Abstract
Full and partial opioid agonists and opioid antagonist medications play an important role in containing the opioid epidemic. However, these medications have not been used to their full extent. Recovery support services, such as recovery residences (RRs), also play a key role. RRs may increase an individual’s recovery capital, facilitate social support for abstinence, and foster a sense of community among residents. These processes may be critical for individuals with opioid use disorder (OUD). In combination these two recovery pathways have the potential to enhance one another and improve outcomes among residents with OUD. Barriers to doing so have resulted in a limited supply of residences that can support residents using opioid agonist and antagonist medications. This perspective describes key interpersonal and structural barriers to medication use among individuals with an OUD seeking support from a recovery residence and discusses measures for reducing these barriers. These measures include workforce development to address stigma and attitudinal barriers and enhancing residence capability to ensure resident safety and reduce potential diversion. The perspective also highlights the need for additional research to facilitate the identification of best practices to improve outcomes among residents treated with medications living in recovery residences.
Keywords: Recovery, recovery residence, opioid use disorder, opioid agonist therapy, policy, implementation
Introduction
Opioid use disorder (OUD) is a significant driver of the opioid epidemic (1,2). Both the National Institute on Drug Abuse and the Substance Abuse and Mental Health Services Administration (SAMHSA) are encouraging states to expand access to medications for OUD (MOUD) and associated treatment services to contain the effects of this crisis, including the use of medications (3,4). The American Society of Addiction Medicine recommends that MOUDs be used in conjunction with psychosocial interventions including recovery support services (5). However, little is known about how MOUDs may best be used in conjunction with recovery support services. This article describes one type of recovery support service, the recovery residence (a continuum of residential, substance-free living environments for individuals in recovery from a substance use disorder (6), and discusses the unique opportunity for addressing the opioid epidemic in these settings.
Background
Medications for opioid use disorder (MOUDs)
Three types of MOUDs are FDA-approved: full agonist (methadone), partial agonist (buprenorphine), and antagonist (naltrexone). Both methadone and buprenorphine activate opioid receptors (7,8) to reduce opioid withdrawal and craving (9), and can result in a similar physiological response as experienced with other illicit and commonly misused opioids. Naltrexone does not activate opioid receptors but instead prohibits opioids from binding to and activating them, preventing a physiological response (7,8). While oral formulations of naltrexone have poor medication adherence, the extended-release injectable formulation has been found to be as effective as buprenorphine in multiple medication trials (10–12). Overall, the evidence base for the effectiveness for all three MOUDs is robust (10,13–15), as evidenced by the significantly lower odds of mortality while receiving MOUDs (16–18).
Despite this evidence, significant gaps in access to treatment remain (19,20). Due to their risk of diversion, both full and partial agonists are classified as Schedule II and Schedule III narcotics, respectively, under the Controlled Substances Act. These medications must be dispensed in either a specialty clinic setting (methadone) or in an office-based outpatient medical setting (buprenorphine) by a physician with a waiver from the Drug Enforcement Agency. Other barriers to access include inadequate physician staffing in specialty treatment settings (21), medication cost (22), patient factors that affect medication adherence (e.g., smoker status, co-occurring psychiatric disorders) (23), and negative perceptions toward full and partial agonist medications (2,24–29). Additional research is needed to better understand for whom these medications are most effective and how best to deploy MOUDs across different settings.
Recovery residences
Psychosocial factors such as employment, peer support, and comorbid health conditions play a key role in the course and management of OUD (30–32). Therefore, additional supports are needed to increase an individual’s psychosocial recovery capital, or the social, financial, cultural, and human capital needed to achieve and maintain recovery long-term (33). These resources are commonly referred to as recovery support services (RSS), and “provide emotional and practical support for continuing remission as well as daily structure and rewarding alternatives to substance use” (34). Examples of RSS include mutual aid groups (e.g., Alcoholics Anonymous, Narcotics Anonymous), peer recovery coaches, and recovery community centers.
Another type of RSS is the recovery residence (RR), commonly referred to as sober homes, therapeutic communities, or Oxford Houses (OH). RRs may increase an individual’s recovery capital (33). Importantly, RRs promote peer support for abstinence by fostering a sense of community among residents (35,36) which may be critical for individuals with OUD struggling with cravings and relapse triggers on their own. While the total number of RRs in the United States is unknown, there are an estimated 4,500 residences supporting approximately 45,000 individuals in a given year among residences that can be identified, i.e., those that are affiliated with the National Alliance for Recovery Residences (NARR) or are chartered by Oxford House (37,38).
NARR developed a typology of RRs that identifies four distinct levels of support (see Figure 1), varying by the staffing, governance, and intensity of clinical services or supports offered on-site, and the stage of recovery for which each level is appropriate (39). The first level of support refers to settings that are wholly peer-run and have no staff or clinical supports and services offered on-site (e.g., OH (40). The second level, such as sober homes or sober living homes in California, may have a house manager that oversees the daily operation of the residence, and often require residents to attend mutual aid meetings in the community as a condition of their stay (41). The third level, such as those that have been studied in Philadelphia, also have a house manager, and may require residents to attend community-based mutual aid meetings and/or outpatient treatment (42). The fourth level, such as therapeutic communities or the Minnesota Model (i.e., 28-day residential treatment), are typically licensed and have trained clinicians that deliver clinical services on-site in addition to peer-supports (43).
Figure 1.
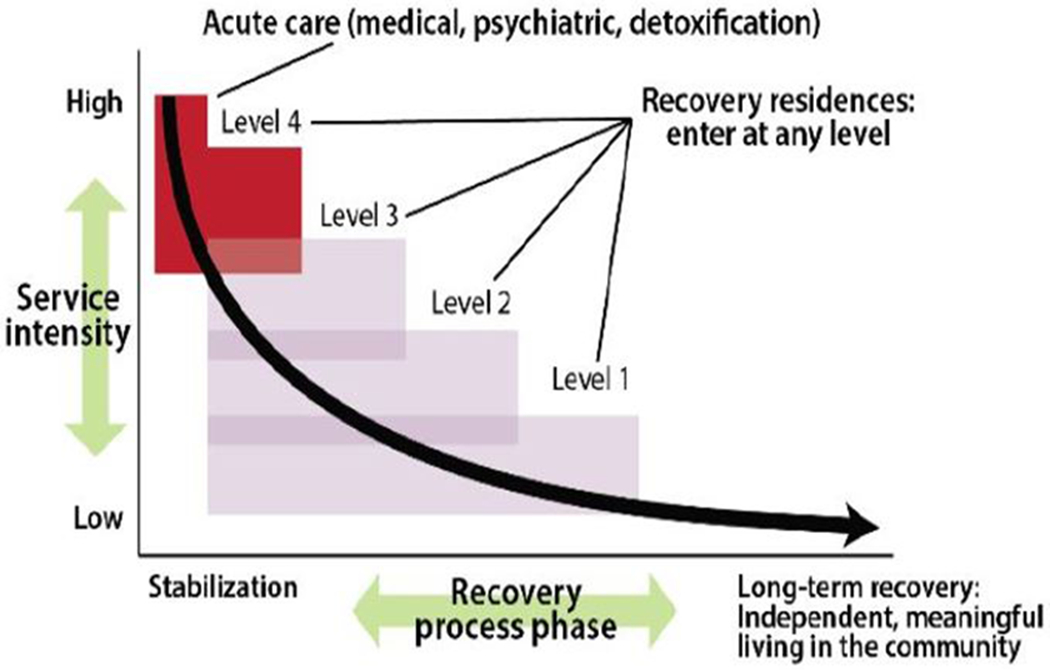
From: “A primer on recovery residences: FAQs”, by the National Alliance for Recovery Residences, 2012, pp. 16, retrieved from https://narronline.org/wp-content/uploads/2014/06/Primer-on-recovery-residences-09-20-2012a.pdf. Copyright 2012 by the National Alliance for Recovery Residences. Reprinted with permission.
The evidence base for these models is growing. A systematic review of the literature on resident outcomes by Reif, George (44) found moderate evidence of reduced substance use and criminal justice involvement and higher rates of employment and higher income levels (44). However, this evidence is limited by small sample sizes, few randomized trials and a lack of control or comparison groups, and little focus on organizational characteristics (44). Empirical evidence of RR effectiveness for individuals with OUD is emerging. Preliminary evidence indicates abstinence rates are higher following opioid detoxification and during psychosocial substance use treatment among individuals residing in RRs compared to those who do not (45,46). However, negative attitudes toward MOUDs among RR residents may present a barrier for those utilizing MOUDs who may concurrently benefit from a stay in a RR (47). While research comparing outcomes for those in MOUD-specific RRs with those in general population RRs has not yet been conducted, some evidence suggests that population-specific RRs may be beneficial (48). No research has been conducted that examines the effects of RRs on MOUD adherence or prescriber attitudes toward or perceptions of RRs.
Combining recovery pathways
Supporting individuals utilizing MOUDs in RRs could have a significant impact on the opioid epidemic, particularly in rural areas (49). Individuals with OUD are more likely to relapse if they received short-term inpatient care only (50), during the period immediately following MOUD initiation (51), and upon MOUD discontinuation (52), and relapse too often results in premature mortality (16). RRs extend the continuum of care beyond detoxification and medication initiation to assist with longer-term symptom management in the community. While a number of barriers work to undermine the support of individuals on MOUD in recovery housing, these barriers may be overcome with adequate infrastructure and guidance from the empirical literature.
Barriers to MOUDs in RRs
Disparate belief systems and stigma
MOUDs and recovery housing evolved out of separate communities and disparate belief systems. The use of medication emerged from the medical model, wherein a trained clinician offers expert advice and treatments primarily targeting underlying biological processes of a disease. In contrast, RRs emerged from a social model approach, wherein non-clinician peers play a central role in the provision of experiential psychosocial support (53). These residences are largely 12-step oriented (42,54), and may espouse an abstinence-based approach which would prohibit resident use of any psychoactive substances, including MOUDs.
These disparate philosophies may lead to mistrust between the two communities, potentially worsening stigma against people treated with MOUDs. Indeed, stigmas may not only come from the general public (55,56), medical providers (57,58), or the criminal justice system (59–61), but may also come from substance use treatment counselors (62), participants of mutual aid groups (63), or RR operators and residents (47,57,62). Additionally, anti-medication stigma varies by type and in some cases has resulted in an overemphasis on antagonist medication despite its limitations for some individuals (59,61).
Residence safety
RR operators may not consider an applicant who is utilizing a full or partial opioid agonist due to their risk of diversion. Factors that increase the risk for diversion include their potential for abuse (64), the difficulty of legally accessing these medications (65), and their potential as a source of income (66). When misuse of these medications occurs in a RR the entire community’s safety is at risk, given the increased risk of relapse caused by drug-seeking environmental cues that can trigger relapse (67,68).
A primary tool for reducing the risk of diversion is direct monitoring of medication use (4). However, monitored administration in RRs presents several challenges. RRs may lack tools that support medication safety such as lock boxes or safes which require upfront capital investment. Staffing is also a concern in some RRs. While higher-intensity settings (e.g., Level III and Level IV residences) may have credentialed staff and closer supervision of residents, staff-to-resident ratios in lower-intensity settings are usually lower, and staff may have little or no training in medication management. Long-acting injectable or implantable buprenorphine with naloxone also show promise in reducing overdose (69), as does maintaining a supply of naloxone on-site. However, access to these medications is often cost-prohibitive (70,71) and varies widely by state (72).
Facilitators of medication use in RRs
Workforce development
Over time the definition of recovery has evolved from one that is abstinence-based to one that is recovery-oriented. SAMHSA describes recovery as occurring across “multiple pathways” (73). While abstinence-based approaches are still dominant, the concept “medication-assisted recovery”, i.e., the use of medications in combination with abstinence-based recovery to support individuals for whom both pathways are appropriate, is gaining acceptance (74). Educating RR operators on MOUDs and how best to support residents using these medications could reduce stigma (62,75), particularly by including the voices of individuals who have had success using MOUDs. At the same time, building collaborations between RR operators and MOUD prescribers requires increasing prescribers’ understanding of RRs and the need to establish resident information exchange protocols.
Preventing diversion and overdose
Even when an operator is supportive of MOUDs the setting must be properly equipped to monitor medication adherence. Some low-cost protocols are already being implemented by RR operators. These include screening protocols that ensure that a prospective resident’s needs can be addressed. For example, a prospective resident who needs to be closely monitored while initial dosage levels are established may be more appropriate for a higher level of support with the accompanying staff who can conduct this monitoring. Regular and random drug testing for all residents – regardless of medication status – is another strategy that is already commonplace in many RRs. Other low-cost strategies include conducting pill counts, keeping medication logs, having staff accompany residents when picking up medications from the pharmacy, and behavioral monitoring by staff and/or fellow residents. Injectable medication formulations also reduce the risk of diversion, although they may not be appropriate for, or available to, everyone.
Conclusions and implications for future research
In response to the ongoing opioid epidemic, states are exploring opportunities to increase the availability of MOUDS and the supply of RRs for individuals using MOUDs. A variety of approaches, as discussed above, may address the challenges of doing so. However, mandating changes without providing financial incentives and operational support limits the ability of key stakeholders to meet these goals. Therefore, we suggest that policymakers and operators work together to create a comprehensive set of policies that account that for local contextual factors that may affect implementation.
Many of the above suggestions are primarily based on anecdotal evidence and practice-based experience. Research is urgently needed to examine the effects of various interventions on rates of medication initiation and maintenance, and the effects of these interventions on proximal and distal recovery outcomes. These research gaps are indicative of the limited research infrastructure needed to conduct rigorous research on recovery residences more broadly. Specifically, there are too few researchers knowledgeable about RRs, there is no central registry of RRs, and few incentives for RR operators to participate in research (76). More training and funding opportunities are needed to address these challenges, and to stimulate new research on RRs, including studies focused on outcomes of individuals with OUD residing in RRs.
Acknowledgments
Funding
This work was supported by the Substance Abuse and Mental Health Services Administration, under requisition number X to X and by the National Institute on Alcohol Abuse and Alcoholism (X). The funding agencies had no role in the writing of this report or in the decision to submit the paper for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the SAMHSA, NIAAA, or the National Institutes of Health; Substance Abuse and Mental Health Services Administration, Center for Substance Abuse Treatment [SAM200916]; National Institute on Alcohol Abuse and Alcoholism [R01AA027782].
Footnotes
Financial disclosures
The authors report no relevant financial conflicts.
References
- 1.Hall AJ, Logan JE, Toblin RL, Kaplan JA, Kraner JC, Bixler D, Crosby AE, Paulozzi LJ. Patterns of abuse among unintentional pharmaceutical overdose fatalities. Jama. 2008;300:2613–20. [DOI] [PubMed] [Google Scholar]
- 2.Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370:2063–66. doi: 10.1056/NEJMp1402780. [DOI] [PubMed] [Google Scholar]
- 3.National Institute on Drug Abuse. Prescription opioids and heroin. Rockville, MD: National Institute for Drug Abuse; 2018. Available from: https://www.drugabuse.gov/node/pdf/19774/prescription-opioids-and-heroin [last accessed 12 Jan 2020]. [Google Scholar]
- 4.Substance Abuse and Mental Health Services Administration. Medications for Opioid Use Disorder. Treatment Improvement Protocol (TIP) Series 63, Full Document. HHS Publication No. (SMA) 18-5063FULLDOCRockville, MD: Substance Abuse and Mental Health Services Administration; 2018. [Google Scholar]
- 5.Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9:358. doi: 10.1097/ADM.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Society for Community Research and Action. The role of recovery residences in promoting long-term addiction recovery. Am J Community Psychol. 2013;52:406–11. doi: 10.1007/s10464-013-9602-6. [DOI] [PubMed] [Google Scholar]
- 7.Pathan H, Williams J. Basic opioid pharmacology: an update. Br J Pain. 2012;6:11–16. doi: 10.1177/2049463712438493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weis WI, Kobilka BK. The molecular basis of G protein–coupled receptor activation. Annu Rev Biochem. 2018;87:897–919. doi: 10.1146/annurev-biochem-060614-033910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Lib. 2009; (3):1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomized trial. Lancet. 2011;377:1506–13. doi: 10.1016/S0140-6736(11)60358-9. [DOI] [PubMed] [Google Scholar]
- 11.Tanum L, Solli KK, Latif Z-EH, JŠ B, Opheim A, Sharma-Haase K, Krajci P, Kunøe N. The effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74:1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JD, EV N Jr, Novo P, Bachrach K, Bailey GL, Bhatt S, Farkas S, Fishman M, Gauthier P, Hodgkins CC, King J. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X: BOT): a multicentre, open-label, randomized controlled trial. Lancet. 2018;391:309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fullerton CA, Kim M, Thomas CP, Lyman DR, Montejano LB, Dougherty RH, Daniels AS, Ghose SS, Delphin-Rittmon ME. Medication-assisted treatment with methadone: assessing the evidence. Psychiatr Serv. 2014;65:146–57. [DOI] [PubMed] [Google Scholar]
- 14.Thomas CP, Fullerton CA, Kim M, Montejano L, Lyman DR, Dougherty RH, Daniels AS, Ghose SS, Delphin-Rittmon ME. Medication-assisted treatment with buprenorphine: assessing the evidence. Psychiatr Serv. 2014;65:158–70. [DOI] [PubMed] [Google Scholar]
- 15.Bart G Maintenance medication for opiate addiction: the foundation of recovery. J Addict Dis. 2012;31:207–25. doi: 10.1080/10550887.2012.694598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Degenhardt L, Bucello C, Mathers B, Briegleb C, Ali H, Hickman M, McLaren J. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction. 2011;106:32–51. [DOI] [PubMed] [Google Scholar]
- 17.Larochelle MR, Bernson D, Land T, Stopka TJ, Wang N, Xuan Z, Bagley SM, Liebschutz JM, Walley AY. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169:137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma J, Bao Y-P, Wang R-J, Su M-F, Liu M-X, Li J-Q, Degenhardt L, Farrell M, Blow FC, Ilgen M, Shi J. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24:1868–83. [DOI] [PubMed] [Google Scholar]
- 19.Mojtabai R, Mauro C, Wall MM, Barry CL, Olfson M. Medication treatment for opioid use disorders in substance use treatment facilities. Health Aff. 2019;38:14–23. doi: 10.1377/hlthaff.2018.05162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones C, Campopiano M, Baldwin G, McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am J Public Health. 2015;105:e55–e63. doi: 10.2105/AJPH.2015.302664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abraham AJ, Knudsen HK, Rieckmann T, Roman PM. Disparities in access to physicians and medications for the treatment of substance use disorders between publicly and privately funded treatment programs in the United States. J Stud Alcohol Drugs. 2013;74:258–65. doi: 10.15288/jsad.2013.74.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aletraris L, Edmond MB, Roman PM. Adoption of injectable naltrexone in US substance use disorder treatment programs. J Stud Alcohol Drugs. 2015;76:143–51. doi: 10.15288/jsad.2015.76.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fareed A, Eilender P, Ketchen B, Buchanan-Cummings AM, Scheinberg K, Crampton K, Nash A, Shongo-Hiango H, Drexler K. Factors affecting noncompliance with buprenorphine maintenance treatment. J Addict Med. 2014;8:345–50. [DOI] [PubMed] [Google Scholar]
- 24.Molfenter T, Sherbeck C, Zehner M, Quanbeck A, McCarty D, Kim J-S, Starr S. Implementing buprenorphine in addiction treatment: payer and provider perspectives in Ohio. Subst Abuse Treat Prev Policy. 2015;10:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volkow ND. Medications for opioid use disorder: bridging the gap in care. Lancet. 2018;391:285–87. doi: 10.1016/S0140-6736(17)32893-3. [DOI] [PubMed] [Google Scholar]
- 26.Sharma A, Kelly SM, Mitchell SG, Gryczynski J, O’Grady KE, Schwartz RP. Update on barriers to pharmacotherapy for opioid use disorders. Curr Psychiatry Rep. 2017;19:35. doi: 10.1007/s11920-017-0783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allen B, Nolan ML, Paone D. Underutilization of medications to treat opioid use disorder: what role does stigma play? Subst Abuse. 2019;40(4):459–65. [DOI] [PubMed] [Google Scholar]
- 28.Woo J, Bhalerao A, Bawor M, Bhatt M, Dennis B, Mouravska N, Zielinski L, Samaan Z. “Don’t judge a book by its cover”: a qualitative study of methadone patients’ experiences of stigma. Subst Abuse. 2017;11:1178221816685087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wakeman SE, Rich JD. Barriers to medications for addiction treatment: how stigma kills. Subst Use Misuse. 2018;53:330–33. doi: 10.1080/10826084.2017.1363238. [DOI] [PubMed] [Google Scholar]
- 30.Brewer DD, Catalano RF, Haggerty K, Gainey RR, Fleming CB. A meta-analysis of predictors of continued drug use during and after treatment for opiate addiction. Addiction. 1998;93:73–92. doi: 10.1046/j.1360-0443.1998.931738.x. [DOI] [PubMed] [Google Scholar]
- 31.Scherbaum N, Specka M. Factors influencing the course of opiate addiction. Int J Methods Psychiatr Res. 2008;17:S39–S44. doi: 10.1002/(ISSN)1557-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz RP, Kelly SM, O’Grady KE, Mitchell SG, Brown BS. Antecedents and correlates of methadone treatment entry: a comparison of out-of-treatment and in-treatment cohorts. Drug Alcohol Depend. 2011;115:23–29. doi: 10.1016/j.drugalcdep.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cloud W, Granfield R. Conceptualizing recovery capital: expansion of a theoretical construct. Subst Use Misuse. 2008;43:1971–86. doi: 10.1080/10826080802289762. [DOI] [PubMed] [Google Scholar]
- 34.US Department of Health and Human Services (HHS) Office of the Surgeon General. Facing addiction in America: the surgeon general’s report on alcohol, drugs, and health. Washington, DC: US Department of Health and Human Services; 2016. [PubMed] [Google Scholar]
- 35.Ferrari JR, Jason LA, Olson BD, Davis MI, Alvarez J. Sense of community among Oxford House residents recovering from substance abuse: making a house a home In: Fisher A, Sonn C, Bishop B, editors. The Plenum series in social/clinical psychology psychological sense of community: research, applications, and implications. New York, NY: Kluwer Academic/Plenum Publishers; 2002:109–22. [Google Scholar]
- 36.Jason LA, Davis MI, Ferrari JR. The need for substance abuse after-care: longitudinal analysis of Oxford House. Addict Behav. 2007;32:803–18. doi: 10.1016/j.addbeh.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 37.Oxford House International. History and accomplishments; 2019. Available from: https://www.oxfordhouse.org/userfiles/file/oxford_house_history.php [last accessed 12 Jan 2020].
- 38.National Alliance for Recovery Residences. About Us; 2019. Available from: https://narronline.org/about-us/[last accessed 12 Jan 2020].
- 39.National Alliance for Recovery Residences. A primer on recovery residences: FAQs; 2012. doi: 10.1094/PDIS-11-11-0999-PDN. [DOI] [Google Scholar]
- 40.Jason LA, Olson BD, Ferrari JR, Lo Sasso AT. Communal housing settings enhance substance abuse recovery. Am J Public Health. 2006;96:1727–29. doi: 10.2105/AJPH.2005.070839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polcin DL, Korcha R, Bond J, Galloway G. What did we learn from our study on sober living houses and where do we go from here? J Psychoactive Drugs. 2010;42:425–33. doi: 10.1080/02791072.2010.10400705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mericle AA, Miles J, Cacciola J. A critical component of the continuum of care for substance use disorders: recovery homes in Philadelphia. J Psychoactive Drugs. 2015;47:80–90. doi: 10.1080/02791072.2014.976726. [DOI] [PubMed] [Google Scholar]
- 43.Malivert M, Fatséas M, Denis C, Langlois E, Auriacombe M. Effectiveness of therapeutic communities: a systematic review. Eur Addict Res. 2012;18:1–11. doi: 10.1159/000331007. [DOI] [PubMed] [Google Scholar]
- 44.Reif S, George P, Braude L, Dougherty RH, Daniels AS, Ghose SS, Delphin-Rittmon ME. Recovery housing: assessing the evidence. Psychiatr Serv. 2014;65:295–300. [DOI] [PubMed] [Google Scholar]
- 45.Tuten M, DeFulio A, Jones HE, Stitzer M. Abstinence-contingent recovery housing and reinforcement-based treatment following opioid detoxification. Addiction. 2012;107:973–82. doi: 10.1111/add.2012.107.issue-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tuten M, Shadur JM, Stitzer M, Jones HE. A comparison of reinforcement based treatment (RBT) versus RBT plus recovery housing (RBTRH). J Subst Abuse Treat. 2017;72:48–55. doi: 10.1016/j.jsat.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Majer JM, Beasley C, Stecker E, Bobak TJ, Norris J, Nguyen HM, Ogata M, Siegel J, Isler B, Wiedbusch E, Jason LA. Oxford House residents’ attitudes toward medication assisted treatment use in fellow residents. Community Ment Health J. 2018;54(5):571–77. [DOI] [PubMed] [Google Scholar]
- 48.Mericle AA, Hemberg J, Stall R, Carrico AW. Pathways to recovery: recovery housing models for men who have sex with men (MSM). Addict Res Theory. 2019;27:373–82. doi: 10.1080/16066359.2018.1538409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rinker B Recovery residences combat addiction in rural communities. Health Aff. 2019;38:1968–70. doi: 10.1377/hlthaff.2019.01489. [DOI] [PubMed] [Google Scholar]
- 50.Andersson HW, Wenaas M, Nordfjærn T. Relapse after inpatient substance use treatment: a prospective cohort study among users of illicit substances. Addict Behav. 2019;90:222–28. doi: 10.1016/j.addbeh.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 51.Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, Ferri M, Pastor-Barriuso R. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. bmj. 2017;357:j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tkacz J, Severt J, Cacciola J, Ruetsch C. Compliance with buprenorphine medication-assisted treatment and relapse to opioid use. Am J Addict. 2012;21:55–62. doi: 10.1111/ajad.2012.21.issue-1. [DOI] [PubMed] [Google Scholar]
- 53.Borkman T, Kaskutas LA, Room J, Bryan K, Barrows D. An historical and developmental analysis of social model programs. J Subst Abuse Treat. 1998;15:7–17. doi: 10.1016/S0740-5472(97)00244-4. [DOI] [PubMed] [Google Scholar]
- 54.Mericle AA, Polcin DL, Hemberg J, Miles J. Recovery housing: evolving models to address resident needs. J Psychoactive Drugs. 2017;49:352–61. doi: 10.1080/02791072.2017.1342154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barry CL, McGinty EE, Pescosolido BA, Goldman HH. Stigma, discrimination, treatment effectiveness, and policy: public views about drug addiction and mental illness. Psychiatr Serv. 2014;65:1269–72. doi: 10.1176/appi.ps.201400140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olsen Y, Sharfstein JM. Confronting the stigma of opioid use disorder—and its treatment. Jama. 2014;311:1393–94. doi: 10.1001/jama.2014.2147. [DOI] [PubMed] [Google Scholar]
- 57.Paquette CE, Syvertsen JL, Pollini RA. Stigma at every turn: health services experiences among people who inject drugs. Int J Drug Policy. 2018;57:104–10. doi: 10.1016/j.drugpo.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haffajee RL, Bohnert AS, Lagisetty PA. Policy pathways to address provider workforce barriers to buprenorphine treatment. Am J Prev Med. 2018;54:S230–S42. doi: 10.1016/j.amepre.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Friedmann PD, R H Jr, Gordon M, Schwartz R, Kinlock T, Knight K, Flynn PM, Welsh WN, Stein LA, Sacks S, O’Connell DJ. Medication-assisted treatment in criminal justice agencies affiliated with the criminal justice-drug abuse treatment studies (CJ-DATS): availability, barriers, and intentions. Subst Abuse. 2012;33:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matusow H, Dickman SL, Rich JD, Fong C, Dumont DM, Hardin C, Marlowe D, Rosenblum A. Medication assisted treatment in US drug courts: results from a nationwide survey of availability, barriers and attitudes. J Subst Abuse Treat. 2013;44:473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wakeman SE. Why it’s inappropriate not to treat incarcerated patients with opioid agonist therapy. AMA J Ethics. 2017;19:922–30. [DOI] [PubMed] [Google Scholar]
- 62.Rieckmann T, Daley M, Fuller BE, Thomas CP, McCarty D. Client and counselor attitudes toward the use of medications for treatment of opioid dependence. J Subst Abuse Treat. 2007;32:207–15. doi: 10.1016/j.jsat.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.White WL. Narcotics Anonymous and the pharmacotherapeutic treatment of opioid addiction in the United States. Chicago, IL: Philadelphia Department of Behavioral Health and Intellectual Disability Services & Great Lakes Addiction Technology Transfer Center; 2011. [Google Scholar]
- 64.Maxwell JC, McCance-Katz EF. Indicators of buprenorphine and methadone use and abuse: what do we know? Am J Addict. 2010;19:73–88. doi: 10.1111/j.1521-0391.2009.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fox AD, Chamberlain A, Sohler NL, Frost T, Cunningham CO. Illicit buprenorphine use, interest in and access to buprenorphine treatment among syringe exchange participants. J Subst Abuse Treat. 2015;48:112–16. doi: 10.1016/j.jsat.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lofwall MR, Walsh SL. A review of buprenorphine diversion and misuse: the current evidence base and experiences from around the world. J Addict Med. 2014;8:315. doi: 10.1097/ADM.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, BEN-SHAHAR OS, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse: neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci. 2001;937:1–26. [DOI] [PubMed] [Google Scholar]
- 68.Volkow ND, Koob GF, McLellan AT. Neurobiologic advances from the brain disease model of addiction. N Engl J Med. 2016;374:363–71. doi: 10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walsh SL, Nuzzo PA, Babalonis S, Casselton V, Lofwall MR. Intranasal buprenorphine alone and in combination with naloxone: abuse liability and reinforcing efficacy in physically dependent opioid abusers. Drug Alcohol Depend. 2016;162:190–98. doi: 10.1016/j.drugalcdep.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huskamp HA, Riedel LE, Barry CL, Busch AB. Coverage of medications that treat opioid use disorder and opioids for pain management in marketplace plans, 2017. Med Care. 2018;56:505–09. doi: 10.1097/MLR.0000000000000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gupta R, Shah ND, Ross JS. The rising price of naloxone—risks to efforts to stem overdose deaths. N Engl J Med. 2016;375:2213–15. doi: 10.1056/NEJMp1609578. [DOI] [PubMed] [Google Scholar]
- 72.Davis CS, Carr D. Legal changes to increase access to naloxone for opioid overdose reversal in the United States. Drug Alcohol Depend. 2015;157:112–20. doi: 10.1016/j.drugalcdep.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 73.Substance Abuse and Mental Health Services Administration (SAMHSA). Working definition of recovery: 10 guiding principles. Rockville, MD: U.S. Department of Health and Human Services; 2012: 1–8. [Google Scholar]
- 74.White W, Kurtz E. The varieties of recovery experience: a primer for addiction treatment professionals and recovery advocates. Int J Self Help Self Care. 2005;3:21. doi: 10.2190/911R-MTQ5-VJ1H-75CU. [DOI] [Google Scholar]
- 75.Schwartz RP, Kelly SM, O’Grady KE, Mitchell SG, Peterson JA, Reisinger HS, Agar MH. and Brown BS Attitudes toward buprenorphine and methadone among opioid-dependent individuals. Am J Addict. 2008;17:396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Polcin DL, Mericle AA, Callahan S, Harvey R, Jason LA. Challenges and rewards of conducting research on recovery residences for alcohol and drug disorders. J Drug Issues. 2016;46:51–63. doi: 10.1177/0022042615616432. [DOI] [PMC free article] [PubMed] [Google Scholar]


