Abstract
Retroperitoneal sarcomas (RPS) are rare heterogeneous tumors arising in the retroperitoneum with unique biological and behavioral patterns that are thought to be closely linked to histology. The aim of the study was to audit our results and analyze various clinico-pathological factors including surgical excision, histology, and their implications on the recurrences and survival outcomes in RPS. Retrospective analysis of patients treated at a tertiary referral center in India from March 2008 to July 2017 was performed. The clinico-pathological variables were analyzed for their association with tumor recurrence and survival with special emphasis on histological subtype. The primary outcome was overall survival (OS). One hundred consecutive patients operated for RPS were analyzed. Of these, 27 were operated for recurrent tumors. Liposarcomas (LPS) and leiomyosarcomas (LMS) constituted 50% (n = 50) and 30% (n = 30) of patients respectively. Complete tumor excision was achieved in 83%, with 43% patients undergoing adjacent organ resection. At a median follow-up of 25.3 months, the median disease-free survival (DFS) and overall survival (OS) were 30 months and 87.8 months respectively. On multivariate analysis, tumor grade was the only factor to significantly affect survival (p = 0.001 for DFS and 0.005 for OS). There was no difference in survival outcomes between infiltrative and adhesive tumors with respect to adjacent organ invasion (p = 0.361 for OS). Tumor grade remains an important prognostic factor affecting disease-free and overall survival in retroperitoneal sarcomas irrespective of tumor size, site, and histology.
Keywords: Retroperitoneal sarcoma, Multivisceral resection, Grade, Histology
Introduction
Retroperitoneal sarcomas (RPS) constitute 15% of all soft tissue sarcomas [1]. They are rare [2], heterogeneous group of tumors with variable biological behavior and outcomes. The peak incidence of RPS is in the 5th decade, although any age can be affected [3]. Liposarcomas (LPS) and leiomyosarcomas (LMS) represent the most common histological types. Complete surgical excision remains the standard of care in order to achieve long-term cure [4].
Patterns of recurrences are closely related to histology and grade, with low-grade tumors showing a predominantly local recurrence pattern. On the contrary, high-grade tumors often fail systemically, the most common site being the lung. Ten to fifteen percent of patients present with synchronous metastases [5] and have a dismal outlook. Local recurrences in low-grade tumors tend to be asymptomatic; these recurrences manifest only when the tumor attains a considerable size. Owing to the anatomical site and the presence of critical structures surrounding the tumor, re-excision may also be inadequate/ incomplete. Multiple recurrences due to inadequate/incomplete surgeries worsen the overall outcome and increase the risk of tumor dissemination [3]. This underscores the principle of first attempt at resection being the best attempt at complete resection.
Both patient and tumor-related prognostic factors influence outcomes. Patient factors include age and gender; tumor-related factors include tumor size, histology, grade, location, and extent of local and distant disease.
The aim of this study is to audit our results and analyze factors affecting recurrence and survival in resected patients of RPS at a single tertiary referral center in India. Over the years, there has been a steady rise in the number of cases of RPS being operated as shown in Fig. 1.
Fig. 1.
Bar graph showing a steady increase in the number of operated patients at our institution over the years
Materials and Methods
This was a retrospective analysis of a prospectively maintained database of patients undergoing retroperitoneal sarcoma excision with a curative intent at Tata Memorial Hospital from March 2008 to July 2017. Patients with gastrointestinal stromal tumors (GIST), desmoids, and visceral and pediatric sarcomas were excluded. All patients underwent a thorough clinical examination with emphasis on pertinent details regarding the type of surgical procedure and recurrence-free intervals. Outside specimens were reviewed by a group of expert sarcoma pathologists at our institute; in cases where establishing a histological diagnosis deemed difficult, these were subjected to a multidisciplinary meeting involving the clinicians, pathologists, and radiologists. Tumors were categorized as upfront or recurrent respectively.
All patients underwent a computed tomography (CT) scan of the abdomen and thorax. The type of treatment (surgical/neoadjuvant therapy) was based on the size and location of the tumor and the ability to achieve R0 resection. Patients whose tumors were deemed such that a safe R0 resection was not feasible were given neoadjuvant therapy (radiation or chemotherapy). Chemotherapy administered was doxorubicin and ifosfamide–based combination. Reassessment after neoadjuvant therapy was performed 4 weeks after last chemotherapy/radiation. Where the tumor was found to be attached/infiltrating adjacent organs, organ excision was done wherever feasible. Histopathology reports were analyzed for tumor size, histology, nodal involvement (if any), margins, grade, and adjacent organs being microscopically involved or not. Tumor size/diameter was calculated on the surgical specimen excised. In case where multiple tumor masses were removed, the diameter of the largest mass was taken into account. Histology was in accordance with the World Health Organization (WHO) classification. Tumor grade was classified based on differentiation, necrosis, and mitotic rate per high-powered field as per the FNCLCC (Fédération Nationale des Centres de Lutte Contre Le Cancer) system [6]. Each adjacent organ removed was counted for except for the kidney and ureter which was considered as single organ. Margins were evaluated both grossly and microscopically and categorized as clear, microscopically positive, or grossly positive. Post-operative morbidity and mortality were graded as per Clavien-Dindo grading system [7].
Patients received adjuvant therapy based on histology, grade, tumor size, and margin status. The decision to administer adjuvant therapy was taken in multidisciplinary clinic. All patients were followed up every 3 months for the first 2 years, every 6 months for the subsequent 3 years, and annually thereafter. At each visit, a complete physical examination was done with imaging of the abdomen and chest. Local recurrence (LR) was defined as recurrences occurring within the retroperitoneum and distant recurrence (DR) was defined as those occurring in the lung, liver, peritoneal lining, and nodes.
Statistical Analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) ver. 25 software (IBM Corporation, NY; formerly SPSS Inc. Chicago, IL). Descriptive statistics including median, frequency, and percentage for categorical variables was used. The Kaplan-Meier method with Cox logistic regression was used to calculate survival. Statistical significance was defined as p value < 0.05. Univariate and multivariate analyses were used to identify prognostic variable which would affect recurrences and survival. Overall survival (OS) was defined as the time period from the date of surgical resection until the date of last follow-up or death while disease-free survival (DFS) was defined as the time period from the date of surgical resection until the development of recurrent disease. Time to tumor recurrence was defined as the date of documented pathologic or radiographic recurrence of disease from the date of previous definitive treatment. The primary outcome was OS and secondary outcome was recurrence rate.
Results
Patient Demographic, Surgical, and Pathological Details
A total of 100 patients were included in the study. The various demographic, surgical, and pathological details are depicted in Table 1. In upfront and recurrent tumors, R0 resection rates were 83% and 77% respectively. The histological subtypes are shown in Table 2. Grade III tumors in particular demonstrated the following histologies: LMS 73.3% (n = 22/30), LPS 44% (n = 22/50), spindle cell sarcoma 85.7% (n = 6/7), and pleomorphic sarcoma 100% (n = 4/4).
Table 1.
Patient demographic, surgical, and pathological parameters
| Parameter | Values | |
|---|---|---|
| Type of tumors at presentation | Upfront | 73 (73%) |
| Recurrent | 27(27%) | |
| Gender | Males | 60 (60%) |
| Females | 40 (40%) | |
| Age (years) | Median | 52 |
| Range | 21–81 | |
| ECOG status | 0 | 16 (16%) |
| 1 | 84 (84%) | |
| Symptoms at presentation | Pain in abdomen | 46% |
| Abdominal lump | 26% | |
| Prior biopsy | Yes | 72 (72%) |
| -Upfront + recurrent | 50 + 22 | |
| No | 28 (28%) | |
| Blood loss (median) | 1350 ml | |
| Median hospital stay (range) | 9 (6–67) days | |
| Resection status | R0 | 83 (83%) |
| R1 | 08 (8%) | |
| R2 | 09 (9%) | |
| Post-operative complications (29%) | II | 2 patients |
| III | 16 patients | |
| IV | 5 patients | |
| V | 1 patient | |
| Median tumor diameter (range) | 15 cm (3.5–60 cm) | |
| Tumor grade | I | 31 (31%) |
| II | 13 (13%) | |
| III | 56 (56%) | |
| pT status | T1b | 13 (13%) |
| T2b | 87 (87%) | |
| pN status | N0/Nx | 97 (97%) |
| N+ | 03 (3%) | |
| Margins | Negative | 66 (66%) |
| Positive | 34 (34%) | |
| Neoadjuvant therapy (11%) (n = 11) | Chemotherapy | 7 (7%) |
| Radiotherapy | 1 (1%) | |
| Both (sequential/concurrent) | 3 (3%) | |
| Adjuvant therapy (63%) (n = 63) | Chemotherapy | 09 (9%) |
| Radiotherapy | 50 (50%) | |
| Concurrent chemoradiation | 04 (4%) | |
Table 2.
Histological subtypes on final pathology, both in upfront and recurrent tumors
| Histology | Number | Percent |
|---|---|---|
| Liposarcoma | 50 | 50 |
| -WDLPS | 29 | 29 |
| -DDLPS | 21 | 21 |
| Leiomyosarcoma | 30 | 30 |
| Spindle cell sarcoma | 7 | 7 |
| Myofibroblastic sarcoma | 2 | 2 |
| Myxoid sarcoma | 5 | 5 |
| Pleomorphic sarcoma | 4 | 4 |
| Desmoplastic round cell | 1 | 1 |
| Follicular dendritic cell sarcoma | 1 | 1 |
| Total | 100 | 100 |
WDLPS Well differentiated liposarcoma, DDLPS Dedifferentiated liposarcoma
Contiguous organ resections were performed in 43% of patients and vascular resections in 7%. Additional 7% of patients underwent both multivisceral and vascular resections in the same sitting. The kidney was the most common organ resected (n = 33) followed by the large bowel (n = 22) and adrenal (n = 15) (Table 3). The total number of organs resected was 72 with median number of organ resected being 2. Sixty-eight patients (68%) had tumors adherent to the adjacent organs while 40 patients (40%) had tumor invasion into the adjacent organs on final histological confirmation that also included 6 patients with inferior vena cava (IVC) sarcomas. Adjacent organ resections were evaluated with respect to histology, grade of the tumor, and quadrant in which the tumor was predominantly located (Table 4). It was found that high-grade tumors, tumors in the right lower quadrant, and those having dedifferentiated liposarcoma and leiomyosarcoma histologies had a higher necessity for adjacent organ resection.
Table 3.
Adjacent organ removed during surgery: adhesion alone versus infiltration on pathology
| Organ removed (n) | Adhesion | Infiltration on final HPR |
|---|---|---|
| Kidney/ureter (33) | 18 | Renal capsule: 1 |
| Renal parenchyma: 3 | ||
| Renal pelvis and hilum: 4 | ||
| Ureteric wall: 2 | ||
| Perirenal/periureteric fat positive: 5 | ||
| Spleen (10) | 9 | Spleen capsule: 1 |
| Pancreas (8) | 6 | Parenchyma: 2 |
| Small intestine (13) | 5 | Serosa involved: 3 |
| Muscularis propria involved: 4 | ||
| Subserosal involvement: 1 | ||
| Adrenal (15) | 14 | 1 |
| Colon* and rectum (22) | 14 | Full thickness involved: 5 |
| Serosa involved: 2 | ||
| Pericolonic fat/mesentery involved: 1 | ||
| Appendix (10) | 9 | 1 |
| Liver (3) | 2 | 1 |
| Vessels only (5) | 1 | Adventitia involved: 3 |
| Vessel wall infiltrated: 1 | ||
| Ovary and tube (1) | - | 1 |
| Gall bladder (6) | 6 | - |
| Stomach (2) | 2 | - |
| Lung (1) | 1 | - |
| Diaphragm (1) | 1 | - |
| Testis and scrotum (1) | 1 | - |
| Iliac bone (1) | 1 | - |
HPR histopathology report. *Both right and left colon
Table 4.
Relationship of adjacent organ removal with various parameters including grade, location, and histology
| Parameter | Infiltration | Adhesion | Total |
|---|---|---|---|
|
1 organ removed 2 organs removed 3 organs removed 4 organs removed 5 organs removed Vessel alone removed |
6 11 5 12 1 5 |
15 9 6 2 0 0 |
21 20 11 14 1 5 |
|
RUQ RLQ LUQ LLQ |
11 12 8 9 |
6 11 7 8 |
17 23 15 17 |
|
Grade I Grade II Grade III |
12 4 24 |
7 3 22 |
19 7 46 |
|
WDLPS DDLPS LMS Myxoid sarcoma UPS Spindle cell sarcoma Sarcoma NOS FDCT Myofibroblastic |
10 11 12 1 2 2 - 1 1 |
7 9 7 3 3 1 1 - 1 |
17 20 19 4 5 3 1 1 2 |
RUQ Right upper quadrant, RLQ Right lower quadrant, LUQ Left upper quadrant, LLQ Left lower quadrant, UPS Undifferentiated pleomorphic sarcoma, FDCT Follicular dendritic cell tumour
Treatment Details
Neoadjuvant Treatment
Eleven patients (11%) received pre-operative treatment. Five patients received ifosfamide and Adriamycin–based chemotherapy. Two patients received vincristine and cyclophosphamide–based chemotherapy.
Adjuvant Treatment
Sixty-three patients (63%) received adjuvant treatment. In patients with grade III tumors, adjuvant chemotherapy, radiation, and concurrent chemoradiotherapy were administered in 8 patients, 33 patients, and 3 patients respectively. Twelve patients with grade III tumors, however, received no adjuvant therapy. The reasons included old age (n = 6), doubtful compliance (n = 1), large tumor at presentation (n = 2), prior concurrent chemoradiation (CTRT) (n = 1), and post-operative complications (n = 2). Four patients with grade II tumors received radiation/chemoradiation. When the two main histological subtypes were considered, 26 patients with LPS received adjuvant therapy (52%) which included radiotherapy in 25 patients. However, most patients with LMS received some form of adjuvant treatment which included RT (n = 18; 60%), chemotherapy (n = 3; 10%), and concurrent chemoradiation (n = 2; 6.67%).
Oncological Outcomes
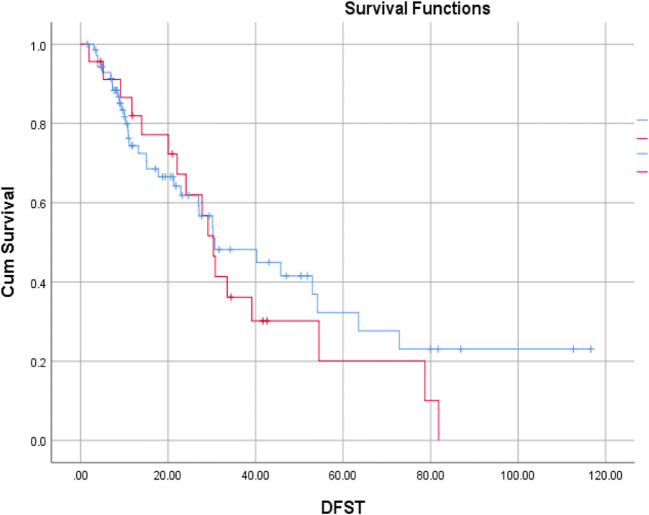
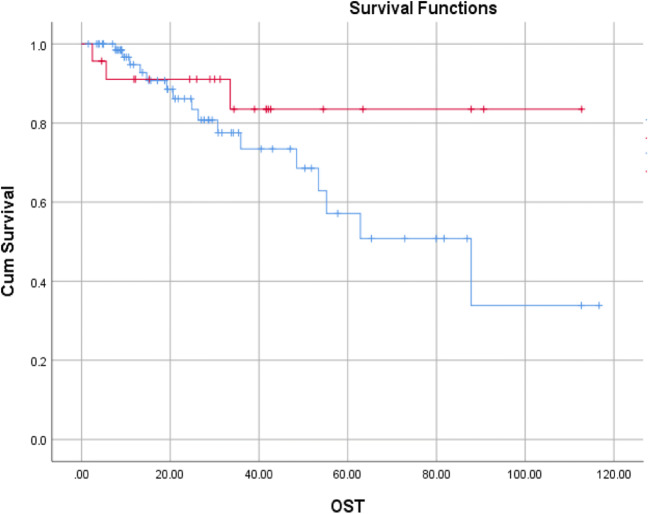
The median follow-up time was 25.3 months (range 1.5 to 117 months). The median DFS was 30 months (range 25 to 35.8 months) and median OS was 87.8 months. Thirty-seven of seventy-three (50.68%) patients in the upfront setting and 18/27 (66.67%) patients in the recurrent setting relapsed at a median time of 30.2 months and 29.1 months respectively. The corresponding DFS at 5 years were 32.3% and 20.1% (Fig. 2). Among the relapsed patients, 35 were local recurrences and 20 distant recurrences (9 patients lung, 9 patients liver, and 7 patients other sites). Seventeen of seventy-three (23.29%) patients and 2/27 (7.4%) patients in the upfront and recurrent settings died of disease at a median time of 62.8 months (upfront) and not reached (recurrent) respectively. The corresponding OS at 5 years were 67.1% and 83.5% respectively (Fig. 3).
Fig. 2.
Kaplan-Meier curves depicting disease-free survival (DFS) in upfront and recurrent tumors (blue line: upfront; red line: recurrent)
Fig. 3.
Kaplan-Meier curves depicting overall survival (OS) in upfront and recurrent tumors (blue line: upfront; red line: recurrent)
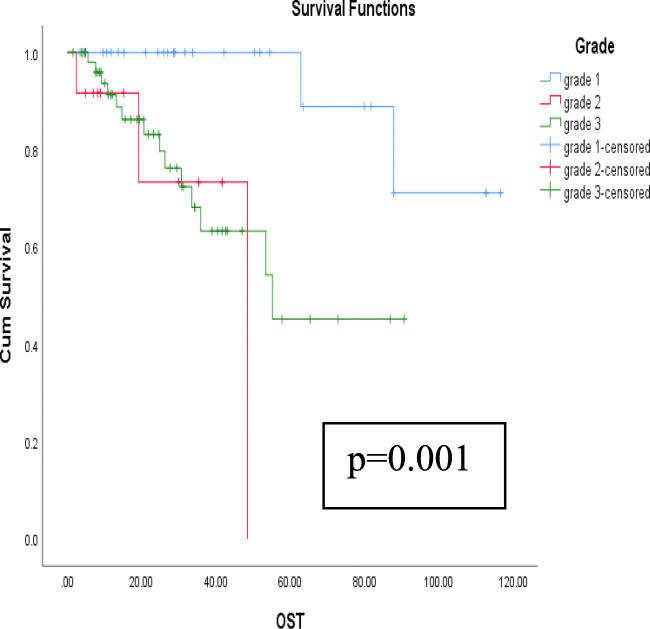
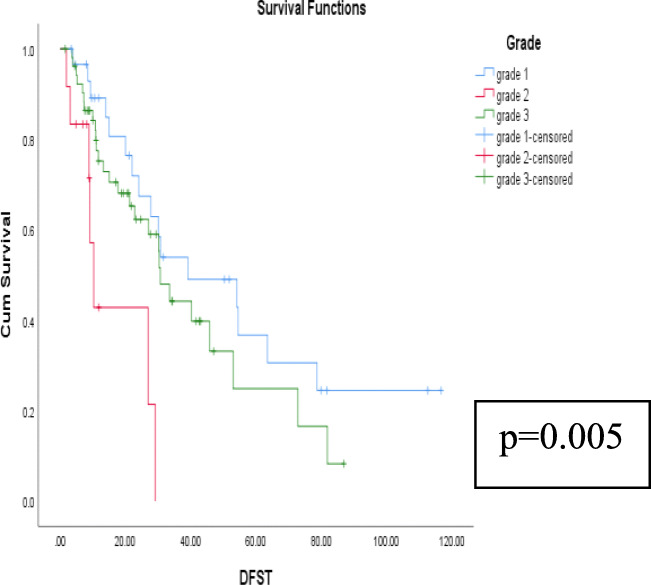
Relationship of survival with respect to various prognostic factors is shown in Table 5. The univariate analyses of gender, age at presentation, tumor size, tumor grade, histological subtype, type of presentation (upfront versus recurrent tumors), surgical margins, presence of contiguous organ resection, and the use of adjuvant treatment were analyzed with regard to DFS and OS. It was found that tumor grade was the main factor associated with both DFS and OS (p = 0.001 and 0.005 respectively) (Figs. 4 and 5). The remaining factors did not affect DFS or OS significantly (Tables 5 and 6). Among the patients undergoing adjacent organ removal, there was no difference between those with adhesion and infiltration with respect to OS (p = 0.361) and DFS (p = 0.139).
Table 5.
Relationship of various prognostic variables on survival: Only tumor grade showed significance
| Variables | Outcomes | p value (OS) | ||||
|---|---|---|---|---|---|---|
| A-D | A+D | D+D | Total | |||
| Grade | Grade I | 17 | 12 | 2 | 31 | 0.005 |
| Grade II | 6 | 4 | 3 | 13 | ||
| Grade III | 26 | 16 | 14 | 56 | ||
| Resection status | R0 | 40 | 26 | 17 | 83 | 0.718 |
| R1 | 5 | 2 | 1 | 8 | ||
| R2 | 4 | 4 | 1 | 9 | ||
| Histology | WDLPS | 14 | 10 | 3 | 27 | 0.15 |
| DDLPS | 8 | 10 | 4 | 22 | ||
| LMS | 17 | 6 | 7 | 30 | ||
| Myxoid sarcoma | 3 | 2 | 1 | 6 | ||
| UPS | 2 | 1 | 1 | 4 | ||
| Spindle cell sarcoma | 4 | - | 3 | 7 | ||
| Others | 1 | 3 | - | 4 | ||
| Presentation | Upfront tumors | 37 | 19 | 17 | 73 | 0.159 |
| Recurrent tumors | 12 | 13 | 2 | 27 | ||
| T status | T1b (< 5 cm) | 9 | 2 | 2 | 13 | 0.433 |
| T2b (> 5 cm) | 40 | 30 | 17 | 87 | ||
| Gender | Males | 33 | 17 | 10 | 60 | 0.966 |
| Females | 16 | 15 | 9 | 40 | ||
| Adjacent organ removal | Infiltration | 15 | 17 | 8 | 40 | 0.361 |
| Adhesion | 13 | 7 | 7 | 27 | ||
| None | 21 | 8 | 4 | 33 | ||
| Total | 49 | 32 | 19 | 100 | ||
A-D alive without disease, A+D alive with disease, D+D dead due to disease
Fig. 4.
Kaplan-Meier curves depicting relationship of tumor grade on OS
Fig. 5.
Kaplan-Meier curves depicting relationship of tumor grade on DFS
Table 6.
Relationship of various prognostic factors on tumor recurrence patterns
| Variables | Recurrences | p value (DFS) | |||
|---|---|---|---|---|---|
| Local | Distant | Total | |||
| Grade | Grade I | 15 | 2 | 17 | 0.001 |
| Grade II | 3 | 4 | 7 | ||
| Grade III | 17 | 14 | 31 | ||
| Resection status | R0 | 29 | 18 | 47 | 0.392 |
| R1 | 3 | 1 | 4 | ||
| R2 | 3 | 1 | 4 | ||
| Histology | WDLPS | 14 | - | 14 | 0.48 |
| DDLPS | 12 | 2 | 14 | ||
| LMS | 3 | 12 | 15 | ||
| Myxoid sarcomas | 2 | 1 | 3 | ||
| Spindle cell sarcoma | 2 | 1 | 3 | ||
| UPS | 1 | 1 | 2 | ||
| Others | - | 3 | 3 | ||
| Presentation | Upfront | 17 | 20 | 37 | 0.55 |
| Recurrent | 18 | - | 18 | ||
| Gender | Males | 21 | 8 | 29 | 0.611 |
| Females | 14 | 12 | 26 | ||
| Adjacent organ removal | Infiltration | 16 | 10 | 26 | 0.139 |
| Adhesion | 11 | 3 | 14 | ||
| None | 8 | 7 | 15 | ||
| Total | 35 | 20 | 55 | ||
Out of the 67 patients with grade II and grade III tumors, 39 patients received radiotherapy. In the radiotherapy group, the 5-year OS was 55.9% (median OS, not reached) while in the no-radiotherapy group, it was 24.9% (median OS, 33.58 months) with a p value of 0.069. The corresponding 5-year DFS in the radiotherapy and no-radiotherapy groups respectively were 27.1% (median DFS, 26.35 months) and 22.5% (median DFS, 12.52 months) with a p value of 0.12.
Pattern of recurrence and prognostic factors are tabulated in Table 6. When comparing LPS and LMS histologies, local recurrences were commonly associated with well-differentiated LPS (n = 14) while distant recurrences were associated with LMS (n = 12). The overall local and distant recurrence rates in LPS and LMS respectively were 92.86%, 7.14%, 20%, and 80%. Grade III tumors were associated with higher local as well as distant recurrences. Patients who needed adjacent organ removal had a higher risk of local recurrences. Although there was no difference in survival between LPS and LMS histologies, local recurrences were higher in the former whereas distant failures were predominant in the latter.
Discussion
Surgery for RPS has undergone a paradigm shift over the years from marginal resections to multivisceral and compartmental excisions. Historically, marginal resections were commonly performed, resulting in margin-positive resections with higher local recurrence rates that paved way for wider resection margins, at the cost of removal of adjacent organs involved grossly by the tumor. The Transatlantic Retroperitoneal Sarcoma Working Group (TARPSWG) [8] published their one-decade-long (2002–2011) data on 1007 patients undergoing resection for RPS. Patients were subjected to radical surgeries that included multivisceral resections. Majority of the patients had at least 2 organs being removed. This led to higher R0 resections and improved outcomes.
In our study, adjacent organ to achieve margin negativity was 42.9%, with kidney being the most common organ removed followed by small/large bowel. Right lower quadrant tumor location, grade III tumors, and histologies like LMS and DDLPS were factors associated with a higher contiguous organ removal during surgery.
Prognosis of patients with adjacent organ removal is related to tumor directly infiltrating the particular organ or just abutting it. Hogg et al. [9], Wan Jie et al. [10], and Fairweather et al. [11] found that such infiltrating tumors had poorer outcomes compared with abutting tumors. Furthermore, Fairweather’s study [11] also correlated histology with invasion and reported that dedifferentiated or high-grade tumors were more likely to be infiltrative in nature. In our study, there was no difference in survival with adhesive or infiltrative tumors. A Resected Organ Score (ROS) has been introduced to account for differences in morbidity in patients undergoing organ resection [12]. Organs such as the pancreas (especially head) and duodenum, whose resections can often lead to serious morbidity, are assigned higher scores. Organs such as the kidney, and appendix whose removal is not so morbid, are assigned lower scores. Furthermore, patients who undergo nephrectomy as part of multivisceral resections are not at a higher risk of developing chronic kidney disease compared with the general population [13].
Table 7 depicts the outcomes of various studies on RPS. Patient’s age, gender, extent of resection, margins, and tumor grade remain important prognostic variables across all studies. Three studies included patients from the National Cancer Database (NCDB) [17, 20, 23]. In our study, only tumor grade remained significant for both DFS and OS. However, there was a trend in the improvement in OS in the radiotherapy group (although not statistically significant) with no effect on DFS. This underscores the fact that, unlike in extremity sarcomas, there is limited benefit of adjuvant radiotherapy in retroperitoneal sarcomas and that striving for a margin-negative resection is the most important factor affecting outcomes.
Table 7.
Published literature on RPS
| Author/year | Period | n | U/R | 5-year OS | 5-year DFS | LR/DR (%) | M:F | R0:R+ (%) | Grade I/II/III | Histology | Age (years) | NACT/NART | Adj RT | Adj CT | Death | T size | MVR (%) | F/U (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hogg 2016 [9] | 1997-2013 | 79 | 63/27 | 55.3%mean | 24.8% mean | 41/12.2 | 45:45 | 88.9:11.1 | 38.9/18.9/42.2% |
WDLPS: 38% DDLPS: 29% LMS: 13.3% |
60* | 16.7%/1.1% | 8.9% | 3.3% | 3 (postop) | 20.5 cm mean | 70 (≥ 1) | 61* |
| Panda 2015 [1] | 2008–2010 | 23 | 20/3 | 60% approx. | NA | 39.1 | 13:10 | 61:39 | -/-/43.5% |
LPS Neuro-fibroma |
39.1 | NA | NA | NA |
1 (post op) D+D: 1 |
10 cm median | 26.1 | 24 |
|
Sandrucci 2018 [14] multi-institute |
2006–2011 | 72/138 | 46/26 |
65% 31%(R+) |
NA | NA | 43:29 | 60:25 |
14/31/ 37.5% |
LPS: 55.5% LMS: 14% Sarcoma, NOS: 11% |
> 60 79% |
NA | NA | NA | NA |
< 10 cm: 30.5% > 10 cm: 69.5% |
NA | 85 |
| Kim 2017 [13] | 1990–2014 | 47 | 37/21 | NA | NA | 36/22 | 29:18 | NA | NA |
WDLPS: 30.2% DDLPS: 32.3% LMS: 24% |
63 |
RT: 52% (overall) CT: 19.8% (overall) |
D+D: 2 | 15 cm (median) | 57.2 (k) | 26.8 | ||
| Fairweat-her 2018 [11] | 2002–2011 | 99/118 | 118/0 | 34–62% | NA | 48/22–46 | 52:47 | 48.3/35.6 | 28.8/15.3/40.7% |
WDLPS: 12.7% DDLPS: 31.4% LMS: 21.1% |
58–59 | 15.3%/21.2% | 7.6% | 4.2% | NA | 15–17 cm (median) | 84 (1) | 33.6 |
| Abdel-fateh 2016 [15] | 1994–2010 | 131 | 131/0 | 48.7 months median | NA | NA | 63:68 | 84.4/15.6 | 17.6/20.6/52.7% |
LMS: 39.7% LPS: 38.2% Sarcoma, NOS: 7.6% |
59 | -/29% | 48% | 23.7% (incl. NACT) | NA |
12.3 cm > 15 cm: 45.8% |
51.3 (1–2) | NA |
| Wang 2018 [16] | 2012–2017 | 25 | 17/8 | 83.2% (1 year) | 91.3% (1 year) | 4/- | 17/8 | 92:8 | 16/40/44% |
LPS: 68% UPS: 16% LMS: 12% |
56 |
12% Both |
NA | NA | 8% |
< 15 cm: 20% > 30 cm: 12% |
96 (1) | 11 |
| Wan Jie 2017 [10] | 2000–2014 | 85 | 55/30 | 45 months median | 21 months median | 59 (all) | 40/45 | 50:50 | 42/24/34% |
LPS: 72% LMS: 14% |
55 | 1.1%/- | 17.6% | 9.4% | NA | 16.5 cm |
50 (1) 9.4 (>1) |
46 |
|
Stahl 2016 [17] NCDB |
1998–2011 | 4015 | NA |
64.7% 54.3% (R+) |
NA | NA | 1821/2194 | 64.6/35.4 | II+III: 52.2% | LPS: 5 9.1% | 60 | -/25.4% | 69% + 5.7% IORT | 11.1% (incl. NACT) | 35.4% | 16 cm | NA | 67 |
|
Maurice 2017 [18] NCDB |
2004–2014 | 3141 | NA | 43.4–84.3 months median | NA | NA | 1507/1634 | 67.6:32.4 | 28.95/9.85/41 |
LPS: 60.8% LMS: 26.3% NOS: 4.1% |
61 | 2.55/11.35 | NA | NA | NA | 17 cm (median) | NA | 31.1 |
|
Guiliano 2016 [19] SEER |
2002–2012 | 2920 | NA | 58.4% | NA | 4.6/14.6 | 1414/1506 | NA | 24.6/9.8/40.7 |
LPS: 46.9% LMS: 25% NOS: 14.3% |
63 | NA | 21.6% | 11.9% | 33.6% | 15 cm (median) | 39.5 | NA |
|
Berger 2018 [20] NCDB |
2004–2013 | 2762 | NA |
70.1–84.2 months median |
NA | NA | 1300/1462 | 52.3:34.5 | 11.7/7.6/20% | NA | 63 | −/9.9% | 19.9% | NA | 6.3% (90 days) | 19.9 cm (mean) | NA | NA |
| Gronchi 2013 [12] | 1999–2009 | 523 | NA | 56.8% | 39.4% | 24.5/17.8 | 280/243 | 90.8:9.2 | 28.1/23.3/48.6% |
WDLPS: 23.1% DDLPS: 29.6% LMS: 17.6% UPS: 13.4% |
57 |
CT: 39.6% (overall) RT: 36.9% (overall) |
D+D: 139/171 | 16 cm (median) |
34 (1) 57 (>1) |
45 | ||
| Tan 2016 [2] | 1982–2010 | 675 | NA | 69% (DSS) | NA | 38/24 | 299/376 | 56:44 | III: 64% |
LGLPS: 28% HGLPS: 32% LMS: 20% SFT: 5% |
60 | 11%4% | 4% | 7% | DSD: 31% | 17 cm (median) |
58 (1) 27 (>1) 10 (v) |
39.6 |
| Rosa 2016 [21] | 1984–2013 | 107 | 92/15 | 71% | 65% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 79.7 |
| Amersi 2016 [22] | 1985–2010 | 68 | NA | 55% | NA | NA | 25/43 | NA | II+III: 75% | NA | 59* | NA | NA | NA | NA | 12 cm (median) |
43 10 (v) |
NA |
| Present study/2019 | 2008–2017 | 100 | 73/27 |
62% 87.8 months median |
30 months median | 35/20 | 60/40 | 83:17 | 34/11/55 |
WDLPS: 29% DDLPS: 21% LMS: 30% Spindle cell: 7% |
52 | 7%/1% | 50% | 9% | 19% | 15 cm (median) | 50 | 25.3 |
(1) one organ resection, (>1) more than one organ resection, (v) vascular resection, NA not available, D+D dead due to disease, (k) nephrectomy, LPS liposarcoma, LMS leiomyosarcoma, UPS undifferentiated pleomorphic sarcoma, MPNST malignant peripheral nerve sheath tumor, LGLPS low-grade LPS, HGLPS high-grade LPS, NOS not otherwise specified sarcomas, WDLPS well-differentiated LPS, DDLPS dedifferentiated LPS, DSS disease-specific survival, U upfront tumors, R recurrent tumors, NACT/NART neoadjuvant chemotherapy/radiation, IORT intraoperative radiotherapy, LR local recurrence, DR distant recurrence, MVR multivisceral resections, DSD disease-specific death, NCDB National cancer, SEER Surveillance, Epidemiology, and End Results. *Mean value
While local recurrences remain significant for low-grade tumors, distant recurrences are common in higher grade tumors. Multiple local recurrences increase risk of distant tumor dissemination even in low-grade tumors. Most studies depict recurrence rates of 20–40%. In our study, the recurrence rates were 55% with a higher local recurrence pattern. Liposarcoma histology and grade III tumors showed higher local recurrence rates.
The strengths of this study include a single-center experience in managing these complex tumors with emphasis on surgical treatment and outcomes. The limitations of this study include the short follow-up time and a relatively small patient cohort.
Conclusions
RPS are rare tumors with ever-evolving treatment strategies. This study represents the largest single-center experience from India. There has been a steady increase in the number of patients undergoing multivisceral and compartmental resections to achieve macroscopic complete excision and minimize marginality. However, diligent pre-operative work up needs to be done to anticipate and reduce morbidity associated with multivisceral resections. Tumor grade remained an important prognostic factor affecting survival in our study. There is an urgent need for centralization in the treatment of RPS tumors with development of multidisciplinary treatment approach, in order to improve the survival outcomes beyond what can be achieved by surgery alone.
Acknowledgments
We would sincerely thank Dr. A. Gronchi, Department of Surgery, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy, for providing valuable inputs during the manuscript drafting and editing.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from the patients for surgery.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shraddha Patkar, Email: drshraddhapatkar@gmail.com.
Abhay K Kattepur, Email: drabhay1985@gmail.com.
Rajesh Shinde, Email: dr.rajeshinde@gmail.com.
Mahesh Goel, Email: drmaheshgoel@gmail.com.
References
- 1.Panda N, Das R, Banerjee S, Chatterjee S, Gumta M, Bandyopadhyay SK. Retroperitoneal sarcoma: outcome analysis in a teaching hospital in Eastern India- a perspective. Indian J Surg Oncol. 2015;6(2):99–105. doi: 10.1007/s13193-015-0404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan MCB, Brennan MF, Kuk D, Agaram NP, Antonescu CR, Qin LX, Moraco N, Crago AM, Singer S. Histology-based classification predicts pattern of recurrence and improves risk stratification in primary retroperitoneal sarcoma. Ann Surg. 2016;263(3):593–600. doi: 10.1097/SLA.0000000000001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Francis IR, Cohan RH, Varma DGK, Sondak VK. Retroperitoneal sarcomas. Cancer Imaging. 2005;5:89–94. doi: 10.1102/1470-7330.2005.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacNeill Andrea J., Fiore Marco. Surgical morbidity in retroperitoneal sarcoma resection. Journal of Surgical Oncology. 2018;117(1):56–61. doi: 10.1002/jso.24902. [DOI] [PubMed] [Google Scholar]
- 5.Matthyssens LE, Creytens D, Ceelen WP. Retroperitoneal liposarcoma: current insights in diagnosis and treatment. Front Surg. 2015;2:4. doi: 10.3389/fsurg.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coindre JM. Grading of soft tissue sarcomas: review and update. Arch Pathol Lab Med. 2006;130:1448–1453. doi: 10.5858/2006-130-1448-GOSTSR. [DOI] [PubMed] [Google Scholar]
- 7.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacNeill AJ, Gronchi A, Miceli R, Bonvalot S, Swallow CJ, Hohenberger P, van Coevorden F, Rutkowski P, Callegaro D, Hayes AJ, Honoré C, Fairweather M, Cannell A, Jakob J, Haas RL, Szacht M, Fiore M, Casali PG, Pollock RE, Barretta F, Raut CP, Strauss DC. Postoperative morbidity after radical resection of primary retroperitoneal sarcoma: a report from the Transatlantic RPS Working Group. Ann Surg. 2018;267(5):959–964. doi: 10.1097/SLA.0000000000002250. [DOI] [PubMed] [Google Scholar]
- 9.Hogg HDJ, Manas DM, Lee D, Dildey P, Scott J, Lunec J, French JJ. Surgical outcome and patterns of recurrence for retroperitoneal sarcoma at a single centre. Ann R Coll Surg Engl. 2016;98:192–197. doi: 10.1308/rcsann.2016.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan Jie NG, Tan GHC, Chia CS, et al. Tumor biology remains the main determinant of prognosis in retroperitoneal sarcomas: a 14-year single-center experience. Asia-Pac J Clin Oncol. 2017;13:e458–e465. doi: 10.1111/ajco.12662. [DOI] [PubMed] [Google Scholar]
- 11.Fairweather M, Wang J, Jo VY, Baldini EH, Bertagnolli MM, Raut CP. Incidence and adverse prognostic implications of histopathologic organ invasion in primary retroperitoneal sarcoma. J Am Coll Surg. 2017;224:876–883. doi: 10.1016/j.jamcollsurg.2017.01.044. [DOI] [PubMed] [Google Scholar]
- 12.Gronchi A, Miceli R, Shurell E, Eilber FC, Eilber FR, Anaya DA, Kattan MW, Honoré C, Lev DC, Colombo C, Bonvalot S, Mariani L, Pollock RE. Outcome prediction in primary resected retroperitoneal soft tissue sarcoma: histology-specific overall survival and disease-free survival nomograms built on major sarcoma center data sets. J Clin Oncol. 2013;31:1649–1655. doi: 10.1200/JCO.2012.44.3747. [DOI] [PubMed] [Google Scholar]
- 13.Kim Daniel B., Gray Richard, Li Zhuo, Wasif Nabil, Bagaria Sanjay P. Effect of nephrectomy for retroperitoneal sarcoma on post-operative renal function. Journal of Surgical Oncology. 2017;117(3):425–429. doi: 10.1002/jso.24875. [DOI] [PubMed] [Google Scholar]
- 14.Sandrucci S, Ponzetti A, Gianotti C, et al. Different quality of treatment in retroperitoneal sarcomas (RPS) according to hospital-case volume and surgeon-case volume: a retrospective regional analysis in Italy. Clin Sarcoma Res. 2018;8:3. doi: 10.1186/s13569-018-0091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdelfatah E, Guzzetta AA, Nagarajan N, Wolfgang CL, Pawlik TM, Choti MA, Schulick R, Montgomery EA, Meyer C, Thornton K, Herman J, Terezakis S, Frassica D, Ahuja N. Long-term outcomes in treatment of retroperitoneal sarcomas: a 15 year single-institution evaluation of prognostic features. J Surg Oncol. 2016;114(1):56–64. doi: 10.1002/jso.24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Wu J, Lv A, Li CP, Tian XY, Hao CY. Anterior approach to en bloc resection in left-sided retroperitoneal sarcoma with adjacent organ involvement: a study of 25 patients in a single center. Med Sci Monit. 2018;24:961–969. doi: 10.12659/MSM.908559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stahl JM, Corso CD, Park HS, An Y, Rutter CE, Han D, Roberts KB. The effect of microscopic margin status on survival in adult retroperitoneal soft tissue sarcomas. Eur J Surg Oncol. 2017;43:168–174. doi: 10.1016/j.ejso.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 18.Klooster B, Rajeev R, Chrabaszcz S, et al. Is long-term survival possible after margin-positive resection of retroperitoneal sarcoma (RPS)? J Surg Oncol. 2016;113:823–827. doi: 10.1002/jso.24232. [DOI] [PubMed] [Google Scholar]
- 19.Guiliano K, Nagarajan N, Canner JK, et al. Predictors of improved survival for patients with retroperitoneal sarcoma. Surgery. 2016;160:1628–1635. doi: 10.1016/j.surg.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 20.Berger NG, Silva JP, Mogal H, et al. Overall survival after resection of retroperitoneal sarcoma at academic cancer centers versus community cancer centers: an analysis of the National Cancer Data Base. Surgery. 2018;163:318–323. doi: 10.1016/j.surg.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Rosa F, Fiorillo C, Tortorelli AP, et al. Surgical management of retroperitoneal soft tissue sarcomas: role of curative resection. Am Surg. 2016;82(2):128–133. [PubMed] [Google Scholar]
- 22.Amersi Farin, Forscher Charles, Silberman Allan W. Surgical Resection of Retroperitoneal Sarcomas: Analysis of Factors Determining Outcome. Critical Reviews™ in Oncogenesis. 2016;21(1-2):105–113. doi: 10.1615/CritRevOncog.2016016967. [DOI] [PubMed] [Google Scholar]
- 23.Maurice MJ, Yih JM, Ammori JB, Abouassaly R. Predictors of surgical quality for retroperitoneal sarcoma: volume matters. J Surg Oncol. 2017;116:766–774. doi: 10.1002/jso.24710. [DOI] [PubMed] [Google Scholar]