Abstract
Introduction
As of March 11th, 2020, the World Health Organization declared the COVID-19 outbreak a pandemic. Articles published after the SARS-CoV-1 (2002) epidemic suggest that the use of an herbal-drug integrative medical approach could have contributed to a lower fatality rate and a more rapid response in controlling the outbreak.
Methods
Pubmed was searched for articles that investigated the antiviral properties and mechanisms of action of herbs or natural compounds against the SARS-coronavirus (CoV).
Results
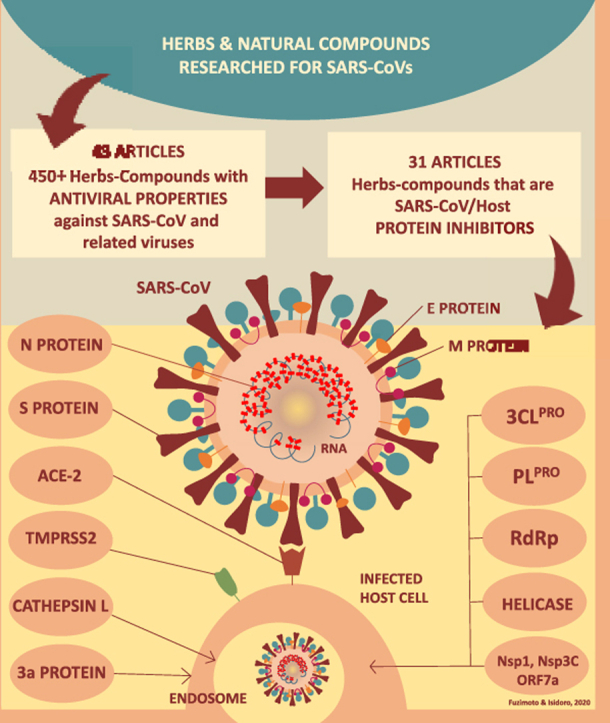
Forty-three (43) relevant papers were located. A general count rendered 450+ herbs and natural compounds with antiviral properties against the SARS-CoV and related viruses. From the 43 articles, thirty-one (31) uncovered the mechanisms of action of the natural substances able to oppose the coronavirus.
Discussion
A series of herbs and natural compounds demonstrated moderate to strong antiviral activity. Research on many herbs-natural compounds also showed potent and significant inhibition of CoV-host protein pathways responsible for different phases of viral replication specifically targeting 3CLPRO, PLPRO, RdRp, helicase protein, S protein, N protein, 3a protein, Cathepsin L, Nsp1, Nsp3c, and ORF7a, and the S protein/ACE-2 interaction.
Conclusion
The herbs-natural compounds with antiviral activity and that caused inhibition/blockade of the CoV-host protein pathways are potential therapeutic candidates. The homology between the SARS-CoV-1 and SARS-CoV-2 is around 80%. Thus, effective herbs-compounds for the former would likely be beneficial for the latter also depending on target protein similarities between the viruses. Here we provide the mechanistic bases supporting an integrative approach that includes natural compounds to fight coronavirus infections.
Keywords: Coronavirus, COVID-19, Herbs, Natural compounds, Antiviral, Pandemic
Graphical abstract
Highlights
-
•
We researched herbs-natural compounds with properties against the SARS-coronavirus (CoV).
-
•
Approximately 450+ herbs-natural compounds showed strong-moderate antiviral properties.
-
•
Many herbs potently-significantly inhibited-blocked SARS-CoVs-host protein pathways.
-
•
These herbs-compounds are potential therapeutic candidates to address the SARS-CoVs.
-
•
The drug-herbal integrative medical approach could support the conventional medical system.
Abbreviations
- ACE-2
angiotensin converting-enzyme-2
- 3CLPRO
3-chymotrysin-like protease
- COVID-19
corona Virus Disease-2019
- CPE assay
cytopathogenic effect assay
- CTSL
cathepsin L
- DENV
dengue virus
- HCoV
human coronavirus
- HRV-3C Protease
human rhinovirus 3C protease
- IBV
infectious bronchitis virus
- IFN
interferon
- IV
intravenous
- IVIG
intravenous immunoglobulin
- MERS-CoV
Middle East Respiratory Syndrome-coronavirus
- MHV
mouse hepatitis virus
- PLPRO
papain-like protease
- N protein
nucleocapsid protein
- Nsps
non-structural proteins
- RdRp
RNA-dependent RNA polymerase
- SARS-CoV
Severe Acute Respiratory Syndrome-coronavirus
- S protein
spike protein
- TCM
Traditional Chinese Medicine
- TMPRSS2
transmembrane protease serine type 2
1. Introduction
As of March 11th, 2020, the World Health Organization (WHO) officially declared the coronavirus (CoV) outbreak a pandemic and the disease was designated Corona Virus Disease-2019 (COVID-19).1 The virus that causes the disease was formally named Severe Acute Respiratory Syndrome (SARS)-CoV-2 because of the genome homology with the SARS-CoV-1 (approx. 80%) that originated a similar syndromic disease during the epidemic in 2002–2003.1, 2, 3 At the time of this manuscript submission, the COVID-19 has reached 115 countries/territories and has caused 1,844,863 confirmed cases and 117,021 fatalities (updated as per April 14th, 2020).4 The COVID-19 has already surpassed the previous outbreaks caused by the SARS-CoV-1 (2002), which infected 8422 people and caused 916 deaths worldwide, and by the MERS-CoV (2012), with a total of 1401 infections and 543 deaths.5
1.1. Conventional medications used for the coronavirus
Currently, there is not a custom-made medication for the treatment of the COVID-19. The development of a vaccine for SARS-CoV-2 is under progress, and there is a substantial effort to design a medication specific for it. Yet, it will require some time to prove the efficacy and safety of any new agents. Drugs such as ribavirin and corticosteroids have significant side-effects.6,7 The systemic use of ribavirin through intravenous or oral administration may cause dose-dependent anemia and bone marrow suppression.6 The aerosol application of ribavirin is associated with nausea, headaches, and rarely to bronchospasm.6 In a retrospective cohort study with SARS patients, the researchers verified that corticosteroid therapy was associated with a 20-fold increase in adverse effects.7 Monoclonal antibody therapies for the CoV are still under investigation. Although not yet proven to be effective for the COVID-19, broad-spectrum antiviral medications and drugs used for the treatment of retrovirus-associated diseases may be repurposed to control the actual CoV infection. The drug recommendations for the COVID-19 such as lopinavir, ritonavir, nebulized α-interferon, chloroquine or hydroxychloroquine, ribavirin, and interferon (IFN) are being made based on retrospective cohort studies, historically controlled studies, case reports and case series that resulted from the SARS-CoV-1 and MERS-CoV outbreaks.8 In a systematic review requested by the WHO (2006), the investigators examined the published literature on ribavirin, corticosteroids, lopinavir/ritonavir, type 1 interferon (IFN), intravenous immunoglobulin (IVIG), and SARS convalescent plasma from both in-vitro studies and SARS patients.9 From the 72 reviewed studies, the authors concluded that it was not possible to determine if the treatments indeed benefitted the patients during the SARS outbreak of 2002-03, and actually, some could have been harmful.9 Thus, the efficacy and safety of these drugs also need to be assessed by future clinical trials.10
1.2. Herbal medicine and the treatment of the SARS-CoV-1 infection
In 2002, the SARS mortality in mainland China was the lowest (7%) when compared with the 15%–27% of other areas such as Canada (17%), France (14%), Malaysia (40%), Philippines (14%), Thailand (22%), Hong Kong (17%), Taiwan (11%), Singapore (14%), and Vietnam (8%).11 It was suggested that the lower mortality rate in mainland China and the relatively rapid response in controlling the SARS 2002 outbreak could have been due to the inclusion of herbal formulations from Traditional Chinese Medicine (TCM) in the treatment protocols.12,13 A statistical study showed a significant low fatality rate caused by the SARS-CoV-1 in Beijing when compared with Hong Kong and Singapore.13 Many hospitals in mainland China used a combination of TCM and conventional (Western) medicine, while Hong Kong and Singapore essentially used conventional drugs throughout the research period.13 Thus, the researchers suggested that the use of an integrative approach could more effectively control the infection and reduce the number of deaths.13 The Beijing You-an Hospital, for example, reported that the death rate of severe patients was 15.4% when a drug-herbal combination was used, and it was 47.7% when only conventional medications were applied.14 Also, one study reported on the administration of a TCM herbal formula to 3160 hospital workers as a preventive measure. Within a two-week period, none of the herb consumers contracted the SARS-CoV-1, and adverse effects were infrequent and mild.15 The short period of follow-up is an obvious weakness of this study. Yet, the subject definitely deserves further investigations.
1.3. Potential benefits of herbal medicine-based treatments for the CoV infection: stand-alone herbal use, drug-herbal combination, symptomatology management, and preventive care
After the outbreak of 2002, there was a surge in research focusing on drug discovery and testing of new medications, herbs, natural and synthetic compounds for the SARS-CoV. Like the conventional drugs, a series of studies investigating the use of herbs for the SARS-CoV-1 were published in the form of case studies, controlled and uncontrolled clinical studies, systematic reviews, meta-analyses, and one randomized double-blind and placebo-controlled clinical pilot study.16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 These studies attracted the interest of researchers and encouraged more investigations on the potential of herbs-compounds for the management of the CoV infection.5,22,24,25,27 Peer-reviewed papers examined how TCM participated in an integrative treatment approach and showed that the combination of herbs with conventional medications was more effective than the use of conventional drugs alone.14,16,17,19,20,23, 24, 25,27,28 Herbs were seen to significantly lower the average daily dosage of drugs such as methylprednisolone and hydrocortisone in severe cases (p < 0.05), and to reduce the serious side effects caused by some medications.14,24 Although there was some conflicting evidence on the mortality and cure rates, in general, the studies showed that the herbs helped reduce the symptoms of the CoV such as dyspnea, non-productive cough, fatigue, and malaise.14,18,21,22,24 In one study, the mean duration of these symptoms, except for headaches and myalgias, was relatively shortened and their intensity lowered in the patients treated with herbal compounds along with conventional drugs.14 In another research, patients receiving an integrative treatment showed a likelihood of a complete or partial pulmonary infiltrate resolution, a shorter defervescence time, and higher CD4+ lymphocyte count.24 In a small clinical study, the use of TCM herbal medicine as a standalone treatment strategy showed promising results to control fever and pulmonary infiltration.18 Another paper warranted the use of TCM in the SARS-CoV clinically cured and discharged patients from the hospital that presented with sequelae such as impairment of heart, lungs, liver, hypo-immune status, and psychological problems.29 Other articles reported the preventive function of TCM herbs in strengthening the immune system to reduce the number of possible onsets of infectious cases.15,30 One recent article revealed that three out of four patients with the SARS-CoV-2 infection showed improvement with a combination of Kaletra (lopinavir/ritonavir) and arbidol associated with the Chinese medicine formula Shu Feng Jie Du capsule.31 In an animal model, this formula protected against acute lung injury through the suppression of the MAPK/NF-Kβ pathway.32 Many other herbal formulas have been proposed to prevent and treat the SARS-CoV-1 infection, and many formulations are also currently being researched and suggested for the SARS-CoV-2 (reviewed in).30,33,34 Limitations of the above-mentioned studies include a small number of participants of some investigations, the poor quality of some studies mentioned by systematic reviews, a low number of controlled clinical trials, and a lack of investigations on drug-herbal interaction. However, one of the advantages of the TCM rationale is to use diagnostic patterns of differentiation to select the remedies for treatment that can be prescribed according to the individual clinical presentation. In general, TCM doctors utilize herbal formulas for the SARS-CoV infection that are composed of herbs known to have a broad-spectrum antiviral, anti-inflammatory, immune-modulatory, and anti-toxicity effects, among other actions. Also, the comorbidities, age, constitution, and many other relevant factors during the diagnostic process are taken into account.
Collectively, these research articles offer a broader perspective on new treatment possibilities that should be explored. There is an urgent need to find therapeutic options in support of the current protocols to assist in the prevention, treatment, control of symptomatology, and decrease the severity of SARS-CoV infections. Herbs are generally consumed as teas prepared from raw herbs, water and ethanol extracts, dry extracts, pills, powder, liposomal, and other forms. Natural products can also be a source of “drugs” in the form of compounds, derivatives, and other refined substances obtained from them. With this review, we aimed to investigate the antiviral properties and mechanisms of action of herbs and natural compounds against the SARS-CoVs. We hope to inspire a fruitful cooperation among medical scientists and clinicians for developing novel and more efficacious therapeutic agents as well as treatment protocols in an integrative medical approach to fighting coronaviruses.
2. Methodological approach
Pubmed was searched for articles in English that investigated the antiviral properties of the Traditional Chinese Medicine (TCM) herbs or natural compounds against the SARS-coronavirus (CoV). The herbs refer to their unaltered and whole form while the natural compounds are active components isolated from the herbs. The articles were screened and selected for the primary experimental and/or clinical evidence of the herbs and natural compounds to effectively target the CoV infection. Particularly, we paid attention to the mechanisms of action and/or signaling pathways involved in the activity of such herbs and natural products that could support the ability to contrast the SARS-CoV infection. Keywords were used to include studies published out of the outbreak in China in 2002-03 (SARS-CoV-1), as well as any publications on the novel 2019 coronavirus (SARS-CoV-2). During the search, the Pubmed “similar articles” section was also screened, and the list of bibliographic references in each article was examined for additional relevant papers. Articles that investigated exclusively conventional drugs or synthetic substances for SARS-CoVs were not included.
Primary search parameters: (coronavirus OR “corona virus” OR SARS OR “severe acute respiratory syndrome” OR SARS-CoV OR 2019-nCov OR nCoV-2019 OR nCoV-19 OR COVID-19) AND (herb OR herbs OR “herbal medicine” OR “herbal medicines” OR “Chinese medicine” OR “medicinal herb” OR “medicinal herbs” OR “medicinal herbal extract” OR “natural compounds”). The search was done at the beginning of March of 2020 but it was last repeated on April 6 of 2020 due to the large number of papers being currently published on the coronavirus subject.
3. Results
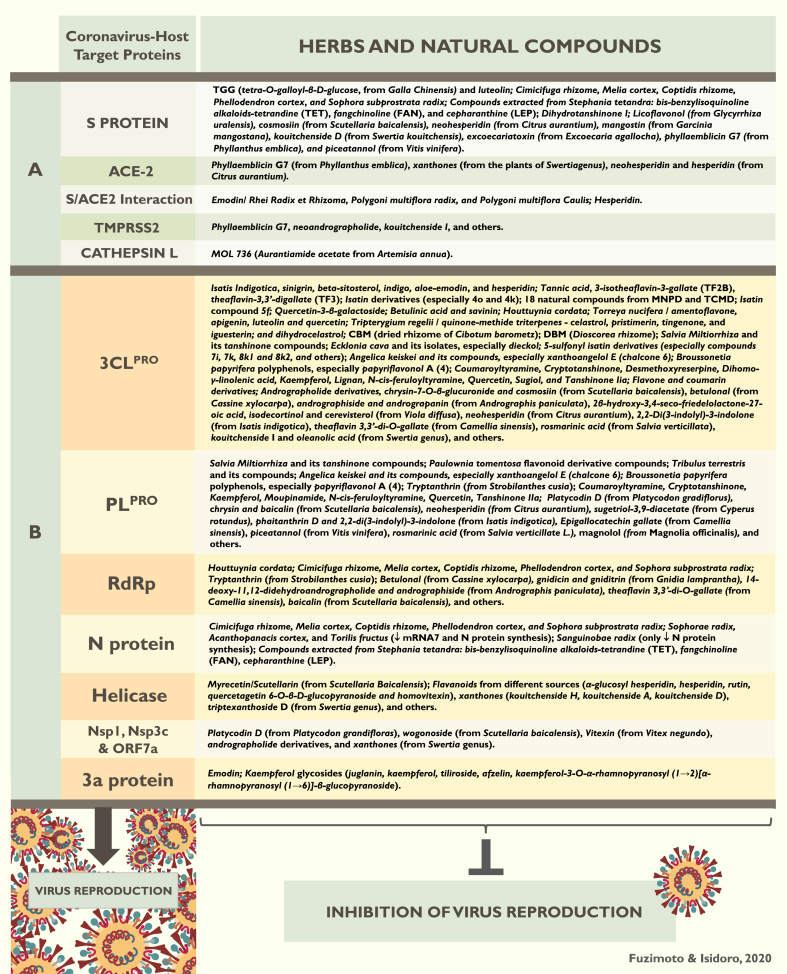
The Pubmed search rendered 201 articles and, after the secondary searches, 43 relevant papers were located. The articles revealed many known and new herbs, natural compounds, and derivatives studied for the SARS-CoV and related viruses. Some of these studies also included conventional drugs and synthetic compounds. Although it is impossible to precisely quantify, we estimated that these 43 articles screened and tested 45,000+ substances, although some were in duplicate. A general hand-count resulted in 450+ herbs and natural compounds with antiviral properties, though some were also duplicated. Drawing the exact numbers was not possible because of the way that some papers displayed the results. From the 43 articles, 31 demonstrated the mechanism(s) of action by which some of the herbs-compounds specifically acted against the SARS-CoVs and associated viruses. The molecular mechanisms refer to the inhibition or blockade of the main SARS-CoV and host protein pathways that are important for the whole process of viral replication. Data were extracted from the articles to provide a summary for the readers, as organized in Table 1 according to the date of publications, the goal of each study, and results. Due to the large amount of data reported by the 43 articles, Table 1 aimed to provide the readers with a quick synopsis of each paper, yet it does not reflect all the accounted results. The mechanism(s) of action of the herbs-natural products reported in the reviewed articles were summarized in Fig. 1.
Table 1.
Summary of the forty three (43) studies that reported antiviral activity of herbs and natural compounds against the SARS-CoV and other related viruses. Out of the 43 articles, thirty one (31) investigated the mechanisms of action by which the herbs-compounds exerted the antiviral activity, and are marked with a ∗. These molecular mechanisms refer to the action on the coronavirus-host protein pathways (See Fig. 1).
| Studies | Tested substances - drugs, medicinal herbs, natural compounds, and synthetic substances | Results - Herbs and natural compounds with antiviral properties |
|---|---|---|
| Cinatl et al., 200351 | Ribavirin, 6-azauridine, pyrazofurin, mycophenolic acid, and glycyrrhizin were tested for their antiviral properties against CoV isolates (FFM-1 and FF-M2). | Glycyrrhizin or GL (from Glycyrrhiza radix): the most active in inhibiting the replication of SARS-associated virus with few toxic effects. Ribavarin and mycophenolic acid did not affect the replication of the SARS-CoV. |
| ∗Yi et al., 200469 | Extracts from 121 Chinese herbs from small molecule libraries that exhibited antiviral activities were screened and tested. | TGG (tetra-O-galloyl-β-D-glucose, from Galla Chinensis) and luteolin avidly binded to SARS-CoV Spike-2 (S2) protein without cytotoxic effects. Quercetin also showed antiviral activity with low cytotoxicity. |
| Chen et al., 200412 | 20 commercially antimicrobial agents and the bioactive compounds from “Qing Fei Jie Du Tang” (“Clear the lung and detoxify decoction”) were screened and tested against SARS-CoV isolates: glycyrrhizin (from Glycyrrhiza uralensis), baicalin (from Scutellaria baicalensis), chlorogenic acid (from Flos lonicerae) and artesunate (from Artemisia apiacea). Additional tested drugs: acyclovir, ganciclovir, cidofovir, foscarnet, ribavirin, IFN-α-2a, IFN-α-2b, IFN-β1a, leukocytic IFN-α, amantadine, rimantadine, zidovudine, stavudine, nevirapine, abacavir, ritonavir, and lopinavir. | Glycyrrhizin, baicalin, chlorogenic acid, IFN-β-1a, leukocytic IFN-α, ribavirin, lopinavir, and rimantadine showed antiviral activity. The combination of ribavirin with the IFNs revealed potentially synergistic action against the SARS-CoV. Baicalin instead of glycyrrhizin should be used for prophylaxis or treatment. |
| Wu et al., 200436 | More than 10,000 compounds were screened and tested to identify antiviral agents against the SARS-CoV. The tested substances included approximately 200 FDA-approved drugs, more than 8000 synthetic compounds, around 1000 TCM herbs, and 500 protease inhibitors. | Approximately 50 compounds showed antiviral activity against the SARS-CoV. The most potent compound was Valinomycin. 15 compounds related to Glycyrrhizin and Aescin, and 6 compounds related to Reserpine showed antiviral activity against the SARS-CoV. The extracts of eucalyptus, Lonicera japonica, and ginsenoside-Rb1 showed antiviral activities against the SARS-CoV at a higher concentration (100 μM). The following drugs and compounds did not show antiviral activity against SARS-CoV: AG7088, Pentoxifylline, melatonin, vitamin C, AZT, didanosine, nevirapine, ritonavir, lopinavir, saquinavir, and ribavirin. Ritonavir and Saquinavir did not inhibit SARS-CoV 3CL-PRO. |
| Hoever et al., 2005111 | The anti-SARS-CoV activity of 15 glycyrrhizin derivatives (from Glycyrrhiza radix) was tested. | Seven (7) Glycyrrhizin (GL) derivatives inhibited SARS-CoV replication in-vitro at lower concentrations when compared to GL. Chemical structure modifications to GL caused from a 10-fold to a 70-fold increase in activity against the SARS-CoV. |
| ∗ Lin et al., 200561 | Water extract of Isatis indigotica, its 5 major compounds, and 7 phenolic compounds were tested as SARS-CoV 3CLPRO inhibitors (indigo, indirubin, indicant, β-sitosterol, sinigrin, aloe-emodin, hesperidin, quercetin, naringenin, daidzein, emodin, and chrysophanol). | Isatis Indigotica significantly inhibited SARS 3CLPRO. Of the 5 compounds, sinigrin, β-sitosterol, and indigo dose-dependently inhibited 3CLPRO. Sinigrin was more efficient than indigo and β-sitosterol. Aloe-emodin and hesperidin also dose-dependently inhibited the cleavage activity of 3CLPRO. Indigo, sinigrin, and hesperidin showed low cytotoxicity. |
| ∗Chen et al., 2005 (a)37 | A library of 720 natural compounds was screened for inhibitory activity against SARS-CoV 3CLPRO. Green, Oolong, Pu’er, and black teas were investigated for the same inhibitory property. | Tannic acid, the polyphenols 3-isotheaflavin-3-gallate, and theaflavin-3,3′-digallate (TF3) found in teas potently inhibited 3CLPRO. The extract from Pu’er and black teas were more potent in inhibiting 3CLPRO than green or oolong teas. Caffeine, theophylline (TP), epigallocatechin gallate (EGCG), epicatechin (EC), catechin (C), epicatechin gallate (ECG), and epigallocatechin (EGC) did not inhibit 3CLPRO at concentrations up to 100 μM. |
| ∗Chen et al., 2005 (b)38 | 26 Isatin derivative compounds (from Isatis Indigotica) were examined for inhibitory activity against SARS-CoV 3CLPRO. | Some Isatin compounds are potent and selective inhibitors against the SARS-CoV 3CLPRO. Isatin 4o and 4k were especially more potent. |
| Li et al., 200567 | 200 Chinese medicinal herbal extracts historically used for the treatment of virus-induced infections were screened for antiviral activities against the SARS-CoV. Interferon-α (IFN-α) was used as a positive control. | Lycoris radiata, Artemisia annua, Pyrrosia lingua, Lindera aggregate, and the compound Lycorine (from L. radiata) showed moderate to potent antiviral activity in a dose-dependent manner. Lycoris radiata was the most potent. Inhibition of the four herbs was stronger than the control IFN-α. |
| ∗Liu & Zhou, 200540 | The study screened and tested 8078 compounds from the Marine Natural Products Database (MNPD) and 9127 compounds from the Traditional Chinese Medicine Database (TCMD) to evaluate their molecular docking affinity to the SARS-3CLPRO. | 18 compounds, 7 compounds from MNPD and 11 compounds from TCMD showed anti-SARS-CoV 3CLPRO activity (M3927, M4367, M4890, M5410, M5789, M6601, M6602, T1434, T1441, T2826, T2831, T4744, T537, T5656, T6791, T8593, T3091, T5242). |
| ∗Zhou et al., 200641 | A series of 23 Isatin derivatives (from Isatis indigotica) were synthesized and tested against the SARS-CoV 3CLPRO. | Isatin compound 5f was the most selective inhibitor against SARS CoV 3CLPRO. It was also a potent inhibitor against HRV-14 3C protease, and it may be used as a broad-spectrum antiviral agent. |
| ∗Chen et al., 200642 | 8 quercetin-3-β-galatoside derivatives were synthesized and studied for their binding affinity to SARS-CoV 3CLPRO. | Quercetin-3-β-galactoside was identified as a new class of drug and a potent inhibitor of SARS-CoV 3CLPRO. |
| ∗Ho et al., 200772 | 312 controlled Chinese medicinal herbs were screened for its inhibitory activity against the SARS-CoV Spike (S) protein. Due to a similar chemical structure, emodin was compared to the promazine, an anti-psychotic drug shown to inhibit the replication of SARS-CoV. | Rhei Radix et Rhizoma, Polygoni multiflora radix, and Polygoni multiflora Caulis inhibited the CoV S protein. Also, emodin, an anthraquinone compound derived from Rheum officinalis and Polygonum multiflorum significantly blocked the S protein/ACE-2 interaction in a dose-dependent manner. When compared to emodin, promazine exhibited a higher inhibition, yet differences between them were not significant. |
| ∗Wang et al., 200743 | The Traditional Chinese Medicine Database (TCMD) was used to screen more than 10,458 natural molecules. MDL28170 was used as a template as it was shown to inhibit CTSL activity during the entry of the SARS-CoV into the target cell. | MOL 736 (Aurantiamide acetate from Artemisia annua) inhibited the activity of CTSL in the molecular docking analysis. MOL 736 was more “matchable” than MDL28170. |
| ∗Wen et al., 200752 | 221 phytocompounds were evaluated for activity against the SARS-CoV. Niclosamide and valinomycin were used as controls for their potent anti-SARS-CoV replicative activity. | 20 newly identified phytocompounds exhibited significant levels of anti-SARS-CoV activity: 10 diterpenoids, 2 sesquiterpenoids, 2 triterpenoids, 5 lignoids, and curcumin, Betulinic acid and savinin were competitive inhibitors of SARS-CoV 3CLPRO. |
| ∗Lau et al., 200839 | The antiviral and immunomodulatory effects of the water extract of Houttuynia cordata were investigated using a murine model. | Houttuynia cordata (HC) exhibited antiviral and immunomodulatory properties that can help prevent the SARS-CoV infection. HC inhibited SARS-CoV 3CLPRO and RdRp, and it was non-toxic after administration to lab animals (16 g/kg). |
| ∗Kim et al., 200893 | The effects of 22 medicinal herbal extracts were evaluated for their antiviral activity against CoV (MHV-A59), porcine epidemic diarrhea virus (PDEV), and vesicular stomatitis virus (VSV) in-vitro. | Cimicifuga rhizome, Melia cortex, Coptidis rhizome, Phellodendron cortex, and Sophora subprostrata radix inhibited MHV activity, and reduced viral RNA synthesis, and S and N protein expression. Coptidis rhizome completely abolished MHV production. RNA synthesis was significantly decreased suggesting that the underlying antiviral mechanism might be RdRp inhibition. |
| Chen et al., 2008112 | Formulas-herbs from the TCM classical medical texts Shang Han Lun and Wen Bing Tiao Bian were screened for their possible antiviral activity against the SARS-CoV (strain FFM1) and HCoV 229E. Several other herbs in addition to a popular vegetable Toona sinensis (AKA Cedrela sinensis) were suggested by experienced by TCM doctors for testing. | Only TSL-1, the extract of Toona sinensis Roem was found to have anti-SARS-CoV activity. |
| Zhuang et al., 200968 | The water extract of 7 medicinal herbs was tested for anti-SARS-CoV and anti-ACE-2 properties: Forsythiae fructus, Scutellaria radix, Bupleuri radix, Astragali radix, and Glycyrrhizae radix, and 4 fractionated samples of Cinnamomi cortex (CC) and Caryophylli Flos (CF). The 4 fractions were ethanol (Fr.1), n-butanol (Fr.2), aqueous (Fr.3), and ethylacetate (Fr.4) extractions. Eight (8) compounds isolated from CC were also tested. | Only the extracts of Cinnamomi cortex (CC) and Caryphylli Flos (CF) showed slight to moderate antiviral activity. All CC and CF fractions showed inhibitory activity, but CC/Fr. 2 was the most potent. The CC fractioned compounds procyanidin A2 and procyanidin B1 showed moderate anti-SARS-CoV activity. The compounds did not affect ACE-2. |
| ∗Ryu et al., 2010 (a)44 | 12 phytochemicals (8 diterpenoids and 4 biflavonoids) were isolated from Torreya nucifera and examined for their anti-3CLPRO activity. Abietic acid, apigenin, luteolin, and quercetin were also evaluated. | The ethanol extract of Torreya nucifera exhibited inhibitory activity against 3CLPRO. From the diterpenoids, ferruginol (3) was the strongest anti-SARS-CoV 3CLPRO. Among the isolated bioflavonoids, amentoflavone (9) was the most potent inhibitor of SARS-CoV 3CLPRO. Apigenin, luteolin, and quercetin were also strong inhibitors. |
| ∗Ryu et al., 2010 (b)45 | Isolated quinone-methide triterpenes (celastrol, pristimerin, tingenone, iguesterin) from Tripterygium regelii, and the semi-synthetic dihydrocelastrol were evaluated for anti-SARS-3CLPRO activity. Curcumin was used as a positive control which is known to inhibit 3CLPRO. | Tripterygium regelli remarkably inhibited SARS-CoV 3CLPRO activity (>70% inhibition at 30 μg/ml). Celastrol, pristimerin, tingenone, and iguesterin showed potent anti-SARS-3CLPRO activity, and dihydrocelastrol also showed SARS-3CLPRO inhibitory activity, although less. |
| ∗Kim et al., 201059 | 19 TCM herbal extracts were evaluated for antiviral activity against MHV-A59. The extracts were also tested against the John Howard Mueller strain of MHV (MHV-JHM), porcine diarrhea virus (PEDV), and vesicular stomatitis virus (VSV). Ribavirin was used as a control. | Sophorae radix, Acanthopanacis cortex, Sanguinobae radix, and Torilis fructus showed in-vitro antiviral activity reducing or completely inhibiting MHV-A59. All 4 herbs were stronger CoV inhibitors than ribavirin. Sophorae radix had the highest selectivity index. Sophorae radix, Acanthopanacis cortex, and Torilis fructus reduced mRNA7 and N protein synthesis, while Sanguinobae radix only decreased N protein synthesis without significant reduction in intracellular RNA levels. |
| ∗Schwarz et al., 201135 | Emodin was investigated for anti-SARS-CoV 3a protein in-vivo and in-vitro. | Emodin is a potent inhibitor of 3a ion channel of SARS-CoV and HCoV-OC43. In the plaque reduction assay emodin showed antiviral property in a dose-dependent manner. Emodin at 100 μM inhibited viral release by 20% and with 100 μM it completely inhibited viral release. |
| ∗Wen et al., 201164 | More than 200 extracts from Chinese medicinal herbs were evaluated for anti-SARS-CoV activity. Valinomycin was used as reference control with high inhibition. For the SARS-CoV 3CL inhibition assay, niclosamide was used as a reference control. | The herbal extracts of Gentiana radix (GSH), Dioscorea rhizome (DBM), Cassiae semen (CTH), Loranthi ramus (TCH), and CBE and CBM (extracts from Rhizoma cibotii, the root of Cibotium barometz) were potent inhibitors of SARS-CoV with little or no cytotoxicity. Although the valinomycin was more potent, these 6 herbs significantly inhibited SARS-CoV. CBM and DBM showed significant inhibition of SARS-CoV 3CLPRO activity. |
| Yin et al., 2011113 | Houttuynia cordata (HC) was investigated in-vitro, in-vivo and in-ovo for antiviral properties against the avian infectious bronchitis virus (IBV). Also, the effect of HC on cell apoptosis induced by IBV was investigated. | Houttuynia cordata had more than 90% inhibition rate against the IBV infection, and decreased more than 90% apoptotic cells caused by the virus. The inhibitory effects of HC on IBV infection in-ovo protected the SPF embryos from death with no adverse effects. However, the treatment of chickens with HC did not fully protect against IBV. |
| ∗Yu et al., 201265 | The researchers examined 64 purified natural compounds against the SARS-CoV helicase (nsP13) protein and the HCV helicase. | Myrecetin and Scutellarin (from Scutellaria Baicalensis) potently inhibited the SARS-CoV helicase protein in-vitro by affecting ATPase activity by more than 90% at a concentration of 10 μM, but with no unwinding activity or cytotoxicity. Other compounds such as myricitrin, amentoflavone, diosmetin-7-O-Glc-Xyl, and taraxerol also inhibited nsP13. |
| Chang et al., 201253 | 23 compounds were isolated from Euphorbia neriifolia L., including 22 triterpenoids and 1 flavonoid glycoside, and tested for their antiviral activity against the human coronavirus (HCoV-229E). Actinomycin D was used as a positive control. | 13 new compounds were isolated from Euphorbia neriifolia L. for the first time. 3β-Friedelanol exhibited more potent anti-viral activity (132.4%) than the positive control, Actinomycin D (69.5%). |
| ∗Park et al., 201263 | Seven tanshinone isolates from Salvia miltiorrhiza were investigated for SARS-CoV 3CLPRO and PLPRO inhibition, and deubiquitinating (DUB) enzyme activities. Tanshinones: tanshinone IIA (1), tanshinone IIB (2), methyl tanshinonate (3), cryptotanshinone (4), tanshinone I (5), dihydrotanshinone I (6), and rosmariquinone (7). | The ethanol extract from Salvia Miltiorrhiza inhibited both proteases SARS-3CLPRO (60%) and PLPRO (80%) at 30 μg/ml. The tanshinone compounds (1-7) showed marked inhibitory activity against the SARS-CoV 3CLPRO and PLPRO. The isolate tanshinone I (5) exhibited the most potent inhibitory activity toward deubiquitinating. |
| ∗Park et al., 201346 | The study investigated 9 phlorotannins (1-9) isolated from the edible algae Ecklonia cava for their anti-SARS-3CLPRO inhibitory activity. Isolated compounds: 1. Phloroglucinol, 2. Triphloretol, 3. Eckol, 4. Dioxinodehydroeckol, 5.2-phloroeckol, 6.7-phloroeckol, 7. Fucodiphloroethol, 8. Diecklo, 9. Phlorofucofuroeckol. | Isolates 2-9 of Ecklonia cava showed SARS-CoV 3CLPRO inhibitory activity in a dose-dependent and competitive manner with no toxicity. Dieckol (8) showed the most potent anti-SARS-CoV 3CLPRO activity. |
| ∗Cho et al., 201395 | The study investigated the SARS-CoV PLPRO inhibitory activities of the methanol extract of Paulownia tomentosa fruits and its 12 flavonoid (1-12) isolates. | Most compounds (1-12) from P. tomentosa inhibited SARS-PLPRO in a dose-dependent manner in-vitro. All the newly discovered geranylated flavonoid compounds (1-5) (tomentin A, tomentin B, tomentin C, tomentin D, tomentin E) showed better inhibition than their parent compounds. |
| ∗Schwarz et al., 201466 | The researchers tested the inhibitory activity of the flavonols kaempferol, kaempferol glycosides, and acylated kaempferol glucoside derivatives against the SARS-CoV 3a protein in-ovo. Other flavonoids such as quercetin, naringenin, and genistein were also tested. | Five (5) kaempferol glycosides (kaempferol, juglanin, tiliroside, afzelin, and Kaempferol-3-O-α-rhamnopyranosyl(1 → 2) [α rhamnopyranosyl(1 → 6)]-β-glucopyranoside) inhibited 3a protein. Juglanin was a potent 3a protein inhibitor and produced nearly complete inhibition (10 μM) or complete inhibition (20 μM). The kaempferol glycosides tiliroside and afzelin, although less potent than juglanin, also inhibited 3a protein. Quercetin, naringenin, and genistein did not affect 3a protein. |
| ∗Liu et al., 201447 | A series of 5-sulfonyl isatin derivatives (from Isatis indigotica) were designed, synthesized and evaluated for anti-SARS-CoV 3CLPRO activity. | A series of compounds showed inhibitory activity against SARS-3CLPRO. Compounds 7a-m, 7i and 7 k showed the highest inhibition. Among the compounds 8, 8k1 and 8k2 were the most potent. Compounds 3f and 3h showed high inhibitory activities (95.32% and 95.37%). |
| ∗Song et al., 201496 | The methanol extract of Tribulus terrestris fruits and the fractioned 6 cinnamic amides (1-6) and ferulic acid (7) were investigated for anti-SARS-CoV PLPRO activity. Compounds: 1. N-trans-caffeoyltyramine, 2. N-trans-coumaroyltyramine, 3. N-trans-feruloyltyramine, 4. terrestriamide, 5. N-trans-feruloyloctopamine, 6. terrestrimine, and 7. ferulic Acid. | T. terrestris showed potent activity against SARS-CoV PLPRO. Compounds 1-6 displayed significant PLPRO inhibitory activity. Terrestrimine (6) was the most potent, and ferulic acid was inactive against PLPRO up to 200 μM. |
| Chiow et al., 201662 | The study evaluated the activities of ethyl acetate (EA) fraction of Houttuynia cordata and three of its flavonoids, quercetin, quercitrin, and rutin against the SARS-CoV and Dengue using the MHV and DEN-2 virus models. The compounds were compared with Cinanserin hydrochloride which was proven to neutralize SARS-CoV in-vitro. | The EA fraction of Houttuynia cordata inhibited both MHV and DEN-2 in-vitro. HC exerted antiviral activity with no cytotoxicity in-vitro and in-vivo. Quercetin also inhibited both MHV and DEN-2. Quercetrin inhibited DENV-2, but not MHV. Rutin did not show an inhibitory effect on both viruses. |
| ∗Park et al., 201648 | The isolates of Angelica keiskei, 9 ankylated chalcones (1-9) and 4 coumarins (10-13) were examined for their anti-SARS-CoV 3CLPRO and PLPRO activity. Compounds: isobavachalcone (1), 4-hydroxyderricin (2), xanthoangelol (3), xanthoagenol F (4), xanthoangelol D (5), xanthoangelol E (6), xanthoangelol B (7), xanthoangelol G (8), xanthokeistal (9), psoralen (10), bergapten (11), xanthotoxin (12), isopimpinellin (13). | The ethanol extract of Angelica keiskei significantly inhibited 3CLPRO (75% inhibition at 30 μg/ml) and PLPRO (88% at 30 μg/ml). All of the isolated compounds (1-13) except the coumarin derivatives (10-13) showed a dose-dependent inhibitory activity against the SARS-CoV 3CLPRO. Chalcone 6 (xanthoangelol E) exhibited the most potent anti-SARS 3CLPRO and PLPRO inhibitory activity. All 13 isolates also showed potent activity against ubiquitin and ubiquitin-like proteins. |
| ∗Park et al., 201790 | The study investigated the inhibitory activity of Broussonetia papyrifera-derived polyphenols against SARS-3CLPRO and PLPRO in-vitro. Isolated compounds: broussochalcone B (1), broussochalcone A (2), 4-hydroxyisolonchocarpin (3), papyriflavonol A (4), 30-(3-methylbut-2-enyl)-30,4,7-trihydroxyflavane (5), kazinol A (6), kazinol B (7), broussoflavan A (8), kazinol F (9), kazinol J (10). Additionally, isoliquiritigenin, kaempferol, quercetin and quercetin-β-galactoside were also tested. | All polyphenols (1-10) were potent inhibitors of SARS-PLPRO, more than against 3CLPRO. Compound 4 (papyriflavonol A) was the most potent against SARS-CoV PLPRO and presented the highest deubiquitination and deISGylation inhibitory activity. |
| ∗Kim et al., 2019108 | The study evaluated the antiviral properties against the compounds isolated from Stephania tetrandra and other related species of Menispermaceae against the HCoV-OC43. Compounds: bis-benzylisoquinoline alkaloids-tetrandine (TET), fangchinoline (FAN), and cepharanthine (LEP). | Stephania tetandra, TET, FAN, and LEP significantly inhibited HCoV-O43 replication in a time- and dose-dependent manner with no cytotoxicity even with high concentrations. The three compounds inhibited S and N protein expression, and experiments indicated that they can be applied for the prevention and treatment of HCoV infection. Compounds also reduced the expression of the cytokines IL-1β, IL-6 and IL-8. |
| Weng et al, 2019109 | The study investigated the antiviral activity of ethanol extract of Sambucus FormosanaNakai stem and some phenolic constituents against the HCoV-NL63 in-vitro. Phenolic acid constituents: caffeic acid, chlorogenic acid, coumaric acid, ferulic acid, and gallic acid. | S. FormosanaNakai inhibited HCoV-NL63 virus yield, plaque formation, and viral attachment with low toxicity in a concentration-dependent manner. The in-vitro antiviral activity was ranked according to virus yield reduction: caffeic acid > chlorogenic acid > coumaric acid. Caffeic acid was the strongest inhibitor of HCoV-NL63 and powerfully reduced the viral attachment to the cell surface and plaque formation. |
| Shen et al., 201960 | A 2000-compound library of FDA-approved drugs and pharmacologically active compounds were screened for an in-vivo and in-vitro study to assess the broad-spectrum antiviral properties against the HCoV-OC43, HCoV-NL63, MERS-CoV, and MHV-A59. Control: dimethyl sulfoxide (DMSO) | The in-vitro antiviral activity of 36 compounds against wild-type HVoV-OC43 was confirmed. 17 compounds inhibited HCoV-NL63, 13 compounds inhibited MERS-CoV, and 12 compounds inhibited MHV-A59. 7 compounds were identified as broad-spectrum inhibitors of the 4 CoVs in-vitro in a dose-dependent manner (lycorine, emetine, monensin sodium, mycophenolate mofetil, mycophenolic acid, phenazopyridine, and pyrvinium pamoate). All 7 compounds significantly inhibited HCoV-OC43 (90% inhibitory effect). Lycorine (from Lycoris radiata) was the most potent antiviral compound against all 4 viruses. |
| ∗Tsai et al., 2020114 | This study investigated the antiviral activity of the methanol extract of Strobilanthes cusia leaf and its chemical components against the anti-HCoV-NL63. The researchers also investigated the anti-RdRp and anti-PLPRO activity of the compounds. S. cusia components: β-sitosterol, indirubin, tryptanthrin, betulin, indigodole A, and indigodole B. | S. cusia potently inhibited virus yield and viral infectivity of HCoV-NL63-infected cells in a concentration-dependent manner. Tryptanthrin showed the strongest antiviral activity. It prevented the early and late stages of viral replication and significantly inhibited RdRp and PLPRO 2 activity. Indigodole B was the second-highest to reduce HCoV-NL63. Both tryptanthrin and indigole B showed low cytotoxicity. |
| ∗Zhang et al, 202050 | A literature search for natural compounds with confirmed anti-SARS-CoV or MERS-CoV activity was conducted. The resulting compounds were cross-checked with the TCM Systems Pharmacology Database. The study aimed to identify compounds that would be potentially protective against the SARS-CoV-2 (2019). | The antiviral activity of 115 natural compounds was confirmed. After Absorption, Distribution, Metabolism, and Excretion (ADME) evaluation, thirteen (13) compounds were found to have anti-SARS-CoV-2 (2019) activity. 125 herbs contained one or more of these 13 compounds. Of these 125 herbs, 26 are classically cataloged to treat viral respiratory infections. For all plants analyzed, nearly half of the top 30 pathways are related to antiviral, immune, inflammatory and hypoxia responses indicating that these herbs are suitable for antiviral use. Molecular docking identified a series of compounds that inhibited 3CLPRO, PLPRO and S protein (see Fig. 1). TCM classical treatments used for viral respiratory infections might contain direct anti-2019-nCoV compounds. |
| ∗Khan et al., 202049 | Database of natural and synthetic molecules and 16 FDA-approved drugs were screened and tested for their inhibitory activity against SARS-CoV-2 3CLPRO. | Remdesivir, Saquinavir, Darunavir, and the natural compounds flavone and coumarin derivatives exhibited inhibitory activity against SARS-CoV 3CLPRO. Saquinavir showed the highest binding affinity. |
| ∗Wu et al., 20202 | Molecular docking simulations were performed to identify the drugs, herbs, natural compounds, and synthetics that would inhibit/block 3CLPRO, PLPRO, RdRp, helicase, Spike protein, ACE-2, and other protein pathways of the SARS-CoV-2. Several databases were screened such as the FDA approved drug database (ZINC drug database) containing 2924 compounds, TCM herbs and natural products database including reported common antiviral components with 1066 substances, and database of commonly used antiviral drugs containing 78 antiviral compounds. The complete genome of the Wuhan-Hu-1 SARS-CoV was downloaded from the NCBI nucleotide database and used for this study. Studies on the homology encoded proteins of the SARS-CoV-2 in comparison with the SARS-CoV-1 were performed. |
For the mechanisms of action of the herbs and natural compounds onSARS-CoV-2, seeFig. 1. Drugs that may inhibit SARS-CoV-2 PLPRO: ribavirin, valganciclovir, β-thymidine (antiviral); doxycycline, chloramphenicol, cefamandole, tigecycline (antibacterial); chlorphenesin carbamate (muscle relaxant); and levodropropizine (anti-tussive), and others. Also, l(+)-Ascorbic acid, glutathione, hesperidin and sildenafil (for erectile dysfunction) showed potential as PLPRO inhibitors. Drugs that may inhibit 3CLPRO:lymecycline, chlorhexidine, demeclocycline, doxycycline, tigecycline, oxytetracycline (anti-bacterial); alfuzosin, nicardipine, telmisartan (anti-hypertensive); and conivaptan (used for hyponatremia), and others. Montelukast (asthma medication) and lutein also showed potential as 3CLPRO inhibitor. Potential drugs inhibitors of SARS-CoV-2 RdRp: valganciclovir (antivirus), itraconazole (anti-fungal); chlorhexidine, ceftibuten, cefuroxime, novobiocin (antibacterial); atovaquone (antimalarial), chenodeoxycholic acid (gall-dissolving drug); cortisone (anti-allergic); fludarabine, idarubicin (anti-tumor); silybin (hepatoprotective); pancuronium bromide (muscle relaxant); dabigatran etexilate (anti-coagulant), and others. Drugs that would inhibit SARS-CoV-2 helicase protein: lymecycline, cefsulodine, rolitetracycline (antibacterial); itraconazole (anti-fungal); saquinavir (anti-HIV-1); dabigatran (anti-coagulant); and canrenoic acid (diuretic). Possible SARS-CoV-2 anti-Spike protein drug inhibitors: rescinnamine, iloprost, prazosin (anti-hypertensive); poconazole, itraconazole (anti-fungal); sulfasalazine, azlocillin, penicillin, cefsulodin (antibacterial); and dabigatran etexilate (anti-coagulant). Drugs that may inhibit Nsp1, Nsp3c, and ORF7a: piperacillin, cefpiramide, streptomycin, lymecycline, and tetracycline. Potential host ACE-2 inhibitors: troglitazone (anti-diabetes), losartan (anti-hypertensive), ergotamine (analgesic), cefmenoxime (anti-bacterial), and silybin (hepatoprotective). Potential TMPRSS2 inhibitors: pivampicillin, hetacillin, cefoperazone, and clindamycin (antibacterial). |
Fig. 1.
Herbs and natural compounds able to inhibit the SARS-CoV-host protein pathways. Panel A corresponds to the proteins involved in the internalization of the CoV into the host cells; Panel B corresponds to further processes involved in translation, transcription, replication, assembly and egress from the host cells.
4. Discussion
We organized the discussion to progressively facilitate the understanding of the extracted data and to specifically explain the mechanisms of action found in the articles. We first clarify the overall methodologies of the papers, the broader antiviral properties, and then the more explicit molecular mechanisms of the antiviral herbs and natural compounds.
4.1. Methodologies used in the studies
Generally, drugs with antiviral properties against the SARS-CoV are tested through in-vitro infected cell lines accompanied by the cytopathic effect (CPE) assay. Since the investigations with SARS-CoVs require a Biosafety Level 3 (BSL-3) laboratory equipped to contain dangerous pathogens,35 very often researchers use pseudotyped viruses known for their inability to replicate. Different viruses can be used as models to study the SARS-CoV such as the mouse hepatitis virus (MHV), HCoV-OC43, HCoV229E, or HIV-luc/SARS pseudotyped virus, to name a few. Experiments with HCoV-OC43, hepatitis, and HIV viruses require a Biosafety Level 2 (BSL-2) lab making them easier to test.35 So, the viruses used as models for the SARS-CoV and that provide a proximity for testing are RNA viruses such as hepatitis C, HIV, different CoVs of the Coronaviridae family, different strains of SARS-CoV, and similar viruses. The influenza viruses are also RNA viruses, yet their manipulation requires a BSL-3 lab. Furthermore, in the studies, different biochemical and immunological assays are applied, and the investigations are developed with a multi-layered methodology. Although the methodologies of the included articles in this review are varied and complex, there is a coherence and a certain commonality in designing the experiments. Ultimately, the goal is to identify the substances with antiviral properties against the SARS-CoV. In addition to detecting the antiviral properties, some studies also aspired to uncover the specific molecular mechanism(s) by which herbs-natural compounds would act against the SARS-CoV. Researchers used complementary techniques to identify these mechanism(s) such as fluorescence resonance energy transfer (FRET), SARS-CoV 3CL protease (3CLPRO) and papain-like protease (PLPRO) inhibition assays, SARS-CoV RdRp assay, SARS-CoV PL deubiquitination and deISGylation assays, and many others.36, 37, 38, 39 Several studies on drug discovery screened a database of hundreds or even thousands of existing substances to identify the best possible options for the CoV treatment (Table 1). To help determine the mechanisms of action, computer molecular docking simulations are often the choice because it is precise in locating the potential remedies. These docking analyses allow scientists to discover the capacity of small-molecule ligands to bind to three-dimensional proteins of the virus and host cells. The present review located fourteen (14) studies that included this methodology.2,36,38,40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50 Importantly, the studies investigating the antiviral properties and molecular mechanism(s) of conventional drugs and herbs-natural compounds against the SARS-CoV adopt the same (or very similar) experimental models and methodologies. As a matter of fact, some of these studies include and compare conventional drugs, whole herbs, natural and synthetic compounds in the same research.2,12,36,51 For example, in a recently published study (2020), databases consisting of 2924 FDA-approved drugs, 1066 TCM herbs and natural products, and 78 antiviral compounds were analyzed by computational molecular docking against the SARS-CoV-22.
Also, we verified that some studies tested the herbs and natural compounds using conventional drugs or other agents known for their antiviral action as a template or positive control.43,52,53 Some examples are given such as niclosamide and valinomycin,52 actinomycin D,53 MDL2817043, and curcumin.44 Niclosamide is an antiparasitic drug and it was shown to inhibit dengue virus infection by hindering endosomal acidification.54 Valinomycin is a potent antibiotic, and it blocked the entrance of poliovirus into the host cells.55 Actinomycin D is a chemotherapy drug used for cancer, and it obstructed influenza virus RNA synthesis in-vivo.56 MDL28170 is a compound known to inhibit the Cathepsin-L endosomal protease activity thus preventing the coronavirus cell entry.57 Finally, curcumin, a compound extracted from Curcuma longa, ameliorated severe pneumonia caused by influenza A virus in-vitro and in-vivo, downregulated inflammatory cytokines and inhibited NF-κβ signaling macrophages.58 Worthy of note, one study took a step further on pharmacokinetics and assessed the absorption, distribution, metabolism, and excretion (ADME) of the orally administered herbs and natural compounds.50
4.2. Antiviral properties of herbs and compounds against the SARS-CoV
The ideal therapeutic choices for the SARS-CoV need to show antiviral properties, have low cytotoxicity, few side effects, and good bioavailability. In general, the studies that used cell-based assays and additional techniques examined some of these requirements to elect the potential candidates (Table 1). As mentioned, the selected 43 studies showed antiviral properties of many herbs and natural compounds. Remarkably, in some experiments, various herbs even exceeded the antiviral properties of some conventional drugs at certain dosages and concentrations while presenting low cytotoxicity. For example, 3β-Friedelanol extracted from Euphorbia neriifolia L. exhibited a more potent antiviral activity than the positive control, actinomycin D.53 In another study, ribavirin did not affect viral replication while glycyrrhizin extracted from Glycyrrhiza radix was the most active in inhibiting the clinical isolates of the CoV (FFM-1 and FF-M2).51 A high concentration of glycyrrhizin (4000 mg/L) completely blocked the CoV replication with no toxic effects, yet the mechanism of action was not clarified.51 However, in another research, baicalin isolated from Scutellaria Baicalensis was suggested for the SARS-CoV instead of glycyrrhizin. A small intravenous dosage of baicalin can achieve a much higher serum concentration when compared to glycyrrhizin.12 In this case, the use of baicalin at 48h and 72h (EC50 12.5–25 and 25-50 μg/ml) against 10 strains of SARS-CoV in the fRhK4 cell line surpassed ribavirin (EC50 12.5–200 and 50-200 μg/ml) as well as glycyrrhizin (EC50 > 400 μg/ml).12 Also, the herbs Sophorae radix (EC50 0.8 μg/ml), Pulsatillae radix (EC50 6.1 μg/ml), Acanthopanacis cortex (EC50 0.9 μg/ml), Sanguisorbae radix (EC50 3.7 μg/ml), Punicae cortex (EC50 11.0 μg/ml) and Torilis fructus (EC50 0.8 μg/ml) were more potent inhibitors of MHV-59/CoV than ribavirin (EC50 17.5 μg/ml).59 In another study, a series of herbal compounds showed strong antiviral activity against 4 types of CoVs when compared with several conventional drugs. Lycorine (EC50 0.15, 0.47, 1.63 and 0.31 μM) and emetine (EC50 0.30, 1.43, 0.34 and 0.12 μM) potently inhibited HCoV-OC43, HCoV-NL63, MERS-CoV and MHV-A59, in contrast with chloroquine (EC50 0.33, 4.89, 16.44 and 15.92 μM), valinomycin (EC50 4.43, 1.89, 6.07 and 6.78 μM), and loperamide (EC50 1.86, 6.47, 4.82 and 10.65 μM), respectively.60 Many other herbs and natural compounds exerted a strong replicative inhibition of the SARS-CoV and related viruses such as theaflavins (IC50 3–9.5 μM),37 Isatis indigotica (IC50 53.8 μg/ml),61 isatins (from IC50 0.37+ μM),38,41,47 Houttuynia cordata (IC50 0.98 μg/mL),62 diekol from Ecklonia cava (IC50 2.7 μM),46 hesperidin (IC50 8.3 μM),61 tanshinone compounds from Salvia Miltiorrhiza (from IC50 0.7+ μM),63 amentoflavone extracted from Torreya nucifera (IC50 8.3 μM),44 the isolates celastrol, pristimerin, tingenone, iguesterin from Tripterygium regelii (IC50 2.6–10.3 μM)45, Gentiana radix, Dioscorea rhizome, Cassiae semen, Loranthi ramus, and CBE and CBM (from Rhizoma Cibotii, the dried rhizome Cibotium barometz) (EC50 5.39 to >10 μg/ml)64, Myrecetin and Scutellarin (from Scutellaria Baicalensis) (IC50 2.71 and 0.86 μM, respectively),65 and juglanin, a Kaempferol-arabinoside present Kaempferia galangal L. (IC50 2.3 μM).66 Lycoris radiata, Pyrrosia lingua, Artemisia annua, Lindera aggregate, and lycorine provided from moderate to potent antiviral activity with Lycoris radiata being the strongest (EC50 2.4 μg/ml).67 In contrast, other natural substances only exerted a moderate effect on the SARS-CoV such as Cinnamoni cortex and Caryphylli Flos (IC50 < 100 μg/ml).68 The studies provided a long list of herbs, isolates, and derivatives to fuel the discussion on the clinical use and future development of antiviral remedies for the SARS-CoVs. Although the majority of the available studies focused on the SARS-CoV-1, three recent articles by Zhang et al. (2020), Khan et al. (2020), and Wu et al. (2020) presented an extended list of antiviral candidates specifically for the SARS-CoV-2.2, 49 Zhang et al. (2020), for example, listed the following herbs with antiviral activity against the SARS-CoV-2: Forsythiae fructus, Licorice (Glycyrrhiza radix), Mori cortex, Chrysanthemi flos, Farfarae flos, Lonicerae japonicae flos, Mori follum, Peucedami radix, Rhizoma fagopyri cymosi, Tamaricis cacumen, Erigenon breviscapus, Radix bupleuri, Coptidis rhizoma, Houttuynniae herba, Hoveniae dulcis semen, Inulae flos, Eriobotryae folium, Hedysarum multijugum maxim., Lepidii semen descurainiae semen, Ardisiae japonicae herba, Asteris radix et rhizoma, Euphorbiae helioscopiae herba, Ginkgo semen, Anemarrhenae rhizoma, Epimrdii herba, and Fortunes bossfern rhizoma. A comprehensive summary of herbs-compounds with antiviral properties is reported in Table 1.
Regarding the cytotoxicity of the natural agents, many articles reported on their low or absent in-vitro cell toxicity. For instance, TGG (EC50 4.5 μM) and luteolin (EC50 10.6 μM) were more potent inhibitors of the wild-type SARS-CoV than glycyrrhizin (EC50 > 607.6 μM), while presenting low/no cytotoxicity (TGG CC50 1.08 μM, SI 240; and luteolin CC50 0.155 μM, SI 14.62).69 Quercetin also showed antiviral activity against the HIV-luc/SARS pseudotyped virus with low cytotoxicity (EC50 83.4 μM; CC50 3.32 mM).69 A variety of potent antiviral compounds exhibited substantially higher selectivity index (SI) than the control valinomycin (SI 41.4), also revealing little or no toxicity. These included ferruginol (SI 58), dehydroabieta-7-one (SI 76.3), 8β-hydroxyabieta-9(11),13-dien-12-one (SI > 510), 7β-hydroxydeoxycryptojaponol (SI 111), 3β,12-diacetoxyabieta-6,8,11,13-tetraene (SI 193), pinusolidic acid (SI 159), forskolin (SI 89.8), betulinic acid (SI 180) and savinin (SI 667).52 The water extract of Houttuynia cordata expressed strong antiviral activity against the SARS-CoV and was considered non-toxic to animals upon oral administration of 16 g/kg.39 In addition to exerting a potent antiviral property, myricetin and scutellarin were non-toxic when tested against breast-derived epithelial MCF10A cells at 2 μM dosages.65 For the confirmed anti-HCoV-OC43 compounds tetrandrine (TET), fangchinoline (FAN) and ceparanthine (CEP) extracted from Stephania tetrandra, the IC50/CC50/SI were 0.33/13.41/40.19 μM, 1.01/11.54/11.46 μM, and 0.83/11.26/13.63 μM, respectively.70 Further clinical studies would verify the efficacy and safety of these compounds for humans through pharmacokinetics and cytotoxicity analyses.
4.3. The SARS-CoV and host protein pathways
In the past years, a lot of progress was made in understanding how the coronavirus invades the host cells and replicate. CoVs are enveloped positive-sense RNA viruses and possess the largest genome of all RNA viruses (30 kilobase genomes).71 Potential therapies against the CoV can be divided into two categories. They can block signaling pathways of human cells that are required by the virus to replicate, or they can act directly on the virus.2 In the first case, therapies could inhibit or block the virus from binding to human cell receptors, preventing the viral entry and the spreading from cell to cell. In the second instance, the therapies could act on structural and functional proteins, enzymes, or the genetic material of the virus to inhibit RNA synthesis, replication or virus self-assembly.2
The CoV Spike (S) glycoprotein, which binds to the ACE-2 receptor of the human host, is essential for viral attachment and cell entry, and it is also involved in the fusion process.72, 73, 74 Stopping the virus entry through a blockade of the S protein is important because it hinders the propagation of the virus at an early stage and prevents drug resistance.69 After receptor binding, the second important step for viral entry is the fusion of the viral envelope with host cellular membranes that frees the virus genome into the cytoplasm.74 The membrane fusion can happen directly with the host cell membrane (cell-surface non-endosomal pathway) or with endosome membrane (endosomal pathway).75 Cathepsin L (CTSL), an endosomal protease is known to process the SARS-CoV S protein and to participate in the activation of membrane fusion.57,74 The CoV membrane fusion happens through a three-step process which includes receptor binding and induced conformational changes of the S protein, followed by the CTSL proteolysis within the endosomes.57 So, the S protein and CTSL play an important role in the internalization of the CoV, and are considered attractive targets for the development of new drugs. In addition, the host transmembrane protease serine-type 2 (TMPRSS2) activates the S protein and was shown, in-vitro, to induce virus cell-membrane fusion.76 3-Chymotrypsin-like protease (3CLPRO or nsp5), papain-like protease (PLPRO or nsp3), RNA-dependent RNA polymerase (RdRp or Nsp12), and helicase protein (nsp13) are the main targets for developing RNA synthesis and replication inhibitors.2 Two-thirds of the CoV RNA is translated into two large polyproteins, pp1a and pp1ab that encode 16 non-structural proteins (nsps1–nsp16)75. These polyproteins are cleaved by the proteases PLPRO and 3CLPRO, a step necessary for the transcription, translation, and replication processes leading to new virions inside the host cells.75,77 The cleavage of protein precursors releases key replicative enzymes such as RdRp and the helicase protein. The role of both 3CLPRO and PLPRO is to process the polyproteins to generate the various viral proteins. To be noted, PLPRO has the additional function of stripping ubiquitin and ISG15 from the host cell proteins to help the CoV escape the innate immune system.77 The ubiquitin-like protein ISG15 is an interferon-induced protein that is believed to have an important role in the host antiviral defense.78 RdRp is an indispensable enzyme for replicating the genome and the transcription cycle of RNA viruses.79 Helicases are motor proteins that separate and rearrange nucleic acid duplexes in processes powered by adenosine triphosphate (ATP) hydrolysis.80 The helicase protein unwinds double-stranded DNA and RNA into single strands so they can be copied.81 DNA helicases play a crucial role in replication, recombination and repair, and RNA helicases are instrumental for transcription, translation and RNA splicing.80 The Nucleocapsid (N) protein is a multifunctional structural protein that is involved in the CoV life cycle and cellular response.82 The N protein plays a key role in viral core formation, viral assembly, viral envelope formation and budding, and genomic mRNA replication and RNA synthesis.82 It also participates in a series of cell processes that support the CoV pathogenesis.82 The encoded ORF 3a protein is a transmembrane protein and it is believed to form an ion channel that modulates viral release.83 Among the CoV virulence factors, Nsp1, Nsp3c, and ORF7a interfere with the host’s innate immune response and assist the CoV immune scape.2 The main target proteins that would intervene with the SARS-CoV-2 replicative process include Nsp1, Nsp3 (Nsp3b, Nsp3c, Papain-like protease (PLpro), and Nsp3e), Nsp7-Nsp8 complex, Nsp9-Nsp10, and Nsp14-Nsp16, E channel (E protein), ORF7a, Spike, angiotensin-converting-enzyme 2 (ACE-2), C-terminal RNA binding domain (CRBD), N-terminal RNA binding domains (NRBD), helicase, RdRp, and TMPRSSS2.2 All these proteins are potential candidates for designing new antiviral drugs capable of interfering with different phases of the viral life cycle.
4.3.1. Herbal medicine and the inhibition/blockade of the coronavirus-host target protein pathways
During previous years, many drugs have been researched as inhibitors of the coronavirus-host protein pathways. For example, the combination of ritonavir and lopinavir exhibited some clinical signs of effectiveness against the SARS-CoV. However, molecular docking simulations failed to prove that these drugs could indeed interact significantly with 3CLPRO 84 Remdesivir, a drug developed for Ebola and Marburg diseases, has been researched as an RdRp inhibitor which would hinder the CoV nucleic acid production. Thus, it is currently being considered as a repurposed drug for the SARS-CoV-2.85 Likewise, many herbs and natural compounds have been investigated for their ability to interfere with these same virus protein pathways. Fig. 1 (panels A and B) summarizes our current knowledge on the herbs-natural compounds able to inhibit CoV 3CLPRO, PLPRO, RdRp, helicase protein, S protein, N protein, 3a protein, Cathepsin L, the virulence factors (Nsp1, Nsp3c, and ORF7a), and blockade the S protein/ACE-2 interaction.
In many studies, the herbs-natural compounds significantly and potently inhibited some of these protein pathways. For example, four studies showed that Isatis indigotica, its compounds isatin, sinigrin, beta-sitosterol, indigo, aloe-emodin, hesperidin, and other compounds and derivatives are potent SARS-CoV 3CLPRO inhibitors.38,41,47,61 In molecular docking analyses, hesperidin demonstrated the potential to inhibit 3CLPRO, helicase protein, S protein, ACE-2 receptor, and S protein/ACE-2 interaction.2 Neohesperidin can also inhibit all these proteins except for the S protein/ACE-2 interaction.2 Phaitanthrin D and 2,2-di(3-indolyl)-3-indolone also from Isatis indigotica inhibited the SARS-CoV PLPRO 2 Isatis indigotica has a long history in the treatment of viral infections. In one article, Isatis indigotica isolate lariciresinol-4-O-β-d-glucopyranoside inhibited influenza A (H1N1) virus-induced pro-inflammatory response in-vitro.86 Indirubin, an alkaloid extracted from Isatis Indigotica reduced the H1N1 susceptibility in an animal model, lowered the mortality rate, and alleviated lung injury. It also promoted the expression of interferon-β (IFN-β) and interferon (IFN) inducible transmembrane 3, and preserved the function and morphology of mitochondria after the infection.87 Epigoitrin, another alkaloid from Isatis indigotica, reduced H1N1 infection in a stress-induced susceptible model in-vivo and in-vitro, evidenced by low mortality rate, improved inflammation, a decreased viral replication in the lungs and increased production of IFN-β and IFN-inducible transmembrane 3.88 Moreover, the crude extract of Isatis indigotica prevented viral attachment to the target cells in an in-vitro model of influenza.89 Thus, I. Indigotica has several isolates of proved antiviral activity and may target different proteins pathways. Other herbs-natural compounds that showed potential inhibition of both SARS-CoV 3CLPRO and PLPRO were Salvia miltiorrhiza and its tanshinone compounds,63 Angelica keiskei and its compounds,48 and Broussonetia papyfera polyphenols.90 The water extract of Salvia miltiorrhiza, for example, demonstrated potent activity against HIV-1 integrase activity in-vitro and viral replication in-vivo with no cytotoxicity.91
Houttuynia cordata showed antiviral activity by inhibiting the SARS-CoV 3CLPRO and RdRp, and stimulated the proliferation of CD4+ and CD8+ T cells in-vitro.39 Houttuynia cordata is a widely used TCM herb for respiratory diseases, bacterial and viral infections. In an animal study with influenza H1N1, Houttuynia cordata polysaccharides ameliorated pneumonia and intestinal injury through inhibition of inflammation, protection of intestinal barrier and regulation of mucosal immunity.92 In another research, Cimicifuga rhizome, Melia cortex, Coptidis rhizome, Phellodendron cortex, and Sophora subprostrata radix were able to inhibit the SARS-CoV RdRp, S and N proteins.93 One of the main isolates of Coptidis rhizome and Phellodendri cortex is berberine. Berberine, a natural isoquinoline alkaloid isolated from plants of the berberis species, exerted anti-inflammatory, antibacterial, antifungal, and anti-helminthic properties.94 As an example, berberine reduced viral replication, pulmonary inflammation, necrosis, inflammatory cell infiltration, and pulmonary edema caused by the infection in a murine model of H1N1.94 Other herbs-compounds that also inhibited PLPRO were Paulownia tomentosa flavonoids,95 Tribulus terrestris and its compounds,96 Platycodin D (from Platycodon gradiflorus), and sugetriol-3,9-diacetate (from Cyperus rotundus).2 Herbs and natural compounds able to inhibit the PLPRO’s deubiquitinating (DUB) and deISGylating activities would also increase their effectiveness against the CoV.48,63,90 In this context, all the 13 isolates from Angelica keiskei showed potent inhibitory effects on deubiquitination (from 2.6 to 73.3 μM) and deISGylation (from 1.1 to 33.1 μM).48 Similarly, all the 10 compounds extracted from Broussonetia papyfera and the substances isoliquiritigenin, kaempferol, quercetin, and quercetin-β-galactoside exhibited moderate deubiquitination and deISGylation inhibition effects (from 7.6 to 136.9, and from 8.5 to 71.7, respectively).90
Rhei Radix et Rhizoma, Polygoni multiflora radix, Polygoni multiflora Caulis, and especially the compound emodin extracted from these plants exerted strong antiviral activity (IC50 1–10 μg/ml; emodin IC50 200 μM) and blocked the SARS-CoV S protein/ACE-2 interaction.72 In a different study, emodin inhibited the SARS-CoV 3a protein and S protein.35 Thus, emodin could hinder the SARS-CoV by blocking both viral entry and release. Interestingly, Emodin increased the survival rate in an animal model of H1N1 infection, reducing edema, pulmonary viral titer and inflammatory cytokines, and improved lung histopathological changes.97 Emodin was shown to inhibit influenza A virus and influenza viral pneumonia through the Nrf2, TLR4, p38/JNK, and NF-Κβ pathways.97 Moreover, juglanin, a kaempferol derivative from Kaempferia galangal L. completely blocked the SARS-CoV 3a protein.66
Another interesting research investigated the SARS-CoV 3CLPRO inhibitory effects of black, Pu’er, green and oolong teas. The authors concluded that tannic acid, 3-isotheaflavin-3-gallate (TF2B), and theaflavin-3,3′-digallate (TF3) were potent SARS-CoV 3CLPRO inhibitors, and that the black and Pu’er teas were more potent at their inhibitory properties than the green and oolong teas.37 However, Caffeine, Epigallocatechin gallate (EGCG), and epigallocatechin (EGC) did not inhibit 3CLPRO.37 In another study,2 the researchers verified that theaflavin 3,3′-di-O-gallate (TF3) from Camellia sinensis green tea effectively inhibited SARS-CoV RdRp and 3CLPRO, and epigallocatechin gallate (also from C. sinensis) blocked PLPRO. Also, three different theaflavins, theaflavin (TF1), theaflavin-3′-monogallate (TF2), and theaflavin-3-3′-digallate (TF3), major polyphenols from black tea, were tested against the hepatitis C virus (HCV). The researchers deduced that the theaflavins are inhibitors of the HCV entry, thus able to inhibit cell-to-cell spread.98 Another paper reported that theaflavin derivatives (particularly from black tea) could inhibit HIV-1 entry into target cells by blocking the retroviral envelope glycoprotein 41 (gp41) that is involved in the internalization of the host cells.99
The compound MOL 736, aurantiamide acetate derived from the herb Artemisia Annua, was found to be a CTSL inhibitor during the SARS-CoV infection.43 Artemisia Annua together with its isolates artemisinin and artesunate have been extensively studied as therapeutic agents for malaria, cancer, and schistosomiasis.100, 101, 102 Artemisinin and artesunate exhibited antiviral properties against the cytomegalovirus, herpes simplex type 1, Epstein-Barr, hepatitis B and C viruses, and bovine diarrhea virus.102 In another research, Myrecetin and scutellarin, the latter extracted from Scutellaria Baicalensis, hindered the SARS-CoV helicase protein activity.65 Baicalin from S. baicalesis also demonstrated inhibition potential of the SARS-CoV PLPRO and RdRp in molecular docking simulations.2 Likewise, the flavonoid cosmosiin from S. baicalensis can inhibit the SARS-CoV 3CLPRO and Spike protein, and wogonoside from the same herb displayed the capacity to inhibit the SARS-CoV Nsp1, Nsp3c, and ORF7a.2 Decreasing the coronavirus virulence factors would attenuate the severity of disease and give the immune system a better fighting chance. S. baicalensis, a very popular herb used in TCM for common cold, fever, and influenza has been extensively researched for many ailments and demonstrated great potential in the treatment for acute lung injury induced by influenza virus A in an animal model.103 Besides the antiviral properties, the flavonoid-enriched extract from S. Baicalensis also showed anti-inflammatory and anti-complement activities.103 Also, wogonin, an isolate extracted from S. baicalensis, displayed antiviral activity against the influenza infection through the modulation of the cyclic adenosine monophosphate (AMP) pathway.104 Another herb of interest is Andrographis paniculata, known for its anti-inflammatory and antibacterial properties.105 Its derivatives andrographiside, andrograpanin and andrographolide can inhibit SARS-CoV 3CLPRO, while the former can also hinder RdRp, and the latter can additionally restrain NsP1, Nsp3c, and ORF7a.2 Neoandrographolide can inhibit TMPRSS2 making Andrographis paniculata another herb that can target multiple protein pathways. In another example, andrographolide inhibited influenza A-virus induced inflammation through the NF-Κβ and JAK-STAT signaling pathways in a murine model.106
Panels A and B in Fig. 1 illustrate the potential of many natural substances as drug and therapeutic candidates for the SARS-CoVs. Some herbs and natural compounds significantly and potently inhibited one or more protein pathways, thus interfering in different phases of the viral life cycle. Noteworthy, SARS-CoV-2 is highly homologous to the SARS-CoV-12. In genomic homology analyses, the SARS-CoV-2 displayed the highest genome sequence identity to the SARS-CoV-1 of 79–80%, and a similarity to MERS-CoV of 50%.2,3 Most of the proteins from the SARS-CoV-1 and the SARS-CoV-2 exhibited an homology of 72%–99%.2 The proteins 3CLPRO, PLPRO, and RdRp of the SARS-CoV 1 and 2 are highly analogous, and the homology of the spike-receptor binding domain (RBD) sequence between the two viruses is 76%.2 Though the SARS-CoV-2 is sufficiently divergent from the SARS-CoV-1 to be considered a new type of human coronavirus, the receptor-binding domain structure of both is similar, and the SARS-CoV-2 can bind to the ACE-2 receptors of the host.3 These studies indicate that, even with the mutations, therapeutic agents that intervene with the SARS-CoV-1 and host pathways can, to some extent, be therapeutically useful for the SARS-CoV-2.
4.4. Limitations of the studies
Some limitations in the studies were observed. In our search, we did not see follow up studies as a result of past herbal and natural compounds discovery. Although not part of the goal of the studies, we also missed investigations on drug-herbal interaction while treating CoVs. As a critical warning, when combining a conventional drug with a natural product it is mandatory to rule out any possible interference with pharmacokinetic and pharmacodynamic processes. For instance, coadministration of lopinavir/ritonavir is not recommended with certain herbal medicines (e.g., St. John’s wort or Hypericum perforatum) that may substantially reduce its plasma concentrations.107 Glycyrrhizin, which inhibits SARS-3CLPRO, could compete with the Kaletra (lopinavir/ritonavir). Agents that block RdRp such as Coptidis rhizome, Phellodendron cortex, and ritonavir could interfere with each other. Also, the interaction between ACE-2 inhibitor drugs and natural substances that potentially block ACE-2 receptors or the S protein/ACE-2 interface should be considered. However, not all drug-herbal interactions are harmful and their combination may also be beneficial. Moreover, only three (3) studies researched and/or discussed drug synergism which is very important to elect useful routes of clinical action.12,62,108
4.5. Critical considerations
Important considerations include the fact that some studies used water and ethanol extract with good results in inhibiting the SARS-CoV and host proteins.39,44,53,61,63,67,68,72,109 For example, the water extract of Isatis indigotica and the ethanol extract of Torreya nucifera significantly inhibited SARS-CoV 3CLPRO. Also, the ethanol extract of Salvia miltiorrhiza significantly inhibited SARS-CoV 3CLPRO and PLPRO. Thus, the use of whole herbs may effectively treat the viral infection as opposed to only the exclusive creation of “drugs” from herbal compounds and derivatives. However, since the extraction of the active components of the ingested whole herbs depends on the health of the digestive system of the patients, the use of isolates and derivatives from the herbs may offer advantages of a more targeted approach. The efficacy of natural substances also depends on the mode of administration. Furthermore, studies highlighted that the herbs and natural compounds acted as the antiviral agents in a dose- and concentration-dependent manner. Thus, verifying concentrations and dosage thresholds of isolated compounds and derivatives to act as independent drugs is essential for efficacy and safety. The clinical efficiency of natural substances may also vary according to the stage of pathogenicity of the infection. For example, bis-benzylisoquinoline alkaloids tetrandrine (TEN), fangchinoline (FAN) and cepharanthine (CEP) demonstrated antiviral activities against the HCoV-OC43 at an early stage of infection.108 Therefore, these substances acted not only in a concentration- and dose-dependent manner but also in a time-dependent manner. All these ideas deserve future investigations. Apart from sequelae of coronaviruses infections, there is evidence of reactivation of cases of the SARS-CoV-2. A recent study showed that from 55 patients with a history of epidemiological exposure to the COVID-19, 5 patients (9%) who were discharged from the hospital had the SARS-CoV-2 reactivation.110 Symptoms of the reactivated patients included fever, cough, sore throat and fatigue. One patient had progressive lymphopenia and neutrophilia.110 Therefore, clinical therapeutic procedures need to also envision possible sequelae and viral reactivation. Additional measures to promote detoxification and increase the immune-modulatory capabilities of the patients with SARS-CoVs should be explored. Many herbs have been potentially proven useful for this purpose.
5. Conclusion and perspectives
The investigation on the CoV treatments represents a big challenge for researchers due to the fast dissemination of the disease and the impossibility to perform randomized clinical treatment trials that are dangerous and pose ethical problems in face of a life-threatening condition.105 Some conventional drugs are tried in infected patients through compassionate use programs.3 Thus, there is an urgent need to explore and discover new treatment options for SARS-CoV-2 and similar pathogens. Approximately 450+ herbs and natural compounds reviewed here have shown their activity as antiviral substances against the SARS-CoV and related viruses. More specifically, many of them demonstrated the ability to inhibit the coronavirus-host protein pathways and interfere in different stages of the coronavirus life cycle such as viral entry into the host cell, membrane fusion, the processes of transcription, translation, replication, viral assembly, and viral release. The evidence on these herbs and natural compounds’ properties was acquired utilizing the same or very similar methodologies as with the research on conventional drugs. The data suggest that these herbs-compounds offer a huge potential as therapeutic options to support the SARS-CoV treatment protocols.
Composing complementary and synergistic herbal remedies that could inhibit/block several viral-host protein pathways would surely render powerful allies in the fight against the SARS-CoV-2 infection. The study of synergistic combinations of conventional drugs and natural substances would also be of great value. Regardless, many herbs and modified herbal formulas habitually used by master herbalists for viral infections might be hitting some of the target proteins of the coronavirus and host. One herbal formula containing several herbs, that in turn comprise dozens or even hundreds of compounds, may target different pathways, some of which not yet known. It is highly credible that the herbal formulas used for the SARS-CoV-2 may inhibit multiple protein pathways while mediating antiviral, anti-inflammatory, antipyretic, immune-modulatory, anti-complement, anti-pulmonary fibrosis, anti-toxicity, and analgesic responses. However, further post-drug discovery research would provide a more in-depth perspective on the efficacy and safety of these herbs and isolated substances for standalone use, herbal-herbal, or drug-herbal combination. As a warning, the results of the in-vitro studies may not correspond to the same clinical effects if applied to human patients. The use of herbs and natural compounds may also represent enormous support for the conventional medical system, alleviating the work of medical staff and hospital workers actively fighting in the front-line against the COVID-19. Thus, this review also indicates the importance of considering the combination of conventional drugs and herbal medicine in an integrative and collaborative medical approach.
Availability of data and material
All data generated or analyzed during this study are included in this published article.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Funding
The authors declare that they obtained no funding for this study.
Authors’ contributions
ADF designed and carried out the literature searches, drafted the entire article, and prepared the table, graphical abstract, and figure. CI revised and finalized the figure and manuscript, adding comments and references. Both authors revised the manuscript in different phases of its preparation, and they have read and approved the final version.
Authors’ information
ADF is a Doctor of Acupuncture and Oriental Medicine (DAOM), a Master of Science of Traditional Chinese Medicine (MSTCM), and a Multiple Sclerosis Certified Specialist (MSCS). CI is a Doctor of Medicine and Surgery, Doctor of Biological Sciences, and Professor of Pathology.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
Not applicable.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Naming the Coronavirus Disease (COVID-19) and the Virus that Causes it. Retrieved on April 14th. World Health Organization (WHO); 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it [Google Scholar]
- 2.Wu C., Liu Y., Yang Y. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Phamaceutica Sin B. 2020 doi: 10.1016/j.apsb.2020.02.008. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu R., Zhao X., Li J. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coronavirus Disease 2019 (COVID-19) Situation Report – 85. Retrieved on Aprill 14. World Health Organization (WHO); 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200414-sitrep-85-covid-19.pdf?sfvrsn=7b8629bb_4 [Google Scholar]
- 5.Li G., Fan Y., Lai Y. Coronavirus infections and immune responses. J Med Virol. 2020;92(4):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koren G., King S., Knowles S., Phillips E. Ribavirin in the treatment of SARS: a new trick for an old drug? CMAJ (Can Med Assoc J) 2003;168(1):1289–1292. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC154189/ [PMC free article] [PubMed] [Google Scholar]
- 7.Auyeung T., Lee J., Lai W. The use of corticosteroid as treatment in SARS was associated with adverse outcomes: a retrospective cohort study. J Infect. 2005;51(2):98–102. doi: 10.1016/j.jinf.2004.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith T., Prosser T. Clinical Drug Information, Clinical Solutions, Elsevier. Retrieved on Mar 16, 2020. 2020. COVID-19 drug therapy — potential options. [Google Scholar]
- 9.Stockman L., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3(9):e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H., Wang Y., Xu J., Cao B. Potential antiviral therapeutics for 2019 novel coronavirus. Zhonghua Jie He He Hu Xi Za Zhi. 2020. 0. 43, E002. [DOI] [PubMed]
- 11.Summary of Probable SARS Cases with Onset of Illness from 1 November 2002 to 31 July 2003. 2020. https://www.who.int/csr/sars/country/table2003_09_23/en/ [Google Scholar]
- 12.Chen F., Chan K., Jiang Y. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. 2004;31(1):69–75. doi: 10.1016/j.jcv.2004.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Z., Nakamura T. Statistical evidence for the usefulness of Chinese medicine in the treatment of SARS. Phytother Res. Published online. July 2004 doi: 10.1002/ptr.1485. [DOI] [PubMed] [Google Scholar]
- 14.Jia W., Gao W. Is traditional Chinese medicine useful in the treatment of SARS? Phytother Res. 2003;17(7):840–841. doi: 10.1002/ptr.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lau T., Leung P., Wong E. Using herbal medicine as a means of prevention experience during the SARS crisis. Am J Chin Med. 2005;33(3):345–356. doi: 10.1142/S0192415X05002965. [DOI] [PubMed] [Google Scholar]
- 16.Lin L., Han Y., Yang Z. Clinical observation on 103 patients of severe acute respiratory syndrome treated by integrative traditional Chinese and Western Medicine. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2003;23(6):4009–4013. https://www.ncbi.nlm.nih.gov/pubmed/?term=Clinical+observation+on+103+patients+of+severe+acute+respiratory+syndrome+treated+by+integrative+traditional+Chinese+and+Western+medicine [PubMed] [Google Scholar]
- 17.Li J., Li S., Du N. Clinical study on treatment of severe acute respiratory syndrome with integrative Chinese and Western medicine approach. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2004;24(1):28–31. https://www.ncbi.nlm.nih.gov/pubmed/14976885 [PubMed] [Google Scholar]
- 18.Tong X., Li A., Zhang Z. TCM treatment of infectious atypical pneumonia — a report of 16 cases. J Tradit Chin Med. 2004;24(4):266–269. https://www.ncbi.nlm.nih.gov/pubmed/?term=TCM+Treatment+of+Infectious+Atypical+Pneumonia+-+A+Report+of+16+Cases [PubMed] [Google Scholar]
- 19.Zhang R., Jiao Q., Wang B. Controlled clinical study on 49 patients of SARS treated by integrative Chinese and Western medicine. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2003;23(9):654–657. https://www.ncbi.nlm.nih.gov/pubmed/?term=Controlled+clinical+study+on+49+patients+of+SARS+treated+by+integrative+Chinese+and+Western+medicine [PubMed] [Google Scholar]
- 20.Liu J., Manheimer E., Gluud C. Chinese herbal medicine for severe acute respiratory syndrome: a systematic review and meta-analysis. J Alternative Compl Med. 2004;10(6):1041–1051. doi: 10.1089/acm.2004.10.1041. [DOI] [PubMed] [Google Scholar]
- 21.Liu X., Zhang M., He L., Li Y. Chinese herbs combined with Western medicine for severe acute respiratory syndrome (SARS) Cochrane Database Syst Rev. 2012;10:CD004882. doi: 10.1002/14651858.CD004882.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung P. The efficacy of Chinese medicine for SARS: a review of Chinese publications after the crisis. Am J Chin Med. 2007;35(4):575–581. doi: 10.1142/S0192415X07005077. [DOI] [PubMed] [Google Scholar]
- 23.Zhang M., Liu X., He L. Effect of integrated traditional Chinese and Western medicine on SARS: a review of clinical evidence. World J Gastroenterol. 2004;10(23):3500–3505. doi: 10.3748/wjg.v10.i23.3500. https://search.crossref.org/?q=Zhang+M%2C+Liu+X%2C+He+L.+Effect+of+integrated+traditional+Chinese+and+Western+medicine+on+SARS%3A+a+review+of+clinical+evidence.+World+J+Gastroenterol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y., Guo J., Healy D., Zhan S. Effect of integrated traditional Chinese medicine and western medicine on the treatment of severe acute respiratory syndrome: a meta-analysis. Pharm Pr Granada. 2007;5(1):1–9. doi: 10.4321/s1886-36552007000100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu C., Hwang K., Chao C. An evaluation of the additive effect of natural herbal medicine on SARS or SARS-like infectious diseases in 2003: a randomized, double-blind, and controlled pilot study. Evid Based Complement Altern Med. 2008;5(3):355–362. doi: 10.1093/ecam/nem035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng R., Yang R., Shi M. Characterization of the 3a protein of SARS-associated coronavirus in infected vero E6 cells and SARS patients. J Mol Biol. 2004;341(1):271–279. doi: 10.1016/j.jmb.2004.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu C., Huwang K., Chao C. The lesson of supplementary treatment with Chinese medicine on severe laboratory-confirmed SARS patients. Am J Chin Med. 2006;34(6):927–935. doi: 10.1142/S0192415X06004405. [DOI] [PubMed] [Google Scholar]
- 28.Jiang Y., Wnag R., Liu J. Effect of integrative Chinese and western medicine on T-lymphocyte subsets in treating patients with severe acute respiratory syndrome. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2004;24(6):514–516. https://www.ncbi.nlm.nih.gov/pubmed/?term=Jiang+2004+Effect+of+integrative+chinese+and+western+medicine+on+T-lymphocyte [PubMed] [Google Scholar]
- 29.Han Y., Geng H., Feng W. A follow-up study of 69 discharged SARS patients. J Tradit Chin Med. 2003;23(3):214–217. https://www.ncbi.nlm.nih.gov/pubmed/14535196 [PubMed] [Google Scholar]
- 30.Luo H., Tang Q., Shang Y. Can Chinese medicine Be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chin J Integr Med. Published online February. 2020;17 doi: 10.1007/s11655-020-3192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z., Chen X., Chen F., Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. 2020;14(1):64–68. doi: 10.5582/bst.2020.01030. [DOI] [PubMed] [Google Scholar]
- 32.Tao Z., Gao J., Zhang G. Shufeng Jiedu Capsule protect against acute lung injury by suppressing the MAPK/NF-κB pathway. Biosci Trends. 2014;8(1):45–51. doi: 10.5582/bst.8.45. [DOI] [PubMed] [Google Scholar]
- 33.Chan K., Wong V., Tang S. COVID-19: an update on the epidemiological, clinical, preventive and therapeutic evidence and guidelines of integrative Chinese–western medicine for the management of 2019 novel coronavirus disease. Am J Chin Med. 2020;48(3):1–26. doi: 10.1142/S0192415X20500378. [DOI] [PubMed] [Google Scholar]
- 34.Yang Y., Islam M., Wang J., Li Y., Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int J Biol Sci. 2020;16(10):1708–1717. doi: 10.7150/ijbs.45538. https://search.crossref.org/?q=+Yang+Y%2C+Islam+M%2C+Wang+J%2C+Li+Y%2C+Chen+X.+Traditional+Chinese+medicine+in+the+treatment+of+patients+infected+with+2019-new+coronavirus+%28SARS-CoV-2%29%3A+a+review+and+perspective [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwarz S., Wang K., Yu W., Sun B., Schwarz W. Emodin inhibits current through SARS-associated coronavirus 3a protein. Antivir Res. 2011;90(1):64–69. doi: 10.1016/j.antiviral.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu C., Jan J., Ma S. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc Natl Acad Sci USA. 2004;101(27):10012–10017. doi: 10.1073/pnas.0403596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen C., Lin C., Huang K. Inhibition of SARS-CoV 3C-like protease activity by theaflavin-3,3’-digallate (TF3) Evid Based Complement Altern Med. 2005;2(2):209–215. doi: 10.1093/ecam/neh081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen L., Wang Y., Lin Y. Synthesis and evaluation of isatin derivatives as effective SARS coronavirus 3CL protease inhibitors. Bioorg Med Chem Lett. 2005;15(12):3058–3062. doi: 10.1016/j.bmcl.2005.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lau K., Lee K., Koon C. Immunomodulatory and anti-SARS activities of Houttuynia cordata. J Ethnopharmacol. 2008;118(1):79–85. doi: 10.1016/j.jep.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu B., Zhou J. SARS-CoV protease inhibitors design using virtual screening method from natural products libraries. J Comput Chem. 2005;26(5):484–490. doi: 10.1002/jcc.20186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou L., Liu Y., Zhang W. Isatin compounds as noncovalent SARS coronavirus 3C-like protease inhibitors. J Med Chem. 2006;49(12):3440–3443. doi: 10.1021/jm0602357. [DOI] [PubMed] [Google Scholar]
- 42.Chen L., Li J., Luo C. Binding interaction of quercetin-3-beta-galactoside and its synthetic derivatives with SARS-CoV 3CL(pro): structure-activity relationship studies reveal salient pharmacophore features. Bioorg Med Chem. 2006;14(24):8295–8306. doi: 10.1016/j.bmc.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang S., Du Q., Zhao K., Li A., Wei D., Chou K. Virtual screening for finding natural inhibitor against cathepsin-L for SARS therapy. Amino Acids. 2007;33(1):129–135. doi: 10.1007/s00726-006-0403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryu Y., Jeong H., Kim J. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CL(pro) inhibition. Bioorg Med Chem. 2010;18(22):7040–7047. doi: 10.1016/j.bmc.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryu Y., Park S., Kim Y. SARS-CoV 3CLpro inhibitory effects of quinone-methide triterpenes from Tripterygium regelii. Bioorg Med Chem Lett. 2010;20(6):1873–1876. doi: 10.1016/j.bmcl.2010.01.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park J., Kim J., Kwon J. Dieckol, a SARS-CoV 3CL(pro) inhibitor, isolated from the edible brown algae Ecklonia cava. Bioorg Med Chem. 2013;21(13):3730–3737. doi: 10.1016/j.bmc.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu W., Zhu H., Niu G. Synthesis, modification and docking studies of 5-sulfonyl isatin derivatives as SARS-CoV 3C-like protease inhibitors. Bioorg Med Chem. 2014;22(1):292–302. doi: 10.1016/j.bmc.2013.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park J., Ko J., Kim D. Chalcones isolated from Angelica keiskei inhibit cysteine proteases of SARS-CoV. J Enzym Inhib Med Chem. 2016;31(1):23–30. doi: 10.3109/14756366.2014.1003215. [DOI] [PubMed] [Google Scholar]
- 49.Khan S., Zia K., Ashraf S., Uddin R., Ul-Haq Z. Identification of chymotrypsin-like protease inhibitors of SARS-CoV-2 via integrated computational approach. J Biomol Struct Dyn. 2020;2:1013. doi: 10.1080/07391102.2020.1751298. [DOI] [PubMed] [Google Scholar]
- 50.Zhang D., Wu K., Zhang X., Deng S., Peng B. In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus. J Integr Med. 2020;18(2):152–158. doi: 10.1016/j.joim.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361(9374):2045–2046. doi: 10.1016/s0140-6736(03)13615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wen C., Kuo Y., Jan J. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J Med Chem. 2007;50(17):4087–4095. doi: 10.1021/jm070295s. [DOI] [PubMed] [Google Scholar]
- 53.Chang F., Yen C., Ei-Shazly M., Lin W., Lin K., Wu Y. Anti-human coronavirus (anti-HCoV) triterpenoids from the leaves of Euphorbia neriifolia. Nat Prod Commun. 2012;7(11):1415–1417. https://www.ncbi.nlm.nih.gov/pubmed/?term=Anti-Human+Coronavirus+(anti-HCoV)+Triterpenoids+from+the+Leaves+of+Euphorbia+neriifolia [PubMed] [Google Scholar]
- 54.Kao J., Fu W., Tsai T. The antiparasitic drug niclosamide inhibits dengue virus infection by interfering with endosomal acidification independent of mTOR. PLoS Neglected Trop Dis. 2018;21(8) doi: 10.1371/journal.pntd.0006715. https://search.crossref.org/?q=Kao+J%2C+Fu+W%2C+Tsai+T%2C+et+al.+The+antiparasitic+drug+niclosamide+inhibits+dengue+virus+infection+by+interfering+with+endosomal+acidification+independent+of+mTOR.+PLoS+Neglected+Trop+Dis [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Irurzun A., Carrasco L. Entry of poliovirus into cells is blocked by valinomycin and concanamycin A. Biochemistry. 2001;40(12):3589–3600. doi: 10.1021/bi002069p. [DOI] [PubMed] [Google Scholar]
- 56.Pons M. The inhibition of influenza virus RNA synthesis by actinomycin D and cycloheximide. Virology. 1973;51(1):120–128. doi: 10.1016/0042-6822(73)90372-3. [DOI] [PubMed] [Google Scholar]
- 57.Simmons G., Gosalia D., Rennekamp A., Reeves J., Diamond S., Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc Natl Acad Sci USA. 2005;102(33):11876–11881. doi: 10.1073/pnas.0505577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han S., Xu J., Guo X., Huang M. Curcumin ameliorates severe influenza pneumonia via attenuating lung injury and regulating macrophage cytokines production. Clin Exp Pharmacol Physiol. 2018;45(1):84–93. doi: 10.1111/1440-1681.12848. [DOI] [PubMed] [Google Scholar]
- 59.Kim H., Eo E., Park H. Medicinal herbal extracts of Sophorae radix, Acanthopanacis cortex, Sanguisorbae radix and Torilis fructus inhibit coronavirus replication in vitro. Antivir Ther. 2010;15(5):697–709. doi: 10.3851/IMP1615. [DOI] [PubMed] [Google Scholar]
- 60.Shen L., Niu J., Wang C. High-throughput screening and identification of potent broad-spectrum inhibitors of coronaviruses. J Virol. 2019;93(12) doi: 10.1128/JVI.00023-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin C., Tsai F., Tsai C. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antivir Res. 2005;68(1):36–42. doi: 10.1016/j.antiviral.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chiow K., Phoon M., Putti T., Tan B., Chow V. Evaluation of antiviral activities of Houttuynia cordata Thunb. extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection. Asian Pac J Tro Med. 2016;9(1):1–7. doi: 10.1016/j.apjtm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park J., Kim J., Kim Y. Tanshinones as selective and slow-binding inhibitors for SARS-CoV cysteine proteases. Bioorg Med Chem. 2012;20(19):5928–5935. doi: 10.1016/j.bmc.2012.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wen C., Shyur L., Jan J. Traditional Chinese medicine herbal extracts of Cibotium barometz, Gentiana scabra, Dioscorea batatas, Cassia tora, and Taxillus chinensis inhibit SARS-CoV replication. J Tradit Complement Med. 2011;1(1):41–50. doi: 10.1016/s2225-4110(16)30055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu M., Lee J., Lee J. Identification of myricetin and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, nsP13. Bioorg Med Chem Lett. 2012;22(12):4049–4054. doi: 10.1016/j.bmcl.2012.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schwarz S., Sauter D., Wang K. Kaempferol derivatives as antiviral drugs against the 3a channel protein of coronavirus. Planta Med. 2014;80(2-3):177–182. doi: 10.1055/s-0033-1360277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li S., Chen C., Zhang H. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antivir Res. 2005;67(1):18–23. doi: 10.1016/j.antiviral.2005.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhuang M., Jiang H., Suzuki Y. Procyanidins and butanol extract of Cinnamomi Cortex inhibit SARS-CoV infection. Antivir Res. 2009;82(1):73–81. doi: 10.1016/j.antiviral.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yi L., Li Z., Yuan K. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J Virol. 2004;78(20):11334–11339. doi: 10.1128/JVI.78.20.11334-11339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim D., Min J., Jang M. Natural bis-benzylisoquinoline alkaloids-tetrandrine, fangchinoline, and cepharanthine, inhibit human coronavirus OC43 infection of MRC-5 human lung cells. Biomolecules. 2019;9(11) doi: 10.3390/biom9110696. pii:E696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fehr A., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015 doi: 10.1007/978-1-4939-2438-7_1. 1282-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ho T., Wu S., Chen J., Hsiang C. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antivir Res. 2007;74(2):92–101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuba K., Imai Y., Rao S. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Millet J., Whittaker G. Host cell proteases: critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015;202:120–134. doi: 10.1016/j.virusres.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Wit E., van Doremalen N., Falzarano D., Munster V. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Iwata-Yoshikawa N., Okamura T., Shimizu Y., Hasegawa H., Takeda M., Nagata N. TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J Virol. 2019;93(6) doi: 10.1128/JVI.01815-18. pii: e01815-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Báez-Santos Y., St John S., Mesecar A. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds. Antivir Res. 2015;115:21–38. doi: 10.1016/j.antiviral.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Perng Y., Lenschow D. ISG15 in antiviral immunity and beyond. Nat Reriew Microbiol. 2018;16:423–439. doi: 10.1038/s41579-018-0020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Venkataraman S., Prasad B., Selvarajan R. RNA dependent RNA polymerases: insights from structure, function and evolution. Viruses. 2018 doi: 10.3390/v10020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shadrick W., Ndjomou J., Kolli R., Mukherjee S., Hanson A., Frick D. Discovering new medicines targeting helicases: challenges and recent progress. J Biomol Screen. 2013;18(7):761–781. doi: 10.1177/1087057113482586. https://search.crossref.org/?q=Shadrick+W%2C+Ndjomou+J%2C+Kolli+R%2C+Mukherjee+S%2C+Hanson+A%2C+Frick+D.+Discovering+new+medicines+targeting+helicases%3A+challenges+and+recent+progress.+J+Biomol+Screen [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ivanov K., Thiel V., Dobbe J., Van Der Meer Y., Snijder E., Ziebuhr J. Multiple enzymatic activities associated with severe acute respiratory syndrome coronavirus helicase. J Virol. 2004;78(11):5619–5632. doi: 10.1128/jvi.78.11.5619-5632.2004. https://search.crossref.org/?q=Ivanov+K%2C+Thiel+V%2C+Dobbe+J%2C+Van+Der+Meer+Y%2C+Snijder+E%2C+Ziebuhr+J.+Multiple+enzymatic+activities+associated+with+severe+acute+respiratory+syndrome+coronavirus+helicase [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McBride R., van Zyl M., Fielding B. The coronavirus nucleocapsid is a multifunctional protein. Viruses. 2014;6(8):2991–3018. doi: 10.3390/v6082991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lu W., Zheng B., Xu K. Severe acute respiratory syndrome-associated coronavirus 3a protein forms an ion channel and modulates virus release. Proc Natl Acad Sci USA. 2006;103(33):12540–12545. doi: 10.1073/pnas.0605402103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nukoolkarn V., Lee V., Malaisree M., Aruksakulwong O., Hannongbua S. Molecular dynamic simulations analysis of ritonavir and lopinavir as SARS-CoV 3CL(pro) inhibitors. J Theor Biol. 2008;254(4):861–867. doi: 10.1016/j.jtbi.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ko W., Rolain J., Lee N. Arguments in favor of remdesivir for treating SARS-CoV-2 infections. Int J Antimicrob Agents. 2020;(5) doi: 10.1016/j.ijantimicag.2020.105933. 105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li J., Zhou B., Li C. Lariciresinol-4-O-β-D-glucopyranoside from the root of Isatis indigotica inhibits influenza A virus-induced pro-inflammatory response. J Ethnopharmacol. 2015;174:379–386. doi: 10.1016/j.jep.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 87.Jie C., Luo Z., Chen H. Indirubin, a bisindole alkaloid from Isatis indigotica, reduces H1N1 susceptibility in stressed mice by regulating MAVS signaling. Oncotarget. 2017;8(62):105615–105629. doi: 10.18632/oncotarget.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Luo Z., Liu L., Wang X. Epigoitrin, an alkaloid from Isatis indigotica, reduces H1N1 infection in stress-induced susceptible model in vivo and in vitro. Front Pharmacol. 2019;10(78) doi: 10.3389/fphar.2019.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang Z., Wang Y., Zhong S. In vitro inhibition of influenza virus infection by a crude extract from Isatis indigotica root resulting in the prevention of viral attachment. Mol Med Rep. 2012;5(3):793–799. doi: 10.3892/mmr.2011.709. [DOI] [PubMed] [Google Scholar]
- 90.Park J., Yuk H., Ryu H. Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. J Enzym Inhib Med Chem. 2017;32(1):504–515. doi: 10.1080/14756366.2016.1265519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abd-Elazem I., Chen H., Bates R., Huang R. Isolation of two highly potent and non-toxic inhibitors of human immunodeficiency virus type 1 (HIV-1) integrase from Salvia miltiorrhiza. Antivir Res. 2002;55(1):91–106. doi: 10.1016/s0166-3542(02)00011-6. [DOI] [PubMed] [Google Scholar]
- 92.Zhu H., Lu X., Ling L. Houttuynia cordata polysaccharides ameliorate pneumonia severity and intestinal injury in mice with influenza virus infection. J Ethnopharmacol. 2018;218:90–99. doi: 10.1016/j.jep.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 93.Kim H., Shin H., Park H. In vitro inhibition of coronavirus replications by the traditionally used medicinal herbal extracts, Cimicifuga rhizoma, Meliae cortex, Coptidis rhizoma, and Phellodendron cortex. J Clin Virol. 2008;41(2):122–128. doi: 10.1016/j.jcv.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yan Y., Fu Y., Wu S. Anti-influenza activity of berberine improves prognosis by reducing viral replication in mice. Phytother Res. 2018;32(12):2560–2567. doi: 10.1002/ptr.6196. [DOI] [PubMed] [Google Scholar]
- 95.Cho J., Curtis-Long M., Lee K. Geranylated flavonoids displaying SARS-CoV papain-like protease inhibition from the fruits of Paulownia tomentosa. Bioorg Med Chem. 2013;21(11):3051–3057. doi: 10.1016/j.bmc.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Song Y., Kim D., Curtis-Long M. Papain-like protease (PLpro) inhibitory effects of cinnamic amides from Tribulus terrestris fruits. Biol Pharm Bull. 2014;37(6):1021–1028. doi: 10.1248/bpb.b14-00026. [DOI] [PubMed] [Google Scholar]
- 97.Dai J., Wang Q., Su Y. Emodin inhibition of influenza A virus replication and influenza viral pneumonia via the Nrf2, TLR4, p38/JNK and NF-kappaB pathways. Molecules. 2017;22(10) doi: 10.3390/molecules22101754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chowdhury P., Sahuc M., Rouillé Y. Theaflavins, polyphenols of black tea, inhibit entry of hepatitis C virus in cell culture. PloS One. 2018 Nov 28;13(11) doi: 10.1371/journal.pone.0198226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu S., Lu H., Zhao Q. Theaflavin derivatives in black tea and catechin derivatives in green tea inhibit HIV-1 entry by targeting gp41. Biochim Biophys Acta. 2005;1723(1-3):270–281. doi: 10.1016/j.bbagen.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 100.Tu Y. Artemisinin-A gift from traditional Chinese medicine to the World (nobel lecture) Angew Chem Int Ed Engl. 2016;55(35):10210–10226. doi: 10.1002/anie.201601967. [DOI] [PubMed] [Google Scholar]
- 101.Efferth T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin Canc Biol. 2017;46:65–83. doi: 10.1016/j.semcancer.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 102.Efferth T., Romero M., Wolf D., Stamminger T., Marin J., Marschall M. The antiviral activities of artemisinin and artesunate. Clin Infect Dis. 2008;47(6):804–811. doi: 10.1086/591195. [DOI] [PubMed] [Google Scholar]
- 103.Zhi H., Zhu H., Zhang Y., Lu Y., Li H., Chen D. In vivo effect of quantified flavonoids-enriched extract of Scutellaria baicalensis root on acute lung injury induced by influenza A virus. Phytomedicine. 2019;57:105–116. doi: 10.1016/j.phymed.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 104.Seong R., Kim J., Shin O. Wogonin, a flavonoid isolated from Scutellaria baicalensis, has anti-viral activities against influenza infection via modulation of AMPK pathways. Acta Virol. 2018;62(1):78–85. doi: 10.4149/av_2018_109. [DOI] [PubMed] [Google Scholar]
- 105.Dai Y., Chen S., Chai L., Zhao J., Wang Y., Wang Y. Overview of pharmacological activities of Andrographis paniculata and its major compound andrographolide. Crit Rev Food Sci Nutr. 2019;59(sup1):S17–S29. doi: 10.1080/10408398.2018.1501657. [DOI] [PubMed] [Google Scholar]
- 106.Ding Y., Chen L., Wu W., Yang J., Yang Z., Liu S. Andrographolide inhibits influenza A virus-induced inflammation in a murine model through NF-κB and JAK-STAT signaling pathway. Microb Infect. 2017;19(12):605–615. doi: 10.1016/j.micinf.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 107.Cvetkovic R., Goa K. Lopinavir/ritonavir: a review of its use in the management of HIV infection. Drugs. 2003;63(8):769–802. doi: 10.2165/00003495-200363080-00004. [DOI] [PubMed] [Google Scholar]
- 108.Kim D., Min J., Jang M. Natural bis-benzylisoquinoline alkaloids-tetrandrine, fangchinoline, and cepharanthine, inhibit human coronavirus OC43 infection of MRC-5 human lung cells. Biomolecules. 2019;9(11) doi: 10.3390/biom9110696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Weng J., Lin C., Lai H. Antiviral activity of Sambucus FormosanaNakai ethanol extract and related phenolic acid constituents against human coronavirus NL63. Virus Res. 2019 doi: 10.1016/j.virusres.2019.197767. 273:197767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ye G., Pan Z., Pan Y. Clinical characteristics of severe acute respiratory syndrome coronavirus 2 reactivation. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.001. pii: S0163-4453(20)30114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hoever G., Baltina L., Michaelis M. Antiviral activity of glycyrrhizic acid derivatives against SARS-coronavirus. J Med Chem. 2005;48(4):1256–1259. doi: 10.1021/jm0493008. [DOI] [PubMed] [Google Scholar]
- 112.Chen C., Michaelis M., Hsu H. Toona sinensis Roem tender leaf extract inhibits SARS coronavirus replication. J Ethnopharmacol. 2008;120(1):108–111. doi: 10.1016/j.jep.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yin J., Li G., Yang Q., Ren X. In vitro and in vivo effects of Houttuynia cordata on infectious bronchitis virus. Avian Pathol. 2011;40(5):491–498. doi: 10.1080/03079457.2011.605107. [DOI] [PubMed] [Google Scholar]
- 114.Tsai Y., Lee C., Yen H. Antiviral action of tryptanthrin isolated from strobilanthes cusia leaf against human coronavirus NL63. Biomolecules. 2020;10(3) doi: 10.3390/biom10030366. pii: E366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.