Abstract
Background:
Despite promising advances in stem cell-based therapy, the treatment of ischemic cardiovascular diseases remains a big challenge due to both the insufficient in vivo viability of transplanted cells and poor angiogenic potential of stem cells. The goal of this study was to develop therapeutic human cardiac progenitor cells (hCPCs) for ischemic cardiovascular diseases with a novel M13 peptide carrier.
Method:
In this study, an engineered M13 peptide carrier was successfully generated using a QuikChange Kit. The cellular function of M13 peptide carrier-treated hCPCs was assessed using a tube formation assay and scratch wound healing assay. The in vivo engraftment and cell survival bioactivities of transplanted cells were demonstrated by immunohistochemistry after hCPC transplantation into a myocardial infarction animal model.
Results:
The engineered M13RGD+SDKP peptide carrier, which expressed RGD peptide on PIII site and SDKP peptide on PVIII site, did not affect morphologic change and proliferation ability in hCPCs. In contrast, hCPCs treated with M13RGD+SDKP showed enhanced angiogenic capacity, including tube formation and migration capacity. Moreover, transplanted hCPCs with M13RGD+SDKP were engrafted into the ischemic region and promoted in vivo cell survival.
Conclusion:
Our present data provides a promising protocol for CPC-based cell therapy via short-term cell priming of hCPCs with engineered M13RGD+SDKP before cell transplantation for treatment of cardiovascular disease.
Electronic supplementary material
The online version of this article (10.1007/s13770-020-00244-w) contains supplementary material, which is available to authorized users.
Keywords: M13 bacteriophage, Cardiac progenitor cell, RGD, SDKP, Cardiovascular diseases
Introduction
The number of patients with cardiovascular disease (CVD) has increased due to increased life span and changed lifestyles [1, 2]. CVD, which is the leading cause of death worldwide, is caused by smoking, diabetes mellitus, obesity, alcohol, high blood pressure and high blood cholesterol [3, 4]. These CVD risk factors can promote atherogenesis, which is a major cause of CVD. An unbalanced diet affects atherogenesis by plaque formation, blood lipid and endothelial dysfunction [5, 6]. Traditionally, the heart was known as a terminally differentiated organ. Thus, the focus was on preventing and treating CVD with antihypertensive treatments [7]. Pharmacological therapies can reduce the symptoms and progression of CVD. According to the treatment technology, various methods were developed for the treatment of CVD, such as stent insertion, gene therapy and stem cell transplantation [8, 9].
Cell transplantation attempts to restore heart function with various cells such as cardiomyocytes, bone marrow-derived mononuclear stem cells, pluripotent stem cells, embryonic stem cells, mesenchymal stem cells, endothelial progenitor cells, and cardiac progenitor cells [10–13]. Embryonic stem cells and pluripotent stem cells can differentiate into cardiomyocytes and promote cardiac regeneration, but there still remains a high risk of teratoma formation and ethical problems [14–16]. Other cells may promote cardiac function through indirect regeneration. Stem cells of different origins cannot compensate for cardiomyocytes [17]. They can promote cardiac function by beneficial effects such as angiogenesis, release to cytokines and remodeling of the extracellular matrix. However, these cell therapies do not directly regenerate the heart [18, 19]. In 2003, Anversa et al. discovered the presence of cardiac progenitor cells (CPCs) in adult rat heart [20]. hCPCs have the capability of self-renewal and can differentiate into cardiomyocytes, endothelial cells and smooth muscle cells [21]. And hCPCs are also known to have a paracrine effect [22, 23]. For these reasons, CPCs have been successfully attempted in clinical trials and have demonstrated the ability of cardiac regeneration [24]. Nevertheless, there are still limitations to their therapeutic effect because of the low viability of cells engrafted into the ischemic area and decreased ability of engrafted cells. Thus, it can be an advanced strategy for the transplantation of enhanced function hCPCs and increased engraftment ability [25, 26].
Emerging evidence suggests that CPCs improved by using small molecules, growth factor and cytokines can be a candidate for applied stem cell therapy [27–32]. These cell-priming strategies revealed limited efficacy because of non-specific reactions between treated material and the ischemic region. In this study, we used an engineered M13 phage as a nano-carrier. The M13 bacteriophage, which is referred to as an M13 nano-carrier, is a bacterial virus composed of single-stranded DNA. It has a long rod shape and nano-scale size. An M13 bacteriophage has several advantages for clinical application. Firstly, phage vectors are easy to engineer and produce at low cost because of the characteristics of M13 phage, such as self-assembly and self-replication [33, 34]. Secondly, the phage vectors can involve more than 2700 copies of peptides. It can be considered that a phage is efficient at gene delivery [35, 36]. Finally, M13 phages are developed as a vector for the safe administration of transgenes for in vivo application. M13 phages have been approved by the Food and Drug Administration [36–38].
In this study, using the advantages of M13 peptide carriers, we genetically engineered an M13 phage with two functional peptides (RGD and SDKP) and characterized the efficacy of the hCPCs based on our previous report that the PIII—RGD peptide (M13RGD) provides cell adhesion ability of hCPCs [39–42]. The PVII-SDKP peptides (M13SDKP) provide anti-fibrotic effects and angiogenic effects [43, 44]. In this study, we investigated the angiogenic effect of hCPC pre-treated with an engineered M13 peptide carrier and hCPC engraftment and cell survival in the ischemic heart region.
Materials and methods
Isolation of human c-kit positive cardiac progenitor cells (hCPCsc−kit+)
hCPCsc−kit+ were isolated from human infant-derived heart tissues after surgical procedures, as described in a previously modified protocol [45]. The Ethical Review Board of Pusan National University Yangsan Hospital, Gyeongsangnam-do, Republic of Korea, approved the protocols. To perform this isolation, the biopsied heart specimens were minced and incubated in 0.2% collagenase type II (Worthington, NJ, USA) at 37 °C for 30 min to obtain single cardiac cells. Single cardiac cells were incubated in Ham’s F12 media (Hyclone, Logan, UT, USA) containing 10% fetal bovine serum (FBS, Gibco, CA, USA), 1X penicillin/streptomycin (P/S, Welgene, Daegu, Republic of Korea), 2.5 U human erythropoietin (hEPO, R&D Systems, Minneapolis, MN, USA), 5 μg basic human recombinant fibroblast growth factor (bFGF, Peprotech, Rocky Hill, NJ, USA), and 0.2 mM glutathione (Sigma-Aldrich, St. Louis, CA, USA). When single cardiac cells were grown to a high enough confluence for sorting, the single cardiac cells were conjugated to the c-kit primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and sorted by magnetic activated cell sorting (MACS).
Genetic engineering of the M13 nano-carrier
To engineer an M13 bacteriophage displaying both RGD and SDKP, we genetically engineered the major coat protein pVIII and minor coat protein pIII of the M13 bacteriophage using a recombinant DNA engineering technique, as reported previously [46–48]. To construct the RGD peptide on the minor coat protein and the SDKP peptide on the major coat protein, an M13KE single-stranded phage (New England Biolabs, Ipswich, MA, USA) was used as a base for the library and insert construction. We used a partially constrained library method to design a stable peptide sequence. This was accomplished by site-directed mutagenesis using methods described in the QuikChange Kit (Stratagene, La Jolla, CA, USA). Following mutagenesis, the resulting phages were verified by DNA sequencing (Cosmo Genetech, Seoul, Korea), and the viability of the phages was assessed by plaque forming units (PFU).
Animal
Experiments were performed on male 8- to 10-week-old BALB/CA-nu/nu mice maintained under a 12-h light/dark cycle in accordance with the regulations of Pusan National University. Standard laboratory chow and water were available ad libitum. These protocols were approved by the guidelines of the Institutional Animal Care and Use Committee of Pusan National University in Pusan, Korea (IACUC090017).
Immunocytochemistry
For immunocytochemistry, hCPCs were seeded at a density of 50,000 cells per well in a 2-well chamber slide (BioTek, Winooski, VT, USA). Before immunofluorescence analysis, the cells were washed in PBS, fixed in 4% paraformaldehyde (USB Corporation, Santa Clara, CA, USA) for 10 min, permeabilized with 0.2% (w/v) Triton X-100 in PBS for 5 min, and then incubated in PBS containing 5% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO, USA) for 2 h to block non-specific binding sites. The cells were then incubated with primary antibody (1:100) overnight at 4 °C, and secondary antibody Alexa-488 (Thermo Fisher Scientific, Waltham, MA, USA). The nuclei were stained with 4′,6-diaminido-2-phenylindol (Sigma-Aldrich), and the immunostained cells were imaged under a Lionheart FX automated microscope (Biotech, Winooski, VT, USA). Primary antibodies: anti-M13 (Abcam, Cambridge, UK); anti-Von Willebrand factor (Abcam, Cambridge, UK); anti-Cardiac Troponin T (Thermo Fisher Scientific, Waltham, MA, USA); anti-alpha smooth muscle actin (Abcam, Cambridge, UK).
Cell viability assay
Cell viability was measured using a WST Kit (Ez-cytox, Dail-lab, Seoul, Republic of Korea). hCPCsc−kit+ were seeded on 96-well plates with M13 bacteriophage at various concentrations. Culture plates were incubated for 24 h. After incubation, the culture medium with M13 bacteriophage was changed to the WST solution. The plates were then incubated for 1 h, and the absorbance was measured at 450 nm using a spectrophotometer (Tecan, Grodig, Austria).
Tube formation assay
96-well plates were coated with 70 μL of Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) and incubated at 37 °C for 30 min. Pretreated hCPCs with M13Wild, M13RGD, and M13RGD+SDKP were seeded in 96-well plates with Matrigel and then incubated for 6 h. After incubation, total tube length was measured by counting the number of tubes visualized in one microscopic field per well (40× magnification) with at least three independent replicates using ImageJ software (free software from National Institutes of Health).
Scratch wound-healing assay
Scratch migration assays were performed by plating cells in 6-well plates. M13Wild, M13RGD, M13RGD+SDKP pre-treated cells were seeded at a density of 3 × 105 cells/well and cultured for 24 h. A sterile blue tip was used to scratch the cells in each well, after which the cells were washed with full media. The cells were then incubated in full media at 37 °C for 6 h. Cell migration activity was expressed as the migration area.
Generation of myocardial infarction
Mice were subjected to myocardial infarction (MI) by ligation of the left anterior descending coronary artery (LAD) as described previously [49, 50]. Immediately after LAD ligation, one set of mice received an intramyocardial injection of CPCs with a total volume of 50 μL at five different sites (basal anterior, mid anterior, mid lateral, apical anterior, and apical lateral) in the peri-infarct area. Cytokine secretion, retention, survival, and migration of transplanted CPCs was assessed after 3 and 28 days. Before transplantation, hCPCs were stained with 1 ug/mL chloromethylbenzamido (CM-DiI, Invitrogen) in PBS for 30 min.
Immunofluorescence staining
Three days after the human CPCs implantation, mice were euthanized, and their hearts were removed. The excised hearts were retrograde perfused with PBS to wash the coronary vasculature and left ventricular (LV) and fixed with 4% paraformaldehyde overnight at 4 °C and then with 30% sucrose overnight at 4 °C. Sections (5 μm thick) were subject to immunofluorescent staining with anti-CD31 (Abcam, Cambridge, MA, USA) and anti-smooth muscle actin (SMA; Abcam) to detect the capillary and arteriole sections, respectively of the ischemic regions subject to immunofluorescent staining. Primary antibodies against proliferating cell nuclear antigen (pHH3; Abcam) and anti-sarcomeric alpha actin (α-SA; Abcam) were used for immunofluorescent staining of ischemic tissues. Sections were counterstained with DAPI (Vector Laboratories, Burlingame, CA, USA) and examined using a FluoView 1000 confocal microscope (Olympus, Tokyo, Japan). Similar to the method previously described, quantitative morphometric analysis with Masson’s trichrome staining was performed (16,547,211). The area of infarct size was calculated by dividing the midline length of the infarcted LV wall by the midline length of total LV wall. The percentage of the fibrotic area of the left ventricle was measured using Image J software.
Induced differentiation of hCPCs
To evaluate the ability of endothelial cells (EC) to differentiate, hCPCs were cultured with DMEM (Welgene, Daegu, Republic of Korea) high glucose medium supplemented with 20% FBS, 1% P/S and 30 ng/mL of b-FGF for 7 days. For differentiation into smooth muscle cells (SMC), hCPCs were cultured with Medium 231 (Gibco, CA, USA) supplemented with 5% FBS, 1% P/S and 1X smooth muscle differentiation supplement (SMDS, Gibco, CA, USA) for 7 days. The medium was changed every 2 days. To induce differentiation into cardiomyocytes (CM), hCPCs were cultured on MEM/EBSS (Hyclone) supplemented with 2% of FBS, 1% P/S and 10 nM Dexamethasone (Sigma-Aldrich, St. Louis, CA, USA) for 7 days. The medium was changed every day. The ability of cells to differentiate was analyzed by immunofluorescence staining.
Statistical analysis
All experimental results are presented as the mean ± standard deviation (SD) using ANOVA. Comparisons between the two groups were performed using the unpaired Student’s t test. p value less than 0.05(*), 0.01(**) and 0.001(***) were considered statistically significant.
Results
Schematic experimental design and analysis of engineered M13 on CPCs
To improve the function and engraftment ability of hCPCs, we genetically engineered an M13 phage that displays SDKP and RGD on major and minor proteins (Fig. 1). To determine the proper concentration of M13 on hCPCs, we verified the cytotoxic effect of M13 phages on hCPCs. Cell viability decreased when exposed to more than 104 PFU/cell (Fig. 2). To next investigate the adhesion ability of the M13 phage, hCPC was confirmed by immunofluorescence staining. The adhesion ability of the M13 phage was significantly increased by more than 102 PFU/cell (Fig. 2B). Therefore, we optimized all experiments using 102 PFU/cell. To assess the effect of the M13 phage on hCPC morphology, we analyzed morphologic changes using a microscope. Morphologic change did not differ under four different culture conditions (Fig. 2C).
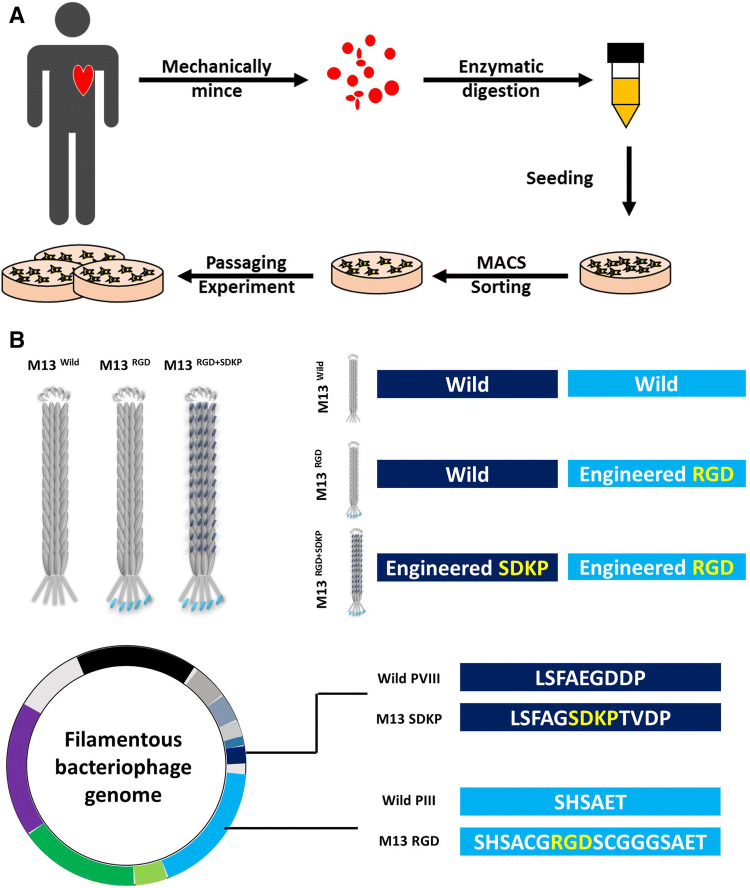
Fig. 1.
Experimental scheme of this study. A Isolation protocol of primary human cardiac progenitor cells. Biopsied heart specimens were minced mechanically into small pieces, digested with collagenase, and sorted by MACS. B Design and characterization of engineered m13 phage. The M13 phage was engineered to express RGD-peptide on PIII site and SDKP on PVIII site
Fig. 2.
Effects of engineered M13 treatment on human CPCs. A hCPCs were treated with different concentrations of M13 nano-carriers for 24 h and viability was measured using a cell viability assay (CCK assay). Data are presented as mean ± standard deviation (SD). *, p < 0.05 versus control. B Expression of M13 nano-carrier in hCPCs pretreated with M13Wild, RGD and RGD+SDKP by immunocytochemistry. Scale bar = 20 µm. C Quantification of adhesion of the engineered M13 phage at various concentrations (0–106 plaque forming unit) on the CPCs. D hCPCs were treated with M13Wild, RGD and RGD+SDKP for 24 h and morphologic changes were confirmed using a microscope. Scale bar = 50 µm. E Quantification of the diameter of the hCPCs treated with the engineered M13 nano-carrier
M13RGD+SDKP enhances in vitro wound healing and tube formation capacity of hCPC
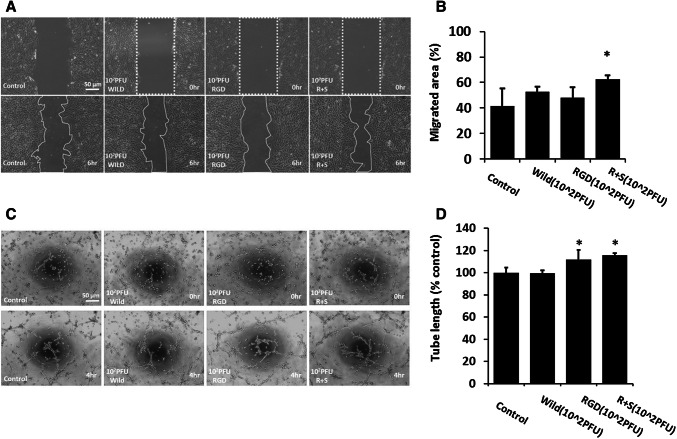
To confirm the migration capacity of hCPCs after treatment with the engineered M13 phage, the migration capacity of hCPCs was confirmed using a scratch wound healing assay (Fig. 3A). hCPCsRGD+SDKP (hCPCs treated with M13RGD+SDKP) revealed significantly enhanced migration capacity compared to the control (Fig. 3B). Moreover, we performed a Matrigel tube formation assay to investigate angiogenic potential in hCPCs (Fig. 3C). The tube formation capacity of hCPC RGD+SDKP was significantly higher than other groups (Fig. 3D). These results suggest that M13RGD+SDKP enhanced the angiogenic potential of hCPCs.
Fig. 3.
Effect of engineered M13 nano-carrier on migration and angiogenic capacity of hCPCs. A The migration capacity of the hCPCs treated with the engineered M13 was investigated using a scratched wound healing assay. Scale bar = 50 µm. B Quantification of migrated area. Data are presented as mean ± standard deviation (SD). *, p < 0.05 versus control. C The tube formation capacity of the hCPCs treated with the engineered M13 nano-carrier was assessed using a Matrigel tube formation assay. Scale bar = 50 µm. D Quantification of total tube length of the hCPCs treated with the engineered M13 nano-carrier
Increased proliferation and survival of M13RGD+SDKP-treated hCPC in the infarct zone of myocardial infarction
Three days following cell transplantation, immunofluorescence analysis confirmed the presence of CM-DiI-positive CPCs in the infarcted region of the mice myocardium. Cell retention is one of the major determining factors for effective and successful cell transplantation. Therefore, we compared the number of cells retained 3 days post-MI for each of the injection groups. We found that the injection of hCPCRGD+SDKP significantly increased hCPC retention even at 3 days post-MI compared with the injection in the other groups. Indeed, IF staining of proliferating cell marker (phh3) positive cells at 3 days was more abundant in the case of hCPCRGD+SDKP than in that of other groups. M13RGD+SDKP would be beneficial for repairing tissue damaged by myocardial injury by preparing cells with a better survival rate at the site of ischemic injury.
hCPCsRGD+SDKP efficiently incorporated into capillaries and protected ischemic vessels at the border zone of the left ventricular (LV) infarct
To elucidate the underlying mechanism that ameliorates cardiac function after infarction, we used immunofluorescence staining of CD31 and smooth muscle actin (SMA) to quantify the border zone capillary and arteriole tissue, respectively. Surprisingly, hCPCsRGD+SDKP efficiently incorporated into capillary tissue (CD31 positive staining) and arteriole tissue (SMA positive staining). Both capillary and arteriole were significantly greater in the hCPCsRGD+SDKP group than in the hCPCs, hCPCsWild, and hCPCsRGD groups, which verifies the cardiac function improvement observed after 3 days. This finding is also in agreement with the better cardiac outcome seen at 3 days with hCPCRGD+SDKP treatment compared with hCPCs, hCPCsWild, and hCPCsRGD treatments.
M13RGD+SDKP enhances cardiac function and angiogenesis of hCPC
The scar size and infarct wall thickness were determined by Masson trichrome staining (Fig. 4). The percentage of scar size in the hCPCsRGD+SDKP group was significantly reduced compared with the hCPCsWild/hCPCsRGD groups, and infarct wall thickness in the hCPCsRGD+SDKP group was significantly greater than in the hCPCs/hCPCsWild groups. Taken together, this demonstrated that hCPCsRGD+SDKP enhanced cardiac function and improved cardiac morphology after myocardial infarction. To detect neovascularization in the infarcted heart, the cardiac tissue sections were stained with smooth muscle cell marker (SMA). The results demonstrated an increased number of SMA positive vessels, respectively, in the peri-infarct regions of the hCPCsRGD+SDKP group compared with the other group.
Fig. 4.
Engraftment of human CPCs RGD + SDKP enhanced heart function recovery after 28 days post-MI. A At 28 days after MI, heart tissue was harvested. Heart sections were stained with Masson’s trichrome dye. Representative whole-heart images for serial sections. Scale bar = 2000 μm. B SMA-positive blood vessels in ischemic samples on day 28. Scale bar = 100 μm. C Quantitative analysis of fibrosis and SMA-positive blood vessels. Average values presented as mean ± SD (n = 5). *p < 0.05, **p < 0.01
Discussion
hCPCs are a promising cell resource for heart regeneration due to such abilities as direct differentiation into cardiac lineage and paracrine effect. Although the therapeutic effect of CPCs in CVD models seems to be clearly demonstrated, there still remain limitations due to the quality of CPCs and engraftment ability. Although several studies have been reported to enhance hCPC function using natural products, growth factors and chemicals, that may cause unexpected side effects in the ischemic region because of an unclear demonstration between molecule and tissue.
We generated an M13 nano-carrier with each protein coated independently, which expressed RGD on PIII site and SDKP on PVIII site (Fig. 1B and Table 1). These characteristics of the M13 phage can deliver a combination of peptides into a cell. In this study, we provided a promising protocol using enhanced CPC therapy against CVDs with a novel peptide carrier, M13RGD+SDKP. Our previous study clearly showed that M13RGD+SDKP promotes endothelial cell function and has an angiogenic effect on a hind-limb model [43]. We demonstrated here that M13RGD+SDKP enhanced the angiogenic potential of hCPC and in vivo engraftment in the ischemic heart region. Interestingly, pretreatment of M13 nano-carrier did not affect morphology, proliferation and differentiation capacity in vitro (Fig. 2 and Figure S1). In contrast, hCPC cultured with M13RGD+SDKP showed enhanced tubular forming capability and migration ability of hCPC, suggesting that M13RGD+SDKP might improve the angiogenic potential of hCPCs (Fig. 3).
Table 1.
Genetically engineered phages as a nano carriers
| Name | PIII | PVIII |
|---|---|---|
| M13Wild | SHSAET | LSFAEGDDP |
| M13RGD | SHSACGRGDSCGGGSAET | LSFAEGDDP |
| M13RGD+SDKP | SHSACGRGDSCGGGSAET | LSFAGSDKPTVDP |
The ischemic region has a special microenvironment due to the loss of blood vessels and endothelial cells. This causes a restriction of blood supply, shortage of oxygen and insufficiency of nutrients [51]. For these reasons, cells transplanted into an ischemic site have a low engraftment rate. Our data suggest that RGD peptide, which is related to adhesion, can promote hCPC engraftment ability. After engraftment of M13RGD and RGD+SDKP treated cells, the proliferated hCPCs were observed in the ischemic site, indicating that RGD peptide might promote their engraftment ability and they will migrate into the ischemic region, which is called homing capacity (Figs. 5, 6). In addition, a mouse model of M13RGD and RGD+SDKP treated cells transplantation exhibited preserved cardiac function, increased capillary density and LV well thickness. Although the engraftment ability and functional recovery were lower in the hCPC treated with M13RGD group than M13RGD+SDKP group, RGD peptide promote hCPC engraftment and function. Ac-SDKP, which is the active site of thymosin β4, has various effect on cardiovascular diseases such as inhibits apoptosis, enhances cell survival, induces angiogenesis and anti-inflammatory activity. There results suggesting that SDKP peptide might promote their function through enhanced engraftment ability. Although RGD and SDKP peptide did not affect on in vitro differentiation capacity and cell proliferation capacity with sufficient nutrients, engraftment ability in ischemic areas with limited blood flow and their function were enhanced by RGD and SDKP peptide.
Fig. 5.
Analysis of survival and proliferation of implanted human CPCs RGD + SDKP. A Representative DiI + CPCs (orange) after injection with M13 (WT, RGD, RGD + SDKP) at 3 days post-infarction. Phosphorylated histone H3 (pHH3; green), sarcomeric alpha-sctin (α-SA; red) and nuclei were stained using DAPI. B, C The statistics of DiI + MNC retention ratio and quantification of pHH3-positive cells at 3 days after MI. Data are presented as mean ± SD (n = 5). *p < 0.05, **p < 0.01. Scale bar = 100 μm
Fig. 6.
Engraftment of human CPCs RGD + SDKP enhanced neovascularization 3 days post-MI. A Representative images of ischemic heart samples at 3 days after immunostaining with anti-CD31 antibody (red) and anti-SMA antibody (green). Nuclei counterstained with DAPI (blue). B, C Quantitative analysis of CD31-positive capillaries. B SMA-positive blood vessels (C) in ischemic samples on day 3. Average values presented as mean ± SD (n = 5). *p < 0.05, **p < 0.01. Scale bar = 100 μm
In conclusion, engineered M13RGD+SDKP might be a novel candidate protocol for clinically applicable stem cell-based therapy for ischemic cardiovascular disease because of its characteristics such as enhanced angiogenic capacities and increased cell viability after transplantation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgement
This work was supported by a Grant from the Korean Health Technology R&D Project, Ministry of Health and Welfare (HI17C1662, HI18C2458), funded by the Korean government.
Compliance with ethical standards
Conflicts of interest
The authors have no financial conflicts of interest.
Ethical statement
The protocols were approved by the guidelines of the Institutional Animal Care and Use Committee of Pusan National University in Pusan, Korea (IACUC090017).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Woong Bi Jang and Seung Taek Ji have contributed equally.
Contributor Information
Sang Hong Baek, Email: whitesh@catholic.ac.kr.
Sang-Mo Kwon, Email: smkwon323@hotmail.com.
References
- 1.Bhatnagar A. Environmental determinants of cardiovascular disease. Circ Res. 2017;121:162–180. doi: 10.1161/CIRCRESAHA.117.306458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen BE, Edmondson D, Kronish IM. State of the art review: depression, stress, anxiety, and cardiovascular disease. Am J Hypertens. 2015;28:1295–1302. doi: 10.1093/ajh/hpv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moran AE, Roth GA, Narula J, Mensah GA. 1990–2010 Global cardiovascular disease Atlas. Glob Heart. 2014;9:3–16. doi: 10.1016/j.gheart.2014.03.1220. [DOI] [PubMed] [Google Scholar]
- 4.Thomas H, Diamond J, Vieco A, Chaudhuri S, Shinnar E, Cromer S, et al. Global Atlas of cardiovascular disease 2000–2016: the path to prevention and control. Glob Heart. 2018;13:143–163. doi: 10.1016/j.gheart.2018.09.511. [DOI] [PubMed] [Google Scholar]
- 5.De Caterina R, Zampolli A, Del Turco S, Madonna R, Massaro M. Nutritional mechanisms that influence cardiovascular disease. Am J Clin Nutr. 2006;83:421S–426S. doi: 10.1093/ajcn/83.2.421S. [DOI] [PubMed] [Google Scholar]
- 6.Booker CS, Mann JI. Trans fatty acids and cardiovascular health: translation of the evidence base. Nutr Metab Cardiovasc Dis. 2008;18:448–456. doi: 10.1016/j.numecd.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Report of the Joint National Committee on Detection Evaluation, and treatment of high blood pressure. A cooperative study. JAMA. 1977;237:255–261. [PubMed] [Google Scholar]
- 8.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 9.Rincon MY, VandenDriessche T, Chuah MK. Gene therapy for cardiovascular disease: advances in vector development, targeting, and delivery for clinical translation. Cardiovasc Res. 2015;108:4–20. doi: 10.1093/cvr/cvv205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duelen R, Sampaolesi M. Stem cell technology in cardiac regeneration: a pluripotent stem cell promise. EBioMedicine. 2017;16:30–40. doi: 10.1016/j.ebiom.2017.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leong YY, Ng WH, Ellison-Hughes GM, Tan JJ. Cardiac stem cells for myocardial regeneration: they are not alone. Front Cardiovasc Med. 2017;4:47. doi: 10.3389/fcvm.2017.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemcke H, Voronina N, Steinhoff G, David R. Recent progress in stem cell modification for cardiac regeneration. Stem Cells Int. 2018;2018:1909346. doi: 10.1155/2018/1909346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braunwald E. Cell-based therapy in cardiac regeneration: an overview. Circ Res. 2018;123:132–137. doi: 10.1161/CIRCRESAHA.118.313484. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 15.Behfar A, Perez-Terzic C, Faustino RS, Arrell DK, Hodgson DM, Yamada S, et al. Cardiopoietic programming of embryonic stem cells for tumor-free heart repair. J Exp Med. 2007;204:405–420. doi: 10.1084/jem.20061916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ménard C, Hagège AA, Agbulut O, Barro M, Morichetti MC, Brasselet C, et al. Transplantation of cardiac-committed mouse embryonic stem cells to infarcted sheep myocardium: a preclinical study. Lancet. 2005;366:1005–1012. doi: 10.1016/S0140-6736(05)67380-1. [DOI] [PubMed] [Google Scholar]
- 17.Stamm C, Nasseri B, Choi YH, Hetzer R. Cell therapy for heart disease: great expectations, as yet unmet. Heart Lung Circ. 2009;18:245–256. doi: 10.1016/j.hlc.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Lai VK, Ang KL, Rathbone W, Harvey NJ, Galiñanes M. Randomized controlled trial on the cardioprotective effect of bone marrow cells in patients undergoing coronary bypass graft surgery. Eur Heart J. 2009;30:2354–2359. doi: 10.1093/eurheartj/ehp262. [DOI] [PubMed] [Google Scholar]
- 19.Stamm C, Klose K, Choi YH. Clinical application of stem cells in the cardiovascular system. Adv Biochem Eng Biotechnol. 2010;123:293–317. doi: 10.1007/10_2010_77. [DOI] [PubMed] [Google Scholar]
- 20.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 21.Sturzu AC, Wu SM. Developmental and regenerative biology of multipotent cardiovascular progenitor cells. Circ Res. 2011;108:353–364. doi: 10.1161/CIRCRESAHA.110.227066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vandergriff AC, de Andrade JB, Tang J, Hensley MT, Piedrahita JA, Caranasos TG, et al. Intravenous cardiac stem cell-derived exosomes ameliorate cardiac dysfunction in doxorubicin induced dilated cardiomyopathy. Stem Cells Int. 2015;2015:960926. doi: 10.1155/2015/960926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amini H, Rezaie J, Vosoughi A, Rahbarghazi R, Nouri M. Cardiac progenitor cells application in cardiovascular disease. J Cardiovasc Thorac Res. 2017;9:127–132. doi: 10.15171/jcvtr.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126:S54–S64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seif-Naraghi SB, Singelyn JM, Salvatore MA, Osborn KG, Wang JJ, Sampat U, et al. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Sci Transl Med. 2013;5:173ra25. doi: 10.1126/scitranslmed.3005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teng L, Bennett E, Cai C. Preconditioning c-kit-positive human cardiac stem cells with a nitric oxide donor enhances cell survival through activation of survival signaling pathways. J Biol Chem. 2018;293:12619. doi: 10.1074/jbc.AAC118.004922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drowley L, McPheat J, Nordqvist A, Peel S, Karlsson U, Martinsson S, et al. Discovery of retinoic acid receptor agonists as proliferators of cardiac progenitor cells through a phenotypic screening approach. Stem Cells Transl Med. 2020;9:47–60. doi: 10.1002/sctm.19-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park JH, Lee NK, Lim HJ, Mazumder S, Kumar Rethineswaran V, Kim YJ, et al. Therapeutic cell protective role of histochrome under oxidative stress in human cardiac progenitor cells. Mar Drugs. 2019;17:E368. doi: 10.3390/md17060368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jang WB, Park JH, Ji ST, Lee NK, Kim DY, Kim YJ, et al. Cytoprotective roles of a novel compound, MHY-1684, against hyperglycemia-induced oxidative stress and mitochondrial dysfunction in human cardiac progenitor cells. Oxid Med Cell Longev. 2018;2018:4528184. doi: 10.1155/2018/4528184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang S, Li X, Jourd’heuil FL, Qu S, Devejian N, Bennett E, et al. Cytoglobin promotes cardiac progenitor cell survival against oxidative stress via the upregulation of the NFκB/iNOS signal pathway and nitric oxide production. Sci Rep. 2017;7:10754. doi: 10.1038/s41598-017-11342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu J, Lian W, Li L, Huang Z. Generation of induced cardiac progenitor cells via somatic reprogramming. Oncotarget. 2017;8:29442–29457. doi: 10.18632/oncotarget.15272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun J, Zhang J, Yan W, Chen C, Wu G, Abbasi S, et al. Iloprost prevents doxorubicin mediated human cardiac progenitor cell depletion. Int J Cardiol. 2014;176:536–539. doi: 10.1016/j.ijcard.2014.07.031. [DOI] [PubMed] [Google Scholar]
- 33.Chung WJ, Oh JW, Kwak K, Lee BY, Meyer J, Wang E, et al. Biomimetic self-templating supramolecular structures. Nature. 2011;478:364–368. doi: 10.1038/nature10513. [DOI] [PubMed] [Google Scholar]
- 34.Oh JW, Chung WJ, Heo K, Jin HE, Lee BY, Wang E, et al. Biomimetic virus-based colourimetric sensors. Nat Commun. 2014;5:3043. doi: 10.1038/ncomms4043. [DOI] [PubMed] [Google Scholar]
- 35.Pranjol MZ, Hajitou A. Bacteriophage-derived vectors for targeted cancer gene therapy. Viruses. 2015;7:268–284. doi: 10.3390/v7010268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lang LH. FDA approves use of bacteriophages to be added to meat and poultry products. Gastroenterology. 2006;131:1370. doi: 10.1053/j.gastro.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 37.Donnelly A, Yata T, Bentayebi K, Suwan K, Hajitou A. Bacteriophage mediates efficient gene transfer in combination with conventional transfection reagents. Viruses. 2015;7:6476–6489. doi: 10.3390/v7122951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atterbury RJ. Bacteriophage biocontrol in animals and meat products. Microb Biotechnol. 2009;2:601–612. doi: 10.1111/j.1751-7915.2009.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin YC, Lee JH, Jin OS, Lee EJ, Jin LH, Kim CS, et al. RGD peptide-displaying M13 bacteriophage/PLGA nanofibers as cell-adhesive matrices for smooth muscle cells. J Korean Phys Soc. 2015;66:12–16. [Google Scholar]
- 40.Shin YC, Lee JH, Jin L, Kim MJ, Kim C, Hong SW, et al. Cell-adhesive matrices composed of RGD peptide-displaying M13 bacteriophage/poly(lactic-co-glycolic acid) nanofibers beneficial to myoblast differentiation. J Nanosci Nanotechnol. 2015;15:7907–7912. doi: 10.1166/jnn.2015.11214. [DOI] [PubMed] [Google Scholar]
- 41.Shin YC, Lee JH, Jin L, Kim MJ, Oh JW, Kim TW, et al. Cell-adhesive RGD peptide-displaying M13 bacteriophage/PLGA nanofiber matrices for growth of fibroblasts. Biomater Res. 2014;18:14. doi: 10.1186/2055-7124-18-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shin YC, Lee JH, Kim MJ, Hong SW, Kim B, Hyun JK, et al. Stimulating effect of graphene oxide on myogenesis of C2C12 myoblasts on RGD peptide-decorated PLGA nanofiber matrices. J Biol Eng. 2015;9:22. doi: 10.1186/s13036-015-0020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JH, Kim SW, Ji ST, Kim YJ, Jang WB, Oh JW, et al. Engineered M13 nanofiber accelerates ischemic neovascularization by enhancing endothelial progenitor cells. Tissue Eng Regen Med. 2017;14:787–802. doi: 10.1007/s13770-017-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang D, Carretero OA, Yang XY, Rhaleb NE, Liu YH, Liao TD, et al. N-acetyl-seryl-aspartyl-lysyl-proline stimulates angiogenesis in vitro and in vivo. Am J Physiol Heart Circ Physiol. 2004;287:H2099–H2105. doi: 10.1152/ajpheart.00592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi SH, Jung SY, Yoo SM, Asahara T, Suh W, Kwon SM, et al. Amine-enriched surface modification facilitates expansion, attachment, and maintenance of human cardiac-derived c-kit positive progenitor cells. Int J Cardiol. 2013;168:100–107. doi: 10.1016/j.ijcard.2012.09.065. [DOI] [PubMed] [Google Scholar]
- 46.Qi D, Scholthof KB. A one-step PCR-based method for rapid and efficient site-directed fragment deletion, insertion, and substitution mutagenesis. J Virol Methods. 2008;149:85–90. doi: 10.1016/j.jviromet.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 47.Merzlyak A, Indrakanti S, Lee SW. Genetically engineered nanofiber-like viruses for tissue regenerating materials. Nano Lett. 2009;9:846–852. doi: 10.1021/nl8036728. [DOI] [PubMed] [Google Scholar]
- 48.Bhattarai SR, Yoo SY, Lee SW, Dean D. Engineered phage-based therapeutic materials inhibit Chlamydia trachomatis intracellular infection. Biomaterials. 2012;33:5166–5174. doi: 10.1016/j.biomaterials.2012.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawamoto A, Tkebuchava T, Yamaguchi J, Nishimura H, Yoon YS, Milliken C, et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation. 2003;107:461–468. doi: 10.1161/01.cir.0000046450.89986.50. [DOI] [PubMed] [Google Scholar]
- 50.Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 51.Heusch G. Myocardial ischemia: lack of coronary blood flow, myocardial oxygen supply-demand imbalance, or what? Am J Physiol Heart Circ Physiol. 2019;316:H1439–H1446. doi: 10.1152/ajpheart.00139.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.