Abstract
Berries of Vaccinium meridionale Swartz contain a variety of phytochemicals, which are believed to account for their bioactive properties. The potential of Vaccinium meridionale Swartz pomace as a source of bioactive compounds was investigated. The dietary fiber (DF) content was assessed by the AOAC method, phenolic compounds were characterized and quantified via HPLC-PDA and UPLC-QTOF-MS. The in vitro antibacterial activity was tested against Gram-positive and Gram-negative bacteria. The antioxidant properties were assessed by the ORAC and the ABTS assays. The DF content was 52.4 ± 3.7%, phenolic compounds comprised anthocyanins (ACNs) (747.6 ± 167.5 mg cyanidin-3-glucoside/100 g FW), hydroxycinammic acids (HCAs) (229.2 ± 68.4 mg chlorogenic acid equivalents/100 g FW), flavonols (335.0 ± 139.5 rutin equivalents/100 g FW), and procyanidins (PACs) (140.9 ± 33.3 mg cocoa procyanidin equivalents/100 g FW). Staphylococcus aureus was more sensitive than E. coli. The ORAC value was 250.0 ± 32.0 μmol TE/g fresh weight (FW). Results suggest that the residue from V. meridionale S. can be utilized to obtain valuable nutraceuticals for the development of functional foods.
Keywords: Food science, Food analysis, Phenolic profile, Vaccinium meridionale Swartz, Pomace, Phenolics, Procyanidins, Antimicrobial, ORAC, ABTS
Food science, Food analysis, Phenolic profile, Vaccinium meridionale swartz, Pomace, Phenolics, Procyanidins, Antimicrobial, ORAC, ABTS.
1. Introduction
Wild bilberries are known for their bioactive properties and their antioxidant capacity, which have been attributed to their high content and variety of polyphenolic compounds (Shahidi and Ambigaipalan, 2015). Phenolic compounds present in berries exhibit anticancer, antioxidant, antineurodegenerative, and antiinflammatory effects as well as antimicrobial properties against pathogenic bacteria (Shahidi and Ambigaipalan, 2015).
Along with ACNs, phenolic acids and flavonols, procyanidins (PACs), are also present in berries from the Vaccinium genus. Procyanidins are flavonoids composed of units of (+)-catechin and/or (-)-epicatechin linked through interflavan A-type and B-type bonds. The structure difference between A and B-type PACs is important because it defines their biological properties. In general, PACs have antibacterial properties against Gram-negative and Gram-positive bacteria (Laplante et al., 2012; Leitão et al., 2005). However, A-type PACs are known for their capacity to inhibit uropathogenic bacteria (Howell et al., 1998), to prevent gastrointestinal infections (Pastene et al., 2014) and for exhibiting anti-diabetic effects (Li et al., 2016). Most Vaccinium species contain primary B-type PACs e.g., Vaccinium angustifolium Ait. and blueberries (Vaccinium corymbosum L. and Vaccinium ashei R.), whereas wild lingonberry (Vaccinium vitis-idaea L.) and cranberry (Vaccinium macrocarpon Ait.) contain A and B-type PACs. Besides cranberries and wild lingonberry, A-type PACs are found in only a few foods such as plums, peanut, cinnamon, and avocado (Prior and Gu, 2005).
Vaccinium merdionale Swartz is a shrub that is wildly distributed along mountain slopes (2800–3000 m above sea level) in Colombia. Its fruit is an edible berry with an acidic and astringent taste. This product is highly valued among consumers and is eaten as whole fruit in the form of dessert, or without the peel in the form of jams, marmalades and wine. Previous studies indicate that these berries exhibit high antioxidant capacity and a variety of bioactive polyphenolics including ACNs, flavonols and hydroxycinnamic acids (HCAs) (Garzón et al, 2010). In fact, the cytotoxic and antiproliferative properties against cancerous cell lines (González et al., 2017) of these berries, as well as their cardioprotective properties in ischemia-induced rats (Shen et al., 2018) have been attributed to such polyphenolics.
The pomace of V. meridionale S. is the byproduct that remains after pulping operations for jam, marmalades and wine processing. This waste product represents about 20% of the fresh fruit weight and it consists of skin and seeds. Generally, fruit pomace (outer layers of the fruit) is rich in polyphenols linked to the dietary fiber (DF) matrix (Saura-Calixto, 1998).
Dietary fiber has several nutraceutical properties including prevention effects against chronic diseases such as diabetes and obesity. Furthermore, DF provides functional properties to food products including water-holding capacity, oil-holding capacity, emulsion stabilization and/or gel formation (Dhingra et al., 2012). Taking these properties into account, the use of V. meridionale S. pomace as source of DF, natural antimicrobial agents and natural antioxidants is a promising approach to give an added value to this byproduct.
Phenolic compounds present in fresh V. meridionale S. have been identified and quantified (Garzón et al., 2010); however, there is no qualitative or quantitative information on the phenolic or DF composition of the fruit pomace. Identification and quantification of polyphenolics are of paramount importance to determine further utilization of this byproduct as source of nutraceuticals or natural additives. Therefore, this study aims to identify and quantify the polyphenolic compounds in extracts obtained from V. meridionale S. pomace. These results were compared with those of the fresh fruit as previously reported (Garzón et al., 2010). Additionally, the DF content of the pomace, total phenolics content (TPC), antibacterial activity against Gram-negative and Gram-positive bacteria, and antioxidant capacity of the extracts were determined.
2. Materials and methods
2.1. Reagents
HPLC reagents were from Fisher Scientific. (Fair Lawn, NJ, USA). Sodium carbonate, potassium persulphate, and Folin–Ciocalteau reagent were purchased from Merck (Darmstadt, Germany). Anthocyanin standards were purchased from Polyphenols Laboratories (Sandnes, Norway). Chlorogenic acid (3-caffeoylquinic acid), rutin, gallic acid, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), fluorescein sodium salt, 2,2-azobis-(2-amidinopropane) dihydrochloride (AAPH), 2,2′- azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), (-)-epicatechin, and (+)-catechin were from Sigma Aldrich (St. Louis, MO, USA). Isolated procyanidin standards were obtained from Mars Inc. (Hackettstown, NJ, USA). Bacterial strains were acquired from American Type Culture Collection (Manassas, VA, USA), Brain-heart infusion (BHI) broth and BHI agar were acquired from Oxoid (Basingstoke, UK).
2.2. Plant material
Ripe berries with 11.4 ± 0.55 Brix and 1.49 ± 0.51% citric acid content were collected at random from wild shrubs in the Boyacá region of Colombia. This species was classified under voucher COL208724 of The National Herbarium of Colombia. Berries were mechanically processed in a cylindrical pulper and the pomace was recovered and spread on a tray at approximately 10-mm thickness. The sample in the tray was frozen at -25 °C for 24 h and transferred to a freeze dryer (Labconco Freezone 6L; Kansas City, MO, USA) with a vacuum pressure of 0.130 mBar, a plate temperature of 20 °C, and a condenser temperature of – 50 °C. The powder obtained was ground to a fine powder and kept at -4 °C for further analysis.
2.3. Determination of moisture and dietary fiber content
Moisture content, total, soluble and insoluble DF were determined by the AOAC method (Association of Official Analytical Chemists, 1990). Final data on DF was converted to FW bases based on the moisture content of lyophilized powder.
2.4. Extraction of phenolic compounds
Phenolic compounds were obtained using ultrasound-assisted extraction according to the protocol described by Kim et al. (2005) with modifications. Ten g of freeze-dried material were mixed with 100 mL of 80% aqueous methanol and sonicated for 20 min at room temperature in a water bath (B-2200R-1; Branson, Shelton, CT, USA). Upon centrifugation at 4000 x g for 20 min, the supernatant was collected, and the pellet was reextracted until a colorless extract was obtained. Methanol was eliminated from the pooled extracts using a Buchi Rotavapor at 40 °C and the extract was taken to volume, freeze-dried and kept at 4 °C for further analysis.
2.5. Determination of total phenolic content
Total phenolics were estimated as mg of gallic acid equivalents (GAE)/100 g FW and mg of GAE/100 g DW using Folin-Ciocalteu reagent (Waterhouse, 2001). Twenty μl of extract or gallic acid standard (50–500 mg/L) was combined with 1.58 mL water and 100 μl of Folin–Ciocalteau's reagent. After 8 min of incubation at ambient temperature, 300 μl of a 20% (w/v) aqueous sodium carbonate mix was added to the previous solution. Samples were kept at room temperature for 2 h before recording their absorbance at 765 nm on a Shimadzu UV–visible spectrophotometer; model UV 160 U (Kyoto, Japan).
2.6. HPLC-PDA analysis
Freeze-dried samples were diluted in 3% aqueous formic acid and filtered with 0.45 μm PTFE filters before HPLC analysis. Anthocyanins, HCAs and flavonols were analyzed following the scheme described by Garzón et al. (2010) using a Waters 2695 gradient chromatograph (Waters Corporation, Milford, MA, USA) equipped with a model 996 photodiode array detector (PDA) and a Symmetry C18 column (C18 250 mm × 4.6 mm id, 5 μm) from Waters Corporation (Milford, MA, USA). The column temperature was set at 21 °C and the mobile phase contained 5% (v/v) formic acid in water (solvent A) and 5% (v/v) formic acid in acetonitrile (solvent B). The flow rate was 1.0 ml/min and elution started with 20% B, which remained for 20 min. The gradient reached 58% B form 20–30 min. Absorbance spectra was recorded from 200 to 700 nm with concurrent detection at 520 nm for ACNs, 320 nm for HCAs and 355 nm for flavonols.
The external standard method was applied to quantify compounds. Individual anthocyanin glycosides were quantified using calibration curves of a mixture of cyanidin, delphinidin, petunidin, peonidin, and malvidine glucoside standards. Standards of rutin and chlorogenic acid stock solutions of varying concentrations were used for quantitation of flavonols and HCAs, respectively.
Separation and monitoring of PACs was achieved according to Kelm et al. (2006) with modifications. The column, a Develosil diol 100A (5 μm, 250 by 4.6 mm) (Phenomenex, Torrance, CA) was attached to a Waters Alliance 2690 HPLC (Waters Corporation, Milford, MA) equipped with a model 474 scanning fluorescence detector with excitation at 276 nm and emission at 316 nm and set at 21 °C. Procyanidins with degrees of polymerization from DP1 (monomer) through DP10 (decamer) were quantified on the basis of an external calibration curve containing mixed procyanidin standards isolated from cocoa. Total PACs were determined by adding individual PACs. Results are given as mg cocoa procyanidin equivalents/100 g FW.
2.7. UHPLC-QTOF-MS of procyanidins
Procyanidin composition was analyzed by UHPLC with an Agilent 1290 UPLC system (Agilent Technologies Inc., Santa Clara, CA, USA) coupled to 6550 quadrupole/time-of-flight (Q-TOF) mass spectrometer (Agilent Technologies Inc., Santa Clara, CA, USA) attached to an electrospray ionization (ESI) source run in the negative ionization mode. The column was a 150 mm × 2.1 mm i.d., 1.8 μm particle size, Zorbax Eclipse Plus C18 HD (Agilent technologies Inc., Santa Clara, CA, USA) was set at 21 °C. The flow rate of the eluent was 0.5 mL/min. The elution profile used two solvents, 0.2% (v/v) acetic acid in water (A) and 0.2% (v/v) acetic acid in acetonitrile (B): 0–1.0 min, 100% A; 1.0–8.0 min, 100−60% A (linear gradient); 8.0–11.0 min, 60−0% A (linear gradient); 11.0–13.0 min, 0% A; 13.-13.1 min 0–100% A: 13.1–15 min 100% A for column wash and stabilization. UV detection was achieved at 280 nm.
ESI conditions for MS analysis were as follows: capillary voltage of 3.5 kV for negative mode, sheath gas temperature of 400 °C with a flow rate of 12 L/min; drying gas temperature of 200 °C with a flow rate of 18 L/min; 345 psi nebulizer; capillary voltage of 3.5 kV; nozzle voltage of 1000 V. Higher voltages of 25–40 eV were applied to generate more fragments for the larger polymers.
2.8. Antibacterial activity
Three bacterial strains were used to evaluate the antibacterial activity of the extracts, including the Gram-positive Staphylococcus aureus ATCC®6538™ and the Gram-negative Escherichia coli P-fimbriated ATCC®25922™ and serotype O157:H7 ATCC®700728™. Bacterial strains were stored in 15% (v/v) aqueous glycerol at -70 °C and subsequently cultured overnight at 37 °C in BHI broth under agitation at 80 RPM in an orbital shaker (Labocon, LTSIO-102, Leicester, UK). Incubation was applied until the bacterial growth reached the exponential phase. The minimum inhibitory concentration (MIC) of the pomace extracts was assessed by harvesting bacteria, which were further diluted in aqueous sodium chloride (0.85 % p/v) until reaching an optical density of 0.08–0.12 at 600 nm (working bacterial stocks), equivalent to 1.0 × 108 colony forming units (CFU)/mL. Subsequently, the working bacterial suspensions (WBS) were obtained by diluting the working bacterial stocks to 6 Log CFU/mL in BHI broth.
Aliquots (100 μL) of each WBS were mixed with 100 μL of phenolic extract (0–4000 μg GAE/mL) in 96-well cell culture plates and incubated for 18 h at 37 °C in an orbital shaker at 80 RPM. The standard plating method was used to establish microbial growth as the total viable counts (CFU/mL) after 24 h of growth in BHI Agar. The MIC was determined as the lowest TPC to inhibit the 50% growth of bacteria (IC50).
2.9. Determination of antioxidant capacity
Antioxidant capacity of the extracts was determined by the ORAC test (Prior et al., 2003) using 48-well microplates and the ABTS (Re et al., 1999) methods. For the ORAC assay, a forty-μL aliquot of diluted sample, Trolox standards (6.25, 12.5, 25, 50 μM) or a blank solution (phosphate buffer) were added to each well. Fluorescein (400 μL, 0.108 μM) followed by 150 μL of 31.6 mM AAPH was automatically injected to each well. Fluorescence was detected at 485 nm (excitation) and 520 nm (emission) after the addition of fluorescein and AAPH and every 192 s thereafter for 112 min to allow for a 95% loss of fluorescence. Results were determined as differences between the blank and the samples. Standard Trolox curves and ORAC values were calculated from the regression equation and presented as μmol Trolox equivalents (TE)/g FW and μmol TE/g DW.
For the ABTS assay, the (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)) ABTS.+ radical was prepared by reacting 7.0 mM ABTS with 2.45 mM potassium persulphate. Upon storage in the dark for 16 h, the solution was diluted in ethanol to reach an absorbance of 0.70 ± 0.01 at 734 nm (working solution). After mixing 1.0 mL of working solution with 10 μL of sample and incubating at 30 °C for 6 min, the absorbance was determined periodically until reaching a plateau. The percentage ABTS·+ inhibition was assessed by equation I = [(AB - AA)/AA] x 100; where: I = ABTS·+ inhibition, %; AB = absorbance of a blank sample (t = 0 min); AA = absorbance of an extract solution at the end of the reaction. Trolox standard solutions (250 μM–1500 μM) were to calculate ABTS·+ inhibition values within the calibration curve. Final values are given as μmol (TE)/g FW and (TE)/g DW.
2.10. Statistical analysis
All experimental results represent mean values with their corresponding standard deviations. Four replications of the experiments were carried out and all analyses were run in triplicate.
IC50 values were determined using the GraphPad Prism8® program (GraphPad Software, San Diego, CA).
3. Results and discussion
3.1. Dietary fiber content
The insoluble DF content in the pomace (44.8 ± 2.2%) was higher than the soluble fiber portion (7.6 ± 1.6%). The higher value of insoluble DF in the pomace agrees with reports from White et al. (2010), who found mainly insoluble DF (65.5%) in dry cranberry (Vaccinium macrocarpon Ait.) pomace and smaller portions soluble fiber (5.7%).
The DF content in V. meridionale S. pomace meets the definitions of a good source of DF as stated by Saura-Calixto (1998) and the first requirement to be considered as an antioxidant DF. According to Dhingra et al. (2012), healthy adults should eat between 20 and 35 g of DF each day; therefore, 50 g of dry V. meridionale S. pomace would fulfill this requirement. Saura-Calixto (1998) defined antioxidant DF as dry matter containing more than 50% fiber and DPPH free radical scavenging capacity equivalent to at least 50 mg vitamin E. Accordingly, our findings suggest the potential use of V. meridionale S. pomace as an additive to develop food products with high DF content.
3.2. Total phenolics content
The pomace of V. meridionale S. had a TPC of 5794.3 ± 169.1 mg GAE/100 g FW or 8277.5 ± 241.6 mg GAE/100 g DW. This content is higher compared to that in the pomace from V. myrtillus L., which contains 1116.24 mg GAE/100 g FW (Vulic et al., 2011).
The higher content of phenolics in the pomace as compared to the content in the whole fruit (758.6 ± 62.3 mg GAE/100 g FW) (Garzón et al., 2010) can be explained by the presence of seeds and pressed peels, which contain larger amount of ACNs, PACs and other phenolic compounds.
3.3. Identification and quantification of anthocyanin and non-anthocyanin polyphenolics
Elution order, retention time (tR), UV/vis spectra and cross-comparison with available standards and with earlier reports (Garzón et al., 2010) on polyphenolic composition of the fruit of V. meridionale S. were used to characterize ACNs, HCAs and flavonols in the pomace.
The HPLC chromatogram of the ACNs present in the V. meridionale S. pomace showed that the most prominent anthocyanin was cyanidin-3-galactoside, followed by cyanidin-3-arabinoside, delphinidin-3-hexoside, delphinidin-3-pentoside, and cyanidin-3-glucoside. This profile was consistent with the one identified in fresh V. meridionale S. fruit (Garzón et al., 2010).
Quantification of the ACNs and non-anthocyanin phenolics present in the pomace is reported in Table 1. The total ACN content was 747.6 ± 167.5 mg cyanidin-3-glucoside/100 g FW or 1245.9 ± 279.2 mg cyanidin-3-glucoside/100 g DW. This value is about twice the ACN content in the fruit (329 ± 28 mg cyanidin-3-glucoside/100 g FW) (Garzón et al., 2010), which confirms that outer layers of the fruit are rich in polyphenols (Saura-Calixto, 1998). In contrast, White et al. (2010) found 121.4 ± 5.9 mg/100 g DW in the acetone/water/acetic acid (70:29.5:0.5 v/v/v) extract from cranberry pomace (V. macrocarpon A.). Differences in berry varieties, growth conditions and extraction methods may have contributed partially to the variability in the ACN concentrations within these Vaccinium species (Vulic et al., 2011).
Table 1.
HPLC analysis of anthocyanins, hydroxycinnamic acids and flavonols present in the pomace of V. meridionale S.a.
|
Peak no |
tR (min) |
% peak area |
Phenolic compound Concentration as corresponding standard (mg/100 g FW) (mg/100 g DW) |
||
|---|---|---|---|---|---|
| Anthocyanins | |||||
| 1 | 12.0 | 5.6 | Delphinidin-3-hexoside | 38.4 ± 17.3 | 64.1 ± 28.9 |
| 2 | 13.3 | 49.6 | Cyanidin-3-galactoside | 357.7 ± 75.5 | 596.2 ± 125.9 |
| 3 | 13.5 | 4.1 | Delphinidin-3-pentoside | 34.7 ± 13.9 | 57.9 ± 23.2 |
| 4 | 14.1 | 1.2 | Cyanidin-3-glucoside | 10.1 ± 1.9 | 16.9 ± 3.2 |
| 5 | 14.8 | 39.5 | Cyanidin-3-arabinoside | 306.6 ± 60.4 | 511.0 ± 100.7 |
| Total | 747.6 ± 167.5 | 1245.9 ± 279.2 | |||
| 1 | 11.0 | 77.0 | Chlorogenic acid isomer 1 | 188.3 ± 62.3 | 313.8 ± 103.8 |
| 2 | 11.6 | 1.5 | Chlorogenic acid isomer 2 | 4.0 ± 2.5 | 6.6 ± 4.2 |
| 3 | 17.2 | 1.1 | Caffeoyl methyl quinate | 1.4 ± 0.6 | 2.4 ± 0.9 |
| 4 | 18.8 | 15.0 | Caffeic acid derivative | 26.8 ± 3.6 | 44.6 ± 5.9 |
| 5 | 24.2 | 1.0 | Caffeic acid derivative isomer 2 | 8.8 ± 1.0 | 14.6 ± 2.6 |
| Total | 229.2 ± 68.4 | 382.1 ± 114.1 | |||
| Flavonols | |||||
| 1 | 19.7 | 47.4 | Quercetin hexoside | 161.2 ± 83.1 | 268.7 ± 138.5 |
| 2 | 20.9 | 3.9 | Quercetin pentoside | 14.2 ± 5.9 | 23.7 ± 9.8 |
| 3 | 21.4 | 10.0 | Quercetin pentoside | 38.9 ± 21.5 | 64.9 ± 35.8 |
| 4 | 21.9 | 21.1 | Quercetin pentoside | 58.8 ± 25.4 | 98 ± 42.4 |
| 5 | 22.4 | 9.4 | Quercetin rhamnoside | 36.9 ± 13.8 | 61.5 ± 23 |
| 6 | 24.9 | 8.2 | Quercetin hydroxymethylglutaryl-α-rhamnoside | 24.9 ± 15.1 | 41.5 ± 25.1 |
| Total | 335.0 ± 139.5 | 558.3 ± 232.5 | |||
Results are expressed as mean ± SD (n = 4).
Cyanidin-based ACNs represented 90% of the total peak area measured at 520 nm; in contrast, the total peak area corresponding to cyanidin-based ACNs reported in the fruit extract accounted for 77% (Garzón et al., 2010). Given the high antioxidant and anti-inflammatory properties of cyanidin glycosides (Shahidi and Ambigaipalan, 2015) and their high concentration in the pomace of V. meridionale S., this byproduct may deliver health benefits to humans when added to food products.
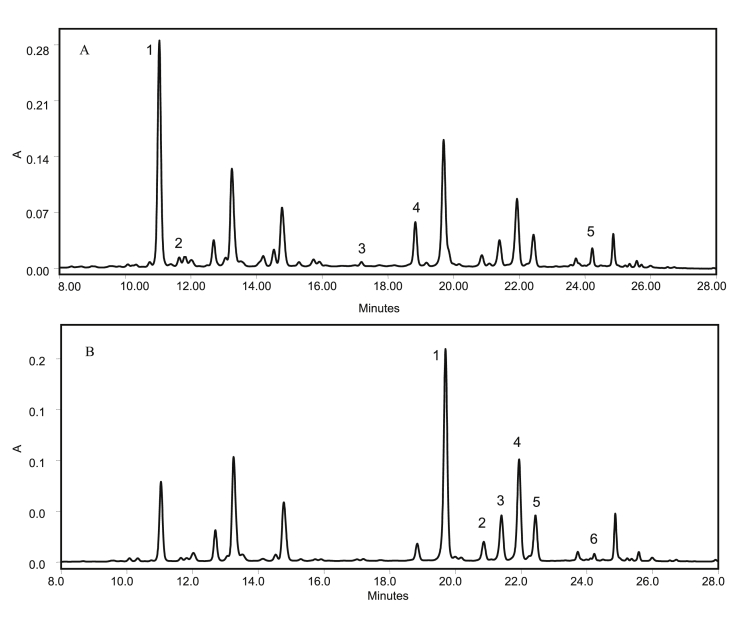
The HPLC profile of HCAs present in the pomace (Figure 1A) extract matched the one from the fruit (Garzón et al., 2010). However, one minor peak corresponding to an isomer of caffeic acid derivative was not detected in the pomace. Chlorogenic acid was present at the highest level, comprising 78.5% of the total amount of HCAs followed by caffeic acid derivatives and caffeoyl methyl quinate.
Figure 1.
A: Representative HPLC chromatogram of hydroxycinnamic detected at 320 nm in wild bilberry (V. meridionale S.) pomace. (1) caffeoylquinic acid isomer 1; (2) caffeoylquinic acid isomer 2; (3) caffeoyl methyl quinate; (4) caffeic acid derivative; (5) caffeic acid derivative. B: Representative HPLC chromatogram of flavonoids detected at 355 nm. (1) quercetin hexoside; (2) quercetin pentoside; (3) quercetin pentoside; (4) quercetin pentoside; (5) quercetin rhamnoside; (6) quercetin hydroxylmethylglutaryl-α-rhamnoside. Unlabelled peaks are anthocyanins.
The total amount of HCAs in the pomace was 229.2 ± 68.4 mg chlorogenic acid equivalents/100 g FW or 382.1 ± 114.1 mg chlorogenic acid equivalents/100 g DW, which is about twice fold the amount in fresh berries (99.2 ± 6.7 mg chlorogenic acid equivalents/100 g FW) (Garzón et al., 2010), This content is comparable to the value delivered by 200-ml serving of coffee (Mills et al., 2013). Coffee is known for its high content of chlorogenic acid, which may deliver potential health effects including reduction of the risk of cardiovascular disease and improvements in cognitive function (Mills et al., 2013).
Regarding flavonols in the pomace extract, quercetin derivatives represented 100% of the total flavonols, with quercetin hexoside being the most abundant flavonol (47.4 % of the total peak area measured at 360 nm) followed by three isomers of quercetin pentoside (35.0 % of the peak area). The profile (Figure 1B) consisting of six peaks was identical to that of the fruit extract (Garzón et al., 2010); however, the total flavonol content (335.0 ± 139.5 mg rutin equivalents/100 g FW or 558.3 ± 232.5 mg rutin equivalents/100 g DW) was considerably higher than the amount of flavonols in fresh berries (41.9 ± 4.9 mg rutin equivalents/100 g FW) and the pomace of other Vaccinium species. For example, bilberry (V. myrtillus L.) pomace contains 187 ± 2 mg rutin equivalents/100 g FW (Aaby et al., 2013). The concentration of flavonols in the pomace form V. meridionale S. indicates that high levels of these compounds are stable during pulping operations and remain in the waste material.
Quercetin is considered an exceptional free radical scavenger and consequently, this flavonol is suggested to be involved in beneficial health effects including the cardioprotective effect of red wines (Formica and Regelson, 1995) and the potential chemopreventive effects against certain types of tumors (Verma et al., 1988).
Procyanidins of DP1-DP10 were detected in V. meridionale S. pomace by normal phase HPLC and data on the identification and quantification is shown in Table 2. The total procyanidin content in the pomace was 140.9 ± 33.3 mg cocoa procyanidin equivalents/100 g FW or 234.8 ± 55.4 mg cocoa procyanidin equivalents/100 g DW. This content is significantly higher than the one found in cranberry pomace (167.3 ± 5.9 mg cocoa procyanidin equivalent/100 g DW) (White et al., 2010).
Table 2.
Normal phase HPLC analysis of procyanidins present in the pomace of V. meridionale S.a
| Procyanidin | Concentration as mg cocoa |
|
|---|---|---|
| procyanidin equivalents | ||
| (mg/100 g FW) | (mg/100 g DW) | |
| Monomer | 20.8 ± 8.0 | 34.7 ± 13.3 |
| Dimer | 13.1 ± 3.6 | 21.9 ± 6.0 |
| Trimer | 23.7 ± 6.2 | 39.5 ± 10.3 |
| Tetramer | 15.2 ± 4.0 | 25.4 ± 6.6 |
| Pentamer | 12.0 ± 3.4 | 20.0 ± 5.7 |
| Hexamer | 13.0 ± 3.2 | 21.6 ± 5.3 |
| Heptamer | 8.5 ± 2.6 | 14.1 ± 4.1 |
| Octamer | 8.5 ± 2.5 | 14.2 ± 4.2 |
| Nonamer | 17.6 ± 37 | 29.3 ± 62 |
| Decamer | 8.5 ± 2.1 | 14.1 ± 3.6 |
| Total | 140.9 ± 33.3 | 234.8 ± 55.4 |
Results are expressed as mean ± SD (n = 4).
Since the estimated daily intake of PACs in the US is 58 mg/person, with 18% coming from chocolate and grapes and 32 % from apples (Gu et al., 2004), the pomace from V. meridionale S. represents a potential source of PACs if included in food products as a functional ingredient.
Trimers (23.7 ± 6.2 mg cocoa procyanidins equivalents/100 g FW) and monomers (20.8 ± 8.0 mg cocoa procyanidins equivalents/100 g FW) were the most abundant procyanidin subunits. These contents were comparable with the contents in black chocolate (21.1 ± 0.8 mg/100 g FW and 31.4 ± 0.2 mg/100 g FW) (Gu et al., 2004). This result is important from the bioavailability point of view because recent studies suggest that PCAs of DP ≤ 4 could be absorbed intact in the gastrointestinal tract and transported across the cells (Gu et al., 2004).
3.4. UPLC-QTOF-MS of procyanidins
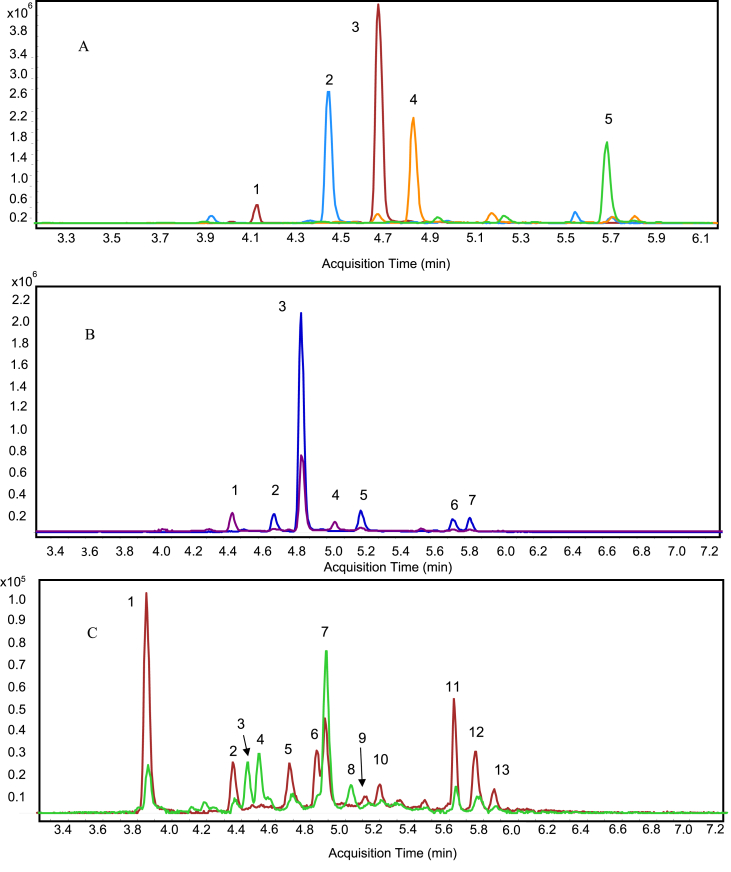
The total ion chromatograms of PACs of DP1-DP3, which were the predominant ones according to MS intensity, are shown in Figure 2A. Larger oligomers produced weak signals. Extracts ion chromatograms of PCAs with DP3 and DP4 are depicted in Figures 2B and 2C, respectively.
Figure 2.
A: Representative UHPLC total ion chromatogram of procyanidins of DP1-DP3 detected in wild bilberry (V. meridionale S.) pomace at 280 nm. (1) catechin; (2) B-type dimer; (3) epicatechin; (4) A-type trimer; (5) A-type dimer. B: Extracted ion chromatograms of procyanidin trimers. Peaks 1 and 4 are B-type isomers; peaks 2, 3, 5, 6, and 7 are A-type isomers. C: Extracted ion chromatograms of procyanidin tetramers. Peaks 1, 2, 5, 6, 9, 10, 11, 12, and 13 are A-type isomers; peaks 3, 4, 7, and 8 are B-type isomers.
Analysis of mass spectra revealed deprotonated ions for PACs from monomers to octamers. Measured m/z signals with their theoretical molecular ions and their fragment ions are shown in Table 3. Mass spectral data supports the presence of A and B-type linkages.
Table 3.
Identification of procyanidins in the pomace of V. meridionale using UPLC-ESI–MS.
| DP | Linkage type | Theoretical [M-H]− |
Observed [M-H]− |
Theoretical [M-2H]2−/2 |
Observed [M-2H]2−/2 |
Product ions |
|---|---|---|---|---|---|---|
| 1 | 289.0719 | 289.0729 | ||||
| 2 | A | 575.1195 | 575.1195 | 125.0238, 285.0404, 289.0717, 407.0761, 423.0711, 449.0867, 539.0975, 163.0036 | ||
| 2 | B | 577.1351 | 577.136 | 289, 125, 407, 245, 161 | ||
| 3 | A | 863.1829 | 863.1831 | 285.0403, 289.0719, 411.0718, 451.1026, 559.0873, 573.1028, 693.1240, 711.1344 | ||
| 3 | B | 865.1985 | 865.1999 | 287, 289, 125, 407, 577, 575, 413, 425, 449, 451, 695, 713, 739 | ||
| 4 | A | 1151.2463 | 1151.2451 | 287.0557, 411.0726, 451.0986, 573.1022, 699.1350, 863.1824, 981.1876, 861.1649, 999.1950, 289.0710, 711.1346, 285.0390 |
||
| 4 | B | 1153.2619 | 1153.262 | 575, 577,287, 863, 865, 125, 413 | ||
| 5 | A | 1439.3097 | 1439.3075 | 287.0531, 575.1185, 711.1363, 863.1781, 1151.2327 | ||
| 5 | B | 1441.3253 | 1441.3223 | 287.0530, 575.1187, 863.1821, 865.1991, 1151.2322, 1153.2635 | ||
| 6 | A | 864.1907 | 864.6915 | |||
| 6 | B | 863.1829 | 863.6847 | |||
| 7 | A | 1008.7267 | 1008.7208 | |||
| 7 | B | 1007.2146 | 1007.7141 | |||
| 8 | A | 1152.7584 | 1152.7513 | |||
| 8 | B | 1151.7506 | 1151.7449 |
Singly charged molecular ions, [M − H]− were observed for monomers through pentamers while doubly charged ions were detected for hexamers through octamers. These findings are in accordance with previous literature reports (Kalili and Villiers, 2009).
Tentative identification using further MS fragmentation was based on three characteristic routes for PACs (Gu et al., 2003); quinone methide fission (fragmentation between two catechin or epicatechin subunits), retro-Diels-Alder fission (Elimination of ring B from the flavan-3-ol) and heterocyclic ring fission. Since there is a 2 Da difference between fragment ions from B-type and A-type linkages, quinone methide fragmentation of dimers leads to pairs of product ions differing by 4 Da or 2 Da, which can be used to distinguish between types of PACs (Gu et al., 2003).
Regarding monomers, two molecular ions at m/z 289.0729, with different retention times, coincident with those from the corresponding standards catechin and epicatechin diastereoisomers were detected.
The typical molecular ions at m/z 575.1195 and 577.136 for the dimers were observed. Exact mass for the dimer at m/z 575.1195 suggested a molecular formula C30H24O12 and was tentatively identified as an A-type proanthocyanidin based on reports for fragmentation of peanut skin (Sarnoski et al., 2012) and lingonberry (Vaccinium vitis-idaea L.) PACs (Sari et al., 2006). This molecular ion resulted in fragments at m/z 125.0238, 285.0404 and 289.0717. These two last fragment ions are formed through quinone methide fission. Other typical fragment ions were produced including one at m/z 423.0711 (loss of a 152 Da fragment from the retro-Diels-Alder fragmentation of the B-ring of flavan-3-ols), 449.0867 (loss of a phloroglucinol molecule from an A-type dimer through heterocyclic ring fission).
Compound with [M − H+]− at m/z 577.136 had a calculated molecular formula of C30H26O12 and has been previously identified as a B-type procyanidin in several Vaccinium species (Jungfer et al., 2012) and peanut skin (Sarnoski et al., 2012). Molecular ion fragmentation produced ions at m/z 289 (quinone methide cleavage of the interflavan bond) and m/z 407, probably from water elimination after the RDA fragmentation of the dimer.
Five isomeric forms of procyanidin trimers at m/z 863.1831 were revealed, suggesting a molecular formula C45H36O18. Product ions at m/z 285.0403, 289.0719 (QM cleavage of the interflavan bond between the middle unit and base units), 411.0718 and 451.1026 (products of heterocyclic ring fission in the middle unit), 559.0873, 573.1018, 693.1240 ([M–152–18-H]- from retro Diels-Alder fission) and successive loss of water, and 711.1344 ([M–152–H], retro Diels-Alder fission) were observed. The same fragmentation pattern was reported in cinnamon PACs, where the fragment ion at m/z 573.3 and its conjugate ion at m/z 289.0 revealed that the A-type linkage of the trimer was between the top and middle units (Gu et al., 2003), and in hybrid bilberry (Vaccinium x intermedium Ruthe), a rare, natural hybrid of bilberry and lingonberry (Hokkanen et al., 2009).
Two isomeric B-type trimers at m/z 865.1999 were found. This molecular ion has been reported in peanut skins (Sarnoski et al., 2012) and cranberry (White et al., 2010).
The fragmentation pattern and characteristic fragment ions of trimers were followed to tentatively identify tetramers and pentamers. Nine procyanidin tetramers with [M − H]− at 1151.2451 suggested the molecular weight of 1152 of procyanidin tetramers with one A-type interflavanoid linkage located between the two internal units, as observed in peanut skins PACs (Sarnoski et al., 2012). Comparison of extracted ions to those from PACs from cinnamon (Gu et al., 2003), specifically extracted ions at m/z 863.1824 ([M−288-H]-), loss of (epi)catechin, 861.1649 and 573.1022, indicate a (epi)Cat–(epi)Cat–A–(epi)Cat–(epi)Cat connection sequence. A-type tetramers have also been identified in Litchi (Litchi chinensis Sonn.) pulp (Lv et al., 2015).
In accordance with previous findings on mass spectra of PACs (Guyot et al., 1997) doubly charged negative molecular ions were detected for higher oligomers (DP > 6). Due to the already reported pattern in decrease in ionization efficacy of PCAs when using medium resolution MS (Gu et al., 2003), the daughter ions were not detected. However, the oligomer identity was tentatively confirmed to be A and B-type PACs according to previous literature (Gu et al., 2003).
3.5. Antibacterial activity
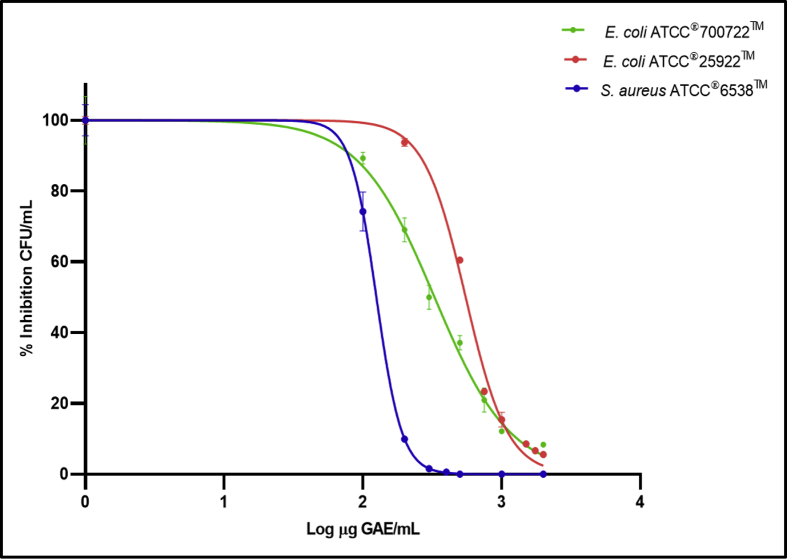
Our results show that S. aureus was the most sensitive strain to the antimicrobial activity of the extract, showing an IC50 value of 126 ± 14 μg GAE/mL, followed by E. coli Ol57:H7 (IC50 = 334 ± 18 μg GAE/mL), and E. coli ATCC 25922 (IC50 528 ± 28 μg GAE/mL) (Figure 3). Other authors have reported similar antimicrobial effects when testing cranberry juice and extracts; for example, Leitão et al. (2005) observed susceptibility of S. aureus to the anthocyanin-rich fraction isolated from cranberries and Laplante et al. (2012) reported inhibition of the growth of the Staphylococcus spp. with cranberry extracts.
Figure 3.
Minimal inhibitory concentration (IC50) of V. meridionale pomace extract against Gram positive and Gram-negative bacteria. Bacteria were grown in BHI media supplemented with varying concentrations of extract. CFU/mL in absence of extract are considered the 100%. Data are mean ± SEM from four independent experiments. Unpaired two-tailed t test at p< 0.0001.
Lacombe et al. (2010) found significant reductions of E. coli O157:H7 with cranberry phenolic extracts and Alshaibani et al. (2017) observed that a solution of cranberry PACs was effective against enteropathogenic E.coli. These authors attributed these effects to the action of A-type PACs as these compounds not only inhibit the attachment of bacteria to the epithelial cells lining, but also increase permeability of bacterial membranes, which results in cellular perforation, disintegration and death. Since V. meridionale S. pomace is rich in A-type PACs, we hypothesize that the observed antibacterial effect against E. coli Ol57:H7 is due to the presence of these compounds.
Our findings are relevant since the tested bacteria are a worldwide risk to public health. S. aureus and E. coli have shown increased antibiotic resistance; furthermore, Staphylococcus aureus enterotoxins represent one of the main causes of food-borne illness in the world, whose symptoms include intense diarrhea, nausea, vomiting, and abdominal pain (Kadariya et al., 2014). P-fimbriated E. coli causes urinary tract infections and enterohemorrhagic E. coli 0157:H7 has been linked to outbreaks of hemorrhagic colitis, some of which included deaths, particularly among children, the elderly, and those with weakened immune systems (Winata Muslimin, 2015).
3.6. Antioxidant capacity
ORAC value in the pomace extracts (250.0 ± 32.0 μmol TE/g FW or 416.8 ± 53.4 μmol TE/g DW) was significantly higher than ORAC values for cranberry pomace extract (281.3 ± 25.8 μmol of TE/g DW) (White et al., 2010), but comparable to the one for berries with outstanding antioxidant capacity. Among 120 studied species/varieties of fruits produced and consumed within the South Andes region of South America, calafate (Berberis microphylla G. Forst) showed the highest ORAC value (256.6 μmol TE/g FW) (Speisky et al., 2012). Antioxidant capacity measured by inhibition of the ABTS radical was 327.9 ± 3.5 μmol TE/g FW or 546.7 ± 5.9 μmol TE/g DW. This antioxidant capacity expressed on a FW basis is about seven-fold the average value (45.5 ± 2.3 μmol TE/100 g) found in fresh fruit (Garzón et al., 2010) and two-fold the ABTS value reported for Vaccinium macrocarpon Ait. L. pomace (Oszmianski et al., 2015).
The outstanding ORAC and ABTS values can be attributed not only to the presence of high amounts of ACNs and other phenolics, but also to their chemical structure. Cyanidin derivatives, quercetin derivatives and chlorogenic acid represent the main phenolic compounds in the pomace. All of these compounds have ortho-dihydroxy structure that have been reported to scavenge 4 moles of radical per ortho-substituted diphenol group (Shahidi and Ambigaipalan, 2015).
4. Conclusions
All in all, the present study is the first one that tentatively characterized and quantified polyphenols present in V. meridionale S. pomace from Colombia as well as its antimicrobial activity. Results indicate that this byproduct contains high levels of DF and polyphenolics with potent antioxidant capacity. It also represents a new source of A-type PACs and exhibits antimicrobial properties against Gram-positive and Gam-negative bacteria, comparable to the one documented for V. macrocarpon A. Given its phytochemical composition and the documented effectiveness of A-type PACs to control pathogenic bacteria, further research is recommended to explore the potential utilization of V. meridionale S. pomace in nutraceutical, pharmaceutical, and food applications. Such applications would contribute to waste management and recovery of health benefit phenolic compounds.
Declarations
Author contribution statement
Gloria A. Garzon: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Carlos Y. Soto, Luke Howard: Contributed reagents, materials, analysis tools or data.
Marcela López-R: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Kenneth M. Riedl: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Cindi R. Browmiller: Performed the experiments; Analyzed and interpreted the data.
Funding statement
This research was supported by Grant No 41646 from the Investigaction Division (DIB) at Universidad Nacional de Colombia, Bogotá.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Aaby K., Grimmer S., Holtung L. Extraction of phenolic compounds from bilberry (Vaccinium myrtillus L.) press residue: effects on phenolic composition and cell proliferation. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2013;54(1):257–264. [Google Scholar]
- Alshaibani D., Zhang R., Wu V.C.H. Antibacterial characteristics and activity of Vaccinium macrocarpon proanthocyanidins against diarrheagenic Escherichia coli. J. Func. Foods. 2017;39:133–138. [Google Scholar]
- Association of Official Analytical Chemists . In: fifteenth ed. Helrich K., editor. Vol. 1. Association of Official Analytical Chemists, Inc; Virginia, USA: 1990. (AOAC: Official Methods of Analysis). [Google Scholar]
- Dhingra D., Michael M., Rajput H., Patil R.T. Dietary fibre in foods: a review. J. Food Sci. Technol. 2012;49:255–266. doi: 10.1007/s13197-011-0365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formica J.V., Regelson W. Review of the biology of quercetin and related bioflavonoids. Food Chem. Toxicol. 1995;33(12):1061–1080. doi: 10.1016/0278-6915(95)00077-1. [DOI] [PubMed] [Google Scholar]
- Garzón G.A., Narváez C.E., Riedl K.M., Schwartz S.J. Chemical composition, anthocyanins, non-anthocyanin phenolics and antioxidant activity of wild bilberry (Vaccinium meridionale Swartz) from Colombia. Food Chem. 2010;122(4):980–986. [Google Scholar]
- González M., Samudio I., Sequeda-Castañeda L.G., Celis C., Iglesias J., Morales L. Cytotoxic and antioxidant capacity of extracts from Vaccinium meridionale Swartz (Ericaceae) in transformed leukemic cell lines. J. Appl. Pharmaceut. Sci. 2017;7:24–30. (03) [Google Scholar]
- Gu L., Kelm M.A., Hammerstone J.F., Beecher G., Holden J., Haytowitz D. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004;134:613–617. doi: 10.1093/jn/134.3.613. [DOI] [PubMed] [Google Scholar]
- Gu L., Kelm M.A., Hammerstone J.F., Zhang Z., Beecher G., Holden J. Liquid chromatographic/electrospray ionization mass spectrometric studies of proanthocyanidins in foods. J. Mass Spectrom. 2003;38:1272–1280. doi: 10.1002/jms.541. [DOI] [PubMed] [Google Scholar]
- Guyot S., Doco T., Moutounet M., Drilleau J. Characterization of highly polymerized procyanidins in cinder apple (Malus sylvestris var. Kermerrien) skin and pulp. Phytochemistry. 1997;44(2):351–357. [Google Scholar]
- Hokkanen J., Mantilla S., Jaakola L., Pirttila A.M., Tolonen A. Identification of phenolic compounds from lingonberry (Vaccinium vitis-idaea L.), bilberry (Vaccinium myrtillus L.) and hybrid bilberry (Vaccinium x intermedium Ruthe L) leaves. J. Agric. Food Chem. 2009;57:9437–9447. doi: 10.1021/jf9022542. [DOI] [PubMed] [Google Scholar]
- Howell A., Vorsa N., Marderosian A.D., Foo L.Y. Inhibition of the adherence of P-fimbriated Escherichia coli to uroepithelial-cell surfaces by proanthocyanidin extracts from cranberries. N. Engl. J. Med. 1998;339(15):1085–1086. doi: 10.1056/NEJM199810083391516. [DOI] [PubMed] [Google Scholar]
- Jungfer E., Zimmermann B.F., Ruttkat A., Galensa R. Comparing procyanidins in selected Vaccinium species by UHPLC-MS with regard to authenticity and health effects. J. Agric. Food Chem. 2012;60:9688–9696. doi: 10.1021/jf303100q. [DOI] [PubMed] [Google Scholar]
- Kadariya J., Smith T.C., Thapaliya D. Staphylococcus aureus and staphylococcal food-borne disease: an ongoing challenge in public health. BioMed Res. Int. 2014;1–9 doi: 10.1155/2014/827965. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalili K.M., De Villiers A. Off-line comprehensive 2-dimensional hydrophilic interaction × reversed phase liquid chromatography analysis of procyanidins. J. Chromatogr. A. 2009;1216:6274–6284. doi: 10.1016/j.chroma.2009.06.071. [DOI] [PubMed] [Google Scholar]
- Kelm M.A., Johnson C., Robbins R.J., Hammerstone J.F., Schmitz H.H. High-performance liquid chromatography separation and purification of cacao (Theobroma cacao L.) procyanidins according to degree of polymerization using a diol stationary phase. J. Agric. Food Chem. 2006;54:1571–1576. doi: 10.1021/jf0525941. [DOI] [PubMed] [Google Scholar]
- Kim D.O., Ho J.H., Young J.K., Hyun S.Y., Lee C.Y. Sweet and sour cherry phenolics and their protective effects on neuronal cells. J. Agric. Food Chem. 2005;53(26):9921–9927. doi: 10.1021/jf0518599. [DOI] [PubMed] [Google Scholar]
- Lacombe A., Wu V.C.H., Tyler S., Edwards K. Antimicrobial action of the American cranberry constituents; phenolics, anthocyanins, and organic acids, against Escherichia coli O157:H7. Int. J. Food Microbiol. 2010;139:102–107. doi: 10.1016/j.ijfoodmicro.2010.01.035. [DOI] [PubMed] [Google Scholar]
- Laplante K.L., Sarkisian S.A., Woodmansee S., Rowley D.C., Seeram N.P. Effects of cranberry extracts on growth and biofilm production of Escherichia coli and staphylococcus species. Phytother Res. 2012;26(9):1371–1374. doi: 10.1002/ptr.4592. [DOI] [PubMed] [Google Scholar]
- Leitão D.P.S., Polizello A.C.M., Ito I.Y., Spadaro A.C.C. Antibacterial screening of anthocyanin and proanthocyanin fractions from cranberry juice. J. Med. Food. 2005;8(1):36–40. doi: 10.1089/jmf.2005.8.36. [DOI] [PubMed] [Google Scholar]
- Li X., Sui Y., Li S., Xie B., Sun Z. A-type procyanidins from litchi pericarp ameliorate hyperglycemia by regulating hepatic and muscle glucose metabolism in streptozotocin (STZ) -induced diabetic mice fed with high fat diet. J. Func. Foods. 2016;27:711–722. [Google Scholar]
- Lv Q., Luo F., Zhao X., Liu Y., Hu G., Sun C. Identification of proanthocyanidins from litchi (Litchi chinensis Sonn.) pulp by LC-ESI- Q-TOF-MS and their antioxidant activity. PloS One. 2015;20:1–17. doi: 10.1371/journal.pone.0120480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills C.E., Oruna-concha M.J., Mottram D.S., Gibson G.R., Spencer J.P.E. The effect of processing on chlorogenic acid content of commercially available coffee. Food Chem. 2013;141(4):3335–3340. doi: 10.1016/j.foodchem.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Oszmianski J., Kolniak-ostek J., Lachowicz S., Gorzelany J. Effect of dried powder preparation process on polyphenolic content and antioxidant capacity of cranberry (Vaccinium macrocarpon L.) Ind. Crop. Prod. 2015;77:658–665. [Google Scholar]
- Pastene E., Parada V., Avello M., Ruiz A., García A. Catechin-based Procyanidins from peumus boldus mol. aqueous extract inhibit helicobacter pylori urease and adherence to adenocarcinoma gastric cells. Phytother Res. 2014;28(11):1637–1645. doi: 10.1002/ptr.5176. [DOI] [PubMed] [Google Scholar]
- Prior R.L., Gu L. Occurrence and biological significance of proanthocyanidins in the American diet. Phytochemistry. 2005;66:2264–2280. doi: 10.1016/j.phytochem.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Prior R.L., Hoang H., Gu L., Wu X., Bacchiocca M., Howard L. Assays for hydrophilic and lipophilic antioxidant capacity oxygen radical absorbance capacity (ORAC FL) of plasma and other biological and food samples. J. Agric. Food Chem. 2003;51:3273–3279. doi: 10.1021/jf0262256. [DOI] [PubMed] [Google Scholar]
- Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free radical biology and medicine. Free Radical Biol. Med. 1999;28:1637–1645. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Sari E., Kartimo H., Mantilla S., Tolonen A. Characterization of phenolic compounds from lingonberry (Vaccinium vitis-idaea) J. Adv. Sci. Arts. 2006;54:9834–9842. doi: 10.1021/jf0623687. [DOI] [PubMed] [Google Scholar]
- Sarnoski P.J., Johnson J.V., Reed K.A., Tanko J.M., Keefe S.F.O. Separation and characterization of proanthocyanidins in Virginia type peanut skins by LC – MS. Food Chem. 2012;131(3):927–939. [Google Scholar]
- Saura-Calixto F. Antioxidant dietary fiber product: a new concept and a potential food ingredient. J. Agric. Food Chem. 1998;46:4303–4306. [Google Scholar]
- Shahidi F., Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: antioxidant activity and health effects–A review. J. Func. Foods. 2015;18:820–897. [Google Scholar]
- Shen M., Li K., Jing H., Zheng L. In vivo therapeutic effect of Vaccinium meridionale swartz in ischemia-reperfusion induced male albino rats. J. Food Sci. 2018;83:221–228. doi: 10.1111/1750-3841.13986. [DOI] [PubMed] [Google Scholar]
- Speisky H., Alarcón-lópez C., Fuentes J., Sandoval-Acuña C. First web-based database on total phenolics and oxygen radical absorbance capacity (ORAC) of fruits produced and consumed within the South Andes region of South America. J. Adv. Sci. Arts. 2012;60:8851–8859. doi: 10.1021/jf205167k. [DOI] [PubMed] [Google Scholar]
- Verma A., Johnson J., Gould M., Tanner M. Inhibition of 7,12-dimethylbenz(a)anthracene- and N-nitrosomethylurea-induced rat mammary cancer by dietary flavonol quercetin. Canc. Res. 1988;48(20):5754–5758. [PubMed] [Google Scholar]
- Vulic J.J., Tumbas V.T., Savatovic S.M., Sonja D.M., Cetkovic G.S., Canadanovic-Brunet J.M. Polyphenolic content and antioxidant activity of the four berry fruits pomace extracts. Acta Period. Technol. 2011;42:1–288. [Google Scholar]
- Waterhouse L. Determination of total phenolics. In: Wrolstad R.E., editor. Handbook of Food Analytical Chemistry. John Wiley and Sons; New Jersey: 2001. pp. 463–470. [Google Scholar]
- White B.L., Howard L.R., Prior R.L. Polyphenolic composition and antioxidant capacity of extruded cranberry pomace. J. Adv. Sci. Arts. 2010;58:4037–4042. doi: 10.1021/jf902838b. [DOI] [PubMed] [Google Scholar]
- Winata Muslimin L. Antimicrobial inhibition on zoonotic bacterial Escherichia coli O157: H7 as a cause of food borne disease. Am. J. Biomed. Life Sci. 2015;2:163. [Google Scholar]