Abstract
Background:
Current in vivo adult stem cell delivery presents limited clinical effects due to poor engraftment and survival. To overcome current challenges in cell delivery and promote surgical cell delivery for soft tissue repair, a multi-spheroid-loaded thin sectioned acellular dermal matrix (tsADM) carrier which preserves loaded spheroids’ three-dimensional (3D) structure, was developed.
Methods:
Adipose-derived stem cells (ASCs) were used for spheroid delivery. After generating spheroids in 3D cell culture dishes, spheroid plasticity and survival in-between coverslips were evaluated. Spheroids were loaded onto tsADM, their shape changes were followed up for 14 days, and then imaged. Spheroid adhesion stability to tsADM against shear stress was also evaluated. Finally, cell delivery efficacy was compared with cell-seeded tsADM by in vivo implantation and histological evaluation.
Results:
Spheroids withstood cyclic compression stress and maintained their 3D shape without fusion after 48 h of culture in-between coverslips. Cell survival improved when spheroids were cultured on tsADM in-between the coverslips. Spheroid-loaded tsADM with coverslips maintained their spheroid outline for 14 days of culture whereas without coverslips, the group lost their outline due to spreading after 4 days in culture. Spheroids loaded onto tsADMs were more stable after six rather than 3 days in culture. Spheroid-loaded tsADMs showed about a 2.96-fold higher ASCs transplantation efficacy than cell-seeded tsADMs after 2 weeks of in vivo transplantation.
Conclusion:
These results indicate that transplantation of spheroid-loaded tsADMs significantly improved cell delivery. These findings suggest that a combined approach with other cells, drugs, and nanoparticles may improve cell delivery and therapeutic efficacy.
Keywords: Cellular spheroids, Adult stem cell, Acellular dermis, Stem cell transplantation, Cell culture techniques
Introduction
Since the demonstration of the extensive differentiating potential of adult stem cells and possibilities of lineage conversion between cells from different germ layers [1–3], diverse studies have shown promising results in clinical application of adult stem cells by obtaining autologous donor cells and injecting them for tissue regeneration [4–6]. However, the results showed limited clinical effects due to poor engraftment and survival of the delivered cells [7, 8]. Even as low as 1% of transplanted cell survival rate could be quantified [9]. Injury caused by cell detachment from the culture dish for in vitro preparation [10], barotrauma and shear stress within the cell transfer device, induced by syringes and needles [11, 12], new harsh environmental condition with inflammatory mediators, oxygen, and nutrient deprivation [13], lack of cell attachment to extracellular matrix induced apoptosis (anoikis) [14], mechanical loss [15] and missing target or unpredictable distribution [16] have been proposed for the cause of poor survival rates.
To clinically apply adult stem cells in soft tissue repair, those problems can be addressed by delivery approach, carrier properties, and loaded cells. Cells can be surgically delivered more precisely at the target, especially if they can be surgically fixed with sutures [17]. Surgical debridement and revascularization can be performed concurrently with cell delivery to improve the hostile environment of the recipient by removing necrotic tissue and improving vascularity, thereby increase cell survival in fresh tissues [18]. For the cell delivery carrier, the carrier should assimilate to soft tissue movement. Because surgical sites will have dissected pocket and external wounds, mismatches in mechanical properties between the carrier and soft tissue can result in carrier migration, extrusion, and covering tissue damage [19]. The carrier would be more suitable when it does not originate from foreign source material and does not have residues from chemical or biological modification. This suitability exists because the carrier itself may cause immunogenic problems and damage the transferred cells in addition to presenting concerns for patient safety and for obtaining approval for clinical application [20]. As a carrier, the material should secure the carrying cells and release them to the target tissue. It would be better to retain as much cell carrying capacity as possible until the tissue-specific required number of cells can be confirmed [21]. For the loaded cells, avoiding enzymatic treatment in harvesting cells from culture and preserving their extracellular matrices is essential for improving cell survival during cell delivery [10, 14].
It is hard to concurrently meet these requirements as a stem cell carrier; therefore, alternative methods have been developed. Currently, numerous biomaterials have been developed and used for cell delivery in order to provide a microenvironment for cell survival and function. Although these biomaterials are biocompatible and their material properties can be modified to assimilate to soft tissue movement, they are basically foreign source material. They still require complicated physical and chemical modification of the material itself, such as cross-linking, arginine/glycine/aspartic acid (RGD) modification, and porosity control, in order to enhance its function as a carrier [20]. Clinically, an allogenic acellular dermal matrix (ADM) have been layered beneath the grafted skin as a dermal supporting layer in skin graft surgery for decades [22]. Although this allograft material requires donation from a cadaver, and the supply of this material is limited, the supply of ADM is still relatively abundant compared to other allogenic grafts. It has been used for adipose-derived stem cell (ASC) delivery to facilitate wound healing; however, the number of transplanted cells was limited [23]. Methods avoiding enzymatic detachment of cells and allowing delivery of complete extracellular matrix have been developed [24]. Cell sheet delivery has the advantages of preserving the complete extracellular matrix without enzymatic detachment; however, it is not mechanically robust, and its cell-carrying capacity is also limited [25, 26].
To overcome those current challenges in cell delivery for soft tissue repair clinically, the authors developed cell carrier by following several principles: (1) to preserve cell adhesion and cellular function while avoiding cellular injury from cell detachment from cell culture dish, cells were delivered in the form of cellular spheroids; (2) to increase cell delivery capacity while preserving its function, multiple cellular spheroids were loaded onto a carrier material thus, preserving its three-dimensional (3D) shape with spaces for nutrient diffusion; and (3) the carrier material should be clinically proven and mechanically robust substance as it will be surgically fixed at the target tissue; it should contain non-foreign material that does not interfere with diffusion and not contain unnecessary chemicals.
The present study addresses a multi-spheroid carrier for cell delivery using ADM inspired by an egg carton. To apply adult stem cells in soft tissue repair, abundant, and clinically easy-to-harvest ASCs were used for loaded cells. The primary objective of this study was to load multiple ASC spheroids onto the ADM in order to preserve its 3D structure and securely attach to its carrier. Additionally, we evaluated the number of surviving and engrafted delivered cells using this multi-spheroid carrier as compared to a cell-seeded acellular dermal matrix for its cell delivery efficacy.
Materials and methods
Thin dermal matrix section
A thick human ADM (CGDerm Implant®, CGBio, Seongnam, Republic of Korea) was used for generating a spheroid carrier. This ADM is an acellular, sterile, and freeze-dried human dermis with a diameter of 12 mm and thickness of 3 to 4 mm. This ADM was fixed on sample stubs in one side with an optimal cutting temperature compound (OCT compound, Tissue-Tek, Torrance, CA, USA) in a cryostat (CM3050S, Leica, Wetzlar, Germany). Target ADM thickness was around 200 μm. However, considering ADM thickness variability by swelling or surface irregularity, 120 μm thick sections were made. Each thin sectioned ADM (tsADM) was put into and sealed in a sterilization pouch and then underwent ethylene oxide (EtO) sterilization (Hanshin Medical Co. Ltd. Incheon, Republic of Korea). Observations and measurements were performed with a digital microscope (AM7915MZT, Dino-Lite, New Taipei City, Taiwan) in dry state. The media-swollen tsADM thickness was measured using a confocal microscope (LSM 800, Carl Zeiss, Oberkochen, Germany) by subtracting end Z- from starting Z-positions of collagen autofluorescence. ADM micro-architecture was evaluated with microcomputed tomography (micro-CT, Skyscan 1272, Bruker, Karlsruhe, Germany). After global thresholding and removing speckles < 125 voxels, morphometric analysis was performed at the level of the sub-volume of the ADM (cylinder of 2 mm in diameter and 1 mm in height). The pixel size was 8 μm. Evaluation indices for quantitative morphometrical analysis were ADM porosity, ADM surface density, surface area, number of pores, pore diameter, and pore diameter distribution.
Cell culture and spheroid generation
Human ASCs (Lonza, Basel, Sweitzerland) were used in experiments. ASCs were cultured in ASC basal medium (Lonza) with 10% fetal bovine serum (Lonza), 1% 2 mM L-glutamine (Lonza), and 0.1% gentamicin sulfate/amphotericin-B (Lonza). Cells were cultured under standard culture conditions in a humidified atmosphere with 5% CO2 at 37 °C.
To generate spheroids, 3D cell culture dishes (Stemfit 3D®, Hanam, Republic of Korea) were used. Use of these types of dishes can generate 389 spheroids per dish. ASCs were harvested after trypsin/ethylenediaminetetraacetic acid (EDTA) treatment and collected by centrifugation at 210 g for 5 min. They were reconstituted with media and 1.2 × 106 cells in 1 ml of medium were seeded into the 3D cell culture dish after microbubble removal as per manufacturer’s instructions. After 2 days of culture, uniform spheroids can be harvested by pipetting and collected within Lo-bind tube (Eppendorf, Hamburg, Germany) for further seeding on tsADM.
Spheroids plasticity and survival in between glass coverslip
The spheroids should fit into the pores and grooves of the tsADM surface without disrupting their original structure. To evaluate spheroid plasticity, several spheroids were seeded between a round glass coverslip (#1.5, World Precision Instruments, Sarasota, FL, USA). Media was then removed so that these spheroids were compressed between the coverslip by the surface tension force of the media and Laplace pressure [27]. They were decompressed by refilling the media and underwent five compression-decompression cycles. The deformation was observed using an EVOS FL imaging system (Thermo Fisher Scientific, Waltham, MA, USA). These spheroids underwent actin green and the DNA DRAQ5 dye staining as described in Sect. 2.5 to assess spheroid shape changes after 48 h of culture in a compressed state and were observed in Z-stack image using a confocal microscope.
Cell survival was evaluated when the spheroids were compressed between coverslips. Spheroids seeded on coverslip, spheroids in-between coverslips, and spheroid-loaded tsADM in-between coverslips were compared. They were stained with the LIVE/DEAD Cell Imaging Kit (Thermo Fisher Scientific) after 48 h of culture in a compressed state and were then observed under the confocal microscope.
Loading spheroids into thin-sectioned acellular dermal matrix
A round coverslip (8 mm in diameter) was placed into the well of a non-tissue culture treated 48-well plate (Fig. 1A). An 8 mm diameter round tsADM was placed over the coverslip (Fig. 1B). tsADM was fixed at the center of the coverslip by focal wetting with a media-dipped pipet tip. To prevent spheroid damage caused by shear stress at the pipet tip during pipetting, a 1000 μL low retention pipette tip was used for spheroid transfer or handling. The harvested spheroids from two 3D cell culture dishes (2.4 × 106 cells) in Lo-bind tube were aspirated. After waiting until the spheroids had settled near the tip, they were carefully dispensed over the tsADM (Fig. 1C). After confirming the proper spheroid distribution over the tsADM, a round coverslip was applied gently over the spheroid-seeded tsADM (Fig. 1D). The top coverslip was gently pressed until it came into contact with the tsADM with the pipette tip at the outer border and center to prevent it from slightly float over the spheroids. The wells were then filled with full medium that was changed every day and cultured under standard culture conditions. Their gross morphology was recorded with a digital microscope (AM7915MZT, Dino-Lite). After 1 week of culture, the top coverslip was gently removed with a fine-tipped tweezer. This spheroid-loaded tsADM was harvested and transported with the bottom coverslip to prevent folding. For the control group, top coverslip application and compression were omitted.
Fig. 1.
Loading spheroids onto thin-sectioned acellular dermal matrices (tsADMs). A A glass coverslip was prepared as a cellular non-adhesive surface. B An 8 mm diameter round tsADM was placed over a coverslip and fixed by focal wetting with media. C Spheroids were carefully dispensed over the tsADM. D After confirming proper distribution of spheroids over the tsADM, a round coverslip was applied gently over the spheroid-seeded tsADM. Because the top-covered coverslip may float, the top coverslip was gently pressed with the pipette tip at each border and in the center until the slip came into contact with the tsADM
Imaging of the spheroid-loaded tsADM
The spheroid-loaded tsADM was fixed with 4% paraformaldehyde in 1X phosphate-buffered saline (PBS) for 25 min and then washed twice with blocking solution (1% bovine serum albumin in 1X PBS). They were permeabilized with 0.1% Triton X-100 in 1X PBS for 5 min and then washed twice. Blocking solution was applied for 40 min at room temperature (RT) and then the tsADMs were washed three times. Both the actin green (R37110, Thermo Fisher) and DRAQ5 (62,251, Thermo Fisher) reagents were applied for 30 min at RT and then the tsADMs were washed with PBS three times. To prevent crushing of the spheroid-loaded tsADM from the coverslip, four corner support was made with nail polish. The stained spheroid-loaded tsADM was then placed on the center of the slide glass and wicked for remnant liquids. After dropping antifade mounting solution (ProLong Diamond, P36965, Thermo Fisher), coverslip was mounted over the supports. The stained spheroid-loaded tsADMs were observed under confocal microscope. Because dermal collagen bundles in tsADM show autofluorescence, they were observed through the 4′,6′-diamidino-2-phenylindole (DAPI) channel.
Spheroids adhesion stability to tsADM
To evaluate stability of tsADM-loaded spheroids against shear stress, spheroid-loaded tsADM from 3- and 6-day cultures were shear-stressed with orbital shaker-generated media flow as previously reported [28]. Briefly, the spheroid-loaded tsADM was placed in a 6-well plate with 2 mL of full medium without a top coverslip. This 6-well plate was sequentially shaken using an orbital shaker at 120, 150, and 200 rpm for 5 min. After each shaking period, detached spheroids were observed and counted under a microscope (EVOS FL, Thermo Fisher). The specimen was transferred to a new well for further shaking. The percentage of detached spheroids per total loaded spheroids was evaluated.
Comparison of cell delivery efficacy by in vivo transplantation of cell-seeded tsADM versus spheroid-loaded tsADM
To generate cell-seeded tsADM, the same ASCs were cultured in T175 flasks until they reached about 80% confluency, harvested with trypsin/EDTA treatment, and collected by centrifugation at 210 g for 5 min. A round coverslip was placed into the non-tissue culture-treated 48-well plate and the tsADM was placed over the coverslip. After cell counting, 2.4 × 106 cells/ml were seeded onto the same tsADM. After 12 h of settlement, a glass coverslip was applied over the cell-seeded tsADM and then compressed. The wells were then filled up with full medium that was changed every day and cultured under standard culture conditions for 7 days.
6-week-old female BALB/c nude mice (n = 5 per group, Orientbio, Gyeonggi-do, Republic of Korea) were used for experiments. They were individually caged and fed ad libitum. All surgical experiments were performed under general anesthesia with isoflurane (Foran, Choongwae Pharmaceutical Corporation, Hwaseong, Republic of Korea). After induction of general anesthesia, the mice were placed prone on the warm operating table. Hair was removed with clipper and depilation cream (Niclean, Ildong Pharmaceutical Corporation, Seoul, Republic of Korea). Skin was cleaned with betadine and 70% ethanol and then draped with sterile surgical drape. To deliver tsADM at the mice flank, a 1.5 cm length midline incision was made with a Metzenbaum scissor at the midback and then dissected beneath the panniculus carnosus. Before delivery, cell-seeded or spheroid-loaded tsADMs were washed three times with sterile PBS and transferred into the pocket with a back of a round coverslip to prevent folding of tsADM. After transplantation of tsADM, the round coverslip was gently removed. The wound was closed with interrupted sutures with 6–0 Nylon (Ethicon, Somerville, NJ, USA). Two weeks after the surgery, mice were euthanized in CO2 chamber and then the overlying skin, transplanted tsADM, and underlying flank muscle were harvested en bloc.
Harvested tissues were fixed with 4% paraformaldehyde for 12 h at RT, embedded in paraffin using conventional methods, sectioned to a thickness of 4 μm and dehydrated with xylene and gradient alcohol. Endogenous peroxidase was blocked with Dako REAL™ peroxidase-blocking solution (Agilent, Santa Clara, CA, USA) for 5 min. Slides were treated for 10 min with 0.1 M citrate buffer (pH 6.0) for antigen retrieval and blocked with 10% normal goat serum (Jackson ImmunoResearch, West Grove, PA, USA) in 0.1 M PBS (pH 7.4) for 1 h. The slides were incubated with anti-human nuclei antibody (Abcam, Cambridge, UK) for 1 h at RT followed by a subsequent rinse with PBS and then incubated with Dako REAL™ EnVision™/HRP (Dako) at RT for 30 min. Next, 3,3′-diaminobenzidine (DAB) chromogen was used to visualize the immunohistochemical reaction after washing with PBS followed by a 5 min incubation of hematoxylin as the counterstain. After staining, the slides were dehydrated in graded alcohols, cleared in xylene, mounted in neutral balsam, and observed under an optical microscope.
To evaluate cell delivery efficacy, the number of cells with/without DAB stain were counted by manual counting with QuPath [29]. Cell counting was performed between panniculus carnosus and tsADM in which the transplanted cells were located. Results are expressed as the mean ± standard deviation (SD). Three high-power field (HPF) counts were averaged per slides (n = 4 per group) and then analyzed using the Mann–Whitney U test in SPSS (IBM, New York, NY, USA). A p-value < 0.05 indicated statistical significance between the groups.
Results
Thin dermal matrix section
The thin dermal section shows interpenetrating pores and grooves with a rough surface texture (Fig. 2A). Under a higher magnification view, interwoven collagen bundle fibers with rough, uneven surfaces can be seen (Fig. 2B). This roughness is more obvious in the oblique view in which spheroids will be fit (Fig. 2C). Because this thin section was interwoven with dermal collagen fiber naturally, it does not become brittle or tear easily when dry or wet. The average thickness of dried tsADM was 176.4 ± 14.9 μm, and media-swollen tsADM was 200.43 ± 35.87 μm (n = 5). Micro-architecture analysis of ADM using micro-CT showed 56.71% of total ADM porosity without closed pores. Both surface density and surface area were 44.84/mm and 566.04 mm2, respectively. In-depth analysis showed 324 pores with various pore diameters that were distributed inside the ADM (Fig. 2D). The mean pore diameter was 53.47 ± 33.24 μm, and median pore diameter was 104 μm. Pore diameter distribution was shown in Fig. 2E. This pore diameter distribution showed pores in ADM that are generally smaller than the average spheroid diameter. However, when ADM was analyzed in thin sections, an overall distribution of larger pores of around 200 μm in diameter was observed (Fig. 2F). Hemisphere of spheroids will fit into these larger pores in tsADM. The thin sectioning process opened the top and bottom of the interconnecting pores and could cause an increase in overall pore diameter.
Fig. 2.
Gross presentation of a thin dermal section (A, scale bar: 1 mm). High magnification view of the thin dermal section. (B, 220 × , scale bar: 200 μm). Oblique view of the thin dermal section showing porous structure (C, 70 × , scale bar: 1 μm). Micro-architecture analysis of ADM using micro-CT revealed 324 pores with various pore diameter were distributed inside the ADM (D, E). In thin section analysis, larger pores of around 200 μm in diameter were predominantly distributed. The spheroid hemispheres are expected to fit into these larger pores in tsADM (F)
Cell culture and spheroid generation
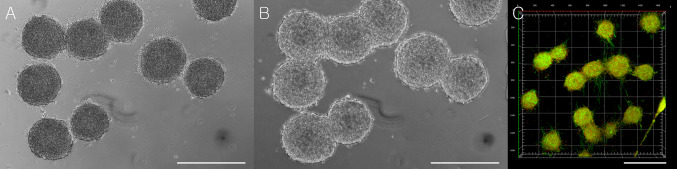
The seeded human adipose derived stem cells in the 3D cell culture dish became spheroids after 2 days of culture (Fig. 3). The average diameter of spheroids was 255.91 ± 16.87 μm (n = 7).
Fig. 3.
Spheroids were generated in the three-dimensional (3D) cell culture dish. Cells (1.2 Mio.) were seeded in the culture dish (A, scale bar: 1000 μm). After 2 days, these cells were compacted together and became spheroids in the non-adherent wells in the 3D cell culture dish (B, scale bar: 1000 μm). Higher magnification view of the spheroid (C, scale bar: 200 μm)
Spheroid plasticity and survival in between glass coverslip
The spheroids between two glass coverslips were decompressed and compressed by filling and removing media (Fig. 4A and B, respectively). Spheroids without indentation or defects maintained their original shapes after five rounds of compression and decompression, implying plasticity during compression stress. After 48 h of culture in-between the coverslips, the spheroids maintained their 3D shape between the glass coverslip without fusing with adjacent spheroids as shown in the 50 × confocal Z-stack image (Fig. 4C).
Fig. 4.
Spheroids between two glass coverslips. Spheroids are in decompressed state when the well is filled with media (A, scale bar: 500 μm). When the media was removed, the spheroids were compressed by the surface tension force of the media and Laplace pressure, resulting in an increase in the two-dimensional (2D) surface area (B, scale bar: 500 μm). The spheroids in-between the coverslip maintained their 3D shape without fusing with adjacent spheroids after 48 h in culture (C, scale bar: 500 μm)
In cell survival test, the spheroids cultured for 48 h on glass coverslips showed spheroid cell spreading on the coverslip. Dead cells (red) at the spheroid core and at the spreading cell periphery were also noted (Fig. 5A). When the spheroids were cultured between the glass coverslip for 48 h, spheroid cell spreading on the coverslip with dead cells at the spreading periphery was observed. However, the observed number of dead cells showed a decrease at the spheroid core (Fig. 5B). When the spheroids were cultured on the tsADM between the glass coverslip, they showed fewer spreading cells and almost no dead cells at the spheroid core and periphery over the tsADM except for cells on the glass through the pores of the tsADM (Fig. 5C).
Fig. 5.
Cell viability evaluation after 48 h of culture by spheroids culture condition. Spheroids cultured on the glass coverslip showed spheroids cell spreading with multiple dead cells at the spheroid core and spreading periphery (A, scale bar: 500 μm). Spheroids cultured in between the glass coverslip showed spheroid cell spreading with multiple dead cells at the spreading periphery. However, fewer dead cells at the spheroids core than cultured on the glass coverslip were noted (B, scale bar: 500 μm). Spheroid-loaded tsADM in-between the coverslip showed more viable cells containing spheroids except for local dead cells at the tsADM pore (C, Scale bar: 500 μm)
Loading spheroids into thin-sectioned acellular dermal matrix
When spheroids were loaded onto the tsADM without a top coverslip, they lost their 3D structure within a few days because the spheroid-containing cells were spread into the porous tsADM. This spreading occurred because spheroids formed spontaneously in areas in which cell–cell interactions dominate over cell-substrate interactions. After 2 weeks in culture, tsADM became opaque as the cell population increased and contracted slightly (Fig. 6 top row). On the other hand, the spheroids loaded on the tsADM with a top glass coverslip maintained their spheroid outline. Pellet formation from remnant spheroids was noted on the eighth and 14th days in culture (Fig. 6 middle row). When the top coverslip was removed on the seventh day, the spheroid outlines became vague on the 14th day compared to the outlines on the eighth day, suggesting a role of the coverslip in preventing spheroid spreading (Fig. 6 bottom row).
Fig. 6.
Spheroids seeded onto the tsADM without a top coverslip lost their 3D structure because of the spreading of spheroid-containing cells into the tsADM in which cell-to-substrate interactions are stronger than cell-to-cell interactions. After 14 days in culture, tsADM became opaque as the cell population increased and contracted slightly (top row). Spheroid-loaded tsADM with a top coverslip retained a spheroid outline during the 14 days in culture. A pellet outside of the tsADM on the eighth and 14th days in culture was noted (middle row). When the top coverslip was removed on the seventh day in culture, the spheroid outline became vague on the 14th day compared to the outline on the eighth day, suggesting a role of the coverslip in preventing spheroid spreading (bottom row). Scale Bar: 2 mm
Imaging of the spheroid-loaded tsADM
The spheroid-loaded tsADMs without and with top coverslips were imaged with the tile scan of confocal microscope after 14 days in culture. The spheroid-loaded tsADM without a top coverslip showed ASCs spreading on the tsADM with multifocal, ill-defined cell clusters (Fig. 7A). However, in spheroid-loaded tsADM with a top coverslip, a well-retained spheroid shape and its distribution along the collagen bundles of tsADM was seen (Fig. 7B). This also suggests a role of the coverslip in maintaining the spheroid shape. Under high magnification, the z-stack view of the spheroid-loaded tsADM, spheroids were anchored into the collagen bundle with basal cell adhesion to tsADM thus preserving its 3D shape (Fig. 7C).
Fig. 7.
Tile scan of the spheroid-loaded tsADMs without and with a top coverslip. Without top coverslip, spheroids were spread out and resulted in ill-defined multifocal cell clusters (A, scale bar: 500 μm). However, with a top coverslip, spheroids maintained their original shapes and distributions along the collagen bundles of tsADM after 14 days in culture (B, scale bar: 500 μm). Under the high magnification z-stack view, spheroids were anchored into the collagen bundle with basal cell adhesion to tsADM while maintaining their 3D shapes (C, scale bar: 200 μm)
Spheroids adhesion stability to tsADM
The detached percentage of spheroids after each orbital shaking session at 120, 150, and 200 rpm were evaluated for the 3- and 6-day cultures of spheroid-loaded tsADMs with a top coverslip (n = 4). After 3 days in culture, the average 10.82 (versus 0.99), 1.91 (versus 1.98), and 6.36 (versus 7.92) percent of detached spheroids were noted at 120, 150, and 200 rpm, respectively, in comparison to 6 days of culture specimen (Fig. 8). After 6 days in culture, a 42.98% decrease in spheroid detachment (10.89% vs. 19.1%) compared to 3 days in culture after sequential orbital shaking was noted. At 120 rpm of orbital shaking on the third day in culture, 10.82% of the spheroids were detached from the tsADM. This value notably decreased to 0.99% on the sixth day in culture. Spheroid detachment at higher rpm on three and six days in culture showed similar percentages between the group. These results indicated that spheroids attachments are more stable after 6 days in culture than after 3 days in culture.
Fig. 8.

Detachment percentage of spheroids after each orbital shaking at 120, 150, and 200 rpm were evaluated with three and 6 days in culture of spheroid-loaded tsADM with a top coverslip (n = 4). After 6 days in culture, the percentage of detached spheroids from tsADM decreased by 42.98% (from 19.1% to 10.89%) compared to 3 days in culture, suggesting more stable spheroid attachment to tsADM after 6 days in culture than after 3 days in culture
Comparison of cell delivery efficacy by in vivo transplantation of cell-seeded tsADM versus spheroid-loaded tsADM
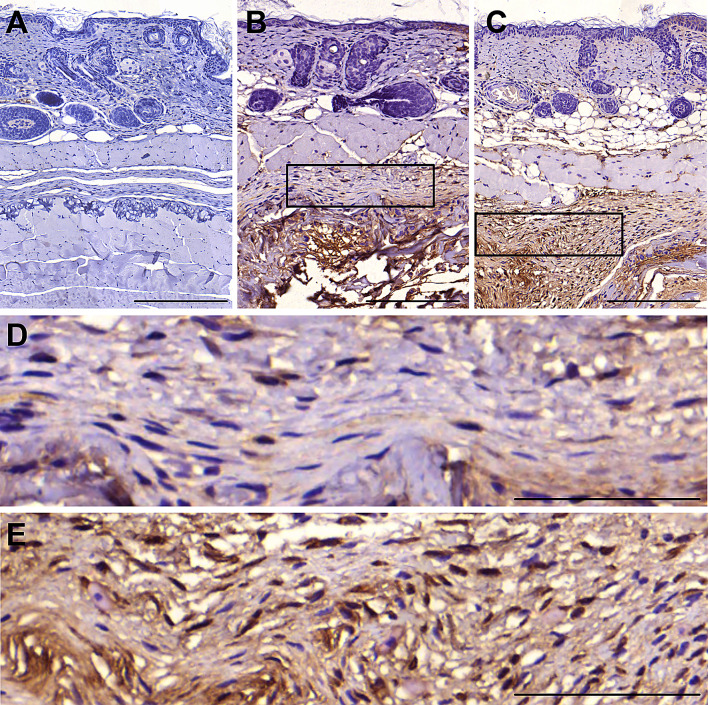
After 14 days of transplantation in nude mice (n = 5 per group), we evaluated human nuclei-positive cells to determine local in vivo transplantation efficacy of ASCs on tsADM. In the cell-seeded and spheroid-loaded tsADMs, human nuclei-positive ASCs were observed in between the panniculus carnosus and underlying muscle (Fig. 9). Immunohistochemically, spheroid-loaded tsADM showed a great increased in the number of human nuclei -positive cells compared with that in the cell-seeded tsADM. There was no difference in average number of total counted cells between cell seeded and spheroids loaded group (n = 4 per group, 293.33 ± 77.6 vs. 280.58 ± 42.34, Fig. 10A). The human nuclei-positive cell per total cell count ratio of the spheroid-loaded tsADM was about 2.96-fold higher with statistical significance than that of the cell-seeded tsADM (55.02% ± 5.33% vs. 18.60% ± 6.64%, Fig. 10B). These findings suggest a greater efficacy of the spheroid-loaded tsADM for cell transplantation than cell-seeded tsADM.
Fig. 9.
Histology of an en bloc section of skin, tsADM and underlying muscle with 3′,3′-diaminobenzidine (DAB) stain. The sham control showed an intact layer between the panniculus carnosus and underlying muscle without DAB signal (A, scale bar: 100 μm). Cell-seeded tsADM showed a stacked layer of transferred cells with DAB-positive staining against anti-human nucleic antibody, suggesting survival of human-origin ASCs beneath the pannuculus carnosus (B, D: higher magnification, scale bar: 100 μm). Spheroid-loaded tsADM showed a much thicker cell construct than cell-seeded tsADM (C, scale bar: 100 μm). The higher magnification view showed a denser and higher population of DAB-positive cells, indicating higher survival and transplantation efficacy of human-origin ASCs than cell-seeded tsADM (E, scale bar: 100 μm)
Fig. 10.
Cell counting results comparing cell-seeded and spheroid-loaded tsADMs. The average total cell count under three high-power field showed minimal differences between the group (293.33 ± 77.6 vs. 280.58 ± 42.34, A). However, the average percentage of human nuclei positive cells per total cell count showed statistically significant differences between the group, indicating an approximately 2.96-fold higher human ASC transplantation efficacy of spheroid-loaded rather than cell-seeded tsADM (B, 55.02% ± 5.33% vs. 18.60% ± 6.64%, n = 4) after 2 weeks of transplantation. Data are expressed as mean ± SD. *p < 0.05
Discussion
When delivering large number of cells into the body, diffusion limitation allows only cells within 100 to 200 μm from the nearest capillary to survive [28]. Although there are numerous studies of engineered vascularized tissue construct, further studies are required to translate them into the clinical application. Because spheroids of 200 to 300 μm in diameter can survive without vasculature, spheroids can be used for the volumetric cell transfer. Spheroids can prevent cell damage from enzymatic detachment of cells from culture dish, increase cell delivery capacity, be preconditioned in hypoxia, and preserve extracellular matrix (ECM) and cellular functions [31]. Moreover, they have shown great potential as a miniature of tissue construct with component cell capabilities [28] and currently are widely used in cancer differentiation, drug screening [32], organoids [33], and other functional studies that cannot be studied under conventional two-dimensional (2D) culture conditions [34]. However, handling of spheroids is not easy. Spheroid formation occurs spontaneously in the environments in which cell–cell interactions dominate over cell-substrate interactions [30]. Therefore, when spheroids come into contact with each other or with the cell adhesive surface, such as tissue culture plastic or ECM, spheroids become fused with each other and form cellular clumps or cells in spheroids spread to the cell adhesive surface, finally lose their 3D structure [35]. Because of these characteristics, spheroids are loaded into a hydrogel carrier matrix for collection and transport. This hydrogel construct is easy to displace with external force or to run down with motion. These two parameters will be the reason why spheroids are not primarily adopted for in vivo applications but are used for in vitro functional studies.
To overcome these problems, the authors embedded multiple spheroids into the pores and grooves of the tsADM in a manner similar to eggs in an egg carton. Because dermal collagen bundles with connecting fibers align with Langer lines [36], the authors used these natural bundle alignments and grooves for spheroid fitting by thin sectioning of dried ADM. Micro-architectural analysis also revealed distribution of larger pores in ADM sections in which the hemisphere of the spheroid will fit. To minimize diffusion distance and lessen the binding of secreted biological molecules to the supporting material, a hemisphere of the spheroid is exposed to the surrounding environment, while the opposite hemisphere is incorporated into a thin-sliced allogenic dermal collagen matrix that supports spheroid structure with minimal immunogenicity. In this way, multiple spheroids can be transferred and fixed with sutures at the target tissue without vasculature. This tsADM has clinically proven its efficacy to take at the recipient site with split-thickness skin as a graft without pre-vascularization. Moreover, tsADM has a naturally intertwined collagen bundle structure and is strong enough to sustain suture fixation. However, when spheroids are transferred onto the tsADM surface, they lose their volumetric structure because of the characteristics of the spheroids. Cells on spheroids spread out and attach to collagen bundles of the tsADM. To overcome these problems, the authors developed a novel approach by providing a weakly cellular adhesive surface to the spheroid so that it could maintain adhesion on the top of the spheroids and not to spread out to the bottom of the collagen bundles while allowing bottom cell adhesion to surrounding collagen bundles. The authors also tested spheroids plasticity by showing their adaptation to compression and survival between coverslips. In this way, spheroids can be attached to tsADM firmly while maintaining their spheroid shapes without spreading out. This top adhesion of the spheroid to a weakly adhesive surface (a coverslip) allows for the maintenance of the spheroid shape for 2 weeks in culture. When the top coverslip is removed after a week in culture, cells in spheroids spread out as expected. This process shows the role of coverslip in maintaining spheroid shape by allowing weak adhesion. On the other hand, the bottoms of the spheroids are stable after 6 days in culture rather than at the 3-day time point according to the shear stress test using the orbital shaker. Because this tsADM construct will be transplanted surgically, withstanding shear stress between dissected planes until the wound heals is important. These results suggest spheroids can be robustly delivered as clinically intended, unlike unstable liquid or hydrogel-based transfer. This delivery is further detailed in in vivo experiments. Although the tsADM construct was implanted at the flank of nude mouse in which continuous motion occurs, it was not displaced or folded in the pocket. Transplanted ASCs were 2.95-fold more than conventional cell-seeded tsADM method after 2 weeks of transplantation. These results are striking compared to previous studies of ASC transplantation survival rates. Human ASCs injected in cell suspensions or spheroids were undetectable at the 3-week time point after transplantation into nude mice [28]. ASC-seeded ADM showed stem cell engraftment and local differentiation in a murine skin injury model. However, in this model, the number of delivered cell was not measured and only showed several engrafted cells [13].
In previous studies, the 3D arrangement of cells show an increase in cell-to-cell interactions and renders better outcomes in in vivo environments [37, 38]. It has been suggested that ASC spheroid cultures can enhance the expressions of angiogenic, anti-apoptotic, anti-inflammatory molecule secretions, hypoxia-resistant proteins, and the preservation of ECM components [39–41]. These results indicate that ASC spheroids could promote cell proliferation and increased their therapeutic potentials.
The results of our study confirm that spheroids can be loaded firmly onto tsADMs while maintaining their spheroid shape without spreading out. This shape maintenance leads to enhancement of ASC survival in in vivo transplantation. However, when ASC spheroids are transplanted with tsADM carriers in bulk, the above-mentioned molecular signaling that mediates the enhanced ASC spheroid properties or leads to improvement in cell survival may be different from the signaling associated with single spheroids. Therefore, further analysis is needed at the molecular level to better understand and optimize ASC spheroids for clinical applications. One limitation of this study is the absence of a comparison of spheroid loading to other biomaterials versus tsADM. The distinctive collagen bundle architecture of tsADM may have a role when firmly loading spheroids onto tsADM. This type of loading possibility can be proven using other biomaterials with surface texturing. Then spheroid-loading and in vivo delivery can be compared to other biomaterials. Future efforts to deploy spheroids in vivo should consider carriers modifications in order to regulate survival, angiogenesis, and tissue regeneration. Moreover, spheroids of other cell types and drug- or nanoparticle-tagged spheroids on tsADM carriers also need to be evaluated further for versatile clinical applications.
This study showed a novel spheroid-loaded thin-sectioned human ADM carrier for surgical cell delivery. The study also presented a spheroid shape maintaining method in loading multiple spheroids onto cell-adhesive surfaces. This carrier delivers more viable cells to target tissues than conventional cell-seeded ADM constructs without displacement in mobile parts of mouse. Because of the simple methods in generating robust allogenic carriers and possibility of loading spheroids with numerous cell type combination, this carrier could be used for cell delivering patch for clinical application with ease.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2017R1C1B5017905). This research was supported by a Grant of Translational R&D Project through Institute for Bio-Medical convergence, Incheon St. Mary’s Hospital, The Catholic University of Korea
Author contributions
JHK performed the experiments, analyzed the data and co-wrote the paper. JYL conceived of the presented idea, designed the study, performed the experiments, analyzed the data and wrote the manuscript.
Compliance with ethical standards
Conflicts of Interest
The authors declare that they have no conflict of interest.
Ethical statement
All in vivo experiments were carried out in accordance with the Institutional Animal Care and Use Committee (IACUC) of Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea (IACUC Approval No. 2019-008).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/28/2020
Unfortunately, the online published article has error in figure��2 caption.
References
- 1.Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999;283:534–537. doi: 10.1126/science.283.5401.534. [DOI] [PubMed] [Google Scholar]
- 2.Galli R, Borello U, Gritti A, Minasi MG, Bjornson C, Coletta M, et al. Skeletal myogenic potential of human and mouse neural stem cells. Nat Neurosci. 2000;3:986–991. doi: 10.1038/79924. [DOI] [PubMed] [Google Scholar]
- 3.Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 4.Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 5.Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 6.Rigotti G, Marchi A, Galiè M, Baroni G, Benati D, Krampera M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409–1422. doi: 10.1097/01.prs.0000256047.47909.71. [DOI] [PubMed] [Google Scholar]
- 7.Burst VR, Gillis M, Pütsch F, Herzog R, Fischer JH, Heid P, et al. Poor cell survival limits the beneficial impact of mesenchymal stem cell transplantation on acute kidney injury. Nephron Exp Nephrol. 2010;114:e107–e116. doi: 10.1159/000262318. [DOI] [PubMed] [Google Scholar]
- 8.Wu KH, Mo XM, Han ZC, Zhou B. Stem cell engraftment and survival in the ischemic heart. Ann Thorac Surg. 2011;92:1917–1925. doi: 10.1016/j.athoracsur.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Hicks AU, Lappalainen RS, Narkilahti S, Suuronen R, Corbett D, Sivenius J, et al. Transplantation of human embryonic stem cell-derived neural precursor cells and enriched environment after cortical stroke in rats: cell survival and functional recovery. Eur J Neurosci. 2009;29:562–574. doi: 10.1111/j.1460-9568.2008.06599.x. [DOI] [PubMed] [Google Scholar]
- 10.Voegele TJ, Voegele-Kadletz M, Esposito V, Macfelda K, Oberndorfer U, Vecsei V, et al. The effect of different isolation techniques on human osteoblast-like cell growth. Anticancer Res. 2000;20:3575–3581. [PubMed] [Google Scholar]
- 11.Aguado BA, Mulyasasmita W, Su J, Lampe KJ, Heilshorn SC. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng Part A. 2012;18:806–815. doi: 10.1089/ten.tea.2011.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendez I, Hong M, Smith S, Dagher A, Desrosiers J. Neural transplantation cannula and microinjector system: experimental and clinical experience. J Neurosurg. 2000;92:493–499. doi: 10.3171/jns.2000.92.3.0493. [DOI] [PubMed] [Google Scholar]
- 13.Altman A, Matthias N, Yan Y, Song YH, Bai X, Chiu E, et al. Dermal matrix as a carrier for in vivo delivery of human adipose-derived stem cells. Biomaterials. 2008;29:1431–1442. doi: 10.1016/j.biomaterials.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 14.Gilmore AP. Anoikis. Cell Death Differ. 2005;12:S1473–S1477. doi: 10.1038/sj.cdd.4401723. [DOI] [PubMed] [Google Scholar]
- 15.Teng CJ, Luo J, Chiu RC, Shum-Tim D. Massive mechanical loss of microspheres with direct intramyocardial injection in the beating heart: implications for cellular cardiomyoplasty. J Thorac Cardiovasc Surg. 2006;132:628–632. doi: 10.1016/j.jtcvs.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 16.Lindvall O, Björklund A. Cell therapy in Parkinson’s disease. NeuroRx. 2004;1:382–393. doi: 10.1602/neurorx.1.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orbay H, Takami Y, Hyakusoku H, Mizuno H. Acellular dermal matrix seeded with adipose-derived stem cells as a subcutaneous implant. Aesthetic Plast Surg. 2011;35:756–763. doi: 10.1007/s00266-011-9683-2. [DOI] [PubMed] [Google Scholar]
- 18.Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003;139:510–516. doi: 10.1001/archderm.139.4.510. [DOI] [PubMed] [Google Scholar]
- 19.Ducheyne P, Healy K, Hutmacher DW, Grainger DW, Kirkpatrick CJ. Comprehensive biomaterials. Amsterdam: Elsevier; 2015. [Google Scholar]
- 20.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moku G, Layek B, Trautman L, Putnam S, Panyam J, Prabha S. Improving payload capacity and anti-tumor efficacy of mesenchymal stem cells using TAT peptide functionalized polymeric nanoparticles. Cancers (Basel) 2019;11:E491. doi: 10.3390/cancers11040491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns. 1995;21:243–248. doi: 10.1016/0305-4179(95)93866-i. [DOI] [PubMed] [Google Scholar]
- 23.Doornaert M, Depypere B, Creytens D, Declercq H, Taminau J, Lemeire K, et al. Human decellularized dermal matrix seeded with adipose-derived stem cells enhances wound healing in a murine model: Experimental study. Ann Med Surg (Lond) 2019;46:4–11. doi: 10.1016/j.amsu.2019.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garg A, Houlihan DD, Aldridge V, Suresh S, Li KK, King AL, et al. Non-enzymatic dissociation of human mesenchymal stromal cells improves chemokine-dependent migration and maintains immunosuppressive function. Cytotherapy. 2014;16:545–559. doi: 10.1016/j.jcyt.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Okano T, Yamada N, Sakai H, Sakurai Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide) J Biomed Mater Res. 1993;27:1243–1251. doi: 10.1002/jbm.820271005. [DOI] [PubMed] [Google Scholar]
- 26.Yamato M, Utsumi M, Kushida A, Konno C, Kikuchi A, Okano T. Thermo-responsive culture dishes allow the intact harvest of multilayered keratinocyte sheets without dispase by reducing temperature. Tissue Eng. 2001;7:473–480. doi: 10.1089/10763270152436517. [DOI] [PubMed] [Google Scholar]
- 27.Cheng S, Robbins MO. Nanocapillary adhesion between parallel plates. Langmuir. 2016;32:7788–7795. doi: 10.1021/acs.langmuir.6b02024. [DOI] [PubMed] [Google Scholar]
- 28.Agrawal H, Shang H, Sattah AP, Yang N, Peirce SM, Katz AJ. Human adipose-derived stromal/stem cells demonstrate short-lived persistence after implantation in both an immunocompetent and an immunocompromised murine model. Stem Cell Res Ther. 2014;5:142. doi: 10.1186/scrt532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, et al. QuPath: open source software for digital pathology image analysis. Sci Rep. 2017;7:16878. doi: 10.1038/s41598-017-17204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehta G, Hsiao AY, Ingram M, Luker GD, Takayama S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J Control Release. 2012;164:192–204. doi: 10.1016/j.jconrel.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhang S, Cho SW, La WG, Lee TJ, Yang HS, Sun AY, et al. Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials. 2011;32:2734–2747. doi: 10.1016/j.biomaterials.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 32.Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA. Spheroid-based drug screen: considerations and practical approach. Nat Protoc. 2009;4:309–324. doi: 10.1038/nprot.2008.226. [DOI] [PubMed] [Google Scholar]
- 33.Freedman BS, Brooks CR, Lam AQ, Fu H, Morizane R, Agrawal V, et al. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun. 2015;6:8715. doi: 10.1038/ncomms9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von der Mark K, Gauss V, von der Mark H, Müller P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature. 1977;267:531–532. doi: 10.1038/267531a0. [DOI] [PubMed] [Google Scholar]
- 35.Jakab K, Neagu A, Mironov V, Markwald RR, Forgacs G. Engineering biological structures of prescribed shape using self-assembling multicellular systems. Proc Natl Acad Sci U S A. 2004;101:2864–2869. doi: 10.1073/pnas.0400164101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ní Annaidh A, Bruyère K, Destrade M, Gilchrist MD, Maurini C, Otténio M, et al. Automated estimation of collagen fibre dispersion in the dermis and its contribution to the anisotropic behaviour of skin. Ann Biomed Eng. 2012;40:1666–1678. doi: 10.1007/s10439-012-0542-3. [DOI] [PubMed] [Google Scholar]
- 37.Hong JK, Yun J, Kim H, Kwon SM. Three-dimensional culture of mesenchymal stem cells. Tissue Eng Regen Med. 2015;12:211–221. [Google Scholar]
- 38.Jeon JH, Yun BG, Lim MJ, Kim SJ, Lim MH, Lim JY, et al. Rapid cartilage regeneration of spheroids composed of human nasal septum-derived chondrocyte in rat osteochondral defect model. Tissue Eng Regen Med. 2020;17:81–90. doi: 10.1007/s13770-019-00231-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park IS, Chung PS, Ahn JC. Enhancement of ischemic wound healing by spheroid grafting of human adipose-derived stem cells treated with low-level light irradiation. PLoS One. 2015;10:e0122776. doi: 10.1371/journal.pone.0122776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng NC, Chen SY, Li JR, Young TH. Short-term spheroid formation enhances the regenerative capacity of adipose-derived stem cells by promoting stemness, angiogenesis, and chemotaxis. Stem Cells Transl Med. 2013;2:584–594. doi: 10.5966/sctm.2013-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng NC, Wang S, Young TH. The influence of spheroid formation of human adipose-derived stem cells on chitosan films on stemness and differentiation capabilities. Biomaterials. 2012;33:1748–1758. doi: 10.1016/j.biomaterials.2011.11.049. [DOI] [PubMed] [Google Scholar]