Abstract
Hormones influence neurodevelopment which can result in vulnerability to endocrine disruptors such as phthalates during both the perinatal period and adolescence. Using a rat model, we have previously shown that perinatal exposure to an environmentally relevant phthalate mixture at low doses results in cognitive flexibility deficits in adults and a reduction in neuron and synapse number within the medial prefrontal cortex. Here, we further examined the behavioral effects of exposure to an environmentally relevant mixture of phthalates at low doses during either perinatal development or adolescence. Using the elevated plus maze, adult females, not males, exposed to phthalates during adolescence showed indications of reduced anxiety-like behavior while perinatal exposed animals were unaffected. There was no effect of adolescent phthalate exposure on cognitive flexibility using the attentional set shift paradigm in either sex, unlike the impairments we have previously reported following perinatal exposure (Kougias et al., 2018b). Finally, there was no effect of phthalate exposure during either time frame on sensorimotor gating measured using prepulse inhibition. Environmentally relevant phthalate exposure during the perinatal period or during adolescence did not induce widespread changes in the adult behaviors measured here.
Keywords: DEHP, DBP, elevated plus maze, neurodevelopment, attentional set shift, PPI
1. INTRODUCTION
Phthalates, a class of lipophilic endocrine-disrupting chemicals, are used widely as plasticizers in consumer goods as well as in food production equipment and packaging. These chemicals are not covalently bound within these products, readily seeping out and leading to ubiquitous human exposure (Wittassek et al., 2011). Individual phthalates vary in pharmacodynamic properties, but as a class have the capacity to act at estrogen and androgen receptors (Takeuchi et al., 2005). Research on the impact of phthalate exposure in humans has shown reprotoxic health effects through their ability to interrupt gonadal hormone signaling (Mariana et al., 2016). Animal models have established that gonadal hormones are also involved in neural development, influencing the formation of cortical neural networks as well as patterns of synaptogenesis and apoptosis (Arai, 1981; Nuñez et al., 2000; Wang, et al., 2003). After a period of quiescence, gonadal hormone levels rise during adolescence, and there is evidence in both humans (Herting and Sowell, 2017) and rats (Drzewiecki et al., 2016; Koss et al, 2015) that this rise influences restructuring of the prefrontal cortex. Both early development and adolescence are potential periods of susceptibility to the endocrine-disrupting effects of phthalates. Recent work has uncovered correlations between developmental phthalate exposure and attentional, emotional, and cognitive deficits in children (Braun, 2017). Moreover, exposure to phthalates during adolescence has been associated with an increased incidence of externalizing behaviors such as physical aggression, impulsivity, and conduct problems, and of internalizing behaviors, including difficulty concentrating, social withdrawal, and symptoms of anxiety and depression (Shoaff et al., 2019).
The effects of disrupted neurodevelopmental processes can be observed through behavioral testing using a rodent model. Given the association between phthalate exposure in humans and internalizing behaviors, which are generally anxiety-like behaviors, the elevated plus maze (EPM) provides a paradigm to assess comparable behaviors in rodents. The EPM measures anxiety-like behavior by comparing the amount of time the animal spends exploring the open, exposed arms to that spent within the enclosed arms of the maze (Pellow et al., 1985). Several studies in rodents have shown a link between perinatal or adolescent phthalate exposure and anxiety-like behavior using the EPM; however, these studies used a single phthalate at relatively high doses. For instance, a few studies administering high doses (10–30 mg/kg/day) of di(2-ethylhexyl) phthalate (DEHP) during the perinatal period demonstrated an increase in anxiety-like behavior in either male or female rats (Carbone et al., 2013; 2019; Wang et al., 2014). Additionally, adolescent exposure to DEHP at 1, 10, 50, or 200 mg/kg/day resulted in female mice spending significantly less time in the open arms compared to controls (Wang et al., 2016). While DEHP is a commonly used phthalate, it is not the only one humans are exposed to. Subacute exposure presumably during adolescence in male mice to high doses (25, 50, 100, & 200 mg/kg/day) of another phthalate, dibutyl phthalate (DBP), also led to increased anxiety-like behavior (Farzanehfar et al., 2016). These studies are informative about the effects of one specific phthalate on anxiety-like behavior, but mimicking human exposure requires the use of a mixture of phthalates and lower doses.
Previous work in our laboratory has shown exposure to an environmentally relevant mixture of phthalates during the perinatal period disrupts the development of the medial prefrontal cortex (mPFC) resulting in a reduction of neurons and synapses in both adult male and female rats (Kougias et al., 2018b). Additional preliminary work has found that exposure to this phthalate mixture during early development leads to increased postnatal apoptosis within the mPFC in males and females, presumably responsible for the lasting deficit in neuron number (Sellinger, Willing, Drzewiecki, and Juraska, unpublished data). The mPFC is one of the last cortical regions to fully develop during the postnatal period (Van Eden et al., 1991), and it undergoes additional maturational restructuring during adolescence in both rats (Cressman et al., 2012; Markham et al., 2007; Willing & Juraska, 2015) and humans (Huttenlocher & Dabholkar, 1997; Lenroot & Giedd, 2006; Petanjek et al., 2011). The mPFC plays a critical role in high-level cognitive processes, including emotional regulation, decision-making, working memory, cognitive flexibility, and impulse control (Brown & Bowman, 2002; Uylings et al., 2003). Cognitive flexibility, a measure of how well subjects adjust their problem-solving strategy to adapt to a changing stimulus, can be assessed in the rat using the attentional set shift paradigm (Birrell et al., 2000). Perinatal exposure to the same mixture and doses (200 and 1000 μg/kg/day) of phthalates that led to reduced neuron and synapse number in the mPFC also impaired performance in the attentional set shift task in adult male and female rats (Kougias et al., 2018b). Given the protracted development of the mPFC, adolescence may be an additional period of susceptibility to phthalate action, leading to altered mPFC-related behaviors.
Another task that requires mPFC functioning is pre-pulse inhibition (PPI) (Schneider & Koch, 2005). PPI tests sensorimotor gating, the attention to and processing of salient stimuli that occur in close succession. PPI measures how well a weak sensory signal inhibits a subsequent startle response to a much larger stimulus. To properly regulate the startle response, top-down inhibition and proper functioning of the inhibitory cortical network, including the mPFC, is required (Li et al., 2009; Swerdlow et al., 2001). Two studies have investigated the potential impact of phthalate exposure during early development on sensorimotor gating, and neither found significant effects (Degroote, et al., 2017). However, a relatively high dose was used and the exposure did not encompass the entire perinatal period so that developmental changes occurring outside these windows may have allowed for compensation.
In this study, we aimed to model human phthalate exposure in rats by exposing them to an environmentally relevant mixture of phthalates at low doses. We continued to explore the behavioral effects of exposure during the perinatal period, and we also examined the behavioral effects following exposure during adolescence when pubertal hormones influence neural development. Existing evidence suggests a link between exposure to a single phthalate and increased anxiety-like behaviors, (Carbone et al., 2013; 2019; Farzanehfar et al., 2016; Wang et al., 2014), so that further work with an environmental relevant mixture is merited at both time points. Similarly, recent evidence has shown exposure to a phthalate mixture perinatally leads to impaired cognitive flexibility, an mPFC-directed function (Kougias et al., 2018). Therefore, exposure during adolescence, the second period of mPFC development, has the potential to impact this cognitive ability. The mPFC is also considered a modulator of prepulse inhibition, a behavioral phenotype associated with schizophrenia (Schneider & Koch, 2005; Li et al., 2009; Swerdlow et al., 2001). The capacity for perinatal phthalate exposure to reduce the number of neurons in the mPFC (Kougias et al., 2018b) suggests the potential for phthalates to disrupt normal prepulse inhibition. As such, the perinatal period and adolescence were examined here as potential windows of susceptibility.
2. MATERIALS & METHODS
2.1. Subjects
Male and female Long-Evans hooded rats were obtained in two groups from Envigo (Indianapolis, IN) at approximately 3 months of age to be used for breeders. In the perinatal phthalate exposure group, pairing of 40 dams occurred in 5 breeding cohorts over the course of 10 months with a variable amount of time between pairings (2 weeks to 2 months). These pairings resulted in 31 litters which were divided into the 3 dosing groups accordingly: 0 μg phthalates/kg body weight (n=11), 200 μg phthalates/kg (n = 9), or 1000 μg phthalates/kg (n = 11). All dosing groups were represented in each cohort. One male and one female per litter were tested on the elevated plus maze (EPM) while one male and one female littermate were tested on prepulse inhibition (PPI). In the adolescent phthalate group, 2–3 different dams and sires were paired approximately every 2 weeks to yield 11 litters. Within each litter, 3 males and 3 females were assigned to each of the 3 doses resulting in the following groups: 0 μg phthalates/kg body weight (n=11 males, 11 females), 200 μg phthalates/kg (n = 10 males, 11 females), or 1000 μg phthalates/kg (n = 11 males, 11 females).
All rats were housed in same-sex pairs on a 12-h light/dark cycle (lights on 7am to 7pm) with food and water available ad libitum for at least two weeks prior to breeding. To minimize exposure to environmental contaminants, all rats were housed in BPA-free polysulfone cages with heat treated-hardwood beta chip bedding (Northeaster Product Corp., NY), given reverse osmosis-filtered water from glass bottles, and fed a low phytoestrogen food (Harlan 2020X; Teklad Diets, Madison, WI). The exception was the dams used in the perinatal exposure time point. At the time that they were given doses of phthalates (pregnancy and first 10 days of lactation), they were fed D10012G (Research Diets Inc., New Brunswick, NJ). This was because littermates of the pups examined here were the diet controls in a study (Kougias et al., 2018a) examining the combination of a high fat diet and phthalate exposure, and therefore they were fed the low-fat diet (D10012G) equivalent. After 10 days of lactation, the dams were switched back to the standard diet of 2020X so that the pups ate only the standard diet. All experiments were carried out in accordance with National Institute of Health guide for the care and use of laboratory animals and were approved by the University of Illinois Institutional Animal Care and Use Committee.
2.2. Phthalate Mixture
The relative concentrations of the phthalates comprising this mixture were based on human exposure data derived from the urinary metabolites of phthalates from pregnant women in the Champaign-Urbana community (unpublished data), which is representative of exposure levels found in the general U.S. population (Corbasson et al., 2016). The phthalate mixture was comprised of approximately 35% diethyl (DEP), 21% di(2-ethylhexyl) (DEHP), 15% dibutyl (DBP), 15% diisononyl (DiNP), 8% diisobutyl (DiBP), and 5% benzyl butyl (BBP) phthalate. To ensure equivalent volumes (1 μL/3g bodyweight) across doses, the mixture was prepared by suspending 0, 0.6, or 3 mg phthalates/mL in tocopherol-stripped corn oil for each of the respective doses: 0 (control), 200, or 1000 μg phthalates/kg bodyweight. The production of tocopherol-stripped corn oil was discontinued during the phase of adolescent phthalate exposure experiments, so corn oil was used as a substitute. While there are limitations in converting estimated human exposure levels to appropriate doses in rodents, the body surface area normalization method (Reagan-Shaw et al., 2008) suggests doses of 200 μg/kg and 1000 μg/kg in a rat equate to roughly 32 and 162 μg/kg in a human. The estimates of median daily exposure levels for individual phthalates in humans are based on metabolite levels collected in urine and reportedly range from 13.8 μg/kg/day for DEHP to 16.2 μg/kg/day for DnBP and 22.1 μg/kg/day for DEP (Koch et al., 2003; Wittassek et al., 2011). Each mixture was coded to conceal treatment and refrigerated for stability.
2.3. Dosing Paradigm
2.3.1. Perinatal phthalate exposure
Male and female pairs were placed in suspended wire-bottom cages and inspected daily for the presence of sperm plugs. On gestational day (GD)0, the day a sperm plug was detected, dams were removed, housed individually in polysulfone tubs, and assigned to one of three groups: 0 μg phthalates/kg body weight (n=11), 200 μg phthalates/kg (n = 9), or 1000 μg phthalates/kg (n = 11).
On GD 0 and 1, the dams were given half a cookie (Newman’s Own organic alphabet cookie, vanilla flavor) with tocopherol-stripped corn oil pipetted onto it for acclimatization. Our Beginning on GD2 through postnatal day (P)10, approximately 30 days, dams readily consumed half a cookie overlaid with the daily dose of phthalate mixture at their corresponding concentration (Fig. 1). This method of oral administration is presumably not stressful, adequate in exposing the fetus to the dose (Mose et al., 2007), and similar to the major route of human exposure to phthalates. Importantly, this is also a suitable postnatal route of exposure as phthalates can be delivered to offspring via lactation (Dostal et al., 1987). Litters were culled to 5 females and 5 males on P1 to control for litter size and sex ratio. Lastly, pups were weaned on P25 and pair-housed with similarly aged animals of the same sex and treatment. Pups were briefly handled for social play observations from P32–40 and for the determination of pubertal onset between P25–50 (reported in Kougias et al., 2018a) and then were not disturbed, except for standard cage changes, until P90 when behavioral testing began.
Figure 1.
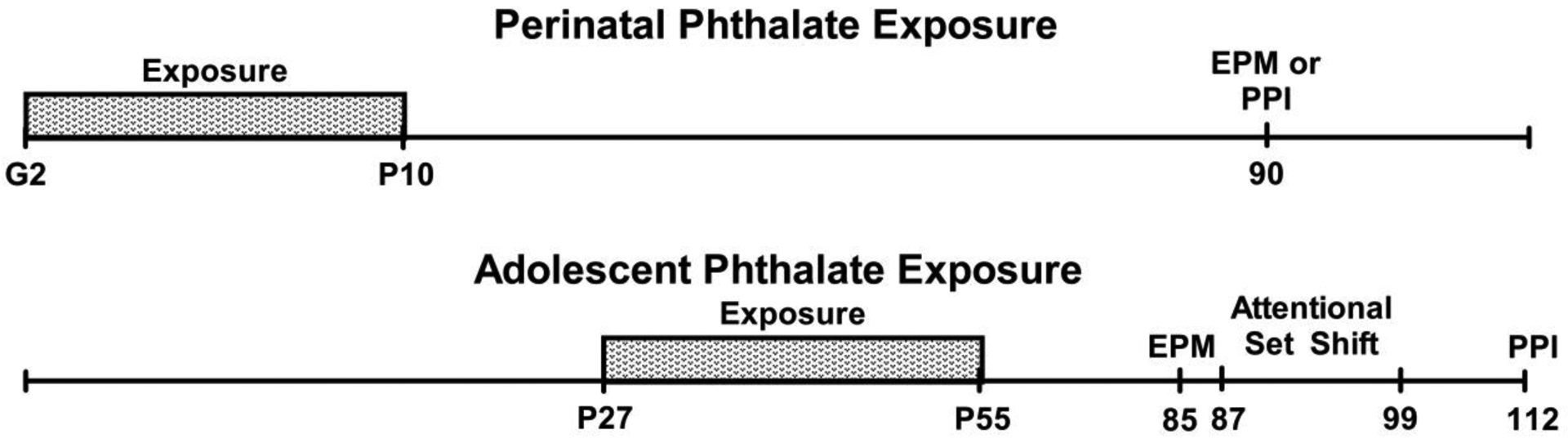
The experimental timelines for perinatal and adolescent phthalate exposure and subsequent behavioral testing.
2.3.2. Adolescent phthalate exposure
Animals were bred by placing male and female pairs together for 10 days, then removing the dams and placing them in individual cages to be monitored for pregnancy. After dams gave birth, litters were culled to 8 pups (n=4 females, n=4 males) on P2. A litter-balanced design was used so that every litter contained 3 females each exposed to a different phthalate dose and 3 males each exposed to a different phthalate dose: 0 μg phthalates/kg body weight (n=11 males, 11 females), 200 μg phthalates/kg (n = 10 males, 11 females), or 1000 μg phthalates/kg (n = 11 males, 11 females). Subjects again were dosed orally with the phthalate mixture pipetted onto a cookie from P27–50.
Knowing subjects would need to be placed into separate cages to be dosed, several stages of cookie exposure were used to acclimate pups to the cookie used in the adolescent dosing paradigm. Moreover, we aimed to reduce any possible novelty-induced stress caused by being placed into a new cage for 5–10 minutes daily by using this exposure paradigm. Beginning when pups reached P15, each dam was given half a cookie while a second half was broken into pieces and sprinkled around the cage every day for 5 days. On P20–22, pups were moved into clean cages with 1–2 sex-matched littermates for 5 minutes and each given a quarter of a cookie to eat. After consuming some or all of the cookie, subjects were placed back in their home cages. Then, on P22–23, pups were placed alone into clean dosing cages and given 5 minutes to consume a quarter cookie overlaid with corn oil. On P25, pups were weaned and housed in triplicate with same-sex littermates. To avoid further stress on this day, juveniles were given a quarter of a cookie overlaid with oil each in their new home cages. On P26, juveniles again were placed alone in a dosing cage and given 5 minutes to consume a quarter cookie overlaid with oil.
Because each subject within a litter (and cage) received a different phthalate dose, rats were ear punched according to dose, placed into a designated dosing cage, and given a quarter of a cookie overlaid with the appropriate phthalate dose, which they readily consumed. Subjects were dosed daily from P27 through P50 with either: 0 μg phthalates/kg body weight (n=11 females, n = 11 males), 200 μg phthalates/kg (n = 11 females, n= 10 males) or 1000 μg phthalates/kg (n = 11 females, n= 11 males) (Fig. 1).
2.4. Pubertal Onset
Previous work from our laboratory has demonstrated that the perinatal phthalate exposure presented in the current study did not show a clear effect on pubertal onset (Kougias et al., 2018a). In the current study, subjects exposed to phthalates during adolescence were monitored for pubertal onset daily beginning on P25 for females and P35 for males. In females, puberty is marked by vaginal opening and in males, by preputial separation (Halász et al.,1988; Korenbrot et al., 1997).
2.5. Behavioral Testing
For subjects exposed perinatally to phthalates, only one male and one female from a litter were used in each behavioral test, which began between P85-P90. For adolescent-exposed subjects, three males and three females from a litter (each receiving a different phthalate dose) were tested on all paradigms with testing beginning between P85-P90 with the elevated plus maze and ending with startle reactivity and pre-pulse inhibition (Fig. 1).
2.5.1. Elevated Plus Maze
The elevated plus maze (EPM) is well-established in assessing anxiety-like behavior by comparing the amount of time an animal spends in the open, exposed arms of the maze to that spent in the enclosed arms. The testing apparatus stood 50 cm tall and consisted of a center with four equiangular arms of 10.5 cm width that extended 50 cm. Two nonadjacent arms were semi-enclosed with walls 33.5 cm tall.
Rats were maintained in their home cage while being habituated to the dimly lit testing room for 5 minutes immediately before the test. Then, rats were placed in the center of the elevated plus maze facing an enclosed arm. Rats were allowed 5 minutes to explore while the experimenter used stopwatches to record the latency to the first open arm entry and the time spent in either open or closed arms. If subjects did not make an open arm entry, which was the case for 5 animals in the perinatal exposure group, latency was recorded as 5 minutes. The number of entries into either open or closed arms was also recorded. Subjects from the perinatal and adolescent exposure groups were run by two different experimenters, resulting in slightly different criteria being used to determine when the rat entered an arm. For the perinatally exposed subjects, a rat was considered to enter an arm when all four limbs crossed the entry. Thus, the time spent in open arms was the accumulation of time between entry and withdrawal of all four limbs from an open arm. For the subjects exposed during adolescence, a rat was considered to enter an arm when the two forelimbs crossed the plane. Additionally, the number of trips to the end of the open arms, counted when the forelimbs of the subject crossed a line drawn 12.5 cm from the end of arm, was recorded to capture exploration of the open arm. When the 5 minutes had elapsed, rats were removed, and the apparatus was cleaned with ethanol before testing the next rat. Testing occurred on one day during the light cycle, and the time of day was recorded. When time of day was included in the analysis, it did not account for a significant amount of variation seen in performance.
2.5.2. Attentional Set Shift
Attentional set shift is a task designed to measure cognitive flexibility. Using a T-shaped maze with a food reward at the end of one arm, the stimulus predicting reinforcement is changed from one modality (i.e. visual – color) to another (i.e. tactile – texture) to assess how readily rats abandon their previously learned association and adopt the new one. The task was performed as described in Kougias et al. (2018b) and showed rats perinatally exposed to the same doses and phthalate mixture used here exhibited impaired performance during the extradimensional shift portion of the task, making significantly more perseverative errors than controls. We therefore sought to investigate if phthalate exposure during adolescence would produce similar deficits.
The apparatus used was a plus maze with a 10 × 10 cm center and four equiangular arms 10 cm wide, 45 cm long with walls 15cm high. The maze was placed on a spinnable table in a small room with indirect, dim lighting. The inside walls and floors of two adjacent arms were white while the remaining two arms were dark grey. Moreover, the floor of one gray arm and the adjacent white arm were textured (“rough”) while the remaining two arms were smooth. Food troughs were at the end of each arm. For training and testing, a black wall piece could be slid into one of four positions to block off an arm, turning the plus-shaped maze into a T-shaped maze.
The 13-day protocol began on P87, with food restriction with the goal of reaching 85% of their free-feeding bodyweights by test days (days 12 and 13 of the protocol). For 5 days, animals were handled for 1 minute, weighed, and given 3–4 reward pellets (TestDiet, LabTab Rodent Tablets 45mg) in their home cages. On days 6–8, animals underwent habituation trials where they were given a maximum of 5 minutes to explore maze and retrieve reward pellets from each of the 4 arms. If a subject failed to consume all reward pellets, the missed arm(s) was recorded to assess potential bias. On days 9–11, rats underwent 8 trials of pre-training where they were placed in the end of one arm (start arm) with the opposite arm blocked off. They then learned to make a choice to enter one of the open arms for a food reward. Trials were rewarded 50% of the time and the starting arm was quasi-randomly varied to avoid subjects establishing an arm preference. Testing on sets 1 and 2 began on days 12 and 13, respectively. The trials of both sets mimicked pre-training trials where rats were placed at the end of a start arm with opposite arm blocked off, leaving the option to enter one of two arms.
Set 1 consisted of training rats to enter a target arm using color as a cue (i.e., white or gray, rule 1) to obtain a reward food pellet. The maze was quasi-randomly rotated between each trial to alter the spatial location of the reinforced arm. Set 1 concluded when an animal reached 8 correct trials in a row with a minimum of 16 total trials. Both the number of trials to criterion and percent correct were analyzed. The maze was cleaned with 70% ethanol between subjects.
Set 2 was performed in a similar fashion the day after set 1 and involved an extradimensional shift where the reward-associated cue was now based on texture (i.e., rough or smooth, Rule 2). Again, the starting arm was changed each trial and the maze was adjusted a T-maze. Every animal completed 80 trials in set 2. Performance in this set reflects a form of cognitive flexibility that is mediated by the PFC (Stefani & Moghaddam, 2005). For each trial, there was a correct choice or an incorrect choice, which could be either a perseverative or an omission error. Perseverative errors were counted when subjects chose an arm that had previously been rewarded in set 1 whereas omission errors consisted of choosing an arm that had never been previously rewarded. The total percent correct, total number of errors, total number of omission errors, total number of perseverative errors, and perseverative errors across the 10 blocks (of 8 trials each) were analyzed. Additionally, the number of perseverative errors performed in each half was analyzed. Again, the maze was cleaned with ethanol between each subject.
As it was not always possible to run all subjects at the same time of day, time of day was recorded, and when included in the statistical model, did not account for significant variance across any measure.
2.5.3. Startle Reactivity & Prepulse Inhibition
Changes in the acoustic startle response (ASR) can be measured as indices of habituation and prepulse inhibition (PPI), a form of sensorimotor gating where the startle response to a loud pulse of sound (“pulse”) is reduced by adding a preceding less intense prepulse (“pulse+prepulse”). The system used to collect these measures was the SR-LAB™ Startle Response System from San Diego Instruments (San Diego, CA). It included a lighted cabinet (Serial Number: SDI 000396) housing a ventilated plexiglass cylinder of 9 cm diameter and 12 cm length secured to a stabilimeter platform (Serial Number: CAL 005799). The platform contained a piezoelectric accelerometer to transduce movement of the subject in response to sound (their ASR) into a digital readout using SR Lab (mxe) software (96.1.5.66).
Subjects exposed perinatally to phthalates began this behavioral paradigm after completing EPM on P90, whereas subjects exposed during adolescence were tested on P112 after completing attentional set shift and subsequently being placed on free feed for two-weeks to allow their weights to stabilize. On the first day of this paradigm, rats underwent an acclimation session consisting of 10 minutes in the cylinder with a constant 65 dB background noise.
The following day, ASR of the rats was tested across four blocks (similar to the protocol discussed in Dulawa and Geyer (2003)). After a 5-minute acclimation period of 65dB background noise, testing began with block 1 consisting of six pulse-alone trials (a 40 msec pulse of 120 dB). The average ASR across these 6 trials was used to measure baseline startle response and compared to the average ASR across the last 6 pulse alone trials (block 4) to measure habituation score. Blocks 2 and 3 consisted of 26 trials each and were used to measure PPI. Each trial consisted of either a pulse alone trial (a 40 msec pulse of 120 dB), a prepulse+pulse trial (a 20 msec prepulse at 3, 6, or 12 dB followed 80 msec later by a pulse of 120 dB), or a no stim trial (no acoustic stimulus). Time between trials (averaging 15 seconds) and stimulus type varied in a pseudorandom and counterbalanced manner.
In order to reduce variance associated with individual differences of startle reactivity, PPI was evaluated as percent PPI (%PPI), the percent reduction in startle response due to the prepulse. %PPI was calculated for each prepulse intensity (3, 6, and 12 dB) using the following equation:
As shown in the equation above, the difference between pulse alone and prepulse+pulse startle responses was divided by the pulse-alone startle response and multiplied by 100. More effective sensorimotor gating is reflected by a higher %PPI, in other words, a greater reduction in startle with a prepulse. Testing concluded with block 4, mirroring block 1 with 6 pulse-alone trials. The average startle response during block 4 was subtracted from that of block 1 to yield a habituation score. Habituation across testing was also analyzed by comparing startle responses in the pulse-alone trials across all blocks.
2.6. Statistical Analysis
Using RStudio, all analyses were performed separately for each sex using a mixed effects linear model (in the “lmerTest” package) with treatment as a main factor and litter or cohort as a random factor. None of the analyses performed revealed a significant effect of cohort. An analysis of variance (ANOVA) was then run on the model and if significant, post hoc tests compared each phthalate dose to the control using a Dunnett’s test (in the “multcomp” package). When analyzing performance across blocks in both PPI and attentional set shift data, block was included in the analysis as a repeated measure. Two data points were considered outliers and removed from the analysis of habituation score as they fell more than 2.5 standard deviations from the mean: one male in the 1000 μg/kg group and one female in the 200 μg/kg group.
3. RESULTS
3.1. Pubertal Onset
Previous work in our laboratory using the same phthalate mixture and doses combined with either a control or high fat diet resulted in an uncertain effect of perinatal exposure on pubertal onset in males, as a main effect of phthalate exposure was detected; however all post hoc tests yielded non-significant results (Kougias et al., 2018a). Here on a standard diet, we observed no significant effect of adolescent phthalate exposure on pubertal onset in males or females (Fig. 2). The average onset in females was P33.5 while the average onset in males was P42.7.
Figure 2.
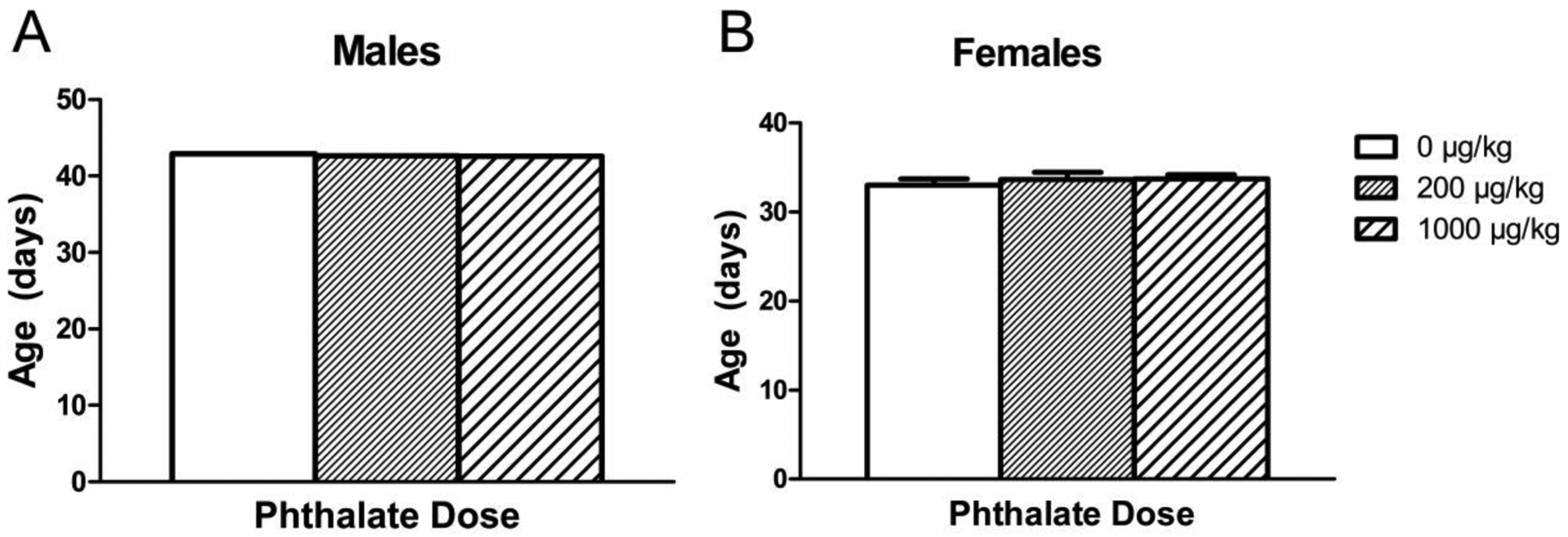
Age of pubertal onset observed in males (A) and females (B) after adolescent phthalate exposure. Bars display means ± standard error of the mean of 11 subjects per group.
3.2. Elevated Plus Maze
3.2.1. Perinatal Exposure
There was no significant effect of perinatal phthalate exposure on the primary measure of anxiety-like behavior, time spent in open (Fig. 3) nor were any significant differences observed in the latency to enter an open arm, time spent in the closed arms, or the number of entries into open or closed arms (data not shown).
Figure 3.
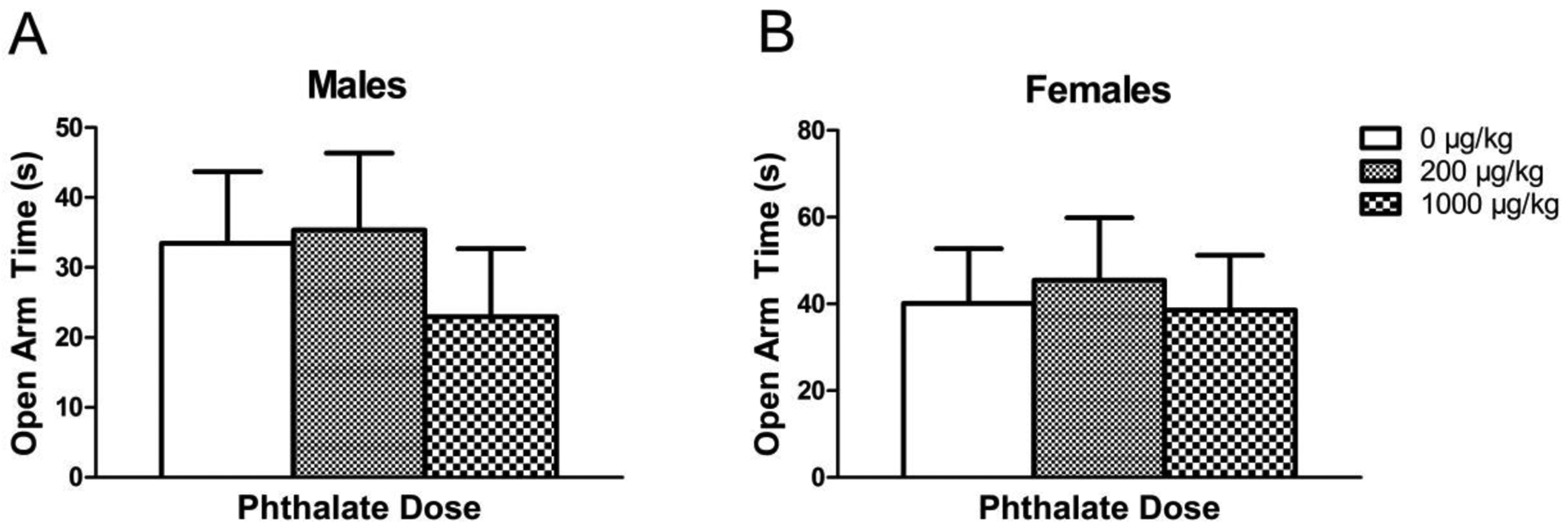
Time (in seconds) spent in the open arms of the elevated plus maze in males (A) and females (B) exposed to phthalates perinatally. Bars display means ± standard error of the mean of 9–11 subjects per group.
3.2.2. Adolescent Exposure
There were no significant effects of adolescent phthalate exposure on time spent in open (Fig. 4a) nor in latency to first open arm entry, time in closed arms, or number of open arm entries in either sex (data not shown). However, there were two effects seen in additional measures. A main effect of adolescent phthalate exposure was seen in the number of closed arm entries in males [F(2,20)=4.576, p = 0.02]. The 1000 μg/kg phthalate group made significantly fewer closed arm entries compared to controls (p = 0.007) (Fig. 4b). This effect was not seen in females.
Figure 4.
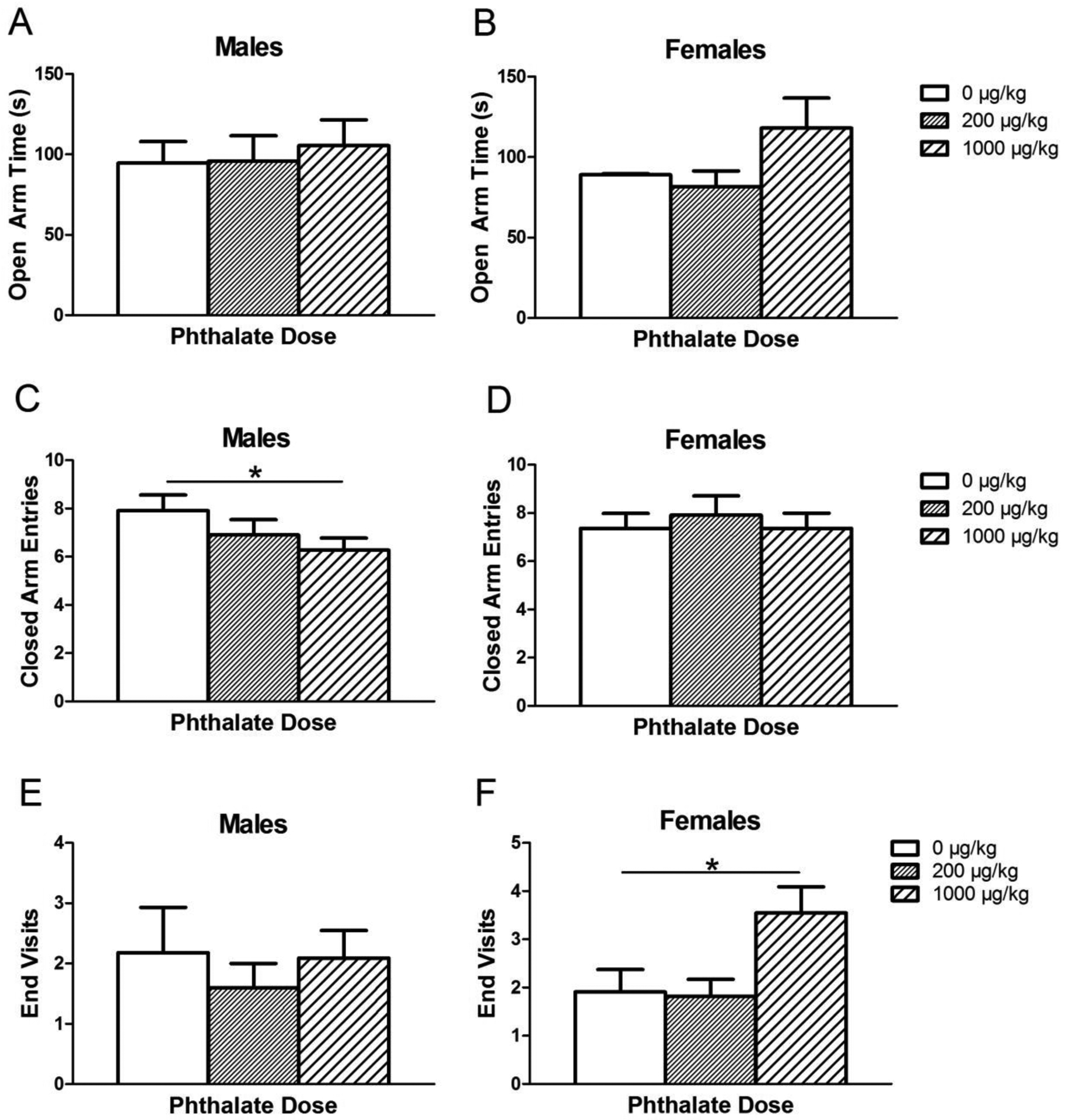
Elevated plus maze measurements after adolescent phthalate exposure. The amount of time male (A) and female (B) subjects spent in the open arm, the number of closed arm entries made by males (C) and females (D) and the number of visits to the end of the open arms observed in males (E) and females (F) exposed to phthalates during adolescence. Bars display means ± standard error of 10–11 subjects per group. *p < 0.05.
The number of trips to the end of the open arms was also measured for adolescent-exposed subjects to more thoroughly explore open arm preference compared to the instances when the subject would extend only forelimbs into the open arm while keeping hindlimbs in the center of the maze (open arm entries). There was a main effect of phthalate exposure on the number of visits to the end of the open arms in females only [F(2,21)=4.47, p = 0.02] (Fig. 4c). In particular, females exposed to 1000 μg/kg/day had significantly more trips to the end of the open arm compared to controls (p = 0.02).
3.3. Attentional Set Shift
Research from our laboratory has previously shown perinatal exposure to the same phthalate mixture and doses used in this study leads to deficits in the attentional set shift task (Kougias et al., 2018b). Specifically, male and female subjects perinatally exposed to both doses made a higher number of perseverative errors during the extradimensional shift trials compared to controls.
3.3.1. Set 1: Training
There was no observed effect of adolescent phthalate exposure on the number of trials completed to reach criterion (Fig. 5). Similar to what our laboratory has observed in the past, there was a trend toward an effect of the rewarded stimulus (i.e., grey or white arms) on the number of trials to reach criterion [F(1,49) = 3.7434, p = 0.06] where rats trained with grey as the rewarded stimulus reached criterion sooner than rats trained to choose white arms. Stimulus type was therefore included in the statistical analysis of set 2 as a random factor as this could have influenced how well the set one rule was learned, potentially impacting the number of perseverative errors. Finally, there was no significant difference in time to completion across dosing groups, suggesting there was not a difference in motivation across phthalate exposure groups.
Figure 5.
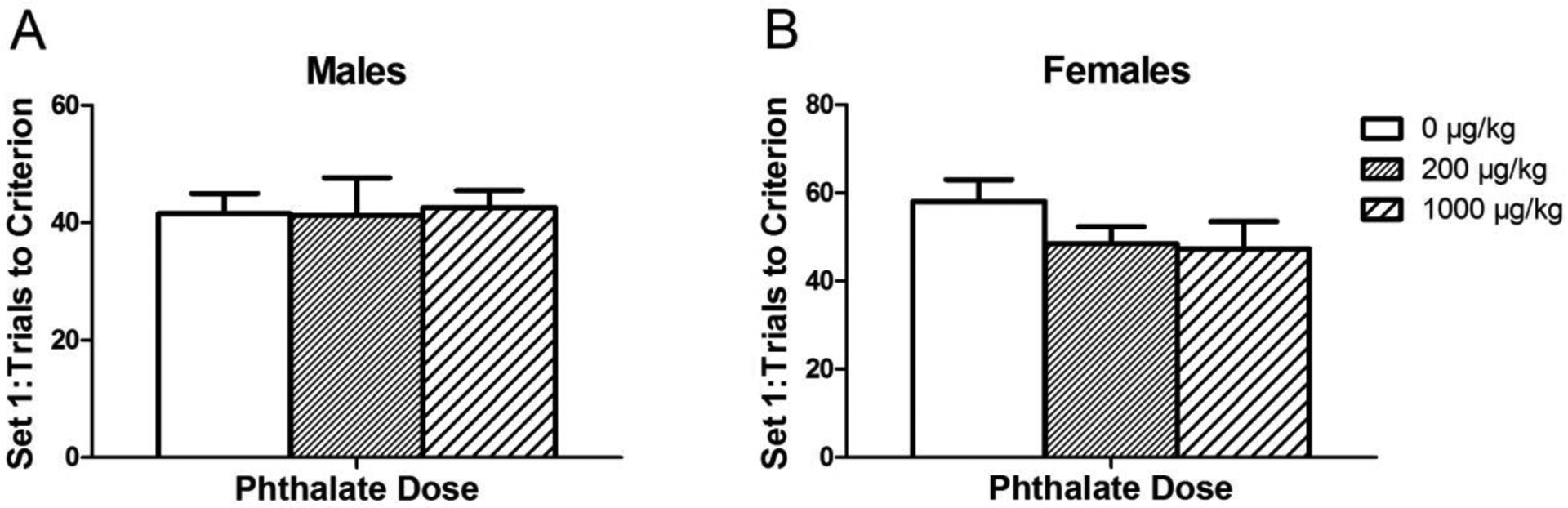
The average number of trials to reach criteria in set 1 of the attentional set shift task in males (A) and females (B) after adolescent phthalate exposure. Bars display means ± standard error of 10–11 subjects per group.
3.3.2. Set 2: Extradimensional shift
As expected, the percent correct increased across the 10 blocks of 8 trials each in set 2 [F(9, 531) = 16.2737, p <0.00001] while the number of perseverative errors decreased across blocks [F(9, 531) = 20.9486, p <0.00001], indicating successful learning of the new rule. There was no effect of phthalate exposure on percent correct (Fig. 6a), total number of errors, total number of omission errors, or number of perseverative errors in set 2 (Fig. 6b).
Figure 6.
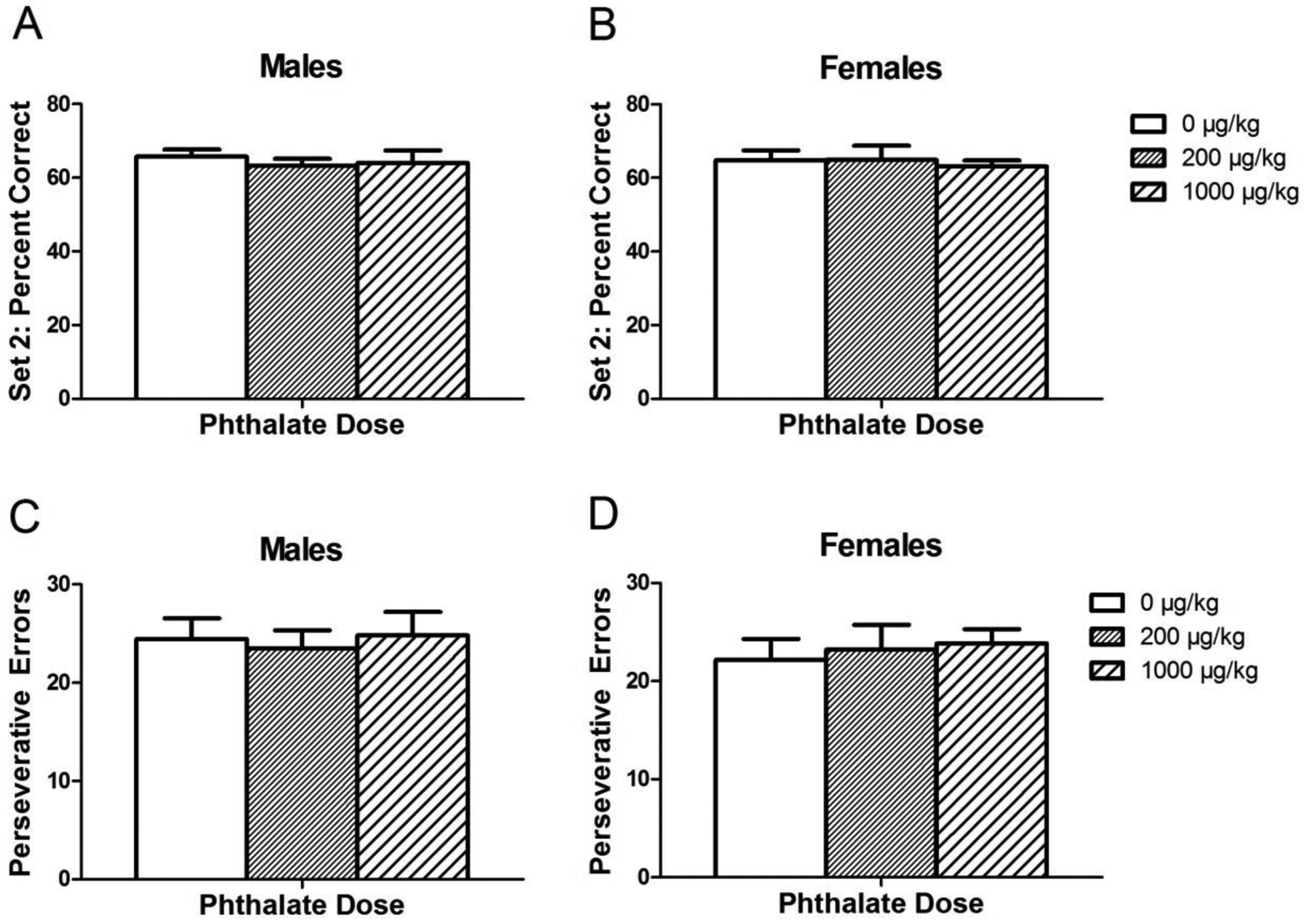
Performance in set 2 of the attentional set shift task after adolescent phthalate exposure. The percentage of correct choices made by males (A) and females (B) and the number of perseverative errors made in set 2 by males (C) and females (D). Bars display means ± standard error of 10–11 subjects per group.
Addressing the potential influence of how well set 1 was learned on the ability of subjects to forget that rule and learn a new one, the first half (40 trials) of set 2 was analyzed. A higher number of perseverative errors seen in the first half of set 2 would likely reflect a well-learned set 1. However, there was no effect of phthalate exposure on the number of perseverative errors over these trials.
3.4. Prepulse Inhibition
3.4.1. Startle Reactivity
3.4.1.1. Perinatal Exposure
As expected, startle reactivity to the pulse alone generally decreased across blocks for both males [F(3, 147) = 29.354, p < 0.001] and females [F(3, 150) = 12.404, p < 0.001] indicating normal acoustic habituation (Fig. 7). However, there were no significant effects of perinatal phthalate exposure on this measure. Additionally, there was no effect of phthalate exposure on the level of habituation, known as the habituation score, which is the difference in startle magnitude in block 1 compared to block 4. There were also no significant effects of phthalate exposure on the initial block of pulse-alone trials indicating that there were no differences between groups in startle reactivity prior to appreciable habituation. In no stim trials, there again were no significant effects of phthalates in males or females indicating overall motor activity did not confound the measurement of startle reactivity or PPI.
Figure 7.
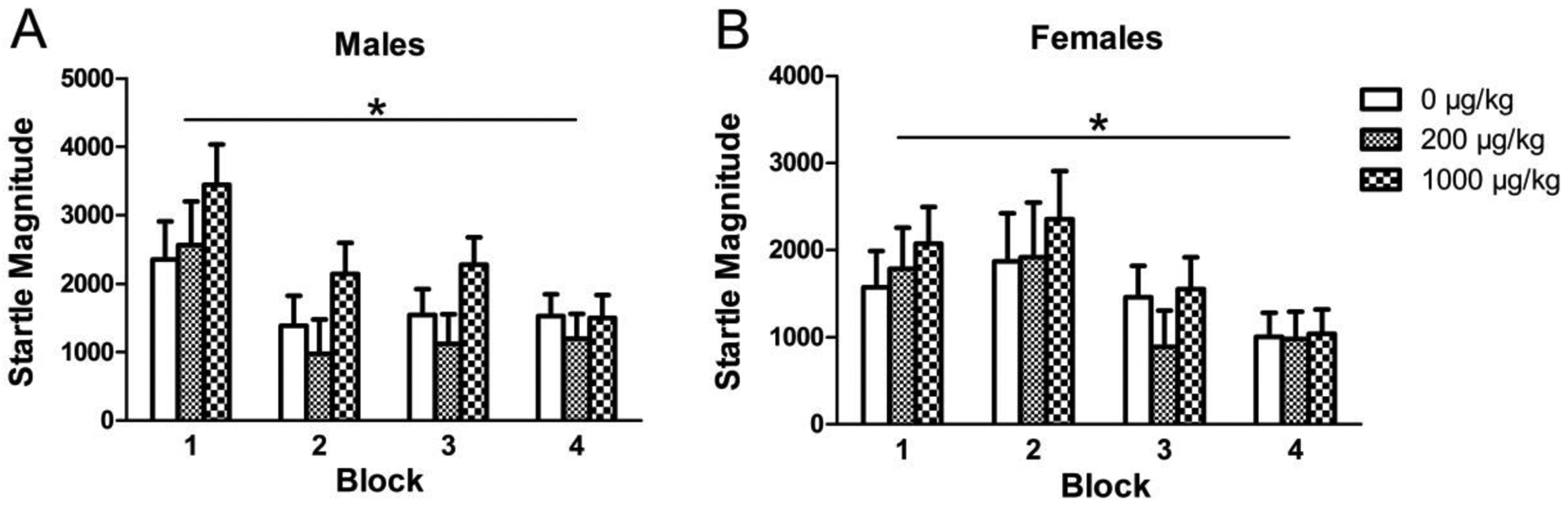
The averaged startle magnitude across the four blocks of the PPI test in males (A) and females (B) perinatally exposed to phthalates. Bars show mean ± standard error of the mean for 9–11 per group. *p<0.001.
3.4.1.2. Adolescent Exposure
Again, as expected, startle reactivity decreased across blocks for both males [F(3, 93) = 19.9985, p < 0.0001] and females [F(3, 99) =8.9826 , p <0.0001], indicating normal acoustic habituation (Fig. 8). In females, phthalates did have a significant effect on the habituation score, the change in average startle magnitude of the last block compared to the first ([F(1, 20) = 5.71, p = 0.03] (Fig. 9). However, post hoc analysis did not show a significant difference between either phthalate dose and the control. Again, there was no effect of phthalate exposure on the startle reactivity during the first block of pulse-alone trials, the nostim trials.
Figure 8.
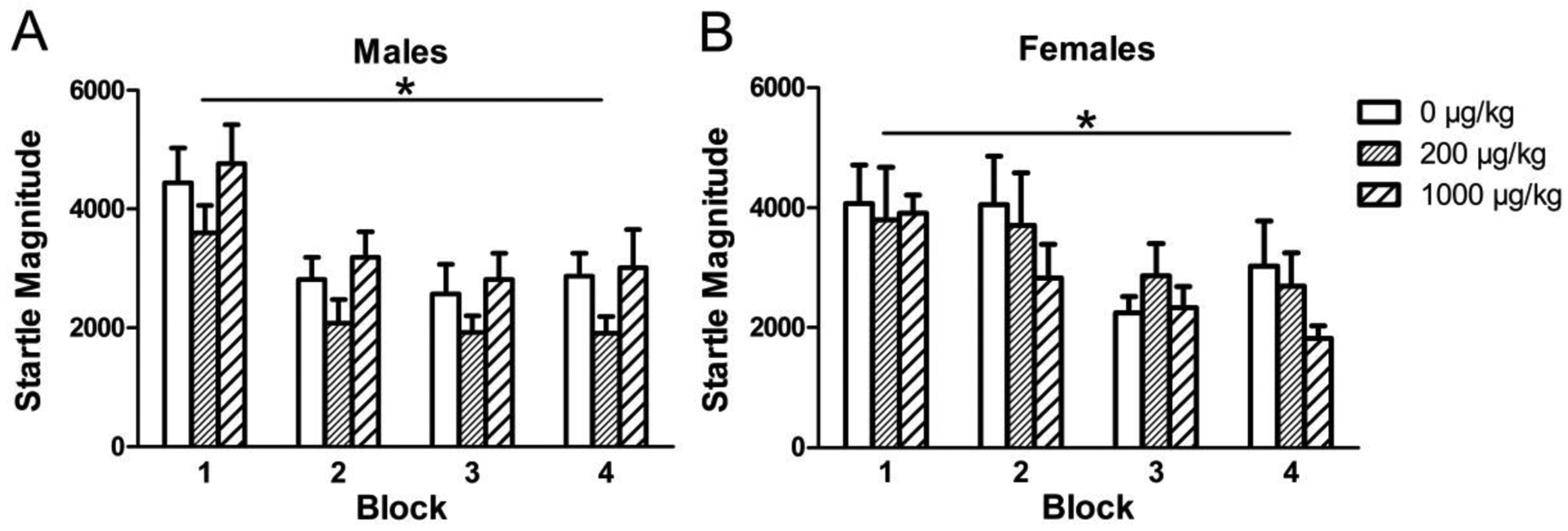
The averaged startle magnitude across the four blocks of the PPI test in males (A) and females (B) exposed to phthalates during adolescence. Bars show mean ± standard error of the mean for 10–11 per group. *p<0.001.
Figure 9.
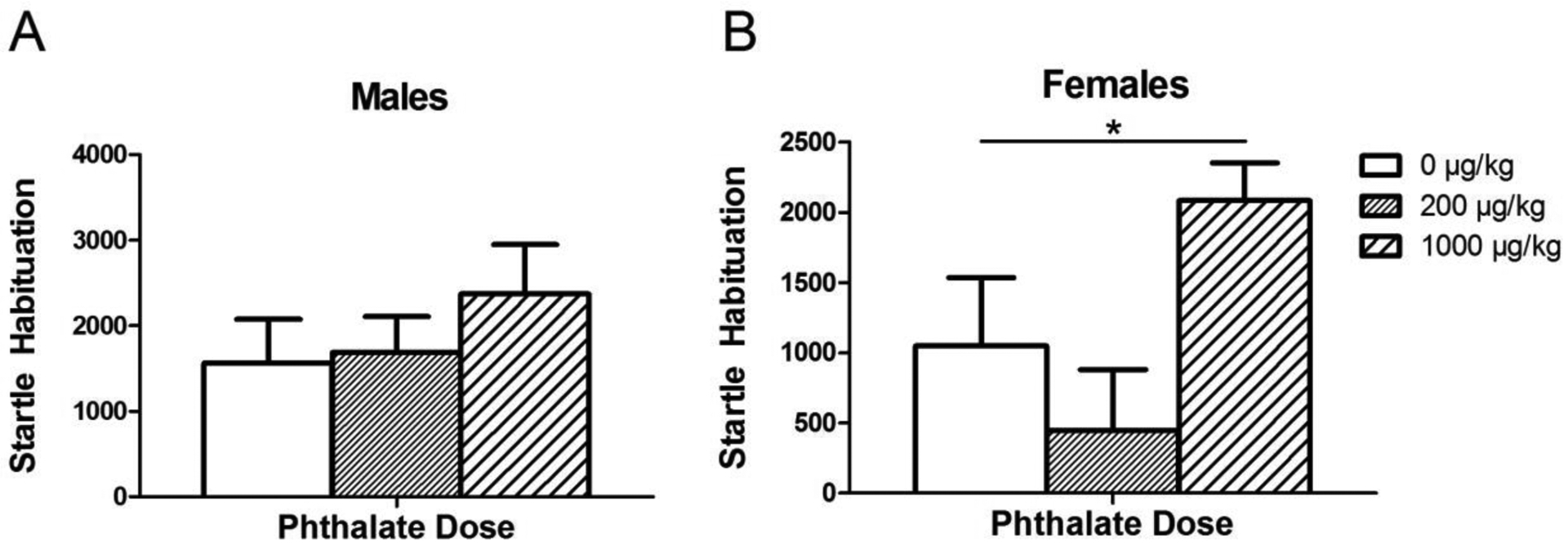
The change in startle magnitude between the first and last 6 trials, termed “habituation score,” in males (A) and females (B) exposed to phthalates during adolescence. Bars show mean ± standard error of the mean for 10–11 per group. *p<0.05.
3.4.2. Percent PPI
3.4.2.1. Perinatal Exposure
As expected, incrementally lower responses (i.e., higher %PPI values) were observed with increasing prepulse intensity for both males [F(2, 58) = 49.2218, p < 0.0001] and females [F(2, 60) = 25.0809, p < 0.0001] (Fig. 10). There were no effects of perinatal phthalate exposure on percent prepulse inhibition in males or females at any of the prepulse intensities.
Figure 10.
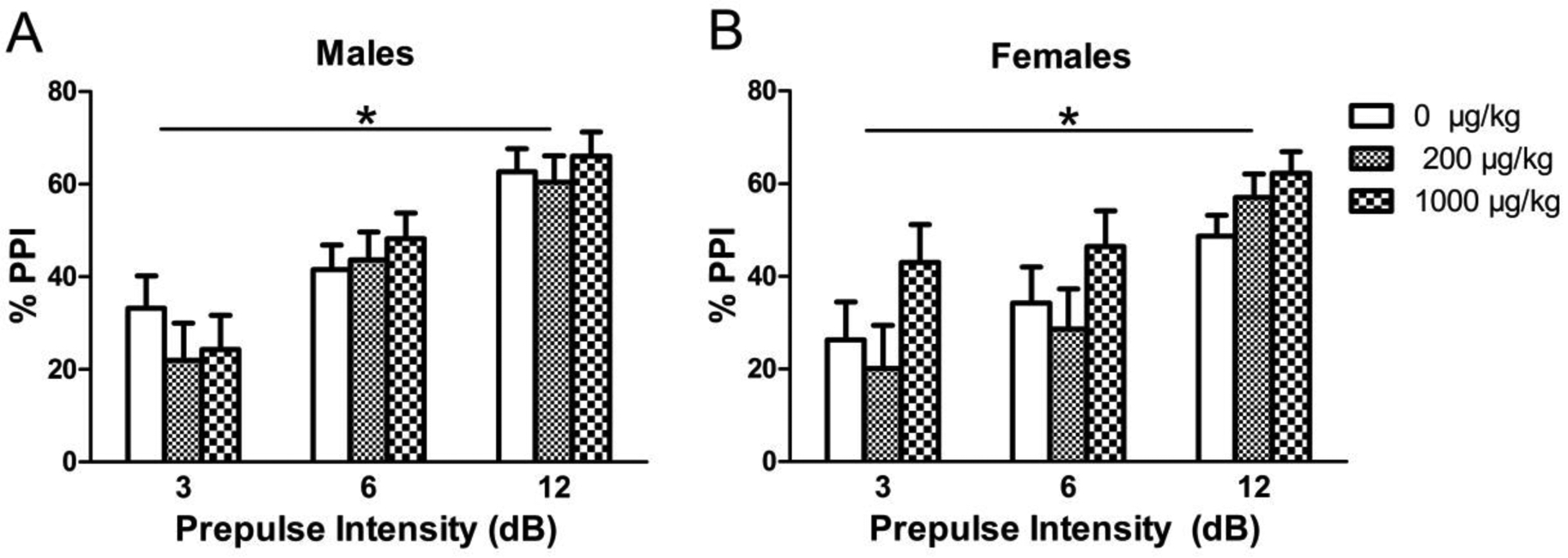
Prepulse inhibition response shown as %PPI at various prepulse intensities (3, 6, and 12 dB) in (A) males and (B) females perinatally exposed to phthalates. Bars show mean ± standard error of the mean for 9–11 per group. *p < 0.0001.
3.4.2.2. Adolescent Exposure
Again, as expected, higher %PPI values were seen with increasing prepulse intensity for both males [F(2,62) = 49.9914, p <0.0001] and females [F(2,64) = 22.2747, p < 0.0001] (Fig. 11). There were no significant effects of adolescent phthalate dose seen in percent prepulse inhibition at any of the prepulse decibels.
Figure 11.
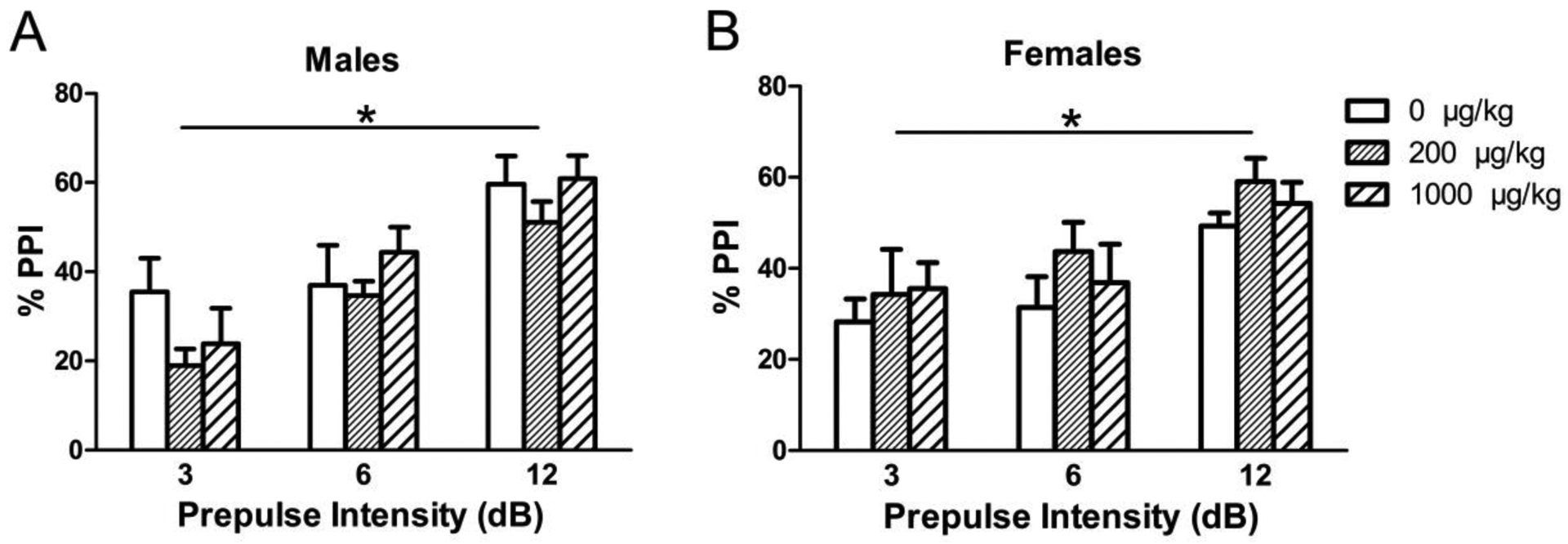
Prepulse inhibition response shown as %PPI at various prepulse intensities (3, 6, and 12 dB) in (A) males and (B) females exposed to phthalates during adolescence. Bars show mean ± standard error of the mean for 10–11 per group. *p < 0.001.
4. DISCUSSION
Perinatal and adolescent exposure to an environmentally relevant mixture and dose of phthalates each had a distinct, albeit not widespread, influence on the emotional and cognitive behaviors measured in adulthood. First, performance in the EPM indicated dose-specific effects of adolescent, but not perinatal, exposure to the phthalate mixture. Second, the detrimental effects of perinatal phthalate exposure previously seen on cognitive flexibility (Kougias et al., 2018b) were not seen in subjects exposed to phthalates during adolescence. Finally, neither perinatal nor adolescent phthalate exposure influenced sensorimotor gating measured using prepulse inhibition, although we did observe enhanced habituation of the startle response in females exposed during adolescence. Furthermore, similar to what has been observed with perinatal phthalate exposure (Kougias et al. 2018a), pubertal onset was unaffected by adolescent exposure to our low dose phthalate mixture.
Correlational studies in humans suggest anxiogenic effects of phthalate exposure both during early development and adolescence (Braun et al., 2017; Ejaredar et al., 2015; Schoaff et al., 2019). Studies conducted in animal models predominantly support this notion; however, these animal models generally rely on the use of a single phthalate at a relatively high dose that is not representative of human exposure. Here, in a study with an animal model that is more representative of the exposures to phthalates in humans, rats with perinatal phthalate exposure did not differ in measures of open arm time, the number of entries into the closed arms or number of entries into the open arms observed during the EPM test, reflecting no impact of phthalates on anxiety-like behavior.
Adolescent exposure to the same doses, on the other hand, resulted in dose-specific effects in the female group. Males did not show altered anxiety-like behavior; however, those exposed to the relatively higher dose of phthalates had significantly fewer closed arm entries than controls. This measure is thought to reflect overall activity level, suggesting adolescent phthalate exposure may lead to decreased activity in males, though additional behavioral testing supporting this claim is needed. Females exposed to the higher dose of phthalates during adolescence made significantly more trips to the ends of the open arms compared to controls, indicating lower levels of anxiety-like behavior. A visit to the end of the open arms was meant to provide an additional measure of open arm exploration given that open arm entries were recorded when the animal’s forelimbs crossed the plane even if their hindlimbs remained in the center. While it is possible this observed increase in trips to the ends of the open arms could be activity-dependent, the non-significant increase in open arm time also seen in this group suggests a potential reduction in anxiety. While these effects do not align with the anxiogenic effects found by other groups, our study is the first to use lower doses, as well as a mixture of phthalates in an attempt to better mimic human exposure patterns. Different phthalates have distinct potencies at target sites and in some cases, dissimilar mechanisms of action (Takeuchi et al., 2005). How phthalates interact with each other in vivo is not well understood and could explain differences in effects seen after exposure to a single phthalate compared to a mixture. To further complicate matters, phthalates, similar to other EDCs, display non-monotonic dose-response curves. Therefore, it is feasible to conclude our relatively lower doses and mixture led to distinct behavioral outcomes compared to those observed after exposure to much higher doses.
Previous work from our laboratory uncovered structural changes occurring within the mPFC after perinatal phthalate exposure that were correlated with impaired performance on attentional set shift, an mPFC-dependent task (Kougias et al., 2018b). Both males and females exposed to phthalates perinatally exhibited deficits in cognitive flexibility paired with a reduction in neurons and synapses within the mPFC. The mPFC undergoes extensive neuronal and synaptic pruning during adolescence, potentially allowing for more complex cognitive processes to emerge. Gonadal hormones are involved in organizing these structural changes (Koss et al., 2015; Drzewiecki et al., 2016) and could be a target for the endocrine-disrupting properties of phthalates during this timeframe. We therefore hypothesized adolescent phthalate exposure would interfere with the neural reorganization during adolescence, leading to impaired mPFC-dependent cognitive abilities. However, adolescent phthalate exposure did not impact cognitive flexibility as there was no difference in performance across groups during the extradimensional shift trials (set 2). The doses used here were the same as those used in our study of perinatal exposure; however, the extent to which the prefrontal cortex is changing during adolescence is less than that occurring during the perinatal period. For this reason, our doses may have not had a large enough impact to result in a detectable change.
We also evaluated sensorimotor-gating ability using the prepulse inhibition paradigm as it relies on mPFC functioning, specifically the inhibitory network (Schneider & Koch, 2005; Li et al., 2009; Swerdlow et al., 2001). Characteristic changes in neuroanatomical structure are seen in patients with schizophrenia in that they have a loss of grey matter and synapses within the mPFC compared to healthy individuals (Glantz & Lewis, 2000; McCarley et al., 1999). Deficits in both startle habituation, a form of non-associative learning, and in prepulse inhibition, a measure of sensorimotor gating reliant on prefrontal cortical inhibitory function, have also been found in non-medicated schizophrenia patients (Ludewig et al., 2003). Given the capacity for perinatal phthalate exposure to produce a decrease in neuron and synapse number in the mPFC, we expected comparable deficits in prepulse inhibition. However, perinatal phthalate exposure did not affect %PPI at any of the prepulse decibels tested in adulthood. The lack of effect was not due to differences in startle reactivity across groups or to differences in habituation. These findings align with the existing literature that also show no or minimal effects of exposure to a single phthalate perinatally on PPI or a related paradigm (Degroote et al., 2017; Kim et al., 2017). The dose given here, which results in alterations to mPFC structure, may not be sufficient to interrupt this processing, or phthalates could be acting in a way that preserves inhibitory cellular function within the mPFC. In assessing startle habituation, we did not observe an effect of phthalate exposure.
Turning to adolescent exposure, we again saw no significant effects on prepulse inhibition. There was, however, an effect of adolescent phthalate exposure on habituation score in females where those exposed to the higher phthalate dose had a greater reduction in startle reactivity by the last block. This enhanced habituation is opposite to the expected schizophrenia-like phenotype (Meincke et al., 2004). However, habituation is mediated predominantly by midbrain and brainstem structures involving to an extent, serotonergic signaling (Dulawa & Geyer, 2000; Koch, 1999), and while the effects of phthalates on the neuroanatomical integrity of the mPFC resembles characteristics of schizophrenia, their effect on deeper midbrain and hindbrain structures is unknown.
Phthalates as a class of endocrine-disrupting compounds are well-recognized for their reprotoxic capacity in animal models (Lovekamp-Swan & Davis, 2003; Martino-Andrade & Chahoud, 2010). Previous studies have demonstrated the influence of exposure to a single phthalate on pubertal onset. For example, postnatal exposure to 10 mg/kg DEHP has been shown to induce precocious puberty in male rats while a higher dose of 750 mg/kg/day results in delayed puberty (Ge et al., 2013). At the same time, perinatal exposure to 15 mg/kg DEHP in female rats has been shown to delay puberty (Grande et al., 2006). Further research examining the effect of exposure encompassing puberty shows doses of 300 or 900 mg/kg/day DEHP delays pubertal onset (Noriega et al., 2009). However, these studies again employ doses much larger than those observed in humans. Our laboratory has previously shown exposure to low doses of a phthalate mixture perinatally do not seem to impact pubertal onset. In the present study, we proposed the sudden elevation in gonadal hormones during puberty may provide a target for both the anti-androgenic as well as (anti-)estrogenic effects of phthalates, both of which are included in our mixture (Takeuchi et al., 2005). However, our relatively low doses of 200 and 1000 μg/kg of the phthalate mixture did not affect pubertal onset in either sex.
5. Conclusions
Though we did not observe substantial effects of phthalates at these doses in the collection of behavioral tasks we selected, we cannot rule out their potential influence on other cognitive functions mediated by non-cortical structures such as spatial memory. The non-significant findings of this study reinforce the importance of using relatively lower doses and a mixture of toxicants that are more representative of human exposure in an animal model in an attempt to more accurately predict potential neurocognitive effects of exposure during two very critical stages of development.
HIGHLIGHTS.
Low doses of a phthalate mixture in rats were used to model human exposure.
Perinatal exposure did not affect anxiety or prepulse inhibition in either sex.
Adolescent exposure reduced a measure of anxiety-like behavior in females.
Adolescent exposure did not alter cognitive flexibility or prepulse inhibition.
Acknowledgements
We would like to thank the Animal Care staff in Psychology and the undergraduate students in the Juraska laboratory for their help with dosing the animals. This research study was supported by the National Institute of Environmental Health Sciences (P01 ES002848-Project3), the United States Environmental Protection Agency (USEPA 83543401 Project 3), and the National Institute of Environmental Health Sciences Grant T32 ES007326 (to D.G.K. and E.P.S.).
Abbreviations
- EPM
Elevated plus maze
- PPI
prepulse inhibition
- %PPI
Percent PPI
- DEHP
Di(2-ethylhexyl) phthalate
- DBP
Dibutyl phthalate
- BBP
Benzyl butyl phthalate
- DiNP
Diisononyl phthalate
- DiBP
Diisobutyl phthalate
- DEP
Diethyl phthalate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
5. REFERENCES
- Arai Yasumasa. Synaptic correlates of sexual differentiation. Trends in Neurosciences 4 (1981): 291–293. [Google Scholar]
- Barakat R, Lin PC, Park CJ, Best-Popescu C, Bakry HH, Abosalem ME, … & Ko C (2018). Prenatal exposure to DEHP induces neuronal degeneration and neurobehavioral abnormalities in adult male mice. Toxicological Sciences, 164(2), 439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell JM, & Brown VJ (2000). Medial frontal cortex mediates perceptual attentional set shifting in the rat. Journal of Neuroscience, 20(11), 4320–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM (2017). Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nature Reviews Endocrinology, 13(3), 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VJ, & Bowman EM (2002). Rodent models of prefrontal cortical function. Trends in neurosciences, 25(7), 340–343. [DOI] [PubMed] [Google Scholar]
- Corbasson I, Hankinson SE, Stanek III EJ, & Reeves KW (2016). Urinary bisphenol-A, phthalate metabolites and body composition in US adults, NHANES 1999–2006. International journal of environmental health research, 26(5–6), 606–617. [DOI] [PubMed] [Google Scholar]
- Carbone S, Ponzo OJ, Gobetto N, Samaniego YA, Reynoso R, Moguilevsky JA, & Cutrera RA (2019). Effect of di (2-ethylhexyl) phthalate on the neuroendocrine regulation of reproduction in adult male rats and its relationship to anxiogenic behavior: Participation of GABAergic system. Human & experimental toxicology, 38(1), 25–35. [DOI] [PubMed] [Google Scholar]
- Carbone S, Ponzo OJ, Gobetto N, Samaniego YA, Reynoso R, Scacchi P, … & Cutrera R (2013). Antiandrogenic effect of perinatal exposure to the endocrine disruptor di-(2-ethylhexyl) phthalate increases anxiety-like behavior in male rats during sexual maturation. Hormones and behavior, 63(5), 692–699. [DOI] [PubMed] [Google Scholar]
- Cressman VL, Balaban J, Steinfeld S, Shemyakin A, Graham P, Parisot N, & Moore H (2010). Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat. Journal of Comparative Neurology, 518(14), 2693–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Yang Y, Xu X, & Hu Y (2015). Effects of uterine and lactational exposure to di-(2-ethylhexyl) phthalate on spatial memory and NMDA receptor of hippocampus in mice. Hormones and behavior, 71, 41–48. [DOI] [PubMed] [Google Scholar]
- Degroote S, Hunting D, & Takser L (2017). Improved assessment of sensorimotor gating in animal models relevant to ASD: A data modelling approach to quantify PrePulse Inhibition of the acoustic startle reflex. Journal of neuroscience methods, 276, 13–22. [DOI] [PubMed] [Google Scholar]
- Dostal LA, Weaver RP, & Schwetz BA (1987). Transfer of di (2-ethylhexyl) phthalate through rat milk and effects on milk composition and the mammary gland. Toxicology and applied pharmacology, 91(3), 315–325. [DOI] [PubMed] [Google Scholar]
- Drzewiecki CM, Willing J, & Juraska JM (2016). Synaptic number changes in the medial prefrontal cortex across adolescence in male and female rats: a role for pubertal onset. Synapse, 70(9), 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa SC, & Geyer MA (2000). Effects of strain and serotonergic agents on prepulse inhibition and habituation in mice. Neuropharmacology, 39(11), 2170–2179. [DOI] [PubMed] [Google Scholar]
- Ejaredar M, Nyanza EC, Ten Eycke K, & Dewey D (2015). Phthalate exposure and childrens neurodevelopment: a systematic review. Environmental research, 142, 51–60. [DOI] [PubMed] [Google Scholar]
- Farzanehfar V, Naderi N, Kobarfard F, & Faizi M (2016). Determination of dibutyl phthalate neurobehavioral toxicity in mice. Food and Chemical Toxicology, 94, 221–226. [DOI] [PubMed] [Google Scholar]
- Ge RS, Chen GR, Dong Q, Akingbemi B, Sottas CM, Santos M, … & Hardy MP (2007). Biphasic effects of postnatal exposure to diethylhexylphthalate on the timing of puberty in male rats. Journal of andrology, 28(4), 513–520. [DOI] [PubMed] [Google Scholar]
- Glantz LA, & Lewis DA (2000). Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Archives of general psychiatry, 57(1), 65–73. [DOI] [PubMed] [Google Scholar]
- Gourevitch R, Rocher C, Le Pen G, Krebs MO, & Jay TM (2004). Working memory deficits in adult rats after prenatal disruption of neurogenesis. Behavioural pharmacology, 15(4), 287–292. [DOI] [PubMed] [Google Scholar]
- Grande SW, Andrade AJ, Talsness CE, Grote K, & Chahoud I (2006). A dose-response study following in utero and lactational exposure to di (2-ethylhexyl) phthalate: effects on female rat reproductive development. Toxicological Sciences, 91(1), 247–254. [DOI] [PubMed] [Google Scholar]
- Halász B, Köves K, Molnár J, Balika K, Stoll V, & Kovács G (1988). Hypothalamus and puberty. Brain research bulletin, 20(6), 709–712. [DOI] [PubMed] [Google Scholar]
- Herting MM, & Sowell ER (2017). Puberty and structural brain development in humans. Frontiers in neuroendocrinology, 44, 122–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, & Dabholkar AS (1997). Regional differences in synaptogenesis in human cerebral cortex. Journal of comparative Neurology, 387(2), 167–178. [DOI] [PubMed] [Google Scholar]
- Kim BJ, Kim J, Keoboutdy V, Kwon HJ, Oh SH, Jung JY, … & Paik KC (2017). The effects of postnatal phthalate exposure on the development of auditory temporal processing in rats. International journal of pediatric otorhinolaryngology, 97, 61–65. [DOI] [PubMed] [Google Scholar]
- Koch M (1999). The neurobiology of startle. Progress in neurobiology, 59(2), 107–128. [DOI] [PubMed] [Google Scholar]
- Koch HM, Drexler H, & Angerer J (2003). An estimation of the daily intake of di (2-ethylhexyl) phthalate (DEHP) and other phthalates in the general population. International journal of hygiene and environmental health, 206(2), 77–83. [DOI] [PubMed] [Google Scholar]
- Korenbrot CC, Huhtaniemi IT, & Weiner RI (1977). Preputial separation as an external sign of pubertal development in the male rat. Biology of reproduction, 17(2), 298–303. [DOI] [PubMed] [Google Scholar]
- Koss WA, Lloyd MM, Sadowski RN, Wise LM, & Juraska JM (2015). Gonadectomy before puberty increases the number of neurons and glia in the medial prefrontal cortex of female, but not male, rats. Developmental psychobiology, 57(3), 305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kougias DG, Cortes LR, Moody L, Rhoads S, Pan YX, & Juraska JM (2018a). Effects of perinatal exposure to phthalates and a high-fat diet on maternal behavior and pup development and social play. Endocrinology, 159(2), 1088–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kougias DG, Sellinger EP, Willing J, & Juraska JM (2018b). Perinatal exposure to an environmentally relevant mixture of phthalates results in a lower number of neurons and synapses in the medial prefrontal cortex and decreased cognitive flexibility in adult male and female rats. Journal of Neuroscience, 38(31), 6864–6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, & Giedd JN (2006). Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neuroscience & biobehavioral reviews, 30(6), 718–729. [DOI] [PubMed] [Google Scholar]
- Li L, Du Y, Li N, Wu X, & Wu Y (2009). Top–down modulation of prepulse inhibition of the startle reflex in humans and rats. Neuroscience & Biobehavioral Reviews, 33(8), 1157–1167. [DOI] [PubMed] [Google Scholar]
- Li Y, Li T, Zhuang M, Wang K, Zhang J, & Shi N (2010). High-dose dibutyl phthalate improves performance of F1 generation male rats in spatial learning and increases hippocampal BDNF expression independent on p-CREB immunocontent. Environmental toxicology and pharmacology, 29(1), 32–38. [DOI] [PubMed] [Google Scholar]
- Lovekamp-Swan T, & Davis BJ (2003). Mechanisms of phthalate ester toxicity in the female reproductive system. Environmental health perspectives, 111(2), 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig K, Geyer MA, & Vollenweider FX (2003). Deficits in prepulse inhibition and habituation in never-medicated, first-episode schizophrenia. Biological psychiatry, 54(2), 121–128. [DOI] [PubMed] [Google Scholar]
- Mariana M, Feiteiro J, Verde I, & Cairrao E (2016). The effects of phthalates in the cardiovascular and reproductive systems: A review. Environment international, 94, 758–776. [DOI] [PubMed] [Google Scholar]
- Markham JA, Morris JR, & Juraska JM (2007). Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience, 144(3), 961–968. [DOI] [PubMed] [Google Scholar]
- Martino-Andrade AJ, & Chahoud I (2010). Reproductive toxicity of phthalate esters. Molecular nutrition & food research, 54(1), 148–157. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, & Shenton ME (1999). MRI anatomy of schizophrenia. Biological psychiatry, 45(9), 1099–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meincke U, Light GA, Geyer MA, Braff DL, & Gouzoulis-Mayfrank E (2004). Sensitization and habituation of the acoustic startle reflex in patients with schizophrenia. Psychiatry research, 126(1), 51–61. [DOI] [PubMed] [Google Scholar]
- Mose T, Knudsen LE, Hedegaard M, & Mortensen GK (2007). Transplacental transfer of monomethyl phthalate and mono (2-ethylhexyl) phthalate in a human placenta perfusion system. International journal of toxicology, 26(3), 221–229. [DOI] [PubMed] [Google Scholar]
- Noriega NC, Howdeshell KL, Furr J, Lambright CR, Wilson VS, & Gray LE Jr (2009). Pubertal administration of DEHP delays puberty, suppresses testosterone production, and inhibits reproductive tract development in male Sprague-Dawley and Long-Evans rats. Toxicological sciences, 111(1), 163–178. [DOI] [PubMed] [Google Scholar]
- Nuñez JL, Jurgens HA, & Juraska JM (2000). Androgens reduce cell death in the developing rat visual cortex. Developmental Brain Research, 125(1–2), 83–88. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, & Briley M (1985). Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. Journal of neuroscience methods, 14(3), 149–167. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judaš M, Šimić G, Rašin MR, Uylings HB, Rakic P, & Kostović I (2011). Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proceedings of the National Academy of Sciences, 108(32), 13281–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, & Kesner RP (1999). Involvement of the prelimbic–infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. Journal of Neuroscience, 19(11), 4585–4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, & Ahmad N (2008). Dose translation from animal to human studies revisited. The FASEB journal, 22(3), 659–661. [DOI] [PubMed] [Google Scholar]
- Schneider M, & Koch M (2005). Behavioral and morphological alterations following neonatal excitotoxic lesions of the medial prefrontal cortex in rats. Experimental neurology, 195(1), 185–198. [DOI] [PubMed] [Google Scholar]
- Shoaff JR, Calafat AM, Schantz SL, & Korrick SA (2019). Endocrine disrupting chemical exposure and maladaptive behavior during adolescence. Environmental research, 172, 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani MR, & Moghaddam B (2005). Systemic and prefrontal cortical NMDA receptor blockade differentially affect discrimination learning and set-shift ability in rats. Behavioral neuroscience, 119(2), 420. [DOI] [PubMed] [Google Scholar]
- Swerdlow N, Geyer MA, & Braff D (2001). Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology, 156(2–3), 194–215. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Iida M, Kobayashi S, Jin K, Matsuda T, & Kojima H (2005). Differential effects of phthalate esters on transcriptional activities via human estrogen receptors α and β, and androgen receptor. Toxicology, 210(2–3), 223–233. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, & Kolb B (2003). Do rats have a prefrontal cortex? Behavioural brain research, 146(1–2), 3–17. [DOI] [PubMed] [Google Scholar]
- Van Eden CG, Kros JM, & Uylings HBM (1991). The development of the rat prefrontal cortex: its size and development of connections with thalamus, spinal cord and other cortical areas In Progress in brain research (Vol. 85, pp. 169–183). Elsevier. [DOI] [PubMed] [Google Scholar]
- Wang L, Andersson S, Warner M, & Gustafsson JÅ (2003). Estrogen receptor (ER) β knockout mice reveal a role for ERβ in migration of cortical neurons in the developing brain. Proceedings of the National Academy of Sciences, 100(2), 703–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DC, Chen TJ, Lin ML, Jhong YC, & Chen SC (2014). Exercise prevents the increased anxiety-like behavior in lactational di-(2-ethylhexyl) phthalate-exposed female rats in late adolescence by improving the regulation of hypothalamus-pituitary-adrenal axis. Hormones and behavior, 66(4), 674–684. [DOI] [PubMed] [Google Scholar]
- Wang R, Xu X, & Zhu Q (2016). Pubertal exposure to di-(2-ethylhexyl) phthalate influences social behavior and dopamine receptor D2 of adult female mice. Chemosphere, 144, 1771–1779. [DOI] [PubMed] [Google Scholar]
- Willing J, & Juraska JM (2015). The timing of neuronal loss across adolescence in the medial prefrontal cortex of male and female rats. Neuroscience, 301, 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittassek M, Koch HM, Angerer J, & Brüning T (2011). Assessing exposure to phthalates–the human biomonitoring approach. Molecular nutrition & food research, 55(1), 7–31. [DOI] [PubMed] [Google Scholar]


