Summary
Background
Patients with cancer are a high-risk population in the COVID-19 pandemic. We aimed to describe clinical characteristics and outcomes of patients with cancer and COVID-19, and examined risk factors for mortality in this population.
Methods
We did a retrospective, multicentre, cohort study of 205 patients with laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and with a pathological diagnosis of a malignant tumour in nine hospitals within Hubei, China, from Jan 13 to March 18, 2020. All patients were either discharged from hospitals or had died by April 20, 2020. Clinical characteristics, laboratory data, and cancer histories were compared between survivors and non-survivors by use of χ2 test. Risk factors for mortality were identified by univariable and multivariable logistic regression models.
Findings
Between Jan 13 and Mar 18, 2020, 205 patients with cancer and laboratory-confirmed SARS-CoV-2 infection were enrolled (median age 63 years [IQR 56–70; range 14–96]; 109 [53%] women). 183 (89%) had solid tumours and 22 (11%) had haematological malignancies. The median duration of follow-up was 68 days (IQR 59–78). The most common solid tumour types were breast (40 [20%] patients), colorectal (28 [14%]), and lung cancer (24 [12%]). 54 (30%) of 182 patients received antitumour therapies within 4 weeks before symptom onset. 30 (15%) of 205 patients were transferred to an intensive care unit and 40 (20%) died during hospital admission. Patients with haematological malignancies had poorer prognoses than did those with solid tumours: nine (41%) of 22 patients with haematological malignancies died versus 31 (17%) of 183 patients with solid tumours (hazard ratio for death 3·28 [95% CI 1·56–6·91]; log rank p=0·0009). Multivariable regression analysis showed that receiving chemotherapy within 4 weeks before symptom onset (odds ratio [OR] 3·51 [95% CI 1·16–10·59]; p=0·026) and male sex (OR 3·86 [95% CI 1·57–9·50]; p=0·0033) were risk factors for death during admission to hospital.
Interpretation
Patients with cancer and COVID-19 who were admitted to hospital had a high case-fatality rate. Unfavourable prognostic factors, including receiving chemotherapy within 4 weeks before symptom onset and male sex, might help clinicians to identify patients at high risk of fatal outcomes.
Funding
National Natural Science Foundation of China.
Introduction
COVID-19, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first detected in December, 2019, in Wuhan, China. It spread rapidly across the world during the following few weeks.1, 2 As of May 27, 2020, 5 491 678 cases have been confirmed worldwide, with 349 190 deaths.3, 4 In Wuhan, the initial centre of the epidemic, 50 340 COVID-19 cases and 3869 deaths have been confirmed, as of May 26, 2020.5
Patients with cancer are a vulnerable population during the COVID-19 pandemic. They are often immunosuppressed because of their underlying illness, poor nutrition, and treatment-related side-effects. Therefore, they are at increased risk of opportunistic infections, developing severe complications, requiring admission to an intensive care unit (ICU), or even death.6, 7, 8, 9 Liang and colleagues10 analysed data from 18 patients with cancer, from a sample of 1590 patients with COVID-19, and found a higher risk of COVID-19 and poorer outcomes in patients with cancer than in those without. Zhang and colleagues11 reported 28 cases of SARS-CoV-2 infection in patients with cancer, with a case-fatality of 28·6%. However, these studies were limited by small sample sizes. Large studies are needed to comprehensively describe the characteristics and outcomes of patients with cancer and COVID-19.
We collected and analysed data from patients with cancer and COVID-19 who were admitted to nine local hospitals in Hubei, China. We aimed to describe the clinical features and outcomes of patients with cancer diagnosed with COVID-19, and to identify risk factors associated with in-hospital mortality.
Research in context.
Evidence before this study
We searched PubMed, and two preprint servers (bioRxiv and medRxiv) on March 28, 2020, for articles describing clinical characteristics, outcomes, and risk factors of patients with cancer and diagnosed with COVID-19. Search terms included “COVID-19 or SARS-CoV-2 or 2019-nCoV or novel coronavirus” and “tumor or carcinoma or cancer”. We found studies showing a higher risk of COVID-19 and poorer outcomes in patients with cancer than in individuals without cancer. Recent studies have shown that receiving antitumour treatments and patchy consolidation on CT scans were associated with development of severe events in COVID-19 (requiring admission to the intensive care unit, the use of mechanical ventilation, or death). One paper reported that a patient with lung cancer who was diagnosed with COVID-19 continued targeted therapy and recovered from pneumonia. However, we found no published multicentre study with a large sample size discussing clinical characteristics, outcomes, and risk factors of mortality of patients with cancer and a confirmed diagnosis of COVID-19.
Added value of this study
In this retrospective, multicentre, cohort study, we report demographic, clinical, laboratory, and radiological findings, as well as treatments and outcomes, for 205 patients with cancer and laboratory-confirmed severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) infection in Hubei, China. Patients with haematological malignancies had poorer prognoses than did those with solid tumours. Receiving chemotherapy within 4 weeks before symptom onset, and male sex were risk factors for death during admission to hospital.
Implications of all the available evidence
Patients with cancer are a vulnerable population with a higher case-fatality from COVID-19 than the general population. Male patients with cancer and those receiving chemotherapy within 4 weeks before symptom onset might require additional medical attention and supportive care once diagnosed with COVID-19, as they appear to be at increased risk of in-hospital mortality.
Methods
Study design and participants
This retrospective, multicentre, cohort study was led by Wuhan Union Hospital (Wuhan, China). The list of participating hospitals is as follows: Cancer Center of Wuhan Union Hospital, West Branch of Wuhan Union Hospital, Jin Yin-tan Hospital, Wuhan Red Cross Hospital, the Central Hospital of Wuhan, Huanggang Central Hospital, the First People's Hospital Affiliated to Yangtze University, Xianning Central Hospital, and Suizhou Central Hospital (appendix p 1). All hospitals involved in this study were officially designated for treatment of patients with COVID-19 after the outbreak was declared. Diagnosis of COVID-19 followed WHO interim guidance.12 From Jan 13 to March 18, 2020, we enrolled 205 patients with a history of cancer, irrespective of when the cancer had been diagnosed. The inclusion criteria were strictly based on pathological diagnosis of a malignant tumour and laboratory confirmation of SARS-CoV-2 infection; patients clinically diagnosed with COVID-19 were excluded. Patients with a pathological diagnosis of a benign tumour were excluded because we chose to focus on malignancies that are usually linked to an immunosuppressive state. The presence of SARS-CoV-2 infection was confirmed by RT-PCR and next-generation sequencing analysis of samples collected from nasopharyngeal swabs.4, 13 We aimed to explore the clinical characteristics and outcomes of patients with COVID-19 who had malignant tumours. There was no formal determination of sample size and all patients meeting the inclusion criteria were recruited. The cutoff date for our study was April 20, 2020.
Ethics approval was obtained from the Ethics Committee of Wuhan Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, at the beginning of the study. Written, informed consent was waived because of the urgency and unprecedented nature of the COVID-19 pandemic.
Data collection
We obtained information about demographic data, clinical manifestations, cancer histories, laboratory findings, chest CT examinations, treatments, and outcomes of all enrolled patients from electronic medical records and patients' interviews. All lesions in CT scans were independently reviewed by two thoracic radiologists to exclude any lung cancer or metastasis. A standardised data collection form modified from the International Severe Acute Respiratory and Emerging Infection Consortium forms14 was used. Any logical error or core data omission was revised and replaced by contacting the attending physicians directly. All data were independently validated by YS and BW.
Data about age, sex, underlying complications (hypertension, diabetes, chronic obstructive pulmonary disease, coronary heart disease, hepatitis B virus infection, and chronic nephrosis), history of malignancies (Eastern Cooperative Oncology Group [ECOG] performance status, cancer type, cancer stage, time since cancer diagnosis, and treatments), vital signs (temperature, breathing rate, heart rate, and blood oxygen saturation), symptoms (fever, cough, sputum, chest congestion, shortness of breath, chills, dyspnoea, fatigue, nausea, vomiting, and diarrhoea), and laboratory tests (haemoglobin, white blood cells, neutrophils, lymphocytes, platelets, albumin, creatinine, blood urea nitrogen, lactate dehydrogenase, creatine kinase, D-dimer, C-reactive protein, procalcitonin, electrolytes, and interleukin-6) were all collected at the time of admission. Based on the TNM staging system, cancer stage was defined as early (I–II) or late (III–IV) stage for solid tumours (staging information for brain cancer was not recorded). Haematological malignancies have various staging systems and are distinct from the TNM staging system, so information about cancer stage was not collected to avoid inaccuracies. We also recorded information about treatments for COVID-19 (administration of antibiotics and antivirals, oxygen therapy, and mechanical ventilation), complications, and outcomes during admission to hospital.
Definitions
Acute respiratory distress syndrome was defined according to the Berlin definition.15 Acute heart failure, acute kidney injury, septic shock, and secondary infection were defined according to previous studies.13 Leucocytosis was defined as a white blood cell count of more than 1010 cells per L. Lymphopenia was defined as a lymphocyte count of less than 109 cells per L. Coagulopathy was defined as an extension of prothrombin time for more than 3 s or of activated partial thromboplastin time for more than 5 s.16 A high neutrophil–lymphocyte ratio (NLR) was defined as a value greater than 4.
Statistical analysis
We hypothesised that differences exist in demographic, clinical, and laboratory characteristics, treatments, and cancer history between survivors and non-survivors of COVID-19 with cancer. Quantitative variables were presented as medians (IQR), and qualitative variables were presented by frequencies and percentages (only available data were calculated).
The Mann-Whitney U test, Fisher's exact test, χ2 test, and Yates' continuity correction were applied to analyse the differences between groups according to the type of data. Kaplan-Meier analysis (log-rank test) and Cox proportional hazards models were applied to analyse survival data. Hazard ratios (HRs) and 95% CIs were estimated with the Cox model. Risk factors associated with death and their odds ratios (ORs) were analysed by the univariable logistic regression model. We chose receiving chemotherapy within 4 weeks before symptom onset as the cutoff according to the number of patients within groupings and the significance of the logistic regression analysis (appendix p 1). For the multivariable logistic regression analysis, we chose four variables to avoid overfitting of the regression model because of the small number of endpoint events (n=40) in our research. Variable selection was based on significance from the univariable logistic regression analysis (p<0·05), the correlation between indicators, basic baseline clinical characteristics, and the accuracy and availability of data. Cancer stage was not chosen for the multivariable analysis as this variable was only collected in solid tumours (except for brain cancer). White blood cell count, lymphocyte count, and NLR were not chosen because they might have been influenced by chemotherapy. Other laboratory tests, including creatinine, lactate dehydrogenase, creatine kinase, D-dimer, interleukin-6, and C-reactive protein can be unavailable in emergency situations. These laboratory tests were not available for all patients in this study, and were excluded from the multivariable analysis. The time period of hospital admission was not included because some patients might have been admitted to other hospitals and been given treatment for COVID-19 (eg, antibiotics, antiviral medication, oxygen therapy) before they were transferred to the current hospital. Therefore, receiving chemotherapy within 4 weeks before symptom onset, cancer type (solid vs haematological), time since cancer diagnosis, and sex were chosen in our multivariable logistic regression model. We used generalised linear mixed-effect models with a logit link function to adjust the between-centre differences. Receiving chemotherapy 4 weeks before symptom onset, time since cancer diagnosis, cancer type (solid vs haematological), and sex were included as fixed effects, and the study centre was treated as a random effect.
We used IBM SPSS Statistics 26.0 software for statistical analysis. The tests we used were all two-sided with less than 5% type I error. The differences between groups were considered to be significant when the p value was less than 0·05.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
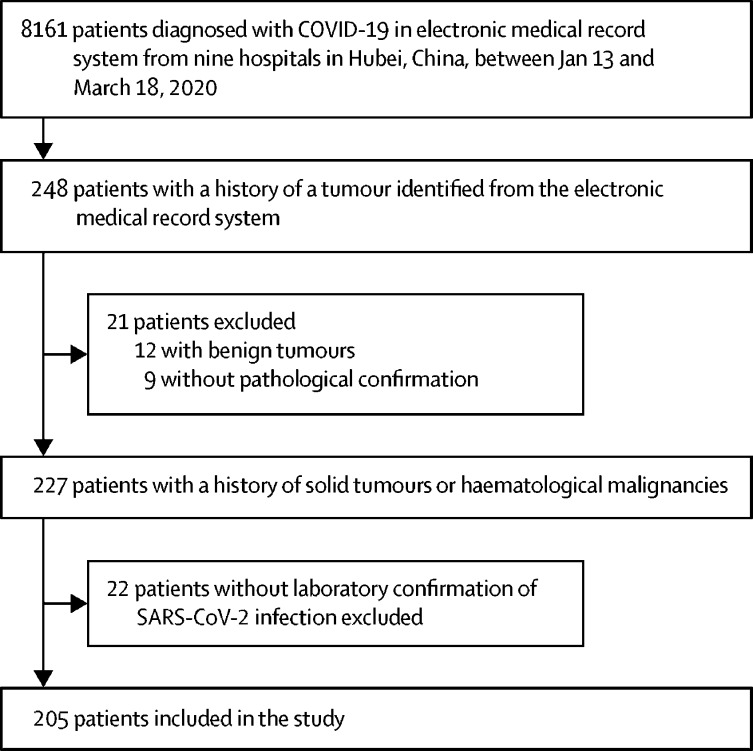
From Jan 13 to March 18, 2020, 227 patients with cancer were screened from 8161 patients with COVID-19 admitted to nine hospitals in Hubei Province. After excluding 22 patients without positive laboratory confirmation of SARS-CoV-2 infection, 205 patients were included in our study (figure ). 11 of 205 patients had positive results of IgM and IgG tests for SARS-CoV-2 infection when they were admitted to hospital. 184 (90%) of 205 patients were identified from among the 6485 patients admitted to five hospitals designated for patients with COVID-19 in the city of Wuhan. None of the 205 patients was lost to follow-up during the study. Among 8161 patients with COVID-19, 205 (2·5%) had cancer.
Figure.
Study profile
SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Of the 205 patients with cancer included (table 1 ), 40 (20%) had died as of April 20, 2020, and 34 (18%) of 184 patients in Wuhan city had died. 109 (53%) patients were female, and the median duration of follow-up for all patients was 68 days (IQR 59–78). The median age was 63 years (IQR 56–70; range 14–96), and half of patients were aged 61–80 years (table 1). The median time from symptom onset to admission was 12 days (IQR 7–19). 200 (98%) of 205 patients had symptoms during the course of COVID-19. Fever was the most common symptom, followed by cough, fatigue, and shortness of breath (table 1). 52 (25%) of 205 patients were categorised as having severe pneumonia on admission, according to the American Thoracic Society guidelines for community acquired pneumonia.17 More than half of patients had other comorbidities besides cancer, including hypertension, diabetes, coronary heart disease, and hepatitis B virus infection (table 1). Compared with survivors, non-survivors had higher respiratory rates and lower levels of blood oxygen saturation. Shortness of breath and dyspnoea were significantly more common in non-survivors than in survivors. No significant differences in age and other comorbidities were observed between survivors and non-survivors.
Table 1.
Demographics and baseline characteristics of patients with cancer and COVID-19
| All patients (n=205) | Survivors (n=165) | Non-survivors (n=40) | p value | ||
|---|---|---|---|---|---|
| Age, years | 63 (56–70) | 62 (57–69) | 63 (53–75) | 0·82 | |
| Age range, years | |||||
| ≤40 | 16 (8%) | 9 (5%) | 7 (18%) | 0·083 | |
| 41–60 | 70 (34%) | 60 (36%) | 10 (25%) | .. | |
| 61–80 | 103 (50%) | 83 (50%) | 20 (50%) | .. | |
| >80 | 16 (8%) | 13 (8%) | 3 (8%) | .. | |
| Sex | |||||
| Female | 109 (53%) | 98 (59%) | 11 (28%) | 0·0006 | |
| Male | 96 (47%) | 67 (41%) | 29 (73%) | .. | |
| Symptoms | |||||
| Fever | 159 (78%) | 126 (76%) | 33 (83%) | 0·53 | |
| Chills | 17 (8%) | 15 (9%) | 2 (5%) | 0·60 | |
| Cough | 151 (74%) | 119 (72%) | 32 (80%) | 0·42 | |
| Sputum | 70 (34%) | 51 (31%) | 19 (48%) | 0·072 | |
| Chest congestion | 67 (33%) | 49 (30%) | 18 (45%) | 0·096 | |
| Shortness of breath | 71 (35%) | 48 (29%) | 23 (58%) | 0·0014 | |
| Dyspnoea | 39 (19%) | 17 (10%) | 22 (55%) | <0·0001 | |
| Nausea or vomiting | 14 (7%) | 10 (6%) | 4 (10%) | 0·59 | |
| Diarrhoea | 24 (12%) | 21 (13%) | 3 (8%) | 0·52 | |
| Fatigue | 75 (37%) | 60 (36%) | 15 (38%) | 1·00 | |
| Median time from onset of symptoms to admission, days* | 12 (7–19) | 12 (8–20) | 10 (5–14) | 0·011 | |
| Disease severity | |||||
| Non-severe pneumonia | 153 (75%) | 148 (90%) | 5 (13%) | <0·0001 | |
| Severe pneumonia | 52 (25%) | 17 (10%) | 35 (88%) | .. | |
| Comorbidities | 106 (52%) | 90 (55%) | 16 (40%) | 0·14 | |
| Hypertension | 67 (33%) | 56 (34%) | 11 (28%) | 0·55 | |
| Diabetes | 22 (11%) | 20 (12%) | 2 (5%) | 0·31 | |
| COPD | 5 (2%) | 5 (3%) | 0 | 0·59 | |
| Coronary heart disease | 16 (8%) | 11 (7%) | 5 (13%) | 0·37 | |
| Hepatitis B virus infection | 13 (6%) | 12 (7%) | 1 (3%) | 0·45 | |
| Chronic kidney disease | 4 (2%) | 4 (2%) | 0 | 0·72 | |
| Hospital admission before Feb 13, 2020† | |||||
| Yes | 123 (60%) | 87 (53%) | 36 (90%) | <0·0001 | |
| No | 82 (40%) | 78 (47%) | 4 (10%) | .. | |
| Median temperature, °C | 36·6 (36·4–36·9) | 36·6 (36·4–36·8) | 36·7 (36·4–37·5) | 0·085 | |
| Median heart rate, beats per min | 86·0 (79·5–99·5) | 86·0 (78·0–98·5) | 88·0 (80·0–103·0) | 0·65 | |
| Median respiratory rate, breaths per min‡ | 20 (20–22) | 20 (20–22) | 23 (20–28) | <0·0001 | |
| Median SpO2, %§ | 97 (95–98) | 98 (95–98) | 91 (85–95) | <0·0001 | |
Data are median (IQR) or n (%). COPD=chronic obstructive pulmonary disease. SpO2 =blood oxygen saturation.
Data on time from onset of symptoms to admission were missing for eight patients.
Hospital admission before Feb 13, 2020, is used to represent the time period of the COVID-19 pandemic.
Data on respiratory rate were missing for one patient.
Data on SpO2 were missing for 17 patients.
Data for abnormal blood cell counts in the 203 patients for whom these data were available included 25 (12%) cases of leucocytosis, 54 (27%) of leucopenia, 32 (16%) of neutropenia, 102 (50%) of lymphocytopenia, and 25 (12%) of thrombocytopenia (table 2 ). 129 (70%) of 185 patients had elevated concentrations of D-dimer, 115 (60%) of 192 patients had elevated concentrations of C-reactive protein, and 78 (49%) of 160 patients had increased lactate dehydrogenase. All 190 patients with available radiological data showed abnormal features. 173 (91%) of 190 patients had bilateral inflammatory infiltration, and 132 (69%) presented with ground-glass opacities. Compared with survivors (table 2), non-survivors had higher NLR and higher concentrations of creatinine, blood urea nitrogen, lactate dehydrogenase, creatine kinase, D-dimer, C-reactive protein, procalcitonin, and interleukin-6, and lower lymphocyte and platelet counts, and albumin and calcium concentrations.
Table 2.
Laboratory and radiological findings of patients with cancer and COVID-19
| All patients (n=205) | Survivors (n=165) | Non-survivors (n=40) | p value | ||
|---|---|---|---|---|---|
| Laboratory findings | |||||
| White blood cells, × 109 cells per L | |||||
| Median | 5·3 (3·9–7·5) | 5·0 (3·9–6·9) | 7·4 (3·8–12·7) | 0·0045 | |
| <4 | 54/203 (27%) | 44/163 (27%) | 10/40 (25%) | <0·0001 | |
| 4–10 | 124/203 (61%) | 108/163 (66%) | 16/40 (40%) | .. | |
| >10 | 25/203 (12%) | 11/163 (7%) | 14/40 (35%) | .. | |
| Neutrophils, × 109 cells per L | |||||
| Median | 3·5 (2·5–5·5) | 3·2 (2·5–4·7) | 6·0 (2·7–11·7) | 0·0003 | |
| <2 | 32/203 (16%) | 23/163 (14%) | 9/40 (23%) | 0·29 | |
| Lymphocytes, × 109 cells per L | |||||
| Median | 0·99 (0·61–1·52) | 1·03 (0·74–1·58) | 0·58 (0·37–0·91) | <0·0001 | |
| <1 | 102/203 (50%) | 71/163 (44%) | 31/40 (78%) | 0·0002 | |
| Platelets, × 109 cells per L | |||||
| Median | 188 (134–246) | 194 (138–252) | 160 (106–213) | 0·015 | |
| <100 | 25/203 (12%) | 16/163 (10%) | 9/40 (23%) | 0·055 | |
| NLR >4 | 90/203 (44%) | 58/163 (36%) | 32/40 (80%) | <0·0001 | |
| Median haemoglobin, g/L* | 117 (105–130) | 118 (107–131) | 115 (92–126) | 0·10 | |
| Median albumin, g/L† | 33·0 (29·8–37·5) | 34·5 (30·8–38·3) | 28·5 (25·7–31·6) | <0·0001 | |
| Creatinine >133 μM | 12/205 (6%) | 6/165 (4%) | 6/40 (15%) | 0·018 | |
| Blood urea nitrogen >7·1 mM | 47/202 (23%) | 27/162 (17%) | 20/40 (50%) | <0·0001 | |
| Lactate dehydrogenase >245 U/L | 78/160 (49%) | 58/131 (44%) | 20/29 (69%) | 0·028 | |
| Creatine kinase >185 U/L | 21/168 (13%) | 12/133 (9%) | 9/35 (26%) | 0·018 | |
| D-dimer >0·5 mg/L | 129/185 (70%) | 94/149 (63%) | 35/36 (97%) | 0·0001 | |
| C-reactive protein >10 mg/L | 115/192 (60%) | 80/155 (52%) | 35/37 (95%) | <0·0001 | |
| Median procalcitonin, ng/mL‡ | 0·08 (0·05–0·14) | 0·06 (0·05–0·13) | 0·22 (0·07–2·50) | <0·0001 | |
| Median interleukin-6, pg/mL§ | 9·5 (5·8–14·4) | 8·8 (5·7–14·3) | 12·8 (10·7–15·4) | 0·022 | |
| Median calcium, mM¶ | 2·1 (2·0–2·2) | 2·1 (2·0–2·3) | 2·0 (1·9–2·1) | 0·0005 | |
| Radiological findings | |||||
| Ground-glass opacity | 132/190 (69%) | 115/155 (74%) | 17/35 (49%) | 0·0056 | |
| Patchy shadowing | 50/190 (26%) | 36/155 (23%) | 14/35 (40%) | 0·068 | |
| Bilateral infiltration | 173/190 (91%) | 138/155 (89%) | 35/35 (100%) | 0·084 | |
| Consolidation | 10/190 (5%) | 7/155 (5%) | 3/35 (9%) | 0·58 | |
Data are median (IQR) or n/N (%), where N is the number of patients with available data. NLR=neutrophil–lymphocyte ratio.
Data on haemoglobin were missing for eight patients.
Data on albumin were missing for 32 patients.
Data on procalcitonin were missing for 35 patients.
Data on interleukin-6 were missing for 82 patients.
Data on calcium were missing for 32 patients.
Of the 205 patients included, 144 (70%) received intravenous antibiotics and 192 (94%) received antiviral medications (table 3 ). Intravenous corticosteroids were given to 62 (30%) of 205 patients. Invasive mechanical ventilation was applied to 21 (66%) of 32 patients requiring mechanical ventilation. 30 (15%) of 205 patients were referred to the ICU. Complications occurred in 126 (63%) of 199 patients, including secondary infection and acute respiratory distress syndrome. Compared with survivors (table 3), non-survivors were more likely to receive intravenous medication (antibiotics, immunoglobulin, or corticosteroids), receive oxygen therapy, require mechanical ventilation, be transferred to the ICU, and develop complications such as acute respiratory distress syndrome, secondary infection, acute renal failure, and septic shock (table 3).
Table 3.
Treatments and complications of patients with cancer and COVID-19
| All patients (n=205) | Survivors (n=165) | Non-survivors (n=40) | p value | |||
|---|---|---|---|---|---|---|
| Median duration of hospital stay, days | 19 (12–33) | 20 (13–33) | 17 (6–29) | 0·032 | ||
| Treatments | ||||||
| Intravenous antibiotics | 144 (70%) | 107 (65%) | 37 (93%) | 0·0012 | ||
| Antiviral medication | 192 (94%) | 153 (93%) | 39 (98%) | 0·45 | ||
| Interferon | 71 (35%) | 48 (29%) | 23 (58%) | 0·0014 | ||
| Oseltamivir | 44 (21%) | 34 (21%) | 10 (25%) | 0·69 | ||
| Umifenovir | 145 (71%) | 116 (70%) | 29 (73%) | 0·94 | ||
| Ribavirin | 52 (25%) | 39 (24%) | 13 (33%) | 0·34 | ||
| Lopinavir plus ritonavir | 49 (24%) | 33 (20%) | 16 (40%) | 0·014 | ||
| Intravenous immunoglobin | 60 (29%) | 33 (20%) | 27 (68%) | <0·0001 | ||
| Intravenous corticosteroids | 62 (30%) | 38 (23%) | 24 (60%) | <0·0001 | ||
| Oxygen therapy | 150 (73%) | 112 (68%) | 38 (95%) | 0·0011 | ||
| Mechanical ventilation | ||||||
| None | 173 (84%) | 162 (98%) | 11 (28%) | <0·0001 | ||
| Non-invasive | 11 (5%) | 3 (2%) | 8 (20%) | .. | ||
| Invasive | 21 (10%) | 0 | 21 (53%) | .. | ||
| Admission to ICU | 30 (15%) | 4 (2%) | 26 (65%) | <0·0001 | ||
| CCRT | 5 (2%) | 1 (1%) | 4 (10%) | 0·0039 | ||
| Complications | 126/199 (63%) | 92/161 (57%) | 34/38 (89%) | 0·0004 | ||
| ARDS | 23/199 (12%) | 2/161 (1%) | 21/38 (55%) | <0·0001 | ||
| Acute renal failure | 13/199 (7%) | 1/161 (1%) | 12/38 (32%) | <0·0001 | ||
| Septic shock | 11/199 (6%) | 0 | 11/38 (29%) | <0·0001 | ||
| Abnormal liver function | 34/199 (17%) | 19/161 (12%) | 15/38 (39%) | 0·0001 | ||
| Coagulopathy | 18/199 (9%) | 5/161 (3%) | 13/38 (34%) | <0·0001 | ||
| Secondary infection | 26/199 (13%) | 5/161 (3%) | 21/38 (55%) | <0·0001 | ||
| Arrhythmia | 5/199 (3%) | 1/161 (1%) | 4/38 (11%) | 0·0034 | ||
| Other complications* | 34/199 (17%) | 13/161 (8%) | 21/38 (55%) | <0·0001 | ||
Data are median (IQR) or n/N (%), where N is the number of patients with available data. ICU=Intensive-care unit. CCRT=continuous renal replacement therapy. ARDS=acute respiratory distress syndrome.
Stroke, acute heart failure, myocardial ischaemia, electrolyte disturbance, and other complications.
38 (20%) of 192 patients had been diagnosed with cancer within the past year, and 20 (12%) of 162 patients had an ECOG score higher than 1 before admission. 183 (89%) of 205 patients were diagnosed with solid tumours (table 4 ; appendix p 2). The most common types of cancers were breast, colorectal, and lung carcinomas. Lymphoma was the most common haematological malignancy. 54 (30%) of 182 patients received antitumour therapy within 4 weeks before symptom onset, and 15 (8%) of 182 received more than one treatment, including seven (4%) of 182 who received both chemotherapy and targeted therapy. Two patients with lung cancer received chest radiotherapy and chemotherapy, and one patient with breast cancer received chest radiotherapy. 40 (27%) of 149 patients with solid tumours were at an advanced stage (stage III/IV). The case-fatality rate in patients with haematological malignancies was 41% (nine of 22 patients) and that in solid tumours was 17% (31 of 183 patients; HR 3·28 [95% CI 1·56–6·91]; log rank p=0·0009; appendix p 3). For example, two of three patients with multiple myeloma died; case-fatality rates were lower for patients with breast cancer, thyroid cancer, and cervical cancer.
Table 4.
History of cancer in patients with COVID-19
| All patients (n=205) | Survivors (n=165) | Non-survivors (n=40) | p value | ||
|---|---|---|---|---|---|
| Cancer type | |||||
| Haematological malignancy | 22 (11%) | 13 (8%) | 9 (23%) | 0·017* | |
| Lymphoma | 7 (3%) | 4 (2%) | 3 (8%) | .. | |
| Acute lymphoblastic leukaemia | 5 (2%) | 2 (1%) | 3 (8%) | .. | |
| Chronic lymphoblastic leukaemia | 4 (2%) | 4 (2%) | 0 | .. | |
| Multiple myeloma | 3 (1%) | 1 (1%) | 2 (5%) | .. | |
| Acute myelogenous leukaemia | 2 (1%) | 1 (1%) | 1 (3%) | .. | |
| Myelodysplastic syndrome | 1 (<1%) | 1 (1%) | 0 | .. | |
| Solid tumour | 183 (89%) | 152 (92%) | 31 (78%) | .. | |
| Breast | 40 (20%) | 37 (22%) | 3 (8%) | .. | |
| Colorectal | 28 (14%) | 22 (13%) | 6 (15%) | .. | |
| Lung | 24 (12%) | 18 (11%) | 6 (15%) | .. | |
| Thyroid | 16 (8%) | 15 (9%) | 1 (3%) | .. | |
| Stomach | 12 (6%) | 9 (5%) | 3 (8%) | .. | |
| Cervical | 9 (4%) | 9 (5%) | 0 | .. | |
| Other† | 54 (26%) | 42 (25%) | 12 (30%) | .. | |
| History of treatments | |||||
| Surgery | 140/182 (77%) | 121/148 (82%) | 19/34 (56%) | 0·0027 | |
| Chemotherapy | 104/182 (57%) | 76/148 (51%) | 28/34 (82%) | 0·0019 | |
| Radiotherapy | 37/182 (20%) | 30/148 (20%) | 7/34 (21%) | 1·00 | |
| Targeted therapy | 18/182 (10%) | 10/148 (7%) | 8/34 (24%) | 0·0084 | |
| Immunotherapy | 7/182 (4%) | 4/148 (3%) | 3/34 (9%) | 0·24 | |
| Treatments within 4 weeks before symptom onset | |||||
| All | 54/182 (30%) | 36/148 (24%) | 18/34 (53%) | 0·0020 | |
| Surgery | 4/182 (2%) | 4/148 (3%) | 0 | 0·75 | |
| Chemotherapy | 31/182 (17%) | 16/148 (11%) | 15/34 (44%) | <0·0001 | |
| Radiotherapy | 9/182 (5%) | 6/148 (4%) | 3/34 (9%) | 0·47 | |
| Targeted therapy | 12/182 (7%) | 6/148 (4%) | 6/34 (18%) | 0·013 | |
| Immunotherapy | 4/182 (2%) | 2/148 (1%) | 2/34 (6%) | 0·33 | |
| Others‡ | 10/182 (5%) | 10/148 (7%) | 0 | 0·25 | |
| Cancer stage | |||||
| I–II | 109/149 (73%) | 98/127 (77%) | 11/22 (50%) | 0·017 | |
| III–IV | 40/149 (27%) | 29/127 (23%) | 11/22 (50%) | .. | |
| Time since cancer diagnosis | |||||
| <1 years | 38/192 (20%) | 24/156 (15%) | 14/36 (39%) | 0·0006 | |
| 1–5 years | 75/192 (39%) | 59/156 (38%) | 16/36 (44%) | .. | |
| >5 years | 79/192 (41%) | 73/156 (47%) | 6/36 (17%) | .. | |
| ECOG performance status score | |||||
| 0 | 101/162 (62%) | 85/132 (64%) | 16/30 (53%) | 0·36 | |
| 1 | 41/162 (25%) | 33/132 (25%) | 8/30 (27%) | .. | |
| 2 | 12/162 (7%) | 9/132 (7%) | 3/30 (10%) | .. | |
| 3 | 7/162 (4%) | 4/132 (3%) | 3/30 (10%) | .. | |
| 4 | 1/162 (1%) | 1/132 (1%) | 0 | .. | |
Data are n (%) or n/N (%), where N is the number of patients with available data. ECOG=Eastern Cooperative Oncology Group.
χ2 test for haematological malignancy and solid tumour.
Other cancer types included liver, ovarian, prostate, and other cancers.
Other therapies included endocrinotherapy and traditional Chinese medicine.
We did a comparative analysis of clinical characteristics, treatments, laboratory data, and radiological data from patients with solid tumours and those with haematological malignancies (appendix pp 4–6). Patients with haematological malignancies were younger than those with solid tumours (median age 55 years [IQR 26–62] vs 64 years [57–70]), and most (15 [68%] of 22) were male. Acute respiratory distress syndrome (six [27%] of 22 patients vs 17 [10%] of 177 patients) and coagulopathy (five [23%] of 22 patients vs 13 [7%] of 177 patients) were more frequently seen in patients with haematological malignancies than in those with solid tumours. 14 (70%) of 20 patients with haematological malignancies received antitumour treatments in the past 4 weeks before symptom onset, compared with 40 (25%) of 162 patients with solid tumours. 11 (55%) of 20 patients with haematological malignancies and 20 (12%) of 162 patients with solid tumours received chemotherapy within 4 weeks before symptom onset.
In univariable logistic regression analysis, cancer type, time since cancer diagnosis, cancer stage, male sex, leucocytosis, lymphopenia, high NLR, thrombocytopenia, lactate dehydrogenase, D-dimer, creatine kinase, C-reactive protein, creatinine, receipt of chemotherapy or targeted therapy within 4 weeks before symptom onset, and hospital admission were associated with death (table 5 ). We included 180 patients with complete data in the multivariable regression analysis (146 survivors and 34 non-survivors), in which we included four variables: sex, cancer type, receipt of chemotherapy within the previous 4 weeks, and time since cancer diagnosis. There was no interaction effect between the selected variables (appendix p 7). Receiving chemotherapy within 4 weeks before symptom onset (OR 3·51 [95% CI 1·16–10·59]; p=0·026) and male sex (3·86 [1·57–9·50]; p=0·0033) were associated with increased odds of death during admission to hospital (table 5). Similar results were shown after adjusting for study centre or different cancer types (appendix p 8).
Table 5.
Bivariate logistic regression analysis of factors associated with death during admission to hospital
| Univariable OR (95% CI) | p value | Multivariable OR (95% CI) | p value | ||
|---|---|---|---|---|---|
| Age, years | 0·99 (0·97–1·01) | 0·44 | .. | .. | |
| Male sex (vs female) | 3·86 (1·80–8·25) | 0·0005 | 3·86 (1·57–9·50) | 0·0033 | |
| Comorbidity | 0·56 (0·28–1·12) | 0·10 | .. | .. | |
| White blood cell count, × 109 cells per L | |||||
| <4 | 1·53 (0·65–3·64) | 0·33 | .. | .. | |
| 4–10 | 1 (ref) | .. | .. | .. | |
| >10 | 8·59 (3·33–22·18) | <0·0001 | .. | .. | |
| Lymphocytes, × 109 cells per L | |||||
| ≥1 | 1 (ref) | .. | .. | .. | |
| <1 | 4·46 (2·00–9·97) | 0·0003 | .. | .. | |
| NLR | |||||
| ≤4 | 1 (ref) | .. | .. | .. | |
| >4 | 7·24 (3·13–16·75) | <0·0001 | .. | .. | |
| Platelets, × 109 cells per L | |||||
| ≥100 | 1 (ref) | .. | .. | .. | |
| <100 | 2·67 (1·08–6·59) | 0·033 | .. | .. | |
| Creatinine, μmol/L | |||||
| ≤133 | 1 (ref) | .. | .. | .. | |
| >133 | 4·68 (1·42–15·38) | 0·011 | .. | .. | |
| Lactate dehydrogenase, U/L | |||||
| ≤245 | 1 (ref) | .. | .. | .. | |
| >245 | 2·80 (1·18–6·60) | 0·019 | .. | .. | |
| Creatine kinase, U/L | |||||
| ≤185 | 1 (ref) | .. | .. | .. | |
| >185 | 3·49 (1·33–9·14) | 0·011 | .. | .. | |
| D-dimer, μg/L | |||||
| ≤0·5 | 1 (ref) | .. | .. | .. | |
| >0·5 | 20·48 (2·73–153·67) | 0·0033 | .. | .. | |
| C-reactive protein, mg/L | |||||
| ≤10 | 1 (ref) | .. | .. | .. | |
| >10 | 16·41 (3·81–70·60) | 0·0002 | .. | .. | |
| Interleukin-6, pg/mL* | 1·00 (0·98–1·01) | 0·52 | .. | .. | |
| Receiving chemotherapy (vs not receiving)† | 6·51 (2·78–15·28) | <0·0001 | 3·51 (1·16–10·59) | 0·026 | |
| Receiving targeted therapy (vs not receiving)† | 5·07 (1·52–16·87) | 0·0081 | .. | .. | |
| Radiotherapy (vs not receiving)† | 2·29 (0·54–9·66) | 0·26 | .. | .. | |
| Cancer stage | |||||
| I–II | 1 (ref) | .. | .. | .. | |
| III–IV | 3·38 (1·33–8·59) | 0·011 | .. | .. | |
| Time since cancer diagnosis | |||||
| <1 year | 7·10 (2·45–20·52) | 0·0003 | 2·89 (0·78–10·76) | 0·11 | |
| 1–5 years | 3·30 (1·21–8·96) | 0·019 | 1·85 (0·63–5·42) | 0·26 | |
| >5 years | 1 (ref) | .. | 1 (ref) | .. | |
| Cancer type | |||||
| Solid tumour | 1 (ref) | .. | 1 (ref) | .. | |
| Haematological malignancy | 3·39 (1·33–8·63) | 0·010 | 2·07 (0·68–6·35) | 0·20 | |
| ECOG score | |||||
| 0 | 1 (ref) | .. | .. | .. | |
| 1 | 1·29 (0·50–3·29) | 0·60 | .. | .. | |
| 2 | 1·77 (0·43–7·26) | 0·43 | .. | .. | |
| 3 | 3·98 (0·81–19·53) | 0·088 | .. | .. | |
| 4 | .. | 1·00 | .. | .. | |
| Hospital admission before Feb 13, 2020‡ | |||||
| Yes | 8·07 (2·75–23·70) | 0·0001 | .. | .. | |
| No | 1 (ref) | .. | .. | .. | |
OR=odds ratio. NLR=neutrophil–lymphocyte ratio. ECOG=Eastern Cooperative Oncology Group.
For each additional unit.
Receiving treatment within 4 weeks before symptom onset.
Hospital admission before Feb 13, 2020, is used as a factor to represent the time period of the COVID-19 pandemic.
Discussion
Patients with cancer are a vulnerable population in the ongoing COVID-19 pandemic. They are at high risk of infection and have a higher probability of severe illness and increased mortality once diagnosed with COVID-19. To our knowledge, this is the first multicentre, retrospective, cohort study to describe the clinical features, outcomes, and risk factors for mortality in patients with cancer and diagnosed with COVID-19. Severe pneumonia occurred in 52 (25%) patients and the in-hospital case-fatality rate in patients with COVID-19 and cancer was 20%, which is much higher than the case-fatality rate for COVID-19 in the overall Chinese population (1%).13 In Wuhan, the case-fatality rate of patients with cancer in our study was 18% (34 of 184 patients), which was higher than the overall case-fatality rate reported for patients with COVID-19 (8%).5 In particular, male sex and receiving chemotherapy within 4 weeks before symptom onset were identified as risk factors for death in patients with cancer who were diagnosed with COVID-19.
The proportion of patients with cancer among those with COVID-19 who were admitted to the nine hospitals in our study was 2·5%, which was higher than that reported in the overall Chinese population (0·29%)18 and in a previous report of patients with COVID-19 (1%).10 This finding suggests that patients with cancer are more susceptible to COVID-19 than the general population. By contrast with four other human coronaviruses (HCoV-NL63, HCoV-229E, HCoV-OC43, and HKU1), which induce only mild upper respiratory diseases,19 SARS-CoV-2 behaves like SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV) to some extent, and leads to higher rates of severe respiratory syndrome.20 Similar to our study, fever and cough were the most common clinical manifestations among patients with COVID-19.16 Although older age and underlying diseases have been found to be risk factors for severe events in a previous study,10 this was not observed in our study. This difference might be due to the fact that our study already comprised an elderly population (median age 63 years) with underlying diseases. We found bilateral lung lesions in 91% of patients with available records, which was higher than the figure reported previously by Xu and colleagues (bilateral lung lesions in 53 [59%] of 90 patients with laboratory-confirmed SARS-CoV-2 infection),21 suggesting that patients with cancer were more vulnerable once they became infected with SARS-CoV-2.
Men were found to be at a higher risk of mortality than women in this study. In addition to sex differences in smoking rate,22 differences in the immune and endocrine systems between men and women23, 24 might exert different responses against SARS-CoV-2 infection. Moreover, case-fatality rates for patients with COVID-19 who had breast, thyroid, or cervical cancer were low in our study. 62 (57%) of 109 women in our study had one of these three types of cancers.
Lymphocytopenia is one of the clinical features of COVID-19,4 indicating that the virus tends to diminish the antiviral immunity of the host. Similar to other studies,10 we found that cytotoxic chemotherapy within 4 weeks before symptom onset was associated with increased risk of mortality. Patients receiving chemotherapy might develop long-lasting myelosuppression and impaired immunity. Since we do not yet have highly effective drugs targeting SARS-CoV-2, a patient's inherent immunity might be a determining factor for their prognosis after effective supportive care. It has been recommended that the mode of administration (from infusion to oral administration) and intervals of chemotherapy should be adjusted according to patients' conditions.25 Although molecular-targeted therapy rarely impairs patients' immunity, those receiving maintenance molecular-targeted therapy all had advanced disease, and seven (58%) of 12 received chemotherapy concurrently within 4 weeks before symptom onset, which might have accounted for the increased risk of death. Immunosuppressive treatments administered more than 4 weeks before symptom onset might not worsen the outcome of COVID-19, which can be partially explained by the recovery of patients from side-effects of cytotoxic treatments. Because of the small number of patients in our study who received chest radiotherapy 4 weeks before onset of COVID-19, we were not able to analyse the effect of recent chest radiotherapy on patient outcomes.
Many haematological malignancies change how blood cells in the immune system function. Lower respiratory tract diseases caused by human coronaviruses in patients with haematological malignancies have been associated with high rates of oxygen use and mortality.26 In our study, patients with haematological malignancies had poorer prognoses than those with solid tumours. Besides the inherent differences between haematological malignancies and solid tumours, more patients with haematological malignancies received chemotherapy within 4 weeks before symptom onset (11 [55%] of 20 vs 20 [12%] of 162), which might partly explain our finding of worse outcomes in these patients.
In addition to lymphocytes, neutrophils are the mainstay in fighting off various infections. NLR is considered to reflect host inflammation and is a predictor of bacterial infection.27 It has also been found to be associated with clinical outcome and treatment efficacy in several cancers.28 In patients with COVID-19, high neutrophil counts have frequently been seen in refractory disease.29 In line with a previous study,30 we found a high NLR to be associated with poor prognosis in patients with cancer and COVID-19. SARS-CoV-2 infection and subsequent bacterial infection might have caused a deterioration of lung function and contributed to death, although this hypothesis requires further investigation.
Our study had some limitations. The sample size was not large enough to derive any firm conclusions. Incomplete documentation and recall bias of cancer history added to the complexity of the study. We were not able to analyse the effect of treatment with immunosuppressants on the outcome of patients with cancer and COVID-19 because this information was not collected. Additionally, we did not compare the case-fatality rate, characteristics, outcomes, and treatment strategies of patients with cancer against a control group of patients without cancer. Dynamic changes in the titre of the IgM and IgG antibodies, SARS-CoV-2 nucleic acid, and other laboratory tests such as tumour-related cytokines besides interleukin-6 (eg, tumour necrosis factor-α and interferon-γ) in the course of disease should be further recorded and analysed. Finally, the impact of COVID-19 on cancer needs to be evaluated with long-term follow-up of survivors.
In conclusion, patients with cancer and COVID-19 require urgent and special attention, since they are a vulnerable population with a much higher case-fatality rate than the general population. Receiving chemotherapy 4 weeks before symptom onset and male sex are two indicators that might help clinicians to identify patients with cancer who are at high risk of fatal outcomes at an early stage.
Acknowledgments
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC 81874218, NSFC 81672978). We thank all front-line health-care workers for their efforts during the COVID-19 pandemic.
Contributors
GW and KY had the idea for and designed the study and provided financial support. JLiu, JY, YD, DP, CS, JLi, JW, YH, LP, MW, and YL were involved in acquisition of the data. KY and YS summarised the data. CH, YJ, NX, KJ, HL, RZ, BW, LC, GL, and TZ were involved in data interpretation. YS drafted the manuscript. GW, KY, BW, CH, YJ, NX, KJ, and HL critically revised the manuscript for important intellectual content.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Holshue ML, DeBolt C, Lindquist S. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothe C, Schunk M, Sothmann P. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO Coronavirus disease (COVID-19) pandemic. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 4.Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinese Center for Disease Control and Prevention Epidemic situation of COVID-19 (in Chinese) March 26, 2020. http://www.chinacdc.cn/jkzt/crb/zl/szkb_11803/jszl_11809/202003/t20200326_215500.html
- 6.Bow EJ. There should be no ESKAPE for febrile neutropenic cancer patients: the dearth of effective antibacterial drugs threatens anticancer efficacy. J Antimicrob Chemother. 2013;68:492–495. doi: 10.1093/jac/dks512. [DOI] [PubMed] [Google Scholar]
- 7.Azoulay E, Mokart D, Lambert J. Diagnostic strategy for hematology and oncology patients with acute respiratory failure: randomized controlled trial. Am J Respir Crit Care Med. 2010;182:1038–1046. doi: 10.1164/rccm.201001-0018OC. [DOI] [PubMed] [Google Scholar]
- 8.Morrison VA. Infectious complications in patients with chronic lymphocytic leukemia: pathogenesis, spectrum of infection, and approaches to prophylaxis. Clin Lymphoma Myeloma. 2009;9:365–370. doi: 10.3816/CLM.2009.n.071. [DOI] [PubMed] [Google Scholar]
- 9.Azoulay E, Lemiale V, Mokart D. Acute respiratory distress syndrome in patients with malignancies. Intensive Care Med. 2014;40:1106–1114. doi: 10.1007/s00134-014-3354-0. [DOI] [PubMed] [Google Scholar]
- 10.Liang W, Guan W, Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, Zhu F, Xie L. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.03.296. published online March 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. Jan 11, 2020. https://www.who.int/internalpublications-detail/clinical-management-of-severe-acute-respiratoryinfection-when-novel-coronavirus-(ncov)-infection-is-suspected
- 13.Guan WJ, Ni ZY, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Severe Acute Respiratory and emerging Infection Consortium COVID-19 clinical research resources. https://isaric.tghn.org/
- 15.Ranieri VM, Rubenfeld GD, Thompson BT. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 16.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metlay JP, Waterer GW, Long AC. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng RS, Sun KX, Zhang SW. Report of cancer epidemiology in China, 2015. Zhonghua Zhong Liu Za Zhi. 2019;41:19–28. doi: 10.3760/cma.j.issn.0253-3766.2019.01.005. (in Chinese). [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Zhao S, Teng T. Systematic comparison of two animal-to-human transmitted human coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses. 2020;12:E244. doi: 10.3390/v12020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen N, Zhou M, Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu X, Yu C, Qu J. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020;47:1275–1280. doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Z, Peto R, Zhou M. Contrasting male and female trends in tobacco-attributed mortality in China: evidence from successive nationwide prospective cohort studies. Lancet. 2015;386:1447–1456. doi: 10.1016/S0140-6736(15)00340-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giefing-Kröll C, Berger P, Lepperdinger G, Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14:309–321. doi: 10.1111/acel.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Wang J, He J. Active and effective measures for the care of patients with cancer during the COVID-19 spread in China. JAMA Oncol. 2020 doi: 10.1001/jamaoncol.2020.1198. published online April 1. [DOI] [PubMed] [Google Scholar]
- 26.Ogimi C, Waghmare AA, Kuypers JM. Clinical significance of human coronavirus in bronchoalveolar lavage samples from hematopoietic cell transplant recipients and patients with hematologic malignancies. Clin Infect Dis. 2017;64:1532–1539. doi: 10.1093/cid/cix160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Shen Y, Wang H, Ge Q, Fei A, Pan S. Prognostic significance of neutrophil-to-lymphocyte ratio in patients with sepsis: a prospective observational study. Mediators Inflamm. 2016;2016 doi: 10.1155/2016/8191254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Templeton AJ, McNamara MG, Seruga B. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 29.Mo P, Xing Y, Xiao Y. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa270. published online March 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin C, Zhou L, Hu Z. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa248. published online March 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.