Abstract
Multiple risk factors that may contribute to the development and severity of pediatric anxiety disorders, one of which is dimensional overcontrol. Overcontrol is a constellation of characteristics including heightened performance monitoring, inflexibility, perfectionism and aversion to making mistakes. In this study, we examined overcontrol in children with anxiety disorders and tested whether the underlying dimension of overcontrol specifically explains altered brain response to errors in pediatric anxiety disorders. Parent-reported scores of child overcontrol were collected in a sample of children (ages 8-12 years) with (n=35) and without (n=34) anxiety disorders and the relationship of overcontrol and anxiety symptoms to neural responding to errors during functional magnetic resonance imaging (fMRI) was examined. Results indicated childhood overcontrol was elevated in pediatric anxiety disorders and was significantly associated with anxiety severity, even when controlling for comorbid depression and ADHD. Additionally, overcontrol was associated with reduced neural response to errors versus correct responses in the bilateral dorsal anterior cingulate cortex (dACC) and insula, even when controlling for anxiety symptoms. Overcontrol may serve as an underlying mechanism associated with clinical pediatric anxiety that demonstrates significant associations with aberrant neural error responding. Overcontrol may be an underlying mechanism contributing to pediatric anxiety that could be targeted for early intervention.
Keywords: overcontrol, errors, pediatric anxiety, dACC, performance monitoring
1. Introduction
Overcontrol is a transdiagnostic phenotype characterized by a constellation of characteristics that cluster together: cognitive inflexibility, anxious apprehension, need for structure, perseverative or obsessive checking behaviors, perfectionism and at the core of this phenotype, heightened performance monitoring and a strong aversion to making mistakes (Donnellan & Robins, 2010; Gilbert, Barch, & Luby, 2019; Lynch et al., 2018). Overcontrol is a feature of multiple psychiatric illnesses, including anorexia nervosa, obsessive compulsive personality disorder, treatment resistant depression, with the earliest emerging presentations being pediatric anxiety disorders (Eisenberg, Spinrad, & Eggum, 2010; Henderson, Pine, & Fox, 2015; Kaye, Wierenga, Bailer, Simmons, & Bischoff-Grethe, 2013; Pinto, Greene, Storch, & Simpson, 2015; Robins, John, Caspi, Moffitt, & Stouthamer-Loeber, 1996). High rates of comorbidity of the abovementioned disorders speak to the importance of identifying transdiagnostic mechanisms that could be targeted in treatment (i.e., Norton & Pasquale, 2017; Pearl & Norton, 2017). As such, identifying and understanding the developmental psychopathology and neurobiology of overcontrol in childhood anxiety disorders may inform the developmental mechanisms and treatment of multiple psychiatric disorders across the lifespan.
Overcontrolled children often engage in high social comparisons, struggle with transition and change and have a strong dislike of making errors (e.g., Vanderbleeck & Gilbert, 2018). In the context of children with high behavioral inhibition or early social fear, overcontrol is a moderating factor, increasing risk for social anxiety (Brooker, Kiel, & Buss, 2016; White, McDermott, Degnan, Henderson, & Fox, 2011). Overcontrol in youth has also been associated with internalizing symptoms (Eisenberg et al., 2004; Robins et al., 1996) and widespread social functioning deficits, including social withdrawal, loneliness, and peer rejection (e.g., Gilbert et al., 2019). Moreover, characteristics that make up the overcontrolled phenotype have repeatedly been implicated in childhood obsessive compulsive disorder (OCD) (Park, Storch, Pinto, & Lewin, 2016) and predict the onset of youth OCD (Gilbert, Barclay, Tillman, Barch, & Luby, 2018).
Initial evidence supports the hypothesis that overcontrol is associated with dysfunction in brain systems involved in performance monitoring and processing errors. These functions are subserved by a distributed set of brain regions (Neta et al., 2015) including the dorsal anterior cingulate cortex (dACC) and anterior insula, which are both components of the brain’s cingulo-opercular network (Dosenbach, Fair, Cohen, Schlaggar, & Petersen, 2008). The small but growing literature indicates overcontrol demonstrates alterations in these brain systems, although the direction of effects is unclear. Specifically, heightened performance monitoring and overcontrol in preschoolers predicts smaller dACC volumes across childhood (Gilbert et al., 2018). Additionally, the error-related negativity (ERN), an event related potential associated with high sensitivity to errors and elevated performance monitoring (Brooker et al., 2016; Henderson et al., 2015; Meyer & Hajcak, 2019), and thought to emerge from the dACC (Garavan, Ross, Murphy, Roche, & Stein, 2002; Ullsperger & von Cramon, 2001), is reduced (i.e., smaller ERN) in relation to overcontrol in early childhood (Gilbert et al., 2019).
Pediatric anxiety disorders are also characterized by aberrant neural error responding (Fitzgerald & Taylor, 2015). For instance, heightened error monitoring, indexed via a larger ERN (i.e., hyperactivity), is associated with pediatric anxiety (Ladouceur et al., 2018; Meyer et al., 2013) and predicts onset of anxiety disorders in school-age children (Meyer, Hajcak, Torpey-Newman, Kujawa, & Klein, 2015). However, emerging work indicates the relationship of the ERN and outcomes may exhibit developmental specificity: adolescent and adult anxiety is characterized by a larger ERN (i.e., hyperreactivity; e.g., Riesel et al., 2019) while a blunted ERN (i.e., smaller or less reactivity) is associated with preschool and early childhood anxiety and temperamental fear (Meyer, Weinberg, Klein, & Hajcak, 2012; Torpey et al., 2013). fMRI evidence of error processing in pediatric anxiety has been minimal and presents mixed findings. Specifically, pediatric anxiety severity is associated with blunted neural activation to errors in the dorsolateral prefrontal cortex (dlPFC) in OCD (Fitzgerald et al., 2013) and the ventromedial prefrontal cortex (vmPFC), but only in the context of elevated behavioral inhibition (Smith, White, et al., 2019). Additionally, pediatric anxiety has demonstrated associations with increased activation in the pregenual ACC (pgACC, rostral ACC), but only in the context of peers (Smith, Kircanski, et al., 2019). Although anxiety disorders demonstrate aberrant error responding (Fitzgerald & Taylor, 2015), preliminary evidence does not implicate the dACC, which is surprising given the dACC is repeatedly involved in error processing (Neta et al., 2015). Rather, mixed findings of neural error responding in pediatric anxiety are often situated within specific constraints or contexts (i.e., only OCD, peers, high behavioral inhibition).
Given overcontrol and pediatric anxiety are both characterized by heightened concern over errors and neural error-processing abnormalities, exploring whether overcontrolled tendencies and pediatric anxiety symptoms are associated with neural error responding may provide pertinent mechanistic information. Moreover, given mixed findings relating pediatric anxiety with fMRI neural error processing, it is important to consider whether a third factor, namely overcontrol, is contributing to relationships between anxiety and aberrant neural error responding.
The aim of the current study was first, to examine the relationship of overcontrol with anxiety severity in pediatric anxiety disorders. Second, we examined associations between parent-reported overcontrol and anxiety severity with neural response to errors. To do this, we first examined associations of overcontrol to dimensional and categorical measures of anxiety as well as depression and attention deficit hyperactivity disorder (ADHD), hypothesizing that overcontrol would be most strongly associated with anxiety. Next, we examined error-related brain activity while a subset of participants underwent fMRI. Because the pediatric literature is mixed, we pulled from the adult literature demonstrating consistent hyperactivity to error processing in anxiety (Norman et al., 2018) and hypothesized that both overcontrol and anxiety would be associated with hyperactivity in the brain regions that most robustly respond to errors during this task, especially the dACC and anterior insula. To determine if anxiety or overcontrol was more central to error related activity, we also included both anxiety and overcontrol measures in the same model.
2. Materials & Methods
2.1. Participants and procedure
Children aged 8-12 years with and without anxiety disorders were recruited from the St. Louis region. Children were oversampled for clinically significant anxiety by providing informational talks about child anxiety delivered by senior author C.S. to local elementary schools and posting brochures and flyers around the community and in pediatrician offices looking for both healthy children and children with anxiety. Eligible participants were invited to the lab, where caregivers and children completed a series of questionnaires and clinical interviews assessing child psychiatric symptoms; children additionally completed an attention task (see description below). The larger study focused on examining attention in healthy and anxious children, and of the 149 children enrolled in the larger study, 70 caregivers completed the measure of overcontrol at baseline (it was added midway through data collection); 1 dyad was excluded due to missing data.
A subset of the entire sample completed a neuroimaging visit (n=63). One participant was excluded due to a neurological contraindication and one was excluded for meeting criteria for ADHD but not anxiety. The remaining 61 participants provided usable data following motion correction. In order to reliably detect brain activation to errors in the error condition, 23 individuals were excluded for making fewer than five behavioral errors; no significant demographic or clinical differences between children excluded and included emerged (all p’s >.05). Data from the remaining 38 participants was used to identify regions demonstrating significant activation to error responses, correct responses, and error versus correct responses. The neuroimaging sample used to assess associations with overcontrol consisted of 29 participants. All materials were approved by the Washington University School of Medicine IRB and conform to Declaration of Helskini standards. Informed consent and assent was obtained from all caregivers and children.
2.2. Overcontrol.
Overcontrol was assessed using the Overcontrol in Youth Checklist (OCYC)(Gilbert et al., 2018), an 18-item yes/no parent report. A total score (α=.90), as well as two subscales, assessing social concern and perfectionism (e.g., frequently compares his her abilities with that of peers and siblings) (α=. 79), and inflexibility and frustration with change (e.g., gets frustrated when s/he can’t seem to get it right the first time) (α=.88) were calculated.
2.3. Clinical symptoms, diagnoses and functioning.
Procedures for the diagnosis and assessment of anxiety were taken from the RUPP Anxiety Study (Isaacs, 2001). Severity of childhood anxiety symptoms was assessed using the interviewer-administered Pediatric Anxiety Rating Scale (PARS) (Research Units on Pediatric Psychopaharmacology Anxiety Study Group, 2002). Both parent and child were interviewed and summary rating scales were used. Parents and children also reported on current anxiety symptoms using the Screen for Child Anxiety Related Emotional Disorders (SCARED) (Birmaher et al., 1999) (α=.93), depressive symptoms using the Child Depression Inventory (CDI)(Kovacs, 1985) (α=.87), and ADHD symptoms (parent-report only) using the Conners 3™-Parent Short (Conners, 2008). Masters’ level clinicians determined DSM-5 diagnoses via parent- and child- interviewed Kiddie Schedule for Affective Disorders Present and Lifetime Version (K-SADS-PL)(Kaufman et al., 1997) and rated child’s functioning via the child global assessment scale (CGAS) (Schaffer et al., 1983) from 1 to 100 (high scores indicate better functioning). Given the measure of overcontrol and ADHD symptoms were only parent-reported, to be consistent in informant ratings across study variables in regression analyses, only parent-report was used in the current study.
2.4. Socioeconomic status.
The Area Deprivation Index (ADI) (Kind & Buckingham, 2018) was used to calculate each child’s national socioeconomic percentile, based on home address. The ADI is a composite measure of socioeconomic status, with higher scores indicating lower socioeconomic status.
2.5. Neuroimaging Error task
Children performed a modified Posner cueing paradigm designed to measure involuntary capture of attention while undergoing fMRI. Each trial began with fixation for 1000ms. Next, one of four cue types was presented for 150ms, 6 degrees to the left or right side (randomly) of fixation. Cues types were a square box, an angry face, a neutral face, or angry and neutral faces. Following a random delay of 50, 350, or 650ms, a target arrow appeared for 1000ms randomly in the left or right screen location. Participants’ task was to indicate whether the arrow was pointing up or down by pressing a button box (Figure S1). Errors were indexed via inaccurate identification of the arrow’s direction. Trials were blocked by cue type, with 5 trials per block. Blocks of 5 trials were separated by fixation periods: for the first 15 participants, blocks were separated by 9, 12, or 15 frames; the subsequent 46 participants blocks were separated by 27, 30, or 33 frames to improve modeling of the baseline. Statistics were unaffected when including time between blocks as a covariate. Four task fMRI runs were obtained, each with 40 trials (160 total trials). A single run was discarded in 3 subjects (2 participants ended early, 1 computer error). Performance on the task was designed to be well above chance; thus, we collapsed errors across cue types.
2.6. Imaging protocol, preprocessing and processing
We utilized a Siemens PRISMA 3T MRI scanner with 32-channel head coil, collecting a T1 (sagittal, 208 slices, 0.8 mm isotropic resolution, TE=2.22ms, TR=2400ms, TI= 1000ms, flip angle=8 degrees) and a T2-weighted structural image (sagittal, 208 slices, 0.8mm isotropic resolution, TE=563ms, TR=3200ms). For functional sequences, we used a blood-oxygen-level dependent (BOLD) multi-band echo-planar sequence (TR=720ms, TE=33ms, flip angle=52 degrees, 2.4mm isotropic resolution, multi-band factor=7). Two spin-echo field maps were obtained (one AP and one PA) during the scanning session with identical parameters. Framewise Integrated Real-time MRI monitoring (FIRMM) monitored motion while scanning was used (Dosenbach, Koller, Earl, & al., 2017).
Preprocessing of BOLD data is described in the Supplement. Freesurfer version 5.0.0 was used to create individual subject surfaces, which were aligned to the common ‘fs_LR32’ surface-space (Van Essen et al., 2012). BOLD preprocessed fMRI volumetric timeseries were sampled to each subject’s surfaces using Connectome Workbench 1.2.3. Timecourses for surface data were smoothed with geodesic 2D Gaussian kernels (σ = 2.55 mm). We focused on cortical structures for these analyses.
Pre-processed, vertexwise BOLD data were subjected to a general linear model (GLM) using in-house software (http://www.nil.wustl.edu/~fidl). BOLD frames with framewise displacement (FD) of >0.9mm were excluded from modeling to reduce motion artifact (Siegel et al., 2014). Functional task runs with <150 frames after censoring were excluded.
Data were modeled as a function of run baseline, run linear trend, and task effects. Task effects were coded as assumed BOLD responses in which the standard hemodynamic response was convolved with trial length. Six task regressors were included: correct responses for each cue type (box, angry face, neutral face, angry and neutral face simultaneously), error responses regardless of cue type, and trials in which subjects failed to respond. One sample t-tests examined significant activity at each vertex for correct trials (collapsed across cue type), error trials, and error minus correct (error>correct) trials. We next constructed regions-of-interest (ROIs) by identifying clusters of vertices with at least 50mm2 of surface area in which each vertex was at least ∣z∣>2.57, p≤.01 for the comparison (error>correct). Finally, we computed activity for correct, error, and error>correct activity in each identified ROI.
2.7. Data Analysis Plan
First, we determined demographic differences related to overcontrol to include as covariates. We then used partial correlations, controlling for significant demographic variables, to test associations between overcontrol and clinical symptoms and functioning. To test incremental validity of anxiety, depressive and ADHD symptoms with overcontrol, we utilized three hierarchical linear regressions predicting overcontrol total and subscale scores, including demographic covariates in the first step of the model and then entering symptoms of anxiety, depression and ADHD simultaneously in the second step. Then, independent samples t-tests examined diagnostic differences on overcontrol for anxiety and depression (ADHD was excluded, due to only having 3 participants reporting clinically significant symptom levels).
We performed correlations between behavioral error and correct responses from the scanner task with overcontrol and anxiety. Overcontrol and anxiety were then correlated with regional brain response to error trials, correct trials, and error minus correct trials in regions showing significant error minus correct response. To test independent relationships of overcontrol and brain activation, we ran three hierarchical linear regressions controlling for demographic covariates in Step 1 and including overcontrol and anxiety symptoms in Step 2. To calculate effect sizes for regressions, we entered all variables in one model and all variables except the variable of interest (i.e., OCYC) in a second model to utilize R2 to convert to Cohen’s f2 (.02, .15, and .35 represent small, medium and large effect sizes; Selya, Rose, Dierker, Hedeker & Mermelstein 2012). Analyses were completed in SPSS® v.26 (IBM Corporation, Armonk NY, USA) for Apple Mac®.
3. Results
3.1. Overcontrol Associations with demographics
Younger children exhibited higher total (r=−.30, p=.01), inflexibility/frustration with change (r=−.26, p=.03) and social concern/perfectionism (r=−.29, p=.02) OCYC scores. The OCYC demonstrated no significant associations with sex, race (Caucasian vs. Minority), or SES (p’s>.05; see Table 1). All subsequent analyses controlled for age.
Table 1.
Participant demographic and clinical information
| Entire sample (n=69) |
Anxiety disorder (n=35) |
No Anxiety Disorder (n=34) |
|
|---|---|---|---|
| Age (Mean(SD)) | 10.23(1.3) | 10.16(1.31) | 10.31(1.31) |
| Sex (% Female) | 36 (52%) | 15 (44%) | 21(60%) |
| Race (%) | |||
| Caucasian | 56 (81%) | 29 (82%) | 27 (79%) |
| Black | 5 (7%) | 3 (9%) | 2(6%) |
| Mixed race | 7 (10%) | 3 (9%) | 4 (12%) |
| Asian | 1 (1%) | 0 (0%) | 1 (3%) |
| Area Deprivation Index (Mean(SD)) | 32.79(19.64) | 34.67(18.30) | 31.76(21.57) |
| Clinical Symptoms (Mean(SD)) | |||
| PARS-Severity | 16.7(8.67) | 24.03(3.90) | 9.15(4.84)** |
| SCARED-P | 19.65(16.81) | 30.11(16.68) | 8.88(7.73)** |
| CDI-P | 8.76(6.43) | 12.03(7.18) | 5.59(3.42)** |
| Connors- Inattention | 2.75(2.80) | 3.39(3.27) | 2.12(2.11) |
| Connors- Hyperactivity | 2.18(2.63) | 2.46(2.98) | 1.91(2.26) |
| Child GAS Functioning | 65.94(19.05) | 49.80(5.58) | 83.06(11.83)** |
| Comorbid Diagnoses (%) | |||
| Depression Diagnosis | 10 (14%) | 7 (20%) | 3 (8%) |
| ADHD Diagnosis | 3 (4%) | 2 (6%) | 1 (3%) |
| OCYC (Mean(SD)) | |||
| Total | 6.69(5.14) | 9.12(4.60) | 4.18(4.45)** |
| Inflexibility/Frustration with change | 3.19(3.00) | 4.51(2.93) | 4.51(2.93)** |
| Social concern/perfectionism | 3.50(2.60) | 2.35(2.41) | 2.35(2.41)** |
Note: Area Deprivation Index= measure of socioeconomic status national percentile; PARS= Pediatric Anxiety Rating Scale; SCARED-P= Screen for Child Anxiety Related Emotional Disorders, parent report; CDI-P= Child Depression Inventory, parent report; GAS= Global Assessment Scale; OCYC= Overcontrol in Youth Checklist
p<.01 for comparison on youth with versus without anxiety disorders.
3.2. Overcontrol Associations with Clinical Presentations
Partial correlations (controlling for age) between OCYC total and subscales with psychiatric symptoms are displayed in Table 2. The OCYC was associated with anxiety, depression and ADHD inattention symptoms. Linear regressions controlling for age and simultaneously including all symptom domains demonstrated that only anxiety symptoms were related to OCYC total (F(4,61)=21.19, ΔR2=.53, p<.001; B(SE)=18(.04)p<.001;f2=.44), inflexibility/frustration with change (F(4,61)=15,64, ΔR2=.47, p<.001; B(SE)=.10(.02)p<.001; f2=.31), and social concern/perfectionism (F(4,61)=14.47, ΔR2=.49, p<.00; B(SE)=.08(.02) p<.001; f2=.29). Additionally, a diagnosis of anxiety disorder was associated with significantly higher total (t(67)= −4.53, p<.001; M=9.13, SD=4.60), inflexibility/frustration with change (t(67)= −4.15, p<.001; M=4.51, SD=1.82) and social concern/perfectionism (t(67)= −3.99, p<.001; M=4.61, SD=2.35) compared with no anxiety disorder (total: M=4.17, SD=4.45; inflexibility/frustration: M=1.82, SD=2.42; social concern/perfectionism: M=2.35, SD=2.41). No differences emerged for a diagnosis of depression versus no depression (p’s>.05).
Table 2.
Partial correlations controlling for age of OCYC and Parent-Reported Clinical Variables (n=69).
| OCYC | |||
|---|---|---|---|
| Total | Inflexibility/Frustration with Change |
Social concern/ Perfectionism |
|
| Anxiety | |||
| PARS- Severity† | .63** | .56** | .58** |
| SCARED-P | .75** | 69** | .67** |
| Depression | |||
| CDI-P | .63** | .58** | .56** |
| ADHD | |||
| Conners Inattention | .38* | .26* | .44* |
| Conners Hyperactivity & Impulsivity | .20 | .13 | .25* |
| Functioning | |||
| Child GAS‡ | −.57** | −.51** | −.53** |
Note:
PARS-Summary incorporates parent and child report
Child GAS is interview rated; OCYC= Overcontrol in Youth Checklist; PARS= Pediatric Anxiety Rating Scale; SCARED-P= Screen for Child Anxiety Related Emotional Disorders, parent report; CDI= Child Depression Inventory, parent report; GAS= Global Assessment Scale.
p<.05
p<.001.
3.3. Identification of brain regions associated with error responding
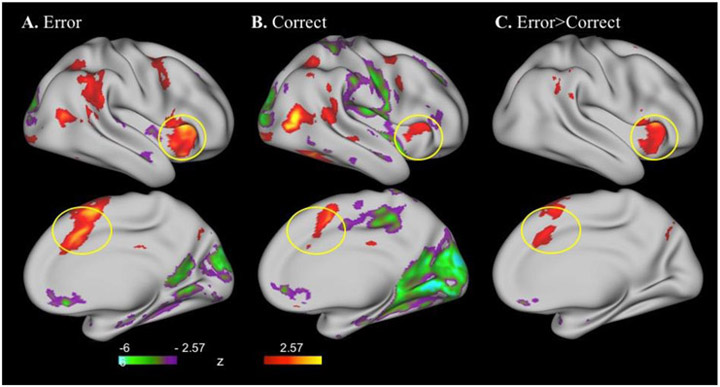
Activity change in responding to error trials, correct trials and the difference between error and correct is depicted in Figure 1. Paired t-tests that survived thresholding revealed 8 ROIs demonstrating differential activation to error>correct. The bilateral dACC, bilateral insula, right medial PFC and dorsal superior pre-motor cortex exhibited higher activation while the bilateral subgenual cingulate cortex demonstrated lower activation to errors compared to correct responses.
Figure 1.
Activity changes across cortex following error trials (A), correct trials (B), and the difference between error and correct trials (C) thresholded (2.57; p< .05). Circled regions include anterior insula (top panel) and dorsal anterior cingulate cortex (dACC) (bottom panel)
Overcontrol and anxiety associations with brain activity in response to errors
The number of behavioral errors (M=12.28, SD=7.29) and correct responses (M=126.45, SD=23.87) were not associated with OCYC (r’s=−.10, .18, −.02, p’s>.05, total, inflexibility/frustration, social concern/perfectionism, respectively) or anxiety (r’s=.02, −.03, p ’s>.05; SCARED, PARS-S, respectively), demographic variables, and did not differ by cue type (all p’s>.05). Correlating OCYC and anxiety with activity in the identified ROIs, four regions demonstrated significant associations with overcontrol: higher OCYC total and both subscale scores were associated with less differential activity for errors>correct responses in the bilateral insula and bilateral dACC (see Table 3 and Figure S2). In additon, the inflexibility/frustration subscale was associated with less left subgenual cingulate error>correct activity. Partial correlations controlling for age demonstrated parallel findings and findings were driven by responses to errors rather than responses to correct trials (Table S1 and Figure S3). No associations between activation in these ROIs and anxiety emerged (Table 3).
Table 3.
Pearson correlations of neural activity with OCYC (n=29).
| Overcontrol | Anxiety | |||||||
|---|---|---|---|---|---|---|---|---|
| Brain Regions With Differential Activity for Error > Correct Trials |
Talairach coordina |
OCYC Total |
OCYC Inflexibility/ Frustration with change |
OCYC Social concern/ Perfectionism |
SCARED- Total |
PARS- Severity |
||
| x | y | z | ||||||
| Left dACC | −8 | 18 | 37 | −.42* | −.42* | −.35 | −.25 | −.23 |
| Right dACC | 9 | 20 | 35 | −.44* | −.38* | −.43* | −.21 | −.14 |
| Left insula | −37 | 19 | −4 | −.44* | −.39* | −.41* | −.20 | −.11 |
| Right insula | 41 | 20 | −1 | −.43* | −.34 | −.45* | −.18 | −.11 |
| Right medial PFC | 11 | 14 | 59 | −.32 | −.24 | −.36 | −.11 | −.17 |
| Dorsal superior pre-Motor | −8 | 4 | 66 | −.26 | −.20 | −.29 | −.16 | −.27 |
| Left subgenual cingulate | −8 | 40 | −11 | −.35 | −.39* | −.25 | −.22 | −.21 |
| Right subgenual cingulate | 6 | 43 | −16 | −.11 | −.12 | −.08 | .02 | −.07 |
Note: OCYC= Overcontrol in Youth Checklist; PARS= Pediatric Anxiety Rating Scale; SCARED-P= Screen for Child Anxiety Related Emotional Disorders, parent report; dACC= dorsal anterior cingulate cortex; PFC= prefrontal cortex.
p<.05.
To determine independent associations of OCYC and anxiety scores with neural activation to errors>correct, four hierarchical linear regressions predicting bilateral dACC and insula BOLD activity were conducted. Child age was entered into the first step and OCYC Total and PARS Severity were entered into the second step of the model. Above age and anxiety, OCYC total scores continued to relate to a reduced difference in activity for error versus correct trials in the right dACC and bilateral insula (Table 4; Cohens f2=.20, .30, .38, and .26 for left and right dACC and left and right insula, respectively). When number of behavioral errors was added to regressions as a covariate, significant associations remained.
Table 4.
Hierarchical regressions of OCYC scores and PARS Anxiety Severity scores predicting brain activation to the difference between responses to errors and correct (n=29).
| dACC | Insula | |||||||
|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | |||||
| ΔR2 | B(SE) | ΔR2 | B(SE) | ΔR2 | B(SE) | ΔR2 | B(SE) | |
| Step 1 | .01 | .01 | .03 | .002 | ||||
| Age | −.03(.08) | −.03(.07) | −.05(.06) | .01(.06) | ||||
| Step 2 | .21 | .25* | .28* | .22* | ||||
| OCYC Total | −.05(.02)* | −.06(.02)* | −.06(.02)** | −.05(.02)* | ||||
| PARS Severity | .002(.01) | .01(.01) | .01(.01) | .01(.01) | ||||
Note: dACC= dorsal anterior cingulate cortex; PFC= prefrontal cortex; OCYC= Overcontrol in Youth Checklist Total Score; PARS= Pediatric Anxiety Rating Scale.
p<.05
p<.01.
4. Discussion
Overcontrol is a constellation of characteristics that emerges early in development and is thought to underlie multiple psychiatric disorders, including pediatric anxiety. Findings demonstrate that overcontrolled characteristics are associated with clinical pediatric anxiety in children aged 8 to 12. Moreover, above and beyond the relationship to anxiety, overcontrol is associated with reduced BOLD activity change in the bilateral dACC and insula following commission of an error.
Results first replicate and extend past work demonstrating that overcontrol is associated with social anxiety, broad internalizing symptoms and worse functioning in youth (Eisenberg et al., 2010; Gilbert et al., 2019; Henderson et al., 2015). Overcontrol total and subscale scores were associated with anxiety, depressive and inattention symptoms, as well as worse global functioning. However, when including all psychiatric symptoms in one model, only associations with anxiety remained significant demonstrating large effect sizes. Findings suggest that overcontrol is a dimensional feature present in childhood anxiety and associations with other symptom domains may be due to underlying anxiety.
Overcontrol also demonstrated significant associations with decreased activity in the dACC and insula in error versus correct responding, indicating medium to large effect sizes. Although involved in multiple cognitive control tasks (Shenhav, Botvinick, & Cohen, 2013), the dACC is consistently implicated in error, conflict and behavioral monitoring and behavioral adaptations following negative feedback (i.e, errors) (Heilbronner & Hayden, 2016; Shane & Weywadt, 2014; Weiss et al., 2018). The insula is involved in detecting salience of internal and external cues and has repeatedly been associated with error responding (Menon & Uddin, 2010; Neta et al., 2015). Moreover, the dACC and insula are both part of the cingulo-opercular network, a group of brain regions implicated in adaptive cognitive control, conflict detection and error responding (Dosenbach et al., 2008).
The current results suggest that the underlying dimension of overcontrol may account for altered neural response to errors noted in pediatric anxiety disorders. While adult anxiety is consistently linked to increased activity in the dACC and insula following commission of errors (Norman et al., 2018; Riesel et al., 2019), the emerging literature in pediatric anxiety is mixed. No study has specifically reported altered error responses in the dACC or insula in pediatric anxiety disorders, but instead altered error responses have been noted in the dlPFC (Fitzgerald et al., 2013), pgACC (Smith, Kircanski, et al., 2019), and vmPFC (Smith, White, et al., 2019). Consistent with these prior results, we likewise did not detect anxiety relationships with error-related activity in brain regions that most strongly respond to errors (dACC, insula). Instead, altered error responses in the dACC and insula were associated with overcontrol, which in turn had a strong independent relation to anxiety severity.
Taken together, results are consistent with a model in which pediatric anxiety is not associated with altered neural error responses per se; rather, dimensional overcontrol is associated with altered error response, and overcontrol appears to be an underlying component of pediatric anxiety. Overcontrol likely interacts with other dimensions such as altered attention, impaired fear learning, and reduced executive control to result in symptoms of anxiety (Sylvester et al., 2012). Each of these underlying dimensions is likely to have its own neurobiology, and uncovering the neurobiology of these multiple interacting constructs may prove more fruitful than attempting to delineate the neurobiology of anxiety disorders as a whole. A long-term goal of the Research Domain Criteria (RDoC) framework is to arrive at more person-centered evaluations and treatments by assessing and treating underlying domains (Insel, 2014). Our results suggest overcontrol may be one dimension necessary for intervention in pediatric anxiety.
Findings from the current study should be considered within the context of limitations. First, the sample size was small, especially examining neuroimaging associations with overcontrol, and as such, findings should be considered preliminary. Second, the fMRI task was not explicitly designed to elicit errors and therefore we collapsed across behavioral cue conditions. While we were able to isolate neural activity in error versus correct responding in regions implicated in error processing, more direct tasks should be used. Third, overcontrol was inversely associated with age and our cross-sectional design did not allow us to assess any moderating effects age might have had on findings (e.g., Smith, White, et al., 2019). It will be important for future work to longitudinally assess the development of overcontrolled tendencies across childhood and adolescence, especially in relation to trajectories of multiple psychiatric disorders.
5. Conclusion
The current findings highlight a dimensional constellation of characteristics that underlies childhood anxiety disorders and appears to be a neural correlate of error responding. Importantly, although overcontrol was associated with clinical anxiety, it was also independently associated with aberrant error responding in the dACC and insula, while anxiety symptoms were not. Overcontrol could be a transdiagnostic intermediate phenotype that leads to trajectories of multiple psychiatric disorders across development, starting with pediatric anxiety. It may be an important mechanism that could be targeted for early intervention to influence neural and symptom change early in development.
Supplementary Material
Highlights.
Pediatric anxiety disorders are characterized by overcontrol
Overcontrol is a phenotype of inflexibility, perfectionism, and error monitoring
Above anxiety, overcontrol was associated with reduced neural response to errors
Overcontrol may be an underlying mechanism associated with pediatric anxiety
Acknowledgements
The authors would like to thank dedicated staff, Jennifer Harper, Qiongru Yu, Kathy Pope, Beth Brunsworth, William Baumel, Shana Santos and Riley Groves, who made this project possible and the parent and child participants for their willingness and help with this study. This work was supported by the National Institute of Mental Health [K.G.: K23MH115074, M.T.P.: T32MH100019, C.M.S: K23MH109983), the Brain and Behavior Research Foundation [C.M.S], the Taylor Family Institute [C.M.S], the McDonnell Foundation [C.M.S], and the Parker Fund [C.M.S]. Funding sources had no involvement in the study design, collection, analysis. interpretation of data, writing of the report, or decision to submit for publication. All authors report no conflicts of interest.
Footnotes
Declaration of Interest: None.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, & Baugher M (1999). Psychometric properties of the screen for child anxiety related emotional disorders (SCARED): A replication study. Journal of the American Academy of Child & Adolescent Psychiatry, 38, 1230–1236. [DOI] [PubMed] [Google Scholar]
- Brooker RJ, Kiel EJ, & Buss KA (2016). Early social fear predicts kindergarteners' socially anxious behaviors: Direct associations, moderation by inhibitory control, and differences from nonsocial fear. Emotion, 16(7), 997–1010. doi: 10.1037/emo0000135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK (2008). Conners 3™ - Parent Short Form. North Tonawanda, NY: Multi-Health Systems Inc. [Google Scholar]
- Donnellan BM, & Robins RW (2010). Resilient, overcontrolled and undercontrolled personality types: Issues and controversies. Social and Personality Psychology Compass, 3, 1–14. [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, & Petersen SE (2008). A dual-networks architecture of top-down control. Trends in Cognitive Sciences, 72(3), 99–105. doi: 10.1016/j.tics.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Koller JM, Earl EA, & al., e. (2017). Real-time motion analytics during brain MRI improve data quality and reduce costs. NeuroImage, 161, 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Spinrad TL, & Eggum ND (2010). Emotion-related self-regulation and its relation to children's maladjustment. Annual Review of Clinical Psychology, 6, 495–525. doi: 10.1146/annurev.clinpsy.121208.131208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Spinrad TL, Fabes RA, Reiser M, Cumberland A, Shepard SA, … Thompson M (2004). The relations of effortful control and impulsivity to children's resiliency and adjustment. Child Development, 75(1), 25–46. doi: 10.1111/j.1467-8624.2004.00652.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Spinrad TL, & Morris AS (2002). Regulation, resiliency, and quality of social functioning. Self and Identity, 1, 121–128. doi: 10.1080/152988602317319294 [DOI] [Google Scholar]
- Fitzgerald KD, Liu Y, Stern ER, Welsh RC, Hanna GL, Monk CS, … Taylor SF (2013). Reduced error-related activation of dorsolateral prefrontal cortex across pediatric anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 52(11), 1183–1191.e1181. doi: 10.1016/j.jaac.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KD, & Taylor SF (2015). Error-processing abnormalities in pediatric anxiety and obsessive compulsive disorders. CNS Spectrums, 20(04), 346–354. doi: 10.1017/s1092852915000036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, & Stein EA (2002). Dissociable executive functions in the dynamic control of behavior: Inhibition, error detection, and correction. NeuroImage, 17, 1820–1829. [DOI] [PubMed] [Google Scholar]
- Gilbert KE, Barch DM, & Luby J (2019). The Overcontrol in Youth Checklist: Validation of a behavioral meausre of overcontrol in preschool aged children Child Psychiatry and Human Development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert KE, Barclay ME, Tillman R, Barch DM, & Luby JL (2018). Associations of observed performance monitoring during preschool with obsessive-compulsive disorder and anterior cingulate cortex volume over 12 yeras. JAMA Psychiatry, 75(9), 940–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronner SR, & Hayden BY (2016). Dorsal Anterior Cingulate Cortex: A Bottom-Up View. Annu Rev Neurosci, 39, 149–170. doi: 10.1146/annurev-neuro-070815-013952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson HA, Pine DS, & Fox NA (2015). Behavioral inhibition and developmental risk: a dual-processing perspective. Neuropsychopharmacology, 40(1), 207–224. doi: 10.1038/npp.2014.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR (2014). The NIMH Research Domain Criteria (RDoC) Project: Precision Medicine for Psychiatry. American Journal of Psychiatry, 171(4), 395–397. doi: 10.1176/appi.ajp.2014.14020138 [DOI] [PubMed] [Google Scholar]
- Isaacs E (2001). Fluvoxamine for the treatment of anxiety disorders in children and adolescents. New England Journal of Medicine, 345, 466–467. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao UMA, Flynn C, Moreci P, … Ryan N (1997). Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry, 36(7), 980–988. doi: 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Kaye WH, Wierenga CE, Bailer UF, Simmons AN, & Bischoff-Grethe A (2013). Nothing tastes as good as skinny feels: the neurobiology of anorexia nervosa. Trends in Neuroscience, 36(2), 110–120. doi: 10.1016/j.tins.2013.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kind AJH, & Buckingham WR (2018). Making Neighborhood-Disadvantage Metrics Accessible — The Neighborhood Atlas. New England Journal of Medicine, 378(26), 2456–2458. doi: 10.1056/NEJMp1802313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M (1985). The Children's Depression Inventory (CDI). Psychopharmacology Bulletin, 21, 995–1124. [PubMed] [Google Scholar]
- Ladouceur CD, Tan PZ, Sharma V, Bylsma LM, Silk JS, Siegle GJ, … Ryan ND (2018). Error-related brain activity in pediatric anxiety disorders remains elevated following individual therapy: a randomized clinical trial. Journal of Child Psychology and Psychiatry, 59( 11), 1152–1161. doi: 10.1111/jcpp.12900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch TR, Hempel RJ, Whalley B, Byford S, Chambra R, Clarke P, … Russell IT (2018). Radically open dialectical behaviour therapy for refractory depression: the RefraMED RCT. Efficacy Mechanism Evaluation, 5(7). [PubMed] [Google Scholar]
- Menon V, & Uddin LQ (2010). Saliency, switching, attention and control: a network model of insula function. Brain structure & function, 214(5-6), 655–667. doi: 10.1007/s00429-010-0262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, & Hajcak G (2019). A review examining the relationship between individual differences in the error-related negativity and cognitive control. International Journal of Psychophysiology, 144, 7–13. [DOI] [PubMed] [Google Scholar]
- Meyer A, Hajcak G, Torpey DC, Kujawa A, Kim J, Bufferd S, … Klein DN (2013). Increased Error-Related Brain Activity in Six-Year-Old Children with Clinical Anxiety. Journal of Abnormal Child Psychology, 41(8), 1257–1266. doi: 10.1007/s10802-013-9762-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Hajcak G, Torpey-Newman DC, Kujawa A, & Klein DN (2015). Enhanced error-related brain activity in children predicts the onset of anxiety disorders between the ages of 6 and 9. J Abnorm Psychol, 124(2), 266–274. doi: 10.1037/abn0000044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Weinberg A, Klein DN, & Hajcak G (2012). The development of the error-related negativity (ERN) and its relationship with anxiety: evidence from 8 to 13 year-olds. Dev Cogn Neurosci, 2(1), 152–161. doi: 10.1016/j.dcn.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta M, Miezin FM, Nelson SM, Dubis JW, Dosenbach NUF, Schlaggar BL, & Petersen SE (2015). Spatial and Temporal Characteristics of Error-Related Activity in the Human Brain. The Journal of Neuroscience, 35(1), 253. doi: 10.1523/JNEUROSCI.1313-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman LJ, Taylor ST, Liu Y, Radua J, Chye Y, De Wit SJ, … Fitzgerald KD (2018). Error Processing and Inhibitory Control in Obsessive-Compulsive Disorder: A Meta-analysis Using Statistical Parametric Maps. Biological Psychiatry, 85, 713–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton PJ, & Roberge P (2017). Transdiagnostic Therapy. Psychiatric Clinics of North America, 40(4), 675–687. [DOI] [PubMed] [Google Scholar]
- Park JM, Storch EA, Pinto A, & Lewin AB (2016). Obsessive-compulsive personality traits in youth with obsessive-compulsive disorder. Child Psychiatry and Human Development, 47, 281–290. doi: 10.1007/s10578-015-0565-8 [DOI] [PubMed] [Google Scholar]
- Pearl SB, & Norton PJ (2017). Transdiagnostic versus diagnosis specific cognitive behavioural therapies for anxiety: A meta-analysis. Journal of Anxiety Disorders, 46, 11–24. [DOI] [PubMed] [Google Scholar]
- Pinto A, Greene AL, Storch EA, & Simpson HB (2015). Prevalence of childhood obsessive-compulsive personality traits in adults with obsessive compulsive disorder versus obsessive compulsive personality disorder. J Obsessive Compuls Relat Disord, 4, 25–29. doi: 10.1016/j.jocrd.2014.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Research Units on Pediatric Psychopaharmacology Anxiety Study Group. (2002). The Pediatric Anxiety Rating Scale (PARS): Development and Psychometric Properties. Journal of the American Academy of Child & Adolescent Psychiatry, 41(9), 1061–1069. [DOI] [PubMed] [Google Scholar]
- Riesel A, Klawohn J, Grützmann R, Kaufmann C, Heinzel S, Bey K, … Kathmann N (2019). Error-related brain activity as a transdiagnostic endophenotype for obsessive-compulsive disorder, anxiety and substance use disorder. Psychological Medicine, 49(7), 1207–1217. doi: 10.1017/S0033291719000199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins RW, John OP, Caspi A, Moffitt TE, & Stouthamer-Loeber M (1996). Resilient, overcontrolled, and undercontrolled boys: Three replicable personality types. Journal of Personality and Social Psychology, 70(1), 157–171. doi: 10.1037/0022-3514.70.1.157 [DOI] [PubMed] [Google Scholar]
- Schaffer D, Gould MS, Brasic J, Abrosini P, Fisher P, Bird H, & Aluwahalia S (1983). A children's global assessment scale (CGAS). Archives of General Psychiatry, 40, 1228–1231. doi:0.1001/archpsyc.1983.01790100074010 [DOI] [PubMed] [Google Scholar]
- Selya AS, Rose JS, Dierker LC, Hedeker D, & Mermelstein RJ (2012). A Practical Guide to Calculating Cohen's f(2), a Measure of Local Effect Size, from PROC MIXED. Frontiers in Psychology, 3, 111–111. doi: 10.3389/fpsyg.2012.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane MS, & Weywadt CR (2014). Voluntary Modulation of Anterior Cingulate Response to Negative Feedback. PLoS ONE, 9(11), e107322. doi: 10.1371/journal.pone.0107322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Botvinick, Matthew M, & Cohen, Jonathan D (2013). The Expected Value of Control: An Integrative Theory of Anterior Cingulate Cortex Function. Neuron, 79(2), 217–240. doi: 10.1016/j.neuron.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JS, Power JD, Dubis JW, Vogel AC, Church JA, Schlaggar BL, & Petersen SE (2014). Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Human brain mapping, 35(5), 1981–1996. doi: 10.1002/hbm.22307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AR, Kircanski K, Brotman MA, Do QB, Subar AR, Silk JS, … Pine DS (2019). Advancing clinical neuroscience through enhanced tools: Pediatric social anxiety as an example. Depression and Anxiety, 36(8), 701–711. doi: 10.1002/da.22937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AR, White LK, Leibenluft E, McGlade AL, Heckelman AC, Haller SP, … Pine DS (2019). The Heterogeneity of Anxious Phenotypes: Neural Responses to Errors in Treatment-Seeking Anxious and Behaviorally Inhibited Youths. Journal of the American Academy of Child & Adolescent Psychiatry. doi: 10.1016/j.jaac.2019.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester CM, Corbetta M, Raichle ME, Rodebaugh TL, Schlaggar BL, Sheline YI, … Lenze EJ (2012). Functional network dysfunction in anxiety and anxiety disorders. Trends in Neurosciences, 35(9), 527–535. doi: 10.1016/j.tins.2012.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpey DC, Hajcak G, Kim J, Kujawa AJ, Dyson MW, Olino TM, & Klein DN (2013). Error-related brain activity in young children: associations with parental anxiety and child temperamental negative emotionality. J Child Psychol Psychiatry, 54(8), 854–862. doi: 10.1111/jcpp.12041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, & von Cramon DY (2001). Subprocesses of performance monitoring: A dissociation of error processing and response competition revealed by event related fMRI and ERPs. NeuroImage, 14, 1387–1401. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Ugurbil K, Auerbach E, Barch D, Behrens TEJ, Bucholz R, … Yacoub E (2012). The Human Connectome Project: A data acquisition perspective. NeuroImage, 62(4), 2222–2231. doi: 10.1016/j.neuroimage.2012.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderbleeck E, & Gilbert KE (2018). Too much versus too little control: The etiology, conceptualizaiton, and treatment implications of overcontrol and undercontrol. the Behavior Therapist, 41(3), 125–131. [Google Scholar]
- Weiss AR, Gillies MJ, Philiastides MG, Apps MA, Whittington MA, FitzGerald JJ, … Green AL (2018). Dorsal Anterior Cingulate Cortices Differentially Lateralize Prediction Errors and Outcome Valence in a Decision-Making Task. Frontiers in Human Neuroscience, 12(203). doi: 10.3389/fnhum.2018.00203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LK, McDermott JM, Degnan KA, Henderson HA, & Fox NA (2011). Behavioral inhibition and anxiety: the moderating roles of inhibitory control and attention shifting. Journal of Abnormal Child Psychology, 39(5), 735–747. doi: 10.1007/s10802-011-9490-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.