Abstract
Background:
Osteochondral injury is a very common orthopaedic pathology, mainly affecting young, active population, with limited current treatment options. Herein we are presenting cellular and early clinical data of a patient series treated for chronic osteochondral lesions in the knee with a filter-based intra-operative bone marrow aspirate (BMA) separation device.
Methods:
Fifteen patients with chronic knee osteochondral lesions (60% females, 19–59 years) were included in this prospective case series. Filtered BMA (f-BMA), containing mesenchymal stem/stromal cells (MSCs), was combined with a biomimetic collagen-hydroxyapatite scaffold (CHAS) and implanted into the site of the lesion. Harvested BMA and post-separation f-BMA were analysed for blood cell counts, flow cytometry, and fibroblast colony forming units (CFU-Fs). Patients were followed for serious adverse events and graft failures. Clinical evaluation was assessed using the knee injury and osteoarthritis outcome score (KOOS). In 8 patients a magnetic resonance imaging (MRI)/arthroscopy were performed.
Results:
Cell suspension contained 0.027% CD271+ CD45− 7-AAD− cells, 0.15% CD73+ CD90+ CD105+ cells and 0.0012% CFU-Fs of all nucleated cells with 86% viability. Filtration process resulted in 12.8 (4.0–40.8) fold enrichment in terms of CFU-F content in comparison to initial BMA. No serious adverse events related directly to the osteochondral treatment were reported. After an average follow-up of 20 months (14–25) all KOOS subscales (Symptoms/Pain/Daily activities/Sport and recreation/Quality of life) increased significantly from pre-operative 55/56/67/30/30 to post-operative 73/76/79/51/52 (p values < 0.05), respectively. MRI or arthroscopic evaluation revealed nearly normal to normal overall International Cartilage Repair Society assessment in 7/8 patients.
Conclusion:
The filter-based BMA separation procedure significantly increased the frequency of mesenchymal stem/stromal cells (MSCs), however their concentration was not increased. The clinical evaluation revealed high safety profile of the treatment and resulted in improved clinical status of the patients.
Keywords: Knee joint, Cartilage, Articular, Bone marrow mesenchymal stem cells, Cell separation, Biomimetic materials
Introduction
Cartilage lesions can be painful and disabling, have poor capacity for repair, and may predispose patients for osteoarthritis [1]. For long-term repair and regeneration of tissue, cells alone or in combination with biomaterials are implanted at the site of injury. Several strategies, including subchondral bone drilling and microfracture techniques are employed to supply blood and bone marrow (BM) to the lesion site [2]. The procedure recruits mesenchymal stem/stromal cells (MSCs, sometimes also referred to as connective tissue progenitors—CTPs [3]) from BM, stimulates fibrin clot formation and subsequent fibrocartilage formation [4]. Another technique, autologous chondrocyte implantation (ACI), is regarded as the first application of a tissue-engineering strategy in orthopaedic surgery [5, 6].
Strategy in chondral/osteochondral repair is moving toward intraoperative single-step procedures to reduce the risks associated with two surgical procedures, to reduce costs and the necessity for a good manufacturing practice (GMP) facility for cell cultivation [7]. In this regard, the use of bone marrow aspirate (BMA), which contains both hematopoietic and mesenchymal stem/stromal cells in addition to other cell types that may play a role in tissue regeneration, represents a viable alternative [8]. The mechanisms of action of MSCs in tissue regeneration are related to the secretion of number of cytokines, chemokines and growth factors, which can improve angiogenesis, reduce inflammation, inhibit apoptosis and stimulate endogenous repair [9]. In addition, MSC differentiation into desired phenotype can be achieved as a result of environmental stimuli [7]. However, in bone marrow aspirates, MSC represent only 0.001%–0.01% of the total mononuclear cells [10]. For increasing the density of MSCs, various concentration methods, like bone marrow density gradient centrifugation or recovery of cells from filters and clotting, were developed. These methods are relatively simple, can be performed in the operation theatre and are therefore not associated with major regulatory obstacles [2].
Cartilage and bone need to be restored for a successful outcome after osteochondral lesion treatment. Traditionally, the autologous bone grafting was combined with a superficial cartilage repair. A nano-structured biomimetic collagen-hydroxyapatite scaffold—CHAS (MaioRegen, Fin-Ceramica, Faenza, Italy) was developed to provide simultaneous osteochondral regeneration. Positive clinical results have been shown by many short to mid-term reports when implanted in a stand-alone manner [11]. A recent randomized clinical trial showed superiority of this device toward bone-marrow stimulation for osteochondral lesions [12]. However, some treatments resulted also in incomplete cartilage and poor subchondral bone repair, despite significant clinical improvements [13]. In the treatment of osteochondral lesions in complex situations, such as older patients, early osteoarthritis, and revision cases, success range of stand-alone scaffolds is rather limited [14].
Herein we present first cellular and clinical data results of a cell filter device for collection of nucleated cells from BMA [15, 16]. Filtered BMA (f-BMA), containing MSCs, was used on patients treated for chronic osteochondral lesion in the knee joints. BM aspiration, cell filtration, their seeding onto CHAS scaffold and surgical implantation were combined intra-operatively in a single step procedure. We hypothesized: (1) the filter-based BMA separation procedure significantly increases concentration and frequency of MSCs; (2) combining f-BMA and CHAS represents a safe therapeutic approach, and (3) improves clinical status of the patient.
Materials and methods
Patient population and surgical procedures
This prospective case series comprises of 15 patients that were operatively treated for chronic knee osteochondral lesions in a single orthopaedic centre between 2014 and 2016. There were 9 females and 6 male patients enrolled in the study with average age of 33 years (from 19 to 59). The study protocol was approved by the National Medical Ethics Committee (No. 0120-14/2016-2). The patients were suffering from the following: three primary large osteochondritis dissecans (OCD) lesions on medial femoral condyle, two recurrent OCD on medial femoral condyle after failed fragment fixation, three isolated post-traumatic osteochondral lesions, two recurrent post-traumatic osteochondral lesions after failed microfractures, two patellofemoral instability related lesions and three cases of multiple femoral lesions due to osteoarthritis. Surgeries were performed under general or spinal anaesthesia. All knee patients had osteochondral graft implanted through a mini-arthrotomy or classical central arthrotomy, depending on the lesion size, location, and concomitant procedures. There were ten isolated cartilage surgeries, two patients required additional patellofemoral stabilization, one patient received a high tibial osteotomy, and one needed an open synovectomy. The site of the osteochondral lesion was first debrided in quadrangular manner to macroscopically intact articular cartilage on sides and toward a non-sclerotic bone in the depth. One patient with subchondral bone cyst in the medial femoral condyle received an additional cyst curettage and autologous bone grafting. A biomimetic osteochondral scaffold was first cut out to press fit into the lesion. On all four sides, as well as on the articular collagen layer, it was stabilized with a fibrin sealant (Beriplast, CSL Behring, Marburg, Germany). A tri-layer CHAS MaioRegen (Fin-Ceramica, Faenza, Italy) was used in 11 cases. A thinner, bi-layer version of same scaffold, MaioRegen Slim (Fin-Ceramica, Faenza, Italy) was used in 4 cases. MaioRegen is a reabsorbable, implantable, multilayer device indicated for the treatment of osteochondral lesions. This substitute is composed of type I collagen fibers (of equine origin) and Mg enriched hydroxyapatite (HA) crystals, nucleated in the fibers. A biologically inspired approach was applied to nucleate bone-like HA nanocrystals in the collagen fibers. After the nucleation/assembling process the suspensions were freeze-dried; controlling the freezing and heating rate, and a preferential collagen orientation in the first layer was achieved [11]. All details on patient population and surgeries are given in Table 1. Post-operatively, the patients were restrained from weight-bearing for 6 weeks followed by progressive rehabilitation for muscle strength, range of motion, and anti-swelling measures. Three months after index surgery they were allowed to get involved with light activities and moderate activities were allowed after 6 months. Participation in strenuous sport activities (such as ball games, martial arts, athletics training) was allowed after one year post-surgery.
Table 1.
Demographic data and surgery details of patients treated with f-BMA
| Patient code | Sex | Age (years) | BMI (kg m−2) | Knee location | Main diagnosis | Lesion size (cm2) | ICRS grade | Scaffold | Concomitant procedures |
|---|---|---|---|---|---|---|---|---|---|
| PM00814M | F | 34 | 24.4 | Patella | Traumatic OC lesion | 2.5 | 4A | MaioRegen | Patella stabilization |
| TG00914M | M | 32 | 25.2 | MFC | OCD | 2.5 | 4B | MaioRegen | None |
| SR00415M | F | 35 | 23.9 | Multiple lesions | Localized OA | 9.5 | 4A | MaioRegen | Knee synovectomy |
| MG00615M | F | 22 | 27.7 | MFC | OCD | 4.0 | 4B | MaioRegen | None |
| MP00915M | F | 36 | 28.3 | MFC | Traumatic OC lesion PF instability | 3.0 | 4A | MaioRegen | Patella stabilization |
| TP01015M | F | 19 | 25.2 | MFC | Recurrent OCD | 3.5 | 4B | MaioRegen | None |
| MG01315M | F | 34 | 21.5 | Trochlea | Recurrent post-traumatic OC lesion | 2.5 | 4A | MaioRegen Slim | None |
| SK01615M | M | 59 | 25.1 | MFC | Recurrent OC lesion, Medial osteoarthritis | 7.0 | 4B | MaioRegen | High tibial osteotomy |
| DM00116M | M | 25 | 25.0 | Trochlea | Traumatic OC lesion after ACL reconstruction | 2.0 | 4A | MaioRegen Slim | None |
| DD00316M | F | 28 | 25.0 | LFC | Traumatic OC lesion | 2.4 | 4A | MaioRegen | None |
| JS00416M | M | 30 | 20.9 | LFC | Recurrent post-traumatic OC lesion | 4.5 | 4B | MaioRegen | None |
| AK00616M | M | 45 | 25.6 | Multiple lesions | Degenerative lesions | 3.5 | 4A | MaioRegen Slim | None |
| PŠ00716 M | F | 33 | 35.6 | MFC | OCD | 6.5 | 4B | MaioRegen | None |
| LL00916MK | F | 53 | 27.3 | Trochlea | Degenerative lesion | 3.5 | 4A | MaioRegen Slim | None |
| 16BK017BK | M | 34 | 23.5 | MFC | Recurrent OCD lesion | 3.5 | 4B | MaioRegen | Bone grafting of subchondral cyst |
ACL anterior cruciate ligament, BMI body mass index, ICRS International Cartilage Repair Society, LFC lateral femoral condyle, MFC medial femoral condyle, OA osteoarthritis, OC osteochondral, OCD osteochondritis dissecans
Bone marrow harvesting, cell isolation and application
BM aspiration needle (13-gauge, HS Hospital Service, Aprilia, Italy) was coated with dalteparin (Pfizer, Luxembourg, Luxembourg) prior to BM aspiration. Between 18 and 35 ml of bone marrow was aspirated from iliac crest into 50-ml or 10-ml syringes, prefilled with acid citrate dextrose solution A (ACD-A, Fresenius Kabi, Bad Homburg, Germany). If 10-ml syringes were used, the needle for BM aspiration was repositioned up to three times. BMA was processed using filter-based cell separation device CellEffic BM (Kaneka Corp., Osaka, Japan). The procedure was performed according to manufacturer’s instructions. Around 50 ml of cell suspension in saline was obtained after the isolation of nuclear cells. Small aliquots (2–4 ml) were sampled for cellular evaluation. The remaining cell suspension was centrifuged for 5 min at 400×g and concentrated to a final volume between 3 and 7 ml. Before the f-BMA administration, the intra-operative tourniquet was released. Osteochondral implant was soaked by fresh blood since this was shown to be crucial for its early stability [18]. Typically, 2/3 of prepared final cell suspension volume was injected diffusely into the upper portion of the scaffold at the very end of surgery. The last 1/3 of the cell suspension volume was injected freely into the joint cavity after the complete wound closure.
Cellular evaluation
To test our hypothesis (1) extensive cellular evaluation was performed.
Samples for cell counting (100 µl), CFU-F assay (1–2 ml) and flow cytometry (1–2 ml) were taken before and after BMA processing with filter-based separation device. The samples were stored at 4 °C and analysed for cell count, CFU-F assay and surface marker expression on the same day, except for the first two samples which were analysed on the following day. The viability analysis for all the samples was performed on the same day.
Blood cell counts
Concentration of white blood cells (WBCs) and red blood cells (RBCs) were determined with haematology analyser COULTER® Ac·T diff2™ (Beckman Coulter, Fullerton, CA, USA).
Fibroblast colony forming units (CFU-F) assay
Cells were seeded at least in duplicates into 6 well culture plates (1.25 × 105, 2.5 × 105 or 5 × 105 WBCs/well, well area 9.026 cm2, TPP, Trasadingen, Switzerland) containing DMEM/Ham’s F12 (Life Technologies Corp., Paisley, UK) supplemented with 10% FBS (Life Technologies Corp., Paisley, UK), 1 ng/ml bFGF (Preprotech, London, UK) and antibiotics: penicillin, streptomycin and gentamycin (Life Technologies Corp., Paisley, UK). Plates were incubated at standard conditions (37 °C, 5% CO2) with media changes every 3 to 4 days. Samples were fixed with 4% formaldehyde and stained with 0.05% Crystal Violet (Sigma Chemical Co., St. Louis, MO, USA) after 14 days of cell culture. Colonies containing ≥ 50 fibroblastic cells were manually counted under stereomicroscope. Frequency and concentration of CFU-Fs was calculated based on average CFU-F plate counts, number of seeded cells and concentration of WBCs in the sample.
Flow cytometry
Estimation of MSC content was achieved by determining CD271 positive and CD45 negative cells, concomitantly assessed for viability using 7-aminoactinomycin D (7-AAD), with the similar procedure as described before [19]. Samples were added into the Trucount tubes, labelled with anti-CD45 (FITC, cat. no. 555482), anti-CD271 (PE, cat. no. 557196) antibodies and 7-AAD (all from BD Biosciences, San Jose, CA, USA), incubated 20 min at room temperature in the dark. After red blood cell lysis, the samples were immediately analysed with flow cytometer FACS Calibur (BD Biosciences, San Jose, CA, USA). By comparing the ratio of counted bead events to CD271 positive and CD45 negative viable cell events, absolute number of MSCs was calculated. The viability of all nucleated cells was assessed by measuring all 7-AAD negative cells.
Additionally, the quantification of MSCs was performed by measuring the cell population coexpressing general mesenchymal markers CD73, CD90 and CD105 [20]. Samples were labeled with anti-CD73 (PE, cat. no. 550257, BD Biosciences or APC, cat. no. 130-097-945, Miltenyi Biotec, Bergisch Gladbach, Germany), anti-CD90 (FITC, cat. no. 555595, BD Biosciences), and anti-CD105 (PerCP-Cy5.5, cat. no. 560819, BD Biosciences) antibodies and incubated 20 min at room temperature in the dark. After incubation red blood cells were lysed with a BD FACS lysing buffer for 10 min, centrifuged at 400×g for 5 min, cell pellet was resuspended with DPBS and samples were analysed with FACSCalibur or FACSAria (BD Biosciences, San Jose, CA, USA). The cells co-expressing all three markers were gated to determine their percentage. Their absolute numbers were calculated indirectly based on the WBC counts measured with haematology analyser.
Calculation of cell separation parameters
To evaluate the proposed hypothesis (1) enrichment and concentration factors were calculated as described below. Additionally, we have also calculated cell recovery rate.
For the measurements of different cell populations, measured with haematology analyser, flow cytometer and CFU-F assay, absolute cell numbers were calculated considering the sample volumes before and after BMA processing with filter-based separation device. For calculation of cell concentrations in the final cell product, potential cell loses during final centrifugation step were neglected. The enrichment factor was calculated in order to compare the frequency of target cells amongst all cells (including RBCs) before and after BMA processing:
| 1 |
The concentration factor was calculated to compare the concentration of cells in the final cell product over the baseline in initial BMA:
| 2 |
To compare the absolute cell numbers before and after cell separation process the recovery rate was determined as follows:
| 3 |
Clinical evaluation
To evaluate the proposed hypothesis (2) and (3) clinical evaluation was robustly focused only for safety and efficacy.
Patients were prospectively followed at one, three, and then every six months after surgery. Unscheduled visits in cases of any unexpected adverse events were allowed. Since this study group was primarily focused on a filter-based bone marrow separation procedure, we aimed to include all patients in whom this f-BMA was used.
All patients were carefully monitored for any serious adverse events (defined as hospitalisation or repetitive surgical intervention) or documented graft failures (revision cartilage repair or arthroplasty, or low clinical outcome together with radiological or arthroscopic evidence for graft failure). Serious adverse events were classified into one out of four categories in relation to the implanted graft: unrelated, possibly related, probably related, and definitely related.
Knee osteoarthritis outcome score (KOOS) was used to assess efficacy of the treatment [21]. Only the data at the time of surgery and at the last evaluation (patients with minimum of one-year follow-up were included). Biological healing response was evaluated non-systematically by magnetic resonance imaging (MRI) or arthroscopy in situations of surgical re-intervention: four patients had MRI only, two patients had arthroscopy only and another two had both. Simple graft evaluation according to ICRS Cartilage Repair Assessment [22] was performed in all these cases.
Statistical analysis
All data averages, except KOOS scores, are presented as geometric mean with 95% confidence interval (CI) or range, as indicated.
In order to test hypothesis (1), the enrichment and concentration factors of evaluated cell populations were compared to value 1, which indicates equal cell frequencies and cell concentrations before and after BMA processing. To test statistical significance of calculated factors, the data was first transformed using function Y = log (Y). Means of transformed data were then compared to hypothetical value of 0 (log (1) = 0) using one sample t test. Differences with p ≤ 0.05 were considered as statistically significant.
KOOS scores are presented as arithmetic mean with 95% CI. In order to test hypothesis (3) paired two-sided t-test was used to determine statistical significance between individual pre-OP and post-OP scores. Differences with p ≤ 0.05 were considered as statistically significant.
Computer software GraphPad Prism (GraphPad Software, La Jolla, CA, USA) was used for all statistical calculations.
Results
Cellular evaluation
Results were obtained by detailed evaluation of blood cell counts, CFU-F assay and flow cytometry analysis. The measured parameters for each individual patient are presented in Table 2. To evaluate hypothesis (1), enrichment and concentration factors were calculated and are presented in Fig. 1.
Table 2.
Characterization of cell populations in bone marrow aspirates and in final cell preparations after separation with filter-based intra-operative bone marrow aspirate separation device
| Patient code | Before separation | After separation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Volume (ml) | WBC (106 ml−1) | RBC (106 ml−1) | CFU-F (ml−1) | CD271+ CD45− 7-AAD− (106 ml−1) | Volume (ml) | WBC (106 ml−1) | RBC (106 ml−1) | CFU-F (ml−1) | CD271+ CD45− 7-AAD− (106 ml−1) | |
| PM00814M | 35 | 19.2 | 3095 | 77 | 0.0040 | 5 | 18.5 | 225 | 148 | 0.0023 |
| TG00914M | 37 | 24.5 | 3930 | 270 | 0.0038 | 5 | 20.7 | 225 | 311 | 0.0042 |
| SR00415M | 37 | 20.0 | 3700 | 40 | 0.0180 | 7 | 26.4 | 214 | 106 | 0.0155 |
| MG00615M | 31 | 34.3 | 3500 | 685 | 0.0130 | 7 | 29.2 | 233 | 322 | 0.0103 |
| MP00915M | 31 | 24.6 | 3705 | 196 | 0.0087 | 5 | 27.4 | 240 | 356 | 0.0168 |
| TP01015M | 32.5 | – | – | – | – | 5 | – | – | – | – |
| MG01315M | 27.5 | 20.5 | 3470 | 150 | 0.0055 | 5 | 27.0 | 250 | 54 | 0.0025 |
| SK01615M | 29 | 13.2 | 3763 | 88 | 0.0010 | 6 | 21.2 | 240 | 127 | 0.0017 |
| DM00116M | 26 | 22.8 | 4947 | 1216 | 0.0024 | 5 | 28.8 | 480 | 1267 | 0.0041 |
| DD00316M | 27 | 10.1 | 3807 | 47 | 0.0058 | 5 | 19.2 | 288 | 128 | 0.0048 |
| JS00416M | 29 | 26.2 | 3807 | 454 | 0.0104 | 4 | 37.2 | 480 | 248 | 0.0055 |
| AK00616M | 19 | 23.3 | 4330 | 699 | 0.0069 | 6 | 20.2 | 323 | 391 | 0.0076 |
| PŠ00716 M | 20 | 36.6 | 3836 | 2584 | 0.0301 | 5 | 36.6 | 289 | 2344 | 0.0303 |
| LL00916MK | 28 | 7.3 | 3875 | 203 | 0.0057 | 5 | 12.0 | 288 | 288 | 0.0097 |
| 16BK017BK | 33 | 38.7 | 4065 | 1754 | 0.0153 | 3 | 69.6 | 640 | 1670 | 0.0096 |
| Geometric mean | 29.0 | 20.9 | 3824 | 286 | 0.0068 | 5.1 | 25.8 | 297 | 307 | 0.0065 |
| Lower 95% CI | 26.0 | 15.9 | 3593 | 133 | 0.0041 | 4.6 | 20.4 | 244 | 164 | 0.0040 |
| Upper 95% CI | 32.3 | 27.5 | 4069 | 616 | 0.0113 | 5.7 | 32.8 | 361 | 576 | 0.0105 |
CFU-F fibroblast colony forming units, CI confidence interval, RBC red blood cells, WBC white blood cells
Fig. 1.
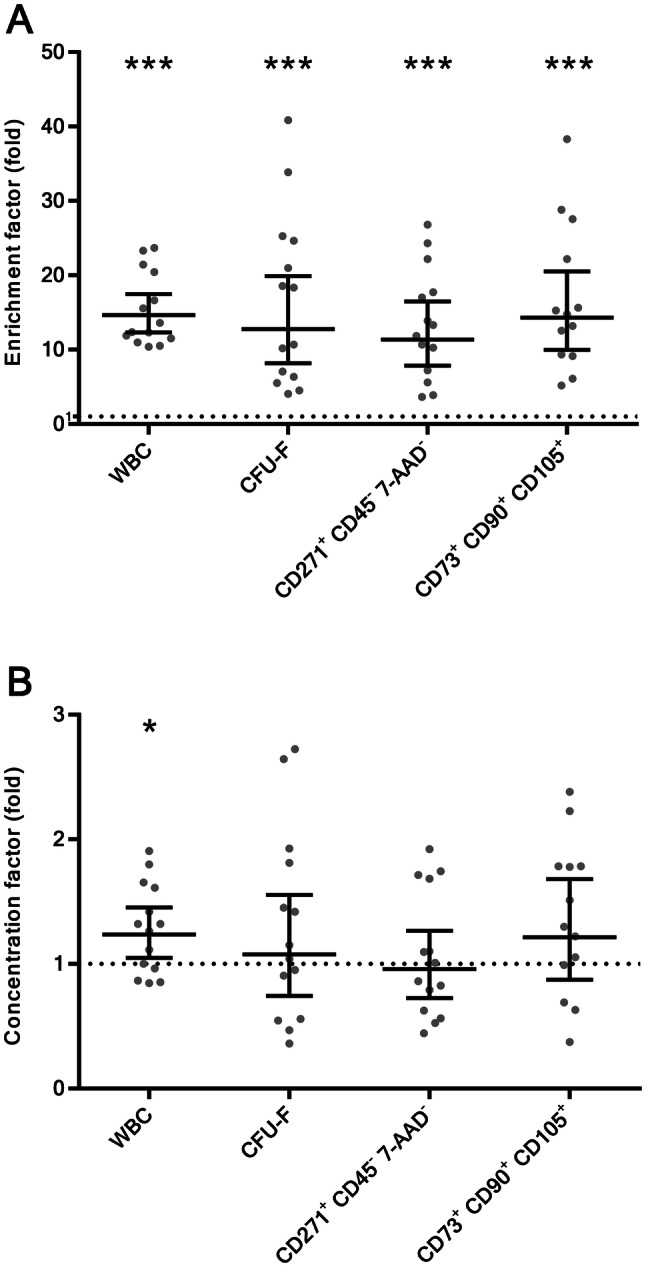
A Enrichment factors and B concentration factors of cell populations after separation with filter-based intra-operative bone marrow aspirate separation device. Data are presented as geometric mean with 95% confidence interval (13 < N < 14). Statistical significance was determined by one sample t-test comparing means of logarithmically transformed data to hypothetical value of 0 (dotted line represents value 1; log (1) = 0), *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. CFU-F, fibroblast colony forming units; WBC white blood cells
Cell suspension volumes before/after separation
The initial BMA (N = 15) with added anticoagulant had an average volume of 29.0 ml (range 19.0–37.0), whereas the final cell suspension had a volume of 5.1 ml (range 3.0–7.0). The volumetric concentration factor was therefore 5.7 (range 3.2–11.0).
Blood cell counts
The BMAs (N = 14) on average contained 20.9 × 106 WBC/ml (range 7.3 × 106–38.7 × 106) and 3824 × 106 RBC/ml (range 3095 × 106–4947 × 106). With the concentration of RBCs being two orders of magnitude higher than the concentration of WBCs, it is evident that RBCs represent the vast majority of cells (99.4%, range 99.0–99.8) in the initial BMAs. Processing of BMA through the separation column removed 98.6% RBCs (range 97.6–99.2), which in the final cell preparation represented 91.7% of all cells (range 88.8–96.0). This resulted in the increase of WBC percentage from initial 0.5% (range 0.2–1.0) to the final 8.0% (range 4.0–11.2). The average absolute number of WBCs before separation was 600.5 × 106 (range 203.0 × 106–1277.1 × 106) and afterwards it decreased to 132.0 × 106 WBC (range 60.0 × 106 –208.8 × 106). Therefore, the average WBC recovery rate was 22.0% (range 11.4–35.3). The average enrichment and concentration factors for WBCs were 14.7-fold (range 10.4–23.7, p < 0.05) and 1.2-fold (range 0.8–1.9, p < 0.05), respectively.
CFU-F assay
The functional assay for measuring the frequency of MSCs with the colony forming abilities was performed (N = 14). The average dosage of CFU-Fs administered to the patients was 1571 CFU-F (range 270–11,722), which presented 0.0012% (range 0.0002–0.0064) of all nucleated cells. The average CFU-F recovery rate was 19.1% (range 6.5–50.4).
The average CFU-F enrichment and concentration factor were 12.8-fold (range 4.0–40.8, p < 0.05) and 1.1-fold (range 0.4–2.7, p = 0.677), respectively.
Flow cytometric analysis
The average viability of all nucleated cells in BMAs was 91% (range 80–98) and has slightly decreased to 86% (range 78–96) after processing through separation column (N = 14).
The estimation of MSC content was performed with flow cytometric analysis for CD271+ CD45− 7-AAD− cells (N = 14). The average frequency of CD271+ CD45− 7-AAD− cells before separation was 0.037% (range 0.008–0.140) of all nucleated cells and has decreased to 0.027% (range 0.008–0.082) of all nucleated cells after separation. The average CD271+ CD45− 7-AAD− cell recovery rate was 15.4% (range 5.7–34.8).
The average enrichment and concentration factors for CD271+ CD45− 7-AAD− cells were 11.4-fold (range 3.6–26.8, p < 0.05) and 1.0 (range 0.4–1.9, p = 0.754), respectively.
Additionally, estimation of MSCs was evaluated by measuring the concentration of CD73+ CD90+ CD105+ cells (N = 13). The average frequency of CD73+ CD90+ CD105+ cells after separation was 0.15% (range 0.01–1.70) of all nucleated cells. The average CD73+ CD90+ CD105+ cell recovery rate was 21.5% (range 6.0–39.8).
The average enrichment and concentration factors for CD73+ CD90+ CD105+ cells were: 14.3-fold (range 5.2–38.3, p < 0.05) and 1.2-fold (range 0.4–2.4, p = 0.223), respectively.
Clinical evaluation
The mean time from surgery to last evaluation was 20 months (range 14–25).
Four patients required surgical re-intervention that was not related to the cartilage repair: removal of an old loosened ACL fixation device, arthroscopy due to a fresh patella lesion, arthroscopic removal of a post-op adhesions in the anterior knee compartment, and HTO plate removal. One patient (SR00415M) encountered partial graft failure due to progression of OA process. Altogether, no serious adverse events related directly to the osteochondral treatment were reported. Therefore, the treatment revealed high safety profile as proposed in hypothesis (2).
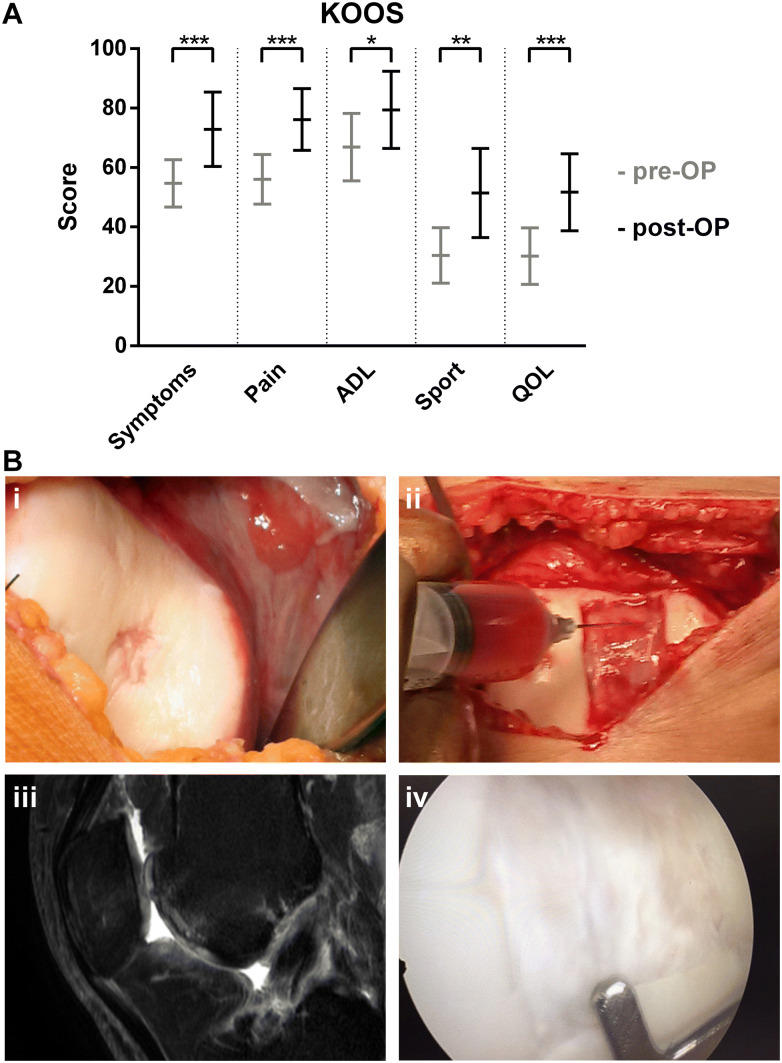
All KOOS subscales increased significantly from pre-operative Symptoms 55/Pain 56/ADL 67/Sport 30/QoL 30 to post-operative Symptoms 73/Pain 76/ADL 79/Sport 51/QoL 52, with all p values < 0.05, confirming the proposed hypothesis (3). Majority of knee patients (14/15) reported improvements in KOOS pain category comparing to the baseline. Refer to Fig. 2A. The results of safety evaluation and clinical outcome are presented in Table 3.
Fig. 2.
A Knee Osteoarthritis outcome scores before and after treatment with f-BMA. Data are presented as arithmetic mean with 95% confidence interval (N = 15). Statistical significance was determined by paired two-sided t-test, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. B A 34-year old female former handball player suffered from an osteochondral lesion of central trochlea femoris (i) 7 years after anterior cruciate ligament reconstruction (case MG01315M). The site of the lesion was debrided and covered with a bi-layered collagen-hydroxyapatite biomimetic scaffold combined with f-BMA (ii). MRI image taken 18 months after surgical repair shows complete reconstruction of the entire osteochondral unit (iii). A knee arthroscopy (performed at 12 months post-surgery due to a loosened ligament fixation device) demonstrated complete filling of the lesion with a repair tissue that was fully integrated to the surrounding cartilage (iv). ADL activities of daily living, KOOS knee osteoarthritis outcome score, QOL quality of life
Table 3.
Clinical outcome and serious adverse events or graft failures following treatment with f-BMA
| Patient code | Follow-up period (months) | KOOS pre-OP | KOOS post-OP | Serious adverse events or graft failure | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symptoms | Pain | ADL | Sport | QOL | Symptoms | Pain | ADL | Sport | QOL | |||
| PM00814M | 24 | 50 | 42 | 94 | 50 | 63 | 86 | 83 | 90 | 60 | 63 | None |
| TG00914M | 24 | 64 | 44 | 82 | 40 | 56 | 90 | 83 | 100 | 90 | 80 | None |
| SR00415M | 21 | 25 | 42 | 50 | 5 | 19 | 18 | 36 | 40 | 0 | 6 | MUA due arthrofibrosis and joint contracture |
| MG00615M | 21 | 71 | 64 | 91 | 30 | 38 | 96 | 92 | 99 | 90 | 75 | None |
| MP00915M | 17 | 57 | 44 | 66 | 25 | 13 | 64 | 72 | 72 | 30 | 25 | None |
| TP01015M | 16 | 64 | 75 | 82 | 40 | 50 | 82 | 97 | 99 | 75 | 69 | None |
| MG01315M | 14 | 57 | 72 | 94 | 55 | 31 | 80 | 90 | 96 | 75 | 80 | Removal of old loosened ACL fixation device |
| SK01615M | 19 | 56 | 64 | 41 | 25 | 12 | 86 | 90 | 90 | 50 | 69 | HTO plate removal |
| DM00116M | 21 | 81 | 57 | 72 | 25 | 19 | 78 | 64 | 68 | 40 | 31 | Arthroscopic debridement of de novo patella lesion |
| DD00316M | 25 | 42 | 50 | 46 | 50 | 25 | 40 | 54 | 40 | 46 | 25 | None |
| JS00416M | 25 | 57 | 61 | 50 | 30 | 38 | 82 | 80 | 96 | 50 | 63 | None |
| AK00616M | 24 | 34 | 32 | 36 | 5 | 32 | 39 | 43 | 38 | 5 | 38 | Revision arthroscopy due to localized adhesions |
| PŠ00716 M | 24 | 42 | 44 | 46 | 37 | 32 | 80 | 83 | 70 | 46 | 69 | None |
| LL00916MK | 20 | 56 | 64 | 82 | 40 | 25 | 89 | 82 | 99 | 75 | 44 | None |
| 16BK017BK | 14 | 64 | 86 | 71 | 0 | 0 | 83 | 93 | 94 | 40 | 38 | None |
| Arithmetic mean | 55 | 56 | 67 | 30 | 30 | 73 | 76 | 79 | 51 | 52 | ||
| Lower 95% CI | 47 | 48 | 56 | 21 | 21 | 60 | 66 | 66 | 36 | 39 | ||
| Upper 95% CI | 63 | 64 | 78 | 40 | 40 | 85 | 87 | 92 | 66 | 65 | ||
ACL anterior cruciate ligament, ADL activities of daily living, HTO high tibial osteotomy, KOOS knee osteoarthritis outcome score, MUA manipulation under anesthesia, QOL quality of life
Eight patients in this case series had an MRI (4), arthroscopic (2), or both (2) follow-up evaluations performed. The failed patient SR00415M presented above, revealed overall ICRS graft repair assessment of 6 points. The remaining seven patients revealed normal (3 cases) or nearly normal (4 cases) overall ICRS repair assessment. Figure 2B shows implantation procedure and outcome of a successful cartilage repair for patient MG01315M.
Discussion
Symptomatic osteochondral lesions require surgical repair to reduce patients’ symptoms and to prevent progression toward early osteoarthritis. Combination of biocompatible scaffolds and autologous active cells is prevailing treatment strategy for midsized to large lesions [23]. The concentration of MSCs is considered one of important parameters that influence clinical success of cell therapies utilizing MSCs isolated within one operative procedure [8]. Bone marrow processing through a separation column offers an alternative approach to conventional centrifuge-based bone marrow processing systems enabling concentration of MSCs. The mechanism of cell isolation on the selected filter device is a combination of cell trapping as result of sieving effect and adherence of MSCs to the nonwoven fabrics [15]. According to DOSES cell therapy communication tool [24] the f-BMAs obtained in the present study represent autologous, bone-marrow derived cells, obtained by minimal manipulation through filtration and centrifugation. Cell suspension contained 0.027% CD271+ CD45− 7-AAD− cells, 0.15% CD73+ CD90+ CD105+ cells and 0.0012% CFU-Fs of all nucleated cells with 86% viability. f-BMA was delivered intra-articularly and injected onto biomimetic collagen-hydroxyapatite scaffold.
Filtration processing of clinical samples removed 98.6% RBCs from initial BMAs. While the majority of RBCs were washed off the column the greater extent of WBCs (evaluated with haematology analyser) and MSCs (evaluated with CFU-F assay and flow cytometry) were harvested from the column which resulted in 11.4–14.7-fold enrichment of these two cell populations in final cell preparation, depending on the evaluation method. The separation column is equally selective for WBCs and MSCs since the enrichment factors for both cellular populations are in the similar range. The high enrichment factors indicate to high selectivity of the cell isolation method. However the concentrations of CFU-Fs before and after separation were very similar, resulting in concentration factors that were not significantly different to 1 (p > 0.05), showing that no concentration of MSCs was achieved with our BMA processing procedure. The average recovery rate for CFU-Fs was 21.5%. Nevertheless, our number of 1571 CFU-F (range 270–11,722) in the final cell preparation falls within range of post-processing CFU-F numbers measured in other commercial systems: 806–8888 CFU-F [25].
Based on our preliminary investigation of flow-through fractions the majority of MSCs is actually retained on the column (data not shown), however they are not efficiently recovered with the current harvesting procedure. With additional optimization of harvesting technique more MSCs could be recovered from the column which could lead to overall improvement of current cell separation process with selected filter-based separation device.
Our proposed hypothesis (1) was partially confirmed as the population of MSCs was significantly enriched. However, the described procedure did not enable concentration of MSCs.
As a most abundant cell population in BM, RBCs can have a great impact of MSC seeding efficiency on 3D scaffolds simply by preventing their physical contact with the surface. With the removal of majority of RBCs the seeding efficiency of MSCs onto decellularized 3D bone construct was significantly increased in the in vitro study [17]. Their BMA filtering device enriched CFU-Fs by 3.7-fold which is lower than 12.8-fold enrichment achieved in our study. Our degree of enrichment is mainly result of 98.6% RBC removal, which is consistent with the previously reported data [16]. In our opinion the most important contribution of filter-based cell separation procedure used in this study is in the removal of RBCs which is expected to be beneficial in terms of MSC seeding efficiency onto biomimetic scaffold. More systematically designed in vitro and in vivo studies should be performed to better understand the impact of MSC frequency in the final cell product on overall tissue regeneration outcome. With this knowledge we could also make more justified decisions which cell isolation strategy to employ for specific application: ones with higher cell recovery rates or others with higher cell enrichment factors.
During the follow-up no serious adverse events related directly to the osteochondral treatment were reported; primary graft failure was 7%. Clinically, the majority of patients significantly improved their post-operative knee function. MRI or arthroscopic evaluation revealed nearly normal to normal lesion repair in the majority of evaluated cases.
The clinical results, showing high safety profile and positive clinical response, are in favour of hypotheses (2) and (3).
It needs to be stressed out that patient pathology was rather complex (more than half were revision cases, one-third required concomitant procedures, one-fourth of the knees already showed degenerative cartilage changes). This particular biomimetic collagen-hydroxyapatite scaffold has been extensively used in a stand-alone manner for the osteochondral repair in the knee [11]. Albeit our results are short-termed, they concur with other clinical reports [26]. These early results also correspond to our series of patients, in whom CHAS was combined with cultivated autologous chondrocyte implantation [27]. There are only limited reports on the usage of single step bone-marrow procedures in combination with other scaffolds for the treatment of focal cartilage lesions. Gobbi et al. [28] were using BMAC covered with collagen I/III matrix, and showed this combination to be viable treatment for knee chondral lesions. They reported complete coverage of lesions seen on MRI with hyaline-like cartilage in 80% of patients, and normal to nearly normal tissues (hyaline cartilage–like tissues) on biopsies at second-look arthroscopy. They also compared the techniques above to the classical MACI techniques based on autologous chondrocyte cultivation, and consider both treatments to be effective for large patellofemoral chondral lesions at 3 year follow-up [29]. Enea et al. [30] used collagen-covered microfractures and bone marrow concentrate for focal cartilage defects in the knee. They consider it safe; it improved knee function, and has a potential to regenerate hyaline-like cartilage. Reconstitution of the original cartilage level was achieved, but bone marrow edema and/or subchondral irregularities were observed in all patients. Second-look arthroscopy was performed in 5 patients, resulting in 1 normal, 3 nearly normal, and 1 abnormal result. Histology showed hyaline-like repair tissue. Skowronski et al. [31] applied BMAC to a collagen membrane. They consider this one stage repair of large chondral lesions as an effective treatment modality. Chahla et al. [32] in their recent systematic review conclude that BMAC treatment appears to be a safe procedure that is growing exponentially, most likely because it represents one of the few categories allowed by the FDA to deliver stem cells (minimally manipulated). All the studies included in their systematic review reported good results, but they used different outcome measures and this heterogeneity does not allow for direct comparison. They also stress out a need for well-conducted randomized controlled trials with large sample sizes and defined end points to further evaluate the efficacy of BMAC for the treatment of knee pathologies. Our clinical results concur to the results above, with one very important difference: only deep chronic osteochondral lesions were treated in our case series. Chronic osteochondral lesions namely cannot be treated with thin collagen membranes that were used in combination with BMAC by other authors. Therefore, usage of a biomimetic osteochondral scaffold and well balanced MSC source is recommended.
The clinical results have to be interpreted in light of limited patient series, short-medium follow-up time and a non-randomized study design. On contrary, the entire two-year patient series operated on the knees by a single surgeon (MD) was included into the study. A single surgeon design avoids possible bias due to different surgical technique. We also cannot delineate what percentage of cartilage repair is attributable to biomaterials versus f-BMA. But we could prove that combination of both offers safe and successful cartilage repair and return to activates, which was a primarily target of this publication. f-BMA obtained by the presented procedure may easily be combined with other cartilage repair scaffolds in the future.
Altogether, this patient case series presents clinical application of filter-based separation device, which enabled efficient removal of RBCs, while achieving 12.8-fold enrichment and 19.1% recovery of CFU-Fs. However, the concentration of MSCs was not increased. Combination of f-BMA and biomimetic scaffold prepared for the treatment of osteochondral lesions resulted in no reported serious adverse events directly related to the graft. One patient (6.7%) in the series had documented graft failure. Clinically, all the other fourteen patients improved their post-operative knee function in all five KOOS categories. All average KOOS subscales increased significantly from pre-operative Symptoms 55/Pain 56/ADL 67/Sport 30/QoL 30 to post-operative Symptoms 73/Pain 76/ADL 79/Sport 51/QoL 52, with all p values < 0.05. MRI or arthroscopic evaluation revealed nearly normal to normal lesion repair in the majority of evaluated cases.
With respect to proposed hypotheses, we could partially confirm hypothesis (1). The population of MSCs was significantly enriched; however, the concentration of MSCs was not achieved. The clinical results, showing high safety profile and positive clinical response, are in favour of hypotheses (2) and (3).
Acknowledgement
The authors acknowledge the financial support from the UKC-LJ Institutional research funding (No. 20150156).
Author contributions
MK and MD conceived and designed the study. MV, JV and MD acquired the data, MV, AB and MD interpreted and analysed the data. MV, JV, AB and MD drafted the article and MK revised it critically for the important intellectual content. All authors approved the final version of the article to be published.
Compliance with ethical standards
Conflict of interest
Educell Ltd., Trzin, Slovenia (co-authors MV, MK, AB) is a commercial cell and tissue institution that is providing separated MSCs and other advanced cell therapies. MD is a clinical consultant to Fin-Ceramica, Faenza, Italy.
Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study protocol was approved by the National Medical Ethics Committee (No. 0120-14/2016-2). Informed consent was confirmed by the National Medical Ethics Committee.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1000 knee arthroscopies. Arthroscopy. 2002;18:730–734. doi: 10.1053/jars.2002.32839. [DOI] [PubMed] [Google Scholar]
- 2.Grässel S, Lorenz J. Tissue-engineering strategies to repair chondral and osteochondral tissue in osteoarthritis: use of mesenchymal stem cells. Curr Rheumatol Rep. 2014;16:452. doi: 10.1007/s11926-014-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muschler GF, Midura RJ, Nakamoto C. Practical modeling concepts for connective tissue stem cell and progenitor compartment kinetics. J Biomed Biotechnol. 2003;2003:170–193. doi: 10.1155/S1110724303209165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richter W. Mesenchymal stem cells and cartilage in situ regeneration. J Intern Med. 2009;266:390–405. doi: 10.1111/j.1365-2796.2009.02153.x. [DOI] [PubMed] [Google Scholar]
- 5.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 6.Martinčič D, Radosavljevič D, Drobnič M. Ten-year clinical and radiographic outcomes after autologous chondrocyte implantation of femoral condyles. Knee Surg Sports Traumatol Arthrosc. 2014;22:1277–1283. doi: 10.1007/s00167-013-2778-3. [DOI] [PubMed] [Google Scholar]
- 7.Veronesi F, Giavaresi G, Tschon M, Borsari V, Nicoli Aldini N, Fini M. Clinical use of bone marrow, bone marrow concentrate, and expanded bone marrow mesenchymal stem cells in cartilage disease. Stem Cells Dev. 2013;22:181–192. doi: 10.1089/scd.2012.0373. [DOI] [PubMed] [Google Scholar]
- 8.Coelho MB, Cabral JMS, Karp JM. Intraopera stem cell therapy. Annu Rev Biomed Eng. 2012;14:325–349. doi: 10.1146/annurev-bioeng-071811-150041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kon E, Filardo G, Di Martino A, Busacca M, Moio A, Perdisa F, et al. Clinical results and MRI evolution of a nano-composite multilayered biomaterial for osteochondral regeneration at 5 years. Am J Sports Med. 2014;42:158–165. doi: 10.1177/0363546513505434. [DOI] [PubMed] [Google Scholar]
- 12.Kon E, Filardo G, Brittberg M, Busacca M, Condello V, Engebretsen L, et al. A multilayer biomaterial for osteochondral regeneration shows superiority vs microfractures for the treatment of osteochondral lesions in a multicentre randomized trial at 2 years. Knee Surg Sports Traumatol Arthrosc. 2018;26:2704–2715. doi: 10.1007/s00167-017-4707-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christensen BB, Foldager CB, Jensen J, Jensen NC, Lind M. Poor osteochondral repair by a biomimetic collagen scaffold: 1- to 3-year clinical and radiological follow-up. Knee Surg Sports Traumatol Arthrosc. 2016;24:2380–2387. doi: 10.1007/s00167-015-3538-3. [DOI] [PubMed] [Google Scholar]
- 14.Marcacci M, Zaffagnini S, Kon E, Marcheggiani Muccioli GM, Di Martino A, Di Matteo B, et al. Unicompartmental osteoarthritis: an integrated biomechanical and biological approach as alternative to metal resurfacing. Knee Surg Sports Traumatol Arthrosc. 2013;21:2509–2517. doi: 10.1007/s00167-013-2388-0. [DOI] [PubMed] [Google Scholar]
- 15.Ito K, Aoyama T, Fukiage K, Otsuka S, Furu M, Jin Y, et al. A novel method to isolate mesenchymal stem cells from bone marrow in a closed system using a device made by nonwoven fabric. Tissue Eng Part C Methods. 2010;16:81–91. doi: 10.1089/ten.tec.2008.0693. [DOI] [PubMed] [Google Scholar]
- 16.Otsuru S, Hofmann TJ, Olson TS, Dominici M, Horwitz EM. Improved isolation and expansion of bone marrow mesenchymal stromal cells using a novel marrow filter device. Cytotherapy. 2013;15:146–153. doi: 10.1016/j.jcyt.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Dawson JI, Smith JO, Aarvold A, Ridgway JN, Curran SJ, Dunlop DG, et al. Enhancing the osteogenic efficacy of human bone marrow aspirate: concentrating osteoprogenitors using wave-assisted filtration. Cytotherapy. 2013;15:242–252. doi: 10.1016/j.jcyt.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Filardo G, Drobnic M, Perdisa F, Kon E, Hribernik M, Marcacci M. Fibrin glue improves osteochondral scaffold fixation: study on the human cadaveric knee exposed to continuous passive motion. Osteoarthritis Cartilage. 2014;22:557–565. doi: 10.1016/j.joca.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Cuthbert R, Boxall SA, Tan HB, Giannoudis PV, McGonagle D, Jones E. Single-platform quality control assay to quantify multipotential stromal cells in bone marrow aspirates prior to bulk manufacture or direct therapeutic use. Cytotherapy. 2012;14:431–440. doi: 10.3109/14653249.2011.651533. [DOI] [PubMed] [Google Scholar]
- 20.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 21.Knee Injury and Osteoarthritis Outcome Score (KOOS), Slovenian version LK1.0. 2007. http://www.koos.nu/KOOSSlovenian.pdf. Accessed 27 Dec 2016.
- 22.Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85:58–69. doi: 10.2106/00004623-200300002-00008. [DOI] [PubMed] [Google Scholar]
- 23.Nooeaid P, Salih V, Beier JP, Boccaccini AR. Osteochondral tissue engineering: scaffolds, stem cells and applications. J Cell Mol Med. 2012;16:2247–2270. doi: 10.1111/j.1582-4934.2012.01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray IR, Chahla J, Safran MR, Krych AJ, Saris DBF, Caplan AI, et al. International expert consensus on a cell therapy communication tool: DOSES. J Bone Joint Surg Am. 2019;101:904–911. doi: 10.2106/JBJS.18.00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hegde V, Shonuga O, Ellis S, Fragomen A, Kennedy J, Kudryashov V, et al. A prospective comparison of 3 approved systems for autologous bone marrow concentration demonstrated nonequivalency in progenitor cell number and concentration. J Orthop Trauma. 2014;28:591–598. doi: 10.1097/BOT.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 26.Filardo G, Kon E, Di Martino A, Busacca M, Altadonna G, Marcacci M. Treatment of knee osteochondritis dissecans with a cell-free biomimetic osteochondral scaffold. Am J Sports Med. 2013;41:1786–1793. doi: 10.1177/0363546513490658. [DOI] [PubMed] [Google Scholar]
- 27.Drobnič M, Martinčič D, Merkač J, Radosavljevič D. Survival rates of various ACI grafts and concomitant procedures. A prospective single-center study over 15 years. In: Book of Abstracts. 13th World Congress of the International Cartilage Repair Society; 2016 Sep 23–27; Sorrento, Italy. ICRS; 2016.
- 28.Gobbi A, Karnatzikos G, Sankineani SR. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med. 2014;42:648–657. doi: 10.1177/0363546513518007. [DOI] [PubMed] [Google Scholar]
- 29.Gobbi A, Chaurasia S, Karnatzikos G, Nakamura N. Matrix-induced autologous chondrocyte implantation versus multipotent stem cells for the treatment of large patellofemoral chondral lesions: a nonrandomized prospective trial. Cartilage. 2015;6:82–97. doi: 10.1177/1947603514563597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enea D, Cecconi S, Calcagno S, Busilacchi A, Manzotti S, Gigante A. One-step cartilage repair in the knee: collagen-covered microfracture and autologous bone marrow concentrate. A pilot study. Knee. 2015;22:30–35. doi: 10.1016/j.knee.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Skowroński J, Skowroński R, Rutka M. Large cartilage lesions of the knee treated with bone marrow concentrate and collagen membrane–results. Ortop Traumatol Rehabil. 2013;15:69–76. doi: 10.5604/15093492.1058409. [DOI] [PubMed] [Google Scholar]
- 32.Chahla J, Dean CS, Moatshe G, Pascual-Garrido C, Serra Cruz R, LaPrade RF. Concentrated bone marrow aspirate for the treatment of chondral injuries and osteoarthritis of the knee: a systematic review of outcomes. Orthop J Sports Med. 2016;4:2325967115625481. doi: 10.1177/2325967115625481. [DOI] [PMC free article] [PubMed] [Google Scholar]