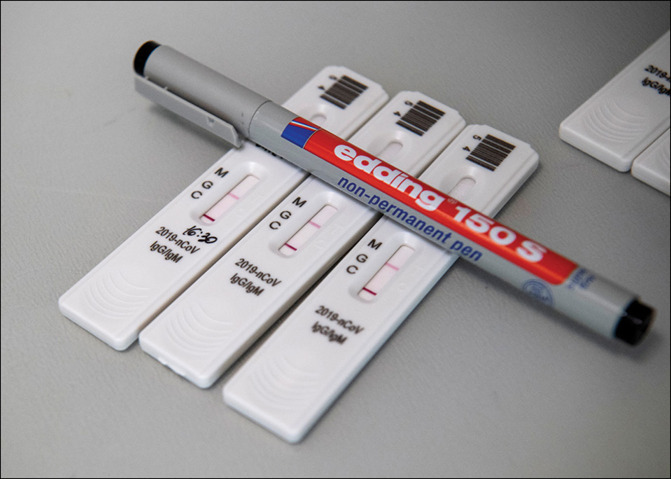
On May 21, 2020, UK Health and Social Care Secretary, Matt Hancock, announced plans to roll out more than 10 million severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody tests. National Health Service (NHS) staff and patients, and workers and residents in care homes, will be the first groups to be offered the tests. Hancock also revealed that an antibody surveillance study led by the Office for National Statistics had found that around 17% of people in London and 5% of people elsewhere in the UK had tested positive for anti-SARS-CoV-2 antibodies. Earlier in the week, the Government confirmed that anyone older than 5 years with symptoms of COVID-19 would be eligible for real time RT-PCR testing.
The antibody tests, which are laboratory-based, will be provided by Roche Diagnostics and Abbott Laboratories. Evaluations by Public Health England (PHE) concluded that each had a specificity of 100%; sensitivity, for samples taken at least 14 days since the onset of symptoms, stood at 93·9% for the Abbott test and 87·0% for the Roche test. The Medicines & Healthcare Products Regulatory Agency has approved both tests. Hancock described the pending rollout as “an important milestone”.
The tests are undoubtedly useful epidemiological tools, particularly for estimating the prevalence of asymptomatic cases of COVID-19. Although, if it is indeed the case that only 5% or so of the population have been infected with SARS-CoV-2, then administering millions of antibody tests might not do much to clarify the picture in terms of overall prevalence. “It is looking as if we have flattened the curve [of new infections]”, explains Phil Beales (University College London, London, UK). “So unless there is a second wave of infections, we are not likely to see much of a change.”
For now, the information provided by antibody tests on an individual level remains limited. The results cannot tell you whether you are currently infected with SARS-CoV-2, nor whether you can infect others. If the test is administered too soon after the infection, there might not be detectable antibodies (although if you are in week 3 of the illness, an antibody test might be better than the RT-PCR test). Crucially, it has yet to be determined whether the presence of antibodies implies immunity. In a briefing to the media on May 20, 2020, NHS England's medical director Stephen Powis said that he “would not want people to think just because you test positive for the antibody that it necessarily means that you can do something different in terms of social distancing, in the way you behave”.
Most experts suspect that infection with SARS-CoV-2 will probably confer a degree of immunity. “I think the presence of antibodies is a reasonable indication that an individual is at least somewhat protected”, said Martin Hibberd (London School of Hygiene and Tropical Medicine, London, UK). “Even if that protection lasts a short while, it is still more likely to be a period of years rather than months.” The other coronaviruses do not offer many clues. There were not enough cases of either severe acute respiratory syndrome (SARS) coronavirus or Middle East respiratory syndrome (MERS) coronavirus to draw conclusions about reinfection, and there is not much data on the common cold.
As the antibody tests are rolled out, researchers will be able to observe whether individuals previously infected with SARS-CoV-2 can be re-infected and what form this re-infection takes. Hibberd believes that a patient's second bout of COVID-19 is likely to be less severe than their first one, though he acknowledged that this remains speculative. “It could be that in few months time, we will know how long the antibodies last, whether we need to be retesting people and, if so, at what intervals”, he added. In the meantime, positive test results could be used as a risk stratification tool.
Private companies have started to offer antibody tests to the general public. Users prick their fingers to acquire a blood sample, which is then sent to the laboratory for analysis. Two major UK vendors, both of which sell test kits for £69, reported that they had run out of stock. Anne Wyllie (Yale School of Public Health, New Haven, CT, USA) points out that the US market has been flooded by antibody tests that have not been approved by the US Food and Drug Administration (FDA). “The risk is that these tests give a false positive”, she said. “We do not want people thinking they are immune to the disease when they are not.” In his press briefing of May 20, Powis echoed this concern. “I would caution against using any tests that might be made available without knowing quite how good those tests are”, he said. Earlier this year, the UK Government purchased 3·5 million at-home test kits which proved too inaccurate to be useable.
To determine whether an individual is currently infected with SARS-CoV-2 requires RT-PCR testing. This was made available soon after the virus had been sequenced in January, 2020. Samples are obtained using a nasopharyngeal swab, which is a challenging proposition. “You do not get anywhere near an acceptable detection rate using a swab test”, said Beales. “You are sticking a piece of equipment into the back of someone's nose and throat; people cannot stand it, it activates the gag reflex.” Operators risk being coughed and spluttered upon. The UK and USA have opened drive-through testing centres. Self-administering the swab in the front seat of the car, using the rear-view mirror for guidance, is not always easy.
© 2020 Sputnik/Science Photo Library
© 2020 Sputnik/Science Photo Library
A saliva test would be preferable. Such tests are easy to administer. As long as the recipient can produce saliva, the presence of the virus can be detected. In contrast, a swab can emerge from an infected person without having picked up any virus. Saliva tests are logistically less complicated than swab tests. The tube can be delivered to the doorstep and subsequently collected or returned by post. “It is a much more stable way of testing; saliva preserves the virus, whereas a swab has to get back to the laboratory within a day or two”, Beales told The Lancet Respiratory Medicine. Assuming users are spitting into a tube with preservatives and additives, it is much easier to deal with a saliva test. The virus is killed, and its RNA preserved; whereas swabs retain infectious particles.
“At the moment, we have to rely on invasive swab testing and laboratory PCR to get accurate data”, said Hibberd. “If we can rollout more widespread testing, using a saliva test, that would allow us to look for asymptomatic patients; that would allow us to isolate people with the virus before they develop symptoms, which is probably when they are at their most infectious.” Wyllie notes that the early indications are that the saliva tests are highly sensitive. The FDA has used emergency provisions to authorise saliva-based tests, one of which is being rolled out to the US Air Force. “We do not yet know if there are antibodies in the saliva”, notes Beales. “If there are, that would be an encouraging development—it is easier to spit than to extract blood, especially if the test is being self-administered.”
In the early stages of the COVID-19 pandemic, the UK struggled to build diagnostic capacity. There was a global shortage of reagents and swabs. On March 12, 2020, the UK discontinued community testing. The Science and Technology Select Committee has written that “amongst other consequences, [the discontinuation] meant that residents in care homes—even those displaying COVID-19 symptoms—and care home workers could not be tested at a time when the spread of the virus was at its most rampant”. On April 2, Hancock announced the ambition to get to 100 000 tests per day by the end of the month. At the time, capacity was around 10 000 per day. The target was achieved, though not without controversy, with suggestions that the figure had been artificially inflated by including tests that had been sent out but not necessarily taken.
The supply issues appear to have been resolved. Hibberd reckons that a decent model for the future would involve a combination of rapid tests (preferably saliva based) that could look for viral proteins or genome, and conventional laboratory testing. “Ideally, we would use rapid testing to routinely identify cases of COVID-19; that opens up the possibility of doing screening at workplaces, airports, and other hubs”, said Hibberd. A weekly testing regimen conducted at the workplace, with a turnaround of 15 min, would enable early detection of the virus. That would reassure workers and allow for the early initiation of contact tracing. The laboratory tests could be reserved for hospitals and for surveillance. Much will depend on whether rapid tests can come close to RT-PCR for sensitivity, as well as the size of the workforce. A 15-min turnaround means 2 h 30 min to test a staff of 10; testing 100 people would take ten times longer. “Testing alone will not be sufficient; you also have to ensure that you have a strong system for contact tracing, and that people comply with the regulations”, stresses Hibberd. If all three components work in tandem, then the virus can be controlled. Hibberd gives the example of South Korea and Singapore. “If you look at those countries, there is one clear lesson”, he said. “If you can identify and quarantine most of the positive cases, then you do not have to lockdown everyone else.”