Abstract
The renoprotective potential of nanoemulsified garlic oil blend (GNE) in alleviating the progressive stages of hyperlipidemia-mediated diabetic nephropathy was examined. The study was carried out in high fat-fed, streptozotocin-induced type 2 diabetic Wistar rats for five months. The diabetic rats showed a significant increase of area under the curve in OGTT (p < 0.01) and IPITT (p < 0.01), increased urinary albumin (p < 0.01), urinary microprotein (p < 0.001), total cholesterol (p < 0.01), triglycerides (p < 0.001) and LDL cholesterol (p < 0.001), with decreased serum albumin (p < 0.01), serum protein (p < 0.001) and HDL-cholesterol levels (p < 0.05) than the control rats. The histopathological analysis evidenced mesangial expansion and hypercellularity at the end of the first and third month, and glomerulosclerosis and tubular atrophy at the end of the fifth month in diabetic rats. Moreover, on disease progression, increase in urinary podocalyxin, NGAL and CD36 was observed, and the renal mRNA and protein expression of podocalyxin decreased significantly with a concomitant increase in NGAL and CD36 expression from first till fifth month end. The treatment with GNE (20 mg/kg) significantly ameliorated the serum albumin (p < 0.001) and urine albumin (p < 0.01) from the end of the third month with significant attenuation in the lipid profile than GO (20 mg/kg) or Ator (8 mg/kg). Moreover, GNE reverted the histopathological alterations and attenuated the aberrant mRNA, protein expression and urinary excretion level of renal CD36, podocalyxin and NGAL in diabetic rats from an early stage of disease till the end of the study period. This study demonstrated the enhanced efficacy of GO in nanoemulsified form in mitigating the progression of nephropathy in type 2 diabetic rats.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02262-w) contains supplementary material, which is available to authorized users.
Keywords: Diabetic nephropathy, Podocalyxin, NGAL, CD36, Nanoemulsified garlic oil blend
Introduction
The epidemic of diabetes mellitus is a major concern worldwide. Diabetes is an association of metabolic disorders marked by persistent hyperglycemia. The micro- and macro-vascular complications of prolonged hyperglycemic state determine the mortality and morbidity associated with diabetes (Aldukhayel 2017). Diabetic nephropathy (DN) is the most prevalent microvascular complication causing a substantial reduction in the life expectancy of diabetic individuals. The increased risk of mortality due to DN is observed in both type 1 and type 2 diabetes, wherein approximately 35% of type 2 diabetic cases progress to DN (Wanner et al. 2016). The subjects with DN were classified as normoalbuminuric, microalbuminuric, and macroalbuminuric, characterized by glomerular hyperfiltration, renal enlargement and nodular glomerulosclerosis that eventually lead to end-stage renal disease (Haneda et al. 2015; Reidy et al. 2014).
The elevated advanced glycation end products (AGE), oxidized low-density lipoprotein (Ox-LDL) and free fatty acids (FFA) play a critical role in the pathogenesis of DN, which leads to the elevated expression of a scavenger receptor, CD36 (Cluster of differentiation 36) (Kawanami et al. 2016). The CD36 expression increases on a variety of cells including epithelial cells, adipocytes, monocytes, platelets, and skeletal muscle cells in diabetic conditions (Silverstein et al. 2009). Apart from these, CD36 expression is also elevated in renal cells that mediate podocyte apoptosis, tubular fibrosis and epithelial–mesenchymal transition (EMT) in DN (Hua et al. 2015). The podocyte injury in type 2 diabetic patients governed by elevated CD36 expression in podocytes leads to lipid accumulation (Herman-Edelstein et al. 2014). Moreover, in dyslipidemia, inflammation led to lipid accumulation, which induced podocyte EMT. Further, the podocyte EMT downregulated the expression of podocalyxin, a negatively charged sialoglycoprotein present on the apical surface of podocyte (Zhang et al. 2015). The podocalyxin functions in limiting the passage of anionic proteins, maintaining the podocyte shape and formation of podocyte foot process (Li et al. 2007; Fang et al. 2013). In case of DN, the neutralized negative charge of podocalyxin caused disruption of foot process architecture and the lack of slit diaphragms led to the passage of plasma proteins (Kostovska et al. 2016; Ling et al. 2018). Besides, the elevated renal CD36 expression due to AGE formation in high-glucose conditions exacerbated the renal tubular dysfunction marked by elevated urinary neutrophil gelatinase-associated lipocalin (NGAL) levels (Feng et al. 2017a, b; Yuan et al. 2017). NGAL is a 25 kDa glycoprotein with 178 amino acids and the increase in NGAL level is recognized as a protective mechanism to repair tubular epithelial damage in chronic kidney diseases (Liu et al. 2015; Papadopoulou-Marketou et al. 2017; Satirapoj 2018). Thus, in DN, the elevated CD36 is directly associated with decreased renal podocalyxin and increased NGAL expression which is examined in our study.
Moreover, the urinary CD36, podocalyxin and NGAL levels were recognized as a diagnostic marker for DN. The previous finding from our lab has shown that the urinary CD36 level can serve as a prognostic marker for type 2 diabetic patients progressing to nephropathy (Shiju et al. 2015). Further, the podocalyxin expression decreased in db/db mice and cultured mouse podocytes in high-glucose conditions (Qi et al. 2007). As podocyte loss is found to occur even before MA could occur, the urinary podocalyxin level is recognized as an early indicator of podocyte injury in diabetes (Hara et al. 2012). The urinary NGAL level is considered as an early detector of tubular damage as it is shown to increase even in patients with normoalbuminuria (Fiseha et al. 2016).
Several drugs are applicated as targeting agent toward the early stage of DN including angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor blockers. These drugs are shown to cause adverse effects in later stages of nephropathy, which demands the need for a natural therapeutic drug (Sonkodi et al. 2003). Allium sativum, commonly termed as garlic has beneficial effects on hyperglycemia, hyperlipidemia, hypertension, platelet aggregation, and blood fibrinolytic activity. Garlic oil has shown to increase GLUT-4 expression in skeletal muscles of streptozotocin (STZ)-induced diabetic rats and exert a renoprotective effect in sodium nitrite-induced liver and renal damage (Liu et al. 2012; Hassan et al. 2009). Besides, hepatorenal protection of GO was ascertained in cisplatin-treated rats. The antihyperlipidemic drug-loaded GO nanoemulsions exerted enhanced hepatorenal protection than GO alone in dyslipidemic animal models (Faran et al. 2019, Abdel-Daim et al. 2020). Further, the diallyl disulfide and diallyl trisulfide in GO ameliorated endothelial nitric oxide (eNOS) degradation that enhanced the CD36 expression in the diabetic state (Lei et al. 2010; Takahashi et al. 2014; Jay et al. 2015; Feng et al. 2017a, b). Moreover, the antioxidant activity of diallyl disulfide attenuated the doxorubicin-induced nephropathy and diallyl sulfide mitigated fipronil-induced oxidative injury (Abdel-Daim et al. 2018; Lin et al. 2019). Thus, we have chosen GO as a natural drug to target hyperlipidemia-mediated nephropathy in type 2 diabetic rats. However, several drawbacks in the use of garlic oil including poor solubility and systemic bioavailability urge for efficient drug delivery systems (Zheng et al. 2013). In our previous study, a nanoemulsion-based delivery system for GO was formulated and was found to be effective toward dyslipidemia prevention (Ragavan et al. 2017). Our present study demonstrates the renoprotective effect of nanoemulsified garlic oil blend (GNE) in comparison with its bulk counterpart GO on type 2 diabetes-mediated nephropathy in association with the renal expression of CD36, podocalyxin and NGAL. Atorvastatin was used as a positive control to ascertain the effect of GNE on type 2 diabetes-induced nephropathy mediated by hyperlipidemic conditions. Subsequently, the effect of GNE against GO on CD36, podocalyxin and NGAL as a urinary diagnostic marker of DN was evaluated.
Materials and methods
Materials
The garlic oil blend (W530316) containing 30–50% Diallyl disulfide, 10–13% Diallyl trisulfide and 5–13% Allyl Sulfide was purchased from Sigma Aldrich (Bengaluru, India). Tween 80 was purchased from Sisco Research Laboratories, Pvt. Ltd, (Mumbai, India). The analytical kits for the estimation of urine and serum biochemistry were purchased from Span Diagnostics Ltd, (Gujarat, India) or Beacon Diagnostics Pvt Ltd (Gujarat, India). All other chemicals of analytical grade were purchased from Himedia or Sisco Research Laboratories, Pvt. Ltd, (Mumbai, India).
Formulation of GNE
Nanoemulsification of the garlic oil blend was carried out as given in our previous study by Ragavan et al. (2017). Briefly, 1.073 g of garlic oil blend and 1.077 g of Tween 80 was mixed in 7 ml of double-distilled water in a magnetic stirrer for 10 min at 600 rpm, which was further subjected to ultrasonication for 20 min.
Characterization of GNE
The mean droplet size, polydispersity index and surface charge of nanoemulsion were determined using the SZ-100 Dynamic Light Scattering (DLS) technique (Horiba Scientific, Japan) and Zeta potential analysis (SZ-100, Horiba Scientific, Japan). The obtained transparent formulation was stored at 25 °C for further use.
Experimental design
Male Wistar rats weighing approximately 150 ± 180 g (n = 90) were used for the study. All animal maintenance and experiments were carried out as per the ethical guidelines suggested by the Institutional Animal Ethics Committee of VIT, Vellore (Registration No—VIT/IAEC/12/July23/8). The animals were housed at VIT animal house under standard conditions of temperature (22 ± 2 °C) and humidity (55 ± 5 °C) with 12 h light/dark cycle and supplied with normal pelleted feed and water ad libitum before altering the dietary regimen. Group 1 was set as Control (Con, n = 18) and supplemented with normal pelleted diet and Group 2 (n = 72) were fed with high-fat diet (HFD) (58% fat, 25% protein and 17% carbohydrate, as a percentage of total kcal), injected once with a low dose of STZ (35 mg/kg) and continued with the respective dietary regimen for the entire duration of the study (Srinivasan et al. 2005). Rats with blood glucose level > 300 mg/dl were considered as diabetic and used for further study. Group 2 after two weeks of STZ injection was further sorted out into four experimental groups of six animals each. Group 2a (n = 30) was considered as diabetic control (Dia), Groups 2b-2d (n = 30) were considered as experimental groups, which orally received GNE at 20 mg/kg bodyweight (0.186 ml of GNE/kg BW) (Dia + GNE), GO at 20 mg/kg bodyweight (0.0186 ml of GO/kg BW) (Dia + GO) and atorvastatin at 8 mg/kg bodyweight (Dia + Ator), respectively, for a period of 5 months on daily basis.
Body mass index and relative weight of organs
The body weight in grams and length of animals in centimeters was measured in all the groups. Body mass index (BMI) was calculated using the following formula (Fraulab et al. 2010):
Besides, the relative weight of kidney (RKI), retroperitoneal fat (RF) and epididymal fat (EF) were measured in euthanized animals, and the values are expressed in % weight ± SEM (n = 6).
Oral glucose tolerance test (OGTT) and Intraperitoneal insulin tolerance test (IPITT)
The animals in all groups were fasted overnight and the blood glucose level was examined. For OGTT, glucose at 2 g/kg body weight was administered to the overnight fasted rats. For IPITT, Insulin Actrapid (1 U/kg body weight) was injected intraperitoneally to the overnight fasted animals. The glucose level before and after 30, 60, 90, 120, 150 and 180 min of glucose or insulin administration was analyzed using a glucometer (Accu chek glucose monitor, active, India) by tail vein incision. The results obtained as mg/dl of blood ± SEM (n = 6) were plotted against time. The area under the curve (AUC) was calculated using the following formula:
where C1 and C2 represent the blood glucose level at 30 min interval, t1 and t2 represent the time interval (30 min) (Fraulab et al. 2010).
Analysis of urinary biomarkers of renal injury
Spot urine was collected and centrifuged at 2500 rpm for 5 min at 4 °C. The supernatant was estimated for creatinine, albumin, and microprotein content using commercially available kits (Span Diagnostics, India). Assays were performed and analyzed as per the manufacturer’s protocol. Urinary albumin–creatinine ratio (UACR) and urinary microprotein–creatinine ratio (UPCR) were estimated from the values obtained. Results were represented in mg/g creatinine ± SEM (n = 6).
Measurement of serum lipid profile and biomarkers of renal injury
As per Institutional Animal Care and Use Committee (IACUC) guidelines, whole blood was collected from each group (n = 6) by retro-orbital plexuses, allowed to clot and centrifuged (REMI instruments, Vasai, India) at 4 °C at 4000 rpm for 20 min (Yuvashree et al. 2018). The supernatant containing serum was pooled out and analyzed for total cholesterol (TC), HDL-cholesterol (HDL-C), triglycerides (TG), albumin and microprotein using commercially available kits (Span diagnostics ltd, India). LDL cholesterol concentration was calculated using the formulae.
Assays were performed and analyzed as per the manufacturer’s protocol. Results were represented in mg/dl of serum ± SEM (n = 6).
Kidney histological examination
Animals were euthanized in all groups at the end of the study (n = 6). Kidneys were excised with care, washed in phosphate buffer saline (PBS) (pH 7.4). One part was fixed in 10% neutral buffered formalin. Tissues were processed, embedded in paraffin and sectioned using Leica RM 2126 microtome at a thickness of 4 μm and mounted on slides. Periodic acid-Schiff (PAS) staining was performed on the sections for histopathological observations and photographed under a trinocular microscope (Olympus BX51; Olympus Optical, Tokyo, Japan) at a magnification of 400× (Shiju et al. 2013). The images were examined by a pathologist to assess the renal abnormalities.
Indirect enzyme-linked immunosorbent assay (ELISA) for podocalyxin, NGAL, and CD36
Indirect ELISA was performed to measure podocalyxin, NGAL and CD36 levels in spot urine samples with slight modifications (Yuvashree et al. 2018; Koenig 1981). The procedure was carried out as given in our previous study (Fraulab et al. 2010). Briefly, Spot urine samples from control rats (n = 10) were pooled, aliquoted and stored at − 80 °C, which served as the standard and was applied in increasing dilutions in triplicates. The urine collected from diabetic rats was used directly without dilution. The 96-well flat-bottom ELISA plates were coated with antigen using 100 mM bicarbonate buffer (pH 9.6) and incubated at 4 °C overnight. Anti-podocalyxin (NBP2-25219, Novus Biologicals, USA) or anti-NGAL (sc-515876, Santa Cruz Biotechnology, Santa Cruz, USA) or anti-CD36 (E-AB-33662, Elabscience, TX, USA) was dissolved in PBS, added to the antigen-coated ELISA plates and incubated at 4 °C overnight. The corresponding secondary antibodies (GeNei, Bangalore, India) were added in respective plates and incubated at room temperature for 2 h. Each incubation step was followed by a wash with PBST (PBS with 0.05% Tween 20). For detection, the substrate solution, TMB/H2O2 (GeNei, Bangalore, India) was incubated with samples in the dark at room temperature for 30 min. The reaction was terminated by adding 2 M sulfuric acid. The absorbance was measured at 450 nm using a plate reader (ELx 800, Biotek, India). The results were calculated relative to the standard urine pool and expressed in relative units.
Quantitative expression of renal podocalyxin, NGAL, and CD36
Quantitative real-time PCR for podocalyxin, NGAL, and CD36 mRNA expression was performed (Sridharan et al. 2016). Total RNA was extracted from kidney lysate using TRIZOL reagent (Sigma Aldrich, Bangalore, India) and quantified using Nanodrop 2000 (Thermofisher Scientific, Bangalore, India). The cDNA was synthesized using prime script 1st strand cDNA synthesis kit (DSS Takara Bio India Pvt. Ltd, Bangalore, India) as per the manufacturer’s protocol. Quantitative real-time PCR was performed using prime script RT-PCR (DSS Takara Bio India Pvt. Ltd Bangalore, India) kit according to the manufacturer’s protocol. The primer used in the study is given in Supplementary Table S1.
Western blot for renal podocalyxin, NGAL, and CD36
Renal tissue protein was extracted by cell lysis with RIPA buffer. The samples were boiled, sonicated and protein contents were estimated using the bicinchoninic acid assay (G-Biosciences, USA). Proteins were separated by SDS-PAGE electrophoresis and transferred to nitrocellulose membranes using a semi-dry transmembrane device (Bio-Rad, Mississauga, ON, USA). The membranes were probed overnight with primary antibody against podocalyxin (NBP2-25219, Novus Biologicals, USA) or NGAL (sc-515876, Santa Cruz Biotechnology, Santa Cruz, USA) or CD36 (bs1100R, Bioss antibody Inc, MA, USA) (1 µg/ml) in TBST followed by incubation with the appropriate HRP conjugated secondary antibody (GeNei, Bangalore, India) as indicated. Western C ultra 2.0 ECL Western Blotting reagent was used to develop the blots. The band intensity was measured by the Fusion FX imaging system (Vilber Lourmat, Torcy, France) and quantified by Image J software (Lee et al. 2014).
Statistical analysis
The statistical analyses of data were performed using statistical software, Graph Pad Prism 5 and the results were expressed as mean ± SD. (standard deviation). The statistical significance of any difference in each parameter among the groups was evaluated by one-way analysis of variance or two-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison tests.
Results and discussion
Formulation and characterization of GNE
The GNE formulated was determined for its mean hydrodynamic size and was found to be 27 ± 0.98 nm with a polydispersity index (PDI) of 0.21 ± 0.10. The surface charge of the nanoemulsion was found to be − 44 mV ± 1.3, and pH was observed to be 4.8 ± 0.03. An insignificant change in mean hydrodynamic size, PDI, and pH, of 28 nm ± 1.21, 0.22 ± 0.1, and 4.7 ± 0.05, respectively, was observed on storage of GNE at 25 °C for 7 weeks, which was consistent with our previous findings (Ragavan et al. 2017). The nanometric droplet size and negative zeta potential ensured the proper Brownian motion and degree of repulsion among dispersed particles (Yuvashree et al. 2018). The PDI value showed the uniformity and homogeneity of nanometric emulsion system (Shah et al. 2019). The presence of phospholipids and free fatty acids in the oil system attributed to the high negative zeta potential (Cretu et al. 2017). Thus, in our study, a stable nanoemulsion was formulated which possesses the characteristics of an ideal nanoemulsion (McClements and Rao 2011).
Effect of GNE on type 2 diabetes-mediated nephropathy in male Wistar rats
Effect of GNE on body mass index and relative organ weight
At the end of the first month, the diabetic group showed an increase in BMI (1.14-fold, p < 0.05), RKI (1.23-fold, p < 0.001) and RFAT (1.49-fold, p < 0.05) than the control group (Table 1). The GNE or GO administration decreased the BMI by 1.30-fold (p < 0.01), and 1.14-fold (p < 0.01), respectively. The RFAT decreased by 2.15-fold (p < 0.001) and 2.13-fold (p < 0.001), respectively, in the GNE or Ator-treated groups. However, no significant change was observed in RKI or EFAT on administering GNE or GO.
Table 1.
BMI and relative weight of organs: control (Con), diabetic (Dia), diabetic rats treated with nanoemulsified garlic oil blend (Dia + GNE) and diabetic rats treated garlic oil blend (Dia + GO), Diabetic rats treated with atorvastatin (Dia + Ator)
| Con | Dia | Dia + GNE | Dia + GO | Dia + Ator | |
|---|---|---|---|---|---|
| BMI | |||||
| M1 | 0.53 ± 0.01 | 0.60 ± 0.03a** | 0.46 ± 0.04b** | 0.53 ± 0.003b**c* | 0.41 ± 0.005b***d** |
| M3 | 0.53 ± 0.014 | 0.61 ± 0.02a** | 0.53 ± 0.003b** | 0.56 ± 0.005b* | 0.44 ± 0.002b***c**d** |
| M5 | 0.51 ± 0.013 | 0.63 ± 0.003a** | 0.5 ± 0.03b*** | 0.62 ± 0.004c**** | 0.52 ± 0.005b**d* |
| RKI | |||||
| M1 | 0.53 ± 0.01 | 0.65 ± 0.01a*** | 0.67 ± 0.01 | 0.69 ± 0.01 | 0.71 ± 0.02 |
| M3 | 0.55 ± 0.09 | 0.79 ± 0.01a*** | 0.77 ± 0.01 | 0.89 ± 0.01b*c* | 0.90 ± 0.015b*c* |
| M5 | 0.49 ± 0.01 | 0.85 ± 0.01a*** | 0.67 ± 0.01b** | 1.41 ± 0.01b****c**** | 1.0 ± 0.03c*d** |
| RFAT | |||||
| M1 | 1.01 ± 0.03 | 1.51 ± 0.02a* | 0.70 ± 0.02b*** | 0.82 ± 0.008b** | 0.71 ± 0.01b***d* |
| M3 | 0.96 ± 0.04 | 2.07 ± 0.05a** | 0.69 ± 0.02b*** | 1.49 ± 0.02b**c*** | 0.68 ± 0.02b***d*** |
| M5 | 0.95 ± 0.03 | 2.7 ± 0.03a**** | 0.75 ± 0.003b**** | 0.77 ± 0.004b****c** | 0.78 ± 0.01b*** |
| EFAT | |||||
| M1 | 0.62 ± 0.01 | 0.6 ± 0.01 | 0.55 ± 0.01 | 0.70 ± 0.01c* | 0.41 ± 0.014b**c*d** |
| M3 | 0.59 ± 0.01 | 1.31 ± 0.03a** | 0.79 ± 0.01b** | 0.89 ± 0.02b* | 0.51 ± 0.02b**c**d* |
| M5 | 0.57 ± 0.01 | 1.8 ± 0.01a**** | 0.64 ± 0.006b**** | 1.4 ± 0.02b***c*** | 0.97 ± 0.03b**c*d** |
M Month. Values represent the mean ± standard error (n = 6) of the samples. Significant difference between Control and Diabetic groups: a*p < 0.05, a**p < 0.001, a***p < 0.001, a****p < 0.0001. Significant difference between Diabetic and Dia + GNE, Dia + GO, Dia + Ator: b**p < 0.01, b***p < 0.001, b****p < 0.0001. Significant difference between Dia + GNE and Dia + GO, Dia + GNE and Dia + Ator: c*p < 0.05, c***p < 0.001, c****p < 0.0001. Significant difference between Dia + GO and Dia + Ator: d*p < 0.05, d**p < 0.01, d***p < 0.001
At the end of the third month, the BMI (1.16-fold, p < 0.05), RKI (1.4-fold, p < 0.001), RF (2.2-fold, p < 0.01) and EFAT (2.2-fold, p < 0.01) increased significantly in the diabetic group than the control group. The GNE or Ator administration decreased the BMI by 1.11-fold (p < 0.05) and 1.39-fold (p < 0.001), respectively, than the GO administration. The RFAT decreased by 3.00-fold (p < 0.001) and EFAT decreased by 1.65-fold (p < 0.01) on administering GNE than GO. The GNE administration showed no significant difference in RKI at third month end; however, GO or Ator administration increased RKI (p < 0.05) significantly (Table 1).
At the end of the fifth month the diabetic group showed a significant increase in BMI, RKI, RF and EFAT by 1.24-fold (p < 0.01), 1.7-fold (p < 0.001), 2.8-fold (p < 0.0001) and 3.2-fold (p < 0.0001), respectively, than the control group. However, the GNE administration significantly decreased BMI by 1.25-fold (p < 0.001), RKI by 1.26-fold (p < 0.01), RF by 3.6-fold (p < 0.0001) and EFAT by 2.8-fold (p < 0.0001) than the GO or Ator administration. In contrary, on administering GO the RKI increased significantly by 1.66-fold (p < 0.0001) in comparison with the diabetic group (Table 1).
The increase in BMI with ectopic fat deposition was observed in the diabetic group. The BMI has a modest relationship with visceral fat, where a change in BMI is minimal compared to the change in the visceral fat (Lamacchia et al. 2011). The visceral fat deposition could impair the organ function either by physical compression or by secreting various locally acting substances (Shah et al. 2014). Further, the renal enlargement observed in our study is a characteristic feature of nephropathy caused by glomerular hypertrophy and nephromegaly (Kiran et al. 2012). Our results corroborate with the earlier findings, in which GO was proven to reduce the FFAs concentration and decrease body weight and fat deposition in obese mice with HFD-induced non-alcoholic fatty liver disease (Lai et al. 2014). However, we observed an improved efficacy of GO on nanoemulsification in attenuating the BMI and relative weight of organs.
Effect of GNE on OGTT and IPITT
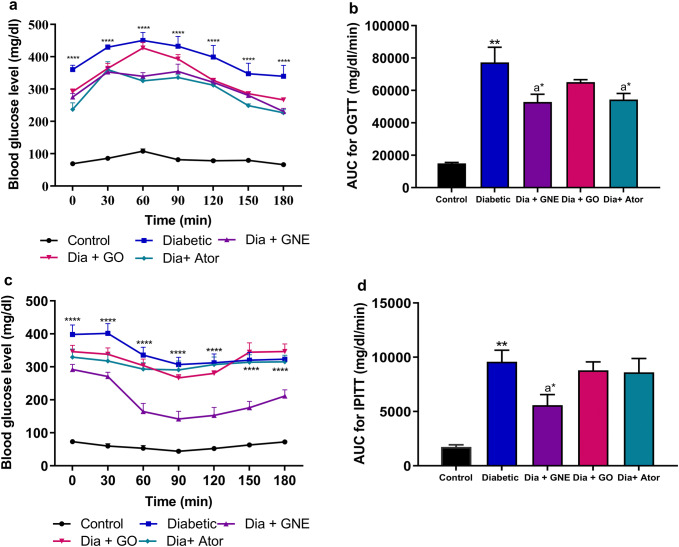
The OGTT and IPITT were carried out at the end of the first, third and fifth month in all the experimental groups (data not shown). At the end of the fifth month, the OGTT in diabetic rats showed a significant increase in blood glucose level and a corresponding increase in AUC by 5.15-fold in comparison with the control group. A significant decrease in the blood glucose level on administering GNE or Ator was illustrated in the diabetic rats with a corresponding decrease in AUC by 1.46-fold (p < 0.05) and 1.42-fold (p < 0.05) was observed. However, GO-administered diabetic rats showed no change in the OGTT curve (Fig. 1a, b).
Fig. 1.
Oral glucose tolerance test and intraperitoneal insulin tolerance test in control, diabetic, diabetic rats treated with nanoemulsified garlic oil blend (Dia + GNE), Diabetic rats treated garlic oil (Dia + GO), and Diabetic rats treated with atorvastatin (Dia + Ator). a Blood glucose level. b Area under curve during OGTT. c Blood glucose level. d Area under curve during IPITT. Values represent the mean ± standard error (n = 6) of the samples. Significant difference between Control and Diabetic groups: ****p < 0.001, **p < 0.01. Significant difference between Diabetic and Dia + GNE, Dia + GO, Dia + Ator: a*p < 0.05
The IPITT in diabetic rats showed a significant increase in blood glucose level with significant increase in AUC by 5.55-fold as compared to the control group. The oral administration of GNE significantly decreased the AUC by 1.71-fold (p < 0.05) when compared to the diabetic group. However, the oral administration of GO or Ator in the diabetic rats did not show any effect in the IPITT in comparison with the diabetic group (Fig. 1c, d).
The OGTT affirmed the insulin resistance in the diabetic rats with declined pancreatic function and compensatory hyperinsulinemia, which is a major feature of T2DM (Srinivasan et al. 2005; Morakinyo et al. 2014). The IPITT is a valuable tool to assess the basal insulin insensitivity, the elevated AUC in IPITT confirms the resistance toward insulin (Morakinyo et al. 2014). Earlier reports have shown that the diallyl trisulfide in GO improved insulin insensitivity at a concentration of 40 mg/kg body weight in diabetic male Wistar rats (Liu et al. 2005). However, our study did not show any effect on glucose intolerance observed in the diabetic group, which may be due to the minimal (13%) concentration of diallyl trisulfide present in GO. However, the efficacy of GO improved in nanoemulsified form as the oral administration of GNE was effective in reverting the glucose intolerance and insulin resistance. Atorvastatin significantly ameliorated AUC in OGTT in diabetic patients but did not show any effect in IPITT, which was consistent with the previous study where atorvastatin showed no effect on the insulinogenic index (Huptas et al. 2006).
Effect of GNE on serum lipid profile
The serum lipid profile was examined at the end of every month (data not shown). At the end of the fifth month the TC, TG, LDL-C increased significantly in diabetic rats by 1.2-fold (p < 0.001), 4.7-fold (p < 0.001) and 9.7-fold (p < 0.01), respectively, with a subsequent decrease in the HDL-C level by 1.6-fold (p < 0.05). The elevated TG, TC and LDL-C in diabetic group implies diabetic dyslipidemia, which is caused due to insulin resistance that impairs the key enzymes and pathways involved in lipid metabolism (Table 2) (Ozder et al. 2014).
Table 2.
Serum lipid profile: control, diabetic, diabetic rats treated with nanoemulsified garlic oil blend (Dia + GNE) and diabetic rats treated garlic oil blend (Dia + GO), diabetic rats treated with atorvastatin (Dia + Ator)
| Con | Dia | Dia + GNE | Dia + GO | Dia + Ator | |
|---|---|---|---|---|---|
| TC | 88 ± 1.0 | 103 ± 2.0a** | 74 ± 0.9b** | 80 ± 1.7b* | 77.6 ± 1.0b** |
| TG | 60.8 ± 0.9 | 285.3 ± 3.3a*** | 57 ± 1.7b**** | 74 ± 0.7b***c* | 57 ± 1.4b****d* |
| HDL-C | 61 ± 1.3 | 39 ± 1.1a* | 48 ± 1.1b* | 44 ± 1.5 | 47 ± 1.0b** |
| LDL-C | 18.3 ± 2.5 | 178 ± 2.0a*** | 22.3 ± 2.2b**** | 37.3 ± 0.9b***c* | 23 ± 2.1b**** |
Values represent the mean ± standard error (n = 6) of the samples. Significant difference between Control and Diabetic groups: a*p < 0.05, a**p < 0.001, a***p < 0.001. Significant difference between Diabetic and Dia + GNE, Dia + GO, Dia + Ator: b*p < 0.05, b**p < 0.01, b***p < 0.001, b****p < 0.0001. Significant difference between Dia + GNE and Dia + GO, Dia + GNE and Dia + Ator: c*p < 0.05. Significant difference between Dia + GO and Dia + Ator: d*p < 0.05
However, the administration of GNE or Ator significantly reduced the TC by 1.4-fold (p < 0.0001) and 1.33-fold (p < 0.0001) than GO administration. Similarly, the TG and LDL-C decreased by 5.0-fold (p < 0.0001) and 8.0-fold (p < 0.05), respectively, on administering GNE than GO. The HDL-C increased significantly in GNE or Ator-administered groups (p < 0.01) than the GO-administered group. The results were consistent with our previous findings where GNE was highly effective in preventing and treating dyslipidemia in HFD fed rats. Further, our study remains the first in examining the effect of GNE in diabetic dyslipidemia-mediated nephropathy in Wistar rats.
Effect of GNE on urine and serum albumin and protein
A gradual increase in urinary albumin concomitant with a decrease in the serum albumin was observed in the diabetic group on disease progression from third till the fifth month end (Table 3). A significant increase by 8.3-fold (p < 0.01) and 11.99-fold (p < 0.01) in urinary albumin level was observed at third and fifth month end, respectively, in comparison with the control group. Subsequently, the diabetic group at the end of the third and fifth month showed 1.20-fold (p < 0.05) and 1.35-fold (p < 0.01) decrease in serum albumin than the control group. However, the GNE administration significantly decreased the urinary albumin level in diabetic rats by 2.89-fold (p < 0.01) at the third month end in correlation with the GO administration. At the fifth month end, the GNE administration decreased the urinary albumin level by 5.82-fold (p < 0.01) than GO (p < 0.05) or Ator (p < 0.05)-administered groups. Moreover, the serum albumin increased significantly in the GNE-administered group (1.09-fold, p < 0.001) than the GO or Ator-administered diabetic group at the end of the third month end. At the fifth month end, 1.35-fold (p < 0.01) increase in the serum albumin was shown than the GO (1.26-fold, p < 0.05) or Ator (1.12-fold, p < 0.05)-administered diabetic group.
Table 3.
Serum and urinary albumin and microprotein: control, diabetic, diabetic rats treated with nanoemulsified garlic oil blend (Dia + GNE) and diabetic rats treated garlic oil blend (Dia + GO), diabetic rats treated with atorvastatin (Dia + Ator)
| Urine and serum parameters | Control | Diabetic | Dia + GNE | Dia + GO | Dia + Ator |
|---|---|---|---|---|---|
| Urinary albumin: creatinine ratio | |||||
| M1 | 40.5 ± 3.52 | 97.3 ± 5.02a* | 101 ± 3.4 | 109.3 ± 3.7 | 114.3 ± 4.69 |
| M3 | 42 ± 2.2 | 355 ± 14.5a** | 123 ± 3.4b** | 341 ± 5.4c*** | 215 ± 12c* |
| M5 | 43 ± 2.3 | 513 ± 30.2a** | 88 ± 6.25b** | 201 ± 13.1b*c* | 250.3 ± 9.0b*c** |
| Urinary microprotein: creatinine ratio | |||||
| M1 | 296 ± 3.92 | 755 ± 24.8a** | 547 ± 6.13b* | 700.2 ± 14.8c** | 282 ± 10.1b**c***d*** |
| M3 | 296 ± 3.53 | 1189 ± 33.4a*** | 932 ± 4.02b* | 931.5 ± 7.02b* | 906.2 ± 10.8b*c*d* |
| M5 | 298 ± 1.82 | 1907 ± 35.6a*** | 518 ± 9.09b*** | 623 ± 4.6b***c* | 1100 ± 8.76b**c****d**** |
| Serum albumin | |||||
| M1 | 4.1 ± 0.04 | 3.8 ± 0.04 | 3.6 ± 0.036 | 3.8 ± 0.03 | 3.39 ± 0.02 |
| M3 | 4.20 ± 0.04 | 3.5 ± 0.04a* | 3.8 ± 0.03b*** | 3.5 ± 0.06 | 3.24 ± 0.02c*d** |
| M5 | 4.05 ± 0.05 | 2.3 ± 0.05a** | 4.05 ± 0.03b** | 3.8 ± 0.044b* | 3.4 ± 0.04b*c*** |
| Serum protein | |||||
| M1 | 8.05 ± 0.05 | 7.7 ± 0.051a* | 7.5 ± 0.02 | 7.3 ± 0.023 | 7.2 ± 0.04 |
| M3 | 8.23 ± 0.04 | 6.77 ± 0.054a** | 6.8 ± 0.03 | 6.4 ± 0.016 | 6.6 ± 0.02 |
| M5 | 8.01 ± 0.04 | 6.4 ± 0.05a*** | 7.6 ± 0.03b** | 7.4 ± 0.016b*c* | 7.2 ± 0.02b*c*d** |
M Month. Values represent the mean ± standard error (n = 6) of the samples. Significant difference between Control and Diabetic groups: a*p < 0.05, a**p < 0.001, a***p < 0.001. Significant difference between Diabetic and Dia + GNE, Dia + GO, Dia + Ator: b*p < 0.05, b**p < 0.01, b***p < 0.001. Significant difference between Dia + GNE and Dia + GO, Dia + GNE and Dia + Ator: c*p < 0.05, c**p < 0.01, c***p < 0.001, c****p < 0.0001. Significant difference between Dia + GO and Dia + Ator: d**p < 0.01, d***p < 0.001, d****p < 0.0001
A gradual elevation in the microprotein excretion level was observed on increase in number of months in the diabetic group than the control group (Table 3). In the diabetic group, at the first, third and fifth month end the urinary microprotein level exceeded by 2.55-fold, 4.01-fold and 6.40-fold, respectively, than the control group. Correspondingly, the serum protein decreased by 1.25-fold (p < 0.0001) at the fifth month end in the diabetic group as compared to the control group. However, the oral administration of GNE significantly attenuated the microprotein excretion at the end of the first month by 1.38-fold (p < 0.01) than the GO-administered group. Similarly, at the end of the third month, the GNE or GO or Ator administration significantly decreased the urinary microprotein level. At the fifth month end, the diabetic rats administered with GNE or GO rats showed a significantly decreased microprotein excretion by 3.69-fold (p < 0.001) and 3.06-fold (p < 0.001), respectively. The decrease in urinary microprotein was associated with an increase in serum protein levels. The GNE administration significantly increased the serum protein by 1.17-fold (p < 0.01) than the GO (1.15-fold, p < 0.05) or Ator (1.12, p < 0.05)-administered group (Table 3).
In diabetic rats, albuminuria was noticed at the end of the third month with reduced serum albumin and protein level. Albuminuria is observed as a prominent biomarker for nephropathy that ensures endothelial dysfunction in the kidney (Huang et al. 2017). Proteinuria is recognized as a non-hemodynamic promoter of progressive stages of diabetic nephropathy, where the glomerular hyperfiltration and albuminuria precedes elevated proteinuria (Williams 2005). In T2DM patients, individuals with ESRD, macroalbuminuria, and microalbuminuria were found to have significantly low serum albumin levels along with decreased creatinine clearance level than normoalbuminuric patients (Viswanathan et al. 2004). Further, the improved potential of GNE in ameliorating albuminuria and proteinuria than GO or Ator was observed. The findings were consistent with our previous study where GNE administration efficiently attenuated microalbuminuria and proteinuria observed in high-fat diet-fed pre-diabetic Wistar rats (Yuvashree et al. 2018). The diallyl disulfide and diallyl trisulfide in GO possess antioxidant activity that suppresses the basement membrane thickening and proteinuria. The improved functioning of the liver and other organs on the GO administration also would have increased the plasma protein level (Hassan et al. 2009).
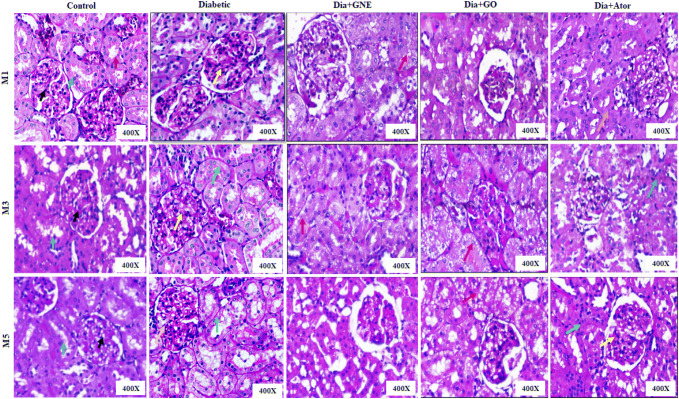
Histopathological analysis
The PAS staining was carried out in the renal sections of all the experimental groups (Fig. 2). The histopathological analysis revealed a mild increase in mesangial cellularity and mesangial expansion at the first month end in diabetic rats, which were reverted to normal on administering GNE or GO or Ator. However, a mild injury in the tubules was observed in the Ator-administered group. At the end of the third month, the renal sections of diabetic rats showed a prominent mesangial expansion and proliferation with protein reabsorption droplets. The mesangial expansion and hyperplasia led to bursting of the capillary lumen and an increase in the glomerular volume (Pourghasem et al. 2015). The elevated protein in the urinary space led to excessive protein reabsorption in the proximal convoluted tubules, which further forms proteinaceous casts leading to tubular dilatation and obstruction. The protein accumulation in the interstitium provokes inflammatory reactions causing the loss of tubular basement integrity (Campion et al. 2017). The GNE or GO administration substantially reverted the mesangial expansion and protein reabsorption observed in the diabetic rats. However, GO administration developed severe vacuolization in the tubules, which was reduced on administering GNE. The protein reabsorption droplets with tubular injury were observed in the Ator-administered group.
Fig. 2.
Renal histopathology images of control, diabetic rats, diabetic rats treated with nanoemulsified garlic oil blend (Dia + GNE), diabetic rats treated garlic oil blend (Dia + GO), and diabetic rats treated with atorvastatin (Dia + Ator) by Periodic acid-Schiff (PAS) staining. M represents month. In control, red arrow indicates distal convoluted tubules, blue arrow indicates proximal convoluted tubules and black arrow indicates mesangium. In diabetic—yellow arrow indicates mesangial proliferation and expansion in M1, green arrow indicates tubular atrophy and yellow arrow indicates mesangial expansion in M3 and in M5 yellow arrow indicates glomerulosclerosis and green arrow indicates tubular basement membrane thickening. Red arrow in Dia + GNE and Dia + GO indicates vacuolization. In Dia + Ator—orange arrow indicates tubular injury in M1, green arrow indicates protein reabsorption in M3, green arrow indicates acute tubular injury and yellow arrow indicates mesangial expansion in M5
At the end of the fifth month, glomerulosclerosis with tubular atrophy was seen in the diabetic group, which is consistent with the previous findings where the heavy proteinuria is associated with diffuse or nodular form of glomerulosclerosis (Williams 2005). A significant recovery of glomerular and tubular injury was observed in the GNE-administered group as observed with our previous findings in pre-diabetic rats with microalbuminuria (Yuvashree et al. 2018). However, mild transient isometric vacuolization was persistent in the tubules of GO-administered group at the end of the fifth month which was found have decreased to a higher degree in the GNE-treated group (Fig. 2). The Ator-administered group showed mild mesangial expansion and proliferation with acute tubular injury (Fig. 2).
Effect of GNE on urinary podocalyxin, NGAL and CD36 excretion
The urinary podocalyxin level increased on disease progression from the first month till the fifth month in the diabetic group than the control group (Fig. 3a). As podocalyxin is present in the apical surface nearer to the capsular space, the loss of podocalyxin is reflected highly in the urine (Hara et al. 2005). The diabetic rats showed a significant increase in urinary podocalyxin by 1.18-fold (p < 0.05), 1.36-fold (p < 0.01) and 1.56-fold (p < 0.01) at the end of the first, third and fifth month, respectively. Podocyte injury indicated by higher urinary podocalyxin excretion was observed even in normoalbuminuric subjects, which was manifested in our study (Hara et al. 2012). The GNE administration significantly decreased the urinary podocalyxin level by 1.21-fold (p < 0.05) than GO or Ator administration at the end of the third month. Similarly, at the fifth month end, a significant decrease in podocalyxin excretion by 1.32-fold (p < 0.05) was observed in GNE than the GO (1.22-fold, p < 0.05) or Ator-administered group. This evidence supports the enhanced efficacy of GO on nanoemulsification in preventing the progression of glomerular injury observed in nephropathy.
Fig. 3.
Urinary podocalyxin, NGAL and CD36 excretion by Indirect ELISA in control, diabetic, diabetic rats treated with nanoemulsified garlic oil blend (Dia + GNE), diabetic rats treated garlic oil blend (Dia + GO) and diabetic rats treated with atorvastatin (Dia + Ator). a Urinary podocalyxin, b urinary NGAL and c urinary CD36 level. Values represent the mean ± standard error (n = 6) of the samples. Significant difference between Control and Diabetic groups: a*p < 0.05, a**p < 0.01. Significant difference between Diabetic and Dia + GNE, Dia + GO, Dia + Ator: b*p < 0.05, b**p < 0.01. Significant difference between Dia + GNE and Dia + GO, Dia + GNE and Dia + Ator: c*p < 0.05. Significant difference between Dia + GO and Dia + Ator: d*p < 0.05
Besides, the diabetic group showed a significant increase in the urinary NGAL level on disease progression (Fig. 3b). In diabetic group, at the end of the first, third and fifth month a significant increase in urinary NGAL by 1.17-fold (p < 0.05), 1.42-fold (p < 0.05) and 1.70-fold (p < 0.01) was observed than the control group. The increased urinary NGAL observed at the end of the first month of diabetes is consistent with the previous report, where urinary NGAL levels increased significantly after seven days of diabetes induction (Arellano-Buendía et al. 2014). This elevated urinary NGAL at the early stage of diabetes reflects the incidence of tubular functional changes ahead of perceptible glomerular lesions or tubular structural changes (Liu et al. 2015). The GNE or Ator-administered group significantly attenuated the urinary NGAL level by 1.02-fold (p < 0.05) and 1.04-fold (p < 0.05) respectively in diabetic rats at the end of the first month than the GO-administered group. However, at the end of the third month the treatments groups showed no significant effect on the urinary NGAL excretion. At the end of the fifth month, the GNE administration showed a significant decrease in NGAL excretion by 1.56-fold (p < 0.01) than GO (1.45-fold, p < 0.05) or Ator-administered group. These results evidenced the improved potential of GNE than GO in alleviating the tubular injury marker, urinary NGAL.
Besides, the urinary CD36 level also elevated significantly in the diabetic group by 1.30-fold (p < 0.05) at the end of the first month which gradually increased to 1.56-fold (p < 0.01) and 2.37-fold (p < 0.01) by third and fifth month end, respectively (Fig. 3c). The circulating soluble form of CD36 in plasma is recognized as a diagnostic marker for type 2 diabetes (Alkhatatbeh et al. 2013). Our results were consistent with the previous finding from our lab where the soluble CD36 level elevated in plasma and urine of DN patients and were linked to albuminuria (Shiju et al. 2015). However, the GNE administration significantly attenuated the urinary CD36 excretion by 1.21-fold (p < 0.05) than GO or Ator-treated diabetic rats at the end of the third month. Similarly, at the end of the fifth month the GNE-administered group showed a significant decrease in CD36 excretion by 1.94-fold (p < 0.01) than GO (1.51-fold, p < 0.05) or Ator (1.49-fold, p < 0.05)-administered diabetic rats. Our study is the foremost in demonstrating the significant effect of GNE on urinary CD36 in diabetic nephropathy. Thus, GO showed an improved effect on urinary excretion of podocalyxin, NGAL and CD36 on nanoemulsification from an early stage of the disease (Fig. 3).
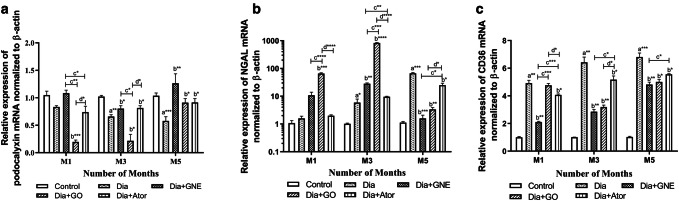
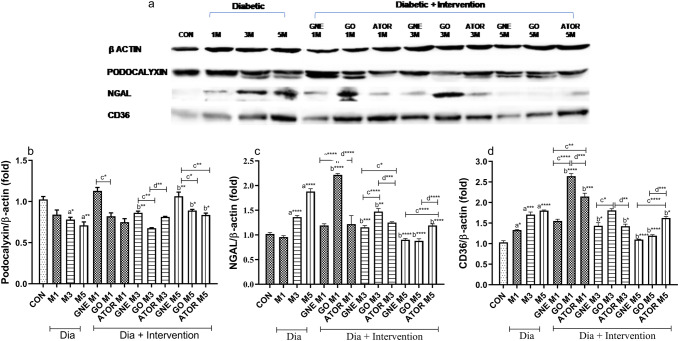
GNE potency toward expression of renal podocalyxin mRNA and protein
In the diabetic group, the renal podocalyxin mRNA downregulated at the end of the third (p < 0.01) and fifth month (p < 0.001) than the control group. Besides, a substantial decrease in the renal podocalyxin expression in diabetic rats in the third (p < 0.05) and fifth month end (p < 0.01) was observed than the control group (Figs. 4a, 5a). Consistent with our results, Zhai et al. (2015) have demonstrated that in type 2 diabetic rats, mRNA and protein levels of renal podocalyxin reduced significantly (Zhai et al. 2015).
Fig. 4.
Relative mRNA expression of podocalyxin, NGAL and CD36 in renal tissues of Control, Diabetic, Diabetic rats treated with nanoemulsified garlic oil blend (Dia + GNE), Diabetic rats treated garlic oil blend (Dia + GO), and Diabetic rats treated with atorvastatin (Dia + Ator). a Podocalyxin, b NGAL and c CD36 mRNA expression. Values represent the mean ± standard error (n = 6) of the samples. Significant difference between Control and Diabetic groups: a*p < 0.05, a**p < 0.01, a***p < 0.001. Significant difference between Diabetic and Dia + GNE, Dia + GO, Dia + Ator: b*p < 0.05, b**p < 0.01, b***p < 0.001, b****p < 0.0001. Significant difference between Dia + GNE and Dia + GO, Dia + GNE and Dia + Ator: c*p < 0.05, c**p < 0.01 c***p < 0.001, c****p < 0.0001. Significant difference between Dia + GO and Dia + Ator: d*p < 0.05, d****p < 0.0001
Fig. 5.
Protein expression of podocalyxin, NGAL and CD36 in renal tissues of control, diabetic, diabetic rats treated with nanoemulsified garlic oil blend (Dia + GNE), Diabetic rats treated garlic oil blend (Dia + GO) and Diabetic rats treated with atorvastatin (Dia + Ator). a Band signal developed using EL system, b renal podocalyxin expression, c renal NGAL expression and d renal CD36 expression normalized to that of β-actin. Results were expressed as mean SEM of three observations. D represents diabetic, M represents month. Significant difference between Control and Diabetic groups: a*p < 0.05, a**p < 0.01, a***p < 0.001, a****p < 0.0001. Significant difference between Dia and Dia + GNE, Dia and Dia + GO, Dia and Dia + Ator: b*p < 0.05, b**p < 0.01, b***p < 0.001, b****p < 0.0001. Significant difference between Dia + GNE and Dia + GO, Dia + GNE and Dia + Ator: c*p < 0.05, c**p < 0.01, c****p < 0.0001. Significant difference between Dia + GO and Dia + Ator: d**p < 0.01, d***p < 0.001, d****p < 0.0001
On oral administration of GNE, the podocalyxin mRNA and protein expression increased significantly (p < 0.01) in diabetic rats at the end of the third month than the GO-administered group. At the end of the fifth month, the mRNA and protein expression of podocalyxin increased significantly (p < 0.01) on GNE administration than the GO (p < 0.05) or Ator (p < 0.05) administration. In contrary, the GO administration showed a substantial downregulation of podocalyxin mRNA at the end of the first and third month in diabetic rats, but it was not correlated with the protein level (Figs. 4a, 5a, b). The poor correlation between mRNA and protein levels can be due to several determining factors like post-translational modifications, protein half-life and the biological differences in the abundance of mRNA and protein (Larsson et al. 2012). Further, our results confirmed the positive effect of GNE in reverting the glomerular dysfunction in diabetic rats from the early phase of DN, which was observed in GO-administered group at later stages. Thus, the nanoform improved the potency of garlic oil in reverting the glomerular dysfunction from an early stage of the disease.
GNE potency toward expression of renal NGAL mRNA and protein
The tissue NGAL mRNA increased substantially at the end of the third (p < 0.01) and fifth month end (p < 0.001) of the diabetic group than the control group with a significant increase in the renal NGAL protein level at the end of the third (p < 0.0001) and fifth month (p < 0.0001) in diabetic rats (Figs. 4b, 5a, c). Consistent with our results, NGAL mRNA expression showed a steady rise in diabetic rats based on the severity of kidney damage (Shiju et al. 2015).
The oral administration of GNE decreased the NGAL protein expression (p < 0.001) at the end of the third month, but no correlation was seen with the NGAL mRNA expression. The NGAL mRNA expression increased significantly on GO administration (p < 0.0001), but it was reduced in the GNE-administered group (p < 0.01) which implies the potency of GNE in reducing the toxicity observed with GO. In diabetic rats at the end of the fifth month, the GNE-administered group significantly decreased the protein (p < 0.0001) and mRNA expression (p < 0.001). Besides, a significant decrease was observed at the end of the fifth month in NGAL mRNA (p < 0.001) and protein (p < 0.0001) expression in the GNE-administered group. The GO or Ator-administered groups decreased the NGAL mRNA (p < 0.01 or p < 0.05, respectively) and protein expression (p < 0.0001 or p < 0.0001, respectively) at the end of the fifth month.
The ameliorative capacity of GNE on NGAL excretion, mRNA and protein expression manifested the potential of GNE in reverting the tubular injury in diabetic rats. The GO administration exacerbated tubular injury in the initial months as evidenced by the elevated renal NGAL expression. This transient tubular injury could be drug-induced, which correlated with the severe vacuolization observed in histopathological analysis (Singh et al. 2003). However, the tubular injury was reduced in GNE-administered group as compared to the GO-administered group. It was demonstrated that the nanoemulsified form of vegetable oils not only enhanced the bioavailability of active compounds but also minimized its toxicity and side effects facilitating the use of active substances at low concentrations (Bajerski et al. 2016). This shows that the toxic effects of GO were suppressed due to the nano emulsification process, which also improved the renal functioning by regulating the NGAL expression.
GNE potency toward expression of renal CD36 mRNA and protein
The CD36 mRNA expression upregulated significantly at the end of the first (p < 0.01), third (p < 0.01) and fifth month (p < 0.01) in the diabetic rats in comparison with the control rats with a concomitant upregulation in the protein expression at the end of the first (p < 0.05), third (p < 0.001) and fifth month (p < 0.0001) in the diabetic group than the control group (Figs. 4c, 5a, d). Consistent with our findings, previous reports showed that the elevated glucose increased the renal CD36 expression that caused lipid-mediated apoptosis, and the elevated soluble CD36 in type 2 diabetic subjects is accompanied with insulin resistance (Hou et al. 2018; Ekici et al. 2018). The GNE administration substantially downregulated CD36 mRNA levels at the first (p < 0.01), third- (p < 0.01) and fifth month end (p < 0.01) in diabetic rats. The GO or Ator-administered group decreased the CD36 mRNA expression at the third (p < 0.01) and fifth month end (p < 0.05) in diabetic rats. Moreover, the GNE administration significantly downregulated the CD36 protein expression at the third (p < 0.05) and fifth month end (p < 0.0001) in diabetic rats. Interestingly, the GO-treated group showed an increased CD36 expression in diabetic rats at the end of the first month. However, at the end of the fifth month, the GO (p < 0.0001) or Ator (p < 0.05)-administered groups decreased CD36 expression significantly in the diabetic rats.
Thus, our study is primary to demonstrate the efficacy of GNE in downregulating the CD36 expression from the early stage of DN. This signifies the improved therapeutic potency of GNE administration in attenuating the CD36 expression as compared to the GO administration. Henceforth, the nanoemulsion forms an ideal drug delivery system as it maximizes the beneficial effect and minimizes the toxicity of the bulk component (Chime et al. 2014).
Conclusion
Diabetic rats progress to nephropathy due to dyslipidemia-mediated hyperglycemic, hyperinsulinemic and dyslipidemic conditions. Our study for the first time manifested the effect of GNE or GO on CD36, podocalyxin and NGAL expression and urinary excretion in type 2 diabetic rats progressing to nephropathy. Moreover, our study focused on attenuating the hyperlipidemia-mediated DN rather than the glycemia-mediated DN which is evident from renal CD36 mRNA or protein expression. The GNE improved the effectiveness of its bulk form in attenuating the renal abnormalities in diabetic rats noticed in the progressive phase of DN, and also reduced the toxic effects of GO. Moreover, our study has shown that the GNE also has the potential to alleviate the renal injury that occurred in both early and later stages in nephropathy without any adverse effects, which is not observed with the synthetic drugs. Thus, the garlic oil blend in nanoemulsified form has better therapeutic relevance to nephropathy than garlic oil that possibly acted by reverting glomerular and tubular injury which is dependent on the downregulation of CD36 expression in type 2 diabetes-induced nephropathy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgement
The authors are thankful to the Council of Scientific and Industrial Research (09/844(0038)/2016 EMR-I) for funding aid and VIT, Vellore, for providing the research amenities.
Compliance with ethical standards
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- Abdel-Daim MM, Shaheen HM, Abushouk AI, Toraih EA, Fawzy MS, Alansari WS, Aleya L, Bungau S. Thymoquinone and diallyl sulfide protect against fipronil-induced oxidative injury in rats. Environ Sci Pollut Res. 2018;25(24):23909–23916. doi: 10.1007/s11356-018-2386-3. [DOI] [PubMed] [Google Scholar]
- Abdel-Daim MM, Abdel-Rahman HG, Dessouki AA, El-Far AH, Khodeer DM, Bin-Jumah M, Alhader MS, Alkahtani S, Aleya L. Impact of garlic (Allium sativum) oil on cisplatin-induced hepatorenal biochemical and histopathological alterations in rats. Sci Total Environ. 2020;25(710):136338. doi: 10.1016/j.scitotenv.2019.136338. [DOI] [PubMed] [Google Scholar]
- Aldukhayel A. Prevalence of diabetic nephropathy among Type 2 diabetic patients in some of the Arab countries. Int J Health Sci. 2017;11(1):1–4. [PMC free article] [PubMed] [Google Scholar]
- Alkhatatbeh MJ, Enjeti AK, Acharya S, Thorne RF, Lincz LF. The origin of circulating CD36 in type 2 diabetes. Nutr Diabetes. 2013;3(2013):e59. doi: 10.1038/nutd.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano-Buendía AS, García-Arroyo FE, Cristóbal-García M, Loredo-Mendoza ML, Tapia-Rodríguez E, Sánchez-Lozada LG, Osorio-Alonso H. Urinary excretion of neutrophil gelatinase-associated lipocalin in diabetic rats. Oxid Med Cell Longev. 2014;2014:961326. doi: 10.1155/2014/961326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajerski L, Michels LR, Colome LM, Bender EA, Freddo RJ, Bruxel F, Haas SE. The use of Brazilian vegetable oils in nanoemulsions: an update on preparation and biological applications. Braz J Pharm Sci. 2016;52:347–363. [Google Scholar]
- Campion CG, Sanchez-Ferras O, Batchu SN. Potential role of serum and urinary biomarkers in diagnosis and prognosis of diabetic nephropathy. Can J Kidney Health Dis. 2017;4:2054358117705371. doi: 10.1177/2054358117705371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chime SA, Kenechukwu FC, Attama AA. Nanoemulsions—advances in formulation, characterization and applications in drug delivery, application of nanotechnology in drug delivery, Ali Demir Sezer. IntechOpen. 2014 doi: 10.5772/58673. [DOI] [Google Scholar]
- Cretu R, Solea LC. Zeta potential and color investigations of vegetable oil based emulsions as eco-friendly lubricants. Scientific Study & Research. Chem Chem Eng Biotechnol Food Ind. 2017;18(2):167–180. [Google Scholar]
- Ekici M, Kisa U, Arikan Durmaz S, Ugur E, Nergiz-Unal R. Fatty acid transport receptor soluble CD36 and dietary fatty acid pattern in type 2 diabetic patients: a comparative study. Br J Nutr. 2018;119(2):153–162. doi: 10.1017/S0007114517003269. [DOI] [PubMed] [Google Scholar]
- Fang J, Wei H, Sun Y, Zhang X, Liu W, Chang Q, Wang R, Gong Y. Regulation of podocalyxin expression in the kidney of streptozotocin-induced diabetic rats with Chinese herbs (Yishen capsule) BMC Complement Altern Med. 2013;5(13):76. doi: 10.1186/1472-6882-13-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faran SA, Asghar S, Khalid SH, Khan IU, Asif M, Khalid I, Gohar UF, Hussain T. Hepatoprotective and renoprotective properties of lovastatin-loaded ginger and garlic oil nanoemulsomes: Insights into serum biological parameters. Medicina (Lithuania) 2019;25(710):136338. doi: 10.3390/medicina55090579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Gu C, Li Y, Huang J. High glucose promotes CD36 expression by upregulating peroxisome proliferator-activated receptor γ levels to exacerbate lipid deposition in renal tubular cells. BioMed Res Int. 2017;2017(2017):1414070. doi: 10.1155/2017/1414070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Gu C, Li Y, Huang J. High glucose promotes CD36 expression by upregulating peroxisome proliferator-activated receptor γ levels to exacerbate lipid deposition in renal tubular cells. Biomed Res Int. 2017;2017:1414070. doi: 10.1155/2017/1414070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiseha T, Tamir Z. Urinary markers of tubular injury in early diabetic nephropathy. Int J Nephrol. 2016;2016(2016):4647685. doi: 10.1155/2016/4647685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraulab JC, Ogg-Diamantino R, Fernandes-Santos C, Aguila MB, Mandarim-de-Lacerda A. A mouse model of metabolic syndrome: insulin resistance, fatty liver and non-alcoholic fatty pancreas disease (NAFPD) in C57BL/6 mice fed a a high fat diet. J Clin Biochem Nutr. 2010;46(3):212–223. doi: 10.3164/jcbn.09-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneda M, Utsunomiya K, Koya D, Babazono T, Moriya T, Makino H, Kimura K, Suzuki Y, Wada T, Ogawa S, Inaba M, Kanno Y, Shigematsu T, Masakane I, Tsuchiya K, Honda K, Ichikawa K, Shide K. A new classification of diabetic nephropathy 2014: a report from Joint Committee on Diabetic Nephropathy. J Diabetes Investig. 2015;6(2):242–246. doi: 10.1111/jdi.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M, Yanagihara T, Kihara I. Apical cell membranes are shed into urine from injured podocytes: a novel phenomenon of Pod injury. J Am Soc Nephrol. 2005;16(2):408–416. doi: 10.1681/ASN.2004070564. [DOI] [PubMed] [Google Scholar]
- Hara M, Yamagata K, Tomino Y, Saito A, Hirayama Y, Ogasawara S, Kurosawa H, Sekine S, Yan K. Urinary podocalyxin is an early marker for podocyte injury in patients with diabetes: establishment of a highly sensitive ELISA to detect urinary podocalyxin. Diabetologia. 2012;55(11):2913–2919. doi: 10.1007/s00125-012-2661-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan HA, El-Agmy SM, Gaur RL, Fernando A, Raj MH, Ouhtit A. In vivo evidence of hepato- and reno-protective effect of garlic oil against sodium nitrite-induced oxidative stress. Int J Biol Sci. 2009;5(3):249–255. doi: 10.7150/ijbs.5.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman-Edelstein M, Scherzer P, Tobar A, Levi M, Gafter U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J Lipid Res. 2014;55(3):561–572. doi: 10.1194/jlr.P040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Shi Y, Han B, Liu X, Qiao X, Qi Y, Wang L. The antioxidant peptide SS31 prevents oxidative stress, downregulates CD36 and improves renalfunction in diabetic nephropathy. Nephrol Dial Transplant. 2018;33(11):1908–1918. doi: 10.1093/ndt/gfy021. [DOI] [PubMed] [Google Scholar]
- Hua W, Huang HZ, Tan LT, Wan JM, Gui HB, Zhao L, Ruan XZ, Chen XM, Du XG. CD36 mediated fatty acid-induced podocyte apoptosis via oxidative stress. PLoS ONE. 2015;10:e0127507. doi: 10.1371/journal.pone.0127507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MJ, Wei RB, Zhao J, Su TY, Li QP, Yang X, Chen XM. Albuminuria and endothelial dysfunction in patients with non-diabetic chronic kidney disease. Med Sci Monit. 2017;23:4447–4453. doi: 10.12659/MSM.903660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huptas S, Geiss HC, Otto C, Parhofer KG. Effect of atorvastatin (10 mg/day) on glucose metabolism in patients with the metabolic syndrome. Am J Cardiol. 2006;98(1):66–69. doi: 10.1016/j.amjcard.2006.01.055. [DOI] [PubMed] [Google Scholar]
- Jay AG, Chen AN, Paz MA, Hung JP, Hamilton JA. CD36 binds oxidized low density lipoprotein (LDL) in a mechanism dependent upon fatty acid binding. J Biol Chem. 2015;290(8):4590–4603. doi: 10.1074/jbc.M114.627026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanami D, Matoba K, Utsunomiya K. Dyslipidemia in diabetic nephropathy. Ren Replace Ther. 2016;2:16. doi: 10.1186/s41100-016-0028-0. [DOI] [Google Scholar]
- Kiran G, Nandini CD, Ramesh HP, Salimath PV. Progression of early phase diabetic nephropathy in streptozotocin-induced diabetic rats: evaluation of various kidney-related parameters. Indian J Exp Biol. 2012;50(2012):133–140. [PubMed] [Google Scholar]
- Koenig R. Indirect ELISA methods for the broad specificity detection of plant viruses. J Gen Virol. 1981;55(1):53–62. [Google Scholar]
- Kostovska I, Trajkovska K, Cekovska S, Spasovski G, Labudovic D. Nephrin and podocalyxin—new podocyte proteins for early detection of secondary nephropathies. BANTAO J. 2016;14(1):11–16. [Google Scholar]
- Lai YS, Chen WC, Ho CT, Lu KH, Lin SH, Tseng HC, Lin SY, Sheen LY. Garlic essential oil protects against obesity-triggered nonalcoholic fatty liver disease through modulation of lipid metabolism and oxidative stress. J Agric Food Chem. 2014;62(2014):5897–5906. doi: 10.1021/jf500803c. [DOI] [PubMed] [Google Scholar]
- Lamacchia O, Nicastro V, Camarchio D, Valente U, Grisorio R, Gesualdo L, Cignarelli M. Para- and perirenal fat thickness is an independent predictor of chronic kidney disease, increased renal resistance index and hyperuricaemia in type-2 diabetic patients’. Nephrol Dial Transplant. 2011;26(3):892–898. doi: 10.1093/ndt/gfq522. [DOI] [PubMed] [Google Scholar]
- Larsson I, Fridberg M, Gaber A, Nodin B, Leveen P, Jonsson G, Uhlen M, Birgisson H, Jirstrom K. Validation of podocalyxin-like protein as a biomarker of poor prognosis in colorectal cancer. BMC Cancer. 2012;12:282. doi: 10.1186/1471-2407-12-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Lee JH, Kim CE, Yang JW. Anti-neovascular effect of chondrocyte-derived extracellular matrix on corneal alkaline burns in rabbits. Graefes Arch Clin Exp Ophthalmol. 2014;252(6):951–961. doi: 10.1007/s00417-014-2633-3. [DOI] [PubMed] [Google Scholar]
- Lei YP, Liu CT, Sheen LY, Chen HW, Lii CK. Diallyl disulfide and diallyl trisulfide protect endothelial nitric oxide synthase against damage by oxidized low-density lipoprotein. Mol Nutr Food Res. 2010;54(Suppl 1):S42–S52. doi: 10.1002/mnfr.200900278. [DOI] [PubMed] [Google Scholar]
- Li JJ, Kwak SJ, Jung DS, Kim JJ, Yoo TH, Ryu DR, Han SH, Choi HY, Lee JE, Moon SJ, Kim DK, Han DS, Kang SW. Podocyte biology in diabetic nephropathy. Kidney Int Suppl. 2007;106:S36–S42. doi: 10.1038/sj.ki.5002384. [DOI] [PubMed] [Google Scholar]
- Lin SC, Chagnaadorj A, Bayarsengee U, Leung TK, Cheng CW. The compound, diallyl disulfide, enriched in garlic, prevents the progression of doxorubicin-induced nephropathy. Food Sci Technol. 2019 doi: 10.1590/fst.15418. [DOI] [Google Scholar]
- Ling L, Chen L, Zhang C, Gui S, Zhao H, Li Z. High glucose induces podocyte epithelial-to-mesenchymal transition by demethylation-mediated enhancement of MMP9 expression. Mol Med Rep. 2018;17(4):5642–5651. doi: 10.3892/mmr.2018.8554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CT, Hse H, Lii CK, Chen PS, Sheen LY. Effects of garlic oil and diallyl trisulfide on glycemic control in diabetic rats. Eur J Pharmacol. 2005;516(2):165–173. doi: 10.1016/j.ejphar.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Liu CT, Hsu TW, Chen KM, Tan YP, Lii CK, Sheen LY. The antidiabetic effect of garlic oil is associated with ameliorated oxidative stress but not ameliorated level of pro-inflammatory cytokines in skeletal muscle of streptozotocin-induced diabetic rats. J Tradit Complement Med. 2012;2(2):135–144. doi: 10.1016/s2225-4110(16)30087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Yang H, Chen H, Zhang M, Ma Q. High expression of neutrophil gelatinase-associated lipocalin (NGAL) in the kidney proximal tubules of diabetic rats. Adv Med Sci. 2015;60(1):133–138. doi: 10.1016/j.advms.2015.01.001. [DOI] [PubMed] [Google Scholar]
- McClements DJ, Rao J. Food-grade nanoemulsions: formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit Rev Food Sci Nutr. 2011;51(4):285–330. doi: 10.1080/10408398.2011.559558. [DOI] [PubMed] [Google Scholar]
- Morakinyo AO, Adekunbi DA, Dada KA, Adegoke OA. Testosterone promotes glucose intolerance, lipid disorder and oxidative stress in type 1 diabetic rats’. JBCPP. 2014;25(1):13–20. doi: 10.1515/jbcpp-2012-0072. [DOI] [PubMed] [Google Scholar]
- Ozder A. Lipid profile abnormalities seen in T2DM patients in primary healthcare in Turkey: a cross-sectional study. Lipids Health Dis. 2014;2014(13):183. doi: 10.1186/1476-511X-13-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou-Marketou N, Margeli A, Papassotiriou I, Chrousos GP, Kanaka-Gantenbein C, Wahlberg J. NGAL as an early predictive marker of diabetic nephropathy in children and young adults with type 1 diabetes mellitus. J Diabetes Res. 2017;2017:7526919. doi: 10.1155/2017/7526919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourghasem M, Shafi H, Babazadeh Z. Histological changes of kidney in diabetic nephropath. Caspian J Intern Med. 2015;6(3):120–127. [PMC free article] [PubMed] [Google Scholar]
- Qi J, Xiao YF, Zhang DJ, Yang GR, Huang HC. High glucose downregulates the expression of podocalyxin protein in glomerular podocytes of mice. Beijing Da Xue Xue Bao Yi Xue Ban. 2007;39:167–170. [PubMed] [Google Scholar]
- Ragavan G, Muralidaran Y, Sridharan B, Nachiappa Ganesh R, Viswanathan P. Evaluation of garlic oil in nano-emulsified form: optimization and its efficacy in high-fat diet induced dyslipidemia in Wistar rats. Food Chem Toxicol. 2017;105:203–213. doi: 10.1016/j.fct.2017.04.019. [DOI] [PubMed] [Google Scholar]
- Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. J Clin Investig. 2014;124(6):2333–2340. doi: 10.1172/JCI72271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satirapoj B. Tubulointerstitial biomarkers for diabetic nephropathy. J Diabetes Res. 2018;2018:2852398. doi: 10.1155/2018/2852398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah RV, Murthy VL, Abbasi SA, Blankstein R, Kwong RY, Goldfine AB, Jerosch-Herold M, Lima JA, Ding J, Allison MA. Visceral adiposity and the risk of metabolic syndrome across body mass index: the MESA study. JACC Cardiovasc Imaging. 2014;7(12):1221–1235. doi: 10.1016/j.jcmg.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J, Nair AB, Jacob S, Patel RK, Shah H, Shehata TM, Morsy MA. Nanoemulsion based vehicle for effective ocular delivery of moxifloxacin using experimental design and pharmacokinetic study in rabbits. Pharmaceutics. 2019;11(5):E230. doi: 10.3390/pharmaceutics11050230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiju TM, Rajesh NG, Viswanathan P. Renoprotective effect of aged garlic extract in streptozotocin-induced diabetic rats. Indian J Pharmacol. 2013;45(1):18–23. doi: 10.4103/0253-7613.106429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiju TM, Mohan V, Balasubramanyam M, Viswanathan P. Soluble CD36 in plasma and urine: A plausible prognostic marker for diabetic nephropathy. J Diabetes Compl. 2015;29(3):400–406. doi: 10.1016/j.jdiacomp.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal. 2009;2:re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NP, Ganguli A, Prakash A. Drug-induced kidney diseases. J Assoc Phys India. 2003;51:970–979. [PubMed] [Google Scholar]
- Sonkodi S, Mogyorosi A. Treatment of diabetic nephropathy with angiotensin II blockers. Nephrol Dial Transplant. 2003;18(5):v21–v23. doi: 10.1093/ndt/gfg1037. [DOI] [PubMed] [Google Scholar]
- Sridharan B, Mehra Y, Ganesh RN, Viswanathan P. Regulation of urinary crystal inhibiting proteins and inflammatory genes by lemon peel extract and formulated citrus bioflavonoids on ethylene glycol induced urolithic rats. Food Chem Toxicol. 2016;94:75–84. doi: 10.1016/j.fct.2016.05.013. [DOI] [PubMed] [Google Scholar]
- Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52(4):313–320. doi: 10.1016/j.phrs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Harris RC. Role of endothelial nitric oxide synthase in diabetic nephropathy: lessons from diabetic eNOS knockout mice. J Diabetes Res. 2014;2014(2014):590541. doi: 10.1155/2014/590541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan V, Snehalatha C, Kumutha R, Jayaraman M, Ramachandran A. Serum albumin levels in different stages of type 2 diabetic nephropathy patients. Indian J Nephrol. 2004;14:89–92. [Google Scholar]
- Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- Williams ME. Diabetic nephropathy: the proteinuria hypothesis. Am J Nephrol. 2005;25(2):77–94. doi: 10.1159/000084286. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Sun H, Sun Z. Advanced glycation end products (AGEs) increase renal lipid accumulation: a pathogenic factor of diabetic nephropathy (DN) Lipids Health Dis. 2017;16(1):126. doi: 10.1186/s12944-017-0522-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuvashree M, Gokulakannan R, Ganesh RN, Viswanathan P. Enhanced therapeutic potency of nanoemulsified garlic oil blend towards renal abnormalities in pre-diabetic rats. Appl Biochem Biotechnol. 2018;188(2):338–356. doi: 10.1007/s12010-018-2919-8. [DOI] [PubMed] [Google Scholar]
- Zhai L, Gu J, Yang D, Wang W, Ye S. Metformin ameliorates podocyte damage by restoring renal tissue podocalyxin expression in type 2 diabetic rats. J diabetes Res. 2015;2015:8. doi: 10.1155/2015/231825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ma KL, Liu J, Wu Y, Hu ZB, Liu L, Lu J, Zhang XL, Liu BC. Inflammatory stress exacerbates lipid accumulation and podocyte injuries in diabetic nephropathy. Acta Diabetol. 2015;52(6):1045–1056. doi: 10.1007/s00592-015-0753-9. [DOI] [PubMed] [Google Scholar]
- Zheng HM, Li HB, Wang DW, Liu D. Preparation methods for monodispersed garlic oil microspheres in water using the microemulsion technique and their potential as antimicrobials. J Food Sci. 2013;78(8):N1301–N1306. doi: 10.1111/1750-3841.12208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.