Abstract
Sustained persistence of chimeric antigen receptor T (CAR-T) cells is a key characteristic associated with long-term remission in patients with hematologic malignancies. Attempts to uncover mechanisms that enhance persistence and thus functionality will have a substantial impact in broadening application of CAR-T cell therapy, especially for solid tumors. In this issue of the JCI, Guedan et al. describe a promising strategy to limit T cell exhaustion and improve persistence by changing a single amino acid in the costimulatory domain of CD28. The authors demonstrated that this single amino acid substitution in CD28-based mesothelin CAR-T cells results in improved persistence and functionality in a xenograft model of pancreatic cancer. Furthermore, reciprocal alteration of the same residue in inducible costimulator–containing (ICOS-containing) CAR-T cells resulted in limited antitumor activity and persistence. These findings suggest that simple alterations in the costimulatory domain may enhance CAR-T cell persistence, warranting future evaluation in other CD28-costimulatory CARs in an effort to improve durable antitumor effects.
Introduction
The overwhelming clinical success of T cells that are genetically modified with chimeric antigen receptors (CARs) against refractory hematologic malignancies (1–3) led to the commercialization of two different CAR-T cell products. Their widespread use has provided key insight into features associated with long-lasting clinical remission, namely (a) robust in vivo expansion and (b) sustained persistence (4–6). Indeed, CAR-T cells that are unable to persist can serve only as a bridge without inducing definitive cures. Persistence-prolonging modifications may prove particularly valuable in improving the efficacy of CAR-T cells directed toward solid tumors, which have yielded less impressive results (7, 8). Thus, ongoing efforts to enable CAR-T cells to avoid exhaustion and persist indefinitely are a priority for the field.
What makes the difference in persistence?
There are two prominent schools of thought regarding the differences in persistence between CAR constructs, both of which revolve around the influence of signal strength on the delicate balance between T cell activation and exhaustion. The predominant hypothesis has focused on how quantitative signaling impacts T cell exhaustion, as exhausted CAR-T cells express more inhibitory receptors, such as PD-1 (9–11). However, qualitative differences in signaling are perhaps as important a factor in CAR-T cell persistence.
Although construct differences may play a role in driving CAR-T cell persistence, the primary predictive factor relates to variations in the costimulatory domain (12–14). CAR-T cells containing CD28 have shorter persistence compared with 4-1BB (irrespective of other construct differences), which has important clinical consequences. In the case of CD19-targeted immunotherapy, most practitioners strongly consider stem cell transplant for patients who achieve remission following CD28-based CAR-T cells. However, patients who remain in remission following treatment with 4-1BB–based CAR-T cells often enter a disease surveillance program, with their likelihood of requiring (or needing to proceed to) a transplant diminishing the longer they remain in remission (4, 11).
The limited persistence of second-generation CARs containing CD28 and CD3ζ tandem signaling domains likely relates to (a) constitutive activation via CAR aggregation that leads to activation of the exhaustion-associated receptor PD-1, which preferentially inhibits CD28 signaling (15); (b) CAR-CD3ζ domain phosphorylation, which is accentuated by CD28 and CD3ζ signaling redundancy (16); as well as the (c) spatiotemporal constraints imparted by the structure of second-generation CARs (17).
CAR-T cells containing 4-1BB costimulatory domains, on the other hand, possess a more memory T cell–like surface phenotype that may ameliorate CAR-T exhaustion (12–14, 18). Mechanism aside, multiple clinical trials have corroborated the decreased persistence of CD28-based CARs versus those with 4-1BB (1, 3, 4, 19). However, costimulatory domains are not one-size-fits-all: 4-1BB, for example, while quite effective in CD19-targeting CARs, may negligibly mediate other CAR constructs, particularly those targeting solid tumors (20). The choice of costimulatory domain therefore has emerged as the premier controllable predictor of CAR-T cell persistence, making it a primary focus of efforts to improve CAR-T cell function.
Can a single residue switch improve persistence?
In this issue of the JCI, Guedan and colleagues (21) substituted a single amino acid of a CD28-based CAR-T cell that targets the mesothelin tumor differentiation antigen. The researchers tested the in vivo persistence and functionality of these CD28-modified T cells in xenograft models of pancreatic cancer. The authors previously showed that CD28-based antimesothelin CARs have limited persistence compared with those containing either the 4-1BB or inducible costimulatory (ICOS) domains (13, 14). ICOS, a T cell costimulatory receptor of the B7-CD28 superfamily, shares the YMXM signaling motif with CD28 in its intracellular domain. The difference between these intracellular domains is a single amino acid at the X position, an asparagine in CD28 and a phenylalanine in ICOS. Previous publications established the impact of alterations in the shared YMXM motif on differential signaling (22, 23). Specifically, evidence suggests that stimulating the asparagine within the YMNM motif of CD28, using a cognate antigen, exaggerates calcium release and increases NFAT signaling, thus driving T cell exhaustion and dysfunction (24). The authors therefore hypothesized that this single amino acid difference may ultimately direct differences in persistence of CAR-T cells containing CD28 versus ICOS intracellular domains. In this study, the authors changed asparagine to phenylalanine to rescue CD28-costimulated CAR-T cells from exhaustion and prolong T cell persistence and durable antitumor effects (21).
Using site-directed mutagenesis, Guedan et al. (21) generated mesothelin-directed CAR-T cells containing single amino acid alterations in the YMXM motif (CD28-YMFM and ICOS-YMNM). In vitro comparison of CD28-YMFM, ICOS-YMNM, and wild-type CD28 CAR-T cells revealed comparable phenotype, differentiation, tonic signaling, and cytotoxicity in response to target cells. Despite similar in vitro characteristics, the mutated CD28-YMFM and ICOS-directed CAR T cells behaved markedly differently in a Capan-2 pancreatic xenograft mouse model compared with wild-type CD28 CAR-T cells (21).
Treatment with wild-type CD28 CAR-T cells or CD28-YMFM CAR-T cells decreased the size of subcutaneous Capan-2 tumors within 23 days. However, tumors of mice treated with CD28 CAR-T cells resumed growth after 23 days. Similarly to mice treated with ICOS-CAR-T cells, CD28-YMFM CAR-T cells durably controlled tumors. As predicted, differences in antitumor efficacy correlated with CAR-T cell persistence. Mice treated with either CD28-YMFM or ICOS CAR-T cells had circulating CD8+ and CD4+ mesothelin CAR-T cells in peripheral blood 30 days after treatment, whereas wild-type CD28 CAR-T cells exhibited limited persistence, similar to nontransduced control T cells (findings similar in both subcutaneous and metastatic models). Phenotypic analysis of peripheral CD28-YMFM CAR-T cells identified as late as day +30 revealed a less exhausted, less terminally differentiated profile, a profile consistent with that of highly activated, resilient CAR-T cells (21).
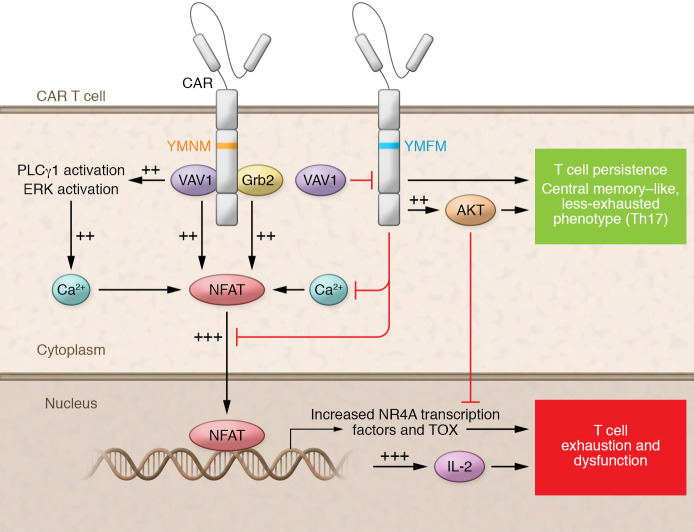
In order to further explore the mechanism by which this single residue switch improved functionality, the authors generated the various CAR-T cell constructs from four healthy donors. Analysis of CD28-YMFM CAR-T cells revealed increases in AKT activation with concomitant decreases in VAV1 activation, in comparison with CD28 CAR-T cells manufactured from the same donors. These are all expected consequences of the disruption in the Grb2-binding domain of CD28 caused by the YMFM alteration. Altered downstream VAV1 signaling results in decreased NFAT activation, IL-2 production, and ultimately calcium release through PLCγ1 and ERK activation, a schematic of which is shown in Figure 1 (21).
Figure 1. Possible mechanisms of improved persistence in CD28-YMFM–costimulatory CAR-T cells.
Signaling pathways proposed by Guedan and colleagues (21) suggest that second-generation CARs with costimulatory domains containing a single amino acid substitution YMNM (yellow) or YMFM (blue) differentially interact with VAV1/Grb2. CD28-YMNM CAR T cells show increased activation (++) and additive activation (+++) from multiple pathways. CD28-YMFM CAR-T cells show decreased NFAT activation, IL-2 production, calcium release, with T cell persistence.
To simulate the chronic antigen stimulation that triggers T cell exhaustion, Guedan et al. studied whether signaling alterations resulted in transcriptional changes in an in vivo model of pancreatic cancer. Interestingly, while T cell infiltration and initial antitumor effects were similar in all CAR constructs, Ki67 proliferation assays revealed that only CD28-YMFM and ICOS CAR-T cells continued to actively proliferate two weeks after treatment. These findings indicate that lack of persistence of CD28 CAR-T cells is at least partly a result of T cell dysfunction and exhaustion following chronic antigen stimulation. Gene expression analysis at this time point revealed clustering of CD28-YMFM with ICOS CAR-T cells (as opposed to CD28 CAR-T cells) (21).
To provide further evidence of the impact this single residue alteration has on the CD28 intracellular signaling domain and CAR-T cell functionality, the authors changed phenylalanine to asparagine in the YMFM motif of the ICOS-based CAR to create a reciprocal ICOS-CAR design. This single residue substitution decreased in vivo functionality. Differences in persistence were even more striking than in antitumor activity. ICOS CAR-T cells persisted up to 36 days after treatment, while ICOS-YMNM CAR-T cell persistence was almost indistinguishable from that of first-generation CAR-T cells (21).
Minor modifications may lead to major mileage gains
In the quest for ideal CAR-T cells, persistence ranks at the top of the list of desired characteristics. In this issue, Guedan and colleagues provide preclinical proof of principal that a single amino acid substitution strategy can enhance the in vivo persistence, and thus antitumor efficacy, of mesothelin-targeted CAR-T cells containing the CD28 costimulatory domain (21). Importantly, their single residue alteration did not alter the length of genetic material in the vector; thus, in stark contrast to some other more complex regulatory approaches (25, 26), this strategy could easily be combined with other genetic modifications and clinically translated.
Despite the convincing mechanistic data, whether the modification described here enhances functionality of all CD28-containing CARs, for example those directed at other solid or brain tumors, remains an important open question. Future studies also should address how this modification works in combination with other genetic modifications or enhancements. Furthermore, prior to broader application, we must improve our understanding of whether this substitution affects motifs outside of YMNM, as well as the role enhanced AKT activation will ultimately play in functionality.
Nevertheless, Guedan et al. (21) provide promising evidence that alterations in the Grb2/VAV signaling pathways enhance CAR-T cell persistence, resulting in durable antitumor effects. Therefore, these experiments should be extended to other CD28-costimulatory CARs, including clinically validated ones, followed by comparison with 4-1BB counterparts.
Acknowledgments
We would like to acknowledge Catherine Gillespie for her scientific editing services.
Version 1. 05/04/2020
Electronic publication
Version 2. 06/01/2020
Print issue publication
Footnotes
Conflict of interest: RHR has received honoraria for advisory board participation from Novartis Pharmaceuticals and research funding from TESSA Pharmaceuticals.
Copyright: © 2020, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2020;130(6):2806–2808. https://doi.org/10.1172/JCI136872.
See the related article at Single residue in CD28-costimulated CAR-T cells limits long-term persistence and antitumor durability.
Contributor Information
Emily M. Hsieh, Email: emhsieh@texaschildrens.org.
Lauren D. Scherer, Email: ldschere@texaschildrens.org.
Rayne H. Rouce, Email: rhrouce@txch.org.
References
- 1.Maude SL, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neelapu SS, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuster SJ, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 4.Maude SL, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramos CA, et al. In vivo fate and activity of second- versus third-generation CD19-specific CAR-T cells in B cell non-Hodgkin’s lymphomas. Mol Ther. 2018;26(12):2727–2737. doi: 10.1016/j.ymthe.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter DL, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louis CU, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118(23):6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown CE, et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375(26):2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feucht J, et al. Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency. Nat Med. 2019;25(1):82–88. doi: 10.1038/s41591-018-0290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salter AI, et al. Phosphoproteomic analysis of chimeric antigen receptor signaling reveals kinetic and quantitative differences that affect cell function. Sci Signal. 2018;11(544):eaat6753. doi: 10.1126/scisignal.aat6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long AH, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21(6):581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawalekar OU, et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity. 2016;44(2):380–390. doi: 10.1016/j.immuni.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 13.Guedan S, et al. ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood. 2014;124(7):1070–1080. doi: 10.1182/blood-2013-10-535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guedan S, et al. Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation. JCI Insight. 2018;3(1):96976. doi: 10.1172/jci.insight.96976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zolov SN, Rietberg SP, Bonifant CL. Programmed cell death protein 1 activation preferentially inhibits CD28.CAR-T cells. Cytotherapy. 2018;20(10):1259–1266. doi: 10.1016/j.jcyt.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Acuto O, Mise-Omata S, Mangino G, Michel F. Molecular modifiers of T cell antigen receptor triggering threshold: the mechanism of CD28 costimulatory receptor. Immunol Rev. 2003;192:21–31. doi: 10.1034/j.1600-065X.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- 17.Frigault MJ, et al. Identification of chimeric antigen receptors that mediate constitutive or inducible proliferation of T cells. Cancer Immunol Res. 2015;3(4):356–367. doi: 10.1158/2326-6066.CIR-14-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cherkassky L, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest. 2016;126(8):3130–3144. doi: 10.1172/JCI83092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park JH, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramakrishna S, Barsan V, Mackall C. Prospects and challenges for use of CAR T cell therapies in solid tumors. Expert Opin Biol Ther. 2020;20(5):503–516. doi: 10.1080/14712598.2020.1738378. [DOI] [PubMed] [Google Scholar]
- 21.Guedan S, et al. Single residue in CD28-costimulated CAR T cells limits long-term persistence and antitumor durability. J Clin Invest. 2020;130(6):3087–3097. doi: 10.1172/JCI133215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harada Y, et al. A single amino acid alteration in cytoplasmic domain determines IL-2 promoter activation by ligation of CD28 but not inducible costimulator (ICOS) J Exp Med. 2003;197(2):257–262. doi: 10.1084/jem.20021305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dennehy KM, Elias F, Na SY, Fischer KD, Hünig T, Lühder F. Mitogenic CD28 signals require the exchange factor Vav1 to enhance TCR signaling at the SLP-76-Vav-Itk signalosome. J Immunol. 2007;178(3):1363–1371. doi: 10.4049/jimmunol.178.3.1363. [DOI] [PubMed] [Google Scholar]
- 24.Posey AD, Kawalekar OU, June CH. Measurement of intracellular ions by flow cytometry. Curr Protoc Cytom. 2015;72:9.8.1–9.8.21. doi: 10.1002/0471142956.cy0908s72. [DOI] [PubMed] [Google Scholar]
- 25.Ajina A, Maher J. Strategies to address chimeric antigen receptor tonic signaling. Mol Cancer Ther. 2018;17(9):1795–1815. doi: 10.1158/1535-7163.MCT-17-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eyquem J, et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543(7643):113–117. doi: 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]