Abstract
The aim of this study was to develop simple sequence repeat (SSR) markers for genetic studies on G. chacoensis, as well as to evaluate their transferability to other bamboo species. Genomic DNA was isolated from G. chacoensis and its partial sequencing was used to find SSR loci. The obtained sequencing data were de novo assembled using the software CLC Genomics Workbench® 8.0v. The SSR loci primers were identified and designed with the software SSRLocator. The selected markers were validated using 56 plants sampled in seven populations from southern Brazil. The markers with potential polymorphism were selected and fluorescently labeled for characterization by capillary electrophoresis. In total, 92 SSR loci were found in G. chacoensis contigs. Suitable primers were designed for 70 SSR loci, and the remaining 22 SSR loci did not have sequences for primer development. Out of 35 selected SSR markers, after PCR optimization, 10 with high polymorphism potential were characterized. These loci can be used in genetic analyses of G. chacoensis and all of them were successfully transferred to other bamboo species. Non-polymorphic loci require further tests with additional plants, from different populations, to identify possibilities of their use.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02268-4) contains supplementary material, which is available to authorized users.
Keywords: Molecular markers, SSR, Genetic diversity, Primer design, Genetic population, Polymorphism
Introduction
Subfamily Bambusoideae (bamboo) is a major Poaceae group that comprises 115 genera and around 1640 species (Kelchner 2013). Natural bamboo populations are distributed all over the world, except for Europe and Antarctica (Clark et al. 2015). Asia has high diversity of bamboo species, particularly in southern China, where they have economic, ecological and social importance (Yuming et al. 2004). Brazil also has high number of bamboo species, including 18 native and five endemic species in the genus Guadua Kunth, which are used in construction, furniture, and handicrafts (Greco et al. 2015; Brazil Flora 2020).
Phylogenetic studies have shown that subfamily Bambusoideae is divided into three main monophyletic lineages that correspond to three tribes: temperate woody (Arundinarieae), tropical woody (Bambuseae) and herbaceous (Olyreae) bamboos (Kelchner 2013, Wysocki et al. 2015). The temperate woody bamboo species diverged from others at the beginning of the evolutionary history of the subfamily (Zang et al. 2011) and are now a distinct group. Among the described species, woody bamboos can be distinguished from others by their infrequent sexual reproduction, with long flowering intervals that range from 7 to 120 years (Janzen 1976). Guadua is a representative woody bamboo genus with many native South America species (Clark 2001), including G. chacoensis (2n = 2x = 46) (Andrada et al. 2007; Rincón and Castillo 2012). This species is ecologically important in its natural habitat and little is known about its reproductive mechanism (Areta et al. 2009). Further, the genetic structure and diversity of G. chacoensis native populations are almost unknown. Reproductive cycle monitoring studies describe that G. chacoensis flowers at intervals of approximately 31 years (Guerreiro 2014).
The advancement of scientific and technological knowledge about molecular genetics, such as SSR markers, facilitates the characterization of genetic diversity and structure, and also assists in the selection of descriptors for neglected species, such as G. chacoensis. Thus, SSR markers overcome the limitations of previous studies, employing dominant molecular markers, and are expected to yield valuable genetic information (Marulanda et al. 2007; Rugeles-Silva et al. 2012). SSR is one of the most informative molecular markers and its uniqueness and value are intrinsic to its multiallelic nature, co-dominant inheritance, relative abundance, broad coverage of the genome, and simple detection by PCR using oligonucleotide primer pairs (forward and reverse) flanking the SSR locus (Powell et al. 1996; Vieira et al. 2016).
Thus, SSRs can potentially be used in many genetic studies (Varshney et al. 2005). However, they can only be used in two ways, either transferred between species that are usually closely related, like species in the same taxon or genus, or developed (identified and validated) for specific species. Specific SSR development requires sequencing the genome, assembling the sequenced fragments, identifying SSR loci, designing primers that flank the SSR region, validation (Powell et al. 1996), and using the DNA of a certain number of individuals. Accordingly, this study aimed to identify, validate and characterize SSR markers for genetic studies about G. chacoensis and examine their transferability among other bamboo species.
Materials and methods
Plant material
Guadua chacoensis leaf samples were collected from natural populations. Collection was authorized (ICMbio-SISBIO nº 45390 and 48802-1) by the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio). The collected leaf samples were dehydrated and stored in silica gel for subsequent genomic DNA extraction. The DNA from a fresh leaf of an individual (voucher FLOR_0058621) grown in Florianópolis, Brazil (27° 35′ 49″ S 48° 32′ 56″ W), was used for sequencing and SSR loci identification. Leaf samples of other bamboo species were also collected from the germplasm maintained at the Federal University of Santa Catarina, namely: Bambusa oldhamii, Chusquea tenella, Dendrocalamus latiflorus, Dendrocalamus yunnanensis, Farguesia gaolinensis, Farguesia yunnanensis, Guadua angustifolia, Guadua paniculata, Guadua paraguaiyana, Merostachys scandens, Merostachys speciosa, Merostachys glauca, Oxytenanthera abyssinica, Phyllostachys aurea, Phyllostachys edulis, Phyllostachys pubescens, Pseudosasa mirabilis and Shibatea kumasasa. Eight plants of G. chacoensis were sampled from six natural populations (1.3 km–12 km apart) in Parque Nacional do Iguaçu, Foz do Iguaçu, Brazil, and one cultivated population from Rancho Queimado, Brazil, which is 570.43 km from the natural populations and was used to validate the SSR markers.
DNA isolation
Genomic DNA was extracted from 100 mg of dried leaves, previously ground using a Precellys® homogenizer (Bertin Technologies, Montigny-le-Bretonneux, France), with NucleoSpin Plant II kit (Macherey–Nagel) according to the manufacturer’s instructions. The 100 µl of extracted DNA was immediately frozen at − 20 °C until analysis. DNA quality and quantity were determined with NanoDrop ND-1000 Spectrophotometer (Thermo Fischer Scientific, Waltham, MA, USA) and 0.8% agarose gel electrophoresis performed at 6 V cm−1 for 60 min and stained with GelRed™ (Biotium Inc., Hayward, CA, USA). For comparison and quantification, Lambda DNA was loaded into gel at concentrations of 12.5 ng µl−1, 25 ng µl−1, 50 ng µl−1, and 100 ng µl−1. For the NanoDrop analysis, 1 µL of DNA was used at the wavelengths 230, 260, and 280 nm, and both the 260/280 and 230/280 ratios were examined for every sample.
DNA sequencing and SSR identification
A total of 1 ng of genomic DNA was used to prepare sequencing libraries with a Nextera XT DNA Sample Prep Kit (Illumina Inc., San Diego, California, USA) according to the manufacturer’s instructions. The libraries were sequenced using a MiSeq Reagent Kit v3 (600 cycles) and an Illumina MiSeq Sequencer (Illumina Inc., San Diego, California, USA). The obtained paired-end reads (2 × 300 bp) were used for de novo assembly with CLC Genomics Workbench 8.0v. Paired-end sequence reads were trimmed of low-quality data with a quality score limit of 0.01 using CLC Genomics Workbench 8.0v and reads of less than 50 bp in length were discarded. Organellar reads were excluded by mapping against two bamboo mitochondrion genomes (gb|JN120789.1, gb|EU365401.1) and the G. chacoensis chloroplast genome (gb|KT373814.1; Vieira et al. 2015) using the Basic Local Alignment Search Tool (BLAST, Altschul et al. 1990). Trimmed read sequences were de novo assembled. The obtained nuclear contigs with depth coverage (higher than 5X) were selected and analyzed with SSRLocator (Maia et al., 2008) for SSR identification, with a threshold of twelve repeat units for mononucleotide SSR, six repeat units for dinucleotide SSR, four repeat units for trinucleotide SSRs, three repeat units for tetra- and pentanucleotide SSR, and two repeat units for hexanucleotide SSR. Primers were designed with SSR Locator using the PRIMER3 algorithm by setting product size ranges from 100 to 350 bp, primer size from 18 to 22 bp, GC content from 40 to 60%, 1 °C as the maximum difference between the melting temperatures of the left and right primers, and a melting temperature (TM) of 57 °C (minimum) and 63 °C (maximum). Primers were analyzed with the software Gene Runner® to ensure the absence of secondary structure formation.
SSR validation by gel electrophoresis
Among the identified SSRs for primer design, 35 with a higher potential for use in population genetic studies were selected. PCR amplifications were carried out to test annealing temperatures (52–62 °C) and reagent concentrations for each of the selected primers. The following thermal cycle conditions were initially used for PCR reactions: 0.2 µM of each primer, 1 unit of Taq DNA polymerase (Invitrogen), 0.2 mM of each dNTP, 2.0 mM MgCl2, 15 ng of template DNA, and 1 × PCR buffer (Invitrogen); the final volume was adjusted to 10 µl. The PCR profile was the following: 95 °C for 3 min; 35 cycles of denaturation at 95 °C for 30 s, annealing temperature (52–62 °C) for 30 s, and 72 °C for 1 min; followed by a final extension at 72 °C for 30 min in a Veriti® 96-Well Thermal Cycler (Applied Biosystems, California, USA). PCR products were submitted to 3% agarose gel electrophoresis at 6 V cm−1 for 120 min and stained with GelRed™. Product sizes were determined by comparison with a 1-kb ladder (Invitrogen). The PCR reactions with dubious results were submitted to new PCR conditions for optimization. After optimization, polymorphism was evaluated in 4% denaturing polyacrylamide gels stained with silver nitrate. Gels were run with 1 × TBE buffer on a vertical electrophoresis apparatus for 90 min at 75 V. Product sizes were determined by comparison with a 100-bp DNA ladder (Invitrogen) (Figure S1).
SSR characterization by multiplex-ready PCR for fluorescence-based genotyping
SSR characterization was made using plant DNA from six natural populations (24 plants of each population) from Paraná State, Brazil, and 12 plants from a population grown in Santa Catarina State, Brazil. The loci with higher polymorphism potential, validated by gel electrophoresis, were selected and primers were fluorescent dye labeled. Genotyping was performed on the ABI Genetic Analyzer 3500xL platform (Applied Biosystems, Forster City, CA). The different fluorescent dye-labeled primers, with different spectra, allowed for multiplex reactions (3–4 primers per reaction). The polymorphism level of each locus was estimated by the polymorphism information content (PIC). Where and pi is the frequency of the i-th allele (Maroof et al. 1994; Anderson et al. 1993).
SSR transferability to other species
SSR transferability was tested using DNA from 18 different bamboo species. Regarding the polymorphic loci, the primers were considered transferred when the amplification resulted in one or two alleles near the expected size. The PCR condition and electrophoresis protocols were the same used for G. chacoensis.
Results
Sequencing the G. chacoensis genomic DNA resulted in 296,699 high-quality paired-end reads. After trimming (0.01 quality threshold) and excluding organellar reads, we obtained 239,621 paired-end reads (average length = 238.79). These reads were submitted to de novo assembly and 6013 contigs were obtained (N50 = 685).
We analyzed the occurrence, type, and distribution of SSRs in G. chacoensis contigs. In total, 92 SSRs were identified. Among them, trinucleotide repeats were the most common with 40 occurrences; whereas, di- (17), tetra- (18), penta- (8), and hexanucleotide repeats (9) occurred with lower frequency (Fig. 1). The most frequent motifs were AT (7.6%), TA (6.5%), TTC (5.4%) and TTA (4.3%). However, only 70 loci were in possible regions to design primers free of secondary structures (Table S1). Among them, 35 loci were selected for validation (Table 1).
Fig. 1.
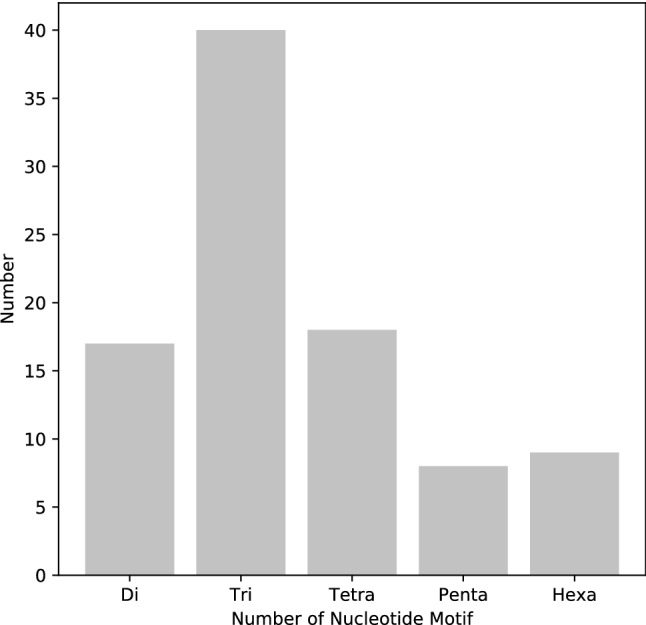
Number of distinct types of genomic simple sequence repeats (SSRs) identified in low-depth sequences of the bamboo Guadua chacoensis, grown in Florianópolis, SC (Brazil), using the Illumina MiSeq Sequencer platform (Illumina Inc., San Diego, California, USA). Di-, tri-, tetra-, penta- and hexanucleotides represent 2, 3, 4, 5 and 6 nucleotides that are the length of an SSR repeat
Table 1.
Features of 35 simple sequence repeat (SSR) markers of Guadua chacoensis selected for validation
| Locus | GenBank accession | Primer sequence (5′–3′) | TM (°C) | Product size (bp) | (Motif) repeat number | |
|---|---|---|---|---|---|---|
| Gcha01 | MN644912 | F | CCAGTTGATATGCAGAACCT | 55 | 238 | (GCC)4 |
| R | TTCTCTCTATCGTCCTCAGC | |||||
| Gcha02 | MN644913 | F | TAGCACGTACCATCAAACAA | 55 | 249 | (GCG)4 |
| R | CCTACCGTAAGCTCTCTGAA | |||||
| Gcha03 | MN644914 | F | TTCACCTAGACTTCTGGCAT | 55 | 289 | (TTC)4 |
| R | TGAGGAGAGAAAGGTTGAGA | |||||
| Gcha04 | MN644915 | F | TTGTGCATGACTTGTTGAGT | 55 | 239 | (AGA)4 |
| R | TCACCTTACAATAATTCGCC | |||||
| Gcha05 | MN644916 | F | TAGTCGGGTGATCATGAAAT | 55 | 291 | (AT)6-(AATT)3 |
| R | GTCGATATATTCTTGGCTGC | |||||
| Gcha06 | MN644917 | F | AGGGGAATACAGATATGCAA | 55 | 312 | (GGA)4 |
| R | CGTTCTCATGATACTCCCAT | |||||
| Gcha07 | MN644918 | F | AACTTCTCCCTCGAAACCT | 55 | 250 | (GGA)4-(GGA)5 |
| R | GTGTCACAGATGACGCAATA | |||||
| Gcha08 | MN644919 | F | AGCTTCACAGATGACGAACT | 55 | 285 | (AATA)3 |
| R | AAGATGACAACGACGATGAT | |||||
| Gcha09 | MN644920 | F | GCGGGTTGTAGAATTAACAG | 55 | 265 | (TGC)4 |
| R | GAGAATGTTGCACTGGATTT | |||||
| Gcha10 | MN644921 | F | CTGTGGACATGAACAATCTG | 55 | 244 | (AAC)4 |
| R | ATGTGCAGCTTTCTCTCAAT | |||||
| Gcha11 | MN644922 | F | GATTGAGGAATGAGATGGAA | 55 | 214 | (CAAG)3 |
| R | GAACTTGGCTTGAAAGATTG | |||||
| Gcha12 | MN644923 | F | CAATCACATCACTGTTCAGC | 55 | 166 | (TA)6 |
| R | CTGCACCTGGAGAGTTTTAC | |||||
| Gcha13 | MN644924 | F | ATGTTGAGGATGAGATCGAC | 55 | 177 | (AGC)4 |
| R | CGAAGAAAATCTGGAACAAG | |||||
| Gcha14 | MN644925 | F | GAGTACTTCGCCTTGCTAGA | 55 | 243 | (GAA)4 |
| R | GTGCTGGTGTTTCTTCTTCT | |||||
| Gcha15 | MN644926 | F | CTCTTCGTTCACACTTCTCC | 55 | 159 | (TCT)4 |
| R | AAGGAAATACCGAAGAGGAC | |||||
| Gcha16 | MN644927 | F | GGTCTGTTCTTCTTCCTTCC | 55 | 246 | (TTC)4 |
| R | CTCCAAAATTGAAGATGAGG | |||||
| Gcha17 | MN644928 | F | GGACCTAAACGCAATTTCTA | 55 | 226 | (TA)18 |
| R | CACCAAAGTTGGAGATGTTT | |||||
| Gcha18 | MN644929 | F | GAACTACGGCAAGAACAAAG | 55 | 305 | (AAG)4 |
| R | AACACACATGAACGTTAGCA | |||||
| Gcha19 | MN644930 | F | GTATTGGGCCGAGTACTGT | 55 | 254 | (CCG)5 |
| R | GGCATAAAAGGTAGCAAATG | |||||
| Gcha20 | MN644931 | F | AATGTCTTTGTTCTGGTTGG | 55 | 194 | (AT)16 |
| R | AGACAGGTTTGCAATAGAGC | |||||
| Gcha21 | MN644932 | F | AACTAGGGAATTGGAAGCTC | 55 | 310 | (TAGC)3 |
| R | TATGAAGTTGACACCCCTTT | |||||
| Gcha22 | MN644933 | F | CGAAGAAAGGAGATCAACTG | 55 | 210 | (CGG)4 |
| R | GGGACGTTACAGCAATAGAG | |||||
| Gcha23 | MN644934 | F | TCCGCCTATATTGTTGAGTT | 55 | 244 | (TTTA)3 |
| R | CTAGTGCTTCTTCCTCATGG | |||||
| Gcha24 | MN644935 | F | GGTGAAGAAGGATGTATGGA | 55 | 304 | (AC)6 |
| R | TATTGCGTGATGTAGTTTGC | |||||
| Gcha25 | MN644936 | F | GAGCCCATATGTCATTGTCT | 55 | 328 | (TTCT)3 |
| R | GATCTTCAATCTCTGCTTGC | |||||
| Gcha26 | MN644937 | F | GAGGGCTGTAAGCAACTAAA | 55 | 309 | (TTC)4 |
| R | ACATGAAGAACAGGGATGAG | |||||
| Gcha27 | MN644938 | F | AACGTATTTTCGACCGC | 55 | 315 | (TCA)4 |
| R | GTAGATGGATCGAAGATGGA | |||||
| Gcha28 | MN644939 | F | TCTATTGTCCTTAGCCAGTCA | 55 | 280 | (GGC)4 |
| R | CTGGAAACATCAATGAGGAC | |||||
| Gcha29 | MN644940 | F | GAGCACAAAAACCTCAAAAC | 55 | 297 | (ACTC)3 |
| R | AAGGAATGGATGAGATGCTA | |||||
| Gcha30 | MN644941 | F | GGGACTACGAGGTAGGACTT | 55 | 318 | (CAAT)3 |
| R | GAGCTTGGGTTAAATGAGTG | |||||
| Gcha31 | MN644942 | F | AGTCCAGTCGCACTCTTCTA | 55 | 167 | (CTTC)3 |
| R | TGTGTAATATAACCCGGAGG | |||||
| Gcha32 | MN644943 | F | ATACCGCCTGGAGAAGTT | 55 | 284 | (GAC)5 |
| R | GACAATCATCCTTGGAGAAA | |||||
| Gcha33 | MN644944 | F | GATCTCAGAAGATGGATTCG | 55 | 208 | (CAAC)3 |
| R | ATACATAATGAATGGGTGGC | |||||
| Gcha34 | MN644945 | F | TTGAGTACAAGGGATGCTCT | 55 | 283 | (GT)7 |
| R | GGATCTAGGTCGAACATTCA | |||||
| Gcha35 | MN644946 | F | AACTCAAGACCCTGGACC | 55 | 187 | (GAG)4 |
| R | GATTTCCAACTACGAAGTGC |
PCR reactions with Gcha17, Gcha19, Gcha22, Gcha23, and Gcha24 revealed no amplification products for any of the tested temperatures (52–62 °C) or plants. Different results were obtained for PCR reactions with primers designed for the Gcha25, Gcha30 and Gcha35 loci, since they showed an abundance of nonspecific bands. These characteristics were used as criteria for selecting the markers to be characterized, as well as the amplicons sharpness and quality revealed after gel electrophoresis. The 12 markers selected for characterization and fluorescent labeling are listed in Table 2.
Table 2.
Multiplex sets of loci used for characterization 12 SSR markers of Guadua chacoensis
| Locus | Motif | Sequence (5′–3′) | ATa | Product size | Fluorescent dye |
|---|---|---|---|---|---|
| Multiplex A | |||||
| Gcha04 | (AGA)4 | F: TTGTGCATGACTTGTTGAGT | 58 | 235–238 | NED |
| R: TCACCTTACAATAATTCGCC | |||||
| Gcha18 | (AAG)4 | F: GAACTACGGCAAGAACAAAG | – | PET | |
| R: AACACACATGAACGTTAGCA | |||||
| Multiplex B | |||||
| Gcha10 | (AAC)4 | F: CTGTGGACATGAACAATCTG | 58 | 241–247 | NED |
| R: ATGTGCAGCTTTCTCTCAAT | |||||
| Gcha21 | (TAGC)3 | F: AACTAGGGAATTGGAAGCTC | 300–308 | VIC | |
| R: TATGAAGTTGACACCCCTTT | |||||
| Gcha33 | (CAAC)3 | F: GATCTCAGAAGATGGATTCG | – | PET | |
| R: ATACATAATGAATGGGTGGC | |||||
| Multiplex C | |||||
| Gcha02 | (GCG)4 | F: TAGCACGTACCATCAAACAA | 58 | 225–249 | FAM |
| R: CCTACCGTAAGCTCTCTGAA | |||||
| Gcha05 | (AT)6-(AATT)3 | F: TAGTCGGGTGATCATGAAAT | 287–291 | NED | |
| R: GTCGATATATTCTTGGCTGC | |||||
| Multiplex D | |||||
| Gcha01 | (GCC)4 | F: CCAGTTGATATGCAGAACCT | 58 | 235–238 | FAM |
| R: TTCTCTCTATCGTCCTCAGC | |||||
| Gcha07 | (GGA)4-(GGA)5 | F: AACTTCTCCCTCGAAACCT | 249–252 | NED | |
| R: GTGTCACAGATGACGCAATA | |||||
| Multiplex E | |||||
| Gcha11 | (CAAG)3 | F: GATTGAGGAATGAGATGGAA | 55 | 202–214 | FAM |
| R: GAACTTGGCTTGAAAGATTG | |||||
| Gcha12 | (TA)6 | F: CAATCACATCACTGTTCAGC | 153–165 | VIC | |
| R: CTGCACCTGGAGAGTTTTAC | |||||
| Gcha13 | (AGC)4 | F: ATGTTGAGGATGAGATCGAC | 174–180 | NED | |
| R: CGAAGAAAATCTGGAACAAG |
aAnnealing temperature (°C)
The SSR characterization showed that the Gcha33 and Gcha18 loci had nonspecific amplification products for all plants, possibly due to instability during amplification reactions or annealing inconsistencies and hence, these loci were discarded. The Gcha01, Gcha02, Gcha04, Gcha05, Gcha07, Gcha10, Gcha11, Gcha12, Gcha13 and Gcha21 loci were polymorphic, of which the Gcha02 locus had the highest allele number (4) and the highest PIC value (0.507). The Gcha04 locus, with only 2 alleles, of which one is rare (frequency < 5%), had the lowest PIC value (0.039) (Table 3).
Table 3.
Allele frequency and polymorphism information content (PIC) for 10 of the characterized SSR markers of Guadua chacoensis
| Locus | GenBank access | Allele frequency (%) | PIC | |||
|---|---|---|---|---|---|---|
| Allele A | Allele B | Allele C | Allele D | |||
| Gcha01 | MN644912 | 0.50 | 0.50 | 0.500 | ||
| Gcha02 | MN644913 | 0.57 | 0.41 | 0.01 | 0.01 | 0.507 |
| Gcha04 | MN644915 | 0.98 | 0.02 | 0.039 | ||
| Gcha05 | MN644916 | 0.51 | 0.49 | 0.500 | ||
| Gcha07 | MN644918 | 0.50 | 0.50 | 0.500 | ||
| Gcha10 | MN644921 | 0.51 | 0.47 | 0.02 | 0.519 | |
| Gcha11 | MN644922 | 0.50 | 0.43 | 0.07 | 0.560 | |
| Gcha12 | MN644923 | 0.50 | 0.50 | 0.500 | ||
| Gcha13 | MN644924 | 0.85 | 0.15 | 0.255 | ||
| Gcha21 | MN644932 | 0.51 | 0.49 | 0.500 | ||
To further characterize the new SSR markers, five multiplexes were formed. Multiplex “A” was composed of the Gcha04 and Gcha18 loci (Figure S2), multiplex “B” of the Gcha08, Gcha10, Gcha21 and Gcha33 loci (Figure S3), multiplex “C” of the Gcha02, Gcha05 and Gcha06 loci, multiplex “D” of Gcha01 and Gcha07 loci (Figure S4), and multiplex “E” of the Gcha11, Gcha12 and Gcha13 loci (Figure S5). All of these multiplexes showed good and unambiguous amplification results.
The transferability to other bamboo species was successfully achieved for all tested SSR loci in 15 (i.e., all but Phyllostachys pubescens, Phyllostachys edulis and M. glauca) of the 15 species (Table 4, Figs. 6S and 7S).
Table 4.
Transferability of SSR loci from Guadua chacoensis to other bamboo species
| Species | SSR loci transferable | Species | SSR loci transferable |
|---|---|---|---|
| Bambusa oldhamii | Yesa | Merostachys glauca | Notb |
| Chusquea tenella | Yes | Merostachys scandens | Yes |
| Dendrocalamus latiflorus | Yes | Merostachys speciosa | Yes |
| Dendrocalamus yunnanensis | Yes | Oxytenanthera abyssinica | Yes |
| Farguesia gaolinensis | Yes | Phyllostachys aurea | Yes |
| Farguesia yunnanensis | Yes | Phyllostachys edulis | Not |
| Guadua angustifolia | Yes | Phyllostachys pubescens | Not |
| Guadua paniculata | Yes | Pseudosasa mirabilis | Yes |
| Guadua paraguaiyana | Yes | Shibatea kumasasa | Yes |
aYes—all tested loci were successfully transferred, 2Not—none SSR loci amplified in these bamboo species
Discussion
AT and GC content of the genome has been used in evolutionary ecology studies about monocots (Smarda et al. 2014). The higher AT content than GC content in the G. chacoensis genome, observed in the present work, is in accordance with previous studies about other bamboos (Liu et al. 2012), as well as other plant species, such as Zea mays, Oryza sativa, Beta vulgaris, and Arabidopsis thaliana. On the contrary, in animal genomes, GC content is higher (Beven et al. 1998; Kubo et al. 2000; Tóth et al. 2000; Barow and Meister 2002; McCouch et al. 2002; Jaillon et al. 2004; Yu et al. 2012).
High di- and trinucleotide repeat frequency was already described for the bamboos D. latiflorus (Bhandawat et al. 2015) and Phyllostachys violascens (Cai et al. 2019), as well as for conifer species, such as Pinus taeda and Picea glauca (Bérubé et al. 2007). These SSR motifs are reported as more informative, due to their greater stability compared to mononucleotides, and higher polymorphism compared to tetra-, penta- and hexanucleotide repeats (Samadi et al. 1998; Bérubé et al. 2007).
The annealing temperature is important to develop protocols with accurate results in molecular genetic analyses. An incorrect annealing temperature can cause unspecific amplifications or a lack of amplifications, resulting in a false positive or false negative, respectively, making it impossible to use (Ishii and Fukui 2001; Sipos et al. 2007). During the annealing temperature test, which ranged from 52 to 62 °C, we were able to discard the primers designed for the Gcha17, Gcha19, Gcha22, Gcha23 and Gcha24 loci, due to the absence of amplification at all tested temperatures. We also discarded the Gcha25, Gcha30 and Gcha35 loci, due to the presence of many unspecific amplicons at all tested temperatures. Further studies are necessary to optimize the protocol of these loci and to develop additional primers without these problems. For all other primer loci, the annealing temperature was 58 °C, except for Gcha11, Gcha12, and Gcha13, where 55 °C resulted in the best amplification (Figure S1). Thus, all the multiplex mixes were standardized with this temperature.
Among the polymorphic loci characterized in this study, only the Gcha05 (dinucleotide + tetranucleotide) and Gcha21 (tetranucleotide) loci were not trinucleotide repeats, which demonstrated that trinucleotide repeats were also informative for G. chacoensis. The allele number and PIC values showed reduced diversity for the characterized loci compared to other species (Hammami et al. 2014; Cubry et al. 2014). However, this seems to be an intrinsic feature of bamboo populations, since similar results were found for Dendrocalamus giganteus (Tian et al. 2012). It is worth mentioning that these estimators and values may be evaluated again for further characterization with additional and distant populations to confirm this feature of G. chacoensis.
The phenological cycle and reproductive biology of G. chacoensis are possible causes of the reduced polymorphism found in this study. Flowering and, consequently, allelic recombination in bamboos are governed by poorly understood environmental factors (Campanello et al. 2007). In G. chacoensis species, flowering occurs at intervals of approximately 31 years (Areta et al. 2009), pollen is predominantly dispersed by wind, seeds are predominantly dispersed by associated fauna, and reproductive behavior is similar to other woody bamboo species (Areta and Bodrati 2008; Montti et al., 2011a). These features do not favor the quick establishment or spread of new alleles (Eriksson 1997) or allelic transgressive combinations, yet little is known about the effects of this reproductive behavior on the ecology and genetic structure of bamboo populations (Budke et al. 2010; Montti et al. 2011b).
This is the first report that characterizes SSR loci for G. chacoensis genetic studies. The informative value of each characterized locus obtained in the present study may vary from the analysis of other populations. Therefore, genetic studies with geographically more distant populations can be based on the molecular markers developed here. With additional data, more accurate estimates of the polymorphic potential and allele number of each locus could be made. The polymorphic loci described here represent an advance in phylogenetic and population genetic studies in G. chacoensis and closely related species, in which primer transferability may be possible.
Conclusion
The shotgun genome sequencing of G. chacoensis with the Illumina platform allowed the identification, validation and characterization of 12 SSR markers for this species. Among them, 10 were polymorphic and can be used in G. chacoensis population genetic studies. These markers could be used in analyses about G. chacoensis genetic diversity, relationships between natural populations and phylogenetics, as well as populations of the 12 bamboo species that were found to be transferable.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1. PCR products amplified on different annealing temperatures (52-62°C) from simple sequences repeat markers (Gcha01–Gcha 5) of Guadua chacoensis. Agarose gels were stained with GelRed. (PDF 144 kb)
Figure S2. Genotyping image of multiplex “A” amplicons in ABI 3500 XL platform. a) Amplicons of loci Gcha04 (black) and Gcha18 (red) of Guadua chacoensis, compared with GeneScan 600 LIZ® dye size standard (orange); b) Locus Gcha04 in multiplex amplification reaction; c) Locus Gcha18 in the multiplex “A” amplification reaction. (PDF 253 kb)
Figure S3. Genotyping image of multiplex “B” amplicons in ABI 3500 XL platform. a) Amplicons of loci Gcha08 (blue), Gcha10 (black), Gcha21(green) and Gcha33 (red) of Guadua chacoensis, compared with GeneScan 600 LIZ® dye size standard (orange); b) Locus Gcha08 in multiplex “B” amplification reaction; c) Locus Gcha10 in multiplex “B” amplification reaction; d) Locus Gcha21 in multiplex “B” amplification reaction; e) Locus Gcha33 in multiplex “B” amplification reaction. (PDF 368 kb)
Figure S4. Genotyping image of multiplex “C” amplicons in ABI 3500 XL platform. a) Amplicons of the loci Gcha02 (blue), Gcha05 (black) and Gcha06 (green) loci of Guadua chacoensis, compared with GeneScan 600 LIZ® dye size standard (orange); b) Locus Gcha02 in multiplex “C” amplification reaction; c) Locus Gcha05 in multiplex “C” amplification reaction; ) Locus Gcha06 in multiplex amplification reaction. (PDF 349 kb)
Figure S5. Genotyping image of multiplex “D” amplicons in ABI 3500xL platform. a) Amplicons of loci Gcha01 (blue) and Gcha07 (black) of Guadua chacoensis, compared with GeneScan 600 LIZ® dye size standard (orange); b) Locus Gcha01 in multiplex “D” amplification reaction; c) Locus Gcha07 in multiplex “D” amplification reaction. (PDF 308 kb)
Figure S6. Multiplex “C” transferability test from Guadua chacoensis to G. paniculata amplicons in ABI 3500 XL platform. a) Amplicons of loci Gcha02 (blue), Gcha05 (black) and Gcha6 (green), compared with GeneScan 600 LIZ® dye size standard (orange); b) Locus Gcha02; c) Locus Gcha05; d) Locus Gcha06. (PDF 298 kb)
Figure S7. Multiplex “C” transferability test from Guadua chacoensis to a) Bambusa oldhamii, b) Chusquea tenella, c) Dendrocalamus latifolius, d) Fargesia yunnanensis, e) G. angustifolia, and f) G. paraguayana. in ABI 3500 XL platform. Amplicons of the loci Gcha02 (blue), Gcha05 (black) and Gcha06 (green) loci, compared with GeneScan 600 LIZ® dye size standard (orange) (PDF 273 kb)
Acknowledgements
The authors thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (Grant no. Finance Code 001) for the scholarship awarded to MDR and LNV, and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the financial support (Proc. 457726/2013-0) and scholarships awarded to MPG, RON, TCT, GHFK, and RP. The authors would also like to thank to Núcleo de Fixação Biológica de Nitrogênio/UFPR for sequencing. We are also grateful to the Parque Nacional do Iguaçu (ICMBio/Brazil), and to the farms Sarakura and Vagalume, for supporting and allowing the collection of plant material.
Author contributions
MDR, MPG, RP and RON conceived the research. MDR and RON designed the experiments. MDR, TCT, GHFK and RFS conducted the lab and statistical analyses. MDR and RON wrote the manuscript. RP, RFS, GHFK, TCT, and LNV revised the draft of the manuscript. MPG coordinated and supported the research and revised the manuscript. All authors read and approved the final manuscript version.
Compliance with ethical standards
Conflict of interest
All authors hereby declare that there is no conflict of interest.
Ethical approval
This article does not include any studies with human participants or animals performed by any of the authors.
Informed consent
This article does not involve any informed consent.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Anderson JA, Churchill GA, Autrique JE, Tanksley SD, Sorrels ME. Optimizing parental selection for genetic linkage maps. Genome. 1993;36:181–186. doi: 10.1139/g93-024. [DOI] [PubMed] [Google Scholar]
- Andrada AR, Lozzia ME, Cristóbal ME. Contribution to cytological knowledge of Guadua chacoensis. Lilloa. 2007;44:3–6. [Google Scholar]
- Areta JL, Bodrati A. Behaviour, identification and relationship with bamboos of the sooty grassquit (Tiaris fuliginosa) in Misiones, Argentina. Hornero. 2008;23:77–86. [Google Scholar]
- Areta JI, Bodrati A, Cockle K. Specialization on Guadua bamboo seeds by three bird species in the Atlantic forest of Argentina. Biotropica. 2009;41:66–73. doi: 10.1111/j.1744-7429.2008.00458.x. [DOI] [Google Scholar]
- Barow M, Meister A. Lack of correlation between AT frequency and genome size in higher plants and the effect of non-randomness of base sequences on dye binding. Cytometry. 2002;47:1–7. doi: 10.1002/cyto.10030. [DOI] [PubMed] [Google Scholar]
- Bérubé Y, Zhuang J, Rungis D, Ralph S, Bohlmann J, Ritland K. Characterization of EST-SSRs in loblolly pine and spruce. Tree Genet Genomes. 2007;3:251–259. doi: 10.1007/s11295-006-0061-1. [DOI] [Google Scholar]
- Beven M, Bancroft I, Bent E, Love K, Goodman H, Dean C, et al. Analysis of 1.9 Mb of contiguous sequence from chromosome 4 of Arabidopsis thaliana. Nature. 1998;391:485–493. doi: 10.1038/35140. [DOI] [PubMed] [Google Scholar]
- Bhandawat A, Singh G, Raina AS, Kaur J, Sharma RK. Development of genic SSR marker resource from RNA-Seq data in Dendrocalamus latiflorus. J Plant Biochem Biotechnol. 2015 doi: 10.1007/s13562-015-0323-9. [DOI] [Google Scholar]
- Brazil Flora G (2020) Brazilian Flora 2020 project—Projeto Flora do Brasil 2020. v393.237. Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Dataset/Checklist. 10.15468/1mtkaw
- Budke JC, Alberti MS, Zanardi C, Baratto C, Zanin EM. Bamboo dieback and tree regeneration responses in a subtropical forest of South America. For Ecol Manag. 2010;260:1345–1349. doi: 10.1016/j.foreco.2010.07.028. [DOI] [Google Scholar]
- Cai K, Zhu L, Zhang K, Li L, Zhao Z, Zeng W, Lin X. Development and characterization of EST-SSR markers from RNA-Seq data in Phyllostachys violascens. Front Plant Sci. 2019;10:50. doi: 10.3389/fpls.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanello PI, Gatti MG, Ares A, Montti L, Goldstein G. Tree regeneration and microclimate in a liana and bamboo-dominated semideciduous Atlantic Forest. For Ecol Manag. 2007;252:108–117. doi: 10.1016/j.foreco.2007.06.032. [DOI] [Google Scholar]
- Clark LG. Diversification and endemism in and woody bamboos (Poaceae: Bambusoideae) Bamboo Sci Cult. 2001;15:14–19. [Google Scholar]
- Clark LG, Londoño X, Ruiz-Sanchez E. Bamboo taxonomy and habitat. In: Liese W, Kohl M, editors. Bamboo, tropical forestry. Berlin: Springer International Publishing; 2015. pp. 1–30. [Google Scholar]
- Cubry P, Pujade-Renaud V, Garcia D, Espeout S, Guen VL, Granet F, Seguin M. Development and characterization of a new set of 164 polymorphic EST-SSR markers for diversity and breeding studies in rubber tree (Hevea brasiliensis Müll. Arg.) Plant Breed. 2014;133:419–426. doi: 10.1111/pbr.12158. [DOI] [Google Scholar]
- Eriksson G. Sampling of genetic resources populations in the absence of genetic knowledge. In: Turok J, Collin E, Demesure B, Erikkson G, Kleinschmit J, Rusanen M, Stephan R, editors. Noble hardwoods network: second meeting. Rome: International Plant Genetic Resources Institute; 1997. pp. 61–75. [Google Scholar]
- Greco TM, Pinto MM, Tombolato FC, Xia N. Diversity of bamboo in Brazil. J Trop Subtrop Bot. 2015;23:1–16. [Google Scholar]
- Guerreiro C. Flowering cycles of woody bamboos native to southern South America. J Plant Res. 2014;127:307–3013. doi: 10.1007/s10265-013-0593-z. [DOI] [PubMed] [Google Scholar]
- Hammami R, Jouve N, Soler C, Frieiro E, González JM. Genetic diversity of SSR and ISSR markers in wild populations of Brachypodium distachyon and its close relatives B. stacei and B. hybridum (Poaceae) Plant Syst Evol. 2014;300:2029–2040. doi: 10.1007/s00606-014-1021-0. [DOI] [Google Scholar]
- Ishii K, Fukui M. Optimization of annealing temperature to reduce bias caused by a primer mismatch in multitemplate PCR. Appl Environ Microbiol. 2001;67(8):3753–3755. doi: 10.1128/AEM.67.8.3753-3755.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillon O, Aury JM, Brunet F, Petit JL, Thomann NS, Maucell E, et al. Genome duplication in the teleost fish Tetraodon nigroviridis reveals the early vertebrate proto-karyotype. Nature. 2004;431:21. doi: 10.1038/nature03025. [DOI] [PubMed] [Google Scholar]
- Janzen DH. Why bamboos wait so long to flower. Annu Rev Ecol Syst. 1976;7:347–391. doi: 10.1146/annurev.es.07.110176.002023. [DOI] [Google Scholar]
- Kelchner SA. Bamboo Phylogenetic Group. Higher level phylogenetic relationships within the bamboos (Poaceae: Bambusoideae) based on five plastid markers. Mol Phylogenet Evol. 2013;67:404–413. doi: 10.1016/j.ympev.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Kubo T, Nishizawa S, Sugawara A, Itchoda N, Estiati A, Mikami T. The complete nucleotide sequence of the mitochondrial genome of sugar beet (Beta vulgaris L.) reveals a novel gene for tRNA Cys (GCA) Nucl Acids Res. 2000;28:2571–2576. doi: 10.1093/nar/28.13.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Qiao G, Jiang J, Yang H, Xie L, Xie J, Zhuo R. Transcriptome sequencing and de novo analysis for Ma bamboo (Dendrocalamus latiflorus Munro) using the Illumina platform. Plos One. 2012;7:10. doi: 10.1371/journal.pone.0046766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia LC, Palmieri DA, Souza VQ, Kopp MM, Carvalho FIF, Oliveira AC. SSR locator: tool for simple sequence repeat discovery integrated with primer design and PCR simulation. Int J Plant Genom. 2008;1:5. doi: 10.1155/2008/412696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroof MAS, Biyashev RM, Yang GP, Zhang Q, Allard W. Extraordinarily polymorphic microsatellite DNA in barley: Species diversity, chromosomal locations, and population dynamics. Proc Natl Acad Sci USA. 1994;91:5466–5470. doi: 10.1073/pnas.91.12.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marulanda ML, López AM, Claroz JL. Analyzing the genetic diversity of Guadua spp. in Colombia using rice and sugarcane microsatellites. Crop Breed Appl Biotechnol. 2007;7:43–51. doi: 10.12702/1984-7033.v07n01a07. [DOI] [Google Scholar]
- McCouch SR, Teytelman L, Xu Y, Katarzyna B, Lobos KB, Clare K, et al. Development and mapping of 2240 new SSR Markers for rice (Oriza sativa L.) DNA Res. 2002;9:199–207. doi: 10.1093/dnares/9.6.199. [DOI] [PubMed] [Google Scholar]
- Montti L, Campanello PI, Goldstein G. Flowering, die-back and recovery of a semelparous woody bamboo in the Atlantic Forest. Acta Oecol. 2011;37:361–368. doi: 10.1016/j.actao.2011.04.004. [DOI] [Google Scholar]
- Montti L, Campanello PI, Gatti MG, Blundo C, Austin AT, Sala OE, Goldstein G. Understory bamboo flowering provides a very narrow light window of opportunity for canopy-tree recruitment in a neotropical forest of Misiones, Argentina. For Ecol Manag. 2011;262:1360–1369. doi: 10.1016/j.foreco.2011.06.029. [DOI] [Google Scholar]
- Powell W, Machray GC, Provan J. Polymorphism revealed by simple sequence repeats. Trends Plant Sci. 1996;1:215–222. doi: 10.1016/1360-1385(96)86898-1. [DOI] [Google Scholar]
- Rincón JCV, Castillo NR. Estimation of cell cycle duration and standardization of cytogenetic protocol Guadua angustifolia Kunth var. angustifolia (Bambusoideae, Poaceae) Rev Invest Univ Quindío (Col) 2012;23:81–91. [Google Scholar]
- Rugeles-Silva PA, Posso-Terranova AM, Lodoño X, Barrera-Marín N, Muños-Flórez JE. Molecular characterization of Guadua angustifolia Kunth using RAM’s. Acta Agron. 2012;61:325–330. [Google Scholar]
- Samadi S, Artiguebielle E, Estoup A, Pointier JP, Silvain JF, Heller J, Cariou ML, Jarne P. Density and variability of dinucleotide microsatellites in the parthenogenetic polyploid snail Melanoides tuberculata. Mol Ecol. 1998;7:1233–1236. doi: 10.1046/j.1365-294x.1998.00405.x. [DOI] [PubMed] [Google Scholar]
- Sipos R, Székely AJ, Palatinszky M, Révész S, Márialigeti K, Nikolausz M. Effect of primer mismatch, annealing temperature and PCR cycle number on 16S rRNA gene-targeting bacterial community analysis. FEMS Microbiol Ecol. 2007;60(2):341–350. doi: 10.1111/j.1574-6941.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- Smarda P, Bures P, Horová L, Leitch IJ, Mucina L, Pacini E, Tichy L, Grulich V, Rotreklová O. Ecological and evolutionary significance of genomic GC content diversity in monocots. Proc Natl Acad Sci USA. 2014;111:E4096–E4102. doi: 10.1073/pnas.1321152111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Yang H-Q, Wong K-M, Liu A-Z, Ruan Z-Y. ISSR analysis shows low genetic diversity versus high genetic differentiation for giant bamboo, Dendrocalamus giganteus (Poaceae: Bambusoideae), in China populations. Genet Resour Crop Evol. 2012;59:901–908. doi: 10.1007/s10722-011-9732-3. [DOI] [Google Scholar]
- Tóth G, Gáspári Z, Jurka J. Microsatellites in different eukaryotic genomes: survey and analysis. Genome Res. 2000;10:967–981. doi: 10.1101/gr.10.7.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney RK, Graner A, Sorrells ME. Genic microsatellite markers in plants: features and applications. Trends Biotechnol. 2005;23:48–55. doi: 10.1016/j.tibtech.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Vieira LN, Anjos KG, Faoro H, Fraga HPF, Greco TM, Pedrosa FO, Souza EM, Rogalski M, Souza RF, Guerra MP. Phylogenetic inference and SSR characterization of tropical woody bamboos tribe Bambuseae (Poaceae: Bambusoideae) based on complete plastid genome sequences. Curr Genet. 2015 doi: 10.1007/s00294-015-0549-z. [DOI] [PubMed] [Google Scholar]
- Vieira MLC, Santini L, Diniz AL, Munhoz CF. Microsatellite markers: what they mean and why they are so useful. Genet Mol Biol. 2016;39(3):312–328. doi: 10.1590/1678-4685-GMB-2016-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki WP, Clark LG, Attigala L, Ruiz-Sanchez E, Duvall MR. Evolution of the bamboos (Bambusoideae; Poaceae): a full plastome phylogenomic analysis. BMC Evol Biol. 2015;15:50. doi: 10.1186/s12862-015-0321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Hon GC, Szulwach KE, Song CX, Zhang L, Kim A, Li X, Dai Q, Shen Y, Park B, Min JH, Jin P, Ren B, He C. Base-revolution analysis of 5-hydroxymethylcytosine in the mammalian genome. Cell. 2012;149:1368–1380. doi: 10.1016/j.cell.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuming Y, Kanglin W, Shengji P, Jiming H. Bamboo diversity and traditional uses in Yunnan, China. BioOne. 2004;24:157–165. doi: 10.1659/0276-4741(2004)024[0157:bdatui]2.0.co;2. [DOI] [Google Scholar]
- Zang YJ, Ma PF, Li DZ. High-throughput sequencing of six bamboo chloroplast genomes: phylogenetic implications for temperature woody bamboos (Poaceae: Bambusoideae) Plos One. 2011;6:5. doi: 10.1371/journal.pone.0020596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. PCR products amplified on different annealing temperatures (52-62°C) from simple sequences repeat markers (Gcha01–Gcha 5) of Guadua chacoensis. Agarose gels were stained with GelRed. (PDF 144 kb)
Figure S2. Genotyping image of multiplex “A” amplicons in ABI 3500 XL platform. a) Amplicons of loci Gcha04 (black) and Gcha18 (red) of Guadua chacoensis, compared with GeneScan 600 LIZ® dye size standard (orange); b) Locus Gcha04 in multiplex amplification reaction; c) Locus Gcha18 in the multiplex “A” amplification reaction. (PDF 253 kb)
Figure S3. Genotyping image of multiplex “B” amplicons in ABI 3500 XL platform. a) Amplicons of loci Gcha08 (blue), Gcha10 (black), Gcha21(green) and Gcha33 (red) of Guadua chacoensis, compared with GeneScan 600 LIZ® dye size standard (orange); b) Locus Gcha08 in multiplex “B” amplification reaction; c) Locus Gcha10 in multiplex “B” amplification reaction; d) Locus Gcha21 in multiplex “B” amplification reaction; e) Locus Gcha33 in multiplex “B” amplification reaction. (PDF 368 kb)
Figure S4. Genotyping image of multiplex “C” amplicons in ABI 3500 XL platform. a) Amplicons of the loci Gcha02 (blue), Gcha05 (black) and Gcha06 (green) loci of Guadua chacoensis, compared with GeneScan 600 LIZ® dye size standard (orange); b) Locus Gcha02 in multiplex “C” amplification reaction; c) Locus Gcha05 in multiplex “C” amplification reaction; ) Locus Gcha06 in multiplex amplification reaction. (PDF 349 kb)
Figure S5. Genotyping image of multiplex “D” amplicons in ABI 3500xL platform. a) Amplicons of loci Gcha01 (blue) and Gcha07 (black) of Guadua chacoensis, compared with GeneScan 600 LIZ® dye size standard (orange); b) Locus Gcha01 in multiplex “D” amplification reaction; c) Locus Gcha07 in multiplex “D” amplification reaction. (PDF 308 kb)
Figure S6. Multiplex “C” transferability test from Guadua chacoensis to G. paniculata amplicons in ABI 3500 XL platform. a) Amplicons of loci Gcha02 (blue), Gcha05 (black) and Gcha6 (green), compared with GeneScan 600 LIZ® dye size standard (orange); b) Locus Gcha02; c) Locus Gcha05; d) Locus Gcha06. (PDF 298 kb)
Figure S7. Multiplex “C” transferability test from Guadua chacoensis to a) Bambusa oldhamii, b) Chusquea tenella, c) Dendrocalamus latifolius, d) Fargesia yunnanensis, e) G. angustifolia, and f) G. paraguayana. in ABI 3500 XL platform. Amplicons of the loci Gcha02 (blue), Gcha05 (black) and Gcha06 (green) loci, compared with GeneScan 600 LIZ® dye size standard (orange) (PDF 273 kb)


