Abstract
Purpose of review:
(Pro)renin receptor (PRR) belongs to type I transmembrane receptor family and binds both prorenin and renin, representing a potential regulator of the activity of the renin-angiotensin system (RAS). Soluble form of PRR (sPRR) is generated by intracellular protease mediated-cleavage of full-length PRR. The purpose of this review is to highlight recent advances in understanding the mechanisms of action and production of sPRR.
Recent findings:
It has recently been demonstrated that site-1-protease (S1P) plays a dominant role in the generation of sPRR. New evidence is also emerging to support a biological function of sPRR in the physiological regulation of fluid homeostasis as well as pathogenesis of chronic kidney disease (CKD).
Summary:
sPRR is a 28 kDa product of PRR cleavage via S1P-mediated protease activity. Not only does sPRR regulate renal tubular water transport but it also mediates pathogenic responses to renal cellular injury. sPRR is likely involved in a wide range of physio-pathological processes.
Keywords: Site-1 protease, soluble (pro)renin receptor, renin-angiotensin system, collecting duct, blood pressure
Introduction
(Pro)renin receptor (PRR), first cloned by Nguyen et al. [1], is a multi-functional molecule that is related to both the renin-angiotensin system (RAS) and vacuolar H⁺-ATPase (v-ATPase), an ATP-dependent multi-subunit proton pump. PRR is a transmembrane protein composed of a large N-terminal extracellular domain, a single transmembrane protein, and a short cytoplasmic domain[2]. In recent years, PRR has been shown to be fundamentally important not only for the development[3] and homeostasis of many organs and tissues[4–8], but also for the pathogenesis of a broad range of diseases including hypertension and CKD[6,8–13].
Soluble form of PRR (sPRR), which consists of the extracellular domain of PRR, is generated by intracellular protease-mediated cleavage of full-length PRR [14]. This process produces three different molecular forms including a full-length PRR, sPRR, and the truncated form composed of the transmembrane and cytoplasmic domains M8.9[14]. However, the cleavage mechanism still remains elusive. sPRR can bind with renin or prorenin to activate the tissue RAS[15] and is also detective in blood and urine[16]. Accumulating evidence shows that sPRR may play a role under various physio-pathological processes. Elevated circulating sPRR has been associated with early pregnancy[17,18], preeclampsia[19,20], gestational diabetes mellitus[17,21], renal dysfunction in patients with heart failure[22], obstructive sleep apnea syndrome[23–25], and CKD due to hypertension and type 2 diabetes[26]. The major objective of this article is to review recent findings regarding the generation mechanism of sPRR and its possible role in renal physiology and pathophysiology.
Generation of sPRR
Furin was first reported to catalyze the production of sPRR in the trans-Golgi.[14] Furin, also known as proprotein convertase, is a key regulator in the activation of the functionally important protein precursors[27,28]. Furin recognizes the full-length PRR with an intact linear amino-acid sequence of R275-K-T-R278 and mutagenesis in this conserved sequence abolishes sPRR production, which suggests that furin is importantly involved in the generation of sPRR[14]. This furin recognizing sequence is conserved among species, which is consistent with the preferential cleavage sequence of R-X-(K/R)-R (where X stands for any amino acid). This study demonstrates an important role of furin in the biogenesis of sPRR.
ADAM19 represents a second cleavage enzyme following furin[29]. It’s been shown that a disintegrin and metalloproteinase 19 (ADAM19) catalyzes the production of sPRR in the Golgi given the observations that transfection of ADAM19 evokes the shedding of PRR, whereas transfection of dominant negative ADAM19 suppresses it[29]. It’s puzzling however that neither furin-deficiency nor furin inhibitor-treatment has any effect on the secretion of sPRR into the media. ADAM19 is a transmembrane metalloprotease functioning in protein ectodomain shedding while its consensus cleavage sequence remains to be determined[29]. These results suggest that ADAM19, rather than furin, mediates the cleavage of PRR although PRR contains a furin cleavage site[29].
Although sPRR is shown to be generated from intracellular cleavage of full-length PRR, our knowledge about the cleavage mechanism is still limited and the detailed contribution of furin versus ADAM19 in the production of sPRR remains to be further explored.
Role of Site-1-protease (S1P) in the generation of sPRR
Previous studies reported that the predicted cleavage sequences of full-length PRR are inconsistent with the proposed furin cleavage site and appear more identical to the consensus sequence recognized by site-1-protease (S1P) [30–33]. A recent study has demonstrated that S1P is required for the generation of sPRR by cleaving full-length PRR[34]. The production of sPRR in Chinese hamster ovary (CHO) cells expressing human PRR is attenuated by inhibiting endogenous S1P level through siRNA and enhanced by overexpression of S1P. The inhibitor of S1P, but not furin and ADAM suppresses sPRR generation in CHO and HeLa cells. These results suggest that S1P is indeed responsible for the generation of sPRR. This study also shows that furin inhibitor produces a large-sized sPRR without affecting the amount of sPRR formation. The large-sized sPRR is increased by the reversible vesicle-trafficking inhibitor brefeldin A (BFA). Moreover, the inhibitor of S1P, not furin suppressed this BFA-induced sPRR production and the size of sPRR generated during BFA treatment is reduced after removal of BFA. The inhibitor of furin, but not ADAM, suppressed this conversion. It is suggested that sPRR may be generated by sequential processing by S1P and furin[34].
A similar discovery concerning S1P as a major PRR cleavage protease was made in our recent study albeit with a different approach[35]. Our study was aimed at determining the role of sPRR in albumin overload-induced responses in cultured human renal proximal tubular cells. We found that bovine serum albumin (BSA) treatment in these cells significantly increased sPRR production due to enhanced cleavage of PRR which was unaffected by inhibition of furin or ADAM19. This result is consistent with previous reports[29,34]. Sequential protease screening leads to the identification of S1P as a dominant protease responsible for BSA-induced sPRR production. In this regard, inhibition of S1P with PF-429242 or siRNA remarkably suppresses sPRR production induced by BSA. Moreover, mutagenesis in cleavage site of the S1P, not furin decreases sPRR production in cultured renal cells overexpressing PRR. These results suggest that S1P plays a dominant role in the generation of sPRR and this S1P-derived sPRR mediates albumin-induced cellular responses[35]. However, our results show that furin inhibition has no effect on the size shift of sPRR as reported by Nagagawa et al. [34]. This discrepancy may be due to the different experiment settings or the distinct roles of different enzymes in the generation of sPRR. Despite of this discrepancy, it is important to note that the two groups independently reach a similar conclusion about the essential role of S1P in the generation of sPRR (Fig. 1).
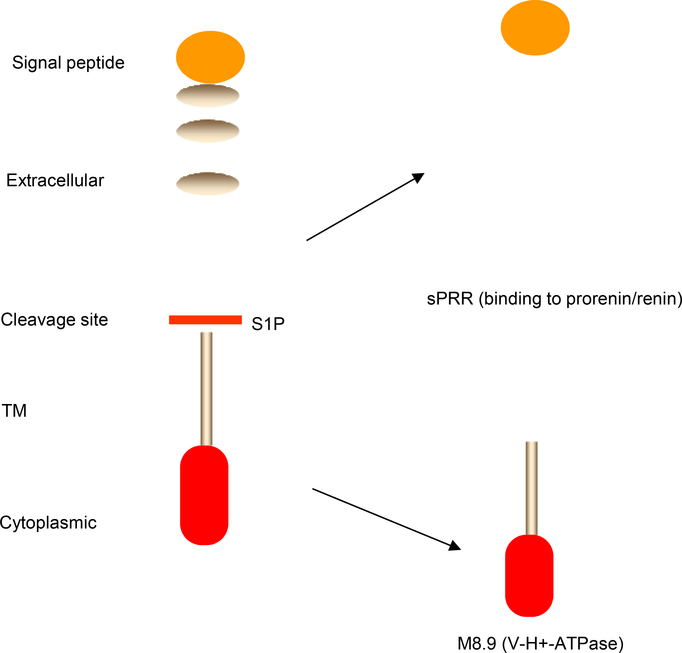
Fig. 1.
Schematic illustration of S1P-mediated cleavage of PRR. The full length PRR is cleaved by S1P at the indicated cleavage site. Following the cleavage, the resulting two fragments, sPRR and M8.9, are generated.
Structure and function of S1P
S1P is formed in the endoplasmic reticulum (ER) as an inactive 1052-amino acid precursor protein consisting of a N-terminal signal peptide, a prodomain, and a catalytic domain[28], which belongs to the proprotein convertase family of nine calcium-dependent serine proteases[28],35,36. Once formed, S1P is translocated into the ER where the signal peptide is removed and the prodomain is autocatalytically cleaved after arrival in the cis-Golgi apparatus leading to the activation of S1P[36].
S1P has been shown to play crucial roles in various cellular processes including lipid biogenesis39,40, ER stress response[37] and lysosome homeostasis[38,39]. In particular, S1P targets the sterol regulatory element binding proteins (SREBPs) and low density lipoprotein receptor to regulate lipid metabolism as highlighted by reduced circulating- and liver-triglyceride along with plasma cholesterol in mice with liver-specific deletion of S1P39,40. Indeed, PRR is involved in lipid metabolism as suggested by the observation that serum sPRR is significantly correlated with lipid dysregulation in patients with essential hypertension[40]. Studies showed that S1P is also important for cartilage development in vertebrates[41–43] since cartilage-specific S1P ablation results in abnormal cartilage in mice[44]. It has been demonstrated that S1P-deficiency leads to embryonic lethality in mice[45], a phenotype similar to that caused by PRR deletion[3]. These results support an intrinsic link between S1P and PRR[34,35]. Given the potential roles of the PRR/sPRR in various physio-pathological processes [9], future research efforts are highly demanded to clarify the contributions of S1P to these conditions dependent or independent of sPRR.
Role of sPRR in regulation of fluid homeostasis
sPRR is generated from protease cleavage of PRR which abundantly expressed in the intercalated cells of the collecting duct (CD) within the kidney[46,47]. sPRR is detected in blood and urine[16]. Although serum sPRR correlated positively with urinary angiotensinogen and negatively with an estimated glomerular filtration rate in essential hypertension and normotensive subjects[40], little is known for a biological function of sPRR under any condition.
Previous work reveals a functional role of PRR in regulating renal aquaporin 2 (AQP2) expression and urine concentrating capability as highlighted by the DI phenotype of mice with conditional deletion of PRR [12,47]. Our lab has recently defined the antidiuretic action of sPRR and its potential interaction with liver X receptors (LXRs), which are known regulators of urine concentrating capability[48]. Our results show that water deprivation consistently elevates urinary sPRR excretion in mice and humans. Recombinant sPRR (sPRR-His) remarkably increases the abundance of renal AQP2 expression in primary rat inner medullary collecting duct cells, indicating antidiuretic property of sPRR.
Our recent data have further shown that sPRR interacts with frizzled-8 (FZD8). It’s known that the AQP2 up-regulation relies on sequential activation of β-catenin signaling and cAMP/PKA pathways. Inhibition of FZD8 or tankyrase in rats induces polyuria, polydipsia, and hyperosmotic urine and administration of sPRR-His alleviates the symptoms of diabetes insipidus (DI) induced in mice by vasopressin type 2 receptor (V2R) antagonism. Moreover, administration of the LXR agonist to mice induces polyuria and suppressed renal AQP2 expression associated with reduced renal PRR expression and urinary sPRR excretion. Administration of sPRR-His reverses most of the effects of LXR agonist. In cultured collecting duct cells, LXR agonist suppressed PRR protein expression, sPRR release, and PRR transcriptional activity, suggesting a physiological function of sPRR.
Taken together, our results demonstrate that sPRR exerts antidiuretic action via FZD8-dependent stimulation of AQP2 expression and that inhibition of this pathway contributes to the pathogenesis of DI induced by LXR agonism. Given the fact that intercalated cells are the potential source of sPRR, and principal cells are the site of its action, it seems reasonable to speculate that sPRR mediates the communication between the two cell types in the CD while the mechanism remains to be further investigated. Additionally, S1P has been demonstrated to be a dominant enzyme for the generation of sPRR[34,35], more rigorous investigation on the possible roles of S1P in the regulation of renal function is needed in the future.
Role of sPRR in pathogenesis of chronic kidney disease (CKD)
sPRR correlated positively with urinary angiotensinogen[40] and serum sPRR is inversely associated with the estimated glomerular filtration rate in patients with CKD caused by hypertension and diabetes[26]. Moreover, increased renal PRR expression contributes to the elevation of sPRR in human kidneys with end-stage renal disease[49]. These findings suggest that sPRR might serve as a biomarker reflecting the intrarenal RAS status[50].
Proteinuria is a hallmark of CKD and a causative factor promoting the disease progression partially through activation of intrarenal RAS. Protein droplets seen in the cytoplasm of proximal tubular cells are associated with acceleration of tubulointerstitial injury via pro-inflammatory response[51,52] and oxidative stress[53], which in turn leads to excretion of chemokines and cytokines, resulting in inflammation, transformation of interstitial fibroblasts, and fibrosis[54]. Our most recent study suggested a potential role of sPRR in albumin-induced cellular responses in cultured human renal proximal tubular epithelial cells[35]. Our results show that albumin overload induces a significant increase in sPRR production along with elevated renin activity and inflammation, which can be attenuated by a PRR decoy inhibitor PRO20. Inhibition of sPRR generation by S1P inhibition suppresses the inflammatory and fibrotic responses induced by albumin overloading. Our data suggest that S1P-derived sPRR mediates the deleterious effect of albumin overload in the renal epithelial cells. Future studies are needed to test the pathogenic role of sPRR in animal models of albumin overload nephropathy as well as other types of CKD. Intervention of sPRR production or activity may offer a novel therapy for treatment of this devastating disease.
Because the level changes of sPRR in renal disease are not striking and not offering a set cut-off line for clinical use[55], the significance of sPRR in certain pathophysiological circumstance still remains elusive. Although there is no doubt that sPRR may play an important role in the regulation of renal injury and CKD, reports on this topic are currently very limited. Apparently, further investigations are expected to determine the relationships between kidney disease and sPRR, PRR, and S1P.
Conclusion
Since the first cloning of PRR more than a decade ago, it has been long recognized that this protein is cleaved by a protease to generate sPRR that is detected in plasma and urine. A large number of clinical studies have shown that circulating sPRR is elevated in patients under various physio-pathological conditions, suggesting its values as disease biomarker. With the first discovery of sPRR as a novel regulator of CD water transport, we have begun to understand its potential physio-pathological functions. Moreover, following the controversial reports on furin and ADAM19 as the PRR cleavage enzymes, solid evidence has emerged to support S1P as the dominant source of sPRR Figure. 2. These results have offered a new platform for future investigations of the biological functions of S1P-derived endogenous sPRR.
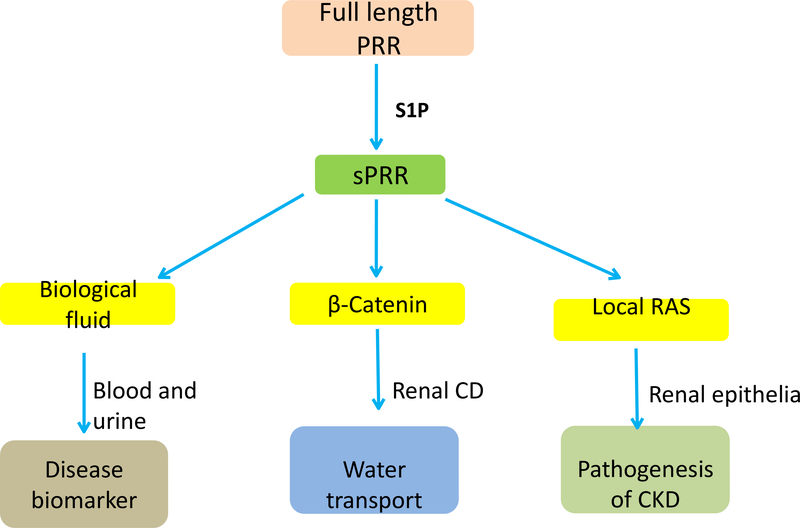
Fig. 2.
Schematic illustration of the functions of sPRR. S1P-derived sPRR is released to biological fluids including blood and urine under various physio-pathological conditions. Within the kidney, sPRR exerts biological functions in regulation of water transport via β-catenin signaling as well as in pathogenesis of CKD likely through activation of local RAS.
Key Points:
Site-1 protease is a predominant source of sPRR.
sPRR exerts antidiuretic action by enhancing renal AQP2 expression.
sPRR mediates renal epithelial cell injury during in vitro albumin overload.
Acknowledgements
None.
Financial support and sponsorship
This work was supported by National Institutes of Health Grants HL13585, DK104072, DK094956, and VA Merit Review from the Department of Veterans Affairs, and National Natural Science Foundation of China Grants (No. 91439205, 31330037, 81630013 and 81770707), and Fundamental Research Funds of Sun Yat-sen University (16YKPY47). T. Yang is Research Career Scientist in the Department of Veterans Affairs.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1. **.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD: Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 2002, 109:1417–1427. This is the first report describing the cloning of (pro)renin receptor in mesangial cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burckle C, Bader M: Prorenin and its ancient receptor. Hypertension 2006, 48:549–551. [DOI] [PubMed] [Google Scholar]
- 3.Sihn G, Rousselle A, Vilianovitch L, Burckle C, Bader M: Physiology of the (pro)renin receptor: Wnt of change? Kidney Int 2010, 78:246–256. [DOI] [PubMed] [Google Scholar]
- 4.Matavelli LC, Huang J, Siragy HM: In vivo regulation of renal expression of (pro)renin receptor by a low-sodium diet. Am J Physiol Renal Physiol 2012, 303:F1652–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang J, Siragy HM: Sodium depletion enhances renal expression of (pro)renin receptor via cyclic GMP-protein kinase G signaling pathway. Hypertension 2012, 59:317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang T: Crosstalk between (Pro)renin receptor and COX-2 in the renal medulla during angiotensin II-induced hypertension. Curr Opin Pharmacol 2015, 21:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trepiccione F, Gerber SD, Grahammer F, Lopez-Cayuqueo KI, Baudrie V, Paunescu TG, Capen DE, Picard N, Alexander RT, Huber TB, et al. : Renal Atp6ap2/(Pro)renin Receptor Is Required for Normal Vacuolar H+-ATPase Function but Not for the Renin-Angiotensin System. J Am Soc Nephrol 2016, 27:3320–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen G: Renin and prorenin receptor in hypertension: what’s new? Curr Hypertens Rep 2011, 13:79–85. [DOI] [PubMed] [Google Scholar]
- 9. *.Yang T, Xu C: Physiology and Pathophysiology of the Intrarenal Renin-Angiotensin System: An Update. J Am Soc Nephrol 2017, 28:1040–1049. This is a comprehensive review of current knowledge in intrarenal renin-agiotensin system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W, Peng H, Mehaffey EP, Kimball CD, Grobe JL, van Gool JM, Sullivan MN, Earley S, Danser AH, Ichihara A, et al. : Neuron-specific (pro)renin receptor knockout prevents the development of salt-sensitive hypertension. Hypertension 2014, 63:316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. *.Wang F, Lu X, Liu M, Feng Y, Zhou SF, Yang T: Renal medullary (pro)renin receptor contributes to angiotensin II-induced hypertension in rats via activation of the local renin-angiotensin system. BMC Med 2015, 13:278 This is the first report presenting in vivo evidence for (pro)renin receptor regulation of intrarenal renin activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramkumar N, Stuart D, Calquin M, Quadri S, Wang S, Van Hoek AN, Siragy HM, Ichihara A, Kohan DE: Nephron-specific deletion of the prorenin receptor causes a urine concentration defect. Am J Physiol Renal Physiol 2015, 309:F48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z, Zhou L, Wang Y, Miao J, Hong X, Hou FF, Liu Y: (Pro)renin Receptor Is an Amplifier of Wnt/beta-Catenin Signaling in Kidney Injury and Fibrosis. J Am Soc Nephrol 2017, 28:2393–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cousin C, Bracquart D, Contrepas A, Corvol P, Muller L, Nguyen G: Soluble form of the (pro)renin receptor generated by intracellular cleavage by furin is secreted in plasma. Hypertension 2009, 53:1077–1082. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez AA, Lara LS, Luffman C, Seth DM, Prieto MC: Soluble form of the (pro)renin receptor is augmented in the collecting duct and urine of chronic angiotensin II-dependent hypertensive rats. Hypertension 2011, 57:859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maruyama N, Segawa T, Kinoshita N, Ichihara A: Novel sandwich ELISA for detecting the human soluble (pro)renin receptor. Front Biosci (Elite Ed) 2013, 5:583–590. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe N, Morimoto S, Fujiwara T, Suzuki T, Taniguchi K, Mori F, Ando T, Watanabe D, Kimura T, Sago H, et al. : Prediction of gestational diabetes mellitus by soluble (pro)renin receptor during the first trimester. J Clin Endocrinol Metab 2013, 98:2528–2535. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe N, Bokuda K, Fujiwara T, Suzuki T, Mito A, Morimoto S, Jwa SC, Egawa M, Arai Y, Suzuki F, et al. : Soluble (pro)renin receptor and blood pressure during pregnancy: a prospective cohort study. Hypertension 2012, 60:1250–1256. [DOI] [PubMed] [Google Scholar]
- 19.Thomason J, Reyes M, Allen SR, Jones RO, Beeram MR, Kuehl TJ, Suzuki F, Uddin MN: Elevation of (Pro)Renin and (Pro)Renin Receptor in Preeclampsia. Am J Hypertens 2015, 28:1277–1284. [DOI] [PubMed] [Google Scholar]
- 20.Nartita T, Ichihara A, Matsuoka K, Takai Y, Bokuda K, Morimoto S, Itoh H, Seki H: Placental (pro)renin receptor expression and plasma soluble (pro)renin receptor levels in preeclampsia. Placenta 2016, 37:72–78. [DOI] [PubMed] [Google Scholar]
- 21.Bonakdaran S, Azami G, Tara F, Poorali L: Soluble (Pro) Renin Receptor is a predictor of gestational diabetes mellitus. Curr Diabetes Rev 2016. [DOI] [PubMed] [Google Scholar]
- 22.Fukushima A, Kinugawa S, Homma T, Masaki Y, Furihata T, Abe T, Suga T, Takada S, Kadoguchi T, Okita K, et al. : Increased plasma soluble (pro)renin receptor levels are correlated with renal dysfunction in patients with heart failure. Int J Cardiol 2013, 168:4313–4314. [DOI] [PubMed] [Google Scholar]
- 23.Nishijima T, Tajima K, Takahashi K, Sakurai S: Elevated plasma levels of soluble (pro)renin receptor in patients with obstructive sleep apnea syndrome: association with polysomnographic parameters. Peptides 2014, 56:14–21. [DOI] [PubMed] [Google Scholar]
- 24.Nishijima T, Tajima K, Yamashiro Y, Hosokawa K, Suwabe A, Takahashi K, Sakurai S: Elevated Plasma Levels of Soluble (Pro)Renin Receptor in Patients with Obstructive Sleep Apnea Syndrome in Parallel with the Disease Severity. Tohoku J Exp Med 2016, 238:325–338. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi K, Ohba K, Tajima K, Nishijima T, Sakurai S: Soluble (Pro)renin Receptor and Obstructive Sleep Apnea Syndrome: Oxidative Stress in Brain? Int J Mol Sci 2017, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamada K, Taniguchi Y, Shimamura Y, Inoue K, Ogata K, Ishihara M, Horino T, Fujimoto S, Ohguro T, Yoshimoto Y, et al. : Serum level of soluble (pro)renin receptor is modulated in chronic kidney disease. Clin Exp Nephrol 2013, 17:848–856. [DOI] [PubMed] [Google Scholar]
- 27.Nakayama K: Furin: a mammalian subtilisin/Kex2p-like endoprotease involved in processing of a wide variety of precursor proteins. Biochem J 1997, 327 (Pt 3):625–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seidah NG, Prat A: The biology and therapeutic targeting of the proprotein convertases. Nat Rev Drug Discov 2012, 11:367–383. [DOI] [PubMed] [Google Scholar]
- 29.Yoshikawa A, Aizaki Y, Kusano K, Kishi F, Susumu T, Iida S, Ishiura S, Nishimura S, Shichiri M, Senbonmatsu T: The (pro)renin receptor is cleaved by ADAM19 in the Golgi leading to its secretion into extracellular space. Hypertens Res 2011, 34:599–605. [DOI] [PubMed] [Google Scholar]
- 30.Elagoz A, Benjannet S, Mammarbassi A, Wickham L, Seidah NG: Biosynthesis and cellular trafficking of the convertase SKI-1/S1P: ectodomain shedding requires SKI-1 activity. J Biol Chem 2002, 277:11265–11275. [DOI] [PubMed] [Google Scholar]
- 31.Marschner K, Kollmann K, Schweizer M, Braulke T, Pohl S: A key enzyme in the biogenesis of lysosomes is a protease that regulates cholesterol metabolism. Science 2011, 333:87–90. [DOI] [PubMed] [Google Scholar]
- 32.Seidah NG, Sadr MS, Chretien M, Mbikay M: The multifaceted proprotein convertases: their unique, redundant, complementary, and opposite functions. J Biol Chem 2013, 288:21473–21481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duncan EA, Brown MS, Goldstein JL, Sakai J: Cleavage site for sterol-regulated protease localized to a leu-Ser bond in the lumenal loop of sterol regulatory element-binding protein-2. J Biol Chem 1997, 272:12778–12785. [DOI] [PubMed] [Google Scholar]
- 34. **.Nakagawa T, Suzuki-Nakagawa C, Watanabe A, Asami E, Matsumoto M, Nakano M, Ebihara A, Uddin MN, Suzuki F: Site-1 protease is required for the generation of soluble (pro)renin receptor. J Biochem 2017, 161:369–379. The authors demonstrate that the S1P plays a major role in the cleavege of (pro) renin receptor to generate soluble PRR. This is a major progress in understanding the regulation of biogenesis of sPRR. [DOI] [PubMed] [Google Scholar]
- 35. **.Fang H, Xu C, Lu A, Zou CJ, Xie S, Chen Y, Zhou L, Liu M, Wang L, Wang W, et al. : (Pro) Renin Receptor Mediates Albumin Induced Cellular Responses: Role of Site-1 Protease-Derived Soluble (Pro) Renin Receptor in Renal Epithelial Cells. Am J Physiol Cell Physiol 2017:ajpcell 00006 02017. The authors not only discover that S1P serve as the cleavege enzyme of PRR but demonstrate that the S1P-derived sPRR plays an essential role in the regulation of renin activity and pro-inflammatory responses in albumin loaded renal epithelial cells. This is probably the most compelling evidence for a functional role of sPRR in chronic kidney disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Espenshade PJ, Cheng D, Goldstein JL, Brown MS: Autocatalytic processing of site-1 protease removes propeptide and permits cleavage of sterol regulatory element-binding proteins. J Biol Chem 1999, 274:22795–22804. [DOI] [PubMed] [Google Scholar]
- 37.Ye J, Rawson RB, Komuro R, Chen X, Dave UP, Prywes R, Brown MS, Goldstein JL: ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell 2000, 6:1355–1364. [DOI] [PubMed] [Google Scholar]
- 38.Velho RV, De Pace R, Klunder S, Di Lorenzo G, Schweizer M, Braulke T, Pohl S: Site-1 protease and lysosomal homeostasis. Biochim Biophys Acta 2017. [DOI] [PubMed] [Google Scholar]
- 39.Klunder S, Heeren J, Markmann S, Santer R, Braulke T, Pohl S: Site-1 protease-activated formation of lysosomal targeting motifs is independent of the lipogenic transcription control. J Lipid Res 2015, 56:1625–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morimoto S, Ando T, Niiyama M, Seki Y, Yoshida N, Watanabe D, Kawakami-Mori F, Kobori H, Nishiyama A, Ichihara A: Serum soluble (pro)renin receptor levels in patients with essential hypertension. Hypertens Res 2014, 37:642–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patra D, DeLassus E, Hayashi S, Sandell LJ: Site-1 protease is essential to growth plate maintenance and is a critical regulator of chondrocyte hypertrophic differentiation in postnatal mice. J Biol Chem 2011, 286:29227–29240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlombs K, Wagner T, Scheel J: Site-1 protease is required for cartilage development in zebrafish. Proc Natl Acad Sci U S A 2003, 100:14024–14029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patra D, Xing X, Davies S, Bryan J, Franz C, Hunziker EB, Sandell LJ: Site-1 protease is essential for endochondral bone formation in mice. J Cell Biol 2007, 179:687–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patra D, DeLassus E, Liang G, Sandell LJ: Cartilage-specific ablation of site-1 protease in mice results in the endoplasmic reticulum entrapment of type IIb procollagen and down-regulation of cholesterol and lipid homeostasis. PLoS One 2014, 9:e105674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitchell KJ, Pinson KI, Kelly OG, Brennan J, Zupicich J, Scherz P, Leighton PA, Goodrich LV, Lu X, Avery BJ, et al. : Functional analysis of secreted and transmembrane proteins critical to mouse development. Nat Genet 2001, 28:241–249. [DOI] [PubMed] [Google Scholar]
- 46.Advani A, Kelly DJ, Cox AJ, White KE, Advani SL, Thai K, Connelly KA, Yuen D, Trogadis J, Herzenberg AM, et al. : The (Pro)renin receptor: site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension 2009, 54:261–269. [DOI] [PubMed] [Google Scholar]
- 47.Wang F, Lu X, Peng K, Fang H, Zhou L, Su J, Nau A, Yang KT, Ichihara A, Lu A, et al. : Antidiuretic Action of Collecting Duct (Pro)Renin Receptor Downstream of Vasopressin and PGE2 Receptor EP4. J Am Soc Nephrol 2016, 27:3022–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. **.Lu X, Wang F, Xu C, Soodvilai S, Peng K, Su J, Zhao L, Yang KT, Feng Y, Zhou SF, et al. : Soluble (pro)renin receptor via beta-catenin enhances urine concentration capability as a target of liver X receptor. Proc Natl Acad Sci U S A 2016, 113:E1898–1906. This is the first report on the antidiuretic function of the sPRR in particular. The authors demonstrate that sPRR acts via frizzled class receptor 8-depdendent β-catenin signaling to increase AQP2 expression in the collecting duct cells. These findings offer an unreported insight into the physiological role of sPRR in regulating fluid homeostasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takahashi K, Yamamoto H, Hirose T, Hiraishi K, Shoji I, Shibasaki A, Kato I, Kaneko K, Sasano H, Satoh F, et al. : Expression of (pro)renin receptor in human kidneys with end-stage kidney disease due to diabetic nephropathy. Peptides 2010, 31:1405–1408. [DOI] [PubMed] [Google Scholar]
- 50.Kobori H, Alper AB Jr., Shenava R, Katsurada A, Saito T, Ohashi N, Urushihara M, Miyata K, Satou R, Hamm LL, et al. : Urinary angiotensinogen as a novel biomarker of the intrarenal renin-angiotensin system status in hypertensive patients. Hypertension 2009, 53:344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu D, Wen Y, Tang TT, Lv LL, Tang RN, Liu H, Ma KL, Crowley SD, Liu BC: Megalin/Cubulin-Lysosome-mediated Albumin Reabsorption Is Involved in the Tubular Cell Activation of NLRP3 Inflammasome and Tubulointerstitial Inflammation. J Biol Chem 2015, 290:18018–18028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao W, Fan Y, Wang N, Chuang PY, Lee K, He JC: Knockdown of RTN1A attenuates ER stress and kidney injury in albumin overload-induced nephropathy. Am J Physiol Renal Physiol 2016, 310:F409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jia Y, Sun Y, Weng L, Li Y, Zhang Q, Zhou H, Yang B: Low molecular weight fucoidan protects renal tubular cells from injury induced by albumin overload. Sci Rep 2016, 6:31759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eddy AA: Proteinuria and interstitial injury. Nephrol Dial Transplant 2004, 19:277–281. [DOI] [PubMed] [Google Scholar]
- 55.Oshima Y, Morimoto S, Ichihara A: Roles of the (pro)renin receptor in the kidney. World J Nephrol 2014, 3:302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]