·0–0·2) | 0·1 (0·0–0·1); n=251 | 0·1 (0·0–0·3); n=161 | 0·0041 | |
| C-reactive protein, mg/L (n=337) | 42·7 (18·5–70·2) | 40·7 (17·3–64·9); n=246 | 46·4 (21·8–94·9); n=91 | 0·047 | |
| Lymphocytes | |||||
| Lymphocytes (n=718) | 16·6% (8·5–24·9) | 16·8% (9·2–25·3); n=515 | 15·9% (7·2–23·5); n=203 | 0·099 | |
| Lymphocyte count per μL (n=746) | 0·9 (0·5–1·3) | 0·9 (0·6–1·3); n=515 | 0·8 (0·5–1·3); n=231 | 0·039 | |
| CD3-CD19+ B-cell count per μL (n=111) | 156·0 (98·0–213·0) | 175·0 (117·0–219·0); n=82 | 102·0 (66·4–154·0); n=29 | 0·0039 | |
| CD4+ T cells (n=136) | 44·3% (38·1–49·7) | 47·7% (42·6–53·9); n=82 | 41·1% (35·6–44·0); n=54 | <0·0001 | |
| CD4+ T-cell count per μL (n=119) | 460·0 (343·0–770·0) | 652·5 (415·0–899·0); n=82 | 370·0 (312·0–400·0); n=37 | <0·0001 | |
| CD8+ T cells (n=136) | 22·4% (17·4–26·9) | 20·7% (17·1–25·7); n=82 | 23·1% (18·4–30·3); n=54 | 0·10 | |
| CD8+ T-cell count per μL (n=125) | 267·0 (145·0–353·0) | 305·0 (184·0–386·0); n=82 | 206·0 (109·0–307·0); n=43 | 0·0081 | |
| CD4+ T cells/CD8+ T cells (n=125) | 2·1 (1·5–2·7) | 2·2 (1·7–2·9); n=82 | 1·8 (1·1–2·5); n=43 | 0·0052 | |
| Organ damage indices | |||||
| Alanine transaminase, U/L (n=464) | 20·0 (14·0–34·0) | 18·0 (13·0–30·0); n=283 | 25·0 (16·0–42·0); n=181 | <0·0001 | |
| Aspartate transaminase, U/L (n=464) | 29·0 (20·0–44·0) | 29·0 (20·0–45·0); n=283 | 27·5 (18·7–43·0); n=181 | 0·19 | |
| Lactate dehydrogenase, U/L (n=458) | 208·0 (176·0–342·0) | 194·0 (171·0–257·0); n=283 | 253·0 (194·0–455·0); n=175 | <0·0001 | |
| Total protein, g/L (n=372) | 68·0 (63·9–71·4) | 68·3 (64·4–71·6); n=279 | 66·0 (61·7–70·6); n=93 | 0·011 | |
| Albumin, g/L (n=462) | 36·8 (33·1–39·9) | 37·2 (34·5–40·0); n=281 | 34·7 (30·1–39·8); n=181 | <0·0001 | |
| Globulin, g/L (n=372) | 31·8 (28·9–35·3) | 32·1 (29·5–35·9); n=279 | 30·7 (26·8–33·4); n=93 | 0·00061 | |
| Albumin–globulin ratio (n=372) | 1·2 (1·0–1·4) | 1·1 (1·0–1·3); n=279 | 1·2 (1·0–1·5); n=93 | 0·40 | |
| NT-proBNP, pg/mL (n=331) | 171·0 (59·0–558·0) | 171·5 (56·0–519·0); n=214 | 158·0 (68·0–678·0); n=117 | 0·28 | |
| Myoglobin, ng/mL (n=234) | 41·4 (29·7–95·6) | 41·4 (28·5–91·8); n=133 | 41·3 (30·6–105·8); n=101 | 0·60 | |
| hs-cTnI, pg/mL (n=368) | 2·7 (1·9–8·1) | 2·4 (1·9–4·5); n=229 | 4·1 (1·9–14·3); n=139 | 0·0083 | |
| Leucocyte count, × 109/L (n=462) | 5·6 (4·2–7·8) | 5·7 (4·3–8·0); n=281 | 5·5 (4·2–7·7); n=181 | 0·58 | |
| Neutrophils (n=462) | 70·6% (62·2–80·7) | 67·8% (61·5–76·4); n=281 | 73·1% (64·2–86·1); n=181 | <0·0001 | |
| Neutrophil count, × 109/L (n=462) | 4·0 (3·0–5·3) | 4·1 (3·4–5·0); n=281 | 3·9 (2·8–6·5); n=181 | 0·84 | |
| Eosinophils (n=462) | 0·7% (0·0–1·6) | 0·8% (0·0–1·8); n=281 | 0·6% (0·0–1·7); n = 181 | 0·72 | |
| Eosinophil count, × 109/L (n=462) | 0·1 (0·0–0·1) | 0·1 (0·0–0·1); n=281 | 0·0 (0·0–0·1); n=181 | 0·034 | |
| Erythrocyte count, × 1012/L (n=462) | 4·0 (3·6–4·5) | 4·1 (3·7–4·5); n=281 | 3·8 (3·3–4·3); n=181 | <0·0001 | |
| Haemoglobin, g/L (n=462) | 123·0 (108·3–134·0) | 126·0 (114·0–137·0); n=281 | 118·0 (98·0–128·0); n=181 | <0·0001 | |
| Platelet count, × 109/L (n=461) | 196·0 (138·0–264·0) | 210·0 (142·0–277·0); n=281 | 182·0 (131·5–237·0); n=180 | 0·0061 | |
| D-dimer, μg/mL (n=447) | 0·9 (0·4–2·6) | 0·8 (0·4–2·1); n=280 | 1·2 (0·5–4·7); n=167 | 0·054 | |
| Prothrombin time, s (n=447) | 13·3 (12·7–13·9) | 13·2 (12·8–13·6); n=278 | 13·6 (12·4–14·8); n=169 | 0·036 | |
| Activated partial thromboplastin time, s (n=394) | 34·6 (30·2–39·9) | 34·1 (30·2–38·2); n=239 | 35·5 (30·2–42·9); n=155 | 0·046 | |
| Prothrombin activity (n=373) | 89·0% (79·0–98·0) | 89·0% (81·0–98·3); n=280 | 88·0% (73·0–96·0); n=93 | 0·092 | |
Data are n (%), n/N (%), or median (IQR), unless specified otherwise. COPD=chronic obstructive pulmonary disease. TNF-α=tumor necrosis factor α. IL=interleukin. IL-2R=IL-2 receptor. CD=cluster of differentiation. NT-proBNP=N-terminal pro-B-type natriuretic peptide. hs-cTnI=high-sensitivity cardiac troponin I.
Table 2.
Demographic, clinical, radiographical, and laboratory findings of patients with cancer with severe and non-severe COVID-19
| All patients with COVID-19 and cancer (n=232) | Patients with cancer and non-severe COVID-19 (n=84) | Patients with cancer and severe COVID-19 (n=148) | p value | ||
|---|---|---|---|---|---|
| Demographics | |||||
| Age, years | 64·0 (58·0–69·0) | 63·0 (57·0–67·0) | 64·0 (58·0–69·0) | 0·024 | |
| Sex | .. | .. | .. | 0·21 | |
| Female | 113 (49%) | 46 (55%) | 67 (45%) | .. | |
| Male | 119 (51%) | 38 (45%) | 81 (55%) | .. | |
| Clinical characteristics and outcomes | |||||
| ECOG performance status | .. | .. | .. | <0·0001 | |
| 0–2 | 141 (61%) | 81 (96%) | 60 (41%) | .. | |
| 3–4 | 91 (39%) | 3 (4%) | 88 (59%) | .. | |
| Tumour stage* | .. | .. | .. | 0·048 | |
| I, II, or III | 192/226 (85%) | 77/84 (92%) | 115/142 (81%) | .. | |
| IV | 34/226 (15%) | 7/84 (8%) | 27/142 (19%) | .. | |
| Clinical cancer grade | .. | .. | .. | 0·43 | |
| High differentiation | 189 (81%) | 72 (86%) | 117 (79%) | .. | |
| Moderate differentiation | 16 (7%) | 5 (6%) | 11 (7%) | .. | |
| Low differentiation | 27 (12%) | 7 (8%) | 20 (14%) | .. | |
| Antitumour treatments | |||||
| Surgery | 197 (85%) | 78 (93%) | 119 (80%) | 0·018 | |
| Chemotherapy or radiotherapy | 214 (92%) | 73 (87%) | 141 (95%) | 0·042 | |
| Targeted therapy or immunotherapy | 32 (14%) | 6 (7%) | 26 (18%) | 0·044 | |
| Comorbidities | |||||
| Hypertension | 96 (41%) | 28 (33%) | 68 (46%) | 0·083 | |
| Diabetes | 55 (24%) | 22 (26%) | 33 (22%) | 0·61 | |
| Coronary heart disease | 22 (9%) | 5 (6%) | 17 (11%) | 0·25 | |
| Chronic kidney disease | 6 (3%) | 2 (2%) | 4 (3%) | 1·0 | |
| Cardiovascular disease | 9 (4%) | 3 (4%) | 6 (4%) | 1·0 | |
| Hepatitis | 6 (3%) | 2 (2%) | 4 (3%) | 1·0 | |
| COPD | 3 (1%) | 1 (1%) | 2 (1%) | 1·0 | |
| Symptoms at admission | |||||
| Fever | 150 (65%) | 51 (61%) | 99 (67%) | 0·42 | |
| Dry cough | 119 (51%) | 33 (39%) | 86 (58%) | 0·0088 | |
| Fatigue | 52 (22%) | 13 (15%) | 39 (26%) | 0·081 | |
| Dyspnoea | 63 (27%) | 11 (13%) | 52 (35%) | 0·00051 | |
| Expectoration | 52 (22%) | 5 (6%) | 47 (32%) | <0·0001 | |
| Chest tightness | 29 (13%) | 6 (7%) | 23 (16%) | 0·098 | |
| Diarrhoea | 26 (11%) | 8 (10%) | 18 (12%) | 0·69 | |
| Sore throat | 3 (1%) | 0 | 3 (2%) | 0·48 | |
| Aversion to cold | 9 (4%) | 1 (1%) | 8 (5%) | 0·21 | |
| Coryza | 4 (2%) | 0 | 4 (3%) | 0·32 | |
| Vomiting | 9 (4%) | 2 (2%) | 7 (5%) | 0·59 | |
| Headache | 8 (3%) | 2 (2%) | 6 (4%) | 0·77 | |
| Follow-up, days | 29 (22–38) | 27 (19–36) | 30 (23–40) | 0·029 | |
| Clinical outcomes | .. | .. | .. | 0·0052 | |
| Survivor | 186 (80%) | 76 (90%) | 110 (74%) | .. | |
| Non-survivor | 46 (20%) | 8 (10%) | 38 (26%) | .. | |
| CT findings | |||||
| Ground-glass opacity | 148/195 (76%) | 45/79 (57%) | 103/116 (89%) | <0·0001 | |
| Patchy shadows | 126/195 (65%) | 34/79 (43%) | 92/116 (79%) | <0·0001 | |
| Fibrous stripes | 77/195 (39%) | 20/79 (25%) | 57/116 (49%) | 0·0014 | |
| Pleural thickening | 60/195 (31%) | 16/79 (20%) | 44/116 (38%) | 0·014 | |
| Nodules | 28/195 (14%) | 9/79 (11%) | 19/116 (16%) | 0·44 | |
| Lymphadenopathy | 35/195 (18%) | 8/79 (10%) | 27/116 (23%) | 0·031 | |
| Bilateral pulmonary | 150/195 (77%) | 43/79 (54%) | 107/116 (92%) | <0·0001 | |
| Right lung | 9/195 (5%) | 4/79 (5%) | 5/116 (4%) | 1·0 | |
| Left lung | 8/195 (4%) | 4/79 (5%) | 4/116 (3%) | 0·85 | |
| Laboratory examinations | |||||
| Cytokines and inflammatory factors | |||||
| TNF-α, pg/mL (n=89) | 8·7 (5·6–10·9) | 6·4 (5·0–7·9); n=21 | 9·4 (6·4–12·6); n=68 | 0·0050 | |
| IL-1β, pg/mL (n=81) | 5·0 (5·0–5·0) | 5·0 (5·0–5·0); n=20 | 5·0 (5·0–5·0); n=61 | 0·87 | |
| IL-6, pg/mL (n=138) | 12·8 (3·7–32·5) | 3·7 (2·4–14·5); n=41 | 16·1 (6·6–49·9); n=97 | <0·0001 | |
| IL-8, pg/mL (n=81) | 11·6 (6·9–23·4) | 9·2 (7·2–12·7); n=20 | 12·7 (6·8–25·5); n=61 | 0·13 | |
| IL-10, pg/mL (n=88) | 5·0 (5·0–7·0) | 5·0 (5·0–5·0); n=21 | 5·0 (5·0–10·4); n=67 | 0·0029 | |
| IL-2R, U/mL (n=79) | 615·0 (428·0–1028·0) | 445·0 (330·3–557·8); n=20 | 749·0 (495·0–1145·0); n=59 | 0·00024 | |
| Procalcitonin, ng/mL (n=161) | 0·1 (0·0–0·3) | 0·1 (0·0–0·1); n=68 | 0·2 (0·1–0·7); n=93 | <0·0001 | |
| Ferritin, μg/L (n=53) | 616·5 (334·3–1613·1) | 497·4 (277·1–625·8); n=13 | 849·7 (347·1–2178·6); n=40 | 0·17 | |
| C-reactive protein, mg/L (n=91) | 46·4 (21·8–94·9) | 42·5 (4·3–53·4); n=31 | 49·4 (28·1–99·0); n=60 | 0·044 | |
| Lymphocytes | |||||
| Lymphocyte count per μL (n=231) | 0·8 (0·5–1·3) | 1·0 (0·6–1·4); n=83 | 0·7 (0·4–1·2); n=148 | 0·0028 | |
| CD3+ T-cell count per μL (n=43) | 605·2 (529·0–643·6) | 637·0 (606·5–672·0); n=12 | 569·0 (455·0–621·0); n=31 | 0·024 | |
| CD3-CD19+ B-cell count per μL (n=29) | 102·0 (66·4–154·0) | 202·0 (147·0–282·0); n=4 | 92·0 (64·0–145·4); n=25 | 0·033 | |
| CD4+ T-cell count per μL (n=37) | 370·0 (312·0–400·0) | 395·3 (370·0–410·3); n=9 | 350·5 (296·0–394·5); n=28 | 0·049 | |
| CD3-CD16+CD56+ natural killer-cell count per μL (n=29) | 105·3 (86·9–123·4) | 132·1 (131·0–134·3); n=4 | 98·0 (86·2–112·0); n=25 | 0·019 | |
| T cell plus B cell plus natural killer-cell count per μL (n=29) | 794·0 (763·0–869·0) | 890·0 (870·0–925·0); n=4 | 782·0 (763·0–852·0); n=25 | 0·015 | |
| Organ damage indices | |||||
| Alanine transaminase, U/L (n=181) | 25·0 (16·0–42·0) | 23·5 (14·0–36·0); n=70 | 26·0 (16·0–49·0); n=111 | 0·11 | |
| Aspartate transaminase, U/L (n=181) | 27·5 (18·7–43·0) | 24·0 (17·9–33·3); n=70 | 31·0 (19·0–48·0); n=111 | 0·012 | |
| Lactate dehydrogenase, U/L (n=175) | 253·0 (194·0–455·0) | 209·0 (182·5–281·3); n=68 | 339·5 (199·5–575·0); n=107 | 0·00010 | |
| Total protein, g/L (n=93) | 66·0 (61·7–70·6) | 66·1 (61·8–71·7); n=32 | 66·0 (61·6–70·4); n=61 | 0·65 | |
| Albumin, g/L (n=181) | 34·7 (30·1–39·8) | 37·6 (32·5–41·6); n=70 | 33·4 (28·2–38·0); n=111 | 0·00021 | |
| Globulin, g/L (n=93) | 30·7 (26·8–33·4) | 30·2 (25·2–33·3); n=32 | 30·7 (27·0–34·4); n=61 | 0·21 | |
| Albumin–globulin ratio (n=93) | 1·2 (1·0–1·5) | 1·3 (1·0–1·6); n=32 | 1·1 (0·9–1·3); n=61 | 0·024 | |
| NT-proBNP, pg/mL (n=117) | 158·0 (68·0–678·0) | 134·3 (68·0–247·0); n=46 | 321·0 (73·0–501·0); n=71 | 0·013 | |
| Myoglobin, ng/mL (n=101) | 41·3 (30·6–105·8) | 37·5 (28·1–50·9); n=36 | 55·0 (33·0–159·3); n=65 | 0·0043 | |
| hs-cTnI, pg/mL (n=139) | 4·1 (1·9–14·3) | 1·9 (0·1–4·2); n=49 | 9·6 (2·3–69·0); n=90 | <0·0001 | |
| Leucocyte count, ×109/L (n=181) | 5·5 (4·2–7·7) | 5·4 (4·3–6·8); n=70 | 5·7 (4·0–8·4); n=111 | 0·17 | |
| Neutrophils (n=181) | 73·1% (64·2–86·1) | 67·5% (61·8–78·7); n=70 | 79·0% (67·6–88·8); n=111 | <0·0001 | |
| Neutrophil count, × 109/L (n=181) | 3·9 (2·8–6·5) | 3·5 (2·6–4·8); n=70 | 4·11 (2·8–7·4); n=111 | 0·039 | |
| Eosinophils (n=181) | 0·6% (0·0–1·8) | 1·0% (0·1–2·0); n=70 | 0·3% (0·0–1·5); n=111 | 0·0092 | |
| Eosinophil count, × 109/L (n=181) | 0·0 (0·0–0·1) | 0·1 (0·0–0·1); n=70 | 0·0 (0·0–0·1); n=111 | 0·018 | |
| Erythrocytes count, × 1012/L (n=181) | 3·8 (3·3–4·3) | 3·9 (3·5–4·3); n=70 | 3·7 (3·0–4·3); n=111 | 0·084 | |
| Haemoglobin, g/L (n=181) | 118·0 (98·0–128·0) | 120·0 (107·0–129·0); n=70 | 114·0 (94·0–127·0); n=111 | 0·037 | |
| Platelet count, × 109/L (n=180) | 182·0 (131·5–237·0) | 200·5 (146·5–256·5); n=70 | 174·0 (122·0–226·0); n=110 | 0·017 | |
| D-dimer, μg/mL (n=167) | 1·2 (0·5–4·7) | 0·7 (0·4–1·7); n=65 | 1·9 (0·5–8·0); n=102 | 0·00033 | |
| Prothrombin time (n=169), s | 13·6 (12·4–14·8) | 13·2 (11·8–14·1); n=66 | 13·9 (12·9–15·4); n=103 | 0·00054 | |
| Activated partial thromboplastin time (n=155), s | 35·5 (30·2–42·9) | 33·2 (27·8–41·0); n=57 | 37·7 (32·5–44·1); n=98 | 0·00060 | |
| Prothrombin activity (n=93) | 88·0% (73·0–96·0) | 90·5% (81·3–99·8); n=32 | 87·0% (70·0–92·5); n=61 | 0·044 | |
ECOG=Eastern Cooperative Oncology Group. COPD=chronic obstructive pulmonary disease. TNF-α=tumor necrosis factor α. IL=interleukin. IL-2R=IL-2 receptor. CD=cluster of differentiation. NT-proBNP=N-terminal pro-B-type natriuretic peptide. hs-cTnI=high-sensitivity cardiac troponin I.
Not including leukaemia (n=3) and multiple myeloma (n=3), due to different judgment standard of tumour stage.
When comparing biochemical indexes of COVID-19 between patients with and without cancer, we found that pro-inflammatory cytokines including TNF-α, IL-6, and IL-2R were higher in patients with cancer than in those without cancer. Infection-related biomarkers procalcitonin and C-reactive protein were also higher in patients with cancer. In terms of immune cells, the decline of lymphocytes, CD4+ T cells, CD8+ T -cell counts, and the ratio of CD4+ T cells to CD8+ T cells were more pronounced in patients with cancer (table 1). Additionally, there was greater evidence of multiple-organ damage in patients with cancer compared with those without cancer, since patients with cancer presented with higher levels of neutrophils, alanine transaminase, lactate dehydrogenase, and hs-cTnI, whereas eosinophils, albumin, globulin, and total protein were decreased (table 1). Furthermore, coagulation-related indicators, such as falling platelet counts and prolonged prothrombin time and activated partial thromboplastin time were also pronounced in patients with cancer (table 1). Notably, aggravated inflammatory responses, lymphopenia, and multiple-organ damage, especially decreased albumin–globulin ratio and elevated NT-proBNP, were more pronounced in patients with cancer who had severe COVID-19 compared with those with non-severe disease (table 2). Other laboratory indices or organ damage biomarkers in both patients with and without cancer, as well as patients with cancer and severe or non-severe COVID-19, are presented in the appendix (pp 11–12).
166 (32%) of 519 patients without cancer and 148 (64%) of 232 patients with cancer had severe COVID-19 at hospital admission (table 1); the risk of having severe illness was higher for patients with cancer (OR 3·61; 95% CI 2·59–5·04; p<0·0001) for patients with cancer. Patients with cancer had a longer follow-up than those without cancer, indicating that they spent more time in hospital, and were also more likely to die during follow-up (table 1). Additionally, the time for viral clearance was longer in patients with cancer (24 [IQR 17–29] days) than in those without cancer (21 days [IQR 15–24]; p=0·045).
When comparing the cancer characteristics between patients with severe and non-severe COVID-19, we found that patients with severe COVID-19 were older, had higher Eastern Cooperative Oncology Group (ECOG) performance status scores, and more advanced stage cancer than those with non-severe COVID-19 (table 2). Patients with severe COVID-19 were more likely to have received chemotherapy, radiotherapy, targeted therapy, or immunotherapy than those with non-severe disease, who were more likely to have had surgical antitumour treatment (table 2). The time interval between last chemotherapy treatment and hospital admission, and time since cancer diagnosis also differed between patients with severe and non-severe COVID-19 (appendix p 13).
184 (79%) of 232 patients with cancer and COVID-19 received antiviral treatment, 204 (88%) patients received antibiotics, and 85 (37%) received immunomodulators. These treatments did not differ significantly between patients with cancer and severe or non-severe COVID-19 (appendix p 14). Ventilation treatment was the conventional non-drug therapy, and, compared with patients without cancer, patients with cancer were more likely to receive high-flow nasal cannula oxygen therapy (77 [33%] of 232 patients with cancer vs 121 [23%] of 519 patients without cancer), non-invasive mechanical ventilation (62 [27%] vs 99 [19%]), or invasive mechanical ventilation (21 [9%] vs 23 [4%]; appendix p 14). Of note, the disease severity statuses of some patients with cancer changed during hospitalisation (appendix p 18): 22 (26%) of 84 patients developed severe disease during hospitalisation, whereas 107 (72%) of 148 patients changed from severe to non-severe disease. Eight (10%) patients with cancer who were admitted with non-severe COVID-19 and 38 (26%) patients with cancer who were admitted with severe COVID-19 died during follow-up (table 2). Treatment processes, dynamic changes of haematological parameters, and clinical outcomes of six typical patients with different types of cancer are shown in the appendix (p 19).
When exploring risk factors of COVID-19 severity in patients with cancer, sensitivity analyses showed no significant difference for most factors between univariable and multivariable analysis (table 3 ). We found that patients with older age, higher ECOG scores, and advanced tumour stage had increased odds of severe COVID-19. Moreover, compared with those who only received surgical treatment for cancer, patients who received targeted or immunotherapy had higher odds of severe illness (table 3). The effects of time interval since last chemotherapy treatment to hospital admission and time since cancer diagnosis on COVID-19 severity and death are shown in the appendix (pp 15–16). Risk of COVID-19 severity and death was highest for patients with last chemotherapy treatment within 2 weeks of admission, and decreased as the time interval since last chemotherapy increased, with significantly reduced risk when last treatment was at least 3 weeks before hospital admission (appendix pp 15–16). Moreover, patients with longer time since cancer diagnosis (1–5 years or >5 years) had lower risk of COVID-19 severity and death compared with patients with less than 1 year of tumour history (appendix pp 15–16).
Table 3.
Factors associated with COVID-19 illness severity in patients with cancer
|
Univariable logistic regression |
Multivariable logistic regression |
||||
|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | ||
| Age, years* | 1·04 (1·00–1·07) | 0·032 | 1·04 (1·00–1·07) | 0·034 | |
| ECOG performance status (per 1-point increase) | 2·32 (1·76–3·07) | <0·0001 | 2·80 (1·96–3·99) | <0·0001 | |
| Tumour stage†‡ | |||||
| I, II, or III | 1 (ref) | .. | 1 (ref) | .. | |
| IV | 2·58 (1·07–6·23) | 0·035 | 2·60 (1·05–6·43) | 0·039 | |
| Antitumour treatments§ | |||||
| Surgery | 1 (ref) | .. | 1 (ref) | .. | |
| Chemotherapy or radiotherapy | 1·27 (0·85–1·89) | 0·25 | 1·28 (0·85–1·94) | 0·24 | |
| Targeted therapy or immunotherapy | 2·84 (1·12–7·22) | 0·028 | 3·29 (1·26–8·61) | 0·015 | |
| Cytokines and inflammatory factors | |||||
| TNF-α, pg/mL | 1·22 (1·04–1·43) | 0·015 | 1·22 (1·01–1·47) | 0·037 | |
| IL-6, pg/mL | 1·03 (1·01–1·06) | 0·014 | 1·03 (1·00–1·05) | 0·019 | |
| IL-2R, U/mL | 1·00 (1·00–1·01) | 0·0081 | 1·00 (1·00–1·01) | 0·093 | |
| Procalcitonin, ng/mL | 2·31 (1·26–3·60) | 0·0013 | 2·76 (1·25–3·93) | 0·0015 | |
| C-reactive protein, mg/L | 1·01 (1·00–1·02) | 0·022 | 1·01 (1·00–1·02) | 0·034 | |
| Lymphocytes | |||||
| Lymphocytes, % | 0·96 (0·93–0·99) | 0·0042 | 0·96 (0·93–0·99) | 0·020 | |
| Lymphocyte count per μL | 0·52 (0·30–0·89) | 0·017 | 0·56 (0·31–1·00) | 0·051 | |
| CD3-CD19+ B-cell count per μL | 0·98 (0·97–1·00) | 0·035 | 0·98 (0·96–1·00) | 0·11 | |
| CD4+ T cells, % | 0·89 (0·79–0·99) | 0·040 | 0·84 (0·71–0·98) | 0·031 | |
| CD3-CD16+CD56+ natural killer cells, % | 0·84 (0·72–0·98) | 0·024 | 0·85 (0·72–0·99) | 0·041 | |
| Haematological tests | |||||
| Leucocyte count, × 109/L | 1·12 (1·02–1·22) | 0·017 | 1·11 (1·01–1·22) | 0·029 | |
| Neutrophils, % | 1·06 (1·03–1·08) | <0·0001 | 1·06 (1·03–1·09) | 0·00017 | |
| Monocytes, % | 0·87 (0·80–0·96) | 0·0040 | 0·88 (0·79–0·98) | 0·023 | |
| Biochemical factors | |||||
| Lactate dehydrogenase, U/L | 1·00 (1·00–1·00) | 0·0040 | 1·00 (1·00–1·00) | 0·018 | |
| Albumin, g/L | 0·92 (0·87–0·97) | 0·0011 | 0·92 (0·87–0·98) | 0·0091 | |
| Albumin–globulin ratio | 0·20 (0·05–0·74) | 0·016 | 0·12 (0·02–0·77) | 0·024 | |
| NT-proBNP, pg/mL | 1·64 (1·02–2·61) | 0·039 | 1·65 (1·03–2·78) | 0·032 | |
| Myoglobin, ng/mL | 1·01 (1·00–1·02) | 0·044 | 1·01 (1·00–1·03) | 0·050 | |
| hs-cTnI, pg/mL | 1·01 (1·00–1·02) | 0·046 | 1·01 (1·00–1·02) | 0·067 | |
| Coagulation function | |||||
| Platelet count, × 109/L | 1·00 (0·99–1·00) | 0·025 | 1·00 (0·99–1·00) | 0·070 | |
| Activated partial thromboplastin time, s | 1·08 (1·03–1·12) | 0·0011 | 1·12 (1·05–1·18) | 0·00016 | |
| Prothrombin time, s | 1·20 (1·04–1·40) | 0·011 | 1·25 (1·07–1·48) | 0·0062 | |
| D-dimer, μg/mL | 1·13 (1·04–1·22) | 0·0029 | 1·12 (1·03–1·21) | 0·0074 | |
All analyses were adjusted for age, sex, comorbidities, tumour stage, cancer type, and antitumour treatment unless otherwise specified. OR=odds ratio. ECOG=Eastern Cooperative Oncology Group. TNF-α=tumor necrosis factor α. IL=interleukin. IL-2R=IL-2 receptor. CD=cluster of differentiation. NT-proBNP=N-terminal pro-B-type natriuretic peptide. hs-cTnI=high-sensitivity cardiac troponin I.
Adjusted for sex, comorbidities, tumour stage, cancer type, and antitumour treatment.
Not including leukaemia (n=3) and multiple myeloma (n=3), due to different judgment standard of tumour stage.
Adjusted for age, sex, comorbidities, cancer type, and antitumour treatment.
Adjusted for age, sex, comorbidities, tumour stage, and cancer type.
After adjusting for age, sex, comorbidities, cancer type, tumour stage, and antitumour treatments in multivariable analyses, we found that pro-inflammatory and infection-related biomarkers, including TNF-α, IL-6, procalcitonin, and C-reactive protein, as well as organ damage indices (leucocytes, neutrophils, and lactate dehydrogenase), coagulation-related indicators (D-dimer, prothrombin time, and activated partial thromboplastin time), and NT-proBNP were significantly associated with worse severity of COVID-19 in patients with cancer (table 3). Conversely, immune cells (lymphocytes, CD4+ T cells, and natural killer cells) and resistant organ damage indices (albumin and albumin–globulin ratio) were significantly associated with lower severity of COVID-19 in patients with cancer (table 3). We found that TNF-α, NT-proBNP, albumin–globulin ratio, and CD4+ T cells were also associated with the risk of death from COVID-19 (appendix p 16), and the dynamic change of these four indices over follow-up are shown in the appendix (p 20). Levels of TNF-α and NT-proBNP were significantly higher in patients with cancer and severe COVID-19 than in those with non-severe COVID-19 on admission, whereas the levels of CD4+ T cells and albumin–globulin ratio were significantly lower (appendix p 20). These differences increased over 4 weeks of hospitalisation, which was a critical period for COVID-19 progression. For patients with non-severe disease, CD4+ T cells decreased in the first 3 weeks and then gradually increased over the following 2 weeks. By contrast, the decrease of CD4+ T cells among patients with severe COVID-19 was more pronounced and prolonged (appendix p 20).
Discussion
In face of the COVID-19 pandemic, research is urgently needed to guide the implementation of effective prevention and control measures for susceptible populations. Here, by integrating clinical data from nine hospitals, we found that patients with cancer infected with SARS-CoV-2 have an increased risk of developing severe COVID-19. Furthermore, in addition to previously reported risk factors for this population, such as older age and elevated IL-6, procalcitonin, and D-dimer, we identified novel risk factors including advanced tumour stage, elevated TNF-α and NT-proBNP, and decreased CD4+ T cells and albumin–globulin ratio.
Our enrolled patients without cancer were statistically matched to those with cancer, which helped to minimise the effects of common confounders such as age, sex, and other comorbidities on the severity of COVID-19. The lower prevalence of sore throat and coryza and increased prevalence of dyspnoea and expectoration in patients with cancer compared with those without cancer are in line with the findings of previous studies,13 and might be due to patients' subjective perception of their disease severity. Moreover, in most situations, CT images of chest lesions in patients with COVID-19 pneumonia is explicitly different from those of lung cancer or metastasis in distribution, density, and size.14 Notably, all patients in our current study were symptomatic for COVID-19; therefore, a screening or an epidemiological study in the patients with cancer is warranted to understand the rate of asymptomatic infection.
We used unconditional logistic regression methods to explore the factors related with COVID-19 severity in patients with cancer. Risk factors reported previously in patients without cancer, such as older age; elevated IL-6, procalcitonin, D-dimer, and C-reactive protein; and decreased lymphocytes were validated in patients with cancer. Additionally, we identified several risk factors for COVID-19 severity in patients with cancer, including advanced tumour stage, elevated TNF-α and NT-proBNP, and decreased CD4+ T cells and albumin–globulin ratio. Older age has previously been reported as an important independent predictor of mortality in severe acute respiratory syndrome (SARS), Middle East respiratory syndrome, and COVID-19.15, 16, 17 Moreover, we observed that advanced tumour stage aggravates COVID-19 progression, which might be due to the tumour burden. Among the measured cytokines, elevation of IL-6 was most pronounced, consistent with recent published studies.18, 19 It is known that IL-6 plays multifaceted roles in regulation of vascular leakage, complement activation, and coagulation pathways, which ultimately causes poor outcomes for acute respiratory distress syndrome, multiple organ dysfunction syndrome, and SARS.20, 21, 22 TNF-α, a potential novel biomarker for COVID-19 identified here, has been reported to facilitate the apoptosis of both lung epithelial cells and endothelial cells, ultimately resulting in vascular leakage, alveolar oedema, and hypoxia.23 TNF-α has also been reported to mediate airway hyper-responsiveness and pathogenesis in influenza and SARS-CoV infection.24 Additionally, TNF-α was originally identified as an effective mediator inducing haemorrhagic necrosis in tumours.25 Collectively, these findings help to explain our results showing that TNF-α, compared with indicators such as C-reactive protein and D-dimer, might be more predictive for COVID-19 severity, especially in patients with cancer.
Reduced CD4+ T cells in patients with COVID-19 has been confirmed by Qin and colleagues, revealing that an immunosuppression feature is pronounced in severe COVID-19 cases.19 A rapid and well coordinated innate immune response is the first line of defence against viral infections. CD4+ T cells can enhance the ability of cytotoxic T cells to clear pathogens.26 However, persistent stimulation by the virus might induce T-cell exhaustion and facilitate host immune response disorders, causing excessive inflammation and even death.23, 27 Notably, in addition to dysregulated inflammatory and immune responses, organ damage biomarkers such as elevated NT-proBNP and decreased albumin–globulin ratio, were also associated with COVID-19 severity, as has been confirmed by other studies.28 Collectively, these findings suggest that aggravated inflammatory storm, dysregulated immune responses, and multiple-organ damage appear to be possible mechanisms in patients with cancer with severe COVID-19.
Successful standardised treatment protocols for patients with COVID-19, especially with severe disease, must be recommended globally to curb poor outcomes. Here, we found that clinical interventions, especially ventilation treatments (high-flow nasal cannula oxygen or mechanical ventilation), were more intensive in patients with cancer than in patients without cancer. In particular, more than 75% of patients with cancer and COVID-19 received antiviral treatment and antibiotics, and approximately a third of patients received immunomodulators. Several clinical trials are underway to define potential roles for antiviral agents (remdesivir, lopinavir, or ritonavir)29 and specific immunomodulators such as IL-6 receptor blockade (tocilizumab; ChiCTR2000029765) in COVID-19. Interactions between several chemotherapy agents and antiviral drugs need to be considered when treating patients with cancer and COVID-19;30 the sample size in our study was too small to permit this analysis. In view of the uncontrolled inflammatory responses, impaired adaptive immune responses, and multiple-organ dysfunction in patients with cancer and COVID-19—especially those with severe COVID-19—the current management of COVID-19 should be focused on inflammatory reaction and immune dysfunction, supportive care including ventilatory support, and treatment of complications.
Based on our findings, three major strategies for the management of patients with cancer during this COVID-19 crisis are warranted. First, robust personal protection provisions should be made for medical staff, patients with cancer, and cancer survivors to avoid cross-infection. Second, intentional postponement of adjuvant chemotherapy or elective surgery for stable cancer should be considered in endemic areas, but oral medications should continue to be administered. Third, more intensive surveillance should be considered for patients admitted with COVID-19 who have cancer, especially older patients or those with other comorbidities.
Our study has several limitations. First, owing to its retrospective design, it lacks dynamic clinical and laboratory data. Second, the potential mechanisms of dysregulated inflammatory cytokines and immune responses were not fully explored and need to be further investigated. Third, our study recruited all available patients with any type of malignant solid tumours and haematological malignancy, but included two patients with basal cell carcinoma and three cancer survivors who had disease-free survival for more than 5 years. Finally, the enrolled patients with cancer represent a mixed sample of multiple cancer types; further studies by cancer type are still needed.
To our knowledge, this study is the largest multicentre cohort study so far among patients with cancer and COVID-19, providing detailed clinical and laboratory information. This study highlights that risk factors including elevated TNF-α and NT-proBNP and decreased CD4+ T cells or albumin–globulin ratio would be helpful for early surveillance of disease progression, in addition to previously reported risk factors of older age; elevated IL-6, procalcitonin, and D-dimer; and decreased lymphocytes.
Acknowledgments
Acknowledgments
This work is supported by the China National Natural Science Foundation (grant number 81974400). We thank all patients and their families involved in the study and all health-care workers who are working against COVID-19. We also thank David Horne (City of Hope, CA, USA), Hui Zhu (Cleveland Clinic Foundation, OH, USA), Gonghong Wei (University of Oulu, Finland), and Jiping Wang (Northwestern University, IL, USA) for their assistance.
Contributors
XM, XY, and ZW were the overall principal investigators who conceived the study and obtained financial support, were responsible for the study design, and supervised the entire study. CY, BL, JingW, YW, SL, BC, JinW, ZY, XD, FZ, WW, QL, XC, WC, JF, and BS recruited participants. JT, JX, CY, BL, YC, and ZL drafted the paper. JT, YC, ZL, MZ, LW, and SN did the statistical analyses. JX, CY, BL, QY, SWu, and WL did the data analysis and interpreted the results. XY, QZ, ZH, SWa, X-PY, XM, and ZW reviewed the manuscript. All authors contributed to data interpretation, manuscript writing, and review of the manuscript. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.WHO Rolling updates on coronavirus disease (COVID-19) https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 2.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Chin J Epidemiol. 2020;41:145–151. (in Chinese). [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma X, Yu H. Global burden of cancer. Yale J Biol Med. 2006;79:85–94. [PMC free article] [PubMed] [Google Scholar]
- 6.Liang W, Guan W, Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veselka J, Faber L, Liebregts M. Short- and long-term outcomes of alcohol septal ablation for hypertrophic obstructive cardiomyopathy in patients with mild left ventricular hypertrophy: a propensity score matching analysis. Eur Heart J. 2019;40:1681–1687. doi: 10.1093/eurheartj/ehz110. [DOI] [PubMed] [Google Scholar]
- 8.Pan A, Liu L, Wang C. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.6130. published online April 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO Clinical management of severe acute respiratory infection when Novel coronavirus (nCoV) infection is suspected: interim guidance. March 13, 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected
- 10.National Health Commission of the People's Republic of China Chinese management guideline for COVID-19 (version 7.0) March 3, 2020. http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf (in Chinese).
- 11.Chen G, Wu D, Guo W. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020 doi: 10.1172/JCI137244. published online April 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prescott RJ. Two-tailed significance tests for 2 × 2 contingency tables: what is the alternative? Stat Med. 2019;38:4264–4269. doi: 10.1002/sim.8294. [DOI] [PubMed] [Google Scholar]
- 13.Guan WJ, Ni ZY, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Dong C, Hu Y. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology. 2020 doi: 10.1148/radiol.2020200843. published online March 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi KW, Chau TN, Tsang O. Outcomes and prognostic factors in 267 patients with severe acute respiratory syndrome in Hong Kong. Ann Intern Med. 2003;139:715–723. doi: 10.7326/0003-4819-139-9-200311040-00005. [DOI] [PubMed] [Google Scholar]
- 16.Hong KH, Choi JP, Hong SH. Predictors of mortality in Middle East respiratory syndrome (MERS) Thorax. 2018;73:286–289. doi: 10.1136/thoraxjnl-2016-209313. [DOI] [PubMed] [Google Scholar]
- 17.Wu C, Chen X, Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. published online March 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D, Hu B, Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin C, Zhou L, Hu Z. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa248. published online March 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saito LB, Diaz-Satizabal L, Evseev D. IFN and cytokine responses in ducks to genetically similar H5N1 influenza A viruses of varying pathogenicity. J Gen Virol. 2018;99:464–474. doi: 10.1099/jgv.0.001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Ma S. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. Am J Emerg Med. 2008;26:711–715. doi: 10.1016/j.ajem.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 23.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nie Z, Scott GD, Weis PD, Itakura A, Fryer AD, Jacoby DB. Role of TNF-alpha in virus-induced airway hyperresponsiveness and neuronal M(2) muscarinic receptor dysfunction. Br J Pharmacol. 2011;164:444–452. doi: 10.1111/j.1476-5381.2011.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Josephs SF, Ichim TE, Prince SM. Unleashing endogenous TNF-alpha as a cancer immunotherapeutic. J Transl Med. 2018;16:242. doi: 10.1186/s12967-018-1611-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Ann Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fenwick C, Joo V, Jacquier P. T-cell exhaustion in HIV infection. Immunol Rev. 2019;292:149–163. doi: 10.1111/imr.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mao R, Liang J, Shen J. Implications of COVID-19 for patients with pre-existing digestive diseases. Lancet Gastroenterol Hepatol. 2020;5:426–428. doi: 10.1016/S2468-1253(20)30076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grein J, Ohmagari N, Shin D. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007016. published online April 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO . 2nd edn. World Health Organization; Geneva: 2016. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.