Abstract
The Ah receptor (AhR) is a ligand-dependent transcriptional factor that mediates the effects of structurally diverse chemicals. Ligand binding stimulates nuclear translocation of the AhR and leads to AhR DNA binding and increased gene expression. Studies of the molecular mechanisms by which ligands bind to and activate the AhR and AhR-dependent signal transduction require methods to easily examine each step of the AhR signaling pathway. While current assays can measure ligand and DNA binding in vitro and gene expression in cells, there is no simple method to monitor AhR nuclear translocation. We developed a stably transfected mouse hepatoma cell line (yAHAYc6) that expresses yellow fluorescent protein-tagged AhR (yAhR) for use in qualitative or semiquantitative assessment of nuclear/cytoplasmic distribution of yAhR in living cells by fluorescent microscopy. yAhR nuclear translocation was stimulated in a concentration- and time-dependent manner by AhR agonists and inhibited by antagonists. Inhibition of nuclear export channels by leptomycin B, resulted in increased nuclear accumulation of yAhR in the absence of added ligand, indicating endogenous nucleocytoplasmic shuttling of unliganded AhR and demonstrating the utility of these cells. This novel cell line can be used to detect and characterize AhR ligands and will facilitate mechanistic studies of AhR signaling.
Keywords: aryl hydrocarbon receptor; AhR; YFP; nuclear translocation 2,3,7,8-tetrachlorodibenzo-p-dioxin; TCDD
Introduction
The aryl hydrocarbon receptor (AhR) is a ubiquitously expressed ligand-dependent transcriptional factor, and a member of bHLH-PAS (basic-helix-loop-helix, Per-Arnt-Sim) family of protein, that regulates the expression of a diverse collection of genes [Denison et al., 2011; Hayes et al., 2007; Prokopec et al., 2017]. While the AhR was initially characterized based on its role in mediating the biochemical and toxic effects of the environmental contaminant 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD, dioxin) and related halogenated aromatic hydrocarbons (HAHs) and nonhalogenated polycyclic aromatic hydrocarbons (PAHs) [Beischlag et al., 2008; Denison et al., 2011; Okey, 2010; Safe, 1990; White and Birnbaum, 2009], recent studies have demonstrated that the AhR plays a key regulatory role in both endogenous processes and human disease [Denison et al., 2011; Esser et al., 2018; Roman et al., 2018; Stockinger et al., 2014]. Although HAHs and PAHs represent the best-studied classes of AhR ligands, the AhR can bind and be activated by a wide range of structurally diverse chemicals [DeGroot et al., 2011; Denison and Nagy, 2003; Denison et al., 1998, 2011; Nguyen and Bradfield, 2008; Stejskalova et al., 2011].
In the absence of exogenous ligands, the AhR is predominantly found in the cytoplasm as part of a multiprotein complex containing hsp90, XAP2, and p23 [Beischlag et al., 2008; DeGroot et al., 2011; Denison et al., 2011; Hankinson, 1995; Okey, 2010]. Upon ligand binding, AhR undergoes a conformational change that facilitates its translocation into the nucleus, where its dimerization with the aryl hydrocarbon receptor nuclear translocator (Arnt) protein, stimulates releases of AhR-associated proteins and conversion of the AhR into its high affinity DNA binding form. The binding of the ligand:AhR:Arnt complex to its specific DNA recognition sequence, the dioxin responsive element (DRE), stimulates transcription of adjacent genes [Beischlag et al., 2008; DeGroot and Denison, 2013; Denison et al., 1988, 1989, 2011; Hankinson, 1995]. Although the ability TCDD and other AhR ligands (agonists) to induce the expression of a wide variety of genes in an AhR-dependent manner has been well documented [Beischlag et al., 2008; Denison et al., 2011; Hayes et al., 2007; Prokopec et al., 2017], numerous aspects of the molecular mechanism of ligand-dependent activation of the AhR and AhR signaling remain to be elucidated.
Nucleocytoplasmic shuttling of the AhR is mediated by its nuclear localization and nuclear export sequences (NLS and NES, respectively) [Berg and Pongratz, 2001; Ikuta et al., 1998]. It has been reported that in the absence of added exogenous ligand, some AhR appears to shuttle between cytoplasmic and nuclear compartments with the NLS and NES regulating this movement [Ikuta et al., 1998; Pollenz and Barbour, 2000; Ramadoss and Perdew, 2005; Richter et al., 2001; Tkachenko et al., 2016]. While the roles of the NLS and NES in modulating the subcellular localization of the AhR are clear, the mechanisms by which ligand can control accessibility to these elements and stimulate changes in AhR localization remains to be elucidated. The availability of a simple method to readily evaluate changes in AhR subcellular localization would facilitate identification of useful chemicals that can be used to study the mechanism of AhR nuclear translocation and to characterize cellular signaling pathways affecting this process.
Common methods to detect AhR nuclear accumulation involve the use of nuclear extracts from ligand-treated cells or tissues for in vitro DNA binding analysis (gel retardation analysis [Denison et al., 1988, 1989]) or quantitative AhR Western blotting [Holmes and Pollenz, 1997]. Alternatively, detection of AhR in fixed cells by immunohistochemical methods and fluorescent microscopy has allowed visualization of the AhR translocation process at selected time points after ligand treatment [Garside et al., 2008; Pollenz et al., 2005, 2006]. While the above methods allow detection and relative quantitation of AhR nuclear translocation, they are somewhat time-consuming, can require use of radioisotopes and are not easily amenable for evaluation or visualization of the time course of AhR nuclear translocation. Transient transfection of an AhR-green fluorescent protein fusion protein or one of its variant has been previously used to examine AhR subcellular localization [Berg and Pongratz, 2001; Ikuta et al., 1998, 2000; Ramadoss and Perdew, 2005; Tkachenko et al., 2016]. However, the transfection efficiency and expression level of AhR can vary dramatically between individual cells, and more importantly, large differences in AhR cellular distribution in the absence of ligand are commonly observed in cells in which the AhR has been overexpressed. Thus, the availability of a simple method to rapidly and inexpensively monitor endogenous and ligand-dependent AhR nuclear translocation would be very useful for AhR mechanistic studies and ligand characterization. Accordingly, here we describe the generation, characterization and utilization of a novel recombinant mouse hepatoma cell line (yAHAYc6) containing a stably transfected yellow fluorescent protein-AhR (YFP-AhR) fusion protein expression plasmid that responds to AhR agonists with the translocation of a functional YFP-AhR fusion protein from the cytosol into the nucleus.
Materials and Methods
Chemicals
TCDD was obtained from Dr. S. Safe (Texas A&M University). 3,3’,4,4’-Tetrachlorobiphenyl (PCB77) and 3,3’,4,4’,5-pentachlorobiphenyl (PCB126) were from AccuStandard (New Haven, CT), CH223191 from Chembridge Corp (San Diego, CA), beta-naphthoflavone (BNF), 3-methylcholanthene (3-MC), dibenz(a,h)anthracene (DBA), indirubin, tenidap and leptomycin B (LMB) were obtained from Sigma-Aldrich (Milwaukee, WI).
Construction of YFP-AhR fusion protein expression plasmids
The construction of N- and C-terminally YFP-tagged mouse AhRb (yAhR/pcDNA3 and AhRy/pcDNA3, respectively (Figure 1A)) was performed using the primers in Table 1. Enhanced YFP (YFP) was amplified from the plasmid pEYFP-1 (Clontech) with pfu DNA polymerase (Strategene) using primers containing a restriction site BstEII site (5’ primer: AS106) and NheI-stop codon-AflII sites (3’ primer: AH19). This PCR product was inserted into the BstEII-AflII site of the plasmid mArnt/pcDNA3 vector [Soshilov and Denison, 2008] replacing the wild type (wt) mouse Arnt cDNA (wtArnt) with the modified YFP to make the plasmid YFP/pcDNA3. cDNA encoding the mouse AhR (mAhR) was amplified from mβAhR/pcDNA3 [Fukunaga and Hankinson, 1996] using primers containing restriction sites for NheI (5’ primer: AH24) and AflII (3’ primer: AH25). This PCR fragment was inserted into the NheI-AflII sites of YFP/pcDNA3 to construct the N-terminal YFP-AhR fusion protein (yAhR) expression plasmid, yAhR/pcDNA3. The mouse AhR gene was also amplified from mAhR/pcDNA3 vector using primers containing restriction sites for BstEII (5’ primer AH20 and 3’ primer AH21), and the resulting fragment was inserted into the BstEII sites of YFP/pcDNA3 to produce the C-terminal YFP-AhR fusion protein (AhRy) expression plasmid, AhRy/pcDNA3. The resulting YFP-AhR cDNA fusion constructs were verified by sequencing.
Figure 1.

The functional analysis of YFP-tagged AhR. (A) Confirmation of the expression of in vitro expressed AhRy and yAhR proteins. Wild-type AhR (wtAhR) and C-term or N-term YFP-tagged AhR (AhRy and yAhR, respectively) were synthesized in vitro with 35S-methionine and an aliquot each protein lysate (1 μl) was analyzed by SDS-PAGE, and 35S-labeled proteins visualized by Phosphoimager analysis. The results shown are representative of three independent experiments. (B) Confirmation of DNA binding of AhR complexes. wtAhR, AhRy, yAhR and mouse wtArnt proteins were expressed in vitro, and each AhR were mixed with Arnt (1:1) and incubated with DMSO (2% (v/v)) or TCDD (20 nM) for 3 h at room temperature. TCDD-inducible protein-DNA complex formation (AhR:Arnt:DRE) were resolved by gel retardation assay as described in Material and Methods. (C) Cos-1 cells were transiently transfected with wtAhR, AhRy or yAhR expression plasmid, the AhR-responsive firefly luciferase reporter plasmid pGudLuc6.1 and Renilla luciferase reporter plasmid pRL-TK (for transfection normalization). After 24 h, transfected cells were incubated in the presence of DMSO (0.1% (v/v) or TCDD (1nM) for 24 h followed by analysis of luciferase activity. Firefly luciferase activity was divided by that of Renilla luciferase and the resulting values expressed as the mean ± SD of three replicate transfections.
Table 1.
Oligonucleotide primers used for constructing N- or C-terminal YFP-tagged AhR.
| Primer | Sequence (5’→3’) |
|---|---|
| AS106 | TAATTAGGTCACCATGGTGAGCAAGGGCGAG |
| AH19 | TTCGCCTTAAGTTCAGCTAGCCTTGTACAGCTCGTCC |
| AH20 | ATCTATGGTCACCATGTCTAGCGGCGCCA |
| AH21 | GCAGCCGGTCACCACTCTGCACCTTGCTT |
| AH24 | TATTTAGCTAGCATGTCTAGCGGCGCCA |
| AH25 | ACATATCTTAAGTTCAACTCTGCACCTTG |
Cells culture and transfection
The AhR deficient monkey kidney cell line (Cos-1) (American Type Culture Collection) and the AhR defective mouse hepatoma cell line, TAOc1BPrc1 (TAO) [Miller et al., 1983] were grown and maintained in α-Minimal essential medium (α-MEM) supplemented with 10% fetal bovine serum (FBS). Cells were transiently transfected with AhRy/pcDNA3 or yAhR/pcDNA3 using Transfectol (Gene Choice) following the manufacturer’s protocol. For stable transfections, TAO and Cos-1 cells were transfected with yAhR/pcDNA3 and following 24 h growth in nonselective medium, cells were split 1 to 10 and replated into medium containing geneticin (G418) (500 mg/l for TAO cells and 250 mg/l for Cos-1). The G418-containing culture medium was replaced every 3 days until colonies were identified by fluorescent microscopy and isolated (about 2 weeks).
In vitro protein expression, SDS-PAGE, and autoradiography
Wild type mAhR (wtAhR), YFP-tagged mAhRs, and wtArnt were synthesized in vitro in the presence of L-35S-methionine (MP-Biomedicals), or unlabeled L-methionine using the TNT Quick-coupled transcription/translation rabbit reticulocyte lysate kit (Promega). For confirmation of protein expression, L-35S-methionine labeled expressed proteins (1 μl of lysate) were resolved in 10% acrylamide SDS-PAGE and proteins in dried gels were analyzed by phosphoimager analysis (Molecular Dynamics, Sunnyvale, CA, or Fujifilm, Japan).
Gel retardation assay
Ligand-dependent AhR protein-DNA complex formation was determined by gel retardation analysis as previously described [Denison et al., 2002; Soshilov and Denison, 2014]. Briefly, complementary synthetic oligonucleotides containing the AhR:Arnt DRE3 DNA binding site (5’-GATCTGGCTCTTCTCACGCAACTCCG-3’ and 5’-GATCCGGAGTTGCGTGAGAAGAGCCA-3’) were reannealed, and end-labeled with [32P]-ATP. In vitro expressed wtAhR, yAhR or AhRy were mixed in a 1:1 (v/v) ratio with mArnt lysate and incubated for 3 h at room temperature in the presence of DMSO (2% (v/v)) or TCDD (20 nM in DMSO). AhR:Arnt:[32P]-DRE complexes were resolved by gel retardation analysis as described [Soshilov and Denison, 2014], and visualized and quantitated by phosphoimager analysis (Molecular Dynamics, Sunnyvale, CA, or Fujifilm, Japan).
Luciferase reporter gene assay
Wild-type Cos-1 and TAO cells or yAHAYc6 cells (TAO cells stably transfected with a yAhR/pcDNA3 expression vector) were plated in 24 well culture plates and transfected with Lipofectamine2000 (Invitrogen) following the manufacturer’s protocol. DNA transfected into Cos-1 or TAO cells included 80 ng of mβAhR/pcDNA3, AhRy/pcDNA3 or yAhR/pcDNA3, and 160 ng of DRE-driven firefly luciferase reporter construct (pGudLuc6.1 [Han et al., 2004]), 40 ng of the control Renilla luciferase reporter gene (pRL-TK) for transfection normalization, and 520 ng of vector control (pcDNA3.1+) to bring the total DNA to 800 ng. DNA transfected into yAHAYc6 cells included 40 ng of pGudLuc6.1, 40 ng of pRL-TK and 720 ng of pcDNA3.1+ (to 800 ng total DNA). Twenty four hours after transfection the cells were mixed 500 μl of α-MEM/FBS containing DMSO (0.1% (v/v)) or TCDD (1 nM) and further incubated for 24 h. Renilla and/or firefly luciferase activities were measured using the dual luciferase assay system or luciferase assay system, respectively (Promega, Madison,WI).
Fluorescent microscopy
For transient transfection experiments, Cos-1 or TAO cells were plated in 4 well Lab-Tek Chambered plates in 600 μl of α-MEM one day before transfection, and cells transfected with YFP-tagged AhR plasmids as described above and incubated for 14 h before chemical treatment and fluorescent microscopy. Stably transfected YFP-AhR expressing TAO cell lines were plated in 8 well Lab-Tek Chambered #1.0 borosilicate coverglass plates (Nunc) (2.2–2.5 × 104 cells in 300 μl of α-MEM/FBS) and grown for 24–36 h before the chemical treatment and microscopy. Cells were rinsed with phosphate-buffered saline (PBS) and treated with Opti-MEM reduced serum media (Invitrogen) containing the indicated chemicals (300 μl for 8-well plates and 600 μl for 4 well plates) and incubated at 37°C for the indicated time, followed by visualization using an Olympus IX71 fluorescence microscope with a YFP filter (excitation: HQ500/20, emission: HQ520lp, beam splitter: Q515lp (Chroma Technology Corp.)) and UPlanApo 40× objective. Live cell images were obtained using an Orca camera (Hamamatsu) with a Lambda automated shutter (Sutter instrument, Novato, CA), controlled by Slidebook 4.2 imaging software (Intelligent Imaging Innovations). Cellular fluorescent images were quantified and the nuclear/cytoplasmic ratio determined using Slidebook 4.2 imaging software. The entire nuclear region and a section of cytoplasmic region in each cell image were manually selected, and the mean fluorescent intensity of each region obtained. The background fluorescence from a comparable region of the slide lacking cells within the same images was subtracted from the regions of interest and the resulting fluorescence of each region used to calculate the nuclear to cytoplasmic (N:C) ratio of YFP fluorescence.
DRAQ5 Fluorescent Microscopy
The relatively high variability commonly observed when quantitating nuclear YFP fluorescence (see Figures 3B and 4A) results in large part from difficulties in defining the exact boundaries of the nucleus with YFP given its fluorescence throughout the cells. Accordingly, latter experiments with yAHAYc6 cells included DRAQ5 (Deep Red Anthroquinone 5 [Edward, 2012; Martin et al., 2005]), a far red fluorescent DNA dye with no excitation or emission spectrum overlap with YFP, to more accurately define nuclear boundaries and subtract background YFP fluorescence. In these experiments, yAHAYc6 cells were plated in 8 well Lab-Tek Chambered #1.0 borosilicate coverglass plates as described above. After 24–36 h of cell growth, cells were rinsed with PBS and treated with 250 μl of Opti-MEM reduced serum media containing the desired chemicals and DRAQ5 (to a final concentration of 0.8 μM). Cells were incubated 37°C for 1 h followed by rinsing of the well with PBS and addition of 250 μl of fresh Opti-MEM medium. YFP fluorescence was visualized as described above and DRAQ% fluorescence was visualized using HQ620/60x excitation and HQ665lp emission filters. DRAQ5 fluorescence defined the boundaries of the cell nucleus that was used for YFP quantitation. YFP fluorescence was determined in a comparable area of the cytosol and the mean intensity of YFP in the nucleus and cytosolic regions used to generate the nuclear:cytosolic YFP (i.e. yAhR) ratio.
Figure 3.
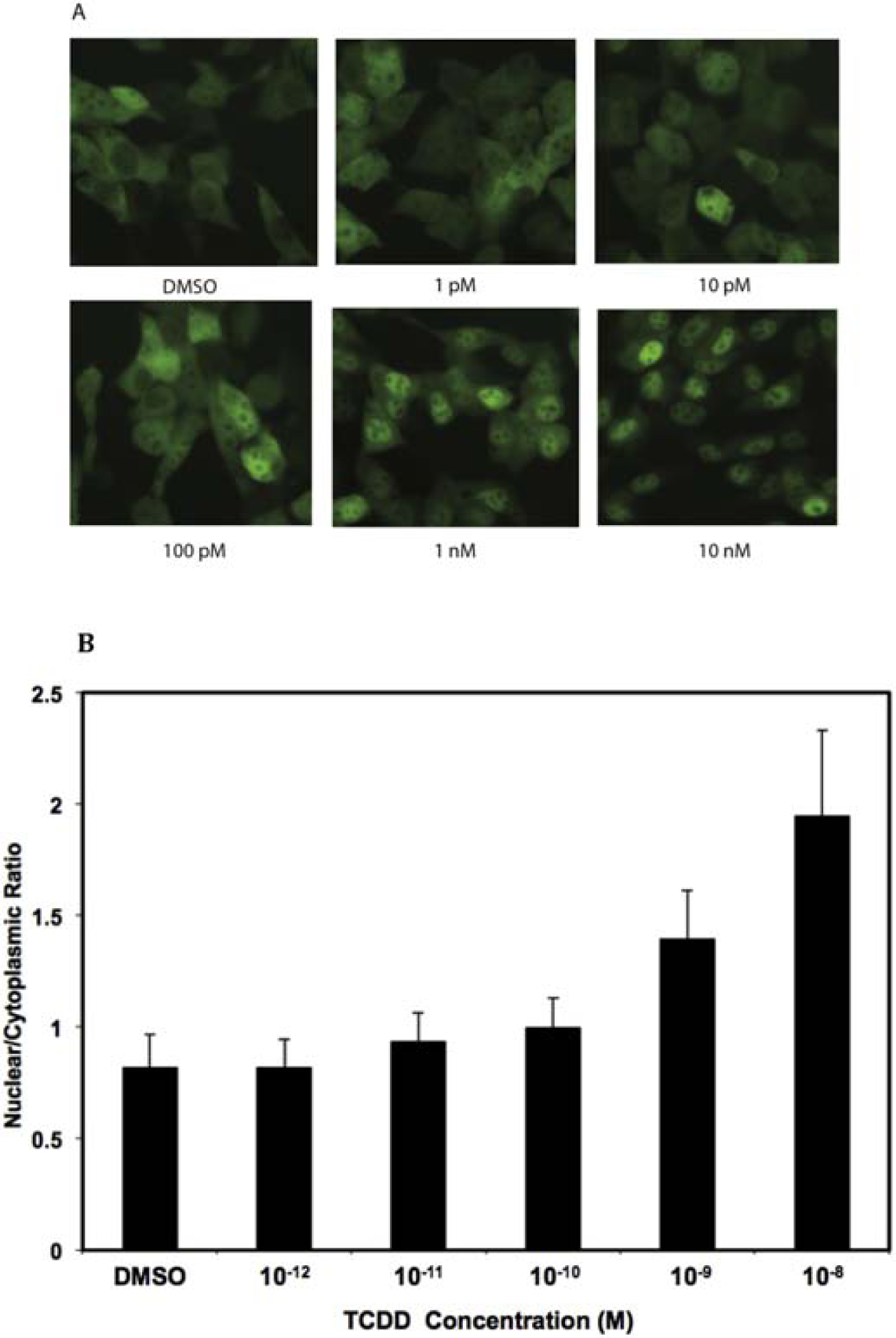
TCDD concentration-dependent nuclear localization of yAHAYc6 cell line. (A) yAHAYc6 cells were incubated with the indicated concentration of TCDD for 1 h at 37°C and fluorescent microscopic images of yAhR localization in cells in response to TCDD are shown. (B) Nuclear/cytoplasmic ratios of YFP fluorescent intensities were measured in individual yAHAYc6 cells incubated with the indicated concentration of TCDD as described in the Materials and Methods and values expressed as the mean ± SD of >100 cell measurements.
Figure 4.

Time-dependent increase in TCDD-stimulated yAhR nuclear translocation in yAHAYc6 cells. (A) yAHAYc6 cells were incubated with 10 nM TCDD at 37°C for the indicated time and fluorescent microscopic images of yAhR localization in cells at selected time points after TCDD treatment are shown. (B) Nuclear/cytoplasmic ratios of fluorescent intensities were measured in individual yAHAYc6 cells at the indicated time points as described in the Materials and Methods and values expressed as the mean ± SD of 30 cell measurements.
Results and Discussion
Characterization of YFP-tagged AhR
To confirm that the YFP-AhR fusion proteins were prepared correctly, each was expressed in vitro, and the size of 35S-labeled fusion protein determined by SDS-PAGE (Figure 1A). Compared to the wtAhR (95 kDa), both of the expressed YFP-AhR fusion proteins were approximately 112 kDa, reflecting an increase of 27 kDa from the addition of YFP to the AhR. These results also revealed that the N-terminal YFP-AhR (yAhR) was expressed at a somewhat lower level comparing wild type AhR or C-terminal YFP-AhR (AhRy). Since functional domains for DNA/hsp90 binding and transactivation are found on the N-and C-terminal ends of the AhR, respectively, whether fusion of YFP onto the N-or C-terminal end of the AhR affects its functionality remains to be determined.
To determine the impact of the N- and C-terminal fusion of YFP on AhR ligand (TCDD)-dependent transformation and DNA binding (which also indirectly assesses the ability of the AhR to bind hsp90, ligand and Arnt) we examined ligand-dependent DNA binding activity of wtAhR, yAhR and AhRy using gel retardation analysis (Figure 1B). Both YFP-AhR fusion proteins could form TCDD-inducible AhR:Arnt:DRE complexes similar to that of the wtAhR. Background (DMSO) DNA binding of the N-terminal YFP-AhR fusion protein (yAhR), was somewhat greater than that of the C-terminal YFP fusion (AhRy) or wtAhR. This slight increase in ligand-independent yAHR DNA binding might not be surprising given that the N-terminal end of the AhR contains both the AhR DNA binding domain and one hsp90 binding domain and the addition of YFP could result in a small degree of constitutive (ligand-independent) Arnt dimerization and DNA binding. To determine whether the addition of YFP affects the transactivation activity of the AhR, we examined the ability of each to stimulate ligand (TCDD)-dependent activation of DRE-driven reporter gene induction. In these experiments, Cos-1 cells (which lack AhR but contain Arnt) were transiently transfected with the AhR constructs of interest (mβAhR/pcDNA3, AhRy/pcDNA3 or yAhR/pcDNA3), and pGudLuc6.1 (a DRE-driven AhR-responsive firefly luciferase reporter gene [Han et al., 2004]), incubated with DMSO (0.1% (v/v)) or TCDD (1 nM) for 24 h, followed by measurement of luciferase activity. These experiments revealed that both YFP-tagged AhRs could stimulate induction of DRE-driven firefly luciferase reporter gene activity in a TCDD-dependent manner (Figure 1C). Interestingly, while the C-terminal YFP fusion AhR resulted in a similar magnitude of induction of luciferase activity as the wtAhR, gene induction was significantly higher with the N-terminal YFP-AhR. Similar to the DNA binding analysis, basal (DMSO) luciferase activity was elevated with both YFP-AhR fusion proteins compared to wtAhR, with the N-terminal fusion exhibiting higher background levels of luciferase activity, consistent with its elevated levels of background DNA binding. Taken together, these results not only demonstrate that the YFP-AhR fusion proteins appear to respond normally to TCDD with an increase in AhR DNA binding and activation of DRE-dependent reporter gene expression, but the increase in gene expression indirectly confirms their ability to translocate into the nucleus.
To demonstrate the potential utility of these cells as an AhR translocation bioassay, we examined the expression of each YFP-AhR for measureable expression in cells (based on intracellular fluorescence levels) and ligand-dependent nuclear translocation in transient transfection experiments. TAO cells were transiently transfected with either AhRy/pcDNA3 or yAhR/pcDNA3, and visualized by fluorescence microscopy. For both constructs, YFP signals were observed predominantly in the cytoplasm in DMSO-treated cells, with some fluorescence present in and/or overlapping the nucleus (Figure 2A). TCDD-treatment of the transfected cells resulted in nuclear localization of both yAhR and AhRy, with the fluorescence intensity of AhRy somewhat lower than that of yAhR. The quantification of the subcellular localization was scored by examining at least 100 YFP-expressing cells and scoring the distribution of YFP-AhR as predominantly cytoplasmic, evenly distributed between cytoplasmic and nucleus (C~N), or predominantly nuclear (C<N). In the absence of ligand, 98% of the cells showed localization of both YFP-AhRs predominantly in the cytoplasm or evenly distributed between the cytoplasm and nucleus (C~N), whereas TCDD treatment resulted in ~70–85% of cells with predominantly nuclear YFP fluorescence (Figure 2B). The subcellular localization of YFP-tagged AhR was also examined in transiently transfected Cos-1 cells in the absence or the presence of TCDD and the subcellular distribution of each YFP-AhR construct was similar to that of the TAO cells (data not shown). These results demonstrate that the ligand-dependent ability of AhRy and yAhR to translocate into the nucleus was comparable. Given the slightly greater activity of the N-terminal YFP-tagged AhR (yAhR) and somewhat lower background C~N signal, coupled with the significantly greater expression and/or fluorescence of yAhR compared to AhRy, the yAhR expression vector was selected for development of a stably transfected cell line.
Figure 2.

TCDD stimulates nuclear accumulation of YFP-AhR fusion proteins in transfected TAO cells. (A.) AhRy or yAhR expression vectors were transiently transfected into TAO cells. Fourteen hours after transfection, cells were incubated with OPTI-MEM medium containing DMSO (0.1% (v/v)) or TCDD (1 nM) for 1 h, followed by analysis by fluorescent microscopy. (B.) Quantification of nucleocytoplasmic subcellular distribution of transiently transfected AhRy or yAhR in DMSO- or TCDD-treated fluorescence-positive TAO cells was determined as described in the Materials and Methods. YFP fluorescence in cells was evaluated as being predominantly cytoplasmic, evenly distributed between cytoplasmic and nucleus (C~N) or predominantly nuclear (N) and represent subcellular localization analysis of at least 100 YFP-positive cells.
Generation of a stably-transfected cell line expressing a YFP-tagged AhR
Transfection of the yAhR/pcDNA3 expression plasmid into Cos-1 and TAO cells initially yielded several hundred yAhR expressing Cos-1 cell clones and ~20–30 TAO cell clones. However, after 8 weeks of selection in G418, only 5 positive TAO stable cell clones remained, whereas no Cos-1 cell clones survived. yAhR expression levels and subcellular localization in the five TAO cell clones (c2, c5, c6, c10, and c13) incubated in the absence and presence of TCDD were examined by fluorescent microscopy and the overall results are summarized in Supplemental Table S1. Except for c13, in which yAhR expression levels appeared to significantly vary between individual cells, each of the clones produced relatively similar levels of yAhR fluorescence among the individual cells. In addition, while expression of yAhR varied somewhat between individual cell clones, nuclear accumulation of yAhR was observed in all cell clones following TCDD treatment, with a greater degree of nuclear translocation observed with clones c2 and c6. These two clones were further examined for their ability to stimulate AhR-dependent gene expression. The c2 and c6 cell lines were transiently transfected with the AhR- and TCDD responsive luciferase reporter plasmid pGudLuc6.1 and pRL-TK (for transfection normalization), followed by incubation with 1 nM TCDD or DMSO for 24 h and measurement of luciferase activity (Supplemental Figure S1). TCDD induced luciferase activity was observed in both cell lines, indicating that the yAhR expressed in these stable lines was transcriptionally active. Although the overall level of TCDD-induced luciferase activity induction was higher in c2 cells, the relative fold induction (e.g., the ratio of TCDD/DMSO) was higher in c6 cells due to their lower background luciferase activity. Although nuclear translocation and transactivation activity were relatively comparable between the c2 and c6 cell lines, given that the yAhR distribution in the absence of TCDD was predominantly more cytoplasmic in the clone c6, providing a better dynamic range of response for a bioassay, the c6 clonal cell line (referred to as yAHAYc6 cells) was selected for the further assay characterization.
To establish the TCDD concentration-dependence of the yAhR nuclear translocation response, yAHAYc6 cells were incubated with increasing concentrations of TCDD (1 pM to 10 nM) for 1 h at 37°C and yAhR localization was detected by fluorescence microscopy. A TCDD concentration-dependent increase in yAhR nuclear localization (fluorescence) was visually readily apparent (Figure 3A). Quantitation of fluorescent intensities in the nucleus and cytosol revealed a significant TCDD concentration-dependent increase in yAhR nuclear accumulation (i.e., an increased nuclear/cytoplasmic ratio) (Figure 3B). The time course of TCDD-dependent AhR nuclear localization was also determined for yAHAYc6 cells incubated for up to 3 h at 37 °C with 10 nM TCDD (Figure 4). These results indicate that nuclear translocation increased linearly in the first 1 h after TCDD treatment, reaching a maximum at ~70 min with a nuclear/cytoplasmic ratio of 2.08 ± 0.43, which was maintained for up to 3 h (Figure 4A). Representative fluorescence microscopic images of yAhR in yAHAYc6 cells at various time points are shown in Figure 4B. The time required for half of maximal nuclear translocation in these cells was estimated to be between 35–40 min. The reason for the increased variability at the later time points time is not clear but may be related to cell differences in overall response, AhR degradation rates, and/or other technical factors. Overall, these results demonstrated that yAhR nuclear translocation in yAHAYc6 cells occurred in both a TCDD-concentration and time- and dependent manner.
Improved quantitative analysis of nuclear translocation using DRAQ5
A relatively high degree of variability in determinations of nuclear yAhR levels was commonly encountered during yAhR fluorescence quantitative analysis. One major contributor to this variation was the difficulty associated with accurate determination of nuclear boundaries when examining YFP fluorescence. While nuclear boundaries were easily distinguished when high degrees of yAhR nuclear translocation occurred, delineating nuclear boundaries was particularly problematic with low to moderate levels of yAhR nuclear translocation. To address this limitation, DRAQ5, a fluorescent DNA stain, was included in assay incubations to more clearly delineate the boundaries of the nucleus, allowing overlay of DRAQ5 and YFP fluorescent images and ultimately allowing a more accurate quantitation of nuclear yAhR levels (Supplemental Figure S2). DRAQ5 does not fluoresce in the excitation/emission wavelengths of YFP [Edward, 2012; Martin et al., 2005], and therefore does not interfere with YFP fluorescent measurements. Quantitation of concentration-response analysis of TCDD- and BNF-induced nuclear accumulation was carried out in yAHAYc6 cells in the presence of DRAQ5 (Figure 5). These results not only reveal greater precision in the quantitation of yAhR nuclear accumulation, but significant increases in yAhR nuclear accumulation could now be detected with as little as 1 pM TCDD (compare results to those of Figure 3B) or 100 pM BNF.
Figure 5.

DRAQ5 nuclear masking improves the precision of yAhR nuclear/cytoplasmic ratio determination in yAHAYc6 cells in response to TCDD and BNF. yAHAYc6 cells were incubated with DRAQ5 and the indicated concentration of TCDD or BNF for 1 h at 37°C and nuclear/cytoplasmic ratios of YFP fluorescent intensities were measured in individual after delineation of nuclear boundaries by DRAQ5 fluorescence measurement as described in the Materials and Methods. Values are expressed as the mean ± SD of >30 cell measurements and an asterisk indicates those values significantly greater than that of DMSO at p<0.5 as determined by the student t-test.
Interestingly, while the potency (EC50) of TCDD and BNF in the yAhR nuclear translocation assay was somewhat similar (2×10−10 M versus 7×10−10 M, respectively (Figure 5)), TCDD is 3-orders of magnitude more potent than BNF in AhR-dependent reporter gene assays (EC50 of 1×10−11 M and 1×10−8 M, respectively (Supplemental Figure S3)). The similarities in relative potencies of TCDD and BNF in the nuclear translocation bioassay were initially surprising given that TCDD reportedly has a significantly greater AhR binding affinity than BNF. However, differences in the metabolic stability of these two ligands are likely responsible for the observed differences in their potency when comparing nuclear translocation and reporter gene assays. While BNF can be metabolically degraded by cytochrome P450s in cells, TCDD is extremely resistant to metabolism. Thus, the decreased potency of BNF in the reporter gene assays likely results primarily from the increased time of incubation in the reporter gene assays compared to the nuclear translocation assays (24 h compared to 1 h, respectively), which results in a greater BNF metabolic degradation and less BNF available to activate the AhR. In contrast, TCDD is metabolically stable and this likely contributes to its increased potency in reporter gene assays, where persistent activation of gene expression is observed. Similar observations and conclusions were reached by Riddick and coworkers [Riddick et al., 1994] in a study comparing the relative AhR activity and potency of TCDD versus the metabolically labile AhR agonist, 3-methylcholanthrene.
Applications of the yAHAYc6 cell nuclear yAhR translocation assay
To confirm the utility of the yAhR nuclear translocation cell bioassay, the ability of these cells to respond to various AhR agonists, antagonists and modulators of the AhR signal transduction pathway was examined. Fluorescent microscopy of yAHAYc6 cells incubated with a variety of known AhR agonists (TCDD, DBA, PCB77, indirubin, 3MC and BNF [Denison et al., 1998, 2011; Faber et al., 2018; Garrison et al., 1996; Safe, 1990]) as well as a proposed novel AhR agonist (tenidap [Hu et al., 2007]) revealed that each compound could stimulate yAhR nuclear accumulation (Figure 6).
Figure 6.

A variety of AhR agonists stimulate yAhR nuclear localization in yAHAYc6 cells. yAHAYc6 cells were incubated with the DMSO (0.1% (v/v)) or the indicated concentration of TCDD, dibenz(a,h)anthracene (DBA), 3,3’4,4’-tetrachlorobiphenyl (PCB77), indirubin (IR), 3-methylcholanthrene (3MC), beta-naphthoflavone (BNF) or tenidap for 1 h at 37°C and yAhR fluorescence visualized by fluorescent microscopy as described in the Materials and Methods. Images are representative of cells from at least three different experimental incubations.
A variety of AhR antagonists have been identified and characterized with regards to their mechanism of action. A complication with most AhR antagonists is that they are not pure antagonists, but partial agonists and exposure to these compounds results a mixed response of induction and inhibition [Henry et al., 1999, Lu et al., 1996]. In a previous study [Zhao et al., 2010], we examined the mechanism of action of the pure AhR antagonist CH223191 [Kim et al., 2006] and found it to be a novel ligand-selective AhR antagonist, inhibiting AhR ligand and DNA binding in vitro and AhR-dependent transcriptional activation in cells by HAHs, such as TCDD, but not by PAHs, and PAH-like compounds such as BNF. To confirm that CH223191 exerted its ligand-specific antagonist effect by also selectively blocking TCDD-dependent AhR translocation, but not that by BNF, yAHAYc6 cells were incubated with DMSO, TCDD (10 nM) or BNF (10 μM) in the absence or presence of 10 μM CH223191 for one hour and yAhR nuclear translocation examined [Zhao et al., 2010]. These experiments revealed that CH223191 significantly reduced (antagonized) TCDD-dependent nuclear translocation of the yAhR, but failed to antagonize the ability of BNF to stimulate yAhR nuclear translocation, consistent with its ligand-selectivity as an AhR antagonist. These analyses provide an additional experimental example of the utility of the yAHAYc6 cell line for investigations into the mechanisms of action of AhR antagonists.
Given the utility of yAhAYc6 cells as a method to easily monitor AhR nuclear translocation, another example of the utility of these cells was their use in examining the hypothesis that the AhR, which contains both nuclear localization and nuclear export sequences [Berg and Pongratz, 2001; Ikuta et al., 1998], undergoes normal nucleocytoplasmic shuttling in cells in the absence of exogenous ligand [Ikuta et al., 1998; Pollenz and Barbour, 2000; Ramadoss and Perdew, 2005; Richter et al., 2001]. One avenue to test this hypothesis was to examine yAhR localization in yAHAYc6 cells that are incubated in the presence of the nuclear export channel inhibitor, leptomycin B (LMB), a chemical previously shown to block AhR nuclear export [Davarinos and Pollenz, 1999; Pollenz and Barbour, 2000; Richter et al., 2001]. If yAhR undergoes nucleocytoplasmic shuttling in the absence of exogenous ligands, then the addition of LMB to yAHAYc6 cells would be expected to result in a progressive nuclear accumulation of yAhR in these cells over time. Incubation of yAHAYc6 cells with 10 nM LMB, a concentration previously shown to completely inhibit nuclear export of the AhR and allow no AhR degradation over the test period [Davarinos and Pollenz, 1999], resulted in a progressive time-dependent nuclear accumulation of yAhR (Figure 7A). These results provide additional experimental support for the hypothesis of nucleocytoplasmic shuttling of the AhR in the absence of added exogenous ligand (LMB is not an AhR agonist [Pollenz and Barbour, 2000]) and could account for the “basal” levels of nuclear yAhR detected by fluorescent microscopy. In addition, while LMB-dependent inhibition of nuclear export channels results in increased nuclear accumulation of yAhR, we observed no increase in AhR-dependent reporter gene expression in LMB treated mouse hepatoma (H1L6.1c3) in the absence of exogenous ligand (Figure 7B). In fact, similar to previous studies, LMB treatment of yAHAHc6 cells was observed to reduce the magnitude of induction of luciferase activity by TCDD, an effect that was previously shown to be due to a mechanism(s) other than inhibition of AhR nuclear export [Pollenz and Barbour, 2000]. Accordingly, these results indicate that the yAhR accumulating within the nucleus of LMB-treated cells is apparently not the result of AhR activation by endogenous AhR agonists, which would be expected to induce AhR-dependent gene expression, but must be regulated by other currently undefined mechanisms [Pollenz and Barbour, 2000; Pollenz et al., 2005; Ramadoss and Perdew, 2005].
Figure 7.

Inhibition of nuclear export channels in yAHAYc6 cells by leptomycin B stimulates a time dependent nuclear accumulation of transcriptionally inactive yAhR. (A) yAHAYc6 cells were incubated with 10 nM leptomycin B (LMB) for the indicated time at 37°C, and the yAhR nuclear/cytoplasmic ratio was determined as described in the Materials and Methods. The values are expressed as the mean ± SD of 30 cell measurements. (B) The effect of LMB on AhR-dependent gene induction in mouse hepatoma H1L6.1c3 cells that contain a stably transfected AhR-responsive luciferase reporter gene [Han et al., 2004]. H1L6.1c3 cells were incubated with DMSO (0.1% (v/v)), TCDD (1 nM), LMB (10 nM), or TCDD (10 nM) and LMB (10 nM) for 6 h at 37°C, and luciferase activity was measured as described in the Materials and Methods. Values were expressed as the mean ± SD of triplicate incubations and are representative of three experiments.
Conclusions
Here we describe the development, optimization and validation of a novel stably transfected cell bioassay method for visualization and relative quantitation of AhR nuclear localization. Not only can these cells be used to characterize the ability of AhR agonists, antagonists and selective AhR modulators to stimulate or inhibit nuclear translocation of the AhR and to identify and characterize novel AhR activators, but they provide an avenue in which to study the molecular mechanisms and signaling pathways involved in this process as well as those regulating endogenous nucleocytoplasmic shuttling in the absence of exogenous ligand.
Supplementary Material
Highlights.
A simple and rapid method is needed to monitor AhR nuclear translocation
Living cells expressing a fluorescently-tagged AhR reveal AhR nuclear translocation.
AhR nuclear translocation was stimulated by agonists and inhibited by antagonists.
Leptomycin B treatment revealed nucleocytoplasmic shuttling of unliganded AhR
Acknowledgements
This research was supported by the National Institute of Environmental Health Sciences (NIEHS) (R01ES007685 and a Superfund Research Grant P42ES004699), the California Agriculture Experiment Station and the American Taxpayers, without whose support this research would not be possible.
Abbreviations
- AhR
Ah receptor
- Arnt
Ah receptor nuclear translocator
- AhRy
AhR with a C-terminal YFP tag
- bHLH-PAS
Basic-helix-loop-helix-Per-Arnt-Sim
- BNF
Beta-Naphthoflavone
- DBA
Dibenz(a,h)Anthracene
- DMSO
Dimethylsulfoxide
- DRAQ5
Deep Red Anthroquinone 5
- DRE
Dioxin responsive element
- G418
Geneticin
- HAHs
Halogenated aromatic hydrocarbons
- LMB
Leptomycin B
- 3MC
3-Methylcholanthrene
- NES
Nuclear export sequence
- NLS
Nuclear localization sequence
- PAHs
Polycyclic aromatic hydrocarbons
- TAO
TAOc1BPrc1 cells
- TCDD
2,3,7,8-Tetrachloridibenzo-p-dioxin
- Wt
Wild-type
- wtAhR
Wild-type AhR
- yAhR
AhR with a N-terminal YFP tag
- YFP
Yellow fluorescent protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH, 2008. The aryl hydrocarbon receptor complex and control of gene expression. Crit. Rev. Eukaryot. Gene Expr 18, 207–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg P, Pongratz I, 2001. Differential usage of nuclear export sequences regulates intracellular localization of the dioxin (aryl hydrocarbon) receptor. J. Biol. Chem 276, 43231–43238. DOI: 10.1006/abbi.2001.2339 [DOI] [PubMed] [Google Scholar]
- Davarinos NA, Pollenz RS, 1999. Aryl hydrocarbon receptor imported into the nucleus following ligand binding is rapidly degraded via the cytoplasmic proteasome following nuclear export. J. Biol. Chem 274, 28708–28715. DOI: 10.1074/jbc.274.40.28708 [DOI] [PubMed] [Google Scholar]
- DeGroot DE, He G, Fraccalvieri D, Bonati L, Pandini A, Denison MS, 2011. AhR ligands: Promiscuity in binding and diversity in response, in: Pohjanvirta R (Ed.), The Ah Receptor in Biology and Toxicology. John Wiley & Sons Inc., Hoboken, pp. 63–79 [Google Scholar]
- DeGroot DE, Denison MS, 2013. Nucleotide specificity of DNA binding of aryl hydrocarbon receptor:ARNT complex is unaffected by ligand structure. Toxicol. Sci 137, 102–113. DOI: 10.1093/toxsci/kft234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison MS, Fisher JM, Whitlock JP Jr., 1988. The DNA recognition site for the dioxin-Ah receptor complex. Nucleotide sequence and functional analysis. J. Biol. Chem 263, 17221–17224. [PubMed] [Google Scholar]
- Denison MS, Fisher JM, Whitlock JP Jr., 1989. Protein-DNA interactions at recognition sites for the Dioxin-Ah receptor complex. J. Biol. Chem 264, 16478–16482. [PubMed] [Google Scholar]
- Denison MS, Seidel SD, Rogers WJ, Ziccardi M, Winter GM, Health-Pagliuso S, 1998. Natural and synthetic ligands for the Ah receptor, in: Puga A and Wallace KB (Eds.), Molecular Biology of the Toxic Response. Taylor & Francis, Philadelphia, pp. 393–410. [Google Scholar]
- Denison MS, Rogers JM, Rushing R, Jones CL, Tetangco SC, Health-Pagliuso S, 2002. Analysis of the aryl hydrocarbon receptor (AhR) signal transduction pathway, Curr. Protocols Toxicol Chapter 4, Unit 4.8 DOI: 10.1002/0471140856.tx0408s11 [DOI] [PubMed] [Google Scholar]
- Denison MS, Nagy SR, 2003. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol 2003 43: p. 309–34. DOI: 10.1146/annurev.pharmtox.43.100901.135828 [DOI] [PubMed] [Google Scholar]
- Denison MS, Soshilov AA, He G, DeGroot DE, Zhao B, 2011. Exactly the same but different: Promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol. Sci 124, 1–22. DOI: 10.1093/toxsci/kfr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edward R, 2012. Red/far-red fluorescing DNA-specific anthraquinones for nucl:cyto segmentation and viability reporting in cell-based assays. Methods Enzymol. 505, 23–45. DOI: 10.1016/B978-0-12-388448-0.00010-3. [DOI] [PubMed] [Google Scholar]
- Esser C, Lawrence BP, Sherr DH, Perdew GH, Puga A, Barouki R, Coumoul X, 2018. Old receptor, new tricks - the ever-expanding universe of aryl hydrocarbon receptor functions. Report from the 4th AHR Meeting, 29–31 August 2018 in Paris, France. Int. J. Mol. Sci 19, E3603 DOI: 10.3390/ijms19113603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber SC, Soshilov AA, Tagliabue SG, Bonati L, Denison MS, 2018. Comparative in vitro and in silico analysis of the selectivity of indirubin as a human Ah receptor agonist. Int. J. Mol. Sci 19, E2692 DOI: 10.3390/ijms19092692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga BN, Hankinson O, 1996. Identification of a novel domain in the aryl hydrocarbon receptor required for DNA binding. J. Biol. Chem 271, 3743–3749. DOI: 10.1074/jbc.271.7.3743 [DOI] [PubMed] [Google Scholar]
- Garrison PM, Tullis K, Aarts JMMJG, Brouwer A, Giesy JP, Denison MS, 1996. Species-specific recombinant cell lines as bioassay systems for the detection of 2,3,7,8-tetrachlorodibenzo-p-dioxin-like chemicals. Fundam. Appl. Toxicol 30, 194–203. DOI: 10.1006/faat.1996.0056 [DOI] [PubMed] [Google Scholar]
- Garside H, Stewart A, Brown N, Cooke EL, Graham M, Sullivan M, 2008. Quantitative analysis of aryl hydrocarbon receptor activation using fluorescence-based cell imaging--a high-throughput mechanism-based assay for drug discovery. Xenobiotica, 38, 1–20. DOI: 10.1080/00498250701668600 [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Vázquez C and Quintana FJ, 2018. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity 48,19–33. DOI: 10.1016/j.immuni.2017.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Nagy SR, Denison MS, 2004. Comparison of recombinant cell bioassays for the detection of Ah receptor agonists. Biofactors 20, 11–22. DOI: 10.1002/biof.5520200102 [DOI] [PubMed] [Google Scholar]
- Hankinson O, 1995. The aryl hydrocarbon receptor complex. Annu. Rev. Pharmacol. Toxicol 35, 307–340. DOI: 10.1146/annurev.pa.35.040195.001515 [DOI] [PubMed] [Google Scholar]
- Hayes KR, Zastrow GM, Nukaya M, Pande K, Glover E, Maufort JP, Liss AL, Liu Y, Moran SM, Vollrath AL, Bradfield CA, 2007. Hepatic transcriptional networks induced by exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Chem. Res. Toxicol 20, 1573–1581. DOI: 10.1021/tx7003294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry EC, kende AS, Rucci G, Totleben MJ, Willey JJ, Dertinger SD, Pollenz RS, Jones JP, Gasiewicz TA, 1999. Flavone antagonists bind competitively with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) to the aryl hydrocarbon receptor but inhibit nuclear uptake and transformation. Mol. Pharmacol 55, 716–725. [PubMed] [Google Scholar]
- Holmes JL, Pollenz RS, 1997. Determination of aryl hydrocarbon receptor nuclear translocator protein concentration and subcellular localization in hepatic and nonhepatic cell culture lines: development of quantitative Western blotting protocols for calculation of aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator protein in total cell lysates. Mol. Pharmacol 52, 202–211. DOI: 10.1124/mol.52.2.202 [DOI] [PubMed] [Google Scholar]
- Hu W, Sorrentino C, Denison MS, Kolaja K, Fielden MR, 2007. Induction of cyp1a1 is a nonspecific biomarker of aryl hydrocarbon receptor activation: Results of large scale screening of pharmaceuticals and toxicants in vivo and in vitro. Mol. Pharmacol 71, 1475–1486. DOI: 10.1124/mol.106.032748 [DOI] [PubMed] [Google Scholar]
- Ikuta T, Eguchi H, Tachibana T, Yoneda Y, Kawajiri K, 1998. Nuclear localization and export signals of the human aryl hydrocarbon receptor. J. Biol. Chem 273, 2895–2904. DOI: 10.1074/jbc.273.5.2895 [DOI] [PubMed] [Google Scholar]
- Ikuta T, Tachibana T, Watanabe J, Yoshida M, Yoneda Y, Kawajiri K, 2000. Nucleocytoplasmic shuttling of the aryl hydrocarbon receptor. J, Biochem 127, 503–509. DOI: 10.1093/oxfordjournals.jbchem.a022633 [DOI] [PubMed] [Google Scholar]
- Kim SH, Henry EC, Kim DK, Kim YH, Shin KJ, Han MS, Lee TG, Kang JK, Gasiewicz TA, Ryu SH, Suh PG, 2006. Novel compound 2-methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazo-phenyl)-amide (CH-223191) prevents 2,3,7,8-TCDD-induced toxicity by antagonizing the aryl hydrocarbon receptor. Mol. Pharmacol 69, 1871–1878. DOI: 10.1124/mol.105.021832 [DOI] [PubMed] [Google Scholar]
- Lu YF, Santostefano M, Cunningham BD, Threadgill MD, Safe S, 1996. Substituted flavones as aryl hydrocarbon (Ah) receptor agonists and antagonists. Biochem. Pharmacol 51, 1077–1087. DOI: 10.1016/0006-2952(96)00063-9 [DOI] [PubMed] [Google Scholar]
- Martin RM, Leonhardt H, Cardoso MC, 2005. DNA labeling in living cells. Cytometry A 67, 45–52. DOI: 10.1002/cyto.a.20172 [DOI] [PubMed] [Google Scholar]
- Miller AG, Israel D, Whitlock JP Jr., 1983. Biochemical and genetic analysis of variant mouse hepatoma cells defective in the induction of benzo(a)pyrene-metabolizing enzyme activity. J. Biol. Chem 258, 3523–3527. [PubMed] [Google Scholar]
- Nguyen LP, Bradfield CA, 2008. The search for endogenous activators of the aryl hydrocarbon receptor. Chem. Res. Toxicol 21, 102–116. doi: 10.1021/tx7001965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okey AB (2010) An aryl hydrocarbon receptor odyssey to the shores of toxicology: the Deichmann Lecture, International Congress of Toxicology-XI. Toxicol. Sci 98, 5–38. DOI: 10.1093/toxsci/kfm096 [DOI] [PubMed] [Google Scholar]
- Pollenz RS, Barbour ER, 2000. Analysis of the complex relationship between nuclear export and aryl hydrocarbon receptor-mediated gene regulation. Mol. Cell. Biol 20, 6095–6104. DOI: 10.1128/mcb.20.16.6095-6104.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollenz RS, Popat J, Dougherty EJ, 2005. Role of the carboxy-terminal transactivation domain and active transcription in the ligand-induced and ligand-independent degradation of the mouse Ahb−1 receptor. Biochem. Pharmacol 70, 1623–1633. DOI: 10.1016/j.bcp.2005.09.006 [DOI] [PubMed] [Google Scholar]
- Pollenz RS, Wilson SE, Dougherty EJ, 2006. Role of endogenous XAP2 protein on the localization and nucleocytoplasmic shuttling of the endogenous mouse Ahb−1 receptor in the presence and absence of ligand. Mol. Pharmacol 70, 1369–1379. DOI: 10.1124/mol.106.027672 [DOI] [PubMed] [Google Scholar]
- Prokopec SD, Houlahan KE, Sun RX, Watson JD, Yao CQ, Lee J, P’ng C, Pang R, Wu AH, Chong LC, Smith AB, Harding NJ, Moffat ID, Lindén J, Lensu S, Okey AB, Pohjanvirta R, Boutros PC, 2017. Compendium of TCDD-mediated transcriptomic response datasets in mammalian model systems. BMC Genomics 18:78 DOI: 10.1186/s12864-016-3446-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss P, Perdew GH, 2005. The transactivation domain of the Ah receptor is a key determinant of cellular localization and ligand-independent nucleocytoplasmic shuttling properties. Biochemistry 44, 11148–11159. DOI: 10.1021/bi050948b [DOI] [PubMed] [Google Scholar]
- Richter CA, Tillitt DE, Hannink M, 2001. Regulation of subcellular localization of the aryl hydrocarbon receptor (AhR). Arch. Biochem. Biophys 389, 207–217. DOI: 10.1006/abbi.2001.2339 [DOI] [PubMed] [Google Scholar]
- Riddick DS, Huang Y, Harper PA, Okey AB, 1994. 2,3,7,8-Tetrachlorodibenzo-p-dioxin versus 3-methylcholanthrene: Comparative studies of Ah receptor binding, transformation and induction of CYP1A1. J. Biol. Chem 269, 12118–12128. [PubMed] [Google Scholar]
- Roman ÁC, Carvajal-Gonzalez JM, Merino JM, Mulero-Navarro S, Fernández-Salguero PM, 2018. The aryl hydrocarbon receptor in the crossroad of signalling networks with therapeutic value. Pharmacol. Ther 185, 50–63. DOI: 10.1016/j.pharmthera.2017.12.003 [DOI] [PubMed] [Google Scholar]
- Safe S, 1990. Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and related compounds: environmental and mechanistic considerations which support the development of toxic equivalency factors (TEFs), Crit. Rev. Toxicol 21, 51–88. DOI: 10.3109/10408449009089873 [DOI] [PubMed] [Google Scholar]
- Soshilov A, Denison MS, 2008. Role of the Per/Arnt/Sim domains in ligand-dependent transformation of the aryl hydrocarbon receptor. J, Biol. Chem 283, 32995–33005. DOI: 10.1074/jbc.M802414200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soshilov AA and Denison MS, 2014. DNA binding (gel retardation assay) analysis for identification of aryl hydrocarbon (Ah) receptor agonists and antagonists, in: Yan A, Caldwell GW (Eds.), Optimization of Drug Discovery: In Vitro Methods. 2nd ed., Humana Press, New York, pp. 207–219. [Google Scholar]
- Stejskalova L, Dvorak Z, Pavek P, 2011. Endogenous and exogenous ligands of aryl hydrocarbon receptor: Current state of art. Curr. Drug Metab 12, 198–212. DOI: 10.2174/138920011795016818 [DOI] [PubMed] [Google Scholar]
- Stockinger B, Di Meglio P, Gialitakis M, Duarte JH, 2014. The aryl hydrocarbon receptor: multitasking in the immune system. Annu. Rev. Immunol 32, 403–432. DOI: 10.1146/annurev-immunol-032713-120245 [DOI] [PubMed] [Google Scholar]
- Tkachenko A, Henkler F, Brinkmann J, Sowada J, Genkinger D, Kern C, Tralau T, Luch A, 2016. The Q-rich/PST domain of the AHR regulates both ligand-induced nuclear transport and nucleocytoplasmic shuttling. Sci. Rep 6, 32009 DOI: 10.1038/srep32009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SS, Birnbaum LS, 2009. An overview of the effects of dioxins and dioxin-like compounds on vertebrates, as documented in human and ecological epidemiology. J. Environ. Sci. Health, C: Environ. Carcinog. Ecotoxicol. Rev 27, 197–211. DOI: 10.1080/10590500903310047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, DeGroot D, Hayashi A, He G, Denison MS, 2010. CH223191 is a ligand-selective antagonist of the Ah (dioxin) receptor. Toxicol. Sci 117, 393–403. DOI: 10.1093/toxsci/kfq217 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


