Abstract
Helicobacter pylori infection is the main risk factor for development of gastric cancer, the third leading cause of cancer death worldwide. H. pylori colonizes the human gastric mucosa and persists for decades. The inflammatory response is ineffective in clearing the infection, leading to disease progression that may result in gastric adenocarcinoma. We have shown that polyamines are regulators of the host response to H. pylori, and that spermine oxidase (SMOX), which metabolizes the polyamine spermine into spermidine plus H2O2, is associated with increased human gastric cancer risk. We now used a molecular approach to directly address the role of SMOX, and demonstrate that Smox-deficient mice exhibit significant reductions of gastric spermidine levels and H. pylori-induced inflammation. Proteomic analysis revealed that cancer was the most significantly altered functional pathway in Smox−/− gastric organoids. Moreover, there was also less DNA damage and β-catenin activation in H. pylori-infected Smox−/− mice or gastric organoids, compared to infected wild-type animals or gastroids. The link between SMOX and β-catenin activation was confirmed in human gastric organoids that were treated with a novel SMOX inhibitor. These findings indicate that SMOX promotes H. pylori-induced carcinogenesis by causing inflammation, DNA damage, and activation of β-catenin signaling.
INTRODUCTION
Gastric cancer is the third most common cause of cancer death worldwide [1] and Helicobacter pylori infection of the stomach is the strongest risk factor for disease development [2]. Although approximately half of the world’s population is colonized by H. pylori [3, 4] only a subgroup of those infected progress through a cascade of histological lesions from atrophic gastritis to intestinal metaplasia, dysplasia, and gastric adenocarcinoma [5, 6].
The polyamines putrescine, spermidine and spermine are generated through a sequential process that starts with the conversion of L-ornithine into putrescine by the enzyme ornithine decarboxylase (ODC). Putrescine is then metabolized to spermidine, spermidine to spermine by spermidine synthase and spermine synthase, respectively [7]. Notably, H. pylori infection increases ODC expression [8] and polyamine levels [9] in gastric tissues of H. pylori-infected individuals as compared with uninfected subjects. Further, ODC-derived putrescine alters the chromatin landscape in macrophages, suppressing gene expression, and thus dampening the gastric immune response [10]. Additionally, inhibition of ODC activity in Mongolian gerbils infected with H. pylori reduces the level of oxidative DNA damage in gastric epithelial cells (GECs) and also decreases cancer incidence [11, 12]. Spermine oxidase (SMOX), specifically back-converts the polyamine spermine to spermidine, generating H2O2 in the process [13, 14], which leads to DNA damage in gastric cells [15, 16]. We have demonstrated that H. pylori infection increases expression of SMOX in human and rodent gastric tissues and this is associated with oxidative DNA damage [17, 18]. Inhibition of SMOX by treatment with the SMOX inhibitor MDL 72527 reduces dysplasia and carcinoma in gerbils infected with H. pylori [11], suggesting that SMOX activity supports H. pylori-mediated carcinogenesis in the stomach. However, the effect of genetic ablation of Smox on the outcome of H. pylori infection has not been explored.
In the present study, we used Smox-deficient mice to directly evaluate the contribution of SMOX to H. pylori-associated pathology. Here we show that Smox deletion reduces spermidine levels in gastric tissues and also decreases inflammation and DNA damage in mice infected with H. pylori. DNA damage and activation of β-catenin, a signaling event strongly linked to gastric carcinogenesis [19–21], were diminished in Smox−/− mice and in murine gastric-derived organoids in response to H. pylori infection. Proteomic analysis implicated multiple cancer pathways as the most affected by Smox deletion in infected gastric organoids. Moreover, treatment of human-derived gastric organoids with a second-generation SMOX inhibitor (SLH150–54) leads to decreased β-catenin activation in response to H. pylori.
RESULTS
SMOX regulates spermidine levels in gastric tissues
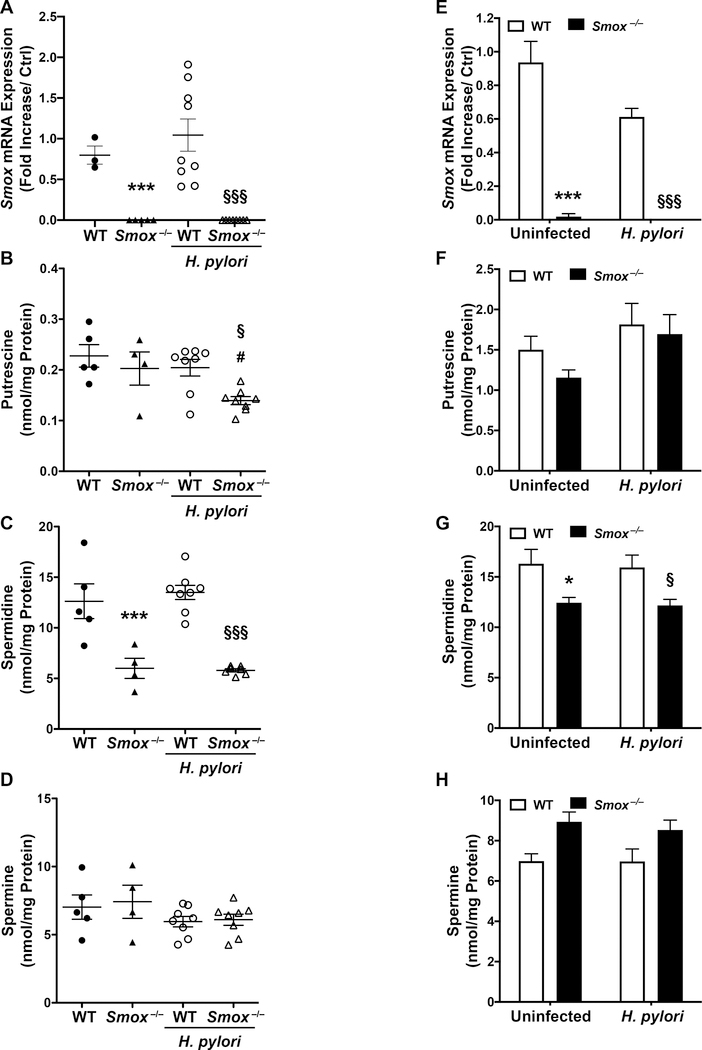
First, we confirmed that Smox mRNA expression is completely eliminated in the gastric tissues of Smox−/− mice (Fig. 1A). We also verified that the deletion of Smox was associated with dysregulation of polyamine metabolism in the stomach (Fig. 1B–D). Spermidine was the most abundant polyamine in tissues from wild-type (WT) mice (Fig. 1C), and deletion of Smox led to significant reductions of gastric spermidine levels in both uninfected and infected mice (Fig. 1C). Putrescine levels were decreased in H. pylori-infected Smox−/− tissues, likely from loss of spermidine back-conversion, and spermine was not significantly altered between the different groups (Fig. 1B and D).
Figure 1.
Smox expression and polyamine concentrations in gastric tissues and murine gastroids. (A) Smox mRNA expression by real time PCR, and (B) putrescine, (C) spermidine, and (D) spermine quantification by mass spectrometry in the stomach tissues of WT and Smox−/− mice, infected or not with H. pylori PMSS1 for 4 weeks. Monolayers of murine gastroids were infected with H. pylori PMSS1 for 24 h, and Smox mRNA expression (E) as well as the three polyamines (F-H) were analyzed in the cell lysates. *P<0.05 and ***P<0.001 versus uninfected WT; §P<0.05 and §§§P<0.001 versus infected WT; #P<0.05 compared to uninfected Smox−/−. In (A-D), each dot represents a mouse and (E-H) is the mean ± SEM of 3 independent experiments, each performed with gastroids from 2 different mice.
To verify that SMOX activity also affects spermidine levels in GECs, we generated monolayers of gastric organoids from WT and Smox−/− mice. The expression of Smox was eliminated in Smox−/− GECs (Fig. 1E). When the monolayers were infected with H. pylori, there were no significant changes in the production of the three polyamines (Fig. 1F–H). However, we confirmed that primary GECs from Smox−/− mice, infected or not with H. pylori, had reduced spermidine levels compared to WT organoids (Fig. 1G). Putrescine concentrations were not significantly affected by Smox deletion and we observed a slight accumulation of spermine in Smox−/− organoids that was not significant (Fig. 1F and H).
Reduction of H. pylori-induced gastritis in Smox−/− mice
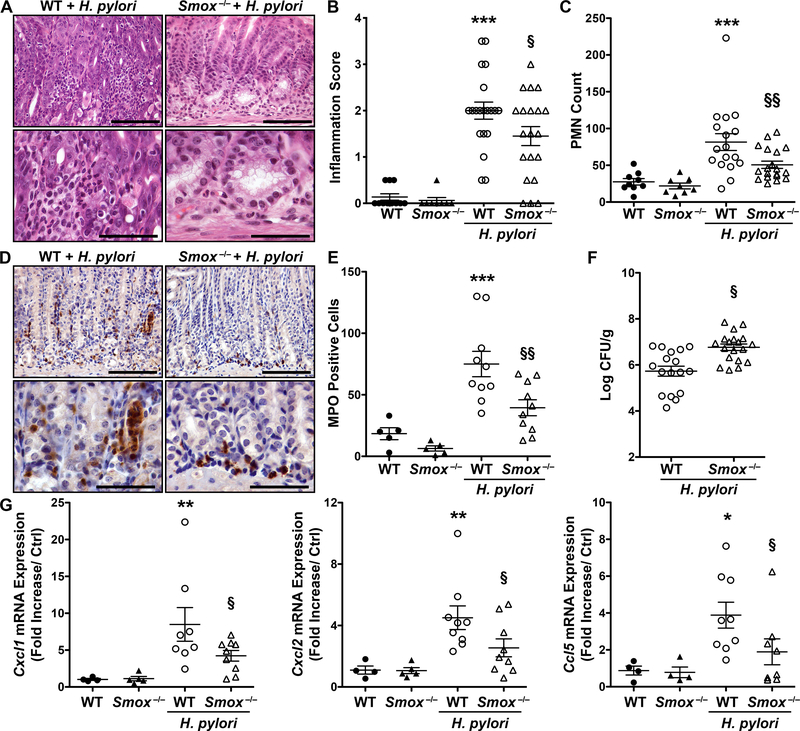
C57BL/6 WT and Smox−/− mice were infected with the H. pylori strain PMSS1 for 4 weeks and gastric inflammation was evaluated by scoring hematoxylin and eosin (H&E) staining (Fig. 2A). There was reduced infiltration of immune cells in the gastric mucosa of H. pylori-infected Smox–/– mice compared to infected WT (Fig. 2A). Using a score combining acute and chronic inflammation in the gastric antrum and corpus [10, 22, 23], we found a significant increase in histologic gastritis in infected WT mice relative to uninfected animals (Fig. 2B). Compared to WT mice, Smox–/– mice showed significantly decreased inflammation (Fig. 2B) and reduced infiltration of polymorphonuclear cells (PMNs; Fig. 2C). When acute and chronic inflammation was assessed individually, H. pylori-infected Smox–/– mice exhibited significantly decreased acute inflammation (Supplementary Fig. 1). We then confirmed these results by assessing myeloperoxidase (MPO) expression in gastric tissues using immunostaining (Fig. 2D). Reduced levels of MPO-positive cells were observed in Smox−/− mice infected for 8 weeks compared with WT animals (Fig. 2D); these observations were confirmed by quantifying the infiltrating cells expressing MPO (Fig. 2E). There was increased H. pylori colonization of the gastric tissue in Smox–/– mice compared with that observed in WT animals (Fig. 2F), an inverse relationship with inflammatory response that we have found in other studies [10, 22, 23]. Finally, in accordance with the level of inflammation in both genotypes, the expression of the genes encoding the chemokines Cxcl1, Cxcl2, and Ccl5 was significantly increased in the WT animals infected with H. pylori and significantly reduced in infected Smox−/− versus WT mice (Figure 2G).
Figure 2.
H. pylori-induced gastritis in C57BL/6 WT and Smox−/− mice. Mice were infected or not with H. pylori strain PMSS1. (A) H&E staining of WT and Smox−/− mouse stomachs after 4 weeks of infection. (B) Inflammation score and (C) number of PMNs per tissue assessed in the antrum from WT and Smox−/− animals at the 4-week time point. (D-E) Gastric tissues from mice infected for 8 weeks were immunostained for MPO (D) and the number of MPO+ cells in the antrum-corpus transition zone was determined (E). (F) Colonization of the stomach determined by serial dilution and culture after 4 weeks of infection. (G) The expression of Cxcl1, Cxcl2, and Ccl5 mRNA in gastric tissues was assessed by RT-real-time PCR in the 4-week infected mice. In (B-C) and (E-G), *P<0.05, **P<0.01, and ***P<0.001 versus control WT mice; §P<0.05 and §§P<0.01 versus WT animals infected with H. pylori. In all panels, each symbol represents a different mouse. In (A) and (D), scale bars are 50 μm (top images) and 100 μm (bottom images).
Oncogenic signaling in H. pylori-stimulated gastric epithelial cells is regulated by SMOX
To determine the involvement of SMOX in the global response of GECs to H. pylori, we used a proteomic approach. Gastric organoids were generated from WT and Smox−/− mice, monolayers of these GECs were infected or not with H. pylori, and the proteins were quantified using isobaric tag for relative and absolute quantification (iTRAQ) technology and LC-MS/MS analysis. There were 44 proteins significantly increased by H. pylori in organoid monolayers from Smox−/− mice as compared with infected WT cells (see data deposition information in Supplementary Methods). We also identified 23 proteins significantly downregulated by H. pylori in Smox−/− organoid monolayers when compared to WT cells infected with H. pylori. We then used Ingenuity Pathway Analysis and found that Cancer and RNA-Post-Transcriptional Modifications were the disease and functions most affected by Smox deletion in H. pylori-infected GECs (Fig. 3A). Importantly, other pathways involved in carcinogenesis, including cell death and survival, cellular movement, cellular function and maintenance, and cell cycle were significantly altered (Fig. 3A). Lastly, the gastrointestinal disease and immunological disease pathways, which are directly related to H. pylori infection, were also regulated by SMOX in infected cells.
Figure 3.
Effect of Smox deletion on H. pylori-stimulated GECs derived from gastric organoids. Cells were infected with H. pylori PMSS1 for 16 h. (A) Ingenuity Pathway Analysis, performed from the iTRAQ data, was used to categorize the pathways related to disease and function in H. pylori-infected monolayers of gastric organoids from WT and Smox−/− mice. (B) The percentage of pH2AFX+ GECs in WT and Smox−/− mice, infected or not with H. pylori for 4 weeks, was determined by flow cytometry. Immunofluorescence (C) and flow cytometry (D) for pH2AFX were performed on the GECs from WT and Smox−/− mice, infected or not with H. pylori for 16 h. In (C), only the merged images are shown; actin is in red, nuclei in blue, and pH2AFX in green; scale bar, 25 μm. (E and G) β-catenin activation was assessed by immunofluorescence in WT and Smox−/− mice infected or not with H. pylori for 4 weeks (E) and in monolayers of primary GECs infected or not for 16 h with H. pylori (G); β-catenin in green and nuclei in blue; scale bar, 100 μm. Mean ± SEM of three experiments. (C and G) Representative image from 3 independent experiments. (F and H) The fluorescence of images in E and G was quantified by measuring the translocation of β-catenin to the cytoplasm and nucleus using ImageJ. **P<0.01, ***P<0.001 versus uninfected WT mice/cells; §§ P<0.01, §§§ P<0.001 compared to infected WT mice/cells.
Based on these data, we assessed the effect of SMOX on different molecular events associated with H. pylori-induced carcinogenesis. We have reported that SMOX is associated with DNA damage in patients infected with H. pylori [11] and that SMOX inhibition by MDL 72527 reduces H. pylori-induced gastric cancer in Mongolian gerbils [11]. Accordingly, the percentage of GECs expressing phosphoserine 139 of H2FA histone family member X (pH2AFX), a reliable marker of DNA damage [24, 25] was reduced in Smox−/− infected mice when assessed by flow cytometry in isolated GECs (Fig. 3B). We then confirmed that H. pylori infection ex vivo of gastric organoid monolayers from WT mice induced DNA damage, as evidenced by the presence of robust punctate nuclear staining for pH2AFX (Fig. 3C). This staining was less abundant in the monolayers of primary GECs from Smox−/− mice infected with H. pylori (Fig. 3C). Further, the quantification of DNA damage by flow cytometry also demonstrated a significant reduction of pH2AFX+ cells in H. pylori-infected Smox−/− monolayers compared with WT cells (Fig. 3D).
β-catenin/WNT signaling plays an important role in cell transformation and H. pylori-positive gastric tissues have increased β-catenin expression [19, 26, 27]. From the proteomic analysis, we identified proteins involved in WNT, NOTCH, and TGF-β signaling pathways, which are linked to β-catenin activation, and were differentially expressed in Smox−/− monolayers compared with WT. In particular, E3 ubiquitin-protein ligase TRIM33 was significantly upregulated in Smox−/− GECs infected with H. pylori compared to infected WT. TRIM33 ubiquitylates β-catenin and promotes its degradation, reducing nuclear β-catenin levels [28]. β-catenin expression was therefore assessed by immunofluorescence in gastric tissues from WT and Smox−/− mice and we observed β-catenin accumulated in the cytoplasm and nuclei in tissues from WT mice infected with H. pylori (Fig. 3E), indicative of activation. In contrast, β-catenin was mainly membrane-associated in the gastric tissues from Smox−/− mice (Fig. 3E), indicating abrogation of β-catenin activation. Quantification of the staining showed a significant increase in β-catenin activation in the infected WT mice and decreased activation in tissues from infected Smox−/− mice (Fig. 3F). Similarly, we observed abundant cytoplasmic β-catenin staining in 2-D monolayers of WT GECs after infection with H. pylori and mostly cell membrane-associated staining in the Smox−/− GECs (Fig. 3G). Image analysis of the staining in organoids replicated our findings in the tissues from WT and Smox−/− mice (Fig. 3H).
SMOX activity contributes to β-catenin signaling pathway activation in human-derived gastric organoids
To test if SMOX affects β-catenin activation in a human model system, gastric organoids were generated from human surgical samples as described [29], cultured as monolayers, and then infected with H. pylori, in the presence or absence of the novel SMOX inhibitor SLH150–54 [30]. H. pylori infection increased spermidine levels in these human GECS, and SLH150–54 significantly inhibited this response (Fig. 4A). Putrescine levels were reduced in H. pylori-infected cells and spermine was not altered (Fig. 4A). The SMOX inhibitor had no effect on putrescine or spermine concentrations (Fig. 4A).
Figure 4.
Effect of SMOX activity on β-catenin activation in human-derived gastric organoids. (A) Polyamine levels measured by mass spectrometry in monolayers of human gastric organoids pre-treated with SLH150–54 (100 μM) for 2 h and then infected with H. pylori PMSS1 for 16 h. (B) TCF/LEF reporter assay in human GECs pre-treated with with SLH150–54 and then infected with H. pylori PMSS1 for 6 h. (C) AXIN2 mRNA expression in human GECs pre-treated with SLH 150–54 and/or infected for 3 h with H. pylori. (D) Immunofluorescence staining for β-catenin was performed on human organoid GEC monolayers pre-treated with SLH150–54 and supplemented or not with spermidine (10 μM), then infected with H. pylori PMSS1 for 16 h; representative image from three experiments, performed with two human organoid lines. The merged images are shown, β-catenin is green, and nuclei are blue; scale bar, 100 μm. (E) The fluorescence of images shown in (D) was quantified by measuring the translocation of β-catenin to the cytoplasm and nucleus using ImageJ. Mean ± SEM of three experiments with two organoid lines. **P<0.01, ***P<0.001 compared to control cells; §§§ P<0.001 versus H. pylori-infected cells; # P<0.05 versus H. pylori-infected cells pre-treated with SLH150–54.
β-catenin activation was assessed in human organoids transduced with a TCF/LEF reporter. Infection with H. pylori significantly increased β-catenin activation and SLH150–54 significantly reduced TCF/LEF reporter activity (Fig. 4B). The expression of AXIN2, a β-catenin target gene [31], was increased in response to H. pylori infection and then significantly decreased when SMOX activity was inhibited by SLH150–54 (Fig. 4C). Immunofluorescence staining for β-catenin also showed that H. pylori infection induced β-catenin activation in human organoids, evidenced by increased cytoplasmic and nuclear staining, which was reduced by SLH150–54 (Fig. 4D). Quantification of the fluorescence confirmed that the H. pylori-induced β-catenin nuclear translocation was decreased by SLH150–54 (Fig. 4E). Spermidine supplementation in the infected SLH150–54-treated cells increased β-catenin activation as compared with the infected cells treated with the inhibitor alone (Fig. 4D–E).
DISCUSSION
Global antibiotic eradication for H. pylori has been proposed as a strategy to decrease gastric cancer incidence, but antibiotic resistance and recurrence and/or recrudescence is becoming more common [32, 33]. Thus, alternative molecular strategies are needed to prevent disease progression in H. pylori-infected subjects. In the present study, we demonstrated that genetic deletion of Smox in mice attenuates H. pylori-induced inflammation and carcinogenic signaling, including oxidative DNA damage and β-catenin activation. In addition, β-catenin activation is also repressed in H. pylori-infected human gastroids by a second-generation SMOX inhibitor, highlighting the potential clinical relevance of our findings.
Using a genetic model of Smox deletion, here we have shown consistent reduction in PMN infiltration and chemokine production in Smox−/− mice infected with H. pylori. These results further support the concept that SMOX is an important mediator of inflammation in response to bacterial infection. In the same way, it has been demonstrated that Smox deletion or a SMOX inhibitor dampen Citrobacter rodentium- and enterotoxigenic Bacteroides fragilis-induced colon inflammation, respectively [34, 35]. Further, we have reported a positive correlation between i) gastric polyamines and gastritis in H. pylori-infected gerbils [11], and ii) colon spermidine concentration and histological damage in C. rodentium-infected WT mice [34]. Taken together with the current results, these findings implicate SMOX as a regulator of mucosal inflammation in infectious models of the gastrointestinal tract, and suggest that the protective effect of Smox deletion is associated with the decreased levels of spermidine. The specific molecular/cellular mechanism responsible for the potentially deleterious effect of spermidine in these infections is under investigation.
Disease progression from gastritis to gastric cancer in H. pylori-infected individuals has been associated with increased levels of pro-inflammatory mediators including reactive oxygen and nitrogen species that induce DNA damage [11, 17, 36]. Previous work from our laboratory demonstrated that SMOX is induced during H. pylori infection in macrophages leading to increased production of H2O2 and oxidative DNA damage [37]. Herein, our data showed that DNA damage was reduced in the infected Smox−/− mice. In these animals, there is less reactive oxygen species (ROS) since there is less recruitment of PMNs, a major source of ROS, and no SMOX activity. Our findings are in agreement with a murine model of enterotoxigenic Bacteroides fragilis infection in which ROS and DNA damage were dependent on SMOX [35]. Our results indicate that SMOX is a main component of the pro-carcinogenic signaling in H. pylori-induced gastric cancer.
Proteomic analysis indicated that SMOX plays a prominent role in pathways activated by H. pylori that drive gastric carcinogenesis. One key signaling pathway linked to carcinogenesis in H. pylori infection is Wnt/β-catenin activation [20]. H. pylori induces β-catenin nuclear accumulation in GECs, promoting the emergence of cells with cancer stem cell-like properties [38]. It has been shown that the cytotoxin-associated gene A (CagA) from H. pylori interacts with E-cadherin disrupting its association with β-catenin [39]. Once this interaction is lost, β-catenin accumulates in the cytoplasm and the nucleus where it induces expression of target genes like AXIN2 [31]. Strikingly, our data show that Smox deletion or chemical inhibition in murine or human gastroids reduces β-catenin activation, suggesting that activation of this oncogenic signaling circuit in the stomach is supported by SMOX. Smox deletion or SMOX inhibition had no effect in uninfected cells, which may indicate that SMOX activity favors, but does not initiate, β-catenin activation. We are describing in this report a novel link between polyamines and β-catenin activation in the context of H. pylori infection. Polyamine quantification in murine tissues and organoids consistently showed reduced levels of spermidine in Smox−/− mice as well as in human organoids treated with SLH150–54. Our result showing that spermidine supplementation can increase β-catenin activation in SLH150–54-treated cells suggests that spermidine could play a role in this process. Supporting our findings, it has been shown that β-catenin tyrosine phosphorylation in colonic epithelial cells is reduced when polyamines are depleted, leading to reorganization of cytoskeletal proteins and inhibition of cell migration [40].
In summary, using a genetic model, we have demonstrated that Smox deletion reduces spermidine levels in gastric tissues, diminishes immune cell infiltration and prevents DNA damage. By using human and mouse gastric-derived organoids we identified β-catenin as one of the oncogenic signaling pathways supported by SMOX activity. Chronic inflammation and dysregulation of homeostatic signaling by H. pylori are essential etiological factors for disease progression. Taken together, our data implicate SMOX as a pro-carcinogenic enzyme in the infected stomach. Thus, targeted inhibition of SMOX could be considered as a chemopreventive strategy for gastric cancer.
Supplementary Material
AKNOWLEDGMENTS
This work was funded by NIH grants R01CA190612 (K.T.W.), P01CA116087 (K.T.W.), P01CA028842 (K.T.W.), and R21AI142042 (K.T.W.); Veterans Affairs Merit Review grant I01BX001453 (K.T.W.); Department of Defense grant W81XWH-18-1-0301 (K.T.W.); the Thomas F. Frist Sr. Endowment (K.T.W.); and the Vanderbilt Center for Mucosal Inflammation and Cancer (K.T.W.). This work was also supported by NIH grants R01AT006896 (C.S.), R03DK107960 (D.B.), R01GM131408 (D.B.), R01CA204345 (R.A.C. and P.M.W.), R01CA235863 (R.A.C.), R01CA100603 (J.L.C.). J.L.C. was also supported by the Cortner-Couch Endowed Chair for Cancer Research from the University of South Florida (J.L.C.) and NCI Comprehensive Cancer Grant P30CA76292 to the H. Lee Moffitt Cancer Center and Research Institute. P.B.L. was supported by a postdoctoral fellowship award from the American Heart Association (16POST27250138). Mass spectrometry analyses were supported in part by Core Scholarships from the Vanderbilt University Medical Center Digestive Disease Research Center funded by NIH grant P30DK058404, and the Vanderbilt Ingram Cancer Center support grant P30CA068485. Immunofluorescence confocal imaging was performed in the Vanderbilt Cell Imaging Shared Resource, supported by the Vanderbilt Digestive Disease Research Center and NIH grant P30DK058404.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
SUPPLEMENTARY INFORMATION
Supplementary information is available at Oncogene’s website.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001; 345: 784–789. [DOI] [PubMed] [Google Scholar]
- 3.Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 2017; 153: 420–429. [DOI] [PubMed] [Google Scholar]
- 4.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med 1991; 325: 1127–1131. [DOI] [PubMed] [Google Scholar]
- 5.Correa P A human model of gastric carcinogenesis. Cancer Res 1988; 48: 3554–3560. [PubMed] [Google Scholar]
- 6.Mera RM, Bravo LE, Camargo MC, Bravo JC, Delgado AG, Romero-Gallo J et al. Dynamics of Helicobacter pylori infection as a determinant of progression of gastric precancerous lesions: 16-year follow-up of an eradication trial. Gut 2018; 67: 1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pegg AE. Functions of polyamines in mammals. J Biol Chem 2016; 291: 14904–14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaturvedi R, Asim M, Hoge S, Lewis ND, Singh K, Barry DP et al. Polyamines impair immunity to Helicobacter pylori by inhibiting L-Arginine uptake required for nitric oxide production. Gastroenterology 2010; 139: 1686–1698, 1698 e1681–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patchett SE, Alstead EM, Butruk L, Przytulski K, Farthing MJ. Ornithine decarboxylase as a marker for premalignancy in the stomach. Gut 1995; 37: 13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardbower DM, Asim M, Luis PB, Singh K, Barry DP, Yang C et al. Ornithine decarboxylase regulates M1 macrophage activation and mucosal inflammation via histone modifications. Proc Natl Acad Sci U S A 2017; 114: E751–E760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaturvedi R, de Sablet T, Asim M, Piazuelo MB, Barry DP, Verriere TG et al. Increased Helicobacter pylori-associated gastric cancer risk in the Andean region of Colombia is mediated by spermine oxidase. Oncogene 2015; 34: 3429–3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sierra JC, Suarez G, Piazuelo MB, Luis PB, Baker DR, Romero-Gallo J et al. alpha-Difluoromethylornithine reduces gastric carcinogenesis by causing mutations in Helicobacter pylori cagY. Proc Natl Acad Sci U S A 2019; 116: 5077–5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Devereux W, Woster PM, Stewart TM, Hacker A, Casero RA, Jr. Cloning and characterization of a human polyamine oxidase that is inducible by polyamine analogue exposure. Cancer Res 2001; 61: 5370–5373. [PubMed] [Google Scholar]
- 14.Vujcic S, Diegelman P, Bacchi CJ, Kramer DL, Porter CW. Identification and characterization of a novel flavin-containing spermine oxidase of mammalian cell origin. Biochem J 2002; 367: 665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu H, Chaturvedi R, Cheng Y, Bussiere FI, Asim M, Yao MD et al. Spermine oxidation induced by Helicobacter pylori results in apoptosis and DNA damage: implications for gastric carcinogenesis. Cancer Res 2004; 64: 8521–8525. [DOI] [PubMed] [Google Scholar]
- 16.Hardbower DM, de Sablet T, Chaturvedi R, Wilson KT. Chronic inflammation and oxidative stress: the smoking gun for Helicobacter pylori-induced gastric cancer? Gut Microbes 2013; 4: 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaturvedi R, Asim M, Piazuelo MB, Yan F, Barry DP, Sierra JC et al. Activation of EGFR and ERBB2 by Helicobacter pylori results in survival of gastric epithelial cells with DNA damage. Gastroenterology 2014; 146: 1739–1751 e1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaturvedi R, Asim M, Romero-Gallo J, Barry DP, Hoge S, de Sablet T et al. Spermine oxidase mediates the gastric cancer risk associated with Helicobacter pylori CagA. Gastroenterology 2011; 141: 1696–1708 e1691–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radulescu S, Ridgway RA, Cordero J, Athineos D, Salgueiro P, Poulsom R et al. Acute WNT signalling activation perturbs differentiation within the adult stomach and rapidly leads to tumour formation. Oncogene 2013; 32: 2048–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clements WM, Wang J, Sarnaik A, Kim OJ, MacDonald J, Fenoglio-Preiser C et al. beta-Catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res 2002; 62: 3503–3506. [PubMed] [Google Scholar]
- 21.Ebert MP, Fei G, Kahmann S, Muller O, Yu J, Sung JJ et al. Increased beta-catenin mRNA levels and mutational alterations of the APC and beta-catenin gene are present in intestinal-type gastric cancer. Carcinogenesis 2002; 23: 87–91. [DOI] [PubMed] [Google Scholar]
- 22.Hardbower DM, Singh K, Asim M, Verriere TG, Olivares-Villagomez D, Barry DP et al. EGFR regulates macrophage activation and function in bacterial infection. J Clin Invest 2016; 126: 3296–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis ND, Asim M, Barry DP, de Sablet T, Singh K, Piazuelo MB et al. Immune evasion by Helicobacter pylori is mediated by induction of macrophage arginase II. J Immunol 2011; 186: 3632–3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sierra JC, Asim M, Verriere TG, Piazuelo MB, Suarez G, Romero-Gallo J et al. Epidermal growth factor receptor inhibition downregulates Helicobacter pylori-induced epithelial inflammatory responses, DNA damage and gastric carcinogenesis. Gut 2018; 67: 1247–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toller IM, Neelsen KJ, Steger M, Hartung ML, Hottiger MO, Stucki M et al. Carcinogenic bacterial pathogen Helicobacter pylori triggers DNA double-strand breaks and a DNA damage response in its host cells. Proc Natl Acad Sci U S A 2011; 108: 14944–14949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franco AT, Israel DA, Washington MK, Krishna U, Fox JG, Rogers AB et al. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci U S A 2005; 102: 10646–10651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014; 513: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xue J, Chen Y, Wu Y, Wang Z, Zhou A, Zhang S et al. Tumour suppressor TRIM33 targets nuclear beta-catenin degradation. Nat Commun 2015; 6: 6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sebrell TA, Sidar B, Bruns R, Wilkinson RA, Wiedenheft B, Taylor PJ et al. Live imaging analysis of human gastric epithelial spheroids reveals spontaneous rupture, rotation and fusion events. Cell Tissue Res 2018; 371: 293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holshouser S, Dunworth M, Murray-Stewart T, Peterson YK, Burger P, Kirkpatrick J et al. Dual inhibitors of LSD1 and spermine oxidase. Medchemcomm 2019; 10: 778–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol 2002; 22: 1172–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence consensus report. Gut 2012; 61: 646–664. [DOI] [PubMed] [Google Scholar]
- 33.Morgan DR, Torres J, Sexton R, Herrero R, Salazar-Martinez E, Greenberg ER et al. Risk of recurrent Helicobacter pylori infection 1 year after initial eradication therapy in 7 Latin American communities. JAMA 2013; 309: 578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gobert AP, Al-Greene NT, Singh K, Coburn LA, Sierra JC, Verriere TG et al. Distinct immunomodulatory effects of spermine oxidase in colitis induced by epithelial injury or infection. Front Immunol 2018; 9: 1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodwin AC, Destefano Shields CE, Wu S, Huso DL, Wu X, Murray-Stewart TR et al. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc Natl Acad Sci U S A 2011; 108: 15354–15359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaiswal M, LaRusso NF, Gores GJ. Nitric oxide in gastrointestinal epithelial cell carcinogenesis: linking inflammation to oncogenesis. Am J Physiol Gastrointest Liver Physiol 2001; 281: G626–634. [DOI] [PubMed] [Google Scholar]
- 37.Chaturvedi R, Cheng Y, Asim M, Bussiere FI, Xu H, Gobert AP et al. Induction of polyamine oxidase 1 by Helicobacter pylori causes macrophage apoptosis by hydrogen peroxide release and mitochondrial membrane depolarization. J Biol Chem 2004; 279: 40161–40173. [DOI] [PubMed] [Google Scholar]
- 38.Yong X, Tang B, Xiao YF, Xie R, Qin Y, Luo G et al. Helicobacter pylori upregulates Nanog and Oct4 via Wnt/beta-catenin signaling pathway to promote cancer stem cell-like properties in human gastric cancer. Cancer Lett 2016; 374: 292–303. [DOI] [PubMed] [Google Scholar]
- 39.Murata-Kamiya N, Kurashima Y, Teishikata Y, Yamahashi Y, Saito Y, Higashi H et al. Helicobacter pylori CagA interacts with E-cadherin and deregulates the beta-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene 2007; 26: 4617–4626. [DOI] [PubMed] [Google Scholar]
- 40.Guo X, Rao JN, Liu L, Rizvi M, Turner DJ, Wang JY. Polyamines regulate beta-catenin tyrosine phosphorylation via Ca(2+) during intestinal epithelial cell migration. Am J Physiol Cell Physiol 2002; 283: C722–734. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.