Abstract
Extensive research over the past decades has identified integrins to be the primary transmembrane receptors that enable cells to respond to external mechanical cues. We reveal here a mechanism whereby syndecan-4 tunes cell mechanics in response to localised tension via a coordinated mechanochemical signalling response that involves activation of two other receptors: epidermal growth factor receptor, and β1 integrin. Tension on syndecan-4 induces cell-wide activation of the kindlin-2/β1 integrin/RhoA axis in a PI3K dependent manner. Furthermore, syndecan-4 mediated tension at the cell-extracellular matrix interface is required for YAP activation. Extracellular tension on syndecan-4 triggers a conformational change in the cytoplasmic domain, the variable region of which is indispensable for the mechanical adaptation to force, facilitating the assembly of a syndecan-4/α-actinin/F-actin molecular scaffold at the bead adhesion. This mechanotransduction pathway for syndecan-4 should have immediate implications for the broader field of mechanobiology.
Keywords: syndecan-4, mechanotransduction, mechanobiology, integrin, kindlin, alpha-actinin, F-actin, RhoA, PI3K, YAP
Syndecans belong to a family of four transmembrane proteoglycan adhesion receptors. Among them, syndecan-4 is the only family member that is ubiquitously expressed across nucleated cells and enriched in focal adhesions1. The extracellular domain of syndecan-4 bears heparan sulphate chains that bind to ECM proteins containing heparin binding domains (e.g. fibronectin) in addition to growth factors2. The transmembrane domain drives dimerization, and the cytoplasmic domain promotes binding to focal adhesion components and intracellular kinases3. Syndecan-4 acts synergistically with integrins to regulate substrate adhesion, focal adhesion assembly and cytoskeletal organisation3–5. Syndecan-4 also complexes with integrin α5β1 to cooperatively bind Thy-1 (CD90), forming a unique dynamic catch bond which strengthens under force to mediate adhesion signalling6. Much like integrins, syndecan-4 provides a direct mechanical linkage from the extracellular matrix (ECM) to the actin cytoskeleton and is thus well positioned to support force transmission and act as a mechanotransducer.
Syndecan-4 null mice exhibit delayed wound healing7, while syndecan-4 null cells show impaired contractility, cytoskeletal organisation, and immature focal adhesions8–12, all suggestive of mechanosensation deficits. Indeed, studies in endothelial cells under fluid shear have implicated syndecan-4 in mechanotransduction both in vitro13,14 and in vivo15,16. Syndecan-4 has additionally been shown to respond to directly applied mechanical tension, activating mitogen-activated protein kinase (MAPK) and protein kinase C-α (PKC-α) signalling17,18. These studies suggest syndecan-4 is directly involved in mechanotransduction, however its specific role and the underlying molecular mechanisms are unknown. Here, we have combined biophysical, cell biology and computational techniques to reveal a role for syndecan-4 in regulating cell mechanics through a previously unidentified mechanotransduction pathway requiring synergistic integrin activation.
Results
Tension on syndecan-4 induces adaptive cell stiffening
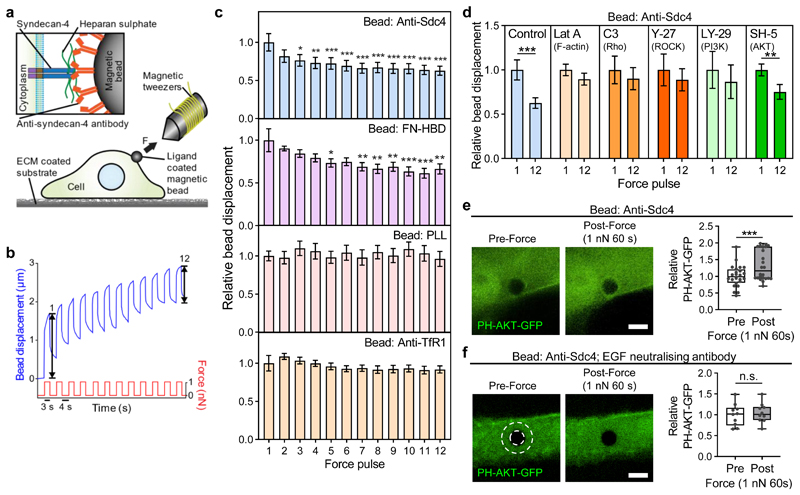
Tensional force on mechanosensitive receptors, such as integrins, triggers rapid cytoskeletal remodelling and adaptive cellular stiffening19. This response has also been observed in cell-cell junction proteins, E-cadherin and platelet endothelial cell adhesion molecule-1 (PECAM-1)20,21, and nuclear envelope component nesprin-122. To investigate if syndecan-4 acts as a mechanotransducer, we used electromagnetic tweezers to apply repeated localised mechanical tension to the syndecan-4 receptor on cells plated on fibronectin via ligand-coated magnetic beads, while tracking bead displacement (Fig. 1a). To ensure specificity of the cellular response to force, we coated the magnetic beads with an anti-syndecan-4 antibody (Anti-Sdc4) that binds specifically to the ectodomain of syndecan-4 core protein (Supplementary Figs. 1,2). Application of 12 successive 1 nN force pulses to Anti-Sdc4 beads triggered an adaptive increase in cellular stiffness evident as a decrease in bead displacement, reaching a relative displacement reduction of 37% by the 12th pulse (Fig. 1b,c, Supplementary Fig. 3). To mimic physiological ligand binding to the heparan sulphate chains of syndecan-4, we used beads coated with the 40 kDa α-chymotryptic fragment of fibronectin which contains the CS-1 heparin binding domain (FN-HBD) and does not bind integrins23. In this case we also observed a reduction in the amplitude of bead oscillation (Fig. 1c). Binding to the heparan sulphate chains of syndecan-4 was also achieved using fibronectin coated beads which were treated with an anti-fibronectin antibody (clone 3E3) which blocks the RGD integrin binding site but leaves the heparin binding domain available23. Again, we observed a decrease in the amplitude of bead oscillation indicative of adaptive cell stiffening (Supplementary Fig. 5). To ensure this response is specific to syndecan-4, we used the same force protocol on beads coated with poly-L-lysine (PLL) which binds the cell membrane non-specifically via electrostatic interactions, and an antibody that binds the extracellular domain of transferrin receptor protein-1 (Anti-TfR1), previously shown to be non-responsive to tensional force19. A reduction in bead displacement over the 12 pulses was not observed in either case (Fig. 1c). These results demonstrate that tensional force applied specifically to syndecan-4 core protein or its associated heparan sulphate chains leads to an adaptive cellular stiffening response.
Figure 1. Tension on syndecan-4 induces Rho-dependent adaptive stiffening mediated by EGFR and PI3K.
a, Schematic representation of electromagnetic tweezers pulling on a ligand-coated magnetic bead bound to the apical cell surface. Inset depicts an anti-syndecan-4 antibody-coated bead binding to the extracellular domain of syndecan-4. b, Representative cell stiffening response apparent as a progressively diminishing bead displacement over 12 force pulses. Bead displacement at force pulse 1 and 12 is indicated. n = 32 cells. c, Relative displacement of beads bound to pancreatic stellate cells (PSCs) plated on fibronectin. Beads were functionalised with anti-syndecan-4 antibody (Anti-Sdc4; n = 32), the heparin binding domain fragment of fibronectin (FN-HBD; n = 31), poly-L-lysine (PLL; n = 20) or anti-transferrin receptor protein-1 antibody (Anti-TfR1; n = 36 cells). Displacement for all pulses was normalised to the average displacement of force pulse 1. Friedman test with Dunn pairwise comparisons: *P ≤ 0.0116, **P ≤ 0.0041, ***P < 0.0001 vs force pulse 1. d, Relative syndecan-4 bound bead displacement at force pulse 1 and 12 in control PSCs (n = 32), or PSCs treated with latrunculin A (Lat A; n = 20), C3 transferase (C3; n = 20), Y-27632 (Y-27; n = 20), LY-294002 (LY-29; n = 20), or SH-5 (n = 24 cells). See Supplementary Fig. 4 for single cell data. Two-sided paired signed rank test: **P = 0.002, ***P < 0.0001. Mean ± s.e.m. e,f, Syndecan-4 bound beads on cells expressing the PIP3 biosensor PH-AKT-GFP were exposed to sustained tension of 1 nN for 60 s in untreated conditions (e) or in the presence of an epidermal growth factor (EGF) neutralising antibody (f). Representative confocal slice images of the area surrounding the bead pre (0 s) and post (60 s) force application. Mean PH-AKT-GFP fluorescent intensity, in a region of interest depicted by white dashed overlay, is presented relative to intensity prior to force application. See Supplementary Fig. 6 for control GFP data. Scale bar: 5 μm. n(e) = 24, n(f) = 10 cells. Two-sided paired signed rank test: ***P = 0.0002, n.s. P = 0.723. Boxes represent median and interquartile range, whiskers extend to the max/min data points, individual values overlaid.
Using a battery of pharmacological inhibitors, we mechanistically investigated this syndecan-4-mediated mechanotransduction response. Pre-treatment of cells with the F-actin polymerisation inhibitor latrunculin A prior to force application prevented the stiffening response, with no reduction in relative bead displacement by force pulse 12 (Fig. 1d). Likewise, pharmacological inhibition of Rho with C3 transferase or Rho-associated protein kinase (ROCK) with Y-27632 also blocked a reduction in bead displacement (Fig. 1d), demonstrating that a functional contractile cytoskeleton is required for syndecan-4 mediated cell stiffening. Phosphoinositide 3-kinase (PI3K) activation has been shown to play a role in cell-cell junction mediated cellular stiffening20,21. Intriguingly, syndecan-4 mediated stiffening also showed a dependency on PI3K, as treatment with the PI3K inhibitor LY-294002 abrogated the cell’s mechanical adaptation to force (Fig. 1d). PI3K activation produces freely diffusible phosphatidylinositol-3,4,5-trisphosphate (PIP3) that acts as a lipid second messenger to propagate signalling cascades throughout the cell24. A major downstream effector of PI3K/PIP3 signalling is AKT. However, selective inhibition of AKT using SH-5 did not prevent cell stiffening in response to force (Fig. 1d), suggesting PI3K acts via an alternative mechanotransduction pathway.
We next investigated whether tension on syndecan-4 activates PI3K activity through the generation of PIP3 in cells expressing a green fluorescent protein (GFP) reporter containing the pleckstrin homology (PH) domain of AKT (PH-AKT-GFP) which binds PIP3. Sustained 1 nN tension for 60 s on syndecan-4 resulted in elevated PI3K activity apparent by the accumulation of PH-AKT-GFP around the bead (Fig. 1e). No such accumulation was observed with the same force applied to PLL-coated beads (Supplementary Fig. 6), indicating that force-induced PI3K activation is specific to syndecan-4.
Receptor tyrosine kinases can activate PI3K in response to ligand stimulation24. Epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase that is known to form a complex with syndecan-425. Activated EGFR recruits GRB2-associated-binding protein 1 (GAB1), which becomes tyrosine phosphorylated at sites that recruit the SH2 domains of the PI3K p85 subunit, providing an indirect mechanism for EGFR to activate PI3K26. To investigate how tension on syndecan-4 regulates PI3K activity, we treated cells with the EGFR inhibitor Gefitinib prior to application of sustained tension to syndecan-4 bound beads; this treatment abolished cell stiffening (Supplementary Fig. 7). As EGFR can be activated by both ligand-dependent and -independent mechanisms, we treated PH-AKT-GFP expressing cells with a neutralising anti-EGF antibody which inhibits EGF ligand-dependent EGFR signalling27. This treatment prevented force induced PI3K activation (Fig. 1f) and these cells failed to exhibit a stiffening response (Supplementary Fig. 7) upon force application to syndecan-4. EGF has been shown to sensitise mechano-responsiveness by enhancing strain mediated mechanotransduction28, and increasing rigidity sensing29, while the dependence of adaptive stiffening on ligand activation of EGFR and PI3K is analogous to a mechanotransduction pathway mediated by E-cadherin21. Hence, ligand-dependent activation of EGFR may amplify syndecan-4 mechanotransduction to facilitate downstream PI3K signalling. Collectively, these results demonstrate that syndecan-4 is a cellular mechanotransducer that tunes cell mechanics in response to localised tension via an adaptive stiffening response involving EGFR and PI3K mediated regulation of cytoskeletal remodelling via Rho.
Tension on syndecan-4 drives cell-wide focal adhesion growth
Most ECM molecules contain binding sites for both integrins and syndecans and there is substantial evidence of a clear synergy between the two receptor families4. Cooperative signalling from both integrins and syndecans potentiates force transduction and is required for the assembly of focal adhesion complexes, associated stress fibre formation, regulation of cytoskeletal dynamics and directional migration3–5,30.
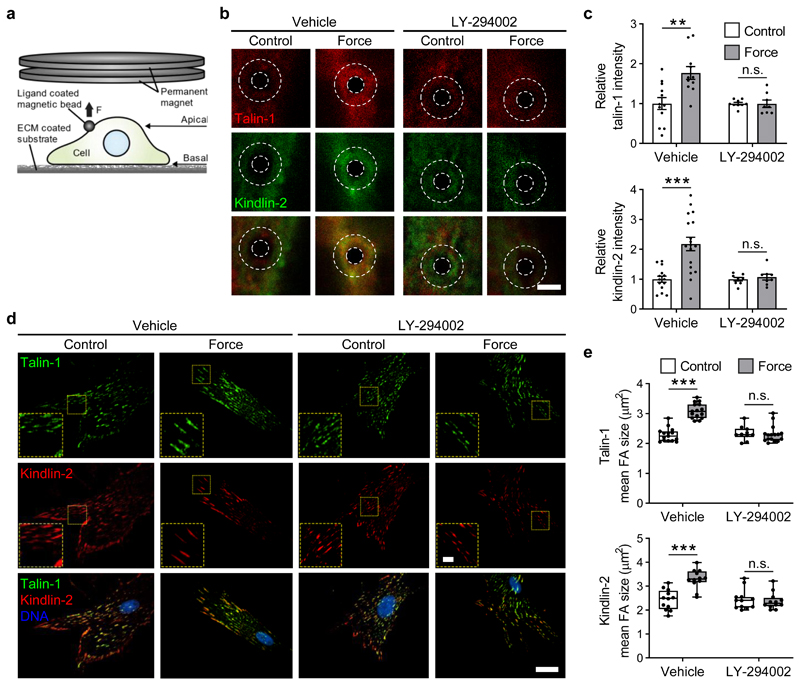
We thus asked if localised tension on syndecan-4 can actively recruit focal adhesion proteins talin and kindlin under force. These two families of FERM-domain proteins bind the cytoplasmic tail of integrins, recruit cytoskeletal and signalling proteins involved in mechanotransduction and synergise to activate integrin binding to the extracellular matrix31. Anti-Sdc4 magnetic beads adhered to the apical surface of cells on fibronectin prior to treatment with PI3K inhibitor LY-294002 or vehicle (DMSO). Using a permanent magnet, a sustained ~200 pN tensile force was applied to syndecan-4 for 5 min (Fig. 2a). In the absence of force, talin-1 and kindlin-2 immunofluorescent staining around the bead was limited. With sustained force application to syndecan-4, both talin-1 and kindlin-2 were recruited to the bead adhesion leading to an approx. 2-fold increase in staining intensity (Vehicle, Fig. 2b,c). However, when PI3K was inhibited, talin-1 and kindlin-2 were not recruited (Fig. 2b,c).
Figure 2. Tension on syndecan-4 leads to PI3K dependent cell-wide growth of focal adhesions.
a, Syndecan-4 bound beads on pancreatic stellate cells plated on fibronectin and pre-treated with vehicle (DMSO) or PI3K inhibitor LY-294002 were exposed to sustained tension of ~200 pN for 5 min using a permanent magnet. b, Representative confocal maximum projection images of talin-1 and kindlin-2 at the bead site. White dashed overlay depicts region of interest (ROI) surrounding bead used for intensity quantification. Scale bar 5 μm. c, Mean fluorescent intensity of talin-1 and kindlin-2 in a ROI surrounding the bead was summed from each confocal section and presented relative to each unstimulated control. nTalin-1 = 8-12 cells (nVehicle-Control = 12, nVehicle-Force = 11, nLY-294002-Control = 9, nLY-294002-Force = 8), nKindlin-2 = 8-18 cells (nVehicle-Control = 14, nVehicle-Force = 18, nLY-294002-Control = 8, nLY-294002-Force = 8); two-sided two-sample t-test: **P = 0.0021, ***P = 0.0001, n.s. P ≥ 0.543. Mean ± s.e.m. d, Representative TIRF microscopy images of talin-1 and kindlin-2 enriched basal focal adhesions. Scale bar 5 μm, inset scale bar 1 μm. e, Mean area of talin-1 and kindlin-2 positive focal adhesions. nTalin-1 = 10-16 cells (nVehicle-Control = 15, nVehicle-Force = 15, nLY-294002-Control = 10, nLY-294002-Force = 16) and nKindlin-2 = 12 cells with ≥ 5 focal adhesions measured per cell; general linear model for analysis of variance with Tukey pairwise comparisons: ***P < 0.0001, n.s. P ≥ 0.9726. Boxes represent median and interquartile range, whiskers extend to the max/min data points, individual values overlaid.
When we assessed talin-1 and kindlin-2 localisation at the basal membrane where the cell contacts the fibronectin ECM, we found a cell-wide increase in the size and number of talin-1 and kindlin-2 containing focal adhesions after sustained force application to apical syndecan-4 (Vehicle, Fig. 2d,e). PI3K inhibition similarly abrogated the force-induced recruitment of talin-1 and kindlin-2 to focal adhesions at the basal surface (Fig. 2d,e). This implies that localised force on syndecan-4 triggers PI3K dependent, cell-wide activation and growth of talin-1 and kindlin-2 enriched focal adhesions to induce a global cellular response even at sites distal from the point of force application.
PIP3 binding to kindlin-2 mediates integrin activation
The cell-wide growth of talin-1 and kindlin-2 containing focal adhesions prompted us to investigate if force on syndecan-4 leads to inside-out activation of integrin receptors. Binding of the talin FERM domain to the β integrin tail provides one key activation signal, but kindlin-2 also plays a central role in this process31. Unlike talin, the FERM domain of kindlin-2 contains a PH domain with a binding site for PIP3, which promotes talin-mediated integrin activation32. Biophysical induction of PI3K activity has been implicated in regulating integrin activation, although the molecular mechanisms have not been studied20,21.
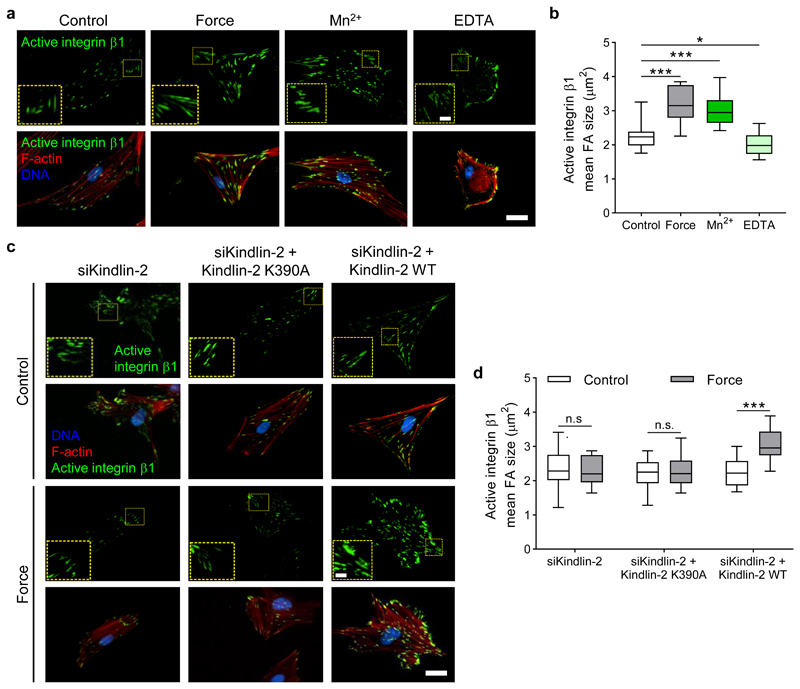
We used a permanent magnet to apply sustained ~200 pN tension for 5 min to syndecan-4 in cells plated on fibronectin. Total internal reflection fluorescence (TIRF) imaging of the basal surface of cells revealed a cell-wide activation of β1 integrins upon force application to syndecan-4, evident as an increase in the size of adhesions positive for the active conformation of integrin β1 (Fig. 3a,b). This is consistent with our findings showing PI3K-dependent cell-wide growth of kindlin-2 and talin-1 enriched focal adhesions (Fig. 2d,e). The force-induced increase of activated β1 integrins mirrored the levels induced by Mn2+ treatment (without force), which shifts integrins into a high-affinity conformation, whereas removal of divalent cations with EDTA treatment reduced β1 integrin activation (Fig. 3a,b). We observed no change in the levels of total integrin β1 incorporation into adhesions under the same conditions (Supplementary Fig. 8).
Figure 3. Tension on syndecan-4 triggers cell-wide integrin activation via PIP3 binding to kindlin-2.
a, Representative TIRF microscopy images of active β1 integrins in the basal plane of pancreatic stellate cells in (i) unstimulated control conditions, (ii) in response to a sustained tension force of ~200 pN for 5 min applied to syndecan-4, (iii) with 1 mM MnCl2 treatment for 30 min (Mn2+), and (iv) with 2 mM EDTA treatment for 30 min, both (iii and iv) in the absence of force application. b, Mean active integrin β1 focal adhesion (FA) area. nControl = 14, nForce = 14, nMn2+ = 46, nEDTA = 86 cells. c, Representative TIRF microscopy images of active β1 integrins in response to sustained tension on syndecan-4 in cells treated with siRNA targeting kindlin-2 (siKindlin-2), re-expressing a kindlin-2 mutant (K390A) which lacks phosphoinositide binding activity (siKindlin-2 + Kindlin-2 K390A), and re-expressing wild type (WT) kindlin-2. Scale bar 10 μm, inset scale bar 2 μm. d, Mean active integrin β1 focal adhesion (FA) area. nsiKindlin-2 = 14, nsiKindlin-2 + Kindlin-2 K390A = 14, nsiKindlin-2 + Kindlin-2 WT - Control = 196, nsiKindlin-2 + Kindlin-2 WT - Force = 52 cells with ≥ 5 focal adhesions measured per cell; two-sided Mann-Whitney test: *P = 0.024, ***P ≤ 0.0002, n.s. P ≥ 0.73. Boxes represent median and interquartile range, whiskers extend to the max/min data points.
Biochemical and structural studies have affirmed that the interaction of PIP3 with kindlin-2 is critical to regulating the latter’s function by promoting membrane anchoring and integrin activation32,33. We thus asked whether force on syndecan-4 regulates integrin activation through an interaction between kindlin-2 and PIP3. siRNA-mediated knockdown of kindlin-2 suppressed the previously observed activation of β1 integrins under force (Fig. 3c,d). Re-expression of a kindlin-2 mutant (K390A), lacking phosphoinositide binding activity34, into kindlin-2 knockdown cells failed to recover cell-wide activation of β1 integrins in response to tension on syndecan-4 (Fig. 3c,d, Supplementary Fig. 9). Force-induced activation of β1 integrins was rescued in kindlin-2 knockdown cells re-expressing wild type kindlin-2. Collectively, these results indicate that activation of PI3K in response to localised tension on syndecan-4 triggers cell-wide inside-out activation of β1 integrins through PIP3 interaction with kindlin-2 at focal adhesions.
RhoA activation requires additional ECM-integrin ligation
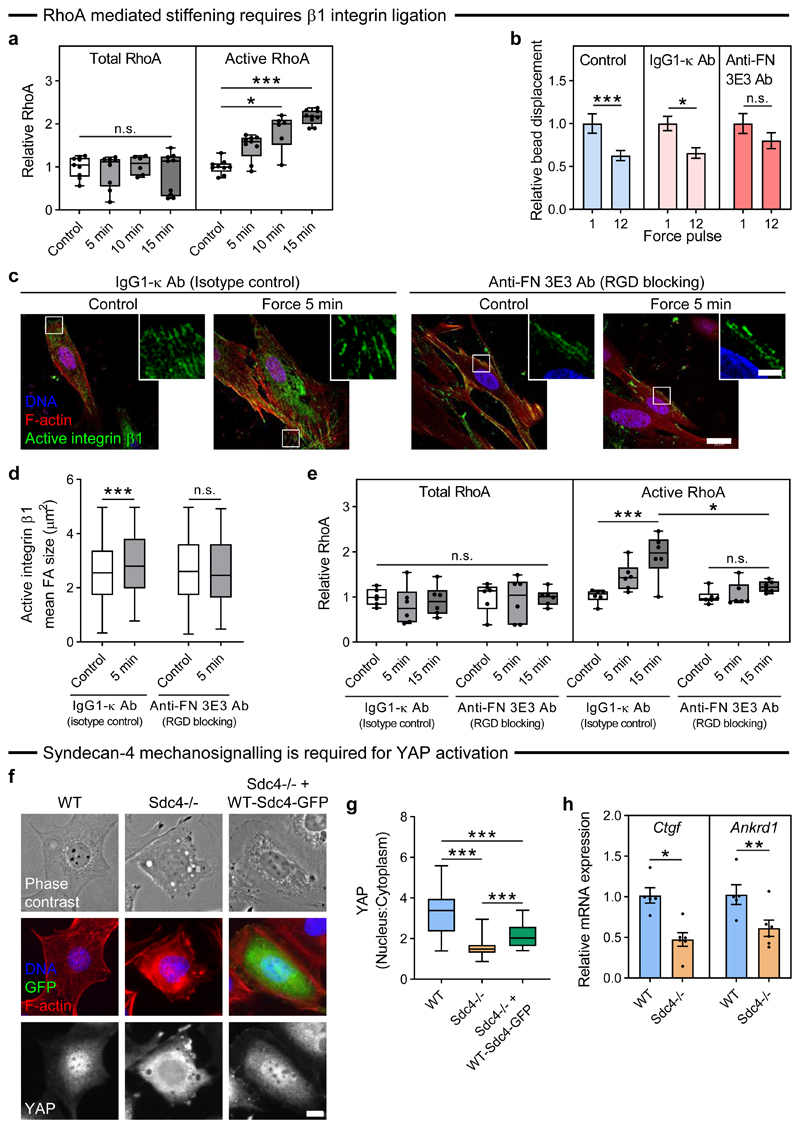
While we have shown that Rho is required for syndecan-4 mediated adaptive cell stiffening (Fig. 1d), the role that RhoA plays in this syndecan-4 mediated mechanotransduction pathway remains unclear. For this reason, we measured activation levels of RhoA and found that sustained ~200 pN tensional force on syndecan-4 increased RhoA activation in a time-dependent manner (Fig. 4a). We next sought to determine whether the observed cell-wide basal activation of β1 integrins and growth of focal adhesions is a consequence, or an essential element in the syndecan-4 mechanotransduction cascade leading to adaptive cell stiffening. Syndecan-4 is known to work in concert with integrins to regulate focal adhesion growth and cytoskeletal organisation4. Thus, we investigated whether force-induced RhoA activation and adaptive cell stiffening downstream of tension on syndecan-4 requires engagement of integrins.
Figure 4. Tension on syndecan-4 activates RhoA via integrin ligation and regulates YAP activity.
a, Pancreatic stellate cells (PSCs) on fibronectin were subjected to sustained tension of ~200 pN on syndecan-4 for 5-15 min, with total and active RhoA presented relative to unstimulated control. nControl/5min/15min = 9, n10min = 6 biologically independent samples; Kruskal-Wallis test with Dunn pairwise comparisons: *P = 0.0104, ***P < 0.0001, n.s. P = 0.823. b, Relative syndecan-4 bound bead displacement in untreated control conditions (n = 32), with IgG1-κ isotype control antibody (n = 15), or with anti-fibronectin antibody (clone 3E3) which blocks the RGD binding site on fibronectin (Anti-FN 3E3 Ab, n = 22 cells). See Supplementary Fig. 10 for single cell data. Two-sided paired signed rank test: *P = 0.035, ***P < 0.0001, n.s. P = 0.134. c, Representative confocal microscopy sections at the cell’s basal surface for active β1 integrin and F-actin in response to sustained ~200 pN tension on syndecan-4 in cells treated with IgG1-κ isotype control antibody, or Anti-FN 3E3 Ab. Scale bar 20 μm, inset scale bar 5 μm. d, Mean active integrin β1 focal adhesion (FA) area. 13-36 cells were analysed with nIgG1-κ-Control = 274, nIgG1-κ-5min = 407, nAnti-FN-Control = 417, nAnti-FN-5min = 203 adhesions; two-sided Mann-Whitney test: ***P = 0.001, n.s. P = 0.581. e, Total and active RhoA relative to unstimulated control for cells subjected to sustained syndecan-4 tension for 5-15 min in the presence of IgG1-κ and Anti-FN 3E3 antibodies. n = 6 biologically independent samples; general linear model for analysis of variance with Tukey pairwise comparisons: ***P = 0.0006, *P = 0.0488, n.s. P ≥ 0.063. f, Representative phase contrast and wide field images for DNA, GFP, F-actin, and YAP in wild type (WT) and syndecan-4 null (Sdc4-/-) mouse embryonic fibroblasts on fibronectin. Scale bar 10 μm. g, Mean nuclear:cytoplasmic YAP intensity. nWT = 43, nSdc4-/- = 64, nSdc4-/-+WT-Sdc4-GFP = 21 cells; general linear model for analysis of variance with Tukey pairwise comparisons: ***P < 0.001. h, Relative mRNA expression of YAP target genes connective tissue growth factor (Ctgf) and ankyrin repeat domain-1 (Ankrd1). nWT = 5, nSdc4-/- = 6 biologically independent samples; two-sided two-sample t-test: *P = 0.011, **P = 0.001. Boxes represent median and interquartile range, whiskers extend to the max/min data points; bar charts represent mean ± s.e.m.; individual values overlaid (a, e, h).
Fibronectin contains domains that can engage both integrins and syndecans via their heparan sulphate chains. The integrin-binding domain of fibronectin lies within the 10th type III module (FN-III10) containing the Arg-Gly-Asp (RGD) motif, while the heparin binding domain lies within type III repeats 12-14 (FN-III12-14). We thus treated cells adherent to fibronectin with an anti-fibronectin antibody (clone 3E3) which blocks the RGD integrin binding site in FN-III10 (ref. 23) to prevent integrin ligation and the formation of new fibronectin connections. We applied a 1 nN pulsatile force to syndecan-4 and observed adaptive cell stiffening in the presence of an isotype control IgG1-κ antibody (Fig. 4b). However, blocking the integrin-binding domain of fibronectin (Anti-FN 3E3 Ab) inhibited the stiffening response (Fig. 4b); suggesting that syndecan-4 mediated force transduction requires engagement of new β1 integrins with the underlying fibronectin substrate. Indeed, when syndecan-4 on cells in the presence of the same antibodies was subjected to sustained force of ~200 pN for 5 min using a permanent magnet, immunofluorescent staining revealed enhanced engagement of new β1 integrins in the presence of the IgG control antibody which did not occur when the RGD integrin binding site was blocked (Anti-FN 3E3 Ab; Fig 4c,d). Consistent with this, blocking of fibronectin’s RGD integrin binding site also suppressed RhoA activation upon sustained force application on syndecan-4 (Fig. 4e). No changes in cell spread area were observed upon blocking of the RGD integrin binding site (Supplementary Fig. 11). Analogous but slightly attenuated adaptive stiffening and RhoA responses were observed in cells on collagen type I (Supplementary Fig. 12), indicating that RGD-specific integrin ligation is not a requirement for RhoA induction. These results demonstrate that activation of RhoA in response to tension on syndecan-4 requires the engagement of new integrin-ECM connections.
Syndecan-4 mediated tension regulates YAP activation
RhoA activity and cytoskeletal tension are known actors in various mechanotransduction pathways which lead to changes in gene expression impacting cell function35. Yes-associated protein (YAP) is a transcription coactivator with a well-established role in converting biophysical inputs into gene expression signatures36. We sought to investigate whether syndecan-4 modulates YAP activity to regulate gene expression and cell function.
Wild-type (WT) and syndecan-4 null (Sdc4-/-) mouse embryonic fibroblasts (MEFs) were plated onto fibronectin-coated glass or 25 kPa polyacrylamide gels. WT cells adopted a spread morphology with prominent F-actin stress fibres and a high nuclear:cytoplasmic YAP ratio (Fig. 4f,g, Supplementary Fig. 13). In contrast, YAP was mostly cytoplasmic in Sdc4-/- cells, and while these cells adhered and spread as normal, they lacked F-actin stress fibres, suggestive of lower cytoskeletal tension (Fig. 4f,g, Supplementary Fig. 13). Nuclear localisation of YAP could be partially rescued with the expression of WT syndecan-4-GFP in Sdc4-/- cells (Fig. 4f,g). These syndecan-4 dependent changes in YAP activity altered the mRNA expression of established YAP target genes as both connective tissue growth factor (Ctgf) and ankyrin repeat domain 1 (Ankrd1) were found to be downregulated in Sdc4-/- cells compared to WT cells (Fig. 4h).
The role of syndecan-4 in the regulation of YAP activity is not limited to fibronectin, as we also observed a reduction in the ratio of nuclear:cytoplasmic YAP in Sdc4-/- compared to WT cells when plated on collagen type I (Supplementary Fig. 14). Consistent with the attenuated syndecan-4 force mediated cell-stiffening and RhoA activation observed in cells on collagen type I as opposed to fibronectin, both WT and Sdc4-/- cells exhibited decreased nuclear:cytoplasmic YAP on collagen type I compared to fibronectin (Supplementary Fig. 14). These results suggest that syndecan-4-mediated tension at the cell-ECM interface is essential for YAP transcriptional signalling.
Syndecan-4’s V-region is required for mechanotransduction
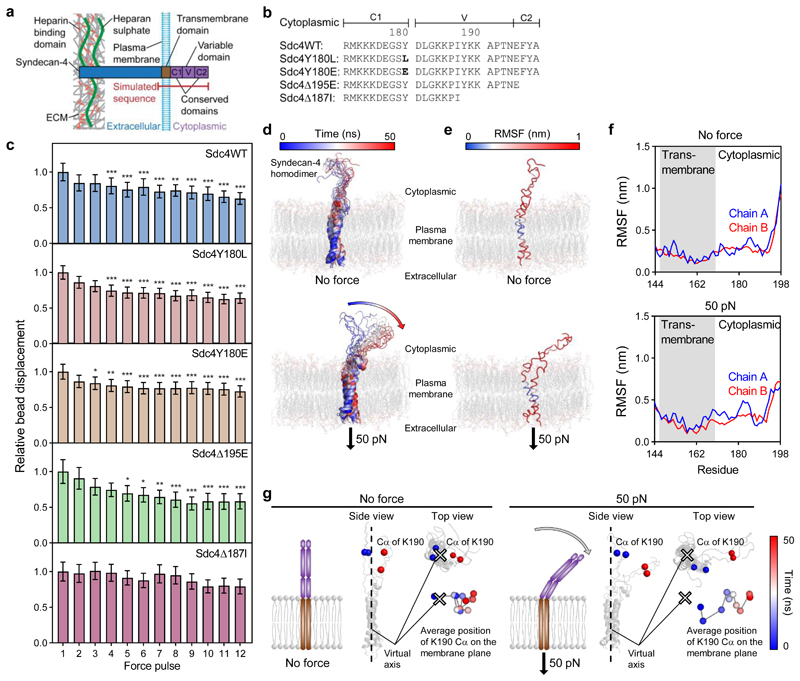
We next sought to identify the molecular mechanism through which tension applied to the extracellular domain of syndecan-4 provokes a mechanoresponse. The cytoplasmic domain of syndecan-4 regulates interactions with scaffolding and signalling proteins and likely plays a role in mediating syndecan-4 mechanotransduction. It comprises a pair of conserved (C1 and C2) regions common to all syndecan isoforms, separated by a variable (V) region specific to syndecan-4 (Fig. 5a).
Figure 5. Syndecan-4 mechanotransduction requires the V-region which changes conformation under force.
a, Schematic representation of syndecan-4 structure. b, Cytoplasmic domain amino acid sequences for wild type syndecan-4 (Sdc4WT), syndecan-4 which cannot be phosphorylated on Y180 (Sdc4Y180L), phospho-mimetic syndecan-4 (Sdc4Y180E), syndecan-4 truncated in the C2 domain (Sdc4Δ195E) and syndecan-4 truncated in the V domain (Sdc4Δ187I). c, Displacement of syndecan-4 bound beads in response to pulsatile 1 nN force in mouse embryonic fibroblast cell lines expressing wild type or mutant syndecan-4 on fibronectin, relative to force pulse 1. See Supplementary Fig. 15 for single cell data. nSdc4WT = 27, nSdc4Y180L = 35, nSdc4Y180E = 43, nSdc4Δ195E = 23, nSdc4Δ187I = 22 cells; Friedman test with Dunn pairwise comparisons: *P ≤ 0.0238, **P ≤ 0.0092, ***P ≤ 0.001 vs force pulse 1. Mean ± s.e.m. d, Molecular structure snapshots of syndecan-4 at 5 ns intervals during a 50 ns simulation with no force applied (molecular dynamics (MD)) and with constant force pulling at 50 pN on the extracellular domain of syndecan-4 (steered molecular dynamics (SMD)). e-f, Root mean square fluctuation (RMSF) of syndecan-4 Cα atoms overlaid on molecular structure (e) and plotted against residue (f). g, Average position of lysine K190 Cα atoms on the membrane plane representing directed movement of the cytoplasmic domain from the virtual axis (located between transmembrane helices) towards the membrane in SMD. Average position was calculated for both K190 Cα atoms (chain A and B) in each 5 ns increment. See Supplementary Fig. 17 for amino acid sequence used in simulations.
Induction of protein phosphorylation is one of the first events that occurs when mechanical force is applied to cells37. We investigated the tyrosine residue Y180 in the C1 region of syndecan-4 which has been identified as a major phosphorylation site controlling integrin trafficking, focal adhesion dynamics and migratory behaviour30. Pulsatile 1 nN tension was applied to syndecan-4 bound beads attached to MEFs expressing either a syndecan-4 mutant which could not be phosphorylated on Y180 (Sdc4Y180L), or a phospho-mimetic syndecan-4 that emulates c-Src-mediated phosphorylation of the Y180 residue (Sdc4Y180E, Fig. 5b)30. The observed reduction in relative bead displacement demonstrated that both phospho-null Sdc4Y180L and phospho-mimetic Sdc4Y180E cells were able to stiffen under force; indicating that the phosphorylation status of Y180 is dispensable for syndecan-4 mechanotransduction (Fig. 5c).
The conserved C2 domain contains a PDZ-binding site that interacts with PDZ-domain containing proteins, which coordinate receptor clustering, exosome biogenesis and the formation of connections to the actin cytoskeleton, mainly via calcium/calmodulin-dependent serine protein kinase (CASK)38,39. We expressed a truncated syndecan-4 core protein lacking the C-terminal C2 (EFYA) domain (Sdc4Δ195E, Fig. 5b, Supplementary Fig. 16) in syndecan-4 null MEFs. Deletion of the C2 domain had no discernible effect on syndecan-4 mediated cell stiffening (Fig. 5c).
The syndecan-4 specific V-region interacts with both PKC-α and the phospholipid phosphatidylinositol (4,5) bisphosphate (PIP2) to transduce downstream signals for cytoskeletal organisation39,40. The V-region further associates with the actin-crosslinking protein α-actinin to provide a direct mechanical link to the cytoskeleton41. MEFs expressing a syndecan-4 core protein truncated in the centre of the V-region (Sdc4Δ187I, Fig. 5b, Supplementary Fig. 16) were unable to mount a stiffening response upon mechanical stimulation of syndecan-4 (Fig. 5c). The Sdc4Δ187I truncation deletes the binding site for PKC-α and α-actinin41,42. Previously, V-region truncation at this site has been associated with defects in cytoskeletal organisation, stress fibre assembly and focal adhesion formation, as well as inhibited myofibroblast differentiation8,11. As expected, adaptive cell stiffening was observed when tension was applied to MEFs expressing wild type syndecan-4 (Sdc4WT; Fig. 5c, Supplementary Fig. 16). These results indicate that interactions between the V-region of syndecan-4’s cytoplasmic domain and its binding partners α-actinin and/or PKC-α is necessary to initiate mechanotransduction and mechanical adaptation to force.
External tension alters cytoplasmic syndecan-4 conformation
To investigate the effect of tensional force on syndecan-4 at the molecular level, we conducted molecular dynamics (MD) simulations. The system consisted of homodimeric transmembrane and cytoplasmic domains of syndecan-4 embedded within a membrane bilayer containing a mixture of lipids, including PIP2. In equilibrium 50 ns MD simulations (no applied force) syndecan-4 structure showed no major conformational changes in the cytoplasmic domain (Fig. 5d). Constant force pulling at 50 pN applied to the extracellular side of syndecan-4 caused directed movement of the cytoplasmic domain towards the membrane (Fig. 5d). Root mean square fluctuations (RMSF) of Cα show that the transmembrane helices in the hydrophobic core of the membrane are mostly stable during the simulation while the C-terminal residues (C2 region) are more flexible (Fig. 5e,f). We chose the lysine residue K190, as one of the most stable positions in the V region, to monitor the conformational changes of the cytoplasmic domain on the membrane plane (Fig. 5g). Directed movement of the cytoplasmic domain led to association of syndecan-4 with the membrane, where electrostatic interactions between lysine residues and heads of PIP2 lipids took place. Using constant force pulling at 50 pN and 100 pN over a 50 ns simulation, we observed similar tilting of the cytoplasmic domain towards the plasma membrane (Supplementary Figs. 17,18). The use of 25 pN constant force caused a similar but slower conformational change within the cytoplasmic domain with 100 ns required to observe an effect like that seen at 50 ns under 50 pN. Using a higher force magnitude of 200 pN caused increased sliding of the protein through the membrane, resulting in non-directed movement of the cytoplasmic domain (Supplementary Fig. 18).
The observed force-dependent orientation of the cytoplasmic domain, leading to membrane interactions, may be important in the modulation of intracellular associations between syndecan-4 and its binding partners, including the scaffolding protein α-actinin which is known to interact with the region.
Force stabilises a syndecan-4/α-actinin/F-actin linkage
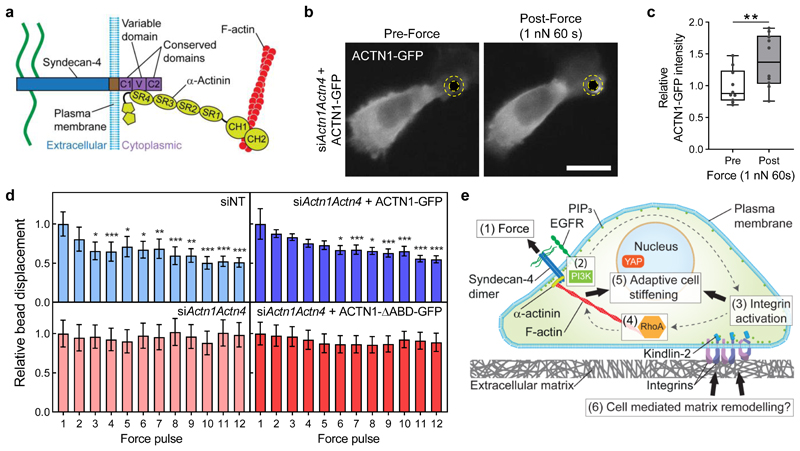
We next sought to investigate how the force-induced conformational change in the cytoplasmic V region is linked to syndecan-4 mechanosignalling. α-actinin, via spectrin repeat 4 (SR4) in its rod domain, interacts with syndecan-4’s cytoplasmic V-region to establish a mechanical linkage between syndecan-4 and the actin cytoskeleton (Fig. 6a)10. We used siRNA to knockdown the endogenous expression of the predominant α-actinin isoforms — α-actinin-1 (Actn1) and α-actinin-4 (Actn4; siActn1Actn4) — in MEFs, and subsequently expressed fluorescently tagged α-actinin-1 (ACTN1)-GFP which localised to focal adhesions throughout the cell (Supplementary Fig. 19). When 1 nN sustained tension was applied to syndecan-4 bound beads with magnetic tweezers, accumulation of ACTN1-GFP could be observed in a region of interest surrounding the magnetic bead after 60 s (Fig. 6b,c).
Figure 6. Tension stabilises a syndecan-4/α-actinin/F-actin linkage to facilitate adaptive stiffening.
a, Schematic representation of the interaction between syndecan-4 and α-actinin which provides a mechanical link to the cytoskeleton. α-actinin spectrin repeat 4 (SR4) binds to the syndecan-4 variable (V) domain, while the calponin-homology (CH) domains of α-actinin bind to F-actin. b, Representative wide field microscopy images of α-actinin-1 (ACTN1)-GFP expressing mouse embryonic fibroblasts (MEFs) pre (0 s) and post (60 s) the application of sustained tension on syndecan-4. MEFs were depleted of endogenous α-actinin-1 and α-actinin-4 (siActn1Actn4) and transfected to express ACTN1-GFP. Imaging conducted in the apical focal plane of the bead attachment. Scale bar: 20 μm. See Supplementary Fig. 20 for GFP control. c, Mean ACTN1-GFP intensity, in a region of interest surrounding the bead depicted by yellow dashed overlay, relative to mean intensity prior to force application. n = 10 cells; two-sided paired signed rank test: **P = 0.002. Boxes represent median and interquartile range, whiskers extend to the max/min data points, individual values are overlaid. d, Relative syndecan-4 bound bead displacement in MEFs treated with non-targeting control siRNA (siNT; n = 18) or siRNAs targeting Actn1 and Actn4 (siActn1Actn4; n = 18) with expression of ACTN1-GFP (siActn1Actn4 + ACTN1-GFP; n = 23) or a mutant form of α-actinin with the actin binding domain (ABD) deleted (siActn1Actn4 + ACTN1-ΔABD-GFP; n = 20 cells). See Supplementary Fig. 21 for single cell data. Friedman test with Dunn pairwise comparisons: *P ≤ 0.0492, **P ≤ 0.0058, ***P ≤ 0.0009 vs force pulse 1. Mean ± s.e.m. e, Schematic representation of the described mechanotransduction pathway where force on syndecan-4 (1) activates PI3K (2) in an EGFR-dependent manner. PIP3 binds kindlin-2 leading to integrin activation (3) and ligation of integrins with ECM which instigates RhoA signalling (4) leading to adaptive cell stiffening (5) with the potential to modulate matrix remodelling (6).
Further to this finding, MEFs with siRNA-mediated knockdown of α-actinin-1 and -4 (siActn1Actn4) were unable to exhibit an adaptive stiffening response to 1 nN pulsatile tension applied on syndecan-4 (Fig. 6d). While adaptive stiffening could be rescued through re-expression of full length ACTN1-GFP into α-actinin depleted cells (siActn1Actn4 + ACTN1-GFP), re-expression of an α-actinin mutant, where the actin-binding domain (ABD) was deleted (siActn1Actn4 + ACTN1-ΔABD-GFP)43, failed to rescue the adaptive stiffening response to tension on syndecan-4 (Fig. 6d). Cells transfected with non-targeting control siRNA (siNT) maintained the adaptive stiffening response (Fig. 6d).
These results imply that force on syndecan-4 stabilises the assembly of a syndecan-4/α-actinin/F-actin molecular scaffold at the cell-ECM junction to facilitate force transmission and downstream syndecan-4 mechanosignalling (Fig. 6e).
Discussion
It has previously been reported that syndecan-4 can initiate mechanochemical signalling without the need for other ECM receptors, exhibiting vinculin recruitment and activation of ERK17 and PKC-α18. Syndecan-4 is also implicated in flow sensing in endothelial cells that ultimately confers atheroprotective effects15,16. We provide evidence that while syndecan-4 is capable of initiating mechanotransduction as a lone ECM receptor, the underlying signalling network integrates distinct plasma membrane receptors; EGFR which augments the mechanoresponse and β1 integrins which are required for adaptive cellular stiffening. Our results demonstrate that localised tension on syndecan-4 initiates an adaptive stiffening response through a mechanosignalling cascade that requires synergistic cell-wide activation of β1 integrins and formation of new integrin-ECM connections, followed by subsequent RhoA induced actomyosin contractility. We have established a force-dependent syndecan-integrin crosstalk with these two mechanosensors acting synergistically to induce RhoA activation, adaptive stiffening and YAP transcriptional signalling.
While adaptive cell stiffening and local adhesion strengthening are common mechanosensitive responses shared among integrins and cadherins, perhaps the most striking aspect of syndecan-4 mechanosensing is how a localised force can elicit a cell-wide response as evidenced by the global growth of focal adhesions and activation of integrins. Intriguingly, our data suggest syndecan-4 mechanosignalling is both chemically and mechanically propagated throughout the cell via diffusion of PIP3 lipid second messengers and mechanical transmission through the tensile cytoskeleton via a syndecan-4/α-actinin/F-actin molecular scaffold. This contrasts with the localised stiffening response which occurs when force is applied to integrin adhesions.
The dependence of adaptive stiffening on PI3K and integrins parallels in many ways the mechanotransduction pathways mediated by PECAM-1 in endothelia20 and E-cadherin in epithelia21. Localised tension on PECAM-1 elicits a cell-wide mechanotransduction response20, which is mediated by a mechanosensory complex additionally comprising vascular endothelial cell (VE-) cadherin and vascular endothelial growth factor receptor 2 (VEGFR2) which activates PI3K44. The analogous global mechanotransduction response observed for these distinct mechanoreceptors—syndecan-4, PECAM-1 and E-cadherin—suggests that the kindlin-integrin-RhoA pathway leading to adaptive cell stiffening may be a conserved mechanism employed by numerous mechanosensory complexes.
The role of integrin-mediated mechanosignalling has been the subject of intense investigation, however a more integrative approach that includes syndecan-4 as a mechanosensor could unearth new disease mechanisms. In summary, our results suggest that syndecan-4 is a key cellular mechanotransducer that responds to localised mechanical tension by eliciting a global response that regulates cell mechanics via cell-wide engagement of a PIP3/kindlin-2/integrin/RhoA axis as well as serving as a potent modulator of YAP activity with important implications for mechanotransduction, syndecan biology, and cell-ECM interactions.
Methods
Cell culture and reagents
Primary human culture activated pancreatic stellate cells (PSCs; Caltag Medsystems SC-3830) were cultured at 37°C, 5% CO2 in Dulbecco’s modified Eagle’s medium/F12 Ham (DMEM/F12; D8437) with 2% foetal bovine serum (FBS), 100 U ml-1 penicillin-100 μg ml-1 streptomycin (all Sigma-Aldrich) and 2.5 μg ml-1 amphotericin B (Gibco) and used at passage 4-8. Immortalised mouse embryonic fibroblast (MEF) cell lines were a gift from Dr Mark Morgan and included wild type, syndecan-4 -/- (ref. 45), and lines expressing mutant syndecan-4 phospho-null Y180L, and phospho-mimetic Y180E (ref. 30). MEFs were cultured at the large T-antigen permissive temperature of 33°C in high glucose DMEM (Gibco) with 10% FBS and 100 U ml-1 penicillin-100 μg ml-1 streptomycin (all Sigma-Aldrich). All experiments and treatments were conducted at 37°C. Cells were plated on substrates coated with 2.5 μg ml-1 fibronectin or 10 μg ml-1 collagen type I (Sigma-Aldrich). 25 kPa polyacrylamide gels were fabricated as described previously46. Expression of syndecan-4 in MEFs and PSCs was confirmed using western blot (Supplementary Fig. 22).
Cells were treated with 0.5 μM latrunculin A (Merck 428026) for 1 h to inhibit F-actin polymerisation, with 2 μg ml-1 C3 transferase (Cytoskeleton Inc.) for 4 h to inhibit Rho, with 10 μM Y-27632 (Merck 688001) for 1 h to inhibit ROCK, with 30 μM LY-294002 (Sigma Aldrich L9908) for 1 h to inhibit PI3K, with 10 μM SH-5 (Abcam ab141442) for 1 h to inhibit AKT, and with 15 μM Gefitinib (Santa Cruz Biotechnology sc-202166) for 2 h to inhibit EGFR. Cells were treated with 1 mM MnCl2 (Fisher Scientific) for 30 min to induce integrin activation, and 2 mM EDTA (Sigma Aldrich) for 30 min to sequester divalent ions responsible for integrin activation. Cells were treated with 5 μg ml-1 mouse anti-human EGF antibody, clone 10825 (R&D Systems MAB236; RRID:AB_2095960) to neutralise EGF. Cells plated on fibronectin substrates were treated with 20 μg ml-1 mouse anti-fibronectin antibody (cell attachment fragment), clone 3E3 (Merck MAB88916; RRID:AB_95479), for 30 min to block integrin ligation to RGD. Mouse IgG1-κ, clone MOPC-21 (Merck MABF1081Z; RRID:AB_395252) was used as an isotype control.
PSCs were transfected by electroporation using the Neon Transfection system (Thermo Fisher Scientific) with one pulse of 1,300 V for 30 ms. WT and Sdc4-/- MEF cell lines were transfected using Lipofectamine 3000 (Thermo Fisher Scientific). After transfection, cells were allowed recover in antibiotic-free media for 24 h. Experiments were conducted 48 h post transfection. Cells were transfected with siRNA targeting kindlin-2 (Mig-2, sc-106786), both α-actinin-1 (Actn1, sc-43096) and α-actinin-4 (Actn4, sc-43102), or non-targeting control (sc-37007, all Santa Cruz Biotechnology). The PH-AKT-GFP plasmid was a gift from Tamas Balla (Addgene plasmid 51465; RRID:Addgene_51465)47. The kindlin-2-GFP plasmid was a gift from Jun Qin32. The kindlin-2-K390A-HA plasmid lacking phosphoinositide binding activity was a gift from Chuanyue Wu34. The syndecan-4 C2 truncation plasmid Sdc4Δ195E (ref. 11) and the syndecan-4 V-domain truncation plasmid Sdc4Δ187I (ref. 8) were gifts from John Couchman. The α-actinin-1 (ACTN1)-GFP plasmid was a gift from Carol Otey (Addgene plasmid 11908; RRID:Addgene_11908)48 and α-actinin-1 (ACTN1)-ΔABD-GFP (Addgene plasmid 66935; RRID:Addgene_66935) was as described previously43. Control empty vector pEGFP-N1 was from Clontech. siRNA mediated knockdown of kindlin-2 was confirmed with immunofluorescent staining (Supplementary Fig. 9) and knockdown of α-actinin confirmed using western blot against α-actinin-4 (Supplementary Fig. 19). Plasmid expression was assessed based on GFP signal or immunofluorescent staining for the HA tag (Supplementary Fig. 16).
Magnetic bead preparation
Antibodies and ECM ligands were coupled to 4.5 μm epoxide paramagnetic beads (Dynabeads M-450 Epoxy, Thermo Fisher Scientific) according to manufacturer’s instructions. Beads were washed twice in 0.1 M sodium phosphate buffer, pH 7.4, before coupling with 40 μg antibody or ECM ligand per 8×107 beads in 0.1 M sodium phosphate buffer pH 7.4 for 30 min followed by addition of 0.1% bovine serum albumin (BSA) and further incubation for 16 h at room temperature with gentle rotation throughout. Beads were washed and subsequently stored in 0.1% BSA-phosphate buffered saline (PBS) pH 7.4 at 4°C. Beads were coated with mouse anti-syndecan-4 antibody, clone 5G9 (Santa Cruz Biotechnology sc-12766; RRID:AB_628314), poly-L-lysine, fibronectin (both Sigma-Aldrich), the 40 kDa α-chymotryptic fragment of fibronectin which contains the CS-1 heparin binding domain (Merck F1903), or mouse anti-transferrin receptor-1, clone 3B8 2A1 (CD71, Santa Cruz Biotechnology sc-32272; RRID:AB_627167). For experiments, ligand-coated magnetic beads were resuspended in media and incubated with cells for 30 min at 37°C. Cells were briefly washed with PBS to remove unbound beads prior to force application. Syndecan-4 binding to functionalised beads was confirmed by immunofluorescent staining (Supplementary Fig. 1) and isolation of the bead adhesion complex in cell lysates and subsequent western blotting (Supplementary Fig. 2).
Force application to cell bound beads
A custom-built electromagnetic tweezers apparatus fitted to an inverted microscope (Eclpise Ti, Nikon) was used to apply controlled pulsatile forces to individual ligand-coated magnetic beads attached to cells plated onto fibronectin or collagen type I coated glass bottomed dishes (Fluorodish, World Precision Instruments). Application of the pulsatile regimen consisting of 12 pulses of 1 nN force for 3 s with intervening 4 s periods of relaxation was achieved through a LabVIEW interface (National Instruments). On force application, bead displacements were recorded with a high-speed CMOS camera (Andor Neo sCMOS, Oxford Instruments) at 50 Hz using a 40× 0.6 NA air objective (Nikon). Displacement was tracked using a custom-written particle tracking algorithm in MATLAB that detects the intensity-weighted centroid of the bead with subpixel accuracy. The electromagnetic tweezers device was calibrated before the experiments using a fluid of known viscosity. Beads that showed displacements of less than 50 nm (detection resolution) and loosely bound beads were not selected for analysis. Application of sustained 1 nN force was also applied to cells for 60 s with concurrent imaging of GFP localisation around the attached bead. To apply a constant force on a larger number of beads, we used a permanent neodymium magnet that allowed us to apply a vertical tensile force on the beads with magnitudes of approx. 200 pN.
Immunofluorescent staining and image analysis
Cells were seeded on ECM coated coverslips for immunofluorescent staining. Following pertinent treatment, cells were fixed with 4% paraformaldehyde (Sigma-Aldrich P6148) in PBS for 10 min, permeabilised with 0.1% Triton X-100 (Sigma, T8787) in PBS for 10 min and blocked with 5% donkey or goat serum in 1% BSA-PBS containing 0.01% Tween 20 (Sigma-Aldrich; PBST). In some cases, required primary antibodies were of the same species as the antibodies used to functionalise beads, or as treatment. To block secondary antibody binding to these sites, cells were incubated with an excess of anti-mouse secondary antibody in 1% BSA-PBST for 1h followed by PBS washing prior to incubation with primary antibodies prepared in 1% BSA-PBST for 1 h at room temperature or overnight at 4°C. Cells were washed in PBST and incubated with donkey or goat Alexa Fluor conjugated secondary antibodies (1:1,000; Thermo Fisher Scientific) and phalloidin (1:40; Thermo-Fisher Scientific, or 1:1,000; Santa Cruz Biotechnology) in 1% BSA-PBST for 30 min to 1 h at room temperature. Finally, coverslips were washed in PBST, and nuclei stained with 1 μg ml-1 DAPI (Sigma Aldrich) prior to mounting using ProLong mounting reagent or mounted in ProLong containing DAPI (Thermo Fisher Scientific, P36931). Primary antibodies: rabbit anti-syndecan-4 (1:100; Abcam ab24511; RRID:AB_448112), mouse anti-integrin β1, clone A-4 (1:100; Santa Cruz Biotechnology sc-374429; RRID:AB_11012020), mouse anti-integrin β1, clone 12G10 (1:100; Santa Cruz Biotechnology sc-59827; RRID:AB_782089), rabbit anti-talin-1 (1:400; Abcam, ab71333; RRID:AB_2204002), mouse anti-kindlin-2, clone 3A3 (1:250; Merck MAB2617; RRID:AB_10631873), mouse anti-β1 integrins, active conformation, clone HUTS-4 (1:100; Merck MAB2079Z; RRID:AB_2233964), mouse anti-YAP, clone 63.7 (1:200; Santa Cruz Biotechnology sc-101199; RRID:AB_1131430), rabbit anti-GFP (1:2,000; Abcam ab6556; RRID:AB_305564), mouse anti-HA.11 epitope tag, clone 16B12 (1:1,000; Biolegend 901513; RRID:AB_2565335), mouse anti-α-actinin, clone BM-75.2 (1:200; Sigma Aldrich A5044; RRID:AB_476737).
Samples were imaged using epifluorescent microscopy (Leica DMI4000B, Leica DMi8, Nikon Eclipse Ti, AE31 Motic), total internal reflection fluorescence (TIRF) microscopy (Nikon Eclipse Ti), confocal microscopy (Zeiss LSM 710, Nikon Eclipse Ti) or structured illumination microscopy (GE OMX) as indicated in figure legends. Image quantification was conducted using NIS Elements (Nikon) or Fiji (ImageJ, NIH)49. A region of interest (ROI) with inner diameter equal to the bead size and outer diameter 9 μm was used to quantify immunofluorescent staining or GFP intensity at the bead adhesion. In live GFP images, this was applied to a single epifluorescent image for each time point. For localisation of adhesion proteins to the bead in immunofluorescently stained cells, the location of the bead was first located in widefield mode, and then imaged using confocal microscopy with a z-spacing of 125 μm. Intensity within the ROI was summed through 5 z-sections expressed as mean fluorescent intensity. Focal adhesion size was assessed from TIRF microscopy images or confocal z-sections of the cell’s basal surface. An intensity-based threshold was applied to images to isolate talin-1, kindlin-2 or active integrin β1 enriched focal adhesions from background for selection. To assess YAP nuclear:cytoplasmic ratio, DAPI stained nuclei were selected based on an intensity threshold which provided ROIs for each nucleus. Morphological outlines of cell area were manually traced on F-actin images providing ROIs for each cell. The nucleus ROI could be subtracted from the cell ROI to obtain the cytoplasmic ROI. The mean intensity within each ROI was used to calculate the YAP nuclear:cytoplasmic ratio.
RhoA activity assay
Total RhoA and active RhoA-GTP were quantified using total RhoA ELISA and active RhoA G-LISA assay kits (Cytoskeleton, Inc. BK150 and BK124) according to the manufacturer’s instructions. Immediately after treatment, cells were rinsed with ice-cold PBS and homogenised in ice-cold lysis buffer. Protein was quantified and sample concentration adjusted to 0.5 mg ml-1. For the G-LISA, binding buffer was added, and assays performed in triplicate using 1.5 μg protein per well. Target binding to the Rho GTP-binding protein coated well was facilitated by 30 min incubation at 4°C with shaking, followed by washing. For the ELISA, protein lysate was added to wells coated with anti-Rho IgY antibody and incubated for 120 min at room temperature followed by washing. Antigen-presenting buffer was added for 2 min, removed, and samples incubated with the anti-RhoA antibody (1:250) for 45 min (G-LISA) or 1 h (ELISA) at room temperature. Samples were washed three times and incubated with HRP conjugated secondary antibody for 45 min or 1 h at room temperature. HRP detection reagent was added, samples incubated briefly, and absorbance measured at 490 nm.
Gene expression analysis
RNA was isolated using the RNeasy Mini Kit (Qiagen 74104). The High-Capacity RNA-to-cDNA Kit (Thermo Fisher Scientific 4387406) was used to reverse transcribe 1 μg of RNA. Real time RT-PCR was performed in a 20 μl reaction with 100 ng cDNA, 300 nM primers and SYBR Green PCR Master Mix (Applied Biosystems 4309155). Primer sequences were: connective tissue growth factor (CTGF): forward 5’-CATACTCCACAGAATTTAGCTC-3’, reverse 5’-TTAAGAAGGGCAAAAAGTGC-3’; ankyrin repeat domain 1 (ANKRD1): forward 5’-TGAGTATAAACGGACAGCTC-3’, reverse 5’-TATCACGGAATTCGATCTGG-3’; and glyceraldehyde 3-phosphate dehydrogenase (GAPDH): forward 5’-ACAGTTGCCATGTAGACC-3’, reverse 5’-TTTTTGGTTGAGCACAGG-3’. Relative expression was determined using the 2−ΔΔCt method with GAPDH used as endogenous control.
Molecular Dynamics (MD) and Steered Molecular Dynamics (SMD) simulations
In simulations, we used the syndecan-4 structure including transmembrane and cytoplasmic domains. The NMR structure of the cytoplasmic domain (ID 1EJP in protein data bank) was used, while the transmembrane domain was modelled with SWISS-MODEL tool using the NMR structure of the dimeric transmembrane domain of human glycophorin A (ID 1AFO in protein data bank) as a template. A lipid membrane with 40 % of POPC (144 residues), 15 % POPS (54 residues), 5 % PIP2 (18 residues) and 40 % cholesterol (144 residues) was generated using Charmm-gui50. Embedding of protein into the membrane was performed by applying high lateral pressure on the system as described before51. All simulations were performed using Gromacs 2016.1 (ref. 52) at the Sisu supercomputer, CSC, Finland. All-atom OPLS-AA force field53 and explicit TIP3P water model54 in 0.15 M KCl neutral solution were used. Energy minimisation of the system was performed in 10,000 steps using the steepest descent algorithm. Equilibration of the system was performed in three phases using harmonic position restraints on lipid/cholesterol heads in the Z dimension and on all heavy atoms of protein. (1) NVT ensemble simulation was performed for 100 ps utilising the Berendsen weak coupling algorithm55 at 100 K. (2) Next, the system was heated from 100 to 310 K with constant heating rate over 1 ns using an NPT ensemble at 1 bar, using the Berendsen algorithm to control both temperature and pressure. Semi-isotropic pressure coupling was used for all the simulations to control pressure in the XY plane (membrane plane) and in the Z dimension separately. Temperature coupling was applied separately for protein/membrane and solution parts. (3) The final equilibration phase was performed using NPT ensemble at 310 K and 1 bar using Nose-Hoover56,57 and Parrinello-Rahman58 algorithms, respectively. These conditions were used in all subsequent simulations. An integration time step of 2 fs was used in all the simulations. Equilibrium MD simulations (with no force applied) for 50 ns were conducted, considering the first 20 ns as a relaxation step. Hence, conformation of the system at 20 ns of the first run of equilibrium MD simulation was used as a starting structure for SMD simulations. The pulling vector was set between the membrane (centre of mass) and Cα of N-terminal residues of the protein. SMD simulations were performed with constant force pulling using different force regimes: 25 pN, 50 pN, 100 pN and 200 pN. Two simulations were conducted for 25 pN, 100 pN and 200 pN, and four simulations for equilibrium MD and SMD with 50 pN of constant force pulling. All the simulations were run for 50 ns, except SMD simulations with 25 pN which were run for 100 ns.
Statistical analysis
Statistical analyses were conducted using GraphPad Prism 8 (RRID:SCR_002798; GraphPad Inc.) or Minitab 18 (RRID:SCR_014483; Minitab Inc.). Data were generated from multiple experimental repeats each containing multiple biological and technical replicates. Details of data presentation, number of replicates, statistical tests and significance is denoted in the figure legends. Where datasets followed a parametric distribution, experimental groups were compared using two-sided two-sample or paired t-tests, or when multiple experimental variables, analysis of variance with Tukey pairwise comparisons. Where datasets were non-parametric, two-sided Mann-Whitney tests, or two-sided paired rank tests were used in place of two-sample and paired t-tests respectively. In the case of multiple experimental groups, non-parametric data was transformed to fit a parametric distribution using a Box-Cox transformation prior to analysis of variance and pairwise comparisons, or the Kruskal-Wallis test used. Non-parametric bead displacement data over successive force pulses was analysed using the Friedman test with Dunn pairwise comparisons. The significance level for all tests was set at 0.05.
Supplementary Material
Acknowledgements
This work was supported by the European Research Council (ERC grant 282051), the Biotechnology and Biological Sciences Research Council (BBSRC grant BB/N018532/1), and the Academy of Finland (grant 290506). VM was supported by an EDUFI (former CIMO) postdoctoral fellowship and Academy of Finland funding for Postdoctoral Researcher (grant 323021). We thank: Dr Mark Morgan (University of Liverpool) for providing MEF cell lines, Prof John Couchman (University of Copenhagen) for providing syndecan-4 cytoplasmic truncation plasmids (C2 and V domains), Prof Jun Qin (Cleveland Clinic) for the kindlin-2-GFP plasmids, Prof Chuanyue Wu (University of Pittsburgh) for the kindlin-2 K390A plasmid, Francesco Di Maggio for help in implementing the initial work with pancreatic stellate cells. We acknowledge CSC – IT Center for Science, Finland for computational resources. We are also grateful to all CMBL members for help and advice throughout this work.
Footnotes
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Code Availability
MATLAB code used to track bead displacements in magnetic tweezers experiments is available from A.D.R.H. upon reasonable request. Code used in molecular dynamics simulations is available from V.P.H. upon reasonable request.
Author contributions
A.C. and A.J.R. conducted magnetic tweezers experiments; A.C. and S.D.T. conducted permanent magnet experiments; S.D.T. and A.C. performed and analysed experiments with mouse embryonic fibroblast cells; A.C. E.C., D.L. and S.D.T. performed and analysed experiments with pancreatic stellate cells; S.D.T. carried out transfections, and western blots, and IF experiments supervised by D.A.L.; E.C. carried out RhoA activity experiments; V.V.M. performed MD and SMD experiments supervised by V.P.H. and T.R.; D.L. performed IF experiments and data analysis; A.C., S.D.T. and A.D.R.H. designed the studies, and wrote and prepared the manuscript with significant inputs from V.P.H.; all authors commented on the manuscript.
Competing financial interests
The authors declare no competing interests.
References
- 1.Kim CW, Goldberger OA, Gallo RL, Bernfield M. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Mol Biol Cell. 1994;5:797–805. doi: 10.1091/mbc.5.7.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elfenbein A, Simons M. Syndecan-4 signaling at a glance. J Cell Sci. 2013;126:3799–3804. doi: 10.1242/jcs.124636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okina E, Manon-Jensen T, Whiteford JR, Couchman JR. Syndecan proteoglycan contributions to cytoskeletal organization and contractility. Scand J Med Sci Sports. 2009;19:479–489. doi: 10.1111/j.1600-0838.2009.00941.x. [DOI] [PubMed] [Google Scholar]
- 4.Morgan MR, Humphries MJ, Bass MD. Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol. 2007;8:957–969. doi: 10.1038/nrm2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saoncella S, et al. Syndecan-4 signals cooperatively with integrins in a Rho-dependent manner in the assembly of focal adhesions and actin stress fibers. Proc Natl Acad Sci USA. 1999;96:2805–2810. doi: 10.1073/pnas.96.6.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiore VF, Ju L, Chen Y, Zhu C, Barker TH. Dynamic catch of a Thy-1-alpha5beta1+syndecan-4 trimolecular complex. Nat Commun. 2014;5 doi: 10.1038/ncomms5886. 4886. [DOI] [PubMed] [Google Scholar]
- 7.Echtermeyer F, et al. Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J Clin Invest. 2001;107:R9–R14. doi: 10.1172/JCI10559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longley RL, et al. Control of morphology, cytoskeleton and migration by syndecan-4. J Cell Sci. 1999;112:3421–3431. doi: 10.1242/jcs.112.20.3421. [DOI] [PubMed] [Google Scholar]
- 9.Cavalheiro RP, et al. Coupling of vinculin to F-actin demands Syndecan-4 proteoglycan. Matrix Biol. 2017;63:23–37. doi: 10.1016/j.matbio.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Okina E, Grossi A, Gopal S, Multhaupt M, Couchman JR. Alpha-actinin interactions with syndecan-4 are integral to fibroblast-matrix adhesion and regulate cytoskeletal architecture. Int J Biochem Cell Biol. 2012;44:2161–2174. doi: 10.1016/j.biocel.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 11.Gopal S, et al. Heparan sulfate chain valency controls syndecan-4 function in cell adhesion. J Biol Chem. 2010;285:14247–14258. doi: 10.1074/jbc.M109.056945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, et al. Matrix Contraction by Dermal Fibroblasts Requires Transforming Growth Factor-β/Activin-Linked Kinase 5, Heparan Sulfate-Containing Proteoglycans, and MEK/ERK. Am J Pathol. 2005;167:1699–1711. doi: 10.1016/s0002-9440(10)61252-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Florian JA, et al. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res. 2003;93:e136–142. doi: 10.1161/01.RES.0000101744.47866.D5. [DOI] [PubMed] [Google Scholar]
- 14.Moon JJ, et al. Role of cell surface heparan sulfate proteoglycans in endothelial cell migration and mechanotransduction. J Cell Physiol. 2005;203:166–176. doi: 10.1002/jcp.20220. [DOI] [PubMed] [Google Scholar]
- 15.Baeyens N, et al. Syndecan 4 is required for endothelial alignment in flow and atheroprotective signaling. Proc Natl Acad Sci USA. 2014;111:17308–17313. doi: 10.1073/pnas.1413725111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, et al. Syndecan 4 controls lymphatic vasculature remodeling during mouse embryonic development. Development. 2016;143:4441–4451. doi: 10.1242/dev.140129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellin RM, et al. Defining the role of syndecan-4 in mechanotransduction using surface-modification approaches. Proc Natl Acad Sci USA. 2009;106:22102–22107. doi: 10.1073/pnas.0902639106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C-P, Cheng C-M, Su H-L, Lin Y-W. Syndecan-4 Promotes Epithelial Tumor Cells Spreading and Regulates the Turnover of PKCalpha Activity under Mechanical Stimulation on the Elastomeric Substrates. Cell Physiol Biochem. 2015;36:1291–1304. doi: 10.1159/000430297. [DOI] [PubMed] [Google Scholar]
- 19.Guilluy C, et al. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol. 2011;13:722–727. doi: 10.1038/ncb2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins C, et al. Localized tensional forces on PECAM-1 elicit a global mechanotransduction response via the integrin-RhoA pathway. Current biology: CB. 2012;22:2087–2094. doi: 10.1016/j.cub.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muhamed I, et al. E-cadherin-mediated force transduction signals regulate global cell mechanics. J Cell Sci. 2016;129:1843–1854. doi: 10.1242/jcs.185447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guilluy C, et al. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat Cell Biol. 2014;16:376–381. doi: 10.1038/ncb2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierschbacher MD, Hayman EG, Ruoslahti E. Location of the cell-attachment site in fibronectin with monoclonal antibodies and proteolytic fragments of the molecule. Cell. 1981;26:259–267. doi: 10.1016/0092-8674(81)90308-1. [DOI] [PubMed] [Google Scholar]
- 24.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Jin H, Rapraeger AC. Syndecan-1 and Syndecan-4 Capture Epidermal Growth Factor Receptor Family Members and the alpha3beta1 Integrin Via Binding Sites in Their Ectodomains: NOVEL SYNSTATINS PREVENT KINASE CAPTURE AND INHIBIT alpha6beta4-INTEGRIN-DEPENDENT EPITHELIAL CELL MOTILITY. J Biol Chem. 2015;290:26103–26113. doi: 10.1074/jbc.M115.679084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakaguchi M, et al. S100A11, an dual mediator for growth regulation of human keratinocytes. Mol Biol Cell. 2008;19:78–85. doi: 10.1091/mbc.E07-07-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muller-Deubert S, Seefried L, Krug M, Jakob F, Ebert R. Epidermal growth factor as a mechanosensitizer in human bone marrow stromal cells. Stem Cell Res. 2017;24:69–76. doi: 10.1016/j.scr.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Saxena M, et al. EGFR and HER2 activate rigidity sensing only on rigid matrices. Nat Mater. 2017;16:775–781. doi: 10.1038/nmat4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan MR, et al. Syndecan-4 phosphorylation is a control point for integrin recycling. Dev Cell. 2013;24:472–485. doi: 10.1016/j.devcel.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calderwood DA, Campbell ID, Critchley DR. Talins and kindlins: partners in integrin-mediated adhesion. Nat Rev Mol Cell Biol. 2013;14:503–517. doi: 10.1038/nrm3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, et al. Structural basis of phosphoinositide binding to kindlin-2 protein pleckstrin homology domain in regulating integrin activation. J Biol Chem. 2011;286:43334–43342. doi: 10.1074/jbc.M111.295352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Zhu Y, Ye S, Zhang R. Crystal structure of kindlin-2 PH domain reveals a conformational transition for its membrane anchoring and regulation of integrin activation. Protein Cell. 2012;3:434–440. doi: 10.1007/s13238-012-2046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qu H, et al. Kindlin-2 regulates podocyte adhesion and fibronectin matrix deposition through interactions with phosphoinositides and integrins. J Cell Sci. 2011;124:879–891. doi: 10.1242/jcs.076976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vining KH, Mooney DJ. Mechanical forces direct stem cell behaviour in development and regeneration. Nat Rev Mol Cell Biol. 2017 doi: 10.1038/nrm.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panciera T, Azzolin L, Cordenonsi M, Piccolo S. Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol. 2017 doi: 10.1038/nrm.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoffman BD, Grashoff C, Schwartz MA. Dynamic molecular processes mediate cellular mechanotransduction. Nature. 2011;475:316–323. doi: 10.1038/nature10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baietti MF, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14:677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 39.Bass MD, Humphries MJ. Cytoplasmic interactions of syndecan-4 orchestrate adhesion receptor and growth factor receptor signalling. Biochem J. 2002;368:1–15. doi: 10.1042/BJ20021228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dovas A, Yoneda A, Couchman JR. PKCalpha-dependent activation of RhoA by syndecan-4 during focal adhesion formation. J Cell Sci. 2006;119:2837–2846. doi: 10.1242/jcs.03020. [DOI] [PubMed] [Google Scholar]
- 41.Greene DK, Tumova S, Couchman JR, Woods A. Syndecan-4 associates with alpha-actinin. J Biol Chem. 2003;278:7617–7623. doi: 10.1074/jbc.M207123200. [DOI] [PubMed] [Google Scholar]
- 42.Lim ST, Longley RL, Couchman JR, Woods A. Direct binding of syndecan-4 cytoplasmic domain to the catalytic domain of protein kinase C alpha (PKC alpha) increases focal adhesion localization of PKC alpha. J Biol Chem. 2003;278:13795–13802. doi: 10.1074/jbc.M208300200. [DOI] [PubMed] [Google Scholar]
- 43.Roca-Cusachs P, et al. Integrin-dependent force transmission to the extracellular matrix by alpha-actinin triggers adhesion maturation. Proc Natl Acad Sci USA. 2013;110:E1361–1370. doi: 10.1073/pnas.1220723110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tzima E, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 45.Bass MD, et al. Syndecan-4-dependent Rac1 regulation determines directional migration in response to the extracellular matrix. J Cell Biol. 2007;177:527–538. doi: 10.1083/jcb.200610076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lachowski D, et al. Substrate Rigidity Controls Activation and Durotaxis in Pancreatic Stellate Cells. Sci Rep. 2017;7 doi: 10.1038/s41598-017-02689-x. 2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Várnai P, Balla T. Visualization of Phosphoinositides That Bind Pleckstrin Homology Domains: Calcium- and Agonist-induced Dynamic Changes and Relationship to Myo-[3H]inositol-labeled Phosphoinositide Pools. J Cell Biol. 1998;143:501–510. doi: 10.1083/jcb.143.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edlund M, Lotano MA, Otey CA. Dynamics of α-actinin in focal adhesions and stress fibers visualized with α-actinin-green fluorescent protein. Cell Motility. 2001;48:190–200. doi: 10.1002/1097-0169(200103)48:3<190::AID-CM1008>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 49.Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jo S, Kim T, Iyer VG, Im W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J Comput Chem. 2008;29:1859–1865. doi: 10.1002/jcc.20945. [DOI] [PubMed] [Google Scholar]
- 51.Javanainen M. Universal Method for Embedding Proteins into Complex Lipid Bilayers for Molecular Dynamics Simulations. J Chem Theory Comput. 2014;10:2577–2582. doi: 10.1021/ct500046e. [DOI] [PubMed] [Google Scholar]
- 52.Van Der Spoel D, et al. GROMACS: fast, flexible, and free. J Comput Chem. 2005;26:1701–1718. doi: 10.1002/jcc.20291. [DOI] [PubMed] [Google Scholar]
- 53.Kaminski GA, Friesner RA, Tirado-Rives J, Jorgensen WL. Evaluation and reparametrization of the OPLS-AA force field for proteins via comparison with accurate quantum chemical calculations on peptides. J Phys Chem B. 2001;105:6474–6487. [Google Scholar]
- 54.Jorgensen WL, Madura JD. Quantum and statistical mechanical studies of liquids. 25. Solvation and conformation of methanol in water. J Am Chem Soc. 1983;105:1407–1413. [Google Scholar]
- 55.Berendsen HJC, Postma JPM, van Gunsteren WF, DiNola A, Haak JR. Molecular-Dynamics with Coupling to an External Bath. J Chem Phys. 1984;81:3684–3690. [Google Scholar]
- 56.Hoover WG. Canonical dynamics: Equilibrium phase-space distributions. Phys Rev A Gen Phys. 1985;31:1695–1697. doi: 10.1103/physreva.31.1695. [DOI] [PubMed] [Google Scholar]
- 57.Nosé S. A unified formulation of the constant temperature molecular dynamics methods. J Chem Phys. 1984;81:511–519. [Google Scholar]
- 58.Parrinello M, Rahman A. Polymorphic Transitions in Single-Crystals - a New Molecular-Dynamics Method. J Appl Phys. 1981;52:7182–7190. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.