Abstract
Background:
Although low-intensity pulsed ultrasound has been reported to be potential cartilage regeneration, there still unresolved treatment due to cartilage fibrosis and degeneration by a lack of rapid and high-efficiency treatment. The purpose of this study was to investigate the effect of a combination therapy of focused acoustic force and stem cells at site for fast and efficient healing on cartilage regeneration.
Methods:
Using a rat articular cartilage defects model, one million adipose tissue-derived stem cells (ASCs) were injected into the defect site, and low-intensity focused ultrasound (LOFUS) in the range of 100–600 mV was used for 20 min/day for 2 weeks. All experimental groups were sacrificed after 4 weeks in total. The gross appearance score and hematoxylin and eosin (H&E), Alcian blue, and Safranin O staining were used for measuring the chondrogenic potential. The cartilage characteristics were observed, and type II collagen, Sox 9, aggrecan, and type X collagen were stained with immunofluorescence. The results of the comprehensive analysis were calculated using the Mankin scoring method.
Results:
The gross appearance scores of regenerated cartilage and chondrocyte-like cells in H&E images were higher in LOFUS-treated groups compared to those in negative control or ASC-treated groups. Safranin O and Alcian blue staining demonstrated that the 100 and 300 mV LOFUS groups showed greater synthesis of glycosaminoglycan and proteoglycan. The ASC + LOFUS 300 mV group showed positive regulation of type II collagen, Sox 9 and aggrecan and negative regulation of type X collagen, which indicated the occurrence of cartilage regeneration based on the Mankin score result.
Conclusion:
The combination therapy, which involved treatment with ASC and 300 mV LOFUS, quickly and effectively reduced articular cartilage defects.
Keywords: Low-intensity focused ultrasound, Adipose tissue-derived stem cell, Articular cartilage defect, Regeneration
Introduction
Cartilage defects, the most common disease of joints, can cause swelling, pain, and subsequent loss of joint function [1]. The cartilage self-repair capability is limited due to the unique structure and a lack of blood supply, nerves, and lymphatic vessels, and cartilage mainly absorbs supplements from synovial fluid. Thus, traumatic joint cartilage damage and early osteoarthritis (OA) cause pain, accelerate arthrosis, and cause severe dysfunction [2]. Cartilage damage and subsequent tissue degeneration can lead to long-term chronic disease, which also consumes large amounts of medical resources [3]. Thus, the field of regenerative medicine has shown promising progress in the recovery of damaged cartilage.
Stem cells have the potential for self-renewal and can differentiate into multiple cell lines [4]. Among adult stem cells isolated from various tissues [4, 5], adipose tissue-derived stem cells (ASCs) have a greater capacity for a 100–500-fold increase in the cell number compared to bone marrow. ASCs are considered a type of mesenchymal stem cell (MSC) in stromal vascular fractions (SVF), which are isolated from fat tissues enzymatically [6] and are an attractive source because of their accessibility, large number, and rapid growth [7]. Chondrogenic differentiation of ASCs have been induced by molecules, cytokines (which are mainly growth factors), and the microenvironment for cell-based regeneration [8, 9]. The common applications for the chondrogenesis of ASCs involve single treatment or treatment with a combination of transforming growth factor beta 1 and 3 (TGF-β1 and -β3), bone morphogenetic protein 4 (BMP 4), sex determining region Y box 9 (SOX 9), and basic fibroblast growth factor (bFGF). However, exogenous treatment to ASCs promotes chondrogenesis into the terminal stage, the accelerated development of the fibrous remodeling of cartilage defects [9, 10].
In previous studies, other types of chondrogenic stimulants have been used, such as shear stress, cellular stretch and centrifugal force, and these have shown positive effects (for cartilage repair and bone formation, etc.) for biomechanical conditioning. Cyclic mechanical compression stimulates chondrogenic differentiation of mesenchymal progenitor cells that fill in the scaffold [11]. Furthermore, cyclic compressive loading promotes chondrogenesis of rabbit bone marrow MSCs in agarose cultures via the endogenous TGF-β signaling pathway, inducing the expression of activating protein 1 (AP-1) and Sox 9 [12].
However, among the mechanical loading methods, ultrasound is known as a noninvasive, cheap, and easy-to-apply tool. The biological effects of ultrasound on chondrocyte and cartilage metabolism have also presented some promising results [13]. In particular, low-intensity pulsed ultrasound (LIPUS) is widely used as an Food and Drug Administration (FDA)-approved intervention in the United States for inducing osteogenic differentiation and has shown the potential of the chondrogenic differentiation of MSCs mediated by biological effects [14, 15]. LIPUS has been found to be effective in inducing the chondrogenesis of bone marrow MSCs. These studies have used ultrasound transducers directly on cells, such as chondrocytes or MSCs, to induce chondrogenic differentiation [16]. By using direct stimulation with pulsed ultrasound, studies have shown that matrix-related regulation can provide effective cartilage repair [17, 18]. MSCs modified by LIPUS stimulation also show enhanced capacity for autophagy regulation, TGF-mediated chondrogenesis and Rho-associated kinase-Cot/Tpl2-MEK-ERK-mediated osteogenesis [15, 19, 20]. Although some studies have demonstrated that ultrasound energy over a wide range is important in regulating chondrogenic differentiation, there have rarely been reports of in vivo studies regarding the rapid and effective use of pulsed ultrasound energy collected in a limited area. To sum up the one focused zone of very short ultrasonic waves, researchers had developed a concave curvature namely low-intensity focused ultrasound (LOFUS) which could concentrate approximately 150 times as much ultrasound energy [21].
In this study, LOFUS treatment with ASC transplantation was examined in terms of its ability to improve cartilage repair in a defect model quickly and effectively. The physical and pathophysiological approach using the LOFUS-ASC therapeutic system promoted the rapid and effective regeneration of cartilage defects, which could lead to progression from fibrocartilage to hyaline cartilage to a greater degree than stem cells alone.
Materials and methods
Culture of ASCs
Human ASCs were cultured as described previously [22]. ASCs from four different human donors (StemPro human adipose-derived stem cells) were purchased from Invitrogen (Carlsbad, CA, USA) and were maintained according to the manufacturer’s instructions. We used low-glucose Dulbecco’s modified Eagle medium (DMEM; Gibco, Waltham, MA, USA) containing 10% fetal bovine serum (FBS; Gibco) and 1% penicillin–streptomycin (Gibco). We used human ASCs from passages 3 to 6 for transplantation.
Induction of cartilage defects
All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of the Catholic Kwandong University International St. Mary’s Hospital in cooperation with the Association for the Assessment and Accreditation of Laboratory Animal Care and were performed in accordance with the Guidelines and Regulations for Animal Care (No. CKU 03-2017-001). A cartilage defect model was induced as described previously [23]. Under Zoletil (20 mg/kg) and Rompum (5 mg/kg) anesthesia, both knee joints of rats were cleaned with 10% betadine solution, and the patellar groove of the distal femur was exposed using an anteromedial approach. Full-thickness articular cartilage defects were produced in the trochlear grooves with a diameter of 2 mm and a depth of 1 mm using a biopsy punch with a metallic drill of 1 mm in diameter. (Kai Industries Co., Ltd., Tokyo, Japan).
ASC treatment and LOFUS induction
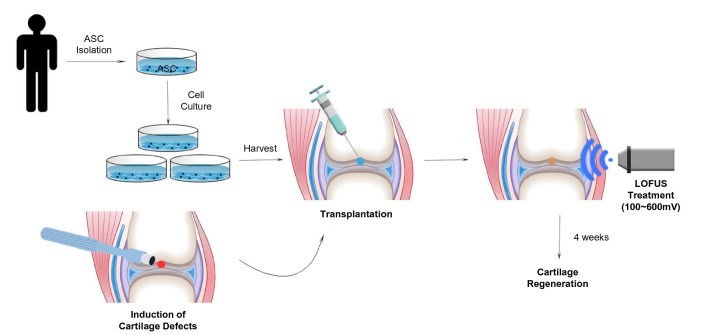
For injection, one million of human ASCs were suspended in 30 µL of PBS. Defects were created in the patellar groove of the knee joints and ASCs were intra-articularly injected using a 23 G needle into the area of the cartilage defect in the space between the femur, tibia, and patella.
The induction of LOFUS used a modified method similar to that described in our previous study [24]. The LOFUS system consists of three major components: a signal generator (#3250A; Agilent Technologies, Santa Clara, CA, USA), an amplifier (#403 LA; Electronics & Innovation, Rochester, NY, USA), and a transducer (#H115; Sonic Concept, Bothell, WA, USA). The LOFUS frequency was 650 kHz, the amplitude was 100–600 mV, and the pulse average is 27–560 mW/cm2. LOFUS treatment was performed for 20 min per day for a total of 2 weeks after cartilage defect modeling and ASC injection. Experimental rats (8-week-old Sprague–Dawley rat;s n = 7 per group) were randomly allocated into five groups: negative control (untreated), ASC (ASC only transplanted), ASC + LOFUS 100 mV, ASC + LOFUS 300 mV, and ASC + LOFUS 600 mV. The detailed treatment method is shown in Fig. 1.
Fig. 1.
Schematic illustration of approaches used to treat stem cells with LOFUS for cartilage regeneration. LOFUS, low-intensity focused ultrasound
Gross appearance evaluation
After sacrifice, each rat was shaved in the knee joint area, and arthrotomy was performed as described for cartilage defect induction. Inflammation, fibrosis, abnormalities, and the degree of cartilage repair in the cartilage defect area as well as any irregularity, depression or bulging of repaired tissues in the border zone were measured, and the gross appearance was scored according to a modified Carranze-Bencano scoring system [25].
Histological analysis
At 4 weeks after defect induction and treatment, the rats were sacrificed by intraperitoneal lethal injection of sodium pentobarbital. The knee joints were separated and then washed with PBS. The obtained cartilage tissue was fixed in 10% buffered formaldehyde for 24 h and decalcified with 10% nitric acid for 3 days. The paraffin blocks were cut into 5-mm sections after embedding the cartilage. The sections were stained with hematoxylin and eosin (H&E) and Alcian blue. Stained slides were viewed at 40 × and/or 200 × magnification with a BX51/dot slide microscope (Olympus, Tokyo, Japan).
Safranin O/fast green staining
Sulfated glycosaminoglycans were stained with Safranin O/Fast green. Five μm sections were stained with Safranin O (ScienCell, Carlsbad, CA, USA) to evaluate the glycosaminoglycan content. Briefly, the sections were dewaxed in xylene, hydrated in ethanol (100%, 95% and 70%) for 2 min each and finally washed distilled in water for 3 min. Slides were immersed in a mixture of 0.2% Safranin O and 1% acetic acid for 10 min followed by rinsing in distilled water to remove excess dye. Counterstaining was performed in a mixture of 0.04% Fast green (ScienCell) and 0.2% acetic acid for 15 s, and the slides were rinsed in distilled water. The slides were then dried with filter paper and rinsed in absolute ethanol until the excess Fast green disappeared. After 3 washes in xylene for 3 min, the slides were mounted with Entellan® (Merck, Darmstadt, Germany) and observed under an optical microscope (Dimis, Anyang, Korea) equipped with a digital camera.
Immunofluorescence staining
After deparaffinization, rehydration, and rinsing with tap water, paraffin sections of separated knee tissue from rats underwent antigen retrieval using 10 mM sodium citrate (pH 6.0) in a microwave for 10 min. Sections were cooled for approximately 20 min and then incubated in 1% H2O2 to quench endogenous peroxidase. Samples were blocked for 1 h with a mixture of 1% (w/v) BSA and 5% (v/v) horse serum and incubated in primary antibody (Type II collagen, #ab34712; Sox 9, #ab26414; aggrecan, #ab36861; Type X collagen, #ab58632; at a ratio of 1:500 each; Abcam, Cambridge, UK) overnight at 4 °C. After three washes with PBS, the sections were incubated with secondary antibody (Alexa Fluor® 488, #ab150077; Alexa Fluor® 647, #ab150075; at a ratio of 1:1000 each; Abcam) for 1 h at room temperature, mounted with DAPI, and viewed under a confocal microscope (LSM 700, Carl Zeiss, Oberkochen, Germany).
Mankin score
The histological data were analyzed by assessing the Mankin scores. The scoring methods were described previously [26, 27]. The semiquantitative Makin scores were significantly correlated and positively associated with exercise duration.
Statistical analysis
Quantitative data were expressed as the means ± SD from at least three independent experiments. For statistical analysis, one-way ANOVA with Bonferroni correction was performed using OriginPro 8 SR4 software (version 8.0951; OriginLab Corporation, Northampton, MA, USA) if there were more than three groups. p < 0.05 was considered statistically significant.
Results
Gross findings in the cartilage defect area
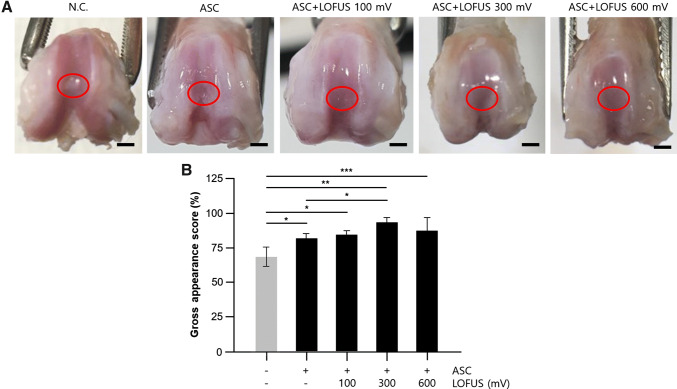
At 4 weeks posttreatment, abnormal findings such as severe inflammation or extensive fibrosis did not appear in any of the groups. In the ASC-treated group, neoplastic tissues were translucent, and smoother surfaces with less irregularities were observed; however, depressed tissues in the repaired site were often present in the knees of the negative control group. Specifically, the articular surface of the ASC group treated with LOFUS (100–600 mV) was smoother than that in the groups not treated with ASC and had a color close to that of the normal cartilage area. In the 300 mV-treated group, border areas and depressions in the cartilage defects were the least noticeable (Fig. 1). The gross appearance scores, which converted the surface morphology into scores, are shown in Fig. 1. This result indicates that LOFUS treatment may improve cartilage defects during stem cell therapy.
Structural outcome in the cartilage defect area
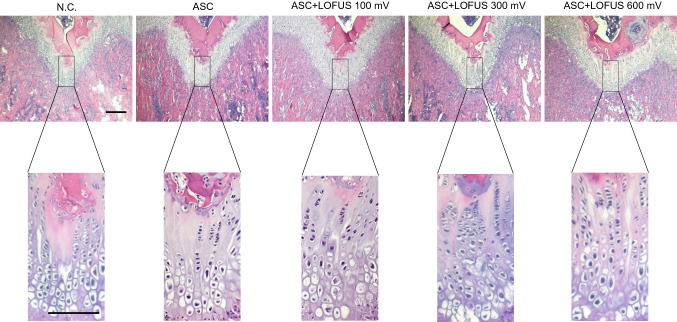
To examine the changes in cartilage regeneration and inflammation, H&E staining was performed in the defect area. According to H&E staining, chondrocyte-like cells and smooth cartilage-like tissue were increased in all ASC + LOFUS groups. Among the ASC + LOFUS groups, the 300 and 600 mV-treated groups had good regenerative performance and little inflammatory response. However, increased fibrous tissue formation was observed in defects of the negative control group compared with that in the other experimental groups (Fig. 2). This result indicated that ASC transplantation combined with LOFUS therapy has a strong effect on the regenerative response in articular cartilage.
Fig. 2.
Pathological analysis of stem cell transplantation and/or LOFUS treatment-mediated cartilage regeneration. H&E-stained tissue sections show the tissue integrity of the repaired area. Magnification: upper panel = 40 ×; lower panel = 200 ×. Scale bar = 200 μm. H&E, hematoxylin and eosin; N.C., negative control. LOFUS, low-intensity focused ultrasound
Effects on cartilage regeneration assessed by histological Alcian blue and Safranin O staining
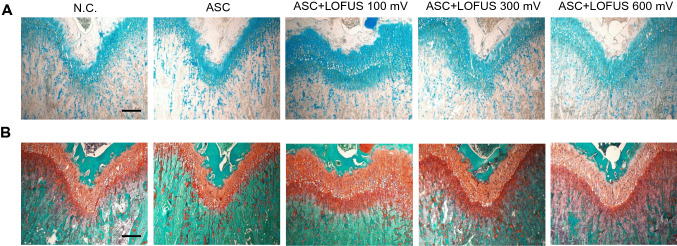
To evaluate cartilaginous matrix regeneration, histopathological staining with Alcian blue and Safranin O was performed at 4 weeks. Representative staining images of cartilage regeneration after defect induction are presented in Fig. 3. In the Alcian blue-stained tissues in Fig. 3A, cartilage-specific glycosaminoglycan (GAG)-positive areas in the ASC + LOFUS groups showed higher expression compared to those in the negative control and ASC-only groups. In particular, Alcian blue produced stronger staining in the 100 mV and 300 mV sections of the ASC + LOFUS group. In the Safranin O-stained tissues in Fig. 3B, a cartilaginous component containing proteoglycan (PG) was revealed. PG was stained dark orange in the cartilage matrix. The ASC + LOFUS groups were more strongly stained with Safranin O in terms of thickness and concentration. In particular, a strongly PG-positive area was found in the combined 300 mV LOFUS and ASC therapy group, whereas there was only weak staining in the negative control and ASC-only groups. Collectively, the results suggested that LOFUS treatment, especially with 100 and 300 mV, increased the positive staining of GAGs and PGs in the cartilage matrix processed with ASCs.
Fig. 3.
Histological analysis of stem cell transplantation and/or LOFUS treatment-mediated cartilage regeneration. A Alcian blue-stained tissue showing cartilage-specific proteoglycan; B Safranin O/Fast Green-stained tissue showing cartilaginous components; magnification = 100 ×. Scale bar = 200 μm. N.C., negative control. LOFUS, low-intensity focused ultrasound
Effects on cartilage regeneration assessed by immunohistological analysis
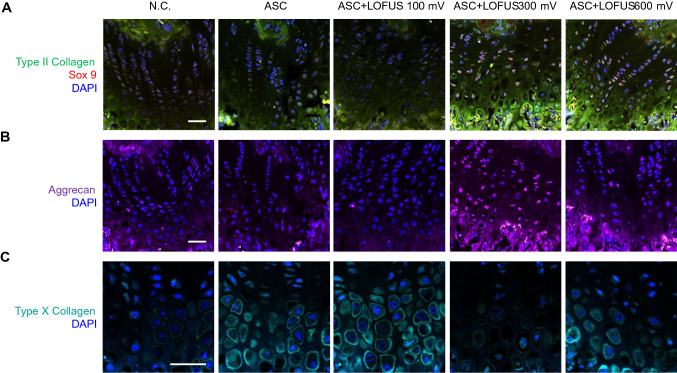
In Fig. 4A, the 300 and 600 mV ASC + LOFUS groups showed evenly distributed staining that indicated the presence of hyaline cartilage via costaining with type II collagen (green fluorescence) and Sox 9 (red fluorescence); by comparison, there were few positive areas in the defective areas of the negative control group. Moreover, the same groups in the upper section had more extended expression of aggrecan, which is extracellular matrix (ECM) protein involved in chondrogenesis, than the negative control and ASC-only groups (Fig. 4B). However, Type X collagen expression was downregulated in the group treated with 300 mV ASC + LOFUS compared with that in the other groups (Fig. 4C). Immunohistological inspection of the defective and regenerated areas revealed that systemic treatment produced hyaline cartilage, whereas fibrocartilage was barely produced.
Fig. 4.
Immunofluorescent image of cartilage regeneration in cell-transplanted and ultrasound-treated sites. Expression of type II collagen costained with Sox 9. B Expression of aggrecan. C Expression of type X collagen. Magnification: A and B = 200 ×; C = 400 ×. Scale bar = 200 μm. N.C., negative control
Mankin scoring according to the histological assessment of cartilage regeneration
We have shown in Figs. 2, 3, 4, 5 and 6 that the effect of stem cell therapy combined with LOFUS treatment on the repair of articular cartilage was assessed by various histological analyses. To evaluate the impact of a combination therapy, the Mankin scoring system was quantitatively used to evaluate these results for (pre)clinical significance (Fig. 6 and Table 1). Three experts individually assessed the parameters of the Mankin score (structure, cellular abnormalities, Safranin O staining, tidemark integrity), which correlated with various results. This result revealed that the ASC + LOFUS 300 mV group showed significant regenerative fibrous cartilage with hyaline and better cartilage repair compared with that in the other groups.
Fig. 5.
Changes in the gross area after stem cell transplantation and/or LOFUS treatment in a rat cartilage defect model. A Gross findings of tissue regeneration in articular cartilage defects in rat knees after 4 weeks. Red circle: defective and/or repaired region; B gross appearance scores of rat knees in five different groups (n = 7, *p < 0.05, **p < 0.001, ***p < 0.01). Data are expressed as the mean ± S.D. Scale bar = 2 mm. N.C., negative control. LOFUS, low-intensity focused ultrasound
Fig. 6.

Mankin scores of cartilage lesions in the group receiving stem cell transplantation and/or LOFUS treatment. Data are expressed as the mean ± S.D. Graphs are representative of experiments performed on seven animals
Table 1.
Mankin grading of cartilage regeneration in five experimental groups. Grade = (1) structure: normal, irregular surface, pannus, superficial cartilage layers absent, slight disorganization, fissures in calcified cartilage, and disorganization; (2) cellular abnormalities: hypercellularity, clusters, and hypocellularity; (3) Safranin O staining: Normal slight reduction, staining reduced in radial layer, reduced in interterritorial matrix, only present in the pencellular matrix, and absent; (4) tidemark integrity: intact and crossed by blood vessels
| Group | Feature | Score | Total |
|---|---|---|---|
| NC | Structure | 5 | 9 |
| Cellular abnormalities | 3 | ||
| Safranin O staining | 1 | ||
| Tidemark integrity | 0 | ||
| ASC | Structure | 3 | 6 |
| Cellular abnormalities | 2 | ||
| Safranin O staining | 1 | ||
| Tidemark integrity | 0 | ||
| ASC + LOFUS 100 mV | Structure | 3 | 3 |
| Cellular abnormalities | 0 | ||
| Safranin O staining | 0 | ||
| Tidemark integrity | 0 | ||
| ASC + LOFUS 300 mV | Structure | 1 | 2 |
| Cellular abnormalities | 1 | ||
| Safranin O staining | 0 | ||
| Tidemark integrity | 0 | ||
| ASC + LOFUS 600 mV | Structure | 2 | 4 |
| Cellular abnormalities | 0 | ||
| Safranin O staining | 2 | ||
| Tidemark integrity | 0 |
NC negative control
Discussion
Various studies of stem cell therapy using animals have been used as defect models for articular cartilage regeneration. Stem cells have been used to directly inject or indirectly treat biocompatible scaffolds (polyglycolic acid, hyaluronic acid, etc.) in induced cartilage defect models such as rat, rabbit, or pig [28–31]. In most cases, the effects were observed 6 to 24 weeks after surgery, and the effects of autologous stem cell therapy on articular cartilage regeneration were studied. Due to advances in technology, research has focused on improving allogeneic stem cell function and chondrogenic differentiation for the treatment of diseases [9, 31, 32]. Despite the therapeutic potential of stem cells in cartilage defects, more effective and rapid therapies that enable clinical application have not been examined in stem cell and engineering studies.
We report here that a combination therapy of stem cell injection and LOFUS treatment induced early and effective cartilage regeneration. Many studies have shown that stem cell-based therapy has an effect on improving cartilage function in terms of key issues such as efficacy, safety, and the stemness of cells cultured ex vivo [28–33]. We also used adipose-derived cells isolated from the SVF of fat tissue with stem cell abilities; however, we further attempted to use acoustic and physical forces for the purpose of the rapid settling and functional improvement of articular cartilage tissue (Fig. 1). Ultrasound is a high frequency acoustical pressure wave and a form of mechanical energy that can be transmitted to biological tissues. As a therapeutic tool, ultrasound achieves bioeffects to relieve muscle pain and reduce joint stiffness in a high energy range. In contrast, a lower level of energy of 0.1–1 W/cm2 has been shown to induce biological effects in cells without thermogenic and destructive action and without any evidence of inertial or stable cavitation being present [34]. In fact, the effect on cellular mechanisms and actions of therapeutic ultrasound were better studied by in vitro than in vivo studies in terms of responses to overall biological effects. However, we used low-intensity LOFUS energy to show the precise migration and activity of stem cells at the in vivo level and the regenerative effects on cartilage defect sites.
LIPUS is a well-known method for treating nonunion fractures and clinically promotes bone union both in vitro and in vivo [15, 35]. According to the report of Harrison et al., the mechanism of LIPUS is known and involves the biochemical response of integrin mechano-receptors to cyclooxygenase 2 (COX-2) [36]. Our unpublished data indicated that LIPUS strengthened MSC migration through the activation of monocyte chemoattractant protein (MCP)-1 and MCP-2. Furthermore, stem cells had enhanced migration effects via C-X-C-receptor 4 (CXCR4), integrin-1β and chemokine-chemokine receptor 2 (CCR-2) by treatment with LIPUS [37]. Migrated stem cells at the defect site promoted chondrogenic effects, represented by increased Type II collagen, aggrecan, and Sox 9 expression (Fig. 5A, B). Similar effects disprove our results that showed that TGF-β1-induced MSCs promoted chondrogenesis through mammalian target of rapamycin (mTOR), an integrin-mechanistic target, by using LIPUS in in vitro experiments [16]. In addition, changes in the integrin/FAK (focal adhesion kinase)/MAPK (mitogen-activated protein kinase) signaling pathways by LIPUS only treatment resulted in cartilage protection in the early osteoarthritis stage in rabbits [38]. These results provide evidence for our findings of rapid cartilage regeneration (week 4) produced by LIPUS treatment. The success of articular cartilage repair avoids fibrous cartilage induction and relies on an increase in functional hyaline-like cartilage. Although it is not the same process as the co-treatment process used for the extracellular matrix components (Matrilin-3) and ASCs [39], our results were superior to the effects of hyaline-like cartilage production compared to that of fibrous cartilage because of the co-treatment process of acoustic radiation force and cell biological factors. The exact mechanisms underlying a more hyaline-like cartilage production and regenerative process are unknown. One possibility is that LOFUS may be induce conformational changes of tissue compression, tension or shear forces, which could open the channels and activate various ion flux [40, 41]. The most important reason for the efficient results of this study is the use of low-intensity 'focused' ultrasound. The stimulation volume size of LOFUS was 8 mm in depth and 18 mm in diameter, which could significantly cover the cartilage defect and ASC distribution area at the site.
This study includes some limitations. First, the process could not be performed while measuring the low-intensity energy in real time. It was not possible to verify the low-intensity energy irradiation site in real time. However, we made in-house devices to immobilize the rat and to stably expose the wound site, and each rat was exposed to a contrast amount of energy at each time each day. Second, the characteristics of the altered stem cells and cartilage require further investigation based on mechanosensitive channel. LOFUS was used to significantly improve the therapeutic function of stem cells; therefore, we found fast and effective results when treating cartilage defects. This study was carried out to examine the preclinical possibility of co-treatment with acoustic radiation force and cell biological factors, namely, combination therapy.
In summary, ASCs co-treated with LOFUS significantly increased cartilage regeneration in fibrous and hyaline-like tissues in the articular cartilage defect model. These results suggest that a combination therapy consisting of acoustic radiation force (LOFUS) and cell biological factors (ASCs) is of substantial clinical interest in terms of fast and effective regeneration.
Acknowledgements
This study was supported by grants from the National Research Foundation of Korea (NRF-2017R1C1B5017159) and from the Ministry of Health and Welfare, Republic of Korea (HI18C0661).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical statement
All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Catholic Kwandong University International St. Mary’s Hospital in cooperation with the Association for the Assessment and Accreditation of Laboratory Animal Care and were performed in accordance with the Guidelines and Regulations for Animal Care (No. CKU 03-2017-001).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dong-Sik Chae, Email: drchae@ish.ac.kr.
Il-Kwon Kim, Email: ilkwonkim@empas.com.
References
- 1.Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192:230–237. doi: 10.1111/j.1749-6632.2009.05240.x. [DOI] [PubMed] [Google Scholar]
- 2.Wang M, Yuan Z, Ma N, Hao C, Guo W, Zou G, et al. Advances and prospects in stem cells for cartilage regeneration. Stem Cells Int. 2017;2017:4130607. doi: 10.1155/2017/4130607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yelin E. Cost of musculoskeletal diseases: impact of work disability and functional decline. J Rheumatol Suppl. 2003;68:8–11. [PubMed] [Google Scholar]
- 4.Song H, Song BW, Cha MJ, Choi IG, Hwang KC. Modification of mesenchymal stem cells for cardiac regeneration. Expert Opin Biol Ther. 2010;10:309–319. doi: 10.1517/14712590903455997. [DOI] [PubMed] [Google Scholar]
- 5.Dulak J, Szade K, Szade A, Nowak W, Józkowicz A. Adult stem cells: hopes and hypes of regenerative medicine. Acta Biochim Pol. 2015;62:329–337. doi: 10.18388/abp.2015_1023. [DOI] [PubMed] [Google Scholar]
- 6.Chu DT, Nguyen Thi Phuong T, Tien NLB, Tran DK, Minh LB, Thanh VV, et al. Adipose tissue stem cells for therapy: an update on the progress of isolation, culture, storage, and clinical application. J Clin Med. 2019;8:917. doi: 10.3390/jcm8070917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han S, Sun HM, Hwang KC, Kim SW. Adipose-derived stromal vascular fraction cells: update on clinical utility and efficacy. Crit Rev Eukaryot Gene Expr. 2015;25:145–152. doi: 10.1615/critreveukaryotgeneexpr.2015013057. [DOI] [PubMed] [Google Scholar]
- 8.Osinga R, Di Maggio N, Todorov A, Allafi N, Barbero A, Laurent F, et al. Generation of a bone organ by human adipose-derived stromal cells through endochondral ossification. Stem Cells Transl Med. 2016;5:1090–1097. doi: 10.5966/sctm.2015-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stromps JP, Paul NE, Rath B, Nourbakhsh M, Bernhagen J, Pallua N. Chondrogenic differentiation of human adipose-derived stem cells: a new path in articular cartilage defect management? Biomed Res Int. 2014;2014:740926. doi: 10.1155/2014/740926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shafaei H, Esfandiari E, Esmaeili A, Razavi S, Hashemibeni B, Nasr Esfahani MH, et al. Optimizing a novel method for low intensity ultrasound in chondrogenesis induction. Adv Biomed Res. 2013;2:79. doi: 10.4103/2277-9175.120867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angele P, Schumann D, Angele M, Kinner B, Englert C, Hente R, et al. Cyclic, mechanical compression enhances chondrogenesis of mesenchymal progenitor cells in tissue engineering scaffolds. Biorheology. 2004;41:335–346. [PubMed] [Google Scholar]
- 12.Huang CY, Reuben PM, Cheung HS. Temporal expression patterns and corresponding protein inductions of early responsive genes in rabbit bone marrow-derived mesenchymal stem cells under cyclic compressive loading. Stem Cells. 2005;23:1113–1121. doi: 10.1634/stemcells.2004-0202. [DOI] [PubMed] [Google Scholar]
- 13.Korstjens CM, van der Rijt RH, Albers GH, Semeins CM, Klein-Nulend J. Low-intensity pulsed ultrasound affects human articular chondrocytes in vitro. Med Biol Eng Comput. 2008;46:1263–1270. doi: 10.1007/s11517-008-0409-9. [DOI] [PubMed] [Google Scholar]
- 14.Padilla F, Puts R, Vico L, Guignandon A, Raum K. Stimulation of bone repair with ultrasound. Adv Exp Med Biol. 2016;880:385–427. doi: 10.1007/978-3-319-22536-4_21. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Lin Q, Zhang T, Wang X, Cheng K, Gao M, et al. Low-intensity pulsed ultrasound promotes chondrogenesis of mesenchymal stem cells via regulation of autophagy. Stem Cell Res Ther. 2019;10:41. doi: 10.1186/s13287-019-1142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia P, Wang X, Qu Y, Lin Q, Cheng K, Gao M, et al. TGF-β1-induced chondrogenesis of bone marrow mesenchymal stem cells is promoted by low-intensity pulsed ultrasound through the integrin-mTOR signaling pathway. Stem Cell Res Ther. 2017;8:281. doi: 10.1186/s13287-017-0733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Li J, Cheng K, Lin Q, Wang D, Zhang H, et al. Effect of low-intensity pulsed ultrasound on MMP-13 and MAPKs signaling pathway in rabbit knee osteoarthritis. Cell Biochem Biophys. 2011;61:427–434. doi: 10.1007/s12013-011-9206-4. [DOI] [PubMed] [Google Scholar]
- 18.Vaughan NM, Grainger J, Bader DL, Knight MM. The potential of pulsed low intensity ultrasound to stimulate chondrocytes matrix synthesis in agarose and monolayer cultures. Med Biol Eng Comput. 2010;48:1215–1222. doi: 10.1007/s11517-010-0681-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kusuyama J, Bandow K, Shamoto M, Kakimoto K, Ohnishi T, Matsuguchi T. Low intensity pulsed ultrasound (LIPUS) influences the multilineage differentiation of mesenchymal stem and progenitor cell lines through ROCK-Cot/Tpl2-MEK-ERK signaling pathway. J Biol Chem. 2014;289:10330–10344. doi: 10.1074/jbc.M113.546382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebisawa K, Hata K, Okada K, Kimata K, Ueda M, Torii S, et al. Ultrasound enhances transforming growth factor beta-mediated chondrocyte differentiation of human mesenchymal stem cells. Tissue Eng. 2004;10:921–929. doi: 10.1089/1076327041348437. [DOI] [PubMed] [Google Scholar]
- 21.Lynn JG, Zwemer RL, Chick AJ, Miller AE. A new method for the generation and use of focused ultrasound in experimental biology. J Gen Physiol. 1942;26:179–193. doi: 10.1085/jgp.26.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J, Lee S, Lee CY, Seo HH, Shin S, Choi JW, et al. Adipose-derived stem cell-released osteoprotegerin protects cardiomyocytes from reactive oxygen species-induced cell death. Stem Cell Res Ther. 2017;8:195. doi: 10.1186/s13287-017-0647-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi E, Lee J, Lee S, Song BW, Seo HH, Cha MJ, et al. Potential therapeutic application of small molecule with sulfonamide for chondrogenic differentiation and articular cartilage repair. Bioorg Med Chem Lett. 2016;26:5098–5102. doi: 10.1016/j.bmcl.2016.08.069. [DOI] [PubMed] [Google Scholar]
- 24.Lee JH, Hong HK, Song BW, Jung YJ, Na YC, Kim NH, et al. Preliminary study on low intensity focused ultrasound system for neuromodulation. Conf Proc IEEE Eng Med Biol Soc. 2017;2017:4545–4548. doi: 10.1109/EMBC.2017.8037867. [DOI] [PubMed] [Google Scholar]
- 25.Carranza-Bencano A, García-Paino L, Armas Padrón JR, Cayuela Dominguez A. Neochondrogenesis in repair of full-thickness articular cartilage defects using free autogenous periosteal grafts in the rabbit. A follow-up in six months. Osteoarthritis Cartilage. 2000;8:351–358. doi: 10.1053/joca.1999.0309. [DOI] [PubMed] [Google Scholar]
- 26.Moussavi-Harami SF, Pedersen DR, Martin JA, Hillis SL, Brown TD. Automated objective scoring of histologically apparent cartilage degeneration using a custom image analysis program. J Orthop Res. 2009;27:522–528. doi: 10.1002/jor.20779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng L, Pi C, Zhang J, Fan Y, Cui C, Zhou Y, et al. Aberrant activation of latent transforming growth factor-β initiates the onset of temporomandibular joint osteoarthritis. Bone Res. 2018;6:26. doi: 10.1038/s41413-018-0027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grande DA, Southerland SS, Manji R, Pate DW, Schwartz RE, Lucas PA. Repair of articular cartilage defects using mesenchymal stem cells. Tissue Eng. 1995;1:345–353. doi: 10.1089/ten.1995.1.345. [DOI] [PubMed] [Google Scholar]
- 29.Im GI, Kim DY, Shin JH, Hyun CW, Cho WH. Repair of cartilage defect in the rabbit with cultured mesenchymal stem cells from bone marrow. J Bone Joint Surg Br. 2001;83:289–294. doi: 10.1302/0301-620x.83b2.10495. [DOI] [PubMed] [Google Scholar]
- 30.Lee KB, Hui JH, Song IC, Ardany L, Lee EH. Injectable mesenchymal stem cell therapy for large cartilage defects–a porcine model. Stem Cells. 2007;25:2964–2971. doi: 10.1634/stemcells.2006-0311. [DOI] [PubMed] [Google Scholar]
- 31.Khanmohammadi M, Golshahi H, Saffarian Z, Montazeri S, Khorasani S, Kazemnejad S. Repair of osteochondral defects in rabbit knee using menstrual blood stem cells encapsulated in fibrin glue: a good stem cell candidate for the treatment of osteochondral defects. Tissue Eng Regen Med. 2019;16:311–324. doi: 10.1007/s13770-019-00189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Windt TS, Vonk LA, Slaper-Cortenbach IC, van den Broek MP, Nizak R, van Rijen MH, et al. Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single-stage cartilage repair in humans upon mixture with recycled autologous chondrons. Stem Cells. 2017;35:256–264. doi: 10.1002/stem.2475. [DOI] [PubMed] [Google Scholar]
- 33.Awad ME, Hussein KA, Helwa I, Abdelsamid MF, Aguilar-Perez A, Mohsen I, et al. Meta-analysis and evidence base for the efficacy of autologous bone marrow mesenchymal stem cells in knee cartilage repair: methodological guidelines and quality assessment. Stem Cells Int. 2019;2019:3826054. doi: 10.1155/2019/3826054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Brien WD., Jr Ultrasound-biophysics mechanisms. Prog Biophys Mol Biol. 2007;93:212–255. doi: 10.1016/j.pbiomolbio.2006.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jingushi S, Mizuno K, Matsushita T, Itoman M. Low-intensity pulsed ultrasound treatment for postoperative delayed union or nonunion of long bone fractures. J Orthop Sci. 2007;12:35–41. doi: 10.1007/s00776-006-1080-3. [DOI] [PubMed] [Google Scholar]
- 36.Harrison A, Lin S, Pounder N, Mikuni-Takagaki Y. Mode & mechanism of low intensity pulsed ultrasound (LIPUS) in fracture repair. Ultrasonics. 2016;70:45–52. doi: 10.1016/j.ultras.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 37.Xiao W, Xu Q, Zhu Z, Li L, Chen W. Different performances of CXCR4, integrin-1β and CCR-2 in bone marrow stromal cells (BMSCs) migration by low-intensity pulsed ultrasound stimulation. Biomed Tech (Berl) 2017;62:89–95. doi: 10.1515/bmt-2015-0166. [DOI] [PubMed] [Google Scholar]
- 38.Xia P, Shen S, Lin Q, Cheng K, Ren S, Gao M, et al. Low-intensity pulsed ultrasound treatment at an early osteoarthritis stage protects rabbit cartilage from damage via the integrin/focal adhesion kinase/mitogen-activated protein kinase signaling pathway. J Ultrasound Med. 2015;34:1991–1999. doi: 10.7863/ultra.14.10016. [DOI] [PubMed] [Google Scholar]
- 39.Muttigi MS, Kim BJ, Choi B, Yoshie A, Kumar H, Han I, et al. Matrilin-3 codelivery with adipose-derived mesenchymal stem cells promotes articular cartilage regeneration in a rat osteochondral defect model. J Tissue Eng Regen Med. 2018;12:667–675. doi: 10.1002/term.2485. [DOI] [PubMed] [Google Scholar]
- 40.Tyler WJ, Tufail Y, Finsterwald M, Tauchmann ML, Olson EJ, Majestic C. Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PLoS One. 2008;3:e3511. doi: 10.1371/journal.pone.0003511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kubanek J, Shi J, Marsh J, Chen D, Deng C, Cui J. Ultrasound modulates ion channel currents. Sci Rep. 2016;6:24170. doi: 10.1038/srep24170. [DOI] [PMC free article] [PubMed] [Google Scholar]