Change history
5/29/2020
Editor’s Note: The Editor in Chief is currently investigating this article as concerns have been raised regarding the integrity of some of data presented here. Further editorial action will be taken as appropriate once the investigation into the concerns is complete and all parties have been given an opportunity to respond in full.
Abstract
10β,17β-dihydroxyestra-1,4-dien-3-one (DHED) which is a brain-selective prodrug of 17β-estradiol has been reported to improve the cognitive function in Alzheimer’s disease (AD) mice model. However, little is known about the potential mechanism for cognitive improvement. In the present study, we used AD mice to investigate the effects and mechanisms of DHED treatment. Female Tg2576 transgenic AD mice were ovariectomized and then treated by implanting Alzet osmotic minipumps containing DHED or vehicle subcutaneously for 8 weeks. Consistent with previous report, DHED treatment ameliorated cognitive function of AD mice with decreasing Aβ levels in the hippocampus. Besides, we also found DHED treatment could reduce oxidative and inflammatory stress and the level of p-tau. The mechanisms underlying the cognitive function improvement may be linked with estrogen receptor (ER)-klf5-NF-κB pathway, demonstrated by decreased expression of klf5 and the secretion of inflammatory cytokines. However, the effects of DHED treatment could be reversed when ERα was inhibited by ICI182780. Taken together, our findings uncovered a new mechanism for DHED to improve the cognitive function of AD mice and may provide a viable therapy to treat AD.
Electronic supplementary material
The online version of this article (10.1007/s00210-019-01639-w) contains supplementary material, which is available to authorized users.
Keywords: Alzheimer’s, klf5, DHED, ICI182780, Inflammation
Introduction
Alzheimer’s disease (AD) is a major cause of dementia which is characterized by a progressive cognitive and neuronal dysfunction clinically, neuroinflammation, and neuronal death (Assoc 2018; Congdon and Sigurdsson 2018). Accumulative deposits of aggregated amyloid-β peptide (Aβ) in the brain is believed to be the primary pathogenic cause of AD (Rajmohan and Reddy 2017; Rangachari et al. 2018). The number the AD patients in America is expected to increase from 5.7 million today to 13.8 million by 2050 according to the World Alzheimer report. However, there has been no effective therapy for AD currently (Vina and Sanz-Ros 2018). Therefore, it is important to develop effective agents to slow or halt the neurodegenerative process and alleviate pathology of AD.
It is well known that estrogen has a wide range of beneficial effects in the maintenance of normal brain function, loss of which in aging may increase the risk of AD (Bimonte-Nelson et al. 2010). The reason for the higher prevalence and greater severity of AD in the postmenopausal women than age-matched men is closely linked with the reduced concentration of estrogen (Baum 2005; Irvine et al. 2012; Pike 2017). Now, the neuroprotective effects of estrogen have been stressed by several investigations, which are associated with decreased neuroinflammation and Aβ accumulation (Li et al. 2014; Yun et al. 2018). Besides, estrogen receptor α (ERα) is thought to be an indispensable element from estrogen to regulate the estrogen-sensitive activities (Audet-Walsh and Giguere 2015; Lan et al. 2015; Tang et al. 2018). Although the mechanism for estrogen ameliorating AD has been extensively studied and huge progresses have been achieved, there is still a long way to go, considering clinical treatment effect.
The Krüppel-like transcription factor 5 (KLF5) which widely expressed in various tissues is a transcriptional factor playing significant roles in cell proliferation, differentiation, carcinogenesis, and inflammation (Diakiw et al. 2013; Gao et al. 2015). In ER-positive breast cancer cells, estrogen could induce a degradation of KLF5 through the E3 ubiquitin ligase EFP (Zhao et al. 2011). Besides, lipopolysaccharide (LPS) could induce and upregulate KLF5 expression in human bronchial epithelial cells and umbilical vein endothelial cells and upregulated KLF5 could induce the expression of nuclear factor-kappaB (NF-κB), thus regulating inflammatory response (Chen et al. 2014). High glucose could induce KLF5 nitration and could activate the expression of inflammatory cytokines tumor necrosis factor α (TNF-α) and interleukin-1β (IL-1β) in vascular smooth muscle cells (VSMCs), while 17β-estradiol could inhibit high glucose-mediated effects in VSMCs (Zhang et al. 2017). However, an actual relationship between KLF5 and inflammation in the context of AD is not fully understood.
In this study, we characterized how estrogen signaling ameliorated cognitive function of AD mice. We found that DHED which is a brain-selective prodrug of 17β-estradiol and produces the hormone only in the brain could improve the memory deficits in Tg2576 transgenic AD model and could decrease Aβ and phosphorylated tau protein levels in the brain of ovariectomized female AD mice (Merchenthaler et al. 2016; Prokai et al. 2015). Besides, DHED could also enhance superoxide dismutase (SOD) in the hippocampus, while decrease malondialdehyde (MDA). Furthermore, the beneficial effects of DHED on AD were achieved by inhibiting KLF5 regulated inflammatory pathway and blocking ERα by ICI182780 could counteract the effects of DHED. Our findings explored a mechanism of estrogen improving AD and may provide a new method for AD treatment.
Material and methods
Reagents and antibodies
17β-dihydroxyestra-1,4-diene-3-one (DHED) and ICI182780 were purchased from Sigma-Aldrich (St. Louis, MO) and Selleck (TX, USA), respectively. The primary antibodies used for Western blot analysis were as follows: anti-TNF-α, anti-IL1β, anti-IL-6, anti-klf5, anti-p-IκB kinase (IKK), anti-inhibitor of NF-κB α (IκBα), anti-tau, anti-p-tau (ser235), and anti-p-tau (ser396) were purchased from Cell Signaling Technology (Beverly, MA, USA). Anti-GAPDH was from Proteintech Group (Wuhan, China).
Animals
Tg2576 mice which carry mutant human gene APPswe (Swedish mutations k670N/M671L) were obtained from the Institute of Laboratory Animal Science, Chinese Academy of Medical Science (Beijing, China). Animals were housed in a controlled environment (temperature 21 ± 1 °C, humidity 50 ± 10%) under a 12-h light/dark cycle, allowed standard rodent chow and water. In this study, only specific pathogen-free AD female mice were used and all experimental procedures were approved by the Ethics Committee of The First Affiliated Hospital of Zhengzhou University.
Treatments and monitoring
Following 1-week habituation, female mice aged 6 months were anesthetized with pentobarbital (sigma) (50 mg/kg) and bilateral ovariectomies or sham surgery (control group) was performed. Alzet osmotic minipumps (model number 2004, 28-days delivery at 0.025 μL/min, DURECT corporation, Cupertino, CA, USA) containing DHED (2 μg/day) or vehicle (propylene glycol) were implanted subcutaneously over the 8-week period of treatment. The concentration of the DHED was 56 μg/ml. Pumps were replaced once, at the 4-week time point. Besides, DHED was dissolved in propylene glycol. ICI182780 at 50 mg/kg dissolved into a mixture of castor oil and ethanol and benzene methanol (7:2:1) was injected into the muscles once a week and the vehicle group mice were injected with castor oil mixture at the same time.
Morris water maze (MWM)
Eight weeks after treatment, the cognitive performance of mice was assessed by Morris Water Maze (MWM) test. The pool was a circular metal tank (120 cm in diameter, 40 cm deep, four quadrants) filled with water and an escape platform (10 cm in diameter) was placed inside the pool, its upper surface 1 cm below the surface of the water, so that a mouse inside the pool would not be able to locate it visually. The first stage is the training period in order to allow mice to adapt to the surrounding environment, mice were subjected to training twice daily for four consecutive days and probe trials and navigation tests were conducted on the fifth day. Each mouse was allowed to swim for up to 60 s in search of the escape platform. Motion parameters were recorded for each mouse using a computer program. A quiet environment was maintained throughout the period of the experiment.
Tissue preparation
Following behavioral testing, animals were anesthetized with pentobarbital and immediately cardiac-perfused with 0.9% saline solution. The hippocampus was removed and frozen in liquid nitrogen and stored at − 80 °C for Western blot, ELISA and PCR.
Aβ ELISA
Hippocampus samples were stored until further processing via homogenization using T-PER reagent (Thermo Fisher) with protease inhibitors (Thermo Fisher). Then, supernatant was collected after centrifuged for 1 h at 14,000g, 4 °C, and the sediments were resuspended in 70% formic acid solution and then centrifuged for 1 h at 14,000g, 4 °C to collect the supernatant to detect the insoluble Aβ40,42. Soluble and insoluble Aβ levels were quantified by Aβ40,42 ELISA kits (Invitrogen, NY) and the assay was performed according to the manufacturer’s instruction.
Determination of oxidative markers
The homogenates supernatants of hippocampus samples were collected after treatment as indicated. Then, the relative level of oxidative markers including the activity of SOD and the level of MDA were measured by using commercial kits (Jiancheng Bioengineering Inst., China) according to the manufacturer’s instruction.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated from hippocampus with Trizol reagent (Invitrogen, Grand Island, NY, USA) and then subjected to reverse transcription using the StarScript first strand cDNA synthesis kit (Transgen Biotech, Beijing, China). Real-time PCR was performed in triplicate using the SYBR Green PCR Master Mix (Applied Biosystems). The β-actin gene served as an endogenous control for normalization. Relative expression levels of different genes were calculated by the 2-ΔΔCt method, and the histogram for fold comparison of different samples was generated by GraphPad Prism 5 (Roche, Switzerland). Experiments were carried out in triplicate three times. The sequences of primers for qRT-PCR were summarized in Supplementary Table 1.
Western blotting
Total proteins were extracted from hippocampus tissues after grinding with liquid nitrogen with radioimmune precipitation assay (RIPA) buffer (Beyotime, Shanghai, China) with protease inhibitor mixture (Thermo Fisher Scientific). Protein concentration was determined by BCA Protein Assay Reagent (Thermo Fisher Scientific). Equal quantities of each protein sample were resolved in SDS-PAGE and transferred to PVDF membranes (Sigma, USA). Membranes were blocked with 5% BSA in TBST for 1 h followed by incubation with diluted primary antibodies and HRP-conjugated secondary antibody separately. Image J software was used to quantify the relative expression level of target proteins, which were normalized to each internal control. Three independent experiments were carried out.
Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM). Statistical significance was performed by using one-way analysis of variance (ANOVA) test or unpaired Student’s t test. Multiple comparisons between the groups were performed using S-N-K method. A P value < 0.05 was considered as statistically significant.
Results
Prevention of memory deficits in ovariectomized female Tg2576 transgenic AD by DHED treatment
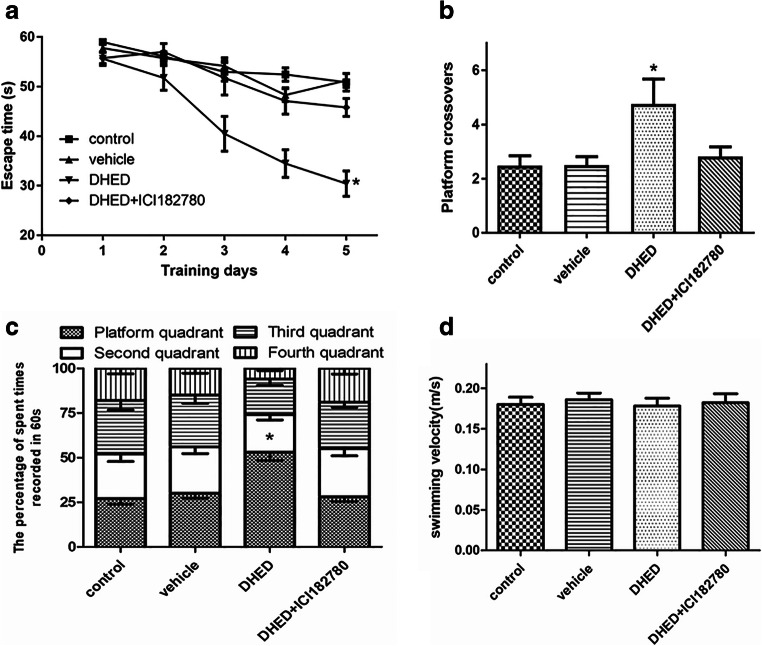
Tg2576 transgenic mice model is a widely used animal model of AD that exhibit severe deficits in spatial memory and high level of amyloid deposits at 6–7 months of age (Bilkei-Gorzo 2014). In the previous studies, DHED which is a bioprecursor prodrug can convert to 17β-estradiol by a short chain dehydrogenase/reductase in the brain and its treatment has been proved to slow the progression of AD characteristics, while, its target is not clear (Prokai et al. 2015; Tschiffely et al. 2018; Tschiffely et al. 2016). Here, we used ICI182780 which is an antiestrogen reagent which competes with estrogen for the ERα (Boer 2017). In our experiments, we treated the ovariectomized female Tg2576 mice with DHED solely or combined with ICI182780 at the same time for 2 months. Then, we assessed spatial learning and memory abilities using the water maze task. As expected, the untreated Tg2576 mice or the vehicle-treated mice exhibited unequivocal learning deficits in the MWM test compared with DHED-treated mice as indicated by significantly longer latency, little crossing numbers, and lower proportion of time spent in the target quadrant (Fig. 1a–c). Besides, ICI182780 could reverse the benefit effects of DHED when treated the mice with DHED and ICI182780 simultaneously (Fig. 1a–c). Meanwhile, the performance of control mice was similar to that in the vehicle-treated group and there were no significant differences in swimming speed among the groups (Fig. 1d).
Fig. 1.
Prevention of memory deficits in ovariectomized female Tg2576 transgenic mice by DHED treatment. Ovariectomized female mice were divided into groups as control (no treatment), vehicle, DHED, or DHED and ICI182780 treatment at the same time for 2 months, then the effects of treatment on spatial learning-memory of AD mice were tested. a Escape time in seconds required for finding the platform. *p < 0.05 vs control or vehicle or DHED+ICI182780. b Frequency of platform crossover. c The percentage of time spent in the four quadrants during 60 s. *p < 0.05 vs control or vehicle or DHED+ICI182780. d Swimming velocity. Four groups of mice were used, n = 10 in each group. Data are presented as mean values ± SEM. ANOVA, *p < 0.05
DHED treatment significantly decreases Aβ level in hippocampus
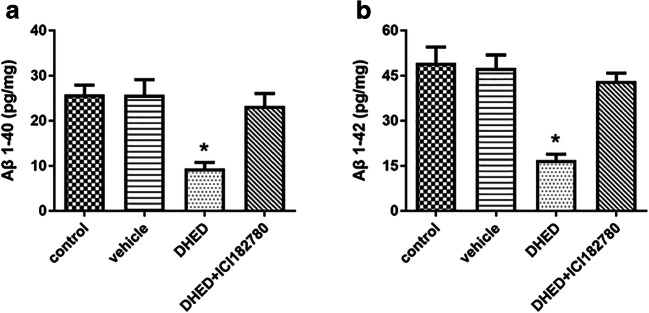
Given that Aβ is a critical pathological feature of AD, so we tested the levels of both soluble and insoluble Aβ40,42 by using ELISA. Our results showed that DHED treatment could significantly decrease both soluble and insoluble Aβ40,42 in hippocampus when compared to the control or vehicle-treated mice (Fig. 2a, b). Besides, ICI182780 could counteract the effects of DHED. These findings indicated the DHED-induced cognitive improvement is associated with a decrease in the expression of Aβ.
Fig. 2.
The effects of DHED and ICI182780 on Aβ level. a, b Aβ 1-40 and Aβ 1-42 peptide levels in the brain of AD mice after treatment were tested. Data are expressed as pg peptide/mg ± SEM (N = 6–9/group) determined by ELISA. ANOVA, *p < 0.05 vs control or vehicle or DHED+ICI182780
DHED treatment significantly decreases phosphorylated tau protein level in hippocampus
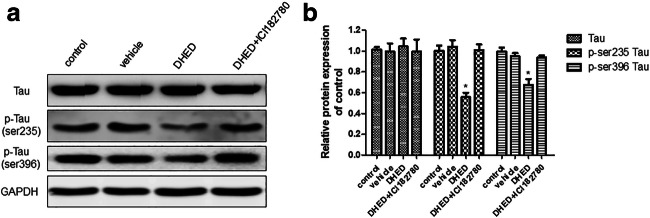
Total proteins were extracted from hippocampus tissues, then total and phosphorylated tau protein were analyzed. Total tau protein had no difference among all groups, and DHED could decrease phosphorylated tau protein. However, when combined with ICI182780, DHED could not decrease phosphorylated tau protein (Fig. 3a). The expression of proteins detected by Western blotting was analyzed by Image J (Fig. 3b).
Fig. 3.
DHED treatment decreased phosphorylated tau protein expression in the hippocampus of AD mice. a Western blot showed the relative expression of total tau, p-ser235 tau, and p-ser396 tau in the hippocampus of the mice. b The protein expressions were normalized to GAPDH and the fold changes were calculated relative to the control. Data are displayed as mean values ± SEM. ANOVA, *p < 0.05 vs control or vehicle or DHED+ICI182780
DHED treatment alleviates oxidative stress in hippocampus of the ovariectomized female Tg2576 mice
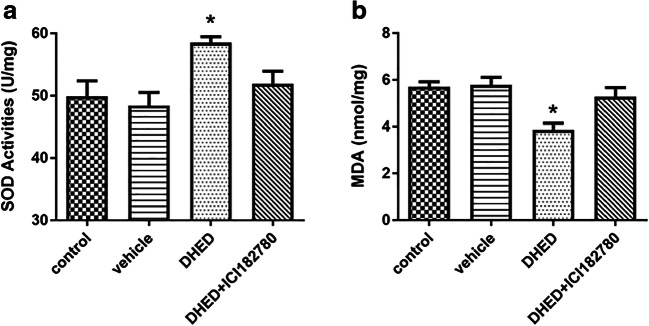
It has been shown that the presence of oxidative damage is one of the pathological hallmarks of AD (Wojsiat et al. 2018); in order to determine the beneficial antioxidative effect of DHED on the ovariectomized female Tg2576 mice, we assessed the levels of oxidative markers in hippocampus. The results showed that the levels of SOD were significantly elevated, and the levels of MDA were significantly reduced in the DHED treated group compared with control or vehicle groups, while treated with DHED and ICI182780 at the same time did not improve the oxidative stress in hippocampus (Fig. 4a, b). That means that DHED treatment could decrease oxidative stress in the hippocampus of the ovariectomized female Tg2576 mice.
Fig. 4.
DHED treatment decreased oxidative stress in the hippocampus of AD mice. The activity of SOD (a) and the level of MDA (b) were measured after the treatment described as above. Four groups of mice were used, n = 6–9 in each group. Data are presented as mean values ± SEM. ANOVA, *p < 0.05 vs control or vehicle or DHED+ICI182780
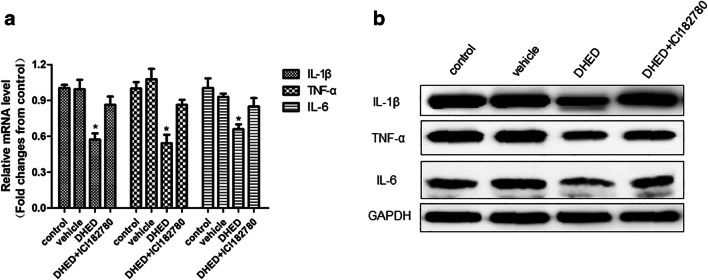
DHED treatment relieves inflammatory stress in hippocampus
The inflammatory factors such as cytokines and chemokines have been reported to play a vital role in the occurrence and development of AD (Heneka et al. 2015; Liu et al. 2014; Patel et al. 2005). Therefore, we detected the levels of inflammatory factors such as IL-1β, TNF-α, and IL-6 by qRT-PCR and Western blot. We found that DHED treatment could relieve inflammatory stress in hippocampus compared with control or vehicle-treated groups (Fig. 5a, b). However, we also found that when ERα is inhibited by ICI182780, it reversed the action of DHED.
Fig. 5.
The effects of DHED and ICI182780 on inflammatory cytokines. The mRNA level (a) and protein level (b) of IL-1β, TNF-α, and IL-6 in the hippocampus were determined by qRT-PCR and Western blot. Gene expressions were normalized to β-actin and quantified relative to that of the control AD mice. (n = 6–9/group) ANOVA, *p < 0.05 vs control or vehicle or DHED+ICI182780
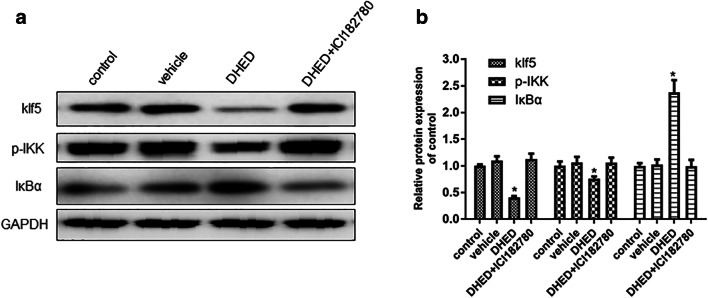
DHED inhibits KLF5-NF-κB pathway in hippocampus
As described above, DHED-treated ovariectomized female Tg2576 mice exhibited enhanced performance in cognitive tests and decreased the levels of Aβ, p-tau, and oxidative and inflammatory stress in hippocampus. Besides, ICI182780 which is a competitor for estrogen could counteract the beneficial effects of DHED. This indicated that the brain-selective 17β-estradiol estrogen prodrug, DHED, improved AD mainly through ER pathway. However, little is known about the molecular mechanism of DHED in regulating AD.
Given that estrogen could degrade klf5 in ER-positive breast cancer cells and klf5 could regulate inflammation through NF-κB pathway, we examined the expression in hippocampus of klf5, p-IKK, and IκBα. The results manifested that DHED could reduce the expression of klf5 and IκB and increase the expression of p-IKK (Fig. 6a, b). ICI182780 could reverse DHED-induced decreasing of klf5 and inflammatory factors (Fig. 6a, b). In conclusion, our results demonstrated that DHED could regulate klf5-NF-κB pathway and decrease the secretion of inflammatory factor, therefore, improving AD symptom.
Fig. 6.
The effect of DHED and ICI182780 on klf5-NF-κB signal pathway. a In hippocampus, the expression of klf5, p-IKK, and IκBα was tested by Western blot. b The protein expressions were normalized to GAPDH and the fold changes were calculated relative to the control. Data are displayed as mean values ± SEM. (n = 6–9/group) ANOVA, *p < 0.05 vs control or vehicle or DHED+ICI182780
Discussion
It has been reported that estrogen treatment has the potential for the treatment of mouse model of AD, especially DHED which is a brain-selective prodrug of 17β-estradiol did not have side effect of increasing uterine tissue weight when compared with 17β-estradiol treatment (Merchenthaler et al. 2016; Prokai et al. 2015). However, the precise mechanism of DHED treatment for AD has not been identified. In the present study, we used ovariectomized female Tg2576 mice, to detect whether DHED treatment could ameliorate cognition and to investigate the underlying mechanism. We confirmed that learning and memory were significantly improved by prolonged treatment with DHED through water maze task. Besides, we also found that Aβ40,42 in hippocampus decreased as reported before. Moreover, our results indicate that ERα is essential for DHED to hinder the progression of AD, which is consistent with some findings. These results suggest that the mechanism of DHED improving cognitive function involves ER pathway. Although several researches have proved that DHED treatment has significant improvements for AD mice model, clinical trials of estrogen-containing hormone therapy in AD patients does not provides credible evidence for Alzheimer prevention (Henderson 2014). Therefore, research that might resolve this issue will have important public health significance.
Previous studies reported that increased p-tau protein, oxidative stress, and neuroinflammation in the hippocampus is closely correlated with cognitive dysfunction of AD (Machado et al. 2014). Therefore, we analyzed the expression of total tau and p-tau protein expression, besides with the activities of SOD and the levels of MDA. Our results demonstrate that DHED treatment increases the activity of SOD and decreases levels of p-tau and MDA. These data manifest that DHED decreases p-tau and oxidative stress. Since oxidative stress deranged signaling pathways leading to tau hyperphosphorylation (Clausen et al. 2012; Kang et al. 2017), the result that DHED decreases the level of p-tau may rely on relieving oxidative stress.
Evidence showed that AD is not restricted to neurodegenerative process but strongly interacts with immunological mechanisms in the brain (Zhang et al. 2013). Cytokines including TNF-α, IL-6, and IL-1β are associated with increased Aβ in aging Tg2576 mice, so we analyzed the RNA and protein levels of TNF-α, IL-6, and IL-1β. We observed a significantly decreasing of TNF-α, IL-6, and IL-1β in the hippocampus. These data demonstrate that DHED might involve in inhibiting the inflammatory pathway to influence Aβ level in hippocampus.
To further investigate the underlying mechanism, we examined the protein level of klf5 which has been reported to be regulated by ER and is associated with NF-κB pathway (Liu et al. 2013). We found that the level of klf5 and IκBα decreased significantly in the hippocampus of DHED-treated group when compared with control or vehicle-treated groups. However, when treated with DHED and ICI182780 simultaneously, the effect of DHED could be reversed. Recent evidence supports the notion that klf5 plays an important role in inhibiting inflammation pathway. Thus, our results indicate that DHED mediates the improvement of cognitive function of ovariectomized female Tg2576 mice mainly by inhibiting klf5-NF-κB pathway and restraining oxidative and inflammatory stress.
Taken together, our findings may suggest that prolonged DHED treatment for AD mice improves cognitive function of AD mice by restraining klf5-related inflammatory pathways, decreasing hippocampal oxidative stress and the level of p-tau. Our results firstly suggest that DHED could inhibit klf5 correlated inflammatory pathway in AD mice model and that might provide a viable therapy to treat AD.
Electronic supplementary material
(DOCX 13 kb)
Author contribution
WH Y and YM X designed the research, WH Y and JW performed the research, BS and QL analyzed the data, WH Y wrote the paper, and YM X supervised the research.
Funding information
This work was supported by the National Natural Science Foundation of China Grant (No. 81530037 and 81471158 to Dr. Yuming Xu; No. 81571158 to Dr. Bo Song).
Compliance with ethical standards
In this study, only specific pathogen-free AD female mice were used and all experimental procedures were approved by the Ethics Committee of The First Affiliated Hospital of Zhengzhou University.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
This article has been retracted. Please see the retraction notice for more detail: https://dx.doi.org/10.1007/s00210-021-02121-2
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/16/2021
A Correction to this paper has been published: 10.1007/s00210-021-02121-2
References
- Assoc A. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 2018;14:367–425. doi: 10.1016/j.jalz.2018.02.001. [DOI] [Google Scholar]
- Audet-Walsh E, Giguere V. The multiple universes of estrogen-related receptor alpha and gamma in metabolic control and related diseases. Acta Pharmacol Sin. 2015;36:51–61. doi: 10.1038/aps.2014.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum LW. Sex, hormones, and Alzheimer’s disease. J Gerontol a-Biol. 2005;60:736–743. doi: 10.1093/gerona/60.6.736. [DOI] [PubMed] [Google Scholar]
- Bilkei-Gorzo A. Genetic mouse models of brain ageing and Alzheimer’s disease. Pharmacol Ther. 2014;142:244–257. doi: 10.1016/j.pharmthera.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Acosta JI, Talboom JS. Neuroscientists as cartographers: mapping the crossroads of gonadal hormones, memory and age using animal models. Molecules. 2010;15:6050–6105. doi: 10.3390/molecules15096050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer K. Fulvestrant in advanced breast cancer: evidence to date and place in therapy (vol 9, pg 465, 2017) Ther Adv Med Oncol. 2017;9:725–725. doi: 10.1177/1758834017743368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HL, et al. Kruppel-like factor 5 mediates proinflammatory cytokine expression in lipopolysaccharide-induced acute lung injury through upregulation of nuclear factor-kappa B phosphorylation in vitro and in vivo. Mediat Inflamm. 2014;2014:281984. doi: 10.1155/2014/281984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen A, Xu X, Bi X, Baudry M. Effects of the superoxide dismutase/catalase mimetic EUK-207 in a mouse model of Alzheimer’s disease: protection against and interruption of progression of amyloid and tau pathology and cognitive decline. J Alzheimers Dis. 2012;30:183–208. doi: 10.3233/JAD-2012-111298. [DOI] [PubMed] [Google Scholar]
- Congdon EE, Sigurdsson EM. Tau-targeting therapies for Alzheimer disease. Nat Rev Neurol. 2018;14:399–415. doi: 10.1038/s41582-018-0013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diakiw SM, D’Andrea RJ, Brown AL. The double life of KLF5: opposing roles in regulation of gene-expression, cellular function, and transformation. IUBMB Life. 2013;65:999–1011. doi: 10.1002/iub.1233. [DOI] [PubMed] [Google Scholar]
- Gao Y, Ding Y, Chen HY, Chen HJ, Zhou J. Targeting Kruppel-like factor 5 (KLF5) for cancer therapy. Curr Top Med Chem. 2015;15:699–713. doi: 10.2174/1568026615666150302105052. [DOI] [PubMed] [Google Scholar]
- Henderson VW. Alzheimer’s disease: review of hormone therapy trials and implications for treatment and prevention after menopause. J Steroid Biochem Mol Biol. 2014;142:99–106. doi: 10.1016/j.jsbmb.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, Khoury JE, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine K, Laws KR, Gale TM, Kondel TK. Greater cognitive deterioration in women than men with Alzheimer’s disease: a meta analysis. J Clin Exp Neuropsychol. 2012;34:989–998. doi: 10.1080/13803395.2012.712676. [DOI] [PubMed] [Google Scholar]
- Kang SW, Kim SJ, Kim MS. Oxidative stress with tau hyperphosphorylation in memory impaired 1,2-diacetylbenzene-treated mice. Toxicol Lett. 2017;279:53–59. doi: 10.1016/j.toxlet.2017.07.892. [DOI] [PubMed] [Google Scholar]
- Lan YL, Zhao J, Li S. Update on the Neuroprotective effect of estrogen receptor alpha against Alzheimer’s disease. J Alzheimers Dis. 2015;43:1137–1148. doi: 10.3233/Jad-141875. [DOI] [PubMed] [Google Scholar]
- Li RN, Cui J, Shen Y. Brain sex matters: estrogen in cognition and Alzheimer’s disease. Mol Cell Endocrinol. 2014;389:13–21. doi: 10.1016/j.mce.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Dong JT, Chen CS. Role of KLF5 in hormonal signaling and breast cancer development. Vitam Horm. 2013;93:213–225. doi: 10.1016/B978-0-12-416673-8.00002-2. [DOI] [PubMed] [Google Scholar]
- Liu C, Cui GH, Zhu MP, Kang XP, Guo H. Neuroinflammation in Alzheimer’s disease: chemokines produced by astrocytes and chemokine receptors. Int J Clin Exp Patho. 2014;7:8342–8355. [PMC free article] [PubMed] [Google Scholar]
- Machado A, Herrera AJ, de Pablos RM, Espinosa-Oliva AM, Sarmiento M, Ayala A, Venero JL, Santiago M, Villarán RF, Delgado-Cortés MJ, Argüelles S, Cano J. Chronic stress as a risk factor for Alzheimer’s disease. Rev Neurosci. 2014;25:785–804. doi: 10.1515/revneuro-2014-0035. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lane M, Sabnis G, Brodie A, Nguyen V, Prokai L, Prokai-Tatrai K. Treatment with an orally bioavailable prodrug of 17beta-estradiol alleviates hot flushes without hormonal effects in the periphery. Sci Rep. 2016;6:30721. doi: 10.1038/srep30721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NS, Paris D, Mathura V, Quadros AN, Crawford FC, Mullan MJ. Inflammatory cytokine levels correlate with amyloid load in transgenic mouse models of Alzheimer’s disease. J Neuroinflammation. 2005;2:9. doi: 10.1186/1742-2094-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike CJ. Sex and the development of Alzheimer’s disease. J Neurosci Res. 2017;95:671–680. doi: 10.1002/jnr.23827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokai L, Nguyen V, Szarka S, Garg P, Sabnis G, Bimonte-Nelson HA, McLaughlin KJ, Talboom JS, Conrad CD, Shughrue PJ, Gould TD, Brodie A, Merchenthaler I, Koulen P, Prokai-Tatrai K. The prodrug DHED selectively delivers 17beta-estradiol to the brain for treating estrogen-responsive disorders. Sci Transl Med. 2015;7:297ra113. doi: 10.1126/scitranslmed.aab1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajmohan R, Reddy PH. Amyloid-Beta and Phosphorylated tau accumulations cause abnormalities at synapses of Alzheimer’s disease neurons. J Alzheimers Dis. 2017;57:975–999. doi: 10.3233/JAD-160612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangachari V, Dean DN, Rana P, Vaidya A, Ghosh P (2018) Cause and consequence of Abeta - lipid interactions in Alzheimer disease pathogenesis. Biochim Biophys Acta. 10.1016/j.bbamem.2018.03.004 [DOI] [PMC free article] [PubMed]
- Tang Y, Min Z, Xiang XJ, Liu L, Ma YL, Zhu BL, Song L, Tang J, Deng XJ, Yan Z, Chen GJ. Estrogen-related receptor alpha is involved in Alzheimer’s disease-like pathology. Exp Neurol. 2018;305:89–96. doi: 10.1016/j.expneurol.2018.04.003. [DOI] [PubMed] [Google Scholar]
- Tschiffely AE, Schuh RA, Prokai-Tatrai K, Prokai L, Ottinger MA. A comparative evaluation of treatments with 17beta-estradiol and its brain-selective prodrug in a double-transgenic mouse model of Alzheimer’s disease. Horm Behav. 2016;83:39–44. doi: 10.1016/j.yhbeh.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschiffely AE, Schuh RA, Prokai-Tatrai K, Ottinger MA, Prokai L. An exploratory investigation of brain-selective estrogen treatment in males using a mouse model of Alzheimer’s disease. Horm Behav. 2018;98:16–21. doi: 10.1016/j.yhbeh.2017.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vina J, Sanz-Ros J. Alzheimer’s disease: only prevention makes sense. Eur J Clin Investig. 2018;48:e13005. doi: 10.1111/eci.13005. [DOI] [PubMed] [Google Scholar]
- Wojsiat J, Zoltowska KM, Laskowska-Kaszub K, Wojda U. Oxidant/antioxidant imbalance in Alzheimer’s disease: therapeutic and diagnostic prospects. Oxid Med Cell Longev. 2018;2018:Artn 6435861. doi: 10.1155/2018/6435861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun J, Yeo IJ, Hwang CJ, Choi DY, Im HS, Kim JY, Choi WR, Jung MH, Han SB, Hong JT. Estrogen deficiency exacerbates Abeta-induced memory impairment through enhancement of neuroinflammation, amyloidogenesis and NF-kB activation in ovariectomized mice. Brain Behav Immun. 2018;73:282–293. doi: 10.1016/j.bbi.2018.05.013. [DOI] [PubMed] [Google Scholar]
- Zhang B, Gaiteri C, Bodea LG, Wang Z, McElwee J, Podtelezhnikov AA, Zhang C, Xie T, Tran L, Dobrin R, Fluder E, Clurman B, Melquist S, Narayanan M, Suver C, Shah H, Mahajan M, Gillis T, Mysore J, MacDonald ME, Lamb JR, Bennett DA, Molony C, Stone DJ, Gudnason V, Myers AJ, Schadt EE, Neumann H, Zhu J, Emilsson V. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell. 2013;153:707–720. doi: 10.1016/j.cell.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ML, Zheng B, Tong F, Yang Z, Wang ZB, Yang BM, Sun Y, Zhang XH, Zhao YL, Wen JK. iNOS-derived peroxynitrite mediates high glucose-induced inflammatory gene expression in vascular smooth muscle cells through promoting KLF5 expression and nitration. Bba-Mol Basis Dis. 2017;1863:2821–2834. doi: 10.1016/j.bbadis.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Zhao KW, Sikriwal D, Dong XY, Guo P, Sun XD, Dong JT. Oestrogen causes degradation of KLF5 by inducing the E3 ubiquitin ligase EFP in ER-positive breast cancer cells. Biochem J. 2011;437:323–333. doi: 10.1042/Bj20101388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 13 kb)