Abstract
Engagement in externalizing behavior is problematic. Deviant peer affiliation increases risk for externalizing behavior. Yet, peer effects vary across individuals and may differ across genes. This study determines gene x environment x development interactions as they apply to externalizing behavior from childhood to adulthood. A sample (n = 687; 68% male, 90% White) of youth from the Michigan Longitudinal Study was assessed from ages 10 to 25. Interactions between γ-amino butyric acid type A receptor γ1 subunit (GABRG1;rs7683876, rs13120165) and maladaptive peer behavior on externalizing behavior were examined using time-varying effect modeling. The findings indicate a sequential risk gradient in the influence of maladaptive peer behavior on externalizing behavior depending on the number of G alleles during childhood through adulthood. Individuals with the GG genotype are most vulnerable to maladaptive peer influences, which results in greater externalizing behavior during late childhood through early adulthood.
Keywords: genes, TVEM, externalizing behavior, peers, GABRG1
Introduction
Externalizing behavior, such as rule-breaking and aggression is a pervasive public health concern. Not only is externalizing behavior associated with a host of negative sequelae to the individual (e.g., substance use problems, mental illness; Farmer et al., 2015), but it also has a deleterious impact on society as externalizing behavior early in life conveys risk for adult work incapacity (Narusyte, Ropponen, Alexanderson, & Svedberg, 2017) and later criminal behavior (Harris-McKoy & Cui, 2013). Thus, gaining a greater understanding of factors contributing to high-risk trajectories of externalizing behavior can inform preventive interventions. A strong predictor of high-risk externalizing trajectories is affiliation with peers engaging in deviant behavior (Epstein et al., 2017). Prior work indicates that youth affiliating with peers that engage in delinquent behavior (e.g., stealing, truancy) and peers that use substances demonstrate high externalizing behavior compared to youth affiliating with more prosocial youth (e.g., Samek, Goodman, Erath, McGue, & Iacono, 2016). Yet, individuals vary in their susceptibility to peer influences as a function of differences in genetic susceptibility (Trucco, Villafuerte, Burmeister, & Zucker, 2017). Moreover, developmental theory posits that the degree of susceptibility to socialization contexts, especially peers, is not static over time (Epstein et al., 2017). Although there is a large literature demonstrating how genetic factors increase susceptibility to environmental factors (i.e., gene x environment [GxE] interactions), few investigations exist examining how GxE interactions change from childhood to adulthood within the same study. In fact, understanding the complex GxE interplay underlying peer relations and social development from a fully developmental perspective is viewed as a challenge hampering continued progress in the field (Trucco, Schlomer, & Hicks, 2018). This is due in part to a lack of quantitative methods able to model nonlinear change in interactions over time (Epstein et al., 2017). This study employs a state-of-the-art approach, time-varying effect modeling (TVEM), to determine possible gene x environment x development (GxExD) interactions as they apply to the patterning of externalizing behavior from childhood to adulthood.
Externalizing Behavior Across Development
Externalizing behavior tends to be variable across development. Moreover, trajectories tend to vary somewhat based on the different facets of externalizing behavior. Aggression reflects behavior that is more overt, such as bullying and fighting, whereas rule breaking reflects behavior that is more covert, such as stealing and truancy (Becht, Prinzie, Deković, van den Akker, & Shiner, 2016). Prior work indicates that aggression is typically characterized by an initial increase from the first year of life to the end of the third year, and by a steady decline thereafter (Broidy et al., 2003). This has led some researchers to conclude that children do not necessarily learn to become physically aggressive; rather, they learn not to be physically aggressive given the combined effects of brain maturation and socialization (Tremblay, 2010). In contrast, rule breaking behavior tends to be infrequent during childhood, increases over the course of adolescence, and then decreases again during the transition into adulthood (Walton et al., 2017). During childhood, youth are socialized to respect and follow rules set by authority figures (LaFontana & Cillessen, 2010). During early adolescence, there is a gradual shift towards engaging in mild forms of rule-breaking behaviors in an effort to explore the boundaries of these rules, followed by an increase over the course of adolescence with externalizing behavior peaking during mid- to late-adolescence (Burt & Neiderhiser, 2009). Despite these early developmental differences, in early adulthood, externalizing behaviors tend to decrease or plateau (Walton et al., 2017) as youth obtain roles that are associated with more responsibility, such as entering the workforce (LaFontana & Cillessen, 2010). However, prior work indicates that a subset of individuals persist in engaging in externalizing behavior well beyond the transition to adulthood (Shaw, Hyde, & Brennan, 2012). It is these youth who are likely to benefit most from early interventions. Despite these important developmental nuances, by and large prior GxE studies have tended to give minimal consideration to age by either controlling for age (Lu & Menard, 2017), examining effects only within a circumscribed age range (Trucco et al., 2017), or collapsing across developmental periods (Simons et al., 2013). There are some notable exceptions. For example, a series of studies recently employed TVEM to examine the longitudinal associations between a preventive intervention and variants in the gene encoding the γ-amino butyric acid α2 receptor subunit (GABRA2) on alcohol misuse (Russell et al., 2018) and delinquency (Schlomer et al., 2019) from ages 11 to 19. Although this work has advanced the field, similar examinations focused on socialization factors outside of intervention settings, such as peer delinquency, may help determine who is most likely to benefit from preventive interventions, as well as optimal timing.
Deviant Peers and Externalizing Behavior
As adolescents gain autonomy, there is a shift from spending time with family to spending an increased amount of time with peers, which results in parents having less of an impact on behavior compared to peers (Vitaro, Boivin, & Poulin, 2018). Adolescents also begin to value social belongingness, fear peer rejection, and become more attuned to behaviors that increase peer acceptance (LaFontana & Cillessen, 2010). Thus, adolescents are more likely to engage in problem behavior if it is rewarded by peer acceptance. A framework that is helpful in understanding the association between externalizing behavior and deviant peer behavior is social learning theory (Akers, 1977). This theory posits that behavior is learned when that behavior is rewarded. For example, if an individual gains popularity from peers for skipping school, they are more likely to continue skipping school. Similarly, the differential association theory posits that an individual’s propensity to engage in delinquent behavior is due primarily to affiliation with others, including peers (Sutherland, 1947). Prior empirical work indicates that affiliating with peers that engage in various deviant behaviors (Burt, McGue, & Iacono, 2009), as well as more specific types of deviant behavior, such as substance use (Barnow et al., 2004), increases risk for externalizing behavior during adolescence and early adulthood. Although there is evidence supporting the increasing salience of peer influence on externalizing behavior (Cleveland, Feinberg, Bontempo, & Greenberg, 2008), several studies support a decrease of peer influence over time (Abadi, Shamblen, Thompson, Collins, & Johnson, 2011), as well as curvilinear trends indicating a peak of peer influence during mid- to late-adolescence and then a plateau during adulthood (Cleveland, Feinberg, & Jones, 2012). Thus, quantitative methods that can model nonlinear change in interactions over time can have significant utility for determining possible critical periods during which certain individuals are particularly sensitive to peer effects.
It is important to note that susceptibility to deviant peers is not uniform across individuals. Several factors have been demonstrated to impact susceptibility to peers. These include an individual’s dispositional characteristics, such as impulsivity and reward sensitivity (Pfeifer et al., 2011). Developmentally, these dispositional characteristics increase from age 10 to ages 13 to 16 (Steinberg & Monahan, 2007), which corresponds to the developmental period where youth are most sensitive to peers. In addition, genetic studies underscore the possibility that genes impact the degree to which youth are susceptible to peers (Villafuerte, Trucco, Heitzeg, Burmeister, & Zucker, 2014b). In fact, the same genetic risk factors demonstrated to increase sensitivity to peers also underlie individual differences in impulsivity and reward sensitivity (Heitzeg et al., 2014; Villafuerte et al., 2014a). Prior work on environmental stressors and the genetics of youth problem behavior indicates that developmental differences in the maturation of brain areas and related behavioral differences in impulsivity and reward sensitivity likely interact in an age-specific way (Zalsman, 2010). Thus, it is critical to understand GxE interactions within a developmental framework. The current study focuses on a specific genetic risk factor located in an area of linkage that has received strong empirical support for its role as a predictor of child externalizing behavior (Trucco, Villafuerte, Heitzeg, Burmeister, & Zucker, 2014), impulsivity (Villafuerte et al., 2014a) and reward sensitivity (Heitzeg et al., 2014), as well as its role in impacting the degree of sensitivity to socialization contexts, such as peers (Trucco et al., 2017; Villafuerte et al., 2014b) and parents (Trucco, Villafuerte, Heitzeg, Burmeister, & Zucker, 2016). This investigation leverages cuttingedge methodology, TVEM, to extend prior work by examining GxE effects from childhood to early adulthood.
Gene x Environment Effects
There is strong evidence demonstrating that individuals differ in vulnerability to peers based on their genotype (Kretschmer, Dijkstra, Ormel, Verhulst & Veenstra, 2013; Latendresse et al., 2011; Mrug and Windle, 2014). Genes involved in the development of neurotransmitter systems, such as gamma-aminobutyric acid receptor genes, have been shown to be correlated with substance use in adults (Enoch et al., 2009), increase risk in externalizing behavior among youth (Dick et al., 2006), and influence vulnerability to social contexts in youth (e.g., Villafuerte et al., 2014b). These include a GABA gene cluster on human chromosome 4, which includes GABRG1, GABRA2, GABRA4 (Edenberg et al., 2004). Prior work has centered around the GABRA2 gene. Namely, GABRA2 has been demonstrated to impact the degree to which youth are susceptible to a variety of socialization contexts that either promote or decrease risk for adolescent externalizing behavior, including parental monitoring (Trucco et al., 2016), deviant peer affiliation (Villafuerte, et al., 2014b), prosocial peer affiliation (Trucco et al., 2017), and preventive interventions (Russell et al., 2018). For example, one study demonstrated a GABRA2 x deviant peer interaction on adolescent externalizing behavior, whereby deviant peer affiliation was a stronger predictor of externalizing behavior for those with the minor (G) allele, compared to those with the major (A) allele (Villafuerte et al., 2014b). Another study supported a GABRA2 x positive peer involvement interaction on adolescent externalizing behavior, as well as more adaptive contexts. Namely, those homozygous for the minor (G) allele were rated as having fewer adaptive outcomes (e.g., high externalizing behavior, low behavioral and social competence) when exposed to low levels of positive peer involvement, but rated as having greater adaptive outcomes when exposed to high levels of positive peer involvement (Trucco, Villafuerte, Burmeister, & Zucker, 2017). Taken together, this provides convincing evidence that this GABA gene cluster impacts the degree to which youth are sensitive to environmental exposures, for better or for worse.
Although recent work has focused on GABRA2, no functional variant has been found to account for these associations (Ittiwut et al., 2012). Moreover, seminal work indicates that the association between GABRA2 and problem behavior could be attributable in part to an adjacent gene in strong linkage disequilibrium (LD), GABRG1, which has demonstrated stronger associations with later problem behavior (Enoch et al., 2009). Thus, the current study focuses on variants of GABRG1. Although biological processes underlying these associations are still unclear, possible mechanisms by which GABA genes impact sensitivity to socialization contexts are emerging. GABRG1 is expressed primarily in the amygdala and areas receiving innervation from the striatum, including the substanta nigra (Schwarzer et al., 2001). These are brain regions implicated in reward-related disinhibited behaviors (Steffensen, Svingos, Pickel, & Henriksen, 1998) and addiction (Enoch et al., 2009). Given that GABRG1 is expressed in the amygdala and the substantia nigra suggests that this gene may impact thresholds for sensitivity to both constructed as well as naturally occurring environments; although the focus has been primarily on constructed contexts (preventative interventions; Brody, Chen, & Beach, 2013). Given developmental changes that occur in reward areas of the brain, the shifting importance of peer influence on an individual’s behavior, and the continued maturation of the GABA system during adolescence (Kilb, 2012), it is likely that these GxE effects are developmentally varying. This study will examine the interplay between GABRG1 variants and maladaptive peer contexts on externalizing behavior over the developmental interval from childhood to early adulthood.
Time-Varying GxE Effects
Progress in the field of GxE effects has been hampered by difficulties replicating effects. One factor that may impact replication across studies is the age of participants (Costello et al., 2013). For example, a series of studies focused on the brain-derived neurotrophic factor (BDNF) gene on response to environmental stressors across different developmental periods in both human and mouse models demonstrated that risk variants associated with low BDNF levels were most pronounced during infancy, but not evident during young adulthood (Casey et al., 2009). Similarly, another study demonstrated that the interaction between variants of the dopamine D4 receptor (DRD4) and peers’ alcohol use on an individual’s own substance use was significant during young adulthood, but not significant during adolescence or emerging adulthood (Mrug & Windle, 2014). Prior work indicates that nonspecific genetic risk factors for externalizing behaviors on a maximal alcohol consumption rose rapidly during early to mid-adolescence, peaked at ages 15–17 years of age, then declined slowly thereafter (Kendler, Gardner, Dick, 2011). Moreover, environmental moderation of genetic effects, including the role of peer group deviance, was also more pronounced in early to mid-adolescence compared to later in life. Another study, focused on interactions between non-specific genetic risk factors, antisocial peer affiliation, and adolescent externalizing disorders from late adolescence to adulthood (i.e., age 17 to age 29) only supported gene x environment interactions on antisocial behavior at age 17 (Samek, Hicks, Keyes, Iacono, & McGue, 2017). Thus, converging evidence indicates that adolescence is a critical period for increased genetic and environmental risk for engaging in externalizing behavior.
Similarly, prior work focused on a specific genetic risk factor using TVEM indicated that the interaction between GABRA2 and a preventative intervention on adolescent delinquency (Schlomer et al., 2019) and alcohol misuse (Russell et al., 2018) was most pronounced during the 13 to 16 age period. Although several researchers state that the next generation of GxE research must include developmental considerations given that some of these interactions may be age-specific (Casey et al., 2009; Costello et al., 2013; Lenroot & Giedd, 2011), progress has been slow-moving. This is due in part to the high cost of longitudinal studies with large enough sample sizes to examine these interactions across development (Costello et al., 2013). Another factor limiting this type of investigation is that conventional methodological approaches are not equipped to adequately capture the inherent complexity of GxE effects across development (Trucco et al., 2018). It will be important to extend existing TVEM studies to include naturally occurring environments, such as deviant peer exposures, as this may help inform who is most likely to benefit from preventative interventions, as well as the optimal timing of these interventions.
Current Study
The current study attempts to extend the current GxE literature by addressing factors that have hampered progression in the field. First, compared to prior work that has focused primarily on specific developmental periods, GxE associations were examined from childhood to adulthood within a large longitudinal dataset. The Michigan Longitudinal Study (MLS) represents an ideal dataset for this investigation as it is one of the largest and longest-running multi-wave studies that encompasses various methodologies (genotyping, observation, survey) across various domains (e.g., social and behavioral development) from childhood to adulthood (Zucker, Ellis, Fitzgerald, & Bingham, 1996). Second, in order to capture the complexity of developmentally varying gene x peer delinquency effects, the time-varying effect model (TVEM; Yang et al., 2017; Yang, Cranford, Li, Zucker, & Buu, 2017) was used to allow the effect of interest, such as GxE, to vary as a complex function of age. Notable benefits of TVEM are that it allows for the flexible estimation of regression coefficients as nonparametric functions of time and that analyses are based on person-age observations instead of sample size; thus, the power to detect significant interactions is enhanced, which is a prominent obstacle in GxE studies (Epstein et al., 2017). More specifically, variants of GABRG1 (rs7683876, rs13120165) were examined as they are not only in high LD with previously examined GABRA2 variants, but they may represent more direct indicators of problem behavior (Enoch et al., 2009). Consistent with prior work demonstrating that individuals carrying the minor allele of specific genes in the same GABA cluster that are in high LD with GABRG1 have increased sensitivity to various socialization contexts (i.e., parenting [Trucco et al., 2016]; peers [Trucco et al., 2017; Villafuerte et al., 2014b]), it was expected that those carrying the G allele of GABRG1 (rs7683876, rs13120165) would be most susceptible to peer effects. However, specific critical periods for these effects were not hypothesized.
Methods
Design and Sample
Participants were taken from the MLS, a multi-wave prospective study of families at high risk for substance use disorder from early childhood to adulthood (Zucker et al., 1996). Participants were families ascertained through two interconnected population-based methods. Ascertainment of the highest risk portion of the sample was by way of the father’s drunk driving conviction with a sufficiently high blood alcohol concentration (0.15% if a first conviction, 0.12% if multiple convictions). The remaining families were systematically recruited door-to-door in the same neighborhoods as the drunk-driver families. The recruitment protocol also required the father to be living with a 3–5-year-old son (the male target child) and the boy’s biological mother. The study families were originally recruited as triads, but thereafter siblings within eight years of the initial male target child were also recruited.
Ethical Considerations
Written informed consent and assent was obtained from the parents and adolescents, respectively. All procedures were performed in accordance with the ethical standards of the institutional committee where the study was conducted (Family Study of Risk for Alcoholism over the Life Course, HUM00039806) and with the 1964 Helsinki declaration and its later amendments.
Procedures
The study assessment schedule is presented in Figure 1. Participating families received extensive in-home assessments at baseline. Assessment waves thereafter took place at three-year intervals (denoted as T). During the critical period of alcohol use (ages 11–26), annual assessments (denoted as A) were also conducted. In order to maximize all available data, data obtained during both the wave and annual assessments were included in the final analyses. Overall, participant retention for the MLS across the 30 years of the study is 89%. This is comparable to retention rates of other large longitudinal studies focused on at-risk youth (Williams et al., 2013) and non-clinical (Orpinas, Lacy, Nahapetyan, Dube, & Song, 2016) adolescent populations. The present study includes 363 families and 687 participants (68% male and 90% White) who provided both genotype and phenotype data, with the median number of two siblings within a family. Further, the median number of time points available for a participant was six. The proportion of racial minorities were: 5% Black/African American, 4% Hispanic/Latino, and 1% biracial.
Figure 1.
Michigan Longitudinal Study Assessment Schedule
Note. Assessments were conducted every three years beginning when the target child was 3 to 5 years of age. T = three-year intervals. Annual assessments were also conducted during late childhood. A = annual assessments. The current study focuses on assessments contained within the box.
Measures
Externalizing behavior.
Participants rated their own social and emotional functioning on the Youth Self-Report (YSR; Achenbach & Rescorla, 2001) during adolescence (T4-T5 & A1-A7), and the Adult Self-Report (ASR; Achenbach & Rescorla, 2003) during adulthood (T6-T8 & A8-A16). The total score of the items on the externalizing behavior subscale was standardized according to the national norm specific to the participant’s age at the assessment (mean = 50, SD = 10). Thus, the standardized scores are comparable developmentally. Both measures have excellent reliability and validity. The internal consistency for the YSR and ASR within this sample was good (Cronbach’s α range = 0.84–0.89 across time points).
Maladaptive peer behavior.
Involvement with delinquent peers was measured as part of the Peer Behavior Profile (Bingham, Fitzgerald, & Zucker, 1995), which asked participants to select how many of their peers (1 = almost none to 5 = almost all) are involved in specific activities. This instrument was administered during adolescence and adulthood (T4-T8 & A1-A16). Nine items focused on peer delinquency, such as being detained by the police; 14 items pertained to peer substance use (e.g., getting drunk, smoking cigarettes, getting high on drugs). The average score of the items in each subscale was used. The internal consistency across time points for peer delinquency (Cronbach’s α range = 0.79– 0.89) and peer substance use (Cronbach’s α range = 0.88–0.96) within this sample was adequate. Separate models were conducted for peer delinquency and peer substance use to determine whether findings generalize to multiple aspects of maladaptive peer influence.
Genotype Data & Quality Control
Single nucleotide polymorphism (SNP) genotyping data of DNA specimens were obtained using Illumina Human Genotyping Arrays HumanCoreExome-12 v1.0 BeadChip. The HumanCoreExome BeadChip has a highly non-uniform distribution of markers and an emphasis on rare coding variants. It contains more than 240K exonic variants, including nearly 220K non-synonymous coding variants. These data were converted to PLINK format (Purcell, Neale, Todd-Bzkker, Daly, & Sham, 2007) and the quality control (QC) of genotypes was carried out based on established best practices (Anderson et al., 2010). Individuals with elevated missing data rates (the genotype failure rate > 0.05) or large heterozygosity rates (larger than ± 3SD from the mean) were eliminated. Duplicated samples from the same participant were identified and the one with a smaller rate of missing data was kept. In addition, SNPs with the minor allele frequency being less than 0.01, the rate of missingness per SNP being larger than 0.05, and the p-value of the Hardy-Weinberg equilibrium test being less than 0.00001 were filtered out. In total, 280K genotyped autosomal SNPs passed QC. Based on the results of quality control and the focus on exon regions, the SNP rs7683876 and rs13120165 mapping to GABRG1 was used to represent the GABAA subunit gene. The genotypes of both SNPs were coded using the additive model, with GG coded as 0; AG as 1; and AA as 2.
Statistical Analysis
This study applied TVEM developed in prior work (Yang et al., 2017; Yang, Cranford, Li, Zucker, & Buu, 2017) to characterize the time-varying effects of peer delinquency and peer substance use on externalizing behaviors by GABRG1 genotypes across two separate models. Let Y(tij)be the j-th observed externalizing score (the outcome variable) from the i-th participant at time tij and Z(tij) be the corresponding environmental factor (i.e., peer delinquency or peer substance use). Let k(k = 0, 1, 2) be the genotype of Participant i with 0 corresponding to the GG group, 1 for the AG group, and 2 for the AA group. Two indicator variables were created to model the differences among the three genotype groups with I{k = 1} contrasting AG with GG and I{k = 2} contrasting AA with GG. Further, two important demographic variables were included as covariates: biological sex X1 (coded as 1 for male and 0 for female) and race X2(coded as 1 for White and 0 for non-White). The TVEM is written mathematically as follows:
where α0(tij), α1(tij), and α2(tij) are the intercept terms; β0(tij) is the time-varying effect of Z(tij)for the GG group; β1(tij) is the difference in the time-varying effect between the AG group and the GG group; β2(tij) is the difference between the AA group and the GG group; γ1 is the effect of biological sex; γ2 is the effect of race; and ai, bi are the random effects accounting for the dependence within family (i.e., family-level clustering) and within participant, respectively. A major strength of TVEM is that the time-varying effects of Z(tij) – β0(tij), β1(tij), and β2(tij)− can be estimated through non-parametric regression functions that do not assume fixed shapes like conventional growth curves, and thus can better characterize developmental changes based on empirical data. Previous methodological work (Yang et al., 2017; Yang, Cranford, Li, Zucker, & Buu, 2017) has proposed statistical methods for parameter estimation and hypothesis testing of group differences, which performed well across different practical settings by simulation studies. Confidence intervals were also estimated. Non-overlapping confidence intervals indicate statistically significant differences across genotypes.
Results
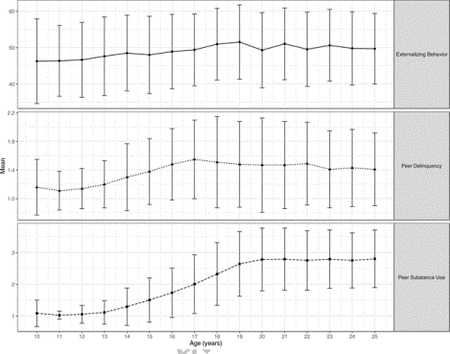
Means and standard deviations for adolescent externalizing behavior, peer delinquency, and peer substance use across age are presented in the Appendix. Externalizing behavior among adolescents and their peers tended to increase from childhood to late adolescence, with a peak in early adulthood. Overall, correlations, calculated as Kendall’s Tau, indicated that GABRG1 was not associated with peer delinquency (r = 0.034, p = 0.56) or peer substance use (r = 0.049, p = 0.41) across ages 10–25. This is notable as gene-environment correlation represents a nonrandom distribution of environments across genotypes, and may confound GxE associations (Trucco, Villafuerte, Burmeister, & Zucker, 2017). We fit the TVEM on the genotype and longitudinal phenotype data. Findings focus on GABRG1 rs7683876 (23.7% = GG; 50.1% = AG, 26.2% = AA) for simplicity and clarity, since a similar pattern of significant effects emerged with GABRG1 rs13120165.
Effects of Peer Delinquency
Figure 2 shows the time-varying effects of peer delinquency on externalizing behavior by GABRG1 rs7683876, controlling for the effects of sex and race. The pattern of effects indicates a sequential risk gradient in the influence of delinquent peers on externalizing behavior depending on the number of G alleles. Greater peer delinquency was associated with greater externalizing behavior among participants carrying a G allele. Participants with the GG genotype were especially susceptible to delinquent peers. Moreover, this association was slightly greater in early adulthood compared to late childhood among those with the GG genotype, whereas this association was relatively consistent across development for those with the AG genotype. In contrast, the association between peer delinquency on externalizing behavior across development was negative among those with the AA genotype. Table 1 depicts the estimated time-invariant effects of sex and race in the TVEM. Sex did not have a significant effect on externalizing behavior, whereas White participants had significantly higher levels of externalizing behavior compared to non-White participants.
Figure 2.
Time-varying effect model for peer delinquency on externalizing behavior
Note. GABRG1 rs7683876 effects depicted. Solid lines represent trajectories, while the dotted lines represent the 95% confidence intervals. Values above the line represent positive associations. Values below the blue line represent negative associations. Confidence intervals that include the blue line are not significant. The y-axes represent estimated effects across time.
Table 1.
The time-invariant effects of sex and race in the TVEM of peer delinquency x GABRG1 on externalizing behavior
| Parameter | Estimate | Std. error | p-value |
|---|---|---|---|
| Male (γ1) | −0.5251 | 0.6075 | 0.3874 |
| White (γ2) | 23.0138 | 1.1568 | <.0000 |
Effects of Peer Substance Use
Similar models were then tested that examined the effect of peer substance use on externalizing behavior. As presented in Figure 3, the fitted TVEMs examining the time-varying effects of peer substance use on externalizing behavior by GABRG1 SNP rs7683876, controlling for the effects of sex and race were similar. Namely, the pattern of effects indicates a sequential risk gradient in the influence of substance using peers on externalizing behavior depending on the number of G alleles. Greater peer substance use was associated with greater externalizing behavior among participants carrying a G allele. Participants with the GG genotype were especially susceptible to substance using peers. Moreover, this association was greater in early adulthood compared to late childhood among those with both the GG and the AG genotype. In contrast, the effect of substance using peers on externalizing behavior was not significant in late childhood or early adulthood among those with the AA genotype, and this association was negative from early adolescence to late adolescence. Table 2 depicts the estimated time-invariant effects of sex and race in the TVEM. Sex did not have a significant effect on externalizing behavior, whereas White participants had significantly higher levels of externalizing behavior compared to non-White participants.
Figure 3.
Time-varying effect model for peer substance use on externalizing behavior
Note. GABRG1 rs7683876 effects depicted. Solid lines represent trajectories, while the dotted lines represent the 95% confidence intervals. Values above the line represent positive associations. Values below the blue line represent negative associations. Confidence intervals that include the blue line are not significant. The y-axes represent estimated effects across time.
Table 2.
The time-invariant effects of sex and race in the TVEM of peer substance use x GABRG1 on externalizing behavior
| Parameter | Estimate | Std. error | p-value |
|---|---|---|---|
| Male (γ1) | 0.1709 | 0.6821 | 0.8022 |
| White (γ2) | 18.3603 | 1.3062 | <.0000 |
Sensitivity Analyses
Additional exploratory analyses were also conducted to examine gene-by-covariate and environment-by-covariate interactions. When examining peer delinquency effects, there was no support for a significant race x peer delinquency interaction. There was evidence of a significant race x genotype interaction indicating that White participants with the AG genotype had a higher level of externalizing behavior (coefficient=0.30, se=0.15, p<.05). In addition, there was evidence for a sex x genotype and a sex x peer delinquency interaction. Among individuals with the AA genotype, males had lower externalizing scores (coefficient=−6.49, se=0.92, p<.05), whereas among those with the AG genotype, males tended to have higher externalizing scores (coefficient=1.91, se=0.40, p<.05). Further, the effect of peer delinquency on externalizing behavior was lower among male participants (coefficient=−7.28, se=0.92, p<.05). Nevertheless, adding these gene-by-covariate and environment-by-covariate interactions to the model did not change the gene-by-environment pattern observed in Figure 2.
Gene-by-covariate and environment-by-covariate exploratory interactions were also examined in the context of peer substance use effects. There was no evidence for a significant race x genotype or race x peer substance use interaction. Conversely, sex had significant interactions with both genotype and peer substance us e. Among the participants with AA, males had lower externalizing scores (coefficient=−4.73, se=1.08, p<.05), whereas among those with AG, males tended to have higher externalizing scores (coefficient=1.11, se=0.48, p<.05). Further, the effect of peer substance use on externalizing behavior was lower among male participants (coefficient=−6.16, se=0.95, p<.05). Nevertheless, adding these gene-by-covariate and environment-by-covariate interactions to the model did not change the gene-by-environment pattern observed in Figure 3.
Lastly, given that prior work also supported interactions between specific GABRA2 variants and positive peer affiliation on adolescent externalizing behavior (Trucco et al., 2017), we also conducted exploratory analyses on this environmental context. Positive peer involvement reflected participants’ perceptions of their peers’ engagement in religious/spiritual activities, scholastic performance, and extracurricular activities as assessed with the Peer Behavior Profile (Bingham. Fitzgerald, & Zucker, 1995). There was no evidence for significant GABRG1 (rs7683876, rs13120165) x positive peer affiliation interactions across development. The lack of significant interactions could indicate that positive peer involvement benefits most individuals similarly (Pluess & Belsky, 2012) or that interactions are specific to GABRA2 variants (Trucco et al., 2017).
Discussion
Although engagement in externalizing behavior, such as stealing and aggression, during adolescence is relatively normative, involvement in these types of behavior in childhood and early adulthood may be indicative of maladaptive outcomes later in life, including mental health problems and incarceration (Farmer et al., 2015). One of the strongest predictors of engagement in externalizing behavior is involvement with deviant peers (Epstein et al., 2017). Yet, susceptibility to peer influence is not static across development (Epstein et al., 2017). Moreover, certain individuals are likely to be more susceptible to peer influences based on biological characteristics. The current study extends prior work by examining how specific genetic variants in the GABAA receptor subunit-encoding genes (i.e., GABRG1 rs7683876, rs13120165) interact with maladaptive peer influence to impact engagement in externalizing behavior from childhood to early adulthood using TVEM. This study addresses a critical gap in the literature; prior work has not used TVEM to examine GxExD interactions outside of constructed environments (e.g., preventative interventions). Examining how genes may impact susceptibility to naturally occurring environments across development, such as peers, will help inform not only who is likely to benefit the most from preventive interventions focused on addressing exposure to deviant peers, but when preventive interventions are likely to have the most utility.
As expected, the impact of peer deviancy and peer substance use on externalizing behavior was strongest for those with the GG genotype. This is consistent with prior work indicating that adolescents carrying the G allele across genetic factors that are closely associated with GABRG1 rs7683876 and rs13120165 (e.g., GABRA2 rs279858, rs279826, rs279827) were most susceptible to maladaptive environmental exposures, including deviant peer affiliation (Villafuerte, et al., 2014b) and poor parenting practices (Trucco et al., 2016). Although biological mechanisms underlying increased sensitivity to environmental exposures remain unclear, there is some evidence indicating that those carrying these specific variants in the GABAA receptor subunit-encoding genes have greater reward sensitivity and impulsivity (Heitzeg et al., 2014; Villafuerte et al., 2014a) and reduced brain activation to emotionally salient stimuli (Trucco, Cope, Burmeister, Zucker, Heitzeg, 2018). Thus, those with the GG genotype may not only find social acceptance by peers as particularly rewarding; they may also find it difficult to refrain from imitating externalizing behavior modeled by deviant peers.
Implications
From a developmental perspective, this study provides a novel contribution to the field by demonstrating that increased vulnerability to peer influence by those carrying the GABRG1 G allele is not confined to the adolescent years. In fact, these results indicate that among those with the GG genotype, the association between maladaptive peer influences and externalizing behavior is present in late childhood and likely increases throughout adulthood. It is possible that the influence of peer deviance and peer substance use on externalizing behavior increases because of increasing mean levels and variability. This pattern of findings may also be due in part to developmental changes that naturally occur in the social world when transitioning from adolescence to adulthood. During adolescence, parents tend to closely monitor an adolescent’s behavior and put limits on whom the child can affiliate. In early adulthood, youth tend to leave the home to attend college, thus gaining more freedom to shape their social worlds. It is likely that this increased autonomy impacts the degree to which genetic vulnerabilities become expressed (Trucco, Schlomer, & Hicks, 2018). Accordingly, the larger discrepancies found across genotypes in the association between maladaptive peer exposures and externalizing behavior in adulthood compared to earlier developmental periods found in the current study may be attributable in part to greater expression of individual differences based on fewer social world constraints.
From a clinical perspective, findings offer preliminary evidence that individuals with the GG genotype may benefit the most from interventions focused on how to navigate negative peer influence. Not only are these individuals most vulnerable to maladaptive peer contexts, but prior work indicates that these same individuals may also reap the most benefit from preventative interventions (Russell et al., 2018). This same work indicates that gene by intervention effects on adolescent delinquency (Russell et al., 2018) and alcohol misuse (Schlomer et al., 2019) is most pronounced starting in early adolescence. Similarly, prior work indicates that preventative interventions initiated during the pre- and early adolescent developmental periods may be particularly beneficial in reducing externalizing behavior and substance use (Spoth, Redmond, Trudeau, & Shin, 2002) compared to early to midadulthood when these processes are more developmentally canalized (Kendler, Gardner, & Dick, 2011). Given that differences across genotypes are present during childhood, there may be particular utility in delivering preventative interventions during pre- and early adolescent developmental periods to disrupt early affiliation with deviant or substance-using peer groups; once youth start forming bonds with deviant peers, it may be more difficult to intervene. Such interventions may target risk factors for exposure to deviant peer processes, such as childhood bullying, peer rejection, and low school connectedness (Van Ryzin, Fosco, & Dishion, 2012).
The current work represents an early stage in a larger body of research that is necessary to gain a greater articulation of the complex mechanisms through which biological differences and environmental exposures interact to impact maladaptive functioning across development. Although the ultimate goal is to develop tailored and time-specific interventions that have the most clinical utility, future work should focus on continued empirical efforts to replicate these findings and to further characterize the inherent complexity of environmental and biological effects on externalizing behavior before specific recommendations can be offered.
Limitations and Future Directions
As large longitudinal datasets that encompass socialization factors and genetic information become available, it will be important to test whether these findings replicate. Until findings are replicated with an independent sample, caution is warranted when drawing inferences. Although this study adopted a hypothesis-driven approach focused on a specific genetic region that has received strong empirical support for its impact on sensitivity to socialization contexts, the focus on single genetic markers provide an incomplete picture regarding the interplay between genetic risk factors and maladaptive peer exposures on externalizing behavior. Complex behavior, such as externalizing behavior, is likely attributable to a number of different genes with small effects as well as a number of different socialization contexts outside of the peer environment (Trucco, Schlomer, & Hicks, 2018). Thus, it will be important that future work consider extending this work to include hypothesis-free approaches, such as polygenic methods based on genome-wide association studies, and other socialization contexts, such as neighborhood, school, and family environment. Although racial differences were accounted for in the models, the sample was primarily White. These findings may not generalize to a more diverse sample. The current sample also represents a group of individuals at high-risk for alcoholism; rates of externalizing behavior and exposure to maladaptive peer influences may be greater in this sample compared to a community sample. Lastly, although the MLS represents an ideal data set for this investigation, only perceptions of peer behavior was available. It will be important for future work to include ratings from multiple reporters to account for shared method variance.
Conclusion
Strong theoretical and empirical support exists for the synergistic effect of specific genetics factors and peer environmental contexts (i.e., GxE) on the emergence of externalizing behavior (Trucco, Schlomer, & Hicks, 2018). Yet, it is likely that GxE effects may wax and wane across the lifespan. Only a few studies have been able to successfully integrate the complexity of interactions between genetic factors and environmental exposures with the nuances that unfold across development. This study employs time-varying effect modeling (TVEM), to determine possible gene x environment x development (GxExD) interactions as they apply to the patterning of externalizing behavior from childhood to adulthood. Study findings support the role of genetic variants in the GABAA receptor subunit-encoding genes (i.e., GABRG1 [rs7683876, rs13120165]) as susceptibility factors to peer socialization contexts across a wider developmental period than previously thought. The results indicate that among those with the GG genotype, the association between maladaptive peer influences and externalizing behavior is present in late childhood throughout adulthood. The pattern of findings may be due in part to developmental changes that naturally occur in the social world and with respect to externalizing behavior when transitioning from childhood to adulthood. Preventative interventions targeting childhood and pre-adolescence are likely to have the most impact on peer influences that may exacerbate externalizing behavior. This work provides preliminary evidence for the utility of conducting similar empirical investigations to inform the design, timing, and implementation of preventive interventions for individuals with genetic predispositions for high-risk externalizing trajectories.
Acknowledgements
We thank all the families that participated in the Michigan Longitudinal Study and their commitment to the project throughout the years.
Funding
This work was supported by the National Institutes of Health (K08 AA023290; U54 MD012393; R01 AA007065; R01 DA035183; P50 DA039838; U19 AI089672; T32 LM012415) and by the National Science Foundation (DMS1512422).
Appendix
Means and Standard Deviations for Study Variables across Age
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Data Sharing Declaration
The datasets generated and/or analyzed during the current study are not yet publicly available but are available from the corresponding author on reasonable request. In addition, these data are currently being prepared for archiving in the publicly available interuniversity consortium for political and social research archiving, and we anticipate the full upload will be available by the end of 2019 (https://www.icpsr.umich.edu).
Conflicts of Interest
The authors report no conflict of interests.
Ethical Approval
All procedures with human participants were in accordance with the ethical standards of the institutional committee where the study was conducted and with the 1964 Helsinki declaration and its later amendments.
Informed Consent
Written informed consent and assent was obtained from the parents and adolescents, respectively.
References
- Abadi MH, Shamblen SR, Thompson K, Collins DA, & Johnson K (2011). Influence of risk and protective factors on substance use outcomes across developmental periods: A comparison of youth and young adults. Substance Use & Misuse, 46, 1604–1612. [DOI] [PubMed] [Google Scholar]
- Achenbach TM, & Rescorla LA (2001). Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- Achenbach TM, & Rescorla LA (2003). Manual for the ASEBA Adult Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- Akers RL (1977). Deviant behavior: A social learning approach. Belmont, California, United States: Wadsworth. [Google Scholar]
- Anderson CA, Pettersson FH, Clarke GM, Cardon LR, Morris AP, & Zondervan KT (2010). Data quality control in genetic case-control association studies. Nature Protocols, 5, 1564–1573. doi: 10.1038/nprot.2010.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnow S, Schultz G, Lucht M, Ulrich I, Preuss U-W, & Freyberger H-J (2004). Do alcohol expectancies and peer delinquency/substance use mediate the relationship between impulsivity and drinking behavior in adolescence? Alcohol and Alcoholism, 39(3), 213–219. doi: 10.1093/alcalc/agh048 [DOI] [PubMed] [Google Scholar]
- Becht AI, Prinzie P, Deković M, van den Akker A, & Shiner RL (2016). Child personality facets and overreactive parenting as predictors of aggression and rule-breaking trajectories from childhood to adolescence. Development and Psychopathology, 28(2), 399–413. doi: 10.1017/S0954579415000577 [DOI] [PubMed] [Google Scholar]
- Bingham CR, Fitzgerald HE, & Zucker RA (1995). Peer Behavior Profile/Peer Activities Questionnaire Unpublished manuscript, Michigan State University. [Google Scholar]
- Brody GH, Chen Y-F, & Beach SRH (2013). Differential susceptibility to prevention: GABAergic, dopaminergic, and multilocus effects. Journal of Child Psychology and Psychiatry, 54(8), 863–871. doi: 10.1111/jcpp.12042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broidy LM, Nagin DS, Tremblay RE, Bates JE, Brame B, … Vitaro F (2003). Developmental trajectories of childhood disruptive behaviors and adolescent rule breaking: A six-site, cross-national study. Developmental Psychology, 39, 222–245. doi: 10.1037/0012-1649.39.2.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, McGue M, & Iacono WG (2009). Nonshared environmental mediation of the association between deviant peer affiliation and adolescent externalizing behaviors over time: Results from a cross - lagged monozygotic twin differences design. Developmental Psychology, 45, 1752–1760. doi: 10.1037/a0016687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, & Neiderhiser JM (2009). Aggressive versus nonaggressive antisocial behavior: Distinctive etiological moderation by age. Developmental Psychology, 45(4), 1164–1176. doi: 10.1037/a0016130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Glatt CE, Tottenham N, Soliman F, Bath K, Amso D, … Lee FS (2009). Brain-derived neurotrophic factor as a model system for examining gene by environment interactions across development. Neuroscience, 164(1), 108–120. doi: 10.1016/j.neuroscience.2009.03.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland MJ, Feinberg ME, Bontempo DE, & Greenberg MT (2008). The role of risk and protective factors in substance use across adolescence. Journal of Adolescent Health, 43, 157–164. doi: 10.1016/j.jadohealth.2008.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland MJ, Feinberg ME, & Jones DE (2012). Predicting alcohol use across adolescence: Relative strength of individual, family, peer, and contextual risk and protective factors. Psychology of Addictive Behaviors, 26, 703–713. doi: 10.1037/a0027583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Eaves L, Sullivan P, Kennedy M, Conway K, Adkins DE, … Patkar AA (2013). Genes, environments, and developmental research: Methods for a multi-site study of early substance abuse. Twin Research and Human Genetics, 16(2), 505–515. doi: 10.1017/thg.2013.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Bierut L, Hinrichs A, Fox L, Bucholz KK, Kramer J, … Foroud T (2006). The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behavior Genetics, 36(4), 577–590. doi: 10.1007/s10519-005-9041-8 [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, … Begleiter H (2004). Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. American Journal of Human Genetics, 74, 705–714. doi: 10.1086/383283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch M-A, Hodgkinson CA, Yuan Q, Albaugh B, Virkkunen M, & Goldman D (2009). GABRG1 and GABRA2 as independent predictors for alcoholism in two populations. Neuropsychopharmacology, 34, 1245–1254. doi: 10.1038/npp.2008.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein M, Hill KG, Roe SS, Bailey JA, Iacono WG, McGue M, … Haggerty KP (2017). Time-varying effects of families and peers on adolescent marijuana use: Person–environment interactions across development. Development and Psychopathology, 29(3), 887–900. doi: 10.1017/S0954579416000559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer RF, Seeley JR, Kosty DB, Gau JM, Duncan SC, Lynskey MT, & Lewinsohn PM (2015). Internalizing and externalizing psychopathology as predictors of cannabis use disorder onset during adolescence and early adulthood. Psychology of Addictive Behaviors, 29(3), 541–551. doi: 10.1037/adb0000059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-McKoy D & Cui M (2013). Parental control, adolescent delinquency, and young adult criminal behavior. Journal of Child and Family Studies, 22, 836–843. doi: 10.1007/s10826-012-9641-x [DOI] [Google Scholar]
- Heitzeg MM, Villafuerte S, Weiland BJ, Enoch M-A, Burmeister M, Zubieta J-K, & Zucker RA (2014). Effect of GABRA2 genotype on development of incentive-motivation circuitry in a sample enriched for alcoholism risk. Neuropsychopharmacology, 39, 3077–3086. doi: 10.1038/npp.2014.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittiwut C, Yang B-Z, Kranzler HR, Anton RF, Hirunsatit R, Weiss RD, … Gelernter J (2012). GABRG1 and GABRA2 variation associated with alcohol dependence in African Americans. Alcoholism: Clinical and Experimental Research, 36(4), 588–593. doi: 10.1111/j.1530-0277.2011.01637.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gardner C, & Dick DM (2011). Predicting alcohol consumption in adolescence from alcohol-specific and general externalizing genetic risk factors, key environmental exposures and their interaction. Psychological Medicine, 41(7), 1507–1516. doi: 10.1017/S003329171000190X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilb W (2012). Development of the GABAergic system from birth to adolescence. The Neuroscientist, 18(6), 613–630. doi: 10.1177/1073858411422114 [DOI] [PubMed] [Google Scholar]
- Kretschmer T, Dijkstra JK, Ormel J, Verhulst FC, & Veenstra R (2013). Dopamine receptor D4 gene moderates the effect of positive and negative peer experiences on later delinquency: The Tracking Adolescents’ Individual Lives Survey study. Development and Psychopathology, 25(4), 1107–1117. doi: 10.1017/S0954579413000400 [DOI] [PubMed] [Google Scholar]
- LaFontana KM, & Cillessen AHN (2010). Developmental changes in the priority of perceived status in childhood and adolescence. Social Development, 19(1), 130–147. doi: 10.1111/j.1467-9507.2008.00522.x [DOI] [Google Scholar]
- Latendresse SJ, Bates JE, Goodnight JA, Lansford JE, Budde JP, Goate A, … Dick DM (2011). Differential susceptibility to adolescent externalizing trajectories: Examining the interplay between CHRM2 and peer group antisocial behavior. Child Development, 82(6), 1797–1814. doi: 10.1111/j.1467-8624.2011.01640.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, & Giedd JN (2011). Annual research review: Developmental considerations of gene by environment interactions. Journal of Child Psychology and Psychiatry, 52(4), 429–441. doi: 10.1111/j.1469-7610.2011.02381.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y-F, & Menard S (2017). The interplay of MAOA and peer influences in predicting adult criminal behavior. Pychiatrict Quarterly, 88, 115–128. doi: 10.1007/s1112 [DOI] [PubMed] [Google Scholar]
- Mrug S, & Windle M (2014). DRD4 and susceptibility to peer influence on alcohol use from adolescence to adulthood. Drug and Alcohol Dependence, 145, 168–173. doi: 10.1016/j.drugalcdep.2014.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narusyte J, Ropponen A, Alexanderson K, & Svedberg P (2017). Internalizing and externalizing problems in childhood and adolescence as predictors of work incapacity in young adulthood. Social Psychiatry and Psychiatric Epidemiology, 52(9), 1159–1168. doi: 10.1007/s00127-017-1409-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orpinas P, Lacy B, Nahapetyan L, Dube SR, & Song X (2016). Cigarette smoking trajectories from sixth to twelfth grade: Associated substance use and high school dropout. Nicotine & Tobacco Research, 18:156–162. doi: 10.1093/ntr/ntv040 [DOI] [PubMed] [Google Scholar]
- Pfeifer Jennifer H., Masten Carrie L., Moore William E. III, Oswald Tasha M., Mazziotta John C., Iacoboni M, & Dapretto M (2011). Entering adolescence: Resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron, 69(5), 1029–1036. doi: 10.1016/j.neuron.2011.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluess M Belsky J (2012). Vantage sensitivity: Individual differences in response to positive experiences. Psychological Bulletin, 139, 901–916. doi: 10.1037/a0030196 [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Bzkker PIW, Daly MJ, & Sham PC (2007). PLINK: A toolset for whole-genome association and population-based linkage analysis. American Journal of Human Genetics, 81, 559–575. doi: 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell MA, Schlomer GL, Cleveland HH, Feinberg ME, Greenberg MT, Spoth RL, … Vandenbergh DJ (2018). PROSPER intervention effects on adolescents’ alcohol misuse vary by GABRA2 genotype and age. Prevention Science, 19(1), 27–37. doi: 10.1007/s11121-017-0751-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samek DR, Goodman RJ, Erath SA, McGue M, & Iacono WG (2016). Antisocial peer affiliation and externalizing disorders in the transition from adolescence to young adulthood: Selection versus socialization effects. Developmental Psychology, 52(5), 813–823. doi: 10.1037/dev0000109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samek DR, Hicks BM, Keyes MA, Iacono WG & McGue M (2017). Antisocial peer affiliation and externalizing disorders: Evidence for Gene x Environment x Development interaction. Development and Psychopathology, 29, 155–172. doi: 10.1017/S0954579416000109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlomer GL, Cleveland HH, Deutsch AR, Vandenbergh DJ, Feinberg ME, Greenberg MT, … Redmond C (2019). Developmental change in adolescent delinquency: Modeling time-varying effects of a preventative intervention and GABRA2 haplotype linked to alcohol use. Journal of Youth and Adolescence, 48, 71–85. doi: 10.1007/s10964-018-0929-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer C, Berresheim U, Pirker S, Wieselthaler A, Fuchs K, Sieghart W, & Sperk G (2001). Distribution of the major γ- aminobutyric acidA receptor subunits in the basal ganglia and associated limbic brain areas of the adult rat. Journal of Comparative Neurology, 433(4), 526–549. doi: 10.1002/cne.1158 [DOI] [PubMed] [Google Scholar]
- Shaw DS, Hyde LW, & Brennan LM (2012). Early predictors of boys’ antisocial trajectories. Development and Psychopathology, 24(3), 871–888. doi: 10.1017/S0954579412000429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons RL, Lei MK, Beach SRH, Brody GH, Philibert RA, Gibbons FX, & Gerrard M (2013). Differential sensitivity to context: GABRG1 enhances the acquisition of prototypes that serve as intermediate phenotypes for substance use In MacKillop J & Munafó MR (Eds.), Genetic influences on addiction: An intermediate phenotype approach. Cambridge, MA: MIT Press. [Google Scholar]
- Spoth RL, Redmon C, Trudeau L, & Shin C (2002). Longitudinal substance initiation outcomes for a universal preventive combining family and school programs. Psychology of Addictive Behavior, 16(2), 129–134. doi: 10.1037/0893-164X.16.2.129 [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Svingos AL, Pickel VM, & Henriksen SJ (1998). Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. Journal of Neuroscience, 18, 8003–8015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, & Monahan KC (2007). Age differences in resistance to peer influence. Developmental Psychology, 43(6), 1531–1543. doi: 10.1037/0012-1649.43.6.1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland EH (1947). Critique of the theory In Edwin H. Sutherland on Analyzing Crime, ed Schuessler K, pp. 30–41. Chicago: University of Chicago Press. [Google Scholar]
- Tremblay RE & Szyf M (2010). Developmental origins of chronic physical aggression and epigenetics, Epigenomics, 2(4), 495–499. doi: 10.2217/epi.10.40 [DOI] [PubMed] [Google Scholar]
- Trucco EM, Cope LM, Burmeister M, Zucker RA, & Heitzeg MM (2018). Pathways to youth behavior: The role of genetic, neural, and behavioral markers. Journal of Research on Adolescence, 28, 26–39. doi: 10.1111/jora.12341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucco EM, Schlomer GL, & Hicks BM (2018). A developmental perspective on the genetic basis of alcohol use disorder In Fitzgerald HE & Puttler LI (Eds.), Alcohol and Other Addictions: A Developmental Analysis of Process over the Life Span (pp. 49–68). Oxford: Oxford Press. [Google Scholar]
- Trucco EM, Villafuerte S, Burmeister M, & Zucker RA (2017). Beyond risk: Prospective effects of GABA receptor subunit alpha-2 (GABRA2) x positive peer involvement on adolescent behavior. Development and Psychopathology, 29(3), 711–724. doi: 10.1017/S0954579416000419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucco EM, Villafuerte S, Heitzeg MM, Burmeister M, & Zucker RA (2014). Rule breaking mediates the developmental association between GABRA2 and adolescent substance abuse. Journal of Child Psychology and Psychiatry, 55(12), 1372–1379. doi: 10.1111/jcpp.12244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucco EM, Villafuerte S, Heitzeg MM, Burmeister M, & Zucker RA (2016). Susceptibility effects of GABA receptor subunit alpha-2 (GABRA2) variants and parental monitoring on externalizing behavior trajectories: Risk and protection conveyed by the minor allele. Development and Psychopathology, 28, 15–26. doi: 10.1017/S0954579415000255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ryzin MJ, Fosco GM, & Dishion TJ (2012). Family and peer predictors of substance use from early adolescence to early adulthood: An 11-year prospective analysis. Addictive Behaviors, 37(12), 1314–1324. doi: 10.1016/j.addbeh.2012.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villafuerte S, Heitzeg MM, Foley S, Wendy Yau W-Y, Majczenko K, Zubieta J-K, … Burmeister M (2014a). Impulsiveness and insula activation during reward anticipation are associated with genetic variants in GABRA2 in a family sample enriched for alcoholism. Molecular Psychiatry, 17, 511–519. doi: 10.1038/mp.2011.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villafuerte S, Trucco EM, Heitzeg MM, Burmeister M, & Zucker RA (2014b). Genetic variation in GABRA2 moderates peer influence on externalizing behavior in adolescents. Brain and Behavior, 4(6), 833–840. doi: 10.1002/brb3.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaro F, Boivin M, & Poulin F (2018). The Interface of Aggression and Peer Relations in Childhood and Adolescence In Bukowski WM, Laursen B, & Rubin KH (Eds.), Handbook of peer interactions, relationships, and groups (2nd ed.) (2 ed., pp. 284–301). New York, NY, United States: Guilford Press. [Google Scholar]
- Walton KE, Krueger RF, Elkins I, D’Accordo C, McGue M, & Iacono WG (2017). Personality traits predict the developmental course of externalizing: A four-wave longitudunal study spanning age 17 to age 29. Journal of Personality, 85(3), 364–375. doi: 10.1111/jopy.12245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P, Chernoff M, Angelidou K, Brouwers P, Kacanek D, …Gadow KD (2013). Participation and retention of youth with perinatal HIV infection in mental health research studies. Journal of Acquired Immune Deficiency Syndromes, 63, 401–409. doi: 10.1097/QAI.0b013e318293ad53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Cranford JA, Jester JM, Li R, Zucker RA, & Buu A (2017). A time-varying effect model for exmaning group differences in trajectories of zero-inflated count outcomes with applications in substance abuse research. Statistics in Medicine, 36, 827–837. doi: 10.1002/sim.7177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Cranford JA, Li R, Zucker RA, & Buu A (2017). A time-varying effect model for studying gender differences in health behavior. Statistical Methods in Medical Research, 26, 2812–2820. doi: 10.1177/0962280215610608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalsman G (2010). Timing is critical: Gene, environment and timing interactions in genetics of suicide in children and adolescents. European Psychiatry, 25, 284–286. doi: 10.1016/j.eurpsy.2010.01.007 [DOI] [PubMed] [Google Scholar]
- Zucker RA, Ellis DA, Fitzgerald HE, & Bingham CR (1996). Other evidence for at least two alcoholism II. Life course variation in antisociality and heterogeneity of alcoholic outcome. Psychopathology, 8(4), 831–848. [Google Scholar]





