Abstract
Objective
To assess the relationship between measures of disease assessment in patients with large-vessel vasculitis.
Methods
Patients with giant cell arteritis (GCA) or Takayasu’s arteritis (TAK) were recruited into a prospective, observational cohort. Assessments within the following outcomes were independently recorded: (a) patient-reported outcomes (PROs) [multi-dimensional fatigue inventory (MFI); patient global assessment (PtGlobal); 36-item short form health survey (SF-36); brief-illness perception questionnaire (BIPQ)], (b) physician-reported outcomes [physician global assessment (PhGlobal)], (c) Laboratory outcomes [CRP, ESR], and (d) imaging outcomes [PETVAS, a qualitative score of vascular FDG-PET activity].
Results
Analyses were performed on 112 patients (GCA=56, TAK=56), over 296 visits, with median follow-up of 6 months. Correlation network analysis revealed assessment measures clustered independently by type of outcome. PhGlobal was centrally linked to all other outcome types, but correlations were modest (ρ=0.12–0.32, p< 0.05). PETVAS, CRP, and PtGlobal were independently associated with clinically active disease. All four PROs strongly correlated with each other (ρ=0.35–0.60, p< 0.0001). PROs were not correlated with PETVAS and only PtGlobal correlated with CRP (ρ=0.16, p< 0.01). Patients whose clinical assessment changed from active disease to remission (n=29) had corresponding significant decrease in ESR, CRP, and PETVAS at the remission visit. Patients whose clinical assessment changed from remission to active disease (n=11) had corresponding significant increase in CRP and PtGlobal at the active visit.
Conclusions
Measures of disease assessment in large-vessel vasculitis consist of independent, yet complementary outcomes, supporting the need to develop composite outcome measures or a standard set of measures covering multiple types of outcomes.
Introduction
Large-vessel vasculitis (LVV) is characterized by inflammation of the aorta and its major branches. The most common forms of LVV include giant cell arteritis (GCA) and Takayasu’s arteritis (TAK) (1). No standardized set of outcome measures currently exist to evaluate treatment response in patients with LVV (2,3). Various outcomes have been proposed for LVV, including patient-reported outcomes (PROs), physician assessment of disease activity, vascular imaging, and laboratory assessment (2–10). However, data examining the relationships between these outcomes is limited.
The lack of standardized outcome measures has hindered clinical trial development in LVV. Only a limited number of randomized trials have been performed in LVV, including recent trials of abatacept and tocilizumab in GCA and TAK (11–15). These trials used a range of outcome measures to assess treatment response, including relapse-free survival, glucocorticoid usage, physician and patient global assessment, acute-phase reactants, and vascular imaging findings. Making comparisons between trials is difficult without a standardized set of outcome measures.
Establishing a set of defined outcome measures was prioritized by the LVV Working Group within the Outcome Measures in Rheumatology (OMERACT) (2,3,16). This group recently completed an international Delphi exercise and interviewed patients to assess what outcomes should be a part of future development efforts (2,3,16). The core outcomes suggested were organ and arterial function, biomarkers, fatigue, pain, and death. Additionally, OMERACT highlighted the importance of including imaging studies for the assessment of disease activity. Including patient-reported measures to assess the psychosocial impact of disease was also emphasized (2,3,16).
Before a common set of outcome measures can be derived, it is important to understand the relationship between potential outcome types. Therefore, the objective of the current study was to assess the relationship structure between patient, physician, imaging, and laboratory-based outcome measures in a prospective, longitudinal cohort of patients with LVV.
Methods
Study population
Patients ≥5 years were recruited into a prospective, observational cohort at the National Institutes of Health (NIH) ( NCT02257866) from November 2015 to November 2018. Patients fulfilled the American College of Rheumatology (ACR) 1990 criteria for the classification of TAK (17) or the modified ACR 1990 criteria for the classification of GCA (18). Patients were enrolled at various stages of disease and not just at time of diagnosis. Patients were evaluated by an investigative team with expertise in LVV at approximately 6-month intervals at the NIH Clinical Center. Patients provided written informed consent and the study was approved by NIH ethics and radiation safety committees.
Data collection
At each visit, patients underwent clinical and laboratory evaluation. FDG-PET imaging studies were performed as part of a previously described clinical imaging protocol [8], but were not necessarily performed at every visit. Patients were asked to complete questionnaires at every visit. Data from multiple different outcome assessments were included. These assessments were classified based on their characteristics. In this study, four types of outcomes were included: (a) Patient reported outcomes, (b) Physician reported outcomes, (c) Laboratory-based outcomes, and (d) Imaging-based outcomes. Within these, one or more outcome measure assessments could be included. For example, the laboratory-based outcome included both ESR and CRP measurements.
Patient-reported outcomes
Patient Global Assessment of Disease Activity (PtGlobal)
Patients were asked to rate the severity of their vasculitis on the day of the study visit. Patient global assessment (PtGlobal) was assessed on a scale of 0 (no disease) to 10 (very severe disease).
Multidimensional Fatigue Inventory (MFI)
Fatigue was measured using the four-item general fatigue scale of the Multidimensional Fatigue Inventory. The MFI is a validated and reliable assessment tool that discriminates between patients with vasculitis and healthy controls (20–23). Total MFI scores ranged from 4 to 20, with higher scores indicating greater fatigue.
Brief-Illness Perception Questionnaire (BIPQ)
Illness perceptions were assessed using the Brief Illness Perception Questionnaire, which is a shortened version of the revised Illness Perception Questionnaire (24). The BIPQ is a valid and reliable assessment tool that has been used to study a range of chronic diseases including vasculitis (24–26). Only the first eight questions of the BIPQ were included in analyses. A composite score was computed, and scores ranged from 0 to 80, with higher scores indicating greater negative illness perception.
36-item short form health survey (SF-36)
Health related quality of life (HRQOL) was assessed using the 36-item short form health survey version 2 (SF-36), which has been validated in multiple diseases and was scored according to the standard scoring guidelines (27). Physical health (SF-36 PCS) and mental health (SF-36 MCS) component summary scores were calculated. For correlation analyses, SF-36 scores were multiplied by −1 (Neg_SF-36), so that higher scores on all of the PRO measures would indicate worse outcomes. For all other analyses, SF-36 scores were not transformed and higher scores on the SF-36 indicate better HRQOL.
Physician-reported outcomes
Physician Global Assessment of Disease Activity (PhGlobal)
Clinical disease activity was assessed using physician global assessment (PhGlobal). PhGlobal was chosen as there are no other validated disease activity measures available for the simultaneous assessment of patients with GCA and TAK. PhGlobal was assessed on a scale of 0 (clinical remission) to 10 (very severe disease). Active disease was defined as having PhGlobal>0 and remission as PhGlobal=0. PhGlobal was performed blinded to imaging studies and PROs. PhGlobal>0 was assigned to patients experiencing any clinical feature directly attributable to vasculitis disease activity (i.e. headache, carotidynia). Patients having fatigue or elevated acute-phase reactants alone were not considered to have active disease.
Laboratory-based outcomes
Serologic Assessment (ESR, CRP)
Laboratory assessments were performed by the NIH Department of Laboratory Medicine. Blood was collected on the same day as the study visit.
Imaging-based outcomes
Imaging Assessment by FDG-PET (PETVAS)
Patients underwent FDG-PET-computed tomography (CT) or FDG-PET-magnetic resonance imaging (MRI) at baseline and at approximately 6 to 12-month intervals. The imaging protocol has been previously described (8,19). A qualitative score of vascular FDG-PET activity, PETVAS, was determined. The calculation of PETVAS has been detailed elsewhere (8). In brief, PETVAS is determined by evaluating nine arterial territories (ascending aorta, aortic arch, descending thoracic aorta, abdominal aorta, carotids, subclavians, and brachiocephalic). A four-point scale is used to evaluate uptake in each region compared to the liver (0=no uptake; 1=less than liver; 2=similar to liver; 3=greater than liver). The nine scores are summed to obtain a PETVAS, which ranges from 0 to 27, with higher scores representing increased burden of arterial inflammation.
Statistical analysis
Demographic characteristics and median scores for each outcome measure at baseline were compared between patients with GCA and TAK using Mann-Whitney test or two-tailed Fisher exact test using JMP 14 or Prism 7. Spearman correlations and p-values were calculated using the rcorr function of the R package Hmisc. Univariable and multivariable nominal logistic regression analyses were performed in R using the glm function from the stats package. To facilitate comparisons on the strength of associations across predictor variables, standardized beta-estimates were calculated in R using the beta function from the reghelper package. Change over time in outcome measures was compared using the Wilcoxon signed rank test in patients with a change in clinical status from active disease (PhGlobal>0) to remission (PhGlobal=0), or from remission to active disease. Median scores on outcome measures were compared between all active and all remission visits using Mann-Whitney test in Prism 7.
Correlation network analysis
Correlation network analysis enables visualization of the strength and directionality of correlations and clusters variables most correlated to one another. To visualize the relationship between the dfferent outcomes, the R package corrr was used to perform correlation network analysis between outcome measures having significant Spearman’s correlations.
Results
Characteristics of the study population
A total of 112 patients (GCA=56, TAK=56) were recruited into the study. Patients were evaluated over a total of 296 study visits, with a median follow-up interval of 6 months (interquartile range (IQR)=5–11). Seventy patients (62.5%) had at least one follow-up visit, with a median of 3 (IQR=2–5) per patient. Patients completed a total of 254 MFI (14.2% missing), 249 PtGlobal (15.9% missing), 241 BIPQ (18.6% missing), and 266 SF-36 (10.1% missing) measures. FDG-PET was performed at 240 visits (18.9% missing). ESR and CRP values were available for 295 visits (0.3% missing). PhGlobal was available for all 296 visits (0% missing). For each outcome measure, there were no significant differences in age, gender, type of vasculitis, treatment status, or disease activity between patients with complete versus missing data (data not shown).
Comparison of patients with GCA and TAK
Demographics and outcome measures were compared between patients with GCA and TAK at their baseline visit (Table 1). Patients with TAK were significantly younger than patients with GCA (34 years, IQR=22.3–43.8 vs. 71 years IQR=63.3–76, p<0.0001). Patients with TAK were on significantly lower doses of prednisone than patients with GCA (2.5 mg/day, IQR=0–10 vs. 5 mg/day, IQR=0–28.8, p=0.05). Additionally, patients with TAK had significantly longer disease duration than patients with GCA (5.3 years, IQR=1.6–15.2 vs. 1.4 years IQR=0.5–2.9, p<0.0001). None of the PRO measures, PhGlobal, or laboratory measures were significantly different between patients with TAK and GCA (p>0.05). Patients with GCA did have significantly higher median PETVAS than patients with TAK (19, IQR=14–26.5 vs. 15, IQR=9–19, p<0.01).
Table 1.
Patient demographics and outcome measure scores at baseline visit
| All patients(n=112) | GCA (n=56) | TAK (n=56) | p-value | |
|---|---|---|---|---|
| Patient Demographics | ||||
| Age | 55 (34–71) | 71 (63.3–76) | 34 (22.3–43.8) | <0.01 |
| Sex (% female) | 79.5% (n=89) | 78.5% (n=44) | 80.4% (n=45) | 1.00 |
| Race (% Caucasian) | 73.2% (n=82) | 78.6% (n=44) | 67.9% (n=38) | 0.29 |
| BMI | 25.8 (22.4–29.4) | 26.3 (24.8–29.2) | 25.7 (21.6–29.5) | 0.31 |
| Prednisone Dose (mg/day) | 5 (0–19.4) | 5 (0–28.8) | 2.5 (0–10) | 0.05 |
| Other immunosuppressant | 54.5% (n=61) | 44.6% (n=25) | 64.3% (n=36) | 0.06 |
| Disease duration (years) | 2.4 (0.7–8.3) | 1.4 (0.5–2.9) | 5.3 (1.6–15.2) | <0.01 |
| Patient Reported Outcomes | ||||
| Patient Global Assessment | 4 (1–6) | 4 (1–5.8) | 4 (1.8–6.3) | 0.58 |
| MFI | 14 (12–17) | 14 (9–16) | 15 (12–17.8) | 0.10 |
| BIPQ | 41 (31–50) | 41 (30–50) | 43 (34.8–50) | 0.24 |
| SF-36 PCS | 50.2 (43.5–57.3) | 50 (42.7–54.3) | 50.7 (44.1–57.9) | 0.37 |
| SF-36 MCS | 50 (40.1–56.7) | 51.1 (41.5–58.9) | 48 (37.5–55.2) | 0.15 |
| Physician Reported Outcomes | ||||
| Physician Global Assessment | 0 (0–3) | 0 (0–3) | 0 (0–3) | 0.60 |
| Active Disease at Visit (Physician Global >0) |
42% (n=47) | 46.4% (n=26) | 37.5% (n=21) | 0.44 |
| Laboratory Outcomes | ||||
| CRP (mg/L) | 4.1 (1.1–11) | 5.0 (1–10.4) | 3.75 (1.2–13.8) | 0.83 |
| ESR (mm/hour) | 18 (7.3–29) | 15.5 (7.3–31.8) | 18 (7.5–29) | 0.90 |
| Imaging Outcomes | ||||
| PETVAS | 17 (13–24) | 19 (14–26.5) | 15 (9–19) | <0.01 |
Values are reported as median and interquartile range or as percentage and n-value. Mann-Whitney test was used to compare continuous variables. Two-tailed fisher exact test was used to compare categorical variables. Values may not be based on total denominations if there were missing responses.
GCA= giant cell arteritis; TAK= Takayasu’s arteritis; BMI= body mass index; MFI= Multi-dimensional fatigue inventory; BIPQ= brief illness perception questionnaire; SF-36 PCS= 36-item short form health survey physical component summary score; SF-36 MCS= 36-item short form health survey mental component summary score; CRP= c-reactive protein; ESR= erythrocyte sedimentation rate; PETVAS= qualitative score of vascular FDG-PET activity; mg= milligram; L=liters; mm=millimeter.
Correlations between outcome measures all patients
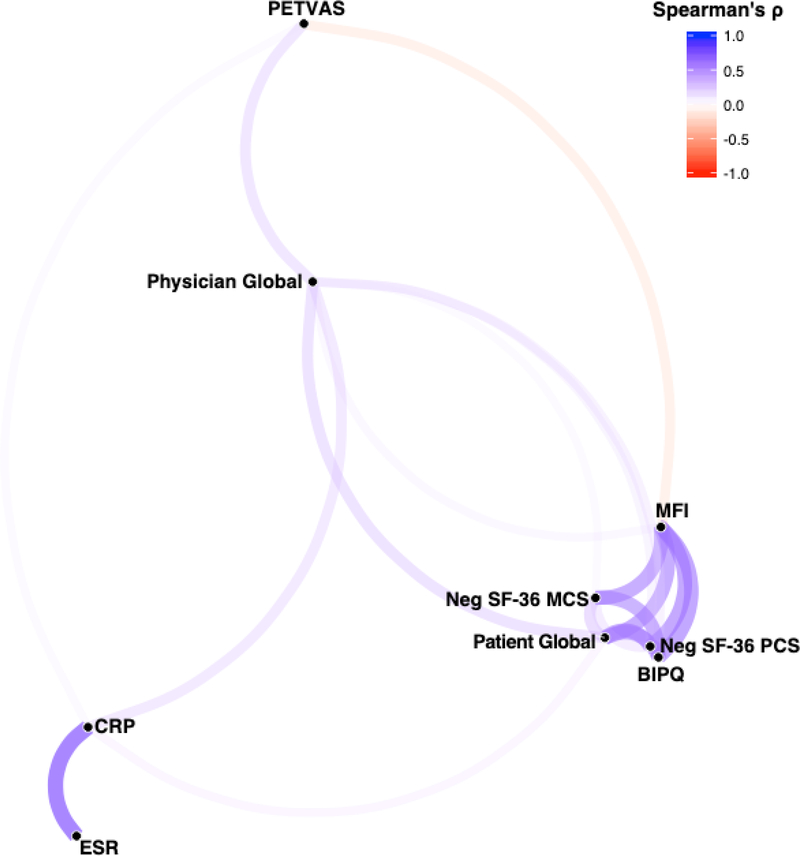
To assess the relationship between outcome measures, correlation network analysis was employed (Figure 1 and Table 2). Network analysis revealed that the outcomes were largely independent, with individual outcome measure assessments clustering by specific type of outcome. The strongest correlations were within individual types of outcomes. The PROs (PtGlobal, MFI, BIPQ, Neg_SF-36 PCS, and Neg_SF-36 MCS) were strongly and significantly correlated with one another (ρ=0.35 to 0.60, p< 0.0001). Acute phase reactants (ESR and CRP) were also strongly correlated (ρ=0.71, p<0.0001).
Figure 1. Correlation network of outcome measures in all patients with LVV.
Correlation network of the significant Spearman ρ correlation coefficients (p<0.05) between outcome measures created using the R package corrr. Color and thickness of the edges between nodes indicates the strength of the correlation. Nodes for each outcome measure are clustered in space using multidimensional scaling of the absolute values of the correlations, such that outcome measures with the highest overall magnitude of correlation with each other are closer in space. For ease of visualization, the SF-36 PCS and MCS measures were multiplied by −1 so that a higher score would indicate a worse outcome. MFI= Multi-dimensional fatigue inventory; BIPQ= brief illness perception questionnaire; Neg SF-36 PCS= negatively transformed 36-item short form health survey physical component summary score; Neg SF-36 MCS= negatively transformed 36-item short form health survey mental component summary score; CRP= c-reactive protein; ESR= erythrocyte sedimentation rate; PETVAS= qualitative score of vascular FDG-PET activity.
Table 2.
Spearman correlations between outcome measures in all patients
| PtGlobal | MFI | BIPQ | Neg SF-36 PCS | Neg SF-36 MCS | PhGlobal | CRP | ESR | PETVAS | |
|---|---|---|---|---|---|---|---|---|---|
| PtGlobal | 1.00 | ||||||||
| MFI | 0.58**** | 1.00 | |||||||
| BIPQ | 0.57**** | 0.60**** | 1.00 | ||||||
| Neg SF-36 PCS | 0.59**** | 0.60**** | 0.55**** | 1.00 | |||||
| Neg SF-36 MCS | 0.35**** | 0.58**** | 0.56**** | 0.25**** | 1.00 | ||||
| PhGlobal | 0.32**** | 0.15* | 0.21** | 0.18** | 0.12* | 1.00 | |||
| CRP | 0.16** | 0.07 | 0.09 | 0.07 | 0.09 | 0.27**** | 1.00 | ||
| ESR | 0.07 | 0.05 | 0.05 | −0.03 | 0.07 | 0.11 | 0.71**** | 1.00 | |
| PETVAS | −0.07 | −0.23*** | −0.06 | −0.05 | −0.12 | 0.29**** | 0.13* | 0.04 | 1.00 |
p<0.05
p<0.01
p<0.001
p<0.0001.
PtGlobal= Patient Global Assessment; MFI= Multi-dimensional fatigue inventory; BIPQ= brief illness perception questionnaire; Neg SF-36 PCS= negatively transformed 36-item short form health survey physical component summary score; Neg SF-36 MCS= negatively transformed 36-item short form health survey mental component summary score; PhGlobal= physician global assessment; CRP= c-reactive protein; ESR= erythrocyte sedimentation rate; PETVAS= qualitative score of vascular FDG-PET activity.
Vascular PET activity was not associated with worse disease outcomes reported by patients. Neither PtGlobal, Neg_SF-36 PCS/MCS, nor BIPQ scores were significantly correlated with PETVAS and MFI was significantly negatively correlated with PETVAS (ρ= −0.23, p<0.0001). Correlation network analysis revealed that PhGlobal was centrally linked to all other outcomes, although the correlations were modest (ρ=0.12 to 0.32, p<0.05). Similarly, CRP was modestly correlated to all other outcome measure types (ρ=0.13 to 0.27, p<0.05). Unlike PhGlobal, CRP was only significantly correlated with PtGlobal and none of the other PRO measures. ESR was not correlated to any outcome measure other than CRP.
Correlations between outcome measures by diagnosis
The relationship between outcome measures was assessed by diagnosis (Supplementary Figure 1 and 2). Correlations between laboratory, physician, and imaging-based outcomes remained largely consistent between patients with GCA and TAK. However, the relationship of PROs to these other outcomes differed in GCA and TAK. Network analysis demonstrated that PROs in TAK were not significantly correlated to either laboratory or physician-reported outcomes (p>0.05). Further, all of the PROs in TAK were negatively correlated to PETVAS (ρ= −0.21 to −0.32, p<0.05).
Associations between patient-reported outcomes and glucocorticoid use
Correlation network analysis was used to understand the relationship of daily prednisone dose to the various PROs across all patients (Supplementary Figure 3). PtGlobal and BIPQ were the only PROs significantly and positively correlated with prednisone dose. In all patients, prednisone dose was most strongly correlated to PhGlobal (ρ=0.34, p<0.0001), without significant correlation to laboratory values or PET findings (Supplementary Table 1). Network analysis also illustrated the relationship between individual PRO measures: PtGlobal clustered most closely to the Neg_SF-36 PCS and not the MCS, while the BIPQ and MFI clustered between the PCS and MCS. These relationships remained largely similar even when considering GCA and TAK separately (Supplementary Table 1).
Associations of outcome measures to the eight SF-36 scales
The SF-36 consists of eight scales reflecting various specific aspects of HRQOL. Correlations between each of these eight scales and the other outcome measures were also studied (Supplementary Table 2 and Supplementary Figure 4). Worse outcomes on each of the eight SF-36 scales were significantly correlated with worse outcomes on each of the other three PROs (PtGlobal, MFI, BIPQ) (ρ= −0.27 to −0.81, p<0.0001). While a worse outcome on PhGlobal was significantly correlated to worse outcomes on the Role Limitations Due to Physical Functioning (ρ= −0.18, p<0.01), Vitality (ρ= −0.21, p<0.001), Social Functioning (ρ= −0.15, p<0.05), Bodily Pain (ρ= −0.24, p<0.001), and General Health (ρ= −0.17, p<0.01) scales only. Worse outcomes on CRP, ESR, and PETVAS were not significantly correlated to worse outcomes on any of the eight SF-36 scales.
Association of outcome measures with clinically active disease
Using PhGlobal>0 to define active disease, 95 total study visits (32.1%) were during active disease while 201 visits (67.9%) were during remission. In comparing all active visits to remission visits (Supplementary Table 3), significantly worse outcomes were observed in all measures during active disease (p<0.05), with the exception of the SF-36 MCS (p=0.06) and ESR (p=0.15) which were not significantly different between active and remission visits.
Logistic regression modeling was then also used to determine which outcome measures had the strongest independent association with clinical assessment of disease activity, as determined by PhGlobal. In univariable regression, worse outcomes on the MFI, PtGlobal, SF-36 PCS, BIPQ, CRP, and PETVAS, were all associated with clinically active disease. Diagnosis (TAK vs GCA) and SF-36 MCS were not associated with clinically active disease. In a multivariable model, only one outcome measure from each type, PETVAS, CRP, and PtGlobal, remained significantly associated with clinically active disease when adjusting for the other measures. Of these three measures, higher PETVAS was the most strongly associated with clinically active disease (PETVAS std ß-estimate = 0.976, p<0.0001; CRP std ß-estimate=0.660, p<0.05; PtGlobal std ß-estimate=0.592, p<0.05) (Table 3).
Table 3.
Nominal logistic regression models showing outcome measures that are associated with active clinical disease in patients with LVV.
| Predictor Variable | Univariable Models |
Multivariable Model |
|||||
|---|---|---|---|---|---|---|---|
| Parameter Estimate | Standard Error | p-value | Parameter Estimate | Std β-Estimate | Standard Error | p-value | |
| MFI | 0.081 | 0.035 | 0.019 | 0.068 | 0.290 | 0.265 | 0.273 |
| PtGlobal | 0.255 | 0.058 | <0.001 | 0.246 | 0.592 | 0.251 | 0.018 |
| SF-36 PCS | −0.040 | 0.013 | 0.003 | −0.001 | 0.011 | 0.252 | 0.965 |
| BIPQ | 0.026 | 0.010 | 0.011 | −0.003 | −0.048 | 0.234 | 0.839 |
| CRP | 0.030 | 0.009 | <0.001 | 0.036 | 0.660 | 0.323 | 0.041 |
| PETVAS | 0.117 | 0.025 | <0.001 | 0.158 | 0.976 | 0.220 | <0.001 |
| SF-36 MCS | −0.018 | 0.012 | 0.129 | ||||
| Diagnosis (TAK) | −0.229 | 0.251 | 0.362 | ||||
MFI= Multi-dimensional fatigue inventory; PtGlobal= Patient Global Assessment; SF-36 PCS= 36-item short form health survey physical component summary score; BIPQ= brief illness perception questionnaire; CRP= c-reactive protein; PETVAS= qualitative score of vascular FDG-PET activity; SF-36 MCS= 36-item short form health survey mental component summary score; TAK= Takayasu’s arteritis.
Outcome measures over time in patients undergoing changes in clinical disease activity
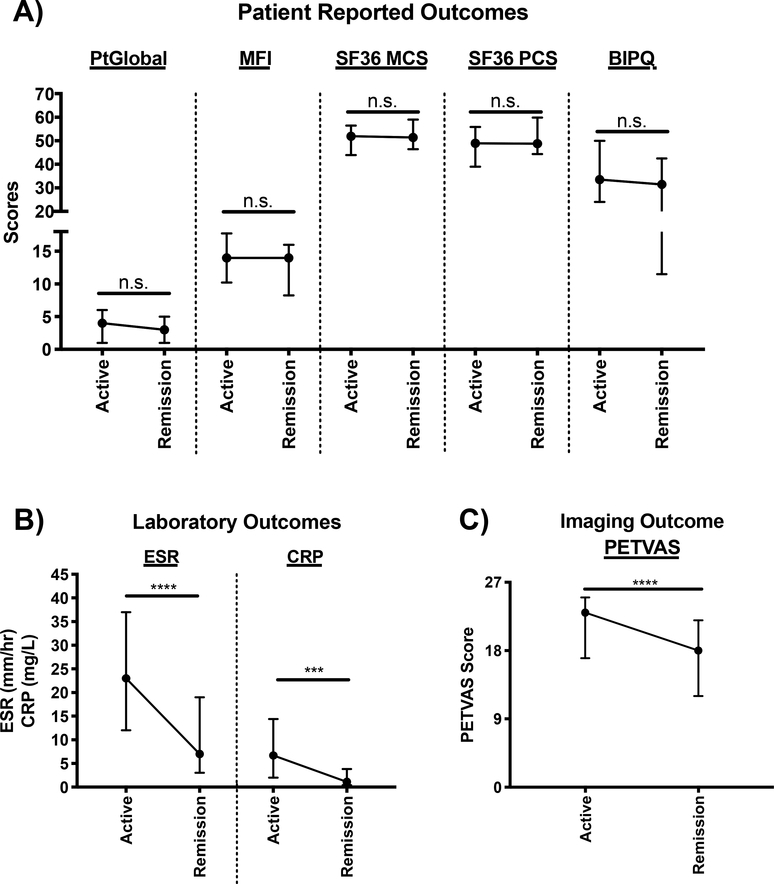
To assess whether change over time in outcomes tracked with changes in disease activity, patients with a change in clinical status were studied. During the study period, 29 patients (GCA=18, TAK=11) experienced a change in clinic disease activity from active disease to remission with a median follow-up of 8 months (IQR=6–15) between their active visit to their first subsequent remission visit. In these patients, PETVAS (23 (IQR=16.5–25) active vs 17.5 (IQR=12–21.3) remission, p<0.0001), ESR (23 (IQR=11–35.5) active vs 5 (IQR=3–117) remission, p<0.0001), and CRP (6.9 (IQR=2–15.3) active vs 1.0 (IQR=0.4–3.8) remission, p<0.001), significantly decreased between the visits. PtGlobal, MFI, BIPQ, SF-36 PCS and SF-36 MCS did not significantly change over these intervals (Figure 2).
Figure 2. Longitudinal analysis of outcome measures in patients experiencing a change in clinical disease activity from active disease to remission.
(A) Patient reported outcomes, (B) Laboratory Outcomes, and (C) Imaging Outcome. Data is plotted as median and interquartile range and n=22–29. Wilcoxon signed rank test was used to compare outcome measure scores between active and remission visits. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, n.s.=not significant. PtGlobal= Patient global assessment; MFI= Multi-dimensional fatigue inventory; SF-36 MCS= 36-item short form health survey mental component summary score; SF-36 PCS= 36-item short form health survey physical component summary score; BIPQ= brief illness perception questionnaire; ESR= erythrocyte sedimentation rate; CRP= c-reactive protein; PETVAS= qualitative score of vascular FDG-PET activity.
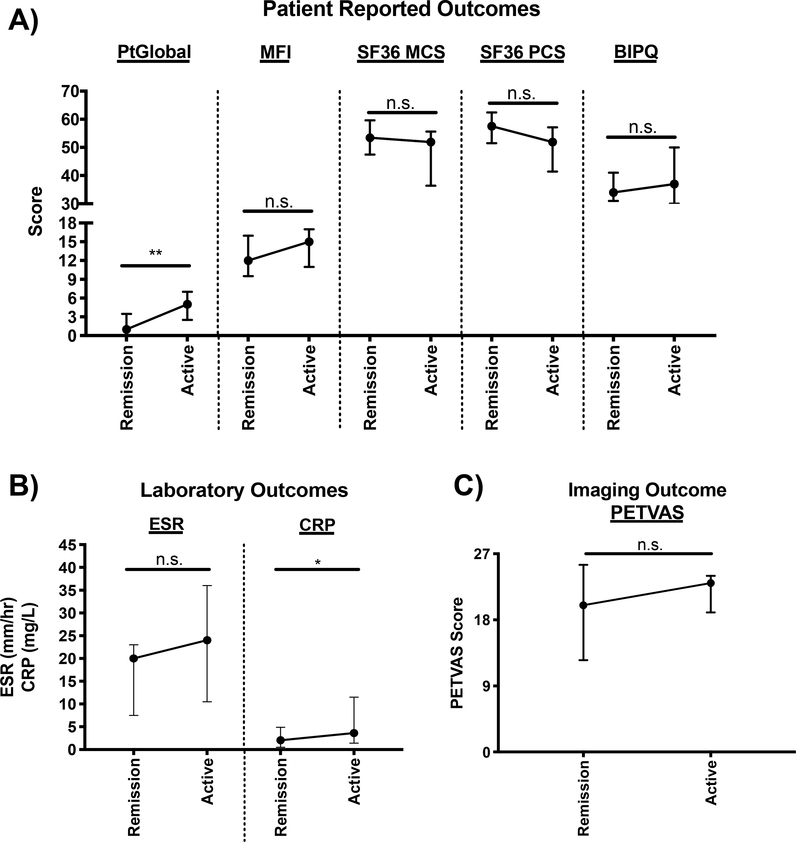
An additional 11 patients (GCA=8, TAK=3) experienced a change in clinical status from remission to active disease with a median follow-up of 7 months (IQR=6–9.5) between their remission visit to their first subsequent active visit. In these patients, PtGlobal (0.5 (IQR=0–2) remission vs 4.5 (IQR=2.3–6.8) active, p=0.02) and CRP (IQR=2.1 (0.8–5.3) remission vs 4.2 (IQR=1.2–13.1) active, p=0.04) significantly increased between the visits (Figure 3).
Figure 3. Longitudinal analysis of outcome measures in patients experiencing a change in clinical disease activity from remission to active disease.
(A) Patient reported outcomes, (B) Laboratory Outcomes, and (C) Imaging Outcome. Data is plotted as median and interquartile range and n=8–11. Wilcoxon signed rank test was used to compare outcome measure scores between remission and active visits. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, n.s.=not significant. PtGlobal= Patient global assessment; MFI= Multi-dimensional fatigue inventory; SF-36 MCS= 36-item short form health survey mental component summary score; SF-36 PCS= 36-item short form health survey physical component summary score; BIPQ= brief illness perception questionnaire; ESR= erythrocyte sedimentation rate; CRP= c-reactive protein; PETVAS= qualitative score of vascular FDG-PET activity.
Discussion
Historically, physician-based assessment has been the predominant means for assessing disease activity in LVV. The most recent randomized clinical trials performed in LVV used clinical-based assessment as primary endpoints to assess treatment efficacy (11–15). Other outcome measures have been proposed for LVV, including acute-phase reactants, imaging-assessment, and PROs. Previous research has demonstrated that these measures may not align with physician-based assessment. Acute-phase reactants are not consistently elevated during active disease (9,28), patient and physician perception of disease does not always align (29), and evidence of vascular inflammation on imaging studies persists even during clinical remission (8). Alignment among physician and patient assessments should not be the goal of outcome measures in LVV, since it is important to capture the burden of disease from all relevant perspectives.
The current study directly assessed the complex relationships between different types of outcome measures in LVV. Standardized data from patient-, physician-, imaging-, and laboratory-based outcomes was collected in a single cohort of patients with LVV. Using correlation network analysis, the structural relationship between these outcomes was identified. Individual outcome measure assessments were highly correlated by type of outcome, while measures across different types of outcomes had only weak to modest correlations. Centrally linking all of the outcomes was physician assessment of disease activity. Taken together, these results suggest that outcome measures in LVV assess independent aspects of disease activity.
This study also further defined relationships between different PRO measures. Even though many of these PRO measures assess seemingly unique outcomes, such as fatigue, physical health, mental health, and illness perceptions, they were all strongly and significantly correlated to one another. The structure of the correlation network provided insight into these relationships. Patient’s assessment of their overall disease activity (PtGlobal) was more strongly associated with physical health (SF-36 PCS), whereas illness perceptions (BIPQ) and fatigue (MFI) were associated with both physical and mental health (SF-36 MCS). Although glucocorticoid therapy is often considered by patients to be a significant burden, daily prednisone dose was only weakly correlated to PROs. Cumulative glucocorticoid exposure, rather than daily dose, may be more tightly linked to patient reported burden of disease; however, these data were not available.
This study has several important strengths. Standardized data acquisition was performed to enable novel comparisons across a range of outcome measures in LVV. For example, no prior study in LVV has compared PROs and FDG-PET findings. Interestingly, findings on FDG-PET were not significantly associated with the PROs, which parallels prior findings demonstrating a lack of strong associations between physician and imaging-based assessments (8), implying that there is a considerable component of clinically “silent” disease in LVV that may require unique measurement methods. Sophisticated analytic techniques were employed, including correlation network analysis. Care was taken to minimize bias by blinding imaging, physician, and patient-based assessments from one another. Finally, the study included an equal number of patients with TAK and GCA, enabling comparisons of outcome measures between patients with different forms of LVV. The relationships between physician-, imaging-, and laboratory-based outcome measures were largely equivalent between patients with GCA and TAK. Patients with TAK, unlike patients with GCA, demonstrated a strong disconnect between PROs and the other types of outcomes. Overall, these findings support the results of an international Delphi exercise, where 67% of experts agreed that a common outcome measure could be developed for both GCA and TAK (2). However, this potential variation in patient-perceived burden of disease in patients with GCA and TAK should remain a consideration.
There are some potential limitations to this study. Since the study was performed at a single center, these results should be replicated in other cohorts. This study was an observational cohort, and the performance of these outcome measures should be assessed in a randomized controlled trial in the future, if they are to be used as standard outcome measures in LVV. Missing data may be a limitation as visits were included if at least one outcome type had data available. However, data was determined to be missing at random and therefore not a major source of bias for correlation-based analyses. In the longitudinal analyses, there were few patients who experienced changes in clinical disease activity, limiting the ability to detect significant changes. However, even with this limitation in sample sizes, significant changes in measures, including CRP, PETVAS, and PtGlobal were detected. Finally, the clinical utility of serial FDG-PET imaging has not been established in LVV, but this study demonstrates in a research context how imaging-based outcome measures relate to other disease assessments in LVV.
In conclusion, potential outcome measures in LVV consist of independent, yet complementary outcomes. These results should inform clinicians about the complex relationships between patient reported outcomes, clinical assessment, vascular imaging findings, and laboratory results in LVV. Different assessments within these outcomes should be incorporated into clinical research in LVV. Adoption of a complementary standard set of measures covering multiple types of outcome assessment would be an important next step in outcome measure development in LVV. Development of a composite multidimensional outcome to comprehensively assess disease activity may eventually provide a more nuanced understanding of drug efficacy in future randomized clinical trials.
Supplementary Material
Significance and Innovations.
Disease activity in large-vessel vasculitis can be defined by clinical assessment, vascular imaging, laboratory tests, and patient-reported outcomes.
The structured relationship between different disease activity measures in large-vessel vasculitis is complex.
Multi-dimensional or composite outcome measures are needed in large-vessel vasculitis.
Acknowledgments
Funding and Conflict of Interest Disclosure
This research was supported by the Division of Intramural Research of the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The authors declare no conflicts of interest.
References
- 1.Gulati A, Bagga A. Large vessel vasculitis. Pediatr Nephrol 2010;25:1037–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sreih AG, Alibaz-Oner F, Kermani TA, Aydin SZ, Cronholm PF, Davis T, et al. Development of a core set of outcome measures for large-vessel vasculitis: Report from OMERACT 2016. J Rheumatol 2017;44:1933–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aydin SZ, Direskeneli H, Merkel PA, Toloza S, Blockmans D, Sato EI, et al. Assessment of disease activity in large-vessel vasculitis: Results of an international delphi exercise. J Rheumatol 2017;44:1928–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yilmaz N, Can M, Oner FA, Kalfa M, Emmungil H, Karadag O, et al. Impaired quality of life, disability and mental health in Takayasu’s arteritis. Rheumatology 2013;52:1898–1904. [DOI] [PubMed] [Google Scholar]
- 5.Akar S, Can G, Binicier O, Aksu K, Akinci B, Solmaz D, et al. Quality of life in patients with Takayasu’s arteritis is impaired and comparable with rheumatoid arthritis and ankylosing spondylitis patients. Clin Rheumatol 2008;27:859–65. [DOI] [PubMed] [Google Scholar]
- 6.Abularrage CJ, Slidell MB, Sidawy AN, Kreishman P, Amdur RL, Arora S. Quality of life of patients with Takayasu’s arteritis. J Vasc Surg 2008;47:131–137. [DOI] [PubMed] [Google Scholar]
- 7.Alibaz-Oner F, Sreih AG, Merkel PA, Direskeneli H. Patient-reported outcomes in Takayasu’s arteritis. Press Medicale 2017;46:e225–e227. [DOI] [PubMed] [Google Scholar]
- 8.Grayson PC, Alehashemi S, Bagheri AA, Civelek AC, Cupps TR, Kaplan MJ, et al. 18 F-Fluorodeoxyglucose-Positron Emission Tomography As an Imaging Biomarker in a Prospective, Longitudinal Cohort of Patients With Large Vessel Vasculitis. Arthritis Rheumatol 2018;70:439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kermani TA, Schmidt J, Crowson CS, Steven R, Hunder GG, Matteson EL, et al. Utility of Erythrocyte Sedimentation Rate and C-Reactive Protein for the Diagnosis of Giant Cell Arteritis. Semin Arthritis Rheum 2012;41:866–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mason JC. Takayasu arteritis advances in diagnosis and management. Nat Rev Rheumatol 2010;6:406–15. [DOI] [PubMed] [Google Scholar]
- 11.Langford CA, Cuthbertson D, Ytterberg SR, Khalidi N, Monach PA, Carette S, et al. A Randomized, Double-Blind Trial of Abatacept (CTLA-4Ig) for the Treatment of Giant Cell Arteritis. Arthritis Rheumatol 2017;69:837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stone JH, Tuckwell K, Dimonaco S, Klearman M, Aringer M, Blockmans D, et al. Trial of Tocilizumab in Giant-Cell Arteritis. N Engl J Med 2017;377:317–328. [DOI] [PubMed] [Google Scholar]
- 13.Villiger PM, Adler S, Kuchen S, Wermelinger F, Dan D, Fiege V, et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: A phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2016;387:1921–1927. [DOI] [PubMed] [Google Scholar]
- 14.Nakaoka Y, Isobe M, Takei S, Tanaka Y, Ishii T, Yokota S, et al. Efficacy and safety of tocilizumab in patients with refractory Takayasu arteritis: results from a randomised, double-blind, placebo-controlled, phase 3 trial in Japan (the TAKT study). Ann Rheum Dis 2018;77:348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langford CA, Cuthbertson D, Ytterberg SR, Khalidi N, Monach PA, Carette S, et al. A Randomized, Double-Blind Trial of Abatacept (CTLA-4Ig) for the Treatment of Takayasu Arteritis. 2017;69:846–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aydin SZ, Direskeneli H, Sreih A, Alibaz-Oner F, Gul A, Kamali S, et al. Update on outcome measure development for large vessel vasculitis: Report from OMERACT 12. J Rheumatol 2015;42:2465–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, et al. The American College of Rheumatology 1990 criteria for the classification of takayasu arteritis. Arthritis Rheum 1990;33:1129–1134. [DOI] [PubMed] [Google Scholar]
- 18.Hunder GG, Bloch DA, Michel BA, Stevens MB, Arend WP, Calabrese LH, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 1990;33:1122–1128. [DOI] [PubMed] [Google Scholar]
- 19.Quinn KA, Ahlman MA, Malayeri AA, Marko J, Civelek AC, Rosenblum JS, et al. Comparison of magnetic resonance angiography and 18F-fluorodeoxyglucose positron emission tomography in large-vessel vasculitis. Ann Rheum Dis 2018;77:1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grayson PC, Amudala NA, McAlear CA, Leduc RL, Shereff D, Richesson R, et al. Illness perceptions and fatigue in systemic vasculitis. Arthritis Care Res 2013;65:1835–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smets EMA, Garssen B, Bonke B, De Haes JCJM. The multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res 1995;39:315–325. [DOI] [PubMed] [Google Scholar]
- 22.Lin J-MS, Brimmer DJ, Maloney EM, Nyarko E, BeLue R, Reeves WC. Further validation of the Multidimensional Fatigue Inventory in a US adult population sample. Popul Health Metr 2009;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lexell J, Jonasson SB, Brogardh C. Psychometric Properties of Three Fatigue Rating Scales in Individuals With Late Effects of Polio. Ann Rehabil Med 2018;42:702–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broadbent E, Petrie KJ, Main J, Weinman J. The Brief Illness Perception Questionnaire. J Psychosom Res 2006;60:631–637. [DOI] [PubMed] [Google Scholar]
- 25.Nowicka-Sauer K, Banaszkiewicz D, Staśkiewicz I, Kopczyński P, Hajduk A, Czuszyńska Z, et al. Illness perception in Polish patients with chronic diseases: Psychometric properties of the Brief Illness Perception Questionnaire. J Health Psychol 2016;21:1739–1749. [DOI] [PubMed] [Google Scholar]
- 26.Brezinova P, Englbrecht M, Lovric S, Sämann A, Strauss B, Wolf G, et al. Coping strategies and depressiveness in primary systemic vasculitis--what is their impact on health-related quality of life? Rheumatology (Oxford) 2013;52:1856–64. [DOI] [PubMed] [Google Scholar]
- 27.Garratt AM, Ruta DA, Abdalla MI, Russell IT. SF 36 health survey questionnaire: II. Responsiveness to changes in health status in four common clinical conditions. Qual Saf Heal Care 1994;3:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salvarani C, Cantini F, Boiardi L, Hunder GG. Laboratory investigations useful in giant cell arteritis and Takayasu’s arteritis. Clin Exp Rheumatol 2003;6:S23–28. [PubMed] [Google Scholar]
- 29.Herlyn K, Hellmich B, Seo P, Merkel PA, Consortium FTVCR. Patient-reported outcome assessment in vasculitis may provide important data and a unique perspective. Arthritis Care Res (Hoboken) 2010;62:1639–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.