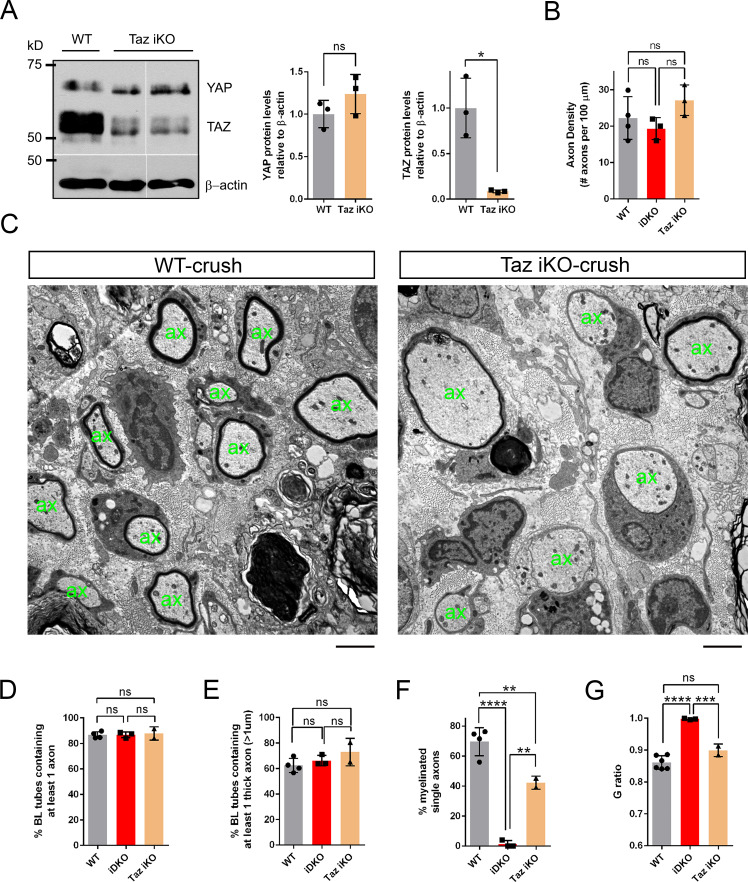
Figure 8. YAP and TAZ are redundantly required for optimal remyelination.
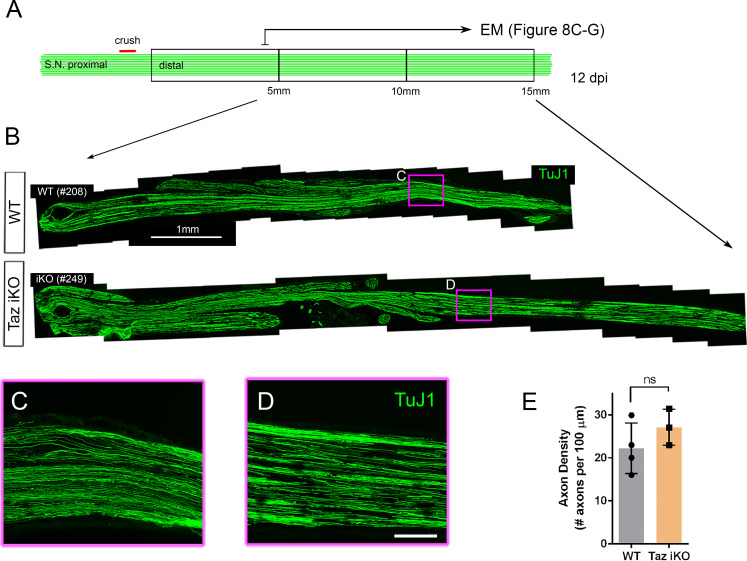
(A) Western blotting of intact sciatic nerve lysates, showing markedly reduced TAZ in Taz iKO, whereas YAP levels remain relatively unchanged. YAP band is tighter and faster migrating in Taz iKO, than in WT, indicative of reduced phosphorylation. Quantification of Yap and Taz in WT and Taz iKO, n = 3 mice per genotype. YAP: ns, not significant, p=0.2752, Mann-Whitney. TAZ: *p=0.0495, Mann-Whitney. (B) Quantification of axon density in WT, Yap/Taz iDKO and Taz iKO nerves at 12 dpi, 8–10 mm distal to crush site (also see Figure 5B,F and Figure 8—figure supplement 1B, (E). n = 4 mice for WT, three mice for iDKO and Taz iKO: WT vs iDKO, p=0.72; WT vs iKO, p=0.41; iDKO vs iKO, p=0.18, all not significant, one-way ANOVA with Tukey’s multiple comparison test. (C–G) Comparative analysis of axon regeneration and remyelination in WT and Taz iKO, 12–13 days after nerve crush. (C) Representative TEM images of WT and Taz iKO nerves, taken at 5 mm distal to the crush site, showing numerous axons that regenerated within basal lamina tubes in Taz iKO, as in WT. ‘ax’ denotes a single axon. Some large axons are myelinated in Taz iKO. (D) Quantification of the percentage of BL tubes containing axons of any diameter in WT, Taz iKO and Yap/Taz iDKO nerves. n = 4 mice for WT, three mice for iDKO and two mice for Taz iKO: WT vs. iDKO, p=0.99; WT vs. iKO, p=0.90; iDKO vs. Taz iKO, p=0.92, all not significant, one-way ANOVA with Tukey’s multiple comparison test. (E) Quantification of the percentage of BL tubes containing at least one axon larger than 1 μm in diameter in WT, Taz iKO and Yap/Taz iDKO nerves. n = 4 mice for WT, three mice for iDKO and two mice for Taz iKO: WT vs. iDKO, p=0.73; WT vs. iKO, p=0.22; iDKO vs. iKO, p=0.52, all not significant, one-way ANOVA with Tukey’s multiple comparison test. (F) Quantification of the percentage of single axons that are remyelinated in WT, Taz iKO and Yap/Taz iDKO nerves. n = 4 mice for WT, three mice for iDKO and two mice for Taz iKO: WT vs. iDKO, ****p<0.0001; WT vs. iKO, **p=0.0094; iDKO vs. Taz iKO, **p=0.0016, one-way ANOVA with Tukey’s multiple comparison test. (G) G-ratios of remyelinated axons in WT and Taz iKO nerves, compared to unmyelinated axons in Yap/Taz iDKO nerve. WT and Taz iKO remyelinated axons have equivalent G-ratios. n = 6 mice for WT, three mice for iDKO and two mice for iKO: WT vs. iDKO, ****p<0.0001; WT vs. iKO, not significant, p=0.074; iDKO vs. iKO, ***p=0.0008, one-way ANOVA with Tukey’s multiple comparison test. Scale bar = 2 μm (C).